Abstract
HMGB1 (High mobility group box 1) expressions in adenocarcinoma (AC) and squamous cell/adenosquamous (SC/ASC) carcinoma of gallbladder, as well as its prognostic significance, have not yet been evaluated. We investigated HMGB1 expression in 80 cases of AC gallbladder cancer and 52 cases of SC/ASC gallbladder cancer. Survival information was concomitantly collected. The association of HMGB1 expression with clinicopathological characteristics and the possible prognostic role of HMGB1 for two aforementioned subtypes of gallbladder cancers were also analyzed. siRNA technique was utilized to explore the role of HMGB1 in proliferation and invasion of gallbladder cancer cells in vitro. HMGB1 overexpression is present in AC and SC/ASC gallbladder cancers. HMGB1 expression significantly associates with growth and metastasis of AC and SC/ASC gallbladder cancers. In vitro cell experiments based on siRNA demonstrated that HMGB1 downregulation inhibits proliferation and invasion of gallbladder cancer cells. Kaplan-Meier analysis revealed that HMGB1 expression is negatively associated with overall survival time of patients with AC or SC/ASC gallbladder cancer. Cox multivariate analysis confirmed that HMGB1 is an independent risk factor for survival of patients with AC or SC/ASC gallbladder cancer. HMGB1 overexpression closely correlates with progression and poor prognosis of AC and SC/ASC gallbladder cancers.
Keywords: Gallbladder cancer, squamous cell carcinoma, adenosquamous carcinoma, HMGB1, prognosis
Introduction
Gallbladder cancer is the most aggressive malignancy of biliary tract characterized by dismal prognosis and high mortality [1]. It is generally diagnosed at advance stage with serosal invasion and metastasis to other organs because absence of noticeable clinical signs or symptoms in early stage [2]. Most gallbladder cancer of early stage is accidentally discovered during or after cholecystectomy. In comparison to palliative chemotherapy and radiotherapy, only surgical resection serves as an effective strategy for improved survival of patient with advanced gallbladder cancer [3].
Histopathologically, over 90% of gallbladder cancer is adenocarcinoma (AC) [4], other subtypes, including mucinous, papillary and squamous cell/adenosquamous (SC/ASC) subtypes, are rarely seen in clinical practice. SC/ASC subtype accounts for up to 10% of various gallbladder cancers [5]. However, only limited information is currently available regarding the clinicopathological and biological characteristics of SC/ASC gallbladder cancer since most prior reports are either single case reports or analyses of small sample size [5]. Studies of large sample are therefore warranted to promote a better understanding about SC/ASC gallbladder cancer. Although several biomarkers have been reported to associate with progression, invasion, metastasis and prognosis of AC gallbladder cancer [6-8], their clinical applications still remain unsatisfactory. More importantly, biomarker related to progression and prognosis of SC/ASC gallbladder cancer has not yet been fully explored.
HMGB1, a highly-conserved yet versatile nuclear protein, is originally discovered as a chromatin-associated factor that binds DNA and facilitates gene transcription [9]. However, in addition to the nuclear function, HMGB1 is also found in the cytoplasm and stroma cells, and able to act as an extracellular signaling molecule in a variety of biological processes, including inflammation, cell migration and tumor metastasis [9]. Some researchers have previously reported that overexpression or cytoplasmic location of HMGB1 correlates with proliferation and metastasis of many malignancies [10-12]. High expression of HMGB1 is present in different solid tumors, such as gastric cancer [13], colon cancer [14], nasopharyngeal cancer [15] and breast cancer [16]. Aberrant expression of HMGB1 also predicts a poor prognosis in some cancer types [17-19]. However, HMGB1 expressions in tissues of AC and SC/ASC gallbladder cancers, as well as its prognostic significance, have not yet been evaluated before.
Considering the important role of HMGB1 in tumor progression, invasion and metastasis, we first investigated the expression of HMGB1 in 80 cases of AC gallbladder cancer and 52 cases of SC/ASC gallbladder cancer by immunohistochemistry. The association of HMGB1 expression with various clinicopathological characteristics and the possible prognostic role of HMGB1 for two aforementioned subtypes of gallbladder cancer were also analyzed. siRNA (Small interfering RNA) was additionally utilized to explore the role of HMGB1 in proliferation and invasion of gallbladder cancer cells in vitro.
Materials and methods
Case selection
This study was approved by The Ethic Committee for Human Research of Fujian Medical College, and conducted in accordance with the Helsinki Declaration. Written informed consents were obtained form all participants. A total of 132 cases of gallbladder cancer that underwent surgical resection or biopsy were recruited from First Hospital of Quanzhou during January 1997 and December 2013. All patients received no prior chemotherapy and radiotherapy before the surgeries. Of 132 cases of gallbladder cancer, 80 were diagnosed as AC and 52 were SC/ASC. Twenty cases of cholelithiasis that underwent simple cholecystectomy were additionally included as the control. Immediately after surgical removal, tissue samples were fixed in 4% formaldehyde, dehydrated through graduated alcohol and then paraffin-embedded for subsequent immunohistochemistry. All diagnoses were based on histopathological examinations and clinical findings. Detailed clinicopathologic characteristics of all specimens were obtained from medical records and presented in Tables S1 and S2 (See Supplementary Materials, Tables S1 and S2). Survival information was collected through letters and phone calls.
Chemicals and antibodies
Mouse anti-human HMGB1 monoclonal antibody was acquired from Santa Cruz Biotech-nology Company (California, USA). Mouse anti-human β-actin, P53, Nm23, Ki-67, ER, PR and Her-2 polyclonal antibodies were obtained from Abcam (Massachusetts, USA). HRP-conjugated goat anti-mouse secondary antibody was procured from Zhongshan Biotechnology Company (Beijing, China).
Immunohistochemistry
Briefly, 4 μm thick sections were cut, deparaffinated in xylene and rehydrated through graduated alcohol. The sections were heated for 20 min in citrate buffer (PH 6.0, Maixin, China) in microwave oven to retrieve antigens. Endogenous peroxidase activity was inactivated in 3% hydrogen peroxide (Maixin, China) for 10 min in darkness, and then blocked in Ultra V Block (Lab Vision Corporation, USA) for 30 min. The sections were incubated with primary antibody in a humid chamber at 4°C overnight. The primary antibody was removed on the following day and the slides were rinsed in PBS (PH 7.0, Maixin, China) and incubated with HRP-conjugated secondary antibody for 1 h at room temperature. Immunohistochemical labeling was revealed using the DAB-Chromogen System (Dako Laboratories, USA) in accordance with the user manual. Substitution of primary antibody with PBS was served as blank control. Microphotographs were captured using an Olympus microscope (Olympus, Japan).
Immunohistochemical scoring
Immunostaining of HMGB1 was analyzed using a semi-quantitative scoring method in which the extent and intensity of immunostaining were concurrently evaluated [20]. In order to explore the association of HMGB1 expression with clinicopathological characteristics and survival, we defined moderate and strong staining as positive, the others as negative. For each section, a total of 800 cells in 20 random microscopic fields at the magnification of × 200 were evaluated. The immunostaining of HMGB1 was evaluated by two independent pathologists in a blinded manner. A consensus score was achieved by reevaluation and discussion if there were any discrepancies.
Cell culture
Two human gallbladder cancer cell lines, TGBC1 and TGBC2, were obtained from Riken BRC Cell Bank (Ibaraki, Japan). Another human gallbladder cancer cell line GBC-SD was procured from Shanghai Institutes of Biological Sciences of China. Cells were maintained in Dulbecco’s Modified Eagle’s Medium (Gibco, USA) supplemented with 10% fetal bovine serum (Thermo Scientific, USA) and 1% penicillin-streptomycin cocktail (Sigma-Aldrich, USA). Cells were cultivated at 37°C in a humidified atmosphere of 5% CO2.
Knockdown of HMGB1 expression by siRNA
siRNA against HMGB1 and the negative control siRNA were synthesized by and purchased from OriGene Technologies (OriGene, USA). siRNA transfection of GBC-SD cells was conducted using Lipofectamine 2000 kit (Invitrogen, USA) according to the user manual. 24 h after the transfection, cells were retrieved, resuspended and subjected to proliferation and invasion assays.
Assessment of cell proliferation
Cell proliferation was measured using the Cell Counting Kit-8 (CCK-8, Dojindo, USA), in accordance with the manufacturer’s instruction. The absorbance at 450 nm was detected with the Infinite M1000 microplate reader (TECAN, Austria).
Matrigel invasion assay
Cell invasion was examined by assessing the movement of cells into an artificial basal membrane of Matrigel-coated Transwell chambers (BD Biosciences, USA) as described previously [21].
Immunoblot
Cells were harvested through trypsinization and centrifugation, lysed subsequently in RIPA buffer (Sigma-Aldrich, USA) supplemented with protease inhibitor cocktail (Roche, Germany). The lysate was then sonicated for 30 sec and centrifuged for 20 min. The supernatant was withdrawn and the protein concentration was assessed using BCA protein assay kit (Takara, Japan). Immunoblot was conducted as previously reported [22]. Immunoreactive signal was developed using the SuperSignal West Pico Chemiluminiscence Kit (Thermo Scientific, USA), visualized and captured with the ChemiDoc XRS Plus System (Bio-Rad, USA).
Statistical analysis
The data are expressed as mean ± s.d., and analyzed by SPSS 16.0 (Illinois, USA). The χ2 test or Fisher’s exact probability test was used to investigate the statistical association between HMGB1 expression and clinicopathological characteristics. Kaplan-Meier method and time series test were applied to performed univariate survival analysis. Cox proportional hazards model was used to performed multivariate analysis and calculate the 95% confidence interval (95% CI). A P value of less than 0.05 was regarded as statistically significant.
Results
Comparison of HMGB1 expression in tissues of cholelithiasis gallbladder, AC gallbladder cancer and SC/ASC gallbladder cancer
With immunohistochemistry, we evaluated the expression of HMGB1 in tissues of AC and SC/ASC gallbladder cancers. Benign tissues of gallbladder with cholelithiasis were included as the control. As illustrated in Figure 1B and 1D, immunoreactivity of HMGB1, a well-known nuclear protein, is predominantly localized to the nuclei but is also diffusively present in the cytoplasm in both AC and SC/ASC gallbladder malignancies. However, no cytoplasmic immunoreactivity of HMGB1 is observed in benign gallbladder tissues of cholelithiasis (Figure 1F). Moreover, as summarized in Tables S1 and S2, of 52 cases of SC/ASC gallbladder cancer, 59.6% (31/52) were determined as positive for HMGB1 expression, showing a slight increase as compared to that (48.8%, 39/80) of AC gallbladder cancer (P>0.05). In contrast, 0% (0/20) of cholelithiasis gallbladder are positive for HMGB1, displaying a markedly decrease when compared to the AC or SC/ASC counterpart (All P<0.05).
Figure 1.
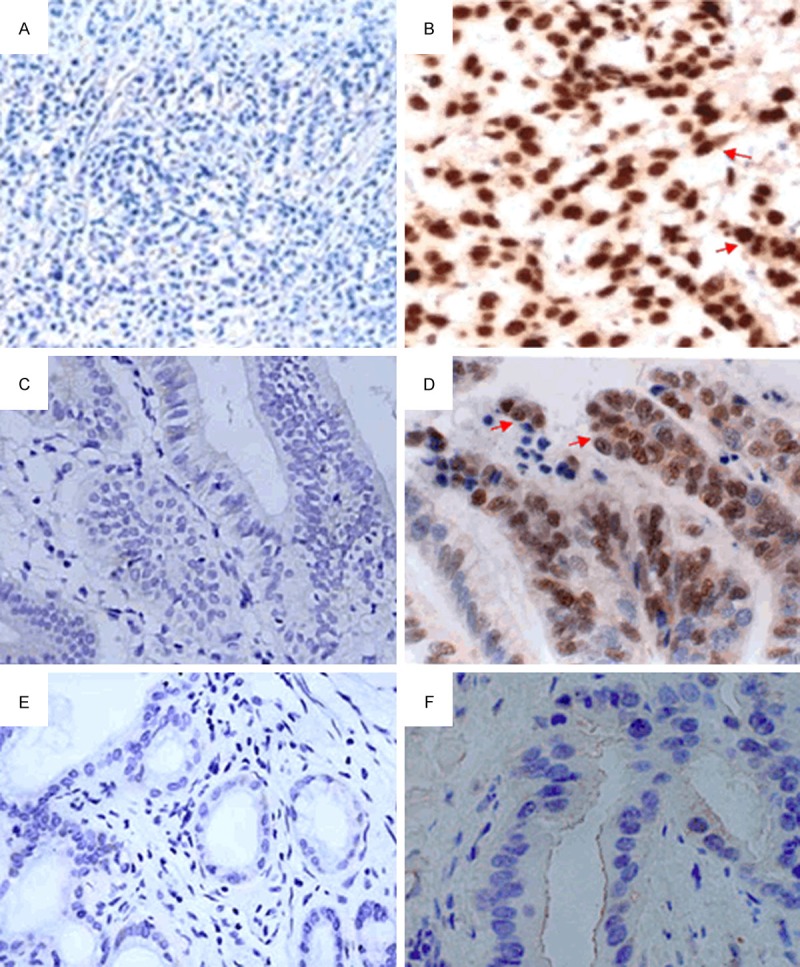
HMGB1 expression in tissues of cholelithiasis gallbladder, AC gallbladder cancer and SC/ASC gallbladder cancer. A. Blank control of Panel B, primary antibody was replaced by PBS. B. Positive HMGB1 expression in poorly-differentiated SC/ASC gallbladder cancer. HMGB1 staining is predominantly localized to the nuclei but also diffusively present in the cytoplasm (Red arrow). C. Blank control of Panel D. D. Positive HMGB1 expression in moderately-differentiated AC gallbladder cancer, HMGB1 staining is mainly found in the nuclei but also present in the cytoplasm (Red arrow). E. Blank control of Panel F. F. Negative HMGB1 expression in benign tissue of cholelithiasis gallbladder. PBS, Phosphate-buffered saline; AC, Adenocarcinoma; SC/ASC, Squamous cell/adenosquamous. Original magnification × 100 for Panel A, C and E; Original magnification × 200 for Panel B, D and F.
Association of HMGB1 expression with clinicopathological characteristics of AC gallbladder cancer
As shown in Table S1, we found that significantly more cases of AC gallbladder cancer with maximal tumor diameter exceeding 3 cm are positive for HMGB1 expression as compared to those with maximal tumor diameter less than 3 cm (64.5% vs. 38.8%, P<0.05). In addition, HMGB1 expression also positively associates with expression of Ki-67, a reliable indicator of cell proliferation (53.8% for HMGB1+ Ki-67+ vs. 26.7% for HMGB1+ Ki-67-, P<0.05). These two lines of findings seem to indicate a promoting effect of HMGB1 on the growth of AC gallbladder cancer. Moreover, the percentage of AC gallbladder cancer concurrently positive for HMGB1 and ER is higher than that of AC gallbladder cancer only positive for HMGB1 (55.7% for HMGB1+ ER+ vs. 26.3% for HMGB1+ ER-, P<0.05). However, HMGB1 expression correlates negatively with the expression of Nm23, a protein capable of repressing metastasis (31.3% for HMGB1+ Nm23+ vs. 53.1% for HMGB1+ Nm23-, P<0.05). This correlation suggests that HMGB1 may be implicated in the metastasis of AC gallbladder cancer. HMGB1 expression shows no significant association with other clinicopathological characteristics of AC gallbladder cancer.
Association of HMGB1 expression with clinicopathological characteristics of SC/ASC gallbladder cancer
As presented in Table S2, we discovered a negative correlation between HMGB1 expression and differentiation grade of SC/ASC gallbladder cancer; significantly more cases of poorly-differentiated SC/ASC gallbladder cancer are positive for HMGB1 expression (86.7%, 13/15) when compared to the counterpart of moderately-differentiated (55.6%, 10/18) or well-differentiated (42.1%, 8/19) SC/ASC gallbladder cancer (P<0.05). Furthermore, HMGB1 expression associates positively with lymph node metastasis (71.0% for HMGB1+ and lymph node metastasis vs. 42.9% for HMGB1+ but no lymph node metastasis, P<0.05). Similar to the abovementioned findings, we found that HMGB1 expression also correlates positively with maximal tumor diameter (80.0% for HMGB1+ and maximal tumor diameter >3 cm vs. 46.9% for HMGB1+ and maximal tumor diameter ≤3 cm), ER expression (67.5% for HMGB1+ ER+ vs. 33.3% for HMGB1+ ER-) and Ki-67 expression (67.4% for HMGB1+ Ki-67+ vs. 22.2% for HMGB1+ Ki-67-), but correlated negatively with Nm23 expression (66.7% for HMGB1+ Nm23- vs. 30.0% for HMGB1+ Nm23+) (All P<0.05). These evidences suggest that HMGB1 expression may be involved in growth and metastasis of SC/ASC gallbladder cancer.
Prognostic value of HMGB1 expression in patients with AC gallbladder cancer
Survival information of 80 cases of AC gallbladder cancer was obtained via letters or phone calls. 24 cases survived more than 1 year and the remaining 56 cases survived less than 1 year, with an average survival time of 12.1 months. Patients that survived more than 1 year have a lower percentage of HMGB1-postive cases as compared to those survived less than 1 year (45.8% vs. 73.2%, P=0.019). As summarized in Table S3 (See Supplementary Materials, Table S3), Kaplan-Meier survival analysis showed that HMGB1 expression, tumor diameter, TNM stage, lymph node metastasis, ER expression, Ki-67 expression and Nm23 expression are associated with average survival time of patients with AC gallbladder cancer (All P<0.05). Average survival time for HMGB1-positve patients of AC gallbladder cancer is significantly shorter than that of HMGB1-negative patients (9.5 months vs. 20.1 months, P<0.05, Table S3; Figure 2B). Cox proportional hazard regression model was additionally used to obtain a more accurate estimate about prognosis. As revealed in Table S4 (See Supplementary Materials, Table S4), the results confirmed that tumor diameter (HR=1.58), HMGB1 expression (HR=3.05), TNM stage (HR=1.77), lymph node metastasis (HR=3.31) and Ki-67 expression (HR=2.43) are negatively associated with post-operative survival (All P<0.05), but Nm23 expression is positively associated with post-operative survival (HR=2.86, P<0.05), demonstrating that high HMGB1 expression is an independent prognostic factor for patient with AC gallbladder cancer.
Figure 2.
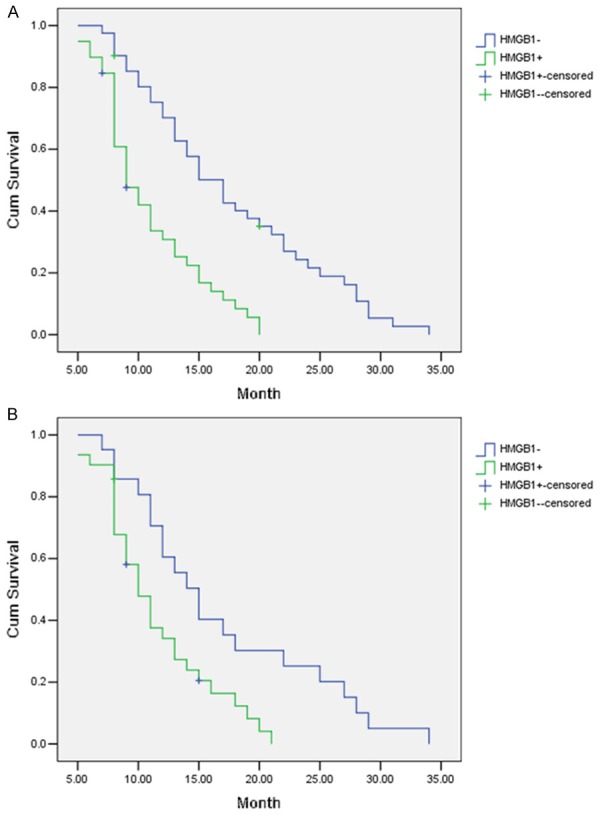
HMGB1 expression and survival in patients with SC/ASC or AC gallbladder cancer. A. Kaplan-Meier plot of overall survival in HMGB1-positive or HMGB1-negative patients with SC/ASC gallbladder cancer. B. Kaplan-Meier plot of overall survival in HMGB1-positive or HMGB1-negative patients with AC gallbladder cancer. AC, Adenocarcinoma; SC/ASC, Squamous cell/adenosquamous.
Prognostic value of HMGB1 expression in patients with SC/ASC gallbladder cancer
Survival information is available for 52 cases of SC/ASC gallbladder cancer. Of which, 16 cases survived longer than 1 year and the other 36 cases survived shorter than 1 year. The average survival time is 11.4 months. Patients that survived longer than 1 year have a lower percentage of HMGB1-postive cases when compared to those survived shorter than 1 year (50.0% vs. 81.3%, P=0.001). Kaplan-Meier survival analysis revealed that HMGB1 expression, differentiation grade, tumor diameter, TNM stage, lymph node metastasis, regional invasion, ER expression, Ki-67 expression and Nm23 expression are significantly associated with average survival time of patients with SC/ASC gallbladder cancer (All P<0.05, Table S5 [See Supplementary Materials, Table S5]). Average survival time for HMGB1-positve patients of SC/ASC gallbladder cancer is significantly shorter than that of HMGB1-negative patients (10.4 months vs. 21.5 months, P<0.05, Table S5; Figure 2A). Cox proportional hazard regression model was also applied to gain a more precise estimate regarding the prognosis. The results confirmed that HMGB1 expression (HR=3.09), differentiation grade (HR=3.20), tumor diameter (HR=1.76), lymph node metastasis (HR=3.25) and Ki-67 expression (HR=2.30) are negatively associated with post-operative survival (All P<0.05, Table S6 [See Supplementary Materials, Table S6]), whereas Nm23 expression is positively associated with post-operative survival (HR=3.81, P<0.05, Table S6), indicating that high HMGB1 expression is indeed an independent risk factor for prognosis of patient with SC/ASC gallbladder cancer.
In vitro effect of HMGB1 downregulation on proliferation and invasion of gallbladder cancer cells
In light of the abovementioned in vivo findings, we went on exploring the in vitro involvement of HMGB1 in gallbladder cancer. Three human gallbladder cancer cell lines, GBC-SD, TGBC-1 and TGBC-2, were selected. Immunoblot analysis showed that HMGB1 expression is indeed present in gallbladder cancer cells (Figure 3A). Next, HMGB1 expression was deliberately downregulated by siRNA technique (Figure 3A); influence of HMGB1 downregulation on proliferation and invasion was subsequently investigated by CCK-8 test and Matrigel invasion assay. We found that siRNA-induced downregulation of HMGB1 results in significant reduction in both proliferation and invasion of GBC-SD, TGBC-1 and TGBC-2 cells (Figure 3B and 3C, all P<0.05). Additionally, morphological result from Giemsa staining also confirmed the inhibitory effect on proliferation and invasion of GBC-SD, TGBC-1 and TGBC-2 cells (See Figure 4A-F for morphology of GBC-SD; Figures not shown for TGBC-1 and TGBC-2). Taken together, these in vitro evidences seem to suggest that elevated expression of HMGB1 may play a promoting role in progression and metastasis of gallbladder cancer.
Figure 3.
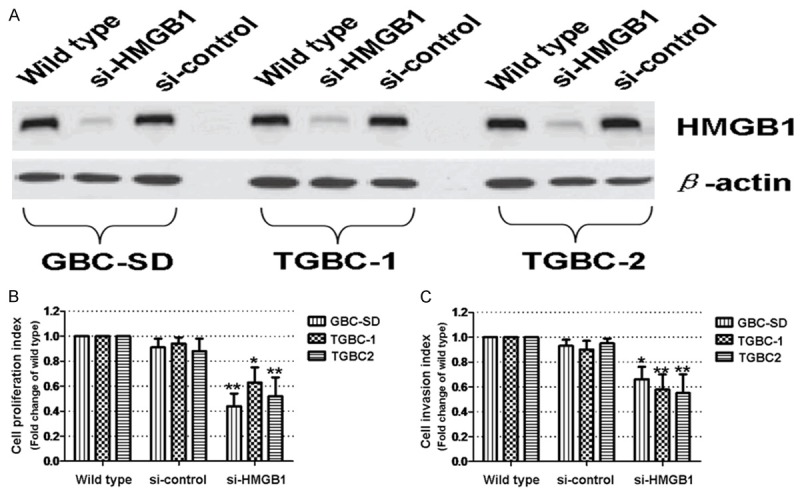
Effect of HMGB1 downregulation on proliferation and invasion of gallbladder cancer cells. A. siRNA-induced downregulation of HMGB1 in GBC-SD, TGBC-1 and TGBC-2 cells. β-actin was applied as the loading control. B. Effect of HMGB1 downregulation on proliferation of GBC-SD, TGBC-1 and TGBC-2 cells. Data are expressed as fold change of the wild type control (Which is assumed as 1). C. Effect of HMGB1 downregulation on invasion of GBC-SD, TGBC-1 and TGBC-2 cells. Data are similarly expressed as aforementioned. The results are representative of at least three separate experiments and shown as mean ± s.d. *P<0.05; **P<0.01, compared to the wild type control.
Figure 4.
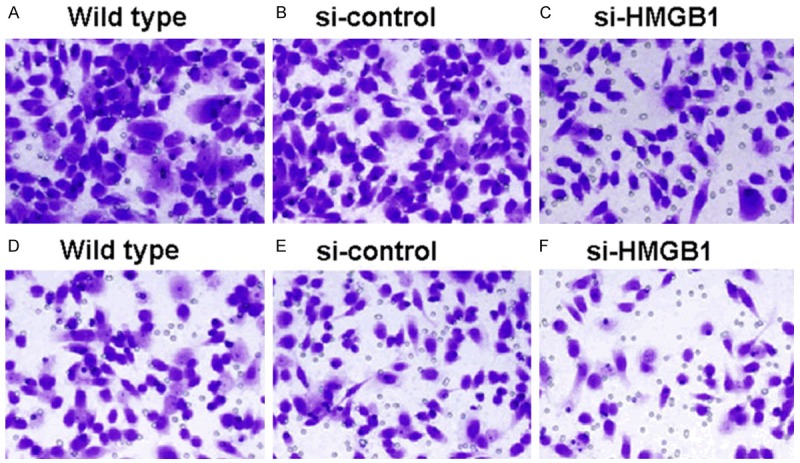
Proliferation and invasion of GBC-SD cells receiving different treatments. A-C. Proliferation of GBC-SD cells receiving indicated treatments. D-F. Invasion of GBC-SD cells receiving indicated treatments. Cells were stained with Giemsa solution, original magnification at × 200.
Discussion
HMGB1 is a highly-conserved chromatin-binding chaperone that bends DNA and promotes access to transcriptional protein assemblies on specific DNA target [9]. HMGB1 has been reported to involve in various disease states, including sepsis [23], ischemia-reperfusion [24], arthritis [25], meningitis [26], neurodegeneration [27], senescence [28] and carcinogenesis [9]. Numerous previous studies demonstrated that HMGB1 expression is significantly elevated in a number of cancer types, including Non-Hodgkin lymphoma [29], colon cancer [18], prostate cancer [30] and cervical cancer [31].
Gallbladder cancer is a malignancy of high aggressiveness, rapid proliferation and poor prognosis. It is therefore necessary to explore reliable biomarkers associated with tumor formation, proliferation and survival. However, studies regarding clinicopathological and biological characteristics of gallbladder cancer, especially the rarely-seen SC/ASC type, are scarce owing to the low incidence. To date, only a few molecular markers have been reported to correlate with tumor invasion, aggression, metastasis and prognosis of gallbladder cancer [6-8]. Due to its rarity, studies of large sample size are thus warranted to systemically explore the clinicopathological and biological signatures of gallbladder cancer.
In this study we recruited substantial specimens of both AC and SC/ASC gallbladder cancers, thus enabling us to gain a more accurate understanding about the molecular signatures of AC and SC/ASC gallbladder cancers. Although prior studies showed that HMGB1 is highly expressed in a numbers of human malignancies [6-8], its expression in different subtypes of gallbladder cancer has not yet been reported before. With immunohistochemistry, we measured the expression of HMGB1 and found that 59.6% of SC/ASC gallbladder cancer and 48.8% of AC gallbladder are respectively positive for HMGB1 expression (Tables S1 and S2). This finding indicates that HMGB1 overexpression associates with gallbladder cancer. Previous studies demonstrated that HMGB1 expression is significantly elevated in a number of cancer types [18,29,31]. Expression of HMGB1 mRNA in prostate cancer is markedly higher than that of the normal prostate tissue [30]. Increased amount of HMGB1 expression is also found in malignant melanoma cells [32]. In agreement with these previous studies, our finding highlights the important role of HMGB1 in gallbladder cancer.
Moreover, we discovered that HMGB1 overexpression associates with tumor growth and metastasis of both AC and SC/ASC gallbladder cancers (Tables S1 and S2), a discovery also substantiated by the results of Kaplan-Meier survival analysis and Cox proportional hazard regression analysis (Tables S3, S4, S5 and S6). Additionally, in vitro cell experiments based on siRNA technique demonstrated that downregulation of HMGB1 inhibits proliferation and invasion of gallbladder cancer cells (Figures 3 and 4), confirming that HMGB1 overexpression correlates with growth and metastasis of gallbladder cancer. Albeit many previous studies have already explored the potential role of HMGB1 in tumor growth and metastasis, it still remains as a controversial hotspot. HMGB1 has been showed to play paradoxical roles in cell survival and death through regulation of multiple signal pathways [33]. HMGB1 exhibits dual functions, and can either be pro-tumor or anti-tumor, depending on the biological context [33]. The in vitro study by Palumbo showed that HMGB1 enhances migration and proliferation of adult and embryonic mesoangioblasts, and disrupts the barrier effect of endothelial monolayers [11]. Additionally, Kuniyasu found that cell growth, migration and invasion are markedly suppressed in Colo320 and WiDr carci-noma cells treated with HMGB1 antisense S-oligodeoxynucleotide when compared to the sense-treated cells [34]. Furthermore, Taguchi demonstrated in a animal study that inhibition of RAGE (Receptors for advanced glycation end-products)-HMGB1 interaction represses tumor growth and metastasis via activation of MAPK and NF-κB pathways, and concurrently up-regulates the expressions of MMP2 and MMP9, proteins that degrade extracellular matrix proteins and promote tumor invasion and metastasis [35]. Todorova J and Pasheva E also proved in their recent study that suppression of HMGB1-RAGE interaction represses tumor growth and metastasis of lung cancer [36]. Our finding, together with these previous studies, seems to argue and underscore that HMGB1 expression may enhance tumor growth and promotes metastasis of AC and SC/ASC gallbladder cancers.
No previous studies have investigated the prognostic role of HMGB1 for gallbladder cancer. We are the first to analyze its prognostic value for gallbladder cancer. Kaplan-Meier analysis revealed that HMGB1 expression is negatively associated with overall survival of patients with AC or SC/ASC gallbladder cancer. Cox multivariate analysis also confirmed that HMGB1 is an independent risk factor in predicting overall survival time of patients with AC or SC/ASC gallbladder cancer. Taken together, these results seem to suggest a potential role of HMGB1 in promoting progression and malignancy of gallbladder cancer, as well as the possible application of HMGB1 as a novel prognostic biomarker for gallbladder cancer. In fact, the systematic review by Tang proposed that HMGB1 plays pivotal roles in cancer [33]. HMGB1 overexpression correlates with each of the hallmarks of cancer, including unlimited replication, re-angiogenesis, evasion of apoptosis, growth signal autonomy, insensitivity to anti-growth signal, pro-inflammation and tissue invasion and metastasis [33]. Survival analysis of patients with colorectal cancer demonstrated a significant poorer prognosis for patients with co-expression of RAGE and HMGB1 as compared to patients without the co-expression [37]. Furthermore, Chung also showed that HMGB1 level increases as gastric cancer progresses, and serum HMGB1 level associates significantly with invasion, lymph node metastasis, tumor size and poor prognosis [38]. These evidences clearly indicate a promoting role of HMGB1 in progression and metastasis of gallbladder cancer, a notion that coincides well with our finding that HMGB1 overexpression associates with poor prognosis of patients with AC or SC/ASC gallbladder cancer.
In summary, our findings suggest that HMGB1 overexpression is closely associated with progression and poor prognosis of AC and SC/ASC gallbladder cancers. HMGB1 may be a novel prognostic biomarker and therapeutic target for gallbladder cancer.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Gourgiotis S, Kocher HM, Solaini L, Yarollahi A, Tsiambas E, Salemis NS. Gallbladder cancer. Am J Surg. 2008;196:252–264. doi: 10.1016/j.amjsurg.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins WG, DeMatteo RP, Jarnagin WR, Ben-Porat L, Blumgart LH, Fong Y. Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol. 2004;11:310–315. doi: 10.1245/aso.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Reid KM, Ramos-De la Medina A, Donohue JH. Diagnosis and surgical management of gallbladder cancer: a review. J Gastrointest Surg. 2007;11:671–681. doi: 10.1007/s11605-006-0075-x. [DOI] [PubMed] [Google Scholar]
- 4.Ootani T, Shirai Y, Tsukada K, Muto T. Relationship between gallbladder carcinoma and the segmental type of adenomyomatosis of the gallbladder. Cancer. 1992;69:2647–2652. doi: 10.1002/1097-0142(19920601)69:11<2647::aid-cncr2820691105>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Roa JC, Tapia O, Cakir A, Basturk O, Dursun N, Akdemir D, Saka B, Losada H, Bagci P, Adsay NV. Squamous cell and adenosquamous carcinomas of the gallbladder: clinicopathological analysis of 34 cases identified in 606 carcinomas. Mod Pathol. 2011;24:1069–1078. doi: 10.1038/modpathol.2011.68. [DOI] [PubMed] [Google Scholar]
- 6.Li M, Shen J, Wu X, Zhang B, Zhang R, Weng H, Ding Q, Tan Z, Gao G, Mu J, Yang J, Shu Y, Bao R, Ding Q, Wu W, Cao Y, Liu Y. Downregulated expression of hepatoma-derived growth factor (HDGF) reduces gallbladder cancer cell proliferation and invasion. Med Oncol. 2013;30:587. doi: 10.1007/s12032-013-0587-7. [DOI] [PubMed] [Google Scholar]
- 7.Li M, Zhang S, Wang Z, Zhang B, Wu X, Weng H, Ding Q, Tan Z, Zhang N, Mu J, Yang J, Shu Y, Bao R, Ding Q, Wu W, Cao Y, Liu Y. Prognostic significance of nemo-like kinase (NLK) expression in patients with gallbladder cancer. Tumour Biol. 2013;34:3995–4000. doi: 10.1007/s13277-013-0988-4. [DOI] [PubMed] [Google Scholar]
- 8.Yuan LW, Liu DC, Yang ZL. Correlation of S1P1 and ERp29 expression to progression, metastasis, and poor prognosis of gallbladder adenocarcinoma. Hepatobiliary Pancreat Dis Int. 2013;12:189–195. doi: 10.1016/s1499-3872(13)60030-2. [DOI] [PubMed] [Google Scholar]
- 9.Muller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A, Beltrame M, Bianchi ME. New EMBO members’ review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 2001;20:4337–4340. doi: 10.1093/emboj/20.16.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusume A, Sasahira T, Luo Y, Isobe M, Nakagawa N, Tatsumoto N, Fujii K, Ohmori H, Kuniyasu H. Suppression of dendritic cells by HMGB1 is associated with lymph node metastasis of human colon cancer. Pathobiology. 2009;76:155–162. doi: 10.1159/000218331. [DOI] [PubMed] [Google Scholar]
- 11.Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164:441–449. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang ZT, Yao YM, Sheng ZY. Effect of high mobility group box 1 protein on proliferation and apoptosis and balance between Th1/Th2 and Tc1/Tc2 of lymphocytes in vitro. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2008;24:324–327. [PubMed] [Google Scholar]
- 13.Choi YR, Kim H, Kang HJ, Kim NG, Kim JJ, Park KS, Paik YK, Kim HO, Kim H. Overexpression of high mobility group box 1 in gastrointestinal stromal tumors with KIT mutation. Cancer Res. 2003;63:2188–2193. [PubMed] [Google Scholar]
- 14.Volp K, Brezniceanu ML, Bosser S, Brabletz T, Kirchner T, Gottel D, Joos S, Zornig M. Increased expression of high mobility group box 1 (HMGB1) is associated with an elevated level of the antiapoptotic c-IAP2 protein in human colon carcinomas. Gut. 2006;55:234–242. doi: 10.1136/gut.2004.062729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu D, Ding Y, Wang S, Zhang Q, Liu L. Increased expression of high mobility group box 1 (HMGB1) is associated with progression and poor prognosis in human nasopharyngeal carcinoma. J Pathol. 2008;216:167–175. doi: 10.1002/path.2391. [DOI] [PubMed] [Google Scholar]
- 16.He Q, Liang CH, Lippard SJ. Steroid hormones induce HMG1 overexpression and sensitize breast cancer cells to cisplatin and carboplatin. Proc Natl Acad Sci U S A. 2000;97:5768–5772. doi: 10.1073/pnas.100108697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuniyasu H, Oue N, Wakikawa A, Shigeishi H, Matsutani N, Kuraoka K, Ito R, Yokozaki H, Yasui W. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J Pathol. 2002;196:163–170. doi: 10.1002/path.1031. [DOI] [PubMed] [Google Scholar]
- 18.Kuniyasu H, Sasaki T, Sasahira T, Ohmori H, Takahashi T. Depletion of tumor-infiltrating macrophages is associated with amphoterin expression in colon cancer. Pathobiology. 2004;71:129–136. doi: 10.1159/000076467. [DOI] [PubMed] [Google Scholar]
- 19.Sasahira T, Akama Y, Fujii K, Kuniyasu H. Expression of receptor for advanced glycation end products and HMGB1/amphoterin in colorectal adenomas. Virchows Arch. 2005;446:411–415. doi: 10.1007/s00428-005-1210-x. [DOI] [PubMed] [Google Scholar]
- 20.Pinheiro C, Longatto-Filho A, Scapulatempo C, Ferreira L, Martins S, Pellerin L, Rodrigues M, Alves VA, Schmitt F, Baltazar F. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch. 2008;452:139–146. doi: 10.1007/s00428-007-0558-5. [DOI] [PubMed] [Google Scholar]
- 21.Kono H, Nakamura M, Ohtsuka T, Nagayoshi Y, Mori Y, Takahata S, Aishima S, Tanaka M. High expression of microRNA-155 is associated with the aggressive malignant behavior of gallbladder carcinoma. Oncol Rep. 2013;30:17–24. doi: 10.3892/or.2013.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geng ZM, Zhang M, Pan XT, Wang L. Bcl-2 gene silencing by RNA interference inhibits the growth of the human gallbladder carcinoma cell line GBC-SD in vitro and in vivo. Oncol Rep. 2013;30:793–800. doi: 10.3892/or.2013.2539. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255:320–331. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson U, Tracey KJ. HMGB1 as a mediator of necrosis-induced inflammation and a therapeutic target in arthritis. Rheum Dis Clin North Am. 2004;30:627–637. xi. doi: 10.1016/j.rdc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Tang D, Kang R, Cao L, Zhang G, Yu Y, Xiao W, Wang H, Xiao X. A pilot study to detect high mobility group box 1 and heat shock protein 72 in cerebrospinal fluid of pediatric patients with meningitis. Crit Care Med. 2008;36:291–295. doi: 10.1097/01.CCM.0000295316.86942.CE. [DOI] [PubMed] [Google Scholar]
- 27.Qi ML, Tagawa K, Enokido Y, Yoshimura N, Wada Y, Watase K, Ishiura S, Kanazawa I, Botas J, Saitoe M, Wanker EE, Okazawa H. Proteome analysis of soluble nuclear proteins reveals that HMGB1/2 suppress genotoxic stress in polyglutamine diseases. Nat Cell Biol. 2007;9:402–414. doi: 10.1038/ncb1553. [DOI] [PubMed] [Google Scholar]
- 28.Enokido Y, Yoshitake A, Ito H, Okazawa H. Age-dependent change of HMGB1 and DNA double-strand break accumulation in mouse brain. Biochem Biophys Res Commun. 2008;376:128–133. doi: 10.1016/j.bbrc.2008.08.108. [DOI] [PubMed] [Google Scholar]
- 29.Meyer A, Staratschek-Jox A, Springwald A, Wenk H, Wolf J, Wickenhauser C, Bullerdiek J. Non-Hodgkin lymphoma expressing high levels of the danger-signalling protein HMGB1. Leuk Lymphoma. 2008;49:1184–1189. doi: 10.1080/10428190802064909. [DOI] [PubMed] [Google Scholar]
- 30.Ishiguro H, Nakaigawa N, Miyoshi Y, Fujinami K, Kubota Y, Uemura H. Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate. 2005;64:92–100. doi: 10.1002/pros.20219. [DOI] [PubMed] [Google Scholar]
- 31.Hao Q, Du XQ, Fu X, Tian J. Expression and clinical significance of HMGB1 and RAGE in cervical squamous cell carcinoma. Zhonghua Zhong Liu Za Zhi. 2008;30:292–295. [PubMed] [Google Scholar]
- 32.Poser I, Golob M, Buettner R, Bosserhoff AK. Upregulation of HMG1 leads to melanoma inhibitory activity expression in malignant melanoma cells and contributes to their malignancy phenotype. Mol Cell Biol. 2003;23:2991–2998. doi: 10.1128/MCB.23.8.2991-2998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang D, Kang R, Zeh HJ 3rd, Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta. 2010;1799:131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuniyasu H, Chihara Y, Kondo H. Differential effects between amphoterin and advanced glycation end products on colon cancer cells. Int J Cancer. 2003;104:722–727. doi: 10.1002/ijc.11016. [DOI] [PubMed] [Google Scholar]
- 35.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 36.Todorova J, Pasheva E. High mobility group B1 protein interacts with its receptor RAGE in tumor cells but not in normal tissues. Oncol Lett. 2012;3:214–218. doi: 10.3892/ol.2011.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuniyasu H, Chihara Y, Takahashi T. Coexpression of receptor for advanced glycation end products and the ligand amphoterin associates closely with metastasis of colorectal cancer. Oncol Rep. 2003;10:445–448. [PubMed] [Google Scholar]
- 38.Chung HW, Lee SG, Kim H, Hong DJ, Chung JB, Stroncek D, Lim JB. Serum high mobility group box-1 (HMGB1) is closely associated with the clinical and pathologic features of gastric cancer. J Transl Med. 2009;7:38. doi: 10.1186/1479-5876-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


