Abstract
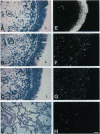
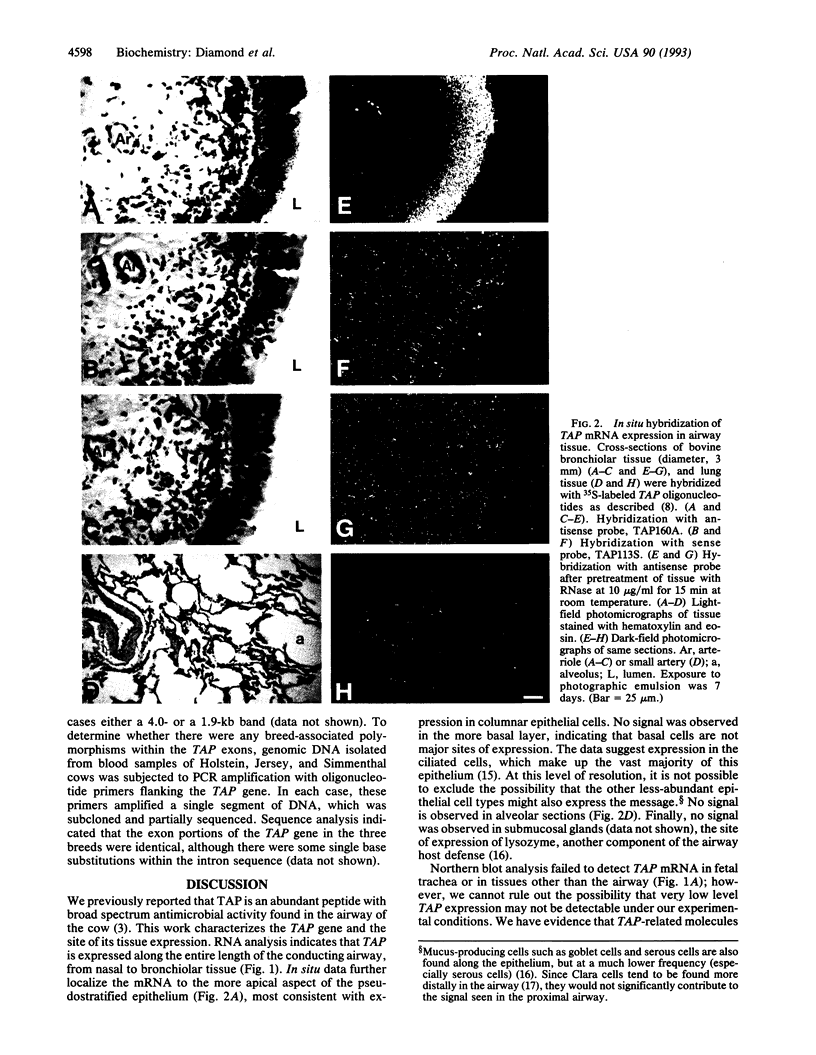
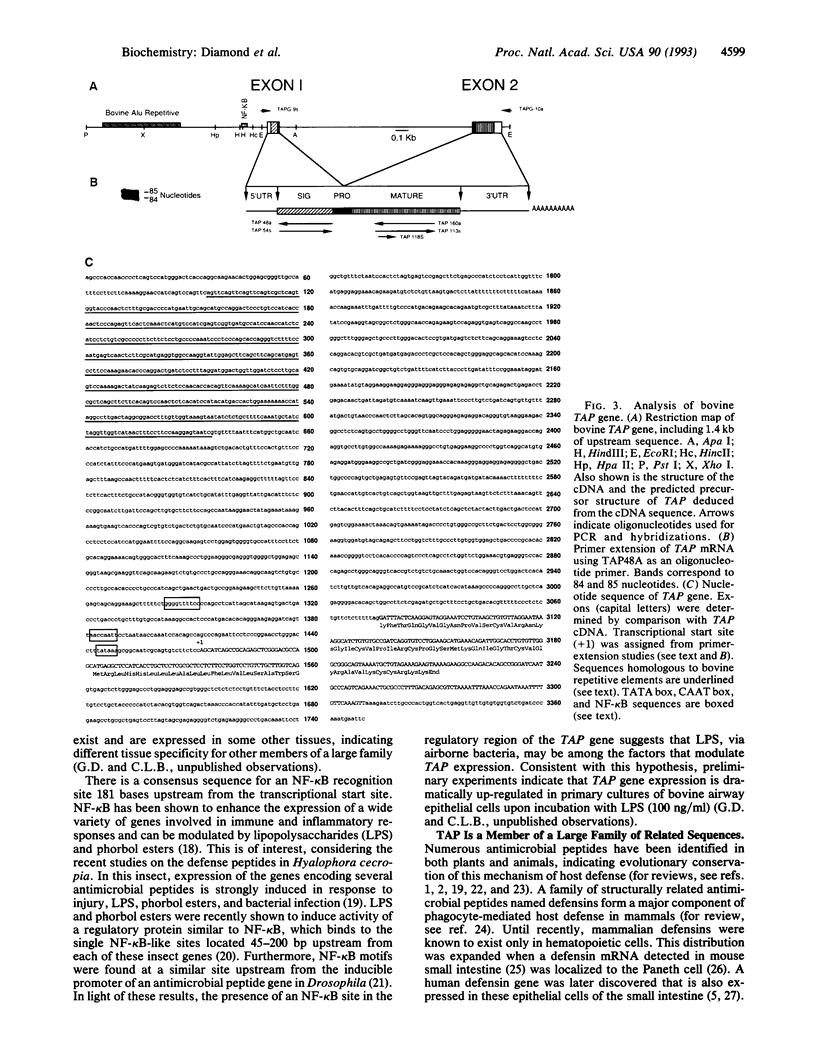
We previously reported the isolation and characterization of a broad-spectrum antimicrobial peptide from the bovine tracheal mucosa, which we called tracheal antimicrobial peptide (TAP). We now show the TAP gene is expressed throughout the adult conducting airway, from nasal to bronchiolar tissue, but not in tissues other than airway mucosa, as determined by Northern blot analysis. In situ hybridization of airway sections localizes TAP mRNA to columnar cells of the pseudostratified epithelium. We report the structural organization of the TAP gene and show that TAP is a member of a large family of related sequences with high nucleotide identity in the 5' exon. The data support the hypothesis that antimicrobial peptides contribute to host defense of the respiratory tract.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge J., Kunkel L., Bruns G., Tantravahi U., Lalande M., Brewster T., Moreau E., Wilson M., Bromley W., Roderick T. A strategy to reveal high-frequency RFLPs along the human X chromosome. Am J Hum Genet. 1984 May;36(3):546–564. [PMC free article] [PubMed] [Google Scholar]
- Bevins C. L., Zasloff M. Peptides from frog skin. Annu Rev Biochem. 1990;59:395–414. doi: 10.1146/annurev.bi.59.070190.002143. [DOI] [PubMed] [Google Scholar]
- Boman H. G. Antibacterial peptides: key components needed in immunity. Cell. 1991 Apr 19;65(2):205–207. doi: 10.1016/0092-8674(91)90154-q. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Hultmark D. Cell-free immunity in insects. Annu Rev Microbiol. 1987;41:103–126. doi: 10.1146/annurev.mi.41.100187.000535. [DOI] [PubMed] [Google Scholar]
- Diamond G., Zasloff M., Eck H., Brasseur M., Maloy W. L., Bevins C. L. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. H. Novel Alu-type repeat in artiodactyls. Nucleic Acids Res. 1987 Feb 11;15(3):1340–1340. doi: 10.1093/nar/15.3.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T., Rayner J. R., Valore E. V., Tumolo A., Talmadge K., Fuller F. The structure of the rabbit macrophage defensin genes and their organ-specific expression. J Immunol. 1989 Aug 15;143(4):1358–1365. [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Lehrer R. I. Defensins. Eur J Haematol. 1990 Jan;44(1):1–8. doi: 10.1111/j.1600-0609.1990.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Jones D. E., Bevins C. L. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 1993 Jan 4;315(2):187–192. doi: 10.1016/0014-5793(93)81160-2. [DOI] [PubMed] [Google Scholar]
- Jones D. E., Bevins C. L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992 Nov 15;267(32):23216–23225. [PubMed] [Google Scholar]
- Kylsten P., Samakovlis C., Hultmark D. The cecropin locus in Drosophila; a compact gene cluster involved in the response to infection. EMBO J. 1990 Jan;9(1):217–224. doi: 10.1002/j.1460-2075.1990.tb08098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T., Selsted M. E. Defensins: endogenous antibiotic peptides of animal cells. Cell. 1991 Jan 25;64(2):229–230. doi: 10.1016/0092-8674(91)90632-9. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Ouellette A. J., Cordell B. Accumulation of abundant messenger ribonucleic acids during postnatal development of mouse small intestine. Gastroenterology. 1988 Jan;94(1):114–121. doi: 10.1016/0016-5085(88)90618-x. [DOI] [PubMed] [Google Scholar]
- Ouellette A. J., Greco R. M., James M., Frederick D., Naftilan J., Fallon J. T. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol. 1989 May;108(5):1687–1695. doi: 10.1083/jcb.108.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette A. J., Lualdi J. C. A novel mouse gene family coding for cationic, cysteine-rich peptides. Regulation in small intestine and cells of myeloid origin. J Biol Chem. 1990 Jun 15;265(17):9831–9837. [PubMed] [Google Scholar]
- Reichhart J. M., Meister M., Dimarcq J. L., Zachary D., Hoffmann D., Ruiz C., Richards G., Hoffmann J. A. Insect immunity: developmental and inducible activity of the Drosophila diptericin promoter. EMBO J. 1992 Apr;11(4):1469–1477. doi: 10.1002/j.1460-2075.1992.tb05191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Tang Y. Q., Morris W. L., McGuire P. A., Novotny M. J., Smith W., Henschen A. H., Cullor J. S. Purification, primary structures, and antibacterial activities of beta-defensins, a new family of antimicrobial peptides from bovine neutrophils. J Biol Chem. 1993 Mar 25;268(9):6641–6648. [PubMed] [Google Scholar]
- Sun S. C., Faye I. Cecropia immunoresponsive factor, an insect immunoresponsive factor with DNA-binding properties similar to nuclear-factor kappa B. Eur J Biochem. 1992 Mar 1;204(2):885–892. doi: 10.1111/j.1432-1033.1992.tb16708.x. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Mezey E., Siegel R. E. Quantitative in situ hybridization histochemistry reveals increased levels of corticotropin-releasing factor mRNA after adrenalectomy in rats. Neurosci Lett. 1986 Oct 8;70(2):198–203. doi: 10.1016/0304-3940(86)90463-5. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Antibiotic peptides as mediators of innate immunity. Curr Opin Immunol. 1992 Feb;4(1):3–7. doi: 10.1016/0952-7915(92)90115-u. [DOI] [PubMed] [Google Scholar]
- de Zabala L. E., Weinman D. E. Prenatal development of the bovine lung. Anat Histol Embryol. 1984 Mar;13(1):1–14. doi: 10.1111/j.1439-0264.1984.tb00391.x. [DOI] [PubMed] [Google Scholar]