Significance
Since its introduction to North America, West Nile virus (WNV) has impacted the health of both human and wildlife populations, but the extent of the burden across host species remains poorly understood. Using extensive mark-recapture data from 49 species spanning nearly two decades, combined with recently developed models of WNV risk, we estimated the impacts of this emergent disease on avian populations. We show that WNV has had significant negative effects on survival of 47% of bird species examined. We provide the first in-depth picture of the extent, duration, and phylogenetic signal of WNV impacts on bird populations. Our results suggest that introduced infectious diseases can have significant persistent effects on populations long after initial concerns have waned.
Keywords: demography, avian populations, West Nile virus, emerging infectious diseases, population biology
Abstract
Since its introduction to North America in 1999, West Nile virus (WNV) has had devastating impacts on native host populations, but to date these impacts have been difficult to measure. Using a continental-scale dataset comprised of a quarter-million birds captured over nearly two decades and a recently developed model of WNV risk, we estimated the impact of this emergent disease on the survival of avian populations. We find that populations were negatively affected by WNV in 23 of the 49 species studied (47%). We distinguished two groups of species: those for which WNV negatively impacted survival only during initial spread of the disease (n = 11), and those that show no signs of recovery since disease introduction (n = 12). Results provide a novel example of the taxonomic breadth and persistent impacts of this wildlife disease on a continental scale. Phylogenetic analyses further identify groups (New World sparrows, finches, and vireos) disproportionally affected by temporary or persistent WNV effects, suggesting an evolutionary dimension of disease risk. Identifying the factors affecting the persistence of a disease across host species is critical to mitigating its effects, particularly in a world marked by rapid anthropogenic change.
The emergence of wildlife diseases in new geographic locations and naïve host populations is the inevitable consequence of a present-day global ecology characterized by unprecedented connectivity. Increases in anthropogenic stressors to the environment, including changes in land use and climate, have made exposure to new diseases more likely (1–3), and a rise in global transport and intercontinental travel has allowed “old” diseases to infect hosts in new environments (4–6). Such introductions may have negative effects on hosts that have never been exposed to a disease (7, 8), and once exposed, the resilience and recovery rates of naïve host populations remain unclear. Although such introductions are most often relegated to isolated regions, such as islands, the devastating impacts of emerging infectious diseases can also reach continental scales.
The rapid spread of West Nile virus (WNV) across North America in just 5 y (1999–2003) has been associated with the death of millions of native wild birds that act as primary hosts to the virus (9). Despite the documented loss of individual birds and apparent impacts on bird populations, no study to date has fully documented the demographic impacts of WNV on avian populations across large regions of North America. Previous analyses identified the negative effects of WNV in roughly one-fifth to one-third of the bird species studied (8, 10). However, these studies used animal count data that likely underestimate impacts because, even in the face of high mortality, recruitment and immigration may mask population declines (11). WNV is hypothesized to influence bird populations through reductions in survival, which can be influenced by various biotic and abiotic factors, including age (12), climate (13, 14), and regional environment heterogeneity (15). In addition, a number of studies suggest that the impact of WNV on bird populations increases with human land use (16, 17). Understanding the contribution of disease to annual variation in survival across space and time requires rigorous demographic analyses that take into account these factors.
We use 16 y of mark-recapture data collected at over 500 bird-banding stations across the United States (Fig. S1), in combination with newly developed spatiotemporal models of WNV risk (18) and land-use patterns, to examine the effects of WNV spread on survival rates of all bird species for which analyses were possible (49 species) (Table S1). Our objectives were threefold. First, we examined the relative contribution of an introduced infectious disease on landbird annual survival across a broad geographical range, controlling for the influence of human land use, climate, and regional covariates on survival (Table S2). Second, we determined how effects of WNV on annual survival have changed through time, by examining impacts of WNV on survival of these species before, during, and after its arrival. Finally, we explored whether species experienced differential effects from WNV depending on their ecological distribution or evolutionary histories.
Table S1.
Species included in survival analysis, number of individuals captured, number of banding stations, and the average WNV risk (SE) at the stations where a species was captured
| Common name | Scientific name | Sample size* | Banding stations | Mean (SE) WNV risk† |
| Song sparrow | Melospiza melodia | 17,641 | 201 | 182.11 (1.24) |
| Gray catbird | Dumetella carolinensis | 17,521 | 129 | 347.83 (1.376) |
| Swainson's thrush | Catharus ustulatus | 15,433 | 114 | 71.09 (0.605) |
| Yellow warbler | Setophaga petechia | 15,111 | 124 | 165.39 (1.35) |
| Common yellowthroat | Geothlypis trichas | 14,497 | 169 | 274.53 (1.536) |
| Wilson's warbler | Cardellina pusilla | 12,057 | 81 | 83.25 (0.76) |
| American robin | Turdus migratorius | 11,041 | 196 | 171.57 (1.794) |
| American goldfinch | Carduelis tristis | 10,735 | 115 | 263.24 (1.882) |
| Northern cardinal | Cardinalis cardinalis | 10,709 | 173 | 370.88 (1.86) |
| Dark-eyed junco | Junco hyemalis | 9,304 | 105 | 72.25 (0.93) |
| MacGillivray’s warbler | Oporornis tolmiei | 8,948 | 89 | 84.34 (0.9) |
| Wood thrush | Hylocichla mustelina | 7,339 | 115 | 314.6 (1.94) |
| Warbling vireo | Vireo gilvus | 7,151 | 77 | 117.16 (1.13) |
| Black-headed grosbeak | Pheucticus melanocephalus | 6,068 | 97 | 228.6 (1.919) |
| Yellow-rumped warbler | Setophaga coronate | 5,679 | 75 | 81.22 (1.33) |
| Red-eyed vireo | Vireo olivaceus | 5,616 | 100 | 269.62 (2.1756) |
| Yellow-breasted chat | Icteria virens | 5,497 | 68 | 281.9 (1.85) |
| White-eyed vireo | Vireo griseus | 5,097 | 66 | 391.84 (2.67) |
| House wren | Troglodytes aedon | 5,086 | 85 | 313.88 (2.38) |
| Ovenbird | Seiurus aurocapilla | 4,861 | 89 | 235.28 (2.2925) |
| Carolina wren | Thryothorus ludovicianus | 4,744 | 93 | 274.77 (2.78) |
| Orange-crowned warbler | Vermivora celata | 4,744 | 61 | 101.79 (1.7937) |
| Purple finch | Carpodacus purpureus | 4,368 | 32 | 111.45 (1.21) |
| Spotted towhee | Pipilo maculatus | 4,342 | 84 | 264.61 (2.34) |
| Acadian flycatcher | Empidonax virescens | 4,065 | 59 | 291.11 (1.70) |
| Black-capped chickadee | Parus atricapillus | 3,945 | 102 | 184.47 (2.82) |
| Western flycatcher | Empidonax occidentalis | 3,890 | 60 | 140.21 (2.19) |
| Traill's flycatcher | Empidonax traillii | 3,861 | 58 | 133.44 (2.86) |
| American redstart | Setophaga ruticilla | 3,285 | 41 | 131.72 (2.37) |
| Dusky flycatcher | Empidonax oberholseri | 3,200 | 33 | 116.15 (1.81) |
| Bewick's wren | Thryomanes bewickii | 3,135 | 75 | 347.84 (2.73) |
| Lincoln's sparrow | Melospiza lincolnii | 3,064 | 37 | 79.66 (1.71) |
| Kentucky warbler | Geothlypis formosa | 2,955 | 51 | 271.44 (2.09) |
| Veery | Catharus fuscescens | 2,776 | 46 | 176.39 (3.12) |
| Wrentit | Chamaea fasciata | 2,763 | 36 | 268.33 (2.6) |
| Field sparrow | Spizella pusilla | 2,569 | 45 | 367.74 (2.78) |
| Painted bunting | Passerina ciris | 2,408 | 27 | 533.58 (1.77) |
| Hermit thrush | Catharus guttatus | 2,145 | 38 | 70.31 (2.37) |
| Brown-headed cowbird | Molothrus ater | 2,128 | 49 | 288.13 (4.6) |
| Western wood-pewee | Contopus sordidulus | 1,937 | 47 | 145.68 (2.93) |
| Tufted titmouse | Baeolophus bicolor | 1,820 | 50 | 307.33 (3.6) |
| Hooded warbler | Setophaga citrina | 1,699 | 31 | 200.7 (4.02) |
| White-crowned sparrow | Zonotrichia leucophrys | 1,611 | 22 | 21.756 (0.88) |
| Blue-winged warbler | Vermivora chrysoptera | 1,285 | 22 | 265.11 (3.44) |
| Fox sparrow | Passerella iliaca | 933 | 22 | 81.94 (2.89) |
| Hammond's flycatcher | Empidonax hammondii | 850 | 18 | 40.04 (1.23) |
| Downy woodpecker | Picoides pubescens | 821 | 31 | 315.51 (5.86) |
| Summer tanager | Piranga rubra | 799 | 20 | 348.18 (4.67) |
| Eastern towhee | Pipilo erythrophthalmus | 576 | 18 | 214.26 (7.21) |
Total number of adult individuals captured and marked for use in survival analyses.
Mean of WNV score for stations used in analyses.
Table S2.
Summary of models used to examine the impacts of WNV on survival of landbirds in the western United States
| Model | Explanation |
| Null | Climate and geographic model from previous analysis (Materials and Methods) |
| ϕ(WNV) | Predicted value from WNV risk model (18) |
| ϕ(WNV + LU1km) | WNV + Human land use (1 km from station) |
| ϕ(WNV + LU1km + WNV × LU1km) | WNV + WNV × Land use (1 km from station) |
| ϕ(WNV_PRE) | WNV presence |
| ϕ(WNV_PRE + LU1km) | WNV presence + Human land use (1 km from station) |
| ϕ(WNV_PRE + LU1km + WNV_PRE × LU1km) | WNV presence +WNV presence × Land use (1 km from station) |
| ϕ(WNV_PRELag1) | Same as WNV_PRE but lagged 1 y |
| ϕ(WNV_ PRELag1 + LU1km) | WNV presence Lag1 + Land use |
| ϕ(WNV_ PRELag1 + LU1km + WNV_ PRELag1 × LU1km) | WNV presence Lag1 + Land use + WNV presence Lag1 × Land use |
| ϕ(WNV_ARR) | Compare survival the year of WNV arrival to all other years (all years set to 0 except year of arrival) |
| ϕ (WNV_ARR + LU1km) | WNV arrival + Land use |
| ϕ(WNV_ARR + LU1km + WNV_ARR × LU1km) | WNV arrival + Land use + WNV arrival × Land use |
| ϕ(WNV_ARRLag1) | Same as WNV arrival but lagged 1 y |
| ϕ(WNV_ARRLag1 + LU1km) | WNV arrival Lag 1 y + Land use |
| ϕ(WNV_ARRLag1 + LU1km + WNV_ARRLag1 × LU1km) | WNV arrival Lag 1 y + Land use + WNV arrival Lag 1 y × Land use |
WNV presence and arrival are based on arrival year of WNV for each state. Land use data were obtained from the US Government Survey LandUse database (Materials and Methods).
Results and Discussion
Effects of WNV on Avian Population Survival Rates.
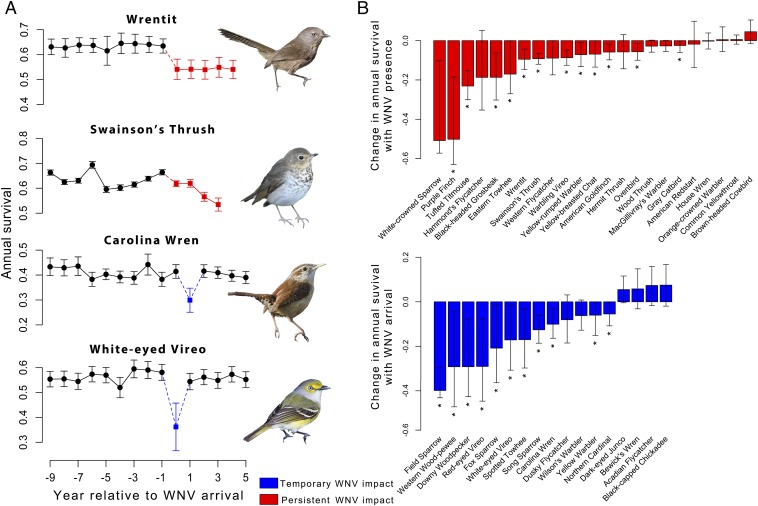
WNV has had large impacts on landbird survival rates (Fig. 1). The relationship between survival and WNV was negative and was included in the top model predicting annual adult survival for 33 of the 49 (67%) species examined. These declines were significant in 23 of the 49 (47%) species (Fig. 1 and Table S3). Although decreases in survival were observed across a broad range of species, we found evidence for two distinct temporal patterns within these declines. Some species, such as the field sparrow (Spizella pusilla), downy woodpecker (Picoides pubescens), and red-eyed vireo (Vireo olivaceus), experienced significant declines in survival associated with the arrival of WNV, followed by recoveries to pre-WNV levels (n = 11) (Fig. 1 and Fig. S2). For others, such as the Swainson’s thrush (Catharus ustulatus), purple finch (Haemorhous/Caprodacus purpureus), and tufted titmouse (Baeolophus bicolor), mean survival declined upon WNV arrival and consistently remained lower than pre-WNV levels (n = 12) (Fig. 1 and Fig. S2).
Fig. 1.
The effects of WNV on landbird species across the continental United States. (A) Examples of temporary and persistent effects of WNV on model-averaged estimates of annual survival. In some species, such as the wrentit and Swainson’s thrush, survival decreased (red) after the arrival of WNV, and remained low in subsequent years. In other species, such as the Carolina wren and white-eyed vireo, survival was reduced during the year of WNV arrival (blue), followed by recovery in subsequent years despite the disease persisting in the community (Materials and Methods). The year that WNV affected survival varied between species when the top-ranked model included a 1-y lag (Carolina wren). Time series were shorter for species where the majority of individuals were captured in the western United States where WNV arrived later (Swainson’s thrush). Photos courtesy of (Top to Bottom) Harjeet Randhawa, Brian Plunkett, Nathan Corry, and Jacob Spendelow. (B) Change in annual survival because of WNV from top-ranked models for 40 landbird species (9 species did not have a WNV covariate in the top survival model). Species are shown as being affected by either the continued presence (red) or the arrival (blue) of WNV based on which variable was in the top-ranked model describing annual survival. Bars represent confidence intervals (±95%), and asterisks indicate a significant effect (P < 0.05) of WNV on survival.
Table S3.
Covariates and associated beta coefficients included in top-ranked mark-recapture model (based on AICc) predicting adult annual survival of 49 bird species from 1992 to 2007
| Species | WNV variable in top-ranked model by AIC | WNV classification (arrival vs. presence) | Cumulative support for WNV classification | Average annual survival | Intercept | WNV effect | 95% CI | Land-use effect | 95% CI | WNV/Land-use Interaction | 95% CI |
| Song sparrow | WNV arrival lag 1 y* | WNV arrival | 0.69 | 0.430 | −0.280 | −0.176 | (−0.32, −0.033) | 0.054 | (0.017, 0.091) | −0.178 | (−0.32, −0.036) |
| Gray catbird | WNV presence* | WNV presence | 0.60 | 0.516 | 0.063 | 0.056 | (−0.017, 0.13) | 0.057 | (0.007, 0.106) | −0.078 | (−0.151, −0.005) |
| Swainson’s thrush | WNV risk* | WNV presence | 1.00 | 0.604 | 0.422 | −0.146 | (−0.202, −0.09) | — | — | — | — |
| Yellow warbler | WNV arrival lag 1 y* | WNV arrival | 0.44 | 0.577 | 0.309 | 0.143 | (−0.041, 0.327) | 0.065 | (0.025, 0.105) | −0.195 | (−0.377, −0.014) |
| Common yellowthroat | WNV presence lag 1 y | WNV presence | 0.92 | 0.496 | −0.018 | 0.023 | (−0.071, 0.116) | −0.131 | (−0.187, −0.075) | 0.162 | (0.06, 0.263) |
| Wilson's warbler | WNV arrival† | WNV arrival | 0.44 | 0.446 | −0.217 | −0.256 | (−0.544, 0.032) | — | — | — | — |
| American robin | — | — | — | 0.489 | −0.044 | — | — | — | — | — | — |
| American goldfinch | WNV risk* | WNV presence | 0.75 | 0.451 | −0.196 | −0.091 | (−0.175, −0.008) | 0.049 | (−0.018, 0.116) | — | — |
| Northern cardinal | WNV arrival lag 1 y* | WNV arrival | 0.78 | 0.564 | 0.257 | −0.221 | (−0.435, −0.007) | — | — | — | — |
| Dark-eyed junco | WNV arrival lag 1 y† | WNV arrival | 0.60 | 0.405 | −0.385 | 0.224 | (−0.019, 0.467) | — | — | — | — |
| MacGillivray’s warbler | WNV presence† | WNV presence | 0.78 | 0.513 | 0.050 | −0.108 | (−0.220, 0.005) | 0.055 | (0.004, 0.107) | — | — |
| Wood thrush | WNV presence† | WNV presence | 0.75 | 0.456 | −0.176 | −0.114 | (−0.239, 0.011) | 0.085 | (0.034, 0.136) | — | — |
| Warbling vireo | WNV presence* | WNV presence | 1.00 | 0.554 | 0.219 | −0.348 | (−0.504, −0.193) | — | — | — | — |
| Black-headed grosbeak | WNV risk* | WNV presence | 0.69 | 0.555 | 0.220 | −0.107 | (−0.210, −0.003) | 0.035 | (−0.030, 0.100) | −0.092 | (−0.217, 0.032) |
| Yellow-rumped warbler | WNV presence lag 1 y* | WNV presence | 0.76 | 0.478 | −0.087 | −0.290 | (−0.547, −0.033) | — | — | — | — |
| Red-eyed vireo | WNV arrival lag 1 y* | WNV arrival | 0.44 | 0.615 | 0.468 | −0.301 | (−0.683, 0.080) | −0.033 | (−0.117, 0.051) | −0.453 | (−0.906, −0.001) |
| Yellow-breasted chat | WNV presence lag 1 y* | WNV presence | 0.54 | 0.494 | −0.026 | 0.036 | (-−.105, 0.177) | 0.016 | (−0.056, 0.088) | −0.157 | (−0.294, −0.019) |
| White-eyed vireo | WNV arrival* | WNV arrival | 0.99 | 0.504 | 0.017 | 0.355 | (0.012, 0.697) | 0.109 | (0.026, 0.193) | −0.525 | (−0.84, −0.209) |
| House wren | WNV presence | WNV presence | 0.69 | 0.377 | −0.501 | −0.012 | (−0.187, 0.163) | −0.090 | (−0.194, 0.013) | 0.225 | (0.059, 0.391) |
| Ovenbird | WNV risk* | WNV presence | 0.68 | 0.564 | 0.256 | −0.088 | (−0.174, −0.002) | −0.069 | (−0.147, 0.009) | — | — |
| Carolina wren | WNV arrival lag 1 y* | WNV arrival | 0.91 | 0.397 | −0.419 | −0.448 | (−0.772, −0.124) | — | — | — | — |
| Orange-crowned warbler | WNV risk† | WNV presence | 0.70 | 0.398 | −0.412 | 0.008 | (−0.123, 0.14) | 0.048 | (−0.075, 0.171) | −0.300 | (−0.628, 0.029) |
| Purple finch | WNV presence* | WNV presence | 0.58 | 0.572 | 0.289 | −0.411 | (−0.861, 0.039) | 0.217 | (-0.099, 0.533) | −0.943 | (−1.707, −0.179) |
| Spotted towhee | WNV arrival* | WNV arrival | 0.60 | 0.529 | 0.118 | −0.122 | (−0.449, 0.205) | 0.146 | (0.067, 0.225) | −0.283 | (−0.559, −0.008) |
| Acadian flycatcher | WNV arrival | WNV arrival | 0.47 | 0.515 | 0.061 | 0.297 | (−0.07, 0.665) | — | — | — | — |
| Black-capped chickadee | WNV arrival | WNV arrival | 0.50 | 0.455 | −0.181 | 0.300 | (−0.079, 0.678) | — | — | — | — |
| Western flycatcher | WNV risk | WNV presence | 0.62 | 0.493 | −0.027 | −0.138 | (−0.319, 0.042) | — | — | — | — |
| Traill's flycatcher | — | — | — | 0.491 | −0.034 | — | — | — | — | — | — |
| American redstart | WNV risk | WNV presence | 1.00 | 0.527 | 0.110 | −0.028 | (−0.268, 0.212) | −0.021 | (−0.187, 0.145) | 0.454 | (0.096, 0.813) |
| Dusky flycatcher | WNV arrival | WNV arrival | 0.55 | 0.506 | 0.023 | −0.325 | (−0.774, 0.123) | −0.113 | (−0.203, −0.023) | 0.311 | (−0.062, 0.684) |
| Bewick's wren | WNV arrival lag 1 y | WNV arrival | 0.57 | 0.436 | −0.256 | 0.233 | (−0.13, 0.596) | 0.049 | (−0.029, 0.128) | 0.353 | (0.006, 0.699) |
| Lincoln's sparrow | — | — | — | 0.408 | −0.373 | — | — | — | — | — | — |
| Kentucky warbler | — | — | — | 0.493 | −0.028 | — | — | — | — | — | — |
| Veery | — | — | — | 0.619 | 0.484 | — | — | — | — | — | — |
| Wrentit | WNV presence* | WNV presence | 0.96 | 0.637 | 0.562 | −0.394 | (−0.605, −0.183) | 0.113 | (0.01, 0.215) | — | — |
| Field sparrow | WNV arrival lag 1 y* | WNV arrival | 1.00 | 0.437 | −0.253 | −1.056 | (−1.709, −0.403) | 0.014 | (−0.109, 0.137) | −0.880 | (−1.525, −0.236) |
| Painted bunting | — | — | — | 0.616 | 0.474 | — | — | — | — | — | — |
| Hermit thrush | WNV presence lag 1 y | WNV presence | 0.61 | 0.521 | 0.085 | −0.230 | (−0.583, 0.122) | −0.101 | (−0.225, 0.023) | — | — |
| Brown-headed cowbird | WNV risk | WNV presence | 0.59 | 0.516 | 0.065 | 0.070 | (−0.053, 0.193) | 0.113 | (−0.00003, 0.226) | — | — |
| Western wood-pewee | WNV arrival lag 1 y* | WNV arrival | 0.52 | 0.549 | 0.198 | −0.174 | (−0.718, 0.37) | 0.149 | (0.045, 0.253) | −0.515 | (−1.029, −0.002) |
| Tufted titmouse | WNV risk* | WNV presence | 1.00 | 0.490 | −0.042 | −0.370 | (−0.543, −0.197) | 0.163 | (0.026, 0.301) | — | — |
| Hooded warbler | — | — | — | 0.410 | −0.366 | — | — | — | — | — | — |
| White-crowned sparrow | WNV risk† | WNV presence | 0.69 | 0.413 | −0.354 | −1.117 | (-2.363, 0.129) | −0.178 | (−0.387, 0.032) | — | — |
| Blue-winged warbler | — | — | — | 0.541 | 0.164 | — | — | — | — | — | — |
| Fox sparrow | WNV arrival* | WNV arrival | 0.47 | 0.558 | 0.233 | −0.853 | (−1.655, −0.05) | −0.127 | (−0.3, 0.046) | — | — |
| Hammond’s flycatcher | WNV risk | WNV presence | 0.68 | 0.446 | −0.216 | −0.305 | (-0.806, 0.197) | −0.092 | (−0.299, 0.114) | 0.500 | (0.096, 0.905) |
| Downy woodpecker | WNV arrival* | WNV arrival | 0.75 | 0.536 | 0.145 | −1.273 | (−2.237, −0.309) | — | — | — | — |
| Summer tanager | — | — | — | 0.692 | 0.811 | — | — | — | — | — | — |
| Eastern towhee | WNV risk* | WNV presence | 0.64 | 0.345 | −0.642 | 0.309 | (−0.065, 0.683) | −0.202 | (−0.499, 0.095) | −0.352 | (−0.703, −0.001) |
WNV classification (WNV arrival or presence; for phylogenetic analyses), cumulative support for those classifications (based on cumulative model weights of all models with WNV arrival or presence covariates, expressed as a proportion), and annual average survival estimates from these top-ranked models for each species are also provided. Standardized β-coefficients are provided for each variable only if present in the top model. Significant effects for each species are boldface; WNV risk signifies continuous WNV risk covariate.
Effect of WNV or climate (including interactions) was significant (P < 0.05).
Effect of WNV or climate (including interactions) was marginally significant (0.05 < P < 0.10).
Population Responses to WNV.
Although negative impacts of WNV on animal populations have been previously documented (8, 10), to our knowledge, this is the first study to show large-scale declines in survivorship resulting from an infectious disease. In addition, our analyses indicate that a larger proportion of species (at a minimum, 47%) may have been affected by WNV than count data alone would suggest (35%) (8). Although we found that tufted titmice were negatively affected by WNV, consistent with a continent-wide count study (8), we also identified an additional five species [downy woodpecker, gray catbird, northern cardinal (Cardinalis cardinalis), eastern towhee (Pipilo erythrophthalmus), and song sparrow (Melospiza melodia)] negatively affected by WNV that were not identified in the previous study. Conversely, we found three species reported to be affected in the previous study that our analyses suggested were not affected [black-capped chickadee (Parus atricapillus), American robin (Turdus migratorius), and house wren (Troglodytes aedon)]. Although no species within the jay family (Corvidae) were sampled thoroughly enough in our study to meet our criteria for inclusion, we did find a significant negative effect of WNV on jays as a group [standardized effect of WNV arrival on annual survival of jay species combined and 95% confidence interval = −1.051 (−1.796, −0.306)], a result common to all previous multispecies, large-scale studies (8, 10).
The declines in annual survival documented here are substantial and may have lasting effects on population abundances and growth rates of affected species. For example, red-eyed vireos have an estimated population size in North America of ∼130 million birds (19). The 29% decrease in annual survival that we document in the year of WNV arrival means that over 30 million red-eyed vireos may have died from WNV as the disease spread across their range. Although red-eyed vireo survival only declined during the year of WNV arrival, mortality of species whose survival was persistently lower in all years following WNV arrival can have even greater impacts on populations. Warbling vireos (Vireo gilvus) had a smaller annual decline in survival (8.7%) from WNV than red-eyed vireos, yet the drop in survival continues to compound every year after the arrival of WNV, suggesting that over 15 million birds, or nearly one-third of the total population (∼49 million birds) (19), may have died across the continent over the 5 y since the arrival of WNV. Of course, increases in reproductive output and dispersal from regions where birds were not infected may help to ameliorate the effect of these massive die-offs on total population abundances, and in fact, the ability of increased reproduction to offset population declines because of adult mortality may be one reason for discrepancies between our study and those only examining abundance trends (20). Nonetheless, the declines in survival from WNV documented here translate into significant levels of adult mortality and potentially large population declines for many species across North America. Moreover, these reductions in survival represent population averages of all individuals of a species at sites with WNV present, and it is likely that the mortality rates of infected individuals were much higher than these estimates indicate.
Previous studies have suggested that peridomestic species (21) may be more affected by WNV presence because of their close associations with urbanized environments and vector populations (8, 22, 23). However, our analyses suggest the effects of WNV on landbirds are not restricted to such species. In fact, some species identified as having the greatest decline in survivorship in our study [i.e., field sparrow (S. pusilla), western wood-pewee (Contopus sordidulus), red-eyed vireo (V. olivaceus)] are not typically associated with human-dominated or urban landscapes during the breeding season. In addition, species that were previously reported as suffering little to no effect from laboratory-induced WNV infections [i.e., Swainson’s thrush, gray catbird (Dumetella carolinenesis) (24)], or that were assigned low-risk scores in terms of WNV threat [i.e., song sparrow (M. melodia), yellow warbler (Dendroicha petechia) (10)], instead showed significant declines in adult survival in our analyses (Fig. 1).
Effects of Land Use.
Results from this study suggest the interaction between invasive diseases and human land-use patterns can lead to complex effects on overall landbird survival. Ten species showed significant positive effects of land use on survival (positive land use covariate estimate with a 95% confidence interval that did not overlap 0), and two showed a significant negative effect. Some of the species that showed a positive effect [e.g., song sparrow and spotted towhee (Pipilo maculatus)] may benefit from food resources provided by humans, which may help to alleviate some of the burden to populations imparted by a novel infectious disease, such as WNV, but others [e.g., yellow warbler, MacGillivray’s warbler (Geothylypis tolmiei)] would not likely benefit from anthropogenic activity. Eleven species showed significantly negative WNV/land-use interactions, suggesting that the effects of WNV increased with increasing land use for these species, consistent with studies suggesting that WNV effects may be amplified in regions with greater land use (17, reviewed in ref. 25).
Host Phylogenetic Signal.
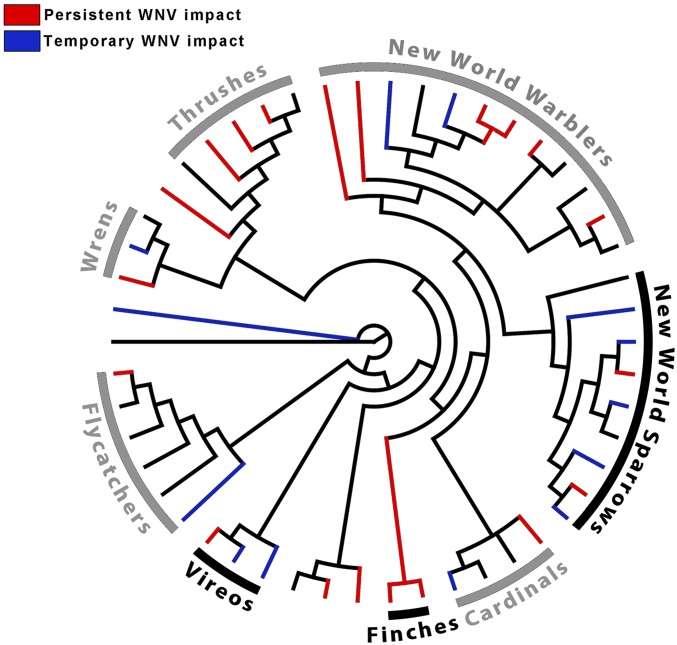
Our results revealed two classes of responses to WNV: (i) temporary decline and recovery, and (ii) persistent declines in survival. To understand the possible drivers of this difference, we examined whether evolutionary history or ecological similarities among closely related species could be a factor. We constructed a phylogenetic tree of all species included in our demographic analyses (26), and then mapped survival response (no effect, arrival, or persistent effect) to WNV on the tree. Although we found no evidence of phylogenetic signal for WNV response across the entire tree, this was not surprising given high rates of immune-response evolution seen in other taxa, including humans (27). We did, however, find several clades that trended toward being nonrandomly affected by temporary or persistent effects (Fig. 2). New World sparrows and allies (P = 0.09) and vireos (P = 0.14) trended toward containing more species affected by temporary effects of WNV than the rest of the tree, whereas finches (P = 0.14) trended toward containing more species experiencing persistent declines in survival in years after WNV outbreak. Although the causes of these patterns are not fully understood, similarities between the impacts of disease on closely related species could be the result of common immunological responses across sister taxa, shared physiological and metabolic functions in closely allied species, or common diets and behavior among clades. It is also possible that these characteristics are associated with common high-risk habitats for WNV in closely related species. A significant phylogenetic signal was found across all species when considering their mean risk to WNV (K = 0.257, P = 0.026) (Fig. S3 and Table S3). In addition, after correcting for phylogeny, a significant but weak negative correlation was found between the average WNV risk of a species and the estimated effect of WNV; species whose habitats were in high-risk WNV areas had larger declines is survival (R = 0.26, P = 0.036). Although the respective roles shaping the response to WNV in various hosts is unclear, this is, to our knowledge, the first evidence to suggest phylogeny may influence whether a species experiences short- or long-term effects of WNV and calls attention to the importance of examining both phylogeny and habitat preferences in understanding the impacts of an introduced disease.
Fig. 2.
Phylogeny of the 49 landbird species used in this study (along with an outgroup, mallard). Tree represents the 70% consensus tree of 1,000 random trees constructed using birdtree.org (26). Terminal branches are colored according to whether species survival was unaffected by WNV (black), only reduced during the year of WNV arrival (blue), or remained low after WNV arrival (red). Names and bars are shown in black for clades for which a nonrandom negative trend with either WNV arrival or presence was observed, and in gray for clades for which no significant trends were observed.
Conclusions
Declines in survival in 47% of species examined at a continental scale, combined with the fact that roughly half of these declines as a result of WNV have persisted over time, demonstrate the widespread and long-term effects that introduced infectious disease may have on naïve host populations. Several important questions are raised by this study. First, it is clear that although our analyses help to explain some of the differential effects of WNV among particular groups of bird species, more work is needed to fully understand why survival of some species rebounded almost immediately from the introduction of a novel infectious disease, while survival of others did not. Second, the broad nature of our sampling strategy necessarily targeted species that are currently not under threat of local or regional extinction (48 of the 49 species examined are listed as Least Concern by the International Union for the Conservation of Nature). However, if such impacts are readily observed on large populations of nonthreatened species, what might the impacts be on species with much smaller distributions that are already threatened? Future research should specifically target the impacts of WNV on threatened populations. Finally, even within large populations, small reductions in survival because of WNV reduce the intrinsic growth rate of populations and make them more susceptible to other natural and anthropogenic impacts. The threat from infectious disease will most likely be magnified with increased deforestation, climate warming, and global connectivity (28), each of which can act as further stressors on populations and catalysts for large-scale introductions to naïve populations. Understanding how these factors act alone or synergistically in driving population dynamics of species will be critical in preserving them.
Materials and Methods
Survivorship Models.
Given the fact that different approaches to examining the impacts of WNV on bird populations are sometimes inconsistent (10), potential biases inherent to each method should be carefully considered. In addition, the species we examined here were dictated by our sampling method (mark-recapture) and not on count data alone (8, 10), suggesting caution should be taken with making direct comparisons to other studies. However, survival analyses examine the demographic parameter affected by WNV directly and therefore may provide a better assessment of potential impacts on bird populations. We developed models of annual survival over the 16-y period for each species or species group using latitude, longitude, and two climate indices, the El Niño-Southern Oscillation and the North Atlantic Oscillation, both known to influence avian reproduction (29) and survival (15) of North American landbirds. After leading regional and climatic models were identified, we tested 15 a priori hypotheses about the relationship between survival and WNV and land-use patterns (Table S2). This two-step approach was used to examine the impacts of WNV while accounting for other factors that may influence year-to-year variation in annual survival. Annual survival was estimated using mark-recapture data collected at Monitoring Avian Productivity and Survivorship (MAPS) stations from 1992 to 2007 (30) in all 48 contiguous states and Alaska that had MAPS stations (Fig. S1). All mist netting and banding of birds was approved and performed under University of California, Los Angeles' Animal Research Committee (ARC, Protocol no. 2008-033-23). Analyses were conducted on all bird species that had sufficient data for analysis. In order for species to be included in analyses, the estimate of the coefficient of variation of survival over the period 1992–2007 from MAPS data had to be less than 5%, as is standard for large-scale analyses of this dataset (30). This threshold criterion is necessary because species with coefficients of variation above 5% have been sampled too poorly to detect spatially and temporally explicit changes in annual survival. Therefore, of a possible 293 species captured at MAPS banding stations, we conducted our analyses on the 49 species from 14 avian families that met this criterion (Table S1). We also limited our analyses to captures of adult (after hatch-year) birds, and used Cormack–Jolly–Seber models to estimate annual adult apparent survival probability between breeding seasons, hereafter referred to as survival. For the climate/regional analyses, we developed an a priori set of candidate models to estimate survival, accounting for transient individuals (31), with covariates for climate indices, latitude, longitude, and time, as well as null models following LaManna et al. (14).
Important to note is that in short-lived bird species that breed in their first year (typical of the species we examined), adult survival is often the largest demographic component of population growth (32, 33) and can have a direct influence on the following year’s growth rate (34). Thus, a decline in survival of 0.3 can result in a decline in the growth rate of the population of 0.3. If a population is stable or increasing slowly, the growth rate of the population (Nt+1/Nt, where N is population size and t is year) will be close to 1.0, and therefore a decline in adult survival may translate into a significant reduction in the population in the following year. However, the degree of population reduction will also be influenced by changes in reproductive success and patterns of dispersal. Thus, differences between the results obtained using these methods compared with those of regional count data may be a result of immigration and reproduction masking effects of WNV on adult survival when using abundance estimates. Moreover, if reductions in annual survival only occur when WNV arrives (species identified as “WNV arrival”), populations can recover quickly assuming that the growth rate exceeds 1.0 in years following WNV arrival. If annual survival remains continuously low after WNV arrival (species identified as “WNV presence”), however, even small reductions in survival can cause the growth rate to remain below 1.0 for an extended period, leading to long-term effects on populations.
Effects of WNV on Avian Population Survival Rates.
The effect of WNV on survival was examined in three different ways. First, we modeled survival as a continuous function of predicted WNV risk at each banding station in each year using a spatially and temporally explicit, continuous WNV risk value (18). We also examined whether survival differed between the years before and after WNV arrived (WNV presence) and whether survival differed between the year of WNV arrival in a state versus all other years (WNV arrival) by coding arrival year of WNV. In our spatially and temporally explicit models, these WNV arrival years were specific to each banding station and determined by the arrival year of WNV in each banding station’s state (according to the year of first wildlife cases reported by CDC). Thus, we were able test if the year of WNV arrival influenced survival for a given species even though WNV arrival year varied spatially across the continent. Finally, to examine whether there was a time lag in WNV effects (sometimes the first case of WNV for a region was reported late in the year or only occurred in a small portion of the state), we considered models where survival declined 1 y after WNV was present (WNV presence +1) or arrived (WNV arrival + 1). We identified species as being only affected by WNV during arrival years when their survivorship estimates were associated with covariates representing WNV arrival. Conversely, we identified species as experiencing no recovery from WNV when their survivorship estimates were associated with covariates representing WNV presence or overall risk (Fig. 1 and Table S3).
Effects of Land Use.
To examine the influence of land use, we computed the proportion of area within 1 km of each station that was urban/suburban or agricultural land. Land-use data were obtained from the United States Geological Survey (USGS, data available from the US Geological Survey). Each measure of WNV included one model with land use and a second with an interaction between land use and WNV, resulting in 15 models (3 for WNV risk, 6 for WNV presence, and 6 for WNV arrival) in addition to the null model, including only climatic and regional effects (Table S2). Species that showed either a significant direct negative effect of WNV or a significant negative interaction between WNV and human land use on annual survival were identified as being negatively affected by WNV. A negative interaction between land use and WNV would support the hypothesis that greater human land use surrounding a station increases the effect of WNV outbreaks on bird survival.
Model Comparison and Selection.
For each species, the top model (based on AICc) from the climate/regional analyses was used as the “null” model to examine the effects of WNV on survival. Because WNV did not arrive in North America until 1999, all years before WNV arrival acted as within-species controls against which the effects of WNV on survival were tested in years following WNV arrival. Model selection results and parameter estimates were computed with the software program MARK as executed in R (35) using the RMark package (36). The 95% confidence intervals of the standardized regression coefficients for WNV variables of the top-performing model were examined to assess whether they included zero. Estimates and errors (Fig. 1) were calculated using the top-ranked model if it included a WNV covariate. These estimates are the difference WNV makes in real annual survival accounting for other variables in the model (i.e., climate, latitude, longitude, and so forth). If the WNV continuous risk covariate was in the top model, we used mean WNV risk value +2 SD for that species to estimate the effect of high WNV risk on survival. In the case of significantly negative WNV×land use interactions, we used the mean land use value +2 SD for that species to estimate the effect of WNV on survival in areas of high human land use. To help visualize the difference between WNV arrival and presence effects on survival, we plotted model-averaged estimates of survival relative to the year of WNV arrival (Fig. 2 and Fig. S2) for all species whose survival was significantly impacted by WNV.
Host Phylogenetic Signal.
We constructed a phylogenetic tree of all 49 species and tested for WNV effects in demographic analyses using species names as input on birdtree.org (26); we also included mallard, Anas platyrhynchos, as an outgroup. We used the dataset “Hackett, All Species” to construct this tree and used the consensus (70% consensus at all nodes) of 1,000 randomly constructed trees as a final phylogeny. Only one of our target species was not listed in the birdtree.org database, the blue-winged warbler (Vermivora cyanoptera), and was replaced by the closest known relative of this species, golden-winged warbler (Vermivora chrysoptera). The full phylogeny was highly resolved and contained no ambiguous nodes after consensus was complete (Fig. S4).
Once our tree was constructed, we assigned terminal nodes one of three states related to the effect that WNV had on survival estimates: (i) no WNV effects, (ii) effects driven by WNV arrival, and (iii) effects driven by the presence of WNV (arrival and subsequent years). Species were assigned as either WNV arrival or WNV presence if an arrival or presence covariate was in the top-ranked model. We also assessed the strength of evidence for these categorizations with the cumulative model weights for that WNV covariate (WNV arrival or WNV presence) (Tables S2 and S3). We used this effect categorization and the phylogeny to test whether there was a global phylogenetic signal of WNV in terms of how it affected species, as well as whether particular clades within the tree had more individuals with a particular effect given the phylogeny and number of species exhibiting that effect. We also used the continuous character of WNV risk (Table S3) and our tree as input in the program phylosignal in phytools (37) within R (35) to determine whether there was phylogenetic signal in the overall risk to a species as determined by Harrigan et al. (18).
Even if differences in WNV response among species are not explained by a phylogenetic signal, groups of closely related species, or clades, might nonetheless have more similar WNV responses than expected by chance. This outcome could be because of rapid evolution of immune response (27) or ecological similarities among species in a clade. We used randomization tests to identify bird clades that showed more similar responses to WNV than expected by chance. We grouped birds into clades according to the phylogeny above, and tested all clades with sufficient sample size for analysis. To assess which clades were more similar in WNV response, the number of species with a given WNV response was compared with the means of 10,000 draws of the same number of species from a random pool of remaining species. Families for whom the number of species with a given response was greater than 95% of these draws were considered significantly affiliated with one type of WNV response (38).
To determine if species occupying riskier habitats (WNV risk, as determined in ref. 18) had larger decreases in survival, we compared the average WNV risk score and the estimated WNV effect on survival (Table S3) across all 49 taxa, corrected for phylogenetic signal within residuals. We used the package caper (39) within the R framework (33) to estimate the values of λ, κ, and δ individually (using the maximum-likelihood criteria, “ML”), and then used these values as input in a phylogenetic generalized linear model (pgls with caper).
Supplementary Material
Acknowledgments
We thank the Lloyd-Smith and T.B.S. laboratories for helpful comments on this manuscript, as well as specific suggestions from Franck Courchamp, Michael Alfaro, and Brian O’Meara; David R. Anderson for providing helpful suggestions on model development; Andy Boyce for assistance with phylogenetic analyses; and the hundreds of Monitoring Avian Productivity and Survivorship station operators and their many volunteer bird banders, who provided the mark-recapture data used in our analyses. This work was supported by US Environmental Protection Agency Grant R 833778 (to T.B.S.); National Science Foundation Grant IIA PIRE–1243524 (to T.B.S.); and STAR Fellowship Assistance Agreement FP-91747701-0 (to J.A.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507747112/-/DCSupplemental.
References
- 1.Wolfe ND, Daszak P, Kilpatrick AM, Burke DS. Bushmeat hunting, deforestation, and prediction of zoonotic emergence. Emerg Infect Dis. 2005;11(12):1822–1827. doi: 10.3201/eid1112.040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lafferty KD. The ecology of climate change and infectious diseases. Ecology. 2009;90(4):888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- 3.Altizer S, Ostfeld RS, Johnson PTJ, Kutz S, Harvell CD. Climate change and infectious diseases: From evidence to a predictive framework. Science. 2013;341(6145):514–519. doi: 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- 4.Tatem AJ, Rogers DJ, Hay SI. Global transport networks and infectious disease spread. Adv Parasitol. 2006;62:293–343. doi: 10.1016/S0065-308X(05)62009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marano N, Arguin PM, Pappaioanou M. Impact of globalization and animal trade on infectious disease ecology. Emerg Infect Dis. 2007;13(12):1807–1809. doi: 10.3201/eid1312.071276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlsson EK, Kwiatkowski DP, Sabeti PC. Natural selection and infectious disease in human populations. Nat Rev Genet. 2014;15(6):379–393. doi: 10.1038/nrg3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Riper C, Van Riper SG, Goff ML, Laird M. The epizootiology and ecological significance of malaria in Hawaiian (USA) land birds. Ecol Monogr. 1986;56:327–344. [Google Scholar]
- 8.LaDeau SL, Kilpatrick AM, Marra PP. West Nile virus emergence and large-scale declines of North American bird populations. Nature. 2007;447(7145):710–713. doi: 10.1038/nature05829. [DOI] [PubMed] [Google Scholar]
- 9.McLean R. West Nile virus in North American birds. Ornithol Monogr. 2006;60:44–64. [Google Scholar]
- 10.Wheeler SS, et al. Differential impact of West Nile virus on California birds. Condor. 2009;111(1):1–20. doi: 10.1525/cond.2009.080013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward MP, et al. Field-based estimates of avian mortality from West Nile virus infection. Vector Borne Zoonotic Dis. 2010;10(9):909–913. doi: 10.1089/vbz.2008.0198. [DOI] [PubMed] [Google Scholar]
- 12.Ricklefs RE. Breeding Biology of Birds. National Academy of Sciences; Washington, DC: 1973. [Google Scholar]
- 13.McClure CJW, Rolek BW, McDonald K, Hill GE. Climate change and the decline of a once common bird. Ecol Evol. 2012;2(2):370–378. doi: 10.1002/ece3.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaManna JA, George TL, Saracco JF, Nott P, DeSante DF. El Niño-Southern Oscillation influences annual survival of a migratory songbird at a regional scale. Auk. 2012;129(4):734–743. [Google Scholar]
- 15.Saracco JF, Royle JA, DeSante DF, Gardner B. Modeling spatial variation in avian survival and residency probabilities. Ecology. 2010;91(7):1885–1891. doi: 10.1890/09-0705.1. [DOI] [PubMed] [Google Scholar]
- 16.Gibbs SEJ, et al. Factors affecting the geographic distribution of West Nile virus in Georgia, USA: 2002–2004. Vector Borne Zoonotic Dis. 2006;6(1):73–82. doi: 10.1089/vbz.2006.6.73. [DOI] [PubMed] [Google Scholar]
- 17.Bradley CA, Gibbs SEJ, Altizer S. Urban land use predicts West Nile virus exposure in songbirds. Ecol Appl. 2008;18(5):1083–1092. doi: 10.1890/07-0822.1. [DOI] [PubMed] [Google Scholar]
- 18.Harrigan RJ, Thomassen HA, Buermann W, Smith TB. A continental risk assessment of West Nile virus under climate change. Glob Change Biol. 2014;20(8):2417–2425. doi: 10.1111/gcb.12534. [DOI] [PubMed] [Google Scholar]
- 19.Partners in Flight Science Committee 2013 Population Estimates Database, version 2013. Available at rmbo.org/pifpopestimates. Accessed July 24, 2015.
- 20.Julliard R. Estimating the contribution of survival and recruitment to large scale population dynamics. Anim Biodivers Conserv. 2004;27(1):417–426. [Google Scholar]
- 21.Sauer JR, et al. 2014. The North American Breeding Bird Survey, Results and Analysis 1966–2013. Version 01.30.201. Available at www.mbr-pwrc.usgs.gov/bbs. Accessed May 31, 2014.
- 22.LaDeau SL, Calder CA, Doran PJ, Marra PP. West Nile virus impacts in American crow populations are associated with human land use and climate. Ecol Res. 2011;26(5):909–916. doi: 10.1007/s11284-010-0725-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Brien VA, Meteyer CU, Reisen WK, Ip HS, Brown CR. Prevalence and pathology of West Nile virus in naturally infected house sparrows, western Nebraska, 2008. Am J Trop Med Hyg. 2010;82(5):937–944. doi: 10.4269/ajtmh.2010.09-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owen J, et al. Migrating birds as dispersal vehicles for West Nile virus. EcoHealth. 2006;3(2):79–85. [Google Scholar]
- 25.LaDeau SL, Marra PP, Kilpatrick AM, Calder CA. West Nile virus revisited: Consequences for North American ecology. Bioscience. 2008;58(10):937–946. [Google Scholar]
- 26.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. The global diversity of birds in space and time. Nature. 2012;491(7424):444–448. doi: 10.1038/nature11631. [DOI] [PubMed] [Google Scholar]
- 27.Kosiol C, et al. Patterns of positive selection in six Mammalian genomes. PLoS Genet. 2008;4(8):e1000144. doi: 10.1371/journal.pgen.1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altizer S, Bartel R, Han BA. Animal migration and infectious disease risk. Science. 2011;331(6015):296–302. doi: 10.1126/science.1194694. [DOI] [PubMed] [Google Scholar]
- 29.Nott MP, DeSante DF, Siegel RB, Pyle P. Influences of the El Niño/Southern Oscillation and the North Atlantic Oscillation on avian productivity in forests of the Pacific Northwest of North America. Glob Ecol Biogeogr. 2002;11(4):333–342. [Google Scholar]
- 30.DeSante DF, Kaschube DR. The Monitoring Avian Productivity and Survivorship (MAPS) program 2004, 2005, and 2006 report. Bird Pop. 2009;9:86–169. [Google Scholar]
- 31.Hines JE, Kendall WL, Nichols JD. On the use of the robust design with transient capture-recapture models. Auk. 2003;120(4):1151–1158. [Google Scholar]
- 32.Sæther B-E, Bakke Ø. Avian life history variation and contribution of demographic traits to the population growth rate. Ecology. 2000;81(3):642–653. [Google Scholar]
- 33.Robinson RA, Morrison CA, Baillie SR. Integrating demographic data: towards a framework for monitoring wildlife populations at large spatial scales. Methods Ecol Evol. 2014;5(12):1361–1372. [Google Scholar]
- 34.Noon BR, Sauer JR. Population models for passerine birds: Structure, parameterization, and analysis. In: McCullough DR, Barrett RH, editors. Wildlife 2001: Populations. Elsevier Applied Science; New York, NY: 1992. [Google Scholar]
- 35.R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. [Google Scholar]
- 36.Laake J. RMark: An R Interface for Analysis of Capture-recapture data with Mark. AFSC Processed Report 2013-1. NOAA, Alaska Fisheries Science Center, National Marine Fisheries Service; Seattle, WA: 2013. [Google Scholar]
- 37.Revell LJ. phytools: An R package for phylogenetic comparative biology (and other things) Methods Ecol Evol. 2012;3(2):217–223. [Google Scholar]
- 38.Frishkoff LO, et al. Loss of avian phylogenetic diversity in neotropical agricultural systems. Science. 2014;345(6202):1343–1346. doi: 10.1126/science.1254610. [DOI] [PubMed] [Google Scholar]
- 39.Orme D, et al. 2013 caper: Comparative Analyses of Phylogenetics and Evolution in R. R package version 0.5.2. Available at https://cran.r-project.org/web/packages/caper/index.html. Accessed April, 2015.
- 40.Kearse M, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.