Abstract
Endocannabinoid (eCB) signaling plays an important role in the stress response pathways of the mammalian brain, yet its role in the avian stress response has not been described. Understanding eCB signaling in avian species (such as the European starling, Sturnus vulgaris) allows a model system that exhibits natural attenuation of hypothalamic-pituitary-adrenal (HPA) responsiveness to stressors. Specifically, seasonally breeding birds exhibit the highest HPA activity during the breeding season and subsequently exhibit a robust HPA down-regulation during molt. Because eCB signaling in mammals has an overall inhibitory effect on HPA activity, we expected shifts in eCB signaling to regulate the seasonal HPA down-regulation during molt. However, our data did not support a role for eCB signaling in the molt-related suppression of HPA activity. For example, injection of the cannabinoid receptor (CB1) antagonist, AM251, did not potentiate molt-suppressed HPA activity. Instead, our data suggest eCB regulation of HPA plasticity as birds transition from breeding to molt. In support of this hypothesis, birds in the late breeding season demonstrated a more dynamic response at the level of avian amygdala eCB content in response to acute stress. The response and directionality of this effect match that seen in mammals. Overall, our data suggest that eCB signaling may allow for a dynamic range in HPA responsiveness (eg, breeding), but the signaling pathway's role may be limited when the HPA response is restrained (eg, molt). This first characterization of eCB signaling in the avian stress response also emphasizes that although the system functions similarly to other species, its exact role may be species specific.
The dynamics of the stress response in a wild animal reflect a balance between the need to respond appropriately to a stressor for immediate survival and the need to maintain other physiological and behavioral processes for long-term fitness. One hallmark of the stress response is the elevation of circulating glucocorticoids (predominantly corticosterone [CORT] in birds) after the stimulation of the hormonal cascade along the hypothalamic-pituitary -adrenal (HPA) axis. Circulating glucocorticoids can affect a range of processes in the body, including reproduction, immunity, and metabolism (1). Whereas connections with these other physiological systems may be adaptive in a time scale of acute stress, overstimulation and chronic stress can lead to maladaptive, pathological conditions (2).
To preserve the balance between immediate survival and long-term fitness, seasonally breeding animals show distinct phases of HPA responsiveness according to their life history stages. In avian species, the most dramatic shift in HPA responsiveness occurs between the breeding season and prebasic molt, when birds will shed and replace their feathers and entire integument. In the breeding season, the HPA axis is at its most responsive (highest baseline and highest stress induced CORT release), whereas during molt the HPA is at its least responsive (lowest baseline and stress induced CORT release) (3). Breeding birds may benefit from exhibiting greater HPA plasticity as suggested from data correlating a more dynamic range in HPA responsiveness during the breeding season with increased individual fitness (4). In contrast, during molt, birds benefit from maintaining a reduced range of HPA responsiveness because elevated CORT can impact feather quality (5) and behavior (6).
Molt presents an interesting model for understanding the value of depressing the HPA axis. Feather building is both energetically expensive and complex (7). The structural complexity of each feather has direct ties to the health and survival of the individual (eg, see reference 8) making the quality of regrown feathers of primary importance when birds undergo molt. Because glucocorticoids can affect protein deposition, it has been suggested that the down-regulation of the HPA axis during this life history stage is a critical component to ensuring the growth of high-quality feathers (5, 9). Similarly, active behavior stimulated by increased HPA activity may be disadvantageous for the energy-limited, feather-growing birds (10). The tradeoff between the preservation of molt vs the ability to respond to stress is reflected in the observation that most seasonal avian species exhibit both suppressed baseline and stress-induced glucocorticoid secretion while undergoing molt (10).
Between breeding and molt, birds, sometimes within a matter of weeks, can exhibit up to 75% reductions in baseline and stress-induced glucocorticoid release (3). Therefore, the transition point between late breeding season and molt can serve as a model for studying dynamic mechanisms of HPA axis plasticity. Here we focus on the potential mechanisms regulating the shift in the HPA axis from a dynamic and highly responsive system to a limited and less responsive system.
One mechanism that may play a role in regulating the transition to HPA nonresponsiveness during molt is the endocannabinoid (eCB) signaling pathway operating in the brain, particularly in limbic structure such as the hippocampus, amygdala, and hypothalamus. In mammals, eCB signaling within these brain regions plays a role in regulating HPA activity (as reviewed in reference 11). The primary role of central eCB signaling is to modulate synaptic transmission and allow for rapid synapse-specific plasticity (12). This action occurs predominantly through the retrograde signaling actions of the two primary eCBs 2-arachidonoylglycerol (2-AG) and anandamide (AEA). Both AEA and 2-AG are believed to be synthesized on demand in the postsynaptic neuron through the cleavage of membrane phospholipids (13). These signaling lipids then travel back across the synapse to bind cannabinoid 1 receptor (CB1) receptors located on the presynaptic neuron, in which they suppress subsequent neurotransmitter release into the synapse. Through this mechanism, the postsynaptic cell can effectively regulate its own afferent stimulation or inhibition by controlling eCB content through synthesis, uptake, and degradation. eCB signaling primarily acts to constrain activation of the HPA axis with eCBs in the amygdala acting as a tonic suppression over the activation of the HPA axis and the stress response, whereas eCBs in the hypothalamus and prefrontal cortex facilitate negative feedback on the axis (11). Considering this role, we hypothesized that eCB signaling is a prime candidate for regulating the seasonal transition in birds.
Because eCB signaling in the avian stress response has yet to be demonstrated, the first aim of this study was to describe the presence and functionality of this system in a wild songbird species. From there, we investigated the potential role for eCB signaling in the modulation of seasonal fluctuations in HPA activity. We predicted that eCB signaling would be present and functioning in the avian brain and that it would play a critical role in the suppression of HPA activity when birds transition into molt.
Materials and Methods
All procedures complied with the University of California Office of Laboratory Animal Care and were approved by the University's Animal Care and Use Committee.
Verification of eCB signaling in the wild avian brain
Gene expression
To demonstrate the presence of specific genes critical to the eCB signaling pathway in the European starling (Sturnus vulgaris) brain, we cloned the CB1 as well as enzymes for ligand synthesis and degradation: fatty acid amide hydrolase (FAAH; metabolizes AEA), and diacylglycerol lipase-α (DAGLα; synthesis of 2-AG).
For CB1, cDNA was synthesized from dissected hypothalamic tissue, and for FAAH and DAGLα, cDNA was synthesized from pooled tissue (obtained from both male and female starlings) dissected from the hypothalamus, hippocampus, and arcopallium. Our cloning protocol, as previously described (14) isolated the genes of interest using primers designed from similar species. For CB1, we designed primers from the zebra finch (Taeniopygia guttata) CB1 mRNA sequence (GenBank accession number NM_001048262). FAAH primers were designed from the zebra finch FAAH mRNA sequence (GenBank accession number XM_002195861.1). DAGLα primers were designed from a zebra finch DAGLα mRNA sequence (GenBank accession number XM_002195915). The resulting PCR products were sequenced at the University of California, Berkeley, DNA Sequencing Facility (Berkeley, California,), and all sequences were compared with the GenBank to find positive matches with similar sequences and determine the percentage of positive identities.
Localization of CB1 expression
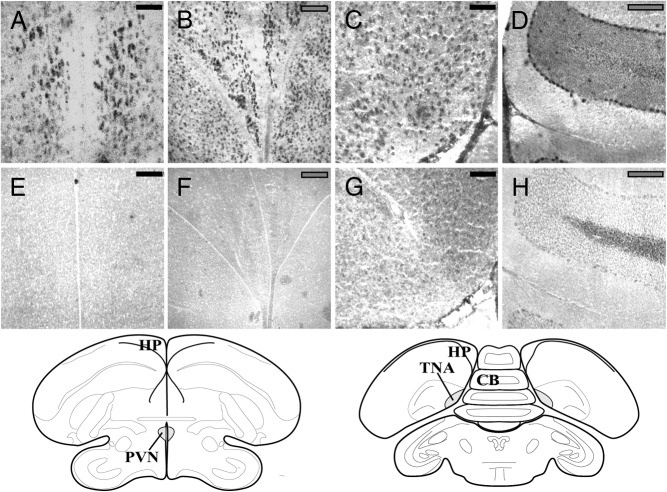
To visualize the location of CB1 expression in the wild avian brain, we used in situ hybridization (ISH) following the protocol described elsewhere (15) with minor modification. Three starlings were used for the visualization. Male and female starlings were shot locally as part of a pest mitigation program run by the US Department of Agriculture. Brains were then removed rapidly (within ∼2 min of death) and were immediately snap frozen on dry ice. The samples were then stored at −80°C until processing. At that time, the brains were sectioned coronally at 20 μm on a cryostat and thaw mounted onto gelatin-coated slides. The slides were again stored at −80°C. Sections on slides were fixed with 4% 0.1 M paraformaldyde solution immediately prior to the ISH. Using a 169-bp section of the cloned CB1 precursor as a template (Figure 1A), we made a digoxigenin (DIG)-labeled RNA antisense probe using a RNA labeling kit according to the manufacturer's instructions (Roche Applied Science). The sections were hybridized with the probes at 50°C overnight (16–20 h). after the wash steps, a labeled sheep anti-DIG antibody (Roche Applied Science) was added to the sections, and the immunoreactive product was visualized using a solution containing nitro blue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate (Roche Applied Science). Specificity of the ISH was confirmed by using a DIG-labeled sense RNA probe (Figure 1B).
Figure 1.
Representative photographs of brain regions containing cells positively expressing the CB1 receptor mRNA (top panel) or nonbinding of the sense probe in negative controls (bottom panel). Expression can be observed in the PVN (A), the hippocampus (HP) (B), TnA (C), and cerebellum (CB) (D) with respective negative controls shown in panels E–H. Scale bars are indicated in top right corner of each photograph. Black bars indicate 100 μm, and gray bars indicate 200 μm. Complementary regions as adapted from the canary stereotaxic atlas (44) are shown below the panels. [Adapted from T. Stokes et al: The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J Comp Neurol. 1974;156(3):337–374 (44), with permission. © 1974 The Wistar Institute of Anatomy and Biology]
Manipulation of CB1 signaling
CB1 signaling has been demonstrated to have a predominantly inhibitory effect over HPA activity. To test the effect of eCB signaling in the avian brain on the HPA axis, we conducted a dose-response test with a commonly used potent CB1 antagonist, AM251 (Tocris Biosciences). This has been used successfully in several species (16, 17), including avian species (18). AM251 was dissolved in dimethylsulfoxide (DMSO) and diluted into three doses (5, 1, and 0.2 mg/kg) using a 1:1:8 ratio of AM251 to DMSO to Tween 80 to 0.9% sterile saline. The vehicle control contained only DMSO in place of the AM251/DMSO solution. Dilutions were freshly made each morning before the injection.
A total of 15 wild-caught male European starlings (Sturnus vulgaris) that had been housed in a large (12 × 6 × 3.5 m) outdoor flight aviary and provided with food and water ad libitum for 10 months prior to the experiment were tested between May 1 and May 4, the end of the breeding season. Birds were housed by single sex but had visual and auditory access to females in an adjacent aviary. Birds were divided between four doses: 0 mg/kg, vehicle control (n = 3), 0.2 mg/kg (n = 4), 1 mg/kg (n = 4), and 5 mg/kg (n = 4). Birds were captured in the flight aviaries using hand nets between 9:00 and 11:00 am, a blood sample was taken immediately by puncturing the brachial vein, and the given dose of AM251 was administered in the pectoral muscle. The average time taken from the moment the aviary was entered until blood samples and injections were completed was 2.6 minutes (median 2.5) with only two birds taking 4 minutes to complete.
Immediately after the injection, the birds were placed inside an opaque bag to induce acute restraint stress. Additional blood sampling occurred at 10, 30, and 60 minutes similar to the timing used in another report (16). Blood was kept on ice until the plasma was separated (1500 × g for 10 min at 4°C), at which point the plasma was frozen at −20°C until assayed.
Seasonal changes in eCB signaling
Pharmacological study
To assess the potential shifts in CB1 signaling during the transition between the breeding season and molt, we sampled a total of 14 starlings (n = 7 for both vehicle and 5 mg/kg of AM251) in a repeated-measures design. Two samples were obtained: the first, at the end of breeding season (May 1 through May 16, including three birds from each group tested during the dose response test), and the second, when the individuals were molting (June 18 through June 21). Molt was determined by visual inspection and all birds showed signs of molt at least up to their third primary feather (starlings molt nine primary feathers sequentially, so they were approximately 33% through molt).
The injection method was identical with that used for the dose-response test. Only the highest dose (5 mg/kg) was used along with the vehicle control. Both solutions were made up fresh immediately prior to the experiment using the same method and proportions reported above, and injections were given im into the pectoral muscle. Sampling methods also remained the same as those used for the dose response in that birds were sampled for baseline within 3 minutes of entering the aviary, injected with either the vehicle or AM251 and then subjected to an acute restraint stress for 60 minutes during which individuals were sampled at 10, 30, and 60 minutes. Blood was kept on ice until plasma was separated (1500 × g for 10 min at 4°C) at which point the plasma was frozen at −20°C until assayed.
Biochemical analysis
To evaluate the eCB response to seasonal changes and to stress, we collected brain tissue from a total of 28 birds (n = 7 or 8 per group) over 2 days during either the late breeding season (mid-May) or midmolt (early July). Birds were captured in the flight aviary and either sampled immediately (baseline) or put into an opaque bag for a restraint stress for 30 minutes. All birds were killed with an overdose of isoflurane, decapitated, and brains rapidly dissected out of the skull and immediately frozen on dry ice. For baseline samples, the process, from entering the aviary to freezing the brains, was accomplished within 5 minutes. Due to the rapid sampling required to avoid stress effects and achieve a true baseline sample, we do not expect alterations occurred in response to anesthesia either (exposure < 45 sec). Trunk blood was collected and plasma separated for CORT analysis..
Brains were later sliced (50 μm) and punched to isolate regions of interest including the avian amygdala, hypothalamus, and hippocampus (described in reference 19). For the region we considered the avian amygdala, dissected tissue included portions of the arcopallium from the nucleus taenaie of the amygdala (TnA) through the predicted location of the posterior pallial amygdala (PoAc). With the current understanding, these regions of the arcopallium, from the lateral bed nucleus stria terminalis to the PoAc are likely limbic in nature (20). Specifically, the PoAc has been described in pigeons as a potential functional equivalent to the mammalian basolateral amygdala, but its specific location in songbirds is still unidentified (personal communication with J. M. Wild and M.J.D.). Therefore, we took a conservative approach with our sample collection.
For the quantification of AEA and 2-AG, samples underwent a lipid extraction as previously described (21). In brief, tissue samples were weighed and placed into borosilicate glass culture tubes containing 2 mL of acetonitrile with 5 pmol of [2H8]anandamide and 5 nmol of [2H8]2-arachidonoylglycerol, homogenized with a glass rod, and sonicated for 30 minutes on ice. Samples were incubated overnight at −20°C to precipitate proteins and were then centrifuged at 1500 × g to remove particulates. The supernatants were removed to a new glass tube and evaporated to dryness under N2 gas. The samples were resuspended in 300 μL of acetonitrile to recapture any lipids adhering to the glass tube and dried again under N2 gas. Finally, lipid extracts were suspended in 20 μL of acetonitrile and stored at −80°C until analysis. Lipid containing acetonitrile samples were then subjected to analysis using liquid chromatography and tandem mass spectrometry as previously described (21).
Gene expression
Two additional sets of male birds were collected locally as described above. The first set was collected in the late breeding season (n = 6), and the second set was collected during molt (n = 6). Gonadal size (see reference 22) and molt status were visually confirmed to be appropriate for the season. Brains were collected immediately and frozen on dry ice. Brains were sectioned on a cryostat (50 μm) and punched as described above. For the arcopallium and hypothalamus, alternate punches, using both sides of the brain, were acquired for two separate analyses; every other punch went toward RNA extraction to quantify expression or the CB1 binding assay (see below).
Extraction, reverse transcription, and quantitative PCR analysis were conducted as described elsewhere (14, 19). Primer sequences for CB1, FAAH, and DAGL were based on the cloning results described above (see Supplemental Table 3 for primer combinations used). For controls, we used GAPDH and 18S and calculated a geometric mean for the individual values. The 18S-GAPDH geometric mean did not change with the season. For analysis, a detection limit was set by using the treatment group with the lowest mean and determining the value 1 SD below that mean (see reference 14). Using this value, we calculated the fold change in expression for that specific gene. We used these values for statistical analysis.
CB1 receptor binding assay
In the samples collected as described above, we performed radioligand CB1 receptor binding assays as previously described (23). To perform CB1 receptor binding assays, we prepared membranes from the dissected brain regions. Specifically, isolated brain regions were homogenized in 20 volumes of a buffer of 50 mM Tris HCl, pH 7.4; 1 mM EDTA; and 3 mM MgCl2 (TME). Homogenates were centrifuged at 18 000 × g for 20 minutes, and the resulting pellet, which contains a crude membrane fraction, was resuspended in 20 volumes of TME buffer. Protein concentrations were determined by the bicinchoninic assay method using a commercially available kit (Pierce Biotechnology).
CB1 receptor agonist binding parameters were determined using a radioligand binding using a multiscreen filtration system with Durapore 1.2-μM filters in 96-well filter plates (Millipore). Incubations (total volume 0.2 mL) were carried out using the TME buffer containing 1 mg/mL BSA. Membranes (10 μg protein per incubate) were added in triplicate to wells containing 0.1, 0.25, 0.5, 1.0, 1.5, or 2.5 nM [3H]CP 55940 (American Radiochemicals), a cannabinoid CB1 receptor agonist, and incubated for 60 minutes at room temperature on an orbital shaker. Ten micromoles of AM251 (Tocris Biosciences) was used to determine nonspecific binding. The maximal binding site density and binding affinity values were determined by a nonlinear curve fitting of specific binding data to the single site binding equation using GraphPad Prism.
CORT assay
For all experiments analyzing CORT, we used a commercially available CORT enzyme immunoassay (Cayman Chemical) thoroughly described elsewhere (19). Samples were run in duplicate and randomly divided between one of two plates. The intraassay coefficient of variability was less than 7.5% and the coefficient of variation between plates was less than 2%.
Data analysis
For the dose-response test, changes in CORT concentrations across time were analyzed as a two-way, repeated-measures ANOVA with dose and time as the independent variables; the total CORT released after the injection was evaluated by determining the individual integrated CORT measurements (24) and was analyzed with a one-way ANOVA. For the seasonal comparison, individual integrated CORT was calculated in a repeated-measures, two-way ANOVA with subjects matched between seasons with dose and season as the independent variables. Seasonal content data were analyzed via a two-way ANOVA with season and treatment (stress or baseline) as variables. To compare content with individual CORT concentrations, a least squares fit model was run for both ligands with treatments separated and season and CORT linked in the regression using JMP Pro software (version 11.0; 2013; SAS Institute). Seasonal binding and gene expression were evaluated with one-way ANOVAs. Measurements were analyzed using Prism, version 5.0c (2009; GraphPad Software, Inc) unless otherwise specified.
Results
Verification of eCB signaling in the wild avian brain
Partial cloning of CB1, FAAH, and DAGLα
For CB1, we were able to identify a partial 381-bp precursor sequence. FAAH cloning yielded a partial 980-bp precursor sequence, and DAGLα cloning yielded a partial 387-bp precursor sequence (Supplemental Table 1 in the Supplemental Material). All sequences have been accepted into GenBank (CB1: number KR425484; FAAH: number KR425485; DAGLα: number KR425486).
Comparing these partial sequences with sequences available on GenBank, we were able to determine the degree of homology (as percentage of identities, matching nucleotides in the sequence) between the starling nucleotide sequence and that of other species (see Supplemental Table 2 in the Supplemental Material). In addition, by translating the nucleotide sequence into a predicted amino acid sequence, we were also able to assess the percentage of identities (amino acids) that matched in other species (Table 2). Each transcribed gene showed varying degrees of homology; CB1 appears to be the most conserved showing homology with humans and rats of greater than 80% for nucleotide identities and greater than 94% for predicted amino acid sequences.
Localization of CB1 expression
In the field-collected starlings, CB1 appears to be expressed in several brain regions predicted to have a role in the avian stress response. We found clear expression patterns in the paraventricular nucleus of the hypothalamus (PVN), the hippocampus, the TnA (Figure 1, A–C). In addition, we saw weaker but evident expression in the lateral part of the bed nucleus of the stria terminalis and the nidopallium caudolaterale, and we found evidence of expression in a region we consider to be the PoAc. As a negative control, we found no evidence of sense probe binding in the concurrent sections (eg, Figure 1, E–G). As a positive control, CB1 expression appears in the cerebellum in a pattern consistent with that demonstrated for immunoreactivity of CB1 protein (25).
Manipulation of CB1 signaling
An im injection of AM251, a CB1 antagonist, had a significant effect on CORT release in the semiwild birds. Comparing means across time (Figure 2A), acute stress resulted in an expected increase in CORT (time: F3,33 = 40.15, P < .0001). The increasing doses of AM251 also had a significant effect on CORT concentrations (dose: F3,33 = 3.75, P = .02) but did not affect how CORT increased across time (time dose interaction: F9,33 = 0.97, P = .48). Integrated CORT concentrations were significantly affected by the dose of AM251 (F3,11 = 4.03, P = .036) (Figure 2B), with a significantly higher concentration when comparing the highest dose, 5 mg/kg, with the vehicle injection (P < .05, post hoc Dunnett's test).
Figure 2.
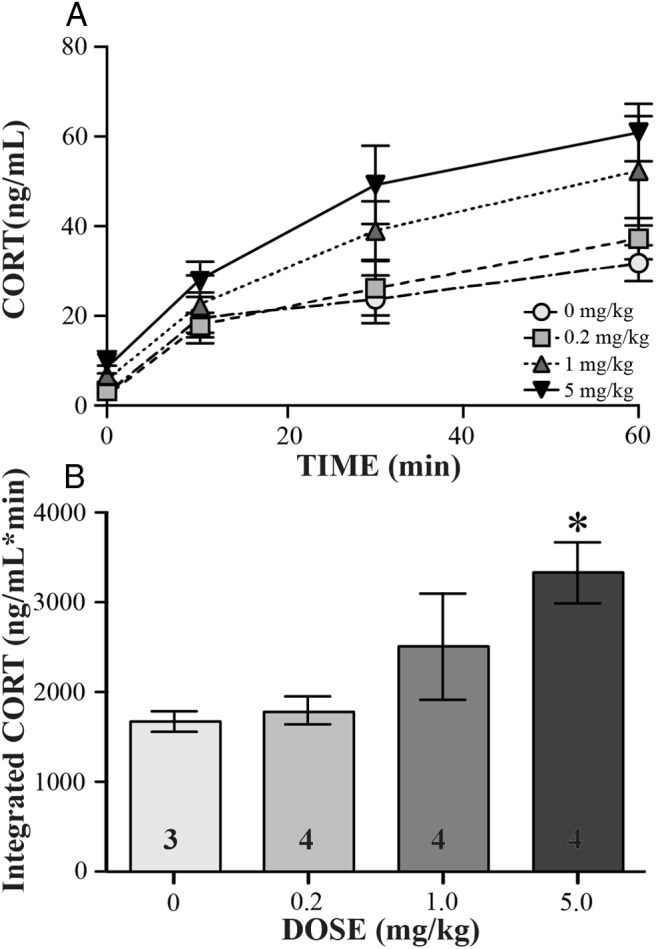
Dose-response curve for CB1 antagonist, AM251. Data are indicated as mean ± SE. Across bleed times (A) and as total (B), integrated CORT released during sampling period is shown. In panel B, the asterisk indicates significance as determined by a post hoc Dunnett's test (P < .05), and sample sizes are indicated as numbers within the bars.
Changes in HPA activity are reflected in eCB signaling pathways
Pharmacological study
In the semiwild birds, AM251 injection and season influenced stress-induced CORT concentrations, although it appears that the effect of AM251 decreased during molt. Comparing the changes in integrated CORT (Figure 3B), birds during breeding season had higher overall concentrations as compared with the molting birds (seasonal effect: F1,12 = 8.95, P = .011), birds injected with AM251 had higher concentrations of integrated CORT than noninjected controls (treatment: F1,12 = 6.18, P = .029), but there was no season treatment interaction (F1,12 = 0.58, P = .46). For the breeding season samples, the AM251-injected group had significantly higher integrated CORT compared with the vehicle-injected group (P < .05, Bonferroni post hoc), but the molting birds did not show a significant difference between AM251 and the vehicle-injected birds. In addition, the AM251-injected birds had higher CORT in the breeding season as compared with the same group during molt (P < .05, Bonferroni post hoc).
Figure 3.
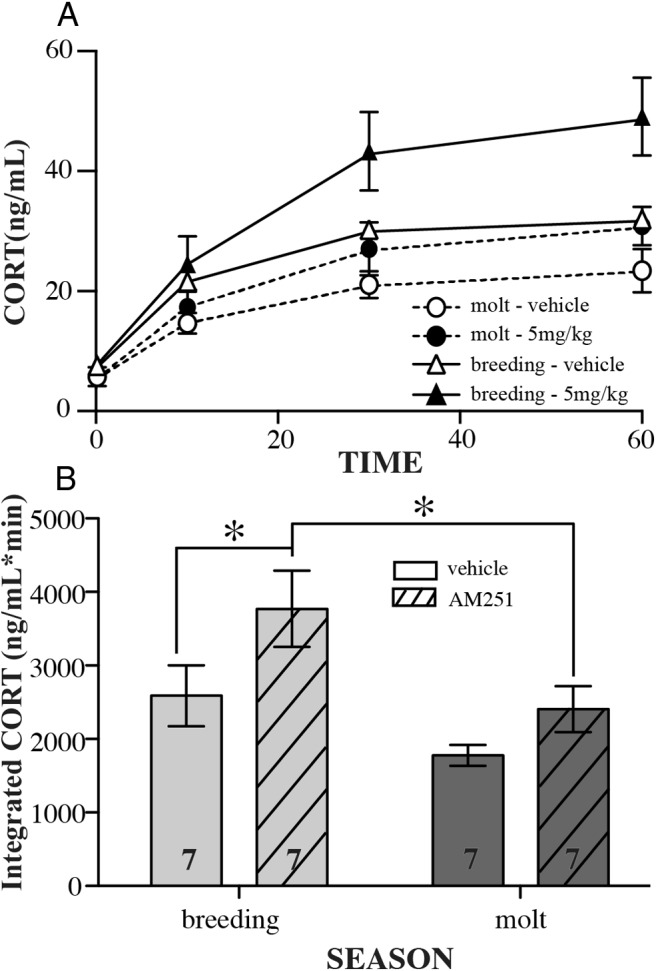
Seasonal responses (late breeding vs molt) to CB1 antagonist injection. Data are indicated as mean ± SE. Across bleed times (A) and as total (B), integrated CORT released during sampling period is shown. Data represent repeated measures between seasons because the same individuals were given the same injection dose during both breeding and molt. Open columns represent vehicle-injected birds, whereas hashed columns represent antagonist AM251-injected birds. In panel B, asterisks indicate significance as determined by a post hoc Bonferroni test (P < .05), and sample sizes are indicated as numbers within the columns.
Biochemical analysis
Tissue content of endocannabinoid ligands was affected by stress and season in a brain region-specific manner in the semiwild birds.
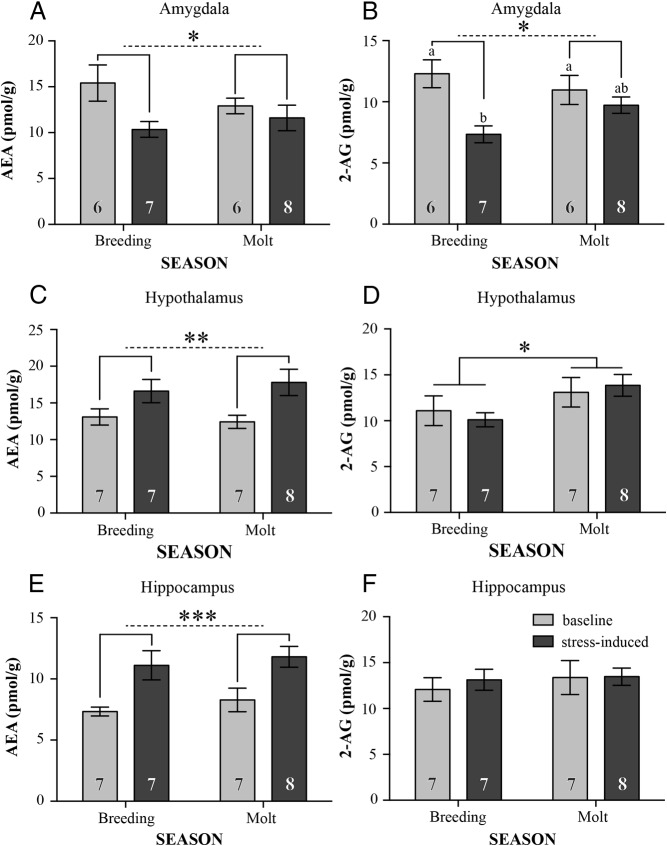
In the avian amygdala samples, (Figure 4A), birds exposed to acute stress exhibited significantly lower AEA concentrations (stress: F1,23 = 5.54, P = .03), but there were no significant effects of season (F1,23 = 0.26, P = .65) or a season stress interaction effect (F1,23 = 1.92, P = .18). For 2-AG in the avian amygdala samples (Figure 4B), birds exposed to acute stress exhibited significantly lower 2-AG concentrations (stress: F1,23 = 11.63, P = .002), and this response was decreased during molt (season stress interaction: F1,23 = 4.15, P = .05). Specifically, during the breeding season, acutely stressed birds had significantly lower 2-AG concentrations (P < .01, Tukey's post hoc), and the stressed group during the breeding season had significantly lower 2-AG than the stressed group during molt (P < .05). There were no significant differences for overall concentrations between seasons (F1,23 = 1.921, P = .18). Comparing individual baseline CORT concentrations with each ligand demonstrated a negative correlation between baseline CORT and AEA (R2 = 0.937, F3,9 = 29.81, P < .0005, Figure 5A). This effect was driven by season (t = −6.53, P = .0006) and CORT (t = −9.44, P < .0001), with a significant season CORT interaction (t = 4.70, P = .003). For baseline CORT vs 2-AG in the avian amygdala, there was no significant directionality (R2 = 0.20, F3,9 = 0.59 P = .64, Figure 5B). Correlations between stress-induced CORT concentrations and ligand levels did not show significant directionality (R2 < 0.25, F3,8 < 1.09, P > .4). We should note that our measurements may include changes in eCB content and expression in the song system because the robust nucleus of the arcopallium could not be isolated from the punched areas. Because we would expect such changes in the song nucleus to occur only at the baseline level, not dynamically in response to an acute stress on the time scale of our measurement, we believe that including portions of the robust nucleus of the arcopallium in our sample did not affect the results.
Figure 4.
Changes in tissue content between seasons (late breeding and molt) in response to acute stress. Data are indicated as mean ± SE. In the amygdala both AEA (A) and 2-AG (B) showed a significant effect of treatment, with 2-AG also exhibiting a significant treatment season interaction and post hoc tests indicating a significant difference between baseline and stress-induced concentrations. In the hypothalamus only, AEA showed a significant effect of treatment(C), whereas 2-AG showed a significant effect of season (D). In the hippocampus, AEA showed a significant effect of treatment (E), whereas 2-AG showed no changes (F). A single asterisk signifies P < .05, double asterisk signifies P < .01, and triple asterisk signifies P < .001. Post hoc Bonferroni test significance (P < .05) is shown as differing letters. Sample sizes are indicated as numbers within the columns. Light gray bars indicate baseline samples, and dark gray bars indicate stress-induced samples.
Figure 5.
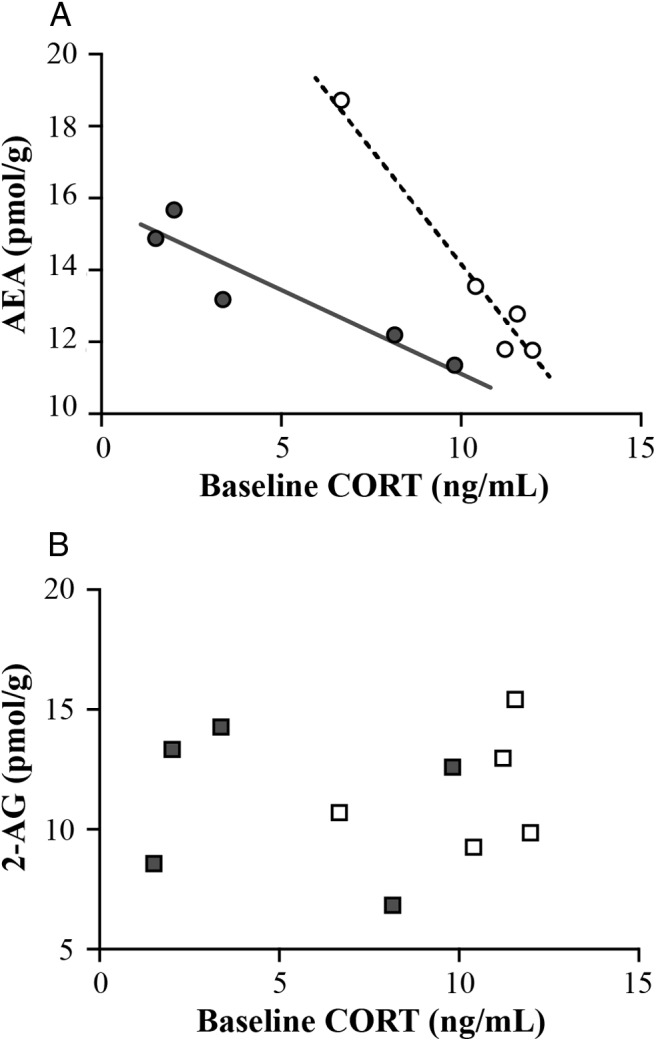
Changes in baseline tissue content in the amygdala vs baseline CORT concentrations. Individual data points exhibited a significantly negative correlation between baseline CORT and AEA (R2 = 0.937 P < .0005) that was driven by season (t = −6.53, P = .0006) and CORT (t = −9.44, P < .0001) A) with a significant season CORT interaction (t = 4.70, P = .003). B, Baseline CORT vs 2-AG. Open shapes represent individuals during breeding season, whereas closed shapes represent individuals during molt.
In the hypothalamus, for AEA (Figure 3C), concentrations were significantly higher in the acutely stressed group than controls (stress: F1,25 = 11.63, P = .002), regardless of season (season: F1,25 = 0.34, P = .57; season treatment interaction: F1,25 = 0.52, P = .42). For 2-AG in the hypothalamus samples (Figure 4D), concentrations were significantly higher in the molt group compared with the breeding season group (season: F1,25 = 4.67, P = .04). There were no significant effects of stress (stress: F1,25 = 0.007, P = .93; season stress interaction: F1,25 = 0.52, P = .43). Correlations between CORT and ligand concentrations in the hypothalamus did not show significant directionality (R2 < 0.38, F3,12 < 1.86, P > .19).
In the hippocampus, for AEA (Figure 4E), concentrations were significantly higher in the acutely stressed group than controls (F1,25 = 16.66, P = .0004), regardless of season (season: F1,25 = 0.84, P = .37; season treatment interaction: F1,25 = 0.02, P = .13). For 2-AG in the hippocampus samples (Figure 3F), there were no significant effects (F1,25 < 0.67, P > .0.13). Correlations between CORT and ligand concentrations in the hippocampus did not show significant directionality (R2 < 0.40, F3,14 < 2.49, P > .11).
Gene expression
In wild starlings, control genes did not change between seasons in any of the brain regions studied (t < 1.18, P > .26), so individual values were calculated relative to the control gene (18S-GAPDH) expression. Overall, there were no changes in expression for any of the genes measured (CB1, DAGL, or FAAH) in the avian amygdala (t < 1.46, df = 12, P > .17), hypothalamus (t < 1.28, df = 12, P > .22), or hippocampus (t < 0.89, df = 11, P > .39). See Supplemental Table 5 for summary of statistical results.
Ligand binding
There were no seasonal effects on ligand binding dynamics, binding affinity, or maximal binding site density in the amygdala (t < 1.08, df = 15, P > .29) or hypothalamus (t < 1.55, df = 11, P > .15) in the wild-caught birds. See Supplemental Table 4 for summary of statistical results.
Discussion
A wild avian model allows direct investigation of naturally driven shifts in HPA responsiveness, and with this model, we are able to show a potential, novel role for eCB signaling in HPA plasticity. Specifically, we observed changes in the dynamics of eCB signaling in response to stress when comparing birds in breeding (high HPA activity) and molting (suppressed HPA activity) condition. In addition, comparing similarities and differences between the avian and mammalian systems provides novel insight into how the pathway functions. As predicted, birds appear to use eCB signaling in the stress response pathways similarly to mammals. However, although functionality appears to be conserved in these different vertebrate classes, specific roles for 2-AG and AEA, in relation to HPA regulation, may differ between species.
Role of endocannabinoid signaling in HPA plasticity
As discussed in the introductory text, molt in avian species represents a key life history stage in which HPA axis stimulation can become detrimental to long-term survival and overall fitness. As a result, nearly all seasonally breeding birds show a strong suppression of HPA activity during molt (5, 10). In contrast, during the breeding season, birds maintain the capacity to respond appropriately to stressors and benefit from exhibiting a dynamic range in HPA responsiveness (26).
eCB signaling is generally inhibitory on the mammalian HPA (27); therefore, we had originally predicted that changes in eCB signaling contributed to seasonal HPA suppression in molting birds. Contrary to our prediction, whereas CB1 receptor antagonism did significantly enhance stress-induced HPA axis activity, this effect was present only during the breeding season. On the one hand, these data indicate that eCB signaling can function as a brake over the HPA axis in avian species, such that disruption of this inhibitory signal facilitates the rapid stress-induced increase in HPA stimulation (similar to mammals, see reference 11). On the other hand, the decreased functionality of this brake in molting birds, as indicated by the reduced capacity for CB1 antagonism to potentiate the stress response, suggests that the HPA does not use the eCB signaling as a primary mode of constraint during molt.
Although we disproved our hypothesis that eCB signaling acting as a brake was responsible for molt-related HPA suppression, our data pointed to another potential role for this pathway: regulating HPA plasticity. At the level of eCB content in the brain, specifically in the avian amygdala, we observed a robust response to acute stress in breeding birds but a muted response in molting birds. As shown in mammals, the amygdala is the primary limbic structure regulating stress-induced drive to the HPA axis via rapid decreases in AEA (28) in the basolateral amygdala, effectively gating the initiation of the HPA response (29). Specifically, at rest, tonic binding of CB1 (via AEA in mammals, but see below for potential discrepancies in birds) results in general restraint of HPA activity, whereas stress-induced declines in eCB signaling within the amygdala allow for rapid excitation of the pathway leading to the hypothalamus and contribute to the manifestation of neurobehavioral responses to stress (29–31).
In our birds, similar directionality is observed, such that AEA levels within the amygdala were found to decrease in response to stress. 2-AG levels within the amygdala were also found to decline in response to stress. Decreases in both AEA and 2-AG after acute stress occurred, regardless of season, but the effect was much more robust during the breeding season. Furthermore, the strong correlation between baseline CORT and AEA in the avian amygdala suggests a role for AEA signaling in maintaining individual baseline HPA activity as seen in mammals (11, 27, 32). Overall, these data suggest the possibility that, as in mammals, a decline in amygdalar eCB signaling in response to stress could contribute to activation of the HPA axis, although we did not perform pharmacological interventions to elevate eCB signaling and establish whether this was capable of dampening HPA responses to stress. This hypothesis thus remains speculative at present with more experiments necessary to show direct effects of eCB fluctuations in the avian amygdala and HPA activity.
The seasonal differences in the responses to the CB1 antagonist and eCB dynamics in the avian amygdala suggest a potential role for eCB signaling in the rapid shift in HPA plasticity in the short transition window (4 wk here) between the late breeding season and molt. Birds during the late breeding season maintain the capacity to mount a stress response while tightly regulating HPA dynamics (33). The breeding group in our study demonstrate a clear inhibitory effect of CB1 binding, unveiled by CB1 antagonism, and rapid decreases in amygdala eCB content presumed to facilitate stress-induced HPA axis excitation. In contrast, during molt, the response is decreased and less responsive to the antagonist, perhaps underlying the limited capacity of HPA axis to respond to stress during this season (34, 35). The rapid stress-induced decreases in eCB content in the avian amygdala in the breeding season only further suggest the shift in the role of eCB signaling and the localization of this effect, when HPA dynamics are confined during molt and therefore less plastic, eCB signaling in the avian amygdala may be essentially turned off and no longer used as a mechanism to regulate rapid HPA stimulation. An HPA that is independent of the rapid eCB effects may act to preserve a more restricted stress response. Such a reverse role for eCB signaling in the avian amygdala should be investigated further.
In the other brain regions studied, dynamic eCB responses to stress do not appear to be influenced by the breeding season. In the hippocampus and hypothalamus, there were no seasonal differences observed in stress-induced changes in eCB tissue content; AEA content increased in response to stress, regardless of the birds' condition. In both of these regions, the predominant role of eCB signaling is in CORT-negative feedback with elevations in eCB signaling promoting inhibition of the HPA axis and recovery after stress (11). Because we did not examine the recovery of the HPA axis after the cessation of stress (during which the negative feedback enhancing effects of eCB signaling are thought to be important [see reference 11]), the possibility exists that the importance of stress-induced increases in eCB signaling for promoting the recovery of the HPA axis could be relevant across both seasons.
Interestingly, changes in ligand concentrations across seasons did not appear to depend on changes in gene expression (CB1, FAAH, DAGL) or binding characteristics of CB1. Although these measurements came from a separate sample population, such comparisons suggest that seasonal changes may occur at the level of enzymatic activity (see reference 29) rather than at the level of enzyme availability for the production/metabolism of ligand or the capacity of CB1 binding.
Similarities with mammals
The components of the eCB pathway in wild starlings appear to be consistent with those in mammals. Our data further confirm that the CB1 receptor appears well conserved across species (36), with high homology between the starling CB1 receptor mRNA sequences and many other species, including humans (Supplemental Table 2). Previously described in a range of species (37), CB1 expression is abundant in the avian brain (38). Such widespread distribution suggests a role for eCB signaling in various functions shared with other species but also in functions specific to birds (eg, see reference 39 for research on the song control system).
Evaluating our data on the fluctuations in eCB content in response to stress, it appears that each brain region responds to acute stress with the same directionality of change in CB1 ligand as seen in mammals (increase in PVN and hippocampus; decrease in amygdala). The amygdala data are especially interesting because the role of avian amygdala in the stress response is poorly understood. As described above, in the mammalian amygdala, a reduction in AEA signaling in response to stress facilitates the HPA response to stress (29–31). Here, for the first time, we describe a response in birds similar to that seen in mammals: decreased ligand concentrations in the avian amygdala after acute stress. Additional studies will continue to explore this similarity and the role of the avian amygdala in the stress response of wild birds. Such experiments will include measurements of changes in enzymatic activity to better describe whether the rapid changes in content are occurring as a result of metabolism or synthesis in relation to HPA activation or CORT feedback.
Differences from mammals
One clear difference between the avian model and the mammalian model may be the specific role of each ligand. Whereas it has been suggested that AEA plays a tonic, gatekeeper role over the HPA axis and 2-AG plays a role in negative feedback (11), the dynamic nature of these ligands in response to stress in avian species suggests that this effect may be different in this species.
In our study, 2-AG (along with AEA) decreased in the avian amygdala in response to stress, suggesting an HPA gatekeeper role for 2-AG. Also, opposing mammalian data, in both the hypothalamus and hippocampus, AEA appears to increase with stress, (in mammals, 2-AG is typically the endocannabinoid, which is found to elevate in response to stress [40–42]). Although mammalian data suggest that hypothalamic 2-AG is the ligand responsible for the fast regulation of negative feedback via membrane glucocorticoid receptor (41, 43), our data suggest that, in birds, this negative feedback signal may rely on rapid changes in hypothalamic AEA. Additional studies will explore this hypothesis.
Furthermore, although AEA appears as the dynamic element in the hypothalamus, 2-AG may act as a tonic brake on baseline HPA activity during molt. Hypothalamic 2-AG content is elevated during molt, regardless of stress exposure, suggesting a role as a final brake on the system to regulate the baseline stimulatory signal traveling to the pituitary. Because our current study was limited to antagonist data acquired poststressor exposure, additional studies will aim to address how 2-AG elevation during molt acts as a local control to reduce baseline HPA activity during this season.
The similarities in directionality but differences in specific eCB ligand suggest that the specific roles for AEA and 2-AG during stress may not be as important as previously expected and may differ across species. Importantly, though, the overall effects of eCB signaling on HPA regulation appears to remain the same, regardless of which ligand takes on which role and the overarching role of eCB signaling in inhibition and suppression of HPA activity in avian species is consistent with the current mammalian literature.
Conclusions
The shift in HPA activity as birds transition from breeding season to molt provides a novel study of how eCB signaling may facilitate changes in HPA axis plasticity. Our data suggest that eCB signaling, especially in the avian amygdala, may play an important role when the HPA response requires a dynamic range (during the breeding season) but may play a limited role when the HPA response is limited (during the molt). Given that eCB signaling can dynamically regulate multiple aspects of HPA axis activity, this may be a signal that becomes relevant during periods of high lability (such as the breeding season). In contrast, during periods in which HPA axis activity requires restriction for survival (such as the molt), additional mechanisms, independent of eCB signaling, may ensure that this response does not exhibit the same lability and remains constrained. In addition, understanding how and where eCB signaling acts on HPA responsiveness in avian species may serve as a new tool for mapping similarities between species and broadening our understanding of the comparative nature of the physiological stress response.
Acknowledgments
We thank the staff at the Field Station for the Study of Behavior, Ecology, and Reproduction for Animal Care and Y. Nguyen, J. Vu, and B. Moon for assistance in the laboratory. We also acknowledge the Southern Alberta Mass Spectrometry Centre, located in and supported by the Cumming School of Medicine, University of Calgary, for their services in targeted liquid chromatography and tandem mass spectrometry.
This work was supported by National Science Foundation Grant IOS 1122044 (to G.E.B.) and National Institutes of Health Grant NRSA F32 HD072732–03 (to M.J.D.). The salary for H.A.V. was provided by the Canadian Consortium for the Investigation of Cannabinoids, and operating funds for M.N.H. came from the Canadian Institutes of Health Research.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AEA
- anandamide
- 2-AG
- 2-arachidonoylglycerol
- CB1
- cannabinoid 1 receptor
- CORT
- corticosterone
- DAGLα
- diacylglycerol lipase-α
- DIG
- digoxigenin
- DMSO
- dimethylsulfoxide
- eCB
- endocannabinoid
- FAAH
- fatty acid amide hydrolase
- HPA
- hypothalamic-pituitary-adrenal
- PoAc
- posterior pallial amygdala
- PVN
- paraventricular nucleus of the hypothalamus
- TME
- buffer of Tris HCl, EDTA, and MgCl2
- TnA
- nucleus taenaie of the amygdala.
References
- 1. Sapolsky R, Romero L, Munck A. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. [DOI] [PubMed] [Google Scholar]
- 2. McEwen B. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583(2–3):174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Romero L. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen Comp Endocrinol. 2002;128(1):1–24. [DOI] [PubMed] [Google Scholar]
- 4. Ouyang JQ, Sharp P, Quetting M, Hau M. Endocrine phenotype, reproductive success and survival in the great tit, Parus major. J Evol Biol. 2013;26(9):1988–1998. [DOI] [PubMed] [Google Scholar]
- 5. Romero L, Strochlic D, Wingfield J. Corticosterone inhibits feather growth: Potential mechanism explaining seasonal down regulation of corticosterone during molt. Comp Biochem Physiol. 2005;142(1):65–73. [DOI] [PubMed] [Google Scholar]
- 6. Landys M, Ramenofsky M, Wingfield J. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen Comp Endocrinol. 2006;148(2):132–149. [DOI] [PubMed] [Google Scholar]
- 7. Murphy ME. Energetics and Nutrition of Molt. In: Avian Energetics and Nutritional Ecology. Boston, MA: Springer US; 1996:158–198. [Google Scholar]
- 8. Swaddle JP, Witter MS, Cuthill IC, Budden A, McCowen P. Plumage condition affects flight performance in common starlings: implications for developmental homeostasis, abrasion and moult. J Avian Biol. 1996;27(2):103–111. [Google Scholar]
- 9. DesRochers D, Reed J, Awerman J, et al. Exogenous and endogenous corticosterone alter feather quality. Comp Biochem Physiol. 2009;152(1):46–52. [DOI] [PubMed] [Google Scholar]
- 10. Cornelius JM, Perfito N, Zann R, Breuner CW, Hahn TP. Physiological trade-offs in self-maintenance: plumage molt and stress physiology in birds. J Exp Biol. 2011;214(16):2768–2777. [DOI] [PubMed] [Google Scholar]
- 11. Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2012;204(C):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76(1):70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Marzo V. The endocannabinoid system: Its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res. 2009;60(2):77–84. [DOI] [PubMed] [Google Scholar]
- 14. Perfito N, Guardado D, Williams TD, Bentley GE. Social cues regulate reciprocal switching of hypothalamic Dio2/Dio3 and the transition into final follicle maturation in European starlings (Sturnus vulgaris). Endocrinology. 2015;156(2):694–706. [DOI] [PubMed] [Google Scholar]
- 15. Ubuka T, Bentley GE, Ukena K, Wingfield JC, Tsutsui K. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc Natl Acad Sci USA. 2005;102(8):3052–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hill MN, Kambo JS, Sun JC, Gorzalka BB, Galea LAM. Endocannabinoids modulate stress-induced suppression of hippocampal cell proliferation and activation of defensive behaviours. Eur J Neurosci. 2006;24(7):1845–1849. [DOI] [PubMed] [Google Scholar]
- 17. Sink KS, Segovia KN, Sink J, et al. Potential anxiogenic effects of cannabinoid CB1 receptor antagonists/inverse agonists in rats: comparisons between AM4113, AM251, and the benzodiazepine inverse agonist FG-7142. Eur Neuropsychopharmacol. 2010;20(2):112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Novoseletsky N, Nussinovitch A, Friedman-Einat M. Attenuation of food intake in chicks by an inverse agonist of cannabinoid receptor 1 administered by either injection or ingestion in hydrocolloid carriers. Gen Comp Endocrinol. 2011;170(3):522–527. [DOI] [PubMed] [Google Scholar]
- 19. Dickens MJ, Bentley GE. Stress, captivity, and reproduction in a wild bird species. Horm Behav. 2014;66(4):685–693. [DOI] [PubMed] [Google Scholar]
- 20. Atoji Y, Saito S, Wild JM. Fiber connections of the compact division of the posterior pallial amygdala and lateral part of the bed nucleus of the stria terminalis in the pigeon (Columba livia). J Comp Neurol. 2006;499(2):161–182. [DOI] [PubMed] [Google Scholar]
- 21. Dincheva I, Drysdale AT, Hartley CA, et al. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun. 2015:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythms. 2001;16(4):365–380. [DOI] [PubMed] [Google Scholar]
- 23. Lee TTY, Hill MN. Age of stress exposure modulates the immediate and sustained effects of repeated stress on corticolimbic cannabinoid CB1 receptor binding in male rats. Neuroscience. 2013;249(C):106–114. [DOI] [PubMed] [Google Scholar]
- 24. Dickens MJ, Earle KA, Romero LM. Initial transference of wild birds to captivity alters stress physiology. Gen Comp Endocrinol. 2009;160(1):76–83. [DOI] [PubMed] [Google Scholar]
- 25. Soderstrom K, Tian Q. Developmental pattern of CB1 cannabinoid receptor immunoreactivity in brain regions important to zebra finch (Taeniopygia guttata) song learning and control. J Comp Neurol. 2006;496(5):739–758. [DOI] [PubMed] [Google Scholar]
- 26. Ouyang JQ, Sharp PJ, Dawson A, Quetting M, Hau M. Hormone levels predict individual differences in reproductive success in a passerine bird. Proc R Soc B Biol Sci. 2011;278(1717):2537–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patel S. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145(12):5431–5438. [DOI] [PubMed] [Google Scholar]
- 28. Herman J, Ostrander M, Mueller N, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1201–1213. [DOI] [PubMed] [Google Scholar]
- 29. Hill MN, McLaughlin RJ, Morrish AC, et al. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009;34(13):2733–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gray JM, Vecchiarelli HA, Morena M, et al. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci. 2015;35(9):3879–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bedse G, Colangeli R, Lavecchia AM, et al. Role of the basolateral amygdala in mediating the effects of the fatty acid amide hydrolase inhibitor URB597 on HPA axis response to stress. Eur Neuropsychopharmacol. 2014;24(9):1511–1523. [DOI] [PubMed] [Google Scholar]
- 32. Newsom RJ, Osterlund C, Masini CV, Day HE, Spencer RL, Campeau S. Cannabinoid receptor type 1 antagonism significantly modulates basal and loud noise induced neural and hypothalamic-pituitary-adrenal axis responses in male Sprague-Dawley rats. Neuroscience. 2012;204(C):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lattin CR, Bauer CM, de Bruijn R, Michael Romero L. Hypothalamus pituitary adrenal axis activity and the subsequent response to chronic stress differ depending upon life history stage. Gen Comp Endocrinol. 2012;178(3):494–501. [DOI] [PubMed] [Google Scholar]
- 34. Romero L, Soma K, Wingfield J. Changes in pituitary and adrenal sensitivities allow the snow bunting (Plectrophenax nivalis), an Arctic-breeding song bird, to modulate corticosterone release seasonally. J Comp Physiol B. 1998;168(5):353–358. [DOI] [PubMed] [Google Scholar]
- 35. Romero L, Wingfield J. Seasonal changes in adrenal sensitivity alter corticosterone levels in Gambel's white-crowned sparrows (Zonotrichia leucophrys gambelii). Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;119(1):31–36. [DOI] [PubMed] [Google Scholar]
- 36. Elphick MR. The evolution and comparative neurobiology of endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 2012;367(1607):3201–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11(2):563–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alonso-Ferrero M, Paniagua MA, Mostany R, et al. Cannabinoid system in the budgerigar brain. Brain Res. 2006:1–9. [DOI] [PubMed] [Google Scholar]
- 39. Soderstrom K, Johnson F. CB1 cannabinoid receptor expression in brain regions associated with zebra finch song control. Brain Res. 2000:1–7. [DOI] [PubMed] [Google Scholar]
- 40. Dubreucq S, Matias I, Cardinal P, et al. Genetic dissection of the role of cannabinoid type 1 receptors in the emotional consequences of repeated social stress in mice. Neuropsychopharmacology. 2012;37(8):1885–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology. 2010;151(10):4811–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang M, Hill MN, Zhang L, Gorzalka BB, Hillard CJ, Alger BE. Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. J Psychopharmacol. 2012;26(1):56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tasker J, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stokes T, Leonard C, Nottebohm F. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J Comp Neurol. 1974;156(3):337–374. [DOI] [PubMed] [Google Scholar]