Abstract
Objectives
To examine N-acetylaspartate (NAA), a general marker of neuronal viability, and total NAA (tNAA), the combined signal of NAA and N-acetylaspartylglutamate, in bipolar depression before and after lamotrigine treatment. Given that NAA is synthesized through direct acetylation of aspartate by acetyl-coenzyme A-L-aspartate-N-acetyltransferase, we hypothesized that treatment with lamotrigine would be associated with an increase in NAA level.
Methods
Patients with bipolar depression underwent two-dimensional proton magnetic resonance spectroscopy of the anterior cingulate at baseline (n = 15) and after 12 weeks of lamotrigine treatment (n = 10). A group of age-matched healthy controls (n = 9) underwent scanning at baseline for comparison.
Results
At baseline, patients with bipolar depression had significantly lower NAA [mean standard deviation (SD) = 1.13 (0.21); p = 0.02] than controls [mean (SD) = 1.37 (0.27)]. Significant increases in NAA [mean (SD) = 1.39 (0.21); p = 0.01] and tNAA [mean (SD) = 1.61 (0.25); p = 0.02] levels were found after 12 weeks of lamotrigine treatment.
Conclusions
These data suggest an NAA deficit in bipolar depression that is normalized after lamotrigine treatment. Future research is warranted to evaluate whether baseline NAA level is a potential biomarker for identifying lamotrigine response patterns and whether this functional brain change has an associated clinical response.
Keywords: bipolar depression, bipolar disorder, lamotrigine, N-acetylaspartate, neuroprotection, two-dimensional proton magnetic resonance spectroscopy
Lamotrigine is an anticonvulsant approved by the US Food and Drug Administration for maintenance mood stabilization of bipolar I disorder (1). Its acute antidepressant properties are recognized increasingly, both through meta-analysis (2, 3) and placebo-controlled evaluation (4–6). The anticonvulsant activity of lamotrigine is thought to be mediated by voltage-dependent sodium channel blockade, with subsequent presynaptic inhibition of aspartate (Asp) and glutamate (Glu) resulting in an overall reduction in neuronal excitability (7–10). Similar to lithium, lamotrigine also has been shown to be neuroprotective against Glu excitotoxicity in a number of cellular and animal models (9, 11, 12). Recent work has highlighted evidence of its neuroprotective properties, mediated through histone deacetylase inhibition and chromatin remodeling, and both dose- and time-dependent increases in antiapoptotic Bcl-2 mRNA and protein levels (9). How these mechanisms confer antidepressant effects in bipolar depression is unclear.
N-Acetylaspartate (NAA) is an abundant neuronal metabolite broadly, conceptualized as a marker of mitochondrial activity and neuronal integrity (13). Putative functions include: maintenance of brain fluid balance as an osmolyte, providing a source of acetate for myelin synthesis (14); serving as a precursor for synthesis of N-acetylaspartylglutamate (NAAG); and regulating Glu metabolism (15, 16). NAA is synthesized in neuronal mitochondria from L-aspartate and acetyl coenzyme A through Asp N-acetyltransferase (17). An oligodendrocytic enzyme, aspartoacylase hydrolyzes NAA and thereby provides acetate for myelin synthesis (14). Glu and NAA metabolism are linked through the Glu-glutamine (Gln) and tricarboxylic acid cycles (18, 19) because such NAA may also serve as a Glu pool and buffer of glutamatergic excitoxcity (16).
Proton magnetic resonance spectroscopy (MRS) is a noninvasive functional imaging technique that can quantify NAA cortical deficits and measure dynamic changes (20, 21). This type of MRS resonance spectra of NAA is a dominant singlet peak at 2.02 ppm. Typically, the NAA signal is total NAA (tNAA), which is comprised of NAA and NAAG (22, 23). However, conventional one-dimensional MRS is limited given the resonance signal overlap. Two-dimensional (2D) localized correlated spectroscopy (L-COSY) facilitates better separation of overlapping cerebral metabolites and allows a more accurate quantification of NAA and tNAA through a novel prior-knowledge fitting algorithm (ProFit; QuantumSoft). Early work in 2D MRS has focused on metabolite discrimination and reproducibility in healthy controls (24, 25), cross-sectional evaluation of frontal white and gray matter in chronic hepatitis C infection (26), and different mood states in bipolar disorder (27).
The aim of this exploratory study was to use 2D MRS and L-COSY to quantify anterior cingulate NAA and tNAA in patients with bipolar depression and evaluate change in NAA and tNAA after 12 weeks of treatment with lamotrigine. We hypothesized that patients with bipolar depression have decreased NAA levels in the anterior cingulate cortex and that lamotrigine therapy corrects this deficit.
Methods
The study protocol was approved by the University of California at Los Angeles (UCLA), Harbor–UCLA Medical Center (Los Angeles, CA, USA), and Mayo Clinic (Rochester, MN, USA) institutional review boards. Every study participant gave written informed consent, with diagnosis confirmed with the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision) (28). Study inclusion criteria involved a diagnosis of bipolar I or bipolar II disorder and a current major depressive episode of at least moderate symptom severity, defined as a score of ≥ 16 on the Montgomery–Åsberg Depression Rating Scale (29). Ratings were conducted by the principal investigator (MAF). Secondary severity measures included the Inventory of Depressive Symptoms (30). Further inclusion and exclusion criteria are reviewed elsewhere (31, 32). Baseline MRS scans were collected in 15 patients with bipolar depression and nine healthy controls. A second scan was obtained in 10 patients after 12 weeks of open-label treatment with lamotrigine. Five patients dropped out of the study before the second scan because of: hospitalization for depression (n = 1), rash [full resolution with drug discontinuation (n = 2)], administrative discharge (n = 1), and declined second scan (n = 1) (31, 32).
Magnetic resonance imaging/MRS acquisition and processing
Scans were performed with a 1.5-T MRS scanner (GE Medical Systems, Inc.) with echo-speed gradients using a head transmit and receive coil. Anatomical landmarks based on a human brain reference atlas were used in a systematic manner for voxel positioning. Acquisitions included a sagittal whole-brain image for orientation and positioning, a T1-weighted axial image [repetition time (TR)/echo time (TE), 800 sec/8 msec; 35 slices with 4-mm thickness] for voxel placement, and a water-suppressed, single-voxel scan (TR/TE, 3 sec/30 msec; 256 averages with 3 × 3 × 3-cm3 voxel size) in the anterior cingulate cortex. Thereafter, for 2D MRS, an L-COSY sequence of 3 slice-selective radio frequency pulses was employed. This technique resembles one-dimensional point-resolved spectroscopy sequence, but a 90° pulse replaces the final 180° pulse for volume localization and coherence transfer. Two-dimensional spectral coding was inserted between the second and third pulses. Spectral recording involved the following parameters: TE at 30 msec; TR at 2,000 msec; total number of scans at 800 and including 8 averages for each T1 increment of 1.6 msec; and 3 × 3 × 3-cm3 voxel size. Total duration was approximately 26 minutes.
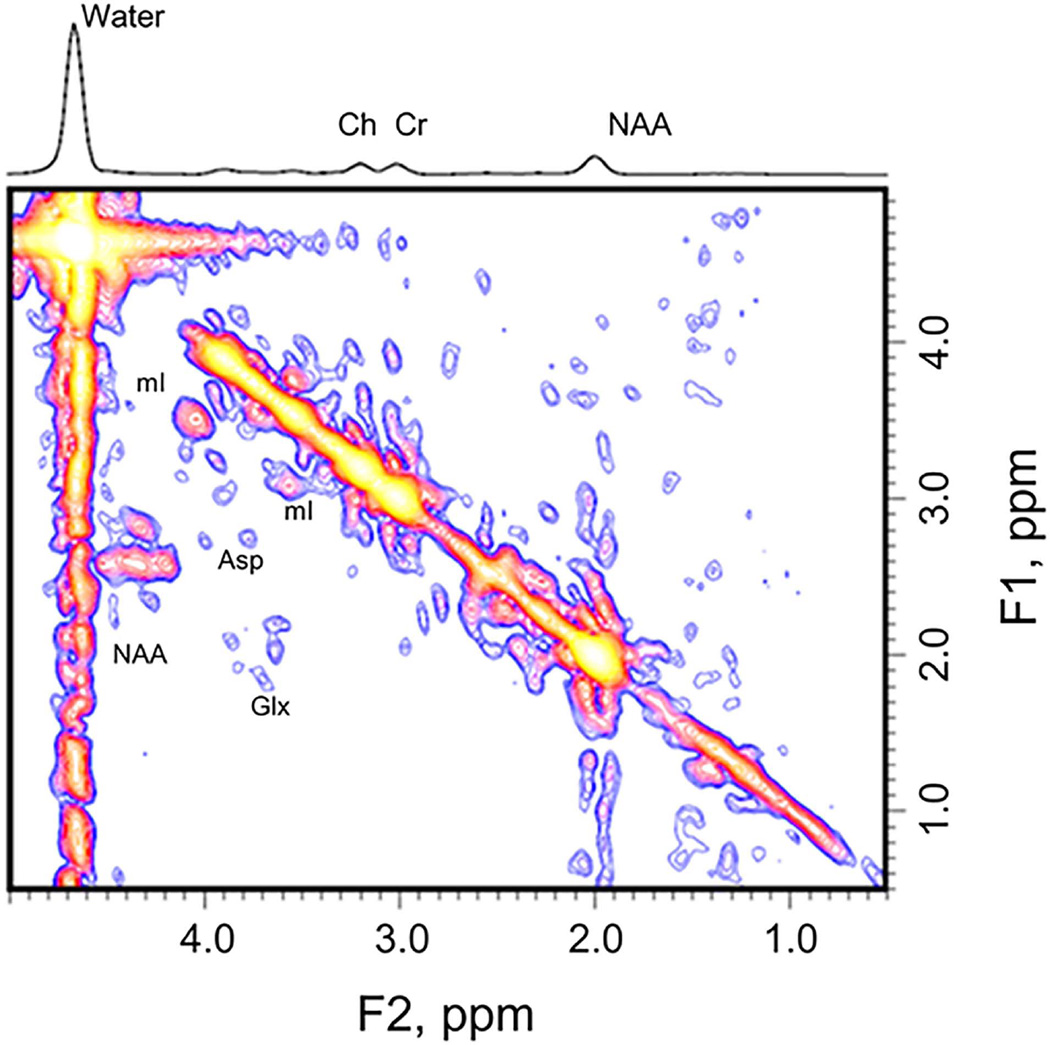
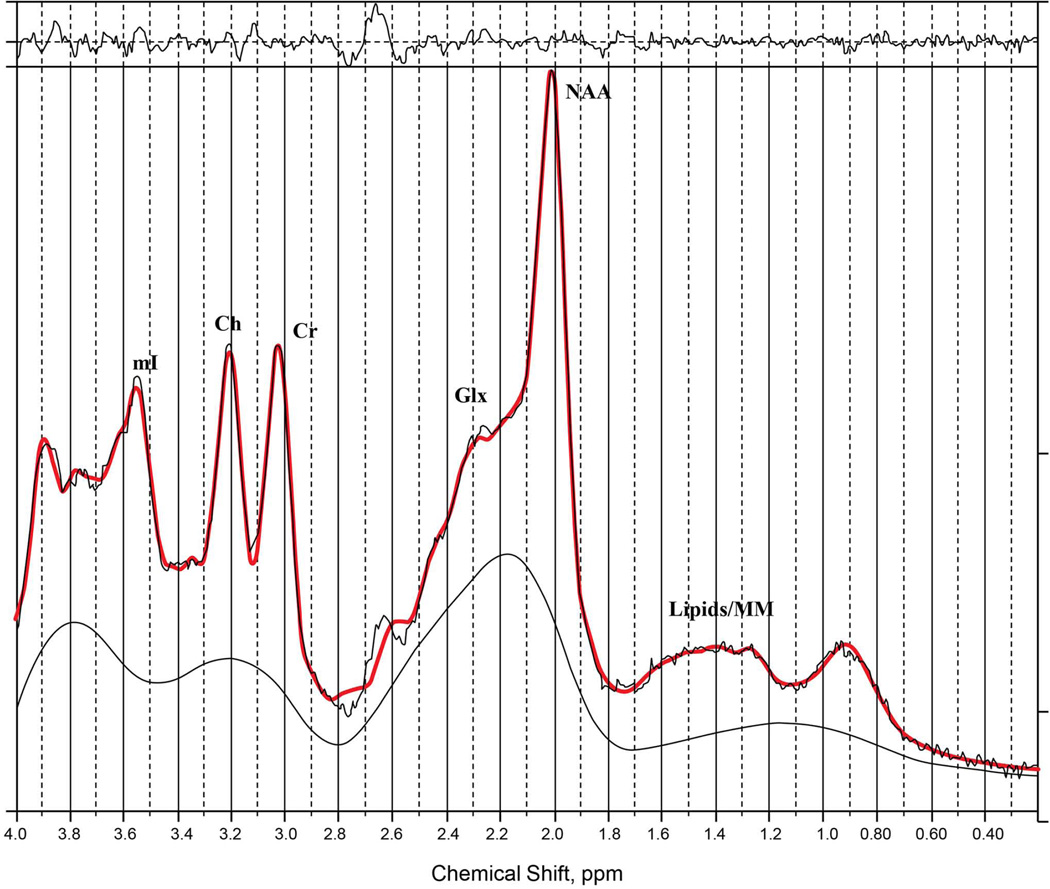
Prior knowledge of the following metabolites was included in the ProFit algorithm (33) for quantification: creatine, NAA, glycerylphosphorylcholine, phosphorylcholine, free choline (Ch), alanine, Asp, γ-aminobutyric acid (GABA), glucose, glutamine (Gln), Glu, glycine, glutathione, lactate, myoinositol (mI), NAAG, phosphoethanolamine, taurine, scyllo-inositol, and ascorbate. The 2D L-COSY spectra from the 1.5-T GE data were then processed with a modified UCLA version of ProFit code (26) because ProFit was originally developed for processing Philips data (Fig. 1). The one-dimensional spectra from the same participant are provided as a reference (Fig. 2). Measurement accuracy was characterized with use of Cramér-Rao lower bound. Further details regarding the methodology for quantification of the L-COSY spectrum can be found in our prior publications (26, 33).
Fig. 1.
A two-dimensional localized correlated spectroscopy spectrum of a patient with bipolar depression. Asp = aspartate; Ch = free choline; Cr = creatine; Glx = the combined signal from glutamate and glutamine; mI = myoinositol; NAA = N-acetylaspartate.
Fig. 2.
A one-dimensional localized correlated spectroscopy spectrum from the same patient with bipolar depression. Image supplied for reference. Ch = free choline; Cr = creatine; Glx = the combined signal from glutamate and glutamine; mI = myoinositol; NAA = N-acetylaspartate.
Demographic characteristics were examined with a χ2 test. Spectroscopic differences between healthy controls, patients with bipolar depression at baseline, and patients with bipolar depression after 12 weeks of lamotrigine treatment were examined with analysis of variance models and the posthoc least significant difference pairwise multiple comparison test. Alpha was set at 0.05 for all statistical tests. Analyses were computed with SPSS Statistics 21.0 (IBM). Remission at posttreatment scan was defined with a Montgomery–Åsberg Depression Rating Scale score of < 8. Spectroscopic metabolite findings among patients with remission and patients without remission were then examined with analysis of variance.
Results
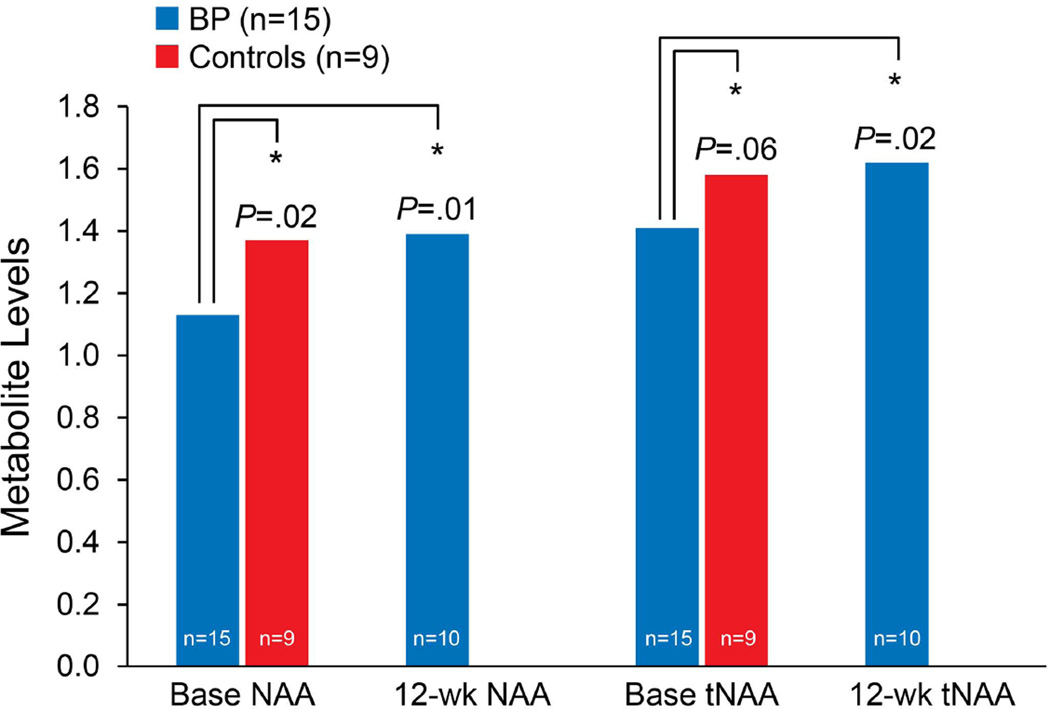
Patients (n = 15) and controls (n = 9) had no significant differences in sex (χ2 = 0.49, p = 0.48) or age (χ2 = 17.12, p = 0.31). Patients with bipolar depression had a mean standard deviation (SD) Montgomery–Åsberg Depression Rating Scale score of 26.27 (5.64) and an Inventory of Depressive Symptomatology mean (SD) score of 31.00 (7.38). Among the three groups, there were significant differences in mean NAA [F(2,31) = 5.15, p = 0.01] and mean tNAA [F(2,31) = 3.49, p = 0.04] levels (Table 1 and Fig. 3). Patients with bipolar depression had significantly lower levels of NAA (p = 0.02) and tNAA (p = 0.06) than healthy controls. After 12 weeks of lamotrigine treatment, patients with bipolar depression had significant increases in NAA (p = 0.01) and tNAA (p = 0.02) compared with patients at baseline after 12 weeks of lamotrigine treatment. No significant differences in glutamate and glutamine (Glx) and Glu levels were found among the healthy controls and participants with bipolar depression at baseline. At posttreatment, Glx and Glu levels were elevated in participants with bipolar depression (Table 1).
Table 1.
Baseline and post-treatment neurometabolites
| Neurometabolitea | Controls (n = 9) |
BP Dep Baseline (n = 15) |
Controls versus BP Dep Baseline LSD p-value (Cohen’s d)b |
BP Dep Post-treatment (n = 10) |
Controls versus BP Dep Post-treatment LSD p-value (Cohen’s d)b |
BP Dep Baseline versus BP Dep Post-treatment LSD p-value (Cohen’s d)b |
|---|---|---|---|---|---|---|
| NAA | 1.37 (0.27) | 1.13 (0.21) | 0.02 (0.99) | 1.39 (0.21) | 0.84 | 0.01 (1.24) |
| tNAA | 1.58 (0.26) | 1.41 (0.14) | 0.06 | 1.61 (0.25) | 0.70 | 0.02 (0.99) |
| Glx | 1.13 (0.20) | 1.27 (0.12) | 0.59 | 1.83 (1.18) | 0.03 (−0.83) | 0.04 (−0.67) |
| Glu | 1.04 (0.16) | 1.13 (0.11) | 0.69 | 1.62 (1.01) | 0.03 (−0.80) | 0.04 (−0.68) |
| tNAA/Glx | 1.44 (0.33) | 1.11 (0.10) | 0.01 (1.35) | 1.10 (0.42) | 0.02 (−0.90) | 0.98 |
| tNAA/Glu | 1.55 (0.34) | 1.26 (0.16) | 0.04 (1.09) | 1.24 (0.48) | 0.05 | 0.92 |
| NAA/Glu | 1.37 (0.44) | 1.00 (0.16) | 0.03 (1.12) | 1.10 (0.52) | 0.14 | 0.50 |
BP Dep = bipolar depression; Glu = glutamate; Glx = glutamine and glutamate; LSD = least significant difference; NAA = N-acetylaspartate; tNAA = total NAA.
Values are presented as mean (standard deviation) unless specified otherwise.
Cohen’s d provided for statistically significant findings.
Fig. 3.
N-acetylaspartate (NAA) and total N-NAA (tNAA) levels at baseline and after 12 weeks of lamotrigine treatment. Base = baseline; BP = bipolar depression.
There were significant differences in the mean tNAA to Glx ratio among these groups [F(2,31) = 4.25, p = 0.02]. Patients with bipolar depression at baseline had a lower tNAA/Glx than healthy controls [mean (SD) = 1.11 (0.10) versus 1.44 (0.33); p = 0.01]. There were no significant differences in NAA to Glu and tNAA to Glu ratios among the three groups. Spearman rho correlation coefficients between changes in tNAA and Glx, in NAA and Glu, and in tNAA and Glu did not show any statistically significant correlation. Of the 10 patients with bipolar depression who had a second MRS scan, five achieved remission with lamotrigine treatment. No statistically significant differences were found among patients whose bipolar depression remitted and those who did not have remission in baseline or post-treatment neurometabolites.
Discussion
The present study is the first, to our knowledge, to show an NAA deficit in bipolar depression that normalized after treatment with lamotrigine. In this relatively small sample, there was no relation between clinical remission with lamotrigine and NAA levels because NAA increased regardless. Hence, this brain change is unrelated to mood changes. Although this is an exploratory study, its strengths include a hypothesis-driven analysis of drug mechanism of action (i.e., increased intraneuronal Asp and acetyl coenzyme A levels). The baseline deficit in NAA corrected with lamotrigine treatment is consistent with preclinical and animal models of lamotrigine-associated neuroprotection (9, 12, 34) and NAA deficit normalization with lithium (20, 35, 36). For example, the NAA deficit, as a state marker, contributes to excessive glutamatergic tone (37, 38) and thereby leads to cell death and resultant decreases in NAA. The normalization of an NAA deficit could be mediated through activating Asp N-acetyltransferase, which promotes NAA synthesis. Also, lamotrigine may inhibit aspartoacylase, a catalytic enzyme in oligodendrocytes, or NAAG synthase in astrocytes. This increase in NAA levels may be associated with a reduction in Glu levels (39, 40).
In prior work examining NAA as a biomarker, decrements were generally thought to be related to neuronal loss. This may be an overly simplistic approach because other pathophysiologic changes could deplete NAA in a manner that is responsive to pharmacologic treatments (41). For example, postmortem brain studies consistently show reduced glial cell density in bipolar disorder (42–44). Rodent models of depression also show that compromised glial cell function induces depressive-like behaviors (45). Although NAA is synthesized in neurons and sensitive to mitochondrial integrity where it is synthesized, it is taken up and metabolized in glial cells (14). Therefore, reduced NAA levels in bipolar depression could relate in part to dysregulations in glial cell functioning or integrity, and this could be amenable to pharmacologic treatments. However, making definitive conclusions is difficult regarding the temporal relation of NAA reductions with compromised glial cell function based on a cross-sectional study.
Previously, investigators have recognized that dynamic change in NAA may occur in relation to other therapeutic drug interventions (46, 47). In a landmark study by Moore et al. (20), 12 adult patients with bipolar disorder showed an increase in NAA after a four-week course of lithium. Other studies (35, 36), but not all studies (48), have reported a lithium-associated increase in NAA. In at least one study, the NAA increase directly correlated with brain lithium levels (49). The lithium-associated increase in NAA appears to be primarily in studies where an initial deficit was reported (40, 50). However, this NAA normalization or deficit amelioration has not been observed with divalproex (49, 51). Early work on the Glu reuptake inhibitor riluzole in bipolar depression reported a drug-associated increase in NAA, which also had a positive association with symptomatic improvement (47).
The present study’s smaller sample size and different MRS methodology did not replicate previous findings of Glx and Glu increases (32, 37, 52). However, at baseline, patients with bipolar depression had lower tNAA to Glx ratios than healthy controls. Prior work shows correlations between NAA and Glu changes (19), indicates that the metabolism of these neurochemicals are coupled (16–18), and contends that comparing their ratios may have merit in quantifying disease burden in psychiatric disorders (13, 19). This result suggests that patients with bipolar depression have a lower NAA level or a higher Glx/Glu level, or both. The present data suggest that further study of NAA and glutamatergic ratios in bipolar depression is warranted because it may provide a sensitive measure to monitor pharmacologic treatments.
Limitations of the present study include its small sample size. This study was able to detect only a brain effect and likely was underpowered to detect any relation with NAA change and clinical effects of lamotrigine therapy. This characteristic does raise questions about the clinical significance of the present findings. Larger studies are needed to examine potential neurochemical and clinical correlations. Further, the size of the voxel extends the region of interest beyond one anatomic region and included bilateral pregenual anterior cingulate, anterior midcingulate cortex, and medial prefrontal cortex (superior frontal gyrus). In addition, cerebrospinal fluid corrections were performed, but tissue segmentation of spectroscopic data was not performed. Fortunately, the midline anterior cingulate placement of the MRS voxel does ensure a low contribution of white matter (≤ 10%). Finally, prior knowledge-based peak fitting programs, such as ProFit and LCModel (LCMODEL, Inc.), do not fit NAAG, Gln, and other weaker metabolites with acceptable Cramér-Rao lower bounds. Hence, a common practice is to report both NAA and tNAA. Even though spectral dispersion is better and metabolite peaks can be resolved less ambiguously with 2D L-COSY than with one-dimensional point-resolved spectroscopy (PRESS) at 1.5 T, peak separation between NAA and NAAG is not sufficient to resolve NAAG. A 2D MRS resolves sensitive metabolites such as NAA, Glu, Ch, and mI more effectively than weaker metabolites (such as NAAG and Gln). More metabolites can be better resolved at 3 T and, more recently with our group’s work, at 7 T (53).
Limitations of the current findings need to be considered in the context of understanding the molecules that contribute to resonance peaks of an MRS spectrum at a given field strength, typical concentrations, correlation times in the brain, and metabolic inter-conversions. For example, the NAA resonance peak may have contributions from N-acetyl sugars, which are at a high level in the gangliosides found in synaptic membranes, and uridine diphosphate-N-acetyl sugars, in the synthetic pathways of gangliosides. Even with L-COSY, Glu, Gln, and GABA cannot be quantified reliably at 1.5 T. The majority of the Glu resonance is from the metabolic pool rather than the neurotransmitter pool. Within the neurotransmitter pool, it is also not possible to differentiate neuronal, synaptic, and glial Glu metabolites. Findings with NAA/Glx ratios must be viewed with caution. These were included as exploratory measures, with the prospect of selecting a more sensitive measure in the future (54).
In conclusion, after 12 weeks of lamotrigine treatment, NAA and tNAA levels normalized in patients with bipolar depression. This lamotrigine-associated brain change (i.e., increase in neuronal viability) may be related to increasing intraneuronal storage of Asp and, subsequently, NAA. Future research is encouraged to evaluate whether baseline NAA could be a potential biomarker for identifying lamotrigine response patterns and whether this functional brain change has an associated clinical response.
Acknowledgements
This study was supported by a grant from GlaxoSmithKline.
PEC has received grant support from Pfizer, the National Institute of Mental Health (K23 MH100266), the Brain and Behavior Research Foundation, and the Mayo Foundation. MAF has received grant support from Assurex Health, Inc., Myriad Genetics, Inc., Pfizer, the National Institute of Mental Health (RO1 MH079261), the National Institute of Alcohol Abuse and Alcoholism (P20AA017830), and the Mayo Foundation; has been a consultant to Janssen Global Services, LLC, Mitsubishi Tanabe Pharma Corp, Myriad, Sunovion Pharmaceuticals, Inc., and Teva Pharmaceuticals; and has received continuing medical education, travel support, or presentation support from CME Outfitters, LLC, and Sunovian Pharmaceuticals, Inc.
Footnotes
Presented at the 68th Annual Scientific Meeting of the Society of Biological Psychiatry, San Francisco, CA, USA, May 16–18, 2013, and at the 10th International Conference on Bipolar Disorders, Miami Beach, FL, USA, June 13–16, 2013.
Disclosures
(Mayo Clinic has a financial interest in Assurex Health Inc). MAT, JDP, JMB, D-SC, and OAA do not have any conflicts of interest to report.
References
- 1.Goodwin GM, Bowden CL, Calabrese JR, et al. A pooled analysis of 2 placebo-controlled 18-month trials of lamotrigine and lithium maintenance in bipolar I disorder. J Clin Psychiatry. 2004;65:432–441. doi: 10.4088/jcp.v65n0321. [DOI] [PubMed] [Google Scholar]
- 2.Vieta E. Role of antidepressants in bipolar depression. J Clin Psychiatry. 2010;71:e21. doi: 10.4088/JCP.8125tx3c. [DOI] [PubMed] [Google Scholar]
- 3.Frye MA. Clinical practice: bipolar disorder: a focus on depression. N Engl J Med. 2011;364:51–59. doi: 10.1056/NEJMcp1000402. [DOI] [PubMed] [Google Scholar]
- 4.Frye MA, Ketter TA, Kimbrell TA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20:607–614. doi: 10.1097/00004714-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry. 2009;194:4–9. doi: 10.1192/bjp.bp.107.048504. [DOI] [PubMed] [Google Scholar]
- 6.van der Loos ML, Mulder PG, Hartong EG, et al. LamLit Study Group. Efficacy and safety of lamotrigine as add-on treatment to lithium in bipolar depression: a multicenter, double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70:223–231. doi: 10.4088/jcp.08m04152. [DOI] [PubMed] [Google Scholar]
- 7.Lees G, Leach MJ. Studies on the mechanism of action of the novel anticonvulsant lamotrigine (Lamictal) using primary neurological cultures from rat cortex. Brain Res. 1993;612:190–199. doi: 10.1016/0006-8993(93)91660-k. [DOI] [PubMed] [Google Scholar]
- 8.Lee CY, Fu WM, Chen CC, Su MJ, Liou HH. Lamotrigine inhibits postsynaptic AMPA receptor and glutamate release in the dentate gyrus. Epilepsia. 2008;49:888–897. doi: 10.1111/j.1528-1167.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 9.Leng Y, Fessler EB, Chuang DM. Neuroprotective effects of the mood stabilizer lamotrigine against glutamate excitotoxicity: roles of chromatin remodelling and Bcl-2 induction. Int J Neuropsychopharmacol. 2013;16:607–620. doi: 10.1017/S1461145712000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delvendahl I, Lindemann H, Heidegger T, Normann C, Ziemann U, Mall V. Effects of lamotrigine on human motor cortex plasticity. Clin Neurophysiol. 2013;124:148–153. doi: 10.1016/j.clinph.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Pisani F, Pedale S, Macaione V, et al. Neuroprotective effects of lamotrigine and remacemide on excitotoxicity induced by glutamate agonists in isolated chick retina. Exp Neurol. 2001;170:162–170. doi: 10.1006/exnr.2001.7681. [DOI] [PubMed] [Google Scholar]
- 12.Deng P, Xu ZC. Contribution of Ih to neuronal damage in the hippocampus after traumatic brain injury in rats. J Neurotrauma. 2011;28:1173–1183. doi: 10.1089/neu.2010.1683. [DOI] [PubMed] [Google Scholar]
- 13.Kraguljac NV, Reid MA, White DM, den Hollander J, Lahti AC. Regional decoupling of N-acetyl-aspartate and glutamate in schizophrenia. Neuropsychopharmacology. 2012;37:2635–2642. doi: 10.1038/npp.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madhavarao CN, Arun P, Moffett JR, et al. Defective N-acetylaspartate catabolism reduces brain acetate levels and myelin lipid synthesis in Canavan’s disease. Proc Natl Acad Sci U S A. 2005;102:5221–5226. doi: 10.1073/pnas.0409184102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baslow MH. Functions of N-acetyl-L-aspartate and N-acetyl-L-aspartylglutamate in the vertebrate brain: role in glial cell-specific signaling. J Neurochem. 2000;75:453–459. doi: 10.1046/j.1471-4159.2000.0750453.x. [DOI] [PubMed] [Google Scholar]
- 16.Clark JF, Doepke A, Filosa JA, et al. N-acetylaspartate as a reservoir for glutamate. Med Hypotheses. 2006;67:506–512. doi: 10.1016/j.mehy.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 17.Ariyannur PS, Madhavarao CN, Namboodiri AM. N-acetylaspartate synthesis in the brain: mitochondria vs. microsomes. Brain Res. 2008;1227:34–41. doi: 10.1016/j.brainres.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 18.Magistretti PJ, Pellerin L. Celluar mechanisms of brain energy metabolism: relevance to functional brain imaging and to neurodegenerative disorders. Ann N Y Acad Sci. 1996;777:380–387. doi: 10.1111/j.1749-6632.1996.tb34449.x. [DOI] [PubMed] [Google Scholar]
- 19.Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 2013;70:1294–1302. doi: 10.1001/jamapsychiatry.2013.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore GJ, Bebchuk JM, Hasanat K, et al. Lithium increases N-acetyl-aspartate in the human brain: in vivo evidence in support of bcl-2’s neurotrophic effects? Biol Psychiatry. 2000;48:1–8. doi: 10.1016/s0006-3223(00)00252-3. [DOI] [PubMed] [Google Scholar]
- 21.Kubas B, Walecki J, Kulak W, Tarsow E, Drozdowski W, Pniewski J. Metabolite profile in pyramidal tracts after ischemic brain stroke assessed by 1H MRS: a multicenter study. Neuroradiol J. 2007;19:699–704. doi: 10.1177/197140090601900602. [DOI] [PubMed] [Google Scholar]
- 22.Maddock RJ, Buonocore MH. MR spectroscopic studies of the brain in psychiatric disorders. Curr Top Behav Neurosci. 2012;11:199–251. doi: 10.1007/7854_2011_197. [DOI] [PubMed] [Google Scholar]
- 23.Mason GF, Krystal JH. MR spectroscopy: its potential role for drug development for the treatment of psychiatric diseases. NMR Biomed. 2006;19:690–701. doi: 10.1002/nbm.1080. [DOI] [PubMed] [Google Scholar]
- 24.Thomas MA, Yue K, Binesh N, et al. Localized two-dimensional shift correlated MR spectroscopy of human brain. Magn Reson Med. 2001;46:58–67. doi: 10.1002/mrm.1160. [DOI] [PubMed] [Google Scholar]
- 25.Prescot AP, Renshaw PF. Two-dimensional J-resolved proton MR spectroscopy and prior knowledge fitting (ProFit) in the frontal and parietal lobes of healthy volunteers: assessment of metabolite discrimination and general reproducibility. J Magn Reson Imaging. 2013;37:642–651. doi: 10.1002/jmri.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagarajan R, Sarma MK, Thames AD, Castellon SA, Hinkin CH, Thomas MA. 2D MR spectroscopy combined with prior-knowledge fitting is sensitive to HCV-associated cerebral metabolic abnormalities. Int J Hepatol. 2012 doi: 10.1155/2012/179365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Dydak U, Harezlak J, et al. Neurochemical abnormalities in unmedicated bipolar depression and mania: a 2D 1H MRS investigation. Psychiatry Res. 2013;213:235–241. doi: 10.1016/j.pscychresns.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research version, Non-patient edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 29.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 30.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 31.Frye MA, Thomas MA, Yue K, et al. Reduced concentrations of N-acetylaspartate (NAA) and the NAA-creatine ratio in the basal ganglia in bipolar disorder: a study using 3-Tesla proton magnetic resonance spectroscopy. Psychiatry Res. 2007;154:259–265. doi: 10.1016/j.pscychresns.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Frye MA, Watzl J, Banakar S, et al. Increased anterior cingulate/medial prefrontal cortical glutamate and creatine in bipolar depression. Neuropsychopharmacology. 2007;32:2490–2499. doi: 10.1038/sj.npp.1301387. [DOI] [PubMed] [Google Scholar]
- 33.Schulte RF, Boesiger P. ProFit: two-dimensional prior-knowledge fitting of J-resolved spectra. NMR Biomed. 2006;19:255–263. doi: 10.1002/nbm.1026. [DOI] [PubMed] [Google Scholar]
- 34.Yi YH, Guo WC, Sun WW, et al. Neuroprotection of lamotrigine on hypoxic-ischemic brain damage in neonatal rats: relations to administration time and doses. Biologics. 2008;2:339–344. doi: 10.2147/btt.s2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hajek T, Bauer M, Pfennig A, et al. Large positive effect of lithium on prefrontal cortex N-acetylaspartate in patients with bipolar disorder: 2-centre study. J Psychiatry Neurosci. 2012;37:185–192. doi: 10.1503/jpn.110097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forester BP, Finn CT, Berlow YA, Wardrop M, Renshaw PF, Moore CM. Brain lithium N-acetyl aspartate and myo-inositol levels in older adults with bipolar disorder treated with lithium: a lithium-7 and proton magnetic resonance spectroscopy study. Bipolar Disord. 2008;10:691–700. doi: 10.1111/j.1399-5618.2008.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuksel C, Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madhavarao CN, Chinopoulos C, Chandrasekaran K, Namboodiri MA. Characterization of the N-acetylaspartate biosynthetic enzyme from rat brain. J Neurochem. 2003;86:824–835. doi: 10.1046/j.1471-4159.2003.01905.x. [DOI] [PubMed] [Google Scholar]
- 40.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demougeot C, Bertrand N, Prigent-Tessier A, et al. Reversible loss of N-acetyl-aspartate in rats subjected to long-term focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:482–489. doi: 10.1097/01.WCB.0000050066.57184.60. [DOI] [PubMed] [Google Scholar]
- 42.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- 44.Savitz JB, Price JL, Drevets WC. Neuropathological and neuromorphometric abnormalities in bipolar disorder: view from the medial prefrontal cortical network. Neurosci Biobehav Rev. 2014;42:132–147. doi: 10.1016/j.neubiorev.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Cao X, Li LP, Wang Q, et al. Astrocyte-derived ATP modulates depressive-like behaviors. Nat Med. 2013;19:773–777. doi: 10.1038/nm.3162. [DOI] [PubMed] [Google Scholar]
- 46.DelBello MP, Cecil KM, Adler CM, Daniels JP, Strakowski SM. Neurochemical effects of olanzapine in first-hospitalization manic adolescents: a proton magnetic resonance spectroscopy study. Neuropsychopharmacology. 2006;31:1264–1273. doi: 10.1038/sj.npp.1300950. [DOI] [PubMed] [Google Scholar]
- 47.Brennan BP, Hudson JI, Jensen JE, et al. Rapid enhancement of glutamatergic neurotransmission in bipolar depression following treatment with riluzole. Neuropsychopharmacology. 2010;35:834–846. doi: 10.1038/npp.2009.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brambilla P, Stanley JA, Sassi RB, et al. 1H MRS study of dorsolateral prefrontal cortex in healthy individuals before and after lithium administration. Neuropsychopharmacology. 2004;29:1918–1924. doi: 10.1038/sj.npp.1300520. [DOI] [PubMed] [Google Scholar]
- 49.Silverstone PH, Wu RH, O’Donnell T, Ulrich M, Asghar SJ, Hanstock CC. Chronic treatment with lithium, but not sodium valproate, increases cortical N-acetyl-aspartate concentrations in euthymic bipolar patients. Int Clin Psychopharmacol. 2003;18:73–79. doi: 10.1097/00004850-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Brambilla P, Stanley JA, Nicoletti MA, et al. 1H magnetic resonance spectroscopy investigation of the dorsolateral prefrontal cortex in bipolar disorder patients. J Affect Disord. 2005;86:61–67. doi: 10.1016/j.jad.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Strawn JR, Patel NC, Chu WJ, et al. Glutamatergic effects of divalproex in adolescents with mania: a proton magnetic resonance spectroscopy study. J Am Acad Child Adolesc Psychiatry. 2012;51:642–651. doi: 10.1016/j.jaac.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gigante AD, Bond DJ, Lafer B, Lam RW, Young LT, Yatham LN. Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: a meta-analysis. Bipolar Disord. 2012;14:478–487. doi: 10.1111/j.1399-5618.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 53.Verma G, Hariharan H, Nagarajan R, et al. Implementation of two-dimensional L-COSY at 7 tesla: an investigation of reproducibility in human brain. J Magn Reson Imaging. 2014;40:1319–1327. doi: 10.1002/jmri.24510. [DOI] [PubMed] [Google Scholar]
- 54.Pettegrew JW, McClure RJ, Panchalingam K. Spectroscopic imaging of schizophrenia. In: Shenton ME, Turetsky BI, editors. Understanding Neuropsychiatric Disorders: Insights from Neuroimaging. Cambridge: Cambridge University Press; 2011. p. 56. [Google Scholar]