Abstract
One of the goals in using cells for tissue engineering (TE) and cell therapy consists of optimizing the medium for cell culture. The present study compares three different blood product supplements for improved cell proliferation and protection against DNA damage in cultured human dental pulp stem cells for tooth TE applications. Human cells from dental pulp were first characterized as adult stem cells (ectomesenchymal mixed origin) by flow cytometry. Next, four different cell culture conditions were tested: I, supplement-free; II, supplemented with fetal bovine serum; III, allogeneic human serum; and IV, autologous human serum. Cultured cells were then characterized for cell proliferation, mineralized nodule formation, and colony-forming units (CFU) capability. After 28 days in culture, the comet assay was performed to assess possible damage in cellular DNA. Our results revealed that Protocol IV achieved higher cell proliferation than Protocol I (p = 0.0112). Protocols II and III resulted in higher cell proliferation than Protocol I, but no statistical differences were found relative to Protocol IV. The comet assay revealed less cell damage in cells cultured using Protocol IV as compared to Protocols II and III. The damage percentage observed on Protocol II was significantly higher than all other protocols. CFUs capability was highest using Protocol IV (p = 0.0018) and III, respectively, and the highest degree of mineralization was observed using Protocol IV as compared to Protocols II and III. Protocol IV resulted in significantly improved cell proliferation, and no cell damage was observed. These results demonstrate that human blood product supplements can be used as feasible supplements for culturing adult human dental stem cells.
Introduction
Current difficulties in identifying compatible organ donors for transplantations in a timely manner, result in considerable financial and psychological burden to the recipient individual.1 The use of biomaterials as substitutes or inducers for tissue formation presents an attractive alternative approach, although biomaterials can be limited by the complexity of their macro and microgeometry when they are applied as scaffolds to guide conductive or inductive strategy.
For tissue loss caused by trauma, agenesis, or disease, some studies conclude that the optimal substitute is either autologous healthy tissue, or autologous tissue produced by tissue engineering (TE) methods.2–5
Embryonic or adult stem cells have great therapeutic potential, representing an unlimited source for the study of TE models. TE applies Engineering techniques and Biological Sciences principles, using knowledge of embryology, tissue regeneration, and formation. The goal is a clinically relevant regenerated replacement tissue that restores function and provides mechanical stability.2–6
Cultured cells need to maintain and mimic their original tissue characteristics, including viability and preservation of function, while not inducing immunogenic phenomena, illnesses, or tumor development in the recipient host. It is also necessary to eliminate the risk of pathogenic agent transmission from the donor, as well as immunological reactions caused by animal antigens, proteins, genes, specific enzymes, that may be present in cell cultures that use animal nutritional supplements.7
Purified mesenchymal stem cells (MSCs) and epithelial stem cells have been shown to proliferate both in vitro and in vivo.8 They are potentially capable of differentiating into multiple tissues using proper differentiation media. The use of autologous MSCs for regenerative therapies to minimize the occurrence of rejection has been the target of much research. Cell cultures in vitro require the simulation of a microenvironment similar to that of the tissue or organ. Therefore, the addition of nutritional medium supplements, including minerals, ions, proteins, growth factors, cell differentiation inducers, and substrates that ensure lack of contaminations, etc., is used to mimic the microenvironmental conditions.7 There is a consensus among researchers on the need for nutritional supplements in culture medium. Often, industrial culture medium used to culture human embryonic or adult human stem cells are supplemented with blood products, such as fetal serum obtained from bovine, equine, swine, goat, and other sources (heterologous). These supplements of animal origin can risk the introduction of pathogens into the recipient host, including swine retrovirus, resulting in immune reactions and infections.7,9,10
Thus, allogeneic and autologous blood product supplements can be used to provide a microenvironment most similar to that of the natural tissue, and minimize the occurrence of immune reactions and the introduction of interspecies virus. Therefore, the aim of this study was to evaluate three different tissue culture protocols using blood product supplements for cell proliferation. The risk of DNA damage of dental human stem cells cultured in each protocol was assessed by comet assay.
Materials and Methods
Extractions of impacted M3 tooth
Five patients aged between 12 and 16 years old, of both genders, healthy, originally from private offices and/or the Department of Plastic Surgery, UNIFESP, Medicine School of São Paulo, Brazil, with indication for extraction of impacted M3 tooth germs, were invited to participate in the study. All patients signed an informed consent form and donated 10 dental germs. In case of minor/children enrolled on this study, the informed consent was signed by guardians or caretaker besides the patient themselves that was informed in writing their participation. All informed consent was obtained and explained, read before the signature. The participant got a copy in writing of the informed consent form. The study was approved by the Ethics Committee in Medical Research of UNIFESP-0968/09.
Human dental pulp stem cells cultures
Extracted tooth germs, tooth development stage 2,11 were immediately placed in Hank's balanced salt solution (HBSS; Gibco BRL, Gaithersburg, MD, USA) at 37°C and transported to the laboratory. The pulp tissue was removed, weighed, and broken up into pieces smaller than 1 mm in fresh HBSS, washed, and digested for 30–40 min with 0.4 mg/mL of type II collagenase (Sigma-Aldrich) and 0.2 mg/mL dispase I (Boehringer Mannheim, Indianapolis, IN). After enzymatic digestion, the tissues were dissociated by trituration, washed in 50% Dulbecco's modified Eagle's medium (DMEM; Gibco BRL), 50 units/mL penicillin, 50 mg/mL streptomycin, and 50% F12 medium (Sigma-Aldrich), filtered through the cell filter Falcon 40 μm, and resuspended. Viable cells were counted by means of inverted light microscopy and hematocytometer. At least 11 × 106 cells were obtained from each patient, of which 1 × 106 were characterized by flow cytometry using the antibodies CD90, CD105, CD73, and CD34 acquired from BD Biosciences (San Jose, CA). The 10 × 106 remaining cells were divided into two new vials. In the first, 6 × 106 cells were distributed into 16-well plates for cell culture (triplicate), four plates for each culture medium of the study (I, II, III, and IV). Cell cultures were maintained at a temperature of 37°C, 95% of relative humidity, and 5% CO2 with exchanges of culture medium twice a week, with weekly analyses during the 28 days of study. The second vial with 4 × 106 cells was distributed into four sets (one for each protocol) of two T-25 tissue culture flasks (25 cm2) for analysis of colony-forming units (CFU) on the 14th and 21st day of cell culture, respectively.
Flow cytometry
Cell characterizations were performed by Flow Cytometry in FACSCanto using FACSDiva software (BD Biosciences), and then analyzed by FlowJo software (Tree Star, San Carlo, CA). Fluorescently labeled cells were sorted from matched unstained cells. Compensation was carried out with CompBeads (BD Biosciences) single-stained with CD3-PerCP, CD4-FITC, CD3-PE, and CD3-APC. Samples were acquired for at least 200,000 events in a live lymphocyte gate.
Monoclonal antibodies used in FACS (Flow Amplified Cytometer) for characterization of mesenchymal cells included CD73, CD90, and CD105, and antibody CD34 for stem cells (BD Biosciences). The following monoclonal antibodies were used in the FACS assays: CD105 peridinin chlorophyll protein (PerCP) (clone 266); CD34 allophycocyanin–cyanine (APC) (clone 581); CD73 phycoerythrin (PE) (clone AD2); and CD90 Fluorescein isothiocyanate (FITC) (clone 5E10) (BD Biosciences). All antibodies were used for cell-surface staining. Fluorescence minus one was used for gating strategy. Sorted cells were centrifuged at 1500 rpm for 5 min and transferred into 96 V bottom well plates (Nunc, Denmark) in 100 μL of staining buffer phosphate buffered saline (PBS supplemented with 0.1% sodium azide [Sigma-Aldrich] and 1% fetal bovine serum [FBS], and pH 7.4–7.6) with the surface monoclonal antibodies panel. Cells were incubated at 4°C in darkness for 30 min, washed twice, and resuspended in 100 μL of the fixation buffer (1% paraformaldehyde [Polysciences, Warrington, PA] in PBS, pH 7.4–7.6).
Obtaining human serum
Five days before tooth extraction, 80 mL of blood was collected from each patient in dry sterile Vacutainer® vials (BD, Franklin Lakes, NJ). The harvested blood sample was processed by centrifugation for 10 min at 500 g, allowed to stand for 5 min, followed by a second centrifugation for 20 min at 2500 g. Under laminar flow, the resulting human serum was drawn into a sterile syringe, subjected to filtration through a 0.2 μ syringe filter, immersed in a serum bath (56°C for 60 min), left to stand for 20 min, and then stored under refrigeration at −4°C until the preparation of the medium.
Culture media
Culture medium I (protocol I) consisted of supplement-free (SF) blood product, 50% (DMEM; Gibco BRL), 50 units/mL penicillin, 50 mg/mL streptomycin, and 50% F12 medium (Sigma-Aldrich). Culture medium II (Protocol II) was supplemented with bovine blood product, heterologous serum (HS) traditionally used in the cultures of dental stem cell (DSCs), consisting of media composed of 50% (DMEM; Gibco BRL) containing 10% FBS, 50 units/mL penicillin, 50 mg/mL streptomycin, and 50% F12 medium (Sigma-Aldrich). Culture medium III (Protocol III) consisted of 50% (DMEM; Gibco BRL) containing 10% allogeneic human serum (AlHS), 50 units/mL penicillin, 50 mg/mL streptomycin, and 50% F12 medium (Sigma-Aldrich). Culture medium IV (Protocol IV) was composed of 50% (DMEM; Gibco BRL) containing 10% autologous human serum (AuHS) from the donor of tooth germ, 50 units/mL penicillin, 50 mg/mL streptomycin, and 50% F12 medium (Sigma-Aldrich).
Cell counting in triplicate
At the end of each week of the study, one plate of each culture medium was analyzed for cell proliferation by means of hematocytometer and inverted light microscopy, and documented through photomicrographs. Viable cell counts were performed in triplicate by counting separately each of the three upper wells and taking a growth average.
Analysis of pH
During weekly cell cultures for each of the four protocols, at the time of cell proliferation of each plate, the pH of the tissue culture medium was recorded, for comparison with the initial recording of pH in fresh medium.
Markers for staining mineralized tissue: von Kossa and Alizarin Red stains
Also at weekly intervals, two wells (A and B) of each plate were analyzed for cell differentiation and mineralized tissue formation. Using the von Kossa (VK) method, applied in the well A, the cells were fixed with 4% paraformaldehyde for 30 min, washed, and impregnated with 5% silver nitrate under UV light for 20 min. Afterward, they were exposed to 5% sodium thiosulfate for further 5 min, stained with 0.1% eosin, washed, dried at room temperature, and analyzed by reversed light microscopy. Alizarin Red (AR) method was performed on well B, where cells were fixed with 10% formalin for 15 min, stained with 2% AR for 10 min under mild agitation, washed, and dried at room temperature for examination by inverted light microscopy. For the nodules quantification, images were taken with a stereo dissection microscope AxioVert® −40°C (Carl Zeiss, Jena, Germany), camera AxioCam® (I Cc1 Zeiss–Germany). Image analysis and quantification of area stained were performed using ImageJ version 1.43U (NIH).
CFUs assay
For each culture condition, two T-25 (25-cm2) tissue culture flasks were seeded with 5.00 × 105 cells, and grown for 14 and 21 days, respectively. At each time point, the cultures were fixed in 10% formalin solution, kept in an incubator at 95% relative humidity, 5% CO2, and 37°C for 20 min, washed and stained with 2.0% Cresyl Violet (Sigma-Aldrich). Colonies larger than 2 mm in diameter were then counted and the effective CFU percentage was calculated.
Comet assay
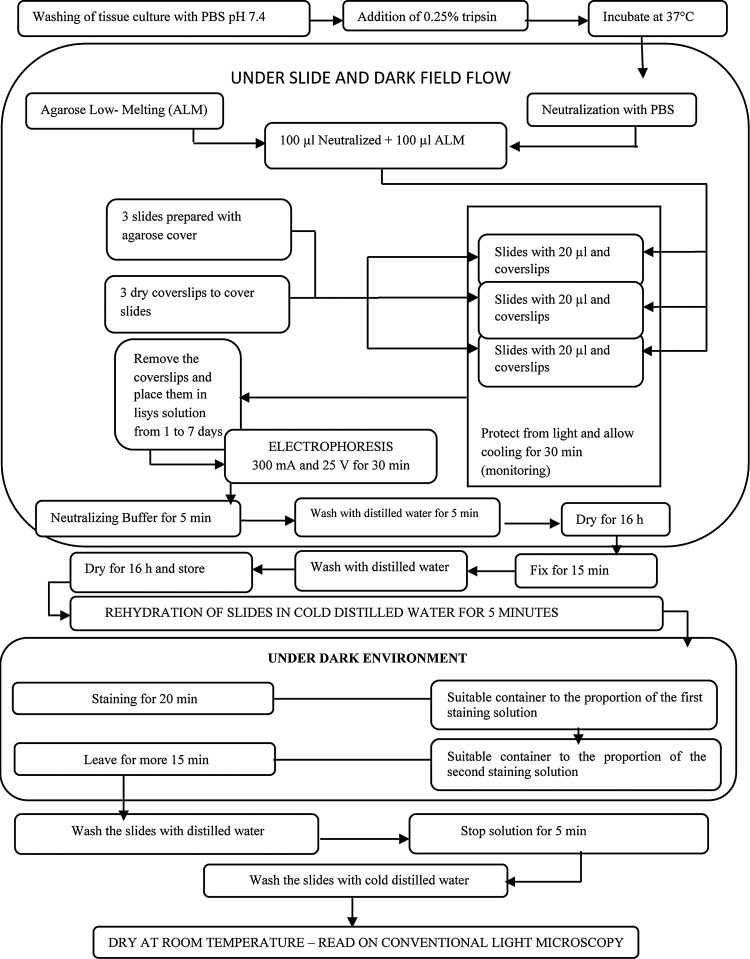
On the 28th day of culture, replicate wells for each culture condition were subjected to Comet Assay. The comet assay is the comparative analysis of the number and size of comets that emerge during electrophoresis of harvested DNAs in agarose gels, which correlates with DNA damage that occurred during each respective cell culture condition (Protocol I, II, III, and IV). Under laminar flow, cultured cells were rinsed with sterile PBS pH 7.4, and then subjected to the action of 0.25% trypsin, incubated at 37°C, until the beginning of detachment of cells from the bottom of a culture well, according to the flowchart of the comet assay (Fig. 1). Cell damage was estimated in five levels (0–4) from each experiment, as follows: Level 0, comet tail length less than one times cell nucleus diameter, indicative of reversible damage and minimal chance of mutations; Level 1, comet tail size 1 to 2 times cell nucleus diameter, indicative of reversible damage with the possibility of some mutations; Level 2, comet tail size 2 to 3 times cell nucleus diameter, indicative of reversible damage with possibility of DNA damage; Level 3, comet tail size 3 to 4 times cell nucleus diameter, indicative of greater chance of mutations; and Level 4, comet tail size greater than 4 times cell nucleus diameter, indicative of irreversible damage and strong indication of DNA damage.
FIG. 1.
Flowchart for implementation of comet assay.
The comet assay assesses DNA damage by immersing cells in an UltraPure Agarose film, and then subjecting them to electrophoresis under darkfield microscopy. The smaller and lighter the DNA fragments are, the greater their migration distance is with respect to the cell nucleus, resulting in an image similar to a comet, when stained with Ethidium Bromide and viewed under UV light.
Statistical analysis
The Friedman analysis of variance was used for the statistical calculations of cell proliferation, culture media pH, and percent CFUs formation. The Mann–Whitney analysis was used for comet assay analyses, with the percent damage between the four protocols calculated using chi-square and chi-square partition. The 0.05 or 5% rejection level was established as the null hypothesis.
Results
Flow cytometry
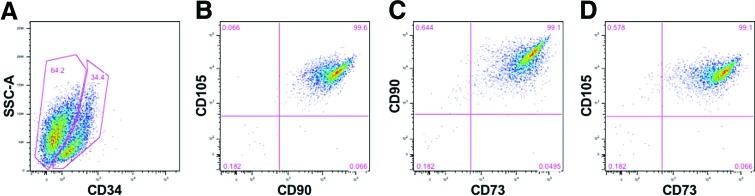
Cultured human dental pulp stem cells (hDPSCs) were collected and characterized by flow cytometry as described. These analyses showed that of the 64.2% mesenchymal cells identified using the specific markers, such as CD105, CD90, and CD73, 34.4% were stem cells. Cells that were gated on CD34-positive expression were found to be 99% positive for the mesenchymal cells markers (Fig. 2).
FIG. 2.
Flow cytometry gate strategy: (A) A human dental stem cell labeled with CD34 stem cell marker, 34.4% of cells were positive. The following gates (B–D), were extracted from positive gate of CD34. (B) The markers CD105 and CD90 with 99.6% of coexpression. (C) CD90 and CD73 were coexpressed in 99.1% of the cells. (D) The markers CD105 and CD73 were also coexpressed in 99.1% of the cells. Color images available online at www.liebertpub.com/tea
Analysis of pH
During the 28 days of cell culture with the medium exchanged twice a week, no significant differences in pH were observed between the protocols studied (Friedman test). Cell culture media maintained an alkaline pH (7.86–9.10) during all periods examined (7, 14, 21, and 28 days) (p = 0.9361, p = 0.2070, p = 0.4826, and p = 0.4202, respectively).
VK and AR stain
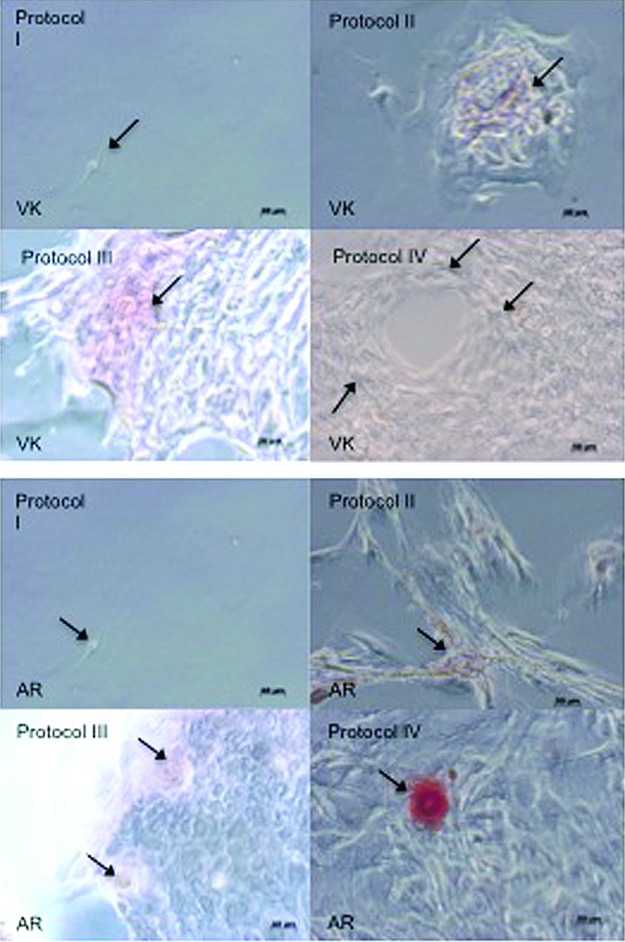
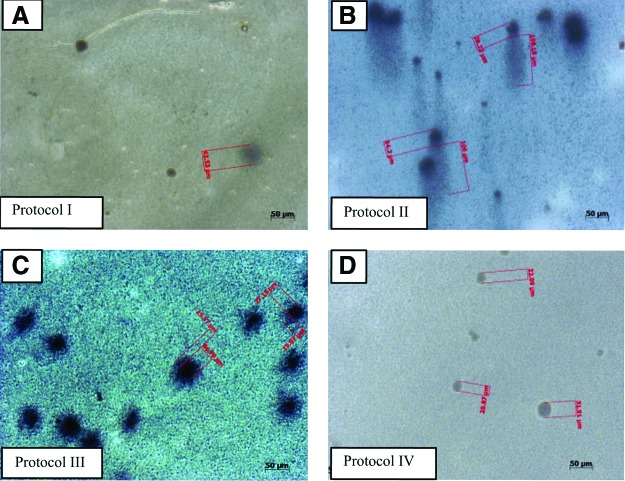
Photomicrographs of VK and AR staining of 28-day cell cultures showed the occurrence of cell clusters and mineralized nodule formation in protocol III and IV cell cultures. Note the reduced cell proliferation in Protocol I conditions, and no evidence of cell clusters (Fig. 3). These data were observed by optical microscope over all flask surfaces.
FIG. 3.
Photomicrographs of cells after 28 days in each culture condition, showing the occurrence of clusters and mineralized nodule formation. Arrows indicate nodules formation in Protocol III (allogeneic human serum) and IV (autologous human serum) cell cultures, as characterized by von Kossa (VK) and Alizarin Red (AR) staining. Note the reduced cell proliferation in Protocol I (SF)-cultured cells, and the lack of cluster formation. Color images available online at www.liebertpub.com/tea
Cell proliferation
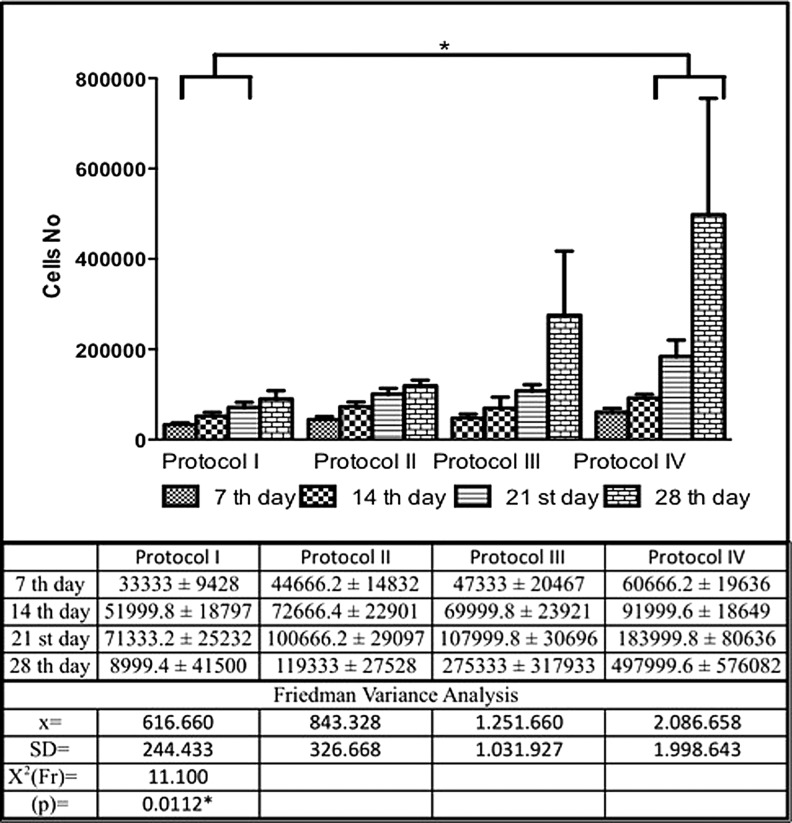
Analysis of five replicate cultures at 4 weeks showed that hDPSCs cultured using Protocol IV conditions were statistically significant as compared to Protocol I, (p = 0.0112). Figures 4 and 6 showed the photomicrographs and proliferation curve.
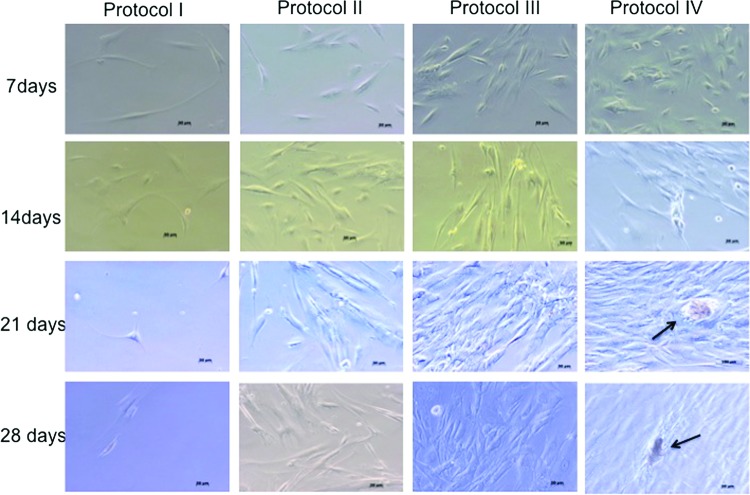
FIG. 4.
Photomicrographs of cells in each of the indicated four Protocols of the study during the 4 weeks culture period, showing the incidence of nodules in Protocol IV (arrows). Reduced cell proliferation was observed in Protocol I (SF)-cultured cells. Cell proliferation in Protocol IV is significantly increased as compared to Protocol I-cultured cells, as indicated by black arrows at 21 and 28 days of culture. Color images available online at www.liebertpub.com/tea
FIG. 6.
Cell proliferation in the four Protocol culture conditions. Five replicate cultures were averaged for each of the four protocols over 28 days in culture. Comparison between the cell proliferation curves in the respective protocol of the study, showing a significant difference between Protocol IV and Protocol I, as calculated using Friedman variance analysis. *statistically significant.
Cell cultures from all four protocols resulted in statistically significant differences in cell proliferation between the 7th and 28th days of culture (Protocol I, p = 0.0029, 7th day <21st and 28th day; Protocol II p = 0.0029; 7th day <21st and 28th day; Protocol III p = 0.0018, 7th day <28th day, and Protocol IV, p = 0.0018, 7th day <28th day).
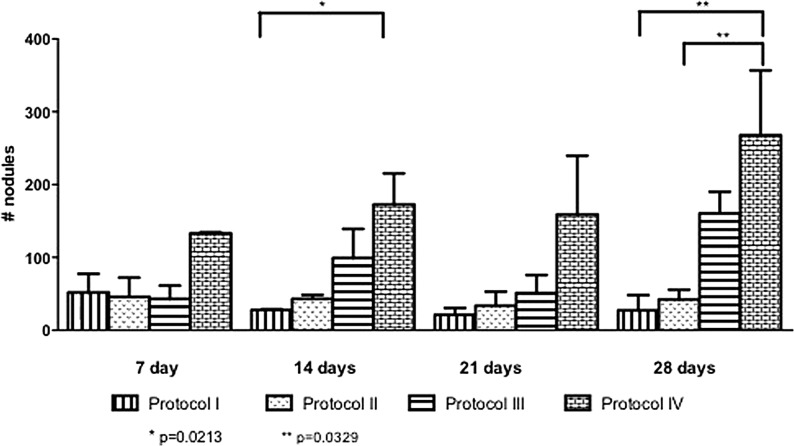
The cells morphologies assumed predominantly mesenchymal-like aspects with cytoplasm elongation and few cells numbers on the Protocol I. The others Protocols (II, III, and IV), had the same morphological aspects of the control, but we can observe clearly the cells proliferation over the time evaluation especially on the Protocol III and IV after 21 days in culture. We also observed by optical microscope, more cell adherence, total confluence on the Protocol III and IV at 28 days, and the presence of little mineralized nodules over all the flasks. The Figure 5 showed the nodules quantification in the four protocols. The Protocol IV showed significant differences between the Protocol I and II on 28 days of the end of the culture.
FIG. 5.
Comparison between the nodules formation on the four protocols of the study, showing a significant difference between Protocol IV and Protocol I, and Protocol IV and Protocol II. *p = 0.0213; **p = 0.0329.
CFU
CFU number was significantly higher in Protocol IV as compared to Protocol I-cultured cells (p = 0.0018), at both 14 and 21 days in culture (Table 1, Fig. 7). The Wilcoxon test was used to show that all four protocols resulted in a significant number of CFU at 21 days as compared to 14 days in culture, demonstrating the viability of the stem cells in culture (Protocol I, z = 2.20, p = 0.0277; Protocol II, z = 2.20, p = 0.0277; Protocol III, z = 2.20, p = 0.0139, and Protocol IV, z = 1.99, p = 0.0464).
Table 1.
Percentage of Colony-Forming Units on the Four Protocols of This Study on the 14th and 21st Day of Culture
| Protocol I | Protocol II | Protocol III | Protocol IV | Friedman variance analysis | |
|---|---|---|---|---|---|
| Percentage of CFUs—14th day | |||||
| N | 5 | 5 | 5 | 5 | x2r calc = 15.00 |
| Average | 5.96 | 14.28 | 16.60 | 22.04 | p = 0.0018* |
| Median | 6.00 | 15.60 | 17.40 | 20.00 | Medium I <Medium IV |
| Standard deviation | 1.02 | 3.12 | 2.58 | 5.26 | |
| Percentage of CFUs—21st day | |||||
| N | 5 | 5 | 5 | 5 | x2r calc = 15.00 |
| Average | 8.04 | 16.96 | 18.68 | 25.44 | p = 0.0018* |
| Median | 8.60 | 17.00 | 19.00 | 23.00 | Medium I <Medium IV |
| Standard deviation | 1.04 | 1.98 | 1.82 | 4.47 | |
CFU, colony-forming units.
FIG. 7.
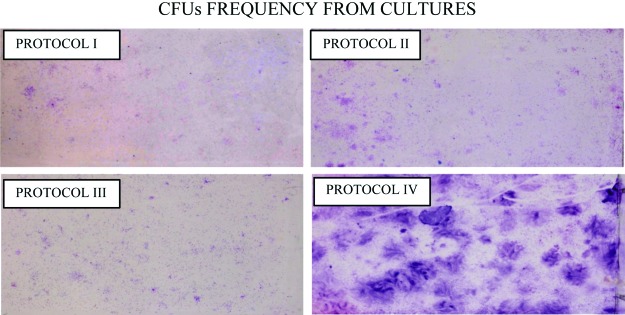
Cell differentiation. Photographs of cells cultured in T-25 flasks. Note the differences in the colony-forming units (CFUs) especially between Protocol IV and Protocol I. The CFUs between Protocol II (heterologous) and Protocol III (allogeneic) were similar. Color images available online at www.liebertpub.com/tea
Comet assay
To obtain the qualitative analysis, we performed the comet test. The cells showing the least amount of DNA damage (levels 0 and 1) were observed using Protocols I and IV cell culture conditions (Fig. 8). Level 2 comet formations was observed in Protocol III-cultured cells, while level 3 and 4 comet formation occurred more frequently in Protocol II-cultured cells. Protocol IV cell culture conditions resulted in no comet or halo formations, and also exhibited a significantly greater cell proliferation as compared to Protocol I-cultured cells (Fig. 8 and Table 2).
FIG. 8.
Comet formation. Microphotoghraphs of comet assay after 28 days in culture. (A) Protocol I, no evidence of comet formation. (B) Presence of comets with tails that are three to four times the cell nuclei diameter. (C) Presence of comets with tail size one to two times of cell nuclei diameter. (D) No evidence of comets. Color images available online at www.liebertpub.com/tea
Table 2.
Average of Cellular Damage Identified on Comets Assay in Five Experiments
| Damage level | Protocol I | Protocol II | Protocol III | Protocol IV |
|---|---|---|---|---|
| 0 | 748.8 ± 65.9 | 1480.0 ± 203.9 | 2240.0 ± 233.2 | 6880.0 ± 652.4 |
| 1 | 448.0 ± 64.0 | 600.0 ± 282.8 | 1200.0 ± 419.5 | 0.0 ± 0.0 |
| 2 | 150.4 ± 46.7 | 1040 ± 233.2 | 448 ± 28.6 | 0.0 ± 0.0 |
| 3 | 150.4 ± 46.7 | 1040 ± 233.2 | 448 ± 20.2 | 0.0 ± 0.0 |
| 4 | 150.4 ± 42.1 | 600.0 ± 219.1 | 150.4 ± 46.7 | 0.0 ± 0.0 |
| Cellular damage | ||||
|---|---|---|---|---|
| Protocol | With | Without | Total | Damage% |
| I | 899.2 | 748.8 | 1650 | 54.55 |
| II | 3280 | 1480 | 4760 | 68.75 |
| III | 2240 | 2240 | 4480 | 50.00 |
| IV | 0 | 6880 | 6880 | 0.00 |
| Total | 6419.2 | 11348.8 | 17850 | 36.13 |
Discussion
The aim of expanding cells for TE applications is to acquire the highest number of healthy cells, while at the same time incurring the least amount of structural and functional damage from expansion in cell culture. The dental pulp is a well-documented and safe source of ectomesenchymal stem cells, which could be used to improve tissue regeneration promoting regenerative medicine in the future.12 The relevance of this work is in the design of reliable methods to obtain high quality and quantity of healthy hDPSC populations, with the objective to promote their therapeutic use in TE applications. The in vitro analyzes presented in this study included cellular proliferation, CFU formation at 14 and 28 days in culture, tissue culture media pH, comet assay formation, and mineralized nodule formation.
This study is highly relevant to that of Stute et al.13 and Bluteau et al.14 which concerns the influence of the microenvironment on tissue culture, and how differences between cell culture conditions and those of the natural microenvironment can directly influence cell viability, quality, and quantity, particularly as evidenced in clinical trials using cells cultured in media supplemented with human serum.
In this study, Protocol I, although supplement free, exhibited reduced efficacy as compared to the supplemented media.7 Protocol I-cultured cells exhibited reduced cell size,15 and cultured mesenchymal cells exhibited stellate, thinner, and sparse cellular aspects, and decreased cell clusters and numbers, and mineralized nodule formations were not observed over the time. All cells cultured using Protocol I conditions did not reach confluence at day 28, and exhibited large gaps between colonies. In contrast, cells cultured using Protocol II, III, and IV conditions exhibited significantly increased cell proliferation between 7th and 28th day, consistent with published reports.10,15
The importance of supplementing cell culture media with human blood products was also analyzed, to determine its potential for cellular differentiation and mineralized tissue formation. In this study, Protocols III and IV exhibited the highest cell proliferation between the 7th and 28th day, similar to that obtained using Protocol II, with no significant changes in cell size, but rather the formation of brownish mineralized nodules suggestive of cell differentiation. Unlike Protocol I, the Protocol II, III, and IV culture conditions produced cell culture performance more markedly from the 14th day. When cultured cells using serum-free conditions were compared between Protocol I versus AlHS (Protocol III), the proliferation of cells appeared to increase in the presence of human allogeneic serum, corroborating with other published reports emphasized in others studies.4,5,8,13,16–27
Similar to Protocol III culture conditions, the Protocol IV resulted in increased cell proliferation between 7 and 28 days of culture (Fig. 4). Also on the Protocol IV, we can observe at 28 days in culture, the presence of mineralized nodules over all the flasks. The Protocol IV-cultured cells exhibited improved cell shape and consequently cell culture morphology appearance as compared to Protocol II- and III-cultured cells, as mentioned by Dolatshahi-Pirouz et al.15
Cell culture quality was improved when the culture medium was supplemented with human serum, corroborating the findings of Kurita et al.26 Inclusion of human blood product supplements, both allogeneic and autologous, resulted in cell culture conditions similar to those obtained using Protocol II, which used animal serum supplements.24,25 Protocol III may, therefore, represent an advantage over Protocol II, due to the lack of immunogenic factors in cell culture media.27,28 Although Friedman analysis of variance did not detect any significant differences between Protocols III and IV, a clear trend of increased cell proliferation was identified in Protocol IV (Fig. 6).13,24,25
The CFUs frequency in supplemented culture medium with human blood products have been analyzed by many other authors, based on the cellular morphology and differentiation capacity10,25–29 (Figs. 6 and 7). In this study, CFU formation between 14 and 21 days in culture (Table 1) was significantly different between Protocol I and Protocol IV, with Protocol IV showing the greatest CFU capability.
Tissue culture media collected from each of the four protocols did not exhibit significant variations in pH that might influence cell proliferation or differentiation potential, likely due to the fact that the culture medium was changed frequently, every 3 days.10,28,30
The odontogenic and osteogenic potential of DSCs was analyzed by means of Alizarin Red and VK staining, which indicate mineralized tissue formation as a red or blackish toned stain, respectively.29,31 A greater tendency for cell differentiation was observed in Protocol IV cell cultures, as compared to Protocol II and III cell cultures, at the 21 day time point. Pigmentation promoted by VK and Alizarin Red staining appeared similar between Protocol II and Protocol III cell cultures, both in the number and size of the mineralized nodules. These results were observed by optical microscopy over all the flasks and they were quantified (Fig. 5), which confirmed mineralization nodule formation of cells of dental origin cultured in supplemented media.10,31 In the Protocol I, due to reduced cell proliferation, no formation of clusters was observed using the same methodology.
The importance of an ideal culture media to promote a rapid cell proliferation without inducing DNA damage is a fundamental strategy to construct biological substitutes in a rapid manner and with less chance of rejection. Cell viability is an important consideration, but the presence of structural and functional DNA damage can invalidate their use in TE applications.7,32
Therefore, in addition to measuring cellular proliferation, DNA integrity must also be validated. Our findings showed that media supplemented with human autologous serum (Protocol IV) resulted in the best cell proliferation and least amount of DNA damage, therefore, performing the best biomimetic activity when compared with others supplements published conventionally in the literature. In fact, cells cultured in HS exhibited suboptimal behavior during 28 days in culture, including reduced cell proliferation and increased presence of level 3 and 4 comets.4,33–36 Comparison of cellular damage present in each of the four cell culture Protocols (Table 2) revealed that the percentage damage observed on the Protocol II was significantly higher as compared with the other protocols. Comet formation was observed in cells cultured in Protocol I and IV conditions, and chi-square analysis showed that the damage percentage observed with the Protocol I was significantly higher than the Protocol III, consistent with the results reported by Augello et al.9
In Protocol II- and III-cultured cells, comets were identified, but only slight increases in nuclear size were observed in Protocol IV-cultured cells, suggesting only mild and reversibility damage in Protocol IV-cultured cells. Few comets were identified in Protocol I-cultured cells, but very little cell proliferation was observed, suggesting that the cells were more fragile, as supported by other studies.7,37,38
The chi-square partition showed significant result with the first partition with comparison Protocol IV in relation to the other Protocols (Protocols I, II, III > Protocol IV, X2 = 6363.97, p < 0.0001); the second partition confirmed significant difference with the comparison of Protocol II and Protocol I and III (II > I and III, X2 = 359.01, p < 0.0001). The third partition also showed significant difference between Protocol I and Protocol III (I > III, X2 = 10.81, p < 0.0001).
In conclusion, this report has demonstrated that AuHS, as tissue culture supplement, proves to be superior to AlHS. Protocol IV supplemented with AuHS resulted in reduced occurrence of cell damage. Protocol III and IV supplemented with human blood products, allogeneic and autologous, respectively, increased cell proliferation in relation to Protocol I, showing the potential for use in cultured human DSCs. Protocol II (HS) resulted in significant cell damage, with the formation of comets in level 3 and 4 indicative of a high degree of cell damage. Also, although the use of cultured human pulp stem cells will be useful in future TE therapies, the results of this report revealed that for the best performance of the cultured cells and validation process for applications in human therapeutic use, one needs to customize the process of collection, storage, and maintenance of biological material.
Acknowledgments
The authors would like to thank Dr. Esper Georges Kallas who provided technical support of the LIM 60, Medical Investigation Laboratories (Kallas' Lab), University of Sao Paulo. The authors thank the Brazilian sponsors UNIFESP, Surgery Plastic Department, FAPESP (07/58856-7; 07/51227-4; 07/59488-1), and CAPES for the RLP's scholarship, CNPq (310049/2011-3; 310048/2011-7; 133745/2012-0), Instituto Nacional de Ciência e Tecnologia (INCT)–Biofabrication (CNPq 5736661/2008-1 and FAPESP 08/57860-3), and NIH/NIDCR/NIBIB for their continued support [DE016132, TW007665 (PCY)].
Disclosure Statement
No competing financial interests exist.
References
- 1.Vats A., Bielby R.C., Tolley N.S., Nerem R., and Polak J.M. Stem cells. Lancet 366, 592, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Langer R., and Vacanti J.P. Tissue engineering. Science 260, 920, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Kim S.S., and Vacanti J.P. The current status of tissue engineering as potential therapy. Semin Pediatr Surg 8, 119, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Stock U.A., and Vacanti J.P. Tissue engineering, current state and prospects. Annu Rev Med 52, 443, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Kaigler D., and Mooney D. Tissue engineering's impact on dentistry. J Dent Educ 65, 456, 2001 [PubMed] [Google Scholar]
- 6.Kneser U., Schaefer D.J., Polykandriotis E., and Horch R.E. Tissue engineering of bone, the reconstructive surgeon's point view. J Cell Mol Med 10, 7, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin M.J., Muotri A., Gage F., and Varki A. Human embryonic stem cells express an immunigenic nonhuman sialic acid. Nat Med 11, 228, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 284, 143, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Augello A., Kurth T.B., and De Bari C. Mesenchymal stem cells, a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater 20, 121, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Backopoulou A., Leyhausen G., Volk J., Tsiftsoglou A., Garefis P., et al. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol 56, 709, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Duailibi M.T., Duailibi S.E., Duailibi Neto E.F., Negreiros R.M., Jorge W.A., et al. Tooth tissue engineering, optimal dental stem cell harvest based on tooth development. Artif Organs. 35, E129, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Jo Y.Y., Lee H.J., Kook S.Y., Choung H.W., Park J.Y., et al. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng 13, 767, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Stute N., Holtz K., Bubenheim M., Lange C., Blake F., and Zander A.R. Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Exp Hematol 32, 1212, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Bluteau G., Luder H.U., De Bari C., and Mitsiadis T.A. Stem cell for tooth engineering. Eur Cell Mater 16, 1, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Dolatshahi-Pirouz A., Jensen T.H.L., Kolind K., Bünger C., Kassem M., et al. Cell shape and spreading of stromal (mesenchymal) stem cell cultured on fibronectin coated gold and hydroxyapatite surfaces. Colloids Surf B Biointerfaces 84, 18, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Harada H., Kettunen P., Jung H.S., Mustonen T., Wang Y.A., and Thesleff I. Localization of putative stem cell in dental epithelium and their association with notch and FGF signaling. J Cell Biol 147, 105, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zvaifler N.J., Marinova-Mutafchieva L., Adams G., Edwards C.J., Moss J., et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res 2, 477, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bianco P., Riminucci M., Gronthos S., and Robey P.G. Bone marrow stromal stem cells, nature, biology and potential applications. Stem Cell 19, 180, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Gronthos S., Brahim J., Li W., Fisher L.W., Cherman N., et al. Stem cell properties of human dental pulp stem cells. J Dent Res 81, 531, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Young C.S., Terada S., Vacanti J.P., Honda M., Bartlett J.D., and Yelick P.C. Tissue engineering of complex tooth structure on biodegradable polymer scaffolds. J Dent Res 81, 695, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Duailibi M.T., Duailibi S.E., Young C.S., Vacanti J.P., Bartlett J.D., and Yelick P.C. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res 83, 523, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Gronthos S., Mrozik K., Shi S., and Bartold P.M. Ovine periodontal ligament stem cells, isolation, characterization, and differentiation potential. Calcif Tissue Int 79, 310, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Duailibi S.E., Duailibi M.T., Zhang W., Asrican R., Vacanti J.P., and Yelick P.C. Bioengineered dental tissues grown in the rat raw. J Dent Res 87, 745, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira C.F., Gomes M.C.C., Scarso Filho J., Granjeiro J.M., Simões C.M.O, and Magini R.S. Platelet-rich plasma influence on human osteoblast growth. Clin Oral Impl Res 16, 456, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Kocaoemer A., Kern S., Klüter H., and Bieback K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells 25, 1270, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kurita M., Aiba-Kojima E., Shigeura T., Matsumoto D., and Suga H. Differential effects of three preparations of human serum on expansion of various types of human cells. Plast Reconstr Surg 122, 438, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Xia W., Li H., Wang Z., Xu R., Fu Y., and Zhang X. Human platelet lysate supports ex vivo expansion and enhances osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Cell Biol Int Cell 35, 639, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Kamil S.H., Kojima K., Vacanti M.P., Zaporojan V., Vacanti C.A., and Eavey R.D. Tissue engineered cartilage, utilization of autologous serum and serum-free media for chondrocyte culture. Int J Pediatr Otorhinolaryngol 71, 71, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Miura M., Gronthos S., Zao M., Lu B., Fisher L.W., Robey P.G., and Shi S. SHED, stem cells from human exfoliated deciduous teeth. PNAS 100, 5807, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nait Lechguer A.N., Kuchler-Bopp S., and Lesot H. Crown formation during tooth development and tissue engineering. J Exp Zool Mol Dev Evol 15312B, 399, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Park B.W., Hah Y.S., Choi M.J., Ryu Y.M., Lee S.G., Kim D.R., et al. In Vitro osteogenic differentiation of cultured human dental papilla-derived cells. J Oral Maxillofac Surg 67, 507, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Katiyar S.K., Mantena S.K., and Meeran S.M. Silymarin protects epidermal keratinocytes from ultraviolet radiation-induced apoptosis and DNA damage by nucleotide excision repair mechanism. PLoS One 6, e21410, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutmacher D.W., Goh J.C., and Teoh S.H. An introduction to biodegradable materials for tissue engineering applications. Ann Acad Med Singapore 30, 183, 2001 [PubMed] [Google Scholar]
- 34.Tuan R.S., Boland G., and Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arth Res Therp 5, 32, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preston S.L., Alison M.R., Forbes S.J., Direkze N.C., Poulsom R., and Wright N.A. The new stem cell biology, something for everyone. J Clin Pathol Mol Pathol 56, 86, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hovatta O. Derivation of human embryonic stem cell lines, towards clinical quality. Reprod Fert Develop 18, 823, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Richards M., Fong C.Y., Chan W.K., Wong P.C., and Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nature Biotech. 20, 933, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Skottman H., and Hovatta O. Culture conditions for human embryonic stem cells. Reproduction 132, 691, 2006 [DOI] [PubMed] [Google Scholar]