Abstract
AIM
To compare the intraoperative performance and postoperative outcome after microcoaxial torsional phacoemulsification using either a Kelman or balanced phaco tip.
METHODS
Cataracts were treated using 2.2 mm microcoaxial torsional phacoemulsification using either a 45-degree mini-flared Kelman® or a 45-degree Intrepid® Balanced phaco tip. Intraoperative measurements included total ultrasound (US) time, cumulative dissipated energy (CDE), torsional US time, and balanced salt solution (BSS) use. The central endothelial cell density (ECD) and central corneal thickness (CCT) were evaluated preoperatively and postoperatively 1, 7, and 30d after surgery using noncontact specular microscopy.
RESULTS
The 116 enrolled eyes (116 patients) were divided equally between the Kelman and balanced tip groups. Intraoperative measurements showed significantly less total US time, torsional US time, CDE, and BSS use in the balanced group than in Kelman group (P<0.05). The total US time, torsional US time, CDE, and BSS use were 17.45±14.53s, 16.63±13.97s, 6.38±5.26, and 48.21±17.21 mL in the Kelman group and 11.39 ± 9.60s, 10.90 ± 9.25s, 4.04 ± 3.42, and 41.36 ± 12.70 mL in the balanced group, respectively.
CONCLUSION
Torsional phacoemulsification performed with a balanced tip provided more effective lens removal with less total US time, torsional time, CDE, and BSS use, as well as similar changes in ECD with a Kelman tip in all cataract grades. This special designed phaco tip for torsional phacoemulsification provides an alternative phaco tip for many surgeons' preference with straight phaco tip.
Keywords: phaco tip, torsional phacoemulsification, Kelman phaco tip, balanced phaco tip
INTRODUCTION
The development of new technologies and techniques in cataract surgery have focused on reducing incision size, phacoemulsification energy, and endothelial cell loss while increasing efficiency[1]–[4]. A relatively new cataract removal modality, torsional mode phacoemulsification, uses OZil® Intelligent Phaco software (Infiniti and Centurion platforms, Alcon) and a torsional handpiece that produces side to side rotary oscillations of the phaco tip. Though the phaco tip moves at a lower resonant frequency than those used in longitudinal phacoemulsification, the side to side oscillations shear material from the lens with less repulsion and cuts with both directions of tip movement, thereby not compromising its emulsifying efficiency[1]–[6].
These phaco platforms also offer pulse, burst, and continuous modes through a tip with a bent design. The software delivers the alternating torsional and longitudinal pulses after a preset maximum vacuum level is reached, which dramatically reduces tip clogging during emulsification of hard cataracts. There is a need for tip configurations other than the flared or mini-flared designs. These modifications could lead to increased safety and efficiency of torsional technology in hard cataracts[7],[8].
The phaco tip used in torsional phacoemulsification includes a shaft and a cutting edge that has one or two bends. The small-angle rotational movement of the shaft is translated into a horizontal stroke at the tip of the needle. Stroke amplification depends on the angle of the bend near the distal end of the needle and the length of the shaft beyond the bend[9],[10]. Consequently, the geometric configuration of the tip is expected to be an important factor in the procedure's efficiency and surgical outcome.
We designed a study to compare the intraoperative performance and postoperative outcomes after microcoaxial torsional phacoemulsification using either the Kelman® phaco tip or Intrepid® Balanced phaco tip because theoretically, the efficiency of torsional phacoemulsification is influenced by the tip's design.
SUBJECTS AND METHODS
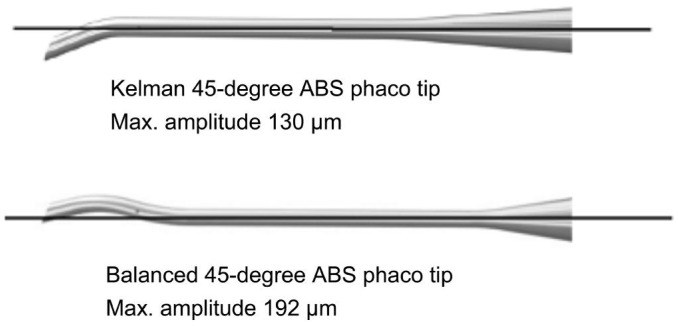
This prospective randomized comparative study was performed at Eye Clinic, Kayseri Training and Research Hospital, Kayseri, Turkey from March to June 2014. The study protocol was approved by the institutional review board and performed according to the Declaration of Helsinki. Written informed consent for participation in the study was obtained from each patient. Patients who applied to the clinic in related with diagnosis of age-related cataracts were enrolled in the study. Exclusion criteria were previous ocular trauma or intraocular surgery, intraocular inflammation, preoperative anterior chamber depth (ACD) less than 2.5 mm centrally, preoperative pupil dilation less than 4 mm after dilation, any corneal pathology, or any condition that impeded corneal evaluation by specular microscopy and pachymetry on follow-up. Patients were randomly assigned to surgery groups after they provided informed consent. All patients underwent a complete ophthalmic examination. A Pentacam nucleus staging (PNS) system was used for nucleus grading[11]–[13]. Equal numbers of eyes were allocated between the groups for each nucleus density grade. Phacoemulsification was performed with a quick chop technique using an Alcon OZil IP system and 0.9 mm mini-flared 45-degree Kelman® aspiration bypass system (ABS) phaco tip or 0.9 mm 45-degree Intrepid® balanced ABS phaco tip. The 2 tips have similar angles at the aperture of the tips but vary in configuration. In both tips, the outer and inner diameters were 0.9 mm and 0.8 mm, respectively (Figure 1). The same energy and fluid settings were used to compare the intraoperative performance and postoperative outcomes after microcoaxial torsional phacoemulsification in all patients.
Figure 1. Configuration of 45 degree balanced and Kelman phaco tip and their maximum amplitudes.
In the quick chop technique, the torsional mode was adjusted as follows: torsional amplitude, linear 85% pulse mode (70% power on ); vaccum limit, 450 mm Hg linear; aspiration flow rate, 32 mL/min fixed with dynamic rise at +1; and bottle height at 110 cm. All surgery was performed under topical anesthesia (proparacaine hydrochloride 0.5%) by the same experienced surgeon (Demircan S). A clear corneal incision was made on the steep axis with the Intrepid® ClearCut 2.2 mm dual-bevel metal keratome blade (Alcon Surgical, Inc.). Viscoat (sodium hyaluronate 3.0%, chondroitin sulfate 4.0%) was used to reform and stabilize the anterior chamber and protect the corneal endothelium. Trypan blue 0.4% was used to improve visualization of the capsule in eyes with dense cataracts. A 5.0 to 5.5 mm continuous curvilinear capsulorhexis was made using an ultrata capsulorhexis forceps (Katena, USA). Intraocular lenses (Acrysof SA60AT, Alcon) were inserted into capsular bags using an injector system (MonarchIII D-Catridge) and sodium hyaluronate 1.0% (Provisc, Alcon). The ocular viscoelastic device was removed from the anterior chamber and the capsular bag using the I/A system. For endophthalmitis prophylaxis, 0.1 mL moxifloxacin ophthalmic solution 0.5% (Vigamox) was injected into the anterior chamber after closure of the port incisions by stromal hydration using a balanced salt solution (BSS). After surgery, all patients used topical prednisolone acetate 1.0% and moxifloxacin 0.5% 6 times daily for 4wk. Intraocular pressure was measured using a noncontact applanation tonometer (NT-510, Nidek). In addition, the central corneal thickness (CCT) was measured using a Pentacam Scheimpflug camera preoperatively and postoperatively 1, 7, and 30d after surgery.
The central endothelial cell density (ECD), polymegathism (coefficient of variation; CV), and pleomorphism (percentage of hexagonal cells) were evaluated preoperatively and postoperatively 1, 7, and 30d after surgery using noncontact specular microscopy (SP 3000P, Topcon). More than 75 endothelial cells per eye were used to calculate the ECD, CV, and percentage hexagonalty with the IMAGEnet i-base imaging system (software version 3.5.6, Topcon). During each visit, 3 photographs of each cornea were taken and analyzed indepedently by another ophthalmologist. The mean of the 3 readings was calculated and used as the final reading for each visit. The percent change in ECD, CCT, and intraocular pressure at each visit were calculated.
The intraoperative parameters total ultrasound (US) time, cumulative dissipated energy (CDE), torsional time, and BSS (Alcon Surgical, Inc.) use were automatically calculated and displayed on the Infiniti OZil IP phacoemulsification system monitor.
Statistical Analysis
Data normality was assessed using the Shapiro-Wilk test, histograms, and q-q plots. Differences between groups were tested using the Chi-squared test for categorical variables and independent samples and the t-test for continuous variables. To compare continuous variables in each group over time, one way repeated measure analysis of variance was used followed by a Bonferroni correction. Values are expressed as n% or mean±SD. Analyses were conducted using SPSS for Windows (Version 16.0, SPSS, Inc.). Statistical significance was at P <0.05.
RESULTS
We enrolled 116 eyes (116 patients) and assigned half to the Kelman group and half to the balanced group. We used the 45-degree mini-flared Kelman® tip on the former and the 45-degree Intrepid® Balanced tip on the latter. Table 1 shows patient characteristics and intraoperative parameters. There were no statistically significant differences in age, sex, cataract density, pupil size, or ACD between the groups. However, intraoperative measurements showed significantly less total US time, torsional US time, CDE, and BSS use in the balanced group than in the Kelman group (P<0.05). The respective values were 17.45±14.53s, 16.63±13.97s, 6.38±5.26, and 48.21±17.21 mL in the Kelman group and 11.39±9.60s, 10.90±9.25s, 4.04±3.42, and 41.36±12.70 mL in the balanced group. Table 2 shows the nucleus density grades in each group. The total US time, torsional US time, and CDE were significantly lower in the balanced group than in the Kelman group for all PNS grades. However, estimated fluid used was lower in the balanced group only for PNS grade 4 (P =0.013). The mean ECD loss was 8.60% in the Kelman group and 14.82% in the balanced group 30d after surgery. The percent change in ECD was higher in the balanced group than in the Kelman group throughout the follow up period. However, it was not reached statistically significant (P>0.05). Clear ECD measurements could not be obtained in 2 patients in the Kelman group and 8 patients in balanced group 1d postoperatively. There was a relative decrease in ECD at 1 and 7d postoperative that can be related to the difficulty in measuring ECD caused by greater corneal edema in both groups. At 1 and 7d after surgery, the percentage change in CCT was higher in the balanced group (P≤0.015). At 30d, there was no difference in CCT between the groups (P>0.05; Table 3). No patient suffered intraoperative complications or postoperatively complications, such as synechiae, fibrin formation, or endophthalmitis.
Table 1. Comparison of patient characteristics and intraoperative parameters.
| Variables | Kelman (n=58) | Balanced (n=58) | P |
| Age (a) | 67.00±8.95 | 68.40±10.04 | 0.459 |
| Gender (F/M) | 25(43.1)/33 (56.9) | 26(44.8)/32 (55.2) | 0.825 |
| Eye (right/left) | 31(53.4)/27 (46.6) | 29(50.0)/29 (50.0) | 0.662 |
| Anterior chamber depth (mm) | 3.19±0.34 | 3.11±0.37 | 0.336 |
| Pupil size (mm) | 7.76±1.43 | 7.72±1.51 | 0.885 |
| Total ultrasound time (s) | 17.45±14.53 | 11.39±9.60 | 0.014 |
| Torsional ultrasound time (s) | 16.63±13.97 | 10.90±9.25 | 0.016 |
| Cumulative dissipated energy | 6.38±5.26 | 4.04±3.42 | 0.009 |
| Estimated fluid use (mL) | 48.21±17.21 | 41.36±12.70 | 0.024 |
n (%), x±s
Table 2. Comparison of intraoperative parameters in PNS groups.
| Variables | PNS 2 |
PNS 3 |
PNS 4 |
||||||
| Kelman (n=12) | Balanced (n=12) | P | Kelman (n=30) | Balanced (n=30) | P | Kelman (n=16) | Balanced (n=16) | P | |
| Total U/S time (s) | 9.61±7.80 | 3.48±2.79 | 0.021 | 12.15±6.30 | 8.36±4.86 | 0.015 | 37.85±14.93 | 20.72±11.42 | 0.002 |
| Torsional U/S time (s) | 8.83±6.90 | 3.41±2.77 | 0.024 | 11.58±11.58 | 7.95±4.62 | 0.013 | 36.44±14.60 | 19.90±11.07 | 0.003 |
| Cumulative dissipated energy | 3.31±2.63 | 1.22±0.96 | 0.021 | 4.70±2.84 | 2.95±1.76 | 0.008 | 13.47±5.39 | 7.37±4.03 | 0.002 |
| Estimated fluid use (mL) | 39.46±13.62 | 36.28±11.62 | 0.609 | 45.59±14.04 | 39.46±11.86 | 0.086 | 63.58±18.41 | 47.26±13.31 | 0.013 |
Grading by PNS groups: PNS 2, PNS 3, PNS 4. PNS: Pentacam nucleus staging.
x±s
Table 3. Comparison of ECD, CCT and percentage differences in ECD and CCT between groups over time.
| Variables | Phaco tip |
P | |
| Kelman (n=58) | Balanced (n=58) | ||
| ECD | |||
| Preoperative | 2462±345 | 2390±369 | 0.322 |
| Postoperative 1d | 2307±437 | 2149±412 | 0.090 |
| Postoperative 7d | 2222±438 | 2023±476 | 0.058 |
| Postoperative 30d | 2236±418 | 2007±463 | 0.019 |
| Δ0_1 | -0.06±0.13 | -0.09±0.14 | 0.281 |
| Δ0_7 | -0.08±0.16 | -0.15±0.17 | 0.063 |
| Δ0_30 | -0.09±0.12 | -0.15±0.18 | 0.067 |
| CCT | |||
| Preoperative | 542±30 | 543±36 | 0.871 |
| Postoperative 1d | 615±96 | 734±142 | 0.0001 |
| Postoperative 7d | 571±40 | 590±65 | 0.147 |
| Postoperative 30d | 547±33 | 549±42 | 0.838 |
| Δ0_1 | 0.13±0.18 | 0.31±0.24 | 0.0001 |
| Δ0_7 | 0.05±0.06 | 0.09±0.08 | 0.015 |
| Δ0_30 | 0.01±0.02 | 0.01±0.03 | 0.622 |
ECD: Endothelial cell density; CCT: Central corneal thickness.
x±s
DISCUSSION
Balanced phaco tips have not been sufficiently available for a prospective comparative study, which is needed to prove the apparent increased performance of these tips over Kelman mini-flared phaco tips. Phaco tips are designed to both deliver US energy and aspirate material from the lens through its open end. Phaco tips impact efficiency and safety in the phaco procedure through their energy output, holdability, followability, and surge suppression. The choice of phaco tip depends on surgeon preference, which is mainly dependent on the surgical technique (i.e. chop or nucleofractis). Many surgeons prefer a straight tip to the Kelman-style tip in longitudinal phacoemulsification. Traditional Kelman phaco tips are widely used for both longitudinal and torsional phacoemulsification. This tip is excellent for use on the hardest nuclei during traditional longitudinal phacoemulsification. However, torsional phacoemulsification requires the Kelman tip to be angled 30° or 45° to be effective. The phaco tip end displacement is dependent on the degree of asymmetry of the tip shaft in torsional phacoemulsification. A straight tip induces very little phaco tip displacement. As the angle of the bend increases, more movement of the phaco tip cutting end occurs. The amplitude of phaco energy is modified by tip selection. Theoretically, there are 2 ways to enhance the cutting efficiency of a tip. First is the stroke length; the 22-degree, bent 30-degree Kelman mini-flared tip cuts longer than the 12-degree, bent 30-degree version. Second is the angulation or bevel; the higher the degree (45 degrees), the better the cutting efficiency[9],[10],[14]. The bevel of the phaco tip focuses power in the direction of the bevel. The 0° tip focuses both jackhammer and cavitational force directly in front of it. The 30° tip focuses these forces at a 30° angle from the phaco tip. The Kelman tip produces broad powerful cavitation directed away from the angle in the shaft. The cavitational force is concentrated into the nucleus rather than away from it. This causes the energy to emulsify the nucleus and be absorbed by it. When the bevel is turned away from the nucleus, the cavitational enegy is directed up and away from the nucleus toward the the iris and endothelium. Flare tips direct cavitation into the opening of the bevel of the tip, minimizing random emission of phaco energy is minimized. The wide opening of the tip makes it easier to minipulate the fragments. The narrow neck of the tip acts as a flow restrictor and reduces the tendency to create surge[15].
A drawback of Kelman tips, when used for torsional US, is significant tip motion of the shaft which can lead to undesired corneal stroma changes. Heat production, which can lead to wound burn and damage to intraocular structures, is an important disadvantage of emulsification. A novel balanced phaco tip has been specifically designed for torsional US and has enhanced sideways displacement at the tip end and greatly reduced tip action along the shaft. Its special structure creates less clogging and increases sharpness. The new phaco system also features balanced energy technology that enhances phacoemulsification efficiency through the software. The new Intrepid® balanced tip is available in both 30° and 45° aperture configurations, but we used only the 45° configuration. This system is particularly useful when nuclei are hard, where, thanks to the unique tip motion, the lens material appears to essentially dissolve. By combining the motion of the balanced tip and new fluidics, nuclear material flows to the tip and stays on it so that it virtually disappears as it is emulsified at the shearing plane.
Application of the minimal phaco power intensity necessary for emulsification of the nucleus is desirable. The power intensity is a source of heat, which can result in wound damage. Moreover, excessive cavitational energy is a cause of endothelial cell damage and iris damage with resultant alteration of the blood–aqueous barrier.
Tjia[16] compared Kelman® mini-tips with the new balanced phaco tips in a preliminary observational study where the same surgeon performed all surgeries with identical fluid dynamics and US settings. Balanced tips showed increased emulsification and cavitation at the sides of the tip but no significant phaco tip shaft motion whereas Kelman® tips showed significant tip action at the incision site. In addition, the CDE levels seemed to be lower and no wound changes were observed. Based on these observations, they concluded that the balanced phaco tips, which were specifically designed for torsional US, seemed to have a significant increased emulsification effect and a greatly reduced shaft action. In a recent study, Aslan[17] reported the balanced tip outperformed the miniflared phaco tip, with less fluid use, less chatter, but a higher mean needle time, higher postoperative pachymetry values. Our intraoperative findings were consistent with those observations. In this study, the percent change in ECD and CCT were used as outcome parameters representing the corneal injury due to the cataract surgery. Though they could be influenced by many factors, such as technique, fluid energy settings, and proximity of emulsification to the cornea during surgery, statistically significant changes in CCT were observed. At 1 and 7d after surgery, the percentage change in CCT was higher in the balanced group (P≤0.015). The mean ECD loss was 8.60% in the Kelman group and 14.82% in the balanced group 30d after surgery. The percent change in ECD was higher in the balanced group than in the Kelman group throughout the follow up period. However, it was not reached statistically significant (P>0.05). In our study, The higher ECD loss in the balanced phaco tip is explained by two possible scenarios. First, amplitude values for the balanced tips are more powerful than those for the kelman tips. The 22-degree bend/ 45-degree bevel Kelman tip has a maximum amplitude of 130 µm. The 45-degree balanced tip has a maximum amplitude of 192 µm. So that applied the same settings cause more produced (more than appoximately 68% power) the energy and turbulance in the eye compared to the Kelman group. CDE, which was used to show the efficiency of the procedures, did not reflect a meaningful intraoperative parameter for indicating postoperative morbidity regarding the corneal endothelium in this study. Because of amplitude differences between both phaco tips in the same settings. Second unwanted cavitational energy emerged from the bend portions of the shaft for the balanced tip and harmed endothelium during phacoemulsification.
Miyoshi and Yoshida[18] found that a swinging motion in the tip created 4 cavitation points that moved from the inside rim to outside rim with each vibration. Watanabe[19] reported that the threaded phaco tip can enhance the cavitation effect and it might contribute to a more effective phacoemulsification. Tognetto et al[20] suggested that the effect of cavitation and generation of the turbulence waves depend on the shape of the phaco tip. Clinical studies show that cavitation can play a role in ECD loss. The application of high-intensity US energy in aqueous media can generate acoustic cavitation with accompanying generation of free radicals and sonoluminesce. It can also result in localized high pressure and temparature elevation. All these factors can cause endothelial cell loss.
In conclusion, we found that the efficiency of torsional phacoemulsification was greater, as well as were the similar changes in ECD loss, in all cataract grades when using the balanced phaco tip compared to the Kelman® tip. This special designed phaco tip for torsional phacoemulsification provides an alternative phaco tip for many surgeons' preference with straight phaco tip.
Acknowledgments
Conflicts of Interest: Demircan S, None; Ataş M, None; Göktaş E, None; Başkan B, None.
REFERENCES
- 1.Liu Y, Zeng M, Liu X, Luo L, Yuan Z, Xia Y, Zeng Y. Torsional mode versus conventional ultrasound mode phacoemulsification; randomized comparative clinical study. J Cataract Refract Surg. 2007;33(2):287–292. doi: 10.1016/j.jcrs.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 2.Christakis PG, Braga-Mele RM. Intraoperative performance and postoperative outcome comparison of longitudinal, torsional, and transversal phacoemulsification machines. J Cataract Refract Surg. 2012;38(2):234–241. doi: 10.1016/j.jcrs.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 3.Atas M, Demircan S, Karatepe Hashas AS, Gulhan A, Zararsiz G. Comparison of corneal endothelial changes following phacoemulsification with transversal and torsional phacoemulsification machines. Int J Ophthalmol. 2014;7(5):822–827. doi: 10.3980/j.issn.2222-3959.2014.05.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng M, Liu X, Liu Y, Xia Y, Luo L, Yuan Z, Zeng Y, Liu Y. Torsional ultrasound modality for hard nucleus phacoemulsification cataract extraction. Br J Ophthalmol. 2008;92(8):1092–1096. doi: 10.1136/bjo.2007.128504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson JA. Cumulative tip travel and implied followability of longitudinal and torsional phacoemulsification. J Cataract Refract Surg. 2008;34(6):986–990. doi: 10.1016/j.jcrs.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Demircan S, Gokce G, Atas M, Baskan B, Goktas E, Zararsiz G. The impact of reused phaco tip on outcomes of phacoemulsification surgery. Curr Eye Res. 2015:1–7. doi: 10.3109/02713683.2015.1039654. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Ratnarajan G, Packard R, Ward M. Combined occlusion triggered longitudinal and torsional phacoemulsification duringcoaxial microincision cataract surgery: effect on 30-degree mini-flared tip behavior. J Cataract Refract Surg. 2011;37(5):825–829. doi: 10.1016/j.jcrs.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 8.Cionni RJ, Crandall AS, Felsted D. Length and frequency of intraoperative occlusive events with new torsional phacoemulsification software. J Cataract Refract Surg. 2011;37(10):1785–1790. doi: 10.1016/j.jcrs.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Helvacioglu F, Sencan S, Yeter C, Tunc Z, Uyar OM. Outcomes of torsional microcoaxial phacoemulsification using tips with 30- degree and 45-degree apertura angles. J Cataract Refract Surg. 2014;40(3):362–368. doi: 10.1016/j.jcrs.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 10.Helvacioglu F, Yeter C, Sencan S, Tunc Z, Uyar OM. Comparison of two different ultrasound methods of phacoemulsification. Am J Ophthalmol. 2014;158(2):221–226.e1. doi: 10.1016/j.ajo.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Kim JS, Chung SH, Joo CK. Clinical application of a Scheimpflug system for lens density measurements in phacoemulsification. J Cataract Refract Surg. 2009;35(7):1204–1209. doi: 10.1016/j.jcrs.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Kirkwood BJ, Hendicott PL, Read SA, Pesudovs K. Repeability and validity of lens densitometry measured with Scheimpflug imaging. J Cataract Refract Surg. 2009;35(7):1210–1215. doi: 10.1016/j.jcrs.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Magalhães FP, Costa EF, Cariello AJ, Rodrigues EB, Hofling-Lima AL. Comparative analysis of the nuclear lens opalescence by the Lens Opacities Classification System III with nuclear density values provided by Oculus Pentacam: a cross-section study using Pentacam Nucleus Staging software. Arq Bras Oftalmol. 2011;74(2):110–113. doi: 10.1590/s0004-27492011000200008. [DOI] [PubMed] [Google Scholar]
- 14.Kim EK, Jo JK, Joo CK. Comparison of tips in coaxial microincision cataract surgery with the bevel-down technique. J Cataract Refract Surg. 2011;37(11):2028–2033. doi: 10.1016/j.jcrs.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 15.Fishkind WJ, Neuhann TF, Steinert RF. The Phaco Machine: The Physical Principles Guiding its Operation. In: Steinert RF, editor. Cataract Surgery. Third Edition. Elsevier Inc; 2010. pp. 75–92. https://www.clinicalkey.com/#!/content/book/3-s2.0-B9781416032250000076. [Google Scholar]
- 16.Tjia KF. Novel Balanced Phacotip for Microcoaxial Torsional Phaco, ASCRS-ASOA Symposium and Congress on Cataract, IOL and Refractive Surgery, Boston, Massachusetts, USA, 2014 http://www.communityy.asoa.org/resources/abstracts?field_abstract_year_value=2014&keys=tJ%C4%B0A.
- 17.Aslan BS. Intraoperative performance and postoperative outcome comparison of two phaco tips in the same phacoemulsification machine, XXXII Congress of the ESCRS, Excel, London, UK, 2014 http://www.escrs.org/abstracts/ [Google Scholar]
- 18.Miyoshi T, Yoshida H. Emulsification action of longitudinal and torsional ultrasound tips and the effect on treatment of the nucleus during phacoemulsification. J Cataract Refract Surg. 2010;36(7):1201–1206. doi: 10.1016/j.jcrs.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe A. New phacoemulsification tip with a grooved threaded-tip construction. J Cataract Refract Surg. 2011;37(7):1329–1332. doi: 10.1016/j.jcrs.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Tognetto D, Sanguinetti G, Sirotti P, Brezar E, Ravalico G. Visualization of fluid turbulence and acoustic cavitation during phacoemulsification. J Cataract Refract Surg. 2005;31(2):406–411. doi: 10.1016/j.jcrs.2004.04.042. [DOI] [PubMed] [Google Scholar]