Abstract
There are several reports suggesting that the pathophysiology of psoriasis may be associated with aberrant circadian rhythms. However, the mechanistic link between psoriasis and the circadian time-keeping system, “the circadian clock,” remains unclear. This study determined whether the core circadian gene, Clock, had a regulatory role in the development of psoriasis. For this purpose, we compared the development of psoriasis-like skin inflammation induced by the Toll-like receptor 7 ligand imiquimod (IMQ) between wild-type mice and mice with a loss-of-function mutation of Clock. We also compared the development of IMQ-induced dermatitis between wild-type mice and mice with a loss-of-function mutation of Period2 (Per2), another key circadian gene that inhibits CLOCK activity. We found that Clock mutation ameliorated IMQ-induced dermatitis, whereas the Per2 mutation exaggerated IMQ-induced dermatitis, when compared with wild-type mice associated with decreased or increased IL-23 receptor (IL-23R) expression in γ/δ+ T cells, respectively. In addition, CLOCK directly bound to the promoter of IL-23R in γ/δ+ T cells, and IL-23R expression in the mouse skin was under circadian control. These findings suggest that Clock is a novel regulator of psoriasis-like skin inflammation in mice via direct modulation of IL-23R expression in γ/δ+ T cells, establishing a mechanistic link between psoriasis and the circadian clock.
Introduction
Psoriasis is a common chronic inflammatory skin disease characterized by increased proliferation, altered differentiation of the epidermis, and infiltration of inflammatory cells such as neutrophils into the dermis (Lowes et al., 2014). Recent studies suggest that IL-23 has a critical role in the pathogenesis of psoriasis by inducing pathological T helper type 17 (Th17) cells that release IL-17 and IL-22 (Kikly et al., 2006; Fitch et al., 2007; Chiricozzi et al., 2014). However, it is not fully understood how the IL-23 pathway in psoriasis is regulated.
The circadian clock is an essential timing system driving daily oscillations of behavior and physiology, such as sleep–wake cycles and hormonal secretions (Dibner et al., 2010; Mohawk et al., 2012). The mammalian circadian clock system consists of the central oscillator, located in the suprachiasmatic nucleus of the hypothalamus, and peripheral oscillators present in virtually all cell types including the skin (Tanioka et al., 2009). The central suprachiasmatic nucleus clock can receive light input from the retina that synchronizes internal clock timing to the external solar day, which it passes on to peripheral clocks via neural and endocrine pathways. The molecular mechanisms of rhythm generation are highly conserved in the suprachiasmatic nucleus and peripheral cells, and created and maintained by interlocked transcriptional–translational feedback loops (Ukai and Ueda, 2010). The core loop is driven by two activators (CLOCK and BMAL1) and two repressors (PERIOD (PER) and CRYPTOCHROME (CRY)) (Dibner et al., 2010; Mohawk et al., 2012). Briefly, CLOCK and BMAL1 heterodimerize and activate transcription of the Per1/2 and Cry1/2 genes, as well as other clock output genes, via the E-box (or E-box-like) elements in the promoter region of the genes. The PER and CRY proteins heterodimerize and, in turn, suppress CLOCK/BMAL1 activity, thereby inhibiting their own transcription.
There have been several reports suggesting that the pathophysiology of psoriasis may be associated with aberrant circadian rhythms (Gelfant et al., 1982; Mozzanica et al., 1988; Bacaksiz et al., 2012). For instance, patients with psoriasis had disrupted circadian rhythms in blood pressure and heart rate (Bacaksiz et al., 2012). Furthermore, a most recent study reported an increased risk of psoriasis in night-shift workers who had aberrant circadian rhythms (Li et al., 2013). However, the mechanistic link between the circadian clock and psoriasis remains unclear.
This study determined whether a core circadian gene, Clock, had a regulatory role in the development of psoriasis. For this purpose, we compared the development of psoriasis-like dermatitis induced by the Toll-like receptor 7 ligand imiquimod (IMQ) (van der Fits et al., 2009) between wild-type mice and mice with a loss-of-function mutation of Clock (ClockΔ19/Δ19 mice) (Vitaterna et al., 1994) or Period2 (Per2) (mPer2m/m mice) (Zheng et al., 1999), another key circadian protein that inhibits CLOCK activity.
Results
Clock-mutated mice do not have obvious developmental defects in the immune system
We first checked whether ClockΔ19/Δ19 mice had any developmental defects in the immune system. ClockΔ19/Δ19 mice showed normal numbers and percentages of CD4+, CD8+ T cells, or B cells in the thymus and spleen (Supplementary Figure S1a online). Serum IgG, IgA, and IgE levels, fecal IgA levels, and peripheral blood white cell counts and differentials were comparable between wild-type and ClockΔ19/Δ19 mice (Supplementary Figure S1b online, data not shown). ClockΔ19/Δ19 mice also showed comparable numbers and percentages of γ/δ+ T cells in the spleen and skin with wild-type mice (Supplementary Figure S1c online). These results suggested that ClockΔ19/Δ19 mice did not have obvious developmental defects in the immune system.
Clock mutation ameliorates IMQ-induced dermatitis
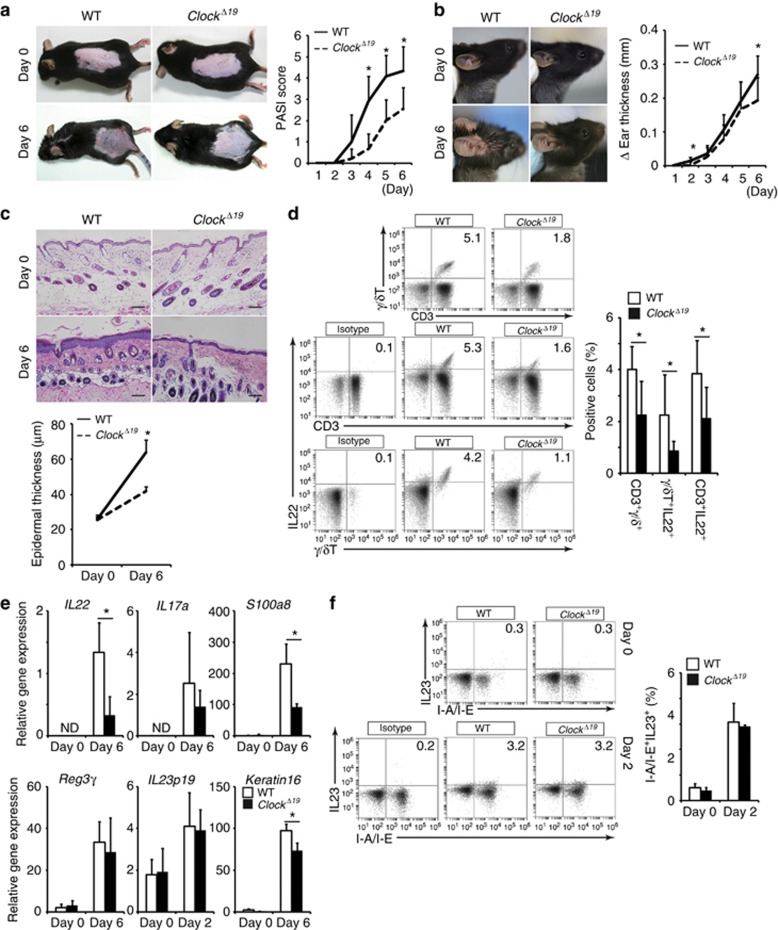
We treated wild-type and ClockΔ19/Δ19 mice daily with IMQ cream or control cream at Zeitgeber time 2 (ZT2) (0800 hours) on both ears and the shaved dorsal skin for 5 consecutive days (days 1–5) as described previously (van der Fits et al., 2009), and the severity of dermatitis was evaluated on day 6. Upon IMQ treatment, ClockΔ19/Δ19 mice exhibited significantly lower levels of dermatitis compared with wild-type mice, as judged by the Psoriasis Area Severity Index (PASI) score in the dorsal skin, ear thickness, epidermal hyperplasia, and frequency of CD3+ γ/δ+ T cells in the cervical lymph nodes (Figure 1a and d). The inflamed skin showed significantly reduced expression of IL-22, S100A8, and keratin 16 mRNAs in IMQ-treated ClockΔ19/Δ19 mice when compared with wild-type mice on day 6, although IL-17A and Reg3γ mRNA levels were comparable between IMQ-treated wild-type mice and ClockΔ19/Δ19 mice (Figure 1e). The number of infiltrating CD3+ T cells into the skin decreased in IMQ-treated ClockΔ19/Δ19 mice compared with IMQ-treated wild-type mice on day 6 (Supplementary Figure S2 online). These results suggested that Clock mutation ameliorated IMQ-induced skin inflammation in mice.
Figure 1.
Clock mutation results in reduced imiquimod (IMQ)-induced skin inflammation. (a) Psoriasis Area Severity Index (PASI) score in the dorsal skin (n=10–12 per group). (b) Ear thickness (n=10–12 per group). (c) Hematoxylin and eosin (HE) staining of the dorsal skin (n=5 per group). Bar=100 μm. (d) Frequency of γ/δ+ T cells (CD3+γ/δ+ cells, IL-22+γ/δ+ cells, CD3+ IL-22+ cells) in the cervical lymph nodes evaluated by FACS analysis at Zeitgeber time 2 (ZT2) (n=7 per group). (e) IL-22, IL-17A, S100A8, Reg3γ, IL-23 p19, and keratin 16 mRNA expression at ZT2 in the inflamed skin evaluated by quantitative reverse transcriptase in real time (qPCR) (n=3–5 per group). Amplification was normalized to GAPDH. (f) Frequency of I-A/I-E+IL-23+ cells in the cervical lymph nodes evaluated by FACS analysis at ZT2 (n=3–4 per group). The values represent means±SD. *P<0.05.
Clock mutation blunts γ/δ+ T cell responses to IL-23
A pivotal role of the IL-23/IL-17/IL-22 axis has been demonstrated in the psoriasis-like skin inflammation induced by IMQ in mice (van der Fits et al., 2009; Cai et al., 2011; Pantelyushin et al., 2012; Van Belle et al., 2012; Wohn et al., 2013; Yoshiki et al., 2014). Treatment of mice with IMQ is thought to activate dermal dendritic cells or Langerhans cells, releasing IL-23. IL-23, in turn, stimulated γ/δ+ T cells or Rorγt+ innate lymphoid cells (type 3 innate lymphoid cells (ILCs)) to produce IL-17 and IL-22, thereby leading to recruitment of neutrophils to the skin, keratinocyte proliferation, and release of anti-microbial peptides (Flutter and Nestle, 2013).
We found that IL-23 p19 mRNA levels in the skin were comparable between IMQ-treated wild-type and ClockΔ19/Δ19 mice on days 0, 2, and 6 (Figure 1e and Supplementary Figure S3 online). In addition, the frequency of I-A/I-E+IL-23+ cells in the cervical lymph nodes was comparable between IMQ-treated wild-type and ClockΔ19/Δ19 mice on day 2 (Figure 1f). We were unable to detect I-A/I-E+IL-23+ cells in the cervical lymph nodes on day 6 (data not shown). These findings suggested that production of IL-23, an innate cytokine that was rapidly induced following topical IMQ treatment, was comparable between wild-type and ClockΔ19/Δ19 mice. Therefore, we hypothesized that Clock mutation affected critical events downstream of IL-23 in the development of IMQ-induced dermatitis.
We thus determined whether Clock mutation affected γ/δ+ T cell responses to IL-23. Interestingly, at basal conditions, IL-23-induced IL-17A and IL-22 production significantly decreased in splenic γ/δ+ T cells isolated from ClockΔ19/Δ19 mice when compared with wild-type mice (Figure 2a). Consistently, γ/δ+ IL-22+ or CD3+ IL-22+ cells decreased in the cervical lymph nodes of IMQ-treated ClockΔ19/Δ19 mice compared with those in IMQ-treated wild-type mice (Figure 1d). Unexpectedly, the number of γ/δ+ IL-17A+ cells or CD3+ IL-17A+ cells in the cervical lymph nodes was comparable between IMQ-treated wild-type and ClockΔ19/Δ19 mice (Supplementary Figure S4 online).
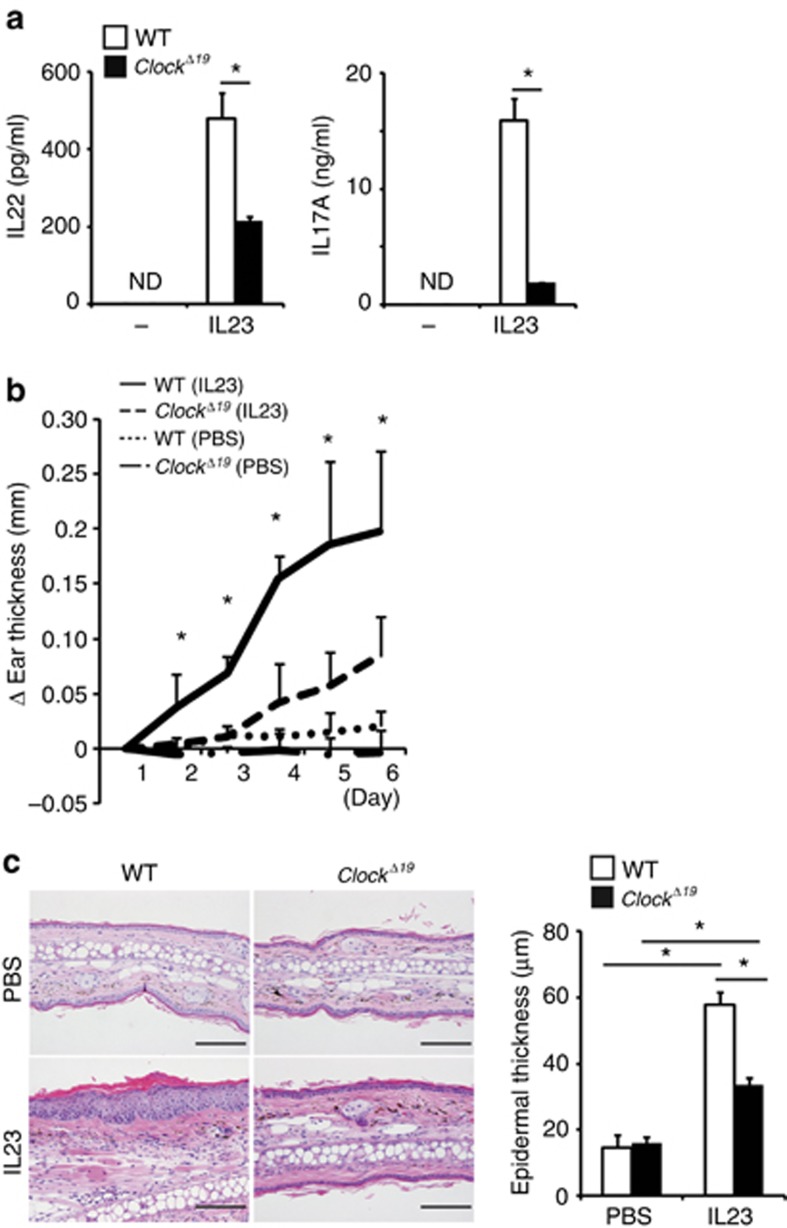
Figure 2.
Clock mutation blunts γ/δ+ T cell responses to IL-23. (a) IL-23-induced IL-22 and IL-17A production in splenic γ/δ+ T cells isolated from wild-type or ClockΔ19/Δ19 mice detected by ELISA (n=3 per group). (b and c) Wild-type or ClockΔ19/Δ19 mice underwent intradermal injection of phosphate-buffered saline (PBS) or IL-23 in the ears on days 1, 3, and 5 at Zeitgeber time 2 (ZT2). (b) Ear thickness (n=4–5 per group) or (c) hematoxylin and eosin (HE) staining of the ear and its quantitative analysis (epidermal thickness) (n=4–5 per group) on day 6 are shown. Bar=100 μm. The values represent means±SD. *P<0.05.
Previously, Chan et al. (2006) reported that direct intradermal administration of IL-23 into the skin induced psoriasis-like skin inflammation in mice, which recapitulated the dermatitis induced by IMQ treatment. Importantly, direct treatment of ClockΔ19/Δ19 mice with mouse recombinant IL-23 also induced significantly lower levels of dermatitis compared with wild-type mice as judged by ear thickness and epidermal hyperplasia in ClockΔ19/Δ19 mice (Figure 2b and c). These results suggested that Clock mutation blunted γ/δ+ T cell responses to IL-23 and resulted in reduced IMQ- or IL-23-induced psoriasis-like skin inflammation in mice.
Clock regulates IL-23R expression in γ/δ T cells
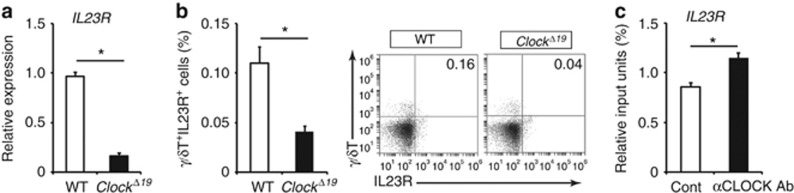
To investigate the mechanisms behind blunted responses to IL-23 in Clock-mutated γ/δ+ T cells, we examined the effects of Clock mutation on IL-23 receptor (IL-23R) expression in γ/δ+ T cells. IL-23R mRNA and protein expression levels decreased in splenic γ/δ+ T cells from ClockΔ19/Δ19 mice compared with wild-type mice (Figure 3a and b), suggesting that CLOCK regulated IL-23R expression in γ/δ+ T cells. Consistently, we noticed that several E-box-like elements that CLOCK could bind to (Hardin, 2004) were present in the promoter regions of the mouse IL-23R (Supplementary Figure S5 online). As expected, chromatin immunoprecipitation assay revealed that CLOCK bound to the promoter region of IL-23R in wild-type splenic γ/δ+ T cells (Figure 3c). These results suggested that CLOCK regulated the transcription of IL-23R in γ/δ+ T cells in a direct manner. Collectively, Clock mutation blunted γ/δ+ T cell responses to IL-23, at least in part, by decreasing IL-23R expression and ameliorated IMQ-induced dermatitis.
Figure 3.
Clock regulates IL-23 receptor (IL-23R) expression in γ/δ T cells. (a) IL-23 receptor (IL-23R) mRNA expression in splenic γ/δ+ T cells isolated from wild-type mice or ClockΔ19/Δ19 mice (n=3 per group). (b) Frequency of splenic γ/δ+IL-23R+ cells isolated from wild-type mice or ClockΔ19/Δ19 mice (n=4 per group). (c) Detection of CLOCK binding to the promoter region of IL-23R in splenic γ/δ+ T cells isolated from wild-type mice evaluated by chromatin immunoprecipitation (ChIP) assay. The values represent means±SD. *P<0.05.
IL-23R expression shows circadian rhythms in the mouse skin
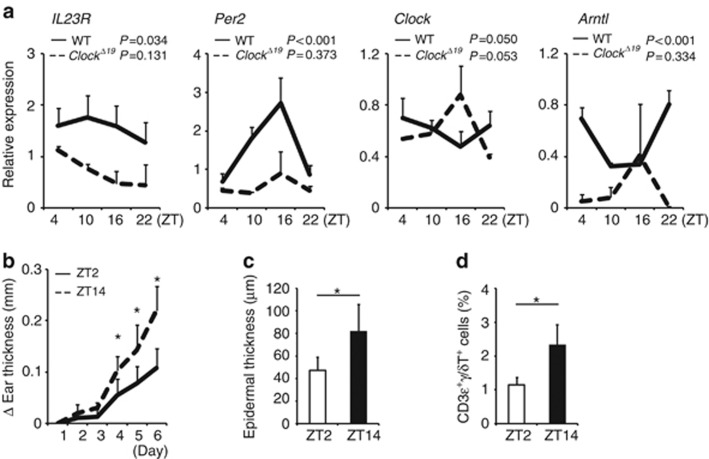
If IL-23R expression is regulated by CLOCK, IL-23R mRNA or protein levels should show circadian variations. Indeed, IL-23R mRNA levels exhibited circadian variations in the wild-type, but not Clock-mutated, mouse skin (Figure 4a). Expression of core clock genes such as Per2 and Bmal1 (Arntl) mRNA levels also showed circadian rhythms in the wild-type, but not Clock-mutated, mouse skin (Figure 4a). The Clock mRNA expression levels appeared to show circadian trend both in the wild-type and Clock-mutated mouse skin, but they were not statistically significant (Figure 4a), which was consistent with previous findings that Clock mRNA did not show daily oscillations in the skin of healthy human subjects (Bjarnason et al., 2001). These results suggested that IL-23R expression was under circadian control in the mouse skin at steady states. Of note, IL-23R mRNA expression levels were comparable between wild-type mice and ClockΔ19/Δ19 mice at ZT2 (0800 hours) (Supplementary Figure S3 online), although IL-23R mRNA expression exhibited circadian variations. In addition, IL-23R mRNA expression levels in the skin were comparable between wild-type mice and ClockΔ19/Δ19 mice on day 6 when the mice were sufficiently exposed to IMQ and substantial inflammation occurred (Supplementary Figure S3 online).
Figure 4.
IL-23 receptor (IL-23R) expression shows circadian rhythms in the mouse skin. (a) Whole-skin samples were obtained from wild-type mice at the indicated time points, mRNA was extracted, and quantitative reverse transcriptase in real time (qPCR) analysis was performed for IL-23R, Period2 (Per2), Clock, and Bmal1(Arntl) mRNA. The values represent the means±SD (n=4–5 per group). *P<0.05. (b–d) Wild-type mice were treated with imiquimod (IMQ) for 5 days (days 1–5) either at Zeitgeber time 2 (ZT2) or at ZT14. (b) Ear thickness (n=5 per group), (c) epidermal thickness (n=5 per group), or (d) frequency of CD3+γ/δ+ cells in the cervical lymph nodes evaluated by FACS analysis (n=5 per group) on day 6 is shown. The values represent the means±SD. *P<0.05.
To determine whether the circadian variation of IL-23R expression in the skin was translated to the susceptibility to IMQ-induced dermatitis in mice, we treated mice with IMQ either at ZT2 (0800 hours) or at ZT14 (1800 hours). We found that mice treated with IMQ at ZT14 exhibited more severe dermatitis than did mice treated with IMQ at ZT2, as judged by ear thickness, epidermal thickness, and frequency of γ/δ+ T cells in the cervical lymph nodes (Figure 4b–d). Collectively, these findings suggested that IMQ-induced dermatitis exhibited a time-of-day-dependent variation in wild-type mice, possibly in association with circadian IL-23R expression.
Per2 mutation exacerbates IMQ-induced dermatitis
As CLOCK and PER2 function in opposite directions as integral clock proteins (i.e., CLOCK or PER2 acts as a positive or negative limb of the circadian clock machinery, respectively), it is possible that mice with a loss-of-function mutation of Per2 exhibit opposite phenotypes to mice with a loss-of-function mutation of Clock upon IMQ treatment.
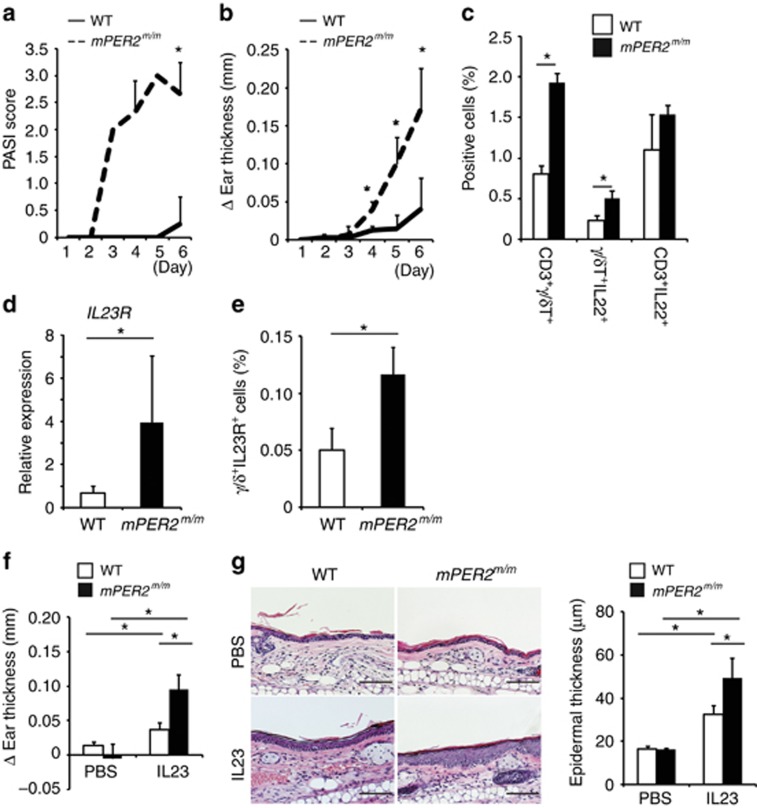
As expected, mice with a loss-of-function mutation of Per2 (mPer2m/m mice) (Zheng et al., 1999) showed exaggerated IMQ-induced dermatitis compared with wild-type mice, as judged by the PASI score in the dorsal skin, ear thickness, and frequency of CD3+ γ/δ+ T cells in the cervical lymph nodes (Figure 5a–c). Frequency of γ/δ+ IL-22+ cells or CD3+IL-22+ cells in the cervical lymph nodes also increased in IMQ-treated mPer2m/m mice compared with IMQ-treated wild-type mice (Figure 5c). In basal conditions, mPer2m/m mice showed increased IL-23R mRNA and protein expression in splenic γ/δ+ T cells compared with wild-type mice (Figure 5d and e). Consistently, direct treatment of mPer2m/m mice with mouse recombinant IL-23 also induced significantly severe levels of dermatitis compared with wild-type mice, as judged by ear thickness and epidermal hyperplasia (Figure 5f and g). These findings suggested that Per2 mutation exaggerated IMQ-induced dermatitis in mice in contrast to Clock mutation, which was associated with increased IL-23R and also IL-22 expression.
Figure 5.
Period2 (Per2) mutation enhances imiquimod (IMQ)-induced skin inflammation. (a) Psoriasis Area Severity Index (PASI) score in the dorsal skin (n=3–4 per group). (b) Ear thickness (n=3–4 per group). (c) Frequency of γ/δ T cells (CD3+γ/δ+, IL-22+γ/δ+s, and CD3+IL-22+ cells) in the cervical lymph nodes evaluated by FACS analysis on day 6 (n=3 per group). (d) IL-23 receptor (IL-23R) mRNA expression evaluated by quantitative reverse transcriptase in real time (qPCR) in splenic γ/δ+ T cells isolated from nontreated wild-type mice or mPer2m/m mice (n=3 per group). (e) Frequency in splenic γ/δ+IL-23R+ cells isolated from nontreated wild-type or mPer2m/m mice evaluated by FACS analysis at Zeitgeber time 2 (ZT2) (n=3 per group). (fand g) Wild-type mice or mPer2m/m mice underwent intradermal injection of phosphate-buffered saline (PBS) or IL-23 in the ears on days 1, 3, 5, 7, and 9 at ZT2. (f) Ear thickness (n=3 per group) or (g) hematoxyin and eosin (HE) staining of the ear and its quantitative analysis (epidermal thickness) (n=3 per group) on day 10 are shown. Bar=100 μm. The values represent the means±SD. *P<0.05.
Mast cells have a marginal role in IMQ-induced dermatitis in the current experimental conditions
Mast cells have a regulatory role in the development of IMQ-induced dermatitis in mice—in particular, in the initiation phase of dermatitis—via regulation of tumor necrosis factor-α and IL-1β (Heib et al., 2007). In addition, we have previously shown that Clock mutation affects IgE–mediated mast cell responses (Nakamura et al., 2011, 2014). Thus, it is possible that Clock mutation affects mast cell functions, thereby influencing IMQ-induced dermatitis.
To determine whether mast cells have a role in IMQ-induced dermatitis in our experimental conditions, we compared the development of IMQ-induced dermatitis between mast cell–deficient W/Wv mice and their control mice. The extents of IMQ-induced dermatitis were comparable between these mice as judged by the PASI score in the dorsal skin, ear thickness, epidermal thickness, and frequency of CD3+γ/δ+ T cells in the cervical lymph nodes (Supplementary Figure S6a and d online). These findings suggested that mast cells had a marginal role in IMQ-induced dermatitis in the current experimental conditions.
Discussion
This study showed that a loss-of-function mutation of the integral circadian gene Clock ameliorated IMQ-induced psoriasis-like skin inflammation in mice, which was associated with decreased IL-23R expression in γ/δ+ T cells. Consistently, CLOCK directly bound to the promoter of IL-23R in γ/δ+ T cells, and IL-23R expression in the mouse skin was under circadian control. In contrast, a loss-of-function mutation of Per2, another key clock protein that inhibits CLOCK activity, exaggerated IMQ-induced skin inflammation associated with increased IL-23R expression in γ/δ+ T cells. Collectively, these findings suggested that Clock regulated IMQ-induced psoriasis-like skin inflammation in mice partly via direct modulation of IL-23R expression in γ/δ+ T cells. Given that IL-23R is a candidate gene involved in the pathogenesis of psoriasis (Capon et al., 2007), Clock may have an important regulatory role in psoriasis, which may provide new insight into previously unknown aspects of the biology of psoriasis.
We showed that Clock mutation blunted γ/δ+ T cell responses to IL-23 in vitro: IL-23-induced IL-17A and IL-22 production decreased in Clock-mutated splenic γ/δ+ T cells (Figure 2a) associated with reduced IL-23R expression (Figure 3a and b). However, in contrast to IL-22 expression, expression levels of skin IL-17A mRNA and frequency of γ/δ+ IL-17A+ cells in the cervical lymph nodes were comparable between IMQ-treated wild-type mice and ClockΔ19/Δ19 mice (Figure 1e and Supplementary Figure S4 online). IL-17A was produced not only by γ/δ+ T cells but also by T helper type 17 cells and type 3 innate lymphoid cells (ILC3) (Artis and Spits, 2015). ILC3 has been recently suggested to have a critical role in human psoriasis by producing IL-17A production via IL-23R signaling (Ward and Umetsu, 2014). So far, the relative contribution of γ/δ+ T cells, T helper type 17 cells, or ILC3 to IL-17A production in the IMQ-treated mouse skin remains unclear. Thus, given that CD3+ IL-17A+ cells in the cervical lymph nodes were comparable between wild-type and ClockΔ19/Δ19 mice (Supplementary Figure S4 online), the comparable IL-17A mRNA and protein expression in the skin between wild-type and ClockΔ19/Δ19 mice could be attributed to IL-17A production by ILC3. In this case, Clock might regulate IL-23R expression selectively in γ/δ+ T cells, but not in ILC3, because it is well accepted that Clock-controlled genes are regulated in a cell-type– or tissue-specific manner (Partch et al., 2014). We would like to address this issue in future studies because it would be beyond the current scope to determine whether Clock regulated induction or activation of ILC3 in general or under particular conditions such as IMQ-induced skin pathology.
The findings that Clock mutation decreased IL-23R expression and CLOCK directly bound to the E-box-like elements in the promoter region of IL-23R in γ/δ+ T cells suggested that IL-23R was a direct target gene by CLOCK. As PER2 inhibits CLOCK activity, it was most likely that Per2 mutation failed to inhibit CLOCK activity, leading to increased IL-23R expression in γ/δ+ T cells. The opposite severity observed in IMQ-treated Clock- or Per2–mutated mice compared with wild-type mice also supported this notion.
IMQ-induced dermatitis exhibited a time-of-day-dependent variation in wild-type mice, possibly in association with circadian IL-23R expression in the skin (Figure 4). However, it remains to be determined whether the circadian variation of IL-23R expression in the skin really determines the optical timings or outcomes of daily treatment with IMQ for the generation of skin lesions, as the fluctuation of mIL-23R appeared to be little.
Of note, we used mice with an ICR background for Per2 mutation experiments (Figure 5) as we failed to have enough numbers of Per2–mutated mice for experiments because of poor fertility of Per2–mutated mice with a C57BL/6 background. The different genetic backgrounds might lead to different sensitivity levels to IMQ treatment (e.g., PASI scoring, ear thickness) in mice. It appeared that ICR mice exhibited less IMQ- or IL-23-induced dermatitis compared with C57BL/6 mice, as judged by the PASI score in the dorsal skin, ear thickness, and epidermal hyperplasia. In addition, ClockΔ19/Δ19 mice exhibited significantly reduced ear thickness on day 2 compared with wild-type mice (Figure 1b), which was not observed in mPer2m/m mice (Supplementary Figure S5b online). It is possible that this early reduction of ear thickness (dermal edema) in ClockΔ19/Δ19 mice might be caused by intrinsic effects of Clock mutation on the skin, such as anomaly, but not by IMQ treatment.
In the current experimental conditions, mast cell deficiency did not affect IMQ-induced skin inflammation in mice (Supplementary Figure S6 online). A previous study reported that mast cells were essential to initiate an early inflammatory reaction in IMQ-induced dermatitis in mice via regulation of tumor necrosis factor-α and IL-1β (Heib et al., 2007). Different experimental conditions such as the concentration of topical IMQ (2.5% in the current study vs. 5% in the others study) and possible skin microbe variations in mice housed in different facilities (Zeeuwen et al., 2013) might explain the discrepancy, although the precise reasons remain unclear.
We have previously shown that ClockΔ19/Δ19 mice exhibit significantly severe levels of allergic contact dermatitis compared with wild-type mice, where the mice were treated with TNCB (2,4,6-trinitro-1-chlorobenzene) on the abdominal skin on day 0 (sensitization) and then with TNCB on the ears on day 5 (challenge) (Takita et al., 2013). The exaggerated allergic contact dermatitis phenotypes in ClockΔ19/Δ19 mice were associated with increased Th2-type responses such as serum IgE levels and mast cell number in the skin. Therefore, it appears that Clock mutation affects the development of IMQ-induced skin inflammation and allergic contact dermatitis in mice differently, possibly because of different influences of Clock mutation on their pathophysiology.
There have been several reports suggesting that psoriasis may be linked to aberrant circadian rhythms. Importantly, a most recent study suggested an increased risk for psoriasis in night-shift workers who had aberrant circadian rhythms (Li et al., 2013). Our findings in mice implicate that Clock may be a potent regulator of psoriasis by affecting IL-23R expression. Thus, we speculate that aberrant circadian rhythms affecting Clock expression or activity might enhance susceptibility to psoriasis by increasing IL-23R expression or by other unknown mechanisms. More human studies are therefore clearly needed to determine how strongly the circadian clock (or its disruption) influences the predisposition, etiology, maintenance, and progression of psoriasis.
In summary, we propose that Clock is a novel regulator of psoriasis-like skin inflammation in mice. To the best of our knowledge, this is the first study to reveal a mechanistic link between psoriasis and the circadian clock, which will provide a novel insight into the pathophysiology of psoriasis.
Materials and methods
Mice
Female C57BL/6 ClockΔ19/Δ19 mice, their wild-type littermates, ICR Per2–mutated mice (mPer2m/m mice) (Zheng et al., 1999), and control ICR mice were bred under specific pathogen-free conditions. All mice were housed under 12-hour light/12-hour dark conditions (light/dark (L/D) 12:12 cycles; the light was turned on at 0600 hours, ZT0, and the light was turned off at 0600 hours, ZT12) with ad libitum access to food and water for at least 2 weeks. All animal experiments were approved by the Institutional Review Board of the University of Yamanashi.
IMQ-induced psoriasis-like skin inflammation
Mice were treated daily for 5 consecutive days on both ears and on the shaved dorsal skin with 31.2 mg of commercially available IMQ cream (5% Beselna cream; Mochida Pharmaceutical, Tokyo, Japan) or Vaseline at ZT2. IMQ cream was mixed with Vaseline in a 1:1 ratio and used.
Intradermal IL-23 injection
To induce IL-23-mediated psoriasis-like skin inflammation, 20 μl phosphate-buffered saline, either alone or containing 500 ng recombinant mouse IL-23 (BioLegend, San Diego, CA), was intradermally injected into the ears of anesthetized mice using a 30-gauge needle, under a microscope (Wraymer, Osaka, Japan) at ZT2.
Scoring severity of skin inflammation
Ear thickness was measured using an engineer's micrometer at indicated time points. The Δ ear thickness is calculated as the changes in ear thickness ((ear thickness at the indicated time points)−(ear thickness before treatment on day 0)). A scoring system based on the clinical Psoriasis Area and Severity Index was used (van der Fits et al., 2009).
Splenic γ/δ+ T cell preparation and culture
γ/δ+ T cells were purified from spleens using a mouse γ/δ+ TCR+ T cell Isolation Kit (Milteny Biotec, Bergisch Gladbach, Germany) at ZT2. Cells were stimulated for 72 hours with IL-23 (10 ng ml−1; R&D, Minneapolis, MN) or medium alone in the presence of anti-CD3 (2 μg ml−1; eBioscience, San Diego, CA). After 72 hours, IL-17A or IL-22 concentrations in the supernatants were determined by ELISA (eBioscience).
Chromatin immunoprecipitation assay
Chromatin Immunoprecipitation assay was performed as described previously (Nakamura et al., 2014).
Statistical analysis
The statistical analyses were performed using the unpaired Student's t-test for two-group comparisons, and analysis of variance for comparison between more than two groups.
For more information, see the Supplementary Methods section in this article's Online Repository.
Acknowledgments
We thank Tomoko Tohno and Mutsuko Hara for general assistance. This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Glossary
- IMQ
imiquimod
- Per2
Period2
- TNCB
2,4,6-trinitro-1-chlorobenzene
- ZT
Zeitgeber time
The authors state no conflict of interest.
Footnotes
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Artis D, Spits H. (2015) The biology of innate lymphoid cells. Nature 517:293–301 [DOI] [PubMed] [Google Scholar]
- Bacaksiz A, Akif Vatankulu M, Sonmez O et al. (2012) Non-dipping nocturnal blood pressure in psoriasis vulgaris. Wien Klin Wochenschr 124:822–829 [DOI] [PubMed] [Google Scholar]
- Bjarnason GA, Jordan RC, Wood PA et al. (2001) Circadian expression of clock genes in human oral mucosa and skin: association with specific cell-cycle phases. Am J Pathol 158:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Shen X, Ding C et al. (2011) Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity 35:596–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon F, Di Meglio P, Szaub J et al. (2007) Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum Genet 122:201–206 [DOI] [PubMed] [Google Scholar]
- Chan JR, Blumenschein W, Murphy E et al. (2006) IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med 203:2577–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiricozzi A, Saraceno R, Chimenti MS et al. (2014) Role of IL-23 in the pathogenesis of psoriasis: a novel potential therapeutic target? Expert Opin Ther Targets 18:513–525 [DOI] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72:517–549 [DOI] [PubMed] [Google Scholar]
- Fitch E, Harper E, Skorcheva I et al. (2007) Pathophysiology of psoriasis: recent advances on IL-23 and Th17 cytokines. Curr Rheumatol Rep 9:461–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flutter B, Nestle FO. (2013) TLRs to cytokines: mechanistic insights from the imiquimod mouse model of psoriasis. Eur J Immunol 43:3138–3146 [DOI] [PubMed] [Google Scholar]
- Gelfant S, Ozawa A, Chalker DK et al. (1982) Circadian rhythms and differences in epidermal and in dermal cel proliferation in uninvolved and involved psoriatic skin in vivo. J Invest Dermatol 78:58–62 [DOI] [PubMed] [Google Scholar]
- Hardin PE. (2004) Transcription regulation within the circadian clock: the E-box and beyond. J Biol Rhythms 19:348–360 [DOI] [PubMed] [Google Scholar]
- Heib V, Becker M, Warger T et al. (2007) Mast cells are crucial for early inflammation, migration of Langerhans cells, and CTL responses following topical application of TLR7 ligand in mice. Blood 110:946–953 [DOI] [PubMed] [Google Scholar]
- Kikly K, Liu L, Na S et al. (2006) The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol 18:670–675 [DOI] [PubMed] [Google Scholar]
- Li WQ, Qureshi AA, Schernhammer ES et al. (2013) Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol 133:565–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes MA, Suárez-Fariñas M, Krueger JG. (2014) Immunology of psoriasis. Annu Rev Immunol 32:227–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS. (2012) Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35:445–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzanica N, Tadini G, Radaelli A et al. (1988) Plasma melatonin levels in psoriasis. Acta Dermatol Venereol 168:312–316 [PubMed] [Google Scholar]
- Nakamura Y, Harama D, Shimokawa N et al. (2011) Circadian clock gene Period2 regulates a time-of-day-dependent variation in cutaneous anaphylactic reaction. J Allergy Clin Immunol 127:1038–1045 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Nakano N, Ishimaru K et al. (2014) Circadian regulation of allergic reactions by the mast cell clock in mice. J Allergy Clin Immunol 133:568–575 [DOI] [PubMed] [Google Scholar]
- Pantelyushin S, Haak S, Ingold B et al. (2012) Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J Clin Invest 122:2252–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partch CL, Green CB, Takahashi JS. (2014) Molecular architecture of the mammalian circadian clock. Trends Cell Biol 24:90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takita E, Yokota S, Tahara Y et al. (2013) Biological clock dysfunction exacerbates contact hypersensitivity in mice. Br J Dermatol 168:39–46 [DOI] [PubMed] [Google Scholar]
- Tanioka M, Yamada H, Doi M et al. (2009) Molecular clocks in mouse skin. J Invest Dermatol 129:1225–1231 [DOI] [PubMed] [Google Scholar]
- Ukai H, Ueda HR. (2010) Systems biology of mammalian circadian clocks. Annu Rev Physiol 72:579–603 [DOI] [PubMed] [Google Scholar]
- Van Belle AB, de Heusch M, Lemaire MM et al. (2012) IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J Immunol 188:462–469 [DOI] [PubMed] [Google Scholar]
- van der Fits L, Mourits S, Voerman JS et al. (2009) Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol 182:5836–5845 [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, King DP, Chang AM et al. (1994) Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264:719–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NL, Umetsu DT. (2014) A new player on the psoriasis block: IL-17A- and IL-22-producing innate lymphoid cells. J Invest Dermatol 134:2305–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohn C, Ober-Blöbaum JL, Haak S et al. (2013) Langerin(neg) conventional dendritic cells produce IL-23 to drive psoriatic plaque formation in mice. Proc Natl Acad Sci USA 110:10723–10728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiki R, Kabashima K, Honda T et al. (2014) IL-23 from langerhans cells is required for the development of imiquimod-induced psoriasis-like dermatitis by induction of IL-17A-producing γδ T cells. J Invest Dermatol 134:1912–1921 [DOI] [PubMed] [Google Scholar]
- Zeeuwen PL, Kleerebezem M, Timmerman HM et al. (2013) Microbiome and skin diseases. Curr Opin Allergy Clin Immunol 13:514–520 [DOI] [PubMed] [Google Scholar]
- Zheng B, Larkin DW, Albrecht U et al. (1999) The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 400:169–173 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.