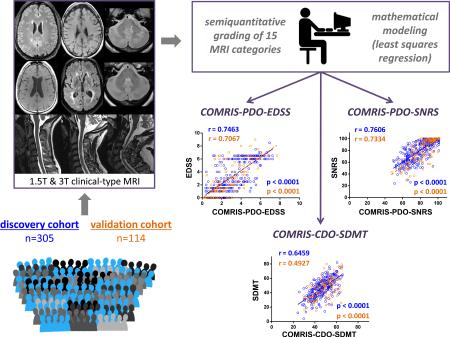
Abstract
Understanding genotype-phenotype relationships or development/validation of biomarkers requires large multicenter cohorts integrated by universal quantification of crucial phenotypical traits, such as central nervous system (CNS) tissue destruction. We hypothesized that mathematical modeling-guided combination of biologically meaningful, semiquantitative MRI elements characterized by high signal-to-noise ratio will provide such reliable, universal tool for measuring CNS tissue destruction. We retrospectively graded 15 elements in MRI scans performed in 419 untreated subjects with or without neurological diseases, while being blinded to their prospectively acquired clinical scores. We then used 305 subjects for disability-guided mathematical modeling to select and combine MRI elements that had non-redundant contributions to clinical disability, resulting in Combinatorial MRI Scale (COMRIS). We validated our model on the remaining 114 independent subjects. COMRIS requires 5-10 minutes per scan on average to compute and demonstrates highly significant (p<0.0001) and validation-consistent Spearman correlation coefficients (0.75, 0.76, and 0.65) for the expanded disability status scale (EDSS), Scripps neurological rating scale (SNRS), and symbol digit modality test (SDMT) measures of neurological disability, respectively. Because COMRIS is not greatly influenced by MRI scanners or protocols and can be computed even in the presence of some motion artifacts, it does not require censoring out patients and it provides comparable results across different cohorts. As such, it represents a broadly available clinical and research tool that can facilitate multicenter research studies and comparative analyses across patient cohorts and research projects.
Keywords: multiple sclerosis, MRI, clinical disability
Graphical Abstract
1. Introduction
Translational research on diseases of the central nervous system (CNS) would be greatly facilitated by the development of a biomarker for CNS tissue destruction that can be utilized across diverse research groups. For example, while disease status itself was sufficient for integrating multinational cohorts in genome-wide association studies, genotype-phenotype analyses or predictive biomarker studies, which also require thousands of patients, cannot be performed without universal measures of disease severity. Clinical scales have played a unifying role in multicenter clinical trials; however, they are not ideal for aforementioned translational research applications for several reasons: 1. Each scale measures only some aspect of clinical disability; 2. Multiple clinical scales are rarely documented in broad clinical practice; and, most importantly, 3. Clinical disability is greatly influenced by the localization of a lesion; so a single strategically located lesion (e.g. in the internal capsule) can have devastating clinical outcome, while many lesions, equally destructive, when located in subcortical white matter may have little impact on disability. In contrast to localization-dependency of clinical outcomes, a biomarker of CNS tissue destruction should reflect damage to CNS cells irrespective of their localization. Consequently, this undesired feature of clinical scales makes alternative measures of CNS tissue destruction, especially those obtained by magnetic resonance imaging (MRI) highly attractive. Indeed, MRI of the brain is routinely performed by neurologists caring for patients with chronic CNS diseases such as multiple sclerosis (MS), making quantitative MRI (qMRI) measures of CNS tissue destruction, such as brain atrophy, favorite candidate(s). Generation of qMRI data of high quality is time- and skill-demanding process that often requires filtering out considerable proportion of scans due to poor technical quality. Additionally, qMRI data are critically influenced by hardware, sequence parameters and post-processing methods. Consequently, data generated by different groups are not readily comparable.1, 2
We hypothesized that it may be possible to devise a more universal (i.e. applicable across different hardware/sequence combinations), reproducible and clinically relevant scale of CNS tissue destruction based on disability-guided combination of multiple semi-quantitative assessments of conventional clinical brain MRIs. We report the construction of such an MRI scale that we named COMRIS (Combinatorial MRI Scale) and its optimization/validation process.
2. Material and Methods
2.1 Participants
The study was approved by the National Institutes of Health Institutional Review Board and all participants provided written informed consent. Two patient cohorts (Supplemental Table 1) were prospectively acquired between 5/2007 and 9/2014. Diagnostic work-up included MRI of the brain and upper cervical spinal cord (SC). The diagnoses of relapsing-remitting MS (RRMS), primary progressive MS (PPMS) and secondary progressive MS (SPMS) were based on published diagnostic criteria.3 Remaining subjects were classified as either other inflammatory neurological disorders (OIND) or non-inflammatory neurological disorders (NIND) based on the evidence of intrathecal inflammation. Subjects were not treated by disease-modifying therapies.
2.2 Clinical scales
The following clinical scales were prospectively collected. The expanded disability status scale (EDSS),4 which represents the “gold standard” in MS. The more universal Scripps neurological rating scale (SNRS),5 which offers a greater range of scores (100 compared to 20 for EDSS) with more linear behavior. Symbol digit modalities test (SDMT), which has been proposed as a reliable scale of cognitive impairment in MS patients.6
2.3 MRI acquisition
MRIs were performed on 1.5T and 3T Signa units (General Electric, Milwaukee, WI) and 3T Skyra (Siemens, Malvern PA) equipped with standard clinical head and spinal cord imaging coils. MRI pulse sequences used for grading included FLAIR, PD/T2 weighted images, pre- and post-contrast (gadopentetate dimeglumine at 0.1 mmol/kg) T1-weighted image for brain scans, T1-weighted scan in the sagittal plane, T2- weighted scan in both sagittal and axial planes, and a short-tau inversion recovery scan in the sagittal plane for the cervical SC. Sequence details are in the Supplemental Methods.
2.4 Grading of MRI scans
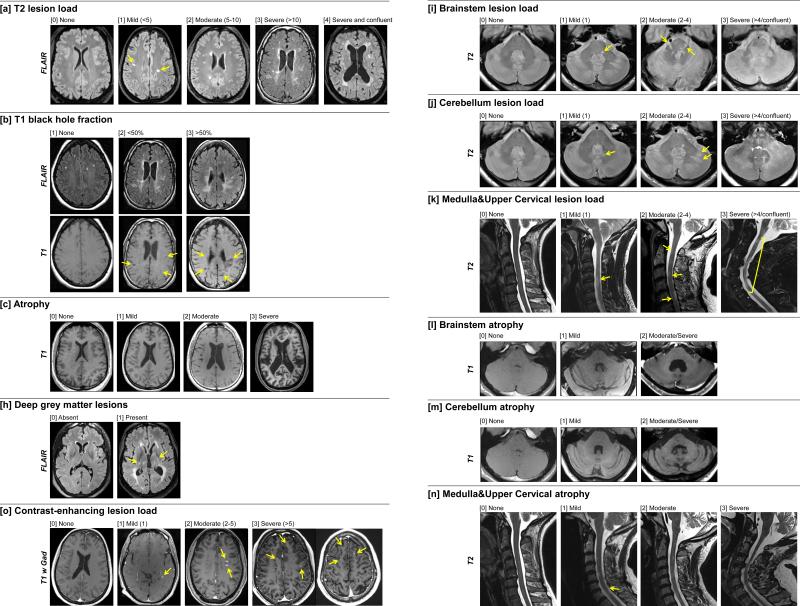
Scoring of telencephalon focused on T2 lesion load (T2LL; [a]), T1 black hole fraction (BHFr; [b]; a proportion of T2 lesions that have appearance of black holes on post-contrast T1WI), cerebral atrophy [c] and qualitative identification of periventricular (PV) lesions [d], juxtacortical/cortical (JC) lesions [e], involvement of corpus callosum (CC) [f], predominance of deep white matter (WM) lesions [g], and presence of deep gray matter (GM) lesions [h]. Evaluation of infratentorium comprised grading of lesion load (LL) and level of atrophy for brainstem [i,l], cerebellum [j,m], and medulla & upper cervical SC [k,n]. Contrast-enhancing lesions (CELs; [o]) were quantified in reference to pre-contrast T1- and T2-weighted scans (more details in Fig. 1 legend).
Figure 1. MRI grading procedure.
Representative MRI images demonstrate different grades for fifteen MRI variables (a – o).
T2LL [a] was defined as number of lesions (larger than 3mm in diameter) showing high signal on T2WI or FLAIR images in the supratentorial brain. The number of T2LL was categorized as: 0 = None, 1 = 1 to 4 lesions, 2 = 5 to 10 lesions, 3 = more than 10 lesions, and 4 = more than 10 and confluent lesions.
BHFr [b] was defined as the percentage of T2 lesions that have appearance of black holes on T1WI (i.e. show comparable intensity as CSF). 1 = None (no black holes present), 2 = less than 50 % of lesions are black holes, and 3 = 50 % or more lesions are black holes.
To assess cerebral atrophy [c], we considered both ventricular size and width of cortical sulci, best appreciated on axial cuts through insular cortex or vertex. Presented examples focus on difference in ventricular size. 0 = None (No visible atrophy of ventricles or sulci), 1 = Mild (mild increase in ventricular size or visible atrophy, but often only focal increase in sulci width), 2 = Moderate (considerable enlargement of lateral ventricles, definite enlargement of the third ventricle or more wide-spread broadening of the sulci) and 3 = Severe (concomitant severe atrophy of ventricles and sulci that leads to diffuse reduction of visible brain tissue).
Presence of deep gray matter lesions (GML) [h] was evaluated in thalamus, lenticulate nuclei and caudate nuclei (both head of the cause and tail) by using T2WI/FLAIR as well as T1WI/MP-RAGE, although only FLAIR images are depicted. 0 = Absent, 1 = Present.
Contrast-enhancing lesions (CEL; [o]) were identified on post-contrast T1WI in reference to pre-contrast T1- and T2WI/FLAIR and quantified as 0 = None, 0.5 = 1 CEL, 0.75 = 2 to 5 CELs, 1 = more than 5 CELs
Evaluation of infratentorial lesion load for brainstem [i], cerebellum [j], and medulla & upper cervical spinal cord [k] were defined on T2WI/proton density images and T2WI/STIR images (for CS lesions). Lesions were verified on T1WI/MP-RAGE. 0 = None, 1 = 1 lesion, 2 = 2 to 4 lesions, 3 = more than 4 lesions or large/confluent lesions.
Level of atrophy of brainstem [l] and cerebellum [m], were evaluated on axial and sagittal of T1WI/MP-RAGE images. For brainstem rating: 0 = None, 1 = Mild (mild flattening of the pons with visible enlargement of 4th ventricle or widening of the interpeduncular cistern with mild flattening of cerebral peduncles), 2 = Moderate/Severe (severe atrophy of the mesencephalon with small cerebral peduncles and large interpeduncular cistern and definite flattening of pons with enlarged cisterna pontis). For cerebellar rating: 0 = None, 1 = Mild (mild widening of cerebellar sulci and narrowing of middle cerebellar peduncles), 2 = Moderate/Severe (clear widening of cerebellar sulci, prominent atrophy of 4th ventricle and middle cerebellar peduncles)
Atrophy of the medulla & upper cervical spinal cord [n] was evaluated on sagittal and axial T2WI and T1WI/MP-RAGE and rated as 0 = None, 1 = Mild (segmental atrophy at a single level) 2 = Moderate (mild diffuse or 1-3 focal atrophy segments visible by naked eye, but with > 50 % of thickness preserved compared to non-affected area), 3 = Severe (diffusely atrophied spinal cord or >3 focal atrophy segments or < 50 % of SC thickness preserved).
Abbreviations: FLAIR = fluid-attenuation inversion recovery, MPRAGE = Magnetization Prepared Rapid Gradient Echo, STIR = Short TI Inversion Recovery), T1WI = T1-weighted image, T2WI = T2-weighted image, T1 w Gad = T1-weighted image after IV injection of contrast agent, yellow arrows = examples of lesions or focal atrophy
2.5 Mathematical modeling
Optimization of the combinatorial scale was performed by the least square methodology described in detail in the Supplemental Methods.
2.6 Statistical analyses
For variables that did not follow the normal distribution, Box-Cox transformation was applied. To evaluate the association between different MRI/clinical measures and diagnostic groups, oneway analysis of variance (ANOVA) was performed, with Tukey's correction for pair-wise multiple comparisons, using SAS software version 9.2. The correlation between different variables was evaluated by Spearman correlation methods (GraphPad Prism 6). Due to multiple-comparison, we considered p<0.01 as statistically significant.
3. Results
3.1 Construction of COMRIS
First, we selected those aspects of MRI imaging that reflect CNS tissue destruction based on publication knowledge7-13 (Table 1). Because we expected that lesion location will have decisive influence on disability, we rated lesion load and atrophy separately for four structures that may differ in their involvement between individual patients: 1. Supratentorial brain; 2. Brainstem (pons, mesencephalon); 3. Cerebellum (including superior, middle and inferior cerebellar peduncles); and 4. Medulla and upper CS. In the supratentorial brain, we also rated BHFr as a measure of lesion-destructiveness7 and added qualitative description of the presence or absence of periventricular, juxtacortical, corpus callosum, deep WM and deep GM lesions. Next, for each selected MRI element, we devised semi-quantitative rating steps (with analogous numerical codes) that seemed logical (e.g. none, mild, moderate and severe atrophy) and time-efficient (i.e. for calculation of T2LL). Consequently, the quantification of T2LL focused on lower number of lesions (i.e. up to ten in the supratentorial brain and up to four in the remaining structures) with the goal to provide discriminatory value in patients in early stages of disease process,14 when atrophy is not yet visible with the naked eye.
Table 1.
Semi-quantitative rating scores of MRIs and coefficients for linear equations determined by mathematical modeling
| MRI category | verbal grade | numerical code | Mathematically-optimized COMRIS models (coefficient “weights” for linear equation) |
|||
|---|---|---|---|---|---|---|
| COMRIS-PDO-EDSS | COMRIS-PDO-SNRS | COMRIS-CDO-SDMT | ||||
| [a] | T2 lesion load (T2LL) | None | 0 | 0 | 0 | −1.94 |
| Mild (<5 small lesions) | 1 | |||||
| Moderate (5-10 lesions) | 2 | |||||
| Severe (>10 lesions) | 3 | |||||
| Severe and confluent | 4 | |||||
| [b] | T1 black hole fraction | None | 1 | 0 | 0 | 0 |
| <50% | 2 | |||||
| >50% | 3 | |||||
| [c] | Atrophy | None | 0 | 0.22 | −1.95 | −3.83 |
| Mild | 1 | |||||
| Moderate | 2 | |||||
| Severe | 3 | |||||
| [d] | Periventricular (PV) lesions | Absent | 0 | 0 | 0 | 0 |
| Present | 1 | |||||
| [e] | Juxtacortical/cortical (JC) lesions | Absent | 0 | 0 | 0 | 0 |
| Present | 1 | |||||
| [f] | Corpus Callosum (CC) involvement | Absent | 0 | 0 | 0 | 0 |
| Present | 1 | |||||
| [g] | Mostly deep white matter (WM) lesions | Absent | 0 | 0 | 0 | 0 |
| Present | 1 | |||||
| [h] | Deep grey matter (GM) lesions | Absent | 0 | 0 | 0 | −1.34 |
| Present | 1 | |||||
| [i] | Brainstem lesion load (LL) | None | 0 | 0.39 | −4.29 | −2.31 |
| Mild (1 lesion) | 1 | |||||
| Moderate (2-4 lesions) | 2 | |||||
| Severe (>4 lesions/confluent) | 3 | |||||
| [j] | Cerebellum lesion load | None | 0 | 0 | 0 | −2.18 |
| Mild (1 lesion) | 1 | |||||
| Moderate (2-4 lesions) | 2 | |||||
| Severe (>4 lesions/confluent) | 3 | |||||
| [k] | Medulla & Upper Cervical lesion load | None | 0 | 0.84 | −5.29 | 0 |
| Mild (1 lesion) | 1 | |||||
| Moderate (2-4 lesions) | 2 | |||||
| Severe (>4 lesions/confluent) | 3 | |||||
| [l] | Brainstem atrophy | None | 0 | 0 | 0 | 0 |
| Mild | 1 | |||||
| Moderate/Severe | 2 | |||||
| [m] | Cerebellum atrophy | None | 0 | 0.32 | −1.27 | 0 |
| Mild | 1 | |||||
| Moderate/Severe | 2 | |||||
| [n] | Medulla & Upper Cervical atrophy | None | 0 | 0.69 | −5.71 | 0 |
| Mild | 1 | |||||
| Moderate | 2 | |||||
| Severe | 3 | |||||
| [o] | Contrast-enhancing lesion load | None | 0 | 0 | 0 | 0 |
| Mild (1 small lesion) | 0.5 | |||||
| Moderate (1-5 lesions) | 0.75 | |||||
| Severe (>5 lesions) | 1 | |||||
| Age | 0.04 | −0.28 | −0.26 | |||
| Intersection at [0,y] | −0.54 | 107.29 | 71.08 | |||
The table summarizes grading scores (verbal and numerical) of 15 MRI scores (a-o) utilized for calculation of different COMRIS scores and coefficients for linear equation for variables selected by mathematical modeling using three different models (EDSS model, SNRS model and SDMT model). EDSS = expanded disability status scale, SNRS = Scripps neurological rating scale, SDMT = symbol digit modalities test, COMRIS-PDO-EDSS = combinatorial MRI scale – physical disability optimized by EDSS, COMRIS-PDO-SNRS= combinatorial MRI scale – physical disability optimized by SNRS, COMRIS-CDO-SDMT = combinatorial MRI scale – cognitive disability optimized by SDMT
We then retrospectively rated MRIs from 419 untreated subjects while being blinded to their prospectively acquired clinical scores. All MRI elements were found to positively correlate with disability (EDSS, SNRS, and SDMT), with the exception of CELs and qualitative description of predominance of deep WM lesions in supratentorial brain (Supplemental Fig. 1). Analysis of the rating steps demonstrated that we were not able to reproducibly differentiate subjects with moderate versus severe atrophy of the brainstem and cerebellum and therefore these two categories were collapsed into a single rating step (Moderate/Severe; Fig. 1).
3.2 Mathematical modeling
We employed mathematical modeling to select/validate those MRI “building steps” (i.e. MRI elements and their rating) of the combinatorial scale of CNS tissue destruction that are biologically meaningful and non-redundant. If selected MRI characteristics are capturing CNS tissue destructions, then mathematical models can “assemble” these non-redundant MRI elements into models that will have high correlations with clinical scales of physical (i.e. EDSS and SNRS) versus cognitive (i.e. SDMT) disability.
Indeed, the least-square methodology combined with leave-one-out-cross-validation revealed redundancy in MRI parameters; the mean square error in cross-validation was the lowest when only five to seven different variables comprised the model (Supplemental Fig. 2A). We therefore selected six variables per model.
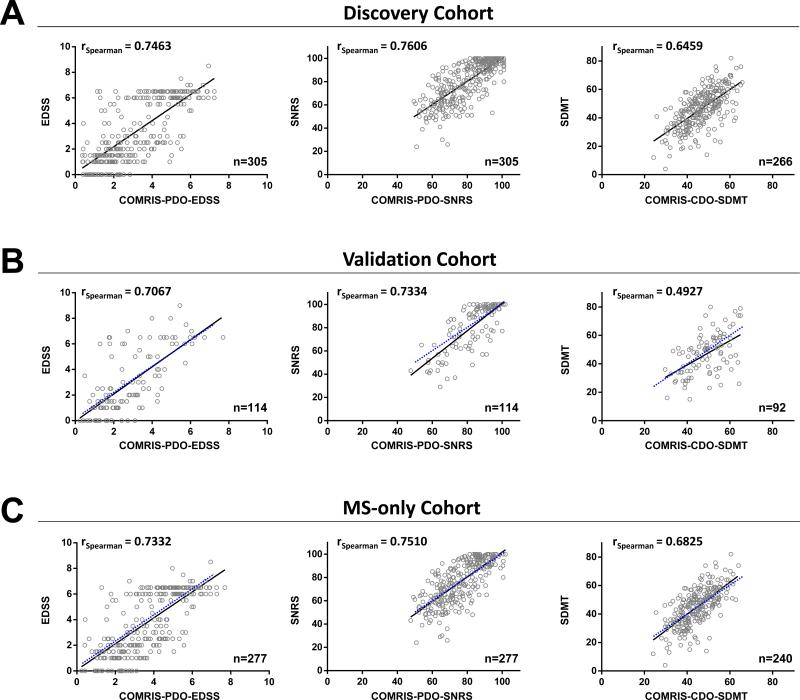
Intriguingly, age ranked as the top variable in every optimized model (Supplemental Fig. 2B), consistent with epidemiological observations that MS-related disability is strongly influenced by age.15 Out of five remaining critical variables in EDSS and SNRS models (which overlapped significantly) infratentorial MRI parameters were the strongest determinants of physical disability, representing four out of five top MRI elements, followed by brain atrophy (Table 1). The main difference between EDSS and SNRS resided in selecting cerebellum atrophy [m] in the EDSS model, whereas deep GM lesions [h] was selected in the SNRS model (Supplemental Fig. 2B). Because of the significant correlation between EDSS and SNRS (Spearman r=0.93, p<0.0001) and the historical preference for EDSS, we chose the six highest ranking variables selected by the EDSS model (Table 1) to calculate two physical disability scales: COMRISPDO-EDSS (Physical Disability Optimized by EDSS) and COMRIS-PDO-SNRS (Physical Disability Optimized by SNRS). The COMRIS-predicted EDSS and SNRS show statistically highly significant (p<0.0001) Spearman correlations (0.75 and 0.76) with clinically measured EDSS and SNRS, respectively (Fig. 2A)
Figure 2. Correlations of mathematically optimized COMRIS scales with clinical disability in different patient cohorts.
Correlation of COMRIS-PDO-EDSS with clinically measured EDSS, COMRIS-PDO-SNRS with clinically measured SNRS, and COMRIS-CDO-SDMT with clinically measured SDMT in the (A) discovery cohort, (B) validation cohort, and (C) MS-only cohort. (A-C) Black lines represent the best linear fit to plotted values, (B-C) blue dotted lines represent the best linear fit from the respective discovery cohort correlation, n = number of patients analyzed. Abbreviations: EDSS = expanded disability status scale, SNRS = Scripps neurological rating scale, SDMT = symbol digit modalities test, COMRIS-PDO-EDSS = combinatorial MRI scale – physical disability optimized by EDSS, COMRIS-PDO-SNRS= combinatorial MRI scale – physical disability optimized by SNRS, COMRIS-CDO-SDMT = combinatorial MRI scale – cognitive disability optimized by SDMT
In contrast, different MRI parameters were selected by the model optimized for SDMT, namely brain atrophy [c], supratentorial T2LL[a], brainstem LL [i], cerebellum LL [j], and supratentorial deep GM lesions [h] (Table 1; Supplemental Fig 2B). Thus, we named this model COMRISCDO-SDMT (Cognitive Disability Optimized by SDMT) and it shows significant (p<0.0001) Spearman correlation (0.65) with clinically measured SDMT (Fig. 2A).
3.3 Validation of disability-optimized COMRIS models
We validated physical- or cognitive-disability optimized versions of COMRIS in an independent cohort of 114 untreated subjects (MRI graded, but not utilized for mathematical modeling). All three MRI scores show highly statistically significant (p<0.0001) Spearman correlation coefficients (0.71, 0.73, and 0.49 for EDSS, SNRS, and SDMT, respectively) comparable to those calculated for the discovery cohort (Fig. 2B).
For the research groups only interested in MS, we assessed performance of COMRIS in an MS sub-cohort: we observed comparable, significant (p<0.0001) correlations of clinical measures with the predicted scores (Spearman correlations of 0.73, 0.75, and 0.68 for EDSS, SNRS, and SDMT, respectively) as in the discovery cohort (Fig. 2C).
To address potential influence of scanner/field/sequences, we re-analyzed correlations between COMRIS-predicted scores and clinical disability scales for the subgroup of subjects that were acquired on the 1.5 T scanner (3-5mm cuts, N=91) versus 3T scanners (1mm3 resolution, N=105). In both sub-cohorts, we validated statistical significance of correlations between COMRIS-predicted and clinical scales, even though the strength of correlations was higher for 3T cohort (Spearman coefficients between predicted and measured clinical scales for EDSS/SNRS/SDMT r=0.58/0.58/0.44 for 1.5T cohort and r=0.82/0.85/0.67 for 3T cohort).
Finally, to address reproducibility of the ratings, we calculated intra- and inter-rater variability for 20 selected subjects that were tested independently by each rater, at two separate time points. The mean intra-rater variability for all COMRIS-predicted clinical scales was less than 5% (0.5-4.8% for EDSS, 0.6-1.7% for SNRS and 0.5-1% for SDMT). The intra-rater variability was larger, but still below 10% for all COMRIS-predicted clinical scales (6.9% for EDSS, 5.0% for SNRS and 2.4% for SDMT).
3.4 Universal COMRIS scale for translational studies
Mathematical modeling confirmed intuitive understanding that utilized clinical scales reflect only some aspects of clinical disability. Furthermore, as postulated in the introduction (and validated by mathematical models), lesion location has decisive influence on physical versus cognitive disability. In contrast, a biomarker of CNS tissue destruction should not be influenced by lesion location.
Therefore, while mathematical modeling selected subgroup of tested MRI elements as biologically meaningful and non-redundant, these endorsed MRI “building blocks” need to be reassembled differently to reflect CNS tissue destruction globally. However, there is no “gold standard” measure of CNS tissue destruction that can be used for mathematical modeling. Consequently, we can device only a conceptual model based on current knowledge, with the understanding that such model inevitably represents only the initial step in subsequent reiterative process of experimentation based model refinement, according to the principles of systems biology.16 To emphasize that following model represents only this initial step, we’ll designate it COMRIS-CTD-v1 (Combinatorial MRI Scale of CNS Tissue Destruction version 1).
Volumetrically, supratentorial brain represents approximately 70% of the CNS, whereas brainstem, cerebellum and spinal cord the remaining 30%. Therefore, we need to re-assemble validated MRI elements into comparable absolute parts (in terms of maximal achievable score), which will then be multiplied by 0.7 for the supratentorial brain and 0.3 for the infratentorial brain. Based on the correlations with disability, atrophy of the supratentorial brain reflects more strongly CNS tissue destruction than T2LL (17, 18 and current study). Additionally T1 hypo-intensities with T1 values similar to CSF (i.e. black holes) have been validated in the context of MRI-pathological correlations as a marker of axonal damage,19 even though corresponding MRI element (i.e. BHFr) was not selected by current mathematical models. We consider two plausible explanations for this discrepancy: First, it is a possibility that a priori selection of the 50% cut-off is not optimal; second possibility, which we deem more relevant, is the fact that BHFr has to be considered within the context of the T2LL, because by definition it represents proportion of T2LL that has an appearance of black holes. Because mathematical models did not consider partial quadratic terms, the biologically most relevant combinatorial marker (i.e. T2LL*BHFr) was not analyzed. Finally, the last supratentorial MRI element selected as biologically-meaningful by modeling is the presence of deep GM lesions; and the models assigned this parameter similar importance (weight) as T2LL. One of the possible explanations for this observation is that presence of deep GM lesions is a surrogate of more wide-spread GM pathology because it's been demonstrated that cortical and deep GM lesions almost always coexist20. This has an important implication, because deep GM represents less than 5% of supratentorial brain volume, whereas all GM is close to 50%. Consequently, the assumption of surrogacy of deep GM lesions for the general GM pathology enhances the proportional “weight” of this contributing element 10-fold in comparison to its pure volumetric consideration, which is consistent with its weight assigned by the mathematical models.
Due to technical difficulties with obtaining reliable volumetric MRI data in the infratentorial CNS compartment, there is lesser knowledge about relationships between T2LL and atrophy in the remaining three sections. Mathematical models assigned comparable weights to T2LL and atrophy in cerebellum and medulla/CS, but actually did not select brainstem atrophy in any of the models. We believe that this reflects inability of the raters to reliably assess brainstem atrophy by naked eye, rather than the lack of its biological relevance.
Synthesizing all of this knowledge and conceptual reasoning, we developed the following formula for the COMRIS-CTD-v1:
COMRIS-CTD-v1 = 0.7*(supratentorial MRI elements; max score 35) + 0.3*(infratentorial MRI elements; max score 35)
COMRIS-CTD-v1 = 0.7*([a]*[b]+6*[c]+5*[h]) + 0.3*(2.5*([i]+[j]+[k]+[m]+[n]))
The “weights” (i.e. multiplication coefficients) in this formula are selected so that the atrophy of the supratentorial brain [c] gives higher absolute number (i.e. 6*3 = 18) than highest possible score obtained by supratentorial lesions (i.e. T2LL*BHFr = 4*3 = 12) and the deep GM lesions [h] contribute approximately ten times higher values than what would be expected from their volumetric proportion. In the infratentorial brain, all five elements selected as biologically-meaningful in the mathematical models are weighted equally and multiplication coefficient is simply selected to give equal maximal absolute value of 35 for the infratentorial CNS, as for the supratentorial brain.
While validation/refinement of COMRIS-CTD-v1 has to come from follow-up studies (e.g. by modeling based on CSF biomarkers of CNS tissue destruction), there are few logical assumptions that one can make about biomarker of CNS tissue destruction: the disease duration should have stronger influence on the biomarker than age and while it should correlate with disability, the correlations should be lower in comparison to disability-optimized COMRIS versions. We tested these expectations and observed that they were all fulfilled (Table 2).
Table 2.
The degree of correlation between COMRIS scales, age, disease duration and clinical disability.
| Age | Disease Duration | EDSS | SNRS | SDMT | ||
|---|---|---|---|---|---|---|
| COMRIS-CTD-v1 | rSpearman | 0.3735 | 0.4424 | 0.6042 | −0.6217 | −0.5356 |
| 95% CI | 0.2854 to 0.4554 | 0.3553 to 0.5218 | 0.5375 to 0.6633 | −0.6787 to −0.5572 | −0.6076 to −0.4549 | |
| COMRIS-PDO-EDSS | rSpearman | 0.5753 | 0.6034 | 0.7388 | −0.7534 | −0.4980 |
| 95% CI | 0.5053 to 0.6377 | 0.5334 to 0.6653 | 0.6904 to 0.7805 | −0.7931 to −0.703 | −0.5742 to −0.4132 | |
| COMRIS-PDO-SNRS | rSpearman | −0.5582 | −0.5982 | −0.7366 | 0.7537 | 0.5056 |
| 95% CI | −0.6225 to −0.4863 | −0.6607 to −0.5275 | −0.7787 to −0.6880 | 0.7076 to 0.7933 | 0.4217 to 0.5810 | |
| COMRIS-CDO-SDMT | rSpearman | −0.6148 | −0.4925 | −0.6206 | 0.6530 | 0.6022 |
| 95% CI | −0.6726 to −0.5495 | −0.5670 to −0.4101 | −0.6777 to −0.5560 | 0.5925 to 0.7061 | 0.5295 to 0.6661 | |
| Number of subjects | 419 | 382 | 419 | 419 | 358 | |
Summary of Spearman correlation coefficients (r) for age, disease duration and three clinical scales (EDSS, SNRS, SDMT). EDSS = expanded disability status scale, SNRS = Scripps neurological rating scale, SDMT = symbol digit modalities test, COMRIS-CTD-v1 = combinatorial MRI scale of CNS tissue destruction verion 1, COMRIS-PDO-EDSS = combinatorial MRI scale – physical disability optimized by EDSS, COMRIS-PDO-SNRS= combinatorial MRI scale – physical disability optimized by SNRS, COMRIS-CDO-SDMT = combinatorial MRI scale – cognitive disability optimized by SDMT
3.5 COMRIS-predicted clinical scales provide comparable differences between disease cohorts as identified by measured clinical scales
Because historical cohorts with available biological samples may not have matched clinical data (especially SNRS and SDMT) it is important to assess how well COMRIS models can reconstruct these missing clinical data from available MRI scans.
Therefore, we compared discriminatory power of COMRIS-predicted clinical scores versus measured clinical scores in detecting significant differences between diagnostic subgroups. We observed that COMRIS-predicted EDSS, SNRS and SDMT differentiated diagnostic groups with equivalent power as prospectively-acquired clinical scales (Fig. 3A; analogous results were observed for discovery and validations cohorts, so only combined analysis is presented).
Figure 3. COMRIS provides novel insight about diagnostic subgroups of neurological diseases.
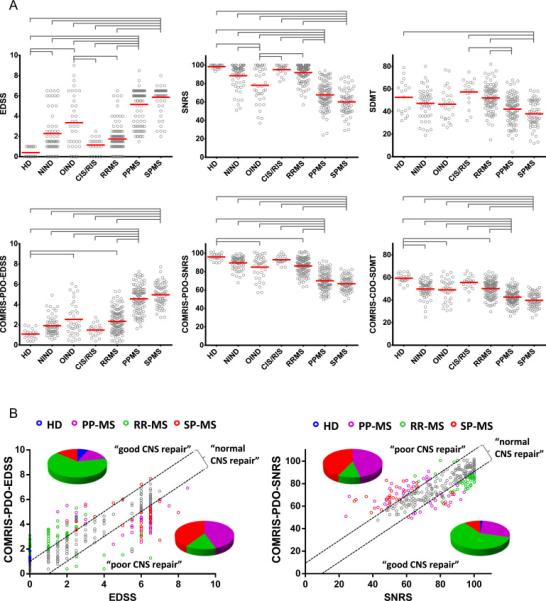
(A) Evaluation of clinical scores and COMRIS-predicted clinical scores (EDSS, SNRS, and SDMT) between diagnostic subgroups. Gray brackets highlight statistically significant differences between seven diagnostic subcategories with adjusted p<0.001. The data (gray circles) are depicted with means (red bars). (B) Correlation between clinically-measured and COMRIS-predicted disability scores (EDSS and SNRS) identifies a group of subjects characterized by lower clinical disability than predicted by the amount of CNS tissue destruction observed on MRI (“good CNS repair”) and a group of subjects with higher clinically measured disability than predicted by MRI (“poor CNS repair”) for both EDSS and SNRS. The black dashed lines identify subjects (grey circles) with “normal CNS repair” with predicted disability within 10% of clinically measured disability. Subjects with good and poor CNS repair are color-coded based on their diagnosis (blue – HD, magenta – PPMS, green – RRMS, red – SPMS), the pie-charts show distribution of diagnoses for subjects with poor or good CNS repair for both EDSS and SNRS. Abbreviations: HD = healthy donors, NIND = non-inflammatory neurological disorders, OIND = other inflammatory neurological disorder, CIS/RIS = clinically-/radiologically isolated syndrome, RRMS = relapsing-remitting multiple sclerosis, PPMS = primary progressive multiple sclerosis, SPMS = secondary progressive multiple sclerosis, EDSS = expanded disability status scale, SNRS = Scripps neurological rating scale, SDMT = symbol digit modalities test, COMRIS-PDO-EDSS = combinatorial MRI scale – physical disability optimized by EDSS, COMRIS-PDO-SNRS= combinatorial MRI scale – physical disability optimized by SNRS, COMRIS-CDO-SDMT = combinatorial MRI scale – cognitive disability optimized by SDMT
In a search for the parameters that may identify or forecast development of progressive MS, we observed an intriguing dichotomy between COMRIS-predicted and clinically-measured physical disability: the difference between COMRIS-projected and measured EDSS and SNRS scores were consistently higher in RRMS in comparison to progressive MS subjects (Fig. 3B). In other words, RRMS patients had generally lower disability in comparison to the amount of visible CNS tissue destruction on MRI, whereas progressive MS subjects had consistently higher disability in comparison to visible CNS tissue damage, suggesting that lack of compensatory (functional) recovery is the decisive factor that determines onset of progressive MS.
4. Discussion
Technological advances in biomedical research, such as genotyping and RNA sequencing, proteomics, lipidomics and metabolomics allow measurements of multimodality data in large patient cohorts. This produces “catalogue” of the elements that constitute a complex system, such as human body affected by MS.16 However, to reach predictive understanding of the system, systems biology needs to evolve from cataloguing system's parts to understanding relationships between them, as these are more important determinants of the system's behavior.21 In concrete terms, this means understanding how genotypes interact with environmental factors to mediate development of phenotypes or which molecular or cellular mechanisms contribute to the destruction of target organ. These questions cannot be answered using traditional reductionists research methods and instead require application of systems biology approaches to large datasets assembled through international consortia.
The presented study originated from the need to develop a universal measure of CNS tissue destruction that can integrate diverse historical cohorts with banked biological samples for these new types of translational studies. While MRI of the CNS tissue seemed to be the logical vehicle for this purpose, use of different scanners and sequences represented a formidable challenge. Based on our experience with MRI analyses in natural history cohorts7, 22 and in clinical trials of MS,23, 24 we recognized that semi-quantitative MRI measures, such as number of CELs or T2 lesions, have an excellent signal-to-noise ratio but limited correlation with clinical outcomes. On the other hand, qMRI measures suffer from considerable non-biological variability25 and are affected by hardware (scanner/field strength, coil), sequence parameters,26 and post-processing methods. At least part of qMRI variability is due to movement, which is inevitable even in the most cooperative subjects due to the long duration of the MRI in comparison to the small changes that qMRI metrics can discern. Even when research groups remove MRIs due to “poor technical quality”, quantitative changes of censored MRI data are reliable across several years but not in shorter intervals.27-32
As an alternative solution that mitigates the aforementioned problems, we considered development of a combinatorial scale based on thoughtful combination of several semi-quantitative MRI measures that are validated through mathematical modeling as biologically meaningful because they can be assembled into models that correlate highly with physical and cognitive disability.9, 33-35 We were actually impressed by the strength and high reproducibility of the developed models and how well the models selected those anatomical structures that underlie physical versus cognitive disability,36-38 without any pre-existing knowledge of biology. This validates the MRI elements selected by mathematical models as biologically meaningful. Our comparison of results between 1.5T and 3T cohorts indicates that while higher field strength and enhanced sequences increase performance of the COMRIS, results obtained from older 1.5T cohorts still remain valid; confirming our presupposition that semi-quantitative MRI scale will moderate problems related to unavoidable differences in the collection of MRI data between diverse cohorts. Finally, while the semi-quantitative nature of COMRIS leads to intra- and inter-rater differences, adherence to the provided rating guide (Fig. 1) assures that these technical errors are below 10%. The strength of independent validation (including the precision of regression slopes, Fig. 2B) on a cohort three times smaller than the modeling cohort, authenticates the robustness of COMRIS models and their MRI “building blocks”.
COMRIS-CTD-v1 is not a diagnostic scale; on the contrary – as a scale of CNS tissue destruction, it should be applicable to more diseases than just MS. While MS MRI studies7-13 guided selection of semi-quantitative elements, we saw no reason to apply limitations of thresholds used by MS diagnostic criteria.3, 39 Instead, to promote broad utilization, simplicity became important consideration. For example, quantification of supratentorial T2LL beyond ten lesions takes disproportionately more of the rater's effort in relation to benefit provided. In patients with supratentorial T2LL above ten, the magnitude of brain atrophy is a more important determinant of disability than presence of additional lesions, as confirmed by modeling.
The main limitation is a priori selection of MRI elements and their rating steps, leading to a possibility that different MRI markers or rating steps may provide a better performance. We expect that current publication will spur further developmental work from interested research groups, which may test a broader combination of MRI elements and potentially improve the scale, analogous to the improvements in MS diagnostic criteria.3 Nevertheless, we also believe that current version of COMRIS represents an excellent starting point for unifying diverse historical cohorts to aforementioned multicenter translational studies, based on the outstanding performance of COMRIS-based models in estimating clinical disability. Because any neurological scale is only an approximation of the true disability and most of the brain tissue is “clinically silent”, the correlation between MRI and clinical scales can never achieve rho=1. As the correlations between COMRIS and disability scales are comparable to those previously reported for qMRI measures,10, 34, 35, 40 including composite qMRI scales33, 41, 42, COMRIS predicts disability exceptionally well.
COMRIS supports the notion that multi-modality MRI imaging and/or development of composite models33, 41, 42, 43 that merge imaging parameters reflecting somewhat different biological processes explains higher proportion of disability variance than any single imaging modality. This notion is also evident from a large meta-analysis of MS clinical trials, when Sormani et al demonstrated that including both T2 lesion load and brain atrophy in mathematical model significantly strengthen correlation between drug-induced changes on MRI and disability.43 Therefore, the emerging question is whether application of analogous systematic modeling used for development of COMRIS to cohorts with fully quantitative MRI measures will lead to composite qMRI scale(s) that outperforms COMRIS in the ability to predict clinical and cognitive disability.
We expect that as a tool that can readily bridge historical patient cohorts to diverse translational research projects, COMRIS will facilitate multicenter studies, such as those being formed under the Progressive MS Alliance. In order to assist such collaborations, we provide a downloadable Excel file (Table S2) with the template of non-redundant MRI elements and macro that calculates all aspects of the COMRIS, including MRI-predicted disability scores.
5. Conclusions
Clinical MRI of the brain routinely performed in vast majority of patients with neurological symptoms represents a unique opportunity to develop a tool for bridging diverse cohorts of patients in multicenter projects. Using a disability-guided mathematical combination of semiquantitative assessments of brain MRI we developed and validated a universal, reproducible and clinically relevant scale of CNS tissue destruction that can be applied across different MRI scanners/coils/sequences. As such this scale is readily available to facilitate multicenter studies and research projects comparing historical patient cohorts.
Supplementary Material
Highlights.
Multicenter clinical studies require a unifying element to bridge patient cohorts
Different COMRIS models correlates highly both with physical and cognitive disability and reflect CNS tissue destruction
COMRIS limits hardware, sequence or post-processing influence on MRI
COMRIS is easy to calculate and ready to support diverse translational research projects
Acknowledgements
This work was supported by the intramural research program of the National Institute of Neurological Disorders and Stroke of the National Institutes of Health. We thank Govind Bhagavatheeshwaran, Alison Wichman, and Muktha Natrajan for critical review of the manuscript and clinical team members who did not fulfill co-authorship criteria (Jenifer Dwyer, Alison Wichman and Anne Mayfield) for expert patient care and support.
Abbreviations
- COMRIS-CDO-SDMT
combinatorial MRI scale – cognitive disability optimized by SDMT
- COMRIS-CTD-v1
combinatorial MRI scale of CNS tissue destruction version 1
- COMRIS-PDO-EDSS
combinatorial MRI scale – physical disability optimized by EDSS
- COMRIS-PDO-SNRS
combinatorial MRI scale – physical disability optimized by SNRS
- CIS
clinically isolated syndrome
- EDSS
expanded disability status scale
- HD
healthy donor
- MS
multiple sclerosis
- NIND
non-inflammatory neurological disorders
- OIND
other inflammatory neurological disorders
- PPMS
primary progressive multiple sclerosis
- RIS
radiologically isolated syndrome
- RRMS
relapsing-remitting multiple sclerosis
- SD
standard deviation
- SDMT
symbol digit modalities test
- SNRS
Scripps neurological rating scale
- SPMS
secondary progressive multiple sclerosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contribution
BB – conceptual study design
PK, MK, BB – study design, data analysis, data interpretation, figures, writing
RW, TG – mathematical modeling, writing
TW – statistical analysis
IC, JO, KF, JC, BB – collection of clinical data
Supplemental information: Supplemental Methods, Supplemental Figure 1, Supplemental Figure 2, Supplemental Table 1, Supplemental Table 2
Disclosure
The authors report no disclosures relevant to the manuscript.
Conflict of interest disclosure
The authors report no disclosures relevant to the manuscript.
References
- 1.Chard DT, Griffin CM, Parker GJ, Kapoor R, Thompson AJ, Miller DH. Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain. 2002 Feb;125(Pt 2):327–37. doi: 10.1093/brain/awf025. [DOI] [PubMed] [Google Scholar]
- 2.Jones BC, Nair G, Shea CD, Crainiceanu CM, Cortese IC, Reich DS. Quantification of multiple-sclerosis-related brain atrophy in two heterogeneous MRI datasets using mixed-effects modeling. NeuroImage Clinical. 2013;3:171–9. doi: 10.1016/j.nicl.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of neurology. 2011 Feb;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 5.Sipe JC, Knobler RL, Braheny SL, Rice GP, Panitch HS, Oldstone MB. A neurologic rating scale (NRS) for use in multiple sclerosis. Neurology. 1984 Oct;34(10):1368–72. doi: 10.1212/wnl.34.10.1368. [DOI] [PubMed] [Google Scholar]
- 6.Parmenter BA, Weinstock-Guttman B, Garg N, Munschauer F, Benedict RH. Screening for cognitive impairment in multiple sclerosis using the Symbol digit Modalities Test. Multiple sclerosis. 2007 Jan;13(1):52–7. doi: 10.1177/1352458506070750. [DOI] [PubMed] [Google Scholar]
- 7.Bielekova B, Kadom N, Fisher E, et al. MRI as a marker for disease heterogeneity in multiple sclerosis. Neurology. 2005 Oct 11;65(7):1071–6. doi: 10.1212/01.wnl.0000178984.30534.f9. [DOI] [PubMed] [Google Scholar]
- 8.Bieniek M, Altmann DR, Davies GR, et al. Cord atrophy separates early primary progressive and relapsing remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2006 Sep;77(9):1036–9. doi: 10.1136/jnnp.2006.094748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filippi M, Paty DW, Kappos L, et al. Correlations between changes in disability and T2-weighted brain MRI activity in multiple sclerosis: a follow-up study. Neurology. 1995 Feb;45(2):255–60. doi: 10.1212/wnl.45.2.255. [DOI] [PubMed] [Google Scholar]
- 10.Fisher E, Rudick RA, Cutter G, et al. Relationship between brain atrophy and disability: an 8-year follow-up study of multiple sclerosis patients. Mult Scler. 2000 Dec;6(6):373–7. doi: 10.1177/135245850000600602. [DOI] [PubMed] [Google Scholar]
- 11.Fisniku LK, Brex PA, Altmann DR, et al. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain. 2008 Mar;131(Pt 3):808–17. doi: 10.1093/brain/awm329. [DOI] [PubMed] [Google Scholar]
- 12.Truyen L, van Waesberghe JH, van Walderveen MA, et al. Accumulation of hypointense lesions (“black holes”) on T1 spin-echo MRI correlates with disease progression in multiple sclerosis. Neurology. 1996 Dec;47(6):1469–76. doi: 10.1212/wnl.47.6.1469. [DOI] [PubMed] [Google Scholar]
- 13.Zivadinov R, Bakshi R. Role of MRI in multiple sclerosis II: brain and spinal cord atrophy. Front Biosci. 2004 Jan 1;9:647–64. doi: 10.2741/1262. [DOI] [PubMed] [Google Scholar]
- 14.Brex PA, Ciccarelli O, O'Riordan JI, Sailer M, Thompson AJ, Miller DH. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med. 2002 Jan 17;346(3):158–64. doi: 10.1056/NEJMoa011341. [DOI] [PubMed] [Google Scholar]
- 15.Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006 Mar;129(Pt 3):595–605. doi: 10.1093/brain/awh714. [DOI] [PubMed] [Google Scholar]
- 16.Bielekova B, Vodovotz Y, An G, Hallenbeck J. How implementation of systems biology into clinical trials accelerates understanding of diseases. Front Neurol. 2014;5:102. doi: 10.3389/fneur.2014.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudick RA, Fisher E, Lee JC, Duda JT, Simon J. Brain atrophy in relapsing multiple sclerosis: relationship to relapses, EDSS, and treatment with interferon beta-1a. Mult Scler. 2000;6(6):365–72. doi: 10.1177/135245850000600601. [DOI] [PubMed] [Google Scholar]
- 18.Barkhof F. MRI in multiple sclerosis: correlation with expanded disability status scale (EDSS). Mult Scler. 1999 Aug;5(4):283–6. doi: 10.1177/135245859900500415. [DOI] [PubMed] [Google Scholar]
- 19.Barkhof F, Karas GB, van Walderveen MA. T1 hypointensities and axonal loss. Neuroimaging Clin N Am. 2000 Nov;10(4):739–52. [PubMed] [Google Scholar]
- 20.Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005 Nov;128(Pt 11):2705–12. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 21.Mesarovic MD, Sreenath SN, Keene JD. Search for organising principles: understanding in systems biology. Syst Biol (Stevenage) 2004 Jun;1(1):19–27. doi: 10.1049/sb:20045010. [DOI] [PubMed] [Google Scholar]
- 22.Markovic-Plese S, Bielekova B, Kadom N, et al. Longitudinal MRI study: the effects of azathioprine in MS patients refractory to interferon beta-1b. Neurology. 2003 Jun 10;60(11):1849–51. doi: 10.1212/01.wnl.0000071218.34009.af. [DOI] [PubMed] [Google Scholar]
- 23.Bielekova B, Howard T, Packer AN, et al. Effect of anti-CD25 antibody daclizumab in the inhibition of inflammation and stabilization of disease progression in multiple sclerosis. Arch Neurol. 2009 Apr;66(4):483–9. doi: 10.1001/archneurol.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borges IT, Shea CD, Ohayon J, et al. The effect of daclizumab on brain atrophy in relapsing-remitting multiple sclerosis. Multiple sclerosis and related disorders. 2013 Apr 1;2(2):133–40. doi: 10.1016/j.msard.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cover KS, van Schijndel RA, van Dijk BW, et al. Assessing the reproducibility of the SienaX and Siena brain atrophy measures using the ADNI back-to-back MP-RAGE MRI scans. Psychiatry research. 2011 Sep 30;193(3):182–90. doi: 10.1016/j.pscychresns.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Neacsu V, Jasperse B, Korteweg T, et al. Agreement between different input image types in brain atrophy measurement in multiple sclerosis using SIENAX and SIENA. J Magn Reson Imaging. 2008 Sep;28(3):559–65. doi: 10.1002/jmri.21501. [DOI] [PubMed] [Google Scholar]
- 27.Bakshi R, Neema M, Healy BC, et al. Predicting clinical progression in multiple sclerosis with the magnetic resonance disease severity scale. Arch Neurol. 2008 Nov;65(11):1449–53. doi: 10.1001/archneur.65.11.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ingle GT, Stevenson VL, Miller DH, Thompson AJ. Primary progressive multiple sclerosis: a 5-year clinical and MR study. Brain. 2003 Nov;126(Pt 11):2528–36. doi: 10.1093/brain/awg261. [DOI] [PubMed] [Google Scholar]
- 29.Kappos L, Moeri D, Radue EW, et al. Predictive value of gadolinium-enhanced magnetic resonance imaging for relapse rate and changes in disability or impairment in multiple sclerosis: a meta-analysis. Gadolinium MRI Meta-analysis Group. Lancet. 1999 Mar 20;353(9157):964–9. doi: 10.1016/s0140-6736(98)03053-0. [DOI] [PubMed] [Google Scholar]
- 30.Khaleeli Z, Ciccarelli O, Manfredonia F, et al. Predicting progression in primary progressive multiple sclerosis: a 10-year multicenter study. Annals of neurology. 2008 Jun;63(6):790–3. doi: 10.1002/ana.21375. [DOI] [PubMed] [Google Scholar]
- 31.Rovaris M, Judica E, Gallo A, et al. Grey matter damage predicts the evolution of primary progressive multiple sclerosis at 5 years. Brain. 2006 Oct;129(Pt 10):2628–34. doi: 10.1093/brain/awl222. [DOI] [PubMed] [Google Scholar]
- 32.Stevenson VL, Ingle GT, Miller DH, Thompson AJ. Magnetic resonance imaging predictors of disability in primary progressive multiple sclerosis: a 5-year study. Mult Scler. 2004 Aug;10(4):398–401. doi: 10.1191/1352458504ms1055oa. [DOI] [PubMed] [Google Scholar]
- 33.Mainero C, De Stefano N, Iannucci G, et al. Correlates of MS disability assessed in vivo using aggregates of MR quantities. Neurology. 2001 May 22;56(10):1331–4. doi: 10.1212/wnl.56.10.1331. [DOI] [PubMed] [Google Scholar]
- 34.Riahi F, Zijdenbos A, Narayanan S, et al. Improved correlation between scores on the expanded disability status scale and cerebral lesion load in relapsing-remitting multiple sclerosis. Results of the application of new imaging methods. Brain : a journal of neurology. 1998 Jul;121:1305–12. doi: 10.1093/brain/121.7.1305. [DOI] [PubMed] [Google Scholar]
- 35.Sailer M, Losseff NA, Wang L, Gawne-Cain ML, Thompson AJ, Miller DH. T1 lesion load and cerebral atrophy as a marker for clinical progression in patients with multiple sclerosis. A prospective 18 months follow-up study. Eur J Neurol. 2001 Jan;8(1):37–42. doi: 10.1046/j.1468-1331.2001.00147.x. [DOI] [PubMed] [Google Scholar]
- 36.Kearney H, Rocca MA, Valsasina P, et al. Magnetic resonance imaging correlates of physical disability in relapse onset multiple sclerosis of long disease duration. Multiple sclerosis. 2014 Jan;20(1):72–80. doi: 10.1177/1352458513492245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazeron RHC, Boringa JB, Schouten M, et al. Brain atrophy and lesion load as explaining parameters for cognitive impairment in multiple sclerosis. Multiple sclerosis. 2005 Oct;11(5):524–31. doi: 10.1191/1352458505ms1201oa. [DOI] [PubMed] [Google Scholar]
- 38.Preziosa P, Rocca MA, Mesaros S, et al. Relationship between damage to the cerebellar peduncles and clinical disability in multiple sclerosis. Radiology. 2014 Jun;271(3):822–30. doi: 10.1148/radiol.13132142. [DOI] [PubMed] [Google Scholar]
- 39.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of neurology. 2001 Jul;50(1):121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 40.Nijeholt GJ, van Walderveen MA, Castelijns JA, et al. Brain and spinal cord abnormalities in multiple sclerosis. Correlation between MRI parameters, clinical subtypes and symptoms. Brain : a journal of neurology. 1998 Apr;121:687–97. doi: 10.1093/brain/121.4.687. [DOI] [PubMed] [Google Scholar]
- 41.Bakshi R, Neema M, Tauhid S, et al. An expanded composite scale of MRI-defined disease severity in multiple sclerosis: MRDSS2. Neuroreport. 2014 Aug 5;25:1156–61. doi: 10.1097/WNR.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moodie J, Healy BC, Buckle GJ, et al. Magnetic resonance disease severity scale (MRDSS) for patients with multiple sclerosis: a longitudinal study. J Neurol Sci. 2012 Apr 15;315(1-2):49–54. doi: 10.1016/j.jns.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sormani MP, Arnold DL, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann Neurol. 2014 Jan;75(1):43–9. doi: 10.1002/ana.24018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.