Abstract
Functional variability at the arylamine N-acetyltransferase genes is associated with drug response in humans and may have been adaptive in the past owing to selection pressure from diet and exposure to toxins during human evolution.
Aims
We have characterized nucleotide variation at the NAT1 and NAT2 genes, and at the NATP1 pseudogene in global human populations, including many previously under-represented African populations, in order to identify potential functional variants and to understand the role that natural selection has played in shaping variation at these loci in globally diverse populations.
Materials & methods
We have resequenced approximately 2800 bp for each of the NAT1 and NAT2 gene regions, as well as the pseudogene NATP1, in 197 African and 132 non-African individuals.
Results & conclusion
We observe a signature of balancing selection maintaining variation in the 3’-UTR of NAT1, suggesting that these variants may play a functional role that is currently undefined. In addition, we observed high levels of nonsynonymous functional variation at the NAT2 locus that differs amongst ethnically diverse populations.
Keywords: Africa, arylamine N-acetyltransferase, drug-metabolizing enzyme loci, natural selection, xenobiotic-metabolizing loci
Characterization of nucleotide variation at genes encoding xenobiotic-metabolizing enzymes in ethnically diverse populations is critical for identifying variants that play a role in xenobiotic response and for understanding the role of these genes in adaptation to diverse environments and diets during human evolution. N-acetylation is a major detoxification route for many drugs and carcinogens [1]. NAT1 and NAT2 are enzymatically distinct, with certain aromatic amine or hydrazine drugs being preferentially acetylated by NAT1 (e.g., p-aminosalicylic acid and p-aminobenzoic acid) and others by NAT2 (e.g., isoniazid and sulfamethazine). NAT isoenzymes are also involved in bioactivation reactions with substrates derived during Phase I metabolism mediated by CYP450 genes. These reactions generate reactive mutagenic compounds that bind to DNA [2]. It is these proposed bioactivation reactions that may result in the hypothesized role of N-acetylation in cancer predisposition [2]. Examples of toxins that are metabolized via N-acetylation include various heterocyclic amine carcinogens present in food, pyrolysis products, tobacco products and products resulting from certain industrial processes (e.g., preparation of various textiles, hair dyes, rubber and plastics) [3–5].
Human NAT1 is expressed ubiquitously and at various stages in development [6,7]. Human NAT2 isozyme shows a more restricted tissue-distribution profile, and is expressed mainly in hepatic tissue [8,9]. Most adverse drug reactions involving the N-acetylation pathway can be linked to the NAT2 isoform, including response to isoniazid commonly used to treat TB. Supplementary Figure 1 (see www.futuremedicine.com/doi/suppl/10.2217/pgs.11.88) illustrates the structure of the human NAT region, located on chromosome 8p21.3–23.1 [10]. NAT1 and NAT2 are separated by a 168-kb region [11,12] that encompasses a nontranscribed pseudogene NATP1 [13]. Both functional NAT genes contain 870-bp intronless, protein-coding exons. NAT1 and NAT2 share 87% sequence similarity with each other and approximately 83–85% sequence homology with NATP1 [13], indicating that these loci arose via gene duplication. Recent studies investigating the exon–intron structure of the NAT1 locus have identified at least eight noncoding exons and have predicted adjacent alternative promoters potentially regulating the tissue-specific expression of the gene [14–17] (Supplementary Figure 1). In addition, three sites have been identified in the 3´-UTR of NAT1, located downstream of the coding region at positions +1085, +1203 and +1242 relative to the +1 ATG of exon 9, which are predicted to influence polyadenylation of the mRNA product (Supplementary Figure 1). By contrast, the majority of NAT2 mRNAs initiate from positions −8682 and −8752 relative to the +1 ATG start site of exon 1 [17]. The NAT2 exon 1 is a short noncoding exon that is separated from the ORF coded by exon 2 by an approximately 8-kb intron (Supplementary Figure 1).
We have surveyed nucleotide sequence variation in both coding and flanking regions of the NAT1 and NAT2 genes, as well as the NAT pseudogene NATP1, in ethnically diverse global populations that include many previously under-represented African populations. Knowledge of the pattern of genetic diversity and haplotype structure at the NAT loci in ethnically diverse population groups has important implications for understanding how NAT genotypes contribute to xenobiotic-metabolism profiles and disease phenotypes. We have identified a number of polymorphisms that show signatures of natural selection and may influence NAT1 and NAT2 function. Our results contribute to understanding of how variation at the NAT loci may have been adaptive for dealing with exposure to toxins during human evolution, and have important implications for understanding individual variation in drug response.
Materials & methods
Population samples
A total of 170 individuals originating from Tanzania, Sudan and Cameroon were included in the study (Table 1). Institutional Review Board approval was obtained from the University of Maryland, College Park (MD, USA) prior to sample collection. Written informed consent was obtained from all participants and research/ethics approval and permits were obtained from the following institutions prior to sample collection: Tanzanian Commission for Science and Technology and Tanzanian National Institute for Medical Research in Dar es Salaam, Tanzania; the University of Khartoum in Sudan; the Nigerian Institute for Research and Pharmacological Development, Abuja, Nigeria; the Ministry of Health and National Committee of Ethics, Cameroon. During field collection 8 ml of peripheral blood was drawn from each individual involved and white blood cells were isolated from the whole blood using a modified salting-out procedure (100 mM Tris-HCL pH 7.6, 40 mM EDTA pH 8.0, 50 mM NaCl, 0.2% sodium dodecyl sulfate, 0.05% 8 mM sodium azide) [18]. DNA was extracted at a later date using the Puregene® DNA purification kit (Gentra Systems, Inc., MN, USA). All extracted DNA was quantified using Pico Green reagent (Invitrogen, CA, USA) and the Wallac Victor2™ 1420 MultiLabel Counter (PerkinElmer Life Sciences, MA, USA) at 1.0 s per well.
Table 1.
Populations included in the study of NAT nucleotide diversity.
| Population | NAT1 2N | NATP1 2N | NAT2 2N | |
|---|---|---|---|---|
| East Africa | ||||
| Tanzania | Burunge6 | 34 | 36 | 34 |
| Hadza4 | 32 | 28 | 28 | |
| Maasai7 | 32 | 26 | 28 | |
| Sandawe6 | 38 | 36 | 36 | |
| Turu6 | 32 | 30 | 30 | |
| Sudan | Dinka1 | 18 | 30 | 26 |
| Central Africa | ||||
| Central African Republic | Biaka pygmy3† | 30 | 30 | 30 |
| West Africa | ||||
| Cameroon | Fulani5 | 22 | 26 | 26 |
| Kanuri8 | 26 | 26 | 24 | |
| Lemande9 | 26 | 28 | 28 | |
| Mada8 | 28 | 28 | 28 | |
| Baka pygmy3 | 18 | 18 | 18 | |
| Bakola pygmy3 | 14 | 14 | 14 | |
| Nigeria | Yoruba9† | 24 | 24 | 24 |
| South Africa | ||||
| Namibia | San2† | 14 | 14 | 14 |
| Total Africa | 388 | 394 | 388 | |
| Europe/Middle East | ||||
| France | French10† | 22 | 22 | 16 |
| Israel | Druze10† | 24 | 22 | 20 |
| Italy | Sardinian10† | 24 | 24 | 22 |
| Pakistan | Brahui11† | 24 | 24 | 22 |
| Russia | Russian10† | 24 | 12 | 12 |
| Total Europe/Middle East | 118 | 104 | 92 | |
| Asia | ||||
| Cambodia | Cambodian13† | 14 | 14 | 14 |
| China | Han13† | 24 | 24 | 20 |
| Japan | Japanese13† | 22 | 20 | 18 |
| New Guinea | Papuan12† | 18 | 4 | 4 |
| Siberia | Yakut13† | 22 | 8 | 6 |
| Total Asia | 100 | 70 | 62 | |
| Americas | ||||
| Brazil | Karitiana14† | 22 | 20 | 22 |
| Mexico | Pima14† | 24 | 20 | 20 |
| Total Americas | 46 | 40 | 40 | |
| Grand total | 652 | 608 | 582 | |
Superscript numbers indicate defined population groups based on the whole-genome structure results [35].
Human Genome Diversity Cell Line Panel–Centre d’Etude du Polymorphisme Humain. 2N: Number of chromosomes.
In addition, a global panel of 166 individuals was included from the Human Genome Diversity Cell Line Panel-Centre d’Etude du Polymorphisme Humain (CEPH) [19,20]. African groups included from the CEPH diversity panel included Biaka pygmy from the Central African Republic, San from Namibia and Yoruba from Nigeria. Population samples used are summarized in Table 1, and grouped according to similar genetic ancestry as determined by structure analysis [21] of genome-wide short tandem repeat polymorphisms, indel marker variants [22], and SNPs [23,24].
PCR amplification & resequencing
PCR and sequencing primers were designed using the program Primer3 version 0.4.0 [101]. All primers used for this project are listed in Supplementary Table 1. All nucleotide positions referenced in this article are numbered according to consensus NAT nomenclature [25–27]. DNA samples obtained from the CEPH diversity panel were subject to whole-genome amplification (WGA) using the GenomiPhi® HY DNA amplification kit (GE Heathcare, Buckinghamshire, UK) prior to PCR amplification. DNA replication with WGA is extremely accurate due to the low error rate of ϕ29 DNA polymerase (1 in 106–107) compared with other enzymes. The total amplified product for each of the NAT1, NAT2 and NATP1 regions was obtained with a single PCR product, with the exception of NAT2 and NATP1 CEPH WGA products that were amplified in six overlapping segments of approximately 500–700 bp in length. Forward primers were used for sequencing in all cases, and reverse primers were used when necessary to allow for sequence confirmation. All amplifications were performed using 1.0 unit of Platinum HiFi enzyme (Invitrogen, CA, USA) and contained 200 µM of each deoxynucleotide triphosphate, 2 mM MgSO4, and 100 ng of genomic DNA in a final volume of 25 µl. Samples were denatured at 94°C for 1 min, followed by 35 cycles of 94°C for 30 s, 55°C for 30 s, and 68°C for 1 min per 1000 bp. The reaction was performed using a Peltier Thermal Cycler (MJ Research, MA, USA). PCR products obtained were run on a 1.6% agarose gel with BenchTop pGEM DNA Marker (Promega, WI, USA).
To examine nucleotide variability and identify novel variants, direct sequencing of the NAT gene regions was performed using the population samples listed in Table 1. PCR products were purified using the ExoSAP-IT process, as described by the manufacturer (US Biochemical Corp., OH, USA). Sequencing reactions were subsequently prepared using this purified DNA. Nucleotide sequences for each gene region were generated in six overlapping sequence reads using the didioxy-BigDye® kit (Applied Biosystems, CA, USA) and analyzed on the ABI 3730×l automated capillary sequencer.
Data analysis
NAT1, NAT2 and NATP1 sequences were edited and assembled into contigs using the programs Sequencher version 4.8 for Macintosh (Gene Codes Corp.) and the Phred, Phrap and Consed suite for the Linux operating system [28–30]. SNPs were called automatically using the Polyphred [31] software program, which tags SNP variants within Consed. All Polyphred SNP calls were then rechecked by eye for accuracy; SNPs identified as occurring once or twice in the dataset (singletons and doubletons, respectively) were then confirmed by resequencing, using both forward and reverse primers. All regions were included for further analysis with the exception of a single problem area of NAT1, which contained high numbers of repetitive elements, where 235 bps of sequence at the 3´ end of primer -1182 was removed from all individuals (Supplementary Table 1). Indels and microsatellite data were not considered in the present analyses.
Diploid haplotypes were inferred across the NAT1, NAT2, and NATP1 regions using the program PHASE version 2.1.1 [32], which reconstructs haplotypes from population genotype data using a coalescence model-based algorithm [33]. PHASE 2.1.1 enables inclusion of triallelic sites under a model of parent-independent mutation. PHASE 2.11 also implements a recombination method (the –MR option), which allows the user to specify the relative physical location of each SNP and accounts for the decay of linkage disequilibrium (LD) with distance [34,35]. No individual was included in phase inference with missing data spanning greater than 200 bp in length. The total number of individuals included for analyses of the coding and noncoding regions is listed for each NAT locus in Table 1. The number of individuals included for analyses of the coding region only for NAT2 are listed in Table 2. The total number of individuals included in phase inference is listed for each NAT locus in Table 1. For each locus, four PHASE runs were performed on samples grouped according to broad geographic regions (i.e., Africa, Europe, Asia and the Americas) (Table 1). PHASE runs for each geographic region were replicated using the ‘−x’ option that runs the algorithm multiple times automatically, starting from different starting points and selecting the run with the best average ‘goodness of fit’, which measures the estimated haplotypes fit to an approximate coalescent model.
Table 2.
NAT2-inferred functional haplotypes by population.
| 2N | NAT2 haplotype designation | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| *4 | *5A | *5B | *5C | *6A | *6B | *6C | *6G | *6H | *6I | *6P† | *6Q† | *7A | *7B | *7C | *12A | *12B | *12E | *12G | *12H | *12K† | *12L† | *13A | *13C† | *14B | *14j† | *22†,‡ | *23†,‡ | *24†,‡ | *25†,‡ | ||
| Africa | |||||||||||||||||||||||||||||||
| Burunge | 34 | 2 | – | 9 | 1 | 10 | – | – | – | – | 1 | – | – | – | 5 | – | 1 | – | 3 | – | – | – | – | – | – | 1 | – | – | 1 | – | – |
| Biaka pygmy | 30 | 3 | – | 4 | – | 1 | – | – | – | – | – | – | – | – | – | – | 14 | 1 | 2 | 1 | – | 1 | – | 2 | – | 1 | – | – | – | – | – |
| Dinka | 26 | 2 | – | 10 | – | 8 | – | – | – | – | – | – | 1 | – | – | – | 2 | – | – | – | 1 | – | 1 | – | – | 1 | – | – | – | – | – |
| Fulani | 26 | . | – | 13 | – | 9 | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | 2 | – | 1 | – | – | – | – | – |
| Hadza | 28 | 2 | – | 4 | – | 16 | – | – | – | – | 1 | – | – | – | 2 | – | 2 | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – |
| Kanuri | 24 | 6 | – | 5 | – | 3 | – | 1 | – | – | – | – | – | – | – | – | 5 | – | – | – | – | – | – | 1 | – | 3 | – | – | – | – | – |
| Lemande | 28 | 2 | 1 | 8 | 1 | 3 | – | – | – | 1 | 2 | – | – | – | – | – | 2 | – | – | – | – | – | – | 3 | – | 4 | 1 | – | – | – | – |
| Mada | 28 | 2 | – | 13 | 1 | 6 | – | 1 | – | – | – | – | – | – | 1 | – | – | – | – | – | 1 | – | – | – | – | 2 | – | 1 | – | – | – |
| Maasai | 28 | 1 | 1 | 8 | 1 | 9 | – | 1 | – | – | 1 | – | – | – | – | 1 | 3 | – | – | – | – | – | – | – | – | 2 | – | – | – | – | – |
| Baka pygmy | 18 | 1 | – | 2 | 2 | 2 | – | – | 1 | – | – | – | – | – | – | – | 2 | – | 1 | 3 | – | – | – | 4 | – | – | – | – | – | – | – |
| Bakola pygmy | 14 | 1 | – | – | – | – | – | – | 2 | – | – | – | – | – | – | – | 11 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| San | 14 | 5 | – | 2 | – | – | – | – | – | – | – | – | – | – | 1 | – | 6 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Sandawe | 36 | 4 | – | 8 | 1 | 13 | – | – | – | 1 | – | 2 | – | – | 1 | – | 5 | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – |
| Turu | 30 | 4 | 1 | 10 | 1 | 4 | – | – | – | – | – | 1 | – | – | 2 | – | 1 | – | 1 | – | – | – | – | 3 | – | 1 | – | – | 1 | – | – |
| Yoruba | 24 | 2 | 2 | – | – | 3 | – | 1 | – | – | 1 | – | – | – | – | – | 5 | – | – | – | 1 | – | – | 5 | – | 2 | – | 1 | – | 1 | – |
| Europe | |||||||||||||||||||||||||||||||
| Druze | 22 | 5 | 2 | 8 | – | 5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | 1 |
| French | 18 | 2 | 4 | 7 | – | 5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Brahui | 24 | 5 | 4 | 2 | 3 | 7 | 2 | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Russian | 12 | – | 2 | 5 | – | 3 | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – |
| Sardinian | 24 | 6 | 3 | 5 | – | 8 | – | – | – | – | – | – | – | – | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Asia | |||||||||||||||||||||||||||||||
| Cambodian | 14 | 4 | – | – | – | 3 | – | – | – | – | – | – | – | 1 | 5 | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – |
| Han | 24 | 11 | 1 | – | – | 5 | – | – | – | – | – | – | – | – | 5 | – | – | – | – | – | – | – | – | 2 | – | – | – | – | – | – | – |
| Japanese | 20 | 14 | – | – | – | 4 | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – |
| Papuan | 4 | 3 | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Yakut | 8 | 4 | 1 | – | – | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Americas | |||||||||||||||||||||||||||||||
| Karitiana | 22 | 10 | 1 | 8 | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | – |
| Pima | 22 | 9 | – | 9 | – | – | – | – | – | – | – | – | – | – | – | – | 3 | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – |
Haplotypes novel to the present study.
Novel haplotypes that bear two SNPs that define phenotypes with conflicting acetylator status.
2N: Number of chromosomes.
General diversity statistics and tests of selective neutrality were calculated using the program DNAsp version 4.20.2 [36,37] at the continental and population group levels for all loci (Table 3 & Supplementary Tables 2–4). Populations with less than ten chromosomes were included in tests of selective neutrality only with pooled geographic groupings, and were not included in individual analyses (refer to Table 1 & Supplementary Tables 2–4). The published sequence for chimpanzee, Pan troglodytes (Ptr8-WGA990) was used for all interspecific analyses. Significance was assessed for all neutrality estimates using the coalescent simulator within DNAsp (10,000 replicates), assuming no recombination. We used the Bonferroni correction for multiple tests in order to obtain an experiment-wise error rate of α, where each individual test obtains a corrected critical probability of α´=α/k, where k is equal to the number of tests carried out for the entire dataset [38]. A sliding window approach for inferring Tajima’s D (TD) statistic was also implemented to assess differing values of the statistic across the gene regions (Supplementary Figure 2). This method makes it possible to visualize variation in the statistic across the region at defined window lengths (by 100 sites at steps of 25 sites), where each window describes a specific topology of the genealogy for that region.
Table 3.
Summary statistics of polymorphism data by population group.
| 2N | Segregating sites (n) |
Synonymous substitutions |
Nonsynonymous substitutions |
Haplotypes (n) |
Haplotype diversity |
Singletons (n) |
Nucleotide diversity per bp (×10−3) |
Watterson’s theta θω(×10−3)† |
Tajima’s D‡ |
Fay and Wu’s H§ |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| NAT1 | |||||||||||
| Africa | 388 | 43 | 6 | 2 | 72 | 0.868 | 4 | 1.220 | 0.002 | −1.417* | −15.340** |
| East Africa | 186 | 36 | 5 | 2 | 47 | 0.881 | 11 | 1.200 | 0.002 | −1.441* | −13.765** |
| West Africa | 158 | 31 | 2 | 2 | 38 | 0.857 | 3 | 1.210 | 0.002 | −1.079 | −15.071** |
| Pygmy groups | 62 | 21 | 3 | 0 | 24 | 0.893 | 7 | 1.280 | 0.002 | −0.562 | −3.101 |
| Europe | 118 | 26 | 3 | 1 | 21 | 0.612 | 9 | 0.950 | 0.002 | −1.300 | −11.113** |
| Asia | 102 | 23 | 2 | 2 | 11 | 0.65 | 15 | 0.780 | 0.001 | −1.374 | −15.057*** |
| Americas | 46 | 7 | 1 | 0 | 7 | 0.675 | 1 | 0.760 | 0.001 | 0.968 | −0.955 |
| NAT2 | |||||||||||
| Africa | 388 | 45 | 3 | 15 | 68 | 0.914 | 9 | 1.860 | 2.320 | −0.556 | 0.822 |
| East Africa | 182 | 38 | 2 | 12 | 40 | 0.875 | 9 | 1.940 | 2.230 | −0.382 | 0.714 |
| West Africa | 106 | 27 | 3 | 9 | 27 | 0.875 | 11 | 1.710 | 1.700 | 0.023 | 0.013 |
| Pygmy groups | 62 | 23 | 2 | 8 | 24 | 0.952 | 4 | 1.620 | 1.620 | 0.014 | 0.167 |
| Europe | 92 | 21 | 2 | 5 | 26 | 0.887 | 3 | 1.730 | 1.360 | 0.797 | 0.323 |
| Asia | 62 | 11 | 2 | 3 | 15 | 0.798 | 0 | 0.930 | 0.770 | 0.586 | 0.876 |
| Americas | 28 | 10 | 2 | 3 | 9 | 0.799 | 0 | 1.04 | 0.85 | 0.717 | −0.529 |
| NATP1 | |||||||||||
| Africa | 394 | 50 | NA | NA | 131 | 0.960 | 6 | 1.800 | 2.640 | −0.896 | −8.200 |
| East Africa | 186 | 44 | NA | NA | 59 | 0.930 | 7 | 1.700 | 2.690 | −1.095 | −10.306* |
| West Africa | 132 | 40 | NA | NA | 59 | 0.964 | 6 | 1.790 | 2.550 | −0.907 | −5.479 |
| Pygmy groups | 32 | 27 | NA | NA | 27 | 0.977 | 5 | 2.810 | 2.530 | 0.403 | −0.702 |
| Europe | 104 | 17 | NA | NA | 23 | 0.915 | 7 | 0.860 | 1.110 | −0.614 | −0.879 |
| Asia | 70 | 14 | NA | NA | 15 | 0.824 | 2 | 0.840 | 0.990 | −0.424 | 1.103 |
| Americas | 40 | 4 | NA | NA | 7 | 0.776 | 1 | 0.550 | 0.400 | 0.972 | −1.274 |
Pairwise population genetic distance (FST) values between populations using phased haplotypes for each NAT locus were generated using Arlequin version 2.0 [39]. Pairwise FST data matrices were used to generate 2D multidimensional scaling (MDS) plots for the NAT1, NAT2 and NATP1 haplotype data (Figure 1), using Statistica version 8 (StatSoft, Inc., 1984–2008). Analyses of molecular variance (AMOVA) calculations were performed using Arlequin version 2.0 [39] to determine the level of within and between population variation.
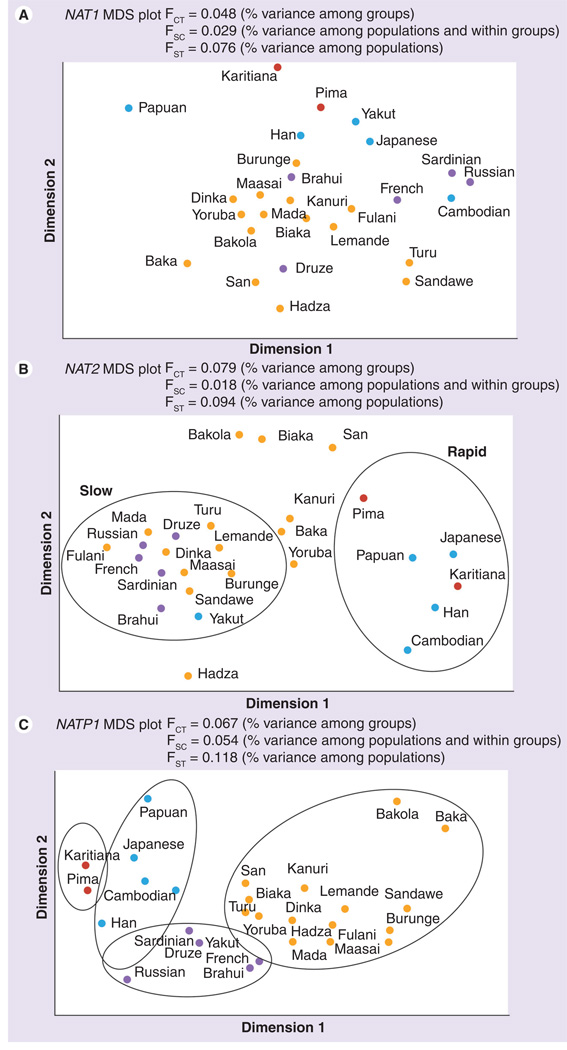
Figure 1. Multidimensional scaling plots of population pairwise FST values for the (A) NAT1, (B) NAT2 and (C) NATP1 gene regions.
Analysis of molecular variance results are indicated in the inset, where variance among groups = FCT, variance among populations within groups = FSC, and variance among populations = FST. Yellow = Africa; Purple = Europe; Blue = Asia; Red = Americas. Population structure is specified according to the population groupings listed in Table 1.
MDS: Multidimensional scaling.
Network version 4.5 was used to construct median-joining (MJ) phylogenetic networks [40] for the NAT2 region (Figure 2). Phylogenetic networks are preferable to simple, bifurcating trees for intraspecies comparison in that differing evolutionary pathways are represented in terms of cycles or hypercubes.
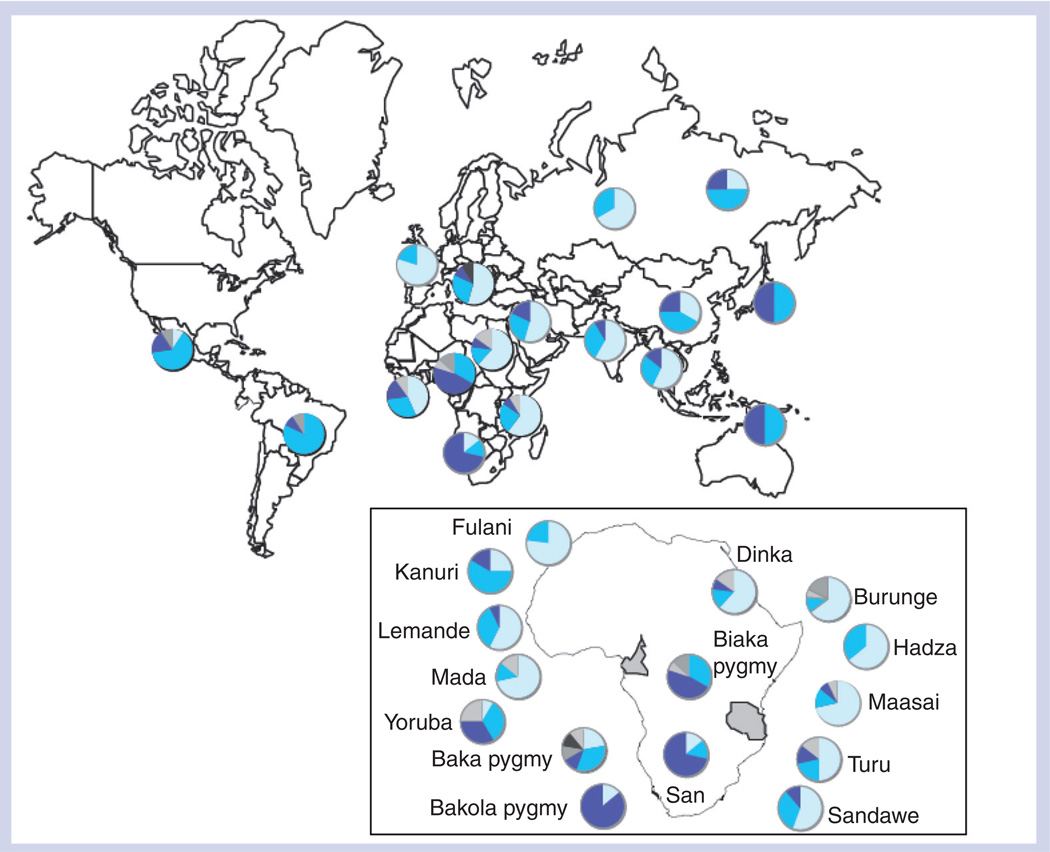
Figure 2. NAT2-inferred acetylator phenotype distribution for all populations in the current study.
Phenotype inference was made based on SNPs known to affect acetylator phenotype [103]. Each individual is represented according to their inferred diploid haplotype, where light blue = slow/slow; turquoise = slow/rapid; dark blue = rapid/rapid; light gray = unknown/slow; dark gray = unknown/rapid; black = unknown/unknown. Inset shows NAT2-inferred acetylator phenotype distribution within Africa for each population group. Populations shown flanking Africa are those sampled from Cameroon and Tanzania (highlighted in gray).
Refer to Table 1 for population counts included in the present study.
Pairwise LD between SNPs across the NAT1 and NAT2 regions was inferred using Haploview version 4.1 (Supplementary Figure 3) [41]. Haploview uses a standard expectation-maximization algorithm to estimate the maximum-likelihood values of gamete frequencies for each SNP, from which pairwise estimates of D´ are calculated. SNPs with minor allele frequencies less that 1% were excluded for analyses of each continental group. LD between NAT loci was analyzed using the Haploview 4.1 map builder and HapMap populations. The LD map of the entire NAT region using HapMap Utah residents with northern and western European ancestry is illustrated in Supplementary Figure 4.
Results
Resequencing & SNP identification
We have characterized nucleotide variation in a total of 326, 301 and 304 globally diverse individuals for NAT1, NAT2, and NATP1, respectively (Table 1). Because Africa is under-represented in prior studies, we have included individuals from 15 geographically and ethnically diverse African populations practicing different subsistence modes (e.g., hunting and gathering, agro-pastoralism, agriculture and pastoralism) and representing all four major linguistic families of Africa.
We resequenced 2723 bp of the NAT1 region, encompassing the 870-bp intronless coding region (exon 9) and 1853 bp of flanking sequence (1048 bp 5´ and 1040 bp 3´) (Supplementary Figure 1) and identified 48 SNPs, 17 of which have not been previously reported (Supplementary Tables 5 & 6). A total of eight SNPs were identified in the 870 bp exon region, two of which were nonsynonymous polymorphisms (+445G>A, +639G>T) that had been previously reported (Supplementary Tables 5 & 6). A SNP located in the 3´ region of NAT1 following a (TAA)n repeat at position +1088, a site thought to play a role in polyadenylation of the NAT1 mRNA, was found to be highly variable, with frequencies of the +1088A variant ranging from 13–83% (Supplementary Figure 5). This SNP was in strong LD with two additional SNPs also at high frequency at positions +1095 and +1191.
We resequenced 2808 bp of the NAT2 region, encompassing the 870-bp intronless coding region (exon 2) and 1938 bp of the flanking regions (1014 bp 5´ and 924 bp 3´) (Supplementary Figure 1) and identified 46 SNPs (including one triallelic SNP at +1362 [Supplementary Table 7]), 16 of which have not been previously reported (Supplementary Table 6). A total of 18 SNPs were identified in the 870 bp coding region of NAT2, three of which have not been previously reported and fifteen nonsynonymous substitutions (Supplementary Tables 6 & 7).
In order to distinguish effects of natural selection and demography on patterns of genetic variation at NAT1 and NAT2 loci, we analyzed nucleotide variation at the NATP1 pseudogene. We resequenced 2834 bp of the NATP1 region, including the 870-bp region homologous to the NAT1/NAT2 coding exons, 1039 bp 5´ and 925 bp 3´ of this region. This region overlaps in part with that analyzed by Patin et al. [42]. We identified 55 SNPs, 30 of which have not been previously reported in dbSNP [102] or by the NAT nomenclature committee (Supplementary Table 7) [101]. We confirmed the presence of eight mutations that result in a nonfunctional protein product as described by Blum [13].
Haplotype variation & population differentiation
A total of 88 distinct haplotypes were observed for the 2723 bp NAT1 region (Supplementary Table 5), indicating that recombination has affected the pattern of diversity at this locus. The pattern of LD for the entire NAT region is presented in Supplementary Figure 4. The LD results for NAT1 (Supplementary Figure 3) confirm lower levels of LD in Africans compared with non-African groups at this locus, a pattern observed for other nuclear loci, [43–45] and consistent with the longer evolutionary history of African groups. Pairwise estimates of D´ indicate statistically significant LD between three 3´ SNPs at positions +1088, +1095 and +1191, which form a single haplotype block in all population groups, with the exception of Europeans.
A total of 100 NAT2 haplotypes were inferred from the 2808 bp region, indicating that recombination has affected the pattern of diversity at the NAT2 locus (Supplementary Table 6 & Supplementary Figure 3). Higher than expected LD exists at only a few sites within the NAT2 region analyzed, some of which are between SNPs known to affect acetylator phenotype (e.g., +290, and +590). LD analysis indicates differing haplotype block structure across the NAT2 region in different populations (Supplementary Figure 3).
Multidimensional scaling plots estimated from pairwise FST values were used to determine how populations cluster based on genetic differentiation (Figure 1). At NATP1, populations clustered based on geographic location as expected for a neutral locus (Figure 1). By contrast, distinct clustering by geography is not observed for the NAT1 and NAT2 loci (Figure 1). The MDS plot for the NAT2 locus reflects clustering that corresponds to rapid and slow acetylator types, where the Bakola, Biaka, San and Hadza groups appear as outliers, likely due to high levels of genetic drift in these small hunter-gatherer populations (Figure 1). Groups clustering in the center of the plot (Kanuri, Baka and Yoruba), as well as the Bakola, Biaka and San hunter–gatherers, have a high proportion of inferred intermediate acetylator phenotypes. AMOVA results, indicating hierarchical levels of variation within and between groups, are given in Figure 1. The level of between relative to within population variation at NATP1 is ~12%, similar to what is observed at other neutral loci in humans [46]. By contrast, the level of between relative to within population variation for NAT2 is 9.4% and for NAT1 is 7.6%.
Nucleotide diversity & tests of selective neutrality
Summary statistics of nucleotide diversity and statistical tests of neutrality based on allele frequency distributions at the NAT1 and NAT2 loci for populations pooled by major geographic region are shown in Table 3, and for individual populations in Supplementary Tables 2–4. Overall, African populations have higher levels of genetic diversity at the NAT loci than non-African populations. TD estimates of neutrality for NAT1, based on the allele frequency distribution, are negative in most cases, signifying an excess of rare variation, although none of these values were significantly different from expectation under a neutral model. However, Fay and Wu’s H statistic [47] is highly negative and significant for NAT1, following Bonferroni correction, for most population groups (Table 3 & Supplementary Table 2). Using a sliding window analysis of TD at NAT1 with 100-bp window lengths, we observed negative, but nonsignificant, values across most regions of the NAT1 gene in all populations and continental groups (Supplementary Figure 2). Notably, we observed a positive peak in TD values in most populations in the 3´-UTR region of the gene, corresponding to the location of SNPs at positions +1088, +1095 and +1191, which was statistically significant in the pooled West African, Asian and Native American populations and in several individual populations (Supplementary Figure 2). At NAT2, we observed negative, but nonsignificant, values of TD in Africans, both pooled and in individual populations, and positive, but nonsignificant, values in non-Africans for pooled and individual populations (Table 3 & Supplementary Table 3).
The Ka:Ks ratio of nonsynonymous to synonymous substitutions was observed to be less than one at NAT1 (Ka:Ks = 0.242), consistent with the effects of purifying selection acting at this locus. The Ka:Ks ratio for NAT2 was observed to be higher than that observed for NAT1, but still less than one (Ka:Ks = 0.813). In addition, we tested for adaptive evolution at NAT1 and NAT2 coding regions using a McDonald–Kreitman test [48], which compares the ratio of polymorphic (among humans) and fixed (between humans and chimpanzee) nonsynonymous and synonymous changes [48]. The McDonald–Kreitman tests were not significant for either the NAT1 (Fisher’s exact test p = 0.659) or NAT2 (Fisher’s exact test p = 0.579) loci. The McDonald–Kreitman test results for NAT2 indicate that high numbers of replacement changes are tolerated at this locus.
We also tested for significant differences in levels of genetic diversity among the NAT1, NAT2, and NATP1 loci using Hudson–Kreitman–Aguade tests [49] to compare ratios of intra- and inter-specific variation between each pair of loci. The Hudson–Kreitman–Aguade tests comparing variation at NAT1 to both NATP1 and NAT2 were not significant (p = 0.248 and 0.251, respectively). By contrast, an Hudson–Kreitman–Aguade test comparing levels of intra- and inter-specific variation at NAT2 relative to NATP1 was significant (p = 0.012).
NAT2 acetylator phenotype inference
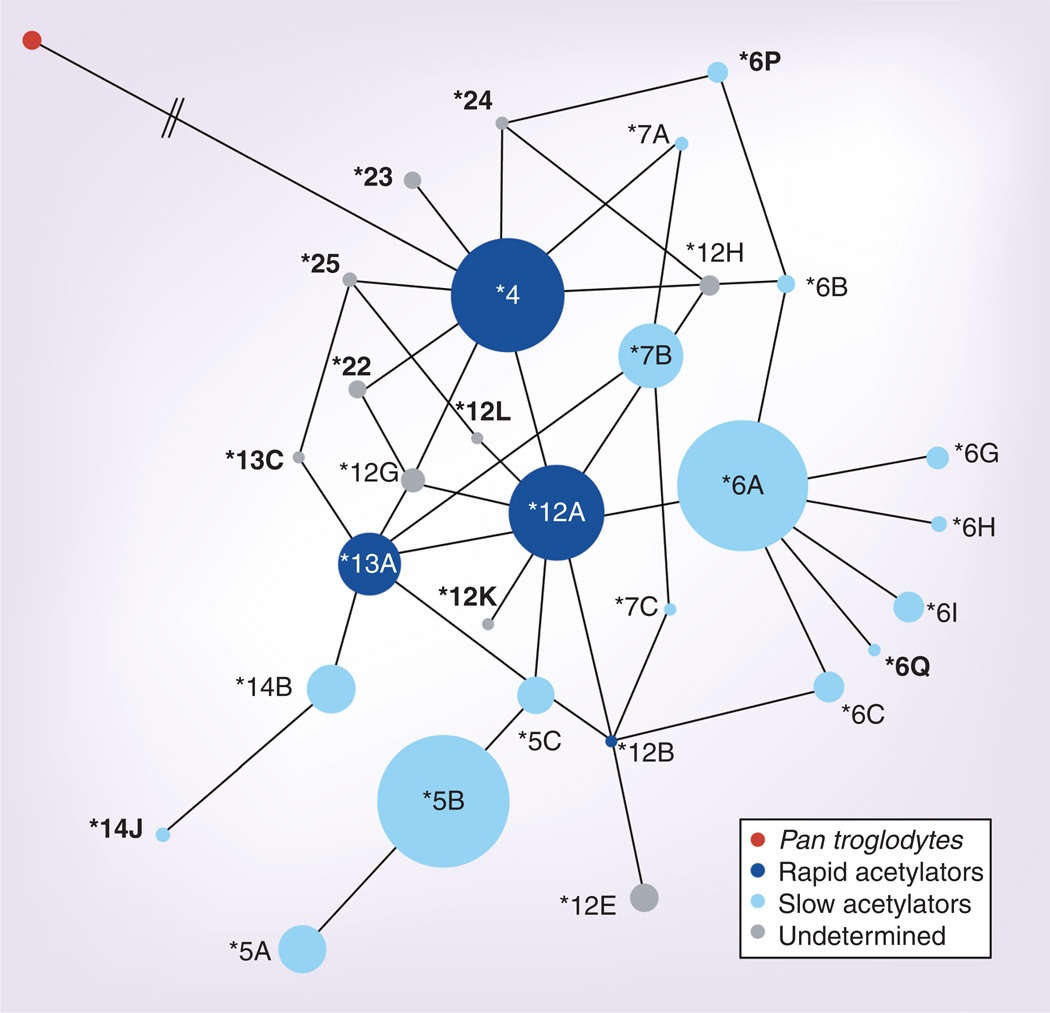
NAT2 acetylator status was inferred for each individual based on phased, diploid haplotypes, and considering only the coding region SNPs with known affect on acetylator phenotype [26]. However, in some cases individuals had novel variants with unknown phenotype (e.g., NAT2*22 and NAT2*23) (SI4). A median-joining network for NAT2 haplotypes is illustrated in Figure 2. The chimpanzee out-group is observed to branch from the human NAT2*4 rapid node, indicating that the rapid acetylator haplotype is ancestral in humans. Inferred frequencies of rapid, intermediate and slow acetylators for global populations are shown in Table 2 & Figure 3. No acetylator phenotype is fixed in any human population. However, rapid acetylators are more prevalent in Asia and the Americas, in concordance with previous findings [50,51]. Interestingly, in Africa rapid acetylators are found at highest frequencies in foraging populations known to have some of the oldest evolutionary lineages according to mtDNA and Y chomosome evidence [52–54], the San of South Africa and the Pygmies from Cameroon (Biaka, Baka and Bakola), an observation consistent with prior studies [42,55–57]. Populations that have a complete absence of rapid acetylator types are the French and Russian, and in Africa the Hadza foragers of Tanzania, and the Mada and Fulani of Cameroon.
Figure 3. Median-joining network for NAT2-inferred acetylator phenotypes.
For all nodes, dark blue = rapid acetylators, light blue = slow acetylators, gray = haplotypes where acetylator phenotype could not be inferred, and red indicates the chimpanzee (Pan troglodytes) outgroup. All nomenclature follows that recommended by the NAT nomenclature committee (e.g., NAT2*Major haplotype/sub-haplotype). Bold sub-haplotypes represent haplotypes that are novel to the current dataset.
Discussion
Here, we present a comprehensive study of global human variation across the NAT1, NAT2, and NATP1 loci, encompassing the 870 bp coding regions, as well as 5´- and 3´-UTRs likely to harbor regulatory elements that may influence the variable, tissue specific expression observed for NAT isozymes. We include a large number of diverse African populations that have been under-represented in prior studies in order to identify novel functional variants and gain a better understanding of the evolutionary history of the NAT loci within Africa. Because African populations are known to show greater genetic diversity when compared with non-African populations [22], we chose direct sequencing, over SNP genotyping of common variants with known effects on acetylator phenotype, in order to provide an unbiased description of NAT sequence variation.
Multiple patterns of selection at the NAT1 locus
Because of the role of the NAT1/NAT2 loci in the metabolism of xenobiotics present in dietary and other environmental sources, and their probable role in epigenetic regulation [58], the NAT loci are potential targets for natural selection. Our study confirms, in concordance with previous studies [42], that NAT1 has only two observed nonsynonymous mutations that reach near fixation levels in most population groups. This is evident in the lack of clustering observed in the MDS plots plots generated from the pairwise population genetic distances (Figure 1). Also, consistent with this observation, tests of neutrality at NAT1 indicate that purifying selection has prohibited nonsynonymous variation from accumulating within the coding region of the gene. In addition, analysis of the allele frequency distribution indicates an excess of rare variation (Table 3 & Supplementary Table 2), as expected under a model of purifying/background selection, consistent with observations from prior study of smaller population sets [42].
In contrast to the pattern observed in the coding region of NAT1, the sliding window analysis of TD indicates highly positive and significant values of TD statistic at three NAT1 SNPs located in the 3´-UTR at positions +1088, +1095 and +1191 in nearly all populations and geographic regions (Supplementary Figure 2). In addition, these sites are in nearly complete LD and form two common haplotypes, A-T-T and T-C-A, which are maintained at high and approximately equal frequencies in nearly every population (Supplementary Figures 3 & 5). Position +1088 is known to be a polyadenylation site [59,60]. However, the functional effects of SNPs in the 3´UTR region of NAT1 (e.g., +1088,+1095 and +1191) are poorly understood [6,61,62]. The two most common NAT1 haplotypes, NAT1*4 (reference sequence) and NAT1*10, differ by two of these three mutations (1088A and 1095A). Whether NAT1*4 (reference sequence) and NAT1*10 confer the same or different acetylator activity has not been confirmed [59,63,64]. These results suggest the possibility that balancing selection may be maintaining haplotype variation at these NAT1 3´-UTR sites, and suggests that they may play some functional role, possibly related to mRNA stability.
NAT2 acetylator frequency variation
Knowledge of patterns of genetic variation at NAT2, which plays a role in metabolism of drugs used to treat several common diseases such as tuberculosis and hypertension, in ethnically diverse populations will be important for developing more efficient treatment approaches for use in ethnically diverse populations. Indeed, we show that the frequency of the NAT2 rapid and slow acetylator genotypes differ across ethnically diverse African populations, even among those from similar geographic regions.
The NAT2 acetylator phenotype is one of the best characterized variable xenobiotic-metabolizing enzymes traits in humans. The acetylator alleles are thought to act in a codominant manner, with the rapid/slow heterozygotes showing intermediate activity. However, depending on the particular substrate administered, the intermediate (rapid/slow) acetylator phenotype may show substantial variation in activity. In addition, modification of the NAT2 substrates may also be influenced by other loci (e.g., CYP1A2, CYP2A6, CYP2A13 and GSTM1) [65].
Based on results of prior studies, we were able to infer acetylator phenotypes for most of the NAT2 haplotypes identified in the current study. NAT2 haplotypes with undetermined phenotype effect were present at low frequencies in both African (8% frequency) and non-African populations (3% frequency), preventing inference of acetylator phenotypes in some cases (Table 2). The most common rapid acetylator haplotypes in our dataset were *4 (reference sequence), *12A and *12B and *13A (Figure 3 & Table 2). The most common slow acetylator haplotypes were observed at appreciable and approximately equal frequencies, in Africans and European groups: NAT2*5B and NAT2*6A. Overall, African populations exhibit a greater diversity of acetylator haplotypes, both rapid and slow, in comparison with non-Africans (Figure 3 & Table 2).
The slow acetylator haplotype NAT2 *14 (+191G>A), which is thought to give rise to an ‘extreme’ slow NAT2 phenotype [66,67], was originally described as ‘African-specific’ because of its high frequency in African–Americans ranging from 48–55%, compared with 10% in populations of European descent [67,68]. We also observe haplotype NAT2*14 to be African-specific, with a high frequency of NAT2*14 in several West African groups (Kanuri [0.125], Lemande [0.1429], Yoruba [0.0833]) and a low frequency in East Africa (Table 2). This observation is consistent with other studies which show a west to east gradient of decreasing frequency of NAT2*14 across subsaharan Africa, consistent with a possible West African origin [68,69].
Population-specific selective pressure at the NAT2 locus
Levels of nucleotide and haplotype diversity are relatively similar between African and European populations at the NAT2 locus (Table 3 & Supplementary Table 3). Asian and Amerindian populations have a slight decrease in diversity at both the continental and population levels (Supplementary Table 3). Tests of neutrality based on the allele frequency distribution at NAT2 show distinct patterns for African and non-African populations, where consistently negative and positive values for estimators of neutrality are observed for Africans and non-Africans, respectively. The high frequency of NAT2*5B and *6A slow acetylator haplotypes in African and European populations, but not in Asian populations (Table 2), may be indicative of the effects of natural selection acting to maintain slow acetylator phenotypes at high frequency in those geographic regions. Given our observation that two main slow acetylator haplotypes are maintained at high frequency in most populations, and that fixation of either rapid or slow acetylator haplotypes has occurred in several ethnically diverse populations, it is possible that long-term balancing selection may be overlaid by the action of positive selection acting on specific acetylator variants in specific populations (Table 2 & Supplementary Figure 6). These patterns, in general, support observations of previous studies [56,57,70,71] which suggest that multiple modes of selection are operating at NAT2 on a population-specific basis.
Future studies to interpret the effects of natural selection on the pattern of variation at the NAT1 locus should focus on understanding the potential functional consequences of the three common 3´ variable sites at positions +1088, +1095 and +1191, particularly in regard to mRNA stability and tissue specific expression. In addition, the elucidation of functional effect on NAT2 enzyme activity of the novel NAT2 haplotypes described in the current study will be informative for understanding differential response to pharmaceutical drugs and other substrates metabolized by NAT2 in ethnically diverse African populations.
Supplementary Material
Executive summary.
-
▪
Knowledge of the pattern of genetic diversity and haplotype structure at the NAT loci in ethnically diverse population groups has important implications for identifying variants that play a role in xenobiotic response and for understanding the role of these genes in adaptation to diverse environments and diets during human evolution.
-
▪
In the present NAT analysis, we survey nucleotide sequence variation in both coding and flanking regions (~2800 bp) of the NAT1 and NAT2 genes, as well as the NAT pseudogene NATP1, in ethnically diverse global populations that include many previously under-represented African populations.
Materials & methods
-
▪
To examine nucleotide variability, direct sequencing of the NAT gene regions was performed using a global panel of 326 individuals, with an emphasis on under-represented African populations.
-
▪
Diploid haplotypes were inferred across the NAT1, NAT2 and NATP1 regions using the program phase version 2.1.1, which reconstructs haplotypes from population genotype data using a coalescence model-based algorithm.
-
▪
General diversity statistics and tests of selective neutrality were calculated. Pairwise population genetic distance values between populations using phased haplotypes for each NAT locus were generated. Median-joining phylogenetic networks for the NAT2 region were constructed. Pairwise linkage disequilibrium (LD) between SNPs within the NAT1 and NAT2 regions was inferred.
Results
-
▪
We have characterized nucleotide variation in a total of 326, 301 and 304 globally diverse individuals for NAT1, NAT2 and NATP1, respectively.
-
▪
We have included individuals from 15 geographically and ethnically diverse African populations practicing different subsistence modes (e.g., hunting and gathering, agro-pastoralism, agriculture and pastoralism) and representing all four major linguistic families of Africa.
-
▪
We resequenced 2723 bp of the NAT1 region, and identified 48 SNPs, 17 of which have not been previously reported. We resequenced 2808 bp of the NAT2 region and identified 46 SNPs (including one triallelic SNP at +1362), eight of which have not been previously reported.
-
▪
A SNP located in the 3’ region of NAT1 following a (TAA)n repeat at position +1088, a site thought to play a role in polyadenylation of the NAT1 mRNA, was found to be highly variable, with frequencies of the +1088A variant ranging from 13–83%.
-
▪
A total of 88 distinct haplotypes were observed for the 2723 bp NAT1 region, indicating that recombination has affected the pattern of diversity at this locus. Pairwise estimates of D’ indicate statistically significant LD between three 3’ SNPs at positions +1088, +1095 and +1191, which form a single haplotype block in all population groups, with the exception of Europeans.
-
▪
A total of 100 distinct NAT2 haplotypes were inferred from the 2808 bp region, indicating that recombination has affected the pattern of diversity at the NAT2 locus. Higher than expected LD exists at only a few sites within the NAT2 region analyzed, some of which are between SNPs known to affect acetylator phenotype (e.g., +290, and +590). LD analysis indicates differing haplotype block structure across the NAT2 region in different populations.
-
▪
We inferred frequencies of NAT2 rapid, intermediate and slow acetylators for this global population dataset, and report ten novel NAT2 haplotypes.
-
▪
Using a sliding window analysis of Tajima’s D at NAT1 we observed a positive peak in Tajima’s D values in most populations in the 3’-UTR region of the gene, corresponding to the location of SNPs at positions +1088, +1095 and +1191, which was statistically significant in the pooled West African, Asian and Native American populations and in several individual populations. This result indicates that SNPs at these sites are being maintained at high frequency.
-
▪
In Africa, the inferred acetylator phenotypes are found at highest frequencies in foraging populations, the San of South Africa and the Pygmies from Cameroon (Biaka, Baka and Bakola).
Conclusion
-
▪
Our NAT1 results suggest the possibility that balancing selection may be maintaining haplotype variation at three NAT1 3’-UTR positions (+1088, +1095 and +1191), and suggests that these SNP variants may play some functional role, possibly related to mRNA stability.
-
▪
These NAT1 3’ sites are in nearly complete LD and form two common haplotypes, A-T-T and T-C-A, which are maintained at high and nearly equal frequencies in most populations.
-
▪
We observe high levels of nonsynonymous functional variation at the NAT2 locus that differs amongst ethnically diverse populations.
-
▪
We show that the frequency of the NAT2 rapid and slow acetylator genotypes differ across ethnically diverse African populations, even among those from similar geographic regions.
-
▪
The most common rapid acetylator haplotypes in our dataset were *4, *12A and *12B and *13A. The most common slow acetylator haplotypes were observed at appreciable and approximately equal frequencies in Africans and European groups: NAT2*5B and NAT2*6A.
-
▪
Given our observation that two main NAT2 slow acetylator haplotypes are maintained at high frequency in most populations, and that fixation of either rapid or slow acetylator haplotypes has occurred in several ethnically diverse populations, it is possible that long-term balancing selection may be overlaid by the action of positive selection acting on specific acetylator variants in specific populations.
Acknowledgements
The authors would first like to thank the Africans who contributed their samples to this study. Special thanks to Floyd Reed for his assistance in writing scripts and creating the program analysis pipeline used in the present study, Alessia Ranciaro for her contribution to the whole-genome re-amplification of samples, as well as Wen-Ya Ko and Michael Campbell for helpful discussion.
This work was supported by the US National Science Foundation (NSF) IGERT grant 9987590 to Holly M Mortensen and Sarah A Tishkoff, NSF grants BCS 0196183, and BCS-0827436, NIH grants R01GM076637 and DP1-OD-006445-01 to Sarah A Tishkoff.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Hein DW. Molecular genetics and function of NAT1 and NAT2: role in aromatic amine metabolism and carcinogenesis. Mutat. Res. 2002;506:65–77. doi: 10.1016/s0027-5107(02)00153-7. [DOI] [PubMed] [Google Scholar]
- 2.Grant DM. Molecular genetics of the N-acetyltransferases. Pharmacogenetics. 1993;3(1):45–50. doi: 10.1097/00008571-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Kufe DM, Pollock RM, Weichselbaum RM, et al. Holland-Frei Cancer Medicine 6 (5th Edition) BC Decker, PA, USA: 2003. [Google Scholar]
- 4.Schut HAJ, Snyderwine EG. DNA adducts of heterocyclic amine food mutagens: implications for mutagenesis and carcinogenesis. Carcinogenesis. 1999;20(3):353–368. doi: 10.1093/carcin/20.3.353. [DOI] [PubMed] [Google Scholar]
- 5.Felton JS, Malfatti MA, Knize MG, et al. Health risks of heterocyclic amines. Mutat. Res. 1997;376(1–2):37–41. doi: 10.1016/s0027-5107(97)00023-7. [DOI] [PubMed] [Google Scholar]
- 6.Boukouvala S, Fakis G. Arylamine N-acetyltransferases: what we learn from genes and genomes. Drug Metab. Rev. 2005;37(3):511–564. doi: 10.1080/03602530500251204. [DOI] [PubMed] [Google Scholar]
- 7.Pacifici GM, Bencini C, Rane A. Acetyltransferase in humans: development and tissue distribution. Pharmacology. 1986;32(5):283–291. doi: 10.1159/000138181. [DOI] [PubMed] [Google Scholar]
- 8.Kilbane AJ, Petroff T, Weber WW. Kinetics of acetyl CoA: arylamine N-acetyltransferase from rapid and slow acetylator human liver. Drug Metab. Dispos. 1991;19(2):503–507. [PubMed] [Google Scholar]
- 9.Husain A, Zhang X, Doll MA, States JC, Barker DF, Hein DW. Identification of N-acetyltransferase 2 (NAT2) transcription start sites and quantitation of NAT2-specific mRNA in human tissues. Drug Metab. Dispos. 2007;35(5):721–727. doi: 10.1124/dmd.106.014621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hickman D, Risch A, Buckle V, et al. Chromosomal localization of human genes for arylamine N-acetyltransferase. Biochem. J. 1994;297(Pt 3):441–445. doi: 10.1042/bj2970441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matas N, Thygesen P, Stacey M, Risch A, Sim E. Mapping AAC1, AAC2 and AACP, the genes for arylamine N-acetyltransferases, carcinogen metabolising enzymes on human chromosome 8p22, a region frequently deleted in tumours. Cytogenet. Cell Genet. 1997;77(3–4):290–295. doi: 10.1159/000134601. [DOI] [PubMed] [Google Scholar]
- 12.Sayers EW, Barrett T, Benson DA, et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2011;39(Database issue):D38–D51. doi: 10.1093/nar/gkq1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blum M, Grant DM, McBride W, Heim M, Meyer UA. Human arylamine N-acetyltransferase genes: isolation, chromosomal localization, and functional expression. DNA Cell Biol. 1990;9(3):193–203. doi: 10.1089/dna.1990.9.193. [DOI] [PubMed] [Google Scholar]
- 14.Barker DF, Husain A, Neale JR, et al. Functional properties of an alternative, tissue-specific promoter for human arylamine N-acetyltransferase 1. Pharmacogenet. Genomics. 2006;16(7):515–525. doi: 10.1097/01.fpc.0000215066.29342.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butcher NJ, Arulpragasam A, Goh HL, Davey T, Minchin RF. Genomic organization of human arylamine N-acetyltransferase Type I reveals alternative promoters that generate different 5´-UTR splice variants with altered translational activities. Biochem. J. 2005;387(Pt 1):119–127. doi: 10.1042/BJ20040903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Husain A, Barker DF, States JC, Doll MA, Hein DW. Identification of the major promoter and non-coding exons of the human arylamine N-acetyltransferase 1 gene (NAT1) Pharmacogenetics. 2004;14(7):397–406. doi: 10.1097/01.fpc.0000114755.08559.6e. [DOI] [PubMed] [Google Scholar]
- 17.Boukouvala S, Sim E. Structural analysis of the genes for human arylamine N-acetyltransferases and characterisation of alternative transcripts. Basic Clin. Pharmacol. Toxicol. 2005;96(5):343–351. doi: 10.1111/j.1742-7843.2005.pto_02.x. [DOI] [PubMed] [Google Scholar]
- 18.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cann HM, de Toma C, Cazes L, et al. A human genome diversity cell line panel. Science. 2002;296(5566):261–262. doi: 10.1126/science.296.5566.261b. [DOI] [PubMed] [Google Scholar]
- 20.Cavalli-Sforza LL. The Human Genome Diversity Project: past, present and future. Nat. Rev. Genet. 2005;6(4):333–340. doi: 10.1038/nrg1596. [DOI] [PubMed] [Google Scholar]
- 21.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tishkoff SA, Reed FA, Friedlaender FR, et al. The genetic structure and history of Africans and African Americans. Science. 2009;324(5930):1035–1044. doi: 10.1126/science.1172257. ▪ Uses a very large multilocus dataset to understand the current population substructure and previous migration patterns of African and African–American populations.
- 23.Rosenberg NA, Mahajan S, Ramachandran S, Zhao C, Pritchard JK, Feldman MW. Clines, clusters, and the effect of study design on the inference of human population structure. PLoS Genet. 2005;1(6):e70. doi: 10.1371/journal.pgen.0010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conrad DF, Jakobsson M, Coop G, et al. A worldwide survey of haplotype variation and linkage disequilibrium in the human genome. Nat. Genet. 2006;38(11):1251–1260. doi: 10.1038/ng1911. [DOI] [PubMed] [Google Scholar]
- 25.Vatsis KP, Weber WW, Bell DA, et al. Nomenclature for N-acetyltransferases. Pharmacogenetics. 1995;5(1):1–17. doi: 10.1097/00008571-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Hein DW, Grant DM, Sim E. Update on consensus arylamine N-acetyltransferase gene nomenclature. Pharmacogenetics. 2000;10(4):291–292. doi: 10.1097/00008571-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Hein DW, Boukouvala S, Grant DM, Minchin RF, Sim E. Changes in consensus arylamine N-acetyltransferase gene nomenclature. Pharmacogenet. Genomics. 2008;18(4):367–368. doi: 10.1097/FPC.0b013e3282f60db0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ewing B, Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998;8(3):186–194. [PubMed] [Google Scholar]
- 29.Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998;8(3):175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- 30.Gordon D, Abajian C, Green P. Consed: a graphical tool for sequence finishing. Genome Res. 1998;8(3):195–202. doi: 10.1101/gr.8.3.195. [DOI] [PubMed] [Google Scholar]
- 31.Nickerson DA, Tobe VO, Taylor SL. PolyPhred: automating the detection and genotyping of single nucleotide substitutions using fluorescence-based resequencing. Nucleic Acids Res. 1997;25(14):2745–2751. doi: 10.1093/nar/25.14.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am. J. Hum. Genet. 2001;68(4):978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephens M, Scheet P. Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. Am. J. Hum. Genet. 2005;76(3):449–462. doi: 10.1086/428594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheet P, Stephens M. A fast and flexible statistical model for large-scale population genotype data: applications to inferring missing genotypes and haplotypic phase. Am. J. Hum. Genet. 2006;78(4):629–644. doi: 10.1086/502802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am. J. Hum. Genet. 2003;73(5):1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozas J, Rozas R. DnaSP, DNA sequence polymorphism: an interactive program for estimating population genetics parameters from DNA sequence data. Comput. Appl. Biosci. 1995;11(6):621–625. doi: 10.1093/bioinformatics/11.6.621. [DOI] [PubMed] [Google Scholar]
- 37.Rozas J, Sanchez-DelBarrio JC, Messeguer X, Rozas R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics. 2003;19(18):2496–2497. doi: 10.1093/bioinformatics/btg359. [DOI] [PubMed] [Google Scholar]
- 38.Sokal RR, Rohlf FJ. Biometry (3rd Edition) NY, USA: WH Freeman and Co.; 1995. The principles and practice of statistics in biological research; p. 887. [Google Scholar]
- 39.Excoffier LGL, Laval G, Schneider S. Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol. Bioinform. Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- 40.Bandelt HJ, Forster P, Rohl A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999;16(1):37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- 41.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 42. Patin E, Barreiro LB, Sabeti PC, et al. Deciphering the ancient and complex evolutionary history of human arylamine N-acetyltransferase genes. Am. J. Hum. Genet. 2006;78(3):423–436. doi: 10.1086/500614. ▪ First comprehensive population genetic analysis of the NAT loci in humans, including the pseudogene NATP1.
- 43.Tarazona-Santos E, Tishkoff SA. Divergent patterns of linkage disequilibrium and haplotype structure across global populations at the interleukin-13 (IL13) locus. Genes Immun. 2005;6(1):53–65. doi: 10.1038/sj.gene.6364149. [DOI] [PubMed] [Google Scholar]
- 44.Tishkoff SA, Dietzsch E, Speed W, et al. Global patterns of linkage disequilibrium at the CD4 locus and modern human origins. Science. 1996;271(5254):1380–1387. doi: 10.1126/science.271.5254.1380. [DOI] [PubMed] [Google Scholar]
- 45.Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science. 2002;296(5576):2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 46.Tishkoff SA, Verrelli BC. Patterns of human genetic diversity: implications for human evolutionary history and disease. Annu. Rev. Genomics Hum. Genet. 2003;4:293–340. doi: 10.1146/annurev.genom.4.070802.110226. [DOI] [PubMed] [Google Scholar]
- 47.Fay JC, Wu CI. Hitchhiking under positive Darwinian selection. Genetics. 2000;155(3):1405–1413. doi: 10.1093/genetics/155.3.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDonald JH, Kreitman M. Adaptive protein evolution at the Adh locus in Drosophila. Nature. 1991;351(6328):652–654. doi: 10.1038/351652a0. [DOI] [PubMed] [Google Scholar]
- 49.Hudson RR. Estimating the recombination parameter of a finite population model without selection. Genet. Res. 1987;50(3):245–250. doi: 10.1017/s0016672300023776. [DOI] [PubMed] [Google Scholar]
- 50.Brockton N, Little J, Sharp L, Cotton SC. N-acetyltransferase polymorphisms and colorectal cancer: a HuGE review. Am. J. Epidemiol. 2000;151(9):846–861. doi: 10.1093/oxfordjournals.aje.a010289. [DOI] [PubMed] [Google Scholar]
- 51.Fuselli S, Gilman RH, Chanock SJ, et al. Analysis of nucleotide diversity of NAT2 coding region reveals homogeneity across Native American populations and high intra-population diversity. Pharmacogenomics J. 2007;7(2):144–152. doi: 10.1038/sj.tpj.6500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knight A, Underhill PA, Mortensen HM, et al. African Y chromosome and mtDNA divergence provides insight into the history of click languages. Curr. Biol. 2003;13(6):464–473. doi: 10.1016/s0960-9822(03)00130-1. [DOI] [PubMed] [Google Scholar]
- 53.Gonder MK, Mortensen HM, Reed FA, de Sousa A, Tishkoff SA. Whole-mtDNA genome sequence analysis of ancient African lineages. Mol. Biol. Evol. 2007;24(3):757–768. doi: 10.1093/molbev/msl209. [DOI] [PubMed] [Google Scholar]
- 54.Tishkoff SA, Gonder MK, Henn BM, et al. History of click-speaking populations of Africa inferred from mtDNA and Y chromosome genetic variation. Mol. Biol. Evol. 2007;24(10):2180–2195. doi: 10.1093/molbev/msm155. [DOI] [PubMed] [Google Scholar]
- 55.Patin E, Harmant C, Kidd KK, et al. Sub-Saharan African coding sequence variation and haplotype diversity at the NAT2 gene. Hum. Mutat. 2006;27(7):720. doi: 10.1002/humu.9438. [DOI] [PubMed] [Google Scholar]
- 56.Sabbagh A, Langaney A, Darlu P, Gerard N, Krishnamoorthy R, Poloni ES. Worldwide distribution of NAT2 diversity: implications for NAT2 evolutionary history. BMC Genet. 2008;9:21. doi: 10.1186/1471-2156-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Luca F, Bubba G, Basile M, et al. Multiple advantageous amino acid variants in the NAT2 gene in human populations. PloS One. 2008;3(9):e3136. doi: 10.1371/journal.pone.0003136. ▪ A model is proposed by these authors to account for increase in NAT2 slow acetylator types, which incorporates dietary shift from foraging to a primarily agricultural existence, and relates to the abundance of folate in the diet and rate of folate catabolism.
- 58. Wakefield L, Boukouvala S, Sim E. Characterisation of CpG methylation in the upstream control region of mouse NAT2: evidence for a gene-environment interaction in a polymorphic gene implicated in folate metabolism. Gene. 2010;452(1):16–21. doi: 10.1016/j.gene.2009.12.002. ▪ Suggests epigenetic regulation of the murine NAT2 expression (orthologous to human NAT1), and provides evidence that this modification varies between tissue types, and is altered by NAT2 depletion or supplementation with folate.
- 59.Bell DA, Badawi AF, Lang NP, Ilett KF, Kadlubar FF, Hirvonen A. Polymorphism in the N-acetyltransferase-1 (NAT1) polyadenylation signal–association of Nat1-asterisk-10 allele with higher N-acetylation activity in bladder and colon tissue. Cancer Res. 1995;55(22):5226–5229. [PubMed] [Google Scholar]
- 60.Bell DA, Stephens EA, Castranio T, et al. Polyadenylation polymorphism in the acetyltransferase 1 gene (NAT1) increases risk of colorectal cancer. Cancer Res. 1995;55(16):3537–3542. [PubMed] [Google Scholar]
- 61.Sim E, Westwood I, Fullam E. Arylamine N-acetyltransferases. Expert Opin. Drug Metab. Toxicol. 2007;3(2):169–184. doi: 10.1517/17425255.3.2.169. [DOI] [PubMed] [Google Scholar]
- 62.Zhu Y, States JC, Wang Y, Hein DW. Functional effects of genetic polymorphisms in the N-acetyltransferase 1 coding and 3´ untranslated regions. Birth Defects Res. A Clin. Mol. Teratol. 2011;91(2):77–84. doi: 10.1002/bdra.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruhn C, Brockmoller J, Cascorbi I, Roots I, Borchert HH. Correlation between genotype and phenotype of the human arylamine N-acetyltransferase type 1 (NAT1) Biochem. Pharmacol. 1999;58(11):1759–1764. doi: 10.1016/s0006-2952(99)00269-5. [DOI] [PubMed] [Google Scholar]
- 64.Yang M, Katoh T, Delongchamp R, Ozawa S, Kohshi K, Kawamoto T. Relationship between NAT1 genotype and phenotype in a Japanese population. Pharmacogenetics. 2000;10(3):225–232. doi: 10.1097/00008571-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Jensen LJ, Kuhn M, Stark M, et al. STRING 8 – a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009;37(Database issue):D412–D416. doi: 10.1093/nar/gkn760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fretland AJ, Leff MA, Doll MA, Hein DW. Functional characterization of human N-acetyltransferase 2 (NAT2) single nucleotide polymorphisms. Pharmacogenetics. 2001;11(3):207–215. doi: 10.1097/00008571-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 67.Bell DA, Taylor JA, Butler MA, et al. Genotype/phenotype discordance for human arylamine N-acetyltransferase (NAT2) reveals a new slow-acetylator allele common in African–Americans. Carcinogenesis. 1993;14(8):1689–1692. doi: 10.1093/carcin/14.8.1689. [DOI] [PubMed] [Google Scholar]
- 68.Bayoumi RA, Qureshi MM, al-Ameri MM, Woolhouse NM. The N-acetyltransferase G191 A mutation among Sudanese and Somalis. Pharmacogenetics. 1997;7(5):397–399. doi: 10.1097/00008571-199710000-00009. [DOI] [PubMed] [Google Scholar]
- 69.Cavaco I, Reis R, Gil JP, Ribeiro V. CYP3A4*1B and NAT2*14 alleles in a native African population. Clin. Chem. Lab. Med. 2003;41(4):606–609. doi: 10.1515/CCLM.2003.091. [DOI] [PubMed] [Google Scholar]
- 70.Magalon H, Patin E, Austerlitz F, et al. Population genetic diversity of the NAT2 gene supports a role of acetylation in human adaptation to farming in Central Asia. Eur. J. Hum. Genet. 2008;16(2):243–251. doi: 10.1038/sj.ejhg.5201963. [DOI] [PubMed] [Google Scholar]
- 71.Sabbagh A, Darlu P, Crouau-Roy B, Poloni ES. Arylamine N-acetyltransferase 2 (NAT2) genetic diversity and traditional subsistence: a worldwide population survey. PloS One. 2011;6(4):e18507. doi: 10.1371/journal.pone.0018507. (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watterson GA. On the number of segregating sites in genetical models without recombination. Theor. Popul. Biol. 1975;7(2):256–276. doi: 10.1016/0040-5809(75)90020-9. [DOI] [PubMed] [Google Scholar]
- 73.Tajima F. Statistical-method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123(3):585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.Primer3 version 0.4.0. http://frodo.wi.mit.edu.
- 102.NCBI dbSNP Short Genetic Variations. www.ncbi.nlm.nih.gov/projects/SNP.
- 103.Hein DW, Grant DM, Sim E, Minchin RF, Boukouvala S. Arylamine N-Acetyltransferase (NAT) Nomenclature. doi: 10.1097/FPC.0b013e3282f60db0. http://louisville.edu/medschool/pharmacology/consensus-human-arylamine-n-acetyltransferase-gene-nomenclature. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.