Significance
In bacterial genomes, chromosomes are distinguished from plasmids by the localization of essential genes. It has been taken for granted that fundamental genes such as the rRNA (rrn) operon should be transmitted faithfully on the chromosome. Here, we found a striking exception: A plant-associated bacterium, Aureimonas sp. AU20, and its close relatives harbor the rrn operon only on a small, high-copy-number replicon but not on the chromosome. Our findings show the existence of novel genome organization in bacteria.
Keywords: ribosomal RNA operon, chromosome, plasmid, genome rearrangement, Aureimonas
Abstract
rRNA is essential for life because of its functional importance in protein synthesis. The rRNA (rrn) operon encoding 16S, 23S, and 5S rRNAs is located on the “main” chromosome in all bacteria documented to date and is frequently used as a marker of chromosomes. Here, our genome analysis of a plant-associated alphaproteobacterium, Aureimonas sp. AU20, indicates that this strain has its sole rrn operon on a small (9.4 kb), high-copy-number replicon. We designated this unusual replicon carrying the rrn operon on the background of an rrn-lacking chromosome (RLC) as the rrn-plasmid. Four of 12 strains close to AU20 also had this RLC/rrn-plasmid organization. Phylogenetic analysis showed that those strains having the RLC/rrn-plasmid organization represented one clade within the genus Aureimonas. Our finding introduces a previously unaddressed viewpoint into studies of genetics, genomics, and evolution in microbiology and biology in general.
Multipartite genomes containing more than one chromosome are not unusual in bacteria. The presence of the main (i.e., largest) chromosome and the second chromosome(s) in one genome is conserved among all members of some genera, such as Burkholderia and Vibrio. Chromosomes are distinguished from plasmids by the localization of essential genes (1, 2). In general, main and second chromosomes are larger (>0.5 Mb) than coresident plasmids, have similar guanine+cytosine (G+C) contents to each other, and are maintained by cell cycle-linked replication and active partitioning systems, whereas plasmids are diverse in that some (i) can be conjugally mobilized, (ii) can replicate within one genus or within many genera (i.e., have a broad host range), (iii) are present in high copy numbers and are segregated stochastically, and (iv) have lower G+C contents than their host chromosomes (1–3). One of the practical ways of designating a replicon as a “chromosome” has been to examine the localization of the rRNA (rrn) operon, which encodes rRNAs (16S, 23S, and 5S rRNAs), because of its functional importance in protein synthesis. Additional copies of the rrn operon are also sometimes found on the second chromosomes (e.g., in species of Rhodobacter, Brucella, Burkholderia, and Vibrio) (4–7) or on plasmids (the 53.9-kb and 23-kb plasmids of Bacillus megaterium and Paracoccus species, respectively) (8, 9). However, so far, no documented bacterial strain lacks the rrn operon on the “main” chromosome.
Here, we report genome analysis of the genus Aureimonas, showing that one clade within this genus carries its sole rrn operon on a small plasmid.
Results and Discussion
Plasmid Localization of the rrn Operon in Aureimonas sp. AU20.
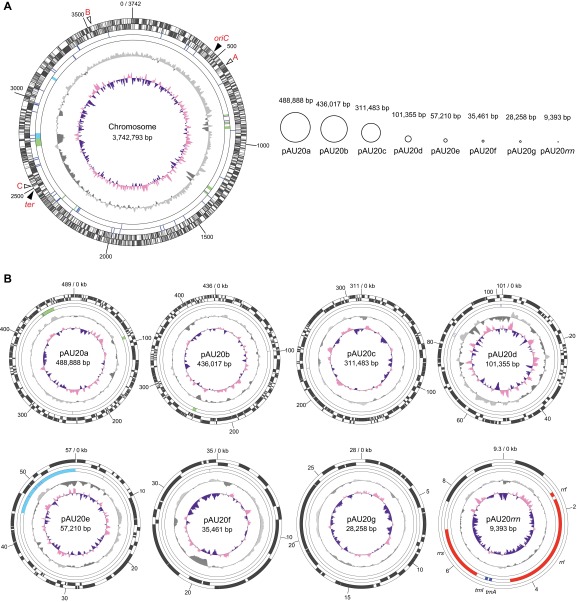
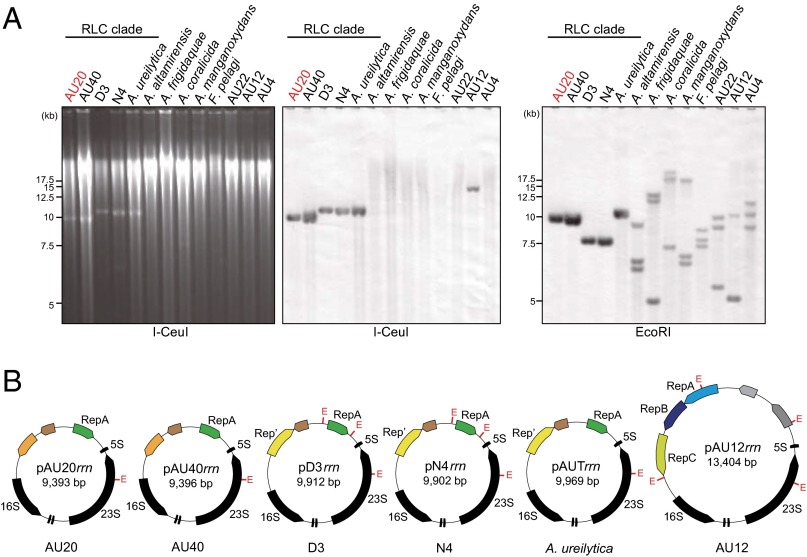
The genus Aureimonas (family Aurantimonadaceae, order Rhizobiales, class Alphaproteobacteria) contains three defined species, Aureimonas altamirensis, Aureimonas ureilytica, and Aureimonas frigidaquae, and its members have been isolated from diverse environments (10). To characterize this bacterial group from a genomic viewpoint, we determined the complete genome sequence of Aureimonas sp. AU20, an isolate from the stem of a soybean plant (11). The result showed that the AU20 genome (5.2 Mb in total) contains nine circular replicons: the main chromosome (3.7 Mb) and eight other replicons (designated pAU20a to pAU20g and pAU20rrn) (Table 1 and Fig. S1). The five smallest replicons, pAU20d to pAU20g and pAU20rrn, could be distinguished from the chromosome by their lower G+C contents, suggesting that they had distinct evolutionary origins. The genome contained a single rrn operon, 55 tRNA genes, and 4,785 protein-coding genes (Table 1). Surprisingly, the rrn operon, which consisted of genes for 16S rRNA, tRNAIle, tRNAAla, 23S rRNA, and 5S rRNA, was located not on the chromosome but on the smallest replicon (9.4 kb), pAU20rrn. The tRNAIle gene on this replicon was also the only one in the genome. The tRNAAla gene on pAU20rrn showed similarities to the tRNAAla gene and the tRNAThr gene on the chromosome (82% and 78% identical, respectively; Fig. S2A), but the two replicons shared no other substantial similarities. Using the 16S rRNA gene as a probe, we performed Southern hybridization analysis of the genomic DNA that was digested with the enzyme I-CeuI, an endonuclease whose cleavage site exists exclusively within the 23S rRNA gene (12) (Fig. 1A). The result showed a single 9-kb band, supporting the localization of the rrn operon on pAU20rrn. A single hybridization band was also detected with EcoRI-digested DNA, consistent with the existence of a single rrn operon in the genome. This rrn operon (with a size of 6 kb) had the typical core promoter consisting of the putative UP element and −35 and −10 consensus hexamers (13) (Fig. S2B) and a putative rho-independent terminator (Fig. S2C).
Table 1.
General features of the Aureimonas sp. AU20 genome
| Replicon | Size, bp | G+C content, % | tRNA gene | rRNA operon | Protein-coding gene | Accession no. |
| Chromosome | 3,742,793 | 66.8 | 53 | 0 | 3,449 | CP006367 |
| pAU20a | 488,888 | 67.8 | 0 | 0 | 425 | CP006368 |
| pAU20b | 436,017 | 67.0 | 0 | 0 | 405 | CP006369 |
| pAU20c | 311,483 | 68.1 | 0 | 0 | 277 | CP006370 |
| pAU20d | 101,355 | 61.0 | 0 | 0 | 98 | CP006371 |
| pAU20e | 57,210 | 59.3 | 0 | 0 | 54 | CP006372 |
| pAU20f | 35,461 | 61.8 | 0 | 0 | 32 | CP006373 |
| pAU20g | 28,258 | 61.2 | 0 | 0 | 42 | CP006374 |
| pAU20rrn | 9,393 | 58.0 (61.2*) | 2 | 1 | 3 | CP006375 |
| Total | 5,210,858 | — | 55 | 1 | 4,785 | — |
Value obtained from a 3.3-kb segment of the replicon when stripped of the rrn operon.
Fig. S1.
Schematic representation of the Aureimonas sp. AU20 genome. (A) Nine replicons are shown on the same relative scale. For the chromosome, circles, from the outermost inward, show (i) predicted protein-coding genes in the clockwise orientation; (ii) predicted protein-coding genes in the counterclockwise orientation; (iii) genes for tRNA (blue) or rRNA (red); (iv) putative genomic islands (yellow green), prophages (light blue), or insertion sequences (purple); (v) G+C content deviation (light gray for values higher than the average and dark gray for values lower than the average); and (vi) GC-skew values (pink for plus values and violet for minus values). (B) Extrachromosomal replicons are shown on different scales. Circles show the same information as the circles for the chromosome in A. Genomic islands, prophage regions, and insertion sequences were predicted by using IslandViewer (38), PHAST (39), and ISsaga (40), respectively. The G+C contents and GC-skew values were calculated by using ArcWithColor (www.ige.tohoku.ac.jp/joho/gmProject/gmdownload.html) with a window of 10 kb (for the chromosome), 5 kb (for pAU20a, pAU20b, and pAU20c), 1 kb (for pAU20d), 0.5 kb (for pAU20e, pAU20f, and pAU20g), or 0.1 kb (for pAU20rrn). Positions marked in red with A, B, and C on the chromosome indicate the loci showing synteny with the rrnA, rrnB, and rrnC regions, respectively, on the chromosomes of other strains (Fig. S5). The origin (oriC) and terminus (ter) of replication are also noted.
Fig. S2.
Sequence comparison between the rrn-plasmids and the chromosomes in the genus Aureimonas. (A) Sequence alignment of the tRNAAla gene on pAU20rrn and the tRNAAla and tRNAThr genes on the AU20 chromosome (locus tags indicated in parentheses). (B) Regions upstream of the 16S rRNA genes. The sequences were from rrn-plasmids (pAU20rrn from AU20 and pAUTrrn from A. ureilytica) and chromosomes (rrnC from AU4 and AU22). For the numbering of nucleotides, the 5′-terminus of the 16S rRNA gene (shown in white letters on a black background with an arrow at the bottom right) is set to +1. Asterisks below the alignment mark nucleotides identical in the four sequences. The positions of the −35 and −10 consensus hexamers of the putative core promoters are marked; these hexamers are similar to the hexamers of the rrn operons in Sinorhizobium meliloti, a species belonging to the order Rhizobiales (41, 42). The position of A+T-rich segments preceding the −35 hexamers, which possibly act as the UP elements (13, 43), is also marked (A and T are shown in white letters). Segments similar to BoxA of the E. coli rrn operons (43) are labeled “BoxA-like,” although an associated stem-loop is present only in the AU22 sequence (indicated by arrows below the alignment upstream of the BoxA-like sequence). A position for the upstream arm of the putative 16S processing stalk (44) is marked (nucleotides complementary to those nucleotides in the other arm downstream of the 16S rRNA gene are shown in white letters). (C) Putative rho-independent terminators located downstream of the 5S rRNA genes. The sequences were from pAU20rrn; pAUTrrn; and the genomes of AU4, AU12, and AU22 (note that the corresponding sequences were identical in all of the rrn operons within each strain). The 3′-terminus of the 5S rRNA gene is shown in white letters with a horizontal line. Nucleotides involved in the stem structure (underlined) and the associated change in free energy (ΔG) (shown on the right) were predicted by GENETYX (GENETYX Corporation).
Fig. 1.
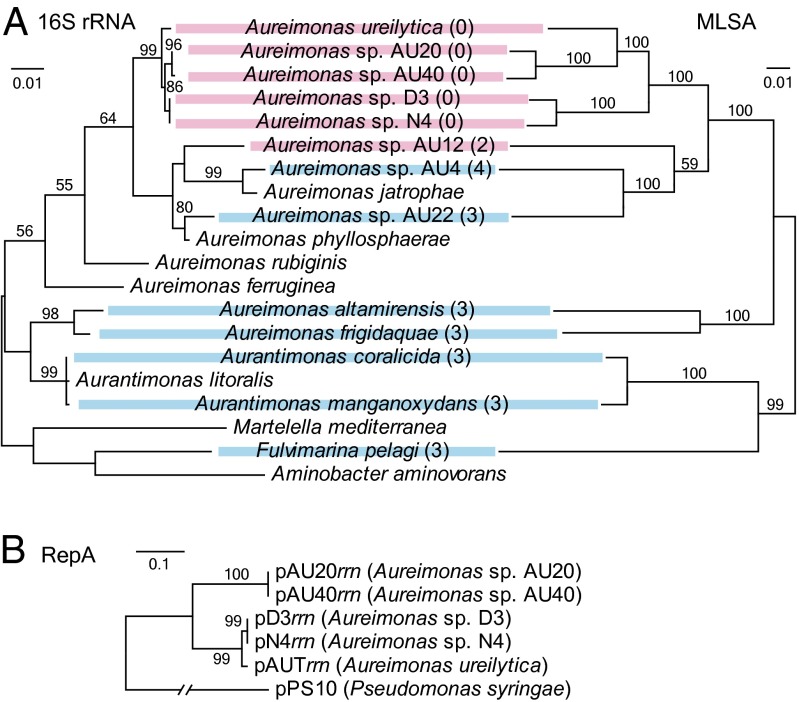
The rrn operons in members of the family Aurantimonadaceae. (A) Southern hybridization analysis. Total DNA was digested with I-CeuI, separated by electrophoresis (Left), transferred to a membrane, and probed with the 16S rRNA gene (Middle). (Right) EcoRI-digested total DNA was separated by electrophoresis, transferred, and probed with the same gene. One of the bands predictable from the genome sequence was not detected in the EcoRI-digested DNA of AU12 and AU4 (corresponding to a 34.3-kb band and a 48.2-kb band, respectively). (B) Maps of the rrn-plasmids in strains AU20, AU40, D3, N4, and A. ureilyltica and another type of rrn-carrying replicon in AU12. Positions of EcoRI sites are marked with a red “E.” The rRNA and tRNA genes are shown in black. RepA and Rep′ encoded on the rrn-plasmids and RepC on pAU12rrn are homologs of different replication initiators. RepA and RepB on pAU12rrn are homologs of partitioning proteins.
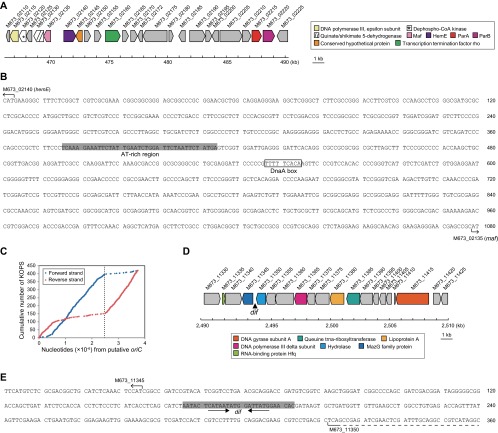
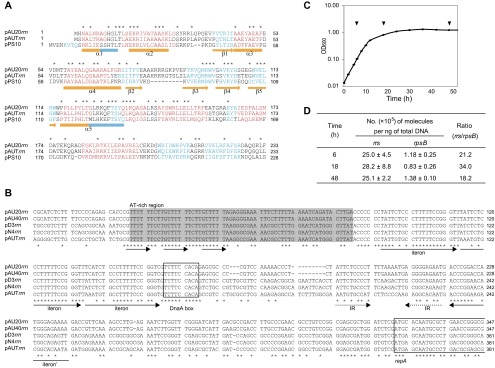
The regions of the origin and terminus of replication of the chromosome (oriC and ter, respectively) were not unusual in alphaproteobacteria (Fig. S3). In contrast, the replication system of pAU20rrn was similar to the replication system of pPS10, a 10-kb high-copy-number plasmid isolated from Pseudomonas syringae that has a stable host range confined to Pseudomonas species (14–16). The pAU20rrn-encoded RepA protein showed similarity to the pPS10 RepA protein in both primary (37% identical in amino acid sequence) and secondary structures (Fig. 1B and Fig. S4A), suggesting that the two RepA proteins behave similarly in replication control. In the case of pPS10, the RepA monomer initiates replication, whereas the RepA dimer represses the transcription of its own gene (16, 17). Upstream of repA on pAU20rrn (a predicted oriV site) were three tandem repeats (iterons) that formed a putative site for the binding of RepA monomers and an inverted repeat that is a putative site for the binding of the RepA dimer. In addition, an AT-rich region and a putative DnaA box (Fig. S4B) could be involved in the initiation of replication. In a quantitative PCR (qPCR) analysis, we determined that the number of pAU20rrn copies in the cell varied from 18 to 34 per chromosome equivalent throughout the growth phases (Fig. S4 C and D). The deduced amino acid sequences of the two ORFs present outside the rrn and repA/oriV regions did not have any motifs that are conserved among proteins involved in active partitioning of replicons, suggesting the stochastic segregation of pAU20rrn.
Fig. S3.
Prediction of the oriC and ter regions on the AU20 chromosome. (A) Map of the predicted oriC region. This region was located at a position showing a GC-skew shift (45) that was in the vicinity of hemE and maf homologs, which are among the genes typically localized near oriC (46). Locus tags for the ORFs are shown above the map. Annotations are shown with the color code on the right. (B) Nucleotide sequence of the intergenic region between hemE and maf homologs. The start positions and the orientations of these ORFs (indicated by arrows), an AT-rich region (shaded), and a putative DnaA box similar to the E. coli consensus sequence 5′-TTATCCACA-3′ (boxed) are marked. (C) Cumulative numbers of FtsK orienting polar sequence (KOPS) motifs (with the sequence 5′-GGGNAGGG-3′) plotted against the distance from the predicted oriC site on both strands of the chromosome. The inflection points on the lines suggest that the ter region was located on the chromosome at coordinates 2,491–2,495 (kb) (47). (D) Map of the predicted ter region. This region was located on the basis of the GC-skew and the distribution of KOPS motifs over the chromosome. Locus tags for the ORFs are shown above the map. Annotations are shown with the color code below. (E) Nucleotide sequence of the intergenic region between M673_11345 and M673_11350. The start position of M673_11345 (indicated by an arrow); the stop position of M673_11350 (indicated by a dashed line); and a predicted dif site [a site for site-specific recombination to resolve dimeric chromosomes; shaded (an inner palindrome is shown by arrows)], which is similar to the 28-nt motif identified in some members of the order Rhizobiales (48), are marked on the sequence.
Fig. S4.
Features of the rrn-plasmid. (A) Sequence comparison among RepA proteins from pAU20rrn (234 aa), pAUTrrn (233 aa), and pPS10 (230 aa; accession no. CAA41700). Asterisks above the alignment mark residues identical in the three sequences. Residues shown in red and blue are those residues predicted by the Jpred 3 server (49) to adopt α-helix and β-sheet secondary structures, respectively. The secondary structure elements evidenced from the crystal structure of the dimeric N-terminal domain of the pPS10 RepA (17) are shown below the sequence; the α-helical segments that can be remodelled into loops or β-strands in the monomeric RepA are indicated in blue. (B) Multiple sequence alignment of putative oriV sites on the five rrn-plasmids. An AT-rich region (shaded), including three 5′-GTTTTT-3′ repeats (indicated by arrows); a putative iteron consisting of three 20-nt tandem repeats and an additional repeat of the opposite orientation with the sequence 5′-(T/A)ATTCT(C/G)CCTTTTTCCGGGT-3′ (indicated by arrows labeled iteron and iteron′, respectively); a putative DnaA box matching at eight of nine positions with the one located at oriC (Fig. S3) (boxed); an inverted repeat with each arm matching half of the repeat in the iteron (indicated by dashed arrows); and the repA ORF (boxed) are shown in the aligned sequences. (C) Growth curve of AU20 harvested to determine copy numbers of pAU20rrn. The optical density at 600 nm (OD600) is shown as the mean of three independent cultures (±SD, as indicated by the error bars, which are not visible because of small variations). Arrowheads indicate the time points at which aliquots of the culture were sampled for DNA extraction. (D) Number of molecules of rrs (pAU20rrn marker) and rpsB (chromosome marker) contained in the total AU20 DNA (mean ± SD; n = 3).
Taken together, pAU20rrn does not match the criteria of a second chromosome because of its small size, high copy number, and lack of partitioning genes. It also differs from rrn-carrying plasmids of B. megaterium and Paracoccus species in that it alone carries the genes for rRNAs and tRNAIle within the genome. Therefore, pAU20rrn represents a distinct class of replicons, which we designated the rrn-plasmid, which is coupled with an rrn-lacking chromosome (RLC).
Distribution of the rrn-Plasmids in the Family Aurantimonadaceae.
To examine whether this RLC/rrn-plasmid genomic organization was specific to AU20, we determined the draft genome sequences of an additional 12 strains in the family Aurantimonadaceae (Tables S1 and S2). We identified rrn operons with the same organization as the organization of AU20 (including genes for tRNAIle and tRNAAla) in all of the strains (the region upstream of the 16S rRNA gene and a putative terminator are shown in Fig. S2). Four of the strains (A. ureilytica and Aureimonas spp. AU40, D3, and N4) (11, 18, 19) were similar to AU20 in that their rrn sequences were assembled into small (9.4–10 kb) circular replicons that contained pPS10 repA-like genes and oriV regions similar to pAU20rrn (Fig. 1B and Fig. S4 A and B). Southern hybridization analysis of the I-CeuI– or EcoRI-digested DNA of each of the four strains resulted in the detection of a single band when using the 16S rRNA gene as a probe (Fig. 1A). Therefore, we considered their rrn-carrying replicons to be rrn-plasmids.
Table S1.
Strains sequenced in this study
| Strain | Source (location) | Ref. |
| Aureimonas sp. AU20 | Soybean stem (Miyagi, Japan) | 11 |
| Aureimonas sp. AU40 | Soybean stem (Miyagi, Japan) | 11 |
| Aureimonas sp. D3 [= Pd-E-(l)-m-D-e (3)] | Rice shoot (Shiga, Japan) | 19 |
| Aureimonas sp. N4 [= Pd-S-(l)-l-N-4 (3)] | Rice shoot (Shiga, Japan) | 19 |
| Aureimonas ureilytica NBRC106430T (= 5715S-12T) | Air dust (Suwon, Korea) | 18 |
| Aureimonas sp. AU12 | Soybean stem (Miyagi, Japan) | 11 |
| Aureimonas sp. AU4 | Soybean stem (Miyagi, Japan) | 11 |
| Aureimonas sp. AU22 | Soybean stem (Miyagi, Japan) | 11 |
| Aureimonas altamirensis DSM21988T (= S21BT) | Terrestrial cave (Altamira Cave, Spain) | 50 |
| Aureimonas frigidaquae JCM14755T (= CW5T) | Water-cooling system (Gwangyang, Korea) | 51 |
| Aurantimonas coralicida DSM14790T (= WP1T) | Diseased coral (Florida Keys, United States) | 52 |
| Aurantimonas manganoxydans DSM21871T (= SI85-9A1T) | Fjord water (Sannich Inlet, Canada) | 53 |
| Fulvimarina pelagi DSM15513T (= HTCC2506T) | Sea water (Western Sargasso Sea) | 54 |
Table S2.
Outline for draft assemblies of the genome sequences
| Strain | No. of reads | Total length, bp | No. of contigs | Estimated genome size, Mb | Coverage | Contig accession no. |
| Aureimonas sp. AU40 | 1,336,160 | 270,613,677 | 90 | 5.5 | 49 | LC066393, BBWL01000001–01000065, 01000067–01000090 |
| Aureimonas sp. D3 | 1,214,449 | 194,144,157 | 127 | 5.4 | 36 | LC066394, BBWM01000001–01000084, 01000086–01000127 |
| Aureimonas sp. N4 | 1,336,419 | 255,523,246 | 85 | 5.3 | 48 | LC066398, BBWS01000001–01000069, 01000071–01000085 |
| A. ureilytica | 1,434,497 | 298,080,307 | 106 | 5.3 | 56 | LC066381, BBWT01000001–01000072, 01000074–01000106 |
| Aureimonas sp. AU12 | 1,290,323 | 234,581,545 | 89 | 4.8 | 49 | LC066382–066384, BBWH01000001–01000015, 01000017–01000028, 01000030–01000034, 01000036–01000041, 01000043–01000057, 01000061–01000094 |
| Aureimonas sp. AU4 | 1,314,008 | 220,880,662 | 121 | 4.7 | 47 | LC066388–066392, BBWK01000002–01000025, 01000027–01000040, 01000042, 01000043, 01000045–01000047, 01000049–01000054, 01000056–01000060, 01000062–01000072, 01000076–01000090, 01000092–01000100, 01000102, 01000103, 01000105, 01000106, 01000108, 01000109, 01000111, 01000114, 01000117, 01000119, 01000121–01000125, 01000129–01000139, 01000141 |
| Aureimonas sp. AU22 | 1,270,908 | 217,991,647 | 84 | 4.7 | 46 | LC066385–066387, BBWJ01000001–01000043, 01000046–01000051, 01000053–01000057, 01000059–01000061, 01000063–01000065, 01000067–01000073, 01000075–01000080, 01000082–01000089 |
| A. altamirensis | 1,308,463 | 278,815,906 | 26 | 4.2 | 66 | LC066369–066371, BBWQ01000002–01000008, 01000010–01000017, 01000020–01000022, 01000024, 01000027, 01000028, 01000030, 01000032 |
| A. frigidaquae | 1,321,097 | 275,827,698 | 22 | 4.1 | 68 | LC066375–066377, BBWR01000004–01000009, 01000011, 01000014–01000017, 01000019–01000026 |
| A. coralicida | 1,363,567 | 282,065,433 | 87 | 4.7 | 60 | LC066372–066374, BBWN01000001–01000015, 01000017, 01000018, 01000020–01000029, 01000031–01000041, 01000043, 01000044, 01000047–01000054, 01000056–01000074, 01000076–01000085, 01000087–01000093 |
| A. manganoxydans | 1,224,134 | 251,756,642 | 85 | 4.3 | 59 | LC066378–066380, BBWP01000001–01000015, 01000019–01000022, 01000024–01000029, 01000031–01000048, 01000050–01000059, 01000061–01000074, 01000076, 01000077, 01000079–01000084, 01000086, 01000087, 01000089–01000091, 01000093, 01000094 |
| F. pelagi | 1,434,753 | 300,888,349 | 50 | 3.8 | 79 | LC066395–066397, BBWO01000001–01000004, 01000006–01000011, 01000013–01000015, 01000018–01000026, 01000028–01000030, 201000032, 01000035–01000044, 01000046, 01000048, 01000050–01000052, 01000054, 01000055, 01000058–01000061 |
In each of the other eight strains, three or four different contigs were initially linked to the same rrn sequence on both its upstream and downstream sides. We investigated which pair of upstream and downstream contigs actually sandwiched the rrn operon by using PCR with primers designed from these contigs. This investigation clarified all of the rrn-flanking contigs in each genome (Tables S3 and S4; the number of rrn operons determined in this way is shown in Fig. 2). The assembled rrn-containing contigs/scaffolds, except for one contig in Aureimonas sp. AU12, were much longer (≥89.5 kb) than the rrn-plasmids. Moreover, one of these contigs/scaffolds in each strain (except Fulvimarina pelagi) contained a predicted oriC site, a chromosomal hallmark. Therefore, we regarded those rrn operons as chromosomally located, with such operons in each strain designated as rrnA, rrnB, rrnC, and/or rrnD. Southern hybridization analysis of the EcoRI-digested DNA of the eight strains confirmed the presence of different copies of the 16S rRNA gene, because all copies of this gene sequenced in this study lacked the EcoRI site (Fig. 1A). More intense hybridization signals from the rrn-plasmids than from the chromosomal signals suggested high copy numbers of the rrn-plasmids per chromosome (Fig. 1A). One of the three rrn-flanking sequences in AU12 was assembled into an exceptional 13.4-kb circular replicon, pAU12rrn (Fig. 1B and Table S3). Unlike pAU20rrn, this replicon carried the repABC cassette, which encodes the replication initiator RepC and the RepA-RepB–based active partitioning system (20).
Table S3.
rrn-carrying contigs and scaffolds in the draft genome assemblies
| Strain | Scaffold | |
| Order of contigs linked in the scaffold* | Length, bp | |
| A. ureilytica | LC066381 (pAUTrrn) | 9,969 |
| Aureimonas sp. AU40 | LC066393 (pAU40rrn) | 9,396 |
| Aureimonas sp. D3 | LC066394 (pD3rrn) | 9,912 |
| Aureimonas sp. N4 | LC066398 (pN4rrn) | 9,902 |
| A. altamirensis | LC066369 (oriC, rrnA) | 235,734 |
| LC066370 (rrnB) | 491,202 | |
| LC066371 (rrnC, dif) | 563,239 | |
| A. frigidaquae | LC066375 (rrnA, oriC) | 709,689 |
| LC066376 (rrnB) | 251,770 | |
| LC066377 (dif, rrnC) | 1,054,539 | |
| Aureimonas sp. AU22 | LC066385 (rrnA)–BBWJ01000053(oriC) | 134,825 |
| LC066386 (rrnB) | 93,746 | |
| LC066387 (dif, rrnC) | 377,401 | |
| Aureimonas sp. AU4 | BBWK01000040(oriC)–LC066388(rrnA) | 208,524 |
| LC066389(rrnD)–BBWK01000060†–BBWK01000059†–BBWK01000058†–BBWK01000057†–BBWK01000056†–LC066390(rrnB)–BBWK01000027 | 352,520 | |
| LC066391(dif)–LC066392(rrnC) | 270,387 | |
| Aureimonas sp. AU12 | LC066382(rrnA)–BBWH01000034†–BBWH01000033(oriC)†–BBWH01000032†–BBWH01000031† | 403,858 |
| LC066383 (rrnB) | 124,947 | |
| LC066384 (pAU12rrn) | 13,404 | |
| F. pelagi | LC066395 (rrnA) | 210,368 |
| LC066396 (rrnB) | 89,502 | |
| LC066397 (rrnC) | 624,009 | |
| A. coralicida | BBWN01000047(oriC)†–LC066372(rrnA) | 209,738 |
| LC066373 (rrnB) | 203,547 | |
| LC066374 (rrnC) | 395,184 | |
| A. manganoxydans | BBWP01000039(oriC)†–BBWP01000038†–BBWP01000071–BBWP01000083–LC066378(rrnA) | 154,368 |
| LC066379 (rrnB) | 195,490 | |
| LC066380 (rrnC) | 426,327 | |
Contigs are listed under their accession numbers. A copy of the rrn operon, a putative oriC or dif site that is present in the contig, is shown in parentheses after the accession number (dif sites are listed in Table S4). In the case of a contig corresponding to a small circular replicon, the replicon name is shown in parentheses.
Contig is arranged in the reverse orientation.
Table S4.
Predicted dif sites in the contigs
| Strain | Sequence (5′→3′)* | Contig | |
| Accession no. | Location (nucleotide no.) | ||
| Aureimonas sp. AU20 | AATACTCATAATATGGATTATGGAACAC | CP006367 | 2,493,549–2,493,576 |
| Aureimonas sp. AU40 | AATCCTCATAATATGGATTATGGAACAC | BBWL01000041 | 393,104–393,077 |
| Aureimonas sp. D3 | TGTTCTCATAATATGGATTATGGAACCA | BBWM01000035 | 69,577–69,604 |
| Aureimonas sp. N4 | TGTTCTCATAATATGGATTATGGAACCA | BBWS01000042 | 121,177–121,204 |
| A. ureilytica | AATACTCATAATATGGATTATGGAACAT | BBWT01000015 | 119,318–119,291 |
| Aureimonas sp. AU12 | CGATGGCATAATATGGATTATGGAACAT | BBWH01000001 | 95,271–95,298 |
| Aureimonas sp. AU4 | AAGTGGCATAATATGCATTATGGAACTA | LC066391 | 52,184–52,211 |
| Aureimonas sp. AU22 | AAATTGCATAATATGGATTATGGAACAT | LC066387 | 180,819–180,792 |
| A. altamirensis | AAATGGCATAAGATAGATTATGGAACCA | LC066371 | 279,206–279,179 |
| A. frigidaquae | AAATGGCATAAGATGGATTATGGAACCA | LC066377 | 676,504–676,531 |
| A. coralicida | AAGTTGCATAAGATAGATTATGGAACCG | BBWN01000036 | 70,610–70,583 |
| A. manganoxydans | AAGTTGCATAAGATAGATTATGGAACCG | BBWP01000052 | 10,286–10,313 |
| F. pelagi | AAGTTGCATAATATAGATTATGGAACTG | BBWO01000001 | 132,315–132,342 |
Nucleotides involved in the palindrome are underlined.
Fig. 2.
Phylogenetic relationship between members of the family Aurantimonadaceae using the maximum likelihood method. (A) Phylogenies based on nucleotide sequences of the 16S rRNA gene (Left) and the chromosomal genes atpD, dnaK, gyrB, rpoB, and rpoC concatenated by multilocus sequence analysis (MLSA) (Right). Colored lines on the back of strain names indicate that the strains were used in both trees; strains with red lines harbor the rrn operon on extrachromosomal replicons. A numeral in parentheses after each strain name is the number of the chromosomal rrn operons. (B) Relationship of the RepA amino acid sequence among the rrn-plasmids and pPS10, with their host names in parentheses. Numerals above branches are the related bootstrap values (%; values ≥ 50 are shown). Scale bars indicate the substitution number per site.
Phylogeny of the Strains Having the rrn-Plasmids.
In both the phylogenetic analysis using 16S rRNA genes and the phylogenetic analysis using five housekeeping protein-coding genes, atpD, dnaK, gyrB, rpoB, and rpoC (all chromosomally located in AU20) (Fig. 2A and Table S4), the five strains with the RLC/rrn-plasmid organization were clustered into one clade (designated the RLC clade) within the genus Aureimonas. The similar topology of the two phylogenetic trees suggested that all of the rrn operons on the rrn-plasmids originated evolutionarily from the common ancestor of the clade. In contrast, the rrn-plasmids in the RLC clade seemed to have diverged themselves after the origination of the first rrn-plasmid (or, alternatively, had independent origins) because they split into two subgroups of AU20/AU40 and D3/N4/A. ureilytica with regard to the region outside the rrn operon. RepA was ≥97% and ≤75% identical within each subgroup and between subgroups, respectively (the RepA genealogy is shown in Fig. 2B). Moreover, the latter subgroup encoded a protein (labeled Rep′ in Fig. 1B) with similarity to the Rep_3-type replication initiator of a Zymomonas mobilis plasmid (21).
Chromosome Rearrangements Observed in the Genus Aureimonas.
We compared the AU20 chromosome with chromosomal rrn contigs from strains AU4, AU12, and AU22, which are non-RLC members close to the RLC clade in the genus Aureimonas. This analysis showed several synteny breaks around each of rrnA, rrnB, and rrnC (Fig. S5). In the rrnC region, tRNA genes were situated at some of the breakpoints (Fig. S5 C and F), suggesting that the tRNA genes were involved in chromosome inversion and integration of alien sequences to generate chromosome variation. In the rrnA and rrnB regions, any characteristic sequences were not found at the synteny breakpoints (Fig. S5 A, B, D, and E). Apparent frequent recombination around rrnC might be related to the ter region, where a stalled replication fork is potentially subject to DNA cleavage by some endonucleases (e.g., as type I and type IV restriction enzymes) and subsequent recombinational repair (22, 23). However, we failed to find any sign of recombination that could explain the loss of the rrn operon; the comparisons between more closely related RLC and non-RLC strains would serve to trace their common ancestral chromosome and the process toward the RLC/rrn-plasmid organization.
Fig. S5.
Chromosomal synteny breaks of the rrn regions in the genus Aureimonas. (A and D) rrnA. (B and E) rrnB. (C and E) rrnC. (A–C) Dot plots comparing the AU20 chromosome (on vertical axes) with scaffolds (or contigs) from strains AU4, AU12, and AU22 (on horizontal axes) by GenomeMatcher (33) using tblastx with a parameter set “–F F –W 3 –e 0.01.” Positions of tRNA genes are indicated by blue lines with three-letter symbols for their charged amino acids, and arrows in parentheses indicate orientations of the genes (otherwise, the opposite orientations). The blue line with a red arrowhead indicates a dif site. (D–F) Multiple alignments comparing sequences of the AU20 chromosome and scaffolds from strains AU12, AU22, AU4, D3, and A. ureilytica (the scaffolds are listed in Table S3) by GenomeMatcher using blastn with a parameter set “–F F –W 21 –e 0.01.” Strain names are shown on the left; maps in D and E are from the same strains when compared horizontally. A line color represents the percentage (%) of nucleotide identity between connected sites. In each map, rrn (oriented rightward in all drawings) is indicated in blue, maf and hemE in black, tRNA genes by blue arrowheads, oriC by a black arrowhead, and dif by an open arrowhead.
Implication of the rrn-Plasmid for Bacterial Genomics.
What evolutionary significance does the emergence of the RLC clade involve? It can first be said that their members have acquired increased rRNA gene dosage. In general, the number of rrn operons on the chromosomes of diverse bacterial species and strains varies, irrespective of their whole-genome sizes; for example, the Bradyrhizobium diazoefficiens genome (9.1 Mb) contains only one copy (24), whereas the genomes of Escherichia coli (4.6 Mb) and Bacillus subtilis (4.2 Mb) contain seven and 10 copies, respectively (25, 26). In E. coli, the presence of multiple rrn operons facilitates a sudden increase in the rate of rRNA synthesis, enabling rapid adaptation to nutritional upshift or favorable temperature shift (27). Therefore, we consider it possible that the high copy number of the rrn-plasmid, compared with four rrn copies at most on the chromosome, confers a selective advantage to the Aureimonas host under changing environmental conditions. In this context, it is noteworthy that the RLC clade overlaps completely with the operational taxonomic unit whose relative abundance in soybean-associated bacterial communities fluctuates drastically in response to phenotypic and nutritional changes of the host plants (11, 28). Second, split of the rrn operon from the chromosome possibly has an additional regulatory influence, for example, on the global transcription profile by redirecting RNA polymerase from the chromosome to the separate replicon. In this sense, it will be interesting to see a subcellular localization pattern of the rrn-plasmid. In eukaryotes, the nucleolus displays functionality of a distinct subnuclear compartment, which is formed around the ribosomal DNA. The nucleolus functions not only in ribosome biogenesis but also in regulation by sequestering key factors of cell-cycle progression and stress responses into the compartment (29). Third, localization of the rrn operon on a small plasmid has possibly lowered a barrier to its horizontal transfer, facilitating its exchange between bacteria residing in common niches. Several studies have indeed suggested the horizontal transfer of the 16S rRNA genes (30). The replication host range and the potential for conjugal transfer of the rrn-plasmid will be very interesting subjects in future studies.
We conducted a survey of the complete genome sequence database for the RLC/rrn-plasmid organization in a wide range of bacterial phyla. We found that Butyrivibrio fibrisolvens 16/4, butyrate-producing bacterium SS3/4 (phylum Firmicutes), and Fretibacterium fastidiosum SGP1 (phylum Synergistetes) have main chromosomes devoid of rRNA-coding genes, although the predicted coresident rrn-carrying replicons have not been included in the database. This result raises the possibility that RLC is not limited to the genus Aureimonas. Altogether, our findings pose a fundamental question of what has shaped the evolution of multipartite genomes in life. In environmental microbiology, moreover, the existence of the RLC/rrn-plasmid organization should be taken into consideration when profiling microbial communities on the basis of rRNA gene sequences.
Methods
Total DNA Extraction.
Total DNA was extracted with a DNeasy Blood & Tissue Kit (QIAGEN) in accordance with the manufacturer’s instructions, with slight modification (11).
Determination and Annotation of the AU20 Genome Sequence.
A fragmented single-end genome library was sequenced by using 454 GS-FLX Titanium (Roche), producing 236,422 reads (92 Mb in total). Then, a 3-kb mate-pair genome library was sequenced by using a HiSeq 2000 (Illumina) instrument (operated by Eurofins Genomics, Inc.). The obtained reads were trimmed based on underrepresented 21-mers by using ShortReadManager (31), and 1,000,000 of the resulting reads (79 Mb in total) were used for subsequent assembly. Finally, an 8-kb paired-end genome library, which was constructed by Takara-Bio, Inc., was sequenced by using 454 GS-FLX Titanium, producing 262,026 reads (121 Mb in total). The reads from these three systems were assembled by using Newbler, version 2.6 (Roche). The finishing was facilitated by using GenoFinisher (31) and AceFileViewer (31), and resulted in the generation of eight circular contigs and one large scaffold containing six gaps. To close all of the gaps, DNA fragments encompassing the gaps were amplified by PCR with primers designed by GenoFinisher, and the reaction products were sequenced with the Sanger method by using a 3730xl DNA Analyzer with a BigDye Terminator Cycle Sequencing Reaction Kit (Life Technologies).
The genome sequence was annotated by using the National Center for Biotechnology Information (NCBI) Prokaryotic Genomes Automatic Annotation Pipeline (32), and the result was manually inspected with respect to positions of start codons for predicted ORFs by using the Microbial Genome Annotation Pipeline (MiGAP; www.migap.org/) and GenomeMatcher (33). All of the ORFs were translated into amino acid sequences, which were then subjected to similarity searches against the RefSeq collection from the NCBI by using BLAST2GO (34) (with an Expect value <10−3 for cutoff). Protein domains were identified by using InterProScan (35).
Southern Hybridization.
Total DNA (1.5 μg) digested with I-CeuI or EcoRI was electrophoresed in a 0.8% agarose gel and blotted onto a nylon membrane. The blot was probed with a fragment of the rrs gene (encoding 16S rRNA) that was amplified from the AU20 DNA by PCR using the primer pair 5′-AGAGTTTGATCCTGGCTCAG-3′ and 5′-AAGGAGGTG(A/T)TCCA(A/G)CC-3′ and labeled with digoxigenin (DIG). Probe labeling, hybridization, and signal detection were performed with a DIG Labeling and Detection Kit (Roche).
Determination of the pAU20rrn Copy Number.
An AU20 culture was grown, with vigorous shaking, at 28 °C in tryptone-yeast extract medium, which contained tryptone (5 g/L), yeast extract (3 g/L), and CaCl2⋅2H2O (0.83 g/L). Aliquots were taken at intervals and centrifuged to collect the cells for DNA extraction. We selected rpsB, which was located close to ter on the chromosome, as a target chromosomal gene for qPCR, and used the primer pair 5′-TGCTGACGAACTGGAAGACG-3′ and 5′-GCAGGTTGAGACGCTCCTTC-3′ to amplify a 112-bp product. We selected rrs as a target pAU20rrn gene for qPCR, and used the primer pair 5′-AACGCGCAGAACCTTACCAG-3′ and 5′-TGCGGGACTTAACCCAACAT-3′ to amplify a 133-bp product. The real-time qPCR assay was performed in a 20-μL reaction mixture (FastStart Essential DNA GreenMaster, version 02; Roche), supplemented with the EcoRI-digested total DNA (0.2 ng) as a template and the above primer pair, by using LightCycler Nano (Roche). The cycling conditions consisted of an initial 10 min at 95 °C, followed by 45 cycles of 95 °C for 10 s, 60 °C for 10 s, and 72 °C for 15 s. A melting curve analysis was then conducted from 60–95 °C at 0.1 °C⋅s−1. Cycle threshold values were determined with the second derivative maximum method by using a LightCycler Nano SW 1.0 (Roche). Standard curves to calibrate the numbers of rpsB and rrs molecules with the threshold cycle numbers were generated by conducting qPCR assays with serial 10-fold dilutions of the respective DNA fragments that had been amplified by PCR using the same primer sets as above. The copy number of pAU20rrn per chromosome equivalent was calculated as the molecular ratio of rrs to rpsB.
Draft Genome Sequencing.
A paired-end genome library was constructed from total DNA and sequenced by using MiSeq (Illumina). Obtained reads were assembled by using Newbler, version 2.6. The resulting contigs were examined for their possible linkage by using GenoFinisher and AceFileViewer. When necessary, PCR was performed using total DNA as a template and the primers designed from the contigs to confirm the actual linkage. The genome sequences were annotated by using MiGAP. Putative oriC or dif sites were located by using BLAST searches for hemE and maf homologs or for a 28-nt dif-like motif, respectively, using the AU20 sequence as a query.
Phylogenetic Analysis.
Nucleotide sequences (Table S5) or amino acid sequences were aligned, and the maximum likelihood trees were built according to the Tamura–Nei model or the Jones–Taylor–Thornton matrix-based model, respectively, by using MEGA version 5.2 (36). For phylogenetic analyses based on the 16S rRNA gene, we used sequences corresponding to nucleotide numbers 109–1,406 of the E. coli gene. Multilocus sequencing analysis was performed by using five genes: gyrB, rpoB, rpoC, atpD, and dnaK. The sequence of each orthologous gene was trimmed to a uniform length in strains (2,372 bp for gyrB, 4,131 bp for rpoC, 4,152 bp for rpoB, 988 bp for recA, and 1,805 bp for dnaK). The five sequences from each strain were concatenated before being aligned. To confirm the tree topology, 1,000 bootstrap trials were performed.
Table S5.
Accession numbers of the genes and contigs used for phylogenetic analyses
| Strain* | rrs | atpD | dnaK | gyrB | rpoB | rpoC |
| Aureimonas sp. AU20 | CP006375 | CP006367 | CP006367 | CP006367 | CP006367 | CP006367 |
| Aureimonas sp. AU40 | AB600141 | BBWL010000033 | BBWL010000030 | BBWL010000030 | BBWL010000064 | BBWL010000064 |
| Aureimonas sp. D3 | AB600180 | BBWM010000034 | BBWM010000047 | BBWM010000047 | BBWM010000083 | BBWM010000083 |
| Aureimonas sp. N4 | AB600181 | BBWS010000006 | BBWS010000008 | BBWS010000008 | BBWS010000062 | BBWS010000062 |
| A. ureilytica | DQ883810 | BBWT010000005 | BBWT010000017 | BBWT010000001 | BBWT010000074 | BBWT010000074 |
| Aureimonas sp. AU12 | AB600133 | BBWH010000021 | BBWH010000031 | BBWH010000029 | BBWH010000057 | BBWH010000057 |
| Aureimonas sp. AU4 | AB600129 | BBWK010000006 | BBWK010000034 | BBWK010000063 | BBWK010000092 | BBWK010000092 |
| Aureimonas jatrophae | JQ346805 | — | — | — | — | — |
| Aureimonas sp. AU22 | AB600138 | BBWJ010000047 | BBWJ010000026 | BBWJ010000051 | BBWJ010000065 | BBWJ010000065 |
| Aureimonas phyllosphaerae | JQ346806 | — | — | — | — | — |
| Aureimonas rubiginis | JQ864241 | — | — | — | — | — |
| Aureimonas ferruginea | JQ864240 | — | — | — | — | — |
| A. altamirensis | DQ372921 | BBWQ010000011 | BBWQ010000025 | BBWQ010000020 | BBWQ010000004 | BBWQ010000004 |
| A. frigidaquae | EF373540 | BBWR010000005 | BBWR010000003 | BBWR010000011 | BBWR010000022 | BBWR010000022 |
| Aurantimonas litoralis HTCC2156 | AY178863 | — | — | — | — | — |
| A. coralicida | AJ786361 | BBWN010000008 | BBWN010000017 | BBWN010000001 | BBWN010000056 | BBWN010000056 |
| Aurantimonas manganoxydans | AJ786360 | BBWP010000013 | BBWP010000006 | BBWP010000004 | BBWP010000026 | BBWP010000026 |
| F. pelagi | AY178860 | BBWO010000012 | BBWO010000012 | BBWO010000001 | BBWO010000032 | BBWO010000032 |
| Martelella mediterranea | AY649762 | — | — | — | — | — |
| Aminobacter aminovorans | AJ011759 | — | — | — | — | — |
Type strains of the respective species were used unless strain names are specified.
Database Survey for RLC.
On November 30, 2014, there were 3,150 RNA gene table (rnt) files, showing replicons carrying rRNA and/or tRNA genes, from 2,767 bacterial strains with complete genome sequences in the NCBI database. We regarded those replicons as including the main chromosomes of the respective strains, from which we selected 228 replicons as lacking rRNA genes. We subsequently selected three replicons, each of which was the largest (i.e., the main chromosome) in each strain. The absence of rRNA genes (or their remnants) was confirmed by RNAmmer (37) and nucleotide-nucleotide BLAST (blastn) searches.
Supplementary Material
Acknowledgments
We thank H. Morisaki for providing Aureimonas strains, S. Sato and T. Kaneko for advice on sequence analysis, K. Chiba-Kakizaki for assistance with sequencing, and the Tohoku University Technical Support Center for use of the 454 GS-FLX Titanium system. This study was supported by a grant from the Bio-oriented Technology Research Advancement Institution (to K.M.) and Grants-in-Aid for Scientific Research Grants 23248052 and 26252065 (to K.M.) and Grant 247034 (to M.A.) from Japan Society for the Promotion of Science (JSPS). M.A. and T.O. were supported by JSPS fellowships.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database, www.ncbi.nlm.nih.gov/genbank/, and in the DNA Data Bank of Japan database, www.ddbj.nig.ac.jp. A list of accession numbers is provided in Table 1 and Table S2.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514326112/-/DCSupplemental.
References
- 1.Egan ES, Fogel MA, Waldor MK. Divided genomes: Negotiating the cell cycle in prokaryotes with multiple chromosomes. Mol Microbiol. 2005;56(5):1129–1138. doi: 10.1111/j.1365-2958.2005.04622.x. [DOI] [PubMed] [Google Scholar]
- 2.Harrison PW, Lower RPJ, Kim NKD, Young JPW. Introducing the bacterial ‘chromid’: Not a chromosome, not a plasmid. Trends Microbiol. 2010;18(4):141–148. doi: 10.1016/j.tim.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Reyes-Lamothe R, Nicolas E, Sherratt DJ. Chromosome replication and segregation in bacteria. Annu Rev Genet. 2012;46:121–143. doi: 10.1146/annurev-genet-110711-155421. [DOI] [PubMed] [Google Scholar]
- 4.Suwanto A, Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: Presence of two unique circular chromosomes. J Bacteriol. 1989;171(11):5850–5859. doi: 10.1128/jb.171.11.5850-5859.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michaux S, et al. Presence of two independent chromosomes in the Brucella melitensis 16M genome. J Bacteriol. 1993;175(3):701–705. doi: 10.1128/jb.175.3.701-705.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodley PD, Römling U, Tümmler B. A physical genome map of the Burkholderia cepacia type strain. Mol Microbiol. 1995;17(1):57–67. doi: 10.1111/j.1365-2958.1995.mmi_17010057.x. [DOI] [PubMed] [Google Scholar]
- 7.Yamaichi Y, Iida T, Park K-S, Yamamoto K, Honda T. Physical and genetic map of the genome of Vibrio parahaemolyticus: presence of two chromosomes in Vibrio species. Mol Microbiol. 1999;31(5):1513–1521. doi: 10.1046/j.1365-2958.1999.01296.x. [DOI] [PubMed] [Google Scholar]
- 8.Kunnimalaiyaan M, Stevenson DM, Zhou Y, Vary PS. Analysis of the replicon region and identification of an rRNA operon on pBM400 of Bacillus megaterium QM B1551. Mol Microbiol. 2001;39(4):1010–1021. doi: 10.1046/j.1365-2958.2001.02292.x. [DOI] [PubMed] [Google Scholar]
- 9.Battermann A, Disse-Krömker C, Dreiseikelmann B. A functional plasmid-borne rrn operon in soil isolates belonging to the genus Paracoccus. Microbiology. 2003;149(Pt 12):3587–3593. doi: 10.1099/mic.0.26608-0. [DOI] [PubMed] [Google Scholar]
- 10.Rathsack K, Reitner J, Stackebrandt E, Tindall BJ. Reclassification of Aurantimonas altamirensis (Jurado et al. 2006), Aurantimonas ureilytica (Weon et al. 2007) and Aurantimonas frigidaquae (Kim et al. 2008) as members of a new genus, Aureimonas gen. nov., as Aureimonas altamirensis gen. nov., comb. nov., Aureimonas ureilytica comb. nov. and Aureimonas frigidaquae comb. nov., and emended descriptions of the genera Aurantimonas and Fulvimarina. Int J Syst Evol Microbiol. 2011;61(Pt 11):2722–2728. doi: 10.1099/ijs.0.027029-0. [DOI] [PubMed] [Google Scholar]
- 11.Anda M, et al. Isolation and genetic characterization of Aurantimonas and Methylobacterium strains from stems of hypernodulated soybeans. Microbes Environ. 2011;26(2):172–180. doi: 10.1264/jsme2.me10203. [DOI] [PubMed] [Google Scholar]
- 12.Liu S-L, Hessel A, Sanderson KE. Genomic mapping with I-Ceu I, an intron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc Natl Acad Sci USA. 1993;90(14):6874–6878. doi: 10.1073/pnas.90.14.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gourse RL, Gaal T, Bartlett MS, Appleman JA, Ross W. rRNA transcription and growth rate-dependent regulation of ribosome synthesis in Escherichia coli. Annu Rev Microbiol. 1996;50:645–677. doi: 10.1146/annurev.micro.50.1.645. [DOI] [PubMed] [Google Scholar]
- 14.Nieto C, Giraldo R, Fernández-Tresguerres E, Díaz R. Genetic and functional analysis of the basic replicon of pPS10, a plasmid specific for Pseudomonas isolated from Pseudomonas syringae patovar savastanoi. J Mol Biol. 1992;223(2):415–426. doi: 10.1016/0022-2836(92)90661-3. [DOI] [PubMed] [Google Scholar]
- 15.Fernández-Tresguerres ME, Martín M, García de Viedma D, Giraldo R, Díaz-Orejas R. Host growth temperature and a conservative amino acid substitution in the replication protein of pPS10 influence plasmid host range. J Bacteriol. 1995;177(15):4377–4384. doi: 10.1128/jb.177.15.4377-4384.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giraldo R, Fernández-Tresguerres ME. Twenty years of the pPS10 replicon: Insights on the molecular mechanism for the activation of DNA replication in iteron-containing bacterial plasmids. Plasmid. 2004;52(2):69–83. doi: 10.1016/j.plasmid.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Giraldo R, Fernández-Tornero C, Evans PR, Díaz-Orejas R, Romero A. A conformational switch between transcriptional repression and replication initiation in the RepA dimerization domain. Nat Struct Biol. 2003;10(7):565–571. doi: 10.1038/nsb937. [DOI] [PubMed] [Google Scholar]
- 18.Weon HY, et al. Aurantimonas ureilytica sp. nov., isolated from an air sample. Int J Syst Evol Microbiol. 2007;57(Pt 8):1717–1720. doi: 10.1099/ijs.0.65035-0. [DOI] [PubMed] [Google Scholar]
- 19.Mano H, Tanaka F, Nakamura C, Kaga H, Morisaki H. Culturable endophytic bacterial flora of the maturing leaves and roots of rice plants (Oryza sativa) cultivated in a paddy field. Microbes Environ. 2007;22(2):175–185. [Google Scholar]
- 20.Pinto UM, Pappas KM, Winans SC. The ABCs of plasmid replication and segregation. Nat Rev Microbiol. 2012;10(11):755–765. doi: 10.1038/nrmicro2882. [DOI] [PubMed] [Google Scholar]
- 21.So LY, Chen WY, Lacap-Bugler DC, Seemann M, Watt RM. pZMO7-Derived shuttle vectors for heterologous protein expression and proteomic applications in the ethanol-producing bacterium Zymomonas mobilis. BMC Microbiol. 2014;14:68. doi: 10.1186/1471-2180-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishikawa K, Handa N, Kobayashi I. Cleavage of a model DNA replication fork by a Type I restriction endonuclease. Nucleic Acids Res. 2009;37(11):3531–3544. doi: 10.1093/nar/gkp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishikawa K, Handa N, Sears L, Raleigh EA, Kobayashi I. Cleavage of a model DNA replication fork by a methyl-specific endonuclease. Nucleic Acids Res. 2011;39(13):5489–5498. doi: 10.1093/nar/gkr153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaneko T, et al. Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res. 2002;9(6):189–197. doi: 10.1093/dnares/9.6.189. [DOI] [PubMed] [Google Scholar]
- 25.Kiss A, Sain B, Venetianer P. The number of rRNA genes in Escherichia coli. FEBS Lett. 1977;79(1):77–79. doi: 10.1016/0014-5793(77)80354-2. [DOI] [PubMed] [Google Scholar]
- 26.LaFauci G, Widom RL, Eisner RL, Jarvis ED, Rudner R. Mapping of rRNA genes with integrable plasmids in Bacillus subtilis. J Bacteriol. 1986;165(1):204–214. doi: 10.1128/jb.165.1.204-214.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Condon C, Liveris D, Squires C, Schwartz I, Squires CL. rRNA operon multiplicity in Escherichia coli and the physiological implications of rrn inactivation. J Bacteriol. 1995;177(14):4152–4156. doi: 10.1128/jb.177.14.4152-4156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeda S, et al. Community shifts of soybean stem-associated bacteria responding to different nodulation phenotypes and N levels. ISME J. 2010;4(3):315–326. doi: 10.1038/ismej.2009.119. [DOI] [PubMed] [Google Scholar]
- 29.Boisvert F-M, van Koningsbruggen S, Navascués J, Lamond AI. The multifunctional nucleolus. Nat Rev Mol Cell Biol. 2007;8(7):574–585. doi: 10.1038/nrm2184. [DOI] [PubMed] [Google Scholar]
- 30.Kitahara K, Miyazaki K. Revisiting bacterial phylogeny: Natural and experimental evidence for horizontal gene transfer of 16S rRNA. Mob Genet Elements. 2013;3(1):e24210. doi: 10.4161/mge.24210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohtsubo Y, Maruyama F, Mitsui H, Nagata Y, Tsuda M. Complete genome sequence of Acidovorax sp. strain KKS102, a polychlorinated-biphenyl degrader. J Bacteriol. 2012;194(24):6970–6971. doi: 10.1128/JB.01848-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angiuoli SV, et al. Toward an online repository of Standard Operating Procedures (SOPs) for (meta)genomic annotation. OMICS. 2008;12(2):137–141. doi: 10.1089/omi.2008.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohtsubo Y, Ikeda-Ohtsubo W, Nagata Y, Tsuda M. GenomeMatcher: A graphical user interface for DNA sequence comparison. BMC Bioinformatics. 2008;9:376. doi: 10.1186/1471-2105-9-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conesa A, et al. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 35.Mulder N, Apweiler R. InterPro and InterProScan: Tools for protein sequence classification and comparison. Methods Mol Biol. 2007;396:59–70. doi: 10.1007/978-1-59745-515-2_5. [DOI] [PubMed] [Google Scholar]
- 36.Tamura K, et al. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lagesen K, et al. RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35(9):3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langille MG, Brinkman FS. IslandViewer: An integrated interface for computational identification and visualization of genomic islands. Bioinformatics. 2009;25(5):664–665. doi: 10.1093/bioinformatics/btp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Liang Y, Lynch KH, Dennis JJ, Wishart DS. PHAST: A fast phage search tool. Nucleic Acids Res. 2011;39(Web Server issue) Suppl 2:W347–W352. doi: 10.1093/nar/gkr485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varani AM, Siguier P, Gourbeyre E, Charneau V, Chandler M. ISsaga is an ensemble of web-based methods for high throughput identification and semi-automatic annotation of insertion sequences in prokaryotic genomes. Genome Biol. 2011;12(3):R30. doi: 10.1186/gb-2011-12-3-r30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gustafson AM, O’Connell KP, Thomashow MF. Regulation of Sinorhizobium meliloti 1021 rrnA-reporter gene fusions in response to cold shock. Can J Microbiol. 2002;48(9):821–830. doi: 10.1139/w02-078. [DOI] [PubMed] [Google Scholar]
- 42.MacLellan SR, MacLean AM, Finan TM. Promoter prediction in the rhizobia. Microbiology. 2006;152(Pt 6):1751–1763. doi: 10.1099/mic.0.28743-0. [DOI] [PubMed] [Google Scholar]
- 43.Condon C, Squires C, Squires CL. Control of rRNA transcription in Escherichia coli. Microbiol Rev. 1995;59(4):623–645. doi: 10.1128/mr.59.4.623-645.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brosius J, Dull TJ, Sleeter DD, Noller HF. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- 45.Lobry JR. Asymmetric substitution patterns in the two DNA strands of bacteria. Mol Biol Evol. 1996;13(5):660–665. doi: 10.1093/oxfordjournals.molbev.a025626. [DOI] [PubMed] [Google Scholar]
- 46.Brassinga AKC, Siam R, Marczynski GT. Conserved gene cluster at replication origins of the α-proteobacteria Caulobacter crescentus and Rickettsia prowazekii. J Bacteriol. 2001;183(5):1824–1829. doi: 10.1128/JB.183.5.1824-1829.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bigot S, Sivanathan V, Possoz C, Barre F-X, Cornet F. FtsK, a literate chromosome segregation machine. Mol Microbiol. 2007;64(6):1434–1441. doi: 10.1111/j.1365-2958.2007.05755.x. [DOI] [PubMed] [Google Scholar]
- 48.Carnoy C, Roten C-A. The dif/Xer recombination systems in proteobacteria. PLoS One. 2009;4(9):e6531. doi: 10.1371/journal.pone.0006531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cole C, Barber JD, Barton GJ. The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 2008;36(Web Server issue) Suppl 2:W197–W201. doi: 10.1093/nar/gkn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jurado V, Gonzalez JM, Laiz L, Saiz-Jimenez C. Aurantimonas altamirensis sp. nov., a member of the order Rhizobiales isolated from Altamira Cave. Int J Syst Evol Microbiol. 2006;56(Pt 11):2583–2585. doi: 10.1099/ijs.0.64397-0. [DOI] [PubMed] [Google Scholar]
- 51.Kim MS, Hoa KT, Baik KS, Park SC, Seong CN. Aurantimonas frigidaquae sp. nov., isolated from a water-cooling system. Int J Syst Evol Microbiol. 2008;58(Pt 5):1142–1146. doi: 10.1099/ijs.0.65421-0. [DOI] [PubMed] [Google Scholar]
- 52.Denner EB, et al. Aurantimonas coralicida gen. nov., sp. nov., the causative agent of white plague type II on Caribbean scleractinian corals. Int J Syst Evol Microbiol. 2003;53(Pt 4):1115–1122. doi: 10.1099/ijs.0.02359-0. [DOI] [PubMed] [Google Scholar]
- 53.Anderson CR, et al. Aurantimonas manganoxydans, sp. nov. and Aurantimonas litoralis, sp. nov.: Mn(II) oxidizing representatives of a globally distributed clade of alpha-Proteobacteria from the order Rhizobiales. Geomicrobiol J. 2009;26(3):189–198. doi: 10.1080/01490450902724840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho JC, Giovannoni SJ. Fulvimarina pelagi gen. nov., sp. nov., a marine bacterium that forms a deep evolutionary lineage of descent in the order “Rhizobiales”. Int J Syst Evol Microbiol. 2003;53(Pt 6):1853–1859. doi: 10.1099/ijs.0.02644-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.