Abstract
Background
Beta1-receptor antagonists (BBs) are commonly administered in the treatment of cardiovascular disease (CVD). The reported benefits of BB use in CVD patients with concomitant obstructive sleep apnea (OSA) may be limited by their impact on apnea-induced bradycardias. Therefore the aim of the study was to test the influence of BBs on periapneic heart rate (HR) fluctuations in hypertensive patients with newly-detected and untreated OSA.
Methods
We studied 88 hypertensive patients (56 on BBs and 32 BB naive) with newly-diagnosed moderate-to-severe OSA who were free of major pulmonary comorbidities and did not require antiarrhythmic therapy. ECGs recorded during sleep were investigated for heart rate (HR) responses to apneas allowing to compare extreme HR accelerations and decelerations between the groups.
Results
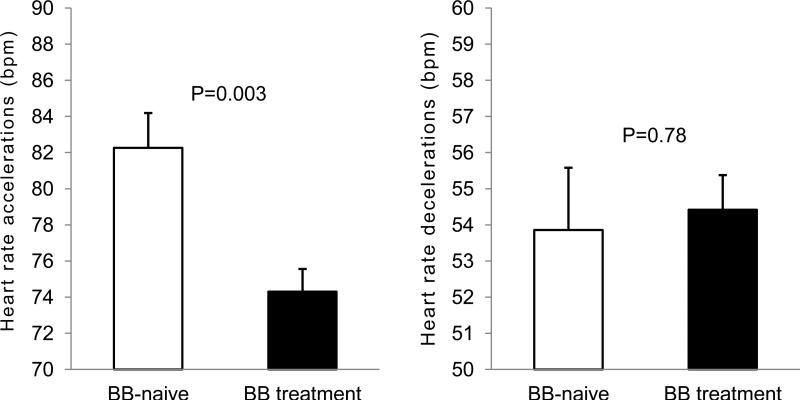
Average sleep-time HR were comparable in BB-naive (BB−) and BB-treated (BB+) patients. Direct comparisons showed that HR decelerations were also similar in the two subgroups (53.8 ±9.6 vs. 54.4 ±7.8 bpm; P=0.78, for BB− and BB+, respectively) however, BBs blunted the OSA-induced HR accelerations (82.3 ±12.2 vs. 74.3±10.0; P=0.003). After adjusting for baseline HR and magnitude of desaturations, HR decelerations were more evident in BB-naive group whereas tachycardic responses remained blunted in the BB+ group. The incidence of ectopies and conduction abnormalities were comparable across two groups.
Conclusions
Beta-blockers do not potentiate apnea-induced HR decelerations, attenuate apnea-induced increases in heart rate and do not influence incidence of ectopies and conduction abnormalities in patients with hypertension and moderate-to-severe, untreated OSA.
Keywords: beta-blockers, obstructive sleep apnea, heart rate control, bradycardia, tachycardia
Introduction
Beta1-receptor antagonists (beta-blockers; BBs) are commonly administered in the treatment of a broad spectrum of cardiovascular diseases. Large clinical trials showed that irrespective of comorbidities, 47% [1] to 57% [2] of all treated hypertensive patients receive BBs on a regular basis. However, the role of BBs in the management of hypertensive OSA patients is unclear. Previous studies in patients with OSA report a superiority of BB therapy as a treatment modality for autonomic imbalance and cardiovascular abnormalities commonly seen in these patients [3, 4]. Much less is known about the effects of BBs on night-time HR and arrhythmias.
Physiologically, apnea triggers powerful and differentiated co-activation of the sympathetic (SNS) and parasympathetic (PNS) branches of the autonomic nervous system leading to marked peripheral vasoconstriction, and bradycardia. Although SNS/PNS co-activation may potentially increase the risk for arrhythmias [5], apparently the overall benefits of the this co-activation outweigh the potential risk. In OSA subjects, each sleep disordered breathing episode may itself evoke reflex bradycardia and tachycardia [6-9], as well as frequent bradyarrhythmias [10,11], which might conceivably be potentiated by the negative chronotropic effects of beta-blockade.
In the absence of CPAP, marked increases in heart rate and cardiac afterload, in the setting of severe hypoxemia, may trigger episodic cardiac ischemia [12] or even infarction [13]. Hence, BBs may mitigate acute neural circulatory responses to obstructive apneas. However, there is a concern that their negative chronotropic effects may potentiate the severity of the acute bradyarrhythmic responses to apnea.
To our knowledge, the effect of BBs on apnea-related cardiac responses has never been systematically studied. We hypothesized that BBs may diminish SNS driven reflex heart rate acceleration episodes in OSA subjects. Concurrently, we speculated that when PNS driven bradycardia prevail, BBs influence might be limited, as the SNS is already effectively blocked by powerful vagal activation.
Material and methods
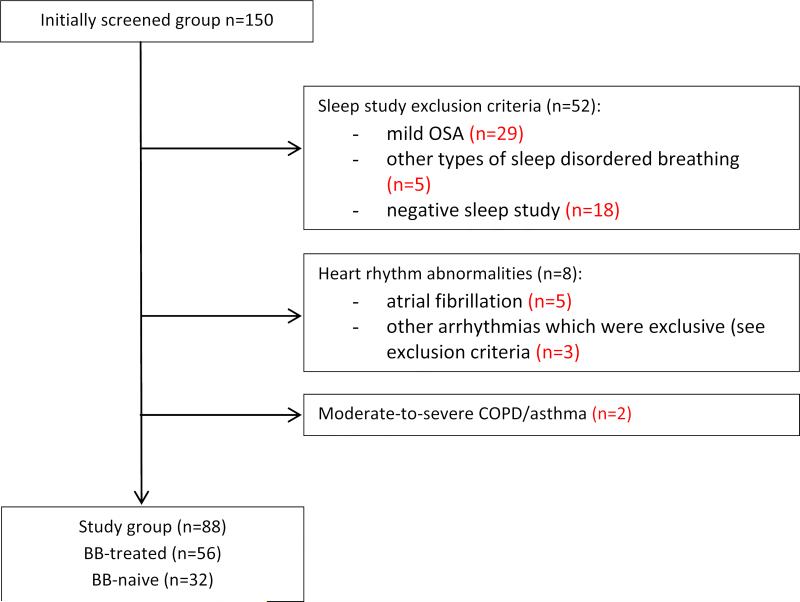
We studied 88 consecutively recruited hypertensive patients screened for OSA at the Medical University of Gdańsk Hospital (Hypertension and Diabetology Clinic, and Pneumonology and Allergology Clinic) in 2009 (Figure 1). All experimental procedures were performed in accordance with the Declaration of Helsinki on the treatment of human subjects and the Ethical Committee of the University of Gdansk approved the study (NKEBN/48/2011). Informed written consent was obtained from each subject.
Figure 1.
Study flow-chart.
Sleep data
The polygraphic recordings (Embletta X30, X100™) were reviewed and scored according to standard rules [14]. Only apneas and hypopnoeas (≥30% reduction in the amplitude of airflow) were analyzed if accompanied with ≥4% oxygen desaturations compared to pre-event baseline. The respiratory events included in the analyses were 10 seconds long at minimum. AHI was defined as the average number of apneas plus hypopneas per hour of study.
Exclusion criteria
Exclusion criteria were as follows: AHI<15; other than obstructive sleep-disordered breathing eg. predominantly central sleep apnea; Cheyne-Stokes respiration during sleep; hypoventilation syndromes; implanted cardiac pacing devices; arrhythmias without evident relation to sleep disordered breathing episodes (sustained supra-, and ventricular arrhythmias incl. atrial fibrillation, sustained 2° or 3° atrio-ventricular block); moderate or severe bronchial asthma or chronic obstructive pulmonary disease (COPD); ongoing therapy with negative chronotropic agents other than BBs including non-dihydropiridine calcium channel blockers (verapamil, diltiazem), ivabradine, digoxin and amiodarone; ongoing treatment with antiarrhythmic agents i.e. sodium channel blockers. ECG signal loss. Following these criteria we excluded 62 patients (Figure 1).
Group dichotomization
Patients were assigned to 2 groups with regard to whether or not BB treatment was administered. The distribution of specific BBs was random and reflected the actual distribution of BB use in patients with hypertension in our Center (bisoprolol, n=24; metoprolol, n=20, bataxolol n=10; carvedilol, n=2). All patients with coronary artery disease (CAD) and heart failure received beta-blockers.
ECG analysis
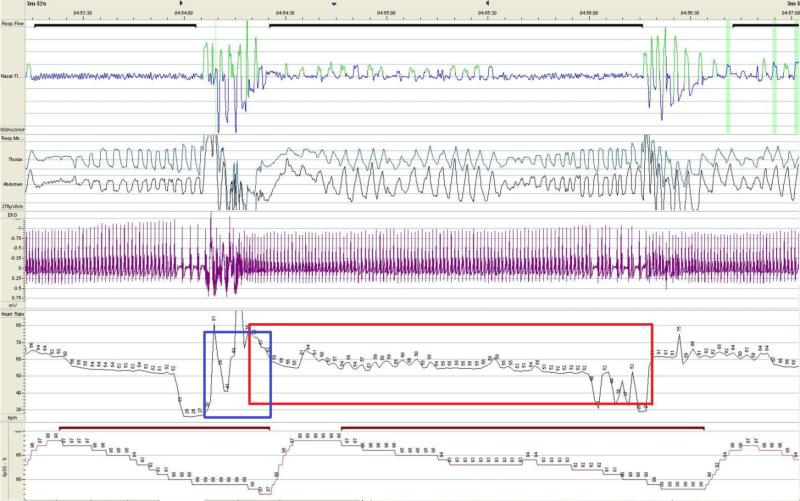
Final analysis was based on ECG tracings (CM5 lead, sampling frequency; f=200Hz) extracted from polygraphic studies of 88 eligible patients. As we sought to analyze extreme cardiac responses, we selected and averaged the 50 fastest reflex heart rate accelerations and 50 slowest reflex heart rate decelerations associated with apneas/hypopneas from each sleep study. Technically, the raw ECG data along with respiratory channels were exported to the MATLAB-based software (written by K.Cz.), which automatically calculated all associated sinus HR acceleration, and deceleration events. The exact evaluation window for each HR deceleration (longest RR-intervals) assessment was set between the onset of the apnea/hypopnea to the moment of the resumption of breathing (Figure 2). Similarly, the apnea-related HR acceleration response was analyzed as the shortest RR intervals recorded immediately after the termination of breathing event but no later than the corresponding desaturation nadir (Figure 2). The average of three RR intervals values clustered with extremes entered the analyses. All cardiac responses were manually reviewed (J.W.) and uploaded to the statistical package. Manual reviewing also allowed for artifact exclusion and capturing ectopies such as supra-, and ventricular extrabeats, non-sinus rhythms, atrioventricular blocks, and pauses. If the electrical impulse conduction impairment and/or ectopy occurred the event was classified either as brady-, or tachyarrhythmia, and was excluded from all sinus HR swings analyses.
Figure 2. Example of recurrent cyclic variations of the heart rate and apnea-related bradyarrhythmias.
Figure depicts reoccurring heart rate accelerations, decelerations, and bradyarrhythmias associated with sleep apneic episodes. The tracings represent following signals (from top to bottom): nasal airflow (pressure cannula), thorax and abdomen movements (inductive belts), ECG, heart rate, and SpO2 (pulse oximetry). The extreme decelerations were calculated as averaged three longest RR intervals while sinus rhythm occurring in apnea-corresponding ECG tracing (red rectangle window), and the HR sinus accelerations were calculated as averaged three shortest RR-intervals while patient attempted rescue breathing (blue rectangle window). The bradyarrhythmias resulting from the atrioventricular blocks were assessed separately (here: AVB 2nd degree 2:1).
Statistical analysis
Statistical tests were computed using Statistica 10.1, Statsoft Inc.®. Skewed data distributions were logarithmically corrected before analyses when appropriate. Descriptive variables were presented as means ±SD or Medians (IQR). Chi-squared test (with Yates correction when appropriate) was used to compare co-morbidities, arrhythmia frequencies, and male-to-female ratios. Unpaired, two-tailed t-tests were used to compare continuous variables between two groups, with and without BB treatment. An analysis of covariance (ANCOVA) was performed to assess the differences in periapneic cardiac responses. P<0.05 was considered significant for all calculations.
Results
Patient demographics and clinical characteristic are presented in Table 1.
Table 1.
Clinical characteristics of BB− and BB+ groups
| BB− | BB+ | P-value | |
|---|---|---|---|
| Anthropometrics | |||
| Males-to-Females ratio | 81%:19% | 80%:20% | 0.86 |
| Age | 55 (46-63) | 57.5 (54-62) | 0.12 |
| BMI | 34.2 (30.5-36.5) | 32.3 (28.1-41.2) | 0.84 |
| Waist circumference | 118.2 ±12.3 | 118.4 ±11.5 | 0.97 |
| Sleep study-derived indices: | |||
| AHI [episodes/hour] | 28.8 (18-52) | 32.9 (22-54) | 0.66 |
| SpO2 [%] | 92.8 (92.0-94.1) | 93.4 (91.8-94.3) | 0.53 |
| T90 [min.] | 23.6 (4-87) | 29.9 (6-75) | 0.59 |
| Desaturation duration (HR deceleration assessment) | 31.7 ± 10.1 | 29.1 ± 7.2 | 0.16 |
| Desaturation duration (HR acceleration assessment) | 33.3 ± 10.5 | 31.1 ± 7.5 | 0.25 |
| Average sleep-study heart rate | 64.9 ±9.0 | 62.6 ±7.5 | 0.20 |
| Co-morbidities: | |||
| Coronary artery disease | 0 (0%) | 16 (29%) | 0.00 |
| Congestive heart failure | 0 (0%) | 3 (5%) | 0.47 |
| Chronic kidney disease | 5 (16%) | 8 (14%) | 0.86 |
| Stroke / TIA | 0 (0%) | 5 (9%) | 0.08 |
| Diabetes Mellitus | 9 (28%) | 14 (25%) | 0.85 |
Normally distributed data are presented as mean values ±SD. Male-to-female ratio, and comorbidities are presented as per cent. Age, BMI, AHI, SpO2, lowest SpO2, T90 (skewed data distribution) are presented as Medians with IQR. P-values for two-tailed t-tests, Chi-squared tests, and Mann-Whitney tests, respectively. Abbreviations: BMI = body mass index, AHI = apnea-hypopnea index, SpO2 = mean blood oxygen saturation, T90 = cumulative time with blood oxygen saturation below 90%, TIA = transient ischemic attack.
Polysomnography-derived indices and average sleep study heart rates were comparable in BB+ and BB− subgroups (Table 1).
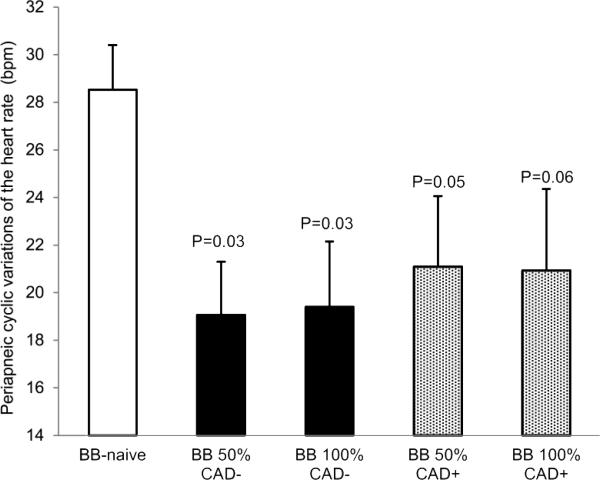
There were differences evident in acute cardiac responses to apneic episodes across subgroups. Reflex HR accelerations were blunted in BB+ patients vs. BB− (Figure 3, left panel). However, BB treatment did not influence reflex bradycardias (Figure 3, right panel). The attenuation of cyclic variations of heart rate observed in BB+ patients was comparable regardless of the BB dosage (Figure 4).
Figure 3.
Comparison of periapneic maximal increases (left panel), and minimal decreases (right panel) of the heart rates with relation to ongoing beta-blocker treatment. Unpaired t-tests.
Figure 4. Comparison of cyclic variations of heart rate and relationships to the dosage of beta-blocker therapy and the presence of coronary artery disease. One-way ANOVA, P=0.001.
Boxes represent mean values, and whiskers standard errors of the mean. BBs = beta-blockers; BBs100% - full registered BBs dosage; BBs50% - half registered dosage according to Summaries of Products Characteristics. CAD - coronary artery disease. P-values refer to post-hoc Dunnett's tests against BBs-naive group (reference group).
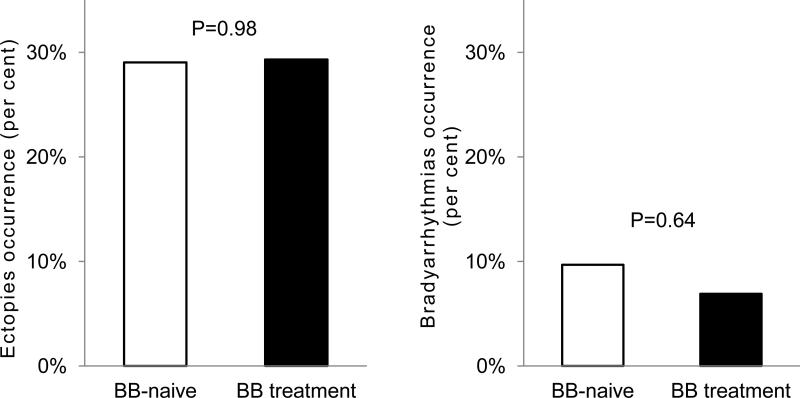
The incidence of apnea-induced pauses, conduction abnormalities and ectopies resulting in increases in non-sinus rhythm were comparable in patients with and without ongoing beta-blocker treatment (Figure 5).
Figure 5. Apnea-related ectopies, and conduction abnormalities occurrence in patients with and without beta-blocker treatment.
Panels represents the comparison of the prevalence of (left) ectopies resulting in acceleration of the HR, and (right) conduction abnormalities causing RR interval prolongation >2000ms or decelerated non-sinus rhythm.
After adjusting for baseline heart rate and magnitude of desaturations (ANCOVA), tachycardic responses were blunted in the BB+ group (Table 2), however, reflex bradycardias were more evident in the BB− vs. BB+ patients.
Table 2.
Comparison of mean periapneic bradycardias and tachycardias and relationships to ongoing beta-blocker therapy, mean sleep-time heart rate and desaturations. Multivariate analysis (ANCOVA).
| BBs-naive | BBs-treatment | P-value | |
|---|---|---|---|
| Reflex bradycardia | 52.7 (51.2-54.3) | 55.2 (54.0-56.3) | 0.02 |
| Reflex tachycardia | 80.1 (78.0-82.2) | 75.7 (74.2-77.2) | 0.001 |
Model adjusted to the mean sleep study heart rates, and desaturations. Data presented as mean values with corresponding 95% confidence intervals. BBs = beta-blockers.
Discussion
There are two novel and important findings in our study. First, in hypertensive patients with moderate-to-severe untreated OSA, beta-blocker treatment is not accompanied by potentiated reflex heart rate decelerations or bradyarrhythmic responses to obstructive apneas comparing to patients without ongoing BB therapy. Second, BB administration attenuates apnea-induced reflex heart rate increases, which is independent of the drug dose.
Autonomic responses to apnea and to recovery breathing, reflected in the cyclical variations of heart rate which are characteristic of the majority of patients with untreated sleep apnea [9,15], may be accompanied by clinically significant bradycardias or bradyarrhythmias [8]. Although continuous positive airway pressure therapy (CPAP) restores autonomic stability and attenuates arrhythmias [16], the common problem with such treatment modality is the long-term patients’ persistence. 15% of eligible patients do not accept CPAP after the first night of usage [17], more than 30% give up treatment by the end of the first month [18], and up to 50% of OSA patients abandon therapy within one year [19].
The impact of beta-blockade on the recurrent apnea-generated heart rate and rhythmic fluctuations has not been systematically studied, and the available analyses have focused on the averaged sleep-time HR only [3, 20]. However, investigation of mean HR alone may be insufficient to fully elucidate the impact of beta-blockers on transient and rapid heart rate changes and arrhythmias in OSA. Given that both severe bradycardias and tachycardias may progress to more complex and prolonged arrhythmias (pauses, escape rhythms) [21], potential consequences of modulation by chronotropic agents are of clear clinical relevance.
Animal studies showed that intravenous infusion of propranolol had a diverse effect on periapneic bradycardias when compared to non-instrumented controls [22]. The cardiac response varied from null effect during NREM sleep to slight decreases of the minimal heart rates when compared to the pre-apnea period in REM. Overall these data did not suggest clinical relevance of the impact of older non-selective beta-blockers on periapneic bradycardias in animals. However, in this animal model, the post-apneic tachycardias were blunted or even absent compared to controls. We now confirm the animal observations in hypertensive patients with OSA who are receiving contemporary beta-blocker therapy. Chronic administration of various beta-blockers at any dose significantly attenuates the magnitude of reflex acceleration of HR, evident as decreased maximal HR and periapneic delta HR (Figure 3 left panel, Figure 4). Furthermore, the incidence of apnea-induced arrhythmias was similar irrespective of beta-blocker treatment despite higher prevalence of CAD in BB+ patients. While the reduction of reflex accelerations of the heart rate in our sample was both statistically and clinically significant, the impact of BBs on apnea-induced bradycardias remains unclear. Surprisingly, there was no evident potentiation of reflex HR decelerations by BBs (Figure3, right panel). Additionally, in multivariate model (ANCOVA) adjusted for the severity of desaturations and baseline heart rate, revealed in fact that slower periapneic heart rates, were present in the BB− patients vs. their BB+ counterparts (approx. 2-3 bpm net difference on average; Table 2). Some of these differences might be possibly attributed to concomitant cardiac disease in patients requiring BB therapy (Table 1). For example ischemic heart disease and congestive heart failure are conditions commonly associated with higher sympathetic tone, and thus higher HR. We were not able to perform a subanalysis of any mediatory role of incident CAD in the bradycardic response, because all patients with hypertension and concomitant CAD were treated with beta-blockers. Nevertheless, attenuation of the bradycardic response to apneas while on BBs was previously reported in one experimental study with acute propranolol administration [9]. Guilleminault et al. reported “slight lessening” of bradycardic responses to apneas during BB infusions (propranolol 5 mg, I.V.) in OSA-patients; however, no quantitative data from that study are available.
The clinical relevance of our findings is that beta-blockers are unlikely to significantly potentiate apnea-generated marked HR decelerations, while reflex increases in HR are attenuated. Our data may be partially explained by the fact that apnea-induced bradycardia is mediated by vagal rather than sympathetic mechanisms and that the powerful vagal responses to apnea obscure any bradycardic effect of cardiac sympathetic blockade by beta-antagonists. It was previously unclear whether the effectiveness of beta-blockers in blood pressure control [3] was partially associated with exacerbated bradyarrhythmic responses. Our study supports the concept that BBs are unlikely to clinically influence blood pressure control via excacerbated bradyarrhythmic responses to apneas. Both, the magnitude of apnea-induced HR decelerations as well as the prevalence of pauses and conduction abnormalities were similar in patients with ongoing BB therapy and their BB-naive counterparts. Furthermore, we can speculate that attenuation of apnea-generated cyclic variations of the heart rate can be one of the mechanisms by which beta-blockers may decrease risk of sudden cardiac death, particularly in patients with CAD.
Limitations of the study
Findings from our cross-sectional studies do not allow for a definitive determination of the nature of the relationships we observed. Confounding by indication of the beta-blocker could explain some of the observed results. Nevertheless, our results are derived from the real life use of beta-blockers in hypertensive patients with and without CAD, and are of clear clinical relevance given the widespread use of beta-blockers and the high prevalence of OSA.
In addition, bisoprolol and metoprolol constituted therapy in 78% of all BB-treated patients; therefore it remains to be determined whether our findings could be considered a drug-class effect. Another possible limitation of our observation is the inclusion of a relatively small group of patients experiencing long apneic episodes (eg. exceeding one minute as indicated in early reports [6, 10]). It has been suggested that it is the duration of apneas and the severity of desaturations [8], rather than the number of apneic and hypopneic episodes per hour (AHI) that increases the odds for incident bradyarrhythmic responses. Nevertheless, as the inclusion criteria were specified by AHI >=15 only, we assume that our sample is representative of obstructive sleep apnea in cardiovascular patients, and our conclusions may be applicable to the vast majority of the CVD-OSA population. However, the ECG signal in patients with documented prolonged apneas and/or severe desaturations, should be reviewed carefully.
Finally, the relatively small sample size and limited adjustments for co-morbid conditions should also be acknowledged making the study results hypothesis-generating.
Conclusion
We conclude, first that beta-blockers are not associated with potentiated apnea-induced heart rate decelerations. Second beta-blockers effectively blunt the heart rate increases resulting from reoccurring apneas during sleep in hypertensive patients with newly-diagnosed untreated moderate-to-severe obstructive sleep apnea.
Acknowledgements
We would like to thank Dr. Joanna Kanarek for her assistance in data collection.
Dr. Virend Somers is supported by NIH R01 HL65176. Drs. Jacek Wolf, Tomas Kara, Krzysztof Narkiewicz and Virend Somers are supported by European Regional Development Fund - Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123) and by European Union - project ICRCERA-HumanBridge (No. 316345). Dr. Kara is supported by Grant of IGA of Ministry of Health No. NT11401-5/2011.
Abbreviations and Acronyms
- AHI
apnea-hypopnea index
- BBs
beta-blockers
- BMI
body mass index
- BP
blood pressure
- CAD
coronary artery disease
- CHF
congestive heart failure
- CPAP
continuous positive airway pressure
- CVD
cardiovascular disease
- HR
heart rate
- OSA
obstructive sleep apnea
- REM
rapid eye movement sleep
- SpO2
mean blood oxygen saturation
- T90
cumulative time with blood oxygen saturation below 90%
- TIA
transient ischemic attack
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Conflicts of Interests: Drs. Virend Somers, Krzysztof Narkiewicz, and Jacek Wolf have served as consultants for ResMed. Dr Virend Somers has served as a consultant for Respicardia, and Neu Pro, Price Waterhouse Coopers, GSK, Sorin Inc., Philips, U-Health, Rhonda Gray, Medtronic. Mayo Foundation has received a gift from the Phillips-Respironics Foundation for the study of sleep apnea and cardiovascular disease. He is also involved in intellectual property development in sleep, obesity and cardiovascular disease with Mayo Health Solutions and their industry partners. All other authors report no relationships that could be construed as a conflict of interest; other authors have no potential conflicts of interests with any company whose products are discussed in this article. The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Weber MA, Bakris GL, Dahlöf B, Pitt B, Velazquez E, Gupte J, Lefkowitz M, Hester A, Shi V, Weir M, Kjeldsen S, Massie B, Nesbitt S, Ofili E, Jamerson K. Baseline characteristics in the Avoiding Cardiovascular events through Combination therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial: a hypertensive population at high cardiovascular risk. Blood Press. 2007;16:13–19. doi: 10.1080/08037050701217643. [DOI] [PubMed] [Google Scholar]
- 2.ONTARGET Investigators. Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 3.Kraiczi H, Hedner J, Peker Y, Grote L. Comparison of atenolol, amlodipine, enalapril, hydrochlorothiazide, and losartan for antihypertensive treatment in patients with obstructive sleep apnea. Am J RespirCrit Care Med. 2000;161:1423–1428. doi: 10.1164/ajrccm.161.5.9909024. [DOI] [PubMed] [Google Scholar]
- 4.Salo TM, Kantola I, Voipio-Pulkki LM, Pelttari L, Viikari JS. The effect of four different antihypertensive medications on cardiovascular regulation in hypertensive sleep apneic patients--assessment by spectral analysis of heart rate and blood pressure variability. Eur J Clin Pharmacol. 1999;55:191–198. doi: 10.1007/s002280050617. [DOI] [PubMed] [Google Scholar]
- 5.Shattock MJ, Tipton MJ. 'Autonomic conflict': a different way to die during cold water immersion?. J Physiol. 2012;590:3219–3230. doi: 10.1113/jphysiol.2012.229864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tilkian AG, Guilleminault C, Schroeder JS, Lehrman KL, Simmons FB, Dement WC. Sleep induced apnea syndrome—prevalence of cardiac arrhythmias and their reversal after tracheostomy. Am J Med. 1977;63:348–358. doi: 10.1016/0002-9343(77)90272-8. [DOI] [PubMed] [Google Scholar]
- 7.Miller WP. Cardiac arrhythmias and conduction disturbances in the sleep apnea syndrome. Prevalence and significance. Am J Med. 1982;73:317–321. doi: 10.1016/0002-9343(82)90716-1. [DOI] [PubMed] [Google Scholar]
- 8.Zwillich C, Devlin T, White D, Douglas N, Weil J, Martin R. Bradycardia during sleep apnea. Characteristics and mechanism. J Clin Invest. 1982;69:1286–1292. doi: 10.1172/JCI110568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guilleminault C, Connolly S, Winkle R, Melvin K, Tilkian A. Cyclical variation of the heart rate in sleep apnoea syndrome. Mechanisms, and usefulness of 24 h electrocardiography as a screening technique. Lancet. 1984;21:126–31. doi: 10.1016/s0140-6736(84)90062-x. [DOI] [PubMed] [Google Scholar]
- 10.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490–494. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 11.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, Sahadevan J, Redline S. Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franklin KA, Nilsson JB, Sahlin C, Näslund U. Sleep apnoea and nocturnal angina. Lancet. 1995;345:1085–1087. doi: 10.1016/s0140-6736(95)90820-x. [DOI] [PubMed] [Google Scholar]
- 13.Kuniyoshi FH, Garcia-Touchard A, Gami AS, Romero-Corral A, van der Walt C, Pusalavidyasagar S, Kara T, Caples SM, Pressman GS, Vasquez EC, Lopez-Jimenez F, Somers VK. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52:343–346. doi: 10.1016/j.jacc.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iber C, Ancoli-Israel S, Chesson AL, Jr., Quan SF, for the American Academy of Sleep Medicine . The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. American Academy of Sleep Medicine; Westchester, IL: 2007. [Google Scholar]
- 15.Junichiro Hayano Eiichi Watanabe, Yuji Saito Fumihiko Sasaki, Keisaku Fujimoto Tetsuo Nomiyama, Kiyohiro Kawai Itsuo Kodama. Hiroki Sakakibara. Screening for Obstructive Sleep Apnea by Cyclic Variation of Heart Rate. Circ Arrhythm Electrophysiol. 2011;4:64–72. doi: 10.1161/CIRCEP.110.958009. [DOI] [PubMed] [Google Scholar]
- 16.Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, Shamsuzzaman AS, Somers VK. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–2594. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 17.Wozniak DR, Lasserson TJ, Smith I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2014 Jan 8; doi: 10.1002/14651858.CD007736.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Poulet C, Veale D, Arnol N, Lévy P, Pepin JL, Tyrrell J. Psychological variables as predictors of adherence to treatment by continuous positive airway pressure. Sleep Medicine. 2009;10:993–999. doi: 10.1016/j.sleep.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Bolling SM. Encouraging CPAP Adherence: It Is Everyone’s Job. Respir Care. 2010;55:1230–1236. [PubMed] [Google Scholar]
- 20.Mayer J, Weichler U, Herres-Mayer B, Schneider H, Marx U, Peter JH. Influence of metoprolol and cilazapril on blood pressure and on sleep apnea activity. J Cardiovasc Pharmacol. 1990;16:952–961. doi: 10.1097/00005344-199012000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Monahan K, Storfer-Isser A, Mehra R, Shahar E, Mittleman M, Rottman J, Punjabi N, Sanders M, Quan SF, Resnick H, Redline S. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol. 2009;54:1797–1804. doi: 10.1016/j.jacc.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirby DA, Pinto JM, Weiss JW, Garpestad E, Zinkovska S. Effects of beta adrenergic receptor blockade on hemodynamic changes associated with obstructive sleep apnea. Physiol Behav. 1995;58:919–923. doi: 10.1016/0031-9384(95)00150-h. [DOI] [PubMed] [Google Scholar]