Abstract
A number of cellular microRNAs (miRNAs) have been identified to have the ability to inhibit HIV-1 replication. In this study, we examined the impact of combination antiretroviral therapy (cART) on the expression of HIV-1 restriction miRNAs in peripheral blood mononuclear cells of HIV-1–infected men who have sex with men (MSM). Compared with male healthy donors, HIV-infected MSM had significantly lower levels of 9 HIV-1 restriction miRNAs. The treatment of HIV-1–infected MSM with cART, however, failed to restore the levels of these miRNAs in peripheral blood mononuclear cells. These observations suggest that the suppression of the cellular restriction miRNAs by HIV-1 may attribute to the virus latency during cART.
INTRODUCTION
MicroRNAs (miRNAs) have been implicated in host cell innate immunity and HIV-1 infection. A number of cellular miRNAs can modulate HIV-1 replication through directly or indirectly targeting HIV-1 RNA or mRNAs that encode HIV-related host cell factors.1 Research from different laboratories have shown that miR-28, miR-125b, miR-150, miR-198, miR-223, miR-382, miR-29a ,2 miR-29b,1 and miR-29c3 have the ability to inhibit HIV-1 replication in CD4+ T cells and macrophages by several mechanisms.1 The miRNAs can suppress HIV expression by directly targeting 3’-UTR (miR-28, miR-125b, miR-150, miR-223, and miR-382)1,4 or nef (miR-29a/b/c)1,5,6 of the HIV genome, or by indirectly targeting the mRNA of Cyclin T1 that stimulates HIV-1 production in the infected cells (miR-198, miR-150, miR-223, and miR-29b).1,7,8 Conversely, HIV-1 infection can alter the miRNA expression profiles in the circulating blood cells from the infected individuals.1,6,9,10 Given the importance of the cellular restriction miRNAs in the host cell innate immunity against HIV-1 infection, it is of great interest to determine whether HIV-1 infection inhibits the expression of these cellular miRNAs in peripheral blood mononuclear cells (PBMCs). More importantly, it is clinically significant to determine whether combination antiretroviral therapy (cART) can restore HIV-1–mediated suppression of cellular viral restriction miRNAs.
MATERIALS AND METHODS
Participants
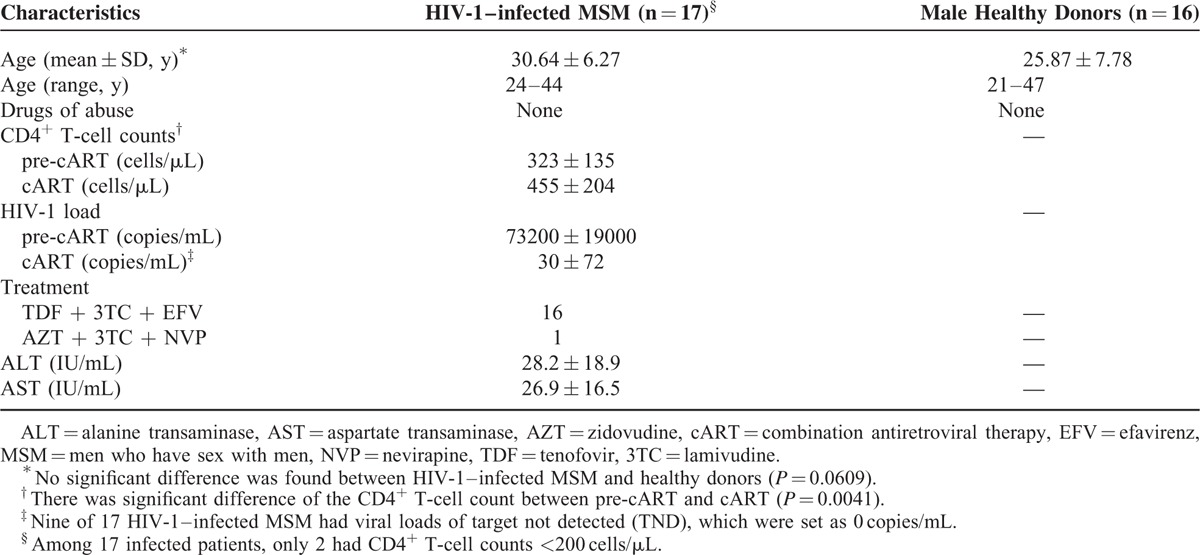
This study is the part of our ongoing program at Wuhan Centers for Disease Prevention and Control (Wuhan CDC), investigating the treatment adherence and the health outcomes in men who have sex with men (MSM) with HIV/AIDS.11 MSM were found to have high rate of HIV infection in Wuhan city.12 To study the impact of ART on the levels of HIV restriction miRNAs in PBMCs, 17 MSM with HIV-1 infection and 16 age-matched male healthy donors were enrolled for this study. The demographic and clinical characteristics of the study participants are shown in Table 1. All the patients with HIV-1 infection were treated with tenofovir (TDF) + lamivudine (3TC) + efavirenz (16 patients) or zidovudine (AZT) + 3TC + nevirapine (NVP) (1 patient) according to the China free ART manual.13 Health screening was conducted before and during the cART. The study was approved by the Ethics Committee of Wuhan Centers for Disease Prevention and Control, and informed consent was obtained from all the participants of this study.
TABLE 1.
Demographic and Clinical Characteristics of HIV-1–infected Men Who Have Sex With Men (MSM) and Male Healthy Donors
Blood Sampling
Blood specimens were collected from the study participants with HIV-1 infection before and after 6-month cART. Absolute CD4+ T-cell counts and plasma HIV-1 loads were tested as described previously.12 Blood samples were also collected from the healthy volunteers, which served as a negative control. Because PBMCs are enriched with the cellular miRNAs,9,14 including the HIV-1 restriction miRNAs,4,15 we isolated PBMCs from the blood specimens of the study participants by standard Ficoll-Paque density gradient centrifugation.2,16
RNA Extraction and RT-Quantitative PCR
Total cellular RNA, including miRNA, was extracted from PBMCs using the TRI Reagent (Molecular Research Center, Clicinnati, OH) according to the manufacturer's instruction. The expression of miRNAs in PBMCs was analyzed by miScript Primer Assays (GIAGEN Sciences, MD). Total RNA (1 μg) was reversely transcribed with a RT kit (miScript II RT Kit, QIAGEN). cDNA (1.5 μL) was subjected for the real-time PCR analysis of miRNA using SYBR Green detection (miScript SYBR Green PCR Kit, QIAGEN). The cellular miRNAs (miR-125b, miR-223, miR-150, miR-382, miR-28, miR-29a, miR-198, miR-29b, and miR-29c) were measured. Glyceraldehydes-3-phosphate dehydrogenase (GAPDH) mRNA was used as an endogenous reference to normalize the quantities of target miRNAs.
Statistical Analysis
When appropriate, data were expressed as mean ± standard deviations (SDs). For comparison of the mean of 2 groups (HIV-1–infected vs health control), unpaired Student t test was used for statistical significance, whereas paired Student t test was applied for the comparison of HIV-1–infected MSM before and after cART. Statistical analyses were performed with GraphPad Instat statistical software (GraphPad Software). Statistical significance was defined as a P value <0.05.
RESULTS
Participants
The study participants with HIV-1 infection had a mean age of 30.64 ± 6.27 (24–44) years, which was insignificant to the healthy donors (25.87 ± 7.78 y, P = 0.0609) (Table 1). The HIV-1–infected patients had a mean CD4+ T-cell counts of 323 ± 135 cells/μL (ranged 62–670 cells/μL) and plasma HIV-1 load of 73,200 copies/mL (ranged 1300–790,000 copies/mL). Only 2 participants had CD4+ T-cell counts <200 cells/μL, and above 50% of participants (9/17) had CD4+ T-cell counts above 350 cells/μL. With 6-month cART, all the participants had a significant increase in CD4+ T-cell counts (455 ± 204 cells/μL; P = 0.0041) and decrease in plasma HIV-1 load (30 ± 72 copies/mL) (Table 1).
cART Fails to Restore HIV-1 Infection-mediated Suppression of the Restriction miRNAs
A number of miRNAs have been identified to have anti-HIV-1 effects by different laboratories.1–3 We therefore examined the levels of these HIV-1 restriction miRNAs in PBMCs of HIV-1–infected individuals and control participants. As shown in Figure 1, the patients with HIV-1 infection had significantly lower levels of 9 miRNAs than the control participants (P < 0.05). The 6-month treatment of the infected patients with cART resulted in the increase of CD4+ T-cell numbers and the decrease of plasma HIV-1 loads (Table 1). However, cART had little or negative impact on the levels of the HIV-1 restriction miRNAs in PBMCs of the HIV-infected MSM (Fig. 1).
FIGURE 1.
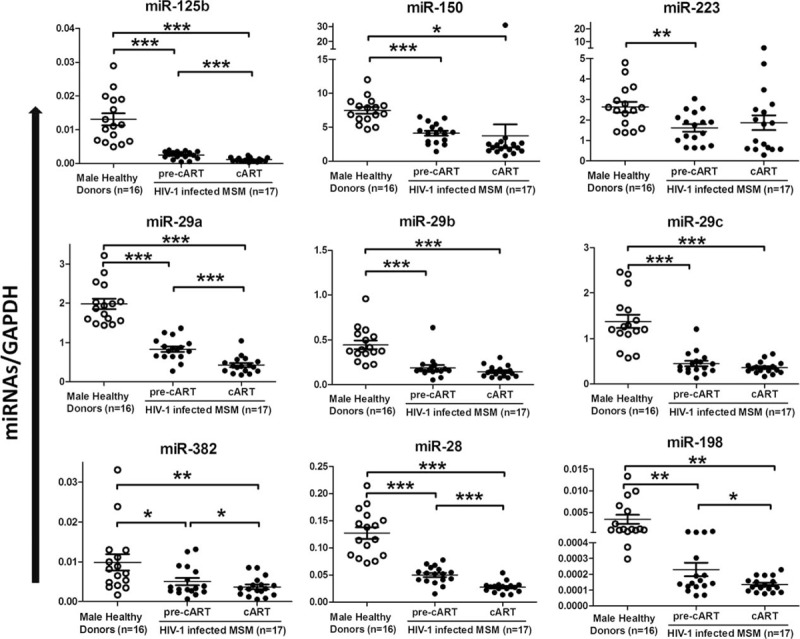
The expression of cellular HIV-1 restriction miRNAs in PBMCs of 17 HIV-1–infected MSM before (pre-cART) or after 6-month cART, when compared with that of 16 age-matched male healthy donors. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. cART = combination antiretroviral therapy, miRNA = microRNA, MSM = men who have sex with men, PBMC = peripheral blood mononuclear cell.
Correlations of HIV-1 Restriction miRNAs with CD4+ T Cells and Viral Loads
CD4+ T-cells count and plasma HIV-1 load are the key indicators of the progression of HIV-1 disease.12 Thus, we examined whether there is an association between the levels of miRNAs and CD4+ T-cell counts or plasma HIV-1 load. Out of the 9 miRNAs tested, the levels of 4 miRNAs (miR-150, miR-29a, miR-29b, and miR-29c) in PBMCs of HIV-1–infected individuals before cART were significantly correlated with CD4+ T-cell counts (Fig. 2). However, the treatment with cART diminished the correlation between the levels of the miRNAs and CD4+ T-cell counts (Fig. 2). There was no correlation, however, between the levels of miRNAs and HIV-1 loads before or after cART (data not shown).
FIGURE 2.
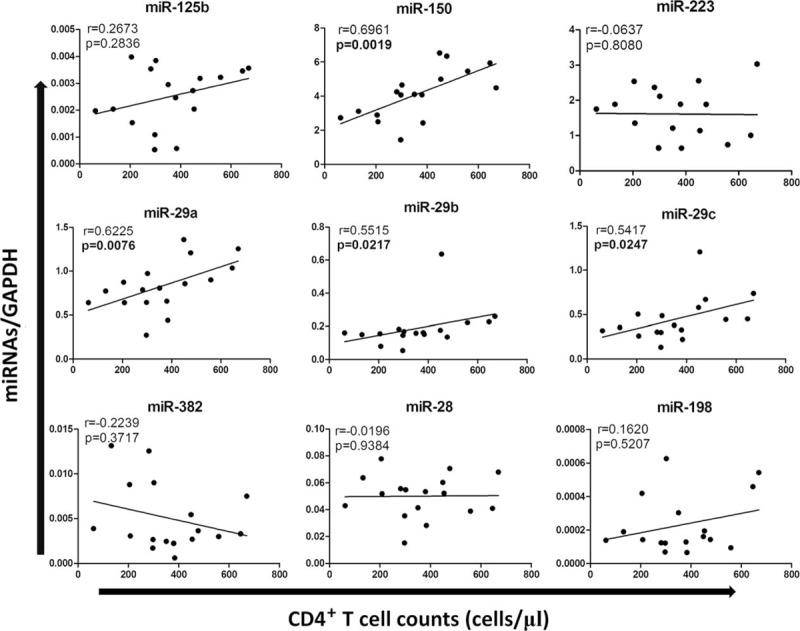
Correlations between the expression of HIV-1 restriction miRNAs and CD4+ T cells in 17 HIV-1–infected MSM before cART. Of 9 anti-HIV-1 miRNAs, 4 (miR-150, miR-29a, miR-29b, miR-29c) showed significant and positive correlation (P < 0.05) to CD4+ T-cell counts. cART = combination antiretroviral therapy, miRNA = microRNA, MSM = men who have sex with men.
DISCUSSION
Cellular miRNAs participate in host cell innate immunity against viral infections, including HIV-1.4,15 Huang et al4 reported that 5 cellular miRNAs play a key role in controlling HIV-1 latency in resting CD4+ T cells. We demonstrated that 4 of these 5 HIV-1 restriction miRNAs are enriched in monocytes as compared to monocyte-derived macrophages, which contributes to differential susceptibilities of these cells to HIV-1 infection in vitro.15 In the present study, we assayed the expression of the HIV restriction miRNAs in PBMCs of HIV-1–infected MSM before and after cART. We found that HIV-1–infected MSM had significantly lower levels of the HIV-1 restriction miRNAs in PBMCs. In addition, the treatment of HIV-1–infected MSM with cART did not restore the levels of these miRNAs in PBMCs. On the contrary, the patients treated with cART for 6 months showed even lower levels of the miRNAs (miR-125b, miR-382, miR-28, miR-29a, and miR-198) in PBMCs than those before cART. These findings are clinically significant as they provide a mechanism for the clinical observation that HIV-1 DNA could be still detected in peripheral system such as PBMC despite cART for as long as 12 months17 or 18 months.18 Given the prominent role of the miRNAs in the host cell innate immunity against HIV-1, the down-regulation of the HIV-1 restriction miRNAs in PBMCs is likely to associate with virus latency in the target cells.17,18
The mechanisms of HIV-1 infection-mediated suppression of the HIV-1 restriction miRNAs remain to be determined. With the limited numbers of the study participants, we did not observe the correlation between viral loads and the levels of the HIV restriction miRNAs. The lack of the association between the viral load and the miRNAs could be to due to the fact that HIV-1 loads are from not only PBMCs but also lymphoid tissues, such as those from gastrointestinal system. However, we did observe a positive correlation between CD4 T-cell counts and some of the HIV restriction miRNAs (Fig. 2), suggesting that the HIV restriction miRNAs are indeed involved in the host immune response to HIV infection. Although cellular and molecular mechanisms for HIV-1 persistence on cART remain to be determined, our observation that cART failed to restore the levels of the HIV-1 restriction miRNAs in PBMCs of HIV-1–infected MSM provides the clinical evidence to support the possibility that the intracellular HIV-1 restriction miRNAs are involved in the elimination of the virus in the target cells. However, our study, with limited clinical specimens, argues for more extensive research to determine the role of the cellular miRNAs in the immunopathogenesis of HIV-1 disease.
Footnotes
Abbreviations: ACC = AIDS Care Center, cART = combination antiretroviral therapy, miRNAs = microRNAs, MSM = men who have sex with men, PBMCs = peripheral blood mononuclear cells, SD = standard deviation.
Funding: This study was supported by Open Research Fund Program of the State Key Laboratory of Virology of China (No. 2014KF002, to MQL), National Natural Science Foundation of China (No. 81001303 to MQL, No. 81271334 to WZH), the Foundation of Young Medical Talents in Wuhan, China (to MQL), National Science and Technology Major Project of the Ministry of Science and Technology of China (No. 2014ZX10001003005, to WZH), and the National Institutes of Health grants (No. DA022177 and DA027550 to WZH).
Conflicts of interest: The authors have no conflict of interest to disclose.
REFERENCES
- 1.Klase Z, Houzet L, Jeang KT. MicroRNAs and HIV-1: complex interactions. J Biol Chem 2012; 287:40884–40890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X, Ye L, Zhou Y, et al. Inhibition of anti-HIV microRNA expression: a mechanism for opioid-mediated enhancement of HIV infection of monocytes. Am J Pathol 2011; 178:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monteleone K, Selvaggi C, Cacciotti G, et al. MicroRNA-29 family expression and its relation to antiviral immune response and viro-immunological markers in HIV-1-infected patients. BMC Infect Dis 2015; 15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J, Wang F, Argyris E, et al. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med 2007; 13:1241–1247. [DOI] [PubMed] [Google Scholar]
- 5.Hariharan M, Scaria V, Pillai B, et al. Targets for human encoded microRNAs in HIV genes. Biochem Biophys Res Commun 2005; 337:1214–1218. [DOI] [PubMed] [Google Scholar]
- 6.Sun G, Li H, Wu X, et al. Interplay between HIV-1 infection and host microRNAs. Nucleic Acids Res 2012; 40:2181–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sung TL. Rice AP. miR-198 inhibits HIV-1 gene expression and replication in monocytes and its mechanism of action appears to involve repression of cyclin T1. PLoS Pathog 2009; 5:e1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang K, Sung TL, Rice AP. Regulation of cyclin T1 and HIV-1 Replication by microRNAs in resting CD4+ T lymphocytes. J Virol 2012; 86:3244–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houzet L, Yeung ML, de Lame V, et al. MicroRNA profile changes in human immunodeficiency virus type 1 (HIV-1) seropositive individuals. Retrovirology 2008; 5:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houzet L, Jeang KT. MicroRNAs and human retroviruses. Biochim Biophys Acta 2011; 1809:686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou W, Zhao M, Wang X, et al. Treatment adherence and health outcomes in MSM with HIV/AIDS: patients enrolled in “one-stop” and standard care clinics in Wuhan China. PLoS One 2014; 9:e113736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu MQ, Tang L, Kong WH, et al. CD4+ T cell count, HIV-1 viral loads and demographic variables of newly identified patients with HIV infection in Wuhan, China. J Med Virol 2013; 85:1687–1691. [DOI] [PubMed] [Google Scholar]
- 13.Zhang FJ. China Free ART Manual. 3rd edBeijing: People's Medical Publishing House; 2012. [Google Scholar]
- 14.Witwer KW, Watson AK, Blankson JN, et al. Relationships of PBMC microRNA expression, plasma viral load, and CD4+ T-cell count in HIV-1-infected elite suppressors and viremic patients. Retrovirology 2012; 9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Ye L, Hou W, et al. Cellular microRNA expression correlates with susceptibility of monocytes/macrophages to HIV-1 infection. Blood 2009; 113:671–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Tan N, Douglas SD, et al. Morphine inhibits CD8+ T cell-mediated, noncytolytic, anti-HIV activity in latently infected immune cells. J Leukoc Biol 2005; 78:772–776. [DOI] [PubMed] [Google Scholar]
- 17.Triboulet R, Mari B, Lin YL, et al. Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science 2007; 315:1579–1582. [DOI] [PubMed] [Google Scholar]
- 18.Han Y, Siliciano RF. Keeping quiet: microRNAs in HIV-1 latency. Nat Med 2007; 13:1138–1140. [DOI] [PubMed] [Google Scholar]


