Abstract
AIM: To study the expression of early growth response gene-1 (Egr-1 gene) and Bcl-X/L protein and its relationship with the cell apoptosis in human esophageal carcinoma(EC) and precancerous lesions.
METHODS: In situ hybridization(ISH), immunohistochemistry (IHC) and TUNEL method were used respectively to detect Egr-1mRNA,Egr-1 protein, apoptosis related-protein Bcl-X/L and cell apoptosis in situ from 66 cases of esophageal squamous cell carcinoma and their upper cut edge and paracancerous mucosa.
RESULTS: Egr-1 gene in situ hybridization, Bcl-X/L immunohistochemistry positive products were located in the cytoplasm, while Egr-1 immunohistochemistry and TUNEL positive signal were located in the nuclei. The apoptosis index(AI) and the frequency of apoptosis occurrence were increased gradually from precancerous lesion to cancer (P < 0.01) and the expression of Egr-1mRNA and Egr-1 protein in dysplasia was the highest among all specimens (P < 0.01). The AI of Egr-1 positive cancer tissues was much higher than that of Egr-1 negative cancer tissues (P < 0.01), while the AI of Bcl-X/L positive cancer tissues was much lower than that of Bcl-X/L negative cancer tissues (P < 0.01). The AI and Egr-1 expression were not correlated with invasiveness and lymphatic metastasis in EC.
CONCLUSION: Cell apoptosis was present through esophageal carcinogenesis. The expression of Egr-1 mRNA and Egr-1 protein were high in precancerous lesion of esophagus. The AI was increased significantly in Egr-1 positive squamous cell carcinoma. Egr-1 might promote apoptotic effect. Egr-1 expression and cell apoptosis may have an important biological significance in esophageal carcinogenesis.
INTRODUCTION
Esophageal carcinoma is one of the most common malignant tumors in China[1-5]. Its pathogenesis and development are closely related to the expression of some proto-oncogenes and their products and apoptosis of the cancer cells[6-19]. The expression of oncogenes and tumor suppressor genes in esophageal carcinoma has been studied; the relationship between Egr-1 and carcinoma has been reported as well[20]. Our previous studies have indicated that Egr-1 gene inhibited the growth of Eca109 after the exogenous introduction of Egr-1 gene[21]. But the relationship between expression of Egr-1 and cell apoptosis in esophageal carcinoma is not well understood so far. In this paper, we have performed the examination of Egr-1mRNA and Egr-1protein expression, apoptosis related-protein Bcl-X/L expression and cell apoptosis of the carcinoma tissue, upper cut edge mucosa and paracancerous lesions from 66 cases esophageal carcinoma using In situ hybridization, immunohistochemistry and terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-digoxigenin nick end labeling method (TUNEL) respectively. The purpose is to understand the correlation of Egr-1 expression and cell apoptosis in esophageal carcinogenesis.
MATERIALS AND METHODS
Sample collecting and processing
Fresh esophagus specimens after operation including esophageal mucosa at the upper cut edge, cancer tissue and mucosa just adjacent to the tumor mass were taken from 66 patients of esophageal carcinomas who had not received accepted chemotherapy or radiotherapy before operation. All specimens were from Department of Pathology Shantou University Medical College, from January to December, 2000. The specimens were fixed in 40 mL/L neutral formalin; the slides were applied by 1/1000 diethyl pyrocarbonate (DEPC, Sigma Chemical Co, USA), paraffin embedded, cut in 4 μm, HE stained.
Histopathology analysis
Histopathological diagnosis of esophageal para-cancer epithelia was made according to Liu et al’s criteria including 39 cases of normal epithelium, 52 cases of simple hyperplasia and 41 cases of dysplasia. 66 cases of esophageal carcinoma were diagnosed using WHO histological tumor classification including 2 cases of carcinoma in situ, 18 cases of grade I squamous cell carcinoma, 33 cases of grade II and 13 cases of grade III. 22 cases had invaded the superficial muscular layer, and others had invaded the serosa. 36 of 66 cases had lymphatic metastasis.
In situ hybridization
Eukaryotic expression vector of PCMV-Egr-1 plasmid was donated by Dr RP Huang (Molecular Medicine, Northwest Hospital, WA, USA). The plasmid was confirmed by amplification, purification and endonuclease cutting, then retrieved the DNA. The expression of Egr-1 was detected by digoxigenin-labeled gene probe from a commercial kit (Boster company, China) according to the manufacturer’s instructions. Sections were dewaxed in xylene, then into graduated ethanol, and then into 30 mL/L hydrogen peroxide methanol for 30 min. Proteinase K at 37 °C for 20 min in 20 µg/mL and then fixed in 40 g/L PFA for 10 min in sequence. 90% ethanol 5 min at -20 °C precold, digoxigenin-labeled cDNA probe (1:40) were denatured in hybridization buffer at 95-100 °C for 10 min, then -20 °C for 3 min, added on tissues and cover slipped at 42 °C overnight. Sections were washed with SSC and then mouse anti-digoxigenin antibody, biotinylated goat anti-mouse and then streptavidin-biotin complex (SABC) for 30 min, finally, with3. 3’-diaminobenzidine (DAB) visualization. Human breast tissue and the mouse brain tissue were used as the positive control. Incubation solution instead of the probe and sections digested by RNase (10 µg/mL) before Egr-1 detection were designed for the negative control.
Immunohistochemistry
Egr-1 and Bcl-XL were analyzed by using Egr-1(588): cat#SC-110 rabbit polyclonal antiserum (1:200,Santa Cruz Biot Co, USA) and Bcl-XL(H-62): sc-7195 polyclonal antibody (ready to use) with the SABC method according to the manufacturer’s instructions (Boster company, China), and finally DAB visualization. The human breast tissue and the esophageal carcinoma tissue were used as the positive control. Negative control was designed by using PBS instead of Egr-1 antiserum or instead of Bcl-XL polyclonal antibody.
Detection of cell apoptosis
Apoptosis was detected by the TdT-mediated dUTP nick end labeling (TUNEL) method using a detection kit from Boster company (China) according to the manufacturer’s instructions. Section were dewaxed in xylene, then into graduated ethanol, and then into Fresh 30 mL/L hydrogen peroxide in room temperature for 10 min, washed with PBS, digested by proteinase K(20 µg/mL) at 37 °C for 10 min, and incubated with TdT and DIG-dUTP reaction mixture at 37 °C for 2 h. Samples were washed with PBS, and then biotinylated mouse anti-digoxigenin antibody , and then streptavidin-biotin complex(SABC) at 37 °C for 30 min,and finally,with DAB visualization. The small intestine mucosa of mouse was used as the positive control. Negative control was designed by PBS instead of TdTase.
Judgement of the results
The Egr-1mRNA positive expression showed brown stained signal in the cytoplasm; the Egr-1 protein positive signal showed brown stained signal in the nucleus. Either Egr-1mRNA positive or Egr-1 protein positive was considered to be positive result. Bcl-XL positive expression showed brown stained signal in the cytoplasm; the result was considered positive if the positive cells accounted for more than 20% in each slide. The nuclei of apoptotic cells were stained brown as detected under light microscopy(Olympus CHK). Apoptotic cells were counted according to the Schepop’s method. Ten optical fields which were the strongest positive areas were counted ( × 400, field diameter 0.545 mm, area 2.33 mm2) and the vicinity of the necrotic areas were not evaluated in each slide. The apoptosis index (AI) was the average of positive cells per mm2 in the slide.
Statistical analysis
Statistical significance was determined by χ2 test or Student’s t test.
RESULTS
Changes of cell apoptosis in esophageal precancerous lesions and cancer tissues
Apoptotic cells were observed in tissues with different lesions of esophageal epithelia and cancer tissues(Figures 1 and 2). The AI and the frequency of apoptosis occurrence were low in the normal epithelia, but they were increased gradually from normal epithelia to dysplasia and to cancer tissues. The AI and the frequency of apoptosis occurrence of dysplasia lesions group was significantly higher than that of normal epithelia group (P < 0.01, P < 0.01, Table 1).
Figure 1.
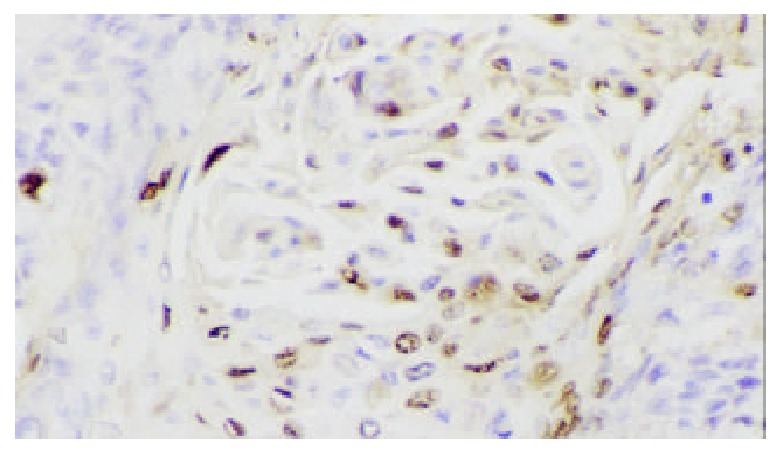
Positive apoptotic cell in nuclei of esophageal epithelial dysplasia. TUNEL × 200.
Figure 2.
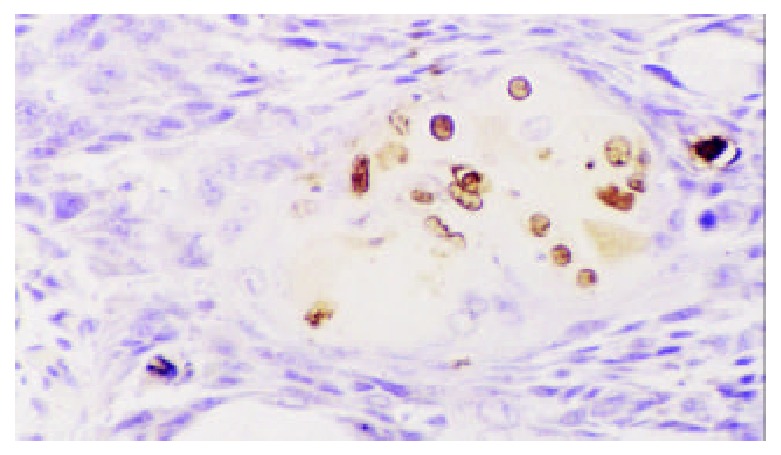
Positive apoptotic cell in nuclei of esophageal squa-mous cell carcinoma. TUNEL × 200.
Table 1.
Changes of cell apoptosis in esophageal precancerous lesions and cancer tissues
| Groups | n | Apoptosis cases (%) | AI (cells/mm2) |
| Normal epithelia | 39 | 8 (20.5) | 8.2 ± 3.1 |
| Simple hyperplasia | 52 | 17 (32.7) | 13.4 ± 4.3 |
| Dysplasia | 41 | 31 (75.6)a | 17.8 ± 8.3b |
| Carcinoma in situ | 2 | 2 (100) | 20.3 ± 5.1 |
| Invasive carcinoma | 64 | 64 (100) | 25.2 ± 9.8 |
P < 0.01, χ2 = 24.29 vs normal epithelia, χ2 test;
P < 0.01, t = 5.19 vs normal epithelia, Student’s t test.
The relationship between the expressions of Egr-1mRNA and Egr-1 protein and cell apoptosis in esophageal precancerous lesion and cancer tissues
The expressions of Egr-1mRNA and Egr-1 protein were observed in the cytoplasm and nuclei in different lesions of esophageal epithelia and cancer lesions respectively (Figure 3). The positivity results of Egr-1 ISH and Egr-1 IHC were nearly identical, but ISH showed slightly higher. The AI and the rate of Egr-1 positivity were increased gradually from normal epithelia to simple hyperplasia and to dysplastia; the AI and the rate of Egr-1 positive expression of dysplasia lesions group was significant higher than that of normal epithelia group (P < 0.01, P < 0.01); the Egr-1 positivity rate of invasive carcinoma group was significant lower than that of dysplasia lesions group (P < 0.01, Table 2).
Figure 3.
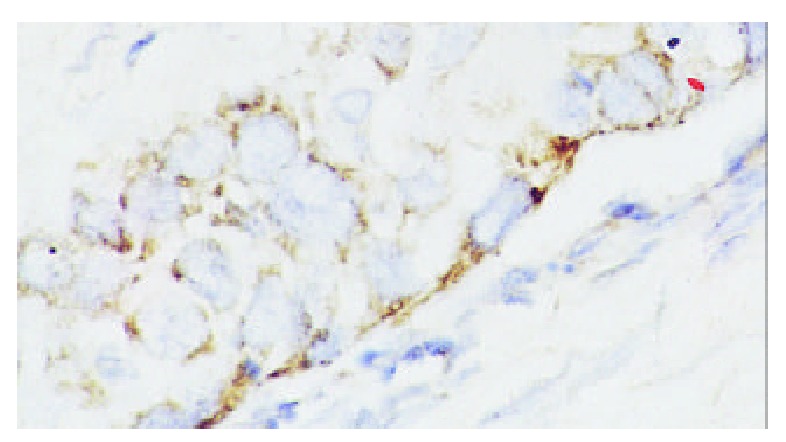
Positive Egr-1 mRNA in cytoplasm of esophageal squamous cell carcinoma. ISH × 400.
Table 2.
The relationship between the expressions of Egr-1 and cell apoptosis in esophageal precancerous lesions and cancer tissues
| Groups | n |
Egr-1 positive |
Egr-1 negative |
||
| n (%) | AI(cells/mm2) | n (%) | AI(cells/mm2) | ||
| Normal epithelia | 39 | 9 (23.1) | 10.2 ± 4.1 | 30 (76.9) | 3.8 ± 2.5 |
| Simple hyperplasia | 52 | 20 (38.5) | 16.4 ± 5.6 | 30 (60.0) | 7.2 ± 4.3 |
| Dysplasia | 41 | 27 (65.9)a | 25.4 ± 9.2b | 14 (34.1) | 10.9 ± 7.4 |
| Carcinoma in situ | 2 | 1 (50.0) | 29.3 ± 5.6 | 1 (50.0) | 11.5 ± 3.7 |
| Invasive carcinoma | 64 | 17 (26.6)c | 30.8 ± 6.8 | 47 (73.4) | 21.4 ± 9.1 |
P < 0.01, χ2 = 14.78 vs normal epithelia, χ2 test;
P < 0.01, t = 6.80 vs normal epithelia, Student’s t test;
P < 0.01, χ2 = 15.84 vs Dysplasia, χ2 test.
The relationship between the protein expression of Bcl-X and cell apoptosis in esophageal precancerous lesions and cancer tissues
The expression of Bcl-X/L protein was observed in tissues with different lesion of esophageal epithelia and cancer tissues. The AI and Bcl-X/L immunostaining positivity rate were increased gradually as the lesion progressed. The AI and the rate of Bcl-X/L positive expression of dysplasia lesions group was significanly higher than that of normal epithelia group (P < 0.01, P < 0.01). In the Bcl-X/L(+) cases (Figure 4) the AI was low, while in the Bcl-X/L(-) cases had higher AI in the cancer tissues (P < 0.01, Table 3).
Figure 4.
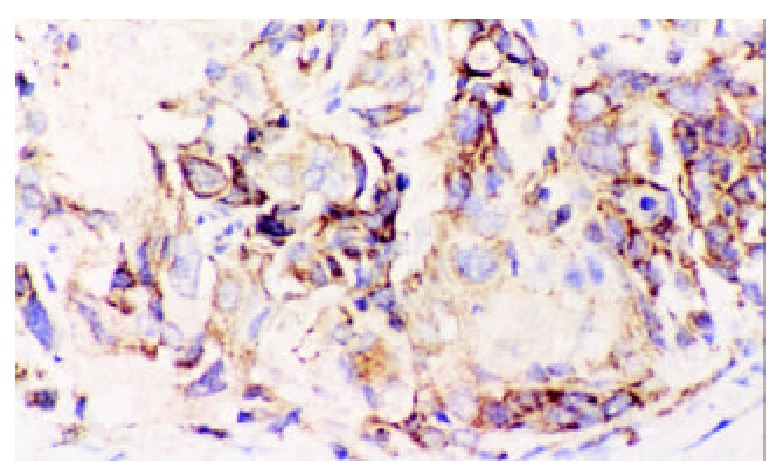
Positive Bcl-X/L protein in cytoplasm of esophageal squamous cell carcinoma. IHC × 200.
Table 3.
The relationship between the protein expression of Bcl-XL and cell apoptosis in esophageal precancerous lesions and cancer tissues
| Groups | n |
Bcl-XL positive |
Bcl-XL negative |
||
| n (%) | AI(cells/mm2) | n (%) | AI(cells/mm2) | ||
| Normal epithelia | 39 | 4 (10.3) | 1.4 ± 1.2 | 35 (89.7) | 8.3 ± 4.3 |
| Simple hyperplasia | 52 | 11 (21.2) | 3.7 ± 3.6 | 41 (82.0) | 14.5 ± 3.7 |
| Dysplasia | 41 | 28 (68.3)a | 18.9 ± 8.2b | 13 (31.7) | 20.6 ± 6.3 |
| Carcinoma in situ | 2 | 1 (50.0) | 11.8 ± 3.3 | 1 (50.0) | 29.0 ± 5.7 |
| Invasive carcinoma | 64 | 46 (71.9) | 14.5 ± 7.5c | 18 (28.1) | 28.6 ± 8.6 |
P < 0.01, χ2 = 28.06 vs normal epithelia, χ2 test;
P < 0.01, t = 10.53 vs normal epithelia, Student’s t test;
P < 0.01, t = 6.10 vs com-pared with Bcl-X/L protein negative group, Student’s t test.
The relationship between the expression of Egr-1 and cell apoptosis and biological behavior in esophageal squamous cell carcinoma
All Egr-1 positive cases were of keratinized squamous cell carcinoma (grade I,II). The AI and Egr-1 positivity rate of keratinized squamous cell carcinoma group were significantly higher than that of the non-keratinized squamous cell carcinoma group (P < 0.05, P < 0.01). In Bcl-X/L protein positive group, the AI and the Egr-1 positivity rate were lower significantly than those in Bcl-X/L(-)group (P < 0.01, P < 0.01, Table 4).
Table 4.
The relationship between the expression of Egr-1 and cell apoptosis and biological behavior in esophageal squamous cell carcinoma
| Clinicopathologic data | n | Egr-1 positive n(%) | AI(cells/mm2) |
| Age(years) | |||
| ≤ 50 | 20 | 6 (30.0) | 21.5 ± 7.9 |
| > 50 | 46 | 12 (26.1) | 24.3 ± 8.8 |
| Tumor site | |||
| Upper segment | 4 | 0 (0) | 20.4 ± 6.2 |
| Middle segment | 50 | 14 (28.0) | 23.7 ± 9.3 |
| Lower segment | 12 | 4 (33.3) | 21.5 ± 8.4 |
| Lesion’s diameter(cm) | |||
| ≤ 5 | 45 | 10 (22.2) | 22.6 ± 8.5 |
| > 5 | 21 | 8 (38.1) | 22.3 ± 9.2 |
| Differentiation degrees | |||
| GradeI | 18 | 7 (38.9) | 31.6 ± 9.3 |
| GradeII | 33 | 10 (30.3) | 28.5 ± 8.9 |
| GradeIII | 13 | 0 (0) | 7.2 ± 3.5 |
| Keratinization | |||
| Negative | 15 | 1 (6.7) | 8.6 ± 3.2 |
| Positive | 51 | 17 (33.3)a | 34.7 ± 9.4b |
| Invasive depth | |||
| Upper muscular layer | 22 | 5 (22.7) | 18.7 ± 7.5 |
| Serosa | 44 | 13 (29.5) | 26.1 ± 9.2 |
| Lymphatic metastasis | |||
| Negative | 30 | 11 (36.7) | 23.4 ± 8.6 |
| Positive | 36 | 7 (19.4) | 20.8 ± 7.5 |
| Bcl-X/L protein | |||
| Negative | 19 | 10 (52.6) | 29.4 ± 8.8 |
| Positive | 47 | 8 (17.0)c | 13.5 ± 4.2d |
P < 0.05, χ2 = 4.15 vs compared with non-keratinized squamous carcinoma group, χ2 test;
P < 0.01, t = 16.79 vs compared with non-keratinized squamous carcinoma group, Student’s t test;
P < 0.01, χ2 = 8.65 vs compared with Bcl-X/L protein negative group, χ2 test;
P < 0.01, t = 7.54 vs compared with Bcl-X/L pro-tein negative group, Student’s t test.
DISCUSSION
Modern molecular biology investigations have indicated that proliferative inhibition of some neoplasm cells is related to apoptosis induction by the oncogene expression of these cells[22-28]. Apoptosis, or programmed cell death, is a process in which a genetic program is activated, may be positively or negatively modulated by several oncogenes and tumor suppressor genes. The cancerigenic course of esophageal carcinoma has been identified as a successive course from simple hyperplasia of basal cell, dysplasia, carcinoma in situ to invasive carcinoma. Our studies suggest that The AI and the frequency of apoptosis occurrence increased gradually in esophageal carcinogenesis.
Egr-1 is one of the immediate early gene family, the Egr-1 gene was localized in human chromosome 5q31.1. Egr-1 is a nuclear protein that contains three zinc-finger domains, which regulate the cellular growth and differentiation by activating Cyclin D1 to promote the cell from the G0/G1 phase into the G2/M phase[29]. It was reported[30,31] that Egr-1 was originally in dormancy, and would be activated by the induction of stress, ischemia, hypoxia, bacterial toxin, cell factors, ionizing radiation and some oncogenic factors through membrane depolarization. We have detected tissue series of esophageal carcinogenesis using In situ hybridization and immunohistochemistry, and found that the rate of Egr-1 positive expression in dysplasia was the highest among all specimens (65.9%). This might be explained that the paracancerous mucosa is much more stimulated, and this is in accordance with that Egr-1 expression is activated by many factors. High expression of Egr-1 might preserve the stabilization of chromosome and suppress proliferation, and also improve differentiation and apoptosis of the cell. Egr-1 expression is decreased significantly from dysplasia to cancer tissues[32,33].
Recent studies have shown[34] that there are several genes which participate in apoptosis regulation. Apoptosis regulation genes are divided into two groups: existence gene and death gene. Living gene includes cell promoting gene, while death gene includes proliferation suppressing gene. Studies using diverse tumor cells suggest that endogenous levels of Egr-1 act to impede proliferation[21,29]. Consistent with its anti-tumor role, Egr-1 has been identified an important gene for impeding proliferation, and apoptosis of certain tumor cells needs Egr-1. Some studies indicated that the mechanism of Egr-1 inducing apoptosis might be involved by the activation of some oncogenes, e.g. wild type P53 gene, TNF-α and the concentration of calcium ions[35,36]. Another gene that implicates apoptotic pathway is bcl-2. Bcl-2 has been identified to be an apoptosis inhibitor. Recently, a new bcl-2 relayed gene Bcl-X was identified. Alternative splicing results in two bcl-X-derived mRNA species, called bcl-XL and bcl-XS. Bcl-XL appears to have functions similar to Bcl-2 in that it is capable of suppressing cell apoptosis in cancer cells[37].
Abnormal hyperplasia and cell apoptosis of cancer cell are the dynamic process in the pathogenesis and development of esophageal carcinoma. Many studies indicated that cell hyperplastic cycle and cell apoptosis were two importance essential portions to maintain homostasis by many genes constituted complex modulation system. Our studies detected that cell apoptosis existed in normal epithelia and different lesion of esophagus, but AI and the frequency of apoptosis occurrence were increased gradually as the lesions progressed. In the similar lesions, the AI in Egr-1 positive group was higher than that in Egr-1 negative group. In contrast, the AI of Bcl-X/L positive group was lower than that of Bcl-X/L negative group. The AI and the expression of Egr-1 were not correlated with invasiveness and lymphatic metastasis of the cancer tissues. Our study suggests that cell apoptosis might be an important process in esophageal carcinogenesis. Egr-1 might promote apoptosis effect while Bcl-X/L inhibit it. But further study is necessary to explore the mechanism and significance of Egr-1 and Bcl-X/L improving or suppressing apoptosis in esophageal carcinogenesis.
Footnotes
Supported by the National Natural Science Foundation of China, No. 39670298
Edited by Xu JY
References
- 1.Su M, Lu SM, Tian DP, Zhao H, Li XY, Li DR, Zheng ZC. Relationship between ABO blood groups and carcinoma of esophagus and cardia in Chaoshan inhabitants of China. World J Gastroenterol. 2001;7:657–661. doi: 10.3748/wjg.v7.i5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao SS, Zhou Q, Li YX, Bai YM, Zheng ZY, Zou JX, Liu G, Fan ZM, Qi YJ, Zhao X, et al. Comparative studies on epithelial lesions at gastric cardia and pyloric antrum in subjects from a high incidence area for esophageal cancer in Henan, China. World J Gastroenterol. 1998;4:332–333. doi: 10.3748/wjg.v4.i4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiao GB, Han CL, Jiang RC, Sun CS, Wang Y, Wang YJ. Overexpression of P53 and its risk factors in esophageal cancer in urban areas of Xi'an. World J Gastroenterol. 1998;4:57–60. doi: 10.3748/wjg.v4.i1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen KN, Xu GW. Diagnosis and treatment of esophageal cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:196–202. [Google Scholar]
- 5.Zheng ZY, Wang LD, Shi ST, Yang GY, Xue ZH, Gao SS, Li YX, Yang CS. p53 gene mutations in multifocal esophageal precan-cerous and cancerous lesions in patients with esophageal cancer in high-risk northern China. Shijie Huaren Xiaohua Zazhi. 1999;7:280–284. [Google Scholar]
- 6.Wang LD, Zhou Q, Wei JP, Yang WC, Zhao X, Wang LX, Zou JX, Gao SS, Li YX, Yang C. Apoptosis and its relationship with cell proliferation, p53, Waf1p21, bcl-2 and c-myc in esophageal carcinogenesis studied with a high-risk population in northern China. World J Gastroenterol. 1998;4:287–293. doi: 10.3748/wjg.v4.i4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Feng CW, Zhao ZG, Zhou Q, Wang LD. A preliminary study on ras protein expression in human esophageal cancer and precancerous lesions. World J Gastroenterol. 2000;6:278–280. doi: 10.3748/wjg.v6.i2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang LJ, Chen KN, Xu GW, Xing HP, Shi XT. Congenital expression of mdr-1 gene in tissues of carcinoma and its relation with pathomorphology and prognosis. World J Gastroenterol. 1999;5:53–56. doi: 10.3748/wjg.v5.i1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casson AG. Molecular biology of Barrett's esophagus and esophageal cancer: role of p53. World J Gastroenterol. 1998;4:277–279. doi: 10.3748/wjg.v4.i4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu CT, Yan XJ. P53 anti-cancer gene and digestive system neoplasms. Shijie Huaren Xiaohua Zazhi. 1999;7:77–79. [Google Scholar]
- 11.Lin J, Deng CS, Sun J, Zhou Y, Xiong P, Wang YP. Study on the genetic susceptibility of HLA-DQB1 alleles in esophageal cancer of Hubei Chinese Hans. Shijie Huaren Xiaohua Zazhi. 2000;8:965–968. [Google Scholar]
- 12.Qin HY, Shu Q, Wang D, Ma QF. Study on genetic polymor-phisms of DCC gene VNTR in esophageal cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:782–785. [Google Scholar]
- 13.Wang LD, Chen H, Guo LM. Alteration of tumor suppressor gene system p53-Rb and human esophageal carcinogenesis. Shijie Huaren Xiaohua Zazhi. 2001;9:367–371. [Google Scholar]
- 14.Yu GQ, Zhou Q, Ivan D, Gao SS, Zheng ZY, Zou JX, Li YX, Wang LD. Changes of p53 protein blood level in esophageal cancer patients and normal subjects from a high incidence area in Henan, China. World J Gastroenterol. 1998;4:365–366. doi: 10.3748/wjg.v4.i4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou YA, Gu ZP, Wang XN, Ma QF. WAF1 gene suppresses growth of human esophageal carcinoma cell line EC109. Shijie Huaren Xiaohua Zazhi. 2002;10:628–632. [Google Scholar]
- 16.Zhou YA, Gu ZP, Wang XN, Ma QF, Huang LJ. Re-expression of p16INK4 gene suppresses growth of human esophageal carcinoma cells. Shijie Huaren Xiaohua Zazhi. 2001;9:877–881. [Google Scholar]
- 17.Liu J, Chen SL, Zhang W, Su Q. P21WAF1 gene expression with P53 mutation in esophageal carcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:1350–1353. [Google Scholar]
- 18.Tan LJ, Jiang W, Zhang N, Zhang XR, Qiu DH. Fas/FasL expres-sion of esophageal squamous cell carcinoma, dysplasia tissues and normal mucosa. Shijie Huaren Xiaohua Zazhi. 2001;9:15–19. [Google Scholar]
- 19.Sarbia M, Bittinger F, Grabellus F, Verreet P, Dutkowski P, Willers R, Gabbert HE. Expression of Bax, a pro-apoptotic member of the Bcl-2 family, in esophageal squamous cell carcinoma. Int J Cancer. 1997;73:508–513. doi: 10.1002/(sici)1097-0215(19971114)73:4<508::aid-ijc9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Levin WJ, Press MF, Gaynor RB, Sukhatme VP, Boone TC, Reissmann PT, Figlin RA, Holmes EC, Souza LM, Slamon DJ. Expression patterns of immediate early transcription factors in human non-small cell lung cancer. The Lung Cancer Study Group. Oncogene. 1995;11:1261–1269. [PubMed] [Google Scholar]
- 21.Wu MY, Chen MH, Liang YR, Meng GZ, Yang HX, Zhuang CX. Experimental and clinicopathologic study on the relationship between transcription factor Egr-1 and esophageal carcinoma. World J Gastroenterol. 2001;7:490–495. doi: 10.3748/wjg.v7.i4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li HL, Chen DD, Li XH, Zhang HW, Lu YQ, Ye CL, Ren XD. Changes of NF-kB, p53, Bcl-2 and caspase in apoptosis induced by JTE-522 in human gastric adenocarcinoma cell line AGS cells: role of reactive oxygen species. World J Gastroenterol. 2002;8:431–435. doi: 10.3748/wjg.v8.i3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403–406. doi: 10.3748/wjg.v7.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun BH, Zhao XP, Wang BJ, Yang DL, Hao LJ. FADD and TRADD expression and apoptosis in primary hepatocellular carcinoma. World J Gastroenterol. 2000;6:223–227. doi: 10.3748/wjg.v6.i2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng ZH, Xing TH, Qiu GQ, Tang HM. Relationship between Fas/FasL expression and apoptosis of colon adenocarcinoma cell lines. World J Gastroenterol. 2001;7:88–92. doi: 10.3748/wjg.v7.i1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Si XH, Yang LJ. Extraction and purification of TGFbeta and its effect on the induction of apoptosis of hepatocytes. World J Gastroenterol. 2001;7:527–531. doi: 10.3748/wjg.v7.i4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu HY, Yang YL, Guan XL, Song G, Jiang AM, Shi LJ. Expression of regulating apoptosis gene and apoptosis index in primary liver cancer. World J Gastroenterol. 2000;6:721–724. doi: 10.3748/wjg.v6.i5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Yang XK, Yu XX, Ge ML, Wang WL, Zhang J, Hou YD. Overexpression of p27(KIP1) induced cell cycle arrest in G(1) phase and subsequent apoptosis in HCC-9204 cell line. World J Gastroenterol. 2000;6:513–521. doi: 10.3748/wjg.v6.i4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair P, Muthukkumar S, Sells SF, Han SS, Sukhatme VP, Rangnekar VM. Early growth response-1-dependent apoptosis is mediated by p53. J Biol Chem. 1997;272:20131–20138. doi: 10.1074/jbc.272.32.20131. [DOI] [PubMed] [Google Scholar]
- 30.Yan SF, Lu J, Zou YS, Soh-Won J, Cohen DM, Buttrick PM, Cooper DR, Steinberg SF, Mackman N, Pinsky DJ, et al. Hypoxia-associated induction of early growth response-1 gene expression. J Biol Chem. 1999;274:15030–15040. doi: 10.1074/jbc.274.21.15030. [DOI] [PubMed] [Google Scholar]
- 31.Bae SK, Bae MH, Ahn MY, Son MJ, Lee YM, Bae MK, Lee OH, Park BC, Kim KW. Egr-1 mediates transcriptional activation of IGF-II gene in response to hypoxia. Cancer Res. 1999;59:5989–5994. [PubMed] [Google Scholar]
- 32.Huang RP, Fan Y, de Belle I, Niemeyer C, Gottardis MM, Mercola D, Adamson ED. Decreased Egr-1 expression in human, mouse and rat mammary cells and tissues correlates with tumor formation. Int J Cancer. 1997;72:102–109. doi: 10.1002/(sici)1097-0215(19970703)72:1<102::aid-ijc15>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 33.Huang RP, Liu C, Fan Y, Mercola D, Adamson ED. Egr-1 negatively regulates human tumor cell growth via the DNA-binding domain. Cancer Res. 1995;55:5054–5062. [PubMed] [Google Scholar]
- 34.Peter ME, Heufelder AE, Hengartner MO. Advances in apoptosis research. Proc Natl Acad Sci USA. 1997;94:12736–12737. doi: 10.1073/pnas.94.24.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed MM, Sells SF, Venkatasubbarao K, Fruitwala SM, Muthukkumar S, Harp C, Mohiuddin M, Rangnekar VM. Ionizing radiation-inducible apoptosis in the absence of p53 linked to transcription factor EGR-1. J Biol Chem. 1997;272:33056–33061. doi: 10.1074/jbc.272.52.33056. [DOI] [PubMed] [Google Scholar]
- 36.Woronicz JD, Calnan B, Ngo V, Winoto A. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature. 1994;367:277–281. doi: 10.1038/367277a0. [DOI] [PubMed] [Google Scholar]
- 37.Schott AF, Apel IJ, Nuñez G, Clarke MF. Bcl-XL protects cancer cells from p53-mediated apoptosis. Oncogene. 1995;11:1389–1394. [PubMed] [Google Scholar]


