Abstract
Salmonella infection, ranging from mild, self-limiting diarrhea to severe gastrointestinal, septicemic disease and enteric fever, is a global health problem both in humans and animals. Rapid development of microbial drug resistance has led to a need for efficacious and affordable vaccines against Salmonella. Microbial heat shock proteins (HSPs), including HSP60 and HSP70, are the dominant antigens that promote the host immune response. Co-administration of these antigens with cytokines, such as IL-22, which plays an important role in antimicrobial defense, can enhance the immune response and protection against pathogens. Therefore, the aim of the present study was to determine the immunogenicity of rGroEL (Hsp60) of S. Typhi, alone or administered in combination with murine rIL-22, and its protective efficacy against lethal infection with Salmonella, in mice. There was appreciable stimulation of the humoral and cell-mediated immune responses in mice immunized with rGroEL alone. However, co-administration of rGroEL with rIL-22 further boosted the antibody titers (IgG, IgG1 and IgG2a), T-cell proliferative responses and the secretion of both Th1 and Th2 cytokines. Additionally, rGroEL alone accorded 65%–70% protection against lethal challenge with S. Typhi and S. Typhimurium, which increased to 90% when co-administered with rIL-22.
Keywords: gene expression, GroEL, immunity, IL-22, vaccine
Introduction
Intestinal bacterial infections are a major cause of mortality worldwide and continue to threaten global health. Salmonella is an important zoonotic pathogen, spreading from contaminated food products to humans. Typhoid fever, an acute life-threatening febrile illness caused by infection with Salmonella enterica serovar Typhi, is still an unsolved problem in most of the world, with an annual global incidence of 22 million cases and nearly 200 000 deaths, predominantly in infants, young children, the elderly and immune-compromised patients,1,2 indicating that the global burden of this disease has increased steadily from a previous estimate of 16 million.3 Non-availability of relevant drugs and rapid development of microbial drug resistance has led to a need for efficacious and affordable vaccines to control typhoid fever. There have been several vaccination strategies against serovar Typhi; however, none of them is optimal in all aspects. Two new-generation typhoid vaccines have replaced the old, reactogenic inactivated whole-cell, vaccines used in the past. These new-generation vaccines, live oral Ty21a and injectable Vi polysaccharide, have been shown in large-scale clinical trials to be moderately efficacious. The single-dose injectable Vi vaccine induces only humoral immunity, provides approximately 65%–70% protection that lasts only three years and is not immunogenic in children less than 2 years of age.4 Moreover, it can lead to side effects such as pain, swelling, redness, tenderness, etc. and sometimes results in a mild fever lasting for 24 h. Ty21a has been used as an orally administered, live, attenuated vaccine and is recommended after the age of 4–6 years. It is contraindicated in immune-compromised hosts as it is a live vaccine. The liquid formulation of Ty21a is given in 3–4 doses and provides 53%–78% protection for 5 years.5 Thus, a potent vaccine capable of inducing humoral and cellular immunity against typhoid fever is an immediate global health need.6
As a novel vaccination approach, heat shock protein (HSP)-based vaccines have become an attractive strategy for disease prevention. HSPs or stress proteins, are among the most highly conserved molecules of the biosphere and help maintain homeostasis in eukaryotic and prokaryotic cells. They function as molecular chaperones, binding to and refolding other cellular polypeptides, preventing their aggregation and misfolding,7 and play an important role in both innate and adaptive immunity.8 Microbial HSPs are the dominant antigens to promote the host immune response.9,10 A number of studies have reported significant protection by using pathogen-derived Hsps as vaccine candidate molecules in various infectious disease models, e.g., recombinant GroES and GroEL from Helicobacter pylori,11 HSP60 from Histoplasma capsulatum,12 HSP60 from Y. enterocolitica13 and HSP60 and 70 from Piscirickettsia salmonis.14 We have already reported the development and efficacy of recombinant GroEL of S. Typhi in protection against Salmonella infection.15 Immunization of mice with rGroEL alone conferred 65%–70% protection against lethal infection with S. Typhi and S. Typhimurium, whereas 80%–90% protection was seen with immunization by rGroEL along with Complete Freud's Adjuvant.15,16
The current adjuvant licensed for human use, alum, has several side effects. Co-immunization with cytokines has been reported to enhance the immune response and protection against pathogens.17,18,19 Cytokines are small secretory protein molecules that are involved in various pro-inflammatory functions against the invading pathogens. They induce the secretion of chemokines and several antimicrobial proteins, thereby creating a protective layer against gastrointestinal pathogens. The use of these key molecules as immune potentiators (adjuvants) is crucial for vaccine effectiveness to obtain the appropriate immune response, thereby ensuring a protective outcome. Several cytokines have already been shown to be efficient adjuvants in animal models and/or in clinical trials.20,21,22
Interleukin 22 (IL-22), a member of the IL-10 family of cytokines discovered in 2000, is an important effector molecule of activated Th17, Th1 and Th22 cells, γδ T cells, natural killer cells and natural killer T cells.23 It has been found to have a critical role in regulating host defense, tissue homeostasis and inflammation. Several researchers have reported anti-inflammatory and tissue protective properties of IL-22 in addition to its protective role against bacterial infections.24,25,26,27 Studies suggest that this mediator might have an important role in the avoidance and clearance of epithelial and mucoepithelial infections, regeneration and protection against damage in some chronic inflammatory cutaneous, pulmonary and intestinal diseases. The beneficial role of IL-22 in host defense has been studied in various infections of the lung and intestine, including Klebsiella pneumonia,24 C. rodentium,25 Salmonella Typhimurium28 and Mycobacterium tuberculosis.29
Recently, we have reported the immunomodulatory effect of IL-22 expressing plasmid either by co-delivery or by fusion with the GroEL gene of S. Typhi in mice.30 In continuation of this study, here we report that co-administration of the rIL-22 protein can modulate the rGroEL-mediated immune response against S. Typhi and can augment protection against lethal Salmonella infection in mice.
Materials and methods
Mice
Four- to six-week-old female BALB/c mice were maintained in the Experimental Animal Facility of the Institute under standard laboratory conditions. Food and sterile water were given ad libitium. Mice were handled and disposed of according to the guidelines of the Institute Animal Ethical Committee.
Bacterial strains, vectors and reagents
S. Typhi MTCC 733 procured from the Institute of Microbial Technology (Chandigarh, India) was used for the isolation of genomic DNA. The E. coli DH5α strain (Invitrogen, Carlsbad, CA, USA) was used for plasmid preparations, and the E. coli BL21 (DE3) strain (Novagen, Dermstadt, Germany) was used as a host for the expression of recombinant proteins. Pathogenic strains of S. Typhi and S. Typhimurium were clinically isolated at All India Institute of Medical Sciences (New Delhi, India). The pTZ57R/T (MBI Fermentas) PCR cloning vector and pET28c (Novagen, Dermstadt, Germany) were used for cloning of PCR products and expression, respectively. All enzymes used in the present study were from MBI Fermentas, and all chemicals and kits, unless otherwise stated, were purchased from Sigma-Aldrich (St. Louis, MO, USA). All of the kits were used as recommended by the manufacturer's instructions.
Bacterial cultivation and DNA purification
The bacterial strains were grown in Luria Bertani (LB) medium (Difco, Sparks, MD, USA) at 37 °C. The rGroEL (HSP60) and IL-22 cultures were supplemented with ampicillin (100 µg/ml) and kanamycin (50 µg/ml), respectively. Genomic and plasmid DNA were isolated using the GenElute genomic DNA isolation kit and GenElute plasmid DNA isolation kit (Sigma, St. Louis, MO, USA), respectively, as per the manufacturer's instructions.
Cloning, expression and purification of recombinant GroEL and IL-22 proteins
Cloning, expression and purification of IL-22
IL-22 cDNA was reverse transcribed from the extracted RNA of mouse splenocytes. In brief, total RNA was isolated from Mus musculus splenocytes activated with concanavalin A (ConA) using an RNeasy Kit (Qiagen, Hilden, Germany). Total RNA was used as a template for the synthesis of first strand cDNA using a cDNA synthesis kit (Fermentas, Hanover, MD, USA) according to the manufacturer's instructions. A 540 bp-long open reading frame of IL-22 was amplified by PCR using gene-specific primers, as specified below with NheI and BamHI at the 5′ and 3′ ends, respectively.
mIL-22 sense: 5′-AAGCTAGCGAGCTCACCATGGCTGTCCTGCAGAAATCTATG-3′
mIL-22 antisense: 5′-AAGGATCCGACGCAAGCATTTCTCAGAGAC-3′.
The PCR product of 540 bp was ligated into the pET28c expression vector and was expressed by transformation into E. coli BL21 (DE3) cells. The recombinant plasmid was confirmed by colony PCR, restriction digest and DNA sequencing. A single colony of transformed E. coli was grown in LB medium supplemented with 50 µg/ml kanamycin and cultured at 37 °C with constant shaking at 200 r.p.m. until the OD600 reached 0.6. Isopropyl-β-𝒹-thiogalactopyranoside was added to a final concentration of 1 mM and the culture was further incubated at 37 °C for 4–5 h.
Bacterial cells were collected by centrifugation at 5000g for 10 min, suspended in lysis buffer (100 mM Tris, 500 mM ethylene diamine tetraacetic acid (EDTA), pH 8.0) and 100 mM phenylmethylsulfonyl fluoride and lysed by sonication (Vibra, Sonics and Materials Inc, CT, USA) in an ice water bath. The IL-22 inclusion bodies were collected by centrifugation at 10 000g for 20 min at 4 °C. Inclusion bodies were washed first with Tris-EDTA buffer with 1% sodium deoxycholate, then in buffer containing 8.5 mM sodium dodecyl sulfate/N-lauryl sarcosine, 6 M urea, 2 M thiourea, 0.5 M NaCl and 30 mM NaH2PO4 (pH 8.0). After 1 h of gentle vortexing, the suspension was centrifuged at 12000g for 20 min at 4 °C to recover inclusion bodies in pellet. The pellet was then solubilized in solubilization buffer containing 100 mM Tris-Cl, 65 mM dithiothreitol and 8 M urea, pH 6.5, for 72 h with constant stirring at 4 °C and refolded in refolding buffer (100 mM Tris-HCl, 2 mM EDTA, 0.5 M L-arginine, 1 mM GSH, 0.1 mM GSSG, pH 8.0). The solution was incubated for 72 h at 4 °C with slow stirring followed by dialysis against Tris-EDTA buffer at 4 °C overnight and finally concentrated using Amicon filters (Millipore, Billerica, MA, USA). The concentration of the purified recombinant protein was estimated by the Folin-Lowry method31 and the protein stored at −80 °C until further use. The amount of lipopolysaccharide in the purified protein was determined by a Limulus amebocyte lysate assay32,33,34 and was found to be negligible (<1 EU/mg protein).
Cloning, expression and purification of GroEL
The full-length 1.6 kb coding region of GroEL was amplified from the genomic DNA of S. Typhi by PCR using the following set of primers with BamHI and HindIII sites at the 5′ and 3′ ends:
Forward primer: 5′-AAGGGAAAGGATCCATGGCAGCTAAAGACG-3′
Reverse primer: 5′-TGCAGGGGGTAAGCTTTTACATCATGC-3′.
The amplicon was cloned into the expression vector pQE30, transformed and expressed in E. coli BL21 (DE3), as reported earlier in our laboratory.15 Briefly, transformed E. coli BL21 cells were grown in LB medium (500 ml) until the OD600 reached 0.5–0.6 and then induced with 0.5 mM isopropyl-β-𝒹-thiogalactopyranoside for 4 h. The expressed recombinant GroEL protein was purified by Ni-NTA chromatography under denaturing conditions according to the manufacturer's instructions (Qiagen). The purified protein was then refolded in vitro using refolding buffer (100 mM Tris-HCl, 2 mM EDTA, 1.0 M L-arginine, 1 mM GSH, 0.1 mM GSSG, pH 8.0), dialyzed against Tris-EDTA buffer at 4 °C for 48 h and concentrated using Amicon filters. The amount of lipopolysaccharide in purified GroEL protein was determined by Limulus amebocyte lysate assay32,33,34 and was found to be negligible (<1 EU/mg protein).
Biological activity of recombinant IL-22
The mitogenic activity of recombinant IL-22 was determined using a 3-(4,5-dimethylthiazolyl-2)-2, 5-diphenyltetrazoliumbromide (MTT) assay and compared to commercial available IL-22 (R&D, Minneapolis, USA).35,36 Splenocytes were collected and cultured in RPMI 1640 medium with 10% (v/v) fetal bovine serum. Approximately 1×106 cells/well were seeded in 96-well tissue culture microtiter plates and stimulated in vitro with varying concentrations of recombinant IL-22 and commercial IL-22 (5 ng–1.0 µg) in each well; 5 µg/ml ConA was used as a positive control. The cells were incubated at 37 °C under 5% CO2 for 72 h. After 72 hrs of incubation, 20 µl MTT was added in each well and the cells were incubated for another 4 h at 37 °C. The supernatant was discarded and 200 µl dimethyl sulfoxide was added in each well to dissolve the purple formazan crystals at the bottom of the wells. Absorbance at 570 nm was measured with the microplate reader (Fluostar Omega, BMG Labtech GmBH, Ortenberg, Germany).
Immunization of mice
Four- to-six week-old female BALB/c mice (n=6/group) were immunized with three doses of antigen/IL-22 on days 0, 7 and 28 intraperitoneally (i.p.). The groups were as follows: Group 1, injected with phosphate-buffered saline (PBS) only, serving as a negative control; Group 2, immunized with rGroEL alone (40 µg/animal); and Group 3, Co-immunized with rGroEL antigen (40 µg) and rIL-22 (20 µg)
Antibody responses in mice immunized with formulations of GroEL alone or with IL-22
On the seventh day after the last immunization, blood was collected and serum separated. Antigen-specific ELISAs were performed in the sera of the control and experimental groups to determine the antibody titers. Briefly, 96-well microtiter plates (Grenier, Frickenhausen, Germany) were coated with antigen (1 µg GroEL) by overnight incubation at 4 °C followed by washing three times with wash buffer (PBS with 0.05% Tween-20) and blocking with 5% bovine serum albumin in PBS–Tween for 2 h at 37 °C. Plates were washed three times and incubated at 37 °C for 2 h with serially diluted serum samples (200 µl) collected from control and immunized animals, followed by incubation with horseradish peroxidase-conjugated goat anti-mouse IgG, IgG1 or IgG2a secondary antibodies (Santacruz, CA, USA) at 37 °C for 1 h. After washing, 100 µl of TMB/H2O2 substrate (BD Biosciences, San Diego, CA, USA) was added to each well and incubated for 20–30 min in the dark. Finally, the reaction was terminated by the addition of 50 µl 2 N H2SO4 and the absorbance was read at 450 nm in a microplate reader (Molecular devices, Sunnyvale, CA, USA).
Cell-mediated immune responses
One week after the last immunization, the animals were sacrificed in each group and the spleens were collected aseptically. Splenocytes (1×106 cells/well) were cultured in duplicate 96-well tissue culture plates for the cell proliferation assay and cytokine estimation, respectively, and stimulated with rGroEL (5 µg/well). Splenocytes stimulated with ConA (5 µg/ml) or RPMI media alone (unstimulated control) were kept as a control. After incubation at 37 °C for 72 h, cell proliferation was studied in one plate using an MTT assay as described above.
Estimation of cytokines
Levels of IFN-γ, IL-4, IL-6 and IL-10 were measured in the supernatants of duplicate tissue culture plate by ELISA according to the manufacturer's instructions (BD Biosciences). IL-1β was assayed in the supernatants of peritoneal macrophages isolated from control and immunized animals.37 Briefly, the animals were injected with 4% thioglycollate i.p. 3 days before killing to induce peritoneal macrophages. The animals were killed and 5 ml of cold PBS was flushed into the peritoneal cavity, gently aspirated and collected in a centrifuge tube. The fluid containing peritoneal macrophages was centrifuged at 2000 r.p.m. for 10 min and the pellet was suspended in RPMI medium. The macrophages were counted and 1×106 cells/ml were cultured in 96-well plates, stimulated with rGroEL as described above and incubated for 72 h at 37 °C and 5% CO2. The supernatant was collected and analyzed for the presence of IL-1β by reading the absorbance at 450 nm, as directed in the manufacturer's instructions (BD Biosciences).
Assessment of protective efficacy against S. Typhi and S. Typhimurium
Two additional sets of mice divided into three groups each (n=10/group) were immunized as described previously. The lethal dose of the pathogenic strains was established by serial dilutions and colony counting. To assess the protective efficacy, 15 days after the last immunization, one set of animals was challenged with a lethal dose of S. Typhi (1×107 colony forming units (CFU)/mouse) and another set with S. Typhimurium (1×104 CFU/mouse) through i.p. injection. The mice were observed for morbidity and mortality for 30 days.
Organ burden estimation
To assess the bacterial load, four animals from each immunized group were killed 5 weeks after challenge and spleen, liver and intestine were removed. The control animals were sacrificed 3 days after challenge and different tissues collected. The tissues of all the groups were homogenized in 5 ml of ice cold PBS containing 0.5% Tween 80 using a tissue homogenizer (Kinetimatica AG, Luzern, Switzerland). The resulting homogenates were 10-fold serially diluted in PBS and 100 µl from each dilution was plated on LB agar plates in duplicate followed by incubation at 37 °C for 16–18 h. The number of CFU was counted and expressed as log10 CFU.
Histopathology
For histopathology studies, the spleen, liver and intestine were collected from another three animals from each group challenged with S. Typhimurium as described above. The tissues were excised, fixed in 10% formalin and embedded in paraffin blocks. Sections were stained with hematoxylin and eosin. Analysis of the sections was performed by microscopic examination.
Statistical analysis
Data are presented as the mean±s.d.. The data were subjected to statistical analysis using SPSS 16.0 by applying one-way analysis of variance with post-hoc Bonferroni analysis or t-test (wherever applicable). P<0.05 was considered as significant. Data from the challenge studies are expressed as Kaplan–Meier survival curves and are analyzed by log-rank test. All the experiments were repeated on three different occasions.
Results
Cloning, expression and purification of recombinant GroEL and IL-22
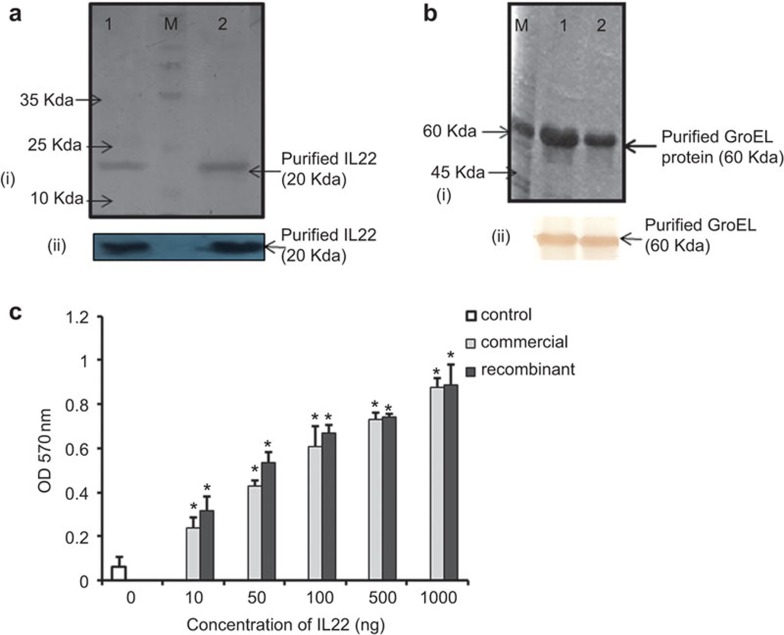
Single bands of 58 kDa and 20 kDa of GroEL and IL-22, respectively, were observed after overexpression and purification of these recombinant proteins and confirmed by western blotting (Figure 1a and b). The biological activity of rIL-22 was tested by in vitro proliferation of mouse spleen cells. MTT results showed that rIL-22 stimulated mouse splenocytes in vitro over a wide range of concentrations, from 10 ng to 1.0 µg (P<0.001 vs. control), and the results were comparable to commercially available IL-22 (Figure 1c)
Figure 1.
Purification and analysis of purified rIL-22 and rGroEL. (a) (i) SDS–PAGE of rIL-22. Lane 1: purified recombinant IL-22; lane 2: commercial IL-22 (positive control). (ii) Western blot of rIL-22. (b) (i) SDS–PAGE of rGroEL. Lane M: molecular weight marker. Lanes 1 and 2 show purified rGroEL protein. (ii) Western blot of rGroEL. (c) rIL-22 promotes murine lymphocyte proliferation. Cell proliferation was evaluated by MTT assay. Significant proliferation was induced by rIL-22 compared to the control (*P<0.001 vs. control). The data are presented as the mean of three independent experiments. SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
GroEL-specific serum antibody response
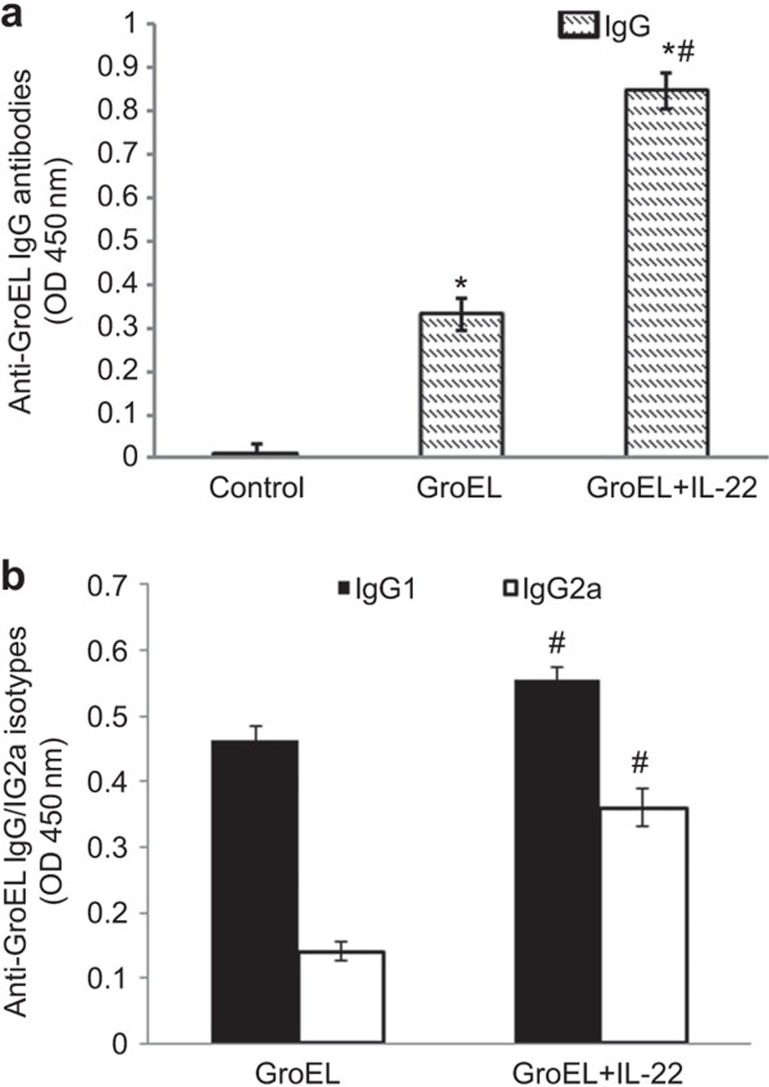
The GroEL-specific serum antibody responses in different groups of immunized and control mice were determined by measuring IgG, IgG1 and IgG2a antibody titers by ELISA. We observed an appreciable increase in the total IgG response in rGroEL-immunized animals compared to controls (Figure 2a). Co-administration of rGroEL antigen with rIL-22 resulted in a further increase in the antibody response compared to mice immunized with rGroEL alone (P<0.001). No detectable IgG response was observed in the PBS control group. To determine the type of immune response, the levels of the antibody isotypes IgG1 and IgG2a were also estimated. Increased levels of both IgG1 and IgG2a were observed in rGroEL+rIL-22-immunized group followed by the rGroEL alone group (Figure 2b). The IgG1 response was predominant in both of the groups, with levels significantly higher in the co-administration group.
Figure 2.
Anti-GroEL antibody response after immunization with rGroEL in the presence of IL-22. Groups of mice (n=6) were immunized on day 0 with rGroEL/rIL-22+rGroEL followed by two booster doses on the seventh and twenty-eighth days. Control animals were injected with PBS. One week after the last immunization, blood was drawn, and antibody titers were measured in the serum of control and immunized animals by ELISA using either (a) goat anti-mouse IgG–HRP conjugate or (b) rabbit anti-mouse IgG1/IgG2a–HRP conjugate. No detectable antibody isotypes (IgG1 and IgG2a) were observed in the control group. The data are presented as the mean±s.d. of six mice per group of three independent experiments, and statistical analysis was performed between the control and different immunized groups by one-way ANOVA. *P<0.001 vs. control, #P<0.001 vs. rGroEL. ANOVA, analysis of variance; HRP, horseradish peroxidase; PBS, phosphate-buffered saline.
Antigen-specific cellular responses
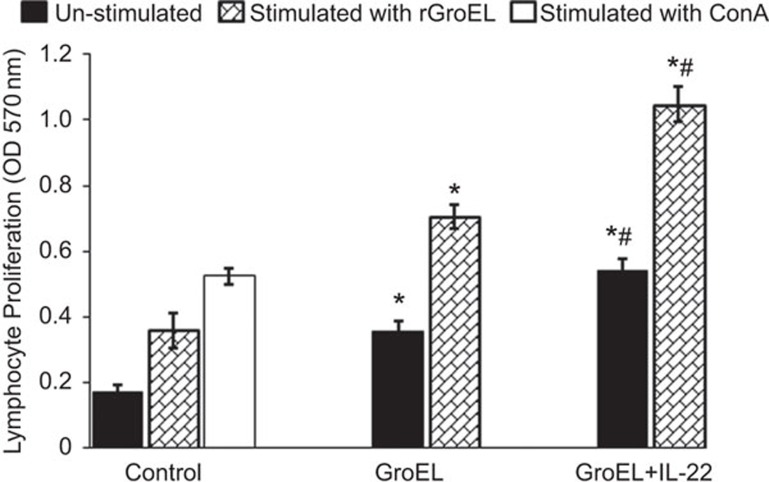
For evaluation of cellular responses, lymphocyte proliferation was studied in response to in vitro stimulation with rGroEL in splenocytes isolated 1 week after the last immunization. Significantly higher proliferation was seen in mice co-administered with rGroEL antigen and IL-22 compared to those immunized with rGroEL alone or PBS control animals (P<0.01) in response to in vitro stimulation with rGroEL (Figure 3) or ConA-stimulated control animals.
Figure 3.
Lymphocyte proliferation in mice immunized with rGroEL in the presence of IL-22. Groups of mice (n=6 each) were immunized with rGroEL/rGroEL+rIL-22 on days 0, 7 and 28. Control animals were injected with PBS. Seven days after the last immunization, splenocytes were isolated from mice and cultured (1×106 cells/well) in the absence (unstimulated) or presence (stimulated) of rGroEL for 72 h in a CO2 incubator at 37 °C. Lymphocyte proliferation was determined by MTT assay and absorbance was measured at 570 nm. The data were compared by one-way analysis of variance between different immunized groups. *P<0.01 vs. control, #P<0.01 vs. rGroEL. MTT, 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazoliumbromide; PBS, phosphate-buffered saline.
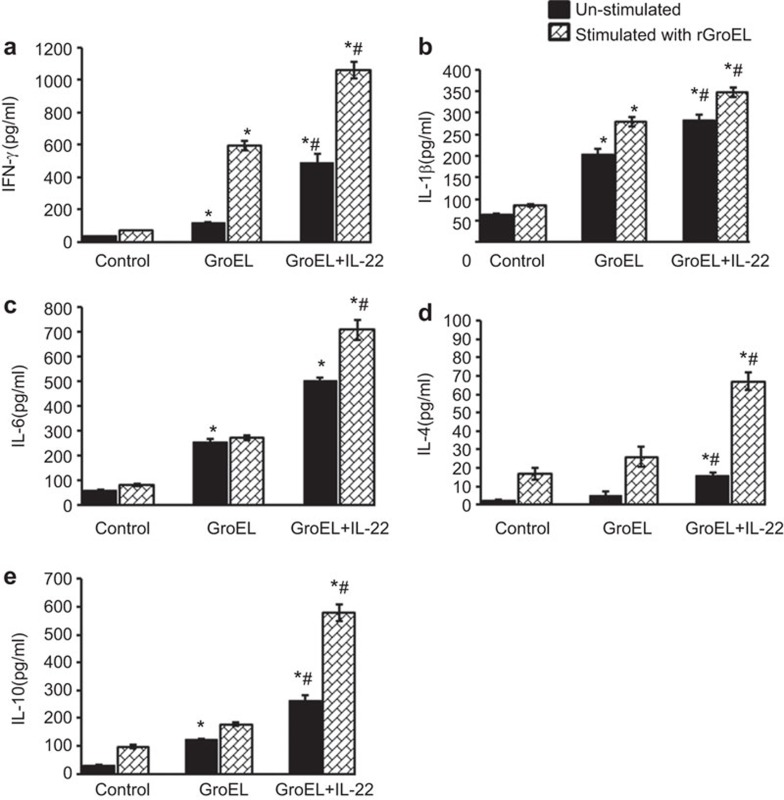
There was a marked increase in interleukins levels in lymphocytes isolated from both of the immunized groups as shown in Figure 4. Furthermore, levels of IL-6, IFN-γ, IL-4, IL-10 and IL-1β were found to be significantly higher in the rGroEL+rIL-22 co-administered group (p<0.01) compared to the group immunized with rGroEL alone. These findings indicate that codelivery of rGroEL with rIL-22 enhances both humoral and cellular immune responses in mice.
Figure 4.
Effect of codelivery of rGroEL antigen and IL-22 on cytokine levels. Mice were immunized with rGroEL or rGroEL+rIL-22. One week after the last immunization, splenocytes/peritoneal macrophages isolated from mice were cultured (1×106 cells/well) in the absence (unstimulated) or presence (stimulated) of rGroEL for 72 h. Cytokines were estimated in culture supernatants collected from control and immunized groups by ELISA: (a) IFN-γ, (b) IL-1β, (c) IL-6, (d) IL-4 and (e) IL-10. The data are presented as the mean concentration (pg/ml)±s.d. and are representative of three independent experiments and compared by one-way ANOVA. *P<0.01 vs. control, #P<0.01 vs. rGroEL. ANOVA, analysis of variance.
Protective efficacy against S. Typhi and S. Typhimurium
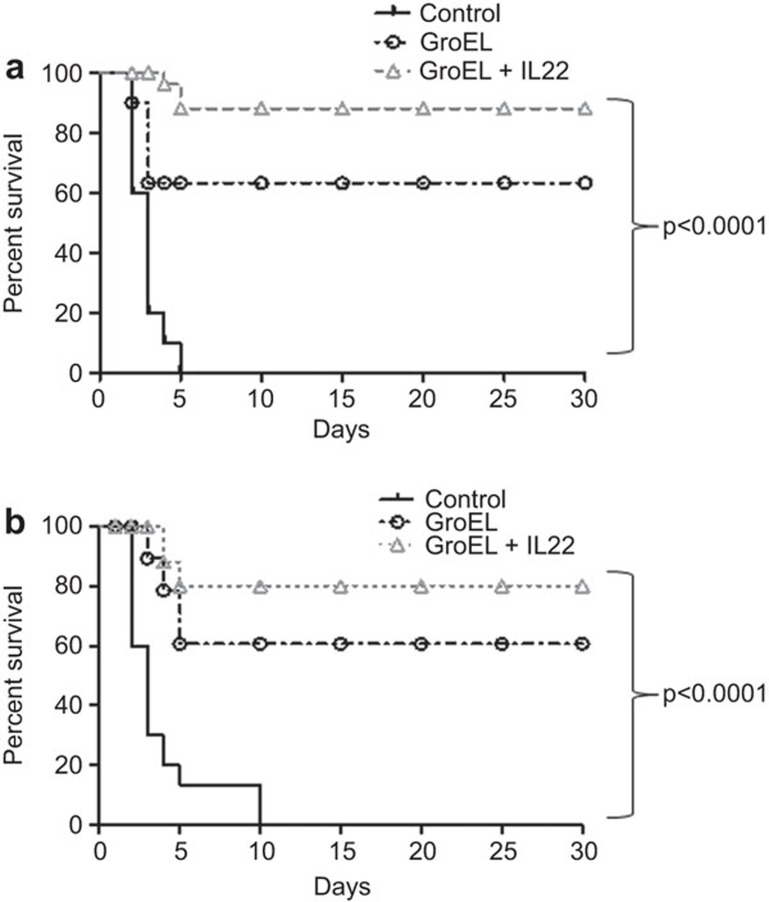
Experiments were carried out to study the protective efficacy of the combined action of rIL-22 and rGroEL against lethal infection of mice with Salmonella. Fifteen days after the last immunization, one set of mice was challenged with 1×107 CFU/mouse of S. Typhi and observed for morbidity and mortality for 30 days. Control mice showed signs of weakness, inactivity, decreased food and water intake and died within 4–5 days of infection. In the group immunized with rGroEL alone, 65%–70% mice survived the lethal infection until the end of the study (Figure 5a). Only 1–2 animals showed signs of lethargy initially and food and water intake were normal. In the group immunized with rGroEL+rIL-22, 85%–90% survival was observed. All of the animals that survived were normal and active. Similarly, another set of mice was challenged with 1×104 cells of S. Typhimurium. Control mice died within 5 days of infection, whereas only 20% mortality was observed in the co-immunized group; rest of the immunized mice survived the infection (Figure 5b). Approximately 60%–65% protection was observed in mice immunized with rGroEL alone.
Figure 5.
Effect of immunization with rGroEL with co-administration of IL-22 on the survival of mice against lethal challenge with Salmonella. Groups of mice (n=10 each) were immunized with rGroEL/rGroEL+rIL-22 on day 0 and followed by two booster doses on the seventh and twenty-eighth day. Control mice were immunized with sterile PBS. Fifteen days after the last immunization, mice were challenged with (a) 1×107 CFU/ml of S. Typhi or (b) 1×104 CFU/ml of S. Typhimurium i.p. Animals were observed for mortality for 30 days. The data were compared by log-rank test and are presented as Kaplan–Meier survival curves. CFU, colony forming units; i.p., intraperitoneally; PBS, phosphate-buffered saline.
Bacterial burden
To determine the efficacy of rGroEL and rGroEL+rIL-22 in reducing the bacterial load, the immunized and control animals were challenged with S. Typhi and S. Typhimurium and the bacterial load was estimated from the spleen, liver and intestine collected from different groups of mice. Co-administration of rGroEL antigen with IL-22 led to a significant decrease in the CFU in different tissues of the animals compared to animals immunized with rGroEL alone and control groups (P<0.001). The level of protection of each treatment was calculated based on the reduction of the bacterial load. As evident from Table 1, mice immunized with rGroEL+rIL-22 elicited significantly higher protection than rGroEL alone. As similar protection was observed against both of the pathogens, the data from the S. Typhimurium infection are shown.
Table 1. Organ burden studies for controls and animals immunized with rGroEL/rGroEL+rIL-22.
| Intestine | Spleen | Liver | ||||
|---|---|---|---|---|---|---|
| Experimental Groups (n=4) | Mean log10 CFU±s.d. | Log10 unit of protection | Mean log10 CFU±s.d. | Log10 unit of protection | Mean log10 CFU±s.d. | Log10 unit of protection |
| Control | 8.00±0.01 | — | 7.69±0.01 | — | 7.65±0.01 | — |
| rGroEL | 7.53±0.01* | 0.47 | 7.47±0.02$ | 0.22 | 7.00±0.04* | 0.65 |
| rGroEL+rIL-22 | 6.30±0.08*,# | 1.71 | 6.00±0.09*,# | 1.70 | 6.47±0.14*,# | 1.18 |
Abbreviation: CFU, colony forming units.
The liver, spleen and intestine of control (injected with PBS) and recombinant protein-immunized mice challenged with S. Typhi and S. Typhimurium were removed aseptically and homogenized in 0.5% Tween 80–PBS buffer, serially diluted and plated on growth medium (LB agar) plates at 37 °C. The mean CFU/organ in each experimental group was determined. Data are presented as the mean log10 CFU±s.d. The level of protection was determined by subtracting the mean log10 CFU value of the experimental group from the mean of log10 CFU value of the negative control group. Statistical significance was determined by one-way analysis of variance between the control group and each immunized group.
P<0.001 vs. control.
P<0.05 vs. control.
P<0.001 vs. rGroEL (as similar protection was observed against both S. Typhi and S. Typhimurium, only the data from S. Typhimurium infection are shown).
Histopathological studies
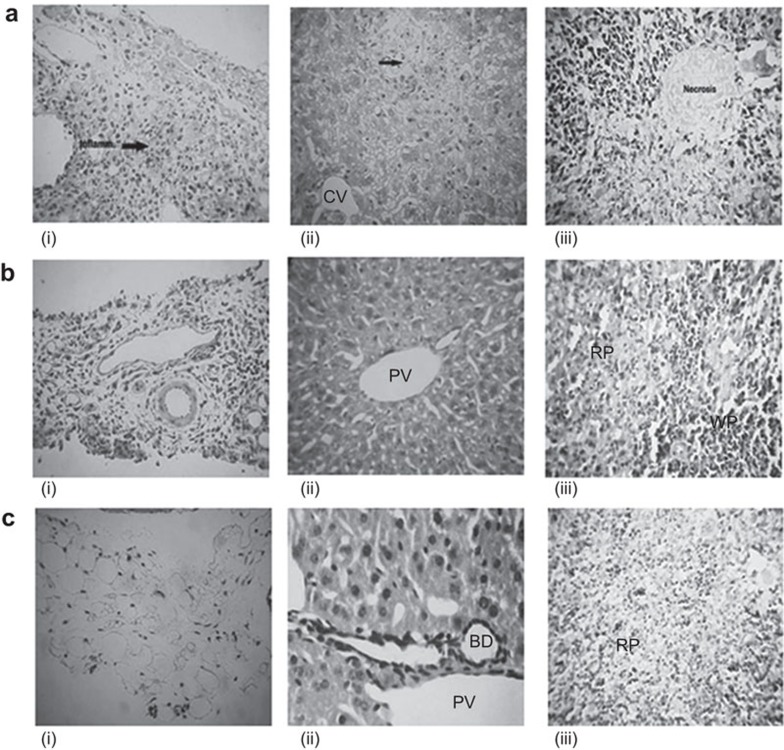
In the control group challenged with Salmonella, intestinal sections showed chronic inflammatory cell infiltration in the serosal layer. The liver cells had inflammation and necrosis in the hepatocytic parenchyma, while spleen sections showed heavy necrosis with loss of cellular outlines (Figure 6ai–iii). In the rGroEL-immunized group, the intestinal cells had acute inflammatory cell infiltration in the serosal layer, the liver showed a normal portal triad and the splenic tissues had normal morphology, with sinusoids containing red blood cells (Figure 6bi–iii). In the group co-administered with rGroEL antigen and rIL-22, normal morphology was observed, with no inflammatory infiltrates in the intestine, normal liver portal triad and normal splenic tissues, showing the red pulp zone with sinusoids containing red blood cells (Figure 6ci–iii).
Figure 6.
Comparative histology of different tissues from mice from the control and immunized groups. Mice from different groups were sacrificed and tissues were fixed in formalin for histopathological analysis. (a) Control mice showing (i) necrosis of villi and a severe degree of inflammation of the serosal layer of the intestine; (ii) necrosis in the hepatic parenchyma; and (iii) necrosis of the lymphoid cells in the spleen with loss of cellular outlines. (b) Mice immunized with rGroEL antigen showing (i) acute inflammatory cell infiltration in the serosal layer; (ii) normal portal triad in the liver; and (iii) improved red and white pulp areas in the splenic parenchyma compared to the control. (c) Mice to which rGroEL and rIL-22 co-administered showing (i) no inflammation of the serosal layer in the intestine; (ii) normal liver portal triad; and (iii) normal red pulp zone in the splenic tissue with sinusoids containing red blood cells (magnification, ×400). CV, central vein; PT, portal vein; RP, red pulp; WP, white pulp.
Discussion
An effective vaccine is composed of a strong immunogen (antigen) and a potent adjuvant (immunopotentiator) that can trigger early innate defense mechanisms to aid in the generation of robust and long-lasting immune responses. However, most known effective adjuvants are unsuitable for human use owing to their toxicity. co-administration of cytokines with recombinant antigens is a novel strategy to enhance immunization and regulate Th1- or Th2-driven cellular and humoral immune responses to promote protective immunity against pathogens, and thus, cytokines have the potential to be used as adjuvants. IL-22 is a novel immune mediator that increases the innate immunity of tissue cells by upregulating the expression of numerous antimicrobial molecules, protects tissues from damage and enhances their regeneration.38,39,40 However, there are few reports of the potential use of IL-22 as an adjuvant. We recently reported that a fusion DNA construct of the IL-22 gene with GroEL of S. Typhi generates robust immune responses and provides protection against lethal infection of mice with Salmonella, thus circumventing the need for an adjuvant.32 Use of IL-22 as an effective adjuvant to enhance the cellular immune responses during HBsAg DNA vaccination and to provide protection against leishmaniasis in mice has also been demonstrated by Wu et al.41 and Hezaejaribi et al.42
In our previous study, we report that codelivery of rIL-22 with rGroEL of S. Typhi modulates the antigen-specific immune responses in mice and increases the protective efficacy of rGroEL against lethal Salmonella infections. Therefore, in this study, we cloned, expressed and purified GroEL of S. Typhi and mouse IL-22 and studied the immunogenicity and protective efficacy of the rGroEL antigen alone or with rIL-22 as an adjuvant against lethal challenge of S. Typhi and S. Typhimurium in mice.
Immunization with rGroEL resulted in a significant increase in antibody titer (IgG) compared to the control group. During microbial infections, HSP determinants are expressed on the cell surface and can be recognized by antibodies. The mechanism required for the translocation of HSPs to the cell surface is still not understood because HSPs are cytosolic proteins and lack the specific leader sequences that are normally required for surface expression. However, studies suggest the existence of a novel antigen processing pathway in which exogenous antigens gain access to the cytosolic MHC class I processing machinery.43 Therefore, a similar mechanism may be involved in presenting GroEL to MHC class I, and the observed antibody-mediated protection against Salmonella infections could be attributed to the surface localization of GroEL. However, the antibody response was further increased with the codelivery of rIL-22 in mice, which indicates that the co-administration of rIL-22 significantly enhanced IgG production. As the type of immune response generated is critical to the effectiveness and safety of a vaccine, we evaluated the antibody isotypes (IgG1 and IgG2a) produced in response to immunization with rGroEL alone and co-administration of rGroEL antigen with IL-22. Production of IgG1 is primarily induced by Th2 type cytokines, whereas the production of IgG2a reflects the induction of Th1 cytokines. We observed the production of both IgG1 and IgG2a isotypes in the immunized groups, indicating the induction of both the Th1 and the Th2 immune responses, though the levels of IgG1 were higher than IgG2a. The responses were augmented by co-administration of rGroEL antigen with rIL-22, as seen by increased IgG1 and IgG2a antibody isotype levels (Figure 2b).
Although antigen-specific antibodies are important for the immune response to Salmonella infections, they are not sufficient for complete protection. The immunity against Salmonella involves both cell-mediated44,45 and humoral immunity.46,47 Additionally, the identification of the antigen-specific cellular immune response provides an important tool for developing effective subunit vaccines against intracellular pathogens such as Salmonella. Therefore, we assessed the cell-mediated immune response by studying lymphocyte proliferation and cytokine levels in the cell supernatants. The data obtained from the cell proliferation assay suggest that co-administration of rGroEL antigen with rIL-22 resulted in higher antigen-specific proliferative T-cell responses than administration of rGroEL antigen alone. The levels of various Th1 (IFN-γ, IL-1β) and Th2 (IL-4, IL-6, IL-10) cytokines were also found to be higher in the rGroEL+rIL-22 group than in the rGroEL alone group. The primary host defense against Salmonella occurs through neutrophils, followed by mononuclear cells. These inflammatory cells produce cytokines such as IFN-γ, IL-1 and IL-6, which help in the neutralization of invasive bacteria. Consequently, the production of macrophage-activating cytokines, particularly IFN-γ, is a major hallmark of the host response against all intracellular bacteria.48 IFN-γ is an important macrophage activating factor that increases the expression of the Fc receptor for the mouse IgG2a subclass and induces potent microbicidal activity. Significant upregulation of IFN-γ was observed in splenocytes collected from the group to which rGroEL and rIL-22 were co-administered compared to the rGroEL alone group (P<0.01). IL-1β has been shown to be protective in several bacterial, viral and fungal infection models. The protective action of IL-1β is mediated by activating several responses, including the rapid recruitment of neutrophils to inflammatory sites, activation of endothelial adhesion molecules, induction of cytokines and chemokines, induction of the febrile response and stimulation of specific types of adaptive immunity, such as the Th17 response.49 However, the inflammatory Th1 response can be destructive for host cells and progressively coincides with an increase in Th2-type immune responses. The increase in the concentrations of IL-6 and IL-4 cytokines in immunized animals shows an induction of the Th2 immune response. IL-4 is a potent helper factor for the generation of cytotoxic T lymphocytes in both the primary and the memory response to alloantigens50 and can stimulate B- and T-cell proliferation. IL-4 signaling is very important for the development of plasma cells from pre-B cells, which is the most important step in the development of effective humoral immunity. IL-6, another Th2 cytokine, is a pleiotropic cytokine capable of having multiple effects on many target cells. IL-6 is involved in the differentiation and proliferation of T and B cells51 and acts in conjunction with other proinflammatory cytokines, such as TNF-α and IL-1, to initiate the early inflammatory response following infection.52 Studies have reported that IL-6 depletion results in the exacerbation of M. avium infection51 and also reduces the protective effect of culture filtrate protein vaccination against aerogenic M. tuberculosis infection.53 Induction of the anti-inflammatory cytokine IL-1054 by IL-22 may also be crucial for fine-tuning local inflammation at epithelial sites of tissue damage. Hence, the development of protective immunity against Salmonella infection is bidirectional, linking the cellular and humoral immune responses, and relies on crosstalk between the two components of the adaptive immune system, as also suggested by Mastroeni.55 Therefore, a balanced Th1–Th2 response induced by IL-22 might be an important factor for the generation of protective immunity against pathogens.
Because we observed robust immune responses in both of the immunized groups, we evaluated the protective efficacy of co-administration of rGroEL with rIL-22 or rGroEL alone by challenging the mice with S. Typhi or S. Typhimurium. The results revealed that immunization with the rGroEL antigen alone conferred 65%–70% protection, while co-administration with rIL-22 provided 85%–90% protection against S. Typhi and 80% against S. Typhimurium. Organ burden studies further revealed a reduction in the bacterial load in different tissues of mice immunized with rGroEL alone or co-administered with rIL-22. The bacterial count was significantly decreased (P<0.001 vs. control) in the intestine, spleen and liver of immunized animals challenged with S. Typhi and S. Typhimurium compared to un-immunized controls. The decrease in the organ burden was more pronounced in the group co-administered with rGroEL and rIL-22, as seen by maximum protection in this group, indicating the adjuvant effect of rIL-22. This also correlated with the results of the histopathological analysis. Animals administered with rGroEL along with rIL-22 had more normal tissue morphology compared to animals immunized with rGroEL alone and the control group. Overall, the results demonstrated that co-administration of rGroEL antigen with rIL-22 as an effective adjuvant can enhance its protective efficacy against Salmonella infection.
In conclusion, this study showed that the administration of rGroEL antigen and rIL-22 to mice resulted in enhanced Th1 and Th2 cell immune responses, together with IgG1 and IgG2a humoral responses, compared with rGroEL alone. The protection against infection conferred by co-administration was also higher (80%–90%) than that of rGroEL alone (70%), signifying that IL-22 holds promise for use as a potent immunopotentiator in recombinant vaccines against bacterial infections. Thus, our study supports the development of IL-22 as a novel and effective adjuvant for HSP60 antigen, enhancing the immune response and protection against Salmonella infection. However, further studies are required to understand the mechanisms of the adjuvanticity of IL-22 against various microbial infections.
Acknowledgments
We thank Mr Bhagwat Singh of the Experimental Animal Facility for his valuable support and technical assistance with animal experiments. The work was supported by grants from the Council of Scientific and Industrial Research, New Delhi, India and Defense Research and Development Organization, Ministry of Defense, Government of India. Ms Gurpreet Kaur was a recipient of a senior research fellowship from the Council of Scientific and Industrial Research.
References
- 1Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Org 2004; 82: 346–353. [PMC free article] [PubMed] [Google Scholar]
- 2Mittrucker HW, Kaufmann SH. Immune response to infection with Salmonella typhimurium in mice. J Leuk Biol 2000; 67: 457–463. [DOI] [PubMed] [Google Scholar]
- 3The World Health report 1996—fighting disease, fostering development. World Health Forum 1997; 18: 1–8. [PubMed] [Google Scholar]
- 4Kossaczka Z, Lin FY, Ho VA, Thuy NT, van Bay P, Thanh TC et al. Safety and immunogenicity of Vi conjugate vaccines for typhoid fever in adults, teenagers, and 2- to 4-year-old children in Vietnam. Infect Immun 1999; 67: 5806–5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Fraser A, Goldberg E, Acosta CJ, Paul M, Leibovici L. Vaccines for preventing typhoid fever. Cochrane Database Syst Rev 2007; (3): CD001261. [DOI] [PubMed]
- 6Marathe SA, Lahiri A, Negi VD, Chakravortty D. Typhoid fever & vaccine development: a partially answered question. Indian J Med Res 2012; 135: 161–169. [PMC free article] [PubMed] [Google Scholar]
- 7Robert J. Evolution of heat shock protein and immunity. Dev Comp Immunol 2003; 27: 449–464. [DOI] [PubMed] [Google Scholar]
- 8Pockley AG. Heat shock proteins as regulators of the immune response. Lancet 2003; 362: 469–476. [DOI] [PubMed] [Google Scholar]
- 9Kaufmann SH. Heat shock proteins and the immune response. Immunol Today 1990; 11: 129–136. [DOI] [PubMed] [Google Scholar]
- 10Young DB. Stress proteins and the immune response. Anton Leeuw 1990; 58: 203–208. [DOI] [PubMed] [Google Scholar]
- 11Perez-Perez GI, Thiberge JM, Labigne A, Blaser MJ. Relationship of immune response to heat-shock protein A and characteristics of Helicobacter pylori-infected patients. J Infect Dis 1996, 174: 1046–1050. [DOI] [PubMed] [Google Scholar]
- 12Gomez FJ, Allendoerfer R, Deepe GS Jr. Vaccination with recombinant heat shock protein 60 from Histoplasma capsulatum protects mice against pulmonary histoplasmosis. Infect Immun 1995; 63: 2587–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Noll A, Autenrieth IB. Immunity against Yersinia enterocolitica by vaccination with Yersinia HSP60 immunostimulating complexes or Yersinia HSP60 plus interleukin-12. Infect Immun 1996; 64: 2955–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Wilhelm V, Soza C, Martinez R, Rosemblatt M, Burzio LO, Valenzuela PD. Production and immune response of recombinant Hsp60 and Hsp70 from the salmon pathogen Piscirickettsia salmonis. Biol Res 2005; 38: 69–82. [DOI] [PubMed] [Google Scholar]
- 15Paliwal PK, Bansal A, Sagi SS, Mustoori S, Govindaswamy I. Cloning, expression and characterization of heat shock protein 60 (groEL) of Salmonella enterica serovar Typhi and its role in protective immunity against lethal Salmonella infection in mice. Clin Immunol 2008; 126: 89–96. [DOI] [PubMed] [Google Scholar]
- 16Bansal A, Paliwal PK, Sagi SS, Sairam M. Effect of adjuvants on immune response and protective immunity elicited by recombinant Hsp60 (GroEL) of Salmonella typhi against S. typhi infection. Mol Cell Biochem 2010; 337: 213–221. [DOI] [PubMed] [Google Scholar]
- 17Cheng G, Zhao X, Yan W, Wang W, Zuo X, Huang K et al. Alpha interferon is a powerful adjuvant for a recombinant protein vaccine against foot-and-mouth disease virus in swine, and an effective stimulus of in vivo immune response. Vaccine 2007; 25: 5199–5208. [DOI] [PubMed] [Google Scholar]
- 18Hung LH, Li HP, Lien YY, Wu ML, Chaung HC. Adjuvant effects of chicken interleukin-18 in avian Newcastle disease vaccine. Vaccine 2010; 28: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 19Arulanandam BP, Lynch JM, Briles DE, Hollingshead S, Metzger DW. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect Immun 2001; 69: 6718–6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Taylor CE. Cytokines as adjuvants for vaccines: antigen-specific responses differ from polyclonal responses. Infect Immun 1995; 63: 3241–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Disis ML, Bernhard H, Shiota FM, Hand SL, Gralow JR, Huseby ES et al. Granulocyte-macrophage colony-stimulating factor: an effective adjuvant for protein and peptide-based vaccines. Blood 1996; 88: 202–210. [PubMed] [Google Scholar]
- 22Kayamuro H, Yoshioka Y, Abe Y, Arita S, Katayama K, Nomura T et al. Interleukin-1 family cytokines as mucosal vaccine adjuvants for induction of protective immunity against influenza virus. J Virol 2010; 84: 12703–12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Wolk K, Sabat R. Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev 2006; 17: 367–380. [DOI] [PubMed] [Google Scholar]
- 24Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 2008; 14: 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 2008; 14: 282–289. [DOI] [PubMed] [Google Scholar]
- 26Adel Galal El-Shemi MA, Kensara OA, Ashshi AM. Interleukin-22 therapy attenuates the development of acute pancreatitis in rats. J Clin Med Res 2011; 3: 82–88. [Google Scholar]
- 27Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 2007; 27: 647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Schulz SM, Kohler G, Schutze N, Knauer J, Straubinger RK, Chackerian AA et al. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J Immunol 2008; 181: 7891–7901. [DOI] [PubMed] [Google Scholar]
- 29Dhiman R, Indramohan M, Barnes PF, Nayak RC, Paidipally P, Rao LV et al. IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J Immunol 2009; 183: 6639–6645. [DOI] [PubMed] [Google Scholar]
- 30Kaur G, Sts C, Nimker C, Singh M, Saraswat D, Saxena S et al. Co-expression of S. Typhi GroEL and IL-22 gene augments immune responses against Salmonella infection. Immunol Cell Biol 2013; 91: 642–651. [DOI] [PubMed] [Google Scholar]
- 31Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265–275. [PubMed] [Google Scholar]
- 32Schwager I, Jungi TW. Effect of human recombinant cytokines on the induction of macrophage procoagulant activity. Blood 1994; 83: 152–160. [PubMed] [Google Scholar]
- 33Urbaschek B, Becker K, Ditter B, Urbaschek R. Quantification of endotoxin and sample-related interferences in human plasma and cerebrospinal fluid by using a kinetic Limulus amoebocyte lysate microtiter test. In:Microbiology. Washington, DC: American Society for Microbiology, 1985: 39–42. [Google Scholar]
- 34Urbaschek R, Mannel DN, Urbanczik R. Isoniazid protects mice against endotoxin lethality without influencing tumor necrosis factor synthesis and release. Antimicrob Agents Chemother 1991; 35: 1666–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Cai X, Wang J, Wang Y, Yang Y, Gao J, Fu W et al. Expression, purification and characterization of recombinant human interleukin-22 in Pichia pastoris. Mol Biol Rep 2010; 37: 2609–2613. [DOI] [PubMed] [Google Scholar]
- 36Tang W, Chen G, Gu Q, Pan J, Wu W. Expression, purification and identification of recombinant mouse interleukin 21 protein in E. coli. Cell Mol Immunol 2006; 3: 311–315. [PubMed] [Google Scholar]
- 37Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. In: Coligan JE (ed.) Current Protocols in Immunology. New York: Wiley, 2008: Chapter 14: Unit 14 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Zindl CL, Lai JF, Lee YK, Maynard CL, Harbour SN, Ouyang W et al. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc Natl Acad Sci USA 2013; 110: 12768–12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev 2013, 252: 116–132. [DOI] [PubMed] [Google Scholar]
- 40Pociask DA, Scheller EV, Mandalapu S, McHugh KJ, Enelow RI, Fattman CL et al. IL-22 is essential for lung epithelial repair following influenza infection. Am J Pathol 2013; 182: 1286–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Wu B, Zou Q, Hu Y, Wang B. Interleukin-22 as a molecular adjuvant facilitates IL-17-producing CD8 T cell responses against a HBV DNA vaccine in mice. Hum Vaccines Immunother 2013; 9: 2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Hezarjaribi HZ, Ghaffarifar F, Dalimi A, Sharifi Z, Jorjani O. Effect of IL-22 on DNA vaccine encoding LACK gene of Leishmania major in BALB/c mice. Exp Parasitol 2013; 134: 341–348. [DOI] [PubMed] [Google Scholar]
- 43Lo WF, Dunn CD, Ong H, Metcalf ES, Soloski MJ. Bacterial and host factors involved in the major histocompatibility complex class Ib-restricted presentation of Salmonella Hsp 60: novel pathway. Infect Immun 2004; 72: 2843–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44Salerno-Goncalves R, Pasetti MF, Sztein MB. Characterization of CD8+ effector T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol 2002; 169: 2196–2203. [DOI] [PubMed] [Google Scholar]
- 45Salerno-Goncalves R, Wyant TL, Pasetti MF, Fernandez-Vina M, Tacket CO, Levine MM et al. Concomitant induction of CD4+ and CD8+ T cell responses in volunteers immunized with Salmonella enterica serovar typhi strain CVD 908-htrA. J Immunol 2003; 170: 2734–2741. [DOI] [PubMed] [Google Scholar]
- 46Mittrucker HW, Raupach B, Kohler A, Kaufmann SH. Cutting edge: role of B lymphocytes in protective immunity against Salmonella typhimurium infection. J Immunol 2000; 164: 1648–1652. [DOI] [PubMed] [Google Scholar]
- 47Mastroeni P, Simmons C, Fowler R, Hormaeche CE, Dougan G. Igh-6−/− (B-cell-deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica serovar typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infect Immun 2000; 68: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48Kaufmann SH. Immunity to intracellular bacteria. Annu Rev Immunol 1993; 11: 129–163. [DOI] [PubMed] [Google Scholar]
- 49Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 2009; 27: 519–550. [DOI] [PubMed] [Google Scholar]
- 50Widmer MB, Grabstein KH. Regulation of cytolytic T-lymphocyte generation by B-cell stimulatory factor. Nature 1987; 326: 795–798. [DOI] [PubMed] [Google Scholar]
- 51Appelberg R, Castro AG, Pedrosa J, Minoprio P. Role of interleukin-6 in the induction of protective T cells during mycobacterial infections in mice. Immunology 1994; 82: 361–364. [PMC free article] [PubMed] [Google Scholar]
- 52Dinarello CA. Role of interleukin-1 in infectious diseases. Immunol Rev 1992; 127: 119–146. [DOI] [PubMed] [Google Scholar]
- 53Leal IS, Smedegard B, Andersen P, Appelberg R. Interleukin-6 and interleukin-12 participate in induction of a type 1 protective T-cell response during vaccination with a tuberculosis subunit vaccine. Infect Immun 1999; 67: 5747–5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54Nagalakshmi ML, Rascle A, Zurawski S, Menon S, de Waal Malefyt R. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int Immunopharmacol 2004; 4: 679–691. [DOI] [PubMed] [Google Scholar]
- 55Mastroeni P. Immunity to systemic Salmonella infections. Curr Mol Med 2002; 2: 393–406. [DOI] [PubMed] [Google Scholar]