Abstract
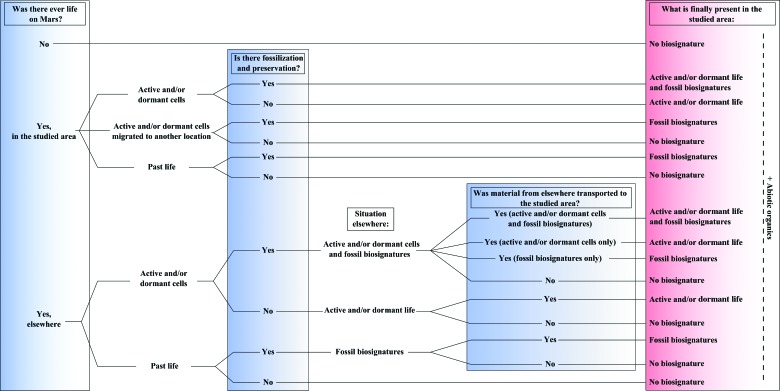
The search for traces of life is one of the principal objectives of Mars exploration. Central to this objective is the concept of habitability, the set of conditions that allows the appearance of life and successful establishment of microorganisms in any one location. While environmental conditions may have been conducive to the appearance of life early in martian history, habitable conditions were always heterogeneous on a spatial scale and in a geological time frame. This “punctuated” scenario of habitability would have had important consequences for the evolution of martian life, as well as for the presence and preservation of traces of life at a specific landing site. We hypothesize that, given the lack of long-term, continuous habitability, if martian life developed, it was (and may still be) chemotrophic and anaerobic. Obtaining nutrition from the same kinds of sources as early terrestrial chemotrophic life and living in the same kinds of environments, the fossilized traces of the latter serve as useful proxies for understanding the potential distribution of martian chemotrophs and their fossilized traces. Thus, comparison with analog, anaerobic, volcanic terrestrial environments (Early Archean >3.5–3.33 Ga) shows that the fossil remains of chemotrophs in such environments were common, although sparsely distributed, except in the vicinity of hydrothermal activity where nutrients were readily available. Moreover, the traces of these kinds of microorganisms can be well preserved, provided that they are rapidly mineralized and that the sediments in which they occur are rapidly cemented. We evaluate the biogenicity of these signatures by comparing them to possible abiotic features. Finally, we discuss the implications of different scenarios for life on Mars for detection by in situ exploration, ranging from its non-appearance, through preserved traces of life, to the presence of living microorganisms. Key Words: Mars—Early Earth—Anaerobic chemotrophs—Biosignatures—Astrobiology missions to Mars. Astrobiology 15, 998–1029.
Key Points
• Conditions for the emergence of life on Mars were temporally and spatially heterogeneous with the result that martian microorganisms were likely to (and may still) be anaerobic chemotrophs.
• Study of the carbonaceous signatures of anaerobic chemotrophs from early Earth demonstrate that these organisms were widely but sparsely distributed—except in the vicinity of hydrothermal vents, where they were abundant.
• Biosignature identification needs to take into account a broad variety of morphological, geochemical, and organic characteristics.
• Searching for signs of past life on Mars will be challenging because the most detectable in situ biosignatures will be organic molecules.
1. Introduction
Considering the environmental, spatial, and temporal conditions required for the appearance of life on Mars, that is, the existence on timescales of several hundreds of thousands to a few million years of an environment conducive to the formation and assemblage of prebiotic molecules from water and carbon into primitive cells (Martin and Russell, 2007) (different from the conditions necessary for sustaining flourishing or dormant life; Westall et al., 2013), it is most likely that, if life ever appeared on the planet, it was during its early history, that is, during the pre-Noachian to Noachian period from which there is evidence of widespread liquid water and standing bodies of water, perhaps even an ocean in the northern hemisphere (Clifford and Parker, 2001; Villanueva et al., 2015). Nevertheless, shorter-lived, habitable areas at the surface could perhaps have been colonized by viable cells transported from a protected habitat, for example in the subsurface, at any time during the Hesperian-Amazonian (or in the earlier epochs as well) [see Cockell (2014) for a recent review of martian habitability].
Many studies have considered the possibility of the emergence of life on Mars (McKay et al., 1992; Brack, 1997; Dartnell, 2007), its possible disappearance from the surface after the degradation of climatic conditions during the Late Noachian/Early Hesperian (Friedmann and Koriem, 1989), as well as the continued existence of life in the subsurface (Michalski et al., 2013; Cockell, 2014) and even at the surface under certain conditions (e.g., Stoker et al., 2010). These conditions imply not only the presence of carbon and liquid water but also suitable electron donors and acceptors to fuel microbial metabolism (cf. Grotzinger et al., 2014; Cockell, 2014). On Mars, it seems that, for most reactions with available electron donors, the acceptors are limited, while for reactions with plentiful acceptors (such as iron and sulfate reduction), the donors (organics and hydrogen) may have been limited. Although O2 is the most common electron acceptor on present-day Earth, it is widely believed that anaerobic respiration preceded aerobic respiration during the early evolution of life (Martin and Sousa, 2015). Indeed, in highly anaerobic terrestrial environments, modern microorganisms have developed a strategy using electrically charged “nanowires” to transport their used electrons to mineral electron acceptors, such as iron oxides (El-Naggar et al., 2010). Nevertheless, the paucity of natural ingredients necessarily would have placed difficult-to-overcome limits on the existence of life at the surface of Mars, especially after the Noachian/Early Hesperian period [although there has been a suggestion that its surface may have been oxidizing, Tuff et al. (2013)]. These possible energy limitations would have had important consequences for the nature of martian life-forms and their preservation and therefore also for present and future missions dedicated to astrobiology. Thus, whatever the scenario for the development of habitability, the relatively short period favorable for the appearance of life—early in the history of Mars (pre-Noachian to Noachian periods)—and the rapid discontinuity of habitable surface conditions (cf. Cockell et al., 2012) imply that, if life appeared on the Red Planet, it probably remained in a very primitive state of evolution, in most cases probably not achieving (anaerobic) photosynthesis. [We note that Noffke (2015) hypothesized the existence of phototrophic microbial mats from observation of photographs from Gale Crater, but based on the lack of direct evidence and on our considerations above, we think that phototrophy at the surface of Mars is very unlikely].
The nature and preservation of biosignatures for both Earth and Mars have been reviewed by Farmer and Des Marais (1999), Westall and Cavalazzi (2011), and Summons et al. (2011). Our objectives in this contribution are to place biosignature preservation in the context of the “punctuated” habitability of Mars and to consider the impact on missions, such as Mars Science Laboratory, ExoMars, and Mars 2020, whose objectives are to search for life at any chosen location on the planet. We use as examples preserved biosignature analogues of the kinds of anaerobic microorganisms that could have lived on, or may still live in the subsurface of, Mars, highlighting the spatial distribution of the biosignatures on the microbial scale within their host sedimentary habitat.
2. Habitability and Life on Mars
2.1. Abiotic/prebiotic carbon on Mars
Given the emphasis on in situ measurements in the search for organic traces of life (e.g., MSL, Mahaffy et al., 2015; ExoMars, http://exploration.esa.int/mars/45103-rover-instruments/?fbodylongid=2132), in this section we examine the possible sources of the kinds of organic molecules that might be preserved on Mars. Whether or not life arose on Mars, it is to be expected that abiotic organic molecules of endogenous and/or exogenous origin could be preserved in rock formations that predate the oxidation of the surface. These molecules, contained in interplanetary dust particles (IDPs), micrometeorites, and meteorites, continue to rain down on the surface of the planet at a rate of 2.4 × 106 kg year−1, or ∼0.1 nm of global coverage per year (Zent and McKay, 1994; Flynn, 1996). Carbonaceous chondrites, for example, contain about 70% insoluble organic matter (IOM) and 30% soluble organic matter (SOM). The IOM consists essentially of polyaromatic hydrocarbons (PAHs) (Pendleton and Allamandola, 2002), while the SOM contains many molecules relevant for life, including amino acids and carboxylic acids (Botta and Bada, 2002; Sephton, 2002; Sephton et al., 2004). The organic matter (OM) in carbonaceous chondrites occurs as discrete, disseminated grains or as an amorphous matrix in which inorganic grains, for example, Mg-rich silicates (olivine, pyroxene) and Fe-Ni sulfides, are embedded (Duprat et al., 2010). Fluid-precipitated inclusions of OM on the order of microns in size exhibit a granular texture (somewhat similar to that observed in IDPs, cf. Duprat et al., 2010) and have a heterogeneous C, H, S, Cl composition (Lin et al., 2014), while micrometer-sized particles of macromolecular carbon are associated with small oxide grains (Steele et al., 2007, 2012). In the former case, the OM is comparable to IOM in CV and CM chondrites (Pizzarello et al., 2006; Alexander et al., 2010), although Lin et al. (2014) also suggested that the OM may be of biogenic origin. However, the apparent co-formation of macromolecular carbon in association with sometimes high-temperature magmatic minerals (Steele et al., 2012) strongly implies an abiotic, magmatic origin. The extraterrestrial OM is also associated with clays, such as saponite (Pearson et al., 2002).
The distribution of exogenous OM of abiotic origin on the surface of Mars today is likely to be widespread and heterogeneous: brought to the surface and distributed via hydrothermal fluids during volcanic/impact events or exposed by impact gardening. However, Summons et al. (2011) noted that the type of carbon associated with IDPs and micrometeorites is likely to be destroyed under the present conditions on Mars' surface, whereas the less labile insoluble organic carbon in ordinary chondrites, well protected by minerals, could possibly survive. During Mars' early history, when water flowed at the surface and water bodies existed, OM released by physical and/or chemical degradation of the meteorites and IDPs could have been concentrated in depositional basins.
In situ formation of prebiotic molecules on Mars also could have arisen and may, under certain circumstances, still occur in the subsurface (cf. Webster et al., 2015). Abiotic production of organics by photochemistry occurs in the atmosphere by Strecker synthesis (Miller, 1953; Johnson et al., 2008), as well as in hydrothermal environments through Fischer-Tropsch synthesis (Shock et al., 1998; Horita and Berndt, 1999; McCollom et al., 1999; see recent review by Konn et al., 2015) where small molecules are formed, such as CH4, alkanes, formic acid, and acetate (Proskurowski et al., 2008; Sherwood Lollar et al., 2008).
Organic molecules on Mars have been difficult to detect on the surface for various reasons. Firstly, a combination of radiation and photochemical processes acting at the surface of the planet contribute to the destruction of the more volatile organic components (Summons et al., 2011). These include solar, galactic, and cosmic radiation affecting the upper few millimeters to about 1.5 m below the surface (Dartnell, 2007; Sephton and Botta, 2008). Physicochemical reactions also form oxidants, for example, hydrogen peroxide, perchlorates, or superoxides derived from mineral reactions (Atreya et al., 2006, 2011). However, the more refractory components of OM at the surface of Mars should survive these processes and, with suitable instrumentation, be measurable. Unfortunately, the instruments on board the Viking landers in their 1976 mission had a relatively limited resolution to the extent that they would not have been able to detect even trace amounts of soil bacteria amino acids (several million cells per gram, Glavin et al., 2001). Nor were they capable of analyzing IOM (Biemann, 2007). The Sample Analysis at Mars (SAM) instrument on Curiosity has also demonstrated the difficulties of analyzing OM in martian materials that naturally contain perchlorate, the presence of which oxidizes any OM during the heating necessary for analysis (François, 2014). Nevertheless, this instrument has been able to definitively identify for the first time on Mars an organic molecule, chlorobenzene (Freissinet et al., 2015).
Despite the difficulty in detecting organics in situ on Mars, small quantities of methane (of abiotic and, possibly, biotic origin) have been detected by the SAM instrument on Curiosity (Webster et al., 2015), and there is evidence for organic carbon in martian meteorites (McKay et al., 1996; Grady et al., 2004; Steele et al., 2012; Lin et al., 2014). Thus, many of the molecules thought to be necessary for abiogenesis were likely present.
2.2. Heterogeneous habitability and martian life
In this section, we consider the potential energy sources for life on Mars and the influence of heterogeneous spatial and temporal habitability on its distribution.
As pointed out by Cockell et al. (2012) and Cockell (2014), the existence of habitable conditions on a planet like Mars does not necessarily imply that a particular habitat was inhabited. To be transported to a newly established habitat, viable life-forms would need to not only overcome stresses related to transport and landing but also survive the spatial and temporal variability of martian conditions, which could have strongly inhibited microbial colonization and reduced microbial viability. Wind-blown transport of viable microorganisms would be an unlikely scenario, especially under post–Early Hesperian environmental conditions, because of the effects of oxidant production during atmospheric dust storms (Atreya et al., 2006).
There is abundant evidence for water at the surface (and possibly in the subsurface) of Mars. Geomorphological features indicate that surface runoff and connected lakes were present for only brief periods of time (Carr, 2006). At Gale Crater, for instance, there is geomorphological, sedimentological, and mineralogical evidence for a small, episodically long-lived lake (Grotzinger et al., 2014). Long debated (Clifford and Parker, 2001; Carr and Head, 2003; DiBiase et al., 2013), D/H isotopic data seem to indicate the past presence of water in the northern plains (Villanueva et al., 2015). Further interpretations of aqueous activity come from orbital identification of minerals indicative of aqueous alteration at the surface (e.g., Bishop et al., 2008; Noe Dobrea et al., 2010; Gaudin et al., 2011; Le Deit et al., 2012) and by long-term subsurface water circulation (Clifford, 1993; Clifford and Parker, 2001; Ehlmann et al., 2011; Andrews-Hanna and Lewis, 2011; Loizeau et al., 2012; Michalski et al., 2013).
After degradation in the surface environment, the subsurface of Mars may have been a refuge for primitive life (cf. Friedmann and Koriem, 1989; Michalski et al., 2013; Cockell, 2014), allowing it to be distributed and to colonize surface oases when, and as, they appeared. Indeed, Villanueva et al. (2015) noted that the equivalent of a 20 m thick global layer of water is missing from Mars' inventory and could be stocked in the subsurface. Subsurface microorganisms, such as chemolithotrophs, may have obtained their carbon from magmatic sources (cf. Steele et al., 2012) or from CO2-rich fluids seeping downward from the surface.
Martian life based on anaerobic respiration could have used a number of electron sources (Table 1) including (1) H2 produced by serpentinization/hydrothermal activity (Shock et al., 1998; Nealson et al., 2005; Reith, 2011), radiolysis (Blair et al., 2007), or mechanoradical chemistry in fault planes (Hirose et al., 2011), as well as microbially mediated mineral alteration (Parkes et al., 2011); (2) Fe2+ and Mg2+ produced by alteration of magmatic rocks; or (3) abiotic methane (Webster et al., 2015). As noted above, identification of electron acceptors on Mars is more problematic. Table 1 lists a variety of potential electron acceptors of which perchlorates, CO2, SO42−, Fe3+, H2O, and organics occur on Mars while oxygen and nitrates are more problematic. Oxygen produced by abiotic reactions (via photolysis/radiolysis of water) should also be considered. Nitrate also appears in this list but, in the form NO3−, has not yet been directly discovered on Mars. However, detection of nitric oxide (NO) by the Curiosity rover (Stern et al., 2015) suggests the possibility of the presence of nitrate, the NO being a degradation product of nitrate produced during heating in the SAM instrument. Stern et al. (2015) noted that the original nitrate could have been formed by abiotic processes including lightning and impacts. Similarly, the detection of Mn distributed in discrete phases in Gale Crater sediments (Lanza et al., 2014) indicates the episodic formation of oxidized phases of Mn. Note that Table 1 also lists redox couples that have not yet been detected on Earth.
Table 1.
Potential Energy Sources for Chemotrophic Life on Mars (Adapted from Cockell, 2014, and Rummel et al., 2014)
| Electron donor | Electron acceptor | Metabolism | Comment |
|---|---|---|---|
| Chemolithotrophy | |||
| H2 | CO2 | Methanogenesis; acetogenesis; C fixation via Wood–Ljungdahl pathway | Hydrogen from hydrothermal alteration of mafic/ultramafic minerals (e.g., olivine) and microbial mediation of H2 from mineral alteration (Parkes et al., 2011) |
| H2 | Fe3+ | Iron reduction | Hydrogen from sources mentioned above |
| H2 | SO42−, S0 | Sulfate reduction | Hydrogen from sources mentioned above |
| H2 | O2 | Hydrogen oxidation | |
| H2 | ClO4− | Perchlorate reduction | |
| CH4 | (Mn4+, Mn3+) | Birnessite reduction | |
| CH4 | Fe3+ | Ferrihydrite reduction | |
| CH4 | NO3− | Anaerobic methane oxidation | |
| CH4 | SO42− | Sulfate reduction | |
| CO | H2O | Carbon monoxide oxidation | |
| CO | O2 | Aerobic carbon monoxide oxidation | “CO oxidizers” are bacteria capable of growing with CO as a sole carbon and energy source |
| CO | NO3− | Aerobic methane oxidation | |
| CO | SO42− | Sulfidogenesis | |
| CO | CO2 | Methanogenesis; acetogenesis | |
| Fe2+ | CO2 | Carbon dioxide reduction | |
| Fe2+(basalt glass) | O2, NO3− | Iron oxidation | Not confirmed for terrestrial microorganisms |
| Fe2+(aqueous) | O2, NO3− | Microaerobic iron oxidation | |
| Fe2+(biotite) | O2, NO3− | Aerobic iron oxidation | |
| Fe2+, Fe3+(magnetite) | NO3− | Aerobic iron oxidation | |
| FeS2 | MnO2, NO3− | Anaerobic pyrite oxidation | |
| S2− | (Mn4+, Mn3+) | Anaerobic sulfides oxidation | |
| HS−(aqueous) | O2, NO3− | ||
| S0 | NO3− | Sulfur oxidation | |
| S0 | Fe3+ | Anaerobic sulfur oxidation | Occurs in acid conditions |
| S0 | MnO2 | Sulfur oxidation | |
| H2S, HS−, S0, S2O32− | O2 | Oxidation of reduced sulfur compound | |
| NH3 | O2 | Oxidation of ammonia | Part of the nitrification process |
| NO2− | H2O | Oxidation of the nitrite | Part of the nitrification process |
| NH4+ | NO2− | Anammox | |
| Chemoorganotrophy | |||
| Organics | Fe3+ | Iron reduction | Carbon from abiotic/prebiotic sources as well as biogenic; hydrogen from sources mentioned above |
| Organics | SO42− | Sulfate reduction | Carbon and hydrogen sources as above |
| Organics | Perchlorates | ||
| Fermentation (disproportionation) | |||
| Organics | Organics | Fermentation | Carbon sources as above |
The fermentation metabolism, whereby OM is both the electron donor and acceptor, is universal on Earth (i.e., present in the three domains of life) and involves a simple biochemical pathway compared to (an)aerobic respiration, which requires an electron transfer chain (absent in fermentation). This kind of metabolism on early Mars could have been fueled by OM of abiotic origin, if degraded and in a bioavailable form and if sufficiently concentrated, as well as biogenic OM.
While colonization of the martian subsurface by microorganisms during the pre-Noachian to Noachian epochs was a possibility, the subsurface could still potentially host microorganisms today (Des Marais, 2010; Ehlmann et al., 2011; Michalski et al., 2013; Cockell, 2014). On Earth, viable subsurface microorganisms utilizing chemotrophic metabolisms can occur at great depths (up to 5300 m) in the terrestrial subsurface (Szewzyk et al., 1994; Chivian et al., 2008) and up to 1922 m below the seafloor within 35 million-year-old marine sediments (Ciobanu et al., 2014). However, the subsurface is likely to be nutrient-poor, that is, oligotrophic, unless in the vicinity of nutrient-bearing hydrothermal fluids.
The energy required to sustain microbial viability within some microbial groups, for example, ultramicrobacteria (Janssen et al., 1997; Schut et al., 1997), can be extremely low (Hoehler and Jørgensen, 2013). Price and Sowers (2004) calculated the amount of carbon necessary for cells to support either cell division, basic cell maintenance (e.g., DNA and protein repairs, protein renewals), or cell survival. They found that 10−7 to 10−12 g C per cell per hour can maintain cell viability. Although these values depend on variables, such as temperature or pressure, interspecies variations, and the possibility of syntrophy, it shows that microbial viability can be maintained with extremely low levels of energy, as demonstrated by the isolation of cells from 5 million-year-old permafrost (Gilichinsky et al., 2007).
It is recognized that terrestrial microorganisms in energy-limited environments catabolize 104 to 106 times more slowly than microorganisms in enriched laboratory cultures (Hoehler and Jørgensen, 2013), with a biomass turnover rate on the order of 102 to 104 years compared to hours to days for laboratory cultures. For example, microorganisms in the present-day deep subseafloor only divide about once every 1000 years (D'Hondt et al., 2002), a situation which has implications for microbial viability in the long term. These deep subseafloor cell division timescales are still relatively rapid compared to the situation that could exist in the martian subsurface. Here, cells will have been exposed to crustal radiation over periods of hundreds of millions to billions (108 to 109) of years which, in the absence of intermittent repairs, imposes a physical hard limit on the survival of life-forms (Kminek et al., 2003) but not on the preservation of their organic remains (Kminek and Bada, 2006; Pavlov et al., 2012). Another limiting factor for subsurface martian life is the availability of fixed nitrogen as an essential nutrient, although, as noted above, nitric oxide was found in the eolian deposits of Gale Crater (Stern et al., 2015). Infiltration of nitrogen-bearing compounds into the suburface would be feasible during some periods in locations when and where there was surface water. But since the Late Noachian/Early Hesperian, such events would have been rare (Mancinelli and Banin, 2003; Cockell, 2014).
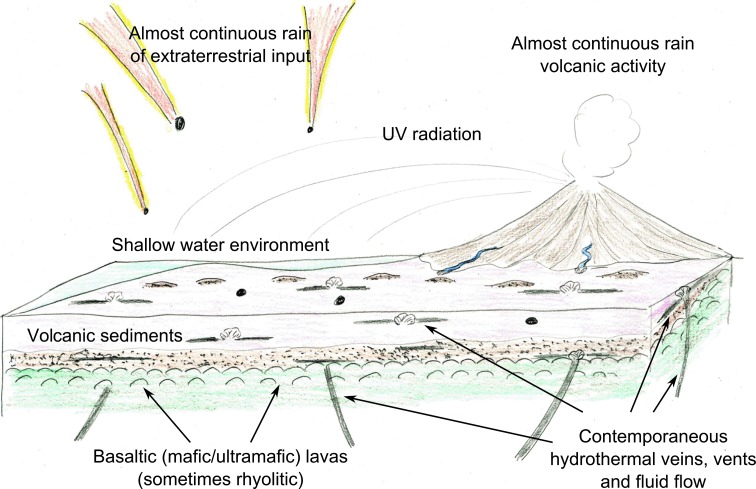
Contrary to early Earth, an ocean planet upon which water circulation allowed the rapid and widespread distribution of life, the conditions for the emergence of life on Mars would have been present during the pre-Noachian to Noachian period (and possibly even later in long-lived impact crater lakes) but would have been restricted to localities that were geographically separated (Westall et al., 2013). These conditions, that is, the simultaneous presence of liquid water and prebiotic molecules in contact with rocks and minerals and potential sources of energy (chemical, heat, light) on long-enough (105 to 106 million-year) timescales, may have occurred at different times and in different places. Note that spatial and temporal scales of habitability for established life are orders of magnitude smaller (10 to 105 μm or more; over hours to days and sometimes up to years) than the relatively long-lasting environments necessary for the appearance of life. Thus environments on Mars that could have hosted already-established life would have been more common than those in which life could have feasibly appeared. Moreover, it is possible that life could have emerged (or could reemerge) in one environment on Mars at the same time as it flourished in another because of the relative lack of spatial connectivity of the habitats. Figure 1 illustrates the temporal and spatial variability of habitable environments on Mars, excluding possible present-day habitats at the poles. We term this situation “punctuated” habitability. These limitations in habitability and on the continuous existence of viable life-forms at the surface of Mars would have placed severe constraints on the evolution of early life on Mars such that photosynthetic metabolisms may not have evolved (Westall et al., 2013). For this reason, we concentrate our discussion on chemotrophic life-forms. Indeed, chemolithoautotrophs are considered to have been the first primary producers on Earth (Nealson et al., 2005; Martin and Sousa, 2015).
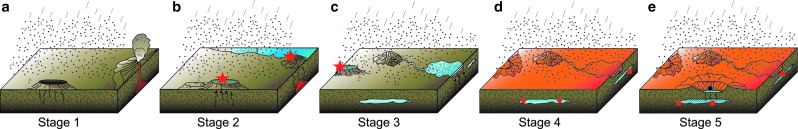
FIG. 1.
Heterogeneous or “punctuated” habitability on Mars. (a) Stage 1, prebiotic period ∼4.4 Ga. Much volcanic and impact activity; heavy rain of IDPs, meteorites, and micrometeorites importing volatiles, including prebiotic molecules. (b) Stage 2, origin of life, ∼4.2 Ga. Bodies of stable water with associated hydrothermal activity; possible origin of life (red star) around hydrothermal vents; continued rain of IDPs, meteorites, and micrometeorites (prebiotic molecules). (c) Stage 3, established life, ∼3.8 Ga. Water bodies are coated with ice; established life in habitable niches, e.g., water-lain sediments (black dots), cracks/fractures in the upper crust, or subsurface aquifers; continued rain of IDPs, meteorites, and micrometeorites (prebiotic molecules); possible emergence of life in another, geographically isolated habitable area (red star). (d) Stage 4, extinct/surviving life (red dots) <3.0 Ga. Surface water has disappeared; former ice-coated water bodies are covered with sediment/lava flows; surviving life in cracks/fractures in the upper crust, subsurface aquifers; continued rain of IDPs, meteorites, and micrometeorites (prebiotic molecules) but oxidation of carbon at the surface. (e) Stage 5, possiblility of ephemeral life as cells in the subsurface (red dots) are brought up to the surface by impact-related hydrothermal activity and survive for as long as there is liquid water.
3. Nature, Preservation, and Identification of Biosignatures on Mars
In the following, we review the kinds of biosignatures that could be produced on Mars and how they could be preserved and identified (cf. Farmer and Des Marais, 1999; Summons et al., 2011; Westall and Cavalazzi, 2011). We illustrate the discussion of biosignatures using two examples of fossilized chemotrophs in terrestrial Mars analog, anaerobic volcanic sediments.
3.1. Biosignatures and their preservation
The signatures of microbial organisms that can be preserved in rocks are related to the morphological, organic, and metabolic characteristics of the organisms. These biosignatures may be preserved and expressed in different ways (Farmer and Des Marais, 1999; Westall, 1999; Westall et al., 2000; Cady et al., 2003; Summons et al., 2011; Westall and Cavalazzi, 2011).
3.1.1. Morphological structures
These can include cells; cellular products, such as extracellular polymeric substances (EPS); and associations of cells, such as colonies, microbial mats, and bioconstructions (e.g., stromatolites, bioherms). Some of these structures can be visible to the naked eye, some require a submillimeter examination of a rock's surface, while in many other cases, substantial sample preparation and micron-scale examination of rocks (spatial, geochemical) is needed to identify the signatures preserved in them.
The possibility for viruslike (or virus-sized) particles should also be seriously considered, as on Earth viruses are present in the three domains of life and may have played a major role in the evolution of early life (Forterre et al., 2014). They are, moreover, fossilizable (Orange et al., 2011).
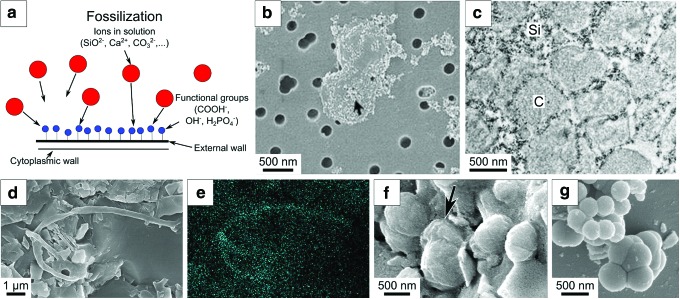
Cellular structures may be preserved as transported, allochthonous, flattened organic features in fine-grained anoxic sediments (e.g., Javaux et al., 2001, 2010), or they may be entombed and/or permeated by a variety of minerals including phosphates, iron oxides, silica, carbonates, sulfates, and halite. Ice can also preserve microbes (Christner et al., 2003). Mineral permeation or encapsulation of microbial structures occurs when minerals precipitate from saturated solutions onto the functional groups (carboxyls COOH−, hydroxyls OH, phosphoryls H2PO4−) of organic substrates (Fig. 2a). The initial precipitation of the mineral is a passive process, the OM acting simply as a template (Fig. 2a–2c). The organic structure thus becomes coated and eventually is permeated with a hydrated mineral phase (Figs. 2b–2f and 4h). Further polymerization and dehydration of the fossilizing mineral is a purely physicochemical reaction. In anaerobic environments, the organic substrate upon which the minerals precipitate degrades, and the degraded molecules (kerogen) may be trapped within the encrusting mineral (Fig. 2d, 2e). In oxidizing environments, for example at the surface of present Earth, the OM may be completely oxidized and only the mineral cast of the microorganism may remain, for example, in hot springs (cf. Cady and Farmer, 1996).
FIG. 2.
Fossilization of microorganisms by mineral replacement. (a) Schematic view of chemical bonding of minerals in solution to functional groups on the organic surface (microbial cell wall, EPS, etc.). (b) Scanning electron microscope and (c) transmission electron microscope view of an artificially fossilized, modern, chemolithotrophic microorganism, Pyrococcus abyssi, an analogue for early life on Earth and possible life on Mars (Orange et al., 2009). Arrow in (b) points to visible nanometer-sized deposits of silica on the cell wall. In (c), note the rugged surface and slightly irregular form of the microfossils (labeled “C”), as well as the fine-scale mineralization (Si, silica) of the cell wall (dark crust). (d, e) Fossilized (silicified), deflated (lysed) filament in the 3.45 billion-year-old Kitty's Gap sediments (see text in Section 3) in secondary electron view and EDX carbon mapping, respectively, showing the presence of carbon in the filament (Westall et al., 2006a). (f) Fossilized (silicified) coccoidal microfossils in the 3.45 billion-year-old Kitty's Gap sediments showing distinguishing features, such as cell division and lysis, as well as two sizes of cells (two species) (Westall et al., 2006a, 2011a). (g) For comparison with the fossilized coccoids in (f), abiotic silica spheres exhibit a similar morphology and, although spheres are juxtaposed, seemingly imitating cell division, they do not have many of the attributes of the biogenic coccoids (carbonaceous composition, fine-scale irregular surface, evidence of lysis, etc.; see text for discussion) (Westall et al., 2006a, 2011a). (Color graphics available at www.liebertonline.com/ast)
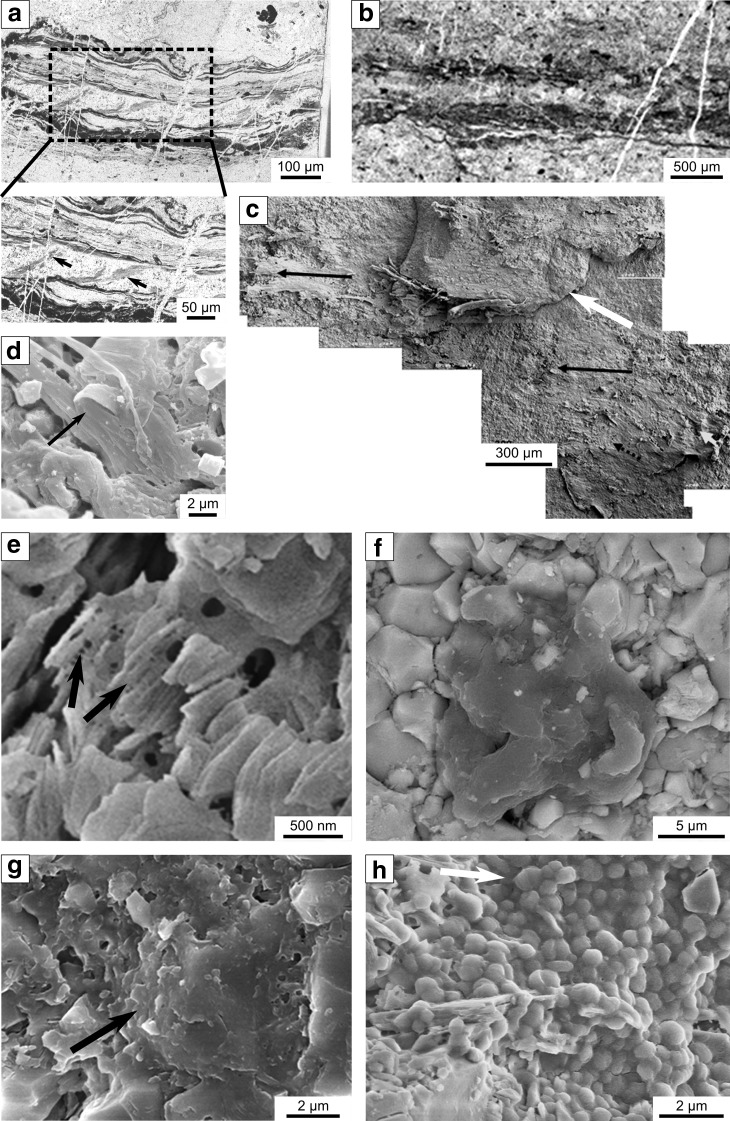
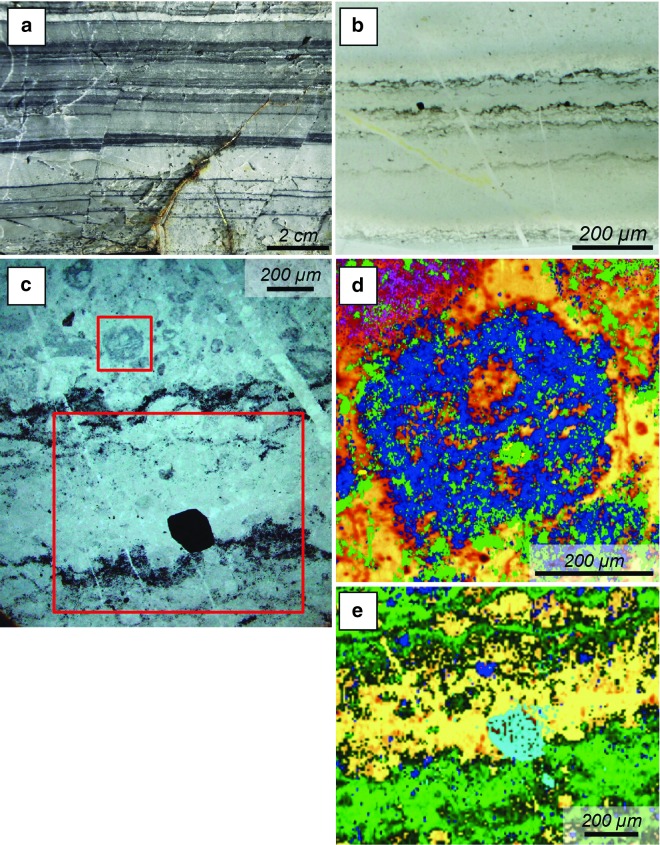
FIG. 4.
Scanning electron microscope (SEM) and light microscope view of carbonaceous features in samples from the 3.33 billion-year-old Josefsdal Chert, Barberton (a–d) and the 3.45 billion-year-old Kitty's Gap Chert, Pilbara (e–h), illustrating their different morphological expressions. (a) Thin section in transmitted light of a packet of thin (∼10 μm), semiparallel, undulating films in primary hydrothermal silica interpreted as fossilized (silicified) anoxygenic phototrophic biofilms. The insert shows details of soft deformation (tearing) of the biofilm due to the force of influx of hydrothermal fluids between the plastically deformable laminae. The excellent preservation of the fine-scale morphological details is due to very rapid solidification and the extremely fine-grained silica matrix. (b) For comparison with the excellent morphological preservation of the phototrophic biofilms in (a), this image shows a transmitted light view of a similar packet of thin, phototrophic laminae that developed on the surface of coarser-grained sediments, which resulted in poorer morphological preservation at the fine scale. (c) SEM view of the surface of one of the phototrophic biofilms, showing a thin, filmy coating with a distinct, striated orientation due to streamlining of the filaments forming the biofilm (black arrows), as also indicated by the small white arrow. Note that different layers of biofilms can be seen in this view (large white arrow). (d) Detail of (c) showing overturning of the filamentous biofilm due to current flow (arrow). (e) SEM view of muscovite that replaced an original volcanic grain. Between the crystalline sheets of the phyllosilicate is a web of mucuslike polymer fibers (arrows). (f) Detrital carbonaceous grain (dark central clot) within silicified volcanic sediments. Note the matrix of small, blocky, microcrystalline quartz crystals. (g) Much of the OM in the Kitty's Gap Chert has an amorphous texture (arrow) and appears to “glue” or cement the fine-grained particulate matrix. (h) Monolayer microcolony of coccoidal, silicified microorganisms on the surface of a volcanic clast (cf., Westall et al., 2006a, 2011a). Note the entrapped mineral particle (white arrow) and apparent two sizes of dividing coccoids.
Morphological indications of life also include physical structures created by living processes, such as parallel laminations [including microbially induced sedimentary structures, MISS, Noffke (2009)] or three-dimensional edifices (stromatolites, microbial mudmounds) produced by microbial biofilms, particularly photosynthetic biofilms (e.g., Fig. 2a–2d). Indeed, Noffke (2015) hypothesized similarities between MISS in modern and ancient terrestrial environments and certain structures observed on the surfaces of sedimentary bedding planes in Gale Crater.
3.1.2. Organic molecules
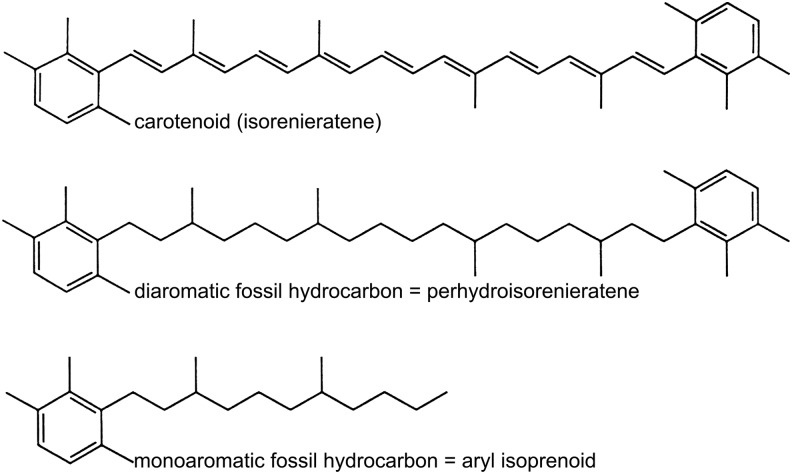
The organic components comprising the cells and their associated materials (e.g., EPS) can be preserved either as degraded molecules trapped in mineralized cells (Fig. 2c, 2e) or as disseminated molecules chemically bonded to mineral particles, such as phyllosilicates. The finely layered structure, high surface area, and the negatively charged surfaces of phyllosilicates make them attractive sites for the fixation of organic molecules (cf., Summons et al., 2011) (e.g., Fig. 4e). Organics also may be trapped in fluid inclusions in minerals such as halite (Winter et al., 2013). Organic cell components break down into smaller fragments and gradually lose their distinctive functional groups (Fig. 3). Up to a certain point, it is still possible to trace a degraded molecule back to its origin and sometimes even to identify microbial domains or phyla. For instance, bacterial hopenoids will degrade to hopanes, steroids to steranes, carotenoids to carotanes (Fig. 3), and so on. These organic molecules are termed biomarkers (Summons, 1993). However, biomarkers become unrecognizable, generic macromolecules with geological time (on a scale of several billions of years) and/or metamorphism. The generally aromaticized OM can have the same molecular composition as abiotic OM in meteorites (e.g., Sephton, 2002) but may be distinguished from the latter on the basis of its more restricted compositional range.
FIG. 3.
Examples of derivatives (biomarkers) of carotenoid from the green sulfur Chlorobium genus that occurs in sediments (after Summons, 1993).
Extracellular polymeric substances or generic amorphous carbonaceous matter is, in fact, more easily preserved in the rock record than intact cellular structures (Fig. 4a–4d, 4f, 4g) (Westall et al., 2000). Rapid fossilization via mineral entombment, burial, or fixation is necessary to preserve the organic signatures (cf. Handley et al., 2008). Both the elemental and molecular composition of the degraded carbon can provide information on its origin and possible biogenicity (Derenne et al., 2008; Summons et al., 2008, 2011; Westall et al., 2011b). A major concern is that, in oxidizing conditions, the organic components may be completely lost (cf. Cady and Farmer, 1996). At the surface of Mars, where oxidizing compounds and ionizing radiation prevail, at least the labile organic compounds are likely to be completely destroyed (Kminek and Bada, 2006; Summons et al., 2011; Pavlov et al. 2012).
The above discussion on the preservation of biogenic OM is also valid for abiotic/prebiotic organics, whether extraterrestrial or endogenic in origin, as well as detrital OM (of biological or abiotic origin) (Fig. 4f).
3.1.3. Metabolic signatures
There are many types of biosignatures that result from the metabolic activity of microorganisms, of which isotopic fractionation is the most commonly used. Carbon and sulfur are frequently utilized on Earth for determination of fractionation during biosynthesis (cf. Des Marais, 2001). However, a number of other nonbiological processes can also fractionate the isotopes of these elements; for example, atmospheric processes can fractionate carbon (Jakosky, 1991) and sulfur (Farquhar et al., 2000), while abiotic synthesis of carbon by Fischer-Tropsch reactions in hydrothermal environments (van Zuilen et al., 2002) may also lead to a range of isotopic signatures overlapping with those produced by life. Abiotic carbon isotopic ratios in carbonaceous chondrites may also overlap with biogenically derived signatures, although the range exhibited by OM in meteorites is much greater (Pearson et al., 2002). Other signals of metabolism include gas production, such as H2S, CH4, and numerous mineralogical phenomena, including direct or indirect microbial precipitation of minerals (Heim, 2011), microbial leaching of elements from rocks and minerals, and the influence of microorganisms and OM on mineral growth (Banfield et al., 2001).
Note that a preserved microbial feature may consist of just one or any combination of biosignatures. Preserved organic remains will be characterized not only by their molecular and elemental composition but also by isotopic signatures indicative of microbial metabolism, a chiral signature, or specific distributions of OM associated with a specific structure having a microbial morphology (e.g., Figs. 2d–2e and 4a–4d, 4h). On the other hand, microbial corrosion features on minerals may not necessarily be related to other biosignatures, in which case demonstration of a biogenic origin would be a challenge. A case in point is the controversy over the biogenicity of corrosion tunnels in the surfaces of 3.45 billion-year-old pillow lavas from Barberton, South Africa (Furnes et al., 2004; Grosch and McLoughlin, 2014).
Whatever the initial type of biosignature—organic, metabolic, or physical—the microbial features need to be rapidly encased in a cement, for example, ice, mineral, or a fine-grained, anaerobic sedimentary matrix that becomes lithified (dehydrated) such that it prevents further destruction by environmental degradation and diagenetic alteration. Rapid lithification of the sediments aids this process. Moreover, through geological time, other factors may contribute to changes to, or destruction of, the biosignatures, including volcanic, hydrothermal, or impact activity; physical/mechanical breakdown processes; diagenesis; or metamorphism.
3.2. Biosignature identification
The successful identification of a feature as a reliable biosignature is often a controversial process. In the first place, it is necessary to establish the biological nature of the signature, as many can be either altered beyond recognition or they may be mimicked by abiotic phenomena (Cady et al., 2003; Westall and Cavalazzi, 2011). Body fossils or microbial bioconstructions can be similar to mineral precipitations (Fig. 2g). If the biological OM is very highly degraded or altered, for example by shock metamorphism, it may be difficult to distinguish from abiotic macromolecules. The chiral signatures so characteristic of biological OM also are lost with geological time. The enzymatic signatures, such as isotope fractionation, leaching, mineral precipitation, and so on, also may be influenced by abiotic processes. These phenomena have been detailed in previous studies (e.g., Westall, 1999; Cady et al., 2003; Westall et al., 2006a, 2011a; Summons et al., 2011; Westall and Cavalazzi, 2011).
The fabrics, textures, and bioconstructions resulting from microbial activities may result in macroscopically visible phenomena that are easier to identify. For example, on an aerobic Earth, carbonate mud mounds that can be up to several tens of meters or more in size are produced through the activity of chemotrophic sulfate-reducing bacteria (e.g., Barbieri et al., 2004; Cavalazzi et al., 2012). Other macroscopic manifestations include “point sources” of hydrocarbon seepage where carbonate deposits are generated by localized anaerobic oxidation of methane by archaea coupled with bacterial sulfate reduction (Campbell, 2006) and finely laminated textures in sediments induced by the formation of photosynthetic microbial mats on sediment surfaces (e.g., Heubeck, 2009), the latter sometimes forming three-dimensional domical features (e.g., Hofmann et al., 1999; Allwood et al., 2006; Van Kranendonk, 2006). All these features, however, would not be expected on an anaerobic world or a world without phototrophs. Note that, although anaerobic chemotrophic mats develop around present-day hydrothermal vents, they occur at the anoxic/oxic interface and use dissolved free oxygen to oxidize reduced sulfur compounds (Teske and Nelson, 2006).
Biosignatures of anaerobic chemotrophic life in rocks from the anaerobic, volcanic, and Mars analog early Earth (Early Archean) are generally subtle, the most common being the presence of OM either disseminated throughout fine-grained sediments or specifically located with respect to volcanic and hydrothermal microhabitats. Despite their great age and the highly degraded nature of the organic molecules, differentiation from abiotic/prebiotic OM can be made based on molecular composition (more restricted in biogenic remains than in abiotic OM), micron-scale spatial microbe-sediment and geochemical associations, and to a certain extent extremely light carbon and sulfur isotope ratios (e.g., Westall et al., 2011a, 2015; Kiyokawa et al., 2014), although thermogenic CH4 production can result in δ13C values as light as −50‰ (McDermott et al., 2015). Body fossils and fossilized colonies of microorganisms exist but are difficult (and controversial) to observe and verify, as are even more subtle features, such as potential microbial corrosion phenomena (Furnes et al., 2004; Westall et al., 2006a, 2006b, 2011a; Foucher and Westall, 2009; Grosch and McLoughlin, 2014).
Given the difficulties that can be encountered when trying to identify fossil biogenic signatures and to differentiate them from abiogenic phenomena, it is frequently necessary to base identification of a potential biosignature on more than one parameter, although for OM, molecular composition, chirality, and other characteristics it may be sufficient to establish a biogenic origin (cf. Summons et al., 2011).
3.3. Anaerobic chemotrophic life and its biosignatures in Mars analog volcanic sediments from early Earth
Habitable environments on early Mars and early Earth had many similarities from the microbial point of view (Westall, 2005; Westall et al., 2013). Heat flow from the mantles of the early planets was higher than at present, estimated at ∼300°C hotter on early Earth for instance (Herzberg et al., 2007; Ruiz, 2014). This high heat flow drove abundant volcanic and hydrothermal activity. Alteration of the ultramafic rocks in the subsurface to serpentinites through subsurface circulation of hydrothermal fluids yielded hydrogen and reduced organic compounds likely implicated in the origin of life (Baross and Hoffman, 1985; Russell and Hall, 1997; Shock et al., 1998; Martin and Russell, 2007; Martin et al., 2008; Lane and Martin, 2012; Holm et al., 2015; McDermott et al., 2015). These are also the main nutrients for chemotrophic communities in the vicinity of hydrothermal vents (Shock et al., 1998). Volcanic terrains (including volcanic sediments) under water and close to hydrothermal activity were habitable for microorganisms that use chemotrophic metabolisms. Thus, early terrestrial volcanic materials (mostly basaltic, although some felsic volcanics occur) and their alteration products deposited in water are relevant analogues for past habitable environments on Mars. In the following, we document the distribution of carbon in ancient (3.5–3.33 billion-year-old), shallow-water, volcanic sedimentary environments from early Earth. Many of the carbonaceous signatures have been interpreted to be biogenic (Westall et al., 2006a, 2006b, 2011a, 2015). While concentrating on chemotrophic biosignatures, we also address the colocated phototrophic remains as a means of addressing possible abiotic film formation and preservation. However, imagining that the rocks studied had been returned to Earth from Mars (or that life had not yet appeared on Earth by 3.5 Ga as is ascertained by some), we make a detailed evaluation of potential abiotic hypotheses for the various carbonaceous features. The terrestrial analog formations used in this study are silicified volcanic sediments in the 3.45 billion-year-old Kitty's Gap Chert from the Pilbara, northwest Australia, and the 3.33 billion-year-old Josefsdal Chert from the Barberton Greenstone Belt, east South Africa (Westall et al., 2006a, 2006b, 2011a, 2015) (Fig. 5). These sediments and their microbial inhabitants share some similarities (and some differences), for instance, with Gale Crater lacustrine sediments (Table 2).
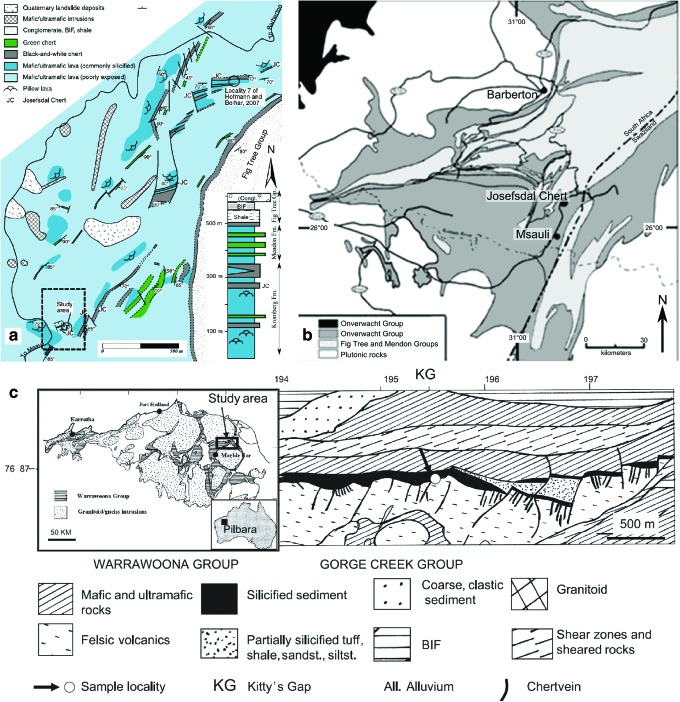
FIG. 5.
Geological maps of the Pilbara and Barberton Greenstone Belts showing the locations of the study areas. (a) Regional geological map of the Barberton Greenstone Belt and (b) local geological map of the Josefsdal Chert. (c) Local geological map of the Kitty's Gap Chert with regional geological map of the Pilbara Greenstone Belt in the inset. (Color graphics available at www.liebertonline.com/ast)
Table 2.
Comparison of Sedimentology, Environmental Conditions, Habitability, and Potential Biosignature Preservation in the 3.5–3.33 Billion-Year-Old Kitty's Gap and Josefsdal Chert Formations and the Sediments in Gale Crater, Yellowknife Bay Formation
| Early Earth: Kitty's Gap, Josefsdal Cherts (3.5–3.33 Ga) | Mars, Yellowknife Bay Formation, Gale Crater (∼3.6 Ga) | |
|---|---|---|
| Depositional environment | Mostly subaqueous, shore face to tidal | Lacustrine to fluvial; eolian |
| Sediment composition | Basaltic (ultramafic, mafic) some more evolved sediments [rhyolitic; primary hydrothermal/evaporitic silica; evaporite minerals (gypsum, aragonite, high-Mg calcite, halite)] | Basaltic sediments (pyroxene, olivine, plagioclase); some more evolved volcanics; amorphous phases |
| Secondary minerals, alteration | Volcanics altered to phyllosilicates and anatase; hydrothermal secondary minerals—silica, siderite, pyrite | Volcanic clasts altered to phyllosilicates (Fe/Mg smectites), magnetite, secondary minerals—Ca, Mg, Fe sulfates … |
| Cement | Silica | Phyllosilicates, amorphous phases? |
| Habitable environment | pH acidic–neutral | pH neutral (possibly partly acidic and partly alkaline) |
| high salinity ∼6% | dilute brines | |
| Anaerobic with micro amounts of abiotic O2 | Anaerobic (with hypothesized micro amounts of abiotic O2) | |
| Moderate-high temperatures >50°C? | Probably low temperatures | |
| Energy source | Organics of abiotic origin (hydrothermal or extraterrestrial broken down and hydrolyzed in water) and biogenic origin; redox reactions at the surfaces of reactive minerals, e.g., olivine, hydrothermal sulfide; H2 (serpentinization) from hydrothermal vents; photons | Organics of abiotic origin (hydrothermal or extraterrestrial broken down and hydrolyzed in water) and possibly biogenic origin, if there was life; redox reactions at the surfaces of reactive minerals, e.g., olivine, hydrothermal sulfide; H2 (serpentinization) from hydrothermal vents if they were active (no sign yet, except possibly CH4); photons |
| Organics | Biological, extraterrestrial (see above), hydrothermal | Certainly extraterrestrial (see above), possibly biological, if life present, possibly hydrothermal, if vents present |
| Life | Life already well established by 3.5–3.33 Ga | Not yet detected in Gale Crater (conditions for the emergence of life here not present), but the environment is potentially habitable if viable cells could have been transported there |
| Potential life-forms | Anaerobic chemolithotrophs, chemoorganotrophs, phototrophs | Anaerobic chemolithotrophs, chemoorganotrophs; little possibility of phototrophs (lack of evolutionary possibilities) |
| Potential distribution | Ubiquitous, on surfaces of volcanic detrital particles; in hydrothermal fluids (including silica gel); on sediment bedding-plane surfaces (phototrophs) including hydrothermal silica gel | Primarily on surfaces of volcanic detrital particles; possibly in hydrothermal fluids if they existed; possibly on sediment bedding-plane surfaces if phototrophs existed |
| Preservation | Rapid silicification | Entombment by cementing phyllosilicates and/or amorphous phases? |
| Biosignatures | Morphological; organic; metabolic | Morphological only if cementation was rapid or if phototrophs developed (MISS, cf. Noffke, 2015); organic (degraded remnants of organisms); possibly metabolic fractionation of carbon |
3.3.1. Early Earth environment
Environmental conditions on early Earth (reviewed in Westall, 2012) were similar to those of Mars on a microbial scale, but there were significant differences on a global scale. Unlike Mars, early Earth was an ocean planet with exposed volcanoes and protocontinents that resembled shallow oceanic plateaus. However, like Mars, it had a CO2-rich atmosphere (probably with other greenhouse gases, such as CH4) and was anaerobic. A small amount of O2 of abiotic origin was produced by photolysis of water vapor in the upper atmosphere, by radiolysis of water molecules at the surface of the oceans by UV radiation whose flux was orders of magnitude greater than today because of the lack of an ozone layer (Cockell and Raven, 2004), and finally by dissociation of boiling, pressurized hydrothermal fluids as they exited shallow vents. The oxygen was immediately consumed by reduced mineral and gas phases. Estimates of temperatures on early Earth vary. However, from the microbiological point of view, temperatures at the rock/sediment interface must have been warm to hot (∼55°C in the Archean; Marin-Carbonne et al., 2012, 2014), judging by the enormous amount of hydrothermal fluids circulating in the upper crust, seeping into the lavas with their overlying sediment layers and into the seawater (van den Boorn et al., 2007; Hofmann and Harris, 2008; Westall et al., 2015). Seawater pH was neutral to slightly acidic, while salinity was double the present values (de Ronde et al., 1997).
The volcanics on early Earth were, for the majority, ultramafic to mafic basalts and included Mg-Fe-rich komatiites (Arndt, 1994), products of a hotter mantle that were closer to martian basalts in composition than those of present-day Earth. Not all the lavas were basaltic, and some fractionated volcanics, such as rhyolites, occurred. The relatively rare remains of early Earth's crust document rapid breakdown and alteration of the volcanic lavas by hydrothermal/metasomatic carbonitization and devitrification on the seafloor and deposition as sediments in mostly shallow-water environments on the oceanic plateaus and around the exposed volcanoes [shore face to upper shore face (intertidal zone), with some sediments deposited below wave base]. The aqueously deposited volcanic sediments were rapidly altered to phyllosilicates, with smectite later transformed to sericite or muscovite by the prevailing lower greenschist metamorphism that affected the Early Archean terrain.
Hydrothermal activity associated with the volcanic activity processes appears to have been prevalent on early Earth (Van Kranendonk, 2006; Hofmann and Harris, 2008). These processes can produce hydrogen and OM in the form of small molecules, such as ketones, fatty acids, and alcohols (Shock, 1997; Peckmann and Thiel, 2004). Methane emanating from vents, long thought to have been the product of Fischer-Tropsch synthesis, may also originate in CH4 inclusions in plutonic rocks through which the hydrothermal fluids circulate (McDermott et al., 2015). Field and geochemical evidence from the Kitty's Gap and Josefsdal Cherts indicates intensive hydrothermal activity with contemporaneous veins transporting potentially organic-rich fluids into the sediments (e.g., Figs. 9c and 11l–11n).
FIG. 9.
Carbon distribution in oligotrophic, clastic sediments, Josefsdal Chert. (a) Outcrop view showing parallel deposits of tephra ash fall separated by thin black horizons. (b, c) Optical micrographs of these sediments showing thin concentrations of mainly microscale pyrite but also some carbon coating the tops of the sediment bedding planes. The large, euhedral black particle is a hydrothermal pyrite. The red boxes denote details shown in the Raman maps (d, e). (d) Distribution of carbon (green) associated with coarse-grained volcanic particles. The volcanic grains have been altered to anatase (blue) or muscovite (pink). The whole sediment was permeated with silica (yellow-orange) during diagenesis. (e) Detail showing the concentration of carbon (green) associated with the black tops of the bedding planes; pyrite (pale blue), anatase (dark blue), quartz (yellow).
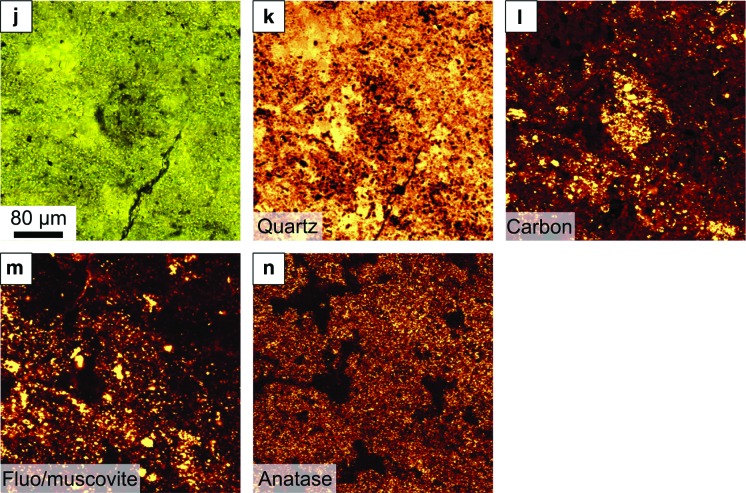
FIG. 11.
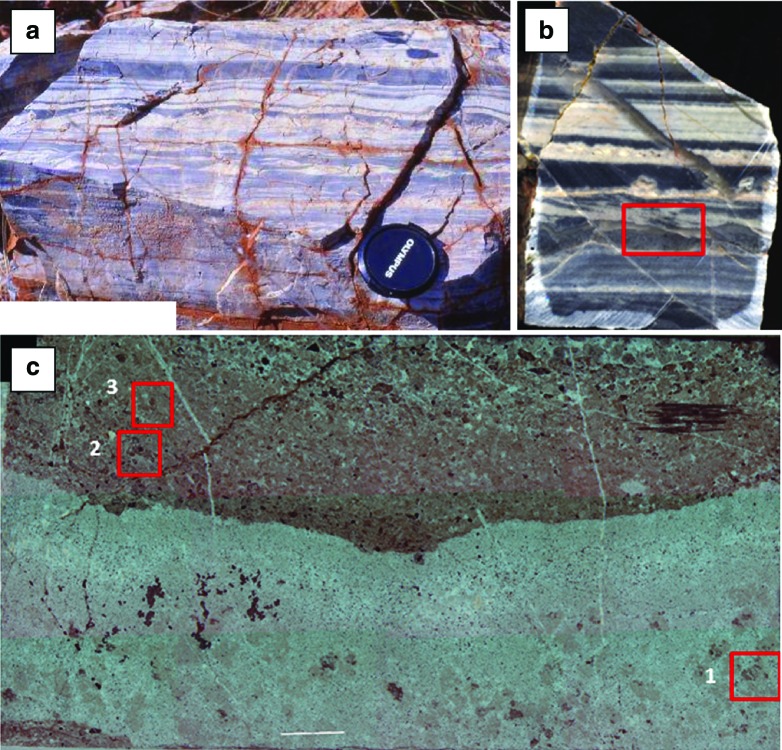
μ-Raman spectral mapping of the red boxed areas in Fig. 10c. (a–c) Optical micrograph (a) and Raman maps (b, c) of the disseminated distribution of carbon in the late diagenetic, cross-cutting hydrothermal chert vein in Zone 1 (Fig. 10c). (d–i) Distribution of carbon around the edges of angular volcanic grains (d) in Zone 2 (Fig. 10c). Particles in Zone 2 are fine sand–sized. Note that the carbon (f) colocates with edges of the volcanic particles (now largely replaced by silica) where the presence of (g) muscovite (the metamorphic equivalent of smectite) and (h) anatase pseudomorph the volcanic particles, indicating aqueous alteration. The SEM micrograph in (i) shows a small colony of coccoidal microorganisms colonizing a muscovite-altered volcanic grain. (j–n) Light micrograph and Raman maps of carbon distribution in Zone 3 (Fig. 10c). This area is characterized by finer-grained and poorly sorted sediment, consisting mostly of silt to clay-sized particles, as well as a few sand-sized grains. Carbon (l) is finely disseminated throughout this layer although concentrated on the surface of the ghost volcanic particle in the middle of the image. The disseminated distribution of (m) muscovite and (n) anatase here documents the original volcanic nature of the fine-grained dust. Here also the sediment has been thoroughly silicified. (Color graphics available at www.liebertonline.com/ast)
Impacts appear to have been relatively common on early Earth, resulting in crustal fracturing (Sleep and Lowe, 2014) and significant layers of spherulites (Lowe et al., 2014; Lowe and Byerly, 2015). Impacts would have caused mechanical breakdown and alteration of the upper crustal materials. The flux of extraterrestrial carbon (principally from IDPs/micrometeorites) during the Late Heavy Bombardment is estimated at 10 to 3 × 103 kg/km2 (Pasek and Lauretta, 2008).
The hydrothermally produced and/or extraterrestrially imported carbon molecules would have been a source of carbon and energy for organotrophs and even may have contributed to the formation of the molecule-thick conditioning layers around the volcanic grains. Abiotic carbon in carbonaceous chondrites consists of 30% soluble matter (e.g., amino acids, dicarboxylic acids) and 70% IOM (mostly aromatic molecules). The IOM, distributed in the meteorites mostly in the form of amorphous matter but also more rarely as discrete grains, can be released into the terrestrial environment by degradation of meteorites in water or by alteration from hydrothermal processes (Pizzarello and Shock, 2010). Exogenous amino acids could have polymerized to larger macromolecules in aqueous environments in Earth's early environment (Pizzarello and Shock, 2010). If in an assimilable form, this OM could be used by organotrophs; it is also a source of accessible nitrogen for microorganisms. Otherwise, it is possible that a certain proportion of non-assimilable extraterrestrial particulate carbon may have constituted part of the total carbon content.
The environment of early Earth is summarized in Fig. 6. On a microbial scale, the early terrestrial environment offered a wide variety of habitats ranging from lava surfaces [purported identifications of microbially produced corrosion tunnels (Furnes et al., 2004) are controversial (Grosch and McLoughlin, 2014)], the surfaces of volcanic sedimentary particles, including scoriaceous pumice fragments (Westall et al., 2006a, 2011a; Foucher et al., 2010), and hydrothermal environments. Sediment bedding planes in the photic zone hosted anoxygenic phototrophic biofilms (Walsh, 1992; Tice and Lowe, 2004; Allwood et al., 2006; Westall et al., 2006b).
FIG. 6.
Sketch showing the geological environments of the Kitty's Gap and Josefsdal Cherts, as representative of shallow-water environments on early Earth. Volcanic sediments were deposited in shore face to upper shore face (tidal) depths on top of lavas of basaltic (Josefsdal) and felsic (Kitty's Gap) origin. Hydrothermal activity was prevalent with hydrothermal fluids seeping into sediments contemporaneously and/or during early- or late-stage diagenesis. The flux of input of extraterrestrial materials was still high after the later heavy bombardment (cf. Lowe and Byerly, 2015) while the flux of UV radiation was also high because of the lack of a protective ozone layer (Cockell and Raven, 2004).
3.3.2. Sedimentary environments of the Kitty's Gap and Josefsdal Cherts
Interpretation of the environment of deposition of both these volcanogenic sedimentary deposits is demonstrated by sedimentary structures documenting shore face to tidal environments (Fig. 6) (de Vries et al., 2006; Westall et al., 2006a, 2006b, 2011a, 2015). In these locations, dynamic aqueous processes resulted in layered alternations of coarse, well-sorted, reworked sediments deposited by relatively strong, combined storm flow or tidal currents, and finer-grained, poorly sorted sediments that settled out in relatively quiet water regimes. Interspersed with these deposits are abundant volcanic ash and sand-sized, graded lapilli horizons representing suspension settling of volcanic ash. The origin of the volcanic ash in the Kitty's Gap Chert is felsic (i.e., from lavas having an originally high SiO2 content, >63%), and small pumice clasts are abundant, deposited at the top of the sediment layers because of their lighter density. In contrast, the origin of the volcanic protoliths in the Josefsdal Chert is mafic/ultramafic (i.e., with an originally low SiO2 content, <45 to ∼52%, and higher Fe and Mg contents).
The sediments were lithified by a fine-grained silica cement (chert) precipitated in situ from silica-saturated seawater and hydrothermal fluids, resulting in metasomatic element and mineral replacement and a final silica content up to >99%. Note that, in both the Kitty's Gap Chert and the Josefsdal Chert locations, field and geochemical evidence indicates the proximity of contemporaneous and post-diagenetic hydrothermal activity as well as strong but spatially and temporally variable influence of hydrothermal fluids on the sediments, the microorganisms they contained, and therefore the alteration (i.e., replacement of the volcanic clasts by silica) and lithification of the sediments (Hofmann and Bolhar, 2007; van den Boorn et al., 2007; Westall et al., 2011a, 2015). Hydrothermal silica infiltrated depositing sediments (and, in places, deformed them) preserving the microbe-sediment systems in situ and the microbial biosignatures in various states of conservation, from pristine to degraded, prior to silicification (cf. Guido et al., 2010; Westall et al., 2015).
It is relevant to note that hydrothermal silica deposits on Mars have been detected in association with volcanic environments (Skok et al., 2010; Ruff et al., 2011). Indeed, the opal A deposit at Home Plate in the Noachian-aged Gusev Crater has been compared to modern hydrothermal siliceous sinter deposits (Ruff, 2015). Further, hydrated silicates interpreted as being of possible hydrothermal origin [although other processes could have formed them as well, Tornabene et al. (2013)] may be associated with other Noachian-age craters (e.g., Mustard et al., 2008; Schwenzer and Kring, 2009; Carter et al., 2010).
The rapid silicification of the terrestrial Early Archean sediments served to preserve the biosignatures in an excellent state of conservation. This kind of preservation could be considered an “ideal” scenario because the resulting cherts were relatively impervious to subsequent alteration and destruction. On the other hand, cherts are extremely indurated and would constitute a challenging target to drill or cut into with the tools that can be carried by a typical robotic mission.
3.3.3. Carbonaceous signatures and their distribution in the Kitty's Gap and Josefsdal sediments
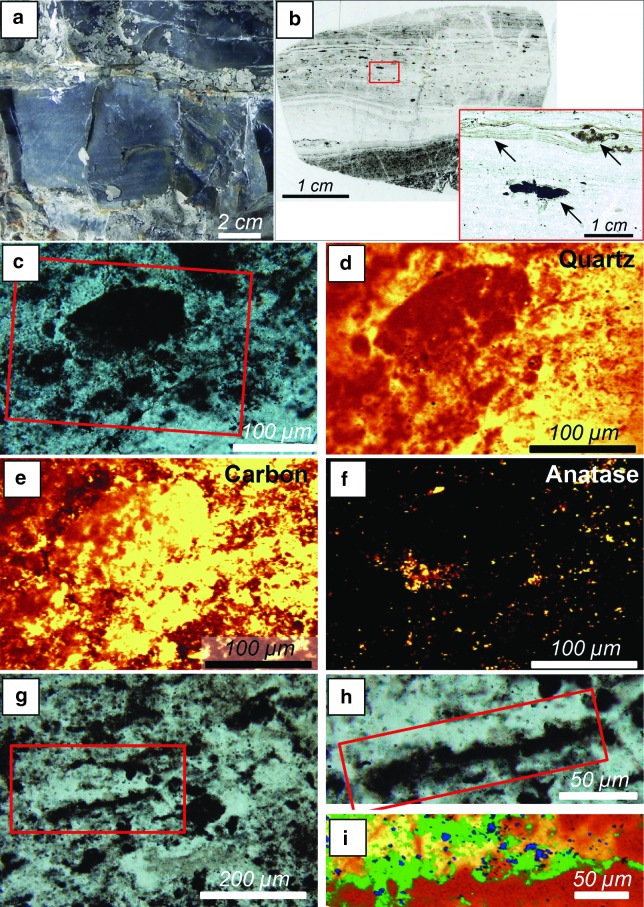
Carbon is relatively common in the Kitty's Gap and Josefsdal sediments but is heterogeneously distributed. In terms of bulk carbon contents, the sediments can be considered to be generally poor in carbon, ∼0.01–0.02%. However, certain horizons strongly influenced by hydrothermal fluids have richer carbon contents up to 0.1–0.5%. The carbonaceous components are generally not visible at the outcrop scale, although cherts characterized by high C contents may present a matte black appearance (Fig. 7a). These carbon-rich deposits form up to centimeter-thick layers characterized by a speckled “clotted” appearance in petrographic thin section, observed by optical microscopy in transmitted light and by μ-Raman mapping (Fig. 7). The clotted fabric consists of two types of features: (1) volcanic particles that are relatively heavily coated with carbon (up to tens of micrometers in thickness), the latter presenting a sometimes “tufted” morphology (Fig. 7c, 7e, 7g–7i) and occurring in a carbon-rich, fine-grained sedimentary matrix; or (2) clots of carbon with three-dimensional tufted or spiky shapes that apparently formed in situ within a very fine-grained sedimentary and/or primary silica gel matrix (Fig. 8).
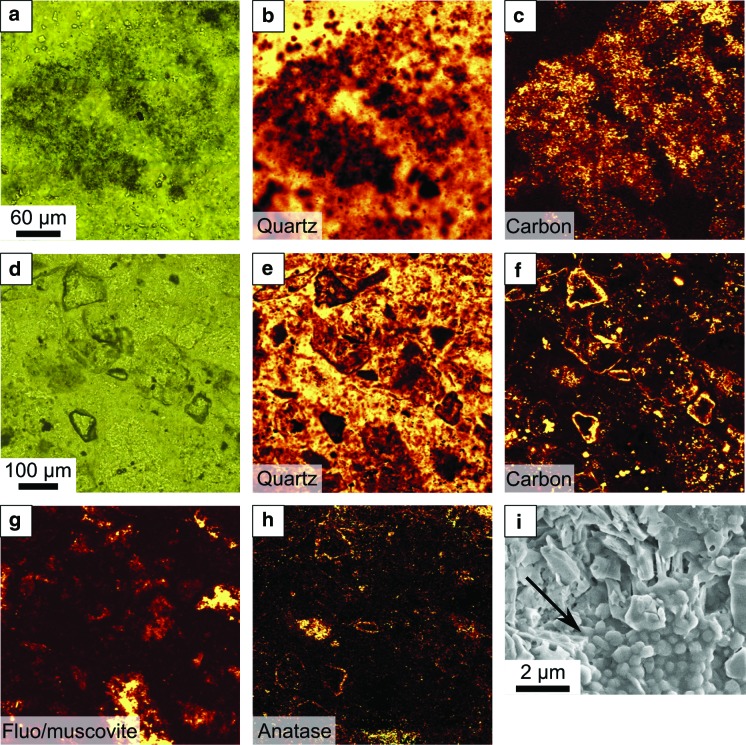
FIG. 7.
Concentrations of interpreted chemotrophic colonies on volcanic clasts in a strongly hydrothermally influenced location, Josefsdal Chert. (a) Field view of black, carbon-rich, silicified volcanic sediments from which the details are shown in micrographs (b–i). (b) Thin section micrographs of this rock showing a dark-colored (carbon-rich, ∼0.5% C), clotted biofilm consisting of thickly carbon-coated volcanic clasts (c–i) overlain by a layer of relatively carbon-poor, fine-grained sediments. In the latter, the bedding plains are outlined by very fine-grained detrital carbon as shown in the inset (small arrow). The large arrows in the inset document larger detrital flocks of carbon. (c) Optical micrograph showing a detail of the clotted biofilm in (b) and, in particular in the red box, a carbon-coated volcanic particle. (d–f) Raman mapping of individual mineral and carbon phases—quartz (d), carbon (e), and anatase (f)—to show the distribution of carbon as a thick layer on the volcanic particle, as well as in the matrix, and the presence of the volcanic clast just visible as an anatase-coated alteration surface beneath the carbon coat in (f). (g–i) Optical views and Raman compositional map of a large, sand-sized, carbon-coated volcanic particle observed in cross section. The silica-replaced volcanic particle exhibits an up to 30 μm thick carbon coat. The Raman map in (i) shows silica (quartz in yellow-orange) permeating the whole sample, carbon (green) coating the grain, and a trace of anatase (blue) indicating surface alteration of the volcanic particle.
FIG. 8.
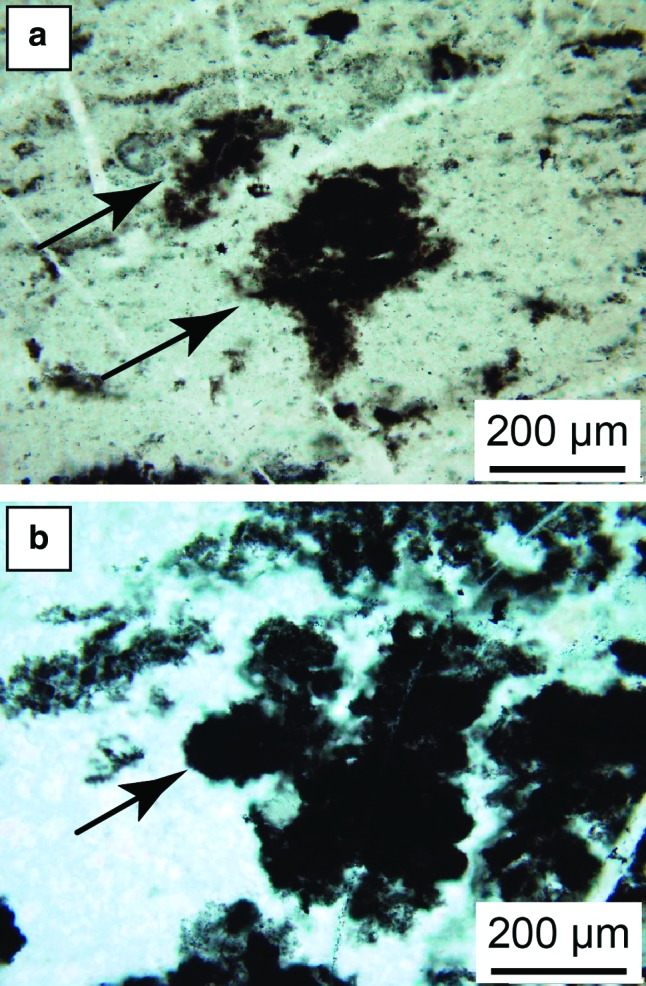
Light micrographs of “free-floating” clots. (a) Clots (arrows) characterized by irregular shapes and spiky protuberances that apparently developed in situ in strongly hydrothermally influenced sediments (same facies as in Fig. 7a). Faint wisps of carbon outline the very fine-grained sediment beds. (b) Clot (arrow) with an irregular but slightly more rounded shape, developed in primary hydrothermal silica. (Color graphics available at www.liebertonline.com/ast)
Sediments at Kitty's Gap and Josefsdal that were not influenced by nutrient-rich hydrothermal fluids can be considered to be oligotrophic. They do not present a clotted fabric (Fig. 9), and carbon is rarely visible in light microscopy, except where concentrated along sediment bedding planes (Fig. 9e). Nevertheless, Raman mapping shows that it is still relatively widespread, in particular, associated with the surfaces of volcanic particles (Figs. 9d, 9e, and 11a–11k).
Carbon is often concentrated at the surfaces of shallow-water sediment bedding planes that were within the photic zone. These concentrations generally consist of packets of thin (≤10 μm) carbonaceous laminae that coat the underlying sediments, have an undulating morphology, entrap organic and/or inorganic detrital particles, and exhibit soft deformation (tearing of plastically deformable organic material). The layers show compensation over obstacles or entrapped particles. Examples of these layers are shown in Figs. 4a–4d and 9. Where formed in the vicinity of hydrothermal fluids, the packets of carbonaceous laminae are very well preserved morphologically because of the almost immediate silicification (Fig. 4a, 4c, 4d). They are less well preserved (but still visible) in moderately coarse sediments (sand-sized) where silicification occurred during diagenesis (Figs. 4b and 9b, 9c).
Because of its low mass, detrital carbon is sedimented out with the finer, silt-clay-sized volcanic detritus and commonly outlines sedimentary structures, as shown in Fig. 7b.
Carbon also occurs as finely disseminated spots within hydrothermal chert veins (Figs. 10 and 11m) or precipitated around hydrothermal silica spherules (Foucher et al., 2015) that are observable in light microscopy and readily revealed by Raman mapping.
FIG. 10.
Kitty's Gap Chert (3.45 Ga), Pilbara, Australia. (a) Field view showing finely layered, volcanic sediments (silicified) exhibiting sedimentary structures including flaser bedding (lenticular-shaped layers), cross bedding (inclined laminations), and suspension settling (parallel laminae). Note the coarser, blocky grains (pumice) at the top of some of the layers. The lens cap is 6.8 cm in diameter. (b) Details of the sedimentary structures showing parallel and cross bedding. The red box marks the details shown in (c). (c) Micrograph of a thin section viewed in transmitted light. The lower, clear part represents an intrusive hydrothermal chert vein that penetrated into the already slightly lithified sediment. The upper part shows inclined, normally graded beds of fine to medium sand at the base grading upward into finer (silt to clay), poorly sorted sediments. The red boxes mark details documented by μ-Raman spectral mapping illustrated in Fig. 11. (Color graphics available at www.liebertonline.com/ast)
The above description of carbon in these Mars analog volcanic sediments illustrates two important points: (1) the macroscale heterogeneity in its distribution and (2) the fact that, although globally heterogeneous, on a microscopic scale, the carbon is very specifically located with respect to substrate and environment. Its distribution is not haphazard. In the following, we will address the origins of the above-described carbonaceous signatures from both abiotic and a biogenic points of view.
3.3.4. Interpretation of the carbonaceous signatures
(1) Carbonaceous films around volcanic clasts. There are two possible explanations for the formation of carbonaceous films around the volcanic particles. They may represent either a macromolecular conditioning film or microbial biofilm formation. In seawater, conditioning films form within minutes of immersion of a surface. On present-day Earth, these molecule-thick layers (angstroms to nanometers) consist of adsorbed organic macromolecules of biological origin (e.g., exopolymers, proteins, humic acids; Jain and Bhosle, 2009). Following conditioning film formation, microbial biofilms develop by adhesion of individual cells via exopolymeric substances to a “conditioned” particle surface, followed by growth of microcolonies and further colonization by one or more microbial species. The distribution of the microcolonies is spatially and temporally heterogeneous (Lehaitre et al., 2008), and the biofilms may vary greatly in thickness, depending on external factors such as temperature and, especially, flow rate affecting nutrient supply (Pedersen, 1982).
However, on early Earth and on Mars, seawater or lake waters probably also contained dissolved abiotic organic molecules of hydrothermal and/or extraterrestrial origin. Hypothesizing that the organic carbon coatings on the volcanic particles in the Kitty's Gap and Josefsdal sediments were simply due to abiotic organic adhesion to the particle surface, the question arises: why do the particles close to hydrothermal vents have such a thick, irregular organic coat? It could be argued that the thicker carbon coats were caused by copious precipitation of organics from hydrothermal fluids. There are, however, a number of observations that appear to refute this hypothesis. The volcanic particles were rapidly sedimented and trapped within a sedimentary matrix. It was not possible for them to be tumbled in the hydrothermal fluids, such that numerous layers of organics could be precipitated. Moreover, microscopic observation of the heavily coated particles shows that the coatings are not simply multiple laminar deposits as would be expected from precipitation from a fluid. Instead, they are complicated, three-dimensional structures that exhibit delicate tufts or protuberances (Figs. 7g, 7h) that suggest in situ growth (Westall et al., 2015).
In situ δ13C values obtained from the thickly carbon-coated volcanic clasts range from ∼−15‰PVDB to −20‰PVDB. These values overlap with those of abiotic meteoritic (Pearson et al., 2006) and hydrothermal (McDermott et al., 2015) carbon. Nevertheless, the range of isotopic ratios measured in situ is limited, a characteristic of microbial fractionation but not abiotic carbon.
A scanning electron microscopy study (Westall et al., 2006a, 2011a) of thin carbon coatings on Kitty's Gap volcanic particles showed that the films consisted of associations of silicified coccoidal structures forming a monolayer at the surfaces of the particles (Figs. 4h and 11d). The simple, spherical morphologies of these features could be produced by abiogenic minerals or by microorganisms. Again, hypothesizing an abiotic origin, it could be argued that the structures consist of abiotic hydrothermal silica spherules (e.g., Fig. 2g) coated with precipitated hydrothermal organic carbon (and then recoated with silica). There are, on the other hand, a number of arguments against this interpretation. In the first place, the Kitty's Gap samples were obtained at a certain distance (a couple of meters) from a cross-cutting hydrothermal vein and not directly adjacent to or within it. There is no evidence in the horizon from which the sample was taken of hydrothermal intrusion leaving soft sediment deformation features that would be indicative of direct, contemporaneous influence. Rather, hydrothermal fluids penetrated parallel to sediment bedding planes in sediments that were already partially lithified (Fig. 9c). Note that van den Boorn et al. (2007) determined that different layers of the Kitty's Gap sediments were influenced to different degrees by hydrothermal fluids mixing with ambient seawater. Thus, precipitation of silica spheres directly from hydrothermal fluids does not appear to be an acceptable explanation. Moreover, the coccoidal associations exhibit physical characteristics and behavior that is more typical of colonies of coccoidal microorganisms than abiotic precipitations. These include association of two sizes of coccoids that exhibit restricted size and shape, likely representing two species, within a filmy substance that resembles EPS; juxtaposed coccoids in various stages of separation, sometimes exhibiting a meniscus between the coccoids; dividing coccoids where one of the cells is full and turgid while the other is deflated indicating lysis or cell death; and a coating of slightly wrinkled, thin films on the coccoids, similar to cell-bound EPS (Westall et al., 2006a, 2011a).
Thus, the combined observations support the interpretation of microbial biofilm development on the volcanic clasts. The direct association of microbial colonies with the volcanic surfaces may be a clue as to the lifestyle of the organisms, whereby they could obtain nutrients from redox reactions at the surfaces of the volcanic substrate. This hypothesis is supported by the observation of corrosion tunnels filled with extracellular polymer (Foucher et al., 2010; Westall et al., 2011a). This association suggests that the fossilized microorganisms are the remains of chemolithotrophic microorganisms. In the environments influenced by hydrothermal fluid, these fluids could have provided nutrients for both lithotrophy and organotrophy, hence the greater biomass development in these locations.
(2) “Free-floating” carbonaceous clots. These irregularly shaped, three-dimensional structures must have formed in situ since there is no way that such delicately shaped features could have been allochthonously transported. They are not supported by clastic particles. Precipitation of carbon from hydrothermal fluids is not likely to produce such delicate, tufted structures. We hypothesize that they represent chemotrophic microbial colonies supplied with nutrients by the hydrothermal fluids.
(3) Carbonaceous laminations on bedding-plane surfaces. Laminated carbonaceous concentrations at the surfaces of sedimentary bedding planes could be explained as sedimented detrital carbon (of abiotic or biotic origin, including floating microbial colonies, cf. Thompson et al., 1990), films of purely abiotic carbon, or microbial biofilms. There are certain features of the undulating laminated films that would be difficult to explain if they were precipitated from fluids enriched in abiotic molecules. In all cases, the films always consist of packets of very fine laminae about 10 μm thick that do not conform completely to the underlying substrate but rather undulate in a finely irregular fashion on top of it. A film that had simply been precipitated should exhibit conformable layering. The undulating, laminated films must have consisted of cohesive material, such as polymer, in order to exhibit the kind of soft deformation phenomena demonstrated in Fig. 4a, 4c, 4d (see also Westall et al., 2006b). Moreover, there are additional features that are difficult to explain by abiotic precipitation. Scanning electron microscope observation (Westall et al., 2006b, 2011b) shows that the upper surface of the films consists of thin filaments (<0.5 μm) thickly coated with a smooth polymerlike substance (similar to EPS). Cross sections of the films show that, below the silicified filaments on the upper surface, the underlying part of the films consists of a network of degraded organics (with enriched S contents typical of the sulfurization process during microbial degradation of OM) in which nanometer-sized crystallites of aragonite were precipitated. Westall et al. (2011b) explained the precipitation of carbonate as being the result of heterotrophic degradation of OM. During this process, the microbial metabolic activity leads to a decrease in local pH causing the release of Ca2+ from the EPS, which then combines with dissolved CO2 in the seawater. It is not certain (a) whether abiotic carbon would present such a network structure as is characteristic of degraded biogenic OM or (b) whether aragonite could precipitate within the abiotic OM. In situ molecular analysis of the OM showed that it had a restricted composition, more indicative of biological than abiotic OM. On the basis of the physical characteristics of the films, their −27‰VPDB δ13C value, and the in situ calcification of the films, Westall et al. (2006b, 2011b) suggested that these structures were the remains of phototrophic microbial biofilms.
The examples of biosignatures in the ∼3.45–3.33 billion-year-old Kitty's Gap and Josefsdal Cherts demonstrate that, where there are volcanic particles in contact with water and a source of OM and energy, chemotrophic microbial life can be potentially ubiquitous on a microbial scale, although biomass (quantity of organic carbon produced) development is limited by the availability of nutrients. This means that, on early Earth, biomass is generally very low (cf. Sleep and Bird, 2007) unless directly associated with hydrothermal activity (Westall et al., 2015).
3.3.5. Microscale distribution of chemotrophic colonies in the Kitty's Gap Chert
A particularly interesting aspect of these sediments is the distribution of the microbial colonies and the way in which their traces were preserved. Westall et al. (2006a, 2011a) studied a 5 cm section of these sediments in detail, analyzing each 2–6 mm thick layer on a microbial scale. Numerous silicified microbial colonies or the degraded remains of colonies were observed within each layer. The characteristics of the microbial remains within different sediment layers are slightly different, and the median size of cells varies between different layers. In some layers, the cells were dead (lysed) and degraded before silicification, whereas in others the cells were flourishing and dividing. Organic carbon contents for each layer vary between 0.01% and 0.05%, while the average isotopic composition based on bulk measurements of each layer varies between −25.9‰ and −27.8‰ (measured by step combustion, Westall et al., 2006a). Note that these concentrations of organic carbon have been diluted by the massive, penecontemporaneous silicification of the sediments, together with their microbial content.
To illustrate the size and distribution of fossilized colonies (Figs. 2f, 4h, and 11i) within one 4 mm thick sediment layer, three specific areas ranging in size from 1500 to 3000 μm2 were examined. Although they were all within one sediment layer, the number and size of microbial colonies (poorly or well preserved) varied greatly (Table 3). For instance, area 1 contained four very large colonies (up to 405 μm2) with an average of 0.3 colonies per 100 μm2; in areas 2 (3000 μm2) and 3 (1800 μm2) the colonies were more frequent (13 each) and smaller, with average sizes of about 10–20 μm2 but ranging up to 205 μm2, and between 0.4 and 0.7 colonies per 100 μm2. The pertinence of these few statistics is that colony size, frequency, and distribution are extremely variable within one and the same microbial-scale habitat.
Table 3.
Semiquantitative Estimation of Chemotrophic Colony Distribution in the Kitty's Gap Sediments
| Colony statistics | ||||||
|---|---|---|---|---|---|---|
| Area measured μm2 | Colonies | No. colonies 100 μm2 | Size μm2 | Av. size μm2 | % total area | Total area covered μm2 |
| 1500 | 1 | 0.3 | 300 | 200 | 20 | 53 |
| 2 | 33 | 2 | ||||
| 3 | 60 | 4 | ||||
| 4 | 405 | 27 | ||||
| 3000 | 1 | 0.43 | 18.4 | 12 | 0.6 | 5.5 |
| 2 | 18.4 | 0.6 | ||||
| 3 | 4.6 | 0.2 | ||||
| 4 | 11.5 | 0.4 | ||||
| 5 | 18.4 | 0.6 | ||||
| 6 | 9.2 | 0.4 | ||||
| 7 | 4.6 | 0.2 | ||||
| 8 | 4.6 | 0.2 | ||||
| 9 | 4.6 | 0.2 | ||||
| 10 | 2.3 | 0.1 | ||||
| 11 | 6.9 | 0.3 | ||||
| 12 | 32.2 | 1 | ||||
| 13 | 20.7 | 0.7 | ||||
| 1800 | 1 | 0.72 | 47 | 32 | 1.3 | 20.5 |
| 2 | 63 | 1 | ||||
| 3 | 205 | 6 | ||||
| 4 | 7.5 | 2 | ||||
| 5 | 21 | 6 | ||||
| 6 | 4.5 | 0.15 | ||||
| 7 | 13.5 | 0.3 | ||||
| 8 | 9 | 0.25 | ||||
| 9 | 4.5 | 0.15 | ||||
| 10 | 33 | 0.9 | ||||
| 11 | 7.5 | 2.1 | ||||
| 12 | 9 | 0.25 | ||||
| 13 | 3 | 0.1 | ||||
The sediments in the Kitty's Gap Chert exposure are laterally continuous over a number of meters. The particular layer described above can be followed over 3 m. In the section studied, its average carbon content is 0.02%, while the carbon isotope signature is −27.3%. We can make a rough estimate of the number of colonies that could occur in this one, 4 mm by 3 m layer by averaging the frequency and sizes of the colonies examined in the layer, that is, 0.5 colonies/100 μm2, 45 μm2 in size, covering about 25% of the total area of view. Over the entire exposure of this layer, this gives us a rough value of 60,000 colonies.
3.4. Consequences for Mars
In scenarios for the origin of life, the first living cells would have had only a minimal number of metabolic and reproductive components and would have been probably very small, similar to micelles and, possibly, similar in size to viruses, that is, ≤200 nm [Madigan et al. (2000), although notably some viruses can be very large, e.g., Mimivirus with a diameter of 750 nm, La Scola et al. (2003)]. On Earth, even with continuous evolution, by 3.45 Ga chemotrophic microorganisms living in, or on, volcanic substrates were still very small, <1 μm in size (Figs. 2d, 2f, 4h, and 11d) (Westall et al., 2006b, 2011a). Even today, subsurface chemotrophic microorganisms are extremely small with diameters ∼0.3–0.8 μm (Ciobanu et al., 2014). Primitive martian microorganisms may have had (and may still have) similar characteristics.
Biosignature preservation on Mars would have proceeded as described for terrestrial microorganisms in Section 3.1. The biosignatures would have been in the form of mineral-replaced and/or coated organisms entombed in sediments and chemical precipitates; organic molecules/structures trapped in fine-grained, anoxic sediments; and corrosion features on volcanic particles. Other signs of metabolic activity, such as inhomogeneous elemental distributions or fractionated isotopic signatures, would have constituted biosignatures as well. However, abiotic carbon isotope ratios in meteorites (Pearson et al., 2002) and the signatures of carbon molecules produced by abiotic Fischer-Tropsch synthesis in hydrothermal environments (McCollom and Seewald, 2006) can overlap considerably with those of terrestrial life (as light as −50‰ δ13C). Other indications of biogenicity include a chiral signature (although racemization through geological time could be a problem) and a complex but relatively restricted molecular composition.
Biosignature identification in situ on Mars is more problematic than on Earth because of the lack of sophisticated instrumentation and sample preparation techniques. The description in Section 3.3 of the terrestrial Early Archean chemotrophic biosignatures places strong emphasis on the microscale distribution and colocation of the organic signatures with other features, such as volcanic clast surfaces or sediment surfaces and primary hydrothermal precipitates, and their molecular composition and δ13C isotopic ratios. Thus, the possibility of documenting the spatial distribution of organic carbon with respect to the mineral matrix [outcrop to micron-scale, e.g., Westall et al. (2015)] would be highly desirable.
Given the much lower geological activity on Mars since the Hesperian period than over the equivalent interval on Earth, martian biosignatures in Noachian rocks, which formed when surface conditions were a priori habitable, have a greater potential for having survived. Nearly two-thirds of the planet's surface is covered by Noachian-age materials. Even though Noachian-age rocks on Mars have generally not been submitted to the often destructive forces of plate tectonic burial and recycling that are typical of the Earth, potential biosignatures in these rocks would be very old, may have been buried beneath accumulated sediment layers and later exhumed by erosion, and certainly would have undergone a certain amount of degradation, mainly with respect to any of the early-formed organic molecules. They may not exhibit the degree of alteration (including metamorphism up to lowermost greenschist facies) of the terrestrial Kitty's Gap and Josefsdal sediments (3.45–3.33 Ga) used as analog examples here. Also, our analog sediments were exposed to aqueous alteration at high water/rock ratios at apparently warm to hot temperatures (van den Boorn et al., 2007; Hofmann and Harris, 2008; Westall et al., 2015), as opposed to the volcanic sediments that were altered at low temperature under low water/rock ratios in the 3.7–3.6 billion-year-old Gale Crater, for instance (Bridges et al., 2015), although the contact between the water and the rocks/particles was restricted in the terrestrial examples because of generally rapid silicification. Despite the fact that the microbial signatures in these terrestrial sediments were particularly well preserved at the microscopic scale due to very rapid silicification, identification of the biosignatures in these rocks is not without controversy, even given the availability of sophisticated analytical capabilities. Two main questions always need to be asked: Is the potential feature a bona fide biosignature and not an abiogenic look-alike and, if it is biogenic, did it form at the same time as the rocks, that is, is it syngenetic (e.g., Westall and Folk, 2003; Rasmussen et al., 2008)?
To answer these questions, analysis of the analog Early Archean biosignatures described in Section 3.3 required the use of many techniques that are, at present, incompatible with space missions: for example, thin section preparation, etching of rock surfaces, high-resolution scanning electron microscopy, even synchrotron techniques. This limitation must be considered during in situ measurement on Mars; that is, nondetection of biosignatures could be due to technical limitations. The instrumentation presently on Mars and to be flown in future missions should be capable of identifying at least organic biomolecules in the bulk of the rock, if they are present (Freissinet et al., 2015). Nonetheless, as demonstrated above, documentation of the distribution of carbon with respect to specific structural or textural features of the rock at the micron-scale level is important and will greatly aid interpretation. Instruments on the Mars2020 mission (SHERLOC and PIXL) plan to map rock surfaces using Raman and X-ray fluorescence spectrometry, respectively, and will be a valuable aid to selecting samples for return to Earth and detailed laboratory analysis.
The surface of Mars is subjected to the combined effects of radiation and oxidation that destroy much of the OM expected to be present (Atreya et al., 2006, 2011; Dartnell, 2007; Sephton and Botta, 2008), the latter primarily in the form of abiotic, extraterrestrial OM but possibly also biogenic organics if a previously inhabited habitat were to become exposed. Moreover, seeking candidate landing sites that experienced active erosion resulting in (relatively) recent exposures would provide access to rocks having a greater potential for the preservation of molecular biosignatures. The recent detection of chlorinated hydrocarbons (Ming et al., 2014; Freissinet et al., 2015) and methane (Webster et al., 2015) in Gale Crater by the SAM instrument on Curiosity hints, for the first time, at the presence of organic molecules on Mars. With respect to methane, it appears that the origin of a part of the gas in Gale Crater cannot be explained by known abiotic processes, leaving open the possibility of a potential subsurface biogenic source.
Although the demonstration of martian life would constitute a result of primary importance, whatever its age and relationship with the host rock, it will also be important to understand the timing of the formation and emplacement of the biosignature within its geological context to better interpret the conditions under which it developed and/or existed. For instance, water-lain sediments may contain the biosignatures of colonies of chemotrophic organisms that inhabited the volcanic sediments and/or associated subaqueous hydrothermal environments, or they may contain detrital or dissolved organic molecules chemically bonded to phyllosilicates, or even eroded detrital fragments of rocks that contained preserved biosignatures. Once lithified, these same sediments and mineral deposits could have hosted endolithic species in cracks or intergrain spaces (Cockell et al., 2002; Westall and Folk, 2003). On Earth, endolithic microorganisms tend to be phototrophic cyanobacteria and eukaryotic fungal species, presumably with associated prokaryotic chemoorganotrophs feeding off the OM of the primary producers. However, on an anaerobic world, chemotrophs could colonize cracks in volcanic rocks under water, for example within exuded lavas or impact-ejected volcanic rocks that fell into water bodies.
Table 4 summarizes the characteristics of the signatures of chemotrophic life-forms as well as abiotic/prebiotic organics, their occurrences, potential preservation, and other features. Following on from the discussion above, it is possible that, for early Mars as on early Earth when the flux of extraterrestrial organics to the surface was greater than today, abiogenic signatures could have co-occurred with those of biogenic origin. Both biogenic and abiogenic signatures could be preserved in situ (i.e., where formed or where they fell, in the case of extraterrestrial OM), or they could have been hydraulically transported and concentrated, or transported in impact ejecta.
Table 4.
Summary of Biosignature and Abiotic/Prebiotic Biosignature Characteristics
| Type of signature | Occurrence | Preservation | In situ or allochthonous | Macroscopic/microscopic | |
|---|---|---|---|---|---|
| Mineralized cells, colonies | Chemotrophic | Apparently widely distributed around volcanic particles (silt to sand-sized) in aqueous environments; reduced biomass development in oligotrophic environments, higher in hydrothermal environments; possibly in fluid inclusions in rapidly precipitated cements of the volcanic environments or evaporitic or hydrothermal environments (yet to be identified); possibly in hydrothermal conduits; possibly in cracks in submerged volcanic or other kinds of rock | By rapid mineralization and occlusion of pore space | In situ in a rock or in a portion of microfossil-containing rock that has been transported (or ejected) from elsewhere | Microscopic |
| Concentrations of colonies can form biofilms | May form macroscopically visible black layers in rocks due to relatively high C content | ||||
| Phototrophic | On the surfaces of bedding planes/rocks in the photic zone | Possibly macroscopic, certainly microscopic | |||
| Biogenic organics | May or may not be associated with body fossils or other microbial remains preserved in situ, as described above; generically disseminated in fine-grained, anaerobic sediments, associated with phyllosilicates; trapped in evaporitic or hydrothermal precipitates | In anaerobic matrices, e.g., fine-grained sediments, mineral cements, or chelated to phyllosilicates | In situ in a rock or in a portion of carbonaceous rock that has been transported or ejected from elsewhere | Microscopic but may be macroscopic if the organic components have been hydraulically concentrated or if there was sufficient energy to support a huge biomass, e.g., in the vicinity of hydrothermal activity | |
| Abiogenic prebiotic organics | Extraterrestrial organics: endogenous organics to be expected in hydrothermal fluids, present as finely disseminated particulate matter, as precipitates around hydrothermal silica spheres, or in other forms not yet identified; exogenous (meteoritic/IDPs) and endogenous (hydrothermal) may be present as particulate organics or chelated to mineral substrates; both possibly concentrated by hydraulic processes and associated with fine-grained sediments | In anaerobic matrices, e.g., fine-grained sediments, mineral cements, or chelated to phyllosilicates | In situ in a rock or in a portion of carbonaceous rock that has been transported or ejected from elsewhere | Microscopic but may be macroscopic if the organic components have been hydraulically concentrated or if there was sufficient energy to support a huge biomass, e.g., in the vicinity of hydrothermal activity | |
4. Scenarios for the Search for Life on Mars
On Earth, life may have appeared in a particular location where the conditions were favorable before colonizing the rest of the planet, distributed by ocean currents. On Mars, as explained in Fig. 1, owing to the absence of a large, global ocean, life may have appeared and disappeared in a particular place without extending to the whole planet. On the other hand, surface and subsurface hydrologic flows and geological and aerial (impact) processes may have transported living, dormant, or fossilized microorganisms from one location to another. While these processes may not be particularly relevant when looking for life on Earth—since life is everywhere—they will have important consequences for the search for life on Mars. For instance, it is possible to envisage the situation where life did not appear in a particular location; but in that same location, an impact crater or ponded depression could have hosted extant or dormant cells transported in impact ejecta or fluvial detritus, which could have survived for the lifetime of the habitable conditions. In an alternative scenario, dormant cells originating from a location that had become inhospitable could have been transported and rapidly colonized a newly inhabitable environment. Such organisms also could have been potentially preserved as fossil traces. Impact ejecta or fluvial detritus may also have deposited fragments of rock containing fossil traces of life, or detrital organic carbon in the case of aqueous transport, to a location that was never habitable. All these scenarios must be considered when looking for life at any particular location (landing site) on Mars. Moreover, as noted above in Section 3 (and in Summons et al., 2011), when looking for past traces of life, the possibilities for, or lack of, fossilization and preservation need to be taken into account. Microbial life may not necessarily have been preserved because of adverse environmental conditions or because the organisms did not support the process of fossilization (cf. Orange et al., 2009) and were destroyed. Fossil biosignatures may also be destroyed by geological processes and impacts.
Finally, several scenarios can lead to the absence or presence of traces of extant, dormant, or fossil life in a given area at the present time as displayed in Fig. 12. In this scheme, note that the presence of abiotic/prebiotic organic molecules is considered to be ubiquitous.
FIG. 12.
Scenarios for life at a given location on Mars taking into account the notion of local habitability, fossilization and preservation, and transportation. (Color graphics available at www.liebertonline.com/ast)
Although 7 of the 16 possible scenarios described in Fig. 12 lead to the absence of biosignatures, only one corresponds to the non-appearance of life on Mars. These scenarios underline the fact that there may be no traces of life in a given place on Mars even if this place had been previously inhabited. On the contrary, due to transportation processes, a locality where life never developed could potentially contain traces of life. These different scenarios for the history of life at a particular location thus have important consequences on the observations made during in situ exploration of the planet. Several scenarios can lead to the same observations and, without the possibility of reconstructing the context, could lead to a number of possible interpretations. Moreover, as explained above, the expected traces of life would probably be very subtle and, due to the technical limitations of instrumentation, difficult to detect in situ. Finally, taking into account the different scenarios, technical limitations, and the potential for contamination (cf. Mahaffy et al., 2015) inherent to any measurement, several possibilities have to be considered while interpreting in situ observations.
Landed planetary missions are complex, expensive enterprises that take many years to develop, and realistically, we can only accomplish a few of them. So, do the above considerations help us in selecting where to search for life? How can we maximize the chance to discover (potential) biosignatures? The obvious answer is to go to the “place and time” having the highest chance of having hosted microorganisms. In the case of Mars, two scenarios stand out: (1) ancient locations (4.2–3.7 billion years old) that have clear indications for prolonged, low-energy, water-rich environments (deposits lain in basins, ponded water, hydrothermal deposits, etc.) and (2) the deep subsurface (∼2 km deep) where even today we can expect liquid water to exist and possibly support extant life. With the type of missions we have at our disposal, Scenario 1 is within our technical reach. Scenario 2, on the other hand, would require a type of drill that has not yet been implemented for planetary exploration but should receive consideration for the future, particularly if the putative atmospheric methane measurements could be connected with the possible presence of active subsurface biology.
Regarding Scenario 1, why favor the Noachian? There are also plentiful indications of surface water having existed during the Hesperian. Whereas both periods may have been habitable, we can argue that the Noachian, and in particular the early Noachian, was more so. Why? Because during the early Noachian it is likely that the lateral connectivity between potential habitats would have been higher. That is, there would have been more opportunity for surface water or subsurface aquifers to transport nutrients and microorganisms. The presence of surface water during the Hesperian, on the other hand, would have gradually become more and more episodic. We do not imply that biosignatures may not be found in Hesperian terrain, but the chance is higher for life to have gained a foothold and moved laterally during the Noachian.
The observations and the possibilities of interpretations displayed in Fig. 13 may be held concurrently. For example, observation of active and dormant life may be due to contamination by living organisms from Earth and/or to the presence of dormant martian cells. This figure highlights the difficulty in detecting and demonstrating the existence of potential past or present microbial life on Mars.
FIG. 13.
Demonstration of the possibilities for interpreting the presence or absence of biosignatures at a particular landing site on Mars. (Color graphics available at www.liebertonline.com/ast)
5. Conclusions
5.1. What?
The lack of continuously hospitable conditions on Mars would have inhibited the evolution of potential microorganisms. It is likely that martian life, necessarily chemotrophic, would have remained (and may still remain) in a very small, primitive state. Preserved chemotrophic life in analog volcanic/hydrothermal environments on anaerobic early Earth indicates that these primitive life-forms could be very widely distributed, although their combined biomass would be low, unless in the vicinity of hydrothermal activity.
The remains of potential martian chemotrophic life would be preserved in the same way as microbial remains on Earth, as physical structures, as complex chiral carbon molecules with specific isotopic signatures, or as other types of biosignatures. Due to the generally low biomass, the quantity of organic compounds preserved would be diluted by the preserving mineral medium. Our missions have to be prepared for this.
5.2. Where?
The specific conditions for the appearance of life may have been met at various times and various places on Mars. The extreme heterogeneity of habitats and their general lack of interconnectivity impose severe constraints on where we should look for potential martian life-forms. The degree of interconnectivity would have depended on the efficiency of the subsurface hydrologic system, the opportunities of transient fluvial activity, or potential transport of viable cells in impact ejecta. It would be preferable to search for biosignatures at a location where they could have been concentrated, as in hydrothermal, lacustrine, or paleosol sedimentary environments. To ensure better chances of preservation, the geological units should have undergone limited diagenesis and metamorphism, and the rocks should have not experienced prolonged contact with the martian surface and its atmosphere, where oxidizing conditions and radiation would have destroyed much of the organic components. The Curiosity rover can retrieve samples of rocks up to 10 cm depth. The drill on the ExoMars 2018 rover, however, will be able to penetrate up to 2 m, thus being able to reach possible organic-containing materials at depths below the reach of long-term cosmic radiation.
5.3. How?
Even if our missions can reach a key location where (well-) preserved molecular biosignatures (at least the heavier fraction of the compounds) can be accessed in the geological record (whether with a deep drill or in a recently, naturally exhumed/excavated area), Mars has thrown one other interesting challenge at us. The in situ instrumentation on Curiosity, ExoMars, and possibly on Mars 2020 could, in principle, have the capacity to identify many molecular biosignatures. However, the detection and identification of organic compounds on Mars is complicated by the presence of perchlorates, which can be activated and degrade organics depending on the method used for extracting and delivering the compounds to the detector (Glavin et al., 2013). Our future missions need to be robust against the possible presence of perchlorates in the deposits and rocks to be studied.
The identification of potential organic biosignatures in a bulk sample will be of the greatest importance. At a minimum, such a finding will indicate that life could have existed on the planet. Such a discovery will pave the way for the more arduous, follow-up investigations into the exact nature of the biosignature, its distribution, and the implications for the mode of life of the original microorganisms. Ultimately, this may require analysis of martian samples with the sophisticated technology available in terrestrial laboratories.
Abbreviations Used
- EPS
extracellular polymeric substances
- IDPs
interplanetary dust particles
- IOM
insoluble organic matter
- MISS
microbially induced sedimentary structures
- OM
organic matter
- SAM
Sample Analysis at Mars
- SEM
scanning electron microscope
- SOM
soluble organic matter
Acknowledgments
Roberta Cecchi is gratefully acknowledged for her editorial aid and construction of Table 1. This work was supported by grants to F.W. from CNES, ANR-09-BLAN-0219-01, CNRS-MI-2014, and the MASE project [supported by European Community's Seventh Framework Programme (FP7/2007-2013) under Grant Agreement n° 607297], to K.C. from the Marsden Fund (RSNZ), to D.G. and K.C. from the National Geographic Society, and to K.C. from a LE STUDIUM Institute for Advanced Studies research fellowship. D.L. worked under the support of the European Research Council under the European Union's Seventh Framework Program (FP7/2007–2013)/ERC Grant agreement n° 280168. The authors thank Norman Sleep for his comments on the manuscript.
References
- Alexander C.M.O'D., Newsome S.D., Fogel M.L., Nittler L.R., Busemann H., and Cody G.D. (2010) Deuterium enrichments in chondritic macromolecular material—implications for the origin and evolution of organics, water and asteroids. Geochim Cosmochim Acta 74:4417–4437 [Google Scholar]
- Allwood A.C., Walter M.R., Kamber B.S., Marshall C.P., and Burch I.W. (2006) Stromatolite reef from the Early Archaean era of Australia. Nature 441:714–718 [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna J.C. and Lewis K.W. (2011) Early Mars hydrology: 2. Hydrological evolution in the Noachian and Hesperian epochs. J Geophys Res 116, doi: 10.1029/2010JE003709 [DOI] [Google Scholar]
- Arndt N. (1994) Archaean komatittes. In Archaean Crystal Evolution, Developments in Precambiran Geology 11, edited by Condie K.C., Elsevier, Amsterdam, pp 11–44 [Google Scholar]
- Atreya S.K., Wong A.-S., Renno N.O., Farrell W.M., Delory G.T., Sentman D.D., Cummer S.A., Marshall J.R., Rafkin S.C.R., and Catling D.C. (2006) Oxidant enhancement in martian dust devils and storms: implications for life and habitability. Astrobiology 6:439–450 [DOI] [PubMed] [Google Scholar]
- Atreya S.K., Witasse O., Chevrier V.F., Forget F., Mahaffy P.R., Price P.B., Webster C.R., and Zurek R.W. (2011) Methane on Mars: current observations, interpretation, and future plans. Planet Space Sci 59:133–136 [Google Scholar]
- Banfield J.F., Moreau J.W., Chan C.S., Welch S.A., and Little B. (2001) Mineralogical biosignatures in search for life on Mars. Astrobiology 1:447–465 [DOI] [PubMed] [Google Scholar]
- Barbieri R., Ori G.G., and Cavalazzi B. (2004) A Silurian cold seep ecosystem from the Middle Atlas, Morocco. Palaios 19:527–542 [Google Scholar]
- Baross J.A. and Hoffman S.E. (1985) Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Orig Life 15:327–345 [Google Scholar]
- Biemann K.R. (2007) On the ability of the Viking gas chromatograph–mass spectrometer to detect organic matter. Proc Natl Acad Sci USA 104:10310–10313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J.L., Dobrea E.Z.N., McKeown N.K., Parente M., Ehlmann B.L., Michalski J.R., Milliken R.E., Poulet F., Swayze G.A., Mustard J.F., Murchie S.L., and Bibring J.-P. (2008) Phyllosilicate diversity and past aqueous activity revealed at Mawrth Vallis, Mars. Science 321:830–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C.C., D'Hondt S., Spivack A.J., and Kingsley R.H. (2007) Radiolytic hydrogen and microbial respiration in subsurface sediments. Astrobiology 7:951–970 [DOI] [PubMed] [Google Scholar]
- Botta O. and Bada J.L. (2002) Extraterrestrial organic compounds in meteorites. Surv Geophys 23:411–467 [Google Scholar]
- Brack A. (1997) Life on Mars: a clue to life on Earth? Chem Biol 4:9–12 [DOI] [PubMed] [Google Scholar]
- Bridges J.C., Schwenzer S.P., Leveille R., Westall F., Wiens R.C., Mangold N., Bristow T., Edwards P., and Berger G. (2015) Diagenesis and clay mineral formation at Gale Crater, Mars. J Geophys Res Planets 120, doi: 10.1002/2014JE004757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady S.L. and Farmer J.D. (1996) Fossilization processes in siliceous thermal springs: trends in preservation along thermal gradients. In Evolution of Hydrothermal Ecosystems on Earth (and Mars?), Ciba Foundation Symposium 202, edited by Bock G.R. and Goode J.A., John Wiley & Sons, Chichester, UK, pp 150–173 [DOI] [PubMed] [Google Scholar]
- Cady S.L., Farmer J.D., Grotzinger J., Schopf J.W., and Steele A. (2003) Morphological biosignatures and the search for life on Mars. Astrobiology 3:351–368 [DOI] [PubMed] [Google Scholar]
- Campbell K.A. (2006) Hydrocarbon seep and hydrothermal vent paleoenvironments and paleontology: past developments and future research directions. Palaeogeogr Palaeoclimatol Palaeoecol 232:362–407 [Google Scholar]
- Carr M.H. (2006) The Surface of Mars, Cambridge University Press, Cambridge, UK [Google Scholar]
- Carr M.H. and Head J.W., III (2003) Oceans on Mars: an assessment of the observational evidence and possible fate. J Geophys Res Planets 108, doi: 10.1029/2002JE001963 [DOI] [Google Scholar]
- Carter J., Poulet F., Bibring J.-P., and Murchie S. (2010) Detection of hydrated silicates in crustal outcrops in the northern plains of Mars. Science 328:1682–1686 [DOI] [PubMed] [Google Scholar]
- Cavalazzi B., Barbieri R., Cady S.L., George A.D., Gennaro S., Westall F., Lui A., Canteri R., Rossi A.P., Ori G.G., and Taj-Eddine K. (2012) Iron-framboids in the hydrocarbon-related Middle Devonian Hollard Mound of the Anti-Atlas mountain range in Morocco: evidence of potential microbial biosignatures. Sediment Geol 263:183–193 [Google Scholar]
- Chivian D., Brodie E.L., Alm E.J., Culley D.E., Dehal P.S., DeSantis T.Z., Gihring T.M., Lapidus A., Lin L.-H., Lowry S.R., Moser D.P., Richardson P.M., Southam G., Wanger G., Pratt L.M., Andersen G.L., Hazen T.C., Brockman F.J., Arkin A.P., and Onstott T.C. (2008) Environmental genomics reveals a single-species ecosystem deep within Earth. Science 322:275–278 [DOI] [PubMed] [Google Scholar]
- Christner B.C., Mosley-Thompson E., Thompson L.G., and Reeve J.N. (2003) Bacterial recovery from ancient ice. Environ Microbiol 5:433–436 [DOI] [PubMed] [Google Scholar]
- Ciobanu M.-C., Burgaud G., Dufresne A., Breuker A., Rédou V., Maamar S.B., Gaboyer F., Vandenabeele-Trambouze O., Lipp J.S., Schippers A., Vandenkoornhuyse P., Barbier G., Jebbar M., Godfroy A., and Alain K. (2014) Microorganisms persist at record depths in the subseafloor of the Canterbury Basin. ISME J 8:1370–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford S.M. (1993) A model for the hydrologic and climatic behavior of water on Mars. J Geophys Res 98:10973–11016 [Google Scholar]
- Clifford S.M. and Parker T.J. (2001) The evolution of the martian hydrosphere: implications for the fate of a primordial ocean and the current state of the northern plains. Icarus 154:40–79 [Google Scholar]
- Cockell C.S. (2014) Trajectories of martian habitability. Astrobiology 14:182–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockell C.S. and Raven J.A. (2004) Zones of photosynthetic potential on Mars and the early Earth. Icarus 169:300–310 [Google Scholar]
- Cockell C.S., Lee P., Osinski G., Horneck G., and Broady P. (2002) Impact-induced microbial endolithic habitats. Meteorit Planet Sci 37:1287–1298 [Google Scholar]
- Cockell C.S., Balme M., Bridges J.C., Davilad A., and Schwenzer S.P. (2012) Uninhabited habitats on Mars. Icarus 217:184–193 [Google Scholar]
- Dartnell L. (2007) Life in the Universe: A Beginner's Guide, Oneworld Publications, Oxford, UK [Google Scholar]
- Derenne S., Robert F., Skryzpczak-Bonduelle A., Gourier D., Binet L., and Rouzaud J.-N. (2008) Molecular evidence for life in the 3.5 billion-year old Warrawoona Chert. Earth Planet Sci Lett 272:476–480 [Google Scholar]
- de Ronde C.E.J., Channer D.M.DeR., Faure K., Bray C.J., and Spooner E.T.C. (1997) Fluid chemistry of Archean seafloor hydrothermal vents; implications for the composition of circa 3.2 Ga seawater. Geochim Cosmochim Acta 61:4025–4042 [Google Scholar]
- Des Marais D.J. (2001) On the origins of photosynthesis. Science 291:436–437 [DOI] [PubMed] [Google Scholar]
- Des Marais D.J. (2010) Exploring Mars for evidence of habitable environments and life. Proc Am Philos Soc 154:402–421 [Google Scholar]
- de Vries S.T., Nijman W., Wijbrans J.R., and Nelson D.R. (2006) Stratigraphic continuity and early deformation of the central part of the Coppin Gap Greenstone Belt, Pilbara, Western Australia. Precambrian Res 147:1–27 [Google Scholar]
- D'Hondt S., Rutherford S., and Spivak A.J. (2002) Metabolic activity of subsurface life in deep sea sediments. Science 295:2067–2070 [DOI] [PubMed] [Google Scholar]
- DiBiase R.A., Limaye A.B., Scheingross J.S., Fischer W.W., and Lamb M.P. (2013) Deltaic deposits at Aeolis Dorsa: sedimentary evidence for a large body of water in the northern plains of Mars. J Geophys Res Planets 118:1285–1302 [Google Scholar]
- Duprat J., Dobrică E., Engrand C., Aléon J., Marrocchi Y., Mostefaoui S., Meibom A., Leroux H., Rouzaud J.-N., Gounelle M., and Robert F. (2010) Extreme deuterium excesses in ultracarbonaceous micrometeorites from central Antarctic snow. Science 328:742–745 [DOI] [PubMed] [Google Scholar]
- Ehlmann B.L., Mustard J.F., Murchie S.L., Bibring J.-P., Meunier A., Fraeman A.A., and Langevin Y. (2011) Subsurface water and clay mineral formation during the early history of Mars. Nature 479:53–60 [DOI] [PubMed] [Google Scholar]
- El-Naggar M.Y., Wanger G., Leung K.M., Yuzvinsky T.D., Southam G., Yang Y., Lau Y.M., Nealson K.H, and Gorby Y.A. (2010) Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc Natl Acad Sci USA 107:18127–18131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J.D. and Des Marais D.J. (1999) Exploring for a record of ancient martian life. J Geophys Res 104:26977–26995 [DOI] [PubMed] [Google Scholar]
- Farquhar J., Bao H., and Thiemens M. (2000) Atmospheric influence of Earth's earliest sulfur cycle. Science 289:756–758 [DOI] [PubMed] [Google Scholar]
- Flynn G. (1996) The delivery of organic matter from asteroids and comets to the early surface of Mars. Earth, Moon, Planets 72:469–472 [DOI] [PubMed] [Google Scholar]
- Forterre P., Krupovic M., and Prangishvili D. (2014) Cellular domains and viral lineages. Trends Microbiol 22:554–558 [DOI] [PubMed] [Google Scholar]
- Foucher F. and Westall F. (2009) Investigating the oldest traces of life by AFM/confocal Raman spectroscopy: applications for the analysis of martian rocks. In Micro-Raman Spectroscopy and Luminescence Studies in the Earth and Planetary Science, AIP Conference Proceedings 1163, edited by Gucsik A., American Institute of Physics, Melville, NY, pp 47–58 [Google Scholar]
- Foucher F., Westall F., Brandstatter F., Demets R., Parnell J., Cockell C.S., Edwards H.G.M., Bény J.-M., and Brack A. (2010) Testing the survival of microfossils in artificial martian sedimentary meteorites during entry into Earth's atmosphere: the STONE 6 experiment. Icarus 207:616–630 [Google Scholar]
- Foucher F., Ammar M.R., and Westall F. (2015) Revealing the biotic origin of silicified Precambrian carbonaceous microstructures using Raman spectroscopic mapping, a potential method for the detection of microfossils on Mars. J Raman Spectrosc 46:873–879 [Google Scholar]
- François P. (2014) Analyse moléculaire in situ de la surface/sous surface de Mars par pyrolyse et CPG: application à la mission spatiale Mars Science Laboratory 2012. PhD thesis, University Paris VII, Paris [Google Scholar]
- Freissinet C., Glavin D.P., Mahaffy P.R., Miller K.E., Eigenbrode J.L., Summons R.E., Brunner A.E., Buch A., Szopa C., Archer P.D., Jr., Franz H.B., Atreya S.K., Brinckerhoff W.B., Cabane M., Coll P., Conrad P.G., Des Marais D.J., Dworkin J.P., Fairén A.G., François P., Grotzinger J.P., Kashyap S., ten Kate I.L., Leshin L.A., Malespin C.A., Martin M.G., Martin-Torres F.J., McAdam A.C., Ming D.W., Navarro-González R., Pavlov A.A., Prats B.D., Squyres S.W., Steele A., Stern J.C., Sumner D.Y., Sutter B., Zorzano M.-P., and the MSL Science Team. (2015) Organic molecules in the Sheepbed Mudstone, Gale Crater, Mars. J Geophys Res Planets 120:495–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann E.I. and Koriem A.M. (1989) Life on Mars: how it disappeared (if it was ever there). Adv Space Res 9:167–172 [DOI] [PubMed] [Google Scholar]
- Furnes H., Banerjee N.R., Muehlenbachs K., Staudigel H., and de Wit M. (2004) Early life recorded in Archean pillow lavas. Science 304:578–581 [DOI] [PubMed] [Google Scholar]
- Gaudin A., Dehouck E., and Mangold N. (2011) Evidence for weathering on early Mars from a comparison with terrestrial weathering profiles. Icarus 216:257–268 [Google Scholar]
- Gilichinsky D.A., Wilson G.S., Friedmann E.I., McKay C.P., Sletten R.S., Rivkina E.M., Vishnivetskaya T.A., Erokhina L.G., Ivanushkina N.E., Kochkina G.A., Shcherbakova V.A., Soina V.S., Spirina E.V., Vorobyova E.A., Fyodorov-Davydov D.G., Hallet B., Ozerskaya S.M., Sorokovikov V.A., Laurinavichyus K.S., Shatilovich A.V., Chanton P., Ostroumov V.E., and Tiedje J.M. (2007) Microbial populations in Antarctic permafrost: biodiversity, state, age and implication for astrobiology. Astrobiology 7:275–311 [DOI] [PubMed] [Google Scholar]
- Glavin D.P., Schubert M., Botta O., Kminek G., and Bada J.L. (2001) Detecting pyrolysis products from bacteria on Mars. Earth Planet Sci Lett 185:1–5 [Google Scholar]
- Glavin D.P., Freissinet C., Miller K.E., Eigenbrode J.L., Brunner A.E., Buch A., Sutter B., Archer P.D., Atreya S.K., Brinckerhoff W.B., Cabane M., Coll P., Conrad P.G., Coscia D., Dworkin J.P., Franz H.B., Grotzinger J.P., Leshin L.A., Martin M.G., McKay C., Ming D.W., Navarro-Gonzalez R., Pavlov A., Steele A., Summons R.E., Szopa C., Teinturier S., and Mahaffy P.R. (2013) Evidence for perchlorates and the origin of chlorinated hydrocarbons detected by SAM at the Rocknest aeolian deposit in Gale Crater. J Geophys Res Planets 118:1955–1973 [Google Scholar]
- Grady M.M., Verchovsky A.B., and Wright I.P. (2004) Magmatic carbon in martian meteorites: attempts to constrain the carbon cycle on Mars. International Journal of Astrobiology 3:117–124 [Google Scholar]
- Grosch E.G. and McLoughlin N. (2014) Reassessing the biogenicity of Earth's oldest trace fossil with implications for biosignatures in the search for early life. Proc Natl Acad Sci USA 111:8380–8385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotzinger J.P., Sumner D.Y., Kah L.C., Stack K., Gupta S., Edgar L., Rubin D., Lewis K., Schieber J., Mangold N., Milliken R., Conrad P.G., Des Marais D., Farmer J., Siebach K., Calef F., Hurowitz J., McLennan S.M., Ming D., Vaniman D., Crisp J., Vasavada A., Edgett K.S., Malin M., Blake D., Geliert R., Mahaffy P., Wiens R.C., Maurice S., Grant J.A., Wilson S., Anderson R.C., Beegle L., Arvidson R., Hallet B., Sletten R.S., Rice M., Bell J., Griffes J., Ehlmann B., Anderson R.B., Bristow T.F., Dietrich W.E., Dromart G., Eigenbrode J., Fraeman A., Hardgrove C., Herkenhoff K., Jandura L., Kocurek G., Lee S., Leshin L.A., Leveille R., Limonadi D., Maki J., McCloskey S., Meyer M., Minitti M., Newsom H., Oehler D., Okon A., Palucis M., Parker T., Rowland S., Schmidt M., Squyres S., Steele A., Stolper E., Summons R., Treiman A., Williams R., Yingst A., and the MSL Science Team. (2014) A habitable fluvio-lacustrine environment at Yellowknife Bay, Gale Crater, Mars. Science 343, doi: 10.1126/science.1242777 [DOI] [PubMed] [Google Scholar]
- Guido D.M., Channing A., Campbell K.A., and Zamuner A. (2010) Jurassic geothermal landscapes and fossil ecosystems at San Agustín, Patagonia, Argentina. J Geol Soc London 167:11–20 [Google Scholar]
- Handley K.M., Turner S., Campbell K.A., and Mountain B. (2008) Silicifying biofilm exopolymers on a hot spring microstromatolite: templating nanometer-thick laminae. Astrobiology 8:747–770 [DOI] [PubMed] [Google Scholar]
- Heim C. (2011) Microbial biomineralization. In Encyclopedia of Geobiology, edited by Reitner J. and Thiel V., Springer, Berlin, pp 1586–1591 [Google Scholar]
- Herzberg C., Asimow P.D., Arndt N., Niu Y., Lesher C.M., Fitton J.G., Cheadle M.J., and Saunders A.D. (2007) Temperatures in ambient mantle and plumes: constraints from basalts, picrites, and komatiites. Geochem Geophys Geosyst 8:1–34 [Google Scholar]
- Heubeck C. (2009) An early ecosystem of Archean tidal microbial mats (Moodies Group, South Africa, ca. 3.2 Ga). Geology 37:931–934 [Google Scholar]
- Hirose T., Kawagucci S., and Suzuki K. (2011) Mechanoradical H2 generation during simulated faulting: implications for an earthquake-driven subsurface biosphere. Geophys Res Lett 38:L17303 [Google Scholar]
- Hoehler T.M. and Jørgensen B.B. (2013) Microbial life under extreme energy limitation. Nat Rev Microbiol 11:83–94 [DOI] [PubMed] [Google Scholar]
- Hofmann A. and Bolhar R. (2007) Carbonaceous cherts in the Barberton Greenstone Belt and their significance for the study of early life in the Archean record. Astrobiology 7:355–388 [DOI] [PubMed] [Google Scholar]
- Hofmann A. and Harris C. (2008) Silica alteration zones in the Barberton Greenstone Belt: a window into subseafloor processes 3.5–3.3 Ga ago. Chem Geol 257:221–239 [Google Scholar]
- Hofmann H.J., Grey K., Hickman A.H., and Thorpe R.I. (1999) Origin of 3.45 Ga coniform stromatolites in Warrawoona Group, Western Australia. Geol Soc Am Bull 111:1256–1262 [Google Scholar]
- Holm N.G., Oze C., Mousis O., Waite J.H., and Guilbert-Lepoutre A. (2015) Serpentinization and the formation of H2 and CH4 on celestial bodies (planets, moons, comets). Astrobiology 15:587–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horita J. and Berndt M.E. (1999) Abiogenic methane formation and isotopic fractionation under hydrothermal conditions. Science 285:1055–1057 [DOI] [PubMed] [Google Scholar]
- Jain A. and Bhosle N.B. (2009) Biochemical composition of the marine conditioning film: implications for bacterial adhesion. Biofouling 25:13–19 [DOI] [PubMed] [Google Scholar]
- Jakosky B.M. (1991) Mars volatile evolution: evidence from stable isotopes. Icarus 94:14–31 [Google Scholar]
- Janssen P.H., Schuhmann A., Mörschel E., and Rainey F.A. (1997) Novel anaerobic ultramicrobacteria belonging to the Verrucomicrobiales lineage of bacterial descent isolated by dilution culture from anoxic rice paddy soil. Appl Environ Microbiol 63:1382–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaux E.J., Knoll A.H., and Walter M.R. (2001) Morphological and ecological complexity in early eukaryotic ecosystems. Nature 412:66–69 [DOI] [PubMed] [Google Scholar]
- Javaux E., Marshall C.P., and Bekker A. (2010) Organic-walled microfossils in 3.2-billion-year-old shallow-marine siliciclastic deposits. Nature 463:934–938 [DOI] [PubMed] [Google Scholar]
- Johnson A.P., Cleaves H.J., Dworkin J.P., Glavin D.P., Lazcano A., and Bada J.L. (2008) The Miller volcanic spark discharge experiment. Science 322:404. [DOI] [PubMed] [Google Scholar]
- Kiyokawa S., Kogea S., Ito T., and Ikehara M. (2014) An ocean-floor carbonaceous sedimentary sequence in the 3.2-Ga Dixon Island Formation, coastal Pilbara terrane, Western Australia. Precambrian Res 255:124–143 [Google Scholar]
- Kminek G. and Bada J. (2006) The effect of ionizing radiation on the preservation of amino acids on Mars. Earth Planet Sci Lett 245:1–5 [Google Scholar]
- Kminek G., Bada J.L., Pogliano K., and Ward J.F. (2003) Radiation-dependent limit for the viability of bacterial spores in halite fluid inclusions and on Mars. Radiat Res 159:722–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konn C., Charlou J.L., Holm N.G., and Mousis O. (2015) The production of methane, hydrogen, and organic compounds in ultramafic-hosted hydrothermal vents of the Mid-Atlantic Ridge. Astrobiology 15:381–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane N. and Martin W.F. (2012) The origin of membrane bioenergetics. Cell 151:1406–1416 [DOI] [PubMed] [Google Scholar]
- Lanza N.L., Fischer W.W., Wiens R.C., Grotzinger J., Ollila A.M., Cousin A., Anderson R.B., Clark B.C., Gellert R., Mangold N., Maurice S., Le Mouélic S., Nachon M., Schmidt M., Berger J., Clegg S.M., Forni O., Hardgrove C., Melikechi N., Newsom H.E., and Sautter V. (2014) High manganese concentrations in rocks at Gale Crater, Mars. Geophys Res Lett 41:5755–5763 [Google Scholar]
- La Scola B., Audic S., Robert C., Jungang L., de Lamballerie X., Drancourt M., Birtles R., Claverie J.M., and Raoult D. (2003) A giant virus in amoebae. Science 299:2033. [DOI] [PubMed] [Google Scholar]
- Le Deit L., Flahaut J., Quantin C., Hauber E., Daniel E., Bourgeois O., Gurgurewicz J., Masse M., and Jaumann R. (2012) Extensive surface pedogenic alteration of the martian Noachian crust suggested by plateau phyllosilicates around Valles Marineris. J Geophys Res 117, doi: 10.1029/2011JE003983 [DOI] [Google Scholar]
- Lehaitre M., Delauney L., and Compère C. (2008) Biofouling and underwater measurements. In Real-Time Coastal Observing Systems for Marine Ecosystem Dynamics and Harmful Algal Blooms: Theory, Instrumentation and Modelling, edited by Babin M., Roesler C.S., and Cullen J.J., UNESCO, Paris, pp 463–494 [Google Scholar]
- Lin Y., Goresy A.E., Hu S., Zhang J., Gillet P., Xu Y., Hao J., Miyahara M., Ouyang Z., Ohtani E., Xu L., Yang W., Feng L., Zhao X., Yang J., and Ozawa S. (2014) NanoSIMS analysis of organic carbon from the Tissint martian meteorite: evidence for the past existence of subsurface organic-bearing fluids on Mars. Meteorit Planet Sci 49:2201–2218 [Google Scholar]
- Loizeau D., Carter J., Bouley S., Mangold N., Poulet F., Bibring J.-P., Costard F., Langevin Y., Gondet B., and Murchie S.L. (2012) Characterization of hydrated silicate-bearing outcrops in Tyrrhena Terra, Mars: implications to the alteration history of Mars. Icarus 219:476–497 [Google Scholar]
- Lowe D.R. and Byerly G.R. (2015) Geologic record of partial ocean evaporation triggered by giant asteroid impacts, 3.29–3.23 billion years ago. Geology doi: 10.1130/G36665.1 [DOI] [Google Scholar]
- Lowe D.R., Byerly G.R., and Kyte F.T. (2014) Recently discovered 3.42–3.23 Ga impact layers, Barberton Belt, South Africa: 3.8 Ga detrital zircons, Archean impact history, and tectonic implications. Geology 42:747–750 [Google Scholar]
- Madigan M.T., Martinko J.M., and Parker J. (2000) Brock Biology of Microorganisms, 9th ed., edited by Cummings B., Prentice Hall, Upper Saddle River, NJ [Google Scholar]
- Mahaffy P.R., Conrad P.G., and the MSL Science Team. (2015) Volatile and isotopic imprints of ancient Mars. Elements 11:51–56 [Google Scholar]
- Mancinelli R.L. and Banin A. (2003) Where is the nitrogen on Mars? International Journal of Astrobiology 2:217–225 [Google Scholar]
- Marin-Carbonne J., Chaussidon M., and Robert F. (2012) Micrometer-scale chemical and isotopic criteria (O and Si) on the origin and history of Precambrian cherts: implications for paleo-temperature reconstructions. Geochim Cosmochim Acta 92:129–147 [Google Scholar]
- Marin-Carbonne J., Robert F., and Chaussidon M. (2014) The silicon and oxygen isotope compositions of Precambrian cherts: a record of ocean paleo-temperatures? Precambrian Res 247:223–234 [Google Scholar]
- Martin W. and Russell M.J. (2007) On the origin of biochemistry at an alkaline hydrothermal vent. Philos Trans R Soc Lond B Biol Sci 362:1887–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W.F. and Sousa F.L. (2015) Early microbial evolution: the age of anaerobes. Cold Spring Harb Perspect Biol doi: 10.1101/cshperspect.a018127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W.F., Baross J., Kelley D., and Russell M.J. (2008) Hydrothermal vents and the origin of life. Nat Rev Microbiol 6:805–814 [DOI] [PubMed] [Google Scholar]
- McCollom T.M. and Seewald J.S. (2006) Carbon isotope composition of organic compounds produced by abiotic synthesis under hydrothermal conditions. Earth Planet Sci Lett 243:74–84 [Google Scholar]
- McCollom T.M., Ritter G., and Simoneit B.R. (1999) Lipid synthesis under hydrothermal conditions by Fischer-Tropsch-type reactions. Orig Life Evol Biosph 29:153–166 [DOI] [PubMed] [Google Scholar]
- McDermott J.M., Seewald J.S., German C.R., and Sylva S.P. (2015) Pathways for abiotic organic synthesis at submarine hydrothermal fields. Proc Natl Acad Sci USA 112:7668–7672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay C.P., Mancinelli R.L., Stoker C.R., and Wharton R.A., Jr. (1992) The possibility of life on Mars during a water-rich past. In Mars, edited by Kieffer H.H., Jakosky B.M., Snyder C.W., and Matthews M.S., University of Arizona Press, Tucson, pp 1234–1245 [Google Scholar]
- McKay D.S., Gibson E.K., Jr., Thomas-Keprta K.L., Vali H., Romanek C.S., Clemett S.J., Chillier X.D.F., Maechling C.R., and Zare R.N. (1996) Search for past life on Mars: possible relic biogenic activity in martian meteorite ALH84001. Science 273:924–930 [DOI] [PubMed] [Google Scholar]
- Michalski J.R., Cuadros J., Niles P.B., Parnell J., Rogers A.D., and Wright S.P. (2013) Groundwater activity on Mars and implications for a deep biosphere. Nat Geosci 6:133–138 [Google Scholar]
- Miller S.L. (1953) A production of amino acids under possible primitive Earth conditions. Science 117:528–529 [DOI] [PubMed] [Google Scholar]
- Ming D.W., Archer P.D., Jr., Glavin D.P., Eigenbrode J.L., Franz H.B., Sutter B., Brunner A.E., Stern J.C., Freissinet C., McAdam A.C., Mahaffy P.R., Cabane M., Coll P., Campbell J.L., Atreya S.K., Niles P.B., Bell J.F., III, Bish B.L., Brinckerhoff W.B., Buch A., Conrad P.G., Des Marais D.J., Ehlmann B.L., Fairén A.G., Farley K., Flesch G.J., Francois P., Gellert R., Grant J.A., Grotzinger J.P., Gupta S., Herkenhoff K.E., Hurowitz J.A., Leshin L.A., Lewis K.W., McLennan S.M., Miller K.E., Moersch J., Morris R.V., Navarro-González R., Pavlov A.A., Perrett G.M., Pradler I., Squyres S.W., Summons R.E., Steele A., Stolper E.M., Sumner D.Y., Szopa C., Teinturier S., Trainer M.G., Treiman A.H., Vaniman D.T., Vasavada A.R., Webster C.R., Wray J.J., and Yingst R.A. (2014) Volatile and organic compositions of sedimentary rocks in Yellowknife Bay, Gale Crater, Mars. Science 343, doi: 10.1126/science.1245267 [DOI] [PubMed] [Google Scholar]
- Mustard J.F., Murchie S.L., Pelkey S.M., Ehlmann B.L., Milliken R.E., Grant J.A., Bibring J.-P., Poulet F., Bishop J., Noe Dobrea E., Roach L., Seelos F., Arvidson R.E., Wiseman S., Green R., Hash C., Humm D., Malaret E., McGovern J.A., Seelos K., Clancy T., Clark R., Des Marais D., Izenberg N., Knudson A., Langevin Y., Martin T., McGuire P., Morris R., Robinson M., Roush T., Smith M., Swayze G., Taylor H., Titus T., and Wolff M. (2008) Hydrated silicate minerals on Mars observed by the Mars Reconnaissance Orbiter CRISM instrument. Nature 454:305–309 [DOI] [PubMed] [Google Scholar]
- Nealson K.H., Inagaki F., and Takai K. (2005) Hydrogen-driven Subsurface Lithoauthotrophic Microbial Ecosystems (SLiMEs): do they exist and why should we care? Trends Microbiol 13:405–410 [DOI] [PubMed] [Google Scholar]
- Noe Dobrea E.Z., Bishop J.L., McKeown N.K., Fu R., Rossi C.M., Michalski J.R., Heinlein C., Hanus V., Poulet F., Mustard R.J.F., Murchie S., McEwen A.S., Swayze C., Bibring J.-P., Malaret E., and Hash C. (2010) Mineralogy and stratigraphy of phyllosilicate-bearing and dark mantling units in the greater Mawrth Vallis/west Arabia Terra area: constraints on geological origin. J Geophys Res 115, doi: 10.1029/2009JE003351 [DOI] [Google Scholar]
- Noffke N. (2009) The criteria for the biogeneicity of microbially induced sedimentary structures (MISS) in Archean and younger, sandy deposits. Earth-Science Reviews 96:173–180 [Google Scholar]
- Noffke N. (2015) Ancient sedimentary structures in the <3.7 Ga Gillespie Lake Member, Mars, that resemble macroscopic morphology, spatial associations, and temporal succession in terrestrial microbialites. Astrobiology 15:169–192 [DOI] [PubMed] [Google Scholar]
- Orange F., Westall F., Disnar J.-R., Prieur D., Bienvenu N., Le Romancer M., and Défarge C. (2009) Experimental silicification of the extremophilic Archaea Pyroccus abyssi and Methanocaldococcus jannaschii. Applications in the search for evidence of life in early Earth and extraterrestrial rocks. Geobiology 7:403–418 [DOI] [PubMed] [Google Scholar]
- Orange F., Chabin A., Gorlas A., Lucas-Staat S., Geslin C., Leromancer M., Prangishvili D., Forterre P., and Westall F. (2011) Experimental fossilisation of viruses from extremophilic Archaea. Biogeosci Discuss 8:2235–2257 [Google Scholar]
- Parkes R.J., Linnane C.D., Webster G., Sass H., Weightman A.J., Hornibrook E.R.C., and Horsfield B. (2011) Prokaryotes stimulate mineral H2 formation for the deep biosphere and subsequent thermogenic activity. Geology 39:219–222 [Google Scholar]
- Pasek M.A. and Lauretta D.S. (2008) Extraterrestrial flux of potentially prebiotic C, N, and P to the early Earth. Orig Life Evol Biosph 38:5–21 [DOI] [PubMed] [Google Scholar]
- Pavlov A.A., Vasilyev G., Ostryakov V.M., Pavlov A.K., and Mahaffy P. (2012) Degradation of the organic molecules in the shallow subsurface of Mars due to irradiation by cosmic rays. Geophys Res Lett 39:L13202 [Google Scholar]
- Pearson V.K., Sephton M.A., Kearsley A.T., Bland P.A., Franchi I.A., and Gilmour I. (2002) Clay mineral-organic matter relationships in the early Solar System. Meteorit Planet Sci 37:1829–1833 [Google Scholar]
- Pearson V.K., Sephton M.A., Franchi I.A., Gibson J.M., and Gilmour I. (2006) Carbon and nitrogen in carbonaceous chondrites: elemental abundances and stable isotopic compositions. Meteorit Planet Sci 41:1899–1918 [Google Scholar]
- Peckmann J. and Thiel V. (2004) Carbon cycling at ancient methane-seeps. Chem Geol 205:443–467 [Google Scholar]
- Pedersen K. (1982) Factors regulating microbial biofilm development in a system with slowly flowing seawater. Appl Environ Microbiol 44:1196–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton J. and Allamandola L.J. (2002) The organic refractory material in the diffuse interstellar medium: mid-infrared spectroscopic constraints. Astrophys J 138:75–98 [Google Scholar]
- Pizzarello S. and Shock E. (2010) The organic composition of carbonaceous meteorites: the evolutionary story ahead of biochemistry. Cold Spring Harb Perspect Biol 2, doi: 10.1101/cshperspect.a002105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzarello S., Cooper G.W., and Flynn G.J. (2006) The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles. In Meteorites and the Early Solar System II, edited by Lauretta D.S. and McSween H.Y., Jr., University of Arizona Press, Tucson, pp 625–651 [Google Scholar]
- Price P.B. and Sowers T. (2004) Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. Proc Natl Acad Sci USA 101:4631–4636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskurowski G., Lilley M.D., Seewald J.S., Früh-Green G.L., Olson E.J., Lupton J.E., Sylva S.P., and Kelley D.S. (2008) Abiogenic hydrocarbon production at Lost City Hydrothermal Field. Science 319:604–607 [DOI] [PubMed] [Google Scholar]
- Rasmussen B., Fletcher I.R., Brocks J.J., and Kilburn M.R. (2008) Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 455:1101–1104 [DOI] [PubMed] [Google Scholar]
- Reith F. (2011) Life in the deep subsurface. Geology 39:287–288 [Google Scholar]
- Ruff S.W. (2015) Assessing the astrobiological potential of silica occurrences on Mars [abstract 7562]. In Astrobiology Science Conference 2015, Lunar and Planetary Institute, Houston [Google Scholar]
- Ruff S.W., Farmer J.D., Calvin W.M., Herkenhoff K.E., Johnson J.R., Morris R.V., Rice M.S., Arvidson R.E., Bell J.F., III, Christensen P.R., and Squyres S.W. (2011) Characteristics, distribution, origin, and significance of opaline silica observed by the Spirit rover in Gusev Crater, Mars. J Geophys Res 116, doi: 10.1029/2010JE003767 [DOI] [Google Scholar]
- Ruiz J. (2014) The early heat loss evolution of Mars and their implications for internal and environmental history. Sci Rep 4, doi: 10.1038/srep04338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel J.D., Beaty D.W., Jones M.A., Bakermans C., Barlow N.G., Boston P.J., Chevrier V.F., Clark B.C., de Vera J.-P., Gough R.V., Hallsworth J.E., Head J.W., Hipkin V.J., Kieft T.L., McEwen A.S., Mellon M.T., Mikucki J.A., Nicholson W.L., Omelon C.R., Peterson R., Roden E.E., Lollar B.S., Tanaka K.L., Viola D., and Wray J.J. (2014) A new analysis of Mars “Special Regions”: findings of the second MEPAG Special Regions Science Analysis Group (SR-SAG2). Astrobiology 14:887–968 [DOI] [PubMed] [Google Scholar]
- Russell M.J. and Hall A.J. (1997) The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front. J Geol Soc Lond 154:377–402 [DOI] [PubMed] [Google Scholar]
- Schut F., Gottschal C.G., and Prins R.A. (1997) Isolation and characterization of the marine ultramicrobacterium Sphingomonas sp. strain RB2256. FEMS Microbiol Rev 20:363–369 [Google Scholar]
- Schwenzer S.P. and Kring D.A. (2009) Impact-generated hydrothermal systems capable of forming phyllosilicates on Noachian Mars. Geology 37:1091–1094 [Google Scholar]
- Sephton M.A. (2002) Organic compounds in carbonaceous meteorites. Nat Prod Rep 19, 292–311 [DOI] [PubMed] [Google Scholar]
- Sephton M.A. and Botta O. (2008) Extraterrestrial organic matter and the detection of life. Space Sci Rev 135:25–35 [Google Scholar]
- Sephton M.A., Love G.D., Watson J.S., Verchovsky A.B., Wright I.P., Snape C.E., and Gilmour I. (2004) Hydropyrolysis of insoluble carbonaceous matter in the Murchison meteorite: new insights into its macromolecular structure. Geochim Cosmochim Acta 68:1385–1393 [Google Scholar]
- Sherwood Lollar B., Lacrampe-Couloume G., Voglesonger K., Onstott T.C., Pratt L.M., and Slater G.F. (2008) Isotopic signatures of CH4 and higher hydrocarbon gases from Precambrian Shield sites: a model for abiogenic polymerization of hydrocarbons. Geochim Cosmochim Acta 72:4778–4795 [Google Scholar]
- Shock E.L. (1997) High temperature life without photosynthesis as a model for Mars. J Geophys Res 102:687–694 [DOI] [PubMed] [Google Scholar]
- Shock E.L., McCollom T.M., and Schulte M.D. (1998) The emergence of metabolism from within hydrothermal systems. In Thermophiles: The Keys to Molecular Evolution and the Origin of Life, edited by Wiegel J. and Adams M.W.W., Taylor & Francis, London, pp 59–76 [Google Scholar]
- Skok J.R., Mustard J.F., Ehlmann B.L., Milliken R.E., and Murchie S.L. (2010) Silica deposits in the Nili Patera caldera on the Syrtis Major volcanic complex on Mars. Nat Geosci 3:838–841 [Google Scholar]
- Sleep N.H. and Bird D.K. (2007) Niches of the prephotosynthetic biosphere and geologic preservation of Earth's earliest ecology. Geobiology 5:101–117 [Google Scholar]
- Sleep N.H. and Lowe D.R. (2014) Physics of crustal fracturing and chert dike formation triggered by asteroid impact, ∼3.26 Ga, Barberton Greenstone Belt, South Africa. Geochem Geophys Geosyst 15:1054–1070 [Google Scholar]
- Steele A., Fries M., Amundsen H.E.F., Mysen B.O., Fogel M.L., Schweizer M., and Boctor N.Z. (2007) Comprehensive imaging and Raman spectroscopy of carbonate globules from martian meteorite ALH84001 and a terrestrial analogue from Svalbard. Meteorit Planet Sci 42:1–18 [Google Scholar]
- Steele A., McCubbin F.M., Fries M.D., Golden D.C., Ming D.W., and Benning L.G. (2012) Graphite in the martian meteorite Allan Hills 84001. Am Mineral 97:1256–1259 [Google Scholar]
- Stern J.C., Sutter B., Freissinet C., Navarro-González R., McKay C.P., Archer P.D., Jr., Buch A., Brunner A.E., Coll P., Eigenbrode J.L., Fairen A.G., Franz H.B., Glavin D.P., Kashyap S., McAdam A.C., Ming D.W., Steele A., Szopa C., Wray J.J., Martín-Torres F.J., Zorzano M.-P., Conrad P.G., Mahaffy M.R., and the MSL Science Teams. (2015) Evidence for indigenous nitrogen in sedimentary and aeolian deposits from the Curiosity rover investigations at Gale Crater, Mars. Proc Natl Acad Sci USA 112:4245–4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker C.R., Zent A., Catling D.C., Douglas S., Marshall J.R., Archer D., Clark B., Kouvanes S.P., Lemmon M.T., Quinn R., Renno N., Smith P.H., and Young S.M.M. (2010) Habitability of the Phoenix landing site. J Geophys Res 115, doi: 10.1029/2009JE003421 [DOI] [Google Scholar]
- Summons R. (1993) Biogeochemical cycles: a review of fundamental aspects of organic matter formation, preservation and composition. In Organic Geochemistry, edited by Engel M.H. and Macko S.A., Plenum Press, New York, pp 3–21 [Google Scholar]
- Summons R.E., Albrecht P., McDonald G., and Moldowan J.M. (2008) Molecular biosignatures. Space Sci Rev 135:115–132 [Google Scholar]
- Summons R.E., Amend J.P., Bish D., Buick R., Cody G.D., Des Marais D.J., Dromart G., Eigenbrode J.L., Knoll A.H., and Sumner D.Y. (2011) Preservation of martian organic and environmental records: final report of the Mars Biosignature Working Group. Astrobiology 11:157–181 [DOI] [PubMed] [Google Scholar]
- Szewzyk U., Szewzyk R., and Stenström T. (1994) Thermophilic, anaerobic bacteria isolated from a deep borehole in granite in Sweden. Proc Natl Acad Sci USA 91:1810–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teske A. and Nelson D.C. (2006) The genera Beggiatoa and Thioploca. In The Prokaryotes, Springer, New York, pp 784–810 [Google Scholar]
- Thompson J.B., Ferris F.G., and Smith D.A. (1990) Geomicrobiology and sedimentology of the mixolimnion and chemocline in Fayetteville Green Lake, New York. Palaios 5:52–75 [Google Scholar]
- Tice M. and Lowe D.R. (2004) Photosynthetic microbial mats in the 3,416-Myr-old ocean. Nature 431:549–552 [DOI] [PubMed] [Google Scholar]
- Tornabene L.L., Osinski G.R., McEwen A.S., Wray J.J., Craig M.A., Sapers H.M., and Christense P.R. (2013) An impact origin for hydrated silicates on Mars: a synthesis. J Geophys Res Planets 118:994–1012 [Google Scholar]
- Tuff J., Wade J., and Wood B.J. (2013) Volcanism on Mars controlled by early oxidation of the upper mantle. Nature 498:342–345 [DOI] [PubMed] [Google Scholar]
- van den Boorn S.H.J.M., van Bergen M.J., Nijman W., and Vroon P.Z. (2007) Dual role of seawater and hydrothermal fluids in Early Archean chert formation: evidence from silicon isotopes. Geology 35:939–942 [Google Scholar]
- Van Kranendonk M.J. (2006) Volcanic degassing, hydrothermal circulation and flourishing of early life on Earth: a review of the evidence from c. 3490–3240 Ma rocks of the Pilbara Supergroup, Pilbara Craton, Western Australia. Earth Sci Rev 74:197–240 [Google Scholar]
- van Zuilen M., Lepland A., and Arrhenius G. (2002) Reassessing the evidence for the earliest traces of life. Nature 418:627–630 [DOI] [PubMed] [Google Scholar]
- Villanueva G.L., Mumma M.J., Novak R.E., Käufl H.U., Hartogh P., Encrenaz T., Tokunaga A., Khayat A., and Smith M.D. (2015) Strong water isotopic anomalies in the martian atmosphere: probing current and ancient reservoirs. Science 348:218–221 [DOI] [PubMed] [Google Scholar]
- Walsh M.M. (1992) Microfossils and possible microfossils from the Early Archean Onverwacht Group, Barberton Mountain Land, South Africa. Precambrian Res 54:271–293 [DOI] [PubMed] [Google Scholar]
- Webster C.R., Mahaffy P.R., Atreya S.K., Flesch G.J., Mischna M.A., Meslin P.Y., Farley K.A., Conrad P.G., Christensen L.E., Pavlov A.A., Martín-Torres J., Zorzano M.P., McConnochie T.H., Owen T., Eigenbrode J.L., Glavin D.P., Steele A., Malespin C.A., Archer P.D., Sutter B., Coll P., Freissinet C., McKay C.P., Moores J.E., Schwenzer S.P., Bridges J.C., Navarro-Gonzalez R., Gellert R., Lemmon M.T., and the MSL Science Team. (2015) Mars methane detection and variability at Gale Crater. Science 347:415–417 [DOI] [PubMed] [Google Scholar]
- Westall F. (1999) The nature of fossil bacteria: a guide to the search for extraterrestrial life. J Geophys Res Planets 104:437–451 [Google Scholar]
- Westall F. (2005) Early life on Earth and analogies to Mars. In Water on Mars and Life, Advances in Astrobiology and Biogeophysics, edited by Tokano T., Springer, Berlin, pp 45–64 [Google Scholar]
- Westall F. (2012) The early Earth. In Astrobiology, edited by Impey C., Lunine J., and Funes J., Cambridge University Press, Cambridge, UK, pp 89–114 [Google Scholar]
- Westall F. and Cavalazzi B. (2011) Biosignatures in rocks. In Encyclopedia of Geobiology, edited by Thiel V. and Reitner J., Springer, Berlin, pp 189–201 [Google Scholar]
- Westall F. and Folk R.L. (2003) Exogenous carbonaceous microstructures in Early Archean cherts and BIFs from the Isua Greenstone Belt: implications for the search for life in ancient rocks. Precambrian Res 126:313–330 [Google Scholar]
- Westall F., Steele A., Toporski J., Walsh M., Allen C., Guidry S., Gibson E., McKay D., and Chafetz H. (2000) Polymeric substances and biofilms as biomarkers in terrestrial materials: implications for extraterrestrial samples. J Geophys Res Planets 105:511–527 [Google Scholar]
- Westall F., de Vries S.T., Nijman W., Rouchon V., Orberger B., Pearson V., Watson J., Verchovsky A., Wright I., Rouzaud J.-N., Marchesini D., and Anne S. (2006a) The 3.466 Ga Kitty's Gap Chert, an Early Archean microbial ecosystem. In Processes on the Early Earth, edited by Reimold W.U. and Gibson R., Geological Society of America Special Paper 405, Geological Society of America, Boulder, CO, pp 105–131 [Google Scholar]
- Westall F., de Ronde C.E.J., Southam G., Grassineau N., Colas M., Cockell C., and Lammer H. (2006b) Implications of a 3.472–3.333 Gyr-old subaerial microbial mat from the Barberton Greenstone Belt, South Africa for the UV environmental conditions on the early Earth. Philos Trans R Soc Lond B Biol Sci 361:1857–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westall F., Foucher F., Cavalazzi B., de Vries S.T., Nijman W., Pearson V., Watson J., Verchovsky A., Wright I., Rouzaud J.N., Marchesini D., and Anne S. (2011a) Early life on Earth and Mars: a case study from ∼3.5 Ga-old rocks from the Pilbara, Australia. Planet Space Sci 59:1093–1106 [Google Scholar]
- Westall F., Cavalazzi B., Lemelle L., Marrocchi Y., Rouzaud J.N., Simionovici A., Salomé M., Mostefaoui S., Andreazza C., Foucher F., Toporski J., Jauss A., Thiel V., Southam G., MacLean L., Wirick S., Hofmann A., Meibom A., Robert F., and Défarge C. (2011b) Implications of in situ calcification for photosynthesis in a ∼3.3 Ga-old microbial biofilm from the Barberton Greenstone Belt, South Africa. Earth Planet Sci Lett 310:468–479 [Google Scholar]
- Westall F., Loizeau D., Foucher F., Bost N., Bertrand M., Vago J., and Kminek G. (2013) Habitability on Mars from a microbial point of view. Astrobiology 13:887–897 [DOI] [PubMed] [Google Scholar]
- Westall F., Campbell K.A., Bréhéret F.G., Foucher F., Gautret P., Hubert A., Sorieul S., Grassineau N., and Guido D.M. (2015) Complex microbe-sediment systems are ancient (3.33 Ga) and flourished in a hydrothermal context. Geology 43:615–618 [Google Scholar]
- Winter Y.D., Lowenstein T.K., and Timofeeff M.N. (2013) Identification of carotenoids in ancient salt from Death Valley, Saline Valley, and Searles Lake, California, using laser raman spectroscopy. Astrobiology 13:1065–1080 [DOI] [PubMed] [Google Scholar]
- Zent A.P. and McKay C.P. (1994) The chemical reactivity of the martian soil and implications for future missions. Icarus 108:146–157 [Google Scholar]