Abstract
Endothelial cells (ECs) that are differentiated from induced pluripotent stem cells (iPSCs) can be used in establishing disease models for personalized drug discovery or developing patient-specific vascularized tissues or organoids. However, a number of technical challenges are often associated with iPSC-ECs in culture, including instability of the endothelial phenotype and limited cell proliferative capacity over time. Early senescence is believed to be the primary mechanism underlying these limitations. Sirtuin1 (SIRT1) is an NAD+-dependent deacetylase involved in the regulation of cell senescence, redox state, and inflammatory status. We hypothesize that overexpression of the SIRT1 gene in iPSC-ECs will maintain EC phenotype, function, and proliferative capacity by overcoming early cell senescence. SIRT1 gene was packaged into a lentiviral vector (LV-SIRT1) and transduced into iPSC-ECs at passage 4. Beginning with passage 5, iPSC-ECs exhibited a fibroblast-like morphology, whereas iPSC-ECs overexpressing SIRT1 maintained EC cobblestone morphology. SIRT1 overexpressing iPSC-ECs also exhibited a higher percentage of canonical markers of endothelia (LV-SIRT1 61.8% CD31+ vs. LV-empty 31.7% CD31+, P < 0.001; LV-SIRT1 46.3% CD144+ vs. LV-empty 20.5% CD144+, P < 0.02), with a higher nitric oxide synthesis, lower β-galactosidase production indicating decreased senescence (3.4% for LV-SIRT1 vs. 38.6% for LV-empty, P < 0.001), enhanced angiogenesis, increased deacetylation activity, and higher proliferation rate. SIRT1 overexpressing iPSC-ECs continued to proliferate through passage 9 with high purity of EC-like characteristics, while iPSC-ECs without SIRT1 overexpression became senescent after passage 5. Taken together, SIRT1 overexpression in iPSC-ECs maintains EC phenotype, improves EC function, and extends cell lifespan, overcoming critical hurdles associated with the use of iPSC-ECs in translational research.
Introduction
Induced pluripotent stem cells (iPSCs) are a novel cell source for disease modeling [1,2], drug discovery [3,4], and potentially patient-specific tissue regeneration [5,6]. Specifically, endothelial cells (ECs) that are derived from iPSCs could be used in vascular repair and regeneration [7]. However, early senescence, limited cell proliferation, and instability of the endothelial phenotype remain significant challenges to the large-scale production and wide use of these cells [8,9]. Therefore, strategies need to be developed to improve the durability and performance of iPSC-EC in culture.
Sirtuin 1 (SIRT1) is a nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylase (HDAC) that functions in mammalian cells to promote cell survival [10] and prevent stress-induced senescence [11]. Moreover, SIRT1 plays various roles in maintaining endothelial function, including angiogenesis through deacetylation of the forkhead transcription factor (FOXO1) [12]; nitric oxide (NO) production through deacetylating and activating endothelial nitric oxide synthase (eNOS) [13]; cell proliferation by targeting the LKB1-AMPK pathway[14]; and inhibiting oxidative stress through p53 deacetylation [15,16]. Based on the various roles of SIRT1 in maintaining endothelial homeostasis, we hypothesize that overexpression of SIRT1 in iPSC-ECs would reduce senescence during in vitro culture, thereby maintaining EC phenotype and improving proliferative capacity.
Materials and Methods
Cells
All iPSC-ECs were purchased from Cellular Dynamics International (iCell Endothelial Cells, Madison, WI) and cultured as per the manufacturer's recommended protocol. Specifically, the cells were cultured in the VascuLife VEGF Medium (Lifeline Cell Technologies, Frederick, MD), supplemented with 10% iCell Endothelial Cells Medium Supplement from Cellular Dynamics International. Every 5–7 days, the cells were trypsinized and plated at 10,000 cells/cm2 on fibronectin-coated surfaces.
Lentivirus
All plasmids DNA were purchased from Addgene (Cambridge, MA). Three lentiviruses (LVs) were constructed: empty vector (LV-empty), LV with human SIRT1 (LV-SIRT1), and SIRT1H363Y (LV-SIRT1H363Y). The SIRT1H363Y protein is a catalytically impaired variant of SIRT1 lacking the deacetylase activity [10]. SIRT1 or SIRT1H363Y was cotransfected with pWPI, pMD2.g, and psPAX2 into HEK-293FT cells [17].
Transduction
LVs (LV-empty, LV-SIRT1, and LV-SIRT1H363Y) at MOI 5 were added to iPSC-EC at the end of P4 in the iPSC-EC growth medium for 48 h. SIRT1 and SIRT1H363Y transduction efficiency was evaluated by quantifying the percentage of cells that stained positive for human SIRT1 (Santa Cruz, Dallas, TX) using ImageJ.
Flow cytometry
EC markers CD31 (PECAM) and CD144 (VE-Cadherin) were stained with FITC-conjugated CD31 antibody (1:200 dilution; Sigma-Aldrich, St. Louis, MO) and PE-conjugated CD144 antibody (1:200 dilution; Life Technologies, Carlsbad, CA). Flow cytometry was performed using BD LSR II flow cytometer (San Jose, CA) and the data analyzed with FlowJo analytical software (Ashland, OR).
Functional analysis
The cellular senescence assay kit (Cell Signaling Technology, Danvers, MA) was used to stain for β-galactosidase (β-gal) in the presence or absence of Ex-527 (10 μM), a SIRT1 inhibitor [18], and the percentage of β-gal-positive cells was quantified with ImageJ. Tube formation assay was performed on Matrigel™ (Corning, Corning, NY) surfaces for evaluating cell angiogenesis in vitro as previously described [19]. The cells were stained with Calcein AM (Life Technologies) and the tube density quantified with ImageJ angiogenesis analyzer plugin.
The HDAC cell-based activity assay kit (Cayman Chemicals, Ann Arbor, MI) was used to assess the iPSC-EC deacetylase activity due to SIRT1 by measuring the difference in the HDAC activity between normal (0 μM Ex-527) and SIRT1-inhibited (10 μM Ex-527) culture conditions. Cell proliferation was determined by counting the cell number every 7 days after LV transduction using a hemocytometer by excluding dead cells with Trypan blue. Cell mitogenic effect in response to vascular endothelial growth factor (VEGF) (100 ng/mL) was assessed by the MTT assay (Sigma-Aldrich) after treating cells in a serum-free (starvation) medium, starvation medium containing 100 ng/mL VEGF or regular growth medium for 24 h. The NO production was assessed by 4,5-diaminofluorescein diacetate (DAF 2-DA) assay (Life Technologies). All results were normalized to cell number by the Alamar blue assay (Sigma-Aldrich).
Results
Influence of SIRT1 overexpression on EC phenotype
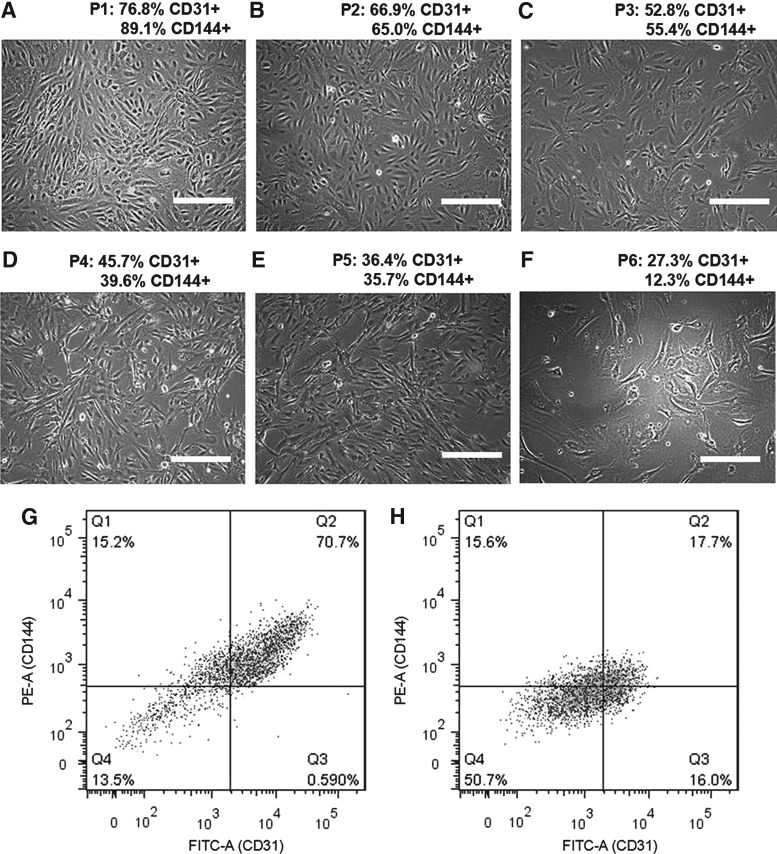
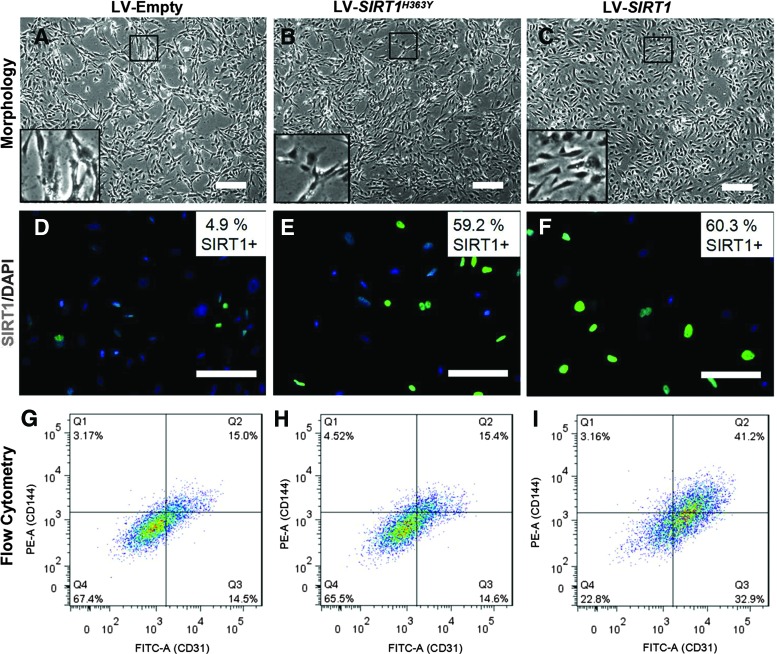
iPSC-ECs exhibit typical EC cobblestone-like morphology between passage 1 and 3, but gradually become fibroblast like with a decreased CD31 and CD144 expression over time (Fig. 1). A transduction efficiency of 60.3% ± 7.3% was measured through immunohistomorphometry of SIRT1-positive cells. Cells overexpressing SIRT1 exhibit a higher degree of EC-like cobblestone morphology compared to the LV-empty and LV-SIRT1H363Y (Fig. 2). The expression of EC markers, such as CD31, was also significantly elevated in the LV-SIRT1 group (61.8% ± 3.6% CD31+) relative to controls (31.1% ± 4.5% CD31+ for LV-empty and 39.8% ±15.4% CD31+ for LV-SIRT1H363Y) at the end of passage 5 (Fig. 2G–I). Moreover, as iPSC-ECs overexpressing SIRT1 continued to proliferate, the percentage of CD31+ cells increased from ∼60% at the end of passage 5 to ∼90% at the end of passage 7 to over 95% by the end of passage 9 (Supplementary Fig. S1; Supplementary materials are available online at http://www.liebertpub.com/scd).
FIG. 1.
Change in morphology and phenotype of iPSC-ECs from passage 1 (A) to passage 6 (F), with representative flow cytometry data from passage 1 (G) and passage 5 (H). Phase contrast images (A–F) show that cells gradually lose the cobble stone-like morphology over time and take on a fibroblast-like appearance. Expression of EC markers (CD31 and CD144) gradually decreased over time. Scale bar = 100 μm. EC, endothelial cell; iPSC, induced pluripotent stem cell.
FIG. 2.
Effect of empty (A, D, G), SIRT1H363Y (B, E, H), and SIRT1 (C, F, I) LV transduction on iPSC-ECs at passage 5, including phase contrast images of iPSC-ECs in culture for morphological assessment (A–C); immunofluorescent staining for SIRT1 (green) as an indication of transduction efficiency (D–F); and flow cytometry analysis for putative markers (x-axis, CD31; y-axis, CD144) of EC phenotype (G–I). Scale bar = 100 μm. LV, lentivirus. Color images available online at www.liebertpub.com/scd
Effect of SIRT1 overexpression on EC function
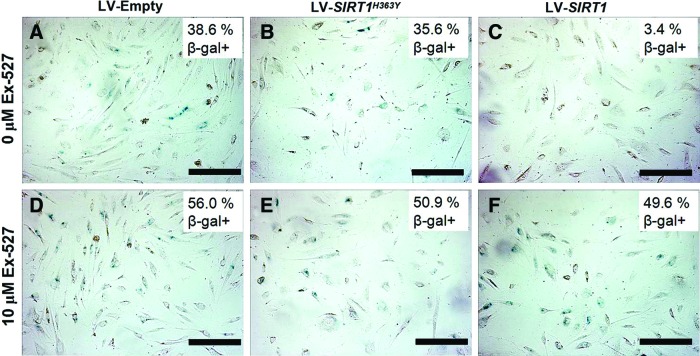
Overexpression of SIRT1 led to a significant reduction of cells entering senescence when compared to control groups (β-gal+ LV-SIRT1: 3.4% ± 2.7%, LV-empty: 38.6% ± 3.3%, and LV-SIRT1H363Y: 35.7% ± 4.9%) (Fig. 3A–C). Blocking SIRT1 with the inhibitor Ex-527 led to a higher percentage of senescent cells in all groups, suggesting a contribution of endogenous SIRT1 (β-gal+ LV-SIRT1: 49.6% ± 10.0%, LV-empty: 56.0% ± 6.2%, and LV-SIRT1H363Y: 50.9% ± 6.8%) (Fig. 3D–F). The tube formation assay showed significantly denser and more organized vascular network formation for cells with SIRT1 overexpression (mesh area LV-SIRT1: 44.4% ± 2.3%, LV-empty: 30.9% ± 5.0%, and LV-SIRT1H363Y: 36.8% ± 3.5%) (Supplementary Fig. S2), suggesting an improved angiogenesis potential.
FIG. 3.
Cellular senescence-associated β-galactosidase (β-gal) staining (blue) of iPSC-EC at passage 6 for empty (A, D), SIRT1H363Y (B, E), and SIRT1 (C, F) LV transduction in the absence (A–C) and presence (D–F) of Ex-527, a SIRT1 inhibitor. Scale bar = 100 μm. Color images available online at www.liebertpub.com/scd
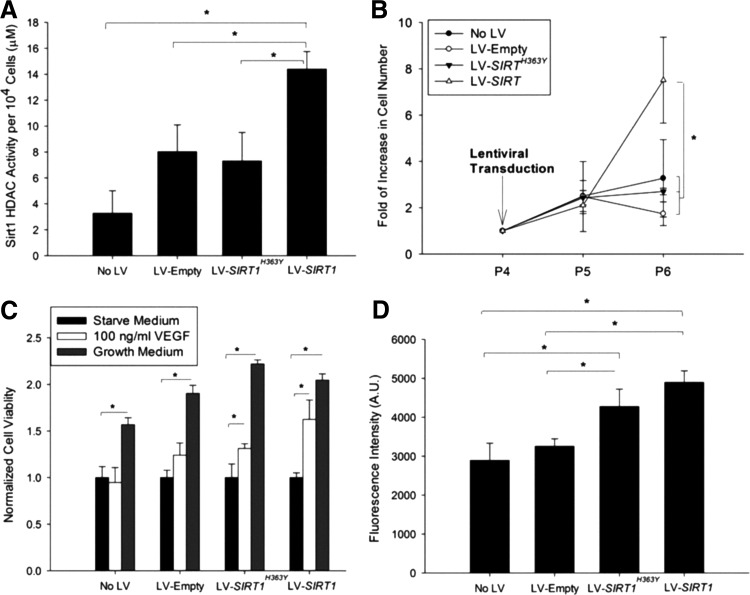
The cell HDAC activity was significantly higher in cells with LV-SIRT1 compared to the endogenous SIRT1 HDAC activity in cells with no viral control, LV-empty and LV-SIRT1H363Y (Fig. 4A). The rate of cell proliferation was significantly increased at two passages following viral transduction with LV-SIRT1, and these cells continued to proliferate throughout passage 10 (Fig. 4B). In contrast, cells from the control groups, including LV-SIRT1H363Y, remained static after passage 5. VEGF stimulation led to an increased mitogenicity in iPSC-ECs overexpressing SIRT1 (Fig. 4C), and these cells also showed significantly higher NO production compared to controls (Fig. 4D).
FIG. 4.
Functional assessment of iPSC-EC with or without viral transduction for (A) HDAC activity, (B) proliferation, (C) response to VEGF, and (D) nitric oxide production. *P < 0.05 (n = 4). HDAC, histone deacetylase; VEGF, vascular endothelial growth factor.
Discussion
The ability of cells derived from iPSCs to maintain a differentiated cell phenotype, function, and proliferative capacity is critical to the use of iPSC technology in disease modeling or tissue regeneration strategies. There has been significant variability regarding the in vitro culture of iPSC-ECs as researchers have reported both satisfactory [7,20] and impaired [8,9] maintenance of EC phenotype. Herein, we report a simple and effective method to prolong EC phenotype, improve cell function, and improve cell proliferation in vitro by overexpressing SIRT1.
The finding that SIRT1 overexpression improves iPSC-EC performance in our investigation agrees with studies by others on primary ECs, which may provide a mechanistic insight to our findings [14,21,22]. A previous study concluded that SIRT1 influences EC proliferation and prevents senescence by promoting the deacetylation, ubiquitination, and proteasome-mediated degradation of LKB1, a serine/threonine kinase and tumor suppressor [14]. In another study, inhibition of SIRT1 activity in ECs resulted in a premature senescent-like phenotype through an increased p53 acetylation in parallel with an increased plasminogen activator inhibitor-1 (PAI-1) expression and decreased eNOS expression [22]. These reports on primary ECs are consistent with our findings using iPSC-ECs demonstrating fewer cells entering senescence upon overexpression of SIRT1 and an increase in senescent cells when SIRT1 was inhibited (Fig. 3). SIRT1 also promotes NO production by activating eNOS through deacetylation [13]. However, how SIRT1 restores or prolongs EC phenotype, including morphology, CD31 and CD144 expression, and response to VEGF in late passage iPSC-ECs, is unclear. Surprisingly, LV-SIRT1H363Y influenced the cellular response to VEGF and NO production, although the effect was not as pronounced as those with LV-SIRT1. In summary, SIRT1 plays critical roles in maintaining iPSC-EC phenotype and function, and its overexpression may be a key step to the large-scale production and wide-scale use of these cells for translational research.
Supplementary Material
Acknowledgments
This work is supported by the Northwestern Memorial Foundation Dixon Translational Research Grants Initiative, the Robert R. McCormick Foundation, National Institute of Health (5R01EB017129-02 and K08DK101757), American Heart Association (AHA) Midwest Affiliate Postdoctoral Fellowship (14POST20160091), and the Chicago Biomedical Consortium (CBC) Postdoctoral Award (PDR-008).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, Feldman O, Gepstein A, Arbel G. and Hammerman H. (2011). Modelling the long QT syndrome with induced pluripotent stem cells. Nature 471:225–229 [DOI] [PubMed] [Google Scholar]
- 2.Stepniewski J, Kachamakova-Trojanowska N, Ogrocki D, Szopa M, Matlok M, Beilharz M, Dyduch G, Malecki MT, Jozkowicz A. and Dulak J. (2015). Induced pluripotent stem cells as a model for diabetes investigation. Sci Rep 5:8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grskovic M, Javaherian A, Strulovici B. and Daley GQ. (2011). Induced pluripotent stem cells—opportunities for disease modelling and drug discovery. Nat Rev Drug Discov 10:915–929 [DOI] [PubMed] [Google Scholar]
- 4.Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH. and Muotri AR. (2010). A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143:527–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan X, Tu Q, Zhang J, Ye J, Sommer C, Mostoslavsky G, Kaplan D, Yang P. and Chen J. (2011). Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. J Cell Physiol 226:150–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu SM. and Hochedlinger K. (2011). Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol 13:497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams WJ, Zhang Y, Cloutier J, Kuchimanchi P, Newton G, Sehrawat S, Aird WC, Mayadas TN, Luscinskas FW. and García-Cardeña G. (2013). Functional vascular endothelium derived from human induced pluripotent stem cells. Stem Cell Reports 1:105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng Q, Lu S-J, Klimanskaya I, Gomes I, Kim D, Chung Y, Honig GR, Kim K-S. and Lanza R. (2010). Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells 28:704–712 [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Hu S, Ghosh Z, Han Z. and Wu JC. (2011). Functional characterization and expression profiling of human induced pluripotent stem cell- and embryonic stem cell-derived endothelial cells. Stem Cells Dev 20:1701–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R. and Sinclair DA. (2004). Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305:390–392 [DOI] [PubMed] [Google Scholar]
- 11.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R. and Cohen HY. (2004). Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303:2011–2015 [DOI] [PubMed] [Google Scholar]
- 12.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E. and Alt FW. (2007). SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev 21:2644–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattagajasingh I, Kim C-S, Naqvi A, Yamamori T, Hoffman TA, Jung S-B, DeRicco J, Kasuno K. and Irani K. (2007). SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci 104:14855–14860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zu Y, Liu L, Lee MY, Xu C, Liang Y, Man RY, Vanhoutte PM. and Wang Y. (2010). SIRT1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circ Res 106:1384–1393 [DOI] [PubMed] [Google Scholar]
- 15.Potente M. and Dimmeler S. (2008). Emerging roles of SIRT1 in vascular endothelial homeostasis. Cell Cycle 7:2117–2122 [DOI] [PubMed] [Google Scholar]
- 16.Zarzuelo MJ, López-Sepúlveda R, Sánchez M, Romero M, Gómez-Guzmán M, Ungvary Z, Pérez-Vizcaíno F, Jiménez R. and Duarte J. (2013). SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol 85:1288–1296 [DOI] [PubMed] [Google Scholar]
- 17.Jen MC, Baler K, Hood AR, Shin S, Shea LD. and Ameer GA. (2013). Sustained, localized transgene expression mediated from lentivirus-loaded biodegradable polyester elastomers. J Biomed Mater Res A 101A:1328–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solomon JM, Pasupuleti R, Xu L, McDonagh T, Curtis R, DiStefano PS. and Huber LJ. (2006). Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol 26:28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnaoutova I. and Kleinman HK. (2010). In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc 5:628–635 [DOI] [PubMed] [Google Scholar]
- 20.Belair DG, Whisler JA, Valdez J, Velazquez J, Molenda JA, Vickerman V, Lewis R, Daigh C, Hansen TD. and Mann DA. (2014). Human vascular tissue models formed from human induced pluripotent stem cell derived endothelial cells. Stem Cell Rev Rep:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moura R, Fadini GP. and Tjwa M. (2010). Induced pluripotent stem (iPS) cells and endothelial cell generation: SIRT-ainly a good idea! Atherosclerosis 212:36–39 [DOI] [PubMed] [Google Scholar]
- 22.Ota H, Akishita M, Eto M, Iijima K, Kaneki M. and Ouchi Y. (2007). Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol 43:571–579 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.