Graphical abstract

Keywords: Dermanyssus gallinae, Vaccine, Serpin, Vitellogenin, Hemelipoglycoprotein
Highlights
-
•
Ten poultry red mite vaccine candidate antigens were identified and recombinant versions produced.
-
•
Mite mortality was monitored after feeding on the blood from vaccinated hens.
-
•
A ⩾1.6-fold increased risk of mite death was observed with four of the vaccine candidates (P < 0.001).
-
•
Best candidates include: a serpin, vitellogenin, hemelipoglycoprotein and a novel protein.
Abstract
An aqueous extract of the haematophagous poultry ectoparasite, Dermanyssus gallinae, was subfractionated using anion exchange chromatography. Six of these subfractions were used to immunise hens and the blood from these hens was fed, in vitro, to poultry red mites. Mite mortality following these feeds was indicative of protective antigens in two of the subfractions, with the risks of mites dying being 3.1 and 3.7 times higher than in the control group (P < 0.001). A combination of two-dimensional immunoblotting and immunoaffinity chromatography, using IgY from hens immunised with these subfractions, was used in concert with proteomic analyses to identify the strongest immunogenic proteins in each of these subfractions. Ten of the immunoreactive proteins were selected for assessment as vaccine candidates using the following criteria: intensity of immune recognition; likelihood of exposure of the antigen to the antibodies in a blood meal; proposed function and known vaccine potential of orthologous molecules. Recombinant versions of each of these 10 proteins were produced in Escherichia coli and were used to immunise hens. Subsequent in vitro feeding of mites on blood from these birds indicated that immunisation with Deg-SRP-1 (serpin), Deg-VIT-1 (vitellogenin), Deg-HGP-1 (hemelipoglycoprotein) or Deg-PUF-1 (a protein of unknown function) resulted in significantly increased risk of mite death (1.7–2.8 times higher than in mites fed blood from control hens immunised with adjuvant only, P < 0.001). The potential for using these antigens in a recombinant vaccine is discussed.
1. Introduction
The poultry red mite, Dermanyssus gallinae (De Geer, 1778), is the most commercially important ectoparasite of laying hens globally. These mites reside in refuges within poultry houses, emerging at night to feed on the blood of hens (Chauve, 1998). The prevalence of the poultry red mite in commercial egg laying premises is high, with an average of 83% of European facilities affected (George et al., 2014. The poultry red mite Dermanyssus gallinae: developing novel management solutions for a complicated and neglected pest. 10th European Congress of Entomology, 3rd–8th August, University of York, UK; George et al., 2015). Poultry red mite is estimated to cost the European poultry industry €130 million per annum which is attributed to production loss, higher feed conversion and control costs (Van Emous, 2005, Sparagano et al., 2009). Severe hen welfare implications are associated with moderate infestation levels (>150,000 mites per hen) and include anaemia, an increase in irritation and restlessness, feather-pecking, cannibalism and hen mortality, although impacts on hen health can occur at typical infestation levels of 50,000 mites per bird (Kilpinen et al., 2005). Poultry red mites have also been implicated as potential reservoirs for a number of commercially important and zoonotic diseases (Valiente et al., 2009, Brannstrom et al., 2010) and can feed adventitiously on mammals, including humans (George et al., 2015).
Acaricidal treatments may give only limited or short-lived reductions in mite numbers in commercial hen houses due to both the difficulties associated with effective application and the emergence of acaricide resistance (Marangi et al., 2009, Sparagano et al., 2014). In spite of these issues, chemical control of poultry red mites through the use of synthetic acaricides remains the dominant method in commercial premises. A recent surge in research activity for novel methods of controlling poultry red mites has encompassed novel biopesticides and plant-derived products, semiochemicals and growth regulators, vaccines, biological control, physical barriers, architectural, engineering and process-management solutions (recently comprehensively reviewed by Sparagano et al., 2014).
Vaccination against blood-feeding ectoparasites can result in effective and sustainable control (De la Fuente and Kocan, 2003, Willadsen, 2004) and offers advantages including prolonged efficacy, freedom from chemical residues/environmental pollution and reduced risk of resistance. In previous studies, we and others (Harrington et al., 2009), showed that vaccination of hens is a feasible strategy to control poultry red mite (Bartley et al., 2009, Bartley et al., 2012, Harrington et al., 2009, Wright et al., 2009) and the identification of suitable antigens for vaccine production is now a principal goal of research in this area.
To date, pragmatic approaches of fractionating native protein extracts of the mites (Wright et al., 2009) or rational approaches for selecting suitable recombinant D. gallinae antigens based on function and/or orthology (Bartley et al., 2009, Bartley et al., 2012) have been used to identify effective autogenous vaccine preparations and recombinant vaccine candidate antigens, respectively. We previously showed that fractionation of D. gallinae extracts into soluble, membrane-associated, membrane-bound and insoluble material enriched protective antigens in the soluble fraction (Wright et al., 2009). This soluble mite extract (SME) is a complex mixture of macromolecules so, in the work described herein, we have taken a combined pragmatic and rational approach in order to identify the immunoreactive components of SME, prioritise their analysis as suitable vaccine antigens in an order of merit, express them as recombinant proteins and test their vaccine efficacy in vitro.
2. Materials and methods
2.1. Collection and conditioning of D. gallinae
Mixed stage and gender D. gallinae mites were collected into 75 cm2 vented tissue culture flasks (Corning, USA) from a commercial egg production unit in Scotland, UK. Mites that were snap frozen in liquid nitrogen within 24 h of collection constituted the “fed” mite population. “Starved” mites were conditioned by initial incubation at room temperature (RT) for 7 days, followed by storage at 4 °C for 14 days (modified from McDevitt et al., 2006). Starved mites were either used in in vitro mite feeding assays or were snap frozen in liquid nitrogen. All snap-frozen mites were stored at −80 °C until required.
2.2. Preparation of SME
Aqueous-soluble proteins were extracted from snap-frozen mites as follows: 1 g of frozen mites was suspended in 10 ml of ice-cold PBS and homogenised, on ice, for two 30 s pulses (Ultra Turrex® T 25 D-S2 with a S25N-8G dispersing element (IKA®-Werke GmbH & Co. KG, Germany). Insoluble material and debris were removed by centrifugation at 25,000g for 20 min at 4 °C. The soluble material was decanted and centrifuged for a second time. The resulting SME was immediately snap-frozen in liquid nitrogen and stored at −80 °C. The concentration of proteins in each SME was measured using a Pierce™ bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, USA), following the manufacturer’s protocol.
2.3. Ion-exchange chromatography (IEX) of SME and pooling of IEX-fractions for use as immunogens
SME from fed and starved mites was desalted using PD-10 columns (GE Healthcare, UK) and eluted in 20 mM Tris–HCl, pH 7.4. IEX was performed at RT using HiTRAP Q HP anion columns (GE healthcare) in conjunction with an ÄKTA fast liquid protein chromatography system (GE Healthcare) eluting with a linear gradient of 0–0.5 M NaCl in 20 mM Tris–HCl, pH 7.4, followed by an isocratic step (20 mM Tris–HCl, 1 M NaCl, pH 7.4). Eluted proteins were analysed by electrophoresis using 12% Bis–Tris Novex gels in NuPAGE® MES SDS Running Buffer (GE Healthcare). Proteins were either visualised by silver staining (SilverQuest™ Silver Staining Kit, Thermo Fisher Scientific) or transferred to a nitrocellulose membrane using an Xcell II blot module (GE Healthcare), following the manufacturer’s procedures. Western blotting was performed as described previously (Bartley et al., 2009). Briefly, blots were incubated in 5% Marvel in PBST (PBS, 0.05% Tween 20 (Sigma, USA)). After washing three times in PBST, the immobilised proteins were probed with yolk-derived IgY (yolk-IgY, purified using the Pierce™ Chicken IgY Purification Kit, Thermo Fisher Scientific) in PBS (100 μg/ml) from hens that had been immunised with SME. Blots were washed and bound IgY was detected by incubating in rabbit-anti-IgY-peroxidase conjugate (diluted at 1:20,000 in PBS, Sigma). Blots were washed five times in PBST prior to developing in SuperSignal™ West Pico Chemiluminescent Substrate (Thermo Fisher Scientific). Blot images were captured using an ImageQuant LAS4000 luminescent image analyzer (GE Healthcare). IEX fractions were thus pooled into five groups (henceforth referred as IEX group 1 to IEX group 5) based on their immunogenic profiles. Each of the five IEX groups and unfractionated SME were concentrated using Ultracel®-10K Amicon® Ultra-15 centrifugal filter units (Merck Millipore Ltd, Germany), filter sterilised through a 0.22 μM Millex-GV 13 mm polyvinyl difluoride (PVDF) unit (Merck Millipore Ltd) and their concentration determined (Pierce™ BCA protein assay) prior to immunisation of hens (see Section 2.8).
2.4. Two-dimensional gel electrophoresis (2-DGE) and identification of immunoreactive spots
IEX group 1 and IEX group 4 proteins were precipitated, then solubilised in 7 M urea, 2% CHAPS, 2 M thiourea, 0.3% DTT, 0.002% bromophenol blue, 2% immobilised pH gradient (IPG) buffer (IPG buffer 7–11 for IEX group 1; 3–10 for IEX group 4), rehydrated on the appropriate Immobiline DryStrips for 16 h at RT and subjected to isoelectric focusing using an Ettan™ IPGphor™ unit (GE Healthcare). The focussed proteins were denatured in equilibration buffer (50 mM Tris–HCl pH 8.8, 6 M urea, 30% glycerol, 2% SDS, 0.002% bromophenol blue) supplemented with 10 mg/ml of DTT for 15 min at RT, followed by incubation for 15 min at RT in equilibration buffer containing 25 mg/ml of iodoacetamide, then separated in the second dimension using ExcelGel™ 2-D homogenous 12.5% (25 cm × 11 cm) gels in a Multiphor™ II Electrophoresis System (GE Healthcare). One replicate of the second dimension gels was stained with SimplyBlue™ SafeStain (Thermo Fisher Scientific) and a further two replicate gels were electroblotted onto Hybond-C nitrocellulose membranes (Thermo Fisher Scientific) and immunoscreened as described above with yolk-IgY from hens that had been immunised with the IEX group 1 or IEX group 4 proteins (positives) or yolk-IgY from the adjuvant-only control hens (group 7 controls). Co-localised stained and immunolabelled protein spots were excised from the SimplyBlue™-stained gel and the proteins identified as described in Section 2.6.
2.5. Immunoaffinity enrichment of SME proteins
Yolk-IgY, derived from hens immunised with IEX group 4 proteins, was cross-linked to a 1 ml HiTrap NHS-activated HP column following the manufacturer’s instructions (GE Healthcare). Following equilibration, 1.4 mg of IEX group 4 proteins were circulated through the column for 16 h at 4 °C. Unbound material was washed from the column with 10 ml of PBS and bound protein eluted in 4 ml of elution buffer (0.1 M Glycine, 6 M Urea, pH 2.5) prior to electrophoresis using a 12% Bis–Tris Novex gel in NuPAGE® MES SDS Running Buffer (GE Healthcare), staining with SimplyBlue™ SafeStain and protein identification as described in Section 2.6.
2.6. Protein identification using liquid chromatography–electrospray ionisation–tandem mass spectrometry (LC–ESI–MS/MS)
Protein spots were picked manually from second dimension SDS–PAGE gels (IEX group 1 and IEX group 4 proteins) using a OneTouch 2D gel spotpicker™ (The Gel Company, USA) and gel lanes resulting from the immunoaffinity enrichment of IEX group 4 proteins were sliced horizontally from top to bottom to yield a series of 24 equal gel slices. Gel slices and excised spots were then subjected to standard in-gel destaining, reduction, alkylation and trypsinolysis procedures (Shevchenko et al., 1996). Digests were subjected to liquid chromatography–electrospray ionisation–tandem mass spectrometry (LC–ESI–MS/MS) analysis employing an Dionex™ Ultimate 3000 nano-HPLC system (Thermo Fisher Scientific) comprising a WPS-3000 well-plate micro auto sampler, a FLM-3000 flow manager and column compartment, a UVD-3000 UV detector, an LPG-3600 dual-gradient micropump and an SRD-3600 solvent rack controlled by Chromeleon™ chromatography software (Thermo Fisher Scientific). A micro-pump flow rate of 246 μl/min−1 was used in combination with a cap-flow splitter cartridge, affording a 1/82 flow split and a final flow rate of 3 μl/min through a 5 cm × 200 μm internal diameter PepSwift™ monolithic reversed phase column (Thermo Fisher Scientific) maintained at 50 °C. Samples of 4 μl were applied to the column by direct injection and bound peptides eluted by the application of a 15 min linear gradient from 8% to 45% solvent B (80% acetonitrile, 0.1% formic acid) and directed through a 3 nl UV detector flow cell. Liquid chromatography (LC) was interfaced directly with a 3-D high capacity ion trap amaZon-ETD mass spectrometer (Bruker Daltonics, USA) via a low volume (50 μl/min maximum) stainless steel nebuliser (Agilent Technologies, USA; Cat. No. G1946-20260) and electrospray ionisation (ESI). Parameters for tandem MS analysis were based on those described previously (Batycka et al., 2006). Deconvoluted MS/MS data in Mascot Generic Format (.mgf) were imported into ProteinScape™ V3.1 (Bruker Daltonics) for downstream database mining of an National Center for Biotechnology Information (NCBI), USA non-redundant (nr) database (09-Jan-2015 release) and a custom D. gallinae transcriptomic database (Bartley et al., 2015, unpublished data) to identify DNA sequences encoding the respective proteins using stringent identification criteria (as described in Taylor and Goodlett, 2005, Nisbet et al., 2010) utilising the Mascot™ V2.3 (Matrix Science, UK) search algorithm.
2.7. Expression and purification of recombinant vaccine candidate proteins
The complete coding sequences (CDS) for the open reading frames (ORFs) of the 10 selected vaccine candidates (summarised in Table 1) were confirmed by direct PCR amplification of the complete ORFs from cDNA or were determined by rapid amplification of cDNA ends (RACE) using the SMART™ RACE cDNA amplification kit (Takara Bio Europe/SAS, France). Amplifications were performed according to the manufacturer’s recommended conditions (the primer sequences and amplification conditions are available upon request), the products were cloned into a pGEM® T-Easy plasmid vector (Promega, USA) and the DNA sequences obtained (Eurofins Genomics, Germany).
Table 1.
Summary of the 10 candidate vaccine antigens selected from Dermanyssus gallinae following a fractionation and immunogenicity analysis of soluble mite extract.
| Candidate antigen | Contig sequence | Predicted functiona | GenBank accession numberb |
|---|---|---|---|
| Deg-PUF-2 variant 1 | 13089 | Protein of unknown function | KR697570 |
| Deg-PUF-2 variant 2 | 13089 | Protein of unknown function | KR697571 |
| Deg-ASP-1 | 00293 | Aspartyl proteinase/cathepsin D | KR697569 |
| Deg-GPD-1 | 00877 | Phosphoglycerate dehydrogenase | KR697574 |
| Deg-SRP-1 | 02564 | Serpin | KR697565 |
| Deg-PUF-1 | 11549 | Protein of unknown function | KR697568 |
| Deg-HGP-1 N-term | 13207 | Hemelipoglycoprotein | KR697566 |
| Deg-HGP-1 C-term | 13207 | Hemelipoglycoprotein | KR697566 |
| Deg-VIT-1 N-term | 12013 | Vitellogenin | KR697567 |
| Deg-PUF-3 | 00186 | Protein of unknown function | KR697575 |
| Deg-SRP-2 | 01514 | Serpin | KR697572 |
| Deg-CPR-1 | 13094 | Peptidase C1A-like cysteine proteinase | KR697573 |
Based on homology searching.
Accession numbers of the 10 vaccine candidate DNA sequences deposited in GenBank.
With the exception of D. gallinae protein of unknown function (Deg-PUF)-2 (Deg-PUF-2) variants 1 and 2, the CDS for each vaccine candidate was subcloned into the Invitrogen™ pET SUMO expression vector (Thermo Fisher Scientific) or into Novagen® pET22b (Merck Millipore Ltd), omitting the ORF-encoded signal peptide sequence where appropriate. Due to its large size, D. gallinae hemelipoglycoprotein-1 (Deg-HGP-1) was cloned and expressed in two sections (N- and C-terminal portions, encoded by nucleotide bases 139–3179 and 3160–4728, respectively) and only the N-terminal portion of D. gallinae vitellogenin-1 (Deg-VIT-1) (encoded by nucleotide bases 43–3157) was expressed. The CDS for both variants of the sequence encoding Deg-PUF-2 were codon optimised for prokaryotic expression and synthesized (Eurofins Genomics) before subcloning into the pET22b vector. Following transformation of each of the plasmids into Escherichia coli BL21-RIPL competent cells (Agilent Technologies), recombinant versions of each vaccine candidate protein were expressed and affinity-purified using His-Trap™ HP columns (GE Healthcare). Dermanyssus gallinae phosphoglycerate dehydrogenase-1 (Deg-GPD-1), D. gallinae serpin-1 (Deg-SRP-1), Deg-PUF-1 and Deg-PUF-3 were recovered from the soluble fraction of lysed E. coli in binding buffer, whereas Deg-PUF-2 variants 1 and 2, Deg-HGP-1, Deg-VIT-1, D. gallinae aspartyl proteinase-1 (Deg-ASP-1), Deg-SRP-2 and D. gallinae Peptidase C1A-like cysteine proteinase-1 (Deg-CPR-1) were recovered from inclusion bodies after solubilisation in 8 M urea. Proteins in 8 M urea were re-folded by sequential dialysis against refolding buffer (100 mM Tris–HCl, pH 8.0; 0.4 M l-Arginine; 2 mM EDTA; 0.5 mM oxidative glutathione; 5 mM reduced glutathione) with decreasing concentrations of urea (6, 4, 2 and 1 M urea), followed by dialysis against 10 mM Tris–HCl; 0.5 M NaCl, pH 7.4 for 16 h at 4 °C.
2.8. Immunisation of hens and the evaluation of acaricidal effects
All experiments using hens were conducted under the regulations of a UK Home Office Project Licence; experimental design was ratified by the Experiments and Ethics Committee of the Moredun Research Institute (MRI), UK. Two separate hen immunisation experiments were performed. In experiment 1, 14 ISA-Warrens hens, 24 weeks old, were randomly assigned into seven groups and were immunised three times, at 2 week intervals, with one of the five IEX groups of proteins (see Section 2.3), unfractionated SME (group 6) or adjuvant only (group 7) (with treatments summarised in Table 2). Each 200 μl immunisation contained 200 μg of the appropriate antigen (groups 1–6) and 200 μg of QuilA adjuvant (Brenntag Biosector, Denmark) (all groups). Experiment 2 was performed in two tranches: in each tranche, 12 ISA-Warrens hens, aged 16 weeks old (tranche 1) and 12 ISA-Warrens hens, aged 21 weeks old (tranche 2), were randomly assigned to six groups and immunised three times, at 2 week intervals, with 50 μg of recombinant vaccine antigen (see Section 2.7), as described in Table 3, formulated with 200 μg of QuilA as adjuvant. An adjuvant-only control group was also included with each tranche. In both experiments, vaccines were administered i.m. into alternate breast muscle. Eggs were collected prior to vaccination and throughout the study as a source of yolk-IgY. Whole blood was withdrawn from the brachial wing vein prior to vaccination, at each vaccination and at three time points following the final vaccination: 3, 5 and 6 weeks post vaccination in experiment 1, and 2, 3 and 4 weeks post vaccination in experiment 2. Blood was withdrawn into heparinised tubes (final concentration 36 United States Pharmacopoeia units (USP)/ml blood). Serum was recovered from a subsample of heparinised blood for immunological evaluation and antigen-specific IgY levels throughout the experiment were monitored by ELISA. In order to assess the effect of blood ingestion from vaccinated hens on mite survival, equal volumes of whole heparinised blood were pooled from the two birds in each group and fed to starved mites using a modified version of the in vitro feeding apparatus described by Wright et al. (2009) (Fig. 1). Ten feeding chambers were set up for each group and the mites were allowed feed for a 24 h period at 39 °C, 75% relative humidity. Mites were removed from the 10 feeding chambers and individual fed mites were isolated in single wells of a 96-well microtitre plate, where they were immediately scored for mortality and again at 48, 72 and 96 h post feeding. The feeding assays were replicated with blood taken on three separate occasions following the final vaccination and involving a minimum number of 326 fed mites on each occasion.
Table 2.
Mortality of Dermanyssus gallinae following ingestion of blood from hens immunised with pooled ion-exchange chromatography fractions (IEX groups 1–5) of soluble mite extract (SME). Hens immunised with SME or adjuvant only served as controls.
| Group | Antigen | Cox’s proportional hazard model |
Generalised linear mixed model |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio a | LCL | UCL | P valuec | Mean mortalityb | LCL | UCL | P valuec | ||
| 1 | IEX-group-1 | 3.06 | 2.08 | 4.52 | <0.001 | 0.37 | 0.20 | 0.57 | 0.006 |
| 2 | IEX-group-2 | 1.21 | 0.78 | 1.89 | 0.400 | 0.16 | 0.08 | 0.31 | 0.696 |
| 3 | IEX-group-3 | 2.31 | 1.51 | 3.54 | <0.001 | 0.30 | 0.15 | 0.50 | 0.041 |
| 4 | IEX-group-4 | 3.72 | 2.51 | 5.51 | <0.001 | 0.40 | 0.22 | 0.61 | 0.003 |
| 5 | IEX-group-5 | 2.52 | 1.70 | 3.72 | <0.001 | 0.30 | 0.16 | 0.50 | 0.034 |
| 6 | Whole SME | 2.42 | 1.63 | 3.60 | <0.001 | 0.35 | 0.19 | 0.55 | 0.012 |
| 7 | Adjuvant only | – | – | – | – | 0.14 | 0.06 | 0.27 | – |
LCL, lower 95% confidence level; UCL, upper 95% confidence level.
The risks of mites dying at any time during the 96 h post feeding (hazard ratio), compared with the adjuvant only control group, were analysed using Cox’s proportional hazard model.
The mean mortality rates of mites at 96 h post feeding in comparison with the adjuvant control group were analysed using a generalised linear mixed model.
P values show the statistical significance of a group compared with the adjuvant only control (group 7).
Table 3.
Mortality of Dermanyssus gallinae following ingestion of blood from hens vaccinated with recombinant poultry red mite antigens.
| Group | Antigen | Cox’s proportional hazard model |
Generalised linear mixed model (24 h post feeding) |
Generalised linear mixed model (96 h post feeding) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratioa | LCL | UCL | P valuec | Mean mortalityb | LCL | UCL | P valuec | Mean mortalityb | LCL | UCL | P valuec | ||
| Tranche 1 | |||||||||||||
| 1 | Deg-PUF-2 | 1.10 | 0.73 | 1.67 | 0.650 | 0.11 | 0.05 | 0.23 | 0.823 | 0.14 | 0.07 | 0.26 | 0.760 |
| 2 | Deg-ASP-1 | 0.89 | 0.60 | 1.33 | 0.570 | 0.10 | 0.05 | 0.21 | 0.624 | 0.13 | 0.07 | 0.24 | 0.971 |
| 3 | Deg-GPD-1 | 1.21 | 0.79 | 1.84 | 0.380 | 0.13 | 0.06 | 0.26 | 0.884 | 0.16 | 0.08 | 0.29 | 0.514 |
| 4 | Deg-SRP-1 | 2.09 | 1.48 | 2.94 | <0.001 | 0.24 | 0.12 | 0.41 | 0.038 | 0.25 | 0.14 | 0.41 | 0.011 |
| 5 | Deg-PUF-1 | 2.79 | 2.01 | 3.88 | <0.001 | 0.29 | 0.16 | 0.48 | 0.005 | 0.31 | 0.18 | 0.48 | 0.001 |
| 6 | Adjuvant only | – | – | 0.12 | 0.06 | 0.24 | – | 0.13 | 0.07 | 0.24 | – | ||
| Tranche 2 | |||||||||||||
| 7 | Deg-HGP-1 | 1.66 | 1.35 | 2.03 | <0.001 | 0.47 | 0.33 | 0.61 | 0.052 | 0.48 | 0.35 | 0.62 | 0.049 |
| 8 | Deg-VIT-1 | 2.03 | 1.66 | 2.48 | <0.001 | 0.50 | 0.37 | 0.64 | 0.021 | 0.52 | 0.38 | 0.66 | 0.020 |
| 9 | Deg-PUF-3 | 1.01 | 0.80 | 1.28 | 0.930 | 0.30 | 0.20 | 0.44 | 0.800 | 0.33 | 0.21 | 0.47 | 0.698 |
| 10 | Deg-SRP-2 | 0.82 | 0.64 | 1.05 | 0.120 | 0.20 | 0.12 | 0.31 | 0.265 | 0.22 | 0.13 | 0.34 | 0.365 |
| 11 | Deg-CPR-1 | 1.89 | 1.53 | 2.34 | <0.001 | 0.43 | 0.30 | 0.58 | 0.117 | 0.45 | 0.32 | 0.60 | 0.094 |
| 12 | Adjuvant only | – | – | – | – | 0.28 | 0.18 | 0.41 | – | 0.29 | 0.19 | 0.42 | – |
LCL, lower 95% confidence level; UCL, upper 95% confidence level.
The risks of mites dying at any time during the 96 h post feeding (hazard ratio), compared with the adjuvant only control groups, were analysed using Cox’s proportional hazard model.
The mean mortality rates of mites at 24 and 96 h post feeding, in comparison with the adjuvant control group, were analysed using a generalised linear mixed model.
P values show the statistical significance of a group compared with the adjuvant only control groups (groups 6 and 12).
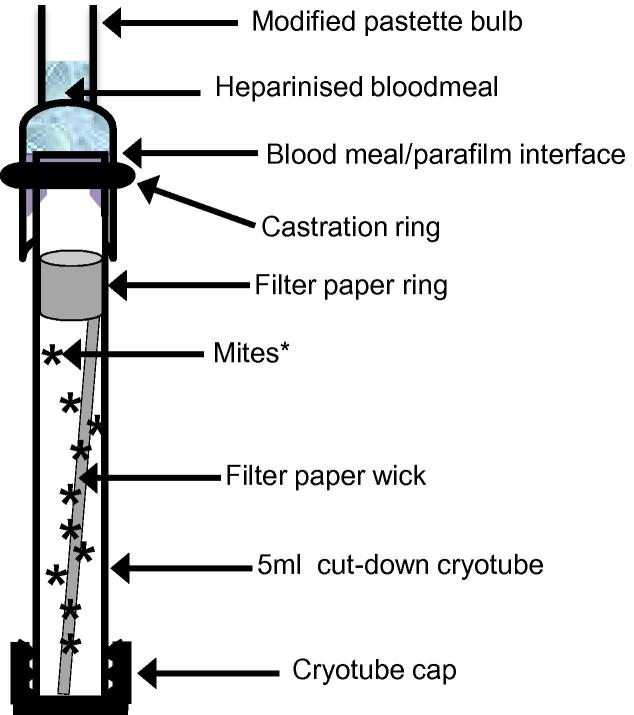
Fig. 1.
An in vitro feeding device for Dermanyssus gallinae. The device is a modification of that described by Wright et al. (2009). Specific modifications include: the replacement of a chick-skin feeding membrane with pre-stretched Parafilm“M” (Bemis® Flexible packaging, USA) and the placement of a lamb rubber castration ring (NetTex, UK) around the pastette bulb to create a tight seal to prevent blood-meal leakage. A ring of filter paper was placed around the internal circumference of the tube near the feeding membrane, together with a filter paper wick, to soak up blood in the event of feeding membrane failure. Mites are indicated as ∗.
2.9. Statistical analyses
Mite mortality data from experiment 1 and the two tranches of experiment 2 were all analysed separately using two different approaches for each of the three data sets. A survival analysis, incorporating the treatment group as a fixed effect, was conducted based on Cox’s proportional hazards model (PHM) with frailty function. Mites that survived after 96 h were treated as censored data. The frailty function considered the replicate (the three blood sampling occasions for each bird pair) within each experiment as a random effect, and the estimate of this random effect was obtained using the restricted maximum likelihood method, assuming a Gaussian distribution. The hazard ratio (the ratio of the instantaneous risk of mites dying at any time in the experiment, which is assumed to be constant at all times in Cox’s PHM) was used to compare the different antigens with the adjuvant. In the second approach, the cumulative mortality rates of mites in each treatment group at 24, 48, 72 and 96 h were analysed with a separate generalised linear mixed model (GLMM) at each time point, using a binomial distribution and logit link function, to examine the mean mortality rates compared with that of the adjuvant only groups at those time points. These models included the treatment group as a fixed effect and the feeding assay as a random effect, and observation level data were used to estimate the over-dispersion in the data. All statistical analyses were carried out using R software version 3.1.0 with appropriate R packages (stats, lme4, survival, frailtypack, ggplot2; http://www.R-project.org). P < 0.05 was deemed statistically significant.
3. Results
3.1. IEX of SME and pooling of IEX fractions for use as immunogens
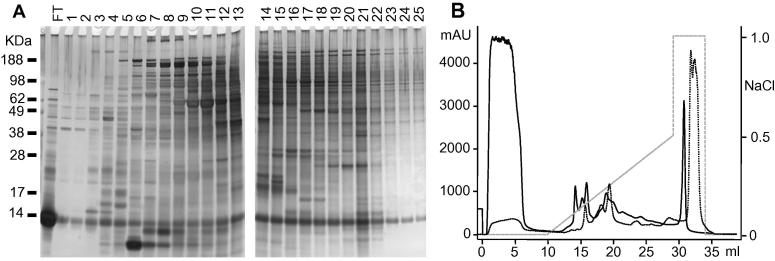
IEX was used to separate the complex mix of protein contained in the SME into a 10 ml flow-through (FT) fraction containing unbound proteins and 25 sequentially eluted 1 ml fractions (Fig. 2). The abundant 14 kDa band present in the FT fraction and, to a lesser extent, in all fed mite eluates (Fig. 2A) was identified by MALDI-ToF-MS analysis as hen haemoglobin subunits. In starved mites, the amplitude of the respective FT peak was reduced approximately 11-fold compared with the fed-mite FT fraction (Fig. 2B), indicating that starvation conditioning of mites was successful in reducing host haemoglobin contamination in the SME. A duplicate gel to that shown in Fig. 2A, was electroblotted onto nitrocellulose and probed with yolk-IgY generated in hens that had been immunised with whole SME (Supplementary Fig. S1). Based upon the immunoreactive profiles, adjacent fractions from the IEX separation were pooled together into five groups. Western blotting of these IEX groups and unfractionated SME (Fig. 3) demonstrated that there was minimal overlap in immunoreactivity between IEX groups and that specific proteins were enriched in the IEX groups compared with the SME. Minimal immunoreactivity was detected in the IEX groups and SME when screened with control IgY (from a hen that had not been immunised with mite extracts), except for two bands which corresponded in size with IgY light and heavy chains. The IgY present in the IEX groups and SME was derived from the blood meal present in the guts of mites and was bound by the anti-IgY-peroxidase conjugate.
Fig. 2.
The separation of Dermanyssus gallinae soluble mite extract using ion exchange chromatography. Soluble mite extract was separated using a 1 ml HiTRAP Q HP anion exchange column (GE Healthcare) and eluted with an increasing NaCl gradient. (A) PAGE separation of unbound column flow-through material and sequentially eluted fractions (1–25) obtained from the ion exchange chromatography of soluble mite extract from fed mites. Proteins were electrophoresed on a 12% Bis–Tris Novex gel (GE Healthcare) and silver stained (SilverQuest, Thermo Fisher Scientific). (B) Chromatograms of the ion exchange chromatography elution profiles of soluble mite extract derived from fed mites (solid line) and starved mites (dotted line) soluble mite extract. The figure depicts the absorbance (mAU at 280 nm) of elution of the unbound flow-through (FT, 0–10 ml) and the 25 sequentially eluted 1 ml fractions (10–35 ml). The broken grey line represents the NaCl content of the elution buffer (moles per litre), which is given on the secondary Y axis (NaCl).
Fig. 3.
Immunoreactive profiles of soluble mite extract of Dermanyssus gallinae separated by ion exchange chromatography. Ion exchange chromatography fractions were pooled into five groups (IEX groups 1–5), with IEX group 1 consisting of the column flow-through (FT) and IEX groups 2–5 of selectively pooled eluted fractions. Two microgram of IEX groups 1–5 (lanes 1–5) and 2 μg of the soluble mite extract (SME, lane 6) were separated on a 12% Bis–Tris Novex gel (GE Healthcare), transferred to nitrocellulose and probed with IgY generated against the soluble mite extract (A) or IgY from a hen that had not been injected with soluble mite extract (B). Bound IgY was detected with rabbit anti-IgY-peroxidase (Sigma) and visualised with SIGMA FAST™ 3,3′-diaminobenzidine substrate (Sigma).
3.2. Generation of IgY responses to IEX groups and their effects on mite mortality
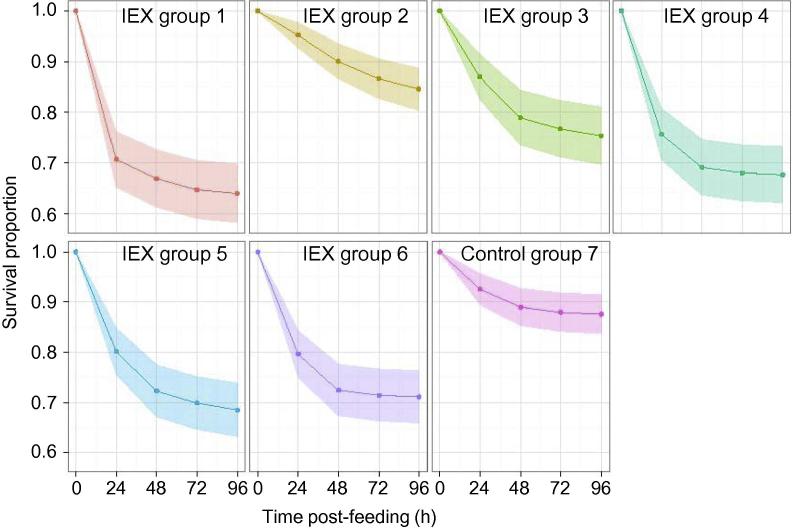
Following immunisation of hens with the five IEX groups or unfractionated SME (group 6), antigen-specific IgY levels peaked at 2 weeks post final vaccination (Supplementary Fig. S2) and did not diminish during the following 4 weeks (Supplementary Fig. S3). The immunoreactive profiles differed markedly between groups, indicating that the strategy used to pool the IEX fractions was successful in maximising the differences between the constituents of the IEX groups. To assess the effect on mite survival after feeding, whole heparinised blood from hens immunised with IEX groups 1–5 or with unfractionated SME was fed to starved mites using the in vitro feeding device on three separate occasions at weeks 3, 5 and 6 post final vaccination. A statistically significant (P < 0.001) reduction in the mean survival proportions of mites fed on the blood from hens immunised with SME and the IEX groups, except IEX group 2, was observed when compared with mites fed on blood from hens in the adjuvant-only control group (group 7) (Fig. 4, results summarised in Table 2). Mites fed on the blood from hens immunised with IEX groups 1 and 4 had the highest risk of dying during the 96 h following feeding and were 3.06 (95% confidence interval (CI): 2.08, 4.52) and 3.72 (95% CI: 2.51, 5.51) times more likely to die than mites in the control group (P < 0.001). GLMM analysis of the cumulative mortality rates of mites fed on blood from immunised hens at 96 h post feeding (Table 2) also identified IEX groups 1 and 4 treatments as producing the highest mite mortality rates at 0.37 (95% CI: 0.20, 0.57) and 0.40 (95% CI: 0.22, 0.61), respectively, and these estimates were significantly (P < 0.004) different from the mean mortality of mites fed on the control group blood (0.14, 95% CI: 0.06, 0.27) at that time point. At the earlier time points of 24, 48 and 72 h post feeding, only mites fed on the blood from hens immunised with IEX groups 1 and 4 showed a consistent and statistically significant (P < 0.030) increase in mortality compared with those fed on blood from the adjuvant-only control group.
Fig. 4.
The observed mean survival proportions of Dermanyssus gallinae following the ingestion of blood from hens immunised with pooled ion exchange chromatograph fractions (IEX groups). Mites were fed with whole heparinised blood from hens immunised with IEX groups 1–5 (IEX groups 1–5), soluble mite extract (IEX group 6) or adjuvant only (Control group 7). The mean proportions of surviving mites at 24, 48, 72 and 96 h post-feeding were calculated using Cox’s proportional hazard model with frailty function and the corresponding 95% confidence intervals (shaded), are plotted.
3.3. Identification and selection of vaccine candidates using 2-DGE immunoblot and immunoaffinity enrichment
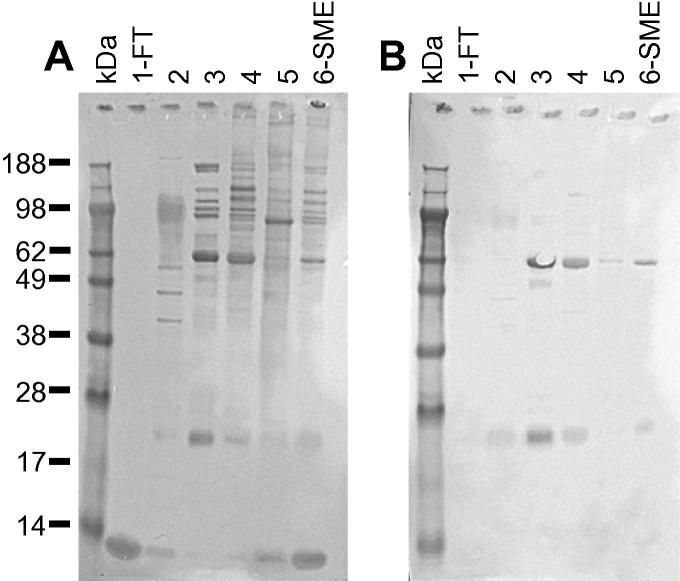
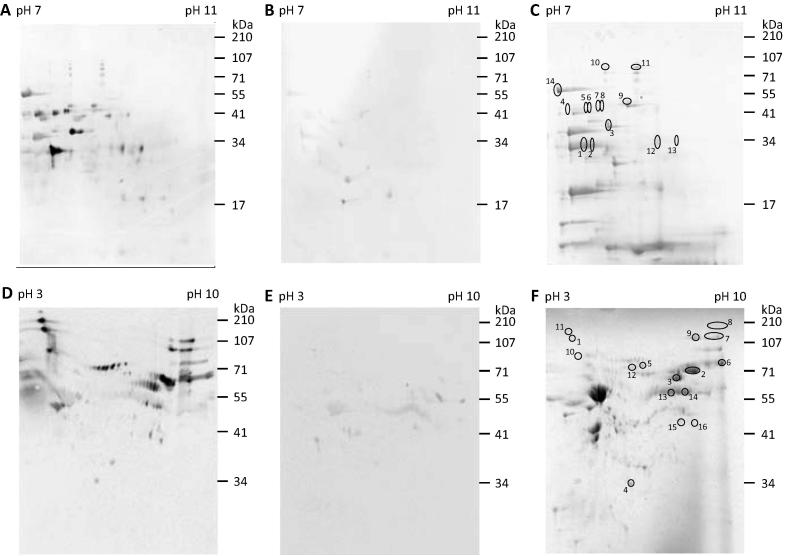
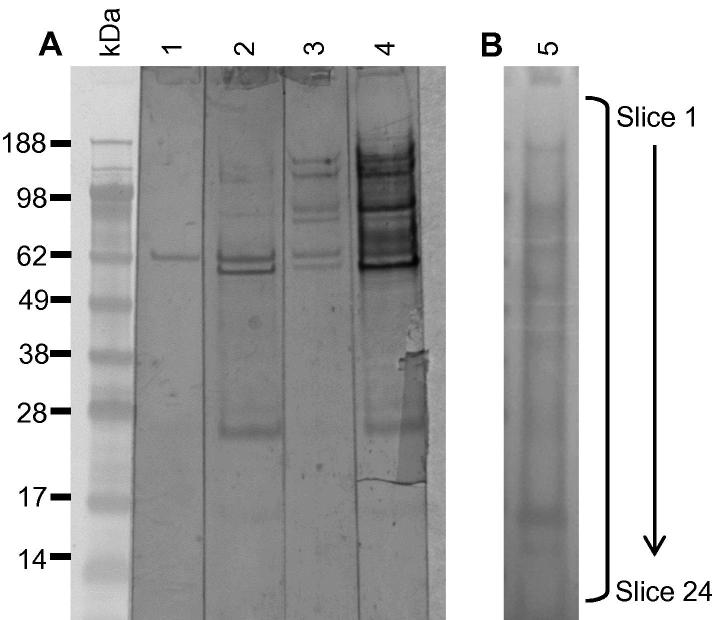
Two complementary approaches, immunoaffinity purification and 2-DGE/immunoblotting, were used to identify the immunoreactive proteins within the IEX groups 1 and 4. Subsequently, protein identification was facilitated by LC–ESI–MS/MS and using the resulting spectral data to mine an annotated red mite transcriptome and the NCBI nr database. The immunoreactive proteins identified from IEX groups 1 and 4 are summarised in Table 4, Table 5, respectively. Full details are available in Supplementary Table S1. The 2-D gel and immunoblot profiles of IEX groups 1 and 4 are shown in Fig. 5. Due to a high abundance of contaminating host haemoglobin amongst the fed mite-derived proteins comprising IEX group 1, the corresponding FT fraction derived from IEX of starved mite SME was used preferentially for these analyses. In total, 15 individual immunoreactive proteins were identified by 2-DGE/immunoblotting of IEX group 1 (Table 4), and immunoaffinity purification of this group of proteins did not identify any further immunoreactive proteins (not shown). Immunoaffinity purification and 2-DGE/immunoblotting of IEX group 4 material identified 35 individual immunoreactive proteins (Table 5). The immunoblot shown in Fig. 6 shows IEX group 4-derived proteins, pre- and post-affinity enrichment (Fig. 6A, lanes 3 and 4, respectively), probed with sera collected from hens immunised with IEX group 4 and demonstrated substantial enrichment of immunoreactive proteins following immunoaffinity purification, particularly of proteins with molecular weights ⩾55 kDa.
Table 4.
Identification of Dermanyssus gallinae proteins present in IgY-immunoreactive spots on a two-dimensional gel immunoblot of the soluble mite extract fractionated by ion exchange chromatography (IEX group 1). Those contigs selected for evaluation as vaccine candidates are given in bold.
| D. gallinae contig number | Top BLASTx hit description and (species) | NCBI accession of top BLASTx hit | % identity | E value | Bit score |
|---|---|---|---|---|---|
| contig00186 | PREDICTED: uncharacterized protein LOC100902161 (Metaseiulus occidentalis) | XP_003741885.1 | 60.8 | 3.09E−53 | 213.4 |
| contig00610 | PREDICTED: fumarate hydratase, mitochondrial-like (Metaseiulus occidentalis) | XP_003742734.1 | 94.3 | 1.65E−70 | 270.8 |
| contig00877 | D-3-phosphoglycerate dehydrogenase, putative (Ixodes scapularis) | XP_002404432.1 | 69.2 | 2.66E−126 | 458.8 |
| contig01284 | PREDICTED: inositol-3-phosphate synthase 1-A-like (Metaseiulus occidentalis) | XP_003740765.1 | 81.5 | 5.43E−42 | 148.3 |
| contig01514 | PREDICTED: serpin B9-like (Metaseiulus occidentalis) | XP_003744662.1 | 36.4 | 0.00025 | 41.2 |
| contig01816 | PREDICTED: aconitate hydratase, mitochondrial-like (Metaseiulus occidentalis) | XP_003744180.1 | 94.0 | 0 | 544.7 |
| contig01795 | PREDICTED: uncharacterized protein LOC100902161 (Metaseiulus occidentalis) | XP_003741885.1 | 56.1 | 2.93E−113 | 415.2 |
| contig02564 | PREDICTED: serpin B9-like (Metaseiulus occidentalis) | XP_003737604.1 | 48.3 | 0 | 544.7 |
| contig02962 | Bifunctional purine biosynthesis protein (Aedes aegypti) | XP_001662952.1 | 75.7 | 1.72E−61 | 142.5 |
| contig03245 | PREDICTED: putative phospholipase B-like 2-like (Metaseiulus occidentalis) | XP_003746012.1 | 57.4 | 0 | 276.9 |
| contig04330 | PREDICTED: inositol-3-phosphate synthase 1-A-like (Metaseiulus occidentalis) | XP_003740765.1 | 78.7 | 4.51E−178 | 631.7 |
| contig05061 | PREDICTED: delta-1-pyrroline-5-carboxylate dehydrogenase, mitochondrial-like (Metaseiulus occidentalis) | XP_003745491.1 | 65.3 | 1.30E−35 | 154.8 |
| contig07527 | PREDICTED: fumarate hydratase, mitochondrial-like (Metaseiulus occidentalis) | XP_003742734.1 | 80.5 | 1.53E−39 | 167.9 |
| contig11549 | PREDICTED: uncharacterized protein LOC100908877 (Metaseiulus occidentalis) | XP_003744327.1 | 34.3 | 2.33E−08 | 64.3 |
| contig12646 | PREDICTED: aconitate hydratase, mitochondrial-like (Metaseiulus occidentalis) | XP_003744180.1 | 86.2 | 5.00E−66 | 256.1 |
Table 5.
Identification of Dermanyssus gallinae proteins present in IgY-immunoreactive spots on a two-dimensional gel immunoblot of the soluble mite extract fractionated by ion exchange chromatography (IEX group 4). Those contigs selected for evaluation as vaccine candidates are given in bold.
| D. gallinae contig number | 2-DGE immuno-blot | Immuno-affinity enrichment | Top BLASTx hit description and (species) | NCBI accession of top BLASTx hit | % identity | E value | Bit score |
|---|---|---|---|---|---|---|---|
| contig00171 | √ | √ | Vitellogenin 2 (Neoseiulus cucumeris) | AGQ56699.1 | 55.3 | 0 | 751.5 |
| contig00200 | √ | Heat shock protein, putative (Ixodes scapularis) | XP_002407132.1 | 82.0 | 0 | 990.7 | |
| contig00293 | √ | PREDICTED: lysosomal aspartic protease-like (Metaseiulus occidentalis) | XP_003738999.1 | 68.4 | 7.29E−154 | 550.8 | |
| contig00394 | √ | √ | PREDICTED: uncharacterized protein LOC100906494 (Metaseiulus occidentalis) | XP_003742265.1 | 52.8 | 0 | 2631.6 |
| contig00411 | √ | PREDICTED: elongation factor 2-like (Metaseiulus occidentalis) | XP_003744110.1 | 91.7 | 0 | 1558.5 | |
| contig00472 | √ | Heat shock protein 60 (Neoseiulus cucumeris) | AGQ50608.1 | 89.6 | 0 | 989.2 | |
| contig01288 | √ | PREDICTED: eukaryotic initiation factor 4A-II-like (Metaseiulus occidentalis) | XP_003740341.1 | 86.5 | 0 | 657.9 | |
| contig01715 | √ | PREDICTED: alpha-aminoadipic semialdehyde synthase, mitochondrial-like (Metaseiulus occidentalis) | XP_003743993.1 | 87.8 | 3.23E−132 | 477.2 | |
| contig02408 | √ | PREDICTED: V-type proton ATPase subunit B-like (Metaseiulus occidentalis) | XP_003739895.1 | 96.1 | 2.27E−172 | 395.2 | |
| contig03001 | √ | PREDICTED: uncharacterized protein LOC100906494 (Metaseiulus occidentalis) | XP_003742265.1 | 52.4 | 1.85E−80 | 305.8 | |
| contig03511 | √ | PREDICTED: GTP-binding nuclear protein Ran-like (Metaseiulus occidentalis) | XP_003738953.1 | 86.4 | 6.05E−81 | 305.4 | |
| contig04115 | √ | PREDICTED: 78 kDa glucose-regulated protein-like isoform 2 (Metaseiulus occidentalis) | XP_003743478.1 | 79.9 | 2.17E−54 | 219.2 | |
| contig04550 | √ | PREDICTED: alpha-aminoadipic semialdehyde synthase, mitochondrial-like (Metaseiulus occidentalis) | XP_003743993.1 | 85.7 | 9.61E−31 | 86.7 | |
| contig04596 | √ | PREDICTED: glutamate dehydrogenase, mitochondrial-like (Metaseiulus occidentalis) | XP_003737714.1 | 96.9 | 5.61E−98 | 209.1 | |
| contig08052 | √ | PREDICTED: isocitrate dehydrogenase [NAD] subunit gamma, mitochondrial-like (Metaseiulus occidentalis) | XP_003738563.1 | 90.0 | 3.71E−30 | 136.7 | |
| contig08304 | √ | PREDICTED: alpha-aminoadipic semialdehyde synthase, mitochondrial-like (Metaseiulus occidentalis) | XP_003743993.1 | 80.8 | 3.44E−57 | 128.3 | |
| contig08760 | √ | PREDICTED: glutathione reductase-like (Metaseiulus occidentalis) | XP_003748614.1 | 71.0 | 2.60E−15 | 87.4 | |
| contig11091 | √ | PREDICTED: 78 kDa glucose-regulated protein-like isoform 2 (Metaseiulus occidentalis) | XP_003743478.1 | 94.9 | 3.55E−153 | 373.6 | |
| contig11504 | √ | Probable ATP-dependent RNA helicase DDX6 (Strongyloides ratti) | CEF64374.1 | 63.5 | 8.94E−153 | 492.7 | |
| contig11553 | √ | MULTISPECIES: ABC transporter substrate-binding protein (Brucella) | WP_008936495.1 | 32.4 | 0.0448883 | 45.1 | |
| contig11846 | √ | √ | PREDICTED: uncharacterized protein LOC100898154 (Metaseiulus occidentalis) | XP_003738142.1 | 47.3 | 6.04E−64 | 250.8 |
| contig11864 | √ | PREDICTED: glycine N-methyltransferase-like (Metaseiulus occidentalis) | XP_003739110.1 | 77.0 | 1.00E−166 | 481.0 | |
| contig11897 | √ | √ | Vitellogenin 2 (Neoseiulus cucumeris) | AGQ56699.1 | 61.5 | 0 | 1466.8 |
| contig12013 | √ | √ | Vitellogenin 1 (Varroa destructor) | AFN88463.1 | 60.5 | 0 | 2327.4 |
| contig12143 | √ | PREDICTED: heat shock 70 kDa protein cognate 4-like (Metaseiulus occidentalis) | XP_003746930.1 | 78.5 | 0 | 664.8 | |
| contig12579 | √ | PREDICTED: uncharacterized protein LOC100898154 (Metaseiulus occidentalis) | XP_003738142.1 | 43.3 | 8.65E−57 | 226.5 | |
| contig13080 | √ | PREDICTED: actin-5C-like (Metaseiulus occidentalis) | XP_003742602.1 | 98.1 | 0 | 748.0 | |
| contig13089 | √ | √ | PREDICTED: uncharacterized protein LOC100898154 (Metaseiulus occidentalis) | XP_003738142.1 | 43.9 | 9.90E−65 | 253.4 |
| contig13090 | √ | PREDICTED: actin, cytoplasmic 2-like isoform 2 (Metaseiulus occidentalis) | XP_003736979.1 | 99.1 | 1.16E−55 | 221.5 | |
| contig13094 | √ | PREDICTED: uncharacterized protein LOC100900885 (Metaseiulus occidentalis) | XP_003745143.1 | 73.2 | 0 | 764.2 | |
| contig13207 | √ | √ | Large lipid transfer protein (Varroa destructor) | AGW50714.1 | 42.7 | 0 | 356.7 |
| contig13301 | √ | Actin 3 (Neoseiulus cucumeris) | AGQ50606.1 | 97.8 | 7.63E−76 | 288.5 | |
| contig13306 | √ | PREDICTED: glutamine synthetase 1, mitochondrial-like (Metaseiulus occidentalis) | XP_003747444.1 | 84.0 | 0 | 339.7 | |
| contig13317 | √ | √ | Vitellogenin 2 (Neoseiulus cucumeris) | AGQ56699.1 | 60.0 | 0 | 1870.5 |
| contig13348 | √ | PREDICTED: ATP synthase subunit beta, mitochondrial-like isoform 1 (Metaseiulus occidentalis) | XP_003745616.1 | 83.6 | 0 | 831.2 |
2-DGE, two-dimensional gel electrophoresis.
Fig. 5.
Two-dimensional gel and immunoblot analyses of the pooled ion exchange chromatograph fractions (IEX groups 1 and 4) derived from Dermanyssus gallinae soluble mite extracts. (A–C) IEX group 1; (D–F) IEX group 4 proteins were separated on replicate gels with pI ranges of 7–11 (IEX group 1) and 3–10 (IEX group 4). Two replicate gels were electroblotted and screened with yolk-IgY from hens vaccinated three times with their respective IEX group 1 or 4 proteins (A and D, respectively) or with yolk-IgY from group 7 hens that had been immunised with adjuvant only (B and E). Bound IgY was detected with rabbit anti-IgY-peroxidase IgG and visualised with chemiluminescent substrate. A third gel replicate of IEX groups 1 and 4 (C and F) was stained with SimplyBlue™ SafeStain (Thermo Fisher Scientific) and the corresponding locations of the immuno-reactive spots and were identified and excised from the gel for liquid chromatography–electrospray ionisation–tandem mass spectrometry (LC–ESI–MS/MS) (circled and numbered).
Fig. 6.
Immunoaffinity enrichment of Dermanyssus gallinae proteins using immobilised antigen-specific IgY. Yolk-IgY generated against ion exchange chromatography group 4 proteins was cross-linked to a HiTrap N-hydroxysuccinimide (NHS)-activated column and IEX group 4 proteins were selectively bound to the column, washed to remove unbound material and then eluted into an affinity enriched fraction with 0.1 M Glycine, 6 M Urea, pH 2.5. (A) The IEX group 4 proteins prior to affinity purification (lanes 1 and 3) and the eluted affinity-enriched material (lanes 2 and 4) were separated on a 12% Bis–Tris Novex gel (GE Healthcare) and transferred to nitrocellulose. Lanes 1 and 2 of the immunoblot were probed with a 1:200 dilution of sera from naive hens that had not been immunised with the soluble mite extract and lanes 3 and 4 were probed with a 1:200 dilution of post-vaccination sera from hens immunised with IEX group 4. Bound IgY was detected with rabbit anti-IgY-peroxidase (Sigma) and visualised using SIGMA FAST™ 3,3′-diaminobenzidine substrate (Sigma). (B) The eluted immunoaffinity enriched IEX group 4 material was concentrated fivefold by lyophilisation and 30 μl was electrophoretically separated as before and stained with SimplyBlue™ SafeStain (lane 5). The gel lane was sectioned into 24 equally-sized horizontal slices and the proteins extracted and subjected to liquid chromatography–electrospray ionisation–tandem mass spectrometry (LC–ESI–MS/MS).
A selection of 10 vaccine candidates was made from the 50 contig sequences identified in IEX groups 1 and 4, based on their predicted functions (inferred from the BLASTx hit annotation). Contigs with a high identity against avian host proteins or predicted to encode for proteins deemed inaccessible to the immune response (e.g. mitochondrial) were immediately eliminated from the selection. Contigs with predicted functions likely to be necessary for the survival of the mite (e.g. feeding, digestion, detoxification or reproduction) or those contigs that exhibited a high level of immunoreactivity, with or without associated gene annotation, were retained.
3.4. Production of recombinant versions of the vaccine candidates
The complete CDS of nine selected antigens and partial coding sequences of two variants of Deg-PUF-2 were obtained using RACE, PCR and DNA sequencing (accession numbers are listed in Table 1). Recombinant, full-length, hexa histidine (6xHis)-tagged versions of seven of the 10 vaccine candidate proteins (minus signal peptides where appropriate) were expressed and purified by nickel-affinity chromatography. The two Deg-PUF-2 variants 1 and 2 were produced as recombinant 6xHis-tagged proteins with C-terminal truncations. Deg-HGP-1 and Deg-VIT-1 were predicted, from transcript sequence, to be very large proteins of 1579 and 1845 amino acids in length, respectively, and prokaryotic expression of full-length versions of these two proteins was unsuccessful. To overcome expression difficulties, the CDS for Deg-HGP-1 and Deg-VIT-1 were divided into two sections (N-terminal and C-terminal domains). Production of recombinant 6xHis-tagged Deg-HGP-1 N- and C-terminal domains and Deg-VIT-1 N-terminal domain was successful.
3.5. Assessment of the acaricidal potential of the recombinant vaccine candidates
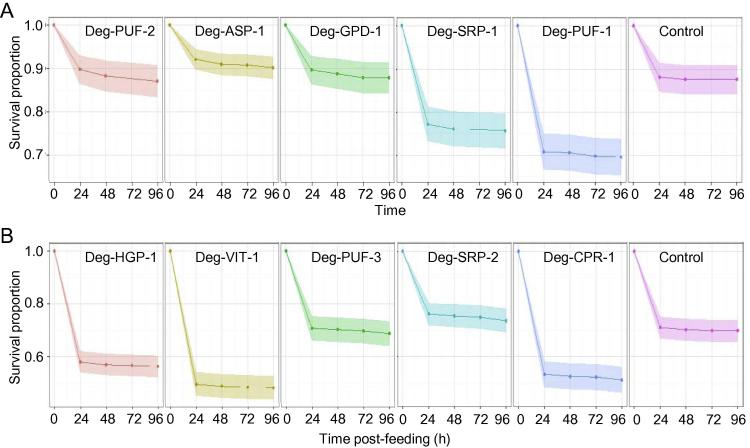
Hens were injected three times with 50 μg of the recombinant vaccine candidates formulated with 200 μg of QuilA in order to generate serum-IgY responses. The generation and longevity of the immune response was monitored using Western blotting and ELISA which showed that hens generated an antigen-specific and sustained IgY response encompassing the period when the mite feeding assays were performed (data not shown). Mites fed on the blood from hens immunised with Deg-SRP-1, Deg-PUF-1 and Deg-VIT-1 were 2.09 (95% CI: 1.48, 2.94), 2.79 (95% CI: 2.01, 3.88) and 2.03 (95% CI: 1.66, 2.48) times more likely to die during the 96 h following feeding than mites fed on blood from hens immunised with adjuvant only (P < 0.001, Table 3). The majority of mite mortality occurred within 24 h after feeding on immunised blood (Fig. 7). For example, mites fed on blood from hens immunised with groups Deg-SRP-1, Deg-PUF-1 and Deg-VIT-1 recorded mean mortality rates as 0.24 (95% CI: 0.12, 0.41), 0.29 (95% CI: 0.16, 0.48) and 0.50 (95% CI: 0.37, 0.64) at 24 h post feeding; these estimates were statistically significantly (P < 0.040) higher than the corresponding control groups at that time point. The mean mortality rate of Deg-HGP-1 group was 0.47 (95% CI: 0.33, 0.61) at 24 h post feeding, and there was weak evidence (P < 0.052) that the estimate was higher compared with the control group at that time point.
Fig. 7.
The observed mean survival proportions of Dermanyssus gallinae following the ingestion of blood from hens immunised with recombinant vaccine candidates. Immunisation of hens with the 10 recombinant vaccine candidates and subsequent mite feeding assays were performed in two tranches. Mites were fed with whole heparinised blood from hens immunised with the tranche 1 recombinant vaccine candidates (A: Deg-ASP-1, Deg-GPD-1, Deg-SRP-1, Deg-PUF-1) or tranche 2 (B: Deg-HGP-1, Deg-VIT-1, Deg-PUF-3, Deg-SRP-2, Deg-CPR-1) or with adjuvant only (control). The feeding assays were performed in three replicates and the mean proportions of surviving mites at 24, 48, 72 and 96 h post-feeding, with corresponding 95% confidence intervals (shaded) calculated using Cox’s proportional hazard model with frailty function, are plotted.
4. Discussion
The work described herein has demonstrated, to our knowledge for the first time, the use of a combined immunological, proteomic and genomic approach to vaccine candidate discovery for the poultry red mite. To complement these methodologies, the resulting candidate molecules were expressed as recombinant proteins and tested for vaccine efficacy in vitro, resulting in the identification of three key vaccine candidate molecules with vaccine efficacy similar to native mite extracts (cf. Table 2, Table 3).
All D. gallinae motile stages (larva, protonymph, deuteronymph and adult), except larva, are haematophagous (Kirkwood, 1968), and as such, all motile stages, except larva, are potentially susceptible to the effects of ingested antibodies present in the blood of vaccinated hens. Therefore we did not selectively target vaccine identification to a specific development stage and instead undertook a mixed-stage approach, which allowed the opportunity of immunoreactive proteins present in the protective SME, regardless of stage or gender bias, to be identified.
Traditional vaccinology has relied upon a pragmatic approach to vaccine candidate identification, where extracts of native proteins are repeatedly subfractionated using column-based protein separation technologies and tested in vivo for immunogenicity and vaccine efficacy. Following several rounds of fractionation and testing, complex mixtures of proteins can be distiled down into fractions containing relatively pure preparations of protective protein(s). The pragmatic approach was instrumental for the identification of the Bm86 antigen, which is the protective component of the Rhipicephalus microplus TickGard vaccine (Willadsen et al., 1988, Willadsen et al., 1989, Willadsen, 2004) and in the identification of the multi-subunit Haemonchus galactose-containing glycoprotein (H-gal-GP) vaccine (Smith et al., 1994, Smith et al., 1999). In recent years, a dramatic increase in publically available proteomic, genomic and transcriptomic data has enabled an in silico approach to be used for vaccine discovery for many pathogens species, with antigen selection based upon criteria such as inferred function, predicted sub-cellular location, transmembrane domains and epitope prediction (Goodswen et al., 2015). Whilst a quicker and cheaper alternative to the pragmatic approach, in silico antigen discovery is reliant on accurate data and precludes antigen selection based on in vivo immunogenicity, and must therefore involve additional in vivo screening at a later stage. By combining the pragmatic approach with newer ‘omic’ technologies, the immunogenicity component of the pragmatic approach can be retained whilst maximising antigen identification by searching peptide data (generated from vaccine candidate proteins using mass spectrometry technologies) against high coverage ‘omic’ datasets. This combined strategy has been successfully applied to vaccine antigen identification for several ecto- and endo-parasite species (Smith et al., 2009, De la Fuente and Merino, 2013, McNulty et al., 2014) and herein with D. gallinae.
While 2-D immunoblotting has previously been widely used to identify vaccine candidate antigens in parasite species (e.g. Nisbet et al., 2010) this technique necessarily requires that proteins are exposed to chaotropic conditions i.e. urea, thiourea and CHAPS in the first (IEF) dimension, and subsequently to a powerful anionic surfactant i.e. SDS, in the second. The ensuing protein denaturation is likely to result in an underestimation of the numbers of immunoreactive proteins actually present in the sample, especially where antibody-based recognition is reliant upon the preservation of conformational epitopes. With this caveat in mind, a complementary non-denaturing immunoaffinity approach was used to enrich proteins whilst preserving their native globular conformation, as proposed by Ellis et al. (2014), leading to an enhanced identification of vaccine candidates from IEX group 4 (Table 5). The identities of more than two-thirds of the resulting vaccine candidates from both IEX groups 1 and 4 were informed by their homology to proteins of the western orchard predatory mite, Metaseiulus occidentalis (Acari: Phytoseiidae), reflecting the availability of annotated genomic data for this species (http://www.ncbi.nlm.nih.gov/bioproject/62309). In spite of the generation of large transcriptomic datasets for D. gallinae (Bartley et al., 2012, Schicht et al., 2013, Schicht et al., 2014), a full annotated genome for D. gallinae remains a priority together with the relevant tools to investigate gene function in this species to inform the targeting of novel interventions and control methodologies (Marr et al., 2014, Viney, 2014). These tools would be of particular relevance to understanding the nature of Deg-PUF-1, a protein of unknown function shown here to be a strong vaccine candidate.
Immunoreactive candidate proteins (Table 4, Table 5) were selected for further evaluation in immunisation experiments using a rational order of merit based on intensity of immune recognition; likelihood of exposure of the antigen to the antibodies in a blood meal (Willadsen, 2004); proposed function and known vaccine potential of orthologous molecules. Mitochondrial enzymes were excluded from consideration as vaccine candidate molecules because, although many were highly immunogenic, they were unlikely to be exposed to host antibody following immunisation. Two proteolytic enzymes, Deg-ASP-1 and Deg-CPR-1, were evaluated here and, although the latter showed some promise, the mite mortalities following feeding on hens immunised with these molecules were not as consistent as those measured previously when using different D. gallinae aspartyl and cysteine proteinases as immunogens (Bartley et al., 2012).
As a result of their profound effects on blood coagulation, homeostasis, fibrinolysis, complement activation and immune effector regulation; serine proteinase inhibitors (serpins) have been advocated as vaccine candidate proteins in a range of blood-borne (e.g. Brugia malayi, Zang et al., 1999) and haematophagous pathogens including other members of the Acari (e.g. in the metastriate tick Amblyomma americanum, Mulenga et al., 2001, Porter et al., 2015). Here, immunisation of hens with the serpin Deg-SRP-1 resulted in a significantly increased (>twofold) chance of mortality in mites feeding on blood from the immunised hens compared with those feeding on blood from hens immunised with adjuvant alone (Table 3). This effect was not universal to all serpins, however, as immunisation with Deg-SRP-2 did not produce the same effects, suggesting distinct biological roles and/or localisation of these two molecules in D. gallinae.
After traversing the gut epithelium, host serum immunoglobulins are capable of entering the haemolymph of haematophagous arthropods and interacting with components of the haemolymph (e.g. vitellogenin) and the membrane receptors involved in processes such as receptor-mediated endocytosis during the incorporation of vitellogenin by developing oocytes (Sauer et al., 1994). This has led to the investigation of vitellogenin and vitellogenin-like molecules (e.g. hemelipoglycoprotein, Donohue et al., 2008) as vaccine candidate molecules in haematophagous arthropods and informed our selection of Deg-VIT-1 and Deg-HGP-1 for vaccine efficacy testing. Previously, immunisation of sheep with purified vitellin (the mature form of vitellogenin) from the tick R. microplus resulted in significantly reduced numbers of engorged ticks with decreased weights and reduced oviposition following challenge (Tellam et al., 2002). We have demonstrated that host IgY is able to enter the haemolymph of D. gallinae following a blood meal from hens (Nisbet et al., 2006). Vitellogenin and hemelipoglycoprotein are therefore accessible to vaccine-induced antibodies following a blood meal on an immunised host, and both Deg-VIT-1 and Deg-HGP-1 induced significant levels of mite mortality when used as immunogens (Table 3). Given the physiological role of vitellogenins in oocyte maturation as well as other functions in arthropods (Morandin et al., 2014), a further benefit of immunisation of hens with D. gallinae vitellogenins may be reduced production or viability of mite eggs, leading to delayed expansion of mite populations in hen houses. Although vitellogenin and hemelipoglycoprotein share many conserved functional domains including a lipoprotein N-terminal domain (heme, lipid and carbohydrate binding) and the von-Willebrand factor type D, their pattern of expression differs in haematophagous arthropods. For example, hemelipoglycoprotein in the tick Dermacentor variabilis is, unlike vitellogenin, expressed in life stages other than mated females (Thompson et al., 2007, Donohue et al., 2008) and is therefore indicative of functions unrelated to oocyte maturation. Hemelipoglycoprotein is undoubtedly multifunctional and is involved in the sequestering of free heme (Maya-Monteiro et al., 2000) and potentially in protection from heme-induced radical damage (Maya-Monteiro et al., 2004). In addition, hemeolipoglycoprotein has been proposed to play a role in innate immunity in ticks (Dupejova et al., 2011, Sterba et al., 2011).
In conclusion, we have demonstrated an integrated methodology for the identification of vaccine candidate molecules from D. gallinae and tested the potential of these molecules in vitro. The ability of the selected vaccine antigens to control mite populations in commercial poultry facilities must now be demonstrated and this requires formulation of the antigens in appropriate adjuvant and delivery vehicles for sustained antibody responses in the host.
Acknowledgements
The authors gratefully acknowledge funding for this project from Biotechnology and Biological the Science Research Council, UK (grant reference BB/J01513X/1), Zoetis Inc. (USA) and Akita Co. Ltd (Japan). We would also like to thank Mr. John Campbell, Glenrath Farms Ltd. (UK) for his continued support throughout this project.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijpara.2015.07.004.
Appendix A. Supplementary data
Immunoreactivity of fractionated Dermanyssus gallinae soluble mite extract (SME) and the pooling of fractions into groups for use as immunogens. SME was separated by ion-exchange chromatography (IEX) through a 1 ml HiTRAP Q HP anion column (GE Healthcare) and eluted with an increasing NaCl gradient. Subsamples (10 μl) of SME, unbound column flow-through (FT) and the eluted fractions 1–25 were electrophoresed on a 12% Bis–Tris Novex gel (GE Healthcare), transferred to nitrocellulose and probed with yolk-IgY previously generated against SME (yolk-anti-SME-IgY). Bound IgY was detected with anti-IgY-peroxidase conjugate (Sigma) and visualised using SuperSignal West Pico chemiluminescence substrate (Thermo Fisher Scientific). SME screened with yolk-IgY (lane −ve, from a naive hen) or with the anti-IgY-peroxidase conjugate only (lane conj) served as controls. The designations of the individual fractions into the five IEX groups (IEX groups 1–5) are indicated by open parentheses. Immunoblotting the IEX fractions with yolk-IgY generated against SME resulted in immuno-reactivity in the FT material and all fractions apart from fractions 1 and 2. A large number of proteins with an estimated MW > 40 kDa were detected. Smaller immuno-reactive proteins were only seen in fraction 9 (discrete 21 kDa protein) and the FT fraction (24 and 29 kDa proteins). An intense immuno-reactivity was also seen in the FT fraction corresponding in size with the haemoglobin and to a lesser intensity in fraction 13–22. Little immuno-reactivity was detected in the negative and conjugate control lanes, except for a 65 kDa protein corresponding in size with the heavy chain of IgY (derived from the blood meal present in the gut of the mite) and a protein approximately 100 kDa of unknown identity. A high degree of variation in the immuno-reactive profiles of adjacent fractions was observed.
Generation of antigen-specific IgY in hens to Dermanyssus gallinae mite proteins. Seven groups of hens (Gp1–7) were immunised three times at weeks 0, 2 and 4. Groups 1–5 (Gp1–5) were immunised with 50 μg per dose of the correspondingly numbered pooled fraction groups from ion-exchange chromatography (IEX groups 1–5) of soluble mite extract (SME). Group 6 (Gp6) hens were immunised with 50 μg per dose of SME and group 7 (Gp7) hens received adjuvant only. The yolks from several eggs collected at weeks 0, 2, 4, 5 and 6 were pooled according to group and time and the IgY purified from these yolks. Immunoblot strips containing 2 μg of the immobilised IEX group proteins (IEX group 1–5 proteins for Gp1-5 respectively) or SME (for Gp 6 and 7), separated on a 12% Bis–Tris SDS–PAGE gel, were probed with 100 μg/ml of their respective pooled yolk-IgY from weeks 0, 2, 4, 5, 6. Bound IgY was detected with anti-IgY-peroxidase conjugate (Sigma) and visualised using SIGMA FAST™ 3,3′-diaminobenzidine substrate (Sigma). Yolk-IgY samples indicted by “/” were not included in the immunoblot analysis if IgY was not available for this time point.
Longevity of serum-IgY responses in hens to Dermanyssus gallinae mite proteins. Seven groups of hens (Gp1–7) were immunised three times at weeks 0, 2 and 4. Groups 1–5 (Gp1–5) were immunised with 50 μg per dose of the correspondingly numbered pooled fraction groups from ion-exchange chromatography (IEX groups 1–5) of soluble mite extract (SME). Group 6 (Gp6) hens were immunised with 50 μg per dose of SME and group 7 (Gp7) received adjuvant only. Serum was sampled pre-vaccination (pre) and at 3, 5 and 6 weeks following final vaccination. A pool of serum from each group and at each time point was prepared by mixing equal volumes of plasma from each hen. Immunoblot strips containing 2 μg of the immobilised IEX pooled proteins (IEX groups 1–5 proteins for Gp1–5 respectively) or SME (for Gp 6 and 7), separated on a 12% Bis–Tris SDS–PAGE gel, were probed with 1/50 dilution of serum pools from pre-vaccination and 3, 5, and 6 weeks post-vaccination. Immunoreactivity was detected with anti-IgY-peroxidase conjugate (Sigma) and visualised using SuperSignal West Pico chemiluminescence substrate (Thermo Fisher Scientific).
Proteomic identification of the immuno-reactive components of the Dermanyssus gallinae soluble mite extract (SME). Immuno reactive proteins identified in the ion-exchange chromatography (IEX) pooled subfraction groups 1 and 4 (IEX groups 1 and 4) using: two-dimensional (2-D) gel electrophoresis coupled with immunoblotting and immuno-affinity enrichment of IEX group 1 proteins; were subjected to liquid chromatography–electrospray ionisation–tandem mass spectrometry (LC–ESI–MS/MS) and the data generated from Mascot™ V2.3 database mining of a custom Dermanyssus gallinae transcriptome database (Bartley, 2015, unpublished data) is presented.
References
- Bartley K., Nisbet A.J., Offer J.E., Sparks N.H., Wright H.W., Huntley J.F. Histamine release factor from Dermanyssus gallinae (De Geer): characterization and in vitro assessment as a protective antigen. Int. J. Parasitol. 2009;39:447–456. doi: 10.1016/j.ijpara.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Bartley K., Huntley J.F., Wright H.W., Nath M., Nisbet A.J. Assessment of cathepsin D and L-like proteinases of poultry red mite, Dermanyssus gallinae (De Geer), as potential vaccine antigens. Parasitology. 2012;139:755–765. doi: 10.1017/S0031182011002356. [DOI] [PubMed] [Google Scholar]
- Batycka M., Inglis N.F., Cook K., Adam A., Fraser-Pitt D., Smith D.G., Main L., Lubben A., Kessler B.M. Ultra-fast tandem mass spectrometry scanning combined with monolithic column liquid chromatography increases throughput in proteomic analysis. Rapid Commun. Mass Spectrom. 2006;20:2074–2080. doi: 10.1002/rcm.2563. [DOI] [PubMed] [Google Scholar]
- Brannstrom S., Hansson I., Chirico J. Experimental study on possible transmission of the bacterium Erysipelothrix rhusiopathiae to chickens by the poultry red mite, Dermanyssus gallinae. Exp. Appl. Acarol. 2010;50:299–307. doi: 10.1007/s10493-009-9317-4. [DOI] [PubMed] [Google Scholar]
- Chauve C. The poultry red mite Dermanyssus gallinae (De Geer, 1778): current situation and future prospects for control. Vet. Parasitol. 1998;79:239–245. doi: 10.1016/s0304-4017(98)00167-8. [DOI] [PubMed] [Google Scholar]
- De la Fuente J., Kocan K.M. Advances in the identification and characterization of protective antigens for recombinant vaccines against tick infestations. Expert Rev. Vaccines. 2003;2:583–593. doi: 10.1586/14760584.2.4.583. [DOI] [PubMed] [Google Scholar]
- De la Fuente J., Merino O. Vaccinomics, the new road to tick vaccines. Vaccine. 2013;31:5923–5929. doi: 10.1016/j.vaccine.2013.10.049. [DOI] [PubMed] [Google Scholar]
- Donohue K.V., Khalil S.M., Mitchell R.D., Sonenshine D.E., Roe R.M. Molecular characterization of the major hemelipoglycoprotein in ixodid ticks. Insect Mol. Biol. 2008;17:197–208. doi: 10.1111/j.1365-2583.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- Dupejova J., Sterba J., Vancova M., Grubhoffer L. Hemelipoglycoprotein from the ornate sheep tick, Dermacentor marginatus: structural and functional characterization. Parasit. Vectors. 2011;4:4. doi: 10.1186/1756-3305-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S., Matthews J.B., Shaw D.J., Paterson S., McWilliam H.E., Inglis N.F., Nisbet A.J. Ovine IgA-reactive proteins from Teladorsagia circumcincta infective larvae. Int. J. Parasitol. 2014;44:743–750. doi: 10.1016/j.ijpara.2014.05.007. [DOI] [PubMed] [Google Scholar]
- George D.R., Finn R.D., Graham K.M., Mul M.F., Maurer V., Moro C.V., Sparagano O.A. Should the poultry red mite Dermanyssus gallinae be of wider concern for veterinary and medical science? Parasit. Vectors. 2015;8:178. doi: 10.1186/s13071-015-0768-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodswen S.J., Barratt J.L., Kennedy P.J., Ellis J.T. Improving the gene structure annotation of the apicomplexan parasite Neospora caninum fulfils a vital requirement towards an in silico-derived vaccine. Int. J. Parasitol. 2015;45:305–318. doi: 10.1016/j.ijpara.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Harrington D., Din H.M., Guy J., Robinson K., Sparagano O. Characterization of the immune response of domestic fowl following immunization with proteins extracted from Dermanyssus gallinae. Vet. Parasitol. 2009;160:285–294. doi: 10.1016/j.vetpar.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Kilpinen O., Roepstorff A., Permin A., Norgaard-Nielsen G., Lawson L.G., Simonsen H.B. Influence of Dermanyssus gallinae and Ascaridia galli infections on behaviour and health of laying hens (Gallus gallus domesticus) Br. Poult. Sci. 2005;46:26–34. doi: 10.1080/00071660400023839. [DOI] [PubMed] [Google Scholar]
- Kirkwood A.C. Some observations on the feeding habits of the poultry mites Dermanyssus gallinae and Liponyssus sylviarum. Exp. Appl. Entomol. 1968;11:315–320. [Google Scholar]
- Marangi M., Cafiero M.A., Capelli G., Camarda A., Sparagano O.A., Giangaspero A. Evaluation of the poultry red mite, Dermanyssus gallinae (Acari: Dermanyssidae) susceptibility to some acaricides in field populations from Italy. Exp. Appl. Acarol. 2009;48:11–18. doi: 10.1007/s10493-008-9224-0. [DOI] [PubMed] [Google Scholar]
- Marr E.J., Sargison N.D., Nisbet A.J., Burgess S.T.G. RNA interference for the identification of ectoparasite vaccine candidates. Parasite Immunol. 2014 doi: 10.1111/pim.12132. [DOI] [PubMed] [Google Scholar]
- Maya-Monteiro C.M., Daffre S., Logullo C., Lara F.A., Alves E.W., Capurro M.L., Zingali R., Almeida I.C., Oliveira P.L. HeLp, a heme lipoprotein from the hemolymph of the cattle tick, Boophilus microplus. J. Biol. Chem. 2000;275:36584–36589. doi: 10.1074/jbc.M007344200. [DOI] [PubMed] [Google Scholar]
- Maya-Monteiro C.M., Alves L.R., Pinhal N., Abdalla D.S., Oliveira P.L. HeLp, a heme-transporting lipoprotein with an antioxidant role. Insect Biochem. Mol. Biol. 2004;34:81–88. doi: 10.1016/j.ibmb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- McDevitt R., Nisbet A.J., Huntley J.F. Ability of a proteinase inhibitor mixture to kill poultry red mite, Dermanyssus gallinae in an in vitro feeding system. Vet. Parasitol. 2006;141:380–385. doi: 10.1016/j.vetpar.2006.05.013. [DOI] [PubMed] [Google Scholar]
- McNulty S.N., Fischer P.U., Townsend R.R., Curtis K.C., Weil G.J., Mitreva M. Systems biology studies of adult paragonimus lung flukes facilitate the identification of immunodominant parasite antigens. PLoS Negl. Trop. Dis. 2014;8:e3242. doi: 10.1371/journal.pntd.0003242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandin C., Havukainen H., Kulmuni J., Dhaygude K., Trontti K., Helanterä H. Not only for egg yolk – functional and evolutionary insights from expression, selection, and structural analyses of Formica ant vitellogenins. Mol. Biol. Evol. 2014;31:2181–2193. doi: 10.1093/molbev/msu171. [DOI] [PubMed] [Google Scholar]
- Mulenga A., Sugino M., Nakajima M., Sugimoto C., Onuma M. Tick-encoded serine proteinase inhibitors (Serpins); potential target antigens for tick vaccine development. J. Vet. Med. Sci. 2001;63:1063–1069. doi: 10.1292/jvms.63.1063. [DOI] [PubMed] [Google Scholar]
- Nisbet A.J., Huntley J.F., Mackellar A., Sparks N., McDevitt R. A house dust mite allergen homologue from poultry red mite Dermanyssus gallinae (De Geer) Parasite Immunol. 2006;28:401–405. doi: 10.1111/j.1365-3024.2006.00862.x. [DOI] [PubMed] [Google Scholar]
- Nisbet A.J., Smith S.K., Armstrong S., Meikle L.I., Wildblood L.A., Beynon R.J., Matthews J.B. Teladorsagia circumcincta: activation-associated secreted proteins in excretory/secretory products of fourth stage larvae are targets of early IgA responses in infected sheep. Exp. Parasitol. 2010;125:329–337. doi: 10.1016/j.exppara.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Porter L., Radulović Ž., Kim T., Braz G.R., Da Silva Vaz I., Jr., Mulenga A. Bioinformatic analyses of male and female Amblyomma americanum tick expressed serine protease inhibitors (serpins) Ticks Tick Borne Dis. 2015;6:16–30. doi: 10.1016/j.ttbdis.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer J.R., McSwain J.L., Essenberg R.C. Cell membrane receptors and regulation of cell function in ticks and blood-sucking insects. Int. J. Parasitol. 1994;24:33–52. doi: 10.1016/0020-7519(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Schicht S., Qi W., Poveda L., Strube C. The predicted secretome and transmembranome of the poultry red mite Dermanyssus gallinae. Parasit. Vectors. 2013;6:259. doi: 10.1186/1756-3305-6-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schicht S., Qi W., Poveda L., Strube C. Whole transcriptome analysis of the poultry red mite Dermanyssus gallinae (De Geer, 1778) Parasitology. 2014;141:336–346. doi: 10.1017/S0031182013001467. [DOI] [PubMed] [Google Scholar]
- Shevchenko A., Wilm M., Vorm O., Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Smith W.D., Smith S.K., Murray J.M. Protection studies with integral membrane fractions of Haemonchus contortus. Parasite Immunol. 1994;16:231–241. doi: 10.1111/j.1365-3024.1994.tb00345.x. [DOI] [PubMed] [Google Scholar]
- Smith S.K., Pettit D., Newlands G.F., Redmond D.L., Skuce P.J., Knox D.P., Smith W.D. Further immunization and biochemical studies with a protective antigen complex from the microvillar membrane of the intestine of Haemonchus contortus. Parasite Immunol. 1999;21:187–199. doi: 10.1046/j.1365-3024.1999.00217.x. [DOI] [PubMed] [Google Scholar]
- Smith S.K., Nisbet A.J., Meikle L.I., Inglis N.F., Sales J., Beynon R.J., Matthews J.B. Proteomic analysis of excretory/secretory products released by Teladorsagia circumcincta larvae early post-infection. Parasite Immunol. 2009;31:10–19. doi: 10.1111/j.1365-3024.2008.01067.x. [DOI] [PubMed] [Google Scholar]
- Sparagano O., Pavlicevic A., Murano T., Camarda A., Sahibi H., Kilpinen O., Mul M., van Emous T., le Bouquin S., Hoel K., Cafiero M.A. Prevalence and key figures for the poultry red mite Dermanyssus gallinae infections in poultry farm systems. Exp. Appl. Acarol. 2009;48:3–10. doi: 10.1007/s10493-008-9233-z. [DOI] [PubMed] [Google Scholar]
- Sparagano O.A., George D.R., Harrington D.W., Giangaspero A. Significance and control of the poultry red mite, Dermanyssus gallinae. Annu. Rev. Entomol. 2014;59:447–466. doi: 10.1146/annurev-ento-011613-162101. [DOI] [PubMed] [Google Scholar]
- Sterba J., Dupejova J., Fiser M., Vancova M., Grubhoffer L. Fibrinogen-related proteins in ixodid ticks. Parasit. Vectors. 2011;4:127. doi: 10.1186/1756-3305-4-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G.K., Goodlett D.R. Rules governing protein identification by mass spectrometry. Rapid Commun. Mass Spectrom. 2005;19:3420. doi: 10.1002/rcm.2225. [DOI] [PubMed] [Google Scholar]
- Tellam R.L., Kemp D., Riding G., Briscoe S., Smith D., Sharp P., Irving D., Willadsen P. Reduced oviposition of Boophilus microplus feeding on sheep vaccinated with vitellin. Vet. Parasitol. 2002;103:141–156. doi: 10.1016/s0304-4017(01)00573-8. [DOI] [PubMed] [Google Scholar]
- Thompson D.M., Khalil S.M., Jeffers L.A., Sonenshine D.E., Mitchell R.D., Osgood C.J., Michael Roe R. Sequence and the developmental and tissue-specific regulation of the first complete vitellogenin messenger RNA from ticks responsible for heme sequestration. Insect Biochem. Mol. Biol. 2007;37:363–374. doi: 10.1016/j.ibmb.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Valiente M.C., De Luna C.J., Tod A., Guy J.H., Sparagano O.A., Zenner L. The poultry red mite (Dermanyssus gallinae): a potential vector of pathogenic agents. Exp. Appl. Acarol. 2009;48:93–104. doi: 10.1007/s10493-009-9248-0. [DOI] [PubMed] [Google Scholar]
- Van Emous R. Wage war against the red mite! Poultry Int. 2005;44:26–33. [Google Scholar]
- Viney M. The failure of genomics in biology. Trends Parasitol. 2014;30:319–321. doi: 10.1016/j.pt.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Willadsen P. Anti-tick vaccines. Parasitology. 2004;129:S367–S387. doi: 10.1017/s0031182003004657. [DOI] [PubMed] [Google Scholar]
- Willadsen P., McKenna R.V., Riding G.A. Isolation from the cattle tick, Boophilus microplus, of antigenic material capable of eliciting a protective immunological response in the bovine host. Int. J. Parasitol. 1988;18:183–189. doi: 10.1016/0020-7519(88)90059-8. [DOI] [PubMed] [Google Scholar]
- Willadsen P., Riding G.A., McKenna R.V., Kemp D.H., Tellam R.L., Nielsen J.N., Lahnstein J., Cobon G.S., Gough J.M. Immunologic control of a parasitic arthropod. Identification of a protective antigen from Boophilus microplus. J. Immunol. 1989;143:1346–1351. [PubMed] [Google Scholar]
- Wright H.W., Bartley K., Nisbet A.J., McDevitt R.M., Sparks N.H., Brocklehurst S., Huntley J.F. The testing of antibodies raised against poultry red mite antigens in an in vitro feeding assay; preliminary screen for vaccine candidates. Exp. Appl. Acarol. 2009;48:81–91. doi: 10.1007/s10493-009-9243-5. [DOI] [PubMed] [Google Scholar]
- Zang X., Yazdanbakhsh M., Jiang H., Kanost M.R., Maizels R.M. A novel serpin expressed by blood-borne microfilariae of the parasitic nematode Brugia malayi inhibits human neutrophil serine proteinases. Blood. 1999;94:1418–1428. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunoreactivity of fractionated Dermanyssus gallinae soluble mite extract (SME) and the pooling of fractions into groups for use as immunogens. SME was separated by ion-exchange chromatography (IEX) through a 1 ml HiTRAP Q HP anion column (GE Healthcare) and eluted with an increasing NaCl gradient. Subsamples (10 μl) of SME, unbound column flow-through (FT) and the eluted fractions 1–25 were electrophoresed on a 12% Bis–Tris Novex gel (GE Healthcare), transferred to nitrocellulose and probed with yolk-IgY previously generated against SME (yolk-anti-SME-IgY). Bound IgY was detected with anti-IgY-peroxidase conjugate (Sigma) and visualised using SuperSignal West Pico chemiluminescence substrate (Thermo Fisher Scientific). SME screened with yolk-IgY (lane −ve, from a naive hen) or with the anti-IgY-peroxidase conjugate only (lane conj) served as controls. The designations of the individual fractions into the five IEX groups (IEX groups 1–5) are indicated by open parentheses. Immunoblotting the IEX fractions with yolk-IgY generated against SME resulted in immuno-reactivity in the FT material and all fractions apart from fractions 1 and 2. A large number of proteins with an estimated MW > 40 kDa were detected. Smaller immuno-reactive proteins were only seen in fraction 9 (discrete 21 kDa protein) and the FT fraction (24 and 29 kDa proteins). An intense immuno-reactivity was also seen in the FT fraction corresponding in size with the haemoglobin and to a lesser intensity in fraction 13–22. Little immuno-reactivity was detected in the negative and conjugate control lanes, except for a 65 kDa protein corresponding in size with the heavy chain of IgY (derived from the blood meal present in the gut of the mite) and a protein approximately 100 kDa of unknown identity. A high degree of variation in the immuno-reactive profiles of adjacent fractions was observed.
Generation of antigen-specific IgY in hens to Dermanyssus gallinae mite proteins. Seven groups of hens (Gp1–7) were immunised three times at weeks 0, 2 and 4. Groups 1–5 (Gp1–5) were immunised with 50 μg per dose of the correspondingly numbered pooled fraction groups from ion-exchange chromatography (IEX groups 1–5) of soluble mite extract (SME). Group 6 (Gp6) hens were immunised with 50 μg per dose of SME and group 7 (Gp7) hens received adjuvant only. The yolks from several eggs collected at weeks 0, 2, 4, 5 and 6 were pooled according to group and time and the IgY purified from these yolks. Immunoblot strips containing 2 μg of the immobilised IEX group proteins (IEX group 1–5 proteins for Gp1-5 respectively) or SME (for Gp 6 and 7), separated on a 12% Bis–Tris SDS–PAGE gel, were probed with 100 μg/ml of their respective pooled yolk-IgY from weeks 0, 2, 4, 5, 6. Bound IgY was detected with anti-IgY-peroxidase conjugate (Sigma) and visualised using SIGMA FAST™ 3,3′-diaminobenzidine substrate (Sigma). Yolk-IgY samples indicted by “/” were not included in the immunoblot analysis if IgY was not available for this time point.
Longevity of serum-IgY responses in hens to Dermanyssus gallinae mite proteins. Seven groups of hens (Gp1–7) were immunised three times at weeks 0, 2 and 4. Groups 1–5 (Gp1–5) were immunised with 50 μg per dose of the correspondingly numbered pooled fraction groups from ion-exchange chromatography (IEX groups 1–5) of soluble mite extract (SME). Group 6 (Gp6) hens were immunised with 50 μg per dose of SME and group 7 (Gp7) received adjuvant only. Serum was sampled pre-vaccination (pre) and at 3, 5 and 6 weeks following final vaccination. A pool of serum from each group and at each time point was prepared by mixing equal volumes of plasma from each hen. Immunoblot strips containing 2 μg of the immobilised IEX pooled proteins (IEX groups 1–5 proteins for Gp1–5 respectively) or SME (for Gp 6 and 7), separated on a 12% Bis–Tris SDS–PAGE gel, were probed with 1/50 dilution of serum pools from pre-vaccination and 3, 5, and 6 weeks post-vaccination. Immunoreactivity was detected with anti-IgY-peroxidase conjugate (Sigma) and visualised using SuperSignal West Pico chemiluminescence substrate (Thermo Fisher Scientific).
Proteomic identification of the immuno-reactive components of the Dermanyssus gallinae soluble mite extract (SME). Immuno reactive proteins identified in the ion-exchange chromatography (IEX) pooled subfraction groups 1 and 4 (IEX groups 1 and 4) using: two-dimensional (2-D) gel electrophoresis coupled with immunoblotting and immuno-affinity enrichment of IEX group 1 proteins; were subjected to liquid chromatography–electrospray ionisation–tandem mass spectrometry (LC–ESI–MS/MS) and the data generated from Mascot™ V2.3 database mining of a custom Dermanyssus gallinae transcriptome database (Bartley, 2015, unpublished data) is presented.