Abstract
AIM: To investigate the role of TR3 in induction of apoptosis in gastric cancer cells.
METHODS: Human gastric cancer cell line, MGC80-3, was used. Expression of TR3 mRNA and its protein was detected by Northern blot and Western blot. Localization of TR3 protein was showed by immunofluorescence analysis under laser-scanning confocal microscope. Apoptotic morphology was observed by DAPI fluorescence staining, and apoptotic index was counted among 1000 cells randomly. Stable transfection assay was carried out by Lipofectamine.
RESULTS: Treatment of MGC80-3 cells with TPA and VP-16 resulted in apoptosis, accompanied by the repression of Bcl-2 protein in a time-dependent manner. At the same time, TPA and VP-16 also up-regulated expression level of TR3 mRNA in MGC80-3 cells that expressed TR3 mRNA. When antisense-TR3 expression vector was transfected into the cells, expression of TR3 protein was repressed. In this case, TPA and VP-16 did not induce apoptosis. In addition, TPA and VP-16-induced apoptosis involved in translocation of TR3. In MGC80-3 cells, TR3 localized concentrative in nucleus, after treatment of cells with TPA and VP-16, TR3 translocated from nucleus to cytosol obviously. However, when this nuclear translocation was blocked by LMB, apoptosis was not occurred in MGC80-3 cells even in the presence of TPA and VP-16.
CONCLUSION: Induction of apoptosis by TPA and VP-16 is through induction of TR3 expression and translocation of TR3 from nucleus to cytosol, which may be a novel signal pathway for TR3, and represent the new biological function of TR3 to exert its effect on apoptosis in gastric cancer cells.
INTRODUCTION
TR3 (also termed as NGFI-B and Nur77) is an orphan receptor that belongs to the member of the steroid/thyroid/retinoid receptor superfamily[1-3]. It is an immediate-early response gene, and its expression is rapidly induced by a variety of growth stimuli, including growth factors, phorbol ester and cAMP-dependent pathways[1,3-5]. Similar to other members of the superfamily, TR3 functions in nucleus as a transcriptional factor to positively or negatively regulate gene expression necessary to alter the cellular phenotype in response to the growth stimuli[6]. We found recently that TR3 heterodimerizes with retinoid X receptor (RXR) that binds to retinoic acid receptor â (RARâ) promoter, and regulates RARâ expression that is critical to inducing apoptosis[7]. In addition, TR3 also heterodimerizes with chicken ovalbumin upstream promoter transcription factor (COUP-TF) to inhibit COUP-TF binding to retinoic acid responsive element (RARE) through direct protein-protein interaction[8]. These evidences suggest that TR3 can mediate diverse signals through its ability either to bind to a variety of response elements or to interact with different protein factors. However, the mechanism by which TR3 exerts its biological functions remains largely unknown.
Apoptosis, as a distinct form of cell death, is an important process that can lead to tumor regression, and suppression of apoptosis is often associated with abnormal cell survival and malignant growth[9-15]. The involvement of TR3 in apoptosis was first demonstrated by showing that TR3 was rapidly induced by T-cell antigen receptor (TCR) signaling in immature thymocytes and T-cell hybridomas[16,17]. Overexpression of a dominant-negative TR3 protein or inhibition of TR3 expression by antisense-TR3 inhibited TCR-induced apoptosis, whereas constitutive expression of TR3 led to massive apoptosis[16,17]. These evidences clearly indicate that TR3 plays an important role in TCR-mediated apoptosis. The involvement of TR3 in apoptosis process is also observed in many cancer cell lines. Treatment of lung cancer cells with AHPN/CD437 strongly induced apoptosis, which was accompanied by a rapid induction of TR3. Inhibition of TR3 by antisense-TR3 effectively abolished apoptosis induction by AHPN/CD437[18]. Rapid induction of TR3 was also found in other cancer cells after stimulation of apoptosis by a variety of apoptosis-inducing agents[4,19-20]. Therefore, these observations suggest that expression of TR3 is required for induction of cell apoptosis.
How TR3 functions to regulate apoptosis in gastric cancer cells is less understood. In this study, we investigated the role of TR3 in inducing apoptosis in gastric cancer cells. The results showed that 12-O-tetradecanoylphorbol-13-acetate (TPA) and VP-16 (also called etoposide) induced apoptosis, accompanied by TR3 expression. More importantly, TR3 protein translocated from nucleus to cytosol, when apoptosis occurred by TPA or VP-16 induction. However, when the translocation was blocked by leptomycin B (LMB), apoptosis was not detected, even in the presence of apoptotic stimuli. Our findings, therefore, have drawn an inspiration that translocation of TR3 from nucleus to cytosol may be one of the essential links involved in the mechanism of apoptosis by apoptotic stimuli in gastric cancer cells.
MATERIALS AND METHODS
Cell line and culture condition
Human gastric cancer cell line, MGC80-3, was established by Cancer Research Center in Xiamen University[21]. The cells were maintained in RPMI-1640 medium, supplemented with 10% FCS, 1 mM glutamine, and 100 u/mL penicillin.
Agents
TPA and VP-16 (Sigma) are used as apoptosis stimuli that can induce apoptosis in a variety of cells, including breast cancer cells, lung cancer cells, prostate cancer cells[22-29]. LMB is an antibiotic with anti-fungal and anti-tumor activity that was first discovered and purified from the fermentation broth and mycelia of streptomyces[30-32]. Recently, this antibiotic has become an important tool for studying nuclear localization[33-35].
Apoptosis analysis[36]
MGC80-3 cells were treated with TPA and VP-16 for different time indicated in figures, trypsinized, washed with PBS, fixed in 3.7% paraformaldehyde, and then stained with 50 μg/mL of 4,6-diamidino-2-phenylindole (DAPI, Sigma) containing 100 μg/mL of DNase-free RNase A to facilitate the examination of nuclei under fluorescence microscope. Apoptotic cells were counted among 1000 cells randomly. The apoptotic index was a mean of three independent experiments.
Immunofluorescence analysis[37]
MGC80-3 cells were cultured on cover glass overnight, and then treated with TPA and VP-16 as required. After washed with PBS, cells were fixed in 4% paraformaldehyde. After fixation, the cells were incubated firstly with anti-TR3 antibody (Santa Cruz), and then reacted with corresponding FITC-conjugated anti-IgG (Pharmingen) as secondary antibody. Fluorescent images were observed and analyzed under laser-scanning confocal microscope (Bio-Rad MRC-1024ES).
Western blot analysis[37,38]
Total 50 μg of nuclear extract was electrophoresed on 8% denaturing gel and electroblotted onto a nitrocellulose membrane. The membrane was probed with anti-TR3 antibody followed by corresponding secondary antibody. The antibody reactivity was detected with an Amersham ECL kit according to the manufacturer’s instruction. For the cytosolic and nuclear fractions, the cells were suspended in 500 μl of 10 mM Tris-Cl (pH7.8), 1% Nonidet P-40, 10 mM mercaptoethanol, 0.5 mM phenylmethylsilfonyl fluoride, 1 μg/mL leupeptin and aprotinin for 2 min at 0 °C, then 500 μl of DDW was added, and the cells were allowed to swell for 2 min. The cells were sheared by 10 passages through 22 gauge needle. Nuclei were recovered by centrifugation at 400Xg for 6 min, and the low-speed supernatant was centrifuged at 100000 g for 30 min. The high-speed supernatant constituted the cytosolic fraction.
Stable transfection
Antisense-TR3 expression vector (provided kindly by Dr. Uemura, and Dr. Chang[39]) was stably transfected into MGC80-3 cells by LipofectamineTM (Gibco/BRL) as described previously[40], then screened with 600 μg of G418. Expression of endogenous TR3 protein was determined by Western blot.
RESULTS
Expression of TR3 mRNA in response to apoptotic stimuli
TPA and VP-16 were both known as the apoptotic stimuli which induced apoptosis in many kinds of cancer cell lines, including the cancer cells of prostate, breast and lung[22-29]. To determine whether these apoptotic stimuli also functioned in gastric cancer cells, a specific, DAPT staining for display of apoptotic nuclear morphology was adopted under fluorescence microscope[37]. The observation indicated that treatment of MGC80-3 cells with TPA and VP-16 caused the classical morphological characteristics of apoptosis, including nuclear condensation and fragmentation (Figure 1A). The apoptotic index was further determined by counting 1000 cells randomly. In MGC80-3 cells, the apoptotic index was merely 3.89%. However, the apoptotic index induced by TPA and VP-16 increased in a time-dependent manner (Figure 1B). The highest apoptotic index reached 44.33% (for TPA induction) and 32.16% (for VP-16 induction), respectively. Thus, the data demonstrated that TPA and VP-16 indeed induced apoptosis in gastric cancer cells.
Figure 1.
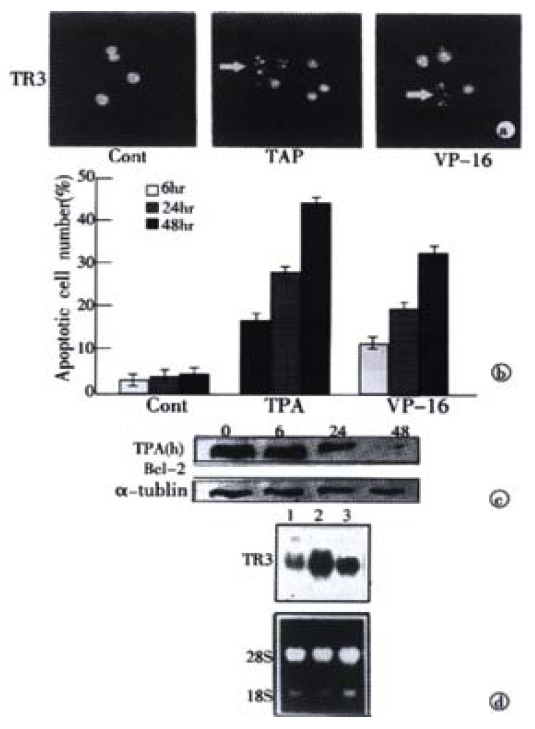
Induction of apoptosis and TR3 expression induced by TPA and VP-16 in MGC80-3 cells. (A) Morphological analysis of apoptotic cells. Cells treated with TPA and VP-16 for 24 hr, and then stained with DAPI. Nuclear mor-phology was visualized under fluorescence microscope. (B) Measure of apoptotic index by counting 1000 cells stained with DAPI under fluorescence microscope. The data shown represents mean of three independent experiments (± SE). (C) Analysis of Bcl-2 protein expression. Cells were treated with TPA for indicated time, and Western blot was preformed as described in materials and methods. α-tubulin was used to quantify the amount of protein used in each lane. (D) Detection of TR3 mRNA expression. Cells were treated with TPA and VP-16 for 24 hr. Preparation of total RNA and Northern blot were carried out as described in materials and methods. 18S and 28S were shown to quantify the loading RNA. Lane 1: control; Lane 2: TPA treatment; Lane 3: VP-16 treatment.
Members of the Bcl-2 family were involved in the regulation of apoptotic process[41,42]. Since TPA and VP-16 induced apoptosis significantly in MGC80-3 cells shown above, it should be interrelated with some proteins that were associated with apoptosis initiation. To investigate this posibility, expression of Bcl-2 was detected. Western blot showed that when TPA treated with cells for different time, the expression of BCL-2 protein was repressed in a time-dependent manner (Figure 1C), which was consistent with the result shown in Figure 1A and B. Similar result was seen in the cells treated with VP-16 (data not shown). This result suggested that inhibition of Bcl-2 might render MGC80-3 cells more susceptible to the apoptosis-inducing effect of TPA and VP-16.
Several evidences showed that TR3 was involved in the regulation of apoptosis in different cell types[16,43,44]. We investigated the role of TR3 in apoptosis of MGC80-3 cells induced by TPA and VP-16. Treatment of cells with TPA and VP-16 not only induced MGC80-3 cell apoptosis and repressed its relative protein, Bcl-2 (Figure 1A-C), but also enhanced expression of TR3 mRNA obviously that was expressed at relative low level in MGC80-3 cells (Figure 1D). Taken together, these results revealed a possible link between expression of TR3 and induction of apoptosis in gastric cancer cells.
Translocation of nuclear TR3 to cytosol induced by apoptotic stimuli
It was reported recently that trnaslocation of protein was closely associated with its biological function[24,37,46]. To determine whether the apoptotic stimuli could cause translocation of TR3 in MGC80-3 cells, the immunofluorescent localization of TR3 protein was conducted by using correspondent TR3-specific antibody and laser-scanning confocal microscope observation. The results illustrated that in MGC80-3 cells, TR3 protein was concentrative in nucleus (Figure 2A). When treatment of TPA for 6 hr, the majority of TR3 protein was still remained in nucleus, little in cytosol. However, after 24 hr of TPA treatment, the majority of TR3 protein translocated from nucleus to cytosol, little in nucleus (Figure 2A). Similar result was also seen in the cells treated with VP-16. After treatment of 24 hr, TR3 protein almost translocated to cytosol (data not shown).
Figure 2.
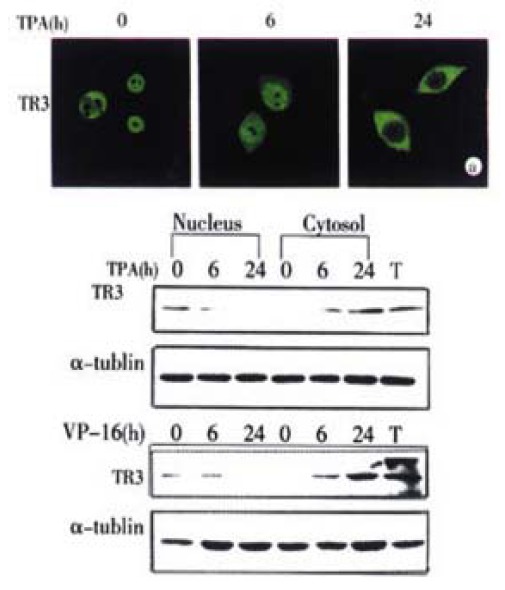
Translocation of TR3 protein from nucleus to cytosol in MGC80-3 cells. The cells treated with TPA for indicated time. (A) Translocation of TR3 protein observed by laser-scanning confocal microscope. TR3 protein was immunostained with anti-TR3 antibody and corresponding FITC-conjugated secondary antibody. (B) Western blot showed the translocation of TR3 protein. Nuclear and cytosolic fractions were prepared as described in materials and methods. α-tubulin was shown to quantify the loading protein. T: total protein.
To further verify this protein translocation, the cytosolic and nuclear fractions were isolated respectively, and TR3 protein level was detected by Western blot. As shown in Figure 2B, TR3 protein was expressed in MGC80-3 cells, which located in nucleus (Figure 2B). TPA and VP-16 treatment led to down-regulation of TR3 protein in nuclear and up-regulation in cytosol with a time-dependent manner. After treatment of the cells for 6 hr with TPA and VP-16, respectively, TR3 protein was distributed both in nucleus and cytosol. However, after 24 hr treatment of TPA and VP-16, TR3 protein appeared more in cytosol and little in nucleus clearly (Figure 2B). This result was in accordance with the result shown in Figure 2A, suggesting that translocation of TR3 protein might be associated with induction of apoptosis, which was a novel signal pathway for TPA and VP-16 to exert their biological functions.
Inhibition of both TR3 protein expression and its translocation
Since TR3 protein was expressed in MGC80-3 cells (Figure 2A, Figure 2B), we transfected antisense-TR3 expression vector into the cells to determine whether inhibition of TR3 protein expression caused TPA and VP-16 fail to induce apoptosis. The stable transfection result was showed in Figure. 3A. Expression of TR3 protein was almost repressed in transfected cells, whereas TR3 protein still expressed in the cells transfected with empty vector, compared to MGC80-3 cells. In this transfected cells, TPA and VP-16 could not induce apoptosis. The apoptotic index only reached 5.14% (for TPA induction) and 4.62% (for VP-16 induction), which were less than that in MGC80-3 cells treated with TPA and VP-16 (Figure 1B) and those transfected with empty vector (apoptotic index amounted to 39.78% for TPA induction and 27.56% for VP-16 induction).
It was then necessary to probe into the intrinsic mechanism of whether there was some inevitable linkage between TR3 protein translocation and apoptosis induction. Since LMB has been used in many systems to demonstrate the nuclear localization and inhibited the export of a number of proteins, including actin, transcription factors, kinases and cell cycle regulators[49-53], we used LMB as inhibitor to block nuclear protein TR3 export from nucleus to cytosol. In MGC80-3 cells, TR3 protein was mainly located in nucleus, after treatment of 24 hr with TPA, the most of TR3 protein was translocated from nucleus to cytosol in the absence of LMB (Figure 3B). However, in the presence of LMB, TR3 protein was remained in nucleus, although MGC80-3 cells were treated by TPA for 24 hr (Figure 3B). The similar result was also observed in VP-16 treated-cells (data not shown). According to this inhibitory result, we detected the apoptotic index to determine the relationship between translocation of TR3 protein and apoptosis induction. As shown in Figure 3C, in the presence of LMB, TPA and VP-16 did not induced apoptosis, compared with that TPA and VP-16 induced apoptosis of MGC80-3 cells obviously in the absence of LMB. Thus, preventing the nuclear TR3 protein export from nucleus to cytosol repressed TPA and VP-16’s ability to induce apoptosis. The result again confirmed that translocation of TR3 protein was associated with induction of apoptosis.
Figure 3.
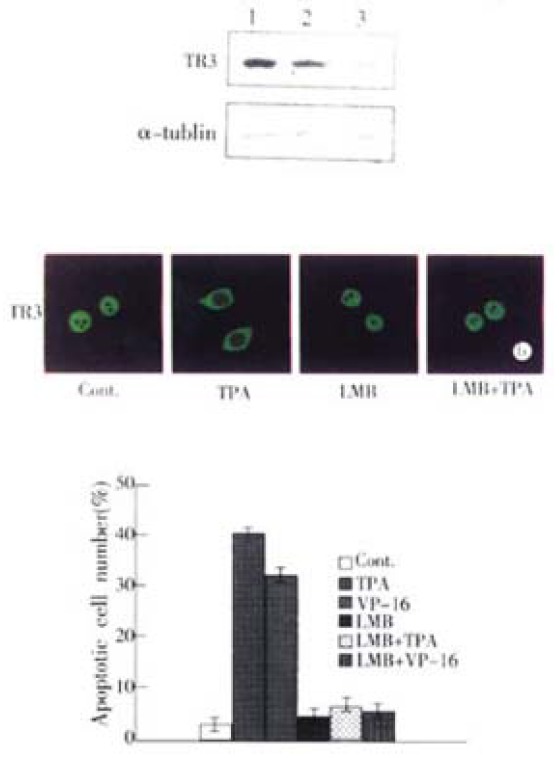
Inhibition of TR3 protein expression and its translocation in MGC80-3 cells. (A) Repression of TR3 protein expression by transfection of antisense-TR3 expression vector into MGC80-3 cells. Endogenous TR3 pro-tein was determined by Western blot. Empty vector was also transfected into cells as a positive control. α-tubulin was shown to quantify the loading protein. Lane 1: protein from MGC80-3 cells; Lane 2: protein from the cells transfected with empty vector; Lane 3: protein from the cells transfected with antisense-TR3 expression vector. Inhibitory effect of LMB on TR3 protein translocation induced by TPA. The cells treated with different agents indicated as in the figure. The reaction with antibody was similar to the description in Figure. 2A. (C) Inhibitory effect of LMB on apoptosis induc-tion by TPA and VP-16. Cells treated with different agents indicated as in the figure. Apoptotic index was measured as described in Figure 1B.
DISCUSSION
The TR3 orphan receptor is required for activation-induced apoptosis of T-cell hybridomas[16]. To gain insight into its function during apoptosis, we, in this study, analyzed the relationship between TR3 expression and its translocation and apoptosis induction in gastric cancer cells.
TR3 is an early response gene whose expression is induced by a variety of stimuli, including signals for cell survival, such as growth factors, and signals for cell death, such as TCR[1,3-5]. To study the role of TR3 in gastric cancer cells, we used TPA and VP-16 to induce apoptosis, and found that when apoptosis occurred and its related protein Bcl-2 was inhibited by TPA and VP-16 in MGC80-3 cells, TR3 mRNA was indeed up-regulated. After transfection of antisense-TR3 expression vector into MGC80-3 cells that expressed TR3 at protein level resulted in the inhibition of TR3 protein expression. In the transfected cells, we could not detect marked apoptosis, even in the presence of TPA and VP-16 induction. The apoptotic index was only 5.14% (for TPA induction) and 4.62% (for VP-16 induction), similar to the control MGC80-3 cells with 3.89% and less than the TPA and VP-16 treated-cells with 44.33% (for TPA induction) and 32.16% (for VP-16 induction). These results indicated that TPA and VP-16 regulated apoptosis through induction of TR3 expression at both mRNA and protein levels.
TR3 functions as a nuclear transcription factor in the regulation of target gene expression[6]. The movement of transcription factors between nucleus and cytoplasm is important in regulating its activity[23,46]. Our observation showed that TPA and VP-16-induced apoptosis involved in translocation of TR3 protein. In MGC80-3 cells, TR3 protein localized mainly in the nucleus in which it might be associated with other nuclear proteins related to stimulation of cell proliferation, such as PKB/AKT, cFos, and AP-1[18,54-57]. However, when cells were treated with TPA and VP-16 for 24 hours, respectively, translocation of TR3 from nucleus to cytosol was observed, with an increase protein expression of TR3 in cytosol and a decrease in nucleus in a time-dependent manner. In this case, apoptosis induced by TPA and VP-16 did happened, also in a time-dependent manner, and during this process, Bcl-2 protein, known to induce cell survival in variety of cell types[58,59], was strongly inhibited by TPA and VP-16. In addition, when this nuclear export was blocked by LMB, apoptosis was not detected, regardless of the presence of TPA and VP-16. As a comparison, all-trans retinoic acid that could not induce TR3 translocation from nucleus to cytosol failed to induction of apoptosis in MGC80-3 cells although it could also induce TR3 expression (data not shown). Taken together, these data clearly demonstrated that induction of apoptosis by TPA and VP-16 was through the translocation of TR3 from nucleus to cytosol, which might be a novel signal pathway for apoptosis and the new function for TR3 to exert its effect on apoptosis induction. Bcl-2 may play an important role in the regulation of TPA and VP-16-induced apoptosis. Of course, the link between the mechanism of TR3 function and the apoptosis induction in gastric cancer cells should be studied further from other aspects.
Footnotes
Supported by the National Outstanding Youth Science foundation of China (B type, 39825502); the National Natural Science Foundation of China (39880015, 30170477); the Natural Science Foundation of Fujian Province (C0110004).
Edited by Zhang JZ
References
- 1.Milbrandt J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988;1:183–188. doi: 10.1016/0896-6273(88)90138-9. [DOI] [PubMed] [Google Scholar]
- 2.Chang C, Kokontis J. Identification of a new member of the steroid receptor super-family by cloning and sequence analysis. Biochem Biophys Res Commun. 1988;155:971–977. doi: 10.1016/s0006-291x(88)80591-6. [DOI] [PubMed] [Google Scholar]
- 3.Hazel TG, Nathans D, Lau LF. A gene inducible by serum growth factors encodes a member of the steroid and thyroid hormone receptor superfamily. Proc Natl Acad Sci USA. 1988;85:8444–8448. doi: 10.1073/pnas.85.22.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams GT, Lau LF. Activation of the inducible orphan receptor gene nur77 by serum growth factors: dissociation of immediate-early and delayed-early responses. Mol Cell Biol. 1993;13:6124–6136. doi: 10.1128/mcb.13.10.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim RW, Zhu CY, Stringer B. Differential regulation of primary response gene expression in skeletal muscle cells through multiple signal transduction pathways. Biochim Biophys Acta. 1995;1266:91–100. doi: 10.1016/0167-4889(94)00226-5. [DOI] [PubMed] [Google Scholar]
- 6.Wilson TE, Fahrner TJ, Johnston M, Milbrandt J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science. 1991;252:1296–1300. doi: 10.1126/science.1925541. [DOI] [PubMed] [Google Scholar]
- 7.Wu Q, Dawson MI, Zheng Y, Hobbs PD, Agadir A, Jong L, Li Y, Liu R, Lin B, Zhang XK. Inhibition of trans-retinoic acid-resistant human breast cancer cell growth by retinoid X receptor-selective retinoids. Mol Cell Biol. 1997;17:6598–6608. doi: 10.1128/mcb.17.11.6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Q, Li Y, Liu R, Agadir A, Lee MO, Liu Y, Zhang X. Modulation of retinoic acid sensitivity in lung cancer cells through dynamic balance of orphan receptors nur77 and COUP-TF and their heterodimerization. EMBO J. 1997;16:1656–1669. doi: 10.1093/emboj/16.7.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson MD, Burne JF, King MP, Miyashita T, Reed JC, Raff MC. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993;361:365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- 11.Douglas RG, John CR. Mitochondrial and Apoptosis. Science. 1998;8:1309–1312. [Google Scholar]
- 12.Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 13.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 14.Zhuang XQ, Yuan SZ, Wang XH, Lai RQ, Luo ZQ. Oncoprotein expression and inhibition of apoptosis during colorectal tumorigenesis. China Natl J New Gastroentero. 1996;2:3–5. [Google Scholar]
- 15.Xue XC, Fang GE, Hua JD. Gastric cancer and apoptosis. Shijie Huaren Xiaohua Zazhi. 1999;7:359–361. [Google Scholar]
- 16.Woronicz JD, Calnan B, Ngo V, Winoto A. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature. 1994;367:277–281. doi: 10.1038/367277a0. [DOI] [PubMed] [Google Scholar]
- 17.Liu ZG, Smith SW, McLaughlin KA, Schwartz LM, Osborne BA. Apoptotic signals delivered through the T-cell receptor of a T-cell hybrid require the immediate-early gene nur77. Nature. 1994;367:281–284. doi: 10.1038/367281a0. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Lin B, Agadir A, Liu R, Dawson MI, Reed JC, Fontana JA, Bost F, Hobbs PD, Zheng Y, et al. Molecular determinants of AHPN (CD437)-induced growth arrest and apoptosis in human lung cancer cell lines. Mol Cell Biol. 1998;18:4719–4731. doi: 10.1128/mcb.18.8.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maruyama K, Tsukada T, Bandoh S, Sasaki K, Ohkura N, Yamaguchi K. Retinoic acids differentially regulate NOR-1 and its closely related orphan nuclear receptor genes in breast cancer cell line MCF-7. Biochem Biophys Res Commun. 1997;231:417–420. doi: 10.1006/bbrc.1997.6122. [DOI] [PubMed] [Google Scholar]
- 20.Yoon JK, Lau LF. Involvement of JunD in transcriptional activation of the orphan receptor gene nur77 by nerve growth factor and membrane depolarization in PC12 cells. Mol Cell Biol. 1994;14:7731–7743. doi: 10.1128/mcb.14.12.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang KH. Establishment of gastricarcinoma cell line MGC80-3 cells. Exp Biol Sinica. 1983;16:257–267. [Google Scholar]
- 22.Yang XJ, Chen SB, Bao JZ, Wang Y, Zhang ZB, Zhang XK, Zhang XR. Effect of HGF on etoposide-induced apoptosis. Chin J New Gastorenterol. 1997;5:518–519. [Google Scholar]
- 23.Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, Chen G, Lu J, Lin F, et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000;289:1159–1164. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- 24.Agadir A, Shealy YF, Hill DL, Zhang X. Retinyl methyl ether down-regulates activator protein 1 transcriptional activation in breast cancer cells. Cancer Res. 1997;57:3444–3450. [PubMed] [Google Scholar]
- 25.Jia L, Patwari Y, Srinivasula SM, Newland AC, Fernandes-Alnemri T, Alnemri ES, Kelsey SM. Bax translocation is crucial for the sensitivity of leukaemic cells to etoposide-induced apoptosis. Oncogene. 2001;20:4817–4826. doi: 10.1038/sj.onc.1204628. [DOI] [PubMed] [Google Scholar]
- 26.Custódio JB, Cardoso CM, Madeira VM, Almeida LM. Mitochondrial permeability transition induced by the anticancer drug etoposide. Toxicol In Vitro. 2001;15:265–270. doi: 10.1016/s0887-2333(01)00019-4. [DOI] [PubMed] [Google Scholar]
- 27.Godlewski MM, Motyl MA, Gajkowska B, Wareski P, Koronkiewicz M, Motyl T. Subcellular redistribution of BAX during apoptosis induced by anticancer drugs. Anticancer Drugs. 2001;12:607–617. doi: 10.1097/00001813-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Kim R, Inoue H, Tanabe K, Toge T. Effect of inhibitors of cysteine and serine proteases in anticancer drug-induced apoptosis in gastric cancer cells. Int J Oncol. 2001;18:1227–1232. doi: 10.3892/ijo.18.6.1227. [DOI] [PubMed] [Google Scholar]
- 29.Friedrich K, Wieder T, Von Haefen C, Radetzki S, Jänicke R, Schulze-Osthoff K, Dörken B, Daniel PT. Overexpression of caspase-3 restores sensitivity for drug-induced apoptosis in breast cancer cell lines with acquired drug resistance. Oncogene. 2001;20:2749–2760. doi: 10.1038/sj.onc.1204342. [DOI] [PubMed] [Google Scholar]
- 30.Hamamoto T, Seto H, Beppu T. Leptomycins A and B, new antifungal antibiotics. II. Structure elucidation. J Antibiot (Tokyo) 1983;36:646–650. doi: 10.7164/antibiotics.36.646. [DOI] [PubMed] [Google Scholar]
- 31.Hamamoto T, Uozumi T, Beppu T. Leptomycins A and B, new antifungal antibiotics. III. Mode of action of leptomycin B on Schizosaccharomyces pombe. J Antibiot (Tokyo) 1985;38:1573–1580. doi: 10.7164/antibiotics.38.1573. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida M, Nishikawa M, Nishi K, Abe K, Horinouchi S, Beppu T. Effects of leptomycin B on the cell cycle of fibroblasts and fission yeast cells. Exp Cell Res. 1990;187:150–156. doi: 10.1016/0014-4827(90)90129-x. [DOI] [PubMed] [Google Scholar]
- 33.Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 34.Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- 35.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Lee MO, Wang HG, Li Y, Hashimoto Y, Klaus M, Reed JC, Zhang X. Retinoic acid receptor beta mediates the growth-inhibitory effect of retinoic acid by promoting apoptosis in human breast cancer cells. Mol Cell Biol. 1996;16:1138–1149. doi: 10.1128/mcb.16.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Q, Liu S, Ding L, Ye X, Su W. PKCalpha translocation from mitochondria to nucleus is closely related to induction of apoptosis in gastric cancer cells. Sci China C Life Sci. 2002;45:237–244. doi: 10.1360/02yc9026. [DOI] [PubMed] [Google Scholar]
- 38.Wu Q. Distinct Ways of Retinoic Acid Receptors on Inhibition of AP-1 Activity in Gastric Cancer Cells. Chin J Biochem Mol Biol. 2001;17:430–435. [Google Scholar]
- 39.Uemura H, Chang C. Antisense TR3 orphan receptor can increase prostate cancer cell viability with etoposide treatment. Endocrinology. 1998;139:2329–2334. doi: 10.1210/endo.139.5.5969. [DOI] [PubMed] [Google Scholar]
- 40.Liu S, Wu Q, Chen ZM, Su WJ. The effect pathway of retinoic acid through regulation of retinoic acid receptor alpha in gastric cancer cells. World J Gastroenterol. 2001;7:662–666. doi: 10.3748/wjg.v7.i5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403–406. doi: 10.3748/wjg.v7.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang XW, Xie H. Presence of Fas and Bcl-2 proteins in BEL-7404 human hepatoma cells. World J Gastroenterol. 1998;4:540–543. doi: 10.3748/wjg.v4.i6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woronicz JD, Lina A, Calnan BJ, Szychowski S, Cheng L, Winoto A. Regulation of the Nur77 orphan steroid receptor in activation-induced apoptosis. Mol Cell Biol. 1995;15:6364–6376. doi: 10.1128/mcb.15.11.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu W, Youn HD, Liu JO. Thapsigargin-induced apoptosis involves Cabin1-MEF2-mediated induction of Nur77. Eur J Immunol. 2001;31:1757–1764. doi: 10.1002/1521-4141(200106)31:6<1757::aid-immu1757>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 45.Steff AM, Trop S, Maira M, Drouin J, Hugo P. Opposite ability of pre-TCR and alpha beta TCR to induce apoptosis. J Immunol. 2001;166:5044–5050. doi: 10.4049/jimmunol.166.8.5044. [DOI] [PubMed] [Google Scholar]
- 46.Katagiri Y, Takeda K, Yu ZX, Ferrans VJ, Ozato K, Guroff G. Modulation of retinoid signalling through NGF-induced nuclear export of NGFI-B. Nat Cell Biol. 2000;2:435–440. doi: 10.1038/35017072. [DOI] [PubMed] [Google Scholar]
- 47.Wada A, Fukuda M, Mishima M, Nishida E. Nuclear export of actin: a novel mechanism regulating the subcellular localization of a major cytoskeletal protein. EMBO J. 1998;17:1635–1641. doi: 10.1093/emboj/17.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sachdev S, Hannink M. Loss of IkappaB alpha-mediated control over nuclear import and DNA binding enables oncogenic activation of c-Rel. Mol Cell Biol. 1998;18:5445–5456. doi: 10.1128/mcb.18.9.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kudo N, Taoka H, Toda T, Yoshida M, Horinouchi S. A novel nuclear export signal sensitive to oxidative stress in the fission yeast transcription factor Pap1. J Biol Chem. 1999;274:15151–15158. doi: 10.1074/jbc.274.21.15151. [DOI] [PubMed] [Google Scholar]
- 50.Huang TT, Kudo N, Yoshida M, Miyamoto S. A nuclear export signal in the N-terminal regulatory domain of IkappaBalpha controls cytoplasmic localization of inactive NF-kappaB/IkappaBalpha complexes. Proc Natl Acad Sci USA. 2000;97:1014–1019. doi: 10.1073/pnas.97.3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukuda M, Gotoh Y, Nishida E. Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 1997;16:1901–1908. doi: 10.1093/emboj/16.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engel K, Kotlyarov A, Gaestel M. Leptomycin B-sensitive nuclear export of MAPKAP kinase 2 is regulated by phosphorylation. EMBO J. 1998;17:3363–3371. doi: 10.1093/emboj/17.12.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taagepera S, McDonald D, Loeb JE, Whitaker LL, McElroy AK, Wang JY, Hope TJ. Nuclear-cytoplasmic shuttling of C-ABL tyrosine kinase. Proc Natl Acad Sci USA. 1998;95:7457–7462. doi: 10.1073/pnas.95.13.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernandez PM, Brunel F, Jimenez MA, Saez JM, Cereghini S, Zakin MM. Nuclear receptors Nor1 and NGFI-B/Nur77 play similar, albeit distinct, roles in the hypothalamo-pituitary-adrenal axis. Endocrinology. 2000;141:2392–2400. doi: 10.1210/endo.141.7.7562. [DOI] [PubMed] [Google Scholar]
- 55.Pekarsky Y, Hallas C, Palamarchuk A, Koval A, Bullrich F, Hirata Y, Bichi R, Letofsky J, Croce CM. Akt phosphorylates and regulates the orphan nuclear receptor Nur77. Proc Natl Acad Sci USA. 2001;98:3690–3694. doi: 10.1073/pnas.051003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang HJ, Song MJ, Choung SY, Kim SJ, Le MO. Transcriptional induction of Nur77 by indomethacin that results in apoptosis of colon cancer cells. Biol Pharm Bull. 2000;23:815–819. doi: 10.1248/bpb.23.815. [DOI] [PubMed] [Google Scholar]
- 57.Meng AH, Ling YL, Zhang XP, Zhao XY, Zhang JL. CCK-8 inhibits expression of TNF-alpha in the spleen of endotoxic shock rats and signal transduction mechanism of p38 MAPK. World J Gastroenterol. 2002;8:139–143. doi: 10.3748/wjg.v8.i1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dai J, Yu SX, Qi XL, Bo AH, Xu YL, Guo ZY. Expression of bcl-2 and c-myc protein in gastric cancinoma and precancerous lesions. World J Gastroenterol. 1998;4(Suppl 2):84–85. [Google Scholar]
- 59.Liu HF, Liu WW, Fang DC, Men RP. Expression of bcl-2 protein in gastric carcinoma and its significance. World J Gastroenterol. 1998;4:228–230. doi: 10.3748/wjg.v4.i3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]


