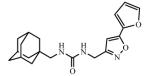
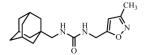
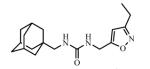
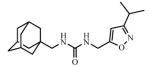
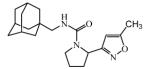
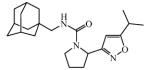
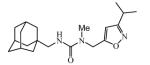
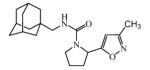
Abstract
Adamantyl ureas are good soluble epoxide hydrolase (sEH) inhibitors; however they have limited solubility and rapid metabolism, thus limiting their usefulness in some therapeutic indications. Herein, we test the hypothesis that nodal substitution on the adamantane will help solubilize and stabilize the compounds. A series of compounds containing adamantane derivatives and isoxazole functional groups were developed. Overall, the presence of methyl on the nodal positions of adamantane yields higher water solubility than previously reported urea-based sEH inhibitors while maintaining high inhibition potency. However, it did not improve microsomal stability.
Keywords: soluble epoxide hydrolase, inhibitor, adamantane, isocyanate, urea, isoxazole
Graphical abstract
The mammalian soluble epoxide hydrolase (sEH, E.C. 3.3.2.10) is involved in the metabolism of epoxy-fatty acids to vicinal diols through a catalytic addition of a water molecule.1,2 Endogenous substrates for the sEH include epoxides of arachidonic acid, epoxyeicosatrienoic acids (EETs), and of docosahexaenoic acid, known as EpDPEs, and other fatty acid epoxides.3,4 EETs are important lipid mediators that have key roles in blood pressure regulation by exerting vasodilatory effects through the activation of the Ca2+-activated K+ channels in endothelial cells, which are beneficial in many renal and cardiovascular diseases.5,6 Furthermore, the EETs and EpDPEs have some anti-inflammatory and analgesic properties.7 Recently, EETs have been reported to increase efficiency of organ transplant.8 Their conversion to dihydroxyeicosatrienoic acids (DHETs) by sEH produces a molecule that is readily conjugated and removed from the site of action. The inhibition of sEH in vivo by highly selective inhibitors results in an increase in the concentration of EETs and EpDPEs, and is accompanied by a reduction in angiotensin driven blood pressure, but also reduction of inflammation and pain, thereby suggesting that sEH is a promising target for the treatment of hypertension, inflammatory diseases and pain.9-11
Early on, small N,N’-disubstituted symmetric ureas, such as 1,3-dicyclohexyl urea, were found to be very potent inhibitors of sEH.12-16 However, these kinds of compounds have poor solubility in many solvents. To improve solubility, asymmetric ureas with a flexible side chain, such as AUDA (12-(3-adamantylureido)-dodecanoic acid) or AEPU (1-adamantanyl-3-{5-[2-(2-ethylethoxy)ethoxy]pentyl]}urea), were developed.17 While this class of sEH inhibitor shows biological effects when tested in vivo, they are rapidly metabolized, limiting their utility.18 Interestingly, a major site of metabolism for these compounds is on the adamantine, although beta oxidation of the side chains by CYPs is also important.19 Therefore, to improve the metabolic stability, a third class of conformationally restricted inhibitors, in which the adamantine was replaced by phenyl derivatives such as TPAU (1-trifluoromethoxyphenyl-3-(1-acetylpiperidin-4-yl) urea) or trans-4-{4-[3-(4-trifluoromethoxyphenyl)-ureido] cyclohexyloxy} benzoic acid (t-TUCB), were designed.20,21 This latest series includes very potent and more metabolically stable sEH inhibitors that permit in vivo studies.22 However, these compounds have in general poorer solubility than the corresponding adamantane containing compounds, and are expensive to synthesize since several steps (up to 5) are required.
A promising way to enhance the water solubility of the urea inhibitors of sEH is the introduction of heterocyclic moieties.23 For example, ureas synthesized with amino-pyridazines, pyridines, pyrimidines, triazines, oxazoles and thiazoles containing amino groups showed high potency against epoxide hydrolases from Mycobacterium tuberculosis.24 Thus, here as a continuation of previous work25,26 we report the testing of adamantyl-ureas containing isoxazoles, however we also tested the hypothesis that nodal substitution on the adamantane will help solubilize and stabilize the resulting compounds because for adamantane containing urea inhibitors hydroxylation of nodal carbon is important metabolic pathway leading to a decrease of inhibitory potency.
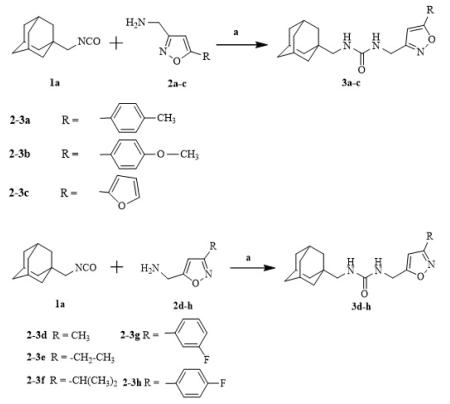
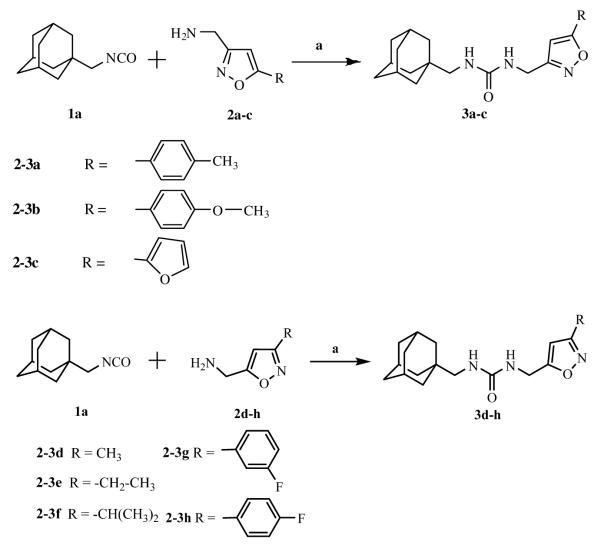
Ureas were synthesized from 1-(isocyanatomethyl)adamantane (1a) or 1-isocyanato-3,5-dimethyladamantane (1b) and 3,5-disubstituted isoxazoles with aminomethyl or pyrrolidine-2-yl as one of the substituents. Compound 1a was selected due to the flexibility-giving methyl spacer. The starting isoxazoles also contain a methylene bridge between the amino group and the isoxazole ring, in some cases this bridge included the pyrrolidine ring. Selected isoxazoles contain electron donor (Me, Et, i-Pr) as well as electron acceptor (4-MeC6H4, 4-MeOC6H4, 3(4)-FC6H4) substituents and amino methyl groups in the 3 and 5 positions.27-33 Such a series of disubstituted isoxazoles will allow us to investigate the influence of its structure on the urea formation reaction and on the inhibition potency of corresponding ureas.
As described in scheme 1, simple (one step) and complementary approaches were used to obtain the desired compounds in high yield (> 95%).
Scheme 1.
Reagents and conditions: (a) DMF, rt, 12 h
The inhibition potency of the synthesized compounds was measured using recombinant purified human sEH and CMNPC (cyano(6-methoxynaphthalen-2-yl)methyl ((3-phenyloxiran-2-yl)methyl) carbonate) as a substrate as described.34 For the isoxazole containing 1,3-disubstituted ureas, the best inhibitory potency (4.9 nM) was recorded for the compound 3c with furyl group in 5th position of isoxasole ring (Table 1). Compound 3d with the methyl substituent in 5th position of isoxasole ring has good inhibition potency (6.7 nM). Further increase of this substituent to the ethyl (3e) and i-propyl (3f) leads to the 30- and 45-fold decrease in potency (higher IC50) and accompanied decrease in water solubility. Potency of the ureas with phenyl substitutents in 5th position of isoxasole ring is only slightly affected by the structure of those groups and ranges from 10.5 to 16.6 nM.
Table 1.
IC50 values for the isoxazole 1,3-disubstituted urea-based sEH inhibitors 3a-h
Due to the low reactivity of secondary amines in the reaction of nucleophilic addition to isocyanates reactions of isoxazoles 2i-m were carried out at 80 °C in presence of Et3N (Scheme 2).
Scheme 2.
Synthesis of 1,3,3-trisubstituted ureas with isoxazole part
Potency of 1,3,3-trisubstituted ureas clearly shows that availability of both NH in the urea group is vital for the satisfactory inhibition of sEH. Disruption of the ability to create one hydrogen bond leads to an up to significant decrease in activity in some cases. For example compound 3k is >5-fold less active than the corresponding 1,3-disubstituted urea 3f. Ureas synthesized from isoxazole with amino methyl group in 3rd position are more potent (up to 5-fold) than those with the amino methyl group in the 5th position. Probably this is due to the formation of the additional hydrogen bonds. The hydrogen bond acceptor in ureas synthesized from 5-amino isoxazoles is probably not able to reach the necessary distance to create such a bond.
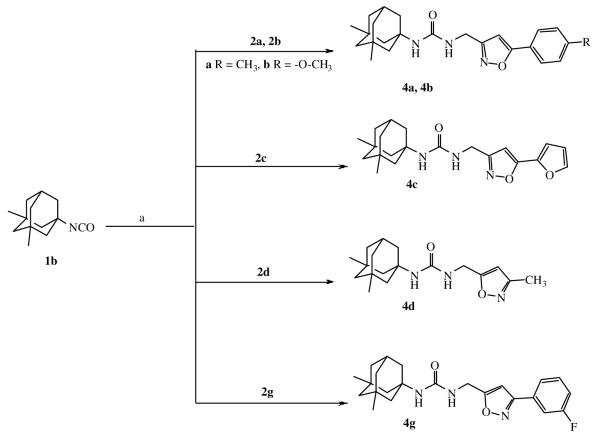
While 1,3-disustituted ureas containing the adamantane moiety were widely investigated as soluble epoxide hydrolase inhibitors only 1-isocyanato adamantane was used for their formation. Introduction of methyl groups into nodal positions of adamantane significantly lowers melting point of the its derivatives. Moreover, the metabolism of adamantyl-containing 1,3-disubstituted ureas in vivo proceeds through the hydroxylation of nodal positions and the corresponding hydroxy derivatives have up to 50 fold less inhibitory activity.23 In this case we decided to synthesize sEH inhibitor from 1-isocyanato-3,5-dimethyladamantane (1b).24 The boiling point of compound 1b (93 °C at 10 torr)36 is lower than that of compound 1a (98 °C at 10 torr)36 so we assumed a corresponding reduction of melting points would occur with the appropriate 1,3-disubstituted ureas.
For the compounds 4a-4c and 4g the replacement of the adamantane part of the molecule did not result in noticeable change of the inhbitory potency. Surprisingly, compound 4d is 10-fold less potent than 3d, which is the second-best inhibitor of this series. However, we have mentioned the tendency of each new methyl group introduced into the aliphatic substituent in 5th position of isoxazole to reduce potency. In this case, we can conclude that the additional methyl groups for this series of ureas caused a decrease in the inhibitory activity as well as water solubility (comparing data in table 1 and 2). But presence of the phenyl or furyl rings negate this effect. Introduction of isocyanate 1b instead of 1a leads to the reduction of melting points of corresponding ureas at about 10 °C but additional methyl groups generally result in 2-fold decreased water solublity. Decreased water solubility is correlated with cLogP (Table 4).
Table 2.
IC50 values for the isoxazole 1,3,3-trisubstituted urea-based sEH inhibitors 3i-m
Table 4.
IC50, water solubility and microsomal stability of selected compounds.
| # | IC50 nMa | Solubilityb (μM) |
cLogPc | Microsomal stability (%)d NADPH |
|
|---|---|---|---|---|---|
| − | + | ||||
| 3a | 15.6 ± 0.7 | 75 < S < 100 | 4.24 | 97 ± 4 | 0.4 ± 0.2 |
| 4a | 28.8 ± 4.5 | 125 < S < 150 |
4.50 | 115 ± 11 | 0.4 ± 0.2 |
| 3b | 12.5 ± 1.8 | 100< S < 125 | 3.85 | 87 ± 6 | 0.8 ± 0.2 |
| 4b | 18.0 ± 2.9 | 25 < S < 50 | 3.89 | 102 ± 11 | 0.4 ± 0.3 |
| 3c | 4.9 ± 0.1 | 75 < S < 100 | 2.59 | 91 ± 8 | 0.4 ± 0.1 |
| 4c | 4.8 ± 0.6 | 75 < S < 100 | 2.63 | 95 ± 1 | 0.3 ± 0.1 |
| 3d | 9.9 ± 2.9 | 450 < S < 475 | 2.36 | 72 ± 4 | 2.9 ± 0.5 |
| 4d | 111.1 ± 10.3 | 200 < S < 225 |
2.62 | 83 ± 3 | 0.1 ± 0.1 |
| 3g | 13.7 ± 3.0 | 75 < S < 100 | 3.91 | 97 ± 10 | 0.3 ± 0.1 |
| 4g | 14.2 ± 2.5 | 75 < S < 100 | 4.17 | 92 ± 6 | 0.4 ± 0.1 |
As determined via a kinetic fluorescent assay.34, 35 Results are average ± standard deviation of three separate measurement.
Solubility was measured in sodium phosphate buffer (pH 7.4, 0.1 M) containing 1% of DMSO.
Calculated using ChemBioDraw Ultra v12.0 (PerkinElmer, Waltham, MA).
Percent of compound (1 μM) remaining after 30 minutes incubation with human liver microsomes (1 mg/mL) at 37°C with or without NADPH generating system. Results are the average of triplicates ± standard deviation.
To further assess the properties of the compounds, we measured their stability in human liver microsomes (Table 4).
However contrary to our assumption introduction of methyl groups to the nodal positions of adamantane did not improve microsomal stability of the corresponding ureas. This effect was observed probably due to the rapid metabolism of isoxazole ring, although isolated methyl groups often are rapidly oxidized by CYP enzymes.
We describe the synthesis and structure-activity relationship of a series of adamantylureas with isoxazole ring containing various substituents. Some ureas have methyl groups in nodal positions of adamantane. The data show that such ureas show very good inhibition potency along with high water solubility (compared to previously reported urea-based sEH inhibitors). We showed that both NH in urea group are essential for the inhibitor binding on the active site of sEH. Introduction of methyl groups to the nodal positions of the adamantane did not affect inhibition potency (which indicates that there is still sufficient space in the cavity of the enzyme) as long as not affect microsomal stability but lead to the reduction of water solubility.
Supplementary Material
Scheme 3.
Reagents and conditions: (a) DMF, rt, 12 h
Table 3.
IC50 values for the isoxazole 1,3-disubstituted urea-based sEH inhibitors with 1,3-dimethyladamantane moiety 4a-d and 4g.
Acknowledgements
The reported study was partially funded by Council for grants under the President of the Russian Federation (program of state support for young PhDs, project MK-5809.2015.3), National Institute of Environmental Health Sciences (NIEHS) grant R01 ES002710, and NIEHS Superfund Research Program grant P42 ES004699.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arand M, Grant DF, Beetham JK, Friedberg T, Oesch F, Hammock BD. FEBS Lett. 1994;338:251. doi: 10.1016/0014-5793(94)80278-5. [DOI] [PubMed] [Google Scholar]
- 2.Oesch F. Xenobiotica. 1973;3:305. doi: 10.3109/00498257309151525. [DOI] [PubMed] [Google Scholar]
- 3.Zeldin DC, Kobayashi J, Falck JR, Winder BS, Hammock BD, Snapper JR, Capdevilla JH. J. Biol. Chem. 1993;268:6402. [PubMed] [Google Scholar]
- 4.Spector AA, Fang X, Snyder GD, Weintraub NL. Prog. Lipid Res. 2004;43:55. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 5.Fleming I, Rueben A, Popp R, Fisslthaler B, Schrodt S, Sander A, Haendeler J, Falck JR, Morisseau C, Hammock BD, Busse R. Arterioscler. Thromb Vasc. Biol. 2007;27:2612. doi: 10.1161/ATVBAHA.107.152074. [DOI] [PubMed] [Google Scholar]
- 6.Imig JD. Expert Opin. Drug Metab. Toxicol. 2008;4:165. doi: 10.1517/17425255.4.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Circ. Res. 2000;87:992. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 8.Li P, Lahvic JL, Binder V, Pugach EK, Riley EB, Tamplin OJ, Panigrahy D, Bowman TV, Barrett FG, Heffner GC, McKinney-Freeman S, Schlaeger TM, Daley GQ, Zeldin DC, Zon LI. Nature. 2015;523:468. doi: 10.1038/nature14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiecker M, Liao JK. Arch. Biochem. Biophys. 2005;433:420. doi: 10.1016/j.abb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Imig JD, Zhao X, Capdevilla JH, Morisseau C, Hammock BD. Hypertension. 2002;39:690. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 11.Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, Kim IH, Watanabe T, Hammock BD. Hypertension. 2005;46:975. doi: 10.1161/01.HYP.0000176237.74820.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morisseau C, Goodrow MH, Dowdy D, Zheng J, Greene JF, Sanborn JR, Hammock BD. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8849. doi: 10.1073/pnas.96.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McElroy NR, Jurs PC, Morisseau C, Hammock BD. J. Med. Chem. 2003;46:1066. doi: 10.1021/jm020269o. [DOI] [PubMed] [Google Scholar]
- 14.Kim IH, Morisseau C, Watanabe T, Hammock BD. J. Med. Chem. 2004;47:2110. doi: 10.1021/jm030514j. [DOI] [PubMed] [Google Scholar]
- 15.Kim IH, Heirtzler FR, Morisseau C, Nishi K, Tsai HJ, Hammock BD. J. Med. Chem. 2005;48:3621. doi: 10.1021/jm0500929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9772. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim IH, Tsai HJ, Nishi K, Kasagami T, Morisseau C, Hammock BD. J. Med. Chem. 2007;50:5217. doi: 10.1021/jm070705c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. J. Med. Chem. 2007;50:3825. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulu A, Davis BB, Tsai HJ, Kim IH, Morisseau C, Inceoglu B, Fiehn O, Hammock BD, Weiss RH. J. Cardiovasc. Pharmacol. 2008;52:314. doi: 10.1097/FJC.0b013e318185fa3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose TE, Morisseau C, Liu JY, Inceoglu B, Jones PD, Sanborn JR, Hammock BD. J. Med. Chem. 2010;53:7067. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang SH, Wecksler AT, Zhang G, Morisseau C, Nguyen LV, Fu SH, Hammock BD. Bioorg. Med. Chem. Lett. 2013;23:3732. doi: 10.1016/j.bmcl.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KSS, Liu JY, Wagner KM, Pakhomova S, Dong H, Morisseau C, Fu SH, Yang J, Wang P, Ulu A, Mate CA, Nguyen LV, Hwang SH, Edin ML, Mara AA, Wulff H, Newcomer ME, Zeldin DC, Hammock BD. J. Med. Chem. 2015;57:7016. doi: 10.1021/jm500694p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulu A, Davis BB, Tsai H-J, Kim I-H, Morisseau C, Inceoglu B, Fiehn O, Hammock BD, Weiss RH. J. Cardiovasc. Pharmacol. 2008;52:314. doi: 10.1097/FJC.0b013e318185fa3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burmistrov V, Morisseau C, Lee KSS, Shihadih DS, Harris TR, Butov GM, Hammock BD. Bioorg. Med. Chem. Lett. 2014;24:2193. doi: 10.1016/j.bmcl.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butov GM, Mohov VM, Burmistrov VV, Saad KR, Pitushkin DA. Russ. J. Org. Chem. 2014;9:1276. [Google Scholar]
- 26.Butov GM, Pershin VV, Burmistrov VV. Russ. J. Org. Chem. 2011;47:606. [Google Scholar]
- 27.Pei Y, Wickham BOS. Tetrahedron Lett. 1993;34:7509. [Google Scholar]
- 28.Zhang D, Jia J, Meng L, Xu W, Tang L, Wang J. Arch. Pharm. Res. 2010;33:831. doi: 10.1007/s12272-010-0605-7. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd J, Finlay HJ, Vacarro W, Hyunh T, Kover A, Bhandaru R, Yan L, Atwal K, Conder ML, Jenkins-West T, Shi H, Huang C, Li D, Sun H, Levesque P. Bioorg. Med. Chem. Lett. 2010;20:1436. doi: 10.1016/j.bmcl.2009.12.085. [DOI] [PubMed] [Google Scholar]
- 30.Garvey DS, Wasicak JT, Decker MW, Brioni JD, Buckley MJ, Sullivan JP, Carrera GM, Holladay MW, Arneric SP, Williams MJ. Med. Chem. 1994;37:1055. doi: 10.1021/jm00034a002. [DOI] [PubMed] [Google Scholar]
- 31.Garvey DS, Wasicak JT, Elliott RL, Lebold S, Hettinger A-M, Carrera GM, Lin N-H, He Y, Holladay MW, Anderson DJ, Cadman ED, Raszkiewicz JL, Sullivan JP, Arneric SP. J. Med. Chem. 1994;37:4455. doi: 10.1021/jm00052a005. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Cui Z, Liu B, Cai B, Li Y, Wang Q. J. Agric. Food Chem. 2010;58:2685. doi: 10.1021/jf902541w. [DOI] [PubMed] [Google Scholar]
- 33.Barrett DG, Catalano JG, Deaton DN, Long ST, Miller LR, Tavares FX, Wells-Knecht KJ, Wright LL, Zhou H-QQ. Bioorg. Med. Chem. Lett. 2004;14:2543. doi: 10.1016/j.bmcl.2004.02.085. [DOI] [PubMed] [Google Scholar]
- 34.Jones PD, Wolf NM, Morisseau C, Whetstone P, Hock B, Hammock BD. Anal. Biochem. 2005;343:66. doi: 10.1016/j.ab.2005.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shihadih DS, Harris TR, Yang J, Merzlikin O, Lee KSS, Hammock BD, Morisseau C. Bioorg. Med. Chem. Lett. 2015;25:276. doi: 10.1016/j.bmcl.2014.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burmistrov VV, Pershin VV, Butov GM. Izvestija VolgGTU. 2012;92:62. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.