SUMMARY
Activation-induced cytidine deaminase (AID), the enzyme mediating class switch recombination (CSR) and somatic hypermutation (SHM) of immunoglobulin genes, is essential for the removal of developing autoreactive B cells. How AID mediates central B-cell tolerance remains unknown. We report that AID enzymes were produced in a discrete population of immature B cells that expressed recombination-activating gene 2 (RAG2), suggesting that they undergo secondary recombination to edit autoreactive antibodies. However, most AID+ immature B cells lacked anti-apoptotic MCL-1 and were deleted by apoptosis. AID inhibition using lentiviral-encoded short hairpin (sh)RNA in B cells developing in humanized mice resulted in a failure to remove autoreactive clones. Hence, B-cell intrinsic AID expression mediates central B-cell tolerance potentially through its RAG-coupled genotoxic activity in self-reactive immature B cells.
Keywords: activation-induced cytidine deaminase, B-cell development, B-cell tolerance, AID-deficient patients
INTRODUCTION
The expression of activation-induced cytidine deaminase (AID), the enzyme mediating class switch recombination (CSR) and somatic hypermutation (SHM) (Muramatsu et al., 2000; Revy et al., 2000), is required for the establishment of central B-cell tolerance (Kuraoka et al., 2011; Meyers et al., 2011). Indeed, AID-deficient patients show an increased frequency of autoreactive clones exiting their bone marrow (BM) (Meyers et al., 2011). In addition, Aicda−/− mice also display central B-cell tolerance defects, suggesting a conserved role for AID during early B-cell development in mice and humans (Kuraoka et al., 2011).
The analysis of patients with primary immunodeficiency due to diverse gene mutations reveals that central B-cell tolerance requires intact B-cell receptors (BCR) and possibly Toll-like receptors (TLR) signaling pathways, perhaps triggered by binding to self-antigens at the immature B-cell stage (Isnardi et al., 2008; Menard et al., 2014; Ng et al., 2004; Romberg et al., 2013; Sauer et al., 2012). These findings, together with the identification of AID transcripts in B-cell precursors in both mice and humans support the idea that AID expression in immature B cells is relevant to tolerance induction (Han et al., 2007; Kuraoka et al., 2009; Mao et al., 2004; Meyers et al., 2011; Ueda et al., 2007; Umiker et al., 2014). However, it remains to be determined if the AID enzyme is in fact expressed in some B-cell precursors or if central B-cell tolerance defects in AID-deficient patients or mice might be related to their susceptibility to infections in the absence of isotype switched, affinity matured antibodies.
Here, we report that AID proteins are expressed during early B-cell development in both human fetal liver and adult bone marrow. AID expression was found restricted to early immature B cells that co-express recombination-activating gene 2 (Rag2) and undergo apoptosis. Furthermore, we show that AID inhibition in B cells developing in humanized mice impaired the counterselection of autoreactive clones, revealing B-cell intrinsic AID requirement to ensure central B-cell tolerance. However, autoreactive B cell deletion through AID and RAG-coupled genotoxic activity occurred at the potential expense of inducing chromosomal translocations that may promote lymphomagenesis.
RESULTS
AID gene dosage regulates central B-cell tolerance
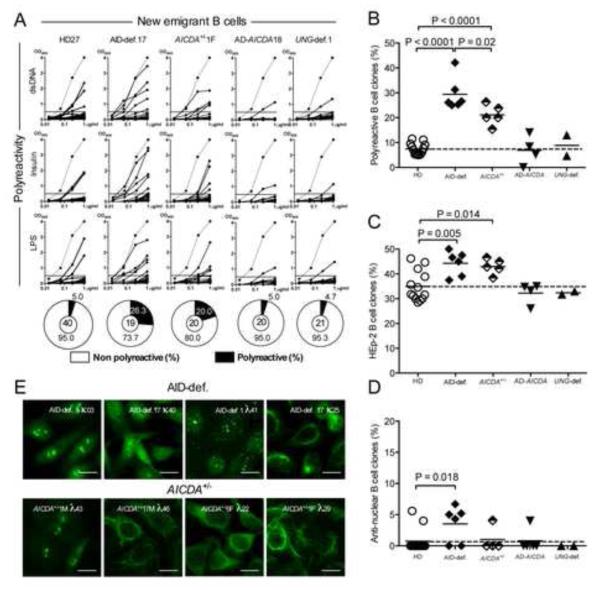
To further assess AID requirement for the central removal of autoreactive clones, we analyzed the reactivity of antibodies expressed by single CD19+CD27−CD10+IgMhiCD21lo transitional B cells that recently emigrated from the bone marrow of additional AID-deficient patients carrying autosomal recessive (AR) mutations and asymptomatic heterozygote relatives, as well as rare patients with autosomal dominant (AD) AICDA mutations or Uracil N-glycosylase (UNG)-deficiency (Tables S1, S2 and S3). AD-AICDA mutations result in the deletion of the last amino acids of AID required for CSR activity; C-terminal truncated AID products also lack the nuclear export signal and therefore remain in the nucleus where they exert a dominant negative role on CSR (Imai et al., 2005; Ito et al., 2004; Zahn et al., 2014). UNG acts downstream of AID and removes AID-induced uracil residues from DNA to create abasic sites (Di Noia and Neuberger, 2007). UNG-deficiency greatly affects CSR but leaves the frequency of SHM intact, albeit with an altered mutation spectrum (Imai et al., 2003). In agreement with these reports, patients with AD-AICDA mutations or UNG-deficiency are similar to AID-deficient patients in that they are virtually devoid of isotype-switched B cells, whereas AICDA+/− asymptomatic relatives display normal isotype switched memory B cell frequencies (Figure S1) (Imai et al., 2003; Imai et al., 2005).
We found that patients with AD-AICDA mutations differed from AID-deficient patients in that they displayed normal frequencies of polyreactive, HEp-2 reactive and anti-nuclear clones, revealing a functional central B-cell tolerance in these individuals in which AID enzymatic activity is preserved (Figure 1 and Figure S2) (Imai et al., 2005; Zahn et al., 2014). Moreover, UNG-deficient patients also showed low proportions of autoreactive new emigrant B cells similar to those in healthy donors (Figure 1 and Figure S2). Hence, impaired CSR, absence of isotype-switched B cells or recurrent infectious episodes characteristic of these patients do not impact the establishment of central B-cell tolerance. In contrast, asymptomatic subjects who carried a heterozygous AR-AICDA mutation showed significantly elevated frequencies of polyreactive and HEp-2 reactive new emigrant B cells, which averaged 21.3 ± 5.6% and 43.0 ± 3.1%, respectively, compared to 7.3 ± 2.4% and 34.9 ± 6.1% in healthy donor counterparts, thereby revealing that these individuals display central B-cell tolerance defects that resembled those in AID-deficient patients (Figure 1, A-C and Figure S2). These frequencies were lower than those in AID-deficient patients carrying 2 recessive mutated AICDA alleles (Figure 1, A-C), demonstrating an AICDA gene dosage dependent regulation of central B-cell tolerance.
Figure 1. AID gene dosage dependent regulation of central B-cell tolerance.
(A) Antibodies from new emigrant B cells from healthy donors (HD, n=12), AID-deficient (AID-def.) patients (n=6), asymptomatic healthy heterozygotes (AICDA+/−, n=5), patients with autosomal dominant (AD) AICDA mutation (n=4) and UNG-deficient (UNG-def.) patients (n=2) were tested by ELISA for reactivity against double-stranded DNA (dsDNA), insulin and lipopolysaccharide (LPS). Antibodies were considered polyreactive when they recognized all 3 analyzed antigens. Dotted lines show ED38-positive control. Horizontal lines show cut-off OD405 for positive reactivity. For each individual, the frequency of autoreactive (filled area) and non autoreactive (open area) clones is summarized in pie charts, with the total number of clones tested indicated in the centers. The frequencies of polyreactive (B), HEp-2 reactive (C) and anti-nuclear (D) new emigrant B cells is summarized. Lines show the mean, dashed line indicates the mean value for the healthy donors (HD). (E) Autoreactive antibodies from AID-def. and asymptomatic healthy AICDA+/− heterozygotes new emigrant B cells show various patterns of HEp-2 staining. Original magnification 40X, scale bar: 10 μm. Please see Figure S2.
Proper AID enzymatic activity is required for central B-cell tolerance
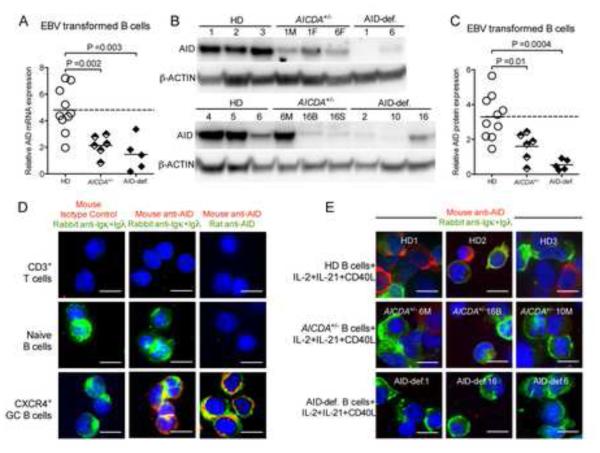
The impact of heterozygote AR-AICDA mutations on the removal of developing autoreactive B cells suggested that AICDA haploinsufficiency may be responsible for central B-cell tolerance defects in asymptomatic AICDA+/− subjects. In agreement with this hypothesis, B cells from Aicda+/− mice display decreased AID expression upon activation or immunization, resulting in reduced SHM and CSR activity (McBride et al., 2008; Takizawa et al., 2008). We therefore assessed AID expression in EBV cell lines derived from six AICDA+/− individuals and five AID-deficient patients and compared it to that in EBV lines from healthy donors (Figure 2). Quantitative PCR and protein expression using western blots revealed that both AICDA+/− and AID-deficient EBV lines displayed significantly decreased AID expression compared to control EBV lines (Figure 2, A-C) (Imai et al., 2005). We also analyzed AID expression by histochemistry in primary B cells isolated from AICDA+/− individuals and AID-deficient patients activated for 5 days with cytokines that induces AID expression in vitro. The reactivity of both rat and mouse anti-human AID monoclonal antibodies was validated by staining various types of cells deposited on slides by cytospin; AID expression was identified in human CXCR4+ germinal center (GC) B cells but not in peripheral CD3+ T cells and naïve B cells as previously reported (Figure 2D) (Muramatsu et al., 2000; Revy et al., 2000; Victora et al., 2012). The specificity of these monoclonal antibodies was demonstrated by the lack of AID detection in AID-deficient activated B cells whereas many activated B cells from healthy donors expressed AID (Figure 2E and data not shown). Similarly to EBV lines, AID expression in activated primary B cells from AICDA+/− subjects was found severely decreased in all tested individuals (Figure 2E). Hence, both B cells from AICDA+/− asymptomatic individuals and AID-deficient patients fail to properly express AID, suggesting that AICDA-haploinsufficiency or AID-deficiency may be responsible for the defective central removal of developing autoreactive B cells.
Figure 2. Decreased AID expression in asymptomatic healthy individuals carrying one mutated AICDA allele.
(A) Quantitative real-time PCR show decreased transcript of AICDA mRNA in EBV-transformed B cell lines from AICDA+/− asymptomatic healthy heterozygotes (n=6), AID-def. patients (n=5) and healthy donors (HD) (n=10). Transcripts were normalized to HPRT1. (B) Immunoblot analysis of total protein lysates from EBV-transformed B cell lines from healthy donors (HD), asymptomatic healthy heterozygotes (AICDA+/−), and AID-def. patients. Immunoblotting against β-Actin was used as loading control. Quantification is summarized in C. (D) Cytospin slides of sorted CD3+ T cells, CD19+IgD+CD38−CXCR4− B cells and CD19+IgD−CD38+CXCR4+ GC B cells were stained for Igκ/Igλ and mouse anti-AID or concentration matched isotype control. (e) cytospin slides of CD20+ B cells isolated from healthy donor (HD), AICDA+/− or AID-def. patients stimulated with CD40L+IL-2+IL-21 were stained for Igκ/Igλ (green) and mouse anti-AID (red). AID expression is detected in GC but not in naive B or T cells and not in AID-deficient B cells stimulated with cytokines that induce AID expression in AID competent B cells. Original magnification 40X, scale bar: 10 μm. Data is representative of 2 independent experiments for (B), (D) and (E).
AID proteins are detected in a discrete population of immature B cells
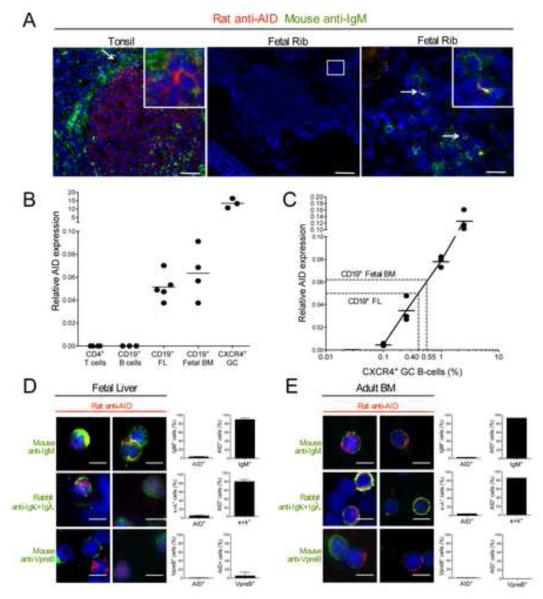
Previous reports identified low amounts of AID transcripts in B-cell precursors but it was unclear how this might support a function for AID during early B-cell development (Han et al., 2007; Kuraoka et al., 2009; Mao et al., 2004; Meyers et al., 2011; Ueda et al., 2007). Since central B-cell tolerance is regulated primarily by B cell-intrinsic pathways (Meffre, 2011), we investigated AID expression in human developing B cells. We first analyzed AID protein expression in situ by immunochemistry by assessing AID expression in B cells developing in the marrow using fetal ribs from 105-115 day old fetuses. We identified some rare AID+ cells that co-expressed IgM heavy chains in fetal ribs whereas AID expression was detected in many GC B cells from tonsil tissues (Figure 3A). Because primary lymphoid organs give rise to many hematopoietic lineages other than B cells, we isolated CD19+ B-cell precursors from human fetal liver and adult marrow to enrich for cells that may express AID for further investigation of AID expression in combination with other molecules produced at different stages of B-cell development. We found that AID+ cells represent 0.9 ± 0.4% of CD19+ B-cell precursors (data not shown). This very low frequency of CD19+ cells expressing AID proteins in fetal liver or adult BM may account for their global low AID transcription amount amplified by quantitative PCRs compared to CXCR4+ GC cells, most of which express AID, whereas AID transcripts were not amplified from peripheral CD19+ B and CD4+ T cells (Figure 3B and 2D) (Han et al., 2007; Kuraoka et al., 2009; Mao et al., 2004; Ueda et al., 2007). If fetal BM or fetal liver B cells express AID at an equal amount to GC B cells and using spike-in experiments in which CXCR4+ GC B cells were diluted in different proportions in AID− peripheral B cells, we estimated that 0.4-0.55% of B cells in fetal BM or fetal liver would be expressing AID, a frequency similar to what was determined by immunofluorescence (Figure 3, B and C).
Figure 3. AID is expressed in immature B cells.
(A) Tonsil and fetal rib (115 day old) sections were stained for AID (red) and IgM (green). Higher magnification (right panel) reveals presence of IgM+AID+ cells in fetal rib. Data is representative of 3 independent fetal rib samples and 1 tonsil sample. scale bar (left to right): 50, 200 and 25 μm. (B) Quantitative real-time PCR shows presence of AICDA mRNA transcript in CD34−CD19+ fetal liver (FL) and fetal BM samples. Transcripts were normalized to HPRT1. (C) 0.1-2.5% of sorted CD19+IgD−CD38+CXCR4+ GC B cells were spiked in peripheral mature naive CD19+ B cells and AICDA mRNA transcript was measured. A relative AICDA expression of 0.05-0.06 in FL and fetal BM as measured in B corresponds to 0.40-0.55% of CXCR4+ GC B cells. (D, E) Cytospin slides of CD34−CD19+ purified fetal liver or adult bone marrow (BM) cells were stained for IgM, Igκ+Igλ or VpreB (green) and AID (red) and quantified for co-staining. AID protein is expressed in 0.9% ± 0.4 of total CD34− CD19+ cells. Data are representative of 3 fetal liver and 1 adult bone marrow sample(s). Error bars represent mean ± SD. Original magnification 40X, scale bar: 10 μm.
We then analyzed the stage of B cell development at which AID was expressed. We found that AID expressing fetal liver and adult BM CD19+ cells co-expressed functional IgM heavy chain products, thereby revealing that AID was not detected in IgM− early B-cell precursors but restricted to pre-B or immature B-cell stages (Figure 3, D and E) (Meffre et al., 2000). We excluded AID expression at the pre-B cell stage because AID and Vpre-B, one of the 2 proteins encoding the surrogate light chain expressed at the early pre-B cell stage were not found in the same cells (Burrows et al., 2002; Martensson et al., 2002). The identification of kappa or lambda light chains with AID showed that AID expression was restricted to the immature B-cell stages at which both heavy and light chain genes are rearranged and encode BCRs (Figure 3, D and E) (Meffre et al., 2000). We conclude that AID proteins appear to be confined to a discrete population of B cells expressing BCRs that are selected by central B-cell tolerance.
AID proteins are expressed in RAG2+ early immature B cells
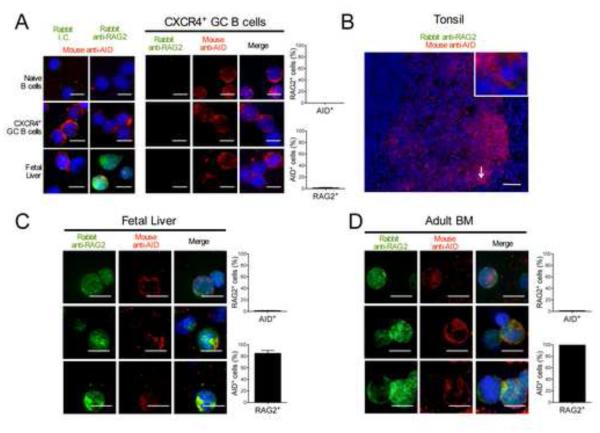
To exclude that AID+ cells were mature B cells, which also express BCRs and are present in CD19+ cells from adult BM though not in fetal liver, we tested the expression by immunohistochemistry of RAG2, that catalyzes V(D)J recombination in lymphocyte precursors when combined with RAG1 (Schatz and Swanson, 2011). Monoclonal rabbit anti-RAG2 antibodies labeled fetal liver B-cell precursors but did not stain naïve or GC B cells either when these B cells were isolated and deposited on cytospin slides or in human tonsil sections (Figure 4, A and B). Most fetal liver and adult BM B cells that expressed AID were found to co-express RAG2, demonstrating that AID+ B cells were true precursors likely undergoing Ig gene rearrangements and not hypothetical recirculating GC B cells (Figure 4, C and D). While RAG2 was detected in both the nucleus and the cytoplasm, AID expression in immature B cells appeared enriched in the cytoplasm, a feature that is also observed in GC B cells (Figure 4) (Rada et al., 2002). Since RAG2 is rapidly degraded when cells enter S phase, AID+ B-cell precursors are likely in a resting or G1 phase (Grawunder et al., 1995; Li et al., 1996). In addition, the expression of RAG2 in AID+ immature B cells expressing both Ig heavy and light chains, suggests that these B cells are undergoing receptor editing, a mechanism involving secondary recombination events that is important for central B-cell tolerance (Goodnow, 1996; Nemazee, 2006; Radic and Weigert, 1995). Thus, AID proteins are expressed in a discrete population of early immature B cells, which may produce self-reactive BCRs and express RAG to keep recombining Ig genes in order to tolerize these clones.
Figure 4. AID is co-expressed with RAG2 in early immature B cells.
Cytospin slides of sorted CD19+IgD+CD38−CXCR4− naïve B cells, CD19+IgD−CD38+CXCR4+ GC B cells and CD34−CD19+ fetal liver B cell precursors were stained for RAG2 or concentration matched isotype control (green) and AID (red) and co-staining was quantified. Data is representative of 2 independent experiments. Original magnification 40X, scale bar: 10 μm (B) Tonsil tissue sections stained for AID (red) and RAG2 (green) showed no RAG2 staining. Data is representative of 1 tonsil sample. Original magnification 20X, scale bar: 50 μm (C, D) Cytospin slides of CD34−CD19+ purified fetal liver or adult bone marrow cells were stained for RAG2 (green) and AID (red) and co-staining was quantified. Most AID+ B cells co-express RAG2. Data are representative of 3 fetal liver and 1 adult bone marrow sample(s). Error bars represent mean ± SD. Original magnification 40X, scale bar: 10 μm.
Most AID-expressing immature B cells are undergoing apoptosis
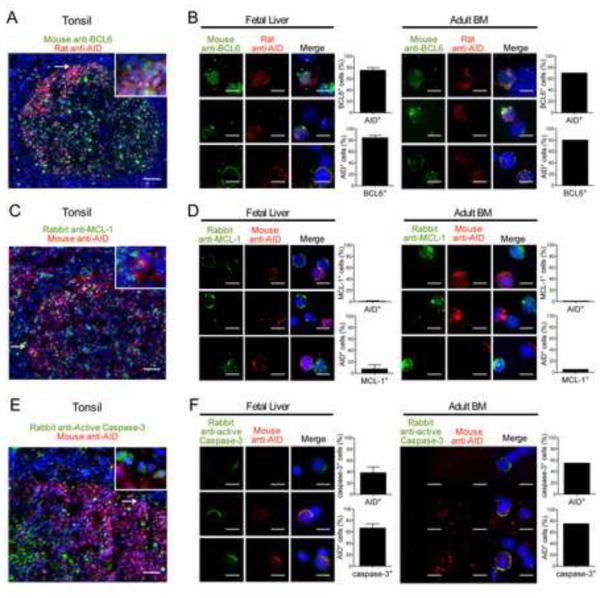
We further characterized AID+ early immature B cells by comparing these cells to GC B cells that also express AID. We first assessed whether BCL6 a transcription factor critical for GC development and regulating AID expression was expressed in human B-cell precursors (Basso and Dalla-Favera, 2012). Anti-BCL6 monoclonal antibody stained many GC B cells but not naïve B cells as previously reported (Figure 5A and Figure S3A) (Basso and Dalla-Favera, 2012). AID expression in CXCR4+ GC B cells was found restricted to cells that co-expressed BCL6 (Figure S3A). We detected BCL6 expression in some B-cell precursors from human fetal liver and adult BM (Figure 5B). Most of these AID+ cells also co-expressed BCL6, suggesting that a similar program potentially induced by BCRs recognizing (self-) antigens may take place in some early immature and GC B cells (Figure 5B). We then investigated the expression of anti-apoptotic factors allowing B-cell precursor and GC B cell survival (Fang et al., 1996; Opferman et al., 2003; Smith et al., 1994; Vikstrom et al., 2010). We could not analyze BCL2 or BCL-XL expression because cell fixation procedures allowing AID detection inhibited the detection of these molecules using several antibodies. However, we were able to detect MCL-1, an anti-apoptotic factor required for both precursor and GC B cell survival in many GC B cells from tonsil sections or cytospins (Figure 5C and Figure S3B) (Opferman et al., 2003). In contrast, we found that AID+ early immature B cells from fetal liver and adult BM were devoid of MCL-1, suggesting that these B-cell precursors may not be protected from apoptosis (Figure 5D). Indeed, they often expressed active caspase-3, characteristic of apoptotic cells, whereas only rare GC B cells were stained for such marker (Figure 5, E and F, and Figure S3C) (Porter and Janicke, 1999). Hence, most AID+ early immature B cells that express RAG2 and undergo receptor editing fail to be rescued from cell death and are likely being deleted. Our observation is in agreement with the dearth of GFP+ B cells exiting the bone marrow of Aicda-cre x Rosa-floxed-GFP mice in which GFP expression is turned on when AID expression is induced (Crouch et al., 2007). Although it was initially postulated that the lack of GFP+ B cells in this mouse model resulted from the absence of AID expression during early B-cell development, our data suggest that AID may be induced in developing autoreactive B cells that eventually get eliminated.
Figure 5. AID+ cells are undergoing apoptosis.
Tonsil tissue section (A) or cytospin slides of CD34−CD19+ purified fetal liver or adult bone marrow (BM) (B) were stained for BCL6 (green) and AID (red) and co-staining was quantified. Most AID+ cells co-express BCL6. Tissue section of tonsil (C) or cytospin slides of CD34−CD19+ purified fetal liver or adult BM (D) were stained for MCL-1 (green) and AID (red) and co-staining was quantified. The vast majority of AID+ cells do not express anti-apoptotic MCL-1. Tissue section of tonsil (E) or cytospin slides of CD34−CD19+ purified fetal liver or adult BM (F) were stained for active caspase-3 (green) and AID (red) and co-staining was quantified. AID and activated caspase-3 co-staining reveals that many AID+ cells fail to be rescued from cell death by apoptosis. Data are representative of 3 fetal liver, 1 adult bone marrow, and 1 tonsil sample(s). Error bars represent mean ± SD. scale bar: 50 μm (A, C, E) and 10 μm (B, D, F). Please see also Figure S3.
Central B-cell tolerance requires B-cell intrinsic AID expression
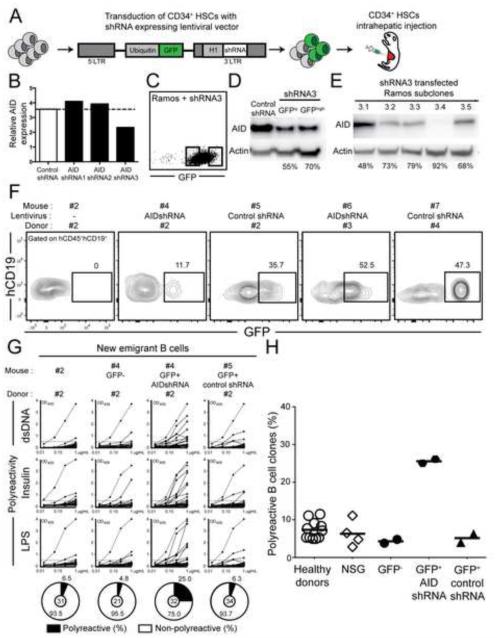
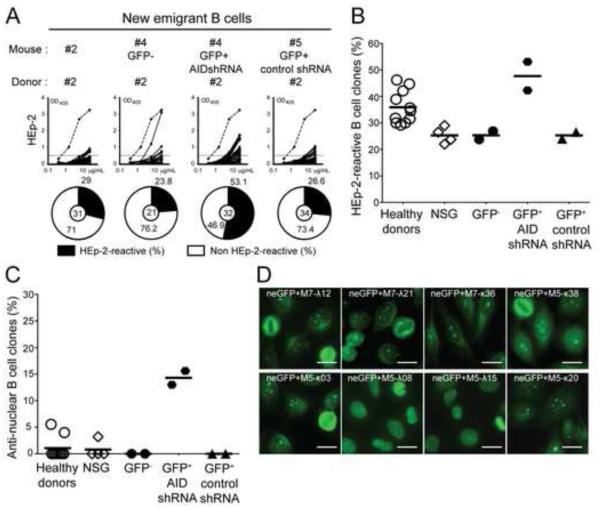
We proceeded to test the B-cell intrinsic requirement of AID expression to eliminate autoreactive immature B cells by developing a model for human central B-cell tolerance in which AID expression could be inhibited using specific shRNA. Humanized mice were produced by engrafting NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) immunodeficient mice with CD34+ hematopoietic stem cells (HSCs) isolated from human fetuses (Shultz et al., 2005). Alternatively, HSCs were transduced overnight with lentiviruses expressing either GFP-tagged AID specific shRNA or control shRNA before transfer into NSG mice (Figure 6A) (Schickel et al., 2012). We characterized an AID specific shRNA, AID shRNA3, that could inhibit 50-70% of AID expression that was detected either by quantitative PCRs (Figure 6B) or by immuno blot using the human RAMOS B cell line transduced with GFP-tagged AID shRNA3 (Figure 6, C and D). Subcloning of GFP+ RAMOS transduced with AID shRNA3 further showed that 48-92% of AID expression could be inhibited using this strategy (Figure 6E). Since the loss of a single AICDA allele, which reduces AID expression by about 50% results in central B-cell selection defects (Figure 1 and 2), the shRNA-induced decrease in AID expression might also interfere with the removal of developing autoreactive B cells. Human CD19+ B cells developed in NSG mice engrafted with HSCs transduced or not with lentiviruses; 11.7-52.5% of new emigrant B cells expressed GFP indicative of shRNA expression, revealing that HSCs transduced with lentiviruses retained engraftment and B-cell development abilities (Figure 6F). The analysis of the reactivity of antibodies cloned from splenic CD19+CD27− CD10+IgMhiCD21lo new emigrant B cells from NSG mice transplanted with HSCs isolated from four distinct fetuses showed that the frequencies of polyreactive clones were similar to those of new emigrant B cells isolated from the blood of healthy donors, demonstrating that central B-cell tolerance is established normally in humanized mice (Figure 6, G and H, Figure S4A and Table S4). The frequencies of new emigrant B cells that produced HEp-2 reactive antibodies were lower in NSG mice compared to healthy donors, a feature that may reflect differences between fetal and adult HSCs (Figure 7, A and B and Figure S4B). Anti-nuclear reactivity in new emigrant B cells was very rare and comparable between humanized mice and healthy donors, further attesting of the proper counterselection of human autoreactive clones in mouse bones (Figure 7C). Altogether, these data reveal that NSG humanized mice represent a good model for human central B-cell tolerance.
Figure 6. Inhibition of AID expression during B cell development interferes with the removal of polyreactive clones.
(A) Schematic diagram depicting the generation of humanized mice. CD34+ hematopoietic stem cells (HSCs) were transduced with lentiviruses allowing the expression of AID or control shRNA before being injected in the liver of 3 day-old recipient NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (B) Relative expression of AICDA in Ramos cells transfected with control shRNA, or 3 different shRNAs targeting human AICDA cDNA sequence. Values are normalized to HPRT1. Data shows the mean of 3 independent experiments. (C) Transfection efficiency and sorting strategy for Ramos cells transfected with GFP-tagged AID shRNA3. (D) AID protein expression in Ramos cells transfected with control shRNA or AID shRNA3 after sorting GFPlo or GFPhi expressing cells. Protein expression of β-actin is used for normalization. Percentage of knock-down is indicated. Data is representative of 2 independent experiments. (E) AID protein expression in subclones of Ramos transfected cells with shRNA3. Protein expression of β-actin is used for normalization. Percentage of gene silencing is indicated. Data is representative of 2 independent experiments (F) Transduction efficiency and sorting strategy of the GFP+ shRNA+ fractions in hCD45+hCD19+ B cells. (G) B-cell intrinsic AID expression is required for central B-cell tolerance. Antibodies from new emigrant B cells isolated from control humanized mice and sorted GFP− as well as GFP+ fractions expressing AID shRNA or control shRNA were tested by ELISA for reactivity against dsDNA, insulin and LPS. Polyreactive antibodies reacted against all 3 antigens. Dotted lines show ED38-positive control. Horizontal lines show cutoff OD405 for positive reactivity. For each mouse, the frequency of polyreactive and non-polyreactive clones is summarized in pie charts, with the number of antibodies tested indicated in the center. The frequencies of polyreactive new emigrant B cells in healthy donors and humanized mice expressing or not the indicated shRNA are summarized in (H). Each symbol represents an individual or mouse, and the horizontal bars show the average. Please see Figure S4.
Figure 7. Central B-cell tolerance requires B-cell intrinsic AID expression.
(A) Antibodies from new emigrant B cells isolated from control humanized mice and sorted GFP− as well as GFP+ fractions expressing AID shRNA or control shRNA were tested by ELISA for reactivity against HEp-2 cell lysate. Solid lines show binding for each cloned recombinant antibody. Dotted lines show ED38-positive control. Horizontal lines show cutoff OD405 for positive reactivity. For each mouse, the frequency of HEp-2 reactive and non HEp-2 reactive B cells are summarized in pie charts, with the number of antibodies tested shown in the center. The frequencies of HEp-2 reactive (B) and anti-nuclear (C) new emigrant B cells are compared between healthy donors and humanized mice expressing or not the indicated shRNA. Each symbol represents an individual or mouse, and the horizontal bars show the average. (D) Anti-nuclear antibodies expressed by new emigrant B cells expressing AID shRNA show various chromatin reactive and non-reactive nuclear reactvity. Original magnification, ×40. scale bar: 10 μm . Please see Figure S4.
In contrast, new emigrant B cells expressing AID shRNA contained many autoreactive clones expressing polyreactive antibodies (Figure 6, G and H, Figure S4 and Table S5). The defective central B-cell tolerance induced by AID shRNA was also evidenced by the high proportion of HEp-2 reactive and anti-nuclear GFP+ new emigrant B cells (Figure 7). Anti-nuclear staining patterns of antibodies from new emigrant B cells that developed with decreased AID expression were very diverse and included chromatin and non-chromatin reactive clones (Figure 7D). While, a high proportion of GFP+ B cells expressing AID shRNA expressed autoreactive antibodies, GFP− new emigrant B cells that did not were properly selected in the same mouse (Figure 6, G and H, Figure S4 and Table S5). In addition, lentiviral transduction per se did not interfere with the counterselection of BM autoreactive B cells because GFP+ new emigrant B cells expressing the control shRNA displayed normal proportions of polyreactive, HEp-2 reactive and antinuclear clones (Figure 6, G and H, Figure S4, Figure 7, A-C, and Table S5). These data therefore demonstrate the B-cell intrinsic requirement of AID expression for the BM removal of developing autoreactive B cells and the establishment of human central B-cell tolerance.
During early B-cell development, DNA double-strand breaks induced by RAG and potentially AID activate DNA damage response controlled by gatekeeper p53, which induces cell-cycle arrest or apoptosis (Green and Kroemer, 2009; Kruse and Gu, 2009). To determine if p53 blockade may interfere with the negative selection of developing B cells, NSG humanized mice engrafted with HSCs from different donors were injected i.p. daily for a week with pifithrin-α (PFTα) p53 inhibitor (Figure S5A) (Komarov et al., 1999). PFTα-treated NSG humanized mice showed elevated frequencies of new emigrant B cells expressing polyreactive and HEp-2 reactive antibodies including ANAs that were similar to those when AID expression was inhibited (Figure S5, B-D and Table S6). In addition, PFTα-treated NSG humanized mice displayed 5 fold-increased frequencies of AID-expressing B-cell precursors in their BM compared to untreated humanized mice, suggesting that p53 inhibition rescue AID-expressing B cell precursors from apoptosis and/or clearance (Figure S6). Hence, the inhibition by PFTα of p53 activation likely induced by RAG- and AID-mediated DNA double-strand breaks in developing autoreactive B cells prevents the couterselection of these B-cell precursors in the BM. Taken together, AID expression in autoreactive immature B cells may lead to their elimination in the bone marrow.
DISCUSSION
The identification of an impaired central B-cell tolerance checkpoint in AR-AID-deficient and AICDA+/− individuals who exhibit no or reduced AID expression, respectively, emphasizes the importance of AID in the removal of developing autoreactive B cells. The absence of BM counterselection defects in patients with rare AD-AICDA mutation that abolishes CSR but preserves enzymatic activity argues that DNA cytidine deamination is a key feature to ensure central tolerance. In agreement with the previous detection of AID transcripts in B-cell precursors, we identified AID protein expression in a discrete population of immature B cells, suggesting that AID may be mediating its tolerogenic function from within developing B cells. In addition, the induction of CSR in mouse pre-B cells in vitro and in vivo previously demonstrated that AID enzyme could be induced in B-cell precursors (Han et al., 2007; Mao et al., 2004; Rolink et al., 1996). The inhibition of AID expression in developing B cells in humanized mice resulted in a failure to remove autoreactive clones, whereas B cells that could express AID in the same mice did not express self-reactive antibodies, demonstrating the B-cell intrinsic requirement for AID expression in the establishment of central tolerance. Hence, AID enzymatic activity is essential not only for mediating CSR and SHM but also for the silencing of BM developing autoreactive B cells.
How does AID expression induced in early immature B cells mediate central B-cell tolerance? Receptor editing, the major mechanism to ensure central B-cell tolerance, is mediated by secondary recombination catalyzed by RAG enzymes and is induced in immature B cells expressing autoreactive BCRs (Goodnow, 1996; Meffre et al., 2000; Nemazee, 2006; Radic and Weigert, 1995). The identification of RAG2 in AID+ immature B cells therefore suggests that these B cells may express autoreactive BCRs and are undergoing secondary recombination to edit such receptors. The binding of self-antigens including nuclear antigens may therefore trigger autoreactive BCRs and TLRs in immature B cells and induce AID expression (Han et al., 2007; Meyers et al., 2011; Perez et al., 2010; Umiker et al., 2014). The co-expression of BCL6, previously shown to be required for the generation of a diverse Ig repertoire, may also play an important role in the induction of AID in autoreactive developing B cells (Basso and Dalla-Favera, 2012; Duy et al., 2010). In addition, BCL6 augments BCR signaling and suppresses anti-apoptotic BCL2 expression (Juszczynski et al., 2009; Saito et al., 2009). BCL6 may therefore favor the silencing of autoreactive immature B cell potentially enhancing deletion associated with strong BCR signaling (Meffre et al., 2000). In humans, early immature B cells, which do not express surface BCRs and were identified in sorted BM CD19+CD34−CD27−CD10+IgM− “pre-B” cells, produce highly self-reactive and anti-nuclear antibodies and may therefore contain clones in which AID is induced (Wardemann et al., 2003). Since most AID+ immature B cells undergo apoptosis as illustrated by the identification of active caspase-3, AID expression may therefore lead to cell death and the deletion of autoreactive B cells that fail to silence self-reactive BCRs. If one mouse model reporting AID-expression failed to detect BM AID-expression without infection (Crouch et al., 2007), another identified rare BM immature B cells that have previously expressed AID, suggesting that some cells may escape deletion potentially after editing their BCRs (Qin et al., 2011). Since AID-deficient B cells have been reported to be less sensitive to apoptosis, autoreactive immature B cells lacking AID may be resistant to deletion and escape central B-cell tolerance, especially since receptor editing has also been reported to be affected by the lack of AID (Zaheen et al., 2009) (Kuraoka et al., 2011; Umiker et al., 2014). Since apoptotic developing B cells are rapidly eliminated by surrounding macrophages, it may explain why AID is only detected in a discrete and transient population of immature B cells but may in fact account for the removal of many autoreactive clones, thereby ensuring central B-cell tolerance. In line with this hypothesis, p53 inhibition by PFTα abrogated central B-cell tolerance and increased the frequency of BM AID-expressing B cells, suggesting that p53 blockade may rescue developing autoreactive B cells from apoptosis (Komarov et al., 1999). We propose that AID may induce DNA lesions that activate p53 function, leading to the elimination of autoreactive clones. The co-expression of RAG in AID+ early immature B cells likely further increases the amount of DNA damage sustained by these cells, as a result of receptor editing and potentially by RAG-induced DNA double-strand breaks at AID-deaminated methyl-CpG motifs (Tsai et al., 2008). Importantly, our identification of a developmental stage at which both AID and RAG are expressed simultaneously provides a rationale for translocation events occurring in pre-B ALL cases that were predicted to be mediated by concerted RAG and AID activity (Tsai et al., 2008). The requirement of both AID and RAG expression for leukemia to emerge from B-cell precursors has now been demonstrated in mice (Swaminathan et al., 2015). Hence, leukemic transformation in B-cell precursors may represent a failure of central B-cell tolerance.
EXPERIMENTAL PROCEDURES
Patients and healthy donor controls
We obtained peripheral blood or frozen PBMC from 16 AR-AID-def. patients, with homozygote or compound heterozygote autosomal recessive AICDA mutations of which 9 were described previously (Meyers et al., 2011). In addition, we collected samples from 12 healthy donors related to these patients carrying one autosomal recessive mutated AICDA allele, 4 AD-AICDA patients, and 3 UNG-deficient patients, one of which was positive for the PTPN22 risk allele and therefore excluded from our analysis since this polymorphism correlates defective early B-cell tolerance checkpoints in healthy donors (Menard et al., 2011). All patients’ information is included in Table S1. Age-matched healthy donors were previously reported, except for healthy donor HD30 (45 y.o. Caucasian female) (Kinnunen et al., 2013; Menard et al., 2014; Romberg et al., 2013). Fetal liver was obtained from 6 different fetuses aged between day 100-115 and bone marrow samples from donors. All samples were collected in accordance with institutional review board-reviewed protocols
Cell staining and sorting
Peripheral B cells were purified from the blood of patients and control donors by positive selection using CD20-magnetic beads (Miltenyi). Enriched B cells were stained with following antibodies: anti-CD19-Pacific Blue, anti-CD27-PercP-Cy5.5, anti-CD10-PE-Cy7, anti-IgM-FITC, anti-CD21-APC and anti-IgG-PE (all from Biolegend). Single CD19+CD21loCD10+IgMhiCD27− new emigrant and CD19+CD21+CD10−IgM+CD27− mature naïve B cells from patients and healthy donors were sorted on a FACSAria flow cytometer (Becton Dickinson, Mountain View, Calif) into 96-well PCR plates. For phenotyping, enriched B cells were stained with anti-CD19-Pacific Blue, anti-CD27-PercP-Cy5.5, anti-CD10-PE-Cy7, anti-IgM-FITC (all from Biolegend), anti-IgG-APC and anti-IgA-PE (BD). Following cell suspensions were isolated, counted and deposited on poly-L-lysine-coated glass slides in a cytospin centrifuge and stored at −80°C until usage. Tonsil was smashed on a 40μm strainer and enriched using CD19 magnetic beads (Miltenyi). Tonsil cells were sorted with following mixes: anti-CD3-FITC (ebioscience), anti-CD38-PE (BD), anti-IgD-PercP-Cy5.5, anti-CD10-PE-Cy7, anti-CXCR4-APC, anti-CD19-Pacific Blue (all from Biolegend). For AID detection in peripheral B cells, CD20+ enriched cells were stimulated with 100 ng/nl of human recombinant trimeric CD40L (Enzo Life Sciences), 50 U/mL of IL-2 (Peprotech) and 50ng/mL of IL-21 (R&D systems) for 2 days. After 48 hours of culture, the medium was replaced by fresh RPMI containing 50 U/mL of IL-2 and 50ng/mL of IL-21 without CD40L and the cells were cultured for another 48 hours. Fetal liver was smashed on a 40μm strainer, depleted of CD34+ cells using magnetic beads (Miltenyi) and enriched for CD19 using magnetic beads (Miltenyi). Adult human bone marrow aspirate was depleted of CD34+ cells using magnetic beads (Miltenyi) and enriched for CD19 using magnetic beads (Miltenyi)
cDNA, RT-PCR, antibody production and purification
RNA from single cells was reverse-transcribed in the original 96 well plate in 12.5□l reactions containing 100U of Superscript II RT (Gibco BRL) for 45 min at 42°C. RT-PCR reactions, primer sequences, cloning strategy, expression vectors, antibody expression, and purification were as described (Wardemann et al., 2003).
ELISAs and IFAs
Antibody concentrations and reactivity were measured as described (Wardemann et al., 2003). Highly polyreactive ED38 was used as positive control in HEp-2-reactivity and polyreactivity ELISAs. Antibodies were considered polyreactive when they recognized all 3 analyzed antigens, which included double-stranded DNA (dsDNA), insulin and lipopolysaccharide (LPS). For indirect immunofluorescence assays, HEp-2 cell coated slides (Bion Enterprises, LTD) were incubated in a moist chamber at room temperature with purified recombinant antibodies at 50-100 μg/mL and detected with FITC-conjugated goat anti-human IgG.
Tissue sections, cytospins and immunofluorescence
Tonsil and Fetal ribs were immediately embedded in Tissue Tek Optimum Cutting Temperature (OCT) (Sakura) and immediately frozen using a mixture of isobuthanol and dry ice, and stored at −80°C. 7μm cryostat sections of fetal rib were transferred to CJ1X adhesive coated slide using the Cryojane® Tape Transfer System (Instrumedics) according to the manufacturer's protocol. Prior to staining, all slides were fixed in 4% paraformaldehyde and permeabilized in 5% donkey serum, 0.5% BSA and 0.05% Tween-20. Following primary antibodies were used for stainings: mouse anti-AID (Invitrogen, clone ZA-001), rat anti-AID (cell signaling, clone EK2-5G9), mouse anti-IgM (Santa Cruz, clone SA-DA4), rabbit anti-Igk (Dako, polyclonal), rabbit anti-L (Dako, polyclonal), mouse anti-VpreB (Biolegend, polyclonal), rabbit anti-RAG2 (gift from D. Schatz), mouse anti-BCL6 (BD bioscience, clone K112-91), rabbit anti-MCL-1 (Abcam, polyclonal), mouse anti-BCL2 (Abcam, clone 4D7), rabbit anti-BCL-XL (Abcam, polyclonal) rabbit anti-active caspase 3 (BD bioscience, clone C92-605). Primary antibodies were revealed with donkey anti-mouse AlexaFluor 647 (Invitrogen), goat anti-rat PE (Santa Cruz Biotechnology) followed by donkey anti-goat PE (Jackson ImmunoResearch), and donkey anti-rabbit AlexaFluor 488. Nuclei were counterstained with DAPI (Invitrogen) and 1000 nuclei per cytospin sample were analyzed. Double-blinded detection of AID+ cells was performed for BM CD19+ cells of NSG control and PFTα-treated mice.
Immunoblot and QPCR
For detection of AID Ramos cellines, RNA was extracted using the Absolutely RNA Microprep Kit (Agilent Technilogies) and 150 ng RNA was reverse transcribed using random hexamers (Applied Biosystems) and SuperScript III Reverse Transcriptase kit (Invitrogen). For mRNA gene expression assays, probes were purchased from Applied Biosystems: HPRT1: Hs02800695_m1, AICDA: Hs00757808_m1. Reactions were run on a 7500 Real-Time PCR system (Applied Biosystems) in duplicate. Values are represented as the difference in Ct values normalized to HPRT1 for each sample. For AID protein detection, cells were lysed in lysis buffer (50mM Tris, 1% NP-40, 2mM EDTA) including protease inhibitor (Roche). Total cell lysates were separated by SDS page, transferred to PDVF membranes, probed with mouse anti-AID (Invitrogen) and anti-mouse HRP (Cell Signaling) and detected by chemiluminescence (Amersham ECL Prime Western Blotting detection Reagent) using a GBox documentation system (Syngene). For quantification, blots were stripped with stripping buffer (Pierce) and reprobed with a mouse anti-β-Actin antibody (Sigma-Aldrich).
AID silencing and CD34+ HSCs transduction
The pTRIP-Ubi-GFP lentiviral vector has been used for short hairpin RNA (shRNA) delivery. For the construction of pTRIP-shAID, a DNA fragment containing the H1 promoter and a AID shRNA sequence was generated by double digestion of pSUPER-shAID plasmid (made by inserting a shRNA targeting human AID cDNA sequence shRNA1: 5’-CTTTGGTTATCTTCGCAATAA-3’, shRNA2: 5’-ACCACGAAAGAACTTTCAAAG-3’ or shRNA3: 5′-AAGCATGGTGAGAGGATCAAA-3′ or control shRNA into pSUPER plasmid) and was subcloned within the 3′ long terminal repeat of pTRIP-Ubi-GFP vector. Lentiviral particles were produced by transient transfection of 293T cells, as previously described (Schickel et al., 2012). Viruses were then used to transduce CD34+ HSCs in the presence of polybrene (Sigma).
Human progenitor cell isolation and injection in NSG mice
Human CD34+ cells were purified from fetal liver samples by density gradient centrifugation followed by positive immunomagnetic selection with anti-human CD34 microbeads (Miltenyi). Newborn NSG mice (within first 3 day of life) were sublethally irradiated (X-ray irradiation with X-RAD 320 irradiator at 180 cGy) and 100,000-150,000 CD34+ cells in 20 μL of PBS were injected into the liver with a 22-gauge needle (Hamilton Company). Mice were used for experiments 10–12 weeks after transplantation. NSG mice treated with Pifithrin-α (PFTα, Sigma) were injected daily i.p. with 50μg PFTα for a week. All animals were treated and experiments were conducted in accordance with the Yale institutional reviewed guidelines on treatment of experimental animals.
Statistics
Statistical analysis was performed using GraphPad Prism (version 5.0; GraphPad, San Diego, CA). Data are reported as mean ± standard deviation. Differences between groups of research subjects were analyzed for statistical significance with unpaired two-tailed Student’s t-tests. A P-value of ≤0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We are very much indebted to the patients and their families. We thank Dr. L. Devine and C. Wang for cell sorting, Dr. P. Soulas-Sprauel for kindly providing the pTRIP-Ubi-GFP lentiviral vector for shRNA delivery, Dr. JC Delpech for help with immunostainings and Dr. D. Schatz for providing the anti-RAG2 antibodies and discussions. We also thank all the members of the Immunodeficiency Clinic of the Centre Hospitalier de l’Université Laval. This work was supported by Grant Number AI061093, AI071087 and AI082713 from NIH-NIAID (to E. M.) and INSERM, CEE EUROPAD-contract 7th Framework Program (#201549) and Assiociation Contre le Cancer (to A. D.). T. Cantaert received support from the Rubicon program, Netherlands Organization for Scientific Research.
Abbreviations
- AID
activation-induced cytidine deaminase
- ANA
anti-nuclear antibody
- AD
autosomal dominant
- AR
autosomal recessive
- BCL6
B-cell lymphoma 6
- BCR
B-cell receptor
- BM
bone marrow
- CSR
class-switch recombination
- GC
germinal centre
- Ig
immunoglobulin
- SHM
somatic hypermutation
- RAG
recombination-activating gene
- UNG
Uracil N-glycosylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
E. M. initiated the collaboration with J. W., A. L., W. A-H., S. S. K., H. O., S. N., and A. D. who provided blood samples from AR-AID-deficient patients, healthy related carriers, AD-AID patients, UNG-def. patients and control donors; T. C. and E. M. designed the experiments. T. C., J.-N. S., J. B., Y. N., C. M., T.O. and R.W. performed the experiments. T. C., J.-N. S. and E. M. interpreted and discussed the data and wrote the manuscript. All authors reviewed the manuscript and provided scientific input.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Basso K, Dalla-Favera R. Roles of BCL6 in normal and transformed germinal center B cells. Immunological reviews. 2012;247:172–183. doi: 10.1111/j.1600-065X.2012.01112.x. [DOI] [PubMed] [Google Scholar]
- Burrows PD, Stephan RP, Wang YH, Lassoued K, Zhang Z, Cooper MD. The transient expression of pre-B cell receptors governs B cell development. Seminars in immunology. 2002;14:343–349. doi: 10.1016/s1044-5323(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Crouch EE, Li Z, Takizawa M, Fichtner-Feigl S, Gourzi P, Montano C, Feigenbaum L, Wilson P, Janz S, Papavasiliou FN, Casellas R. Regulation of AID expression in the immune response. The Journal of experimental medicine. 2007;204:1145–1156. doi: 10.1084/jem.20061952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annual review of biochemistry. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- Duy C, Yu JJ, Nahar R, Swaminathan S, Kweon SM, Polo JM, Valls E, Klemm L, Shojaee S, Cerchietti L, et al. BCL6 is critical for the development of a diverse primary B cell repertoire. The Journal of experimental medicine. 2010;207:1209–1221. doi: 10.1084/jem.20091299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W, Mueller DL, Pennell CA, Rivard JJ, Li YS, Hardy RR, Schlissel MS, Behrens TW. Frequent aberrant immunoglobulin gene rearrangements in pro-B cells revealed by a bcl-xL transgene. Immunity. 1996;4:291–299. doi: 10.1016/s1074-7613(00)80437-9. [DOI] [PubMed] [Google Scholar]
- Goodnow CC. Balancing immunity and tolerance: deleting and tuning lymphocyte repertoires. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grawunder U, Leu TM, Schatz DG, Werner A, Rolink AG, Melchers F, Winkler TH. Down-regulation of RAG1 and RAG2 gene expression in preB cells after functional immunoglobulin heavy chain rearrangement. Immunity. 1995;3:601–608. doi: 10.1016/1074-7613(95)90131-0. [DOI] [PubMed] [Google Scholar]
- Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009;458:1127–1130. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Akira S, Calame K, Beutler B, Selsing E, Imanishi-Kari T. Class switch recombination and somatic hypermutation in early mouse B cells are mediated by B cell and Toll-like receptors. Immunity. 2007;27:64–75. doi: 10.1016/j.immuni.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Slupphaug G, Lee WI, Revy P, Nonoyama S, Catalan N, Yel L, Forveille M, Kavli B, Krokan HE, et al. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nature immunology. 2003;4:1023–1028. doi: 10.1038/ni974. [DOI] [PubMed] [Google Scholar]
- Imai K, Zhu Y, Revy P, Morio T, Mizutani S, Fischer A, Nonoyama S, Durandy A. Analysis of class switch recombination and somatic hypermutation in patients affected with autosomal dominant hyper-IgM syndrome type 2. Clinical immunology. 2005;115:277–285. doi: 10.1016/j.clim.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Isnardi I, Ng YS, Srdanovic I, Motaghedi R, Rudchenko S, von Bernuth H, Zhang SY, Puel A, Jouanguy E, Picard C, et al. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29:746–757. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Nagaoka H, Shinkura R, Begum N, Muramatsu M, Nakata M, Honjo T. Activation-induced cytidine deaminase shuttles between nucleus and cytoplasm like apolipoprotein B mRNA editing catalytic polypeptide 1. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1975–1980. doi: 10.1073/pnas.0307335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juszczynski P, Chen L, O'Donnell E, Polo JM, Ranuncolo SM, Dalla-Favera R, Melnick A, Shipp MA. BCL6 modulates tonic BCR signaling in diffuse large B-cell lymphomas by repressing the SYK phosphatase, PTPROt. Blood. 2009;114:5315–5321. doi: 10.1182/blood-2009-02-204362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen T, Chamberlain N, Morbach H, Cantaert T, Lynch M, Preston-Hurlburt P, Herold KC, Hafler DA, O'Connor KC, Meffre E. Specific peripheral B cell tolerance defects in patients with multiple sclerosis. The Journal of clinical investigation. 2013;123:2737–2741. doi: 10.1172/JCI68775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraoka M, Holl TM, Liao D, Womble M, Cain DW, Reynolds AE, Kelsoe G. Activation-induced cytidine deaminase mediates central tolerance in B cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11560–11565. doi: 10.1073/pnas.1102571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraoka M, Liao D, Yang K, Allgood SD, Levesque MC, Kelsoe G, Ueda Y. Activation-induced cytidine deaminase expression and activity in the absence of germinal centers: insights into hyper-IgM syndrome. Journal of immunology. 2009;183:3237–3248. doi: 10.4049/jimmunol.0901548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Dordai DI, Lee J, Desiderio S. A conserved degradation signal regulates RAG-2 accumulation during cell division and links V(D)J recombination to the cell cycle. Immunity. 1996;5:575–589. doi: 10.1016/s1074-7613(00)80272-1. [DOI] [PubMed] [Google Scholar]
- Mao C, Jiang L, Melo-Jorge M, Puthenveetil M, Zhang X, Carroll MC, Imanishi-Kari T. T cell-independent somatic hypermutation in murine B cells with an immature phenotype. Immunity. 2004;20:133–144. doi: 10.1016/s1074-7613(04)00019-6. [DOI] [PubMed] [Google Scholar]
- Martensson IL, Rolink A, Melchers F, Mundt C, Licence S, Shimizu T. The pre-B cell receptor and its role in proliferation and Ig heavy chain allelic exclusion. Seminars in immunology. 2002;14:335–342. doi: 10.1016/s1044-5323(02)00066-0. [DOI] [PubMed] [Google Scholar]
- McBride KM, Gazumyan A, Woo EM, Schwickert TA, Chait BT, Nussenzweig MC. Regulation of class switch recombination and somatic mutation by AID phosphorylation. The Journal of experimental medicine. 2008;205:2585–2594. doi: 10.1084/jem.20081319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffre E. The establishment of early B cell tolerance in humans: lessons from primary immunodeficiency diseases. Annals of the New York Academy of Sciences. 2011;1246:1–10. doi: 10.1111/j.1749-6632.2011.06347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffre E, Casellas R, Nussenzweig MC. Antibody regulation of B cell development. Nature immunology. 2000;1:379–385. doi: 10.1038/80816. [DOI] [PubMed] [Google Scholar]
- Menard L, Cantaert T, Chamberlain N, Tangye SG, Riminton S, Church JA, Klion A, Cunningham-Rundles C, Nichols KE, Meffre E. Signaling lymphocytic activation molecule (SLAM)/SLAM-associated protein pathway regulates human B-cell tolerance. The Journal of allergy and clinical immunology. 2014;133:1149–1161. doi: 10.1016/j.jaci.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard L, Saadoun D, Isnardi I, Ng YS, Meyers G, Massad C, Price C, Abraham C, Motaghedi R, Buckner JH, et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. The Journal of clinical investigation. 2011;121:3635–3644. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers G, Ng YS, Bannock JM, Lavoie A, Walter JE, Notarangelo LD, Kilic SS, Aksu G, Debre M, Rieux-Laucat F, et al. Activation-induced cytidine deaminase (AID) is required for B-cell tolerance in humans. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11554–11559. doi: 10.1073/pnas.1102600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nature reviews. Immunology. 2006;6:728–740. doi: 10.1038/nri1939. [DOI] [PubMed] [Google Scholar]
- Ng YS, Wardemann H, Chelnis J, Cunningham-Rundles C, Meffre E. Bruton's tyrosine kinase is essential for human B cell tolerance. The Journal of experimental medicine. 2004;200:927–934. doi: 10.1084/jem.20040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- Perez MA, Gordon A, Sanchez F, Narvaez F, Gutierrez G, Ortega O, Nunez A, Harris E, Balmaseda A. Severe coinfections of dengue and pandemic influenza A H1N1 viruses. The Pediatric infectious disease journal. 2010;29:1052–1055. doi: 10.1097/INF.0b013e3181e6c69b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell death and differentiation. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- Qin H, Suzuki K, Nakata M, Chikuma S, Izumi N, Huong le T, Maruya M, Fagarasan S, Busslinger M, Honjo T, Nagaoka H. Activation-induced cytidine deaminase expression in CD4+ T cells is associated with a unique IL-10-producing subset that increases with age. PloS one. 2011;6:e29141. doi: 10.1371/journal.pone.0029141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada C, Jarvis JM, Milstein C. AID-GFP chimeric protein increases hypermutation of Ig genes with no evidence of nuclear localization. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7003–7008. doi: 10.1073/pnas.092160999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radic MZ, Weigert M. Origins of anti-DNA antibodies and their implications for B-cell tolerance. Annals of the New York Academy of Sciences. 1995;764:384–396. doi: 10.1111/j.1749-6632.1995.tb55853.x. [DOI] [PubMed] [Google Scholar]
- Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- Rolink A, Melchers F, Andersson J. The SCID but not the RAG-2 gene product is required for S mu-S epsilon heavy chain class switching. Immunity. 1996;5:319–330. doi: 10.1016/s1074-7613(00)80258-7. [DOI] [PubMed] [Google Scholar]
- Romberg N, Chamberlain N, Saadoun D, Gentile M, Kinnunen T, Ng YS, Virdee M, Menard L, Cantaert T, Morbach H, et al. CVID-associated TACI mutations affect autoreactive B cell selection and activation. The Journal of clinical investigation. 2013;123:4283–4293. doi: 10.1172/JCI69854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Novak U, Piovan E, Basso K, Sumazin P, Schneider C, Crespo M, Shen Q, Bhagat G, Califano A, et al. BCL6 suppression of BCL2 via Miz1 and its disruption in diffuse large B cell lymphoma. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11294–11299. doi: 10.1073/pnas.0903854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer AV, Morbach H, Brigida I, Ng YS, Aiuti A, Meffre E. Defective B cell tolerance in adenosine deaminase deficiency is corrected by gene therapy. The Journal of clinical investigation. 2012;122:2141–2152. doi: 10.1172/JCI61788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz DG, Swanson PC. V(D)J recombination: mechanisms of initiation. Annual review of genetics. 2011;45:167–202. doi: 10.1146/annurev-genet-110410-132552. [DOI] [PubMed] [Google Scholar]
- Schickel JN, Pasquali JL, Soley A, Knapp AM, Decossas M, Kern A, Fauny JD, Marcellin L, Korganow AS, Martin T, Soulas-Sprauel P. Carabin deficiency in B cells increases BCR-TLR9 costimulation-induced autoimmunity. EMBO molecular medicine. 2012;4:1261–1275. doi: 10.1002/emmm.201201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. Journal of immunology. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- Smith KG, Weiss U, Rajewsky K, Nossal GJ, Tarlinton DM. Bcl-2 increases memory B cell recruitment but does not perturb selection in germinal centers. Immunity. 1994;1:803–813. doi: 10.1016/s1074-7613(94)80022-7. [DOI] [PubMed] [Google Scholar]
- Swaminathan S, Klemm L, Park E, Papaemmanuil E, Ford A, Kweon SM, Trageser D, Hasselfeld B, Henke N, Mooster J, et al. Mechanisms of clonal evolution in childhood acute lymphoblastic leukemia. Nature immunology. 2015;16:766–774. doi: 10.1038/ni.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa M, Tolarova H, Li Z, Dubois W, Lim S, Callen E, Franco S, Mosaico M, Feigenbaum L, Alt FW, et al. AID expression levels determine the extent of cMyc oncogenic translocations and the incidence of B cell tumor development. The Journal of experimental medicine. 2008;205:1949–1957. doi: 10.1084/jem.20081007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AG, Lu H, Raghavan SC, Muschen M, Hsieh CL, Lieber MR. Human chromosomal translocations at CpG sites and a theoretical basis for their lineage and stage specificity. Cell. 2008;135:1130–1142. doi: 10.1016/j.cell.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Liao D, Yang K, Patel A, Kelsoe G. T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. Journal of immunology. 2007;178:3593–3601. doi: 10.4049/jimmunol.178.6.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umiker BR, McDonald G, Larbi A, Medina CO, Hobeika E, Reth M, Imanishi-Kari T. Production of IgG autoantibody requires expression of activation-induced deaminase in early-developing B cells in a mouse model of SLE. European journal of immunology. 2014;44:3093–3108. doi: 10.1002/eji.201344282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora GD, Dominguez-Sola D, Holmes AB, Deroubaix S, Dalla-Favera R, Nussenzweig MC. Identification of human germinal center light and dark zone cells and their relationship to human B-cell lymphomas. Blood. 2012;120:2240–2248. doi: 10.1182/blood-2012-03-415380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikstrom I, Carotta S, Luthje K, Peperzak V, Jost PJ, Glaser S, Busslinger M, Bouillet P, Strasser A, Nutt SL, Tarlinton DM. Mcl-1 is essential for germinal center formation and B cell memory. Science. 2010;330:1095–1099. doi: 10.1126/science.1191793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Zaheen A, Boulianne B, Parsa JY, Ramachandran S, Gommerman JL, Martin A. AID constrains germinal center size by rendering B cells susceptible to apoptosis. Blood. 2009;114:547–554. doi: 10.1182/blood-2009-03-211763. [DOI] [PubMed] [Google Scholar]
- Zahn A, Eranki AK, Patenaude AM, Methot SP, Fifield H, Cortizas EM, Foster P, Imai K, Durandy A, Larijani M, et al. Activation induced deaminase C-terminal domain links DNA breaks to end protection and repair during class switch recombination. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E988–997. doi: 10.1073/pnas.1320486111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.