Abstract
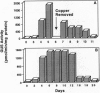
We describe a system for gene expression in plants based on the regulation mechanism of the yeast metallothionein (MT) gene. The system consists of two elements: (i) the yeast ace1 (activating copper-MT expression) gene encoding a transcription factor under control of the cauliflower mosaic virus (CaMV) 35S RNA promoter, and (ii) a gene of interest under control of a chimeric promoter consisting of the 90-base-pair domain A of the CaMV 35S RNA promoter linked to the ACE1 transcription factor-binding site. At elevated copper ion concentrations, the ACE1 protein changes conformation, binds to, and activates transcription from the chimeric promoter. To test the functioning of the system in plants, a construct containing the beta-glucuronidase (GUS) reporter gene under control of the chimeric promoter was prepared, and transgenic tobacco plants were produced. It was shown that GUS activity in the leaves of transgenic plants increased up to 50-fold, either after addition of 50 microM CuSO4 to the nutrient solution or after application of 0.5 microM CuSO4 to the plants in a foliar spray. This GUS expression was repressed after the removal of copper ions. The results show that the activity of the described chimeric promoter directly depends on copper ion concentration and that this system can be used in experiments that demand precise timing of expression.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back E., Dunne W., Schneiderbauer A., de Framond A., Rastogi R., Rothstein S. J. Isolation of the spinach nitrite reductase gene promoter which confers nitrate inducibility on GUS gene expression in transgenic tobacco. Plant Mol Biol. 1991 Jul;17(1):9–18. doi: 10.1007/BF00036801. [DOI] [PubMed] [Google Scholar]
- Benfey P. N., Chua N. H. The Cauliflower Mosaic Virus 35S Promoter: Combinatorial Regulation of Transcription in Plants. Science. 1990 Nov 16;250(4983):959–966. doi: 10.1126/science.250.4983.959. [DOI] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Butt T. R., Sternberg E. J., Gorman J. A., Clark P., Hamer D., Rosenberg M., Crooke S. T. Copper metallothionein of yeast, structure of the gene, and regulation of expression. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3332–3336. doi: 10.1073/pnas.81.11.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dameron C. T., Winge D. R., George G. N., Sansone M., Hu S., Hamer D. A copper-thiolate polynuclear cluster in the ACE1 transcription factor. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6127–6131. doi: 10.1073/pnas.88.14.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X. W., Finlay D. R., Guiney D., Helinski D. R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985 Mar;13(2):149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Evans I. M., Gatehouse L. N., Gatehouse J. A., Robinson N. J., Croy R. R. A gene from pea (Pisum sativum L.) with homology to metallothionein genes. FEBS Lett. 1990 Mar 12;262(1):29–32. doi: 10.1016/0014-5793(90)80145-9. [DOI] [PubMed] [Google Scholar]
- Frohberg C., Heins L., Gatz C. Characterization of the interaction of plant transcription factors using a bacterial repressor protein. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10470–10474. doi: 10.1073/pnas.88.23.10470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst P., Hu S., Hackett R., Hamer D. Copper activates metallothionein gene transcription by altering the conformation of a specific DNA binding protein. Cell. 1988 Nov 18;55(4):705–717. doi: 10.1016/0092-8674(88)90229-2. [DOI] [PubMed] [Google Scholar]
- Gallagher T. F., Ellis R. J. Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J. 1982;1(12):1493–1498. doi: 10.1002/j.1460-2075.1982.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz C., Frohberg C., Wendenburg R. Stringent repression and homogeneous de-repression by tetracycline of a modified CaMV 35S promoter in intact transgenic tobacco plants. Plant J. 1992 May;2(3):397–404. doi: 10.1111/j.1365-313x.1992.00397.x. [DOI] [PubMed] [Google Scholar]
- Grill E., Winnacker E. L., Zenk M. H. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci U S A. 1987 Jan;84(2):439–443. doi: 10.1073/pnas.84.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schena M., Lloyd A. M., Davis R. W. A steroid-inducible gene expression system for plant cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10421–10425. doi: 10.1073/pnas.88.23.10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczypka M. S., Thiele D. J. A cysteine-rich nuclear protein activates yeast metallothionein gene transcription. Mol Cell Biol. 1989 Feb;9(2):421–429. doi: 10.1128/mcb.9.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D. J., Hamer D. H. Tandemly duplicated upstream control sequences mediate copper-induced transcription of the Saccharomyces cerevisiae copper-metallothionein gene. Mol Cell Biol. 1986 Apr;6(4):1158–1163. doi: 10.1128/mcb.6.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D. J. Metal-regulated transcription in eukaryotes. Nucleic Acids Res. 1992 Mar 25;20(6):1183–1191. doi: 10.1093/nar/20.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W., Blevins D. G., Randall D. D. Allantoic Acid Synthesis in Soybean Root Nodule Cytosol via Xanthine Dehydrogenase. Plant Physiol. 1980 Jun;65(6):1203–1206. doi: 10.1104/pp.65.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Töpfer R., Schell J., Steinbiss H. H. Versatile cloning vectors for transient gene expression and direct gene transfer in plant cells. Nucleic Acids Res. 1988 Sep 12;16(17):8725–8725. doi: 10.1093/nar/16.17.8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. C., Howard E. A., Dennis E. S., Peacock W. J. DNA sequences required for anaerobic expression of the maize alcohol dehydrogenase 1 gene. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6624–6628. doi: 10.1073/pnas.84.19.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde R. J., Shufflebottom D., Cooke S., Jasinska I., Merryweather A., Beri R., Brammar W. J., Bevan M., Schuch W. Control of gene expression in tobacco cells using a bacterial operator-repressor system. EMBO J. 1992 Apr;11(4):1251–1259. doi: 10.1002/j.1460-2075.1992.tb05169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. F., Hamer D. H., McKenney K. Autoregulation of the yeast copper metallothionein gene depends on metal binding. J Biol Chem. 1988 Jan 25;263(3):1570–1574. [PubMed] [Google Scholar]
- de Framond A. J. A metallothionein-like gene from maize (Zea mays). Cloning and characterization. FEBS Lett. 1991 Sep 23;290(1-2):103–106. doi: 10.1016/0014-5793(91)81236-2. [DOI] [PubMed] [Google Scholar]
- de Miranda J. R., Thomas M. A., Thurman D. A., Tomsett A. B. Metallothionein genes from the flowering plant Mimulus guttatus. FEBS Lett. 1990 Jan 29;260(2):277–280. doi: 10.1016/0014-5793(90)80122-y. [DOI] [PubMed] [Google Scholar]