Abstract
We conducted a systematic review of the published literature to examine the impact of new vaccine introduction on countries’ immunization and broader health systems. Six publication databases were searched using 104 vaccine and health system-related search terms. The search yielded 15,795 unique articles dating from December 31, 1911 to September 29, 2010. Based on review of the title and abstract, 654 (4%) of these articles were found to be potentially relevant and were referred for full review. After full review, 130 articles were found to be relevant and included in the analysis. These articles represented vaccines introduced to protect against 10 different diseases (hepatitis A, hepatitis B, Haemophilus influenzae type b disease, human papilloma virus infection, influenza, Japanese encephalitis, meningococcal meningitis, Streptococcus pneumoniae disease, rotavirus diarrhea and typhoid), in various formulations and combinations. Most reviewed articles (97 [75%]) reported experiences in high-income countries. New vaccine introduction was most efficient when the vaccine was introduced into an existing delivery platform and when introduced in combination with a vaccine already in the routine childhood immunization schedule (i.e., as a combination vaccine). New vaccine introduction did not impact coverage of vaccines already included in the routine childhood immunization schedule. The need for increased cold chain capacity was frequently reported. New vaccines facilitated the introduction and widespread use of auto-disable syringes into the immunization and the broader health systems. The importance of training and education for health care workers and social mobilization was frequently noted. There was evidence in high-income countries that new vaccine introduction was associated with reduced health-care costs. Future evaluations of new vaccine introductions should include the systematic and objective assessment of the impacts on a country’s immunization system and broader health system, especially in lower-income countries.
Keywords: New vaccines, Under-utilized vaccines, Introduction, Immunization program, Health system
1. Introduction
The Expanded Program on Immunization (EPI) was established by the World Health Organization (WHO) in 1974 to provide protection against six vaccine-preventable diseases (tuberculosis, poliomyelitis, diphtheria, tetanus, pertussis, and measles) through routine infant immunization. Since then, many new vaccines have become available, and global public funding for immunization, including the GAVI Alliance, has increased accessibility to these vaccines [1]. Most of the new vaccines, including hepatitis B (HepB) vaccine, Haemophilus influenzae type B (Hib) vaccine, pneumococcal conjugate vaccine (PCV), and rotavirus (RV) vaccine are intended to be included in the routine childhood immunization schedule. Other new vaccines, such as human papillomavirus (HPV) vaccine meningococcal vaccine, yellow fever vaccine, and typhoid vaccine are intended for older or at-risk populations.
The introduction of a new vaccine can have both positive and negative impacts on the immunization system and the broader health system. It may provide opportunities and resources to strengthen an existing system, or it may add stress to an already weak infrastructure. Based on a request in 2010 from the Strategic Advisory Group of Experts (SAGE) on Immunization that guides the World Health Organization (WHO) on global immunization policies, we conducted a systematic review of the published literature to examine evidence of impact of new vaccine introduction on health and immunization systems reported in the medical literature.
2. Methods
2.1. Search strategy
We developed search terms to identify articles that included information describing the impact of new vaccine introduction on immunization systems and health systems [2] (Table 1). The terms were selected to be inclusive and were developed with input from immunization experts. The search terms were divided into two broad categories: (1) vaccines and (2) immunization and health systems, and databases were searched to identify articles captured by at least one search term in each category (Table 1).
Table 1.
Search terms used for systematic literature review of the impact of new vaccine introduction on the immunization and health system.
| Vaccine search term category | |
| 1 | exp *Hepatitis B Vaccines/ |
| 2 | exp *Haemophilus Vaccines/ |
| 3 | exp *Pneumococcal Vaccines/ |
| 4 | exp *Rotavirus Vaccines/ |
| 5 | exp *Meningococcal Vaccines/ |
| 6 | exp *Yellow Fever Vaccine/ |
| 7 | exp *Japanese Encephalitis Vaccines/ |
| 8 | exp *Papillomavirus Vaccines/ |
| 9 | exp *Typhoid-Paratyphoid Vaccines/ |
| 10 | exp *Cholera Vaccines/ |
| 11 | (HPV vaccine or HPV vaccines).ab,ti. |
| 12 | (HBV vaccine or HBV vaccines).ab,ti. |
| 13 | (hib vaccine or Hib vaccines).ab,ti. |
| 14 | new vaccine.ab,ti. |
| 15 | new vaccines.ab,ti. |
| 16 | ((under utilised or under-utilised or under utilised or under-utilised or underutilised or underutilised) and (vaccine or vaccines)).ab,ti. |
| Immunization and health system search term category | |
| 17 | exp Immunization Programs/ |
| 18 | health planning/or health care rationing/or health plan implementation/or health planning guidelines/or health planning technical assistance/or health priorities/or health resources/or national health programs/or exp regional health planning/ |
| 19 | Capacity Building/ |
| 20 | exp Inservice Training/ |
| 21 | capacity building.ab,ti. |
| 22 | building capacity.ab,ti. |
| 23 | skill development.ab,ti. |
| 24 | “delivery of health care”/or health services accessibility/or healthcare disparities/ |
| 25 | equity.ab,ti |
| 26 | “quality of health care”/or clinical competence/or guideline adherence/or exp “outcome and process assessment (health care)”/or program evaluation/or quality assurance, health care/or benchmarking/or clinical audit/or medical audit/or nursing audit/or total quality management/ |
| 27 | health system.ab,ti. |
| 28 | health systems.ab,ti. |
| 29 | (health service or health services).ab,ti. |
| 30 | Health Manpower/ |
| 31 | exp “Patient Acceptance of Health Care”/ |
| 32 | (community mobilisation or community mobilisation).ab,ti. |
| 33 | community advocacy.ab,ti. |
| 34 | information systems/or integrated advanced information management systems/or management information systems/or ambulatory care information systems/or clinical pharmacy information systems/or database management systems/or healthcare common procedure coding system/or “personnel staffing and scheduling information systems”/ |
| 35 | safety.ab,ti. |
| 36 | systems integration/ |
| 37 | employee incentive plans/or personnel loyalty/or “personnel staffing and scheduling”/or personnel turnover/or physician incentive plans/or “salaries and fringe benefits”/or workload/ |
| 38 | Curriculum/ |
| 39 | Forecasting/ |
| 40 | Group Purchasing/ |
| 41 | procurement.ab,ti. |
| 42 | logistics.ab,ti. |
| 43 | cold chain.ab,ti. |
| 44 | financial management/or exp budgets/or fund raising/or risk management/or financial support/ |
| 45 | Health Services/ |
| 46 | Health Personnel/ |
| 47 | Social Change/ |
| 48 | “Organization and Administration”/ |
| 49 | Product Surveillance, Postmarketing/ |
| 50 | medical waste/or medical waste disposal/ |
| 51 | Decision Making, Organizational/ |
| 52 | policy making/or advisory committees/ |
| 53 | Government Regulation/ |
| 54 | stock.ab,ti. |
| 55 | Immunologic Surveillance/ |
| 56 | population surveillance/or sentinel surveillance/ |
| 57 | (strain surveillance or serotype surveillance or virological surveillance or epidemiological surveillance).ab,ti. |
| 58 | access to services.ab,ti. |
| 59 | (affordability or affordable).ab,ti. |
| 60 | ((timeliness or timely) adj3 vaccination).ab,ti. |
| 61 | delivery strateg*.ab,ti. |
| 62 | integrated disease control.ab,ti. |
| 63 | social mobilization.ab,ti. |
| 64 | (incentiv* adj3 health care worker*).ab,ti. |
| 65 | pre-training.ab,ti. |
| 66 | (in-service training or inservice training).ab,ti. |
| 67 | (career path or career paths).ab,ti. |
| 68 | wages.ab,ti. |
| 69 | supportive supervision.ab,ti. |
| 70 | data quality.ab,ti. |
| 71 | data collection.ab,ti. |
| 72 | data management.ab,ti. |
| 73 | health management information system.ab,ti. |
| 74 | impact monitoring.ab,ti. |
| 75 | adverse events following immunization.ab,ti. |
| 76 | AEFl.ab,ti. |
| 77 | (post marketing adj3 evaluation).ab,ti. |
| 78 | (demand and supply forecasting).ab,ti. |
| 79 | (demand adj3 forecasting).ab,ti. |
| 80 | (supply adj3 forecasting).ab,ti. |
| 81 | (stock* adj3 manag*).ab,ti. |
| 82 | pooled procurement*.ab,ti. |
| 83 | (effective adj3 vaccine management).ab,ti. |
| 84 | financing.ab,ti. |
| 85 | (vaccine* adj3 price*).ab,ti. |
| 86 | healthy market*.ab,ti. |
| 87 | fiscal space.ab,ti. |
| 88 | budget support.ab,ti. |
| 89 | (donor* adj3 pool*).ab,ti. |
| 90 | SWAp.ab,ti. |
| 91 | opportunity cost*.ab,ti. |
| 92 | (national regulatory agenc* or national immunization technical advisory group* or national immunisation technical advisory group* or legislation or governance or accountability or inter-agency coordinating committee* or interagency coordinating committee*).ti,ab. |
| 93 | ((treatment adj3 cost*) or (hospitalization adj3 cost*) or (hospitalisation adj3 cost*) or (norms adj3 standards)).ti,ab. |
| 94 | (storage adj3 capacity).ab,ti. |
| 95 | (storage adj3 volume).ab,ti. |
| 96 | (vaccine* adj3 stor*).ab,ti. |
| 97 | (vaccine* adj3 handl*).ab,ti. |
| 98 | (vaccine* adj5 distribut*).ab,ti. |
| 99 | (vaccine* adj5 transport*).ab,ti. |
| 100 | ((supply or supplies) adj5 (frequen* or interval*)).ab,ti. |
| 101 | logistic*.ab,ti. |
| 102 | ((immunization adj3 expenditure*) or (immunisation adj3 expenditure*)).ab,ti. |
| 103 | exp “health care economics and organizations”/ |
| 104 | (economics or legislation jurisprudence).fs. |
We searched seven publication databases (Medline®, EmbaseTM, Nursing Update, West African Journal of Nursing, CINAHL®, Web of Science®, and Global Health) that are relevant to vaccines and immunization programs, and are likely to contain reports from low income countries. We attempted to use identical terms to search each database; however, as each database had certain specifications, it was sometimes necessary to modify some terms. We limited the search to reports involving human subjects, published in any language. The final search date was September 29, 2010 and was not limited to a beginning year.
2.2. Inclusion criteria
We reviewed the title and abstract of each article identified using the search strategy to determine whether the article was potentially relevant (i.e., contained quantitative or qualitative information on the impact of new vaccine introduction on the immunization system, the broader health system, or both). Potentially relevant articles were referred for a full abstraction. Articles that discussed disease incidence, disease burden, vaccination coverage, serotype replacement, immunization campaigns, or adverse events following immunization were included if they contained data or discussion of vaccine impact on the health or immunization system and assessed outcome within the first five years after vaccine introduction, unless the assessed outcome manifested more than five years after vaccine introduction (e.g., HepB vaccine and hepatocellular carcinoma or cirrhosis, or HPV vaccine and cervical cancer). Cost effectiveness studies were considered if they included real-time data, and real situations and savings and were not based on results of modeling. Expert opinion articles were included if data were reported. We excluded clinical trials, because they did not show impact on the immunization and health systems following vaccine introduction.
2.3. Abstraction process
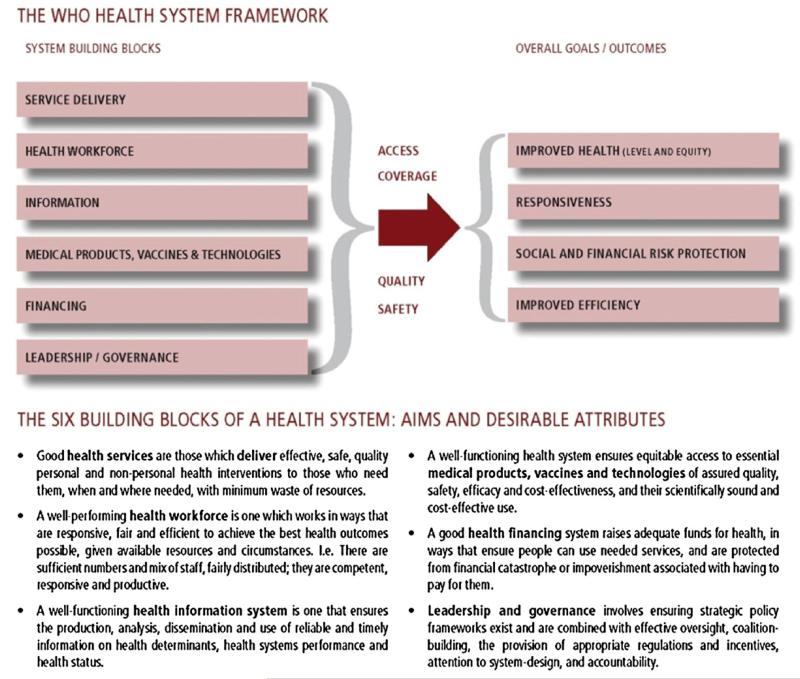
We used EndNote X3.0.1S (Thompson Reuters) to organize and track the articles, adding databases sequentially beginning with Medline, and performing automated and manual de-duplication following the addition of each subsequent database. Each article was reviewed to determine if it addressed the impact of the vaccine introduction on the immunization and health system. Sixteen immunization experts from the World Health Organization (WHO), the Centers for Disease Control and Prevention (CDC), The United Nations Children’s Fund (UNICEF), the London School of Hygiene and Tropical Medicine and Hygiene (LSHTM), the Program for Appropriate Technology in Health (PATH), and Maternal and Child Health Integrated Program (MCHIP) participated in the abstraction process. Information from relevant articles was abstracted using a Microsoft Access® 2007 data collection form and were organized according to the WHO Framework for Action (Fig. 1); this framework was created by WHO in 2007 to promote a common understanding of health care systems by providing a systematic means for considering the essential functions [2]. The Framework comprises six building blocks: service delivery; health workforce; information; medical products, vaccines and technologies; financing; and leadership and governance. Countries were grouped by gross national income into categories according to the World Bank Atlas Method [http://data.worldbank.org/about/country-classifications/country-and-lending-groups]. Non-English articles were reviewed and abstracted by native speakers, whenever possible. A random sample of 10% of the articles were reviewed and abstracted by a second reviewer. If discordance was found, the article was reviewed by the first author to resolve discrepancies.
Fig. 1.
Health systems framework.
Source: [2]. http://www.who.int/healthsystems/strategy/everybodys_business.pdf.
3. Results
3.1. Search and abstraction
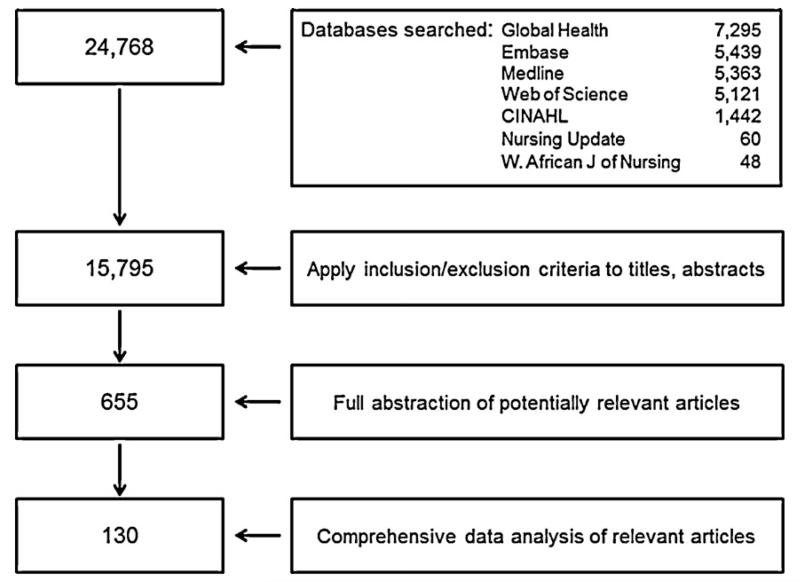
The search yielded 24,768 articles from December 31, 1911 to September 29, 2010, among which 8973 (36%) were found to be duplicates (Fig. 2). Reviewers applied the inclusion criteria to the remaining 15,795 titles and abstracts. Among these, 654 (4%) articles met the inclusion criteria and were referred for full abstraction; 49 (7%) of these were in languages other than English. One hundred twenty-nine (20%) of the 654 abstracted articles were found to be relevant for the analysis. In addition, one key article known to the authors that was not identified by the systematic search of the published literature was included, resulting in a total of 130.
Fig. 2.
Database search algorithm and review criteria used for systematic literature review of the impact of new vaccine introduction on the immunization and health systems.
3.2. General overview
Among the 130 studies included in this review (Table 2), 97 (75%) were from high income countries; 21 (16%) were from middle income countries; and 4 (3%) were from low income countries. The studies included vaccines targeting 10 diseases (hepatitis A (3 [2%]), hepatitis B (24 [19%]), Hib disease (28 [22%]), HPV infection (13 [10%]), influenza (1 [1%]), Japanese encephalitis (1 [1%]), meningococcal meningitis (17 [13%]), Streptococcus pneumoniae disease (28 [22%]), rotavirus diarrhea (14 [11%]) and typhoid (2 [2%])). Multiple vaccine formulations (i.e., antigens, adjuvants, stabilizers, preservatives and other components) and vaccine combinations were represented in the studies. Overall, 64 (56%) studies considered vaccine introductions that took place between 2000 and 2008.
Table 2.
Literature cited according to World Bank income level, country, and vaccine(s) discussed for vaccines introduced 1980–2008.a
| World Bank income levelb | Country | No. of references | Vaccine(s)c [Reference] |
|---|---|---|---|
| High | Australia | 10 | Hib [92,93]; HPV [5,16,32,45,124]; PCV [30,106]; RV [123] |
| Austria | 1 | RV [117] | |
| Belgium | 1 | HPV [128] | |
| Canada | 14 | HepB [18,25]; Hib [24,95]; Influenza [70]; MenPS/Conj [9,110-112]; PCV [17,43,77,78]; PPV23 [52] |
|
| Finland | 1 | Hib [66] | |
| Germany | 2 | HAV [12]; PCV [102] | |
| Great Britain | 13 | Hib [26,62,96]; HPV [23,34]; MenPS/Conj [4,7,28,109,113,114]; PCV [72]; PPV23 [33] |
|
| Greece | 1 | HepB [129] | |
| Israel | 2 | Hib [29,91] | |
| Italy | 9 | HepB [41,79,89,130,131,137]; PCV [88]; PPV23 [87], RV [75] | |
| New Zealand | 1 | MCV-B [46] | |
| Singapore | 1 | HBV [19] | |
| Spain | 3 | HAV/HepB [58]; Hib [97]; MenPS [108] | |
| Sweden | 1 | Hib [31] | |
| Taiwan | 4 | HepB [21,80,81,115] | |
| USA | 28 | Combo (DTP/Hib/HepB/IPV) [54]; Hib [85]; HPV [35]; PCV [11,59-61,63-65,67,68,71,74,83,86,90,94,101,103,104,107]; PPV23 [76]; RV [86,119-122] |
|
| Multiple countriesf | 1 | Hib [69] | |
| Multiple countriesg,h,i | 3 | HPV [8,27,125] | |
| Total high income country refs. (% of total) |
96 | (75) | |
| Middle | Brazil | 1 | RV [82] |
| Chile | 1 | Hib [53] | |
| Cuba | 1 | Hib [98] | |
| Egypt | 1 | MenPS [3] | |
| El Salvador | 1 | RV [118] | |
| Indonesia | 1 | Typhoid [10] | |
| Mexico | 1 | RV [116] | |
| Nicaragua | 1 | RV [51] | |
| Peru | 1 | HepB [15] | |
| People’s Republic of China | HepB [20,36,48]; JE [47]; HAV/JE [38] | ||
| Senegal | 1 | Combo (DTP/Hib/HepB/IPV) [99] | |
| South Africa | 2 | HepB [44]; Hib [50] | |
| Thailand | 1 | HepB[13] | |
| Multiple countriesj,k | 1 | Hib [39] | |
| Multiple countriesl | 1 | Typhoid [6] | |
| Multiple countriesm | 1 | RV [56] | |
| Total middle income country refs (% of total) |
21 | (16) | |
| Low | Ethiopia | 1 | Combo (DTP/Hib/HB/IPV) [42] |
| Gambia | 1 | Hib [55] | |
| Malawi | 1 | Hib [49] | |
| Zimbabwe | 1 | HepB [37] | |
| Total low income country refs. (% of total) |
(3) | ||
| Not classifiedd | 18 European countries | 1 | HPV [57] |
| 50 GAVI-eligible countries | 1 | HepB & Hib [126] | |
| Americas | 1 | Hib [41] | |
| Multiple countriesn,o | Hib [22,127] | ||
| Global | 1 | HepB [14] | |
| Globalp | 1 | Hib [100] | |
| Total not classified refs. (% of total) | 7 | (5) | |
| Literature review | Multiple countriesq | 1 | PCV[73] |
| Total literature reviews (% of total) | 1 | (0.8) | |
| Total refs | 129e |
Year of vaccine introduction (number [%] of references): 1980–1989 – 14(12%); 1990–1999 – 37 (32%); 2000–2007 – 64 (56%); 115/129 (89%) specified studies the year of introduction.
Based on Gross National Income (GNI) per capita. Low: <USD1005; middle: USD1006-12,275 (includes lower middle [USD1006-3975] and upper middle [USD3976-12,275]); high: >USD12,276.
Diphtheria–tetanus–pertussis (DTP), hepatitis A (HAV) vaccine, hepatitis B (HepB) vaccine, Haemophilus influenzae type B (Hib) vaccine, human papillomavirus (HPV) vaccine, inactivated polio vaccine (IPV), Japanese Encephalitis (JE), meningococcal polysaccharide (MenPS) vaccine, meningococcal conjugate vaccine type B (MCV-B) pneumococcal conjugate vaccine (PCV), 23-valent pneumococcal polysaccharide vaccine (PPV-23), and rotavirus (RV) vaccine.
Studies done in multiple countries with different World Bank income levels.
1 reference identified by the authors and not by the literature search for inclusion is not included in the table [136].
Finland, Iceland, Germany, Switzerland.
USA & Canada.
USA, Puerto Rico, Canada.
Italy & Belgium.
Chile & Uruguay.
South Africa & Argentina.
Thailand, China, Vietnam, India, Indonesia, Pakistan.
Brazil, Ecuador, El Salvador, Panama, Mexico, Nicaragua, Venezuela.
Ghana, Mozambique, Tanzania, Lesotho.
Qatar, Uruguay, Chile, Kuwait.
US Army beneficiaries.
US, UK, Norway, Netherlands, Germany, Canada, Switzerland, Spain, Australia.
3.3. Service delivery
In a number of countries, introduction of the new vaccines required new delivery strategies or modification of existing delivery strategies. While many new vaccines have been introduced through routine vaccination programs and mass campaigns, other delivery strategies, including one-time catch up vaccination, school-based vaccination, or a combination of strategies, depending on the vaccine and age recommendation for vaccination have been utilized to introduce new vaccines [3-10]. Novel delivery strategies, and the need for social promotion and education about the new vaccine’s safety and potential adverse events, may also affect the delivery of other immunization and health services. In several high and middle income countries coverage with existing vaccines administered through routine childhood or school-based strategies increased (Australia, Germany and Peru) or remained the same (Thailand and the United States) when new vaccines were introduced [11-16]. In Canada, offering PCV at no cost helped to remove existing inequities in PCV distribution [17], increased PCV acceptance and improved on-time vaccination [17,18]. Australia’s government-funded HPV vaccination strategy consisted of school-based routine vaccination of 12-year-old girls, including a 2-year catch-up strategy [5]. The catch-up strategy for 13–18 year olds was largely school-based, and for 18–26-year-old women was carried out by general practitioners. The general practitioner visit was also intended to provide an opportunity for physicians to broaden sexual health education and discuss cervical cancer prevention [5]. However, ensuring completion of the series for those who missed school-based vaccination required additional efforts, including coordination with general practitioners, additional mop-up campaigns at the end of the year, or establishing an individual recall system.
Communication and education activities were often coupled with delivery of new vaccines. Social mobilization to publicize vaccination – including through mothers’ clubs and professional organizations – was part of successful efforts to ensure wide acceptance of HepB vaccine in Singapore [19], China [20], Taiwan [21] and Peru [15], and of Hib vaccine in Uruguay, Chile, Qatar, and Kuwait [22]. Introduction of HPV vaccine in the UK involved engagement of multiple stakeholders and was facilitated through the establishment of an HPV vaccine implementation group [23]. Knowledge and education about the disease and the vaccine, including safety issues, aided the successful introduction of Hib [24] vaccine and HepB vaccine [25] vaccine in Canada and Hib in the United Kingdom [26]. In the United States and Canada, HPV vaccine, in contrast to other routinely recommended childhood immunizations, was actively marketed through direct consumer advertising and public awareness campaigns that targeted legislators and policy makers in addition to consumers [27]. In the United Kingdom, funding for media communication about meningococcal conjugate C vaccine was included in the budget for vaccine introduction [28]. Because adding a new immunization can result in an additional injection during a routine childhood vaccination visit, immunization planners in Israel chose a Hib vaccine with a 2-dose rather than a 3-dose series, in part to avoid three injections at the 6 month visit [29]. In Australia, some vaccine providers were reported to be reluctant to administer three injections at one time, resulting in lower uptake of PCV in a high-risk population [30].
3.4. Health workforce
There was variability in the impact of new vaccine introduction on the health workforce. In settings where vaccination was introduced into regularly scheduled clinics, little impact on staffing and appointment times was observed, as was the case with Hib vaccine introduction in Sweden [31], HPV vaccine introduction in Australia [32], and PCV introduction in the United States [11]. Vaccine introduction into adolescent and adult immunization programs sometimes required additional staff or adjustment of appointment times: in Scotland, adding pneumococcal vaccine to appointments for influenza vaccination among older adults increased consultation times by about 2 min [33]. Additional staff were needed for HPV vaccine introduction in the United Kingdom [34], where public health nurses, pediatric nurses, health visitors, and managers were recruited into teams, and additional funding was made available to address a shortage of school nurse vaccinators. Lack of staff was reported as a barrier to HPV vaccine introduction in remote areas of Australia [5], and HepB vaccine introduction in juvenile justice facilities in the United States [35].
Training of health staff at national, regional and local levels was required for successful HepB vaccine introduction in China [36] and Zimbabwe [37], hepatitis A and Japanese encephalitis (JE) vaccine in China [38], Hib vaccine in the Americas [39,40], and meningococcal polysaccharide vaccine in Egypt [3]. When the new vaccine was introduced as a combination product that included a vaccine already used in the childhood vaccination schedule [22], training was relatively straightforward. In Zimbabwe the introduction of HepB vaccine also included development of communication materials, and training on community mobilization activities [37]. In Indonesia, training, and periodic re-training in the local language were needed to successfully implement the school-based typhoid program [10]. Continuing education was conducted in Italy to promote the birth dose of HepB vaccine [41]. To ensure funding for training, the vaccine introduction budget for HepB and Hib vaccines in Ethiopia included a training and education component [42].
3.5. Information
In other countries, vaccine introduction stimulated the development of new surveillance or vaccine registry systems, including comprehensive surveillance for meningococcal meningitis in the United Kingdom [4], pediatric invasive pneumococcal disease in Canada [43], hepatitis B virus-associated nephropathy in South Africa [44], and an HPV immunization registry in Australia [16,45]. In Chile and Uruguay, Hib case definitions and disease reporting forms were standardized, and a technology transfer program was developed following Hib vaccine introduction [39]. A national immunization register, implemented at the same time as a meningococcal conjugate B vaccination campaign in New Zealand, was used to monitor coverage, safety and effectiveness assessments [46]. In Australia’s HPV vaccination program, a time-limited incentive payment of $6 per notification of vaccination was offered to general practitioners to improve completeness of the vaccine register [45]. In China [47,48], Malawi [49], South Africa [50], Nicaragua [51], Canada [52] and Egypt [3], existing disease surveillance systems were used for impact evaluation, policy formulation, and program advocacy related to immunization against HepB [48], JE [47], Hib [49,50], rotavirus diarrhea [51], pneumococcal pneumonia [52] and meningococcal meningitis [3,3].
3.6. Medical products, vaccines and technologies
The availability and use of new technologies, including combination vaccines and auto-disable (AD) syringes, had an impact on the immunization system. In Chile (DTP-Hib) [53], the United States (DTP-Hib) [54] and Zimbabwe (DTP-HepB) [37], use of combination vaccines resulted in fewer injections, and the need for fewer needles and syringes; lower administrative costs; and reduced storage capacity, compared with introducing a new vaccine as a separate vaccine injection. Introduction of DTP-Hib vaccine in The Gambia; however, resulted in initial interruption of routine DTP immunization due to irregular supply of the combination vaccine [55].
In some instances, introduction of new vaccines created additional requirements for cold chain and logistics systems. In Ethiopia, the replacement of 10-dose vials of whole cell diphtheria–tetanus–pertussis (DTwP) vaccine with single-dose vials of pentavalent (DTwP–Hep B–Hib) increased transport and cold chain requirements [42]. The need for additional cold chain capacity was also reported for rotavirus in several Latin American countries [56], hepatitis A and B vaccine introduction in China[20,38], HPV vaccine introduction in the United States [35], and 23-valent pneumococcal polysaccharide vaccine (PPV23) for older adults in Scotland [33]. In other cases, as was the case for Hib introduction, existing infrastructure was adequate for introduction [39].
As part of new vaccine introduction, AD syringes, safety boxes, and injection safety policies were introduced into immunization programs. In an evaluation of 58 countries eligible for funding from the GAVI Alliance that received injection safety support, all but two continued exclusive use of AD syringes and safety boxes after funding ended [57,58]. In some countries, the use of AD syringes, safety boxes, and injection safety policies were expanded beyond the immunization program to other health services. Most countries were able to continue the use of AD syringes after GAVI funding for injection safety support ended.
3.7. Financing and sustainability
Cost was a consideration in planning new vaccine introduction, including introduction in developed countries [57]. Utilizing existing infrastructure or combination vaccines reduced the costs for introduction, as was documented with introduction of DTP-Hib in the US [54], combination HepA/HepB vaccines in Spain [58], and Hib vaccine in Sweden [31]. In a number of settings, insufficient funding [22] and loss of donor support [37] resulted in vaccine shortages and program interruptions.
A decrease in ambulatory consultations and hospitalizations, disease-related complications, and long-term sequelae associated with diseases prevented by the newly introduced vaccines was reported from developing and industrialized countries; these led to reductions in health care utilization, and in some cases resulted in changes in treatment recommendations [31,44,55,59-89] (Table 3). The introduction of PCV in the United States resulted in a decrease in the mortality in sickle cell patients; however, guidelines for penicillin prophylaxis remained unchanged, because not all pneumococcal serotypes are covered by currently available vaccines [90]. In Brazil, all-cause diarrhea costs declined following introduction of RV vaccine, but they were not sufficient to offset the costs of program implementation [82]. Targeted strategies to vaccinate high-risk populations reduced or eliminated racial and ethnic disparities in rates of Hib disease incidence in Israel [91] and Australia[92,93] and of pneumococcal disease in the US [94] were decreased or eliminated following vaccine introduction. Populations not targeted by the vaccine experienced reductions in morbidity and mortality associated with Hib disease [24,39,91,95-100], Streptococcus pneumoniae disease [43,83,101-107], meningococcal disease [3,9,108-114], hepatitis A [58], hepatitis B [115], typhoid fever [6], rotavirus diarrhea [116-123], human papilloma virus infection [124,125], and Japanese encephalitis [47]; these reductions were attributed to a herd protective effect.
Table 3.
Impact on health care utilization and economic impact (where documented) following new vaccine introduction in selected countries.
| Vaccine(s) | Outcome(s) | Country(ies) | Reference(s) | Reported economic impact |
|---|---|---|---|---|
| HepBa | Decrease in acute hepatitis B; hepatocellular carcinoma, HBV-associated glomerulonephritis |
Italy, South Africa, Taiwan |
[44,79-81,89] | Estimated US$224M savings in acute health care costs [79,89] per year |
| Hib | Decrease in ambulatory consultations and hospitalizations for meningitis, epiglottitis, orbital and periorbital cellulitis and septic arthritis; change in empiric antibiotic recommendations |
Gambia, Sweden, Finland, Wales, US, Iceland, Germany, Switzerland, Canada, UK, Netherlands, Australia, New Zealand |
[31,55,62,66,69,85] | Modeled cost savings to society [31] |
| Influenza | Decrease in hospitalizations |
Canada | [70] | |
| PCV7, 10,13 | Fewer antibiotic-resistant infections; decrease in hospitalizations for pneumonia, outpatient and emergency department visits for otitis media, pneumonia, and other respiratory infections, fever; invasive pneumococcal disease in HIV-infected persons; decrease in antibiotic prescriptions, insurance claims for otitis media, tympanostomy tube placement; change in recommendations for fever evaluation among vaccinated children |
US, Canada, Italy, England, multi-country literature review |
[59,61,63-65,67,68,71-74,77,78,83,86,88] | Vaccine cost-effective, and in some cases, cost-saving [83] |
| PPV23 | Decrease in otitis media and pneumonia |
Italy, US | [76,87] | Vaccination cost-saving [87] |
| RV | Decrease in hospitalizations, outpatient and emergency department visit for all-cause and rotavirus gastroenteritis |
Brazil, Italy, US | [60,75,82,84] | Decreased curative health care costs [60,75,82]; increase [82] or no decrease [75,75] in health system costs. |
Hepatitis B (HepB), Haemophilus influenzae type b (Hib), pneumococcal conjugate vaccine (PCV), 23-valent pneumococcal polysaccharide vaccine (PPV23), rotavirus (RV).
The introduction of new vaccines has created interest in the development of diversified and innovative funding sources and mechanisms for vaccine introduction and sustainability. For example, innovative mechanisms such as bridge funding from the Vaccine Fund [49]; World Bank, UNICEF, USAID and WHO [42]; and the GAVI alliance [126] have accelerated and increased the coverage of new vaccines in low income countries. However, lack of financial planning impacted program sustainability in some countries [49,127].
3.8. Leadership and governance
New vaccine introduction impacted various aspects of the vaccine recommendation and regulatory processes. In Australia, Belgium, Canada, Chile, Greece, Taiwan, United Kingdom, United States, and Uruguay [4,21,27,39,128,129], existing national regulatory institutions and advisory committees were used to license and develop recommendations for new vaccines. Frequently, subcommittees were formed to develop recommendations for the specific vaccine, often in collaboration with academic pediatric and infectious disease organizations [39] and disease-specific societies[21,129].
Planning of vaccination campaigns has gone beyond the immunization program to include the departments of education, health, and defense; academic institutions and local government [10,21]. In some countries, legislation was enacted to promote vaccine implementation or evaluation. For example, Italy had a law requiring routine infant HepB vaccination and catch-up vaccination of unvaccinated adolescents [130], and failure to comply had the potential to result in the temporary suspension of paternal authority to ensure immunization of the minor [131]. In Australia, legislation enabled the establishment of a national registry to collect data to assess HPV vaccination coverage [45].
4. Discussion
In this comprehensive review of the published literature, we found that new vaccine introduction had a mixed effect on – and often provided opportunities to strengthen – existing components of the immunization system. Findings related to impact on the larger health system, however, were more limited. Few reviewed papers were designed to evaluate impacts on immunization systems or health systems, and information relevant to our review was often an incidental finding noted in the discussion section of the papers. In addition, most of the reviewed papers were from high- or middle-income countries, whose experiences may not represent those from lower-income countries, where the impact related to new vaccine introduction – both positive and negative – is likely to be greater. Our conclusions, therefore, need to be interpreted in the context of these caveats.
The impact of vaccine introduction differed according to the delivery platform and vaccine formulation. When vaccine introduction made use of existing delivery strategies, such as with routine infant immunization, costs and impact on staffing were substantially less than when vaccines were introduced through newly created platforms. School-based programs were documented to be effective platforms for introducing new vaccines to school-aged children and adolescents, although additional staff was required, even for existing programs. Venues outside the school were sometimes needed to complete the vaccination series in a timely fashion. Combination vaccines that added the new antigen or antigens to an existing vaccine were less costly and more efficiently introduced than those that required an additional injection.
Disruptions in routine vaccination services were reported, related to insufficient on-hand stock of the new vaccine when programs commenced, or to global vaccine shortages [132]. In low-income countries, service disruption also occurred due to vaccine program funding shortfalls, some of which were resolved through partner and donor contributions and infrastructure strengthening [22,37]. New vaccine introductions have highlighted existing gaps in logistics and cold chain systems; increased cold chain capacity needs were commonly reported with the introduction of the early formulations of RV vaccine [56,133]. As a result of early introduction experiences with inadequate infrastructure, effective vaccine management [134] assessments and regular cold chain inventories are now a precondition for new GAVI support in order to assure system readiness for the new vaccine. UNICEF has developed a new communication framework for new vaccine introduction that emphasizes cold chain readiness, improved adapted vaccines, presentations with reduced cold chain volumes, out-of-cold-chain use for outreach sessions, new delivery technologies, and enhanced training of health care workers [135]. A comprehensive assessment of cold chain capacity should be included in all pre-vaccine introduction assessments.
Reduced disease incidence following new vaccine introduction, which was primarily reported from high-income countries, led to declines in the use of vaccine preventable disease-related curative health services (Table 3). Less antibiotic use, reduced antimicrobial resistance, and herd immunity extended these benefits to populations not targeted by the vaccines. There was some evidence in high-income countries that new vaccine introduction was associated with less use of ambulatory and hospital services and reduced costs. An important benefit to the health system facilitated by new vaccine introduction has been the widespread use of AD syringes and awareness of the importance of injection safety [136,137].
In high income countries, existing infrastructures were utilized and often strengthened to provide information for vaccine introduction into the early childhood vaccine schedule. In some countries, established health information and disease surveillance systems were enhanced to collect data for policy development, program advocacy, and impact assessment. In other countries, new systems were developed to monitor vaccine safety, vaccine effectiveness, and coverage with vaccines administered to age groups beyond early childhood, such as HPV vaccine. These new or enhanced systems can be expanded and adapted to facilitate introduction of other vaccines, and to improve disease surveillance.
National immunization advisory committees, often referred to as National Immunization Technical Advisory Groups (NITAGs), assist Ministries of Health develop evidence-based decisions regarding vaccine and immunization policy, including the introduction of new vaccines [138,139]. While such committees are more common in high and middle income countries, they will likely play increasingly important roles in low-income countries. The importance of social mobilization for the public and training and education for health care workers was frequently noted. The introduction of new vaccines led to the establishment of legislation intended to improve vaccine delivery or program assessment, including mandatory newborn vaccination [131] or school entry laws and national vaccine registries.
This review was subject to a number of limitations. Although much of the information about the impact of new vaccine introduction is contained in the gray literature [132] only published papers were included in this review. We originally proposed to systematically rate the quality and strength of evidence presented in the reviewed articles according to the GRADE method [140,141]. However, because information related to the impact of new vaccine introduction was rarely the main focus of the studies, and because of differences in study design and specific data elements collected, we were unable to compare studies or evaluate data quality. Because most papers were from high-income countries, it is difficult to generalize those experiences to low-income countries, which often have weaker infrastructure, and require donor support to fund immunization programs. While changes in the immunization and health systems occurred in conjunction with new vaccine introduction, we recognize that other health initiatives occurring in the countries during the same time period may have also contributed to these changes. Although the majority of papers we reviewed were published during the past decade, we also included reports of introductions from more than 20 years ago, and these reports may be less relevant to current introductions of new vaccines. While it is likely that the impact of vaccine introduction on a country’s existing immunization and health system reflected the underlying system strength, evaluating this was beyond the scope of this review.
Donor funding for immunization programs in developing countries is not always consistent or predictable; however, in recent years, a number of new immunization funding mechanisms have been introduced to provide more stable vaccine financing, and will likely facilitate new vaccine introductions in the future. While new vaccine introduction often includes an assessment of disease burden and impact on morbidity and mortality, a component of future evaluations should include the systematic and objective assessment of how the vaccine introduction affects the country’s immunization system and broader health system, especially in low-income countries.
Acknowledgements
CDC, WHO, and PATH librarians for assisting with the searches and locating articles; Cristina Averhoff (CDC student intern) and Samantha Wu (WHO student intern) for their organizational skills; Steven Wassilak for review of Italian articles; Jacek Skarbinski for review of Polish articles; Adina Hirsch for review of Hebrew articles; Enbo Ma for review of Chinese articles; Ahmet Afsar for review of Turkish articles; and David Durham, Narendra Arora, Maria Otelia Costales, Tracie Gardener, and Benjamin Dahl for abstracting articles.
Appendix A. The New Vaccine Introduction Impact Published Literature Working Group
Jacqueline Gindler (Centers for Disease Control and Prevention 1600 Clifton Road NE, MS A04, Atlanta, GA 30333 USA, jgindler@cdc.gov)
Susan T. Goldstein (Centers for Disease Control and Prevention 1600 Clifton Road NE, MS A04, Atlanta, GA 30333 USA, sgoldstein@cdc.gov)
W. Scott Gordon (Program for Appropriate Technology in Health (PATH) P. O. Box 900922, Seattle, WA 98109 USA, sgordon@path.org)
Logan Brenzel (Cascadia Health and Development, 7A The Mews, Cascade, Port of Spain, Trinidad, loganbrenzel@gmail.com)
Jessica C. Shearer (Centre for Health Economics and Policy Analysis, McMaster University, 1280 Main Street W, Hamilton, ON Canada, shearejc@mcmaster.ca)
Michael Favin (The Manoff Group, 4301 Connecticut Avenue, NW, Suite 454, Washington, DC, 20008, USA, mfavin@manoffgroup.com; Maternal and Child Health Integrated Program (MCHIP), 1776 Massachusetts Avenue, NW, Suite 300, Washington, DC 20036, USA)
Footnotes
CDC - The findings and conclusions in this report are those of the author and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
WHO - the authors are staff members of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the World Health Organization.
References
- [1].Hadler SC, Dietz VJ, Okwo-Bele JM, Cutts FT. Immunization in developing countries. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Elsevier Inc.; Philadeplhia: 2008. pp. 1541–72. [Google Scholar]
- [2].World Health Organization . Everybody business: strengthening health systems to improve health outcomes: WHO’s framework for action. WHO Press; Geneva: 2007. [Google Scholar]
- [3].Nakhla I, Frenck RW, Jr, Teleb NA, El Oun S, Sultan Y, Mansour H, et al. The changing epidemiology of meningococcal meningitis after introduction of bivalent A/C polysaccharide vaccine into school-based vaccination programs in Egypt. Vaccine. 2005 May;23(25):3288–93. doi: 10.1016/j.vaccine.2005.01.084. [DOI] [PubMed] [Google Scholar]
- [4].Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine. 2001 Oct;20(Suppl. 1):S58–67. doi: 10.1016/s0264-410x(01)00299-7. [DOI] [PubMed] [Google Scholar]
- [5].Leask J, Jackson C, Trevena L, McCaffery K, Brotherton J. Implementation of the Australian HPV vaccination program for adult women: qualitative key informant interviews. Vaccine. 2009 Sep;27(40):5505–12. doi: 10.1016/j.vaccine.2009.06.102. [DOI] [PubMed] [Google Scholar]
- [6].DeRoeck D, Ochiai RL, Yang J, Anh DD, Alag V, Clemens JD. Typhoid vaccination: the Asian experience. Expert Rev Vaccines. 2008 Jul;7(5):547–60. doi: 10.1586/14760584.7.5.547. [DOI] [PubMed] [Google Scholar]
- [7].Campbell H, Borrow R, Salisbury D, Miller E. Meningococcal C conjugate vaccine: the experience in England and Wales. Vaccine. 2009 Jun;27(Suppl. 2):B20–9. doi: 10.1016/j.vaccine.2009.04.067. [DOI] [PubMed] [Google Scholar]
- [8].Borruto F, Hoppenbrouwers K. HPV vaccination in adolescents: from clinical data to implementation and practice. Curr Cancer Ther Rev. 2010;6(2):138–42. [Google Scholar]
- [9].Bettinger JA, Scheifele DW, Le Saux N, Halperin SA, Vaudry W, Tsang R, et al. The impact of childhood meningococcal serogroup C conjugate vaccine programs in Canada. Pediatr Infect Dis J. 2009 Mar;28(3):220–4. doi: 10.1097/INF.0b013e31819040e7. [DOI] [PubMed] [Google Scholar]
- [10].Agtini MD, Ochiai RL, Soeharno R, Lee HJ, Sundoro J, Hadinegoro SR, et al. Introducing Vi polysaccharide typhoid fever vaccine to primary school children in North Jakarta, Indonesia, via an existent school-based vaccination platform. Public Health. 2006 Nov;120(11):1081–7. doi: 10.1016/j.puhe.2006.06.008. [DOI] [PubMed] [Google Scholar]
- [11].Szilagyi PG, Griffin MR, Shone LP, Bartha R, Zhu Y, Schaffer S, et al. The impact of conjugate pneumococcal vaccination on routine childhood vaccination and primary care use in 2 counties. Pediatrics. 2006 Oct;118(4):1394–402. doi: 10.1542/peds.2006-0314. [DOI] [PubMed] [Google Scholar]
- [12].Kalies H, Grote V, Verstraeten T, Hessel L, Schmitt H-J, von Kries R. The use of combination vaccines has improved timeliness of vaccination in children. Pediatr Infect Dis J. 2006 Jun;25(6):507–12. doi: 10.1097/01.inf.0000222413.47344.23. [DOI] [PubMed] [Google Scholar]
- [13].Chunsuttiwat S, Biggs BA, Maynard J, Thamapalo S, Laoboripat S, Bovornsin S, et al. Integration of hepatitis B vaccination into the expanded programme on immunization in Chonburi and Chiangmai provinces, Thailand. Vaccine. 1997 Apr-May;15(6-7):769–74. doi: 10.1016/s0264-410x(96)00226-5. [DOI] [PubMed] [Google Scholar]
- [14].Centers for Disease Control Prevention Global progress toward universal childhood hepatitis B vaccination, 2003. MMWR Morb Mortal Wkly Rep. 2003 Sep;52(36):868–70. [PubMed] [Google Scholar]
- [15].Cabezas C, Echevarria C, Gomez G, Gotuzzo E. Pilot program of immunization against viral hepatitis B, integrated in the extended immunization program in Abancay (peru) Rev Gastroenterol Peru. 1995 Sep-Dec;15(3):215–22. [PubMed] [Google Scholar]
- [16].Brotherton JML, Deeks SL, Campbell-Lloyd S, Misrachi A, Passaris I, Peterson K, et al. Interim estimates of human papillomavirus vaccination coverage in the school-based program in Australia. Commun Dis Intell. 2008 Dec;32(4):457–61. doi: 10.33321/cdi.2008.32.45. [DOI] [PubMed] [Google Scholar]
- [17].De Wals P, Boulianne N, Sevin E, Ouakki M, Deceuninck G, Guay M. Uptake of pneumococcal conjugate vaccine: methodological issues in measurement and impact of publicly funded programs. Can J Public Health. 2009;100(6):413–6. doi: 10.1007/BF03404335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dobson S, Scheifele D, Bell A. Assessment of a universal, school-based hepatitis B vaccination program. JAMA. 1995 Oct;274(15):1209–13. [PubMed] [Google Scholar]
- [19].Goh KT. Prevention and control of hepatitis B virus infection in Singapore. Annals Acad Med Singapore. 1997 Sep;26(5):671–81. [PubMed] [Google Scholar]
- [20].Jiang H, Zhang M, Liang J, Deng C, Cheng A. Evaluation on immunization effect of hepatitis B vaccine of GAVI project in Fukang City, Xinjiang. Endemic Dis Bull/Di Fang Bing Tong Bao. 2010;25(2):27–8. [Google Scholar]
- [21].Chien Y-C, Jan C-F, Kuo H-S, Chen C-J. Nationwide hepatitis B vaccination program in Taiwan: effectiveness in the 20 years after it was launched. Epidemiol Rev. 2006;28:126–35. doi: 10.1093/epirev/mxj010. [DOI] [PubMed] [Google Scholar]
- [22].Wenger JD, DiFabio J, Landaverde JM, Levine OS, Gaafar T. Introduction of Hib conjugate vaccines in the non-industrialized world: experience in four ‘newly adopting’ countries. Vaccine. 1999 Nov;18(7-8):736–42. doi: 10.1016/s0264-410x(99)00269-8. [DOI] [PubMed] [Google Scholar]
- [23].Green D, Catlow D. Immunisation against human papilloma virus in a primary care trust: a report on the first three months of the national campaign. J Infect Prev. 2009;10(3):112–5. [Google Scholar]
- [24].Scheifele D. Hib conjugate vaccines: lessons learned. Int J Clin Pract Suppl. 2001 Feb;(Suppl. 118):8–9. [PubMed] [Google Scholar]
- [25].Bigham M, Remple VP, Pielak K, McIntyre C, White R, Wu W. Uptake and behavioural and attitudinal determinants of immunization in an expanded routine infant hepatitis B vaccination program in British Columbia. Can J Public Health. 2006 Mar-Apr;97(2):90–5. doi: 10.1007/BF03405322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Salisbury DM. The introduction of Haemophilus influenzae type b immunization into the United Kingdom: practical steps to assure success. Pediatr Infect Dis J. 1998 Sep;17(9 Suppl.):S136–9. doi: 10.1097/00006454-199809001-00009. [DOI] [PubMed] [Google Scholar]
- [27].Shefer A, Markowitz L, Deeks S, Tam T, Irwin K, Garland SM, et al. Early experience with human papillomavirus vaccine introduction in the United States, Canada and Australia. Vaccine. 2008 Aug;26(Suppl. 10):K68–75. doi: 10.1016/j.vaccine.2008.05.065. [DOI] [PubMed] [Google Scholar]
- [28].Salisbury D. Introduction of a conjugate meningococcal type C vaccine programme in the UK. J Paediatr Child Health. 2001 Oct;37(5):S34–6. doi: 10.1046/j.1440-1754.2001.00738.x. discussion 7. [DOI] [PubMed] [Google Scholar]
- [29].Dagan R, Fraser D, Roitman M, Slater P, Anis E, Ashkenazi S, et al. The Israeli Pediatric Bacteremia and Meningitis Group Effectiveness of a nationwide infant immunization program against Haemophilus influenzae b. Vaccine. 1999 Jan;17(2):134–41. doi: 10.1016/s0264-410x(98)00165-0. [DOI] [PubMed] [Google Scholar]
- [30].Hanna JN, Bullen RC, Ziegler CL, Akee T, Dostie BG, Lort-Phillips K. An assessment of the implementation of the pneumococcal conjugate vaccination program for Aboriginal and Torres Strait infants in north Queensland. Commun Dis Intell. 2003;27(2):262–6. doi: 10.33321/cdi.2003.27.50. [DOI] [PubMed] [Google Scholar]
- [31].Garpenholt O, Silfverdal SA, Levin LA. Economic evaluation of general childhood vaccination against Haemophilus influenzae type b in Sweden. Scand J Infect Dis. 1998;30(1):5–10. doi: 10.1080/003655498750002222. [DOI] [PubMed] [Google Scholar]
- [32].Brotherton JML, Leask J, Jackson C, McCaffery K, Trevena LJ. National survey of general practitioners experience of delivering the national human papillomavirus vaccination program. Sexual Health. 2010;7(3):291–8. doi: 10.1071/SH09135. [DOI] [PubMed] [Google Scholar]
- [33].Breen D. Pneumococcal vaccination programme in over 65s and at-risk groups: the Dumfries and Galloway experience. Commun Dis Public Health. 2003 Sep;6(3):228–30. [PubMed] [Google Scholar]
- [34].More Nurses Needed for HPV Scheme. Community Pract. 2008;81(10):4–7. [Google Scholar]
- [35].Henderson CE, Rich JD, Lally MA, Henderson CE, Rich JD, Lally MA. HPV vaccination practices among juvenile justice facilities in the United States. J Adolesc Health. 2010 May;46(5):495–8. doi: 10.1016/j.jadohealth.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ushiur F, Patigul, Liu Q. Survey and analysis of hepatitis B virus vaccine application in Xinjiang from 2003 to 2006. Endemic Dis Bull/Di Fang Bing Tong Bao. 2008;23(6):25–6. 9. [Google Scholar]
- [37].Chihota V, Tswana SA. Hepatitis B prevention and vaccination in Zimbabwe. S Afr J Epidemiol Infect. 2002;17(3/4(Supplement)):60–2. [Google Scholar]
- [38].Yuan J, Wang M, Liang J, Xu J, Li T, Wang D, et al. Analysis of the vaccination program after Wenchuan earthquake in Sichuan, 2008. J Trop Med (Guangzhou) 2008;8(8):833–5. [Google Scholar]
- [39].Landaverde M, Di Fabio JL, Ruocco G, Leal I, de Quadros C. Introduction of a conjugate vaccine against Hib in Chile and Uruguay. Rev Panam Salud Publica. 1999 Mar;5(3):200–6. doi: 10.1590/s1020-49891999000300022. [DOI] [PubMed] [Google Scholar]
- [40].Danovaro-Holliday MC, Garcia S, de Quadros C, Tambini G, Andrus JK. Progress in vaccination against Haemophilus influenzae type b in the Americas. PLoS Med. 2008 Apr;5(4):e87. doi: 10.1371/journal.pmed.0050087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stroffolini T, Pasquini P, Mele A. A nationwide vaccination programme in Italy against hepatitis B virus infection in infants of hepatitis B surface antigen-carrier mothers. Vaccine. 1989;7(2):152–4. doi: 10.1016/0264-410x(89)90056-x. [DOI] [PubMed] [Google Scholar]
- [42].Griffiths UK, Korczak VS, Ayalew D, Yigzaw A. Incremental system costs of introducing combined DTwP-hepatitis B-Hib vaccine into national immunization services in Ethiopia. Vaccine. 2009 Feb;27(9):1426–32. doi: 10.1016/j.vaccine.2008.12.037. [DOI] [PubMed] [Google Scholar]
- [43].Bettinger JA, Scheifele DW, Kellner JD, Halperin SA, Vaudry W, Law B, et al. The effect of routine vaccination on invasive pneumococcal infections in Canadian children, Immunization Monitoring Program, Active 2000-2007. Vaccine. 2010 Feb;28(9):2130–6. doi: 10.1016/j.vaccine.2009.12.026. [DOI] [PubMed] [Google Scholar]
- [44].Bhimma R, Coovadia HM, Adhikari M, Connolly CA. The impact of the hepatitis B virus vaccine on the incidence of hepatitis B virus-associated membranous nephropathy. Arch Pediatr Adolesc Med. 2003 Oct;157(10):1025–30. doi: 10.1001/archpedi.157.10.1025. [DOI] [PubMed] [Google Scholar]
- [45].Brotherton JML, Mullins RM. Estimating coverage of the National HPV Vaccination Program: where are we at? Med J Aust. 2009 Aug;191(3):188. doi: 10.5694/j.1326-5377.2009.tb02738.x. [DOI] [PubMed] [Google Scholar]
- [46].O’Hallahan J, McNicholas A, Galloway Y, O’Leary E, Roseveare C. Delivering a safe and effective strain-specific vaccine to control an epidemic of group B meningococcal disease. N Z Med J. 2009;122(1291):48–59. [PubMed] [Google Scholar]
- [47].Pan XH, Sun LY, Wang CL, Zhu J, Fu ZW, Zeng ZC, et al. Epidemiological characteristics and related influencing factors on Japanese encephalitis in Hainan Province. Zhonghua liu xing bing xue za zhi=Zhonghua liuxingbingxue zazhi. 2009 May;30(5):471–4. [Chinese] [PubMed] [Google Scholar]
- [48].Gong J, Li R-C, Yang J-Y, Li Y-P, Chen X-R, Xu Z-Y, et al. Long-term efficacy of infant hepatitis B immunization program. Chung Hua Kan Tsang Ping Tsa Chih. 2003 Apr;11(4):203–5. [PubMed] [Google Scholar]
- [49].Daza P, Banda R, Misoya K, Katsulukuta A, Gessner BD, Katsande R, et al. The impact of routine infant immunization with Haemophilus influenzae type b conjugate vaccine in Malawi, a country with high human immunodeficiency virus prevalence. Vaccine. 2006 Sep;24(37-39):6232–9. doi: 10.1016/j.vaccine.2006.05.076. [DOI] [PubMed] [Google Scholar]
- [50].von Gottberg A, de Gouveia L, Madhi SA, du Plessis M, Quan V, Soma K, et al. Impact of conjugate Haemophilus influenzae type b (Hib) vaccine introduction in South Africa. Bull World Health Organ. 2006 Oct;84(10):811–8. doi: 10.2471/blt.06.030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Patel M, Pedreira C, De Oliveira LH, Tate J, Orozco M, Mercado J, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009 Jun;301(21):2243–51. doi: 10.1001/jama.2009.756. [DOI] [PubMed] [Google Scholar]
- [52].Ndiaye AA, De Wals P, Proulx J-F, Ouakki M, Jette L, Dery S. Impact of a mass immunization campaign to control an outbreak of severe respiratory infections in Nunavik, northern Canada. Int J Circumpolar Health. 2006 Sep;65(4):297–304. doi: 10.3402/ijch.v65i4.18120. [DOI] [PubMed] [Google Scholar]
- [53].Lagos R, Levine OS, Avendano A, Horwitz I, Levine MM. The introduction of routine Haemophilus influenzae type b conjugate vaccine in Chile: a framework for evaluating new vaccines in newly industrializing countries. Pediatr Infect Dis J. 1998 Sep;17(9 Suppl.):S139–48. doi: 10.1097/00006454-199809001-00010. [DOI] [PubMed] [Google Scholar]
- [54].Freed GL, Clark SJ, Konrad TR, Pathman DE. Factors affecting physicians’ early adoption of combined DTP-Hib vaccine. Ambulatory Child Health. 1997;3(1 I):27–33. [Google Scholar]
- [55].Adegbola RA, Secka O, Lahai G, Lloyd-Evans N, Njie A, Usen S, et al. Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunisation with a Hib conjugate vaccine: a prospective study. Lancet. 2005 Jul;366(9480):144–50. doi: 10.1016/S0140-6736(05)66788-8. [DOI] [PubMed] [Google Scholar]
- [56].de Oliveira LH, Danovaro-Holliday MC, Matus CR, Andrus JK. Rotavirus vaccine introduction in the Americas: progress and lessons learned. Expert Rev Vaccines. 2008;7(3):345–53. doi: 10.1586/14760584.7.3.345. [DOI] [PubMed] [Google Scholar]
- [57].Dorleans F, Giambi C, Dematte L, Cotter S, Stefanoff P, Mereckiene J, et al. The current state of introduction of human papillomavirus vaccination into national immunisation schedules in Europe: first results of the VENICE2 2010 survey. Eurosurveillance. 2010;15(47):19730. doi: 10.2807/ese.15.47.19730-en. [DOI] [PubMed] [Google Scholar]
- [58].Dominguez A, Salleras L, Carmona G, Batalla J. Effectiveness of a mass hepatitis A vaccination program in preadolescents. Vaccine. 2003 Jan;21(7-8):698–701. doi: 10.1016/s0264-410x(02)00583-2. [DOI] [PubMed] [Google Scholar]
- [59].Zhou F, Shefer A, Kong Y, Nuorti JP. Trends in acute otitis media-related health care utilization by privately insured young children in the United States, 1997-2004. Pediatrics. 2008 Feb;121(2):253–60. doi: 10.1542/peds.2007-0619. [DOI] [PubMed] [Google Scholar]
- [60].Wang FT, Mast TC, Glass RJ, Loughlin J, Seeger JD. Effectiveness of the pentavalent rotavirus vaccine in preventing gastroenteritis in the United States. Pediatrics. 2010 Feb;125(2):e208–13. doi: 10.1542/peds.2009-1246. [DOI] [PubMed] [Google Scholar]
- [61].Talbot TR, Poehling KA, Hartert TV, Arbogast PG, Halasa NB, Mitchel E, et al. Reduction in high rates of antibiotic-nonsusceptible invasive pneumococcal disease in tennessee after introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2004 Sep;39(5):641–8. doi: 10.1086/422653. [DOI] [PubMed] [Google Scholar]
- [62].Shoaib A, Rethnam U, Bansal R, Clay N. The effects of mass immunization on Haemophilus influenzae type B-related orthopaedic disease. J Pediatr Orthop B. 2007 May;16(3):236–8. doi: 10.1097/BPB.0b013e3280925703. [DOI] [PubMed] [Google Scholar]
- [63].Ramilo O, Brunton SA, Gooch IWM, Block SL., Jr PCV-7 vaccine: changing the epidemiology of acute otitis media. Adv Stud Med. 2004 Dec;4(10 E):S928–S936. S52–S53. [Google Scholar]
- [64].Poehling KA, Szilagyi PG, Grijalva CG, Martin SW, LaFleur B, Mitchel E, et al. Reduction of frequent otitis media and pressure-equalizing tube insertions in children after introduction of pneumococcal conjugate vaccine. Pediatrics. 2007 Apr;119(4):707–15. doi: 10.1542/peds.2006-2138. [DOI] [PubMed] [Google Scholar]
- [65].Poehling KA, Lafleur BJ, Szilagyi PG, Edwards KM, Mitchel E, Barth R, et al. Population-based impact of pneumococcal conjugate vaccine in young children. Pediatrics. 2004;114(3):755–61. doi: 10.1542/peds.2003-0592-F. [DOI] [PubMed] [Google Scholar]
- [66].Peltola H, Kallio MJ, Unkila-Kallio L. Reduced incidence of septic arthritis in children by Haemophilus influenzae type-b vaccination. Implications for treatment. J Bone Joint Surg Br. 1998 May;80(3):471–3. doi: 10.1302/0301-620x.80b3.8296. [DOI] [PubMed] [Google Scholar]
- [67].Nigrovic LE, Malley R. Evaluation of the febrile child 3 to 36 months old in the era of pneumococcal conjugate vaccine: focus on occult bacteremia. Clin Pediatric Emerg Med. 2004 Mar;5(1):13–9. [Google Scholar]
- [68].Nelson JC, Jackson M, Yu O, Whitney CG, Bounds L, Bittner R, et al. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine. 2008 Sep;26(38):4947–54. doi: 10.1016/j.vaccine.2008.07.016. [DOI] [PubMed] [Google Scholar]
- [69].Madore DV. Impact of immunization on Haemophilus influenzae type b disease. Infect Agents Dis. 1996;5(1):8–20. [PubMed] [Google Scholar]
- [70].Kwong JC, Stukel TA, Lim J, McGeer AJ, Upshur REG, Johansen H, et al. The effect of universal influenza immunization on mortality and health care use. PLoS Med. 2008 Oct;5(10):1440–52. doi: 10.1371/journal.pmed.0050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kourtis AP, Ellington S, Bansil P, Jamieson DJ, Posner SF. Hospitalizations for invasive pneumococcal disease among human immunodeficiency virus-1 infected children, adolescents and young adults in the United States in the era of highly active antiretroviral therapy and the conjugate pneumococcal vaccine. Pediatr Infect Dis J. 2010 Jun;29(6):561–3. doi: 10.1097/INF.0b013e3181cfb65f. [DOI] [PubMed] [Google Scholar]
- [72].Koshy E, Murray J, Bottle A, Sharland M, Saxena S. Impact of the seven-valent pneumococcal conjugate vaccination (PCV7) programme on childhood hospital admissions for bacterial pneumonia and empyema in England: National time-trends study, 1997-2008. Thorax. 2010 Sep;65(9):770–4. doi: 10.1136/thx.2010.137802. [DOI] [PubMed] [Google Scholar]
- [73].Isaacman DJ, Fletcher MA, Fritzell B, Ciuryla V, Schranz J. Indirect effects associated with widespread vaccination of infants with heptavalent pneumococcal conjugate vaccine (PCV7; Prevnar) Vaccine. 2007 Mar;25(13):2420–7. doi: 10.1016/j.vaccine.2006.09.011. [DOI] [PubMed] [Google Scholar]
- [74].Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007 Apr;369(9568):1179–86. doi: 10.1016/S0140-6736(07)60564-9. [DOI] [PubMed] [Google Scholar]
- [75].Giammanco MD, Coniglio MA, Pignato S, Giammanco G. An economic analysis of rotavirus vaccination in Italy. Vaccine. 2009 Jun;27(29):3904–11. doi: 10.1016/j.vaccine.2009.04.002. [DOI] [PubMed] [Google Scholar]
- [76].Gable CB, Holzer SS, Engelhart L, Friedman RB, Smeltz F, Schroeder D, et al. Pneumococcal vaccine. Efficacy and associated cost savings. JAMA. 1990;264(22):2910–5. doi: 10.1001/jama.264.22.2910. [DOI] [PubMed] [Google Scholar]
- [77].De Wals P, Robin E, Fortin E, Thibeault R, Ouakki M, Douville-Fradet M. Pneumonia after implementation of the pneumococcal conjugate vaccine program in the province of Quebec, Canada. Pediatr Infect Dis J. 2008 Nov;27(11):963–8. doi: 10.1097/INF.0b013e31817cf76f. [DOI] [PubMed] [Google Scholar]
- [78].De Wals P, Carbon M, Sevin E, Deceuninck G, Ouakki M. Reduced physician claims for otitis media after implementation of pneumococcal conjugate vaccine program in the Province of Quebec, Canada. Pediatr Infect Dis J. 2009;28(9):e271–5. doi: 10.1097/INF.0b013e3181bad212. [DOI] [PubMed] [Google Scholar]
- [79].Da Villa G. Rationale for the infant and adolescent vaccination programmes in Italy. Vaccine. 2000 Feb;18(Suppl. 1):S31–4. doi: 10.1016/s0264-410x(99)00459-4. [DOI] [PubMed] [Google Scholar]
- [80].Chang MH, Shau WY, Chen CJ, Wu TC, Kong MS, Liang DC, et al. Hepatitis B vaccination and hepatocellular carcinoma rates in boys and girls. JAMA. 2000 Dec;284(23):3040–2. doi: 10.1001/jama.284.23.3040. [DOI] [PubMed] [Google Scholar]
- [81].Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, et al. Taiwan Childhood Hepatoma Study Group Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. N Engl J Med. 1997 Jun;336(26):1855–9. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- [82].Centenari C, Gurgel RQ, Bohland AK, Oliveira DM, Faragher B, Cuevas LE, et al. Rotavirus vaccination in northeast Brazil: a laudable intervention, but can it lead to cost-savings? Vaccine. 2010 Jun;28(25):4162–8. doi: 10.1016/j.vaccine.2010.04.015. [DOI] [PubMed] [Google Scholar]
- [83].Black S, Shinefield H, Baxter R, Austrian R, Bracken L, Hansen J, et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J. 2004 Jun;23(6):485–9. doi: 10.1097/01.inf.0000129685.04847.94. [DOI] [PubMed] [Google Scholar]
- [84].Bégué RE, Perrin K. Reduction in gastroenteritis with the use of pentavalent rotavirus vaccine in a primary practice. Pediatrics. 2010;126(1):e40–5. doi: 10.1542/peds.2009-2069. [DOI] [PubMed] [Google Scholar]
- [85].Barone SR, Aiuto LT. Periorbital and orbital cellulitis in the Haemophilus influenzae vaccine era. J Pediatr Ophthalmol Strabismus. 1997 Sep;34(5):293–6. doi: 10.3928/0191-3913-19970901-08. [DOI] [PubMed] [Google Scholar]
- [86].Balazs GC, Garcia FJ, Yamamoto LG. Conjugate heptavalent pneumococcal vaccine outcome improvements. Hawaii Med J. 2006 Oct;65(10):288–9. [PubMed] [Google Scholar]
- [87].Ansaldi F, Turello V, Lai P, Bastone G, De Luca S, Rosselli R, et al. Effectiveness of a 23-valent polysaccharide vaccine in preventing pneumonia and non-invasive pneumococcal infection in elderly people: a large-scale retrospective cohort study. J Int Med Res. 2005 Sep-Oct;33(5):490–500. doi: 10.1177/147323000503300503. [DOI] [PubMed] [Google Scholar]
- [88].Ansaldi F, Sticchi L, Durando P, Carloni R, Oreste P, Vercelli M, et al. Decline in pneumonia and acute otitis media after the introduction of childhood pneumococcal vaccination in Liguria, Italy. J Int Med Res 2008. 2008;36(6):1255–60. doi: 10.1177/147323000803600612. [DOI] [PubMed] [Google Scholar]
- [89].Da Villa G, Sepe A. Immunization programme against hepatitis B virus infection in Italy: cost-effectiveness. Vaccine. 1999 Jan;17(13-14):1734–8. doi: 10.1016/s0264-410x(98)00414-9. [DOI] [PubMed] [Google Scholar]
- [90].Adamkiewicz TV, Silk BJ, Howgate J, Baughman W, Strayhorn G, Sullivan K, et al. Effectiveness of the 7-valent pneumococcal conjugate vaccine in children with sickle cell disease in the first decade of life. Pediatrics. 2008 Mar;121(3):562–9. doi: 10.1542/peds.2007-0018. [DOI] [PubMed] [Google Scholar]
- [91].Gortzak-Uzan L, Fraser D, Dagan R. Epidemiology of invasive Hemophilus influenzae B infections in Bedouins and Jews; conjugate Hib vaccines. Harefuah. 1998 Sep;135(5-6):175–80. 256. [in Hebrew] [PubMed] [Google Scholar]
- [92].McIntyre PB, Chey T, Smith WT. The impact of vaccination against invasive Haemophilus influenzae type B disease in the Sydney region. Med J Aust. 1995 Mar;162(5):245–8. doi: 10.5694/j.1326-5377.1995.tb139877.x. [DOI] [PubMed] [Google Scholar]
- [93].Bower C, Condon R, Payne J, Burton P, Watson C, Wild B. Measuring the impact of conjugate vaccines on invasive Haemophilus influenzae type b infection in Western Australia. Aust N Z J Public Health. 1998 Feb;22(1):67–72. doi: 10.1111/j.1467-842x.1998.tb01147.x. [DOI] [PubMed] [Google Scholar]
- [94].Talbot TR, Poehling KA, Hartert TV, Arbogast PG, Halasa NB, Mitchel E, et al. Elimination of racial differences in invasive pneumococcal disease in young children after introduction of the conjugate pneumococcal vaccine. Pediatr Infect Dis J. 2004 Aug;23(8):726–31. doi: 10.1097/01.inf.0000133046.60555.de. [DOI] [PubMed] [Google Scholar]
- [95].Scheifele DW, Jadavji TP, Law BJ, Gold R, MacDonald NE, Lebel MH, et al. Recent trends in pediatric Haemophilus influenzae type b infections in Canada. Can Med Assoc J. 1996;154(7):1041–7. [PMC free article] [PubMed] [Google Scholar]
- [96].Ladhani S, Slack MP, Heys M, White J, Ramsay ME. Fall in Haemophilus influenzae serotype b (Hib) disease following implementation of a booster campaign. Arch Dis Child. 2008 Aug;93(8):665–9. doi: 10.1136/adc.2007.126888. [DOI] [PubMed] [Google Scholar]
- [97].Diez-Domingo J, Pereiro I, Morant A, Gimeno C, San-Martin M, Gonzalez A, et al. Impact of non-routine vaccination on the incidence of invasive Haemophilus influenzae type b (Hib) disease: experience in the autonomous region of Valencia, Spain. J Infect. 2001 May;42(4):257–60. doi: 10.1053/jinf.2001.0832. [DOI] [PubMed] [Google Scholar]
- [98].Dickinson FO, Perez AE, Galindo MA, Quintana I. Impact of vaccination against Haemophilus influenzae type b in Cuba. Rev Panam Salud Publica. 2001 Sep;10(3):169–73. doi: 10.1590/s1020-49892001000900004. [DOI] [PubMed] [Google Scholar]
- [99].Cisse MF, Breugelmans JG, Bg M, Diop MB, Faye PC, Mhlanga B, et al. The elimination of Haemophilus influenzae type b meningitis following conjugate vaccine introduction in senegal. Pediatr Infect Dis J. 2010 Jun;29(6):499–503. doi: 10.1097/INF.0b013e3181ccb0a0. [DOI] [PubMed] [Google Scholar]
- [100].Broadhurst LE, Erickson RL, Kelley PW. Decreases in invasive Haemophilus influenzae diseases in US Army children, 1984 through 1991. JAMA. 1993 Jan;269(2):227–31. [PubMed] [Google Scholar]
- [101].Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003 May;348(18):1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- [102].van der Linden M, Reinert RR, Imohl M. The National Immunization Programme for pneumococcal conjugate vaccine in Germany—first signs of a herd immunity effect in adults. Int J Med Microbiol. 2009 Sep;299:85. [Google Scholar]
- [103].Shafinoori S, Ginocchio CC, Greenberg AJ, Yeoman E, Cheddie M, Rubin LG. Impact of pneumococcal conjugate vaccine and the severity of winter influenza-like illnesses on invasive pneumococcal infections in children and adults. Pediatr Infect Dis J. 2005 Jan;24(1):10–6. doi: 10.1097/01.inf.0000148930.41928.0d. [DOI] [PubMed] [Google Scholar]
- [104].Poehling KA, Talbot TR, Griffin MR, Craig AS, Whitney CG, Zell E, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA. 2006 Apr;295(14):1668–74. doi: 10.1001/jama.295.14.1668. [DOI] [PubMed] [Google Scholar]
- [105].McBean AM, Park Y-T, Caldwell D, Yu X. Declining invasive pneumococcal disease in the U.S. elderly. Vaccine. 2005 Dec;23(48-49):5641–5. doi: 10.1016/j.vaccine.2005.05.043. [DOI] [PubMed] [Google Scholar]
- [106].Jardine A, Menzies RI, McIntyre PB. Reduction in hospitalizations for pneumonia associated with the introduction of a pneumococcal conjugate vaccination schedule without a booster dose in Australia. Pediatr Infect Dis J. 2010 Jul;29(7):607–12. doi: 10.1097/inf.0b013e3181d7d09c. [DOI] [PubMed] [Google Scholar]
- [107].Flannery B, Schrag S, Bennett NM, Lynfield R, Harrison LH, Reingold A, et al. Impact of childhood vaccination on racial disparities in invasive Streptococcus pneumoniae infections. JAMA. 2004 May;291(18):2197–203. doi: 10.1001/jama.291.18.2197. [DOI] [PubMed] [Google Scholar]
- [108].Salleras L, Domínguez A, Prats G, Parron I, Muñoz P. Dramatic decline of serogroup C meningococcal disease incidence in Catalonia (Spain) 24 months after a mass vaccination programme of children and young people. J Epidemiol Commun Health. 2001;55(4):283–7. doi: 10.1136/jech.55.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Ramsay ME, Andrews NJ, Trotter CL, Kaczmarski EB, Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ. 2003 Feb;326(7385):365–6. doi: 10.1136/bmj.326.7385.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kinlin LM, Jamieson F, Brown EM, Brown S, Rawte P, Dolman S, et al. Rapid identification of herd effects with the introduction of serogroup C meningococcal conjugate vaccine in Ontario, Canada, 2000-2006. Vaccine. 2009 Mar;27(11):1735–40. doi: 10.1016/j.vaccine.2009.01.026. [DOI] [PubMed] [Google Scholar]
- [111].De Wals P, Erickson L. Economic analysis of the 1992-1993 mass immunization campaign against serogroup C meningococcal disease in Quebec. Vaccine. 2002 Jun;20(21-22):2840–4. doi: 10.1016/s0264-410x(02)00161-5. [DOI] [PubMed] [Google Scholar]
- [112].De Wals P. Meningococcal C vaccines: the Canadian experience. Pediatr Infect Dis J. 2004 Dec;23(12 Suppl.):S280–4. [PubMed] [Google Scholar]
- [113].Bergman BP, Hayton JC, Green AD. Effectiveness of the meningococcal vaccination programme for British Armed Forces recruits. Commun Dis Public Health. 2000 Dec;3(4):298–9. [PubMed] [Google Scholar]
- [114].Balmer P, Borrow R, Miller E. Impact of meningococcal C conjugate vaccine in the UK. J Med Microbiol. 2002;51(9):717–22. doi: 10.1099/0022-1317-51-9-717. [DOI] [PubMed] [Google Scholar]
- [115].Chan CY, Lee SD, Lo KJ. Legend of hepatitis B vaccination: the Taiwan experience. J Gastroenterol Hepatol. 2004 Feb;19(2):121–6. doi: 10.1111/j.1440-1746.2004.03153.x. [DOI] [PubMed] [Google Scholar]
- [116].Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, Esparza-Aguilar M, Johnson B, Gomez-Altamirano CM, et al. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med. 2010 Jan;362(4):299–305. doi: 10.1056/NEJMoa0905211. [DOI] [PubMed] [Google Scholar]
- [117].Paulke-Korinek M, Rendi-Wagner P, Kundi M, Kronik R, Kollaritsch H, Paulke-Korinek M, et al. Universal mass vaccination against rotavirus gastroenteritis: impact on hospitalization rates in austrian children. Pediatr Infect Dis J. 2010 Apr;29(4):319–23. doi: 10.1097/INF.0b013e3181c18434. [DOI] [PubMed] [Google Scholar]
- [118].de Palma O, Cruz L, Ramos H, de Baires A, Villatoro N, Pastor D, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ. 2010;340:c2825. doi: 10.1136/bmj.c2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Cortese MM, Tate JE, Simonsen L, Edelman L, Parashar UD. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatr Infect Dis J. 2010 Jun;29(6):489–94. doi: 10.1097/INF.0b013e3181d95b53. [DOI] [PubMed] [Google Scholar]
- [120].Clark HF, Lawley D, Mallette LA, DiNubile MJ, Hodinka RL. Decline in cases of rotavirus gastroenteritis presenting to The Children’s Hospital of Philadelphia after introduction of a pentavalent rotavirus vaccine. Clin Vaccine Immunol. 2009 Mar;16(3):382–6. doi: 10.1128/CVI.00382-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Centers for Disease Control Prevention Reduction in rotavirus after vaccine introduction—United States, 2000-2009. MMWR Morb Mortal Wkly Rep. 2009 Oct;58(41):1146–9. [PubMed] [Google Scholar]
- [122].Centers for Disease Control Prevention Delayed onset and diminished magnitude of rotavirus activity—United States. November 2007-May 2008. MMWR Morb Mortal Wkly Rep. 2008 Jun;57(25):697–700. [PubMed] [Google Scholar]
- [123].Belshaw DA, Muscatello DJ, Ferson MJ, Nurkic A. Rotavirus vaccination one year on. Commun Dis Intell. 2009 Sep;33(3):337–40. doi: 10.33321/cdi.2009.33.37. [DOI] [PubMed] [Google Scholar]
- [124].Fairley CK, Hocking JS, Gurrin LC, Chen MY, Donovan B, Bradshaw CS. Rapid decline in presentations of genital warts after the implementation of a national quadrivalent human papillomavirus vaccination programme for young women. Sex Transm Infect. 2009 Dec;85(7):499–502. doi: 10.1136/sti.2009.037788. [DOI] [PubMed] [Google Scholar]
- [125].Barr E, Gause CK, Bautista OM, Railkar RA, Lupinacci LC, Insinga RP, et al. Impact of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 virus-like particle vaccine in a sexually active population of North American women. Am J Obstet Gynecol. 2008 Mar;198(3):11. doi: 10.1016/j.ajog.2007.09.001. [DOI] [PubMed] [Google Scholar]
- [126].Lydon P, Levine R, Makinen M, Brenzel L, Mitchell V, Milstien JB, et al. Introducing new vaccines in the poorest countries: what did we learn from the GAVI experience with financial sustainability? Vaccine. 2008 Dec;26(51):6706–16. doi: 10.1016/j.vaccine.2008.10.015. [DOI] [PubMed] [Google Scholar]
- [127].Brugha R, Starling M, Walt G. GAVI, the first steps: lessons for the Global Fund. Lancet. 2002 Feb;359(9304):435–8. doi: 10.1016/S0140-6736(02)07607-9. [DOI] [PubMed] [Google Scholar]
- [128].Simoens C, Sabbe M, Van Damme P, Beutels P, Arbyn M. Introduction of human papillomavirus (HPV) vaccination in Belgium, 2007-2008. Euro Surveill. 2009;14(46) [PubMed] [Google Scholar]
- [129].Papaevangelou G. Hepatitis B immunization programme: lessons learnt in Greece. Vaccine. 1998 Nov;16(Suppl.):S45–7. doi: 10.1016/s0264-410x(98)00293-x. [DOI] [PubMed] [Google Scholar]
- [130].Bonanni P, Crovari P. Success stories in the implementation of universal hepatitis B vaccination: an update on Italy. Vaccine. 1998 Nov;(Suppl.):S38–42. doi: 10.1016/s0264-410x(98)00291-6. [DOI] [PubMed] [Google Scholar]
- [131].Bonanni P, Colombai R, Gasparini R, Lo Nostro A, Tiscione E, Tomei A, et al. Impact of routine infant and adolescent hepatitis B vaccination in Tuscany, Central Italy. Pediatr Infect Dis J. 1999 Aug;18(8):677–82. doi: 10.1097/00006454-199908000-00005. [DOI] [PubMed] [Google Scholar]
- [132].Favin M, Macabasco R, Steinglass R. Impact of new vaccine introduction on developing country immunization programs and broader health systems: a review of the grey literature. In: Organization WH, editor. WHO Strategic Group of Experts on Immunization Meeting of April 2012. USAID-funded Maternal and Child Health Integrated Program (MCHIP); Geneva: 2012. [Google Scholar]
- [133].World Health Organization . In: Introduction of rotavirus vaccines into national immunization programmes Management manual, including operational information for health workers. Department of Immunization VaB, editor. WHO Press; Geneva: 2009. [Google Scholar]
- [134].World Health Organization [cited 06.03.12];Effective Vaccine Management Initiative. 2010 Available from: http://www.who.int/immunizationdelivery/systemspolicy/logistics/en/index6.html.
- [135].UNICEF Communication Framework for New Vaccines and Child Survival. 2011 Available from: https://sites.google.com/site/commframe/ cited 05.05.12.
- [136].Levin A, Fang A, Hansen PM, Pyle D, Dia O, Schwalbe N. A global health partnership’s use of time-limited support to catalyze health practice change: the case of GAVI’s Injection Safety Support. PLoS One. 2010;5(9):e12986. doi: 10.1371/journal.pone.0012986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Cui F-Q, Gong X-H, Chen Y-S. Evaluation on impact of hepatitis B vaccine integrated into routine immunization in the areas of Ministry of Health/Global Alliance for Vaccine and Immunization (GAVI) Cooperation Project P.R, China. Zhongguo Ji Hua Mian Yi. 2009 Aug;15(4):289–93. [PubMed] [Google Scholar]
- [138].Gessner BD, Duclos P, Deroeck D, Nelson EA. Informing decision makers: experience and process of 15 National Immunization Technical Advisory Groups. Vaccine. 2010 Apr;28(Suppl. 1):A1–5. doi: 10.1016/j.vaccine.2010.02.025. [DOI] [PubMed] [Google Scholar]
- [139].Duclos P. National Immunization Technical Advisory Groups (NITAGs): guidance for their establishment and strengthening. Vaccine. 2010 Apr;28(Suppl. 1):A18–25. doi: 10.1016/j.vaccine.2010.02.027. [DOI] [PubMed] [Google Scholar]
- [140].Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008 Apr;336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004 Jun;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]