Abstract
Disrupted daily or circadian rhythms of lung function and inflammatory responses are common features of chronic airway diseases. At the molecular level these circadian rhythms depend on the activity of an autoregulatory feedback loop oscillator of clock gene transcription factors, including the BMAL1:CLOCK activator complex and the repressors PERIOD and CRYPTOCHROME. The key nuclear receptors and transcription factors REV-ERBα and RORα regulate Bmal1 expression and provide stability to the oscillator. Circadian clock dysfunction is implicated in both immune and inflammatory responses to environmental, inflammatory, and infectious agents. Molecular clock function is altered by exposomes, tobacco smoke, lipopolysaccharide, hyperoxia, allergens, bleomycin, as well as bacterial and viral infections. The deacetylase Sirtuin 1 (SIRT1) regulates the timing of the clock through acetylation of BMAL1 and PER2 and controls the clock-dependent functions, which can also be affected by environmental stressors. Environmental agents and redox modulation may alter the levels of REV-ERBα and RORα in lung tissue in association with a heightened DNA damage response, cellular senescence, and inflammation. A reciprocal relationship exists between the molecular clock and immune/inflammatory responses in the lungs. Molecular clock function in lung cells may be used as a biomarker of disease severity and exacerbations or for assessing the efficacy of chronotherapy for disease management. Here, we provide a comprehensive overview of clock-controlled cellular and molecular functions in the lungs and highlight the repercussions of clock disruption on the pathophysiology of chronic airway diseases and their exacerbations. Furthermore, we highlight the potential for the molecular clock as a novel chronopharmacological target for the management of lung pathophysiology.
Keywords: PER2, BMAL1, REV-ERBα, RORα, biomarkers, asthma, COPD, fibrosis
chronic obstructive pulmonary disease (COPD) and asthma are major health concerns worldwide with considerable and well-documented influence on quality of life and mortality (60, 153). Patients with airway diseases including COPD and asthma develop more frequent and severe exacerbation with an increased rate of emergency room visits and hospitalization, mostly at night and in the early morning hours when lung function is lowest (119, 149, 182, 184, 186). Exacerbations lead to deterioration of the disease state and are associated with a rapid decline in lung function (119, 148, 149, 182, 186). Environmental exposomes (measure of all exposures) or individual exposure, such as by air pollutants/particulates, allergens/pollens, tobacco/cigarette smoke (CS), as well as respiratory viral (influenza and rhinoviruses) and bacterial infections can lead to exacerbations of COPD and asthma (149, 184–186). The circadian timing system drives daily changes of airway caliber, airway resistance, respiratory symptoms, mucus hypersecretion and immune-inflammatory responses that underlie this daily variation in exacerbation frequency and occurrence. The effect of virus-induced COPD/asthma exacerbations on clock function in the lung and/or the role of the lung's clock in the pathogenesis of COPD/asthma and its associated exacerbations are largely unknown. Nonetheless, there is a connection between the circadian timing system and the decline in lung function/exacerbations common to both COPD and asthma. Recently, we have defined the role of the molecular clock in regulation of lung cellular and molecular functions, particularly in response to environmental insults [CS and influenza A virus (IAV)] (77, 177, 178, 210). However, the basic role of the molecular clock and the impact of circadian disruption in various lung pathophysiological conditions, the use of clock molecules as biomarkers for pulmonary dysfunction, and the potential for novel chronopharmacological approaches for the treatment of lung disease have yet to be described.
Despite numerous therapeutic advancements, the strength of pharmacotherapy for COPD continues to be reliance on corticosteroids and/or bronchodilators (e.g., β-adrenoreceptor agonists) that do little to reduce mortality, inflammation, or the daily decline in lung function in patients with COPD and in patients with severe asthma who smoke. A considerable number of recent studies support the fact that other environmental factors and exposomes, including air pollutants/particulates (151), xenobiotic detoxification (147, 180), CS (54, 77, 177, 192), shift work (107, 173, 198), jet lag (42, 65), hypoxia/hyperoxia (100, 203), ventilator-induced lung injury (106), and pathogens (bacteria/virus) (57, 174) can disturb molecular clock function in the lungs and hasten the development of lung pathophysiology (Tables 1 and 2). One can conclude from these findings that perturbation of clock function is responsible for changes in various cellular and molecular lung functions during the pathogenesis of chronic airway disease. This concept was recently the focus of an NHLBI workshop (http://www.nhlbi.nih.gov/research/reports/2014-circadian-clock-lung-health), and our own brief translational perspective on molecule clock function in airway diseases (178). Thus there is a growing interest within the lung biology community regarding the mechanism whereby lung physiology and pathology are influenced by circadian clock function.
Table 1.
Role of molecular clock in lung physiology
| Circadian Clock Studies | Experimental Design | Major Outcomes/Findings | Reference |
|---|---|---|---|
| Role of the timing system in rhythmic respiratory function | C57BL/6 and Cry1,2−/− mice. Cervical vagotomy and sympathectomy were performed. Bilateral electrolytic lesion of the SCN (DD and LD 12:12). | Examined the timing system in the respiratory tract and the role of acetylcholine neurotransmission in rhythms of respiratory function. | 9 |
| Role of bronchiolar epithelial cells in lung clock function | C57BL/6J and PER2::LUC transgenic mice ex vivo. Lung explants cultured from PER2::LUC mice. Primary culture of mouse Clara/club cells. (LD 12:12). | Clara/club cells are critical for maintaining clock function in lung; coexpression of the glucocorticoid receptor and clock proteins establishes a likely interface between SCN output and lung physiology. | 58 |
| Molecular link between the circadian clock and xenobiotic detoxification | WT and clock mutant (Clk/Clk) mice injected with benzo[α]pyrene at 09:00 or 21:00. (LD 12:12). | CLOCK protein affects the expression of detoxification enzymes through modulating the activity of AhR. | 180 |
| Diurnal oscillations in the lung transcriptome | Male Wistar rats (LD 12:12). | Genes involved in the metabolism and transport of endogenous compounds, xenobiotics, and therapeutic drugs; other biomarkers or potential therapeutic targets genes for lung disease exhibit diurnal oscillations, suggesting clock control of lung physiology and pathophysiology. | 173 |
AhR, aryl hydrocarbon receptor; CRY, cryptochrome; DD, dark-dark; LD, light-dark; SCN, suprachiasmatic nucleus; WT, wild-type.
Table 2.
Role of molecular clock in lung pathology by environmental agents using animal models
| Circadian Clock Disruption Models/Agents | Experimental Design | Major Outcomes/Findings | Citation |
|---|---|---|---|
| Cigarette smoke (CS) | · Sprague-Dawley rats exposed for 2, 7, and 13 wk (LD 12:12). | · Transcriptomic changes in specific program of defense (phase I and II detoxifying), innate and adaptive immune, inflammation and circadian clock gene expression. | 54 |
| · C57BL/6J and AJ (male) mice (LD 12:12) exposed to whole smoke for a period of 10 days. | · Nr1d1 gene expression is suppressed by CS, which may affect clock function and play a complementary role in CS-induced lung respiratory tract pathobiology and/or lung tumorigenesis. | 192 | |
| · C57BL/6J, Bmal1 Cre-CC10 knockout, Sirt1+/− and Sirt1 transgenic mice exposed to acute (3 days and 10 days) or chronic CS (6 mo) TPM: 100–300 mg/m3). Inverted LD 12:12 for acute 10 days and regular LD 12:12 for 3 days and 6 mo. | · CS exposure alters clock gene expression and reduced locomotor activity by disrupting central and peripheral clocks and augments lung inflammation, leading to COPD/emphysema via the SIRT1-BMAL1 pathway. | 77 | |
| · C57BL/6J mice exposed to acute (3 days and 10 days) or chronic CS (6 mo) (TPM: 100–300 mg/m3). Inverted LD 12:12 for acute 10 days and regular LD 12:12 for 3 days and 6 mo. | · CS exposure affected both the timing and amplitude of the daily rhythms of plasma corticosterone and serotonin (stress hormones). | 177 | |
| Lipopolysaccharide (LPS) | · Conditional club cell Bmal1-knockout mice (Ccsp-Bmal1−/−) and LysM-Bmal1−/− mice were treated with intraperitoneal LPS (1 mg/kg) at CT0. (LD 12:12 and DD 12:12). | · Genetic ablation of the clock gene Bmal1 (also called Arntl or MOP3) in bronchiolar cells disrupts rhythmic Cxcl5 expression demonstrating circadian clock role in host defense to bacterial infection and pulmonary inflammation. | 57 |
| · C57BL/6J and Bmal1−/− mice were given a single intraperitoneal LPS (12 mg/kg) (LD 12:12; LL 12:12 and DD 12:12). | · Genome-wide approach to investigate alterations in clock gene expression during LPS-induced lung inflammation produces a complex reorganization of cellular and molecular circadian rhythms (reprogramming) that are important in early events that occur during lung injury. | 70 | |
| Bleomycin | · C57BL/6J, Nrf2−/−, PER2∷Luc and ClockΔ19 mice intratracheally instilled with bleomycin (1 mg/kg) or vehicle at either ZT0 or ZT12. Rescue studies, sulforaphane (10 mg/kg ip) or vehicle administered at ZT6 prior to a bleomycin challenge at ZT12 and every other day for 7 days. (LD 12:12 and DD 12:12). | · Circadian clock as an endogenous regulatory mechanism controlling the rhythmic activity of the redox-sensitive transcription factor Nrf2/glutathione-mediated antioxidant defense pathway. | 147 |
| Chronic jet lag models (Chronic shift-lag/simulated jet lag/chronic phase advance) | · Male Fischer (F344) rats were exposed to LD 12:12 or a chronic shift-lag (10 repeated 6-h photic advances occurring every 2 days, followed by 5–7 days of constant darkness). | · Chronic shift-mediated circadian disruption promotes tumorigenesis, alters gene expression of Per2 and Bmal1 and cytolytic factors that correlate with suppressed circadian expression of NK cytolytic activity. | 107 |
| · C57BL/6J female and male mice were maintained on a LD (12:12) control or exposed for 4 wk to a shifting light regimen (SJL). | · Circadian disruption alters lung mechanics by shifting light regimen (SJL) and clock gene expression in a sexually dimorphic manner. | 65 | |
| · Chronic phase advance (SJL) protocol involved 6-h phase advances (earlier light onset) every 4 days for 8 wk. (LD 12:12 and DD 12:12) in WT and Bmal1−/−, Per2::Luc mice. | · Chronic phase advance alters physiological rhythms and peripheral molecular clocks; SJL advanced the phase of the rhythm immediately after a shift and mice placed into free-running conditions (DD) for 2 wk after SJL also showed molecular clock shifted in the lung. | 198 | |
| Hyperoxia | · WT and NALP3−/− mice were exposed to room air or hyperoxia for 24, 48, or 72 h. Different concentrations of O2 were used for hyperoxia (50, 75, or 100%) or room air for 72 h. | · Alterations in clock genes are associated with increased inflammatory markers in bronchoalveolar lavage fluid of hyperoxic mice and a dose-dependent increase in clock gene expression with increased O2 concentrations. | 100 |
| · Neonatal (<12-h-old) or adult mice (2-month-old) WT and p50−/− mice exposed to either 21% O2 or room air or 95% O2 for 72 h. | · Clock genes are regulated by oxidative stress and inflammation in the neonatal lung hyperoxia model; REV-ERBα play an essential role in lung cellular function and injury | 203 | |
| Ventilator-induced lung injury (VILI) | · Sprague-Dawley rats endotracheally intubated and placing on a mechanical ventilator (tidal volume of 40 ml/kg or 10 ml/kg without positive end-expiratory pressure). | · Rev-erbα mRNA and protein is decreased in high tidal volume mechanical ventilation group compared with spontaneous group; treatment with REV-ERBα agonists greatly diminished VILI-induced lung edema, inflammatory cell infiltration and proinflammatory cytokine (TNF-α) release. | 106 |
| Cigarette smoke + Influenza A virus (IAV) | · C57BL/6J exposed to chronic air or CS (6 mo), followed by IAV infection (postinfection days 1–9) and WT littermates and Bmal1−/− mice infected with IAV (postinfection days 1–9). (LD 12:12). | · Chronic CS exposure combined with IAV infection altered the timing of clock gene expression, reduced locomotor activity along with increased lung inflammation, emphysema, and disrupted rhythms of lung function; BMAL1 KO mice infected with IAV showed pronounced detriments in behavior, survival, lung inflammatory and profibrotic responses in vivo. | 174 |
CS, cigarette smoke; CT, circadian time; CPA, chronic phase advance; CRY, cryptochrome; DD, dark-dark; HEPA, high-efficiency particulate air; IAV, influenza A virus; LD, light-dark; Nr1d1, nuclear receptor subfamily 1, group D; NK cells, natural killer cells; PER, period; SJL, simulated jet lag; TPM, total particulate matter; VILI, ventilator-induced lung injury; ZT, zeitgeber time.
The development of novel small molecule activators or inhibitors of clock function has stimulated interest in the potential clinical application of chronopharmacology. Chronopharmacology refers to the use of small molecules to target the timing and amplitude of clock or clock-dependent gene expression for treating disease (41). In this review, we have amalgamated the findings in chronobiology and cell and molecular biology of lung pathophysiology and merged them in light of emerging details regarding the regulation of clock function by environmental stressors and and/or the molecular clocks role in the development of chronic airway disease. We propose that the levels of molecular clock components in cells from systemic circulation and/or sputum may be useful as novel biomarkers of disease severity and exacerbations and for assessing the viability and effectiveness of circadian-based therapy for disease management. Finally, we elucidate the potential therapeutic targets and approaches of interest for the management of chronic lung diseases using chronotherapy.
The Circadian Timing System and Pulmonary Physiology
Circadian rhythms are intrinsic biological oscillations with a period near 24 h driven in mammals by the circadian timing system (3). Circadian rhythms are generated at the cellular level by an autoregulatory feedback loop of interlocked transcription factors referred to collectively as clock genes (124) (Fig. 1). In mammals, the BMAL1:CLOCK activator complex regulates expression of period (Per1-3) and cryptochrome (Cry1-2) genes. PER and CRY form heterodimers, are phosphorylated, and translocate back to the nucleus, where they repress their own transcription by blocking the activity of the BMAL1:CLOCK complex (64). The counteracting key nuclear receptors REV-ERBα (Nr1d1; nuclear receptor subfamily 1, group D, member 1) and retinoic acid-related orphan receptor-α (RORα) provide stability to the oscillator by regulating the timing and amplitude of Bmal1 expression. Molecular clock proteins are heavily influenced by posttranslational modifications, including acetylation and phosphorylation, which affect both their activity and stability (73, 129). The detailed molecular mechanisms of each modification and their impacts on clock-dependent cellular functions in peripheral organs, including the lungs, are largely unknown.
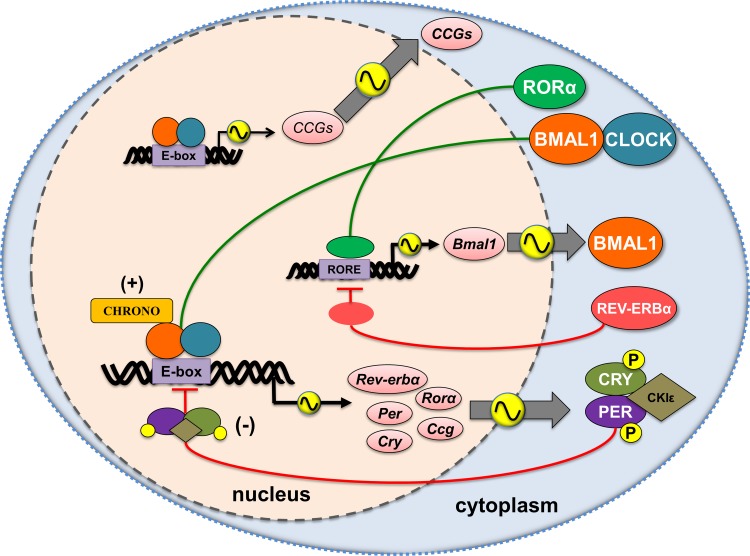
Fig. 1.
Schematic representation of the core clock mechanism and rhythmic output of key target genes that regulate the molecular clock function in the lung. The molecular clock in mammals is composed of a heterodimer of the transcription factors CLOCK and BMAL1, which regulates the transcription of Per (Period) and Cry (Cryptochrome) via E-box sequences within their promoters. PER and CRY heterodimerize and block E-box-dependent transcriptional activity of the CLOCK:BMAL1 complex. The timing of BMAL1 expression depends on the counteracting activity of REV-ERBs and ROR (retinoic acid receptor-related orphan receptor) proteins. CHRONO negatively regulates circadian oscillations by directly interacting with BMAL1. Environmental agents influence lung cells (e.g., epithelial cells) and macrophages through activation of kinases (MAP kinase, casein kinase2, CKII, glycogen synthase kinase GSK3 β, protein kinase C, and PI-3 kinase) that lead to posttranslational modification (phosphorylation/acetylation) of molecular clock proteins (e.g., BMAL1 and PER2). Molecular clock dysfunction negatively affects lung functions and may be a significant factor in the pathobiology of chronic airway diseases. CKIε/δ, casein kinase 1 ε/δ; CHRONO, ChIP-derived repressor of network oscillator; CLOCK, circadian locomotor output cycles kaput; CCG, clock controlled genes; CRY 1,2, cryptochrome 1,2; REV-ERBα, nuclear receptor subfamily 1, group D, member 1; PER1,2, period 1,2; RORα, retinoic acid receptor-related orphan receptor α.
The central pacemaker of the timing system is located within the suprachiasmatic nucleus (SCN) of the basal hypothalamus, though peripheral tissues including the lung are also comprised of cell autonomous oscillators (3, 9). The circadian clock in the lung plays a critical role in optimizing the organization of cellular function and responses to environmental stimuli (103, 156). Any significant change in the timing or amplitude of clock gene expression, commonly referred to as circadian disruption, can affect downstream clock-controlled output genes or physiological processes and has been implicated in chronic disease (50, 112). In addition to clock disruption at the tissue level, desynchrony between central and peripheral oscillators is known to increase the risk of metabolic, endocrine, and cardiovascular disease. In healthy individuals, circadian misalignment results from an acute change in the phase of light-exposure (photoperiod), dissociating internal timing from the external environment (e.g., “jet lag” or shift work) (198). Circadian misalignment influences physiology through its impact on internal circadian organization, defined as the coordinated timing of clock-dependent cell and molecular functions. Internal desynchrony occurs as a result of the differential resetting or “shifting” rates (also called entrainment kinetics) of central and peripheral oscillators (50). Although the malaise of jet lag is generally considered a minor inconvenience, it has been shown that a single 6-h advance repeated over 8 wk can increase mortality in older mice (43). Mice exposed to non-24-h days for short duration (20-h day; ∼10 wk), a treatment that mimics the effects of a daily 4 h advance, exhibit features of metabolic, endocrine, and neurophysiological disease (82). Moreover, human subjects exposed to similar non-24-h days for short duration show a dramatic decline in metabolic, endocrine, and autonomic nervous function (22). Notably, internal desynchrony may also arise in response to other environmental exposomes or physiological perturbations (e.g., CS exposure) independent of changes in the photoperiod and may be a major factor in the etiology of clock-dependent pathophysiology (50, 74, 158, 198). Although evidence has accumulated for significant negative effects of circadian misalignment on metabolism, immune function, cognition, and fertility, similar evidence for an impact on pulmonary physiology or lung pathophysiology has yet to be acquired.
Rhythms of clock gene expression have been reported in the lungs (138, 139), including bronchial epithelial cells (58). Emerging studies show the role of clock dysfunction in pulmonary physiology and pathology, particularly in response to proinflammatory mediators like CS. The timing and amplitude of clock genes and putative clock-controlled gene (CCG) expression in rodent lungs is altered by exposure to CS (54) and proinflammatory mediators like LPS and TNF-α (29). The magnitude of phasic responses to proinflammatory factors can be quite significant. Data have shown that neutrophil influx recorded during LPS-mediated lung inflammation is three- to sixfold higher in Ccsp-Bmal1−/− mice (which specifically lack Bmal1 in the epithelial/club cells) compared with Bmal1fl/fl littermate controls, resulting in a complete loss of rhythmicity (57). This increased neutrophil response was associated with a loss of barrier function as confirmed by elevated IgM levels in bronchoalveolar lavage (BAL) fluid and increased CXCL5 expression in phase with the peak of LPS-induced neutrophil influx. CXCL5 in BAL fluid of LPS-treated Ccsp-Bmal1−/− mice was also two- to threefold higher compared with Bmal1fl/fl controls at both CT0 and CT12 (CT; circadian time, subjective time base relative to the activity period such that CT12=activity onset) (57). These findings are in agreement with early reports revealing that LPS-induced serum cytokines [IL-6, IL-12(p40), CXCL1, CCL5, and CCL2] measured at either CT0 or CT12 showed significant time-dependent variation in response magnitude (59). Similarly, CS-induced lung inflammation in lung epithelial cell-specific BMAL1 knockout (KO) displayed a two- to threefold increase in proinflammatory cytokine gene expression (ccl1/mcp1 and cxcl1/kc) and cytokine release (MCP-1 and KC) compared with air exposed controls (ZT0 vs. ZT12; ZT, zeitgeber time, objective time base relative to the 12:12 L:D cycle such that ZT12=lights off) (77). We recently observed similar effects on proinflammatory MCP-1 release (twofold increase; ZT6/12 vs. ZT18/24) in chronic CS-exposed mice infected with influenza virus compared with air-exposed mice infected with influenza virus (174). We have also recently reported that lung function varies significantly during time of day (day vs. night) among chronic air, CS, and their combination with virus-infected mice (77, 174). Together, these results highlight the involvement of a local/peripheral clock in lung cells of both mouse and humans that can both be modified by inflammation and regulate the timing of inflammatory responses. Accumulating evidence also suggests that circadian disruption has a profound influence on pulmonary function and lung pathophysiology, particularly in lung epithelium (50, 57, 58, 65, 70, 77, 79, 147, 174). Clock function in lung tissue is clearly altered by CS stress both in vivo and in vitro. Furthermore, using targeted deletion with conditional KO mice, these studies have begun to define the functional relationship between the molecular clock and pulmonary physiology, particularly as it relates to airway disease produced by environmental factors.
Normally, pulmonary function exhibits a robust circadian rhythm, with a noon maximum (12:00 h) and an early morning minimum (04:00 h) in healthy individuals. The early morning surge in lung function coincides with exacerbations of COPD/asthma in susceptible individuals (11). Other symptoms of airway diseases also vary as a function of time (11). These symptoms include increased hoarseness in the morning hours and increased wheezing in the middle of the night. Though the mechanisms behind these daily variations in symptomology are complex, it has been speculated that autonomic input to airway tissues via the vagal nerve plays an essential role (127). The autonomic nervous system communicates timing signals from the SCN to peripheral oscillators via two routes: 1) sympathetic (e.g., intermediolateral cell column) and 2) parasympathetic (e.g., dorsal motor nucleus of the vagus and the region nearby the nucleus ambiguous) innervation (17). Bando et al. (9) for the first time showed that vagal nerves convey rhythmic signals to the respiratory clock in airway tissues (i.e., larynx, trachea, bronchus, and lung). These oscillations were abolished in arrhythmic Cry1−/−Cry2−/− KO mice and also in wild-type mice after electrolytic lesion of the SCN (9). Hemivagotomy completely suppressed clock gene expression in the (operated) ipsilateral submucosal glandular cells but only moderately suppressed clock gene expression in epithelial cells. In contrast, clock gene expression was not affected after sympathectomy. Since SCN lesions abolish respiratory rhythms it is presumed that the vagal nerve is the dominant route whereby circadian timing signals are conveyed from SCN to the respiratory tract. These findings support the assertion that cells involved in pulmonary system contain a functional clock that is influenced by the SCN (9) but may regulate cell-specific function semi-independently of central timing cues (Table 1).
Chronobiology of Lung Pathophysiology and Inflammation
Though circadian rhythms of lung function in healthy individuals have been documented, the data remain somewhat controversial and the majority of evidence actually stems from the study of lung function in asthmatic patients (23). More pronounced troughs at night in forced vital capacity, forced expiratory volume in 1 s (FEV1), and peak expiratory flow are found in smokers than in nonsmokers (16, 27, 152). This could be due to oxidative/carbonyl stress imposed by CS stress-dependent effects on rhythms of surfactant protein levels, mucus retention/secretion, and lung inflammation (87). The early morning deterioration in pulmonary function observed in asthmatic patients is associated with decreased serum epinephrine levels, increased vagal nerve tone and cholinergic activity, esophageal reflux, and increased circulating eosinophils due to time-of-the-day response (23). It has also been reported that airway resistance peaks in the morning and reaches its nadir at noon, followed by an increase in the afternoon (75). This pattern is very similar in healthy individuals and also in patients with restrictive and stable obstructive disease (75).
In addition to altered patterns of lung function, patients with COPD or asthma also have poor sleep quality, increased sleep latency, decreased total sleep time, increased waking after sleep onset, and decreased rapid eye movement and non-rapid eye movement sleep episodes (104, 144). These individuals are also commonly diagnosed with sleep disorders including obstructive sleep apnea (113). Psychological distress in those with COPD/asthma is associated with a decline in lung function, increased exacerbation frequency, and worsening of cardiovascular disease, further disrupting sleep in these patients (114). Insomnia, excessive daytime sleepiness, and nocturnal oxygen desaturation are also common in patients with COPD/asthma. Disrupted sleep in patients with COPD/asthma correlates with respiratory symptoms (cough, sputum production, wheezing), nocturnal oxygen desaturation, hypercapnia, and circadian changes in airway caliber and resistance (105). A recent report revealed that disruption of circadian molecular clock function following bacterial endotoxin challenge alters the innate immune response without marked sleep loss, suggesting that the effects of circadian disruption on immune function are independent of their impact on sleep/wake rhythms (150). Similarly, changes in the timing and amplitude of lung function also occur in nocturnal asthmatic patients. Importantly, there is evidence that steroids and bronchodilators may be less potent at night, when the circadian nadir in FEV1 renders these patients most likely to experience an exacerbation (18, 52, 115, 142). Furthermore, corticosteroids and/or bronchodilators (e.g., β-adrenoreceptor agonists), the mainstays of pharmacotherapy for COPD, fail to reduce mortality or inflammation or to improve the rhythmic decline of lung function in patients with COPD (24, 25). These results highlight the need for development of novel strategies based on circadian molecular clock for the treatment of these pulmonary disorders that account for the rhythmic variation of lung function across the 24-h day.
Circadian Disruption and Redox Regulation by Oxidants
The impact of molecular clock dysfunction on lung inflammatory responses via redox cellular changes has been investigated by using mice with loss-of-function clock gene mutations. Antioxidant redox-sensitive transcription factor Nrf2 activity in the lungs was altered in mice expressing a dominant negative mutation of the CLOCK protein (ClockΔ19). This effect of the clockΔ19 mutation on Nrf2 is complemented by reduced glutathione levels, increased protein oxidation, and a spontaneous fibrotic-like phenotype (147). In the same study, rhythms of Nrf2 levels/activity in Nrf2/GSH pathway were demonstrated in vivo in mouse lungs (147). Recently, it was revealed that dioxin-mediated activation of the aryl-hydrocarbon receptor (Ahr) signaling pathway is influenced by CLOCK, suggesting clock-dependent regulation of phase I detoxifying genes (159, 180). Clock mutant mice, which express a dominant negative CLOCK protein lacking histone acetyltransferase (HAT) activity, also lack a rhythm of Ahr gene expression (180). This highlights the role of circadian disruption in abnormal metabolism of inhaled toxicants. As such, the levels of Ahr mRNA in the lungs of ClockΔ19 mutant mice remained lower compared with wild-type mice (180). Arrhythmic bmal1−/− mice also show signs of advanced aging and underlying pathologies, correlated with increased levels of reactive oxygen species and inflammation (92, 93).
It has been reported that circadian clock genes regulate oxidative stress (e.g., markers of lipid peroxidation are increased in Bmal1 KO mice), scavengers of reactive aldehydes during mitochondrial respiration (including ALDH2), and NADPH dehydrogenases [such as NQO1, which encodes quinone 1 (126)]. The cellular redox state alters circadian oscillation through its influence on Bmal1 expression and several mitochondrial proteins are rhythmically expressed, suggesting a potential reciprocal relationship between the core molecular clock mechanism and the cellular redox state (72, 94, 137, 214). Circadian clock-dependent cycles of cellular NAD+ levels can drive mitochondrial oxidative metabolism via SIRT1 and regulate rhythm patterns of oxidative enzyme expression and cellular respiration. This implicates the involvement of NAD+ bioavailability in modulating both mitochondrial oxidative metabolism and circadian clock function (146). REV-ERBα modulates oxidative capacity by regulating mitochondrial biogenesis in skeletal muscle (197). Muscle dysfunction is known to occur in COPD, further linking chronic inflammatory lung diseases, molecular clock function, and oxidative stress. Additional data suggest that the timing of clock gene expression in mammalian tissues is affected by redox modulation [e.g., regulation of cytochrome P-450 and regulation of ROR elements via interaction with REV-ERBα (95)]. Similarly, alcohol consumption, which alters the redox status of the cells and phase II genes, can affect the molecular clock. This effect may be augmented in smokers due to the formation of aldehyde adducts on molecular clock proteins. Overall, this suggests that redox status of the cells or redox modulation of molecular clock gene expression plays an integral role in various cellular and molecular functions in the lung.
Circadian Disruption and Its Influence on Inflammatory and Immune Responses in the Lungs
Immune-inflammatory parameters fluctuate across the 24-h day, and disturbance of these rhythms has been associated with infectious and inflammatory diseases (30, 38). Recent evidence suggest that the molecular clock regulates fundamental aspects of the immune-inflammatory response (38), including Toll-like receptor 9 (TLR9) signaling and repressing chemokine (C-C motif) ligand 2 (CCL2) expression (163, 166). The clock protein and nuclear heme receptor REV-ERBα attenuates the activation of IL-6 expression (59). REV-ERBα binds to nuclear factor-κB (NF-κB) RelA/p65 and can activate NF-κB-dependent transcription (172). Transcription factors activator protein 1 (AP-1) and NF-κB share unique motifs that overlap consensus sequences from the REV-ERBα promoter, suggesting a role for REV-ERBα in regulation of oxidative stress and inflammation (203). REV-ERBα regulates clock-output genes by binding to and recruiting NCoR and HDAC3 to the promoter region of target inflammatory genes. BMAL1 KO animals display upregulation of the prostaglandin synthase gene (ptgs2), TNF-α, and IL-6, suggesting that molecular clock disruption can lead directly to inflammatory responses (126). Targeted deletion of Bmal1 in myeloid cells enhances the number of specific monocytes (Ly6Chi) in the spleen and circulation during peritoneal inflammation in a time-dependent manner (134). This study also revealed that BMAL1 binds to E-boxes in the promoters of Ccl2, Ccl8, and S100a8 (encoding S100 calcium binding protein A8) and recruits members of the polycomb repressor complex (PRC2). This action stimulates repressive histone marks and blocks transcription, thereby suppressing Ly6Chi monocyte numbers and inflammation at the site of damage (134). Together, these data confirm a role for the clock in the control of the inflammatory response in the lungs and suggest that diminished clock function can attenuate normal responses to environmental inflammatory agents like CS.
Recent data show that circadian clocks regulate immune function (99) since molecular clock disruption can lead to both pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) responses. PAMPs are typically microbial or viral macromolecular components. Evidence suggests that several endogenous molecules are released from damaged cells and tissues and these molecules were collectively termed DAMPs (123). These DAMPs are also capable of inducing inflammatory responses similar to that produced by PAMPs (10, 123). Some of the signaling pathways central to PAMPs and DAMPs show a daily variation in the response to their ligands. For example, TLR4 is shown to oscillate and have differential inflammatory responses during a specific period of its activation. TLR9, which is bound by unmethylated repeats of the dinucleotide CpG, shows a daily variation of lung function particularly in animal models of sepsis. The molecular clock in immune cells influences the release of cytokines and chemokines through pattern recognition receptors and cellular functions including phagocytosis and migration of inflammatory/immune cells to the site of infection. Support for circadian control of innate immunity comes from recent work in macrophages (39). Myeloid cells show temporal regulation of inflammatory responses and BMAL1 inhibits activation of NF-κB and miR-155 induction, protecting mice against LPS-induced sepsis. Finally, circadian disruption leads to altered sleep and immune responses in mice (150). This suggests that circadian disruption-induced modulation of inflammatory and immune responses may underlie the reduced quality of sleep reported in patients with chronic lung disease.
Bacterial/Virus Infections and Molecular Clock Dysfunction in the Lungs
Recent studies have also shown that bacterial infection alters the timing and amplitude of clock gene expression in the lungs (14, 59, 134), though the mechanism of this response is largely unknown. The clock appears to play a role in the degree of inflammation, the timing of cytokine gene expression, and bacterial colonization (14, 59). This could be in part due to increased leukocyte numbers and/or dependence on circadian regulation of antimicrobial peptides (14). Similarly, mouse macrophages showed enhanced ability to ingest particles near the onset of activity, indicating significant daily variation in regulating host defense (71). The Toll-like receptor 4 (TLR4) pathway in peritoneal macrophages is regulated by the clock and may drive rhythmic variation in immune responses (86). In lung tissue, the epithelial clock and glucocorticoid hormones control diurnal variation of pulmonary inflammatory responses to bacterial infection (57). Furthermore, endotoxemia-induced lung inflammation reorganizes circadian rhythms of leukocytes in a Bmal1-dependent manner (70). Likewise, rhythms of pulmonary function define the time-dependent sensitivity to steroids and β2-agonists in patients with nocturnal asthma and asthmatic patients who smoke (18, 52). It is likely that the mechanism that couples the molecular clock and bronchiolar glucocorticoid receptors to pulmonary immune-inflammatory responses plays an important role during COPD exacerbations and respiratory infections (178). Viral respiratory infection (influenza A) also causes molecular clock dysfunction in the lungs and increases mortality in bmal1 KO mice, suggesting that diminished clock function depresses the immune response to respiratory infection (174). Although we have observed direct effects of infection on clock function, inflammatory responses, and mortality, it is unclear whether these effects are core clock dependent or indirectly related to dysfunction-mediated changes in non-clock-dependent cellular programming in lung tissue.
Molecular Clock as Biomarkers of Lung Inflammatory and Therapeutic Responses
Recent literature implies that systemic monitoring of the molecular clock may be used as a biomarker or chronobiomarker (time-of-day sampling of genomic/transcriptomic, proteomic, and metabolomic biomarkers that was termed CircadiOmics) (118, 145, 187) of disease and or the response to treatment. These emerging biomarkers may provide key information about the inflammatory response (e.g., recruitment of inflammatory/immune cells) and the release of proinflammatory mediators at baseline or in response to treatments.
As previously noted, circadian misalignment during chronic jet lag alters clock gene expression and pulmonary function in mouse lungs (65). Circadian disruption may also occur as a direct result of tissue and/or cell specific abnormalities in the timing and amplitude of clock gene expression, independent of changes in the external environment (e.g., photic shifts). This form of circadian disruption can perpetuate internal circadian misalignment due to dissociation between the effected tissue and remainder of the animal. Specifically, molecular clock dysfunction is known to affect pulmonary physiology and injurious/damaging responses (57, 65, 77, 174, 177). Similarly, environmental CS exposure or exposomes and viral infection result in circadian disruption, producing molecular clock dysfunction and enhanced inflammatory responses in the lungs (55, 77, 174). Recent data reveal that passive tobacco/cigarette smoking can also affect molecular clock function (135). These data suggest that different forms of circadian disruption can have converging effects on the molecular clock in lung tissue, leading to enhanced injurious and inflammatory responses.
The effects of CS on the molecular clock appear to be Sirtuin 1 (SIRT1)-dependent as they lead to increased acetylation and degradation of BMAL1 in lung tissue. SIRT1 is also known to affect the expression of PER2, and the combined effects of SIRT1 on both BMAL1 and PER2 expression underlies the pathological responses to CS. We have recently shown that molecular clock dysfunction along with SIRT1 reduction contributes to abnormal inflammatory responses in smokers and COPD patients (210). The levels of clock proteins including BMAL1, PER2, and REV-ERBα are reduced in human peripheral blood mononuclear cells (PBMCs), sputum cells, and lung tissues associated with inflammatory responses in patients with COPD compared with nonsmokers (210). This highlights the role of the molecular clock in the pathogenesis of airway disease, making it an excellent candidate as a biomarker of disease severity [e.g., in terms of regulating local/systemic inflammatory responses (44)]. We and others have established that environmental CS, shiftwork, and chronic jet lag can have near parallel influences on the timing system and the inflammatory response (43, 65, 77, 107, 164, 177, 192). Other reports indicate that inflammation due to septic shock also leads to circadian disruption (28, 70, 140, 163, 198). Hence, it is likely that circadian disruption-induced molecular clock dysfunction augments lung inflammatory responses to environmental factors including CS and infectious agents. Altered clock function following exposure to various environmental factors may predispose the system to exaggerated inflammatory responses and hasten the development of pathophysiology in chronic airway disease. The cellular metabolome, which is affected as a result of nutrient intake, is regulated by the molecular clock and SIRT1 (141). Recently, it has been shown that metabolites produced by oxidation of fatty acids can regulate SIRT expression and the clock (132, 133, 183). As an example, palmitate inhibits the SIRT1-dependent Bmal1-Clock interaction and disrupts clock gene oscillations. Furthermore, assessment of molecular clock function may be used as a biomarker of ongoing inflammatory responses and for monitoring the severity of lung pathophysiology (Table 2).
Recently, the role of the circadian clock in mouse physiology and behavior was assessed by use of RNA-seq and transcriptome arrays. This analysis revealed that key drugs used in respiratory medicine are linked to circadian regulatory genes (including Advair Diskus for asthma and COPD; Serpina6, Pgr, Nr3c2, Adrb2, Pla2g4; Combivent for asthma and COPD; Slc22a5, Slc22a4, Chrm2, Adrb1, Adrb2 and ProAir HFA for asthma and COPD; Adrb1 and Adrb2) (212). These targets are potentially clock dependent and may show gene- and time-specific responses to treatment, supporting the notion that clock-driven genes may be useful as biomarkers for drug efficacy in respiratory diseases. These authors have characterized the role of the circadian clock in physiology and found that the expression of many oscillating genes peaked during transcriptional “rush hours” preceding dawn and dusk. Strikingly, heart and lung were notable exceptions, with phase distributions that diverged substantially from other organs. They also found oscillations in the expression of more than 1,000 known and novel noncoding RNAs (ncRNAs). It was found that the majority of best-selling drugs and World Health Organization essential medicines directly target rhythmic genes. Many of these drugs have short half-lives and may benefit from timed dosage or chronotherapeutic strategies (212).
Circadian Disruption and Its Impacts on Stress-Induced Lung Cellular Senescence
Aging differentially affects central and peripheral clock function (74, 164). Conversely, it is likely that cellular senescence due to molecular clock dysfunction is enhanced by chronic CS exposure, persistent shiftwork, or respiratory infection in susceptible smokers. Tobacco smoking is a well-known factor in premature lung aging reflected as a rapid decline in lung function. Circadian disruption has established adverse effects on the elderly population and susceptible smokers (60, 191). As mentioned above, clock dysfunction significantly increases mortality in aged mice. Thus circadian disruption may cause and/or enhance stress-induced cellular senescence via a molecular clock-dependent mechanism, particularly in aged population/patients with chronic airway diseases and susceptible smokers that suffer from frequent exacerbations. In fact, data suggest that clock disruption in Bmal1 KO mice leads to enhanced rates of cellular senescence in multiple tissues including the lungs (92, 93). Though certainly related, a clear link between altered circadian organization, circadian clock function, and aging at the cellular, molecular, and physiological level has yet to be established.
Environmental tobacco smoke is shown to cause molecular clock dysfunction in the lungs in connection with DNA damage and impaired double-strand break (DSB) repair, which is aggravated in COPD (26, 45, 189, 190). Lungs of COPD patients show persistent DNA damage associated with stress-induced premature senescence (SIPS) and a senescence-associated secretory phenotype (SASP) (160, 161). DNA damage leads to an inflammatory phenotype in senescent cells (160, 199). Nonhomologous end joining (NHEJ) in response to stress also occurs in lung cells (110). The molecular clock is shown to regulate the strengths of cellular responses to DNA damage (53, 162). Mice deficient in Bmal1 exhibit early signs of aging (92, 93) via augmented DNA damage/impaired DNA repair (NHEJ), cellular senescence, and inflammatory responses (reviewed in Ref. 178). Circadian clock proteins also interact with other factors including Ku70, Ku80, and ataxia-telangiectasia mutated (ATM) involved in DNA damage checkpoints following genotoxic stress, thereby mediating DNA damage/repair responses (53, 56, 80, 90, 112, 162). Recently, it has been demonstrated that PER1 interacts with ATM/Chk2 complex to modulate DSB damage induced by ionizing radiation (56). It has been shown that BMAL1 negatively regulates prosenescent gene p21, which is required for cell cycle arrest upon DNA damage, further supporting a role for BMAL1-dependent gene expression in cellular senescence (63). Expression of XPA, the rate-limiting factor in nucleotide excision repair, displays a clear circadian rhythm (81). Hence, it is possible that CS-mediated clock dysfunction in the lungs may enhance the susceptibility to DNA damage, thus promoting lung cellular senescence during the pathogenesis of COPD and other chronic airway diseases by inhaled toxicants.
Further role of NAD utilization in the control of circadian rhythms during DNA damage response via SIRT1/PARP1 axis is recently shown (108, 109). It has been reported that genotoxic stress-mediated DNA damage response affects circadian clocks directly/indirectly via the key DNA damage response proteins, SIRT1 and PARP1, which utilize NAD (108, 109). Increased PARP1 activity triggers SIRT1-induced phase advances by decreasing SIRT1 activity in response to reduced NAD+ levels. This competitive inhibition may operate through protein acetylation and phosphorylation to alter the timing of the clock during DNA damage (108, 109). The molecular clock also plays a role in apoptosis and cell cycle arrest by altering the expression of apoptosis-related genes. The protein levels of c-Myc and Cyclin B1 are both downregulated and p53 levels are upregulated during circadian disruption. Data suggest that the clock may play an important role in carcinogenesis by inhibiting apoptotic cell death via attenuating proapoptotic signaling (194). Certain genes that function in cell proliferation and tumor suppression are CCG. Several of these, including c-myc and the tumor suppressor p53, are involved in regulating the cell cycle and apoptosis. Other putative CCG involved in cell cycle regulation include the caspases, c-myc, and cyclins. The deregulation of c-myc is associated with various cancers and with the hyperplastic growth of mammalian tissues. Previous studies have also indicated that c-myc overexpression may enhance cell cycle progression in the presence of genomic DNA damage as seen in lungs of patients with COPD and idiopathic pulmonary fibrosis (IPF).
A model has been developed to connect the molecular clock to DNA damage responses and metabolism via the regulation of chromatin remodeling. This bioinformatics model predicts a molecular signaling mechanism through which multiple forms of perturbation, as a result of DNA damage, lead to alteration in molecular circadian clock (109). Importantly, SIRT1 and PARP1 appear to modulate the timing of the molecular clock in response to genotoxic stress (109). This model further revealed the integration of multiple forms of posttranslational modifications by environmental stress, including acetylation of NAMPT and other kinases including CHK2. It also predicts the role of SIRT1 in regulating histone/nonhistone protein deacetylation in relation to core clock function. The SIRT1-PARP1 system regulates clock function in respiratory diseases via the effects of posttranslational modifications, such as acetylation, methylation, sumoylation, and ubiquitination.
The Molecular Clock and the Temporal Control of “Omics”
The CircadiOmics (http://circadiomics.igb.uci.edu/) provides a consolidated model that integrates metabolomic data along with exposomics, genomics, transcriptomics, and proteomics, to better understand time-of-day coordination of pathophysiology. It is possible to include additional -omics approaches, such as lipidomics or breathomics, that could also be developed in the future as valuable clinical tools for personalized medicine in respiratory disease (145, 196). Epigenomic alterations, DNA methylation, histone modifications, proteomics, lipidomics, and recent data on the role of long noncoding RNAs in rhythmic changes have recently been shown (37). In this aspect, long noncoding RNAs (lncRNAs) are recently identified genetic elements involved in gene expression. It has been shown that lncRNAs are involved in circadian biology, e.g., differential night/day expression of lncRNAs (0.3 to >50 kb) occurs in the rat pineal gland. Approximately one-half of these changes reflect nocturnal increases. Organ culture studies indicate that expression of these lncRNAs in the pineal is regulated by norepinephrine acting through cAMP (37). These findings point to a dynamic role of lncRNAs in the circadian system especially when cAMP level is altered in conditions of chronic inflammation. Varying levels of cAMP regulate apoptosis and efferocytosis in exacerbations of chronic lung disease.
We have recently identified a role for mitochondrial alterations in the pathogenesis of COPD (1), and a circadian acetylome (which is affected by environmental stresses) appears to regulate mitochondrial metabolic pathways. This suggests that rhythmic acetylation plays a significant role in the timing of oxidative metabolism, a cellular function that is dramatically altered by environmental stressors like CS (116).
Stress Response and Regulation of Molecular Clock Proteins by Posttranslational Modifications
Phosphorylation/dephosphorylation.
Posttranslational modifications of molecular clock proteins can affect their stability, activity, and subcellular localization (3) and are directly linked to intracellular signaling pathways (Fig. 2). Phosphorylation and acetylation of both BMAL1 and PER2 are critical and fundamental activities in the basic operation of the molecular clock (64, 73, 129). It is possible that signal transduction in response to extracellular stimuli, such as oxidative/carbonyl stress imposed by CS, results in the activation of a kinase cascade and phosphorylation of molecular clock proteins. Extracellular regulated kinase (ERK), MAP kinase, casein kinase I ε/δ, CK2, and GSK-3 are reported to produce changes in phasing/rhythmicity of the clock and phosphorylate clock proteins in flies, plants, and mammals (2, 4, 66). In mammals, phosphorylation of CLOCK and BMAL1 is regulated during the circadian cycle by rhythmic kinase activity (2, 102). Phosphorylation leads to nuclear translocation and degradation of CLOCK (91), but the identities and function of the kinases responsible are unknown in lung cells. Phosphorylation of PER2 and CRY2 regulates nucleocytoplasmic shuttling of the repressor complex and largely defines the rate of protein degradation, though specific evidence for this activity in lung cells is lacking (3). It is possible that phosphorylation of BMAL1 and PER2 by kinases occurs in response to CS in lung cells, whereas dephosphorylation by ser/thr phosphatase leads to ubiquitination and proteasomal degradation (Fig. 2). It is reasonable to assert that drugs designed to target the activity of enzymes like CK1ε either directly or indirectly may be able to alter the timing of the clock (151) or correct perturbations to the oscillator induced by proinflammatory mediators like CS.
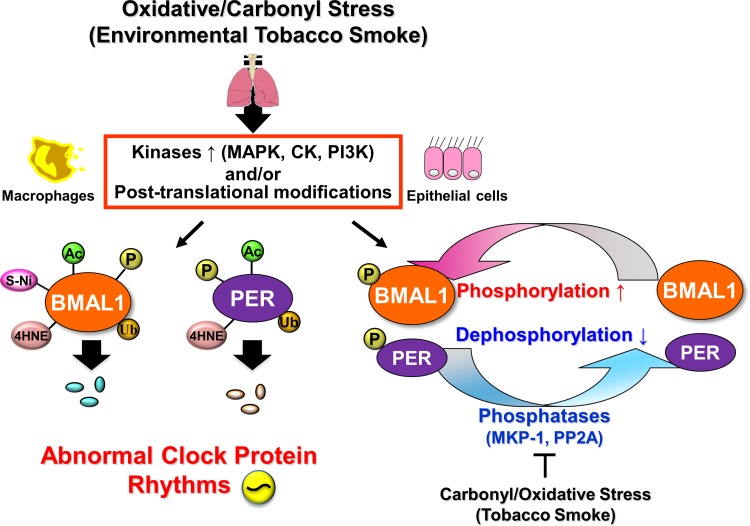
Fig. 2.
Cigarette smoke-induced modification of molecular clock function in the lungs depends on extracellular control of posttranslational modifications. Oxidative/carbonyl stress (environmental tobacco smoke)-induced activation of extracellular regulated kinase signaling stimulates posttranslational modification of molecular clock proteins in lung epithelial cells and macrophages, altering their activity and degradation rates. As reported, posttranslational modifications as phosphorylation (P), ubiquitination (Ub), acetylation (Ac), SUMOylation (SUMO), S-nitrosylation (S-Ni), and carbonylation (4-HNE) in clock protein play an essential role in its regulation. For example, phosphorylation of BMAL1 and PER2 leads to ubiquitination and eventually degradation, which influences both the timing of the clock and downstream clock-dependent gene expression. CLOCK (circadian locomotor output cycles protein kaput), HAT (histone acetyltransferases), HDAC (histone deacetylases), and SIRT1 (Sirtuin 1) are involved in the acetylation and deacetylation processes respectively. MAPK (mitogen-activated protein kinase), CK1δ/ε (casein kinase 1δ/ε), CKII (casein kinase II), GSK3β (glycogen synthase kinase 3β), PKC (protein kinase C), and PP1 (protein phosphatase I) are involved in phosphorylation of clock proteins.
Acetylation.
Acetylation plays a central role in circadian timing as CLOCK has intrinsic HAT activity (46). CLOCK acetylates histone H3 at Lys14, histone H4 at Lys16, BMAL1 at Lys537, and Per2 (7, 13, 46, 73) (Fig. 2). Acetylation of BMAL1 potentiates its binding with CRY1 which leads to transcriptional inhibition (73). CLOCK interacts with p300/CBP and regulates rhythmic acetylation of histone H3 and specific gene expression (40, 68). Thus acetylation plays a role in both activation and repression of clock gene expression (40). CS exposure increases histone H3 (Lys9) and H4 (Lys12) acetylation in vitro in human bronchial epithelial cells (H292) and in mouse lung (36, 175, 176, 205). Our data suggest that CS reduces BMAL1 levels in large part through reduced deacetylase activity of SIRT1 (77). This appears to affect the timing of the clock in both the lungs and the brain. Recently, independent reports have clearly demonstrated a direct BMAL1:CLOCK target gene, gene model 129 (Gm129), later renamed CHRONO (computationally highlighted repressor of the network oscillator), as a novel regulator of the feedback loop (6). CHRONO interacts with BMAL1 and represses the activity of the BMAL1:CLOCK transcriptional complex (5) (Fig. 1). Bimolecular fluorescence complementation and coimmunoprecipitation analysis revealed that CHRONO acts by abrogating the binding of BMAL1 to its transcriptional coactivator CBP (5). CHRONO KO mice showed prolonged locomotor activity rhythms (5, 61), and endogenous CHRONO is rhythmically expressed antiphase with BMAL1 (61). Overexpression of CHRONO leads to suppression of BMAL1:CLOCK activity in a histone deacetylase-dependent manner (61). CHRONO KO mice also show alterations in the rhythmic expression of core clock genes and an impaired response to stress (61). CHRONO forms a complex with the glucocorticoid receptor and modulates the response to circulating glucocorticoids (61). CHRONO is a part of the negative repressor complex and is a potential link between the clock, environmental circadian disruption, and chronic airway disease, though this relationship remains unexplored. Although these data represent a significant advance, there remains a great deal we do not yet understand regarding the influence of environmental stress (oxidative/carbonyl stress by CS) on intrinsic CLOCK-HAT activity, direct clock protein acetylation, and/or histone acetylation at CCG promoters in the lung.
Role of Sirtuins in Regulating Clock Protein Deacetylation During Cellular Senescence and Inflammatory Responses
Evidence suggests that the molecular clock is involved in the DNA damage response, cellular senescence, and cellular/organismal aging. It is well established that the timing system, as a unit, declines in amplitude and robustness as a function of age. SCN-dependent rhythms decline with age in parallel with, and most likely as a function of, a decline in the robustness of the molecular clock. How aging at each level (SCN network, molecular clock) influences the rate and amount of cellular senescence is a matter of conjecture. In Bmal1 KO mice, it was reported that cellular aging is attributed to molecular clock disruption. However, in the case of environmental stresses via DNA damage, this mechanism cannot be reconciled especially when considered in light of chronic lung disease. For example, CS alters the molecular clock through its influence on SIRT1 activity but it also directly affects DNA damage and cellular senescence. It is not known which comes first, whether the effects are parallel and additive, and how much overlap there is between the pathways (i.e., via kinase signaling). The role of mitochondria and reactive oxygen species in the regulation of cellular senescence occurs via the cytochrome oxidase system in cell cycle survival pathways. This system involves metabolism via NAD+-dependent pathways, which also influence the level and activity of SIRT1 (12).
The NAD+-dependent deacetylase-SIRT1 has been reported to counteract the HAT activity of CLOCK (13, 128, 129). The NAD+ substrate dependency of SIRT1 suggests that it constitutes a functional link between metabolism and clock function (129, 130). SIRT1 mediates deacetylation of BMAL1 at Lys537, PER2, and CRY1, thereby regulating the transcription of clock genes (7, 13). SIRT1 level/activity shows daily variation in the lungs, correlated with rhythmic acetylation of BMAL1, PER2, and histone H3 (13). SIRT1 activity is decreased in human lung epithelial cells, macrophages, and lungs of mice exposed to CS and in the lungs of patients with COPD (154, 206). SIRT1 activation reduces CS-induced acetylation and degradation of BMAL1 in mouse lungs and monocytes. Thus targeting circadian-dependent pharmacological activation of SIRT1 may have potential as a chronopharmacological intervention for the management of chronic airway disease (210).
CS causes SIRT1 reduction and chromatin modifications due to histone acetylation on proinflammatory gene promoters (8, 154, 204, 206). There is emerging evidence that circadian rhythms govern proinflammatory cytokine gene expression (193, 211). It is also known that inflammation and alteration in clock gene expression are intimately associated with fatigue and reduced activity (29, 32, 140). Hence, it is possible that SIRT1 regulates acetylation/activation of RelA/p65 (NF-κB) and the inflammatory response indirectly through its influence on the molecular clock (32, 154, 204, 206) (Fig. 3). SIRT1-deficient mice develop exaggerated lung inflammation and cellular senescence, whereas SIRT1-overexpressing transgenic mice are protected against CS-induced lung premature aging (i.e., cellular senescence, inflammation, lung function decline, and air space enlargement/emphysema) (207). It is likely that SIRT1 can affect core clock function via its regulation of clock protein levels, particularly in the lungs. Acetylation of BMAL1 potentiates its binding with the repressor CRY1, which leads to suppression of CLOCK:BMAL1 mediated transcription (73). Hence, it is possible that CS-mediated reduction of SIRT1 level/activity leads to hyperacetylation of clock gene-encoded proteins, culminating in abnormal circadian periodicity and acetylation of histones on proinflammatory and prosenescent genes.
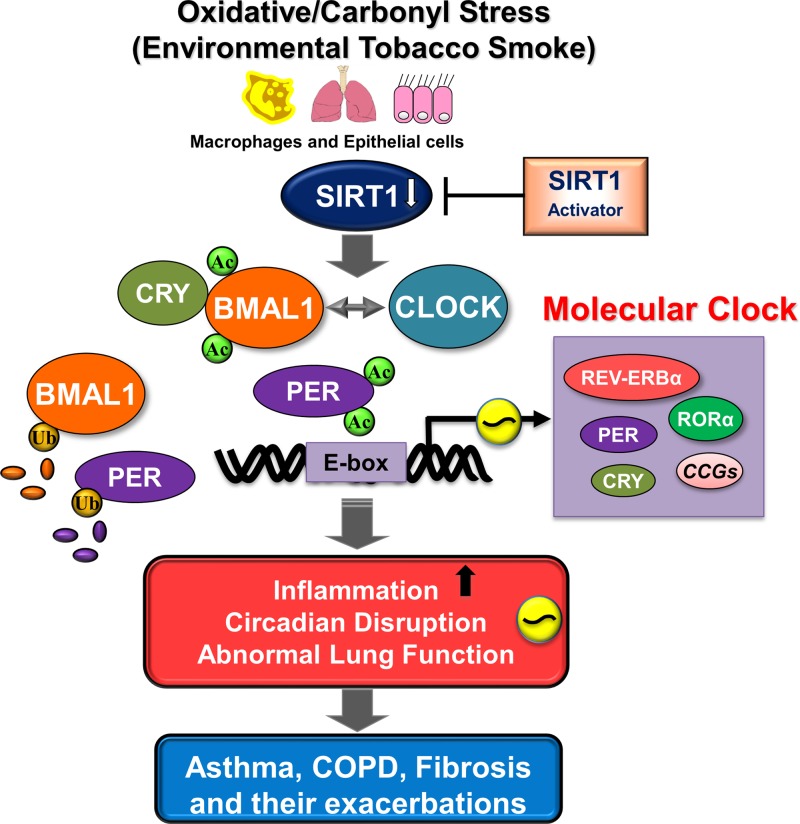
Fig. 3.
Molecular mechanisms of SIRT1-mediated regulation of BMAL1 and PER2 during inflammation. Oxidative/carbonyl stress (cigarette smoke)-induced reduction in SIRT1 levels/activity in the lungs leads to hyperacetylation of clock proteins including BMAL1 and PER2 and transcription factor NF-κB RelA/p65. Acetylation of BMAL1 and PER2 results in increased ubiquitination and degradation and reduced E-box-mediated transcriptional activity. Circadian clock disruption occurs due to enhanced clock gene acetylation leads to reduced efficacy of anti-inflammatory drugs including steroids and β-agonists. Activation of SIRT1 in the presence of NAD+ by using specific SIRT1 activators (e.g., SRT1720) can enhance its deacetylase activity and facilitate normal molecular clock function. Moreover, deacetylation of RelA/p65 (NF-κB) by SIRT1 activation can modulate inflammatory response indirectly through its influence on the molecular clock. Hence, modulation of SIRT1 levels in the lung plays a protective role against inflammation and circadian disruption in the pathogenesis of chronic airway diseases.
SIRT1 regulates cellular senescence (SIPS and SASP) in response to CS and toxicant stress by protecting genomic DNA against double-strand break in lung cells (136, 207, 208). SIRT1 KO mice show increased lung cell senescence possibly via DNA damage and impaired DNA damage repair (DDR), associated with augmented inflammatory responses (207). SIRT1 functions in DDR to promote DNA repair and downregulates inflammation by deacetylating specific histones. The role of SIRT1 in molecular clock function as it relates to DNA damage/repair, as well as to SIPS and SASP in the lungs, is currently unknown. Furthermore, it is not known whether there is a relationship between the decline of SCN function in aging and SIRT1 activity in SCN pacemaker neurons. Furthermore, it is unclear whether this role for SIRT1 is a factor in the increased rate of COPD/IPF among the aged (31).
As in the lungs, SIRT1 also regulates BMAL1:CLOCK activity in the pacemaker neurons of the SCN. In aged mice, SIRT1 levels in the SCN are decreased (similar to its reduction by CS and in patients with COPD), giving rise to a longer intrinsic period, a more disrupted activity pattern, and an inability to readily adapt to acute perturbation in the environment (i.e., phase shifts). Hence, SIRT1 is a significant part of the molecular oscillator regulating the activity of SCN pacemaker neurons. SIRT1 is critical for the maintenance of robust circadian rhythms in young animals (which may be the case in the lung), and decay in this activity by smoking/environmental stresses may play an important role in aging, particularly in the pathogenesis of chronic inflammatory lung diseases. Resveratrol and other dietary proanthocyanidins are known to modulate the molecular clock via a SIRT1/NAD/NAMPT pathway and may be the mechanism whereby these compounds attenuate cellular senescence (157).
SIRT6 is the only sirtuin constitutively localized to chromatin and is prominently present at the transcription start sites of active genomic loci (155). SIRT6 has been reported to be dynamic in its chromatin binding in response to TNF-α, thereby altering the transcriptional landscape of aging- and stress-related genes (85). SIRT6 is known for its deacetylase activity at histones, acting primarily at H3K9 (84, 121) and H3K56 (122, 202) to modulate gene expression, telomere maintenance, and genomic instability (111, 181). SIRT6 activity has been linked to metabolic homeostasis with studies showing that Sirt6−/− mice die at 2–4 wk of age owing to accelerated aging and persistent hypoglycemia. This increase in mortality is largely due to altered rates of glycolysis, glucose uptake, and mitochondrial respiration (125, 200). Analysis of the hepatic circadian transcriptome revealed that SIRT1 along with SIRT6 influences distinct cohorts of oscillating transcripts, suggesting a SIRT-specific clustering of rhythmic genes in the liver (117). SIRT6 interacts directly with BMAL1:CLOCK and SREBP-1, forming a cis-regulatory complex that regulates cyclic expression of genes involved in fatty acid and cholesterol metabolism (117). The role of SIRT6 in the timing of gene expression, cellular senescence, and DNA damage/repair in the lungs remains to be determined.
Molecular Clock Dysfunction in Specific Lung Diseases
Recent studies reveal that molecular clock dysfunction can occur in lung cells from patients or in experimental animal models of lung disease. Various environmental agents that are involved in the pathogenesis of airway diseases can alter molecular clock function, influencing both the timing system and inflammatory responses. Study of the regulation or alterations of molecular clock function in various airway diseases is rapidly developing with many recent and exciting discoveries.
Chronic Obstructive Pulmonary Disease
COPD is a common and devastating chronic airway disease marked by a decline in lung function and marked daily variation in exacerbation frequency and intensity. Using a mouse model of COPD we have shown that tobacco smoke/CS exposure reduces SIRT1 activity and produces clock dysfunction in the lungs (77). A recent study from our group has also determined that a reduction in SIRT1 activity is associated with telomere attrition and inflammosenescence in patients with COPD (209). Recently, a rat model of passive CS exposure was used to demonstrate CS-mediated changes in molecular clock function within intervertebral discs (IVD) (135). Passive smoking for 8 wk in rats altered the timing of clock gene expression in IVD, producing marked phase shifts of peak clock gene expression (135). It was also revealed that CS exposure (both acute and chronic) produced marked changes in the sleep/wake rhythm and timing of clock gene expression in the SCN (77). Sleep disorders, not limited to hypopneas, are strongly comorbid with COPD (113, 114). These data suggest that the altered quality and duration of sleep in those with COPD results from direct impacts of CS on the sleep/wake centers in the brain (77). Thus the bulk of evidence supports the notion that molecular clock function in the lungs is altered during the etiology of COPD, in large part owing to extended periods of exposure to CS. Of note is the finding in rat IVDs indicating that even secondhand smoke exposure can lead to changes in molecular clock function. It remains to be seen whether molecular clock dysfunction is a causative factor in the timing and amplitude of exacerbations in those with COPD. There is clearly significant potential for molecular clock gene expression as a biomarker of disease severity and/or the effectiveness of treatment strategies for COPD.
Asthma
Abnormal rhythms of molecular clock gene expression have been detected in the surface epithelium and isolated BAL cells from patients with asthma (51). It was found that bronchial airway epithelial cells from severe asthmatic patients have increased gene expression of ARNTL2 (BMAL2) compared with mild asthmatic patients and healthy subjects (51). In contrast, Per2 gene expression was higher in healthy subjects compared with mild and severe asthmatic patients, which could impact both the incidence and severity of asthma symptoms (51). ARNTL2/PER2 ratio was increased in severe asthmatic patients compared with healthy subjects and an increase in daytime ARNTL2 was associated with nocturnal symptoms (51). The impact of asthma on Arntl2 and Per2 gene expression rhythms in bronchial airway epithelial cells was further supported by experiments in vitro using cultured BAL cells. It is possible that restoring the balance between repressor (Per2) and activator (Bmal2) expression in lung cells could reduce the frequency and severity of nocturnal exacerbations in asthmatic patients (51).
RORα, a member of the nuclear receptor superfamily of transcription factors and a component of the core clock, was reported to influence the Th17 and Th2 responses in asthma (78), enhance fibrosis through the innate immune lymphoid type 2 cells (ILC2) (67), and play a role in DNA damage-mediated apoptosis and emphysema in patients with COPD (165). Finally, murine studies suggest molecular clock disruption may drive circadian rhythms in IgE/mast cell-mediated allergic reactions (131). Thus the regulation of molecular clock function in various immune-inflammatory cells may both predict the severity/exacerbations of asthma and provide a novel pathway toward new and effective treatments.
Idiopathic Pulmonary Fibrosis
A recent study reports the cell-type dependent upregulation of RORα in fibrotic lung tissues from IPF and cryptogenic organizing pneumonia patients. Though expression of RORα was enhanced in fibroblasts present in fibrotic areas, fibroblasts away from these foci did not show any RORα expression (188). The localization of RORα expression was limited to the nucleus in normal Type II epithelial cells of controls or unaffected areas of the lungs from fibrotic patients. Nevertheless, RORα expression changes dramatically in the lung epithelial cells lining the fibroblastic foci (188). Future studies should examine the role of RORα in pulmonary fibrosis, through its interaction with other canonical signaling pathways such as WNT/β-catenin and the AKT/PI3K pathways previously linked to cell proliferation and the epithelial to mesenchymal transition.
Acute Lung Injury
Acute lung injury (ALI)-associated inflammation due to septic shock can lead to circadian disruption. For example, studies indicate that the core clock protein PER2 regulates the expression of the hypoxia-inducible factor-1 (HIF-1) and may play a role in the activation of mucosal HIF-1 in ALI (47, 49). Furthermore, it has been shown that PER1 interacts with the α-subunit of HIF-1 (34). Prior studies have clearly shown that PER2 levels were induced similar to HIF-1 during mechanical ventilation. Induction of Per2 expression was reported in a murine model of ALI by mechanical ventilation (ventilator-induced lung injury) (48). Additionally, to test the hypothesis that constant light can induce Per2 expression in the lungs, mice were maintained under constant light (LL) for a period of 1 wk. Results revealed an enhanced Per2 rhythm in the lungs over a 48 h time period in LL (48). A recent review highlights the importance of “Chronomics”: timing and rhythm in intensive care unit (ICU), circadian physiology during acute stress, and the effects of ICU milieu upon circadian rhythms to stress the value of considering circadian-immune disturbance as a vital tool for personalized treatment (143). Overall, these observations suggest that activation of PER2-dependent gene expression during ALI represents a novel pathway for targeting the treatment and management of ALI (48).
Infections
To examine the impact of circadian disruption on the severity of responses to pulmonary infection, investigators exposed wild-type and BMAL1 KO mice to viral pneumonia (Sendai virus) (69). Bmal1-null mice exhibited 100% mortality to viral pneumonia by postinfection day 8 compared with 0% mortality for wild-type littermates (69). Increased morbidity was apparent as early as postinfection day 3 and was characterized by a drop in absolute body weight that continued until day 21 postinfection. These data strongly support the notion that circadian disruption exerts a significant effect on acute responses to respiratory viral infection in vivo (69). This observation concurs with a recent study from our group showing that the influenza A virus alters clock function via a Bmal1-dependent pathway (174). The impact of circadian disruption on the immune response to infection is well known (28). It is clear that persistent exposure to external or internal chronodisruption, as experienced during chronic jet lag or shift work or in animal models after genetic manipulation of the clock, dramatically reduces the effectiveness of the immune response to infection. Pharmacological manipulations of molecular clock function in chronic airway diseases or during respiratory infections represent novel and potentially effective therapies for chronic lung diseases and associated exacerbations.
Sleep Apnea
The role of respiratory physiology and its alterations in the progression of sleep apnea and its comorbidity are well known. Recent advances in our understanding of molecular clock function and its role in sleep biology may shed new light on the etiology of sleep disturbances in patients with hypoventilation syndromes, including sleep apnea. It was reported that the timing system contributes to the timing and duration of apneic periods during obstructive sleep apnea. This may be due to a direct influence of the molecular clock and could be in some way regulated by the activity of the SIRT1-BMAL1 pathway (21). This area is emerging and more research is needed to address the role of the molecular clock in the pathophysiological sleep disruption that occurs during sleep apnea.
Chronopharmacology for the Treatment of Lung Pathophysiological Conditions
Recently, a circadian gene expression atlas in mammals and its implications in biology and medicine suggest that more than 50% of all (top-selling) drugs target circadian clock genes or their protein products. Many of these drugs have direct influence in pulmonary physiology. However, there is a fundamental gap between the limited, scattered research on circadian genes and their importance in regulation of lung pathophysiological responses. A detailed study characterizing the role of the circadian clock noted key pharma drugs used in asthma and COPD (Advair Diskus regulating circadian-influenced genes Serpina6, Pgr, Nr3c2, Adrb2, Pla2g4; Combivent regulating Slc22a5, Slc22a4, Chrm2, Adrb1, Adrb2; and ProAir HFA for Adrb1 and Adrb2) have been shown (212). Furthermore, circadian-dependent targets based on molecular clock function in the lung and during pathological conditions have been discussed. Understanding various genes and targets may aid in developing the chronotherapeutics for the treatment of pulmonary conditions (83).
Role of REV-ERBα
Several novel compounds that regulate the timing and amplitude of clock gene expression either directly or indirectly via SIRT1 have been studied (Table 3). We have shown that SIRT1 and REV-ERBα activators/agonists can have an important role in regulating certain abnormalities seen in COPD and may be protective in a mouse model of emphysema (77, 210). Important advancements have been made for the role of molecular clock output nuclear receptor REV-ERBα in coupling molecular clock, metabolism, and inflammation (20, 213). Recent evidence highlighted the role of REV-ERBα in adipogenesis possibly in lung cell regeneration upon noxious stresses. This highlights that molecular clock protein may be intimately linked to repair of lung cellular dysfunction (132, 133) (Table 3).
Table 3.
Chronotherapeutic drugs that can modulate molecular clock proteins
| Small Molecules/Compounds/Drugs | Molecular Targets | Major Outcomes/Findings | Citation |
|---|---|---|---|
| SIRT1 activators | CLOCK:BMAL1 | Altered CLOCK:BMAL1-mediated transcription of clock-controlled genes. Activation of SIRT1 represses the circadian gene expression and decreases H3 K9/K14 acetylation. | 15 |
| SRT 1720/SRT2183 | |||
| REV-ERBα and BMAL1 | Attenuates REV-ERBα and BMAL1 protein levels and inhibition of LPS-induced proinflammatory cytokine release in PBMCs of nonsmokers compared to smokers and patients with COPD | 210 | |
| BMAL1 | Unable to attenuate CS-induced lung inflammatory and injurious responses in Bmal1 epithelial cell specific KO mice | 77 | |
| REV-ERB agonists SR9009/SR9011 | REV-ERBα and REV-ERBβ | REV-ERBs are crucial for the coordination of genes involved in metabolism either directly or indirectly, through effects on the circadian clock. | 171 |
| REV-ERBα and REV-ERBβ | REV-ERB agonist alters the expression of metabolic genes in liver, skeletal-muscle and adipose tissue, and increases energy expenditure. Synthetic REV-ERB ligands are promising candidates for the treatment of metabolic disease. | 35 | |
| REV-ERB agonist GSK4112 | REV-ERBα | Inhibits expression of the circadian target gene Bmal1. Also represses the expression of gluconeogenic genes in liver cells and reduced glucose output in primary hepatocytes. | 62 |
| REV-ERBα | Reduces LPS induced IL-6 but not IL-8 release in primary human monocyte-derived macrophages (MDMs) and primary human alveolar macrophages. In primary human MDMs, combined application of LPS and GSK4112 attenuated transcription of cytokine genes such as il-6, ccl2, Cxcl11, Cxcl6, and il19. | 59 | |
| REV-ERB antagonist SR8278 | REV-ERBα | Stimulates the expression of REV-ERBα target genes involved in gluconeogenesis whereas GSK4112 (agonist) suppresses their expression. REV-ERB antagonist, SR8278 represents a unique chemical tool for probing REV-ERB function to explore the role of circadian clock and metabolic pathways in vitro and in vivo. | 88 |
| RORα/γ agonist SR1078 | RORα and RORγ | Functions as RORα/RORγ agonist by expressing two important ROR target genes, glucose-6-phosphatase (G6Pase) and fibroblast growth factor 21 (FGF21) in the liver. Synthetic RORα/γ agonist can be used as a chemical tool to probe the function of RORs | 195 |
| RORα/γ inverse agonist SR1001 | RORα and RORγ | A high-affinity synthetic ligand that is specific to both RORα/γ, which inhibits TH17 cell differentiation and function. SR1001 treatment in nonobese diabetic mice reduces diabetes incidence and insulitis by targeting TH17 cells. | 170 |
| RORα and RORγ | Inhibits the development of murine TH17 cells and reduces proinflammatory cytokine expression, particularly TH17-mediated cytokines, autoantibody production, and increases the frequency of CD4(+)Foxp3(+) T regulatory cells | 168 | |
| RORα/γ inverse agonist T0901317 | RORα and RORγ (other receptor preference LXRα, LXRβ, PXR, FXR) | Represses RORα/γ -dependent transactivation of ROR-responsive reporter genes and reduces recruitment of steroid receptor coactivator-2 by RORα at an endogenous ROR target gene (G6Pase). | 98 |
| RORα selective inverse agonist SR3335 | RORα | SR3335 directly binds to RORα, suppresses the expression of endogenous RORα target genes involved in hepatic gluconeogenesis including glucose-6-phosphatase and phosphoenolpyruvate carboxykinase in HepG2. SR3335-treated mice displayed lower plasma glucose levels following the pyruvate challenge. | 96 |
| RORγ-specific synthetic ligand SR1555 | RORγ | Inhibits TH17 cell development and function but also increases the frequency of T regulatory cells. | 169 |
| RORγ-specific synthetic ligand SR2211 | RORγ | SR2211, a selective RORγ modulator that potently inhibits both the gene expression and production of IL-17 in EL-4 cells. SR2211 is identified as a potent modulator of RORγ. | 97 |
BMAL1, brain and muscle ARNT-like 1; CLOCK, circadian locomotor output cycles kaput; COPD, chronic obstructive pulmonary disease; IL-6; interleukin 6; LPS, lipopolysaccharide; MDMs, monocyte-derived macrophages; PBMCs, peripheral blood mononuclear cells; RORα/γ, retinoic acid receptor-related orphan receptor α/γ; TH17 cells, T helper cells.
Interestingly, REV-ERBα functions as a core repressor in the molecular clock, a critical regulator of metabolic/inflammatory gene expression, and a metabolic sensor. Zhang et al. (213) report that multitasking transcription factor REV-ERBα plays an essential role in the regulation of both clock and metabolic genes through distinct mechanisms. REV-ERBα binds directly to specific DNA sequences of clock genes by displacing its competing transcription factor ROR. REV-ERBα does not interact with DNA sequences in metabolic genes but instead interacts with other transcription factors that are involved in the regulation of metabolic gene expression in a tissue-specific manner (20, 213). Clock control requires REV-ERBα to bind directly to the genome at its cognate sites, where it competes with the activator RORα to regulate the timing and amplitude of Bmal1 expression. REV-ERBα is a receptor for heme, an indicator of energy status that is depleted during periods of negative energy balance. REV-ERBα regulates metabolic/inflammatory genes primarily by recruiting the HDAC3 corepressor to sites to which it is tethered by cell type-specific transcription factors. These two mechanisms interplay with the availability of REV-ERBα in the nucleus. It is possible that SIRT1 regulates the acetylation and decacetylation of REV-ERBα and its binding to respective promoters. The direct competition between REV-ERBα and RORα provides a mechanism for self-sustained control of the universal molecular clock, whereas REV-ERBα uses epigenomic factors to regulate metabolic/inflammatory genes that may be required during the inflammatory responses.
Recently, there has been a new focus on small molecules that target the REV-ERB and ROR nuclear receptors with an emphasis on clinical applications in pulmonary and cardiac disease (89, 167). Since REV-ERBα also plays a key role in regulating mitochondrial content and the oxidative capacity of skeletal muscle, it may be prudent that pharmacological activation of REV-ERBα will be useful in skeletal muscle related diseases (197). Dysfunction of skeletal muscle is the hallmark in the pathogenesis of COPD. Dual function can be achieved since REV-ERBα agonists can regulate sleep architecture and emotion in mice, and thus these compounds may be useful in the treatment of sleep disorders and anxiety as seen as a comorbid conditions in various lung diseases (10) (Table 3).
Among the other most effective treatments for alleviating inflammation in chronic airway diseases are drugs that modulate glucocorticoid (GC) signaling and sympathomodulatory drugs including the β2-adrenergic agonists. Not surprisingly, activated glucocorticoid receptors (GR) modulate transcription of molecular clock genes through glucocorticoid response elements (8, 101). Clock gene expression is induced by adrenal steroids in human bronchial epithelial cells, PBMCs, lymphocytes and rat fibroblasts (8, 19). Bronchodilators, including β2-adrenoreceptor agonists also induce Per gene expression in bronchial epithelial cells (179). Hence, the induction of clock-dependent output genes may be mechanistically linked to the anti-inflammatory effects of GCs and β2-adrenoreceptor agonists. These data further highlight the potential for the molecular clock, in particular the role of REV-ERBα as a therapeutic target for devising novel therapeutic strategies for minimizing inflammatory responses in chronic airway disease.
It is worth noting that daily rhythms of GC secretion and adrenergic signaling via the autonomic nervous output are widely considered pathways whereby the SCN coordinates and synchronizes central and peripheral oscillators (120). Thus although these drugs may acutely alleviate inflammation they could have previously undefined long-term effects on the timing system. A recent report revealed the inefficacy of GC/β2-adrenoreceptor agonists in treatment of COPD exacerbations or asthmatic patients that smoke. Because GR expression in lung tissue does not vary across the day, it is most likely a downstream response, or lack thereof, to GR agonists that is responsible for this lack of efficacy (58). It is reasonable to implicate phasic variation in clock-dependent responses to GR and β2-adrenoreceptor signaling in this diminished response, particularly in light of our own data showing that the expression and timing of CCG expression are altered in an animal model of COPD. Thus chronopharmacological agents that increase the amplitude and normalize the phase of clock and clock-dependent gene expression in the lungs may be particular effective at regulating inflammatory responses and hold considerable promise for the management of chronic airway diseases and their exacerbations.
Small molecular effectors of oscillator function have been shown to target various components of the core clock, leading to altered timing and amplitude of clock and CCG expression (Table 3). These molecules may be given in conjunction with steroids or β2-adrenoreceptor agonists as part of a novel chronopharmacological treatment regimen (33). It was recently reported that selenium, a trace metal found in most commercial rodent diets and many foods, increases BMAL1 levels and activity (76). Rodents provided a diet high in selenium displayed considerably higher levels of BMAL1 in the liver and showed resistance to mortality following exposure to chemotherapeutic drugs (76). These effects are specific to peripheral oscillators, since selenium did not affect the timing or amplitude of BMAL1 expression in the SCN. Natural ligands for REV-ERBα, e.g., heme precursor 5-aminolevulinic acid, have also been described to have similar effects (201). Finally, synthetic ligands for REV-ERBα (e.g., GSK4112, SR9011, SR9009) may also regulate inflammation, improve respiratory function, and normalize sleep/wake rhythm in patients with asthma and COPD (88, 89, 171).
Conclusions and Future Directions
In summary, abnormal rhythms of lung function in patients with COPD and asthma are associated with molecular clock dysfunction and enhanced airway inflammation in response to environmental agents (Fig. 1). Moreover, exposomes such as environmental tobacco smoke, airborne particulates, and bacterial/viral infections trigger oxidative stress, premature cellular senescence, and DNA damage response that can also cause molecular clock dysfunction in the lungs. CS stress-induced oxidative/carbonyl stress reduces SIRT1 expression in the lungs, leading to changes in the acetylation and degradation of the clock protein BMAL1 and PER2. These posttranslational modifications of clock protein levels can further enhance lung inflammation and cellular senescence. Thus the timing system may act under normal circumstances as a rheostatic modulator of lung inflammoimmune responses that when challenged (as in response to CS-associated stress) can also act to increase the amplitude and duration of proinflammatory impact on lung function. This bidirectional relationship reveals that the clock is poised to act as a target for novel therapeutic interventions. Assessment of molecular clock function itself can be used as a biomarker of chronotherapeutics in lung pathophysiological conditions. Further work is needed to understand the “omics” of the pulmonary clock as it relates to the progression of airway diseases. This approach to understand the influence of the timing system on cellular and molecular lung function is likely to expose novel targets for chronopharmacotherapy. Evidence links the molecular clock to NF-κB, TLR4, and Nrf2 pathways in the lungs, particularly in the context of pulmonary responses to exposomes, environmental agents, virus/bacteria, and “double hits” (combination of environmental agents/toxins, e.g., smoke, and infectious agents, e.g., viral/bacterial). We are already on the cusp of exciting discoveries in this area. Novel clock-targeting compounds like the SIRT1 activators, upcoming small molecular clock-enhancing molecules, and REV-ERB ligands may soon be used to target the molecular clock in lung cells, thus normalizing the lung inflammatory and prosenescence responses and improving the efficacy of glucocorticoids and β2-agonists. Recent evidence also suggests that REV-ERBα ligands may be used for the treatment of sleep and anxiety disorders (10). Clinical evidence suggests that SIRT1 also affects the clock-dependent metabolome and mitochondrial functions/biogenesis, which are altered during the pathogenesis of airway diseases like COPD (123). Thus clock-targeting drugs may provide multimodal global benefit by targeting pulmonary physiology and inflammation, sleep quality, and glucose metabolism. Understanding the mechanism whereby the dysfunctional molecular clock regulates lung pathophysiological functions could lead to new and effective avenues for treatment based on chronopharmacological agents. Furthermore, assessment of molecular clock components in systemic monocytes and/or sputum cells may be useful biomarkers of therapeutic efficacy and outcomes in chronic airway disease.
GRANTS
This study was supported by the NIH 1R01HL097751 (to I. Rahman), 1R01HL092842 (to I. Rahman), American Lung Association RG-266456-N (to H. Yao), American Lung Association RG-305393 (to I. K. Sundar), University of Rochester Drug Discovery Pilot Award, and National Institute of Environmental Health Sciences Environmental Health Science Center Pilot Award (under NIEHS grant P30-ES01247 to M. T. Sellix).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
I.K.S. prepared figures; I.K.S., H.Y., M.T.S., and I.R. drafted manuscript; I.K.S., H.Y., M.T.S., and I.R. edited and revised manuscript; I.K.S., H.Y., M.T.S., and I.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We apologize to those authors whose excellent contribution in the field could not be cited here because of space limitations.
REFERENCES
- 1.Ahmad T, Sundar IK, Lerner CA, Gerloff J, Tormos AM, Yao H, Rahman I. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB J 29: 2912–2929, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akashi M, Nishida E. Involvement of the MAP kinase cascade in resetting of the mammalian circadian clock. Genes Dev 14: 645–649, 2000. [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron 74: 246–260, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Allada R, Meissner RA. Casein kinase 2, circadian clocks, and the flight from mutagenic light. Mol Cell Biochem 274: 141–149, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Anafi RC, Lee Y, Sato TK, Venkataraman A, Ramanathan C, Kavakli IH, Hughes ME, Baggs JE, Growe J, Liu AC, Kim J, Hogenesch JB. Machine learning helps identify CHRONO as a circadian clock component. PLoS Biol 12: e1001840, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annayev Y, Adar S, Chiou YY, Lieb JD, Sancar A, Ye R. Gene model 129 (Gm129) encodes a novel transcriptional repressor that modulates circadian gene expression. J Biol Chem 289: 5013–5024, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134: 317–328, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289: 2344–2347, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Bando H, Nishio T, van der Horst GT, Masubuchi S, Hisa Y, Okamura H. Vagal regulation of respiratory clocks in mice. J Neurosci 27: 4359–4365, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banerjee S, Wang Y, Solt LA, Griffett K, Kazantzis M, Amador A, El-Gendy BM, Huitron-Resendiz S, Roberts AJ, Shin Y, Kamenecka TM, Burris TP. Pharmacological targeting of the mammalian clock regulates sleep architecture and emotional behaviour. Nat Commun 5: 5759, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes PJ. Circadian variation in airway function. Am J Med 79: 5–9, 1985. [DOI] [PubMed] [Google Scholar]
- 12.Bass J. Circadian topology of metabolism. Nature 491: 348–356, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Belden WJ, Dunlap JC. SIRT1 is a circadian deacetylase for core clock components. Cell 134: 212–214, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellet MM, Deriu E, Liu JZ, Grimaldi B, Blaschitz C, Zeller M, Edwards RA, Sahar S, Dandekar S, Baldi P, George MD, Raffatellu M, Sassone-Corsi P. Circadian clock regulates the host response to Salmonella. Proc Natl Acad Sci USA 110: 9897–9902, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellet MM, Nakahata Y, Boudjelal M, Watts E, Mossakowska DE, Edwards KA, Cervantes M, Astarita G, Loh C, Ellis JL, Vlasuk GP, Sassone-Corsi P. Pharmacological modulation of circadian rhythms by synthetic activators of the deacetylase SIRT1. Proc Natl Acad Sci USA 110: 3333–3338, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borsboom GJ, van Pelt W, van Houwelingen HC, van Vianen BG, Schouten JP, Quanjer PH. Diurnal variation in lung function in subgroups from two Dutch populations: consequences for longitudinal analysis. Am J Respir Crit Care Med 159: 1163–1171, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci 2: 521–526, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Burioka N, Fukuoka Y, Takata M, Endo M, Miyata M, Chikumi H, Tomita K, Kodani M, Touge H, Takeda K, Sumikawa T, Yamaguchi K, Ueda Y, Nakazaki H, Suyama H, Yamasaki A, Sano H, Igishi T, Shimizu E. Circadian rhythms in the CNS and peripheral clock disorders: function of clock genes: influence of medication for bronchial asthma on circadian gene. J Pharm Sci 103: 144–149, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Burioka N, Takata M, Okano Y, Ohdo S, Fukuoka Y, Miyata M, Takane H, Endo M, Suyama H, Shimizu E. Dexamethasone influences human clock gene expression in bronchial epithelium and peripheral blood mononuclear cells in vitro. Chronobiol Int 22: 585–590, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Butler AA, Burris TP. Segregation of clock and non-clock regulatory functions of REV-ERB. Cell Metab 22: 197–198, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler MP, Smales C, Wu H, Hussain MV, Mohamed YA, Morimoto M, Shea SA. The circadian system contributes to apnea lengthening across the night in obstructive sleep apnea. Sleep 2015. March 28 pii: sp-00796-14. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 22.Buxton OM, Cain SW, O'Connor SP, Porter JH, Duffy JF, Wang W, Czeisler CA, Shea SA. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 4: 129ra143, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calhoun WJ. Nocturnal asthma. Chest 123: 399S–405S, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 356: 775–789, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Calverley PM, Lee A, Towse L, van Noord J, Witek TJ, Kelsen S. Effect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary disease. Thorax 58: 855–860, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caramori G, Adcock IM, Casolari P, Ito K, Jazrawi E, Tsaprouni L, Villetti G, Civelli M, Carnini C, Chung KF, Barnes PJ, Papi A. Unbalanced oxidant-induced DNA damage and repair in COPD: a link towards lung cancer. Thorax 66: 521–527, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Casale R, Natali G, Colantonio D, Pasqualetti P. Circadian rhythm of peak expiratory flow in children passively exposed and not exposed to cigarette smoke. Thorax 47: 801–803, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol 185: 5796–5805, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, Fontana A. TNF-α suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci USA 104: 12843–12848, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cermakian N, Lange T, Golombek D, Sarkar D, Nakao A, Shibata S, Mazzoccoli G. Crosstalk between the circadian clock circuitry and the immune system. Chronobiol Int 30: 870–888, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang HC, Guarente L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 153: 1448–1460, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-κB action regulated by reversible acetylation. Science 293: 1653–1657, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, Yoo SH, Takahashi JS. Small molecule modifiers of circadian clocks. Cell Mol Life Sci 70: 2985–2998, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chilov D, Hofer T, Bauer C, Wenger RH, Gassmann M. Hypoxia affects expression of circadian genes PER1 and CLOCK in mouse brain. FASEB J 15: 2613–2622, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, Liddle C, Auwerx J, Downes M, Panda S, Evans RM. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 485: 123–127, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung S, Sundar IK, Hwang JW, Yull FE, Blackwell TS, Kinnula VL, Bulger M, Yao H, Rahman I. NF-κB inducing kinase, NIK mediates cigarette smoke/TNFα-induced histone acetylation and inflammation through differential activation of IKKs. PLoS One 6: e23488, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coon SL, Munson PJ, Cherukuri PF, Sugden D, Rath MF, Moller M, Clokie SJ, Fu C, Olanich ME, Rangel Z, Werner T, Program NCS, Mullikin JC, Klein DC. Circadian changes in long noncoding RNAs in the pineal gland. Proc Natl Acad Sci USA 109: 13319–13324, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curtis AM, Bellet MM, Sassone-Corsi P, O'Neill LA. Circadian clock proteins and immunity. Immunity 40: 178–186, 2014. [DOI] [PubMed] [Google Scholar]
- 39.Curtis AM, Fagundes CT, Yang G, Palsson-McDermott EM, Wochal P, McGettrick AF, Foley NH, Early JO, Chen L, Zhang H, Xue C, Geiger SS, Hokamp K, Reilly MP, Coogan AN, Vigorito E, FitzGerald GA, O'Neill LA. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc Natl Acad Sci USA 112: 7231–7236, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curtis AM, Seo SB, Westgate EJ, Rudic RD, Smyth EM, Chakravarti D, FitzGerald GA, McNamara P. Histone acetyltransferase-dependent chromatin remodeling and the vascular clock. J Biol Chem 279: 7091–7097, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Dallmann R, Brown SA, Gachon F. Chronopharmacology: new insights and therapeutic implications. Annu Rev Pharmacol Toxicol 54: 339–361, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davidson AJ, Castanon-Cervantes O, Leise TL, Molyneux PC, Harrington ME. Visualizing jet lag in the mouse suprachiasmatic nucleus and peripheral circadian timing system. Eur J Neurosci 29: 171–180, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol 16: R914–916, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davidson WJ, Wong LE, The S, Leigh R. The impact of diurnal variation on induced sputum cell counts in healthy adults. Clin Transl Allergy 3: 8, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deslee G, Adair-Kirk TL, Betsuyaku T, Woods JC, Moore CH, Gierada DS, Conradi SH, Atkinson JJ, Toennies HM, Battaile JT, Kobayashi DK, Patterson GA, Holtzman MJ, Pierce RA. Cigarette smoke induces nucleic-acid oxidation in lung fibroblasts. Am J Respir Cell Mol Biol 43: 576–584, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell 125: 497–508, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Eckle T, Brodsky K, Bonney M, Packard T, Han J, Borchers CH, Mariani TJ, Kominsky DJ, Mittelbronn M, Eltzschig HK. HIF1A reduces acute lung injury by optimizing carbohydrate metabolism in the alveolar epithelium. PLoS Biol 11: e1001665, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eckle T, Gobel M, Bonney S. Light therapy at the interface of circadian proteins and lung disease. In: B96 VILI and Regional Lung Effects of Mechanical Ventilation, American Thoracic Society International Conference, May 15–20, 2015, Denver, Colorado, p. A3647. [Google Scholar]
- 49.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med 18: 774–782, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evans JA, Davidson AJ. Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Transl Sci 119: 283–323, 2013. [DOI] [PubMed] [Google Scholar]
- 51.Fajt ML, Tedrow JR, Milosevic J, Trudeau JB, Bleecker ER, Meyers DA, Erzurum SC, Gaston BM, Busse WW, Jarjour NN, Kaminski N, Wenzel SE. Circadian rhythm and clock gene expression are altered in asthmatic epithelium and bronchoalveolar lavage (BAL) cells. In: B93 Genetic Signatures of Asthma: Key to Endotypes, American Thoracic Society International Conference, May 15–20, 2015, Denver, Colorado, p. A3628. [Google Scholar]
- 52.Ferraz E, Borges MC, Terra-Filho J, Martinez JA, Vianna EO. Comparison of 4 AM and 4 PM bronchial responsiveness to hypertonic saline in asthma. Lung 184: 341–346, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Gaddameedhi S, Reardon JT, Ye R, Ozturk N, Sancar A. Effect of circadian clock mutations on DNA damage response in mammalian cells. Cell Cycle 11: 3481–3491, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gebel S, Gerstmayer B, Kuhl P, Borlak J, Meurrens K, Muller T. The kinetics of transcriptomic changes induced by cigarette smoke in rat lungs reveals a specific program of defense, inflammation, and circadian clock gene expression. Toxicol Sci 93: 422–431, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Gentner NJ, Weber LP. Secondhand tobacco smoke, arterial stiffness, and altered circadian blood pressure patterns are associated with lung inflammation and oxidative stress in rats. Am J Physiol Heart Circ Physiol 302: H818–H825, 2012. [DOI] [PubMed] [Google Scholar]
- 56.Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell 22: 375–382, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Gibbs J, Ince L, Matthews L, Mei J, Bell T, Yang N, Saer B, Begley N, Poolman T, Pariollaud M, Farrow S, DeMayo F, Hussell T, Worthen GS, Ray D, Loudon A. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med 20: 919–926, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gibbs JE, Beesley S, Plumb J, Singh D, Farrow S, Ray DW, Loudon AS. Circadian timing in the lung; a specific role for bronchiolar epithelial cells. Endocrinology 150: 268–276, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, Loudon AS. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA 109: 582–587, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax 64: 728–735, 2009. [DOI] [PubMed] [Google Scholar]
- 61.Goriki A, Hatanaka F, Myung J, Kim JK, Yoritaka T, Tanoue S, Abe T, Kiyonari H, Fujimoto K, Kato Y, Todo T, Matsubara A, Forger D, Takumi T. A novel protein, CHRONO, functions as a core component of the mammalian circadian clock. PLoS Biol 12: e1001839, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grant D, Yin L, Collins JL, Parks DJ, Orband-Miller LA, Wisely GB, Joshi S, Lazar MA, Willson TM, Zuercher WJ. GSK4112, a small molecule chemical probe for the cell biology of the nuclear heme receptor Rev-erbα. ACS Chem Biol 5: 925–932, 2010. [DOI] [PubMed] [Google Scholar]
- 63.Grechez-Cassiau A, Rayet B, Guillaumond F, Teboul M, Delaunay F. The circadian clock component BMAL1 is a critical regulator of p21WAF1/CIP1 expression and hepatocyte proliferation. J Biol Chem 283: 4535–4542, 2008. [DOI] [PubMed] [Google Scholar]
- 64.Grimaldi B, Nakahata Y, Kaluzova M, Masubuchi S, Sassone-Corsi P. Chromatin remodeling, metabolism and circadian clocks: the interplay of CLOCK and SIRT1. Int J Biochem Cell Biol 41: 81–86, 2009. [DOI] [PubMed] [Google Scholar]
- 65.Hadden H, Soldin SJ, Massaro D. Circadian disruption alters mouse lung clock gene expression and lung mechanics. J Appl Physiol 113: 385–392, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamilton EE, Kay SA. SnapShot: circadian clock proteins. Cell 135: 368–368.e1, 2008. [DOI] [PubMed] [Google Scholar]
- 67.Hams E, Armstrong ME, Barlow JL, Saunders SP, Schwartz C, Cooke G, Fahy RJ, Crotty TB, Hirani N, Flynn RJ, Voehringer D, McKenzie AN, Donnelly SC, Fallon PG. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci USA 111: 367–372, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hardin PE, Yu W. Circadian transcription: passing the HAT to CLOCK. Cell 125: 424–426, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Haspel J, Agapov E, Holtzman MJ. Effects of circadian rhythm disruption on viral pneumonia severity. In: A32 Microbial Interactions and Host Defense, American Thoracic Society International Conference, May 15–20, 2015, Denver, Colorado, p. A1316. [Google Scholar]
- 70.Haspel JA, Chettimada S, Shaik RS, Chu JH, Raby BA, Cernadas M, Carey V, Process V, Hunninghake GM, Ifedigbo E, Lederer JA, Englert J, Pelton A, Coronata A, Fredenburgh LE, Choi AM. Circadian rhythm reprogramming during lung inflammation. Nat Commun 5: 4753, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hayashi M, Shimba S, Tezuka M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol Pharm Bull 30: 621–626, 2007. [DOI] [PubMed] [Google Scholar]
- 72.Hirayama J, Cho S, Sassone-Corsi P. Circadian control by the reduction/oxidation pathway: catalase represses light-dependent clock gene expression in the zebrafish. Proc Natl Acad Sci USA 104: 15747–15752, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature 450: 1086–1090, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Hirota T, Fukada Y. Resetting mechanism of central and peripheral circadian clocks in mammals. Zoolog Sci 21: 359–368, 2004. [DOI] [PubMed] [Google Scholar]
- 75.Hruby J, Butler J. Variability of routine pulmonary function tests. Thorax 30: 548–553, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu Y, Spengler ML, Kuropatwinski KK, Comas-Soberats M, Jackson M, Chernov MV, Gleiberman AS, Fedtsova N, Rustum YM, Gudkov AV, Antoch MP. Selenium is a modulator of circadian clock that protects mice from the toxicity of a chemotherapeutic drug via upregulation of the core clock protein, BMAL1. Oncotarget 2: 1279–1290, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hwang JW, Sundar IK, Yao H, Sellix MT, Rahman I. Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway. FASEB J 28: 176–194, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jaradat M, Stapleton C, Tilley SL, Dixon D, Erikson CJ, McCaskill JG, Kang HS, Angers M, Liao G, Collins J, Grissom S, Jetten AM. Modulatory role for retinoid-related orphan receptor α in allergen-induced lung inflammation. Am J Respir Crit Care Med 174: 1299–1309, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jesudason EC, Keshet E, Warburton D. Entrained pulmonary clocks: epithelium and vasculature keeping pace. Am J Physiol Lung Cell Mol Physiol 299: L453–L454, 2010. [DOI] [PubMed] [Google Scholar]
- 80.Kang TH, Leem SH. Modulation of ATR-mediated DNA damage checkpoint response by cryptochrome 1. Nucleic Acids Res 42: 4427–4434, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kang TH, Lindsey-Boltz LA, Reardon JT, Sancar A. Circadian control of XPA and excision repair of cisplatin-DNA damage by cryptochrome and HERC2 ubiquitin ligase. Proc Natl Acad Sci USA 107: 4890–4895, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci USA 108: 1657–1662, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kathale ND, Liu AC. Prevalence of cycling genes and drug targets calls for prospective chronotherapeutics. Proc Natl Acad Sci USA 111: 15869–15870, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY, Chua KF. SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell 136: 62–74, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kawahara TL, Rapicavoli NA, Wu AR, Qu K, Quake SR, Chang HY. Dynamic chromatin localization of Sirt6 shapes stress- and aging-related transcriptional networks. PLoS Genet 7: e1002153, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci USA 106: 21407–21412, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim CM, Sohn JW, Yoon HJ, Shin DH, Park SS. Circadian rhythm of surfactant protein A, B and C mRNA in rats. Korean J Intern Med 18: 76–82, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kojetin D, Wang Y, Kamenecka TM, Burris TP. Identification of SR8278, a synthetic antagonist of the nuclear heme receptor REV-ERB. ACS Chem Biol 6: 131–134, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kojetin DJ, Burris TP. REV-ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov 13: 197–216, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kondratov RV, Antoch MP. Circadian proteins in the regulation of cell cycle and genotoxic stress responses. Trends Cell Biol 17: 311–317, 2007. [DOI] [PubMed] [Google Scholar]
- 91.Kondratov RV, Chernov MV, Kondratova AA, Gorbacheva VY, Gudkov AV, Antoch MP. BMAL1-dependent circadian oscillation of nuclear CLOCK: posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev 17: 1921–1932, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev 20: 1868–1873, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kondratov RV, Vykhovanets O, Kondratova AA, Antoch MP. Antioxidant N-acetyl-l-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging (Albany NY) 1: 979–987, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kondratova AA, Kondratov RV. The circadian clock and pathology of the ageing brain. Nat Rev Neurosci 13: 325–335, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Korencic A, Kosir R, Bordyugov G, Lehmann R, Rozman D, Herzel H. Timing of circadian genes in mammalian tissues. Sci Rep 4: 5782, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kumar N, Kojetin DJ, Solt LA, Kumar KG, Nuhant P, Duckett DR, Cameron MD, Butler AA, Roush WR, Griffin PR, Burris TP. Identification of SR3335 (ML-176): a synthetic RORα selective inverse agonist. ACS Chem Biol 6: 218–222, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kumar N, Lyda B, Chang MR, Lauer JL, Solt LA, Burris TP, Kamenecka TM, Griffin PR. Identification of SR2211: a potent synthetic RORγ-selective modulator. ACS Chem Biol 7: 672–677, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kumar N, Solt LA, Conkright JJ, Wang Y, Istrate MA, Busby SA, Garcia-Ordonez RD, Burris TP, Griffin PR. The benzenesulfoamide T0901317 [N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethy l]phenyl]-benzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-α/γ inverse agonist. Mol Pharmacol 77: 228–236, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Labrecque N, Cermakian N. Circadian clocks in the immune system. J Biol Rhythms 30: 277–290, 2015. [DOI] [PubMed] [Google Scholar]
- 100.Lagishetty V, Parthasarathy PT, Phillips O, Fukumoto J, Cho Y, Fukumoto I, Bao H, Cox R Jr, Galam L, Lockey RF, Kolliputi N. Dysregulation of CLOCK gene expression in hyperoxia-induced lung injury. Am J Physiol Cell Physiol 306: C999–C1007, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Le Minh N, Damiola F, Tronche F, Schutz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J 20: 7128–7136, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 107: 855–867, 2001. [DOI] [PubMed] [Google Scholar]
- 103.Levi F, Schibler U. Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol 47: 593–628, 2007. [DOI] [PubMed] [Google Scholar]
- 104.Levin AM, Wang Y, Wells KE, Padhukasahasram B, Yang JJ, Burchard EG, Williams LK. Nocturnal asthma and the importance of race/ethnicity and genetic ancestry. Am J Respir Crit Care Med 190: 266–273, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lewis DA. Sleep in patients with asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med 7: 105–112, 2001. [DOI] [PubMed] [Google Scholar]
- 106.Li H, Wang C, Hu J, Tan J. A study on circadian rhythm disorder of rat lung tissue caused by mechanical ventilation induced lung injury. Int Immunopharmacol 18: 249–254, 2014. [DOI] [PubMed] [Google Scholar]
- 107.Logan RW, Zhang C, Murugan S, O'Connell S, Levitt D, Rosenwasser AM, Sarkar DK. Chronic shift-lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. J Immunol 188: 2583–2591, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luna A, Aladjem MI, Kohn KW. SIRT1/PARP1 crosstalk: connecting DNA damage and metabolism. Genome Integr 4: 6, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luna A, McFadden GB, Aladjem MI, Kohn KW. Predicted role of NAD utilization in the control of circadian rhythms during DNA damage response. PLoS Comput Biol 11: e1004144, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mao Z, Bozzella M, Seluanov A, Gorbunova V. Comparison of nonhomologous end joining and homologous recombination in human cells. DNA Repair (Amst) 7: 1765–1771, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mao Z, Hine C, Tian X, Van Meter M, Au M, Vaidya A, Seluanov A, Gorbunova V. SIRT6 promotes DNA repair under stress by activating PARP1. Science 332: 1443–1446, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marcheva B, Ramsey KM, Peek CB, Affinati A, Maury E, Bass J. Circadian clocks and metabolism. Hand Exp Pharmacol 217: 127–155, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med 182: 325–331, 2010. [DOI] [PubMed] [Google Scholar]
- 114.Marrone O, Salvaggio A, Insalaco G. Respiratory disorders during sleep in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 1: 363–372, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martin RJ, Banks-Schlegel S. Chronobiology of asthma. Am J Respir Crit Care Med 158: 1002–1007, 1998. [DOI] [PubMed] [Google Scholar]
- 116.Masri S, Patel VR, Eckel-Mahan KL, Peleg S, Forne I, Ladurner AG, Baldi P, Imhof A, Sassone-Corsi P. Circadian acetylome reveals regulation of mitochondrial metabolic pathways. Proc Natl Acad Sci USA 110: 3339–3344, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Masri S, Rigor P, Cervantes M, Ceglia N, Sebastian C, Xiao C, Roqueta-Rivera M, Deng C, Osborne TF, Mostoslavsky R, Baldi P, Sassone-Corsi P. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell 158: 659–672, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mauvoisin D, Dayon L, Gachon F, Kussmann M. Proteomics and circadian rhythms: it's all about signaling! Proteomics 15: 310–317, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McNicholas WT, Fitzgerald MX. Nocturnal deaths among patients with chronic bronchitis and emphysema. Br Med J (Clin Res Ed) 289: 878, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Menaker M, Murphy ZC, Sellix MT. Central control of peripheral circadian oscillators. Curr Opin Neurobiol 23: 741–746, 2013. [DOI] [PubMed] [Google Scholar]
- 121.Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TL, Barrett JC, Chang HY, Bohr VA, Ried T, Gozani O, Chua KF. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 452: 492–496, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Michishita E, McCord RA, Boxer LD, Barber MF, Hong T, Gozani O, Chua KF. Cell cycle-dependent deacetylation of telomeric histone H3 lysine K56 by human SIRT6. Cell Cycle 8: 2664–2666, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mirrakhimov AE. Chronic obstructive pulmonary disease and glucose metabolism: a bitter sweet symphony. Cardiovasc Diabetol 11: 132, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35: 445–462, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124: 315–329, 2006. [DOI] [PubMed] [Google Scholar]
- 126.Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, Roh JH, Ortiz-Gonzalez X, Dearborn JT, Culver JP, Herzog ED, Hogenesch JB, Wozniak DF, Dikranian K, Giasson BI, Weaver DR, Holtzman DM, Fitzgerald GA. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest 123: 5389–5400, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nadel JA, Barnes PJ. Autonomic regulation of the airways. Annu Rev Med 35: 451–467, 1984. [DOI] [PubMed] [Google Scholar]
- 128.Nakahata Y, Grimaldi B, Sahar S, Hirayama J, Sassone-Corsi P. Signaling to the circadian clock: plasticity by chromatin remodeling. Curr Opin Cell Biol 19: 230–237, 2007. [DOI] [PubMed] [Google Scholar]
- 129.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134: 329–340, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324: 654–657, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nakamura Y, Nakano N, Ishimaru K, Hara M, Ikegami T, Tahara Y, Katoh R, Ogawa H, Okumura K, Shibata S, Nishiyama C, Nakao A. Circadian regulation of allergic reactions by the mast cell clock in mice. J Allergy Clin Immunol 133: 568–575, 2014. [DOI] [PubMed] [Google Scholar]
- 132.Nam D, Chatterjee S, Yin H, Liu R, Lee J, Yechoor VK, Ma K. Novel function of Rev-erbα in promoting brown adipogenesis. Sci Rep 5: 11239, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nam D, Guo B, Chatterjee S, Chen MH, Nelson D, Yechoor VK, Ma K. The adipocyte clock controls brown adipogenesis through the TGF-β and BMP signaling pathways. J Cell Sci 128: 1835–1847, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 341: 1483–1488, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Numaguchi S, Esumi M, Sakamoto M, Endo M, Ebihara T, Soma H, Yoshida A, Tokuhashi Y. Passive cigarette smoking changes the circadian rhythm of clock genes in rat intervertebral discs. J Orthop Res 2015. May 4. doi: 10.1002/jor.22941 [Epub ahead of print] [DOI] [PubMed]
- 136.Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, Hartlerode A, Stegmuller J, Hafner A, Loerch P, Wright SM, Mills KD, Bonni A, Yankner BA, Scully R, Prolla TA, Alt FW, Sinclair DA. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 135: 907–918, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ohsawa I, Nishimaki K, Murakami Y, Suzuki Y, Ishikawa M, Ohta S. Age-dependent neurodegeneration accompanying memory loss in transgenic mice defective in mitochondrial aldehyde dehydrogenase 2 activity. J Neurosci 28: 6239–6249, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oishi K, Sakamoto K, Okada T, Nagase T, Ishida N. Antiphase circadian expression between BMAL1 and period homologue mRNA in the suprachiasmatic nucleus and peripheral tissues of rats. Biochem Biophys Res Commun 253: 199–203, 1998. [DOI] [PubMed] [Google Scholar]
- 139.Oishi K, Sakamoto K, Okada T, Nagase T, Ishida N. Humoral signals mediate the circadian expression of rat period homologue (rPer2) mRNA in peripheral tissues. Neurosci Lett 256: 117–119, 1998. [DOI] [PubMed] [Google Scholar]
- 140.Okada K, Yano M, Doki Y, Azama T, Iwanaga H, Miki H, Nakayama M, Miyata H, Takiguchi S, Fujiwara Y, Yasuda T, Ishida N, Monden M. Injection of LPS causes transient suppression of biological clock genes in rats. J Surg Res 145: 5–12, 2008. [DOI] [PubMed] [Google Scholar]
- 141.Oosterman JE, Kalsbeek A, la Fleur SE, Belsham DD. Impact of nutrients on circadian rhythmicity. Am J Physiol Regul Integr Comp Physiol 308: R337–R350, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Panzer SE, Dodge AM, Kelly EA, Jarjour NN. Circadian variation of sputum inflammatory cells in mild asthma. J Allergy Clin Immunol 111: 308–312, 2003. [DOI] [PubMed] [Google Scholar]
- 143.Papaioannou V, Mebazaa A, Plaud B, Legrand M. ‘Chronomics’ in ICU: circadian aspects of immune response and therapeutic perspectives in the critically ill. Intensive Care Med Exp 2: 19, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Parish JM. Sleep-related problems in common medical conditions. Chest 135: 563–572, 2009. [DOI] [PubMed] [Google Scholar]
- 145.Patel VR, Eckel-Mahan K, Sassone-Corsi P, Baldi P. CircadiOmics: integrating circadian genomics, transcriptomics, proteomics and metabolomics. Nat Methods 9: 772–773, 2012. [DOI] [PubMed] [Google Scholar]
- 146.Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, Levine DC, Bacsik DJ, Gius D, Newgard CB, Goetzman E, Chandel NS, Denu JM, Mrksich M, Bass J. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 342: 1243417, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pekovic-Vaughan V, Gibbs J, Yoshitane H, Yang N, Pathiranage D, Guo B, Sagami A, Taguchi K, Bechtold D, Loudon A, Yamamoto M, Chan J, van der Horst GT, Fukada Y, Meng QJ. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev 28: 548–560, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Perera WR, Hurst JR, Wilkinson TM, Sapsford RJ, Mullerova H, Donaldson GC, Wedzicha JA. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur Respir J 29: 527–534, 2007. [DOI] [PubMed] [Google Scholar]
- 149.Petty TL. Circadian variations in chronic asthma and chronic obstructive pulmonary disease. Am J Med 85: 21–23, 1988. [DOI] [PubMed] [Google Scholar]
- 150.Phillips DJ, Savenkova MI, Karatsoreos IN. Environmental disruption of the circadian clock leads to altered sleep and immune responses in mouse. Brain Behav Immun 47: 14–23, 2015. [DOI] [PubMed] [Google Scholar]
- 151.Pilorz V, Cunningham PS, Jackson A, West AC, Wager TT, Loudon AS, Bechtold DA. A novel mechanism controlling resetting speed of the circadian clock to environmental stimuli. Curr Biol 24: 766–773, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Price D, Small M, Milligan G, Higgins V, Gil EG, Estruch J. Impact of night-time symptoms in COPD: a real-world study in five European countries. Int J Chron Obstruct Pulmon Dis 8: 595–603, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rabe KF, Beghe B, Luppi F, Fabbri LM. Update in chronic obstructive pulmonary disease 2006. Am J Respir Crit Care Med 175: 1222–1232, 2007. [DOI] [PubMed] [Google Scholar]
- 154.Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 177: 861–870, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ram O, Goren A, Amit I, Shoresh N, Yosef N, Ernst J, Kellis M, Gymrek M, Issner R, Coyne M, Durham T, Zhang X, Donaghey J, Epstein CB, Regev A, Bernstein BE. Combinatorial patterning of chromatin regulators uncovered by genome-wide location analysis in human cells. Cell 147: 1628–1639, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Reilly DF, Westgate EJ, FitzGerald GA. Peripheral circadian clocks in the vasculature. Arterioscler Thromb Vasc Biol 27: 1694–1705, 2007. [DOI] [PubMed] [Google Scholar]
- 157.Ribas-Latre A, Baselga-Escudero L, Casanova E, Arola-Arnal A, Salvado MJ, Blade C, Arola L. Dietary proanthocyanidins modulate BMAL1 acetylation, Nampt expression and NAD levels in rat liver. Sci Rep 5: 10954, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Richards J, Gumz ML. Mechanism of the circadian clock in physiology. Am J Physiol Regul Integr Comp Physiol 304: R1053–R1064, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Richardson VM, Santostefano MJ, Birnbaum LS. Daily cycle of bHLH-PAS proteins, Ah receptor and Arnt, in multiple tissues of female Sprague-Dawley rats. Biochem Biophys Res Commun 252: 225–231, 1998. [DOI] [PubMed] [Google Scholar]
- 160.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11: 973–979, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rodier F, Munoz DP, Teachenor R, Chu V, Le O, Bhaumik D, Coppe JP, Campeau E, Beausejour CM, Kim SH, Davalos AR, Campisi J. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci 124: 68–81, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sancar A, Lindsey-Boltz LA, Kang TH, Reardon JT, Lee JH, Ozturk N. Circadian clock control of the cellular response to DNA damage. FEBS Lett 584: 2618–2625, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, Taniguchi N, Ohno H, Kizaki T. A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol 192: 407–417, 2014. [DOI] [PubMed] [Google Scholar]
- 164.Sellix MT, Evans JA, Leise TL, Castanon-Cervantes O, Hill DD, DeLisser P, Block GD, Menaker M, Davidson AJ. Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators. J Neurosci 32: 16193–16202, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shi Y, Cao J, Gao J, Zheng L, Goodwin A, An CH, Patel A, Lee JS, Duncan SR, Kaminski N, Pandit KV, Rosas IO, Choi AM, Morse D. Retinoic acid-related orphan receptor-α is induced in the setting of DNA damage and promotes pulmonary emphysema. Am J Respir Crit Care Med 186: 412–419, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Silver AC, Arjona A, Walker WE, Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity 36: 251–261, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sitaula S, Billon C, Kamenecka TM, Solt LA, Burris TP. Suppression of atherosclerosis by synthetic REV-ERB agonist. Biochem Biophys Res Commun 460: 566–571, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Solt LA, Banerjee S, Campbell S, Kamenecka TM, Burris TP. ROR inverse agonist suppresses insulitis and prevents hyperglycemia in a mouse model of type 1 diabetes. Endocrinology 156: 869–881, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Solt LA, Kumar N, He Y, Kamenecka TM, Griffin PR, Burris TP. Identification of a selective RORγ ligand that suppresses T(H)17 cells and stimulates T regulatory cells. ACS Chem Biol 7: 1515–1519, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, Istrate MA, Kamenecka TM, Roush WR, Vidovic D, Schurer SC, Xu J, Wagoner G, Drew PD, Griffin PR, Burris TP. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 472: 491–494, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, Yoo SH, Takahashi JS, Butler AA, Kamenecka TM, Burris TP. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485: 62–68, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Spengler ML, Kuropatwinski KK, Comas M, Gasparian AV, Fedtsova N, Gleiberman AS, Gitlin II, Artemicheva NM, Deluca KA, Gudkov AV, Antoch MP. Core circadian protein CLOCK is a positive regulator of NF-κB-mediated transcription. Proc Natl Acad Sci USA 109: E2457–2465, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sukumaran S, Jusko WJ, Dubois DC, Almon RR. Light-dark oscillations in the lung transcriptome: implications for lung homeostasis, repair, metabolism, disease, and drug action. J Appl Physiol 110: 1732–1747, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sundar IK, Ahmad T, Yao H, Hwang JW, Gerloff J, Lawrence BP, Sellix MT, Rahman I. Influenza A virus-dependent remodeling of pulmonary clock function in a mouse model of COPD. Sci Rep 4: 9927, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sundar IK, Chung S, Hwang JW, Lapek JD Jr, Bulger M, Friedman AE, Yao H, Davie JR, Rahman I. Mitogen- and stress-activated kinase 1 (MSK1) regulates cigarette smoke-induced histone modifications on NF-κB-dependent genes. PLoS One 7: e31378, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sundar IK, Nevid MZ, Friedman AE, Rahman I. Cigarette smoke induces distinct histone modifications in lung cells: implications for the pathogenesis of COPD and lung cancer. J Proteome Res 13: 982–996, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sundar IK, Yao H, Huang Y, Lyda E, Sime PJ, Sellix MT, Rahman I. Serotonin and corticosterone rhythms in mice exposed to cigarette smoke and in patients with COPD: implication for COPD-associated neuropathogenesis. PLoS One 9: e87999, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sundar IK, Yao H, Sellix MT, Rahman I. Circadian clock-coupled lung cellular and molecular functions in chronic airway diseases. Am J Respir Cell Mol Biol 53: 285–290, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Takata M, Burioka N, Ohdo S, Fukuoka Y, Miyata M, Endo M, Suyama H, Shimizu E. Beta2-adrenoceptor agonists induce the mammalian clock gene, hPer1, mRNA in cultured human bronchial epithelium cells in vitro. Chronobiol Int 22: 777–783, 2005. [DOI] [PubMed] [Google Scholar]
- 180.Tanimura N, Kusunose N, Matsunaga N, Koyanagi S, Ohdo S. Aryl hydrocarbon receptor-mediated Cyp1a1 expression is modulated in a CLOCK-dependent circadian manner. Toxicology 290: 203–207, 2011. [DOI] [PubMed] [Google Scholar]
- 181.Tennen RI, Chua KF. Chromatin regulation and genome maintenance by mammalian SIRT6. Trends Biochem Sci 36: 39–46, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tirlapur VG. Nocturnal deaths among patients with chronic bronchitis and emphysema. Br Med J (Clin Res Ed) 289: 1540, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tong X, Zhang D, Arthurs B, Li P, Durudogan L, Gupta N, Yin L. Palmitate inhibits SIRT1-dependent BMAL1/CLOCK interaction and disrupts circadian gene oscillations in hepatocytes. PLoS One 10: e0130047, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Traves SL, Proud D. Viral-associated exacerbations of asthma and COPD. Curr Opin Pharmacol 7: 252–258, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Traylor ZP, Aeffner F, Davis IC. Influenza A H1N1 induces declines in alveolar gas exchange in mice consistent with rapid post-infection progression from acute lung injury to ARDS. Influenza Other Respir Viruses 7: 472–479, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tsai CL, Brenner BE, Camargo CA Jr. Circadian-rhythm differences among emergency department patients with chronic obstructive pulmonary disease exacerbation. Chronobiol Int 24: 699–713, 2007. [DOI] [PubMed] [Google Scholar]
- 187.Tsimakouridze EV, Alibhai FJ, Martino TA. Therapeutic applications of circadian rhythms for the cardiovascular system. Front Pharmacol 6: 77, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tsitoura E, Koutsopoulos A, Lagoudaki E, Charalambous E, Sourvinos G, Siafakas N, Antoniou KM. Retinoic acid-related orphan receptor-α (RORα) expression and subcellular localization in lung fibroblasts and pneumocytes is associated with fibroblast activation and fibrosis in fibrotic lung tissue specimens. In: C64 Having a Blast with Fibroblasts, American Thoracic Society, 2015, p. A4934.
- 189.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med 174: 886–893, 2006. [DOI] [PubMed] [Google Scholar]
- 190.Tuder RM, Kern JA, Miller YE. Senescence in chronic obstructive pulmonary disease. Proc Am Thorac Soc 9: 62–63, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Van Someren EJ. Circadian and sleep disturbances in the elderly. Exp Gerontol 35: 1229–1237, 2000. [DOI] [PubMed] [Google Scholar]
- 192.Vasu VT, Cross CE, Gohil K. Nr1d1, an important circadian pathway regulatory gene, is suppressed by cigarette smoke in murine lungs. Integr Cancer Ther 8: 321–328, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, Prolo P, Wong ML, Licinio J, Gold PW, Hermida RC, Mastorakos G, Chrousos GP. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab 84: 2603–2607, 1999. [DOI] [PubMed] [Google Scholar]
- 194.Wang F, Li C, Yongluo, Chen L. The circadian gene clock plays an important role in cell apoptosis and the DNA damage response in vitro. Technol Cancer Res Treat 2015. May 14 pii: 1533034615585433. [Epub ahead of print] [DOI] [PubMed]
- 195.Wang Y, Kumar N, Nuhant P, Cameron MD, Istrate MA, Roush WR, Griffin PR, Burris TP. Identification of SR1078, a synthetic agonist for the orphan nuclear receptors RORα and RORγ. ACS Chem Biol 5: 1029–1034, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang Y, Zhang XS, Chen L. A network biology study on circadian rhythm by integrating various omics data. OMICS 13: 313–324, 2009. [DOI] [PubMed] [Google Scholar]
- 197.Woldt E, Sebti Y, Solt LA, Duhem C, Lancel S, Eeckhoute J, Hesselink MK, Paquet C, Delhaye S, Shin Y, Kamenecka TM, Schaart G, Lefebvre P, Neviere R, Burris TP, Schrauwen P, Staels B, Duez H. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med 19: 1039–1046, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wolff G, Duncan MJ, Esser KA. Chronic phase advance alters circadian physiological rhythms and peripheral molecular clocks. J Appl Physiol 115: 373–382, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-κB signaling in response to genotoxic stimuli. Science 311: 1141–1146, 2006. [DOI] [PubMed] [Google Scholar]
- 200.Xiao C, Kim HS, Lahusen T, Wang RH, Xu X, Gavrilova O, Jou W, Gius D, Deng CX. SIRT6 deficiency results in severe hypoglycemia by enhancing both basal and insulin-stimulated glucose uptake in mice. J Biol Chem 285: 36776–36784, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yamashita K, Hagiya Y, Nakajima M, Ishizuka M, Tanaka T, Ogura S. The effects of the heme precursor 5-aminolevulinic acid (ALA) on REV-ERBα activation. FEBS Open Bio 4: 347–352, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yang B, Zwaans BM, Eckersdorff M, Lombard DB. The sirtuin SIRT6 deacetylates H3 K56Ac in vivo to promote genomic stability. Cell Cycle 8: 2662–2663, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yang G, Wright CJ, Hinson MD, Fernando AP, Sengupta S, Biswas C, La P, Dennery PA. Oxidative stress and inflammation modulate Rev-erbα signaling in the neonatal lung and affect circadian rhythmicity. Antioxid Redox Signal 21: 17–32, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-κB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol 291: L46–L57, 2006. [DOI] [PubMed] [Google Scholar]
- 205.Yang SR, Valvo S, Yao H, Kode A, Rajendrasozhan S, Edirisinghe I, Caito S, Adenuga D, Henry R, Fromm G, Maggirwar S, Li JD, Bulger M, Rahman I. IKK α causes chromatin modification on pro-inflammatory genes by cigarette smoke in mouse lung. Am J Respir Cell Mol Biol 38: 689–698, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-κB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol 292: L567–L576, 2007. [DOI] [PubMed] [Google Scholar]
- 207.Yao H, Chung S, Hwang JW, Rajendrasozhan S, Sundar IK, Dean DA, McBurney MW, Guarente L, Gu W, Ronty M, Kinnula VL, Rahman I. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest 122: 2032–2045, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yao H, Rahman I. Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem Pharmacol 84: 1332–1339, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yao H, Sundar IK, Gerloff J, Sellix MT, Sime PJ, Rahman I. SIRT1 reduction is involved in clock dysfunction, telomere attrition, and inflammosenescence in COPD. In: C108 Aging: The Common Denominator, American Thoracic Society, 2015, p. A5324.
- 210.Yao H, Sundar IK, Huang Y, Gerloff J, Sellix MT, Sime PJ, Rahman I. Disruption of SIRT1-mediated control of circadian molecular clock and inflammation in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2015. April 23 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 211.Young MR, Matthews JP, Kanabrocki EL, Sothern RB, Roitman-Johnson B, Scheving LE. Circadian rhythmometry of serum interleukin-2, interleukin-10, tumor necrosis factor-α, and granulocyte-macrophage colony-stimulating factor in men. Chronobiol Int 12: 19–27, 1995. [DOI] [PubMed] [Google Scholar]
- 212.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA 111: 16219–16224, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhang Y, Fang B, Emmett MJ, Damle M, Sun Z, Feng D, Armour SM, Remsberg JR, Jager J, Soccio RE, Steger DJ, Lazar MA. Discrete functions of nuclear receptor Rev-erbα couple metabolism to the clock. Science 348: 1488–1492, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zheng X, Yang Z, Yue Z, Alvarez JD, Sehgal A. FOXO and insulin signaling regulate sensitivity of the circadian clock to oxidative stress. Proc Natl Acad Sci USA 104: 15899–15904, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]