Abstract
Purpose
Follicle-stimulating hormone (FSH) and its receptor play a major role in the development of follicles and regulation of steroidogenesis in the ovary and spermatogenesis in the testis. We aim to analyze the role of FSHR gene variants (single nucleotide polymorphisms (SNPs) in exon 10 (codon 307 and 680) and in the core promoter region (at position −29) and Ala189Val inactivating mutation) in Turkish infertile women. There were studies analyzing the effects of the SNPs in exon 10 (codon 307 and 680) and in the core promoter region (at position −29) of the FSHR gene on spermatogenesis, but to our knowledge, there were no studies analyzing the effects of these three SNP combinations on female fertility.
Methods
In this study, the allelic, genotype, and haplotype frequency distributions of these three SNPs in the FSHR gene were analyzed in 102 infertile women and 99 unrelated healthy control individuals. The distribution of the polymorphisms was conformed by Hardy–Weinberg equilibrium test.
Results
There were no statistical differences (P > 0.05) in the allele, genotype, and haplotype frequencies of the polymorphisms and FSH, luteinizing hormone (LH), estradiol (E2), and prolactin (PRL) levels between the infertile patients and the controls. However, a significant relation was found between 307 SNP GA genotype and FSH level ≥12. We did not find any homozygous or heterozygote mutations in infertile patients and healthy fertile controls.
Conclusion
The present study was the first study analyzing gma mutation and the polymorphism of the FSHR core promoter at position −29 alone and in combination with the two common SNPs in exon 10 in Turkish infertile women population. These findings indicate the significance of Ala307Thr GA genotype may be a predictive marker for poor ovarian reserve and infertility.
Keywords: Follicle-stimulating hormone receptor, Single nucleotide polymorphisms, Infertility
Introduction
Follicle-stimulating hormone (FSH) plays a major role in the development of follicles and regulation of steroidogenesis in the ovary, and in spermatogenesis in the testis, by binding to a specific receptor, located exclusively on the surface of Sertoli cells in the testis and granulosa cells in the ovary [1, 2]. The interaction between FSH and its receptor is crucial for follicular development and maturation which makes it indispensable for female fertility.
The follicle-stimulating hormone receptor (FSHR) is a G protein-coupled receptor, and although it has been suggested that other signal transduction pathways are also involved in FSH action, its main signal transduction mechanism involves activation of adenylate cyclase and elevation of intracellular cyclic AMP (cAMP) [3].
The FSHR can be divided into three regions: the extracellular domain, a transmembrane domain, and the intracellular domain [4, 5]. The FSHR gene, which consisted of ten exons and nine introns, has been mapped to chromosome 2p21. The activity of this gene is driven by a core promoter spanning 225 bp, which represents a TATA-less promoter with no evident regulatory elements beside an E-box [4]. Exons 1 to 9 encode the extracellular domain, whereas the C-terminal part of the extracellular domain, the transmembrane domain, and intracellular domain are encoded by exon 10 [3].
Defects in the FSHR may diminish the ability of the receptor either to bind FSH or to activate signal transduction pathways. Any variation in the genotype of FSHR may also contribute towards the altered ability of the receptor. The first inactivating FSHR mutation was found in the extracellular ligand-binding domain of the protein in individuals from several Finnish relatives who presented with poorly developed secondary sexual characteristics, primary amenorrhea, and recessively inherited hypergonadotropic ovarian failure [6, 7]. Functional studies have demonstrated a C566T transition in exon 7 of FSHR predicting an Ala to Val substitution at residue 189 resulting in a functionally inactive FSHR, consistent with the severe phenotype reported in affected patients [6].
Previous studies analyzing the impact of single nucleotide polymorphisms (SNPs) on reproduction have demonstrated that polymorphisms can exert their effects via at least two mechanisms. One is by directly changing the biochemical properties of a certain gene product, and the other is by acting at the transcriptional level by changing the activity of a promoter [8, 9].
During the screening for FSH receptor mutations, several groups described polymorphisms in this gene and tried to correlate them with particular reproductive phenotypes. FSHR gene includes 731 single nucleotide polymorphisms. One SNP is located in the 5′ untranslated region of FSHR messenger RNA (mRNA) at position −29. The five SNPs in the coding region occur in exon 10. Two of them, Ala307Thr and the Ser680Asn polymorphisms, are well characterized with respect to frequency and ethnic distribution. The Ala307Thr and Ser680Asn SNPs in exon 10 are strong linkage disequilibrium in different populations [10, 11].
SNP at position −29 results in a G→A exchange in a potential GGAA binding domain for an E-twenty-six specific transcription factor [12, 13]. Further studies are required to show whether this changes the DNA binding capacity for this transcription factor, thereby leading to altered receptor mRNA expression.
Currently, two SNPs, originally described by Aittomaki et al., are well known [6]. These two non-synonymous SNPs with a frequency of >30 % in the normal population have been identified in the coding region of exon 10 in the FSH receptor gene. The first is located at position 919 (numbering according to the translational start codon with ATG as 1) in which A is substituted by G, changing codon 307 from threonine (ACT) to alanine (GCT). The second is located at nucleotide position 2039 in which G is replaced by A. This leads to an amino acid change at position 680 from serine (AGT) to asparagine (AAT).
Studies on the distribution of these two variants have produced varying results. Some studies in normal as well as infertile men and women have revealed no difference [12, 14, 15], whereas some other investigations have found significant differences in the distribution of allelic variants between patients and controls [16, 17], suggesting that ethnic differences could be involved.
We aim to analyze the role of FSHR gene variants (SNPs in exon 10 (codon 307 and 680) and in the core promoter region (at position −29) and Ala189Val inactivating mutation) in Turkish infertile women. There are studies investigating the effects of the SNP in exon 10 (codon 307 and 680) and in the core promoter region (at position −29) of the FSHR gene on spermatogenesis [13, 18, 19]; however, to our knowledge, there is no study analyzing the combined effects of these three SNPs on female fertility and also the present study is the first to analyze Ala189Val inactivating mutation in a population of infertile Turkish women.
Materials and methods
A total of 102 infertile women with a mean age of 28.3 ± 5.3 (range 19–43 years) and 99 unrelated healthy controls in whom fertility was confirmed without assisted reproductive techniques were recruited in the study. Haplotype and genotype analyses were performed on 102 infertile women and 99 controls; however, correlation analyses between hormone levels and genotypes were performed on 99 women aged 19–38 years, while 3 women aged >38 years were excluded. The control group consisted of pregnant women with a mean age of 27.2 ± 4.8 (range 20–35 years) who were admitted to the pregnancy outpatient clinic. These women had had regular menstruation cycles and had conceived at least 1 child before their pregnancy at the time of the study.
Subjects with male or tubal factor infertility, ovarian and endometrial lesions (endometriomas, cysts, myomas, endometriosis), as well as those with a history of surgical intervention concerning the uterine adnexa (tubes and ovaries), were not included. All the serum hormone samples were collected on the third day of the cycle prior to the IVF/ICSI treatment. We used an immunoenzymometric assay (Beckman Coulter UniCel DxI 800 Immunoassay System, USA) for the quantitative measurement of follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), and prolactin (PRL) in serum. Our study was approved by the Institutional Ethical Committee of the Faculty of Medicine at Ankara University (approval no. 154-4948), and written informed consent was obtained from all the study subjects. This study has been conducted as part of ongoing research related to a master thesis.
Genotyping and RFLP
Genomic DNA was prepared from peripheral blood by the standard phenol–chloroform method [20]. In all subjects, the polymorphisms at positions 919, 2039 (codon 307 and 680), and −29 were analyzed by restriction fragment length polymorphism (RFLP), as previously described (Figs. 1, 2, and 3) [13, 16]. The mutation at position 566 was also analyzed by RFLP, as previously described (Fig. 4) [21].
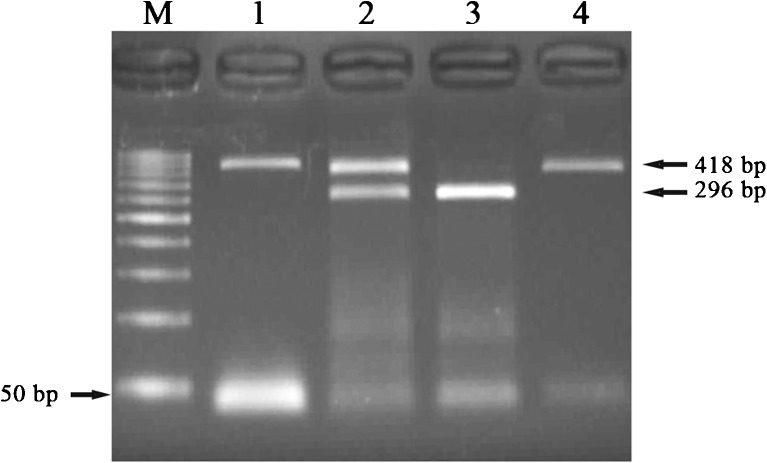
Fig. 1.
PCR-RFLP results of −29G/A polymorphism. M marker (50 bp), 1 Pcr product (418 bp), 2 GA (418/296 bp), 3 GG (296 bp), 4 AA (418 bp)
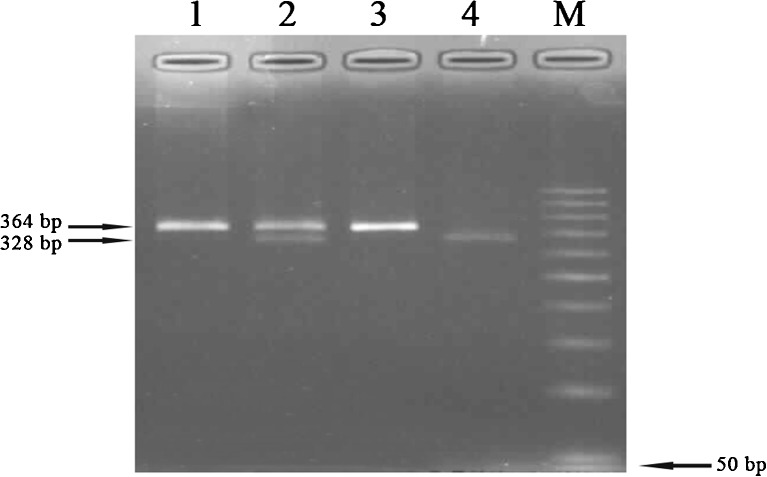
Fig. 2.
PCR-RFLP results of Ala307Thr polymorphism. 1 Pcr product (364 bp), 2 GA (364/328 bp), 3 AA (364 bp), 4 GG (328 bp), M marker (50 bp)
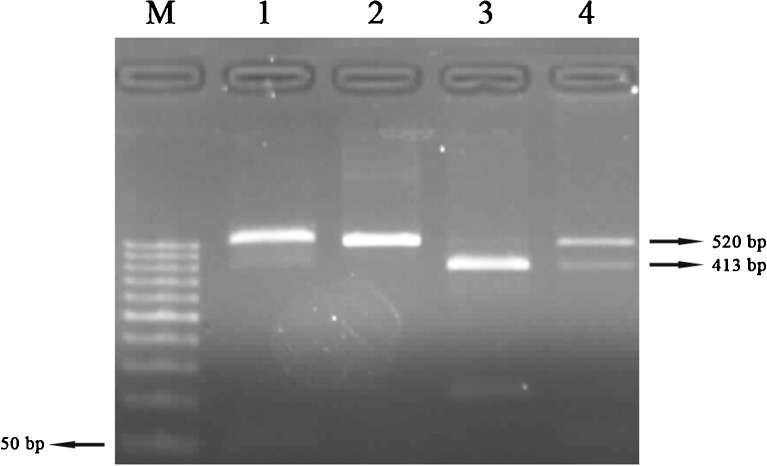
Fig. 3.
PCR-RFLP results of Ser680Asn polymorphism. 1 Pcr product (520 bp), 2 AA (520 bp), 3 GG (413 bp), 4 GA (520/413 bp), M marker (50 bp)
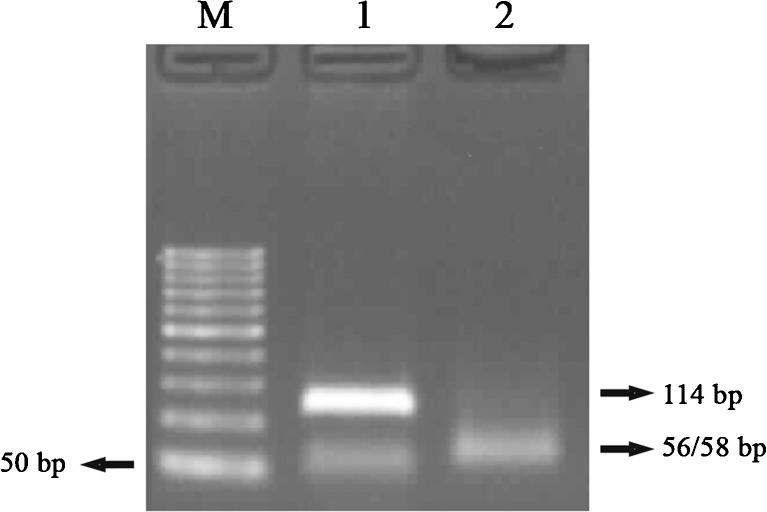
Fig. 4.
PCR-RFLP results of Ala189Val mutation. 1 Pcr product (114 bp), 2 CC (56/58 bp), M marker (50 bp)
PCR was performed on the resulting genomic DNA (1 μL) by using 0.2 μL of Taq DNA polymerase (Vivantis) in 25 μL of reaction mixture containing 2.5 μL of dNTP (Vivantis), 1 μL of each specific primer, 2.5 μL of 10× buffer (Fermantas), 3 μL of MgCl2 (25 mM, Fermentas), and 13.8 μL of H2O. The cycling program for the G29A amplification polymorphism was as follows: denaturation at 95 °C for 10 min; 39 cycles at 94 °C for 45 s, 57 °C for 45 s, and 72 °C for 1 min; and the final extension at 72 °C for 5 min. The cycling program for the G919A amplification polymorphism was as follows: denaturation at 95 °C for 8 min; 39 cycles at 94 °C for 45 s, 53 °C for 45 s, and 72 °C for 1 min; and the final extension at 72 °C for 10 min. The cycling program for the G919A amplification polymorphism was as follows: denaturation at 95 °C for 8 min; 39 cycles at 94 °C for 45 s, 53 °C for 45 s, and 72 °C for 1 min; and the final extension at 72 °C for 10 min. The cycling program for the G2039A amplification polymorphism was as follows: denaturation at 95 °C for 10 min; 37 cycles at 94 °C for 1 min, 61 °C for 1 min, and 72 °C for 1 min; and the final extension at 72 °C for 10 min. The cycling program for the C566T amplification mutation was as follows: denaturation at 95 °C for 5 min; 37 cycles at 94 °C for 1 min, 62 °C for 1 min, and 72 °C for 45 s; and the final extension at 72 °C for 10 min.
The PCR products were then digested with the restriction enzyme, according to the manufacturer's protocol. The restriction enzymes, restriction sites, fragments, and genotypes are shown in Table 1.
Table 1.
Restriction enzymes, restriction sites, fragments, and genotypes
| Polymorphisms/mutation | Restriction enzyme | Restriction site | Fragments (bp) | Genotype |
|---|---|---|---|---|
| −29 G/A | Mbo II | 5′…GAAGA(N)8…3′ 3′…CTTCT(N)7…5′ |
296, 122 418, 296, 122 418 |
GG GA AA |
| Ala307Thr;919 G>A | Bse21I | 5′…CCTNAGG…3′ 3′…GGANTCC…5′ |
328, 36 364, 328, 36, 364 |
GG GA AA |
| Ser680Asn; 2039 G>A | Bse1I | 5′…ACTGGNN…3′ 3′…TGACCNN…5′ |
413, 107 520, 413, 107, 520 |
GG GA AA |
| Ala189Val; 566 C>T | PctI | 5′…GAATGCNN…3′ 3′…CTTACGNN…5′ |
58, 56 114, 58, 56, 114 |
CC CT TT |
Statistical analysis
Statistical analysis was performed using SPSS Statistics version 13.0. Student’s t test or Mann–Whitney U test was used to analyze the differences of two continuous variables. ANOVA and Kruskal–Wallis tests were used to analyze the differences among multiple (2) groups. Results were expressed as percentage, median (min–max), and/or mean ± standard deviation (SD) values. A P value <0.05 was recognized as statistically significant. SHEsis was used to analyze the differences in genotype, haplotype, and allele frequencies between infertile and control groups. On the basis of the observed frequencies of three SNPs, we used the SHEsis analysis platform to calculate linkage disequilibrium index. Linkage disequilibrium parameters and Hardy–Weinberg equilibrium D', r2 were calculated by using the online SHEsis software.
Results
In this study, the infertile group was compared with the fertile control group for the same three SNPs and Ala189Val inactivating mutation of the FSHR gene. The allelic, genotype, and haplotype frequency distributions of these genetic variants in the FSHR gene were analyzed in 102 infertile women and 99 unrelated healthy controls.
We did not find any homozygous or heterozygote Ala189Val inactivating mutations in infertile patients and healthy fertile controls.
The distribution of the FSHR genotypes and allele frequencies were evaluated in infertile patients and controls (Table 2). The distribution of the polymorphisms was conformed by Hardy–Weinberg equilibrium test. The linkage disequilibrium among the three SNPs (−29, 307, 680) was examined. Our results indicated that only 307 and 680 SNPs had near complete linkage disequilibrium (D' = 0.902) (r2 = 0.743). However, no linkage disequilibrium was found between −29 and 307 SNPs (D' = 0.084) (r2 = 0.004) and between −29 and 680 SNPs (D' = 0.107) (r2 = 0.005). However, there were no statistical differences between the infertile patients and the controls with regard to the allele or genotype frequencies of the polymorphisms.
Table 2.
−29, 307, 680 polymorphisms and infertility risk in patient and control
| Genotype | Patient n (freq%) |
Control n (freq%) |
x 2 | P value | Allele | Patient n (freq%) |
Control n (freq %) |
x 2 | P value | |
|---|---|---|---|---|---|---|---|---|---|---|
| −29A/G | ||||||||||
| AA | 9 (9) | 5 (5) | 1.109 | 0.574 | A | 65 (32) | 57 (29) | 0.449 | 0.502 | |
| AG | 47 (46) | 47 (48) | G | 139 (68) | 141 (71) | |||||
| GG | 46 (45) | 47 (48) | ||||||||
| 307A/G | ||||||||||
| AA | 23 (23) | 23 (23) | 0.052 | 0.974 | A | 94 (46) | 91 (46) | 0.0005 | 0.980 | |
| AG | 48 (47) | 45 (46) | G | 110 (54) | 107 (54) | |||||
| GG | 31 (30) | 31 (31) | ||||||||
| 680A/G | ||||||||||
| AA | 24 (24) | 24 (24) | 0.014 | 0.992 | A | 98 (48) | 96 (49) | 0.0079 | 0.928 | |
| AG | 50 (49) | 48 (49) | G | 106 (52) | 102 (52) | |||||
| GG | 28 (27) | 27 (27) |
−29 SNP genotypes and allele frequencies
The infertile group was compared with the fertile control group for the −29 SNP. The frequencies of the A and G alleles of the −29 loci were found to be 32 % (n = 65) and 68 % (n = 139) in the patient group, respectively, and 29 % (n = 57) and 71 % (n = 141) in the control group, respectively. The frequencies of the −29 AA, AG, and GG genotypes were 9 % (n = 9), 46 % (n = 47), and 45 % (n = 46) in the patient group and 5 % (n = 5), 48 % (n = 47), and 48 % (n = 47) in the control group, respectively. However, the observed genotype frequencies did not show significant differences in either of the groups (P > 0.05). The genotype frequency distribution of the −29 locus was consistent with the HWE in both groups (P > 0.05) (Table 2).
Asn680Ser SNP genotypes and allele frequencies
The infertile group was compared with the fertile control group for the Asn680Ser SNP. The frequencies of the alleles A and G of locus 680 were found to be 48 % (n = 98) and 52 % (n = 106) in the patient group and 49 % (n = 96) and 52 % (n = 102) in the control group, respectively. The frequencies of the 680 AA, AG, and GG genotypes were 24 % (n = 24), 49 % (n = 50), and 27 % (n = 28) in the patient group, respectively, and 24 % (n = 24), 49 % (n = 48), and 27 % (n = 27) in the control group, respectively. However, the observed genotype frequencies did not show significant differences in either group (P > 0.05). The genotype frequency distribution of locus 680 was consistent with the HWE in both groups (P > 0.05) (Table 2).
Ala307Thr SNP genotypes and allele frequencies
The infertile group was compared with the fertile control group for the Ala307Thr SNP. The frequencies of the alleles A and G of locus 307 were found to be 46 % (n = 91) and 54 % (n = 107) in the patient group and 49 % (n = 96) and 52 % (n = 102) in the control group, respectively. The frequencies of the 307 AA, AG, and GG genotypes were 23 % (n = 23), 47 % (n = 48), and 30 % (n = 31) in the patient group, respectively, and 23 % (n = 23), 46 % (n = 45), and 31 % (n = 31) in the control group, respectively. However, the observed genotype frequencies did not show significant differences in either group (P > 0.05). The genotype frequency distribution of locus 307 was consistent with the HWE in both groups (P > 0.05) (Table 2).
Haplotype frequencies of loci −29, 307, and 680
The haplotype frequencies of loci −29, 307, and 680 are presented in Tables 3, 4, and 5. Each of the eight possible haplotypes was noted both in the patient and control groups. When the frequency distributions of the estimated haplotypes were compared between the infertile and control groups, frequencies did not show significant differences in either group (P > 0.05).
Table 3.
−29, 307, 680 haplotype frequencies and the association with the risk of fertility in patients and controls
| Haplotype | Patient (freq) | Control (freq) | Chi2 | Fisher's P | Pearson's P | Odds ratio [95 % CI] |
|---|---|---|---|---|---|---|
| A A A | 31 (0.16) | 28 (0.15) | 0.06 | 0.803370 | 0.80 | 1.07 [0.62~1.86] |
| A A G | 0 (0.000) | 1 (0.01) | – | – | – | – |
| A G A | 3 (0.02) | 1 (0.01) | – | – | – | – |
| A G G | 30 (0.15) | 25 (0.13) | 0.43 | 0.51 | 0.51 | 1.21 [0.69~2.14] |
| G A A | 56 (0.28) | 59 (0.30) | 0.27 | 0.61 | 0.61 | 0.90 [0.58~1.38] |
| G A G | 6 (0.03) | 1 (0.01) | 2.44 | 0.12 | 0.12 | 3.51 [0.66~18.86] |
| G G A | 7 (0.04) | 6 (0.03) | 0.03 | 0.86 | 0.86 | 1.10 [0.37~3.26] |
| G G G | 69 (0.34) | 73 (0.37) | 0.50 | 0.49 | 0.49 | 0.87 [0.57~1.30] |
Global result: total control = 198.0, total case = 204.0; global chi2 is 3.331192, while df = 5 (frequency <0.03 was dropped in both control and case groups). Fisher's P value is 0.649086; Pearson's P value is 0.649079
Table 4.
Demographics and endocrine profiles of the patient group (n = 99)
| Variable | Mean ± Std |
|---|---|
| Age (years) | 27.81 (19–38) |
| Body mass index (kg/m2) | 23.43 ± 3.4 |
| Median (min–max)a | |
| Estradiol (E2) (pg/mL) | 55.6 ± 26.1 |
| Luteinizing hormone (LH) (mIU/mL) | 5.25 (0.4–27.2) |
| Follicle-stimulating hormone (FSH) (mIU/mL) | 6.8 (0.6–60) |
| Prolactin (Prl) (ng/mL) | 13.0 (1.3–38.7) |
aMedian values are given due to absence of normal distribution
Table 5.
FSHR polymorphisms vs endocrine parameters in patients
| FSH (mIU/mL) Mean ± std |
LH (mIU/mL) Mean ± std |
Estradiol (pg/mL) Mean ± std |
Prolactin (ng/mL) Mean ± std |
BMI (kg/m2) Mean ± std |
|
|---|---|---|---|---|---|
| −29(G→A) | |||||
| GG | 10.6 ± 10.3 | 7.1 ± 5.02 | 53.1 ± 25.1 | 14.7 ± 6.6 | 23.1 ± 2.8 |
| GA | 9.06 ± 9.2 | 6.03 ± 4.6 | 55.8 ± 23.7 | 15.6 ± 7.1 | 23.4 ± 3.9 |
| AA | 25.3 ± 4.0 | 10.1 ± 8.5 | 66.1 ± 40.6 | 13.2 ± 5.4 | 24.9 ± 2.4 |
| P value | 0.646 | 0.238 | 0.400 | 0.655 | 0.362 |
| Ala307Thr (G→A) | |||||
| GG | 7.1 ± 3.0 | 5.9 ± 3.1 | 61.0 ± 25.4 | 14.1 ± 7.2 | 23.1 ± 3.3 |
| GA | 11.7 ± 11.8 | 7.6 ± 6.4 | 52.7 ± 24.3 | 14.8 ± 6.7 | 23.3 ± 3.5 |
| AA | 11.1 ± 12.5 | 6.7 ± 5.3 | 53.9 ± 30.2 | 16.6 ± 6.0 | 23.9 ± 3.0 |
| P value | 0.389 | 0.665 | 0.333 | 0.149 | 0.701 |
| Ser680Asn (G→A) | |||||
| GG | 7.3 ± 3.5 | 5.8 ± 3.5 | 59.7 ± 23.2 | 13.6 ± 7.3 | 23.4 ± 3.0 |
| GA | 12.0 ± 11.5 | 7.5 ± 6.0 | 53.6 ± 25.1 | 15.0 ± 6.5 | 23.4 ± 3.8 |
| AA | 10.6 ± 12.3 | 6.7 ± 5.1 | 53.5 ± 30.5 | 16.36.0 ± 6.07 | 23.9 ± 3.1 |
| P value | 0.365 | 0.524 | 0.574 | 0.114 | 0.792 |
BMI body mass index, FSH follicle-stimulating hormone, LH luteinizing hormone, E2 estradiol, PRL prolactin
However, in our study group, the genotype frequency distribution of the Asn680Ser SNP did not show any significant difference from those in Caucasian, Hindustani, Creole, or Mediterranean populations (P > 0.05), while being significantly different from those in Asian populations (P = 0.005) (Table 6).
Table 6.
Ser680Asn polymorphisms comparison of Turkish society (our study) and other societies
| Ser680Asn (G→A) | Caucasian | Hindustani | Creole | Asian | Mediterranean |
|---|---|---|---|---|---|
| GG | 29.6 % | 27.3 % | 33.8 % | 50.7 % | 26.3 % |
| GA | 48.9 % | 51.5 % | 47.7 % | 38.8 % | 51.4 % |
| AA | 21.5 % | 21.2 % | 18.5 % | 10.4 % | 22.3 % |
| P value | 0.852 | 0.956 | 0.598 | 0.005* | 0.927 |
* Statistically significance (p < 0.05)
FSHR genotypes and serum baseline hormone levels were compared in 99 patients aged <39 years. There was no significant difference between the −29, 307, and 680 SNPs of the FSHR gene and mean hormone levels. The following were recognized as cutoff values: FSH 12 IU/L, LH 2.9 IU/L, PRL 29 IU/L, and E2 66 ng/L [22]. No significant correlation was found between genotypes and hormone concentrations for the −29 and 680 SNPs. When 307 SNP was compared, genotypes showed no relationship with prolactin and LH; however, genotypes exhibited a significant correlation with FSH and E2 concentrations. According to the Dr. Zekai Tahir Burak Women's Health Teaching and Research Hospital criteria where our infertile patients were assessed, the FSH cutoff value of IU/I >12 was used to categorize the women in two groups: high FSH (IU/I >12) and normal FSH (FSH IU/I ≤12). In the patient group with a FSH level of IU/I >12, GA genotype was more common than GG genotype (P = 0.041). Moreover, in those with an E2 value of <66 ng/mL, GA genotype was again more common than GG genotype (P = 0.046) (Tables 7 and 8).
Table 7.
Genotype frequencies and serum baseline FSH concentrations relative to cutoff level 12 IU/I in patients
| Allele combination | Genotypic frequency, n (%) FSH (IU/I ≤ 12) |
Genotypic frequency, n (%) FSH (IU/I > 12) |
P value |
|---|---|---|---|
| −29(G→A) | |||
| GG | 35 (41.7 %) | 9 (60.0 %) | 0.246 |
| GA | 42 (50.0 %) | 4 (26.7 %) | |
| AA | 7 (8.3 %) | 2 (13.3 %) | |
| 307(G→A) | |||
| GG | 30 (35.7 %) | 1 (6.7 %) | 0.041 |
| GA | 36 (42.9 %) | 9 (60.0 %) | |
| AA | 18 (21.4 %) | 5 (33.3 %) | |
| 680(G→A) | |||
| GG | 27 (32.1 %) | 1 (6.7 %) | 0.109 |
| GA | 38 (45.2 %) | 9 (60 %) | |
| AA | 19 (22.6 %) | 5 (33.3 %) | |
Table 8.
Genotype frequencies and serum baseline E2 concentrations relative to cutoff level 66 ng/mL in patients
| SNPs | Genotypes | Genotypic frequency, % (n) E2 (≤66 ng/mL) |
Genotypic frequency, % (n) E2 (>66 ng/mL) |
P value |
|---|---|---|---|---|
| −29A/G | GG vs AA+ GA | 73.9 % (34) vs 69.6 % (39) | 26.1 % (12) vs 30.4 % (17) | 0.634 |
| 307A/G | GG vs AA+ GA | 58.15 (18) vs 77.5 % (55) | 41.9 % (13) vs 22.5 % (16) | 0.046* |
| 680A/G | GG vs AA+ GA | 64.3 % (18) vs 74.3 % (55) | 35.7 % (10) vs 25.7 % (19) | 0.316 |
* Statistically significance (p < 0.05)
Discussion
Since it binds to a specific receptor, FSH is essential for normal reproductive functions [3, 23, 24]. The interaction of FSH with its receptor is crucial for the follicular development and maturation. Any variation in the genotype of FSHR may contribute to the altered ability of the receptor to bind FSH and to induce signal transduction pathway, and could affect reproductive ability, especially in women [10, 25].
The first inactivating mutation of the FSHR gene was described in individuals from several Finnish relatives who presented with poorly developed secondary sexual characteristics, primary amenorrhea, and recessively inherited hypergonadotropic ovarian failure [6, 7]. Aittomaki et al. [26] reported six Finnish families containing two or more women suffering from gonadal dysgenesis, primary amenorrhea, and infertility. It was demonstrated that a missense Ala189Val mutation in the FSH receptor was responsible for their symptoms [6, 26]. When the inactivating Ala189Val mutation is present in a homozygous form, it can cause primary hypergonadotropic amenorrhea with impairment of follicular development in women, and can block spermatogenesis in men [27]. Women who are homozygous for Ala189Val reveal a block in follicular maturation and scarce apoptosis in granulosa cells, a sign reflecting poor ability for cell replication. Absence of this FSHR gene mutation was shown in studies including American, German, Brazilian, Mexican, and Argentinian infertile populations [14, 28–32], However, in 1998, Jiang et al. [9] identified only one mutation (C566T) carrier in a large-scale screening of 1.162 subjects from Switzerland. We also did not find any homozygous or heterozygous mutations in the infertile patients and healthy fertile controls. To our knowledge, this is the first study analyzing this mutation in a population of Turkish women. Thus, our results further strengthen the observation that C566T mutation is restricted to Finland and may represent a founder effect of this country.
Single nucleotide polymorphisms (SNP) are the most common forms of genetic variants. Some recent studies show that phenotypic effects of haplotypes are more prominent than individual polymorphisms on prevention or susceptibility of diseases. There are series of studies showing the association between haplotypes and diseases. In this study, we analyze individual polymorphisms, while also analyzing the haplotypes.
The FSH receptor and its promoter bear several very common SNPs which seem to be important in reproductive functions and determining the response to FSH stimulation [33].
In the core promoter region, a frequent (>30 %) G→A polymorphism is located at position −29. This position corresponds to a potential binding domain GGAA for an E-26 transcription factor.
In our study, we detected 45 % GG, 46 % AG, and 9 % AA, and 48 % GG, 48 % AG, and 5 % AA genotypes at the −29 position in patients and controls, respectively. The distribution of the SNP at position −29 of the FSH receptor gene did not differ significantly between the controls and the patients (P = 0.502, P > 0.05); moreover, there was no significant correlation of the SNP in the promoter region with baseline FSH, LH, E2, and PRL levels (P > 0.05), which were consistent with the results of the studies of [34–37].
Acherekar et al. [32] studied the −29 position in patients suffering from primary and secondary amenorrhea and found that the prevalence of AA genotypes at the −29 position was significantly higher in both amenorrheic groups as compared to the control group. The primary amenorrhea subjects with AA genotype at the −29 position of the FSHR gene showed significantly higher serum FSH levels. We found no difference between FSH level, menstrual regularity, and SNP in the promoter region. To our knowledge, our study is the first to detect the FSHR gene polymorphic variants at the −29 position in a population of Turkish women.
Currently, only two SNPs, originally described by Aittomaki et al. [6], in the coding region at nucleotides 919 and 2039 in exon 10 in which A/G transitions cause amino acid exchange from threonine to alanine at codon 307 and from asparagine to serine at codon 680, respectively, are well known. However, studies on the frequency distribution of follicle-stimulating hormone receptor (FSHR) polymorphisms report conflicting results. It has been suggested that ethnicity may be influential in the outcomes.
Kuijper et al. [38] studied 1771 women of different ethnic origins (Caucasian, Asian, Hindustani, Creole, and Mediterranean) with fertility problems and determined the frequency distribution of FSHR polymorphisms at position 680 in exon 10. Associations between genotypes and FSH levels were compared between different ethnic groups. A significantly lower number of Asians (10.5 %) were found to have the Ser680Ser receptor variant as compared to the Caucasians (21.5 %) and Mediterraneans (22.3 %) (P = 0.010). This result showed that allelic frequency distributions of the FSHR polymorphism may vary according to ethnicity. In our study, the frequencies of the 680 AA, AG, and GG genotypes were 24 % (n = 24), 49 % (n = 50), and 27 % (n = 28) in the patient group, and 24 % (n = 24), 49 % (n = 48), and 27 % (n = 27) in the control group, respectively. The frequencies of FSHR genotypes in infertile women were consistent with those in Caucasian, Hindustani, Creole, and Mediterranean infertile women. However, they were different from those of the Asian infertile women. The FSH concentrations did not differ with regard to the FSHR polymorphisms in any ethnic group [6], which was a result consistent with our study, as well. Earlier studies have shown that women with the Ser680Ser genotype are characterized by a higher ovarian threshold for FSH, resulting in higher serum FSH concentrations [17, 33, 39]. We found no significant difference between fertile and infertile groups in terms of frequency of alleles and genotypes of codon 680 in exon 10, while we also found no correlation between genotypes and FSH, LH, E2, and PRL levels.
As the two single nucleotide polymorphisms (SNPs) at 307 and 680 reside on the same exon, they have been suggested and even shown to be linked in several studies. In our study, the heterozygous genotype frequency of 307 and 680 SNPs was high but not statistically significant: AA 23 %, GA 47 %, and GG 30 % in infertile women, and AA 23 %, GA 46 %, and GG 31 % in controls, respectively. These results were consistent with those of Unsal et al. [40] and Sever et al. [41], which had been performed in populations of Turkish women suffering from PCOS and infertility, respectively, as well as with those of Acherar et al. [37], performed in a population of Hindustani infertile women. As in several other studies [15, 17, 30], we found no statistical differences in the allele or genotype frequencies of the polymorphisms between the patients and the controls for the SNPs at 307 and 680.
Gene haplotypes have better predictive power to disclose the existing associations between the gene variants and disease conditions that would remain hidden if only individual SNPs are analyzed. In several studies, the polymorphism in FSHR gene core promoter at position 29 was evaluated in combination with A919G (Thr307Ala) and A2039G (Asn680Ser) SNPs for the assessment of male infertility [13, 18].
However, to our knowledge, there is no study evaluating the association of female infertility with the haplotypes formed by three SNPs at position −29, Thr307Ala and Asn680Ser in exon 10 of the FSHR gene. The haplotype frequencies of loci −29, 307, and 680 are presented in Table 3. Each of the eight possible haplotypes was noted both in the patient and the control groups. There was no significant difference between the infertile and control groups with regard to frequency distributions of the estimated haplotypes (P > 0.05).
Women who present to infertility clinics are commonly screened for baseline FSH level which is used as a predictor of ovarian reserve. In females, these SNPs have been shown to influence serum FSH levels during the follicular phase of the menstrual cycle [42]. Our results indicated that there was no association between these SNPs and the mean value of baseline FSH, which was a result inconsistent with the findings of Sudo et al. [16], and also, we found no association between 307 and 680 SNPs and the mean value of LH, E2, and PRL levels in infertile women. However, according to our results, it was shown that the heterozygous genotype GA in infertile women for SNP at 307 was associated with a baseline FSH level >12 (P = 0.041) (Table 7) and baseline E2 level <66 (P = 0.046) (Table 8), while there was also a tendency to have a GA heterozygous genotype in the infertile group. In infertile women, high FSH and low estrogen levels are an indicator of diminished ovarian reserve and are among the reasons for unsuitable endometrial window and first trimester loss, while being the major cause of infertility [43]. The loss of normal ovarian function before age 40 is called premature ovarian failure (POF) and is characterized with low levels of estrogen and high levels of FSH with impaired ovarian folliculogenesis. The phenotype of the FSHR knockout mice was observed to be similar to that observed in POF [44]. Therefore, the phenotype of females with POF can be attributed to FSHR, serving as a strong candidate gene. While there was no clear relationship between the FSHR gene polymorphism and POF, Kim et al. reported [45] that epistasis between FSHR and CYP19A1 polymorphisms was strongly related to POF. To our knowledge, our study was the first study to show that high FSH and low E2 levels, indicative of poor ovarian reserve, had a statistically significant association with FSHR 307 GA genotype in infertile women (P = 0.046). Similarly, several studies reported significant association with FSHR 307 GA genotype in PCOS women, generally suffering from infertility because of impaired folliculogenesis [16, 46, 47]. Women with PCOS are unlikely to undergo a rapid depletion of their ovarian reserve too early [48]. In several studies, the frequency of GA genotype for 307 SNPs had been found to be significantly higher in women with PCOS than in controls [16, 46, 47]. Therefore, it may be speculated that FSHR 307 GA genotypes might be important parameters for impaired folliculogenesis and early diminished ovarian reserve.
In conclusion, although ethnicity may influence the results pertaining to the association between FSHR polymorphisms and infertility, GA heterozygous genotypes at position 307 may be responsible for impaired folliculogenesis and diminished ovarian reserve, thereby leading to infertility. Thus, further studies including larger series of different populations are required to clarify the role of FSHR polymorphisms on folliculogenesis and ovarian reserve.
Acknowledgments
We thank Zeynep Biyikli Gencturk for assistance in statistical analysis.
Footnotes
Capsule FSHR 307 GA genotypes is associated with impaired folliculogenesis and early diminished ovarian reserve.
References
- 1.Richards JS. Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol Rev. 1980;60:51–89. doi: 10.1152/physrev.1980.60.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Hsueh AJW, Bicsak TA, Jia XC, Dahl KD, Fauser BC, Galway AB, et al. Granulosa cells as hormone targets: the role of biologically active follicle stimulating hormone in reproduction. Recent Prog Horm Res. 1989;45:209–77. doi: 10.1016/b978-0-12-571145-6.50009-1. [DOI] [PubMed] [Google Scholar]
- 3.Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology and pathophysiology. Endocrinol Rev. 1997;18:739–73. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]
- 4.Gromoll J, Dankbar B, Gudermann T. Characterization of the 5’ flanking region of the human follicle-stimulating hormone receptor gene. Mol Cell Endocrinol. 1994;102:93–102. doi: 10.1016/0303-7207(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 5.Gromoll J, Pekel E, Nieschlag E. The structure and organization of the human follicle-stimulating hormone receptor (FSHR) gene. Genomics. 1996;35:308–11. doi: 10.1006/geno.1996.0361. [DOI] [PubMed] [Google Scholar]
- 6.Aittomaki K, Lucena JL, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82:959–68. doi: 10.1016/0092-8674(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 7.Aittomaki K, Herva R, Stenman UH. Clinical features of primary ovarian failure caused by a point mutation in the follicle-stimulating hormone receptor gene. J Clin Endocrinol Metab. 1996;81:3722–6. doi: 10.1210/jcem.81.10.8855829. [DOI] [PubMed] [Google Scholar]
- 8.Lamminen T, Huhtaniemi I. A common genetic variant of luteinizing hormone; relation to normal and aberrant pituitary-gonadal function. Eur J Pharmacol. 2001;414:1–7. doi: 10.1016/S0014-2999(01)00756-7. [DOI] [PubMed] [Google Scholar]
- 9.Jiang M, Pakarinen P, Zhang FP, El-Hefenawy T, Koskimies P, Pettersson K, et al. A common polymorphic allele of the human luteinizing hormone b-subunit gene: additional mutations and diffferential function of the promoter sequence. Hum Mol Genet. 1999;11:2037–46. doi: 10.1093/hmg/8.11.2037. [DOI] [PubMed] [Google Scholar]
- 10.Conway GS. Clinical manifestations of genetic defects affecting gonadotrophins and their receptors. Clin Endocrinol. 1996;45:657–63. doi: 10.1046/j.1365-2265.1996.8680879.x. [DOI] [PubMed] [Google Scholar]
- 11.Du J, Zhang W, Guo L, Zhang Z, Shi H, Wang J, et al. Two FSHR variants, haplotypes and meta-analysis in Chinese women with premature ovarian failure and polycystic ovary syndrome. Mol Genet Metab. 2010;100:292–5. doi: 10.1016/j.ymgme.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Simoni M, Gromoll J, Hoppner W, Kamischke A, Krafft T, Stahle D, et al. Mutational analysis of the follicle-stimulating hormone (FSH) receptor in normal and infertile men: identification and characterization of two discrete FSH receptor isoform. J Clin Endocrinol Metab. 1999;84:751–5. doi: 10.1210/jcem.84.2.5500. [DOI] [PubMed] [Google Scholar]
- 13.Pengo M, Ferlin A, Arredi B, Ganz F, Selice R, Garolla A, et al. FSH receptor gene polymorphisms in fertile and infertile Italian men. Reprod Biomed Online. 2006;13:795–800. doi: 10.1016/S1472-6483(10)61026-7. [DOI] [PubMed] [Google Scholar]
- 14.Liu JY, Gromoll J, Cedars MI, La Barbera AR. Identification of allelic variants in the follicle-stimulating hormone receptor genes of females with or without hypergonadotropic amenorrhea. Fertil Steril. 1998;70:326–31. doi: 10.1016/S0015-0282(98)00151-4. [DOI] [PubMed] [Google Scholar]
- 15.Conway GS, Conway E, Walker C, Hoppner W, Gromoll J, Simoni M. Mutation screening and isoform prevalence of the follicle stimulating hormone receptor gene in women with premature ovarian failure, resistant ovary syndrome and polycystic ovary syndrome. Clin Endocrinol (Oxf) 1999;51:97–9. doi: 10.1046/j.1365-2265.1999.00745.x. [DOI] [PubMed] [Google Scholar]
- 16.Sudo S, Kudo M, Wada S, Sato O, Hsueh AJ, Fujimoto S. Genetic and functional analysis of polymorphisms in the human FSH receptor gene. Mol Hum Reprod. 2002;8:893–9. doi: 10.1093/molehr/8.10.893. [DOI] [PubMed] [Google Scholar]
- 17.Laven JSE, Mulders AGMGJ, Suryandari DA, Gromoll J, Nieschlag E, Fauser BCJM, et al. Follicle-stimulating hormone receptor polymorphisms in women with normogonadotropic anovulatory infertility. Fertil Steril. 2003;80:986–92. doi: 10.1016/S0015-0282(03)01115-4. [DOI] [PubMed] [Google Scholar]
- 18.Ahda Y, Gromoll J, Wunsch A, Asatiani K, Zitzmann M, Nieschlag E, et al. Follicle-stimulating hormone receptor gene haplotype distribution in normozoospermic and azoospermic men. J Androl. 2005;26(4):494–9. doi: 10.2164/jandrol.04186. [DOI] [PubMed] [Google Scholar]
- 19.Lend AK, Belousova A, Haller-Kikkatalo K, Punab M, Poolamets O, Peters M, et al. Follicle-stimulating hormone receptor gene haplotypes and male infertility in Estonian population and meta-analysis. Syst Biol Reprod Med. 2010;56:84–90. doi: 10.3109/19396360903456676. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning a laboratory manual. 2. NY: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 21.Etem E, Kuyucu F, Ardıçoğlu A, Yüce H. İnfertilite ve Anormal Semen Analizleri Gösteren Erkeklerde FSHR Ala189Val Gen Polimorfizminin Analizi. 2006;11:vol. 1, p. 033–035.
- 22.Sever B, Simsek M, Akar ME, Alper O, Leblebici IL. Comparison of FSH receptor polymorphisms between infertile and fertile women. Biomed Res. 2014;25(1):121–6. [Google Scholar]
- 23.Chappel SC, Howles C. Reevaluation of the roles of luteinizing hormone and follicle-stimulating hormone in the ovulatory process. Hum Reprod. 1991;6:1206–12. doi: 10.1093/oxfordjournals.humrep.a137513. [DOI] [PubMed] [Google Scholar]
- 24.Simoni M, Nieschlag E. FSH in therapy: physiological basis, new preparations and clinical use. Reprod Med Rev. 1995;4:163–77. doi: 10.1017/S0962279900001150. [DOI] [Google Scholar]
- 25.Gromoll J, Simoni M, Nordhoff V, Behre HM, De Geyter C, Nieschlag E. Functional and clinical consequences of mutations in the FSH receptor. Mol Cell Endocrinol. 1996;125:177–82. doi: 10.1016/S0303-7207(96)03949-4. [DOI] [PubMed] [Google Scholar]
- 26.Aittomaki K. The genetics of XX gonadal dysgenesis. Am J Hum Genet. 1994;54:844–51. [PMC free article] [PubMed] [Google Scholar]
- 27.Tapanainen JS, Vaskivuo T, Aittomaki K. Inactiving FSH receptor mutation and gonadal dysfunction. Mol Cell Endocrinol. 1998;145:129–35. doi: 10.1016/S0303-7207(98)00179-8. [DOI] [PubMed] [Google Scholar]
- 28.Kohek MB, Batista MC, Russell AJ, Vass K, Giacaglia LR, Mendonca BB, et al. No evidence of the inactivating mutation (C566T) in the follicle-stimulating hormone receptor gene in Brazilian women with premature ovarian failure. Fertil Steril. 1998;70:565–7. doi: 10.1016/S0015-0282(98)00203-9. [DOI] [PubMed] [Google Scholar]
- 29.Chesnaye E, Canto P, Ulloa-Aguirre A, Méndez JP. No evidence of mutations in the follicle-stimulating hormone receptor gene in Mexican women with 46, XX pure gonadal dysgenesis. Am J Med Genet. 2001;98:125–8. doi: 10.1002/1096-8628(20010115)98:2<125::AID-AJMG1020>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 30.Sundblad V, Chiauzzi VA, Escobar ME, Dain L, Charreau EH. Screening of FSHR gene in Argentine women with premature ovarian failure (POF) Mol Cell Endocrinol. 2004;222:53–9. doi: 10.1016/j.mce.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Layman LC, Amde S, Cohen DP, Jin M, Xie J. The Finnish follicle-stimulating hormone receptor gene mutation is rare in North American women with 46, XX ovarian failure. Fertil Steril. 1998;69:300–2. doi: 10.1016/S0015-0282(97)00480-9. [DOI] [PubMed] [Google Scholar]
- 32.Achrekar SK, Modi DN, Meherji PK, Patel ZM, Mahale SD. Follicle stimulating hormone receptor gene variants in women with primary and secondary amenorrhea. J Assist Reprod Genet. 2010;27(6):317–26. doi: 10.1007/s10815-010-9404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayorga PM, Gromoll J, Behre HM, Gassner C, And NE, Simoni M. Ovarian response to FSH stimulation depends on the FSH receptor genotype. J Clin Endocrinol Metab. 2000;85:3365–9. doi: 10.1210/jcem.85.9.6789. [DOI] [PubMed] [Google Scholar]
- 34.Wunsch A, Ahda Y, Banaz-Yaşar F, Sonntag B, Nieschlag E, Simoni M, et al. Single-nucleotide polymorphisms in the promoter region influence the expression of the human follicle-stimulating hormone receptor. Fertil Steril. 2005;84(2):446–53. doi: 10.1016/j.fertnstert.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 35.Desai SS, Achrekar SK, Pathak BR, Desai SK, Mangoli VS, Mangoli RV, et al. Follicle-stimulating hormone receptor polymorphism (G-29A) is associated with altered level of receptor expression in granulosa cells. J Clin Endocrinol Metab. 2011;96(9):2805–12. doi: 10.1210/jc.2011-1064. [DOI] [PubMed] [Google Scholar]
- 36.Desai SS, Roy BS, Mahale SD. Mutations and polymorphisms in FSH receptor: functional implications in human reproduction. Reproduction. 2013;146(6):R235–48. doi: 10.1530/REP-13-0351. [DOI] [PubMed] [Google Scholar]
- 37.Achrekar SK, Modi DN, Desai SK, Mangoli VS, Mangoli RV, Mahale SD. Poor ovarian response to gonadotrophin stimulation is associated with FSH receptor polymorphism. Reprod Biomed Online. 2009;18:509–15. doi: 10.1016/S1472-6483(10)60127-7. [DOI] [PubMed] [Google Scholar]
- 38.Kuijper EA, Blankenstein MA, Luttikhof LJ, Roek SJ, Overbeek A, Hompes PG, et al. Frequency distribution of polymorphisms in the FSH receptor gene in infertility patients of different ethnicity. Reprod Biomed Online. 2010;20:588–93. doi: 10.1016/j.rbmo.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Koning CH, Benjamins T, Harms P, Homburg R, van Montfrans JM, Gromoll J, et al. The distribution of FSH receptor isoforms is related to basal FSH levels in subfertile women with normal menstrual cycles. Hum Reprod. 2006;21(2):443–6. doi: 10.1093/humrep/dei317. [DOI] [PubMed] [Google Scholar]
- 40.Unsal T, Konac E, Yesilkaya E, Yilmaz A, Bideci A, Ilke Onen H, et al. Genetic polymorphisms of FSHR, CYP17, CYP1A1, CAPN10, INSR, SERPINE1 genes in adolescent girls with polycystic ovary syndrome. J Assist Reprod Genet. 2009;26:205–16. doi: 10.1007/s10815-009-9308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falconer H, Andersson E, Aanesen A, Fried G. Follicle-stimulating hormone receptor polymorphisms in a population of infertile women. Acta Obstet Gynecol Scand. 2005;84:806–11. doi: 10.1111/j.0001-6349.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 42.Greb RR, Grieshaber K, Gromoll J, Sonntag B, Nieschlag E, Kiesel L, et al. A common single nucleotide polymorphism in exon 10 of the human follicle stimulating hormone receptor is a major determinant of length and hormonal dynamics of the menstrual cycle. J Clin Endocrinol Metab. 2005;90:4866–72. doi: 10.1210/jc.2004-2268. [DOI] [PubMed] [Google Scholar]
- 43.Dolfin E, Guani B, Lussiana C, Mari C, Restagno G, Revelli A. FSH-receptor Ala307Thr polymorphism is associated to polycystic ovary syndrome and to a higher responsiveness to exogenous FSH in Italian women. J Assist Reprod Genet. 2011;28:925–30. doi: 10.1007/s10815-011-9619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dierich A, Sairam R, Monaco L, Fimia M, Gansmuller A, LeMeur M, et al. Impairing follicle- stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. PNAS. 1998;95:13612–7. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim S, Pyun JA, Cha DH, Ko JJ, Kwack K. Epistasis between FSHR and CYP19A1 polymorphisms is associated with premature ovarian failure. Fertil Steril. 2011;95:2585–8. doi: 10.1016/j.fertnstert.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 46.Abdel-Aziz AF, El-Sokkary AMA, El-Refaeey AA, El-Sokkary MMA, Osman HG, El-Saeed RA. Association between follicle stimulating hormone receptor (FSHR) polymorphism and polycystic ovary syndrome among Egyptian women. Int J Biochem Res Rev. 2015;5(3):198–206. doi: 10.9734/IJBCRR/2015/13896. [DOI] [Google Scholar]
- 47.Khafagi Z, Mozdarani H, Behmanesh M, Distribution AA, Of FSHR. ESR1 and ESR2 SNPs among women with polycystic ovary syndrome undergoing in vitro fertilization. Bull Environ Pharmacol Life Sci. 2014;3(8):128–34. [Google Scholar]
- 48.Nikolaou D, Gilling-Smith C. Early ovarian ageing: are women with polycystic ovaries protected? Hum Reprod. 2004;19(10):2175–9. doi: 10.1093/humrep/deh419. [DOI] [PubMed] [Google Scholar]