Abstract
An updated model of the GABA(A) benzodiazepine receptor pharmacophore of the α5-BzR/GABA(A) subtype has been constructed prompted by the synthesis of subtype selective ligands in light of the recent developments in both ligand synthesis, behavioral studies, and molecular modeling studies of the binding site itself. A number of BzR/GABA(A) α5 subtype selective compounds were synthesized, notably α5-subtype selective inverse agonist PWZ-029 (1) which is active in enhancing cognition in both rodents and primates. In addition, a chiral positive allosteric modulator (PAM), SH-053-2′F-R-CH3 (2), has been shown to reverse the deleterious effects in the MAM-model of schizophrenia as well as alleviate constriction in airway smooth muscle. Presented here is an updated model of the pharmacophore for α5β2γ2 Bz/GABA(A) receptors, including a rendering of PWZ-029 docked within the α5-binding pocket showing specific interactions of the molecule with the receptor. Differences in the included volume as compared to α1β2γ2, α2β2γ2, and α3β2γ2 will be illustrated for clarity. These new models enhance the ability to understand structural characteristics of ligands which act as agonists, antagonists, or inverse agonists at the Bz BS of GABA(A) receptors.
1. Introduction
The gamma-amino butyric acid A (GABAA) receptor is a heteropentameric chloride ion channel. This channel is generally made up of two α-subunits, two β-subunits, and a single γ-subunit arranged in an αβαβγ fashion. The GABAA receptors (GABAAR) are responsible for a myriad of brain functions. Positive allosteric modulators (PAMs) and negative allosteric modulators (NAMs) act on the benzodiazepine (BZ) site of the GABAAR which can change the conformation of the receptor to inhibit or excite the neurons associated with the ion channel. To date, researchers have been unable to get an X-ray crystal structure of a functional Bz/GABAAR ion channel. Recently, Miller and Aricescu [1] have reported the crystal structure of a homopentameric GABAAR containing the β3-subunit at 3 Å resolution. Although this work provides great promise that other heteropentameric GABAARs will be crystallized in the near future, molecular modeling and structure-activity-relationships (SARs) still remain key tools to find better subtype-selective binding agents.
2. Subtype Selective Ligands for α5 GABA(A)/Bz Receptors
Interest in BzR/GABA(A) α5 subtypes began years ago when it was realized that α5β3γ2 Bz/GABA(A) subtypes are located primarily in the hippocampus. More recently this interest has been confirmed by the report of Möhler et al. [2–5] on α5 “knock-in mice.” This group has provided strong evidence that hippocampal extrasynaptic α5 GABA(A) receptors play a critical role in associative learning as mentioned above [6–11].
Earlier we synthesized a series of α5 subtype selective ligands (RY-023, RY-024, RY-079, and RY-080) based on the structure of Ro 15-4513 and reported their binding affinity [6], as well as several ligands by Atack et al. [12]. These ligands are benzodiazepine receptor (BzR) negative modulators in vivo and a number of these compounds have been shown to enhance memory and learning [13]. One of these ligands was shown by Bailey et al. [6] to be important in the acquisition of fear conditioning and has provided further evidence for the involvement of hippocampal GABA(A)/BzR in learning and anxiety [13]. This is in agreement with the work of DeLorey et al. [7] in a memory model with a ligand closely related to α5 subtype selective inverse agonists RY-024 and RY-079 including PWZ-029 (1).
In order to enhance the α5 subtype selectivity, the bivalent form of RY-80 (3) was prepared to provide XLi-093 (4) [13]. The binding affinity of XLi-093 in vitro was determined on α 1–6 β3γ2 LTK cells and is illustrated in Figure 1. This bivalent ligand exhibited little or no affinity at α 1–4,6 β3γ2 BzR/GABA(A) subtypes, but this α5 ligand had a K i of 15 nM at the α5β3γ2 subtype [14]. Since this receptor binding study indicated bivalent ligand XLi-093 bound almost exclusively to the α5 subtype, the efficacy of this ligand on GABA(A) receptor subtypes expressed in Xenopus oocytes was investigated by Sieghart, Furtmueller, Li, and Cook [14, 15]. Analysis of the data indicated that XLi-093 up to a concentration of 1 μM did not trigger chloride flux in any one of the GABA(A) subtypes tested. At 1 μM XLi-093 did not modulate GABA induced chloride flux in α1β3γ2, α2β3γ2, or α3β3γ2 receptors, but very slightly inhibited chloride flux in α5β3γ2 subtypes. At 1 μM, XLi-093 barely influenced benzodiazepine (Valium) stimulation of GABA-induced current in α1β3γ2, α2β3γ2, and α3β3γ2 BzR but shifted the diazepam dose response curve to the right in α5β3γ2 receptors in a very significant manner [16]. Importantly, bivalent ligand XLi-093 was able to dose dependently and completely inhibit diazepam-stimulated currents in α5β3γ2 receptors. This was the first subtype selective benzodiazepine receptor site antagonist at α5 receptors. This bivalent ligand XLi-093 provided a lead compound for all of the bivalent ligands in this research [16].
Figure 1.
Alpha 5 selective compounds [13]. This figure is modified from that reported in [13].
Illustrated in Figure 2 is XLi-093 (4) aligned excellently within the pharmacophore-receptor model of the α5β3γ2 subtype [14, 16–19]. The fit to the pharmacophore-receptor and the binding data indicate that bivalent ligands will bind to BzR subtypes [14, 19]. It is believed that the dimer enters the binding pocket with one monomeric unit docking while the other monomer tethered by a linker extends out of the protein into the extracellular domain. If this is in fact true that the second imidazole unit is protruding into the extracellular domain of the BzR/GABA(A) α5 binding site, it could have a profound effect on the ligand design. This means other homodimers or even heterodimers may bind to BzR/GABA(A)ergic sites.
Figure 2.

XLi-093 (4) aligned in the included volume of the pharmacophore receptor model for the α5β3γ2 subtype [17, 18] (this figure is modified from the figure in Clayton et al., 2007) [23].
In this vein, Wenger, Li, and Cook et al. [13, 20, 21] earlier described preliminary data that XLi-093, an α5 subtype selective antagonist, enhances performance of C57BL/6J mice under a titrating delayed matching to position schedule of cognition, as illustrated in Figure 3 [14, 16–19]. This indicates, however, that this agent does cross the blood brain barrier.
Figure 3.

XLi-093 (4) effects on cognition enhancement by Wenger et al. (data on statistical significance not shown, unpublished results). Effects of 4 on cognition from the mean delay achieved by C57BL/6J mice titrating delayed matching-to-position schedule.
Bivalent ligands have a preferred linker of 3–5 methylene units, between the two pharmacophores (see XLi-093). This was established by NMR experiments run at low temperatures, X-ray crystallography, and molecular modeling of the ligands in question and will be discussed [14, 17, 18].
Based on this data, additional α5-subtype selective ligands have been prepared (see Figure 4). The basic imidazobenzodiazepine structure has been maintained [7]; however substituents were varied in regions around the scaffold based on molecular modeling [6]. These are now the most α5 subtype selective ligands ever reported [22]. Moreover, the ability to increase the subtype selectivity can be done by selecting specific substituents on these ligands to new agents with 400–1000-fold α5-selectivity over the remaining 5 subtypes. This is an important step forward to understanding the true, unequivocal physiological responses mediated by α5 subtypes in regard to cognition (amnesia), schizophrenia, anxiety, and convulsions, all of which in some degree are influenced by α5 subtypes. Based on the ligands in Figures 4 and 5, affinity has occurred principally at α5 subtypes. In addition, since XLi-093 bound very tightly only to α5 BzR subtypes, the bivalent nature and functionality presented here can be incorporated into other dimeric ligands.
Figure 4.
Binding data of selected imidazobenzodiazepines [22].
Figure 5.
Binding data of selected imidazobenzodiazepines substituted with an E-ring as compared to XLi-356 (10).
As shown previously in Figure 3, α5-antagonist XLi-093 (4) was shown to enhance cognition. In another study, a reduction of the two acetylenic groups of XLi-093 resulted in ethyl groups [14], providing a new bivalent ligand (XLi-356, 10) which shows α5-selective binding with very low affinity for α1 subtypes (Figure 5). Efficacy (oocyte) data shows XLi-356 is an α5 negative allosteric modulator [7, 13]. DeLorey et al. have recently shown in mice that XLi-356 does potently reverse scopolamine induced memory deficits [7]. This bivalent α5 inverse agonist enhanced cognition in agreement with work reported from our laboratory on monovalent inverse agonists RY-10 [6] and RY-23 [7].
The dimers XLi-093 (4) and XLi-356 (10) were sent to Case Western Reserve (NIMH supported PDSP program, Roth et al.) for full panel receptor binding and they do not bind to other receptors at levels of concern (Table 1).
Table 1.
Full PDSP panel receptor binding reported (Roth [138]) for XLi-093 and XLi-356.
| Cook code | 5ht1a | 5ht1b | 5ht1d | 5ht1e | 5ht2a | 5ht2b | 5ht2c | 5ht3 | 5ht5a | 5ht6 | 5ht7 | α1A | β1B | α2A | α2B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XLi093 | ∗ | Repeat | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
| XLi356 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
|
| |||||||||||||||
| Cook code | α2C | Beta1 | Beta2 | CB1 | CB2 | D1 | D2 | D3 | D4 | D5 | DAT | DOR | H1 | H2 | H3 |
|
| |||||||||||||||
| XLi093 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
| XLi356 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
|
| |||||||||||||||
| Cook code | H4 | Imidaz oline | KOR | M1 | M2 | M3 | M4 | M5 | MDR | MOR | NET | NMDA | SERT | σ1 | σ2 |
|
| |||||||||||||||
| XLi093 | ∗ | ∗ | 2,024.00 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
| XLi356 | ∗ | ∗ | 6,118.00 | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
Data (“secondary binding”) are K i values. K i values are reported in nanomolar concentration, Case Western Reserve University. “∗” indicates “primary missed” (<50% inhibition at 10 µM). See full data of the PDSP screen in the report of Clayton [22].
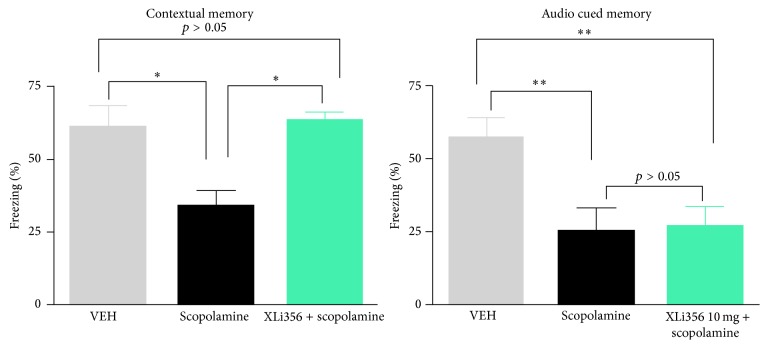
Although XLi-093 (4) was found to be an antagonist at the α5 subtype, XLi-356 (10) was found to be a weak agonist-antagonist. XLi-356 was found to reverse scopolamine induced memory deficits in mice. When XLi-356 was looked at in audio cued fear conditioning, the results show no activity. This suggests that the effect of XLi-356 is selective through α5 receptors which are abundant in the hippocampus which is highly associated with contextual memory. Audio cued memory instead is amygdala-based and should not be affected by an α5 subtype selective compound [39–42].
As illustrated in Figure 6, scopolamine (1 mg/kg) reduced freezing (i.e., impairs memory) generally due to coupling the context (the cage) with a mild shock. XLi-356 (10 mg/kg) attenuated the impairment of memory returning the freezing to the levels on par with subjects dosed with vehicle. In audio cued memory the response was activated by sound, not the context. XLi-356 was not able to reverse this type of memory effect which is amygdala driven. A similar effect was observed for XLi-093 by Harris et al. [43]. XLi-093 is the most selective antagonist for α5 subtypes reported to date [13, 43] and is a very useful α5 antagonist used by many in vivo [22, 44, 45].
Figure 6.
Visual and audio cued data for XLi-356 (10). This figure was modified from that in [22].
Molecular modeling combined with this knowledge was used to generate new lead compounds aimed at the development of α5-subtype selective positive and negative allosteric modulators to study cognition as well as amnesia mediated by the hippocampus. All of these compounds have been prepared based on the structure of current α5-subtype selective ligands synthesized in Milwaukee [46] (see Figures 4 and 5), as well as the binding affinity (15 nM)/selectivity of bivalent α5 antagonist XLi-093 (4) [13].
In efforts to enhance α5-selectivity in regards to cognition, Cook, Bailey, and Helmstetter et al. have employed RY-024 to study the hippocampal involvement in the benzodiazepine receptor in learning and anxiety [14, 19]. Supporting this Harris, DeLorey et al. show in mice that α5 NAMs (1) and RY-10 potently reversed scopolamine-induced memory impairment. These α5 NAMs provide insight as to how GABAARs influence contextual memory, an aspect of memory affected in age associated memory impairment and especially in Alzheimer's disease [13, 62–64]. In addition, Savić et al. have used the α1 preferring antagonist, BCCt, in passive avoidance studies, in which midazolam's amnesic effects are shown to be due to interaction of agonist ligands at α5 in addition to α1β3γ2 BzR subtypes [24, 65].
3. PWZ-029: A Negative Allosteric Modulator
PWZ-029 (1) has been studied extensively as an α5-GABAAR inverse agonist and in certain experimental models has been shown to enhance cognition. The binding data from three separate laboratories (Table 2) have all shown that it exhibits remarkable selectivity for the α5 subunit-containing receptors, all greater than 60-fold compared to the next subunit.
Table 2.
Affinity of PWZ-029 (1); K i (nM)a.
| Code | MW | α1 | α2 | α3 | α4 | α5 | α6 |
|---|---|---|---|---|---|---|---|
| PWZ-029 (1) | 291.73 | >300 | >300 | >300 | ND | 38.8 | >300 |
| PWZ-029 (1) | 291.73 | 920 | ND | ND | ND | 30 | ND |
| PWZ-029 (1) | 291.73 | 362 | 180 | 328 | ND | 6 | ND |
aData from three separate laboratories.
Electrophysiological efficacy testing done by Sieghart et al. in oocytes demonstrated that PWZ-029 (1) acts as a negative allosteric modulator at the α5-subunit, with a very weak agonist activity at the α1, α2, and α3 subunits (Figure 7). At a pharmacologically relevant concentration of 0.1 μM, PWZ-029 exhibits moderate negative modulation at the α5-subunit, while showing little or no effect at the α1, α2, or α3-subunits.
Figure 7.
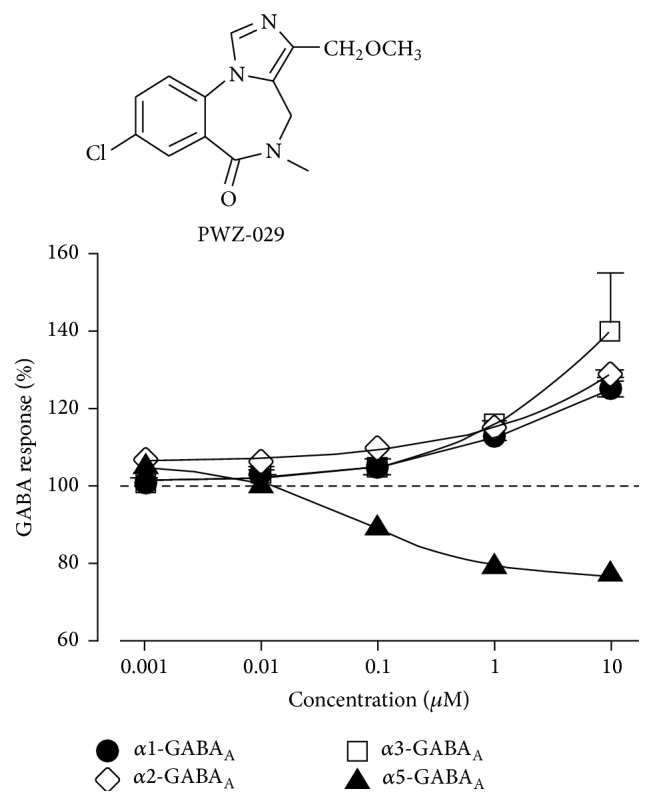
Oocyte electrophysiological data of PWZ-029 (1) [24].
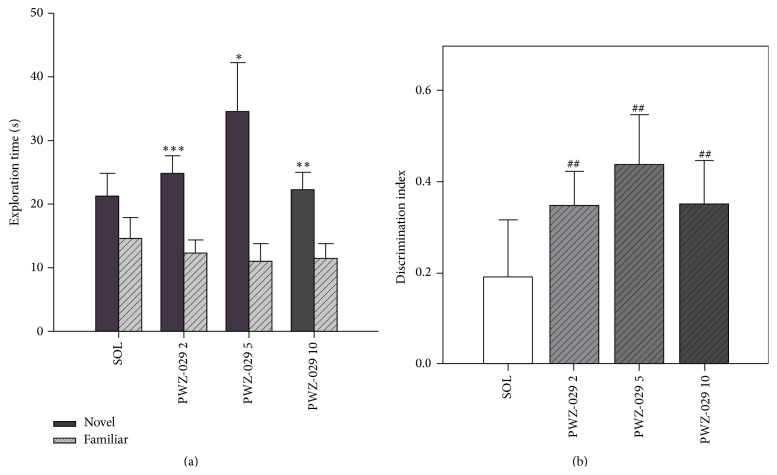
Milić et al. reported on the effects of PWZ-029 in the widely used novel object recognition test, which differentiates between the exploration time of novel and familiar objects. As shown by significant differences between the exploration times of the novel and familiar object (Figure 8(a)), as well as the respective discrimination indices (Figure 8(b)), all the three tested doses of PWZ-029 (2, 5 and 10 mg/kg) improved object recognition in rats after the 24 h delay period. Additionally, in the procedure with the 1 h delay between training and testing, the lowest of the tested doses of PWZ-029 (2 mg/kg) successfully reversed the deficit in recognition memory induced by 0.3 mg/kg scopolamine (Figure 9) [25].
Figure 8.
The effects of PWZ-029 (1) (2, 5 and 10 mg/kg) on (a) time exploring familiar and novel objects and (b) discrimination indices in the novel object recognition test using a 24 h delay (mean + SEM). Significant differences are indicated with asterisks (paired-samples t-test, novel versus familiar, ∗ p < 0.05, ∗∗ p < 0.01, ∗∗∗ p < 0.001). A significant difference from zero is indicated with hashes (one sample t-test, ## p < 0.01). The number of animals per each treatment group was 10. SOL = solvent [25].
Figure 9.
The effects of 0.3 mg/kg scopolamine (SCOP 0.3) and combination of 0.3 mg/kg scopolamine and PWZ-029 (1) (2, 5, and 10 mg/kg) on the rats' performance in the object recognition task after a 1 h delay: (a) time exploring familiar and novel objects and (b) discrimination index. Data are represented as mean + SEM. Significant differences are indicated with asterisks (paired-samples t-test, novel versus familiar, ∗ p < 0.05, ∗∗ p < 0.01). A significant difference from zero is indicated with hashes (one sample t-test, # p < 0.05). The number of animals per each treatment group was 12–15. SAL = saline, SOL = solvent [25].
The results of the described study showed for the first time that inverse agonism at α5-GABAA receptors may be efficacious in both improving cognitive performance in unimpaired subjects and ameliorating cognitive deficits in pharmacologically impaired subjects, as assessed in two protocols of the same animal model [25].
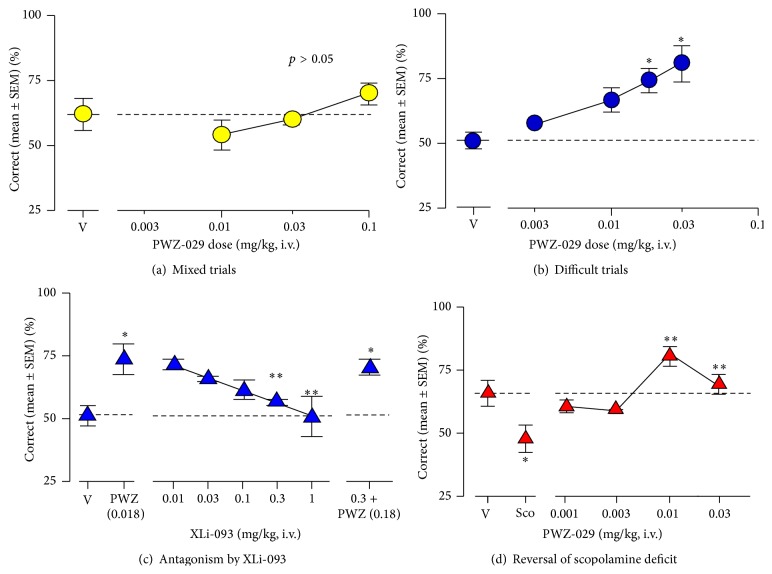
In a recent by Rowlett et al. [26], negative allosteric modulator PWZ-029 was evaluated in female rhesus monkeys (n = 4) in an Object Retrieval test with Detours (ORD; Figure 10 for details). 1 was administered via i.v. catheters in ORD trained monkeys and evaluated for cognition enhancement. A successful trial was determined by the ability of the subject to obtain a food reward within a transparent box with a single open side, with varying degrees of difficulty (“easy” or “difficult” or “mixed” as a combination of both) based on food placement within the box. In “mixed” trials using PWZ-029, no significant results were observed when compared to vehicle (Figure 11(a)). “Difficult” trials, however, exhibited an increasing dose-dependent curve for successful trials (Figure 11(b)). These results were attenuated by a coadministration α5-antagonist XLi-093 (Figure 11(c)). PWZ-029 was also shown to dose-dependently reverse the cholinergic deficits that were induced by scopolamine (Figure 11(d)) [26].
Figure 10.
ORD methods and procedure [26].
Figure 11.
Cognitive-enhancing effects of PWZ-029 in the rhesus monkey Object Retrieval with Detours (ORD) task (n = 5 monkeys). (a) Effects of PWZ-029 on ORD tests consist of both easy and difficult trials: (b) PWZ-029 enhanced performance on the ORD task when tested with difficult trials only; (c) enhancement of ORD performance by 0.018 mg/kg of PWZ-029 was attenuated by the α5 GABAA-preferring antagonist XLi-093 and this antagonism was surmountable by increasing the PWZ-029 dose; (d) PWZ-029 reversed performance impaired by 0.01 mg/kg of scopolamine [26]. ∗ p < 0.05 versus vehicle, ∗∗ p < 0.05 versus Scopolamine.
These findings suggest that PWZ-029 can enhance performance on the ORD task, only under conditions in which baseline performance is attenuated. The effects of PWZ-029 were antagonized in a surmountable fashion by the selective α5-GABAA ligand, XLi-093, consistent with PWZ-029's effects being mediating via the α5-GABAA receptor. The results are consistent with the view that α5 GABAA receptors may represent a viable target for discovery of cognitive enhancing agents.
In addition, we have new data showing that modulation of α5-GABAARs by PWZ-029 rescues Hip-dependent memory in an AD rat model [PMID: 23634826] as evidenced by a significant decrease in the latency to reach the hidden platform (memory probe trials) on spatial water maze task (Figure 12). Roche has employed a similar strategy at α5 subtypes and recently has a drug in the clinic to treat symptoms of dementia in Down syndrome patients. It is well known many Down syndrome patients develop Alzheimer's disease or a dementia with a very similar etiology. This is aimed at treating early onset Alzheimer's patients.
Figure 12.

PWZ-029 rescues spatial memory deficits in AD model as evidenced by a decrease in the latency to reach the hidden platform (probe test) in the water maze relative to vehicle (VEH, ∗ p < 0.05).
4. PWZ-029 Docking within α5γ2 GABAA Receptor Subunit Homology Model
These studies with PWZ-029 led to the molecular model rendering of the compound docked within the α5γ2 BzR subtype (Figures 13– 16). The model figures have the following features:
Figure 13.
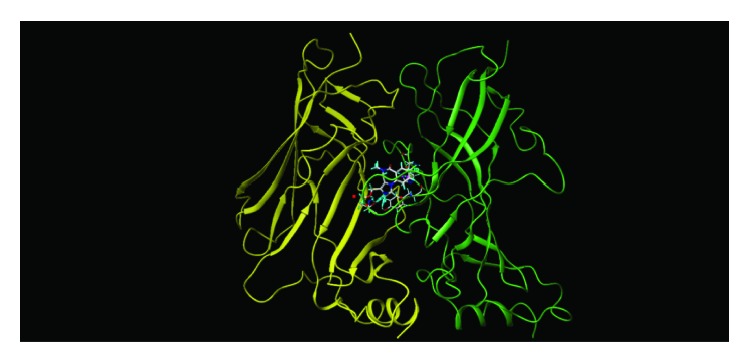
PWZ-029 docked within α5γ2 BzR binding site (BS).
Figure 14.
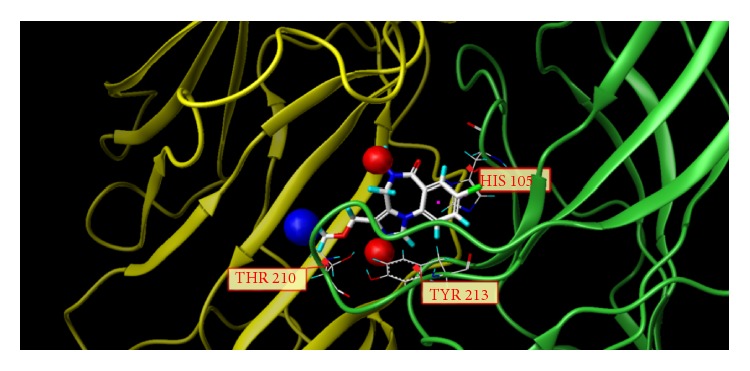
PWZ-029 docked with amino acid residues.
Figure 15.
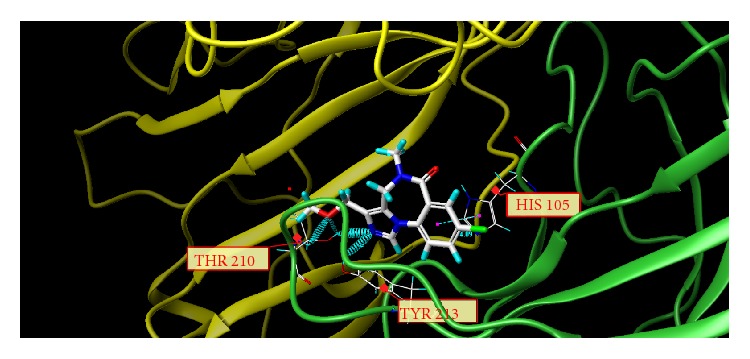
PWZ-029 docked with A.A. residue interactions.
Figure 16.
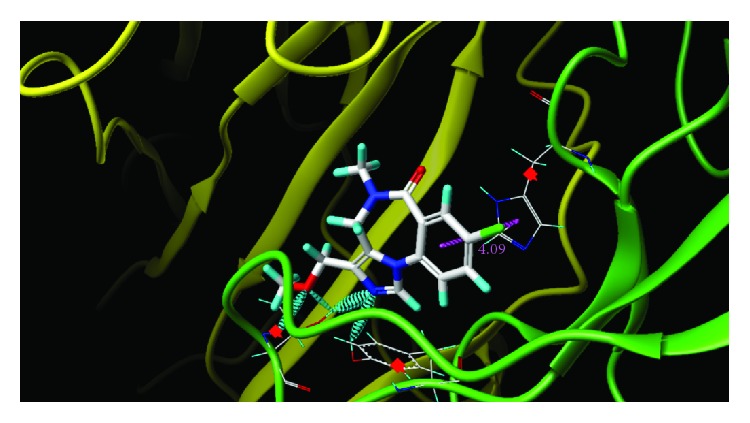
PWZ-029 docked with interactions. (1) HIS 105 π-stacking interaction with centroid of PWZ-029. (2) TYR 213 phenol OH hydrogen bonding to imidazole nitrogen lone pair. (3) THR 210 OH and lone pair on methoxy of PWZ029. (4) α5 ribbon being green. (5) γ2 ribbon being yellow. (6) Hydrogen bonding being aqua blue. (7) π-stacking being magenta.
The docking of PWZ-029 within the GABAA/BzR shows the molecule bound and interacting with specific amino acids. The A and B rings of the benzodiazepine framework undergo a π-stacking interaction with HIS 105, indicated by the magenta coloring. At the other end of the molecule the methoxy lone pair and imidazole nitrogen lone pair act as a hydrogen bond acceptors with THR 210 and TYR 213, respectively. These interactions are shown by the aqua-blue descriptors.
5. Subtype Selective Agonists for α5 GABAA/Bz Receptors
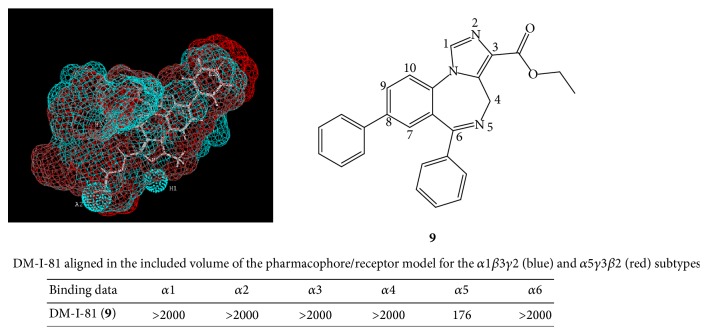
Möhler has proposed that α5 selective inverse agonists or α5 selective agonists might enhance cognition [5, 13, 16–18, 86]. This is because of the extrasynaptic pyramidal nature of α5β3γ2 subtypes, located almost exclusively in the hippocampus. Because of this, a new “potential agonist” which binds solely to α5β3γ2 subtypes was designed by computer modeling (see Figure 17). This ligand (DM-I-81, 9) has an agonist framework and binds only to α5β3γ2 subtypes [13, 17, 18, 86]. The binding potency at α5 subtypes is 176 nM. Although the 8-pendant phenyl of DM-I-81 was lipophilic and bound to the L 2 pocket, additional work on the 8-position of this scaffold has been abandoned and generally left as an acetylene or halide function, with a few exceptions. The steric bulk of the 8-phenyl moiety was felt detrimental to activity and potency which may have led to the weak binding affinity.
Figure 17.
The α5 selective agonist DM-I-81 (9), bound within the α1 and α5 subtypes. Binding data shown as K i (nM).
6. Alpha 5 Positive Allosteric Modulators in Schizophrenia
In addition to inverse agonists, a number of other α5-GABAAR positive allosteric modulators (PAMs) have been synthesized. These compounds, such as SH-053-2′F-R-CH3 (2), have been shown to decrease the firing rate of synapses controlling cognition and can be used to treat schizophrenia.
The following is reported by Gill, Cook, and Grace et al. [27–38].
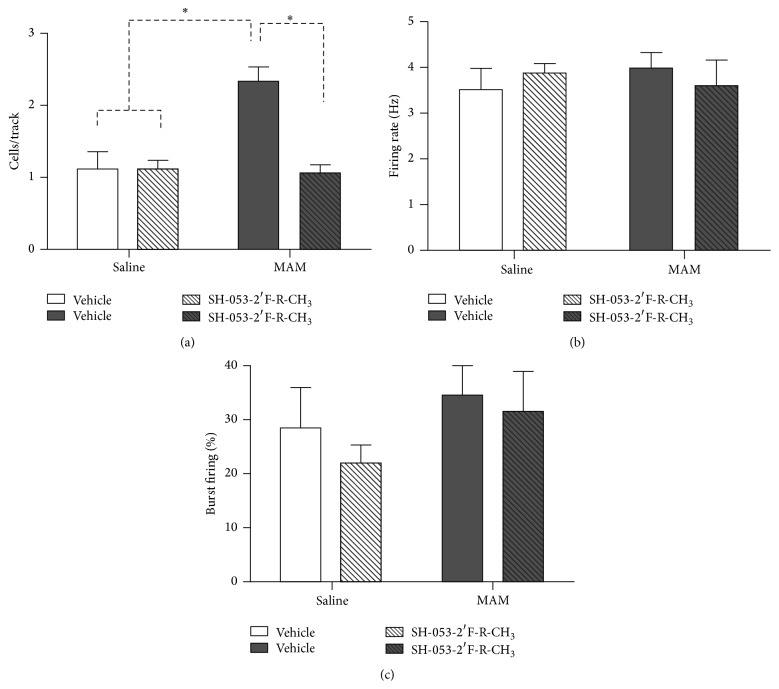
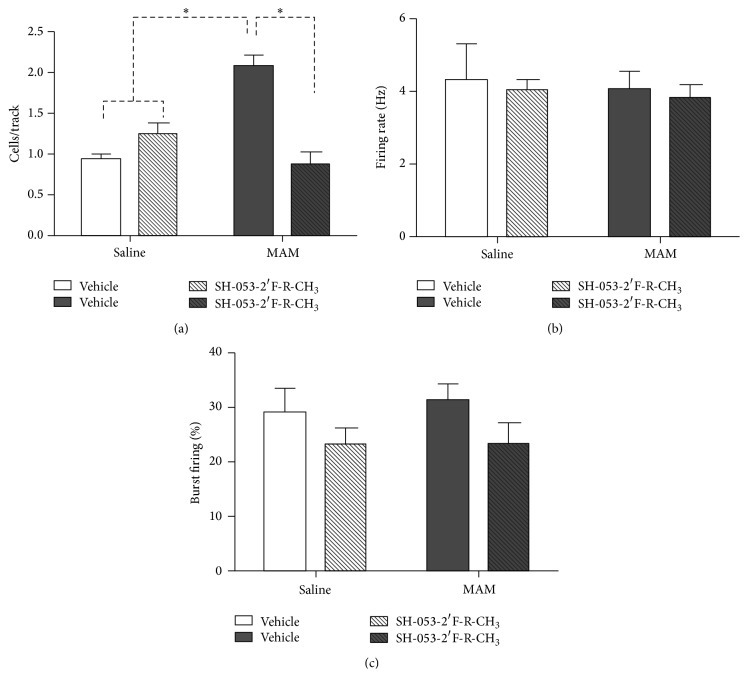
There are a number of novel benzodiazepine-positive allosteric modulators (PAMs), selective for the α5 subunit of the GABAA receptor, including SH-053-2′F-R-CH3 (2), which has been tested for its ability to effect the output of the HPC (hippocampal) in methylazoxymethanol- (MAM-) treated animals, which can lead to hyperactivity in the dopamine system [27–38]. In addition, the effect of this compounds (2) response to amphetamine in MAM-animals on the hyperactive locomotor activity was examined. Schizophrenic-like symptoms can be induced into rats when treated prenatally with DNA-methylating agent, methylazoxymethanol, on gestational day (GD) 17. These neurochemical outcomes and changes in behavior mimic those found in schizophrenic patients. Systemic treatment with (2) resulted in a reduced number of spontaneously active DA (dopamine) neurons in the VTA (ventral tegmental area) of MAM animals (Figure 18) to levels seen in animals treated with vehicle (i.e., saline). To confirm the location of action, 2 was also directly infused into the ventral HPC (Figure 19) and was shown to have the same effect. Moreover, HPC neurons in both SAL and MAM animals showed diminished cortical-evoked responses following α5-GABAAR PAM treatment. This study is important for it supports a treatment of schizophrenia that targets abnormal HPC output, which in turn normalized dopaminergic neuronal activity [27–38]. This is a novel approach to treat schizophrenia.
Figure 18.
Treatment with SH-053-2′F-R-CH3 (0.1 mg/kg, i.v.; patterned bars) normalizes the aberrant increase in the number of spontaneously firing dopamine neurons (expressed as cells/track) in methylazoxymethanol acetate- (MAM-) treated animals (a). There was no effect of SH-053-2′F-R-CH3 treatment in control animals (open bars, (a)–(c)) or on firing rate and burst activity in MAM animals (dark bars; (b)-(c)) (∗ p < 0.05, two-way ANOVA, Holm-Sidak post hoc; N = 5–7 rats/group) [27–38].
Figure 19.
Hippocampal (HPC) infusion of SH-053-2′F-R-CH3 (1 μM/side; patterned bars) normalizes the aberrant increase in the number of spontaneously firing dopamine neurons (expressed as cells/track) in methylazoxymethanol acetate- (MAM-) treated animals (a). There was no effect of SH-053-2′F-R-CH3 treatment in control animals (open bars, (a)–(c)) or on firing rate in MAM animals (dark bars; (b)). Hippocampal (HPC) infusion of SH-053-2′F-R-CH3 significantly reduced the percentage of spikes occurring in bursts of dopamine (DA) neurons in MAM and control animals (c) (∗ p < 0.05, two-way ANOVA, Holm-Sidak post hoc; N = 7 rats/group) [27–38].
The pathophysiology of schizophrenia has identified hippocampal (HPC) dysfunction as a major mediator as reported by many including Anthony Grace [27–38]. This included morphological changes, reduced HPC volume, and GAD67 expression [27, 28] that have been reported after death in the brains of patients with schizophrenia. Both HPC activation and morphology changes have been identified that can precede psychotic symptoms or correlate with severity of cognitive deficits [29–33]. This has been shown in a cognitive test during baseline and activation.
Many animal models of schizophrenia were essential to behavioral pathology and have delivered new knowledge about the network disturbances that contribute to CNS disorder. This study shows that the offspring of MAM-treated animals showed both structural and behavioral abnormalities. These were consistent with those observed in patients with schizophrenia. The animals had reduced limbic cortical and HPC volumes with increased cell packing density and showed increased sensitivity to psychostimulants [34–36]. In addition, the startle response in prepulse inhibition was reduced in MAM-treated animals and deficits in latent inhibition were observed [35]. Furthermore, a pathological rise in spontaneous dopamine (DA) activity by the ventral tegmental area (VTA) was observed that can be attributed to aberrant activation within the ventral HPC [36]. It was suggested that reductions in parvalbumin- (PV-) stained interneurons might be the reason for the hyperactivation of the HPC and disruption of normal oscillatory activity in the HPC and cortex of MAM animals [38, 61]. At least this is the prevailing hypothesis at the moment put forth by many investigators (see references cited in [27–38]).
Selective α5-GABAAR positive allosteric modulator (2) was successful in reversing the pathological increase in tonic DA transmission in methylazoxymethanol rats by targeting abnormal hippocampal activity. In addition, the α5-PAM was able to reduce the behavioral sensitivity to psychostimulants observed in MAM rats (Figures 20 and 21). This suggests that novel α5-partial allosteric modulators should be effective in alleviating dopamine-mediated psychosis. However, if this drug can also restore rhythmicity within HPC-efferent structure, it may also affect other aspects of this disease state such as cognitive disabilities and negative symptoms. This study, using the MAM-model to induce symptoms of schizophrenia, shows that the use of α5-GABAAR targeting compounds could be an effective treatment in schizophrenic patients. The selective targeting solely of α5β3γ2 subunits, as opposed to unselective BZDs such as diazepam, could provide relief from the psychotic symptoms without producing adverse effects such as sedation [27–38].
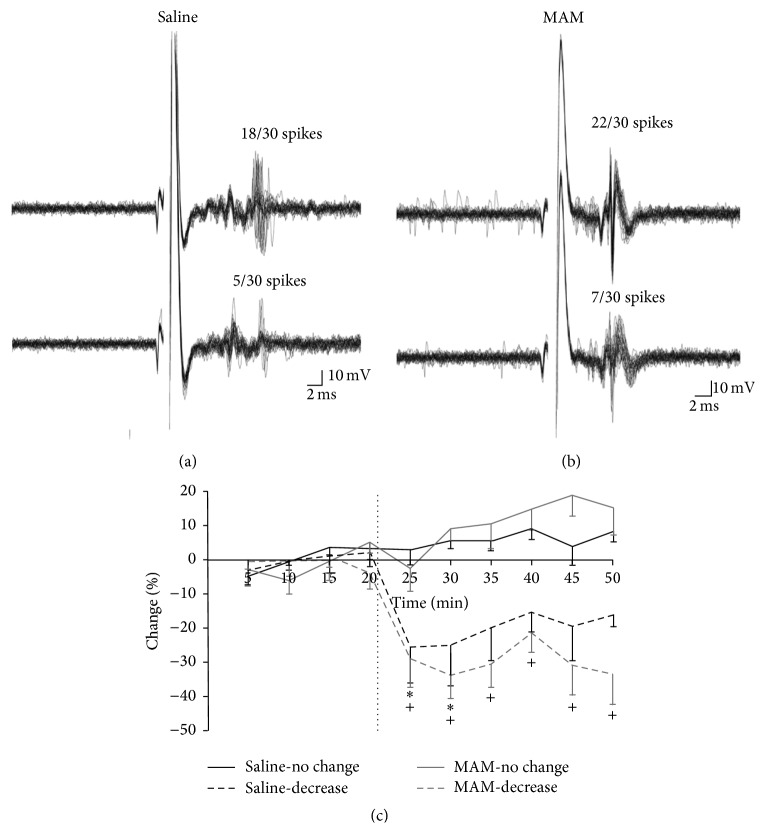
Figure 20.
Extracellular recording traces illustrate the reduction in evoked responses in the ventral hippocampal (HPC) to entorhinal cortex stimulation in both MAM- and saline-treated animals (a, b). Treatment with SH-053-2′F-R-CH3 (0.1 mg/kg, i.v.) decreases the evoked excitatory response (dashed lines) of ventral HPC neurons to entorhinal cortex stimulation in both MAM- and saline-treated animals (c) (∗ p < 0.05 for saline and + p < 0.05, two-way repeated measures ANOVA, Holm-Sidak post hoc) [27–38].
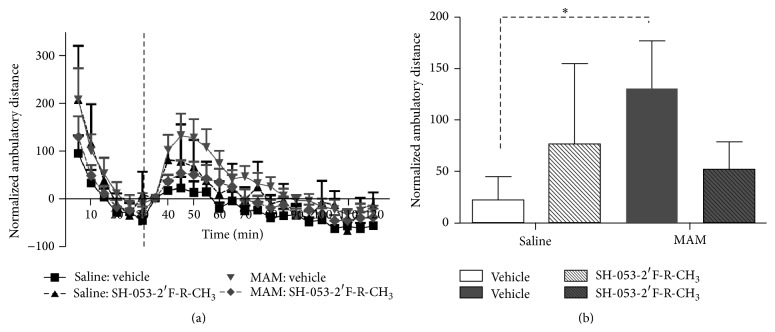
Figure 21.
Treatment with SH-053-2′F-R-CH3 (10 mg/kg, i.p.) reduced the aberrant increased locomotor response to D-amphetamine (0.5 mg/kg i.p.) observed in MAM rats (a). MAM animals demonstrated a significantly larger peak locomotor response than both saline-treated animals and MAM animals pretreated with the alpha-5 PAM (b) (there was a significant difference between MAM-vehicle and all other groups, ∗ p < 0.05, two-way repeated measures ANOVA, Holm-Sidak post hoc) [27–38].
As reported by Gill, Grace et al. [36, 38, 47–61].
Often initial antipsychotic drug treatments (APD) for schizophrenia are ineffective, requiring a brief washout period prior to secondary treatment. The impact of withdrawal from initial APD on the dopamine (DA) system is unknown. Furthermore, an identical response to APD therapy between normal and pathological systems should not be assumed. In another study by Gill, Grace et al., α5 positive allosteric modulator SH-053-2′F-R-CH3 (2) was used in the MAM neurodevelopmental model of schizophrenia which was used to study impact of withdrawal from repeated haloperidol (HAL) on the dopamine system [36, 38, 47–61].
The following studies were designed to provide insight as to why a new drug to treat schizophrenia may be effective in Phase II clinical trials but fail in Phase III because of the large number of patients required for the study. Many of these patients in Phase III studies have altered neuronal pathways in the CNS because of long-term treatment with antipsychotics (sometimes 10–20 years) [36, 38, 47–61].
Importantly, spontaneous dopamine activity reduction was observed in saline rats withdrawn from haloperidol with an enhanced locomotor response to amphetamine, indicating the development of dopamine supersensitivity. In addition, PAM treatment, as well as ventral HPC inactivation, removed the depolarization block of DA neurons in withdrawn HAL treated SAL rats. In contrast, methylazoxymethanol rats withdrawn from HAL displayed a reduction in spontaneous dopamine activity and enhanced locomotor response that was unresponsive to PAM treatment with SH-053-2′F-R-CH3 or ventral HPC inactivation [36, 38, 47–61].
Prior HAL treatment withdrawal can restrict the efficacy of subsequent pharmacotherapy in the MAM model of schizophrenia. This is an extremely important result indicating that testing a new drug for schizophrenia in humans treated for years with both typical and atypical antipsychotics may result in a false negative with regard to treatment. Studies that support this hypothesis follow here [36, 38, 47–61].
Novel therapeutics for the treatment of schizophrenia that exhibit initial promise in preclinical trials often fail to demonstrate sufficient efficacy in subsequent clinical trials. In addition, relapse or noncompliance from initial treatments is common, necessitating secondary antipsychotic intervention [47, 48]. Studies have shown that between 49 and 74% of schizophrenia patients discontinue the use of antipsychotic drug (APD) treatments within 18 months due to adverse side-effects [48, 49]. Current pharmacotherapies for schizophrenia target the pathological increase in dopamine system activity, as mentioned above. Common clinical practice for secondary antipsychotic application involves a brief withdrawal period from the initial APD. Unfortunately, the success of even secondary treatments is far from being optimal with the rehospitalization of patients being a common occurrence. The impact of repeated antipsychotic treatment and subsequent withdrawal on the dopamine system has not been adequately assessed [36, 38, 47–61].
As indicated above, schizophrenia is a complex chronic psychiatric illness characterized by frequent relapses despite ongoing treatment. The search for more effective pharmacotherapies for the treatment of schizophrenia continues unabated. It is not uncommon for novel pharmaceuticals to demonstrate promise in preclinical trials but fail to show an adequate response in subsequent clinical trials. Indeed, evaluating the benefits of one APD versus another is complicated by clinical trials beset with high attrition rates and poor efficacy in satisfactorily reducing rehospitalization [47, 49–52].
Previous work from the Gill, Grace et al.'s laboratory [36, 38, 47–61] with the MAM model of schizophrenia has identified a potential novel therapeutic, a α5GABAAR PAM. The dopamine system pathology in the MAM model is likely the result of excessive output from the ventral HPC [36]. The α5GABAAR PAM was identified as a potential therapeutic due to the relatively selective expression of α5GABAAR in the ventral HPC and its potential for reducing HPC activity [53–60]. When either administered systemically or directly infused into the ventral HPC, the α5GABAAR PAM (SH-053-2′F-R-CH3) was effective in reducing the dopamine system activation in MAM rats [38]. Anthony Grace, Gill et al. showed α5GABAAR PAM treatment was also effective in reducing the enhanced behavioral response to amphetamine in MAM rats, as stated above. Data from the present study sought to delineate whether the α5GABAAR PAM (SH-053-2′F-R-CH3) would remain effective in MAM rats withdrawn from prior neuroleptic treatment, a common occurrence in the patient population. In both SAL and MAM rats, there was a reduction in the spontaneous activity of dopamine neurons in the VTA after 7 days withdrawal from repeated HAL treatment. However, MAM rats continued to exhibit a greater activation of the dopamine system in comparison to SAL rats. Treatment with the α5GABAAR PAM was no longer effective in reducing the activity of dopamine neurons in the VTA in withdrawn HAL treated MAM rats. In contrast, α5GABAAR PAM treatment in the withdrawn HAL treated SAL rats instead increased the spontaneous activity of dopamine in the VTA (Figures 22 –25) [36, 38, 47–61].
Figure 22.
Repeated haloperidol treatment caused a reduction in the number of spontaneously active dopamine neurons in both SAL and MAM rats injected with vehicle compared to untreated control animals. Treatment with SH-053-2′F-R-CH3 (0.1 mg/kg, i.v.) reversed the haloperidol-induced reduction in cells/track in SAL, but not MAM, rats (a). Repeated haloperidol treatment had no effect on the firing rate of dopamine neurons recorded in SAL or MAM rats treated with vehicle. However, SH-053-2′F-R-CH3 caused an increase in firing rate of dopamine neurons in repeatedly haloperidol-treated SAL rats (b). Repeated haloperidol treatment, as well as SH-053-2′F-R-CH3 injection, had no impact on the percentage of spikes occurring in bursts for dopamine neurons recorded in SAL and MAM rats (c) [36, 38, 47–61]. ∗ p < 0.05.
Figure 23.
Repeated haloperidol treatment caused a reduction in the number of spontaneously active dopamine neurons in both SAL and MAM rats microinfused with vehicle in the ventral HPC compared to untreated control animals. Infusion of TTX in the ventral HPC reversed the haloperidol-induced reduction in cells/track in SAL, but not MAM, rats (a). Repeated haloperidol treatment had no effect on the firing rate of dopamine neurons recorded in SAL or MAM rats infused with vehicle or TTX in the ventral HPC (b). Repeated haloperidol treatment had no effect on the percentage of spikes occurring in bursts for dopamine neurons recorded in SAL or MAM rats infused with vehicle or TTX in the ventral HPC (c) [36, 38, 47–61]. ∗ p < 0.05.
Figure 24.

Administration of apomorphine (80 mg/kg i.v.) increased the number of spontaneously active dopamine neurons in SAL rats withdrawn from repeated HAL, while having no effect on the number of active dopamine neurons in MAM rats withdrawn from repeated HAL [36, 38, 47–61]. ∗ p < 0.05.
Figure 25.
Repeated haloperidol treatment causes an enhancement in the locomotor response to D-amphetamine (0.5 mg/kg, i.p.) in SAL animals that is reduced by pretreatment with SH-053-2′F-R-CH3 (10 mg/kg, i.p.) (a). MAM rats treated repeatedly with haloperidol exhibit a locomotor response following D-amphetamine similar to untreated MAM rats. However, repeated haloperidol treatment blocks the effect of SH-053-2′F-R-CH3 pretreatment in decreasing the locomotor response in MAM rats (b). Untreated MAM rats demonstrated a significantly larger peak locomotor response than untreated SAL rats. In addition, SH-053-2′F-R-CH3 pretreatment significantly reduced the peak locomotor response in untreated MAM rats, while having no effect in repeatedly haloperidol-treated MAM rats. In contrast, repeated haloperidol treatment enhanced the peak locomotor response to amphetamine in SAL rats that was reduced by SH-053-2′F-R-CH3 pretreatment (c) [36, 38, 47–61]. ∗ p < 0.05.
Similar to the effects seen following α5GABAAR PAM treatment, ventral HPC inactivation in withdrawn HAL treated SAL rats restored normal dopamine system activity by increasing the number of spontaneously active dopamine neurons. The disparate effect of withdrawal from HAL on the dopamine system between SAL and MAM rats provides a vital clue for the inconsistencies between preclinical trials for novel therapeutics that utilize normal subjects and subsequent clinical trials in a patient population [36, 38, 47–61].
The data suggests underlying dopamine system pathology alters the impact of withdrawal from prior repeated HAL in the MAM model of schizophrenia. In addition, subsequent novel APD treatment loses efficacy following withdrawal from repeated HAL in MAM animals. This certainly has relevance to Phase III clinical trials of new drugs to treat schizophrenia [36, 38, 47–61].
7. GABAA α5 Positive Allosteric Modulators Relax Airway Smooth Muscle
Emala, Gallos, et al. [66–75] have found that novel α5-subtype selective GABAA positive allosteric modulators relax airway smooth muscle from rodents and humans. The clinical need for new classes of bronchodilators for the treatment of bronchoconstrictive diseases such as asthma remains a major medical issue. Few novel therapeutics have been approved for targeting airway smooth muscle (ASM) relaxation or lung inflammation in the last 40 years [66]. In fact, several asthma-related deaths are attributed, in part, to long-acting β-agonists (LABA) [67]. Adherence to inhaled corticosteroids, the first line of treatment for airway inflammation in asthma, is very poor [68, 69]. Therapies that break our dependence on β-agonism for ASM relaxation would be a novel and substantial advancement.
These ASM studies were undertaken due to a pressing clinical need for novel bronchodilators in the treatment of asthma and other bronchoconstrictive diseases such as COPA. There are only three drug classes currently in clinical use as acute bronchodilators in the United States (methylxanthines, anticholinergics, and β-adrenoceptor agonists) [70]. Thus, a novel therapeutic approach that would employ cellular signaling pathways distinct from those used by these existing therapies involves modulating airway smooth muscle (ASM) chloride conductance via GABAA receptors to achieve relaxation of precontracted ASM [71, 72]. However, widespread activation of all GABAA receptors may lead to undesirable side effects (sedation, hypnosis, mucus formation, etc.). Thus, a strategy that selectively targets a subset of GABAA channels, those containing α subunits found to be expressed in airway smooth muscle, may be a first step in limiting side effects. Since human airway smooth muscle contains only α4 or α5 subunits [72], ligands with selectivity for these subunits are an attractive therapeutic option. Concern regarding nonselective GABAA receptor activation is not limited to the airway or other peripheral tissues. GABAA receptor ligands are classically known for their central nervous system effects of anxiolysis, sedation, hypnosis, amnesia, anticonvulsion, and muscle relaxant effects. Such indiscriminate activation of GABAA receptors in the CNS is exemplified by the side effects of classical benzodiazepines (such as diazepam) which were the underpinning for the motivation of a search for benzodiazepine (BZD) ligands that discriminate among the α subunits of GABAA receptors [73–75].
A novel approach to identify novel benzodiazepine derivatives to selectively target GABAA channels containing specific α subunits was developed by Cook et al. in the 1980s that employed a pharmacophore receptor model based on the binding affinity of rigid ligands to BDZ/GABAA receptor sites (as reviewed in 2007 [23]). From this series of receptor models for α 1–6 β3γ2 subtypes a robust model for α5 subtype selective ligands emerged, the result of which included the synthesis of a novel α5β3γ2 partial agonist modulator, SH-053-2′F-R-CH3 (2). The discovery of this and related ligands selective for α5 BDZ/GABAA-ergic receptors and the realization that only α4 and α5 subunits are expressed in GABAA channels on human airway smooth muscle yielded an ideal opportunity for targeting these α5-subunit containing GABAA channels for bronchorelaxation [66–75].
The GABAA α5 subunit protein was first localized to the ASM layer of human trachea while costaining for the smooth muscle specific protein α actin (Figure 26). The first panel of Figure 26 shows GABAA α5 protein stained with fluorescent green and blue fluorescent nuclear staining (DAPI). The second panel is the same human tracheal smooth muscle section simultaneously stained with a protein specific for smooth muscle, α actin, and the third panel is a merge of the first two panels showing costaining of smooth muscle with GABAA α5 and α actin proteins. The fourth panel is a control omitting primary antibodies but including nuclear DAPI staining [66–75].
Figure 26.
Protein expression of the GABAA α5 subunit in intact human trachea-bronchial airway smooth muscle. Representative images of human tracheal airway smooth muscle sections using confocal microscopy are depicted following single, double, and triple immunofluorescence labeling. The antibodies employed were directed against the GABAA α5 subunit (green), α-smooth muscle actin (SMA; red), and/or the nucleus via DAPI counterstain (blue). Panels illustrate the following staining parameters from left to right: (1st) costaining of DAPI and GABAA α5 subunit; (2nd) α-SMA staining alone; (3rd) triple-staining of GABAA α5, α-SMA, and DAPI; (4th) DAPI nucleus counterstain, with primary antibodies omitted as negative control. Modified from [66–75].
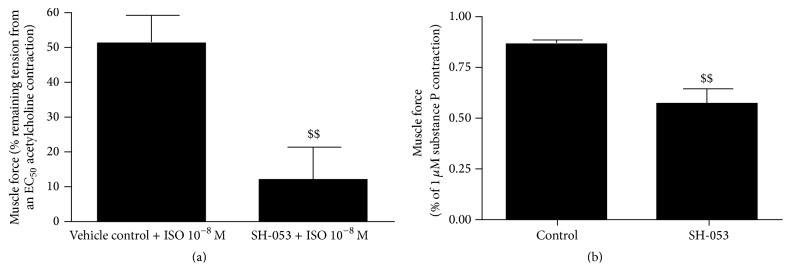
After demonstrating the protein expression of GABAA receptors containing the α5 subunit, functional studies of isolated airway smooth muscle were performed in tracheal airway smooth muscle from two species. Human airway smooth muscle suspended in an organ bath was precontracted with a concentration of acetylcholine that was the EC50 concentration of acetylcholine for each individual airway smooth muscle preparation. The induced contraction was then relaxed with a β-agonist (isoproterenol) in the absence or presence of the GABAA α5 ligand SH-053-2′F-R-CH3 (2). Figure 27(a) shows that the amount of relaxation induced by 10 nM isoproterenol was significantly increased if 50 μM SH-053-2′F-R-CH3 (2) was also present in the buffer superfusing the airway smooth muscle strip. Studies were also performed in airway smooth muscle from another species, guinea pig, that measured direct relaxation of a different contractile agonist, substance P. As shown in Figure 27(b), the amount of remaining contractile force 30 minutes after a substance P-induced contraction was significantly reduced in airway smooth muscle tracheal rings treated with SH-053-2′F-R-CH3 (2) [66–75].
Figure 27.
SH-053-2′F-R-CH3 (2) mediated activation of α5 subunit containing GABAA channels induces relaxation of precontracted airway smooth muscle. (a) SH-053-2′F-R-CH3 (2) (SH-053) potentiates β-agonist-mediated relaxation of human airway smooth muscle. Cotreatment of human airway smooth muscle strips with SH-053-2′F-R-CH3 (2) (50 μM) significantly enhances isoproterenol (10 nM) mediated relaxation of an acetylcholine EC50 contraction compared to isoproterenol alone (N = 8/group, $$ = p < 0.01). Modified from [66–75]. (b) SH-053-2′F-R-CH3 (2) activation of α5 containing GABAA receptors induces direct relaxation of substance P-induced airway smooth muscle contraction. Compiled results demonstrating enhanced spontaneous relaxation (expressed as % remaining force at 30 minutes following a 1 μM substance P mediated contraction) following treatment with SH-053-2′F-R-CH3 (2) compared to treatment with vehicle control (n = 4-5/group, $$ = p < 0.01) [66–75].
Following these studies in intact airway smooth muscle, cell based studies were initiated in cultured human airway smooth muscle cells to directly measure plasma membrane chloride currents and the effects of these currents on intracellular calcium concentrations. SH-053-2′F-R-CH3 (2) induced a Cl− current in vitro using conventional whole cell patch clamp techniques [66–75]. These electrophysiology studies were then followed by studies to determine the effect of these plasma membrane chloride currents on intracellular calcium concentrations following treatment of human airway smooth muscle cells with a ligand whose receptor couples through a Gq protein pathway, a classic signaling pathway that mediates airway smooth muscle contraction.
SH-053-2′F-R-CH3 (2) attenuated an increase in intracellular calcium concentrations induced by a classic Gq-coupled ligand, bradykinin (Figure 28(a)) [66–75]. The attenuation by SH-053-2′F-R-CH3 (2) was significantly blocked by the GABAA antagonist gabazine (Figure 28(b)) indicating that SH-053-2′F-R-CH3 (2) was modulating GABAA receptors for these effects on cellular calcium [66–75].
Figure 28.
SH-053-2′F-R-CH3 (2) mediated activation of α5 containing GABAA receptors attenuates bradykinin-induced elevations in cytosolic Ca2+ in human airway smooth muscle cells. (a) Representative Fluo-4 Ca2+ fluorescence (RFU) tracing illustrating pretreatment with SH-053-2′F-R-CH3 (2) (SH-053) (10 μM) reduces cytosolic Ca2+ response to bradykinin (1 μM). This effect is reversed in the presence of gabazine (200 μM, GABAA receptor antagonist). Modified from [66–75]. (b) Compiled results illustrating SH-053-2′F-R-CH3 (2) pretreatment of GABAA α5 receptors on human airway smooth muscle cells attenuates bradykinin-induced elevations in intracellular Ca2+ compared to levels achieved following pretreatment with vehicle control ($$ = p < 0.01). While gabazine-mediated blockade of GABAA channels does not significantly affect bradykinin-induced intracellular calcium increase compared to vehicle control, gabazine treatment did reverse SH-053-2′F-R-CH3 (2) ability to attenuate bradykinin-induced elevations in intracellular calcium thereby illustrating a GABAA channel specific effect (n.s. = not significant). Modified from [66–75].
The major findings of these studies are that human airway smooth muscle expresses α5 subunit containing GABAA receptors that can be pharmacologically targeted by a selective agonist. The GABAA α5 subunit selective ligand SH-053-2′F-R-CH3 (2) relaxed intact guinea pig airway smooth muscle contracted with substance P and augmented β-agonist-mediated relaxation of intact human airway smooth muscle. The mechanism for these effects was likely mediated by plasma membrane chloride currents that contributed to an attenuation of contractile-mediated increases in intracellular calcium, a critical event in the initiation and maintenance of airway smooth muscle contraction [66–75].
8. Recent Discovery of Alpha 5 Included Volume Differences: L 4 Pocket as Compared to Other Bz/GABAergic Subtypes
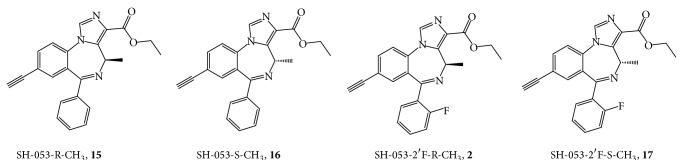
The findings in both the MAM-model of schizophrenia and the relaxation of airway smooth muscle have led to the study of SH-053-2′F-R-CH3 and related compounds bound within the α5-GABAA/BzR (Figure 29). The SH-053-R-CH3 (15) and SH-053-S-CH3 (16) isomers have been previously described [23]. These compounds along with SH-053-2′F-R-CH3 and SH-053-2′F-S-CH3 have been tested for binding affinity and show selectivity for the α5-subunit (Table 3).
Figure 29.
Structures of enantiomers with 2′H (15, 16) and 2′F (2, 17).
Table 3.
Binding affinity at αxβ2γ2 GABAA receptor subtypes (values are reported in nM).
| Compounda | α1 | α2 | α3 | α4 | α5 | α6 |
|---|---|---|---|---|---|---|
| SH-053-R-CH3, (15) | 2026 | 2377 | 1183 | >5000 | 949.1 | >5000 |
| SH-053-S-CH3, (16) | 1666 | 1263 | 1249 | >5000 | 206.4 | >5000 |
| SH-053-2′F-R-CH3, (2) | 759.1 | 948.2 | 768.8 | >5000 | 95.17 | >5000 |
| SH-053-2′F-S-CH3, (17) | 350 | 141 | 1237 | >5000 | 19.2 | >5000 |
aData shown here are the means of two determinations which differed by less than 10%.
From examination of Figure 30 and Tables 3 and 4, it is clear the (R)-isomers bound to the α5 subtype while the (S)-isomers were selective for α2/α3/α5 subtypes.
Figure 30.
Included volume and ligand occupation of the SH-053-2′F-S-CH3 17 and SH-053-2′F-R-CH3 2 enantiomers in the α5 and γ2 pharmacophore/receptor models. This figure was modified and reproduced from that reported by Clayton et al. in [22, 23].
Table 4.
Oocyte electrophysiological data of benzodiazepinesa [87].
| Compound | α1 | α2 | α3 | α5 |
|---|---|---|---|---|
| SH-053-2′F-R-CH3 (2) | 111/154 | 124/185 | 125/220 | 183/387 |
| SH-053-2′F-S-CH3 (17) | 116/164 | 170/348 | 138/301 | 218/389 |
aEfficacy at αxβ3γ2 GABAA receptor subtypes as % of control current at 100 nM and 1 μM concentrations. Data presented as percent over baseline (100) at concentrations of 100 nM/1 μM.
From this data, these compounds were then used in examining the α5-binding pocket, most specifically the fluoroseries. In regard to molecular modeling, depicted in Figure 30 is the included volume and ligand occupation of the SH-053-2′F-S-CH3 (17) and SH-053-2′F-R-CH3 (2) enantiomers in the α5 subtype as well as the α2 subtype. It is clear a new pocket (L 4) has been located in the α5 subtype permitting 2 as well as 17 to bind to the α5 subtype. Examination of both ligands in the α2 subtype clearly illustrates the analogous region in the α2 subtype is not present and thus does not accommodate 2 for the pendant phenyl which lies outside the included volume in the space allocated for the receptor protein itself [23].
9. BzR GABA(A) Subtypes
In terms of potency, examination of the values in Table 4 [87], it is clear the R-isomer (2) shows more selectivity towards the α5-subunit, while the S-isomer (17) is potent at the α2/3/5 subunits. It is important, as postulated earlier [23], that the major difference in GABA(A)/Bz receptors subtypes stems from differences in asymmetry in the lipophilic pockets L 1, L 2, L 3, L 4, and L Di in the pharmacophore/receptor model and indicates even better functional selectivity is possible with asymmetric BzR ligands.
The synthetic switching of chirality at the C-4 position of imidazobenzodiazepines to induce subtype selectivity was successful. Moreover, increase of the potency of imidazobenzodiazepines can be achieved by substitution of the 2′-position hydrogen atom with an electron rich atom (fluorine) on the pendant phenyl ring in agreement with Haefely et al. [88], Fryer [89, 90], and our own work [22, 91]. The biological data on the two enantiomeric pairs of benzodiazepine ligands confirm the ataxic activity of BZ site agonists is mediated by α1β2/3γ2 subtypes, as reported in [23, 91–93]. The antianxiety activity in primates of the S isomers was preserved with no sedation. In only one study in rodents was any sedation observed; the confounding sedation was observed in both the S isomer (functionally selective for α2, α3, and α5 receptor subtypes) and R isomer (essentially selective for α5 subtype) and may involve at least, in part, agonist activity at α5 BzR subtypes. There are some α5 BzR located in the spinal cord which might be the source of the decrease in locomotion with SH-053-2′F-R-CH3 and SH-053-2′F-S-CH3; however, this is possibly some type of stereotypical behavior. Hence in agreement with many laboratories including our own [23, 92, 93] the best potential nonsedative, nonamnesic, antianxiety agents stem from ligands with agonist efficacy at α2 subtypes essentially silent at α1 and α5 subtypes (to avoid sedation) [91]. It must be pointed out again; however, in primates Fischer et al. [87] observed a potent anxiolytic effect with no sedation with the 2′F-S-CH3 (17) isomer, while the 2′F-R-CH3 (2) isomer exhibited only a very weak anxiolytic effect.
Numerous groups have done modeling and SAR studies on different classes of compounds which have resulted in a few different pharmacophore models based on the benzodiazepine binding site (BS) of the GABAA receptor [94]. These models are employed to gain insight in the interactions between the BS and the ligand. These have been put forth by Loew [7, 95, 96], Crippen [97, 98], Codding [76, 77, 99–101], Fryer [89, 90, 94], Gilli and Borea [102–105], Tebib et al. [106], and Gardner [107], as well as from Professors Sieghart, Cromer, and our own laboratory [21, 39, 40, 76, 78–82, 108–118].
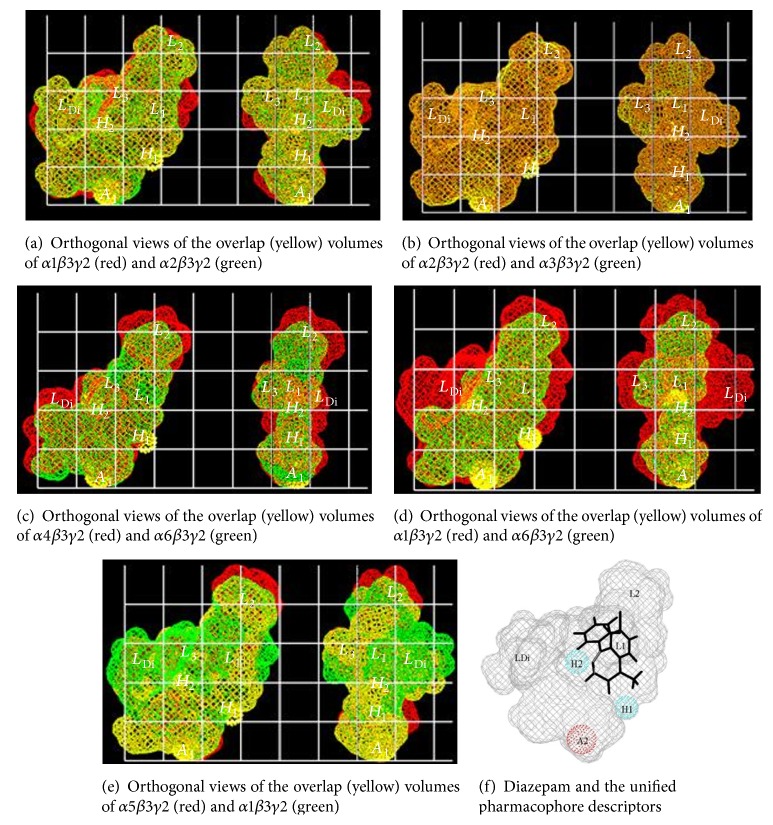
The Milwaukee-based pharmacophore/receptor model is a comprehensive building of the BzR using radioligand binding data and receptor mapping techniques based on 12 classes of compounds [20, 23, 39, 40, 42, 111, 119–122]. This model (Figure 31) [79] has brought together previous models which have used data from the activity of antagonists, positive allosteric modulators, and negative allosteric modulators and included the new models for the “diazepam-insensitive” (DI) sites [123]. Four basic anchor points, H 1, H 2, A 2, and L 1, were assigned, and 4 additional lipophilic regions were defined as L 2, L 3, L Di, and the new L 4 (see captions in Figure 31 for details); regions S 1, S 2, and S 3 represent negative areas of steric repulsion. As previously reported, the synthesis of both partial agonists and partial inverse agonists has been achieved by using parts of this model [99, 100, 104, 105, 119, 124–127].
Figure 31.
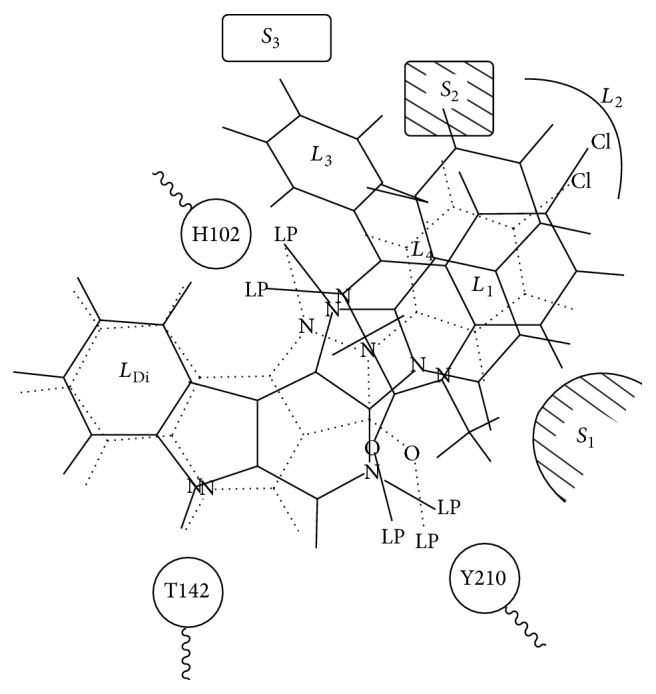
The two-dimensional representation of the Milwaukee-based unified pharmacophore with 3 amino acids in the binding site based on the rigid ligand template [23, 39, 42, 76–80]. This figure has been modified from that reported for PAMs, NAMs, and antagonists in [22, 23].
The cloning, expression, and anatomical localization of multiple GABA(A) subunits have facilitated both the identification and design of subtype selective ligands. With the availability of binding data from different recombinant receptor subtypes, affinities of ligands from many different structural classes of compounds have been evaluated.
Illustrated in Figure 31 is the [3,4-c]quinolin-3-one CGS-9896 (18) (dotted line), a diazadiindole (19) (thin line), and diazepam (20) (thick line) fitted initially to the inclusive pharmacophore model for the BzR. Sites H 1 (Y210) and H 2 (H102) represent hydrogen bond donor sites on the receptor protein complex while A 2 (T142) represents a hydrogen bond acceptor site necessary for potent inverse activity in vivo. L 1, L 2, L 3, L 4, and L Di are four lipophilic regions in the binding pharmacophore. Descriptors S 1, S 2, and S 3 are regions of negative steric repulsion.
Based on SAR data obtained for these ligands at 6 recombinant BzR subtypes [128–132], an effort has been undertaken to establish different pharmacophore/receptor models for BzR subtypes. The alignment of the twelve different structural classes of benzodiazepine receptor ligands was earlier based on the least squares fitting of at least three points. The coordinates of the four anchor points (A 2, H 1, H 2, and L 1) employed in the alignment are outlined in Figure 32. Herein are described the results from ligand-mapping experiments at recombinant BzR subtypes of 1,4-benzodiazepines, imidazobenzodiazepines, β-carbolines, diindoles, pyrazoloquinolinones, and others [126]. Some of the differences and similarities among these subtypes can be gleaned from this study and serve as a guide for future drug design.
Figure 32.
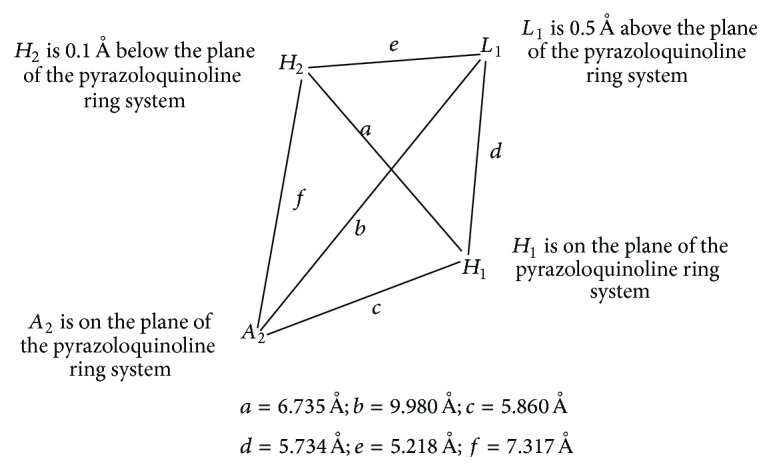
The schematic representation of the descriptors for the initial inclusive BzR pharmacophore based on the rigid ligands (diindoles) [79, 81–84]. This figure has been modified from that reported in [79].
10. α1 Updates
10.1. Beta-Carbolines
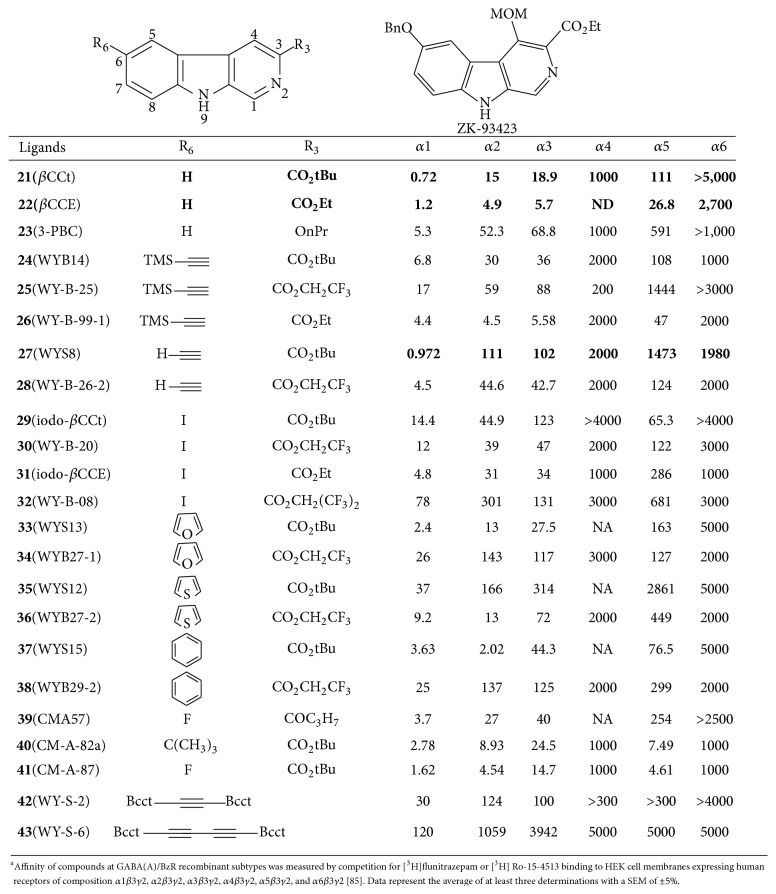
A series of 3,6-disubstituted β-carbolines was prepared and evaluated for their in vitro affinity at αxβ3γ2 GABA(A)/BzRr subtypes by radioligand binding assays in search of α1β3γ2 subtype selective compounds (Figure 33). A potential therapeutic application of such antagonist analogs is to treat alcohol abuse [133, 134]. Analogues of βCCt (21) were synthesized via a carbonyldiimidazole-mediated method by Yin et al. [85] and the related 6-substituted β-carboline-3-carboxylates including WYS8 (27) were synthesized from 6-iodo βCCt (29). Bivalent ligands (42 and 43) were also synthesized to increase the scope of the structure-activity relationships (SAR) to larger ligands. An initial SAR on the first analogs demonstrated that compounds with larger side-groups at C6 were well tolerated as they projected into the L Di domain (see 42 and 43) [85]. Moreover, substituents located at C3 exhibited a conserved stereo interaction in lipophilic pocket L 1, while N2 likely participated in hydrogen bonding with H 1. Three novel β-carboline ligands (21, 23, and 27) permitted a comparison of the pharmacological properties with a range of classical benzodiazepine receptor antagonists (flumazenil, ZK93426) from several structural groups and indicated these β-carbolines were “near GABA neutral antagonists.” Based on the SAR, the most potent (in vitro) α1 selective ligand was the 6-substituted acetylenyl βCCt (WYS8, 27). In a previous study both 21 and 23 were able to reduce the rate at which rats self-administrated alcohol in alcohol preferring and HAD rats but had little or no effect on sucrose self-administration [85]. 3-PBC (23) was also active in baboons [134]. This data has been used in updating the pharmacophore model in the α1-subtype.
Figure 33.
aAffinities (K i = nM) of 3,6-disubstituted β-carbolines at αxβ3γ2 (x = 1–3, 5, 6) receptor subtypes [85]. The structures versus code numbers of all ligands in the tables of this review can be found in the Ph.D. thesis of Terry Clayton (Ph.D. thesis, University of Wisconsin-Milwaukee, Milwaukee, WI, December, 2011) [22] and in the Supporting Information.
11. The Updated Included Volume Models
Illustrated in Figure 34 is the included volume of the updated pharmacophore receptor model of the α1β3γ2 subtype of Clayton [22]. The current model for the α1β3γ2 subtype has several new features. The cyclopropyl group of CD-214 extended 2 Å past the A 2 descriptor slightly increasing its volume. The trimethylsilyl group of QH-II-82 and WYS7 illustrates how well bulky groups are tolerated near the entrance of the binding pocket. Despite not being as potent, dimers of beta carbolines, WYS2 and WYS6, bound to α1 subtypes at 30 nM and 120 nM, respectively. Their ability to bind, albeit weakly, supports the location of the binding site entrance from the extracellular domain. The included volume of the α1β3γ2 subtype was previously 1085.7 cubic angstroms. The volume has now been measured as 1219.2 cubic angstroms. Volume measurements should be used carefully as the binding site is not enclosed and the theoretical opening near L DI is not clearly demarcated. Dimers were excluded from the included volume exercise because although they bound to the receptor, they represented compounds which were felt to extend outside the receptor binding pocket when docked to the protein. Where appropriate, their monomers were included in the included volume analysis. Ligands considered for the included volume in Table 5 exhibited potent binding at α1 subtypes (K i ≤ 20 nM) but were not necessarily subtype selective. The binding data for ligands at α 2–6-subtypes follow (Tables 6–10; structures located in Clayton [22] and Supporting Information, Appendix III in Supplementary Material available online at http://dx.doi.org/10.1155/2015/430248).
Figure 34.
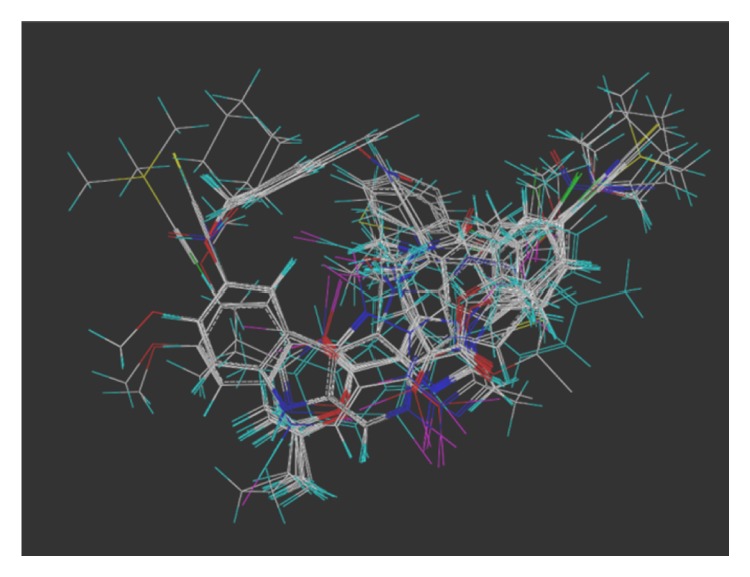
Overlay of selected compounds for α1β3γ2 subtype from Table 5.
Table 5.
These ligands bound with potent affinity for α1; ligands bound with K i values <20 nM at this subtype.
| Cook codea | α1 | α2 | α3 | α4 | α5 | α6 |
|---|---|---|---|---|---|---|
| WY-TSC-4 (WYS8) | 0.007 | 0.99 | 1.63 | 51.04 | ||
| SH-TSC-2 (BCCT) | 0.03 | 0.0419 | 0.035 | 69.32 | ||
| QH-II-090 (CGS-8216) | 0.05 | 0.08 | 0.12 | 0.25 | 17 | |
| XLI-286 | 0.051 | 0.064 | 0.118 | 0.684 | ||
| QH-II-077 | 0.06 | 0.08 | 0.05 | 0.12 | 4 | |
| QH-II-092 | 0.07 | 0.03 | 0.04 | ND | 0.17 | ND |
| JYI-57 | 0.076 | 0.076 | 0.131 | ND | 0.036 | ND |
| QH-II-085 | 0.08 | 0.06 | 0.02 | ND | 0.08 | ND |
| XHE-II-024 | 0.09 | 0.18 | 0.32 | 14 | 0.24 | 11 |
| PWZ-007A | 0.11 | 0.1 | 0.09 | ND | 0.2 | 10 |
| CGS8216 | 0.13 | ND | ND | ND | ND | 46 |
| SPH-121 | 0.14 | 1.19 | 1.72 | ND | 4 | 479 |
| QH-II-075 | 0.18 | 0.21 | 0.25 | ND | 1.3 | 40 |
| PZII-028 | 0.2 | ND | 0.2 | ND | 0.32 | 1.9 |
| CGS9895 | 0.21 | ND | ND | ND | ND | 9.3 |
| PWZ-0071 | 0.23 | 0.17 | 0.12 | ND | 0.44 | 17.31 |
| XHE-III-24 | 0.25 | ND | 8 | 222 | 10 | 328 |
| JYI-42 | 0.257 | 0.146 | 0.278 | ND | 0.256 | ND |
| CGS9896 | 0.28 | ND | ND | ND | ND | 181 |
| JYI-64 (C17H12N4FBr) | 0.305 | 1.111 | 0.62 | ND | 0.87 | 5000 |
| PZII-029 | 0.34 | ND | 0.79 | ND | 0.52 | 10 |
| BRETAZENIL | 0.35 | 0.64 | 0.2 | ND | 0.5 | 12.7 |
| FG8205 | 0.4 | 2.08 | 1.16 | ND | 1.54 | 227 |
| YT-5 | 0.421 | 0.6034 | 36.06 | ND | 1.695 | ND |
| 6-PBC | 0.49 | 1.21 | 2.2 | ND | 2.39 | 1343 |
| QH-146 | 0.49 | ND | 0.76 | ND | 7.7 | 10000 |
| DM-II-90 (C17H12N4BrCl) | 0.505 | 1 | 0.63 | ND | 0.37 | 5000 |
| SPH-165 | 0.63 | 2.79 | 4.85 | ND | 10.4 | 1150 |
| BCCt | 0.72 | 15 | 18.9 | ND | 110.8 | 5000 |
| SH-I-048A | 0.774 | 0.1723 | 0.383 | ND | 0.11 | ND |
| alprazolam | 0.8 | 0.59 | 1.43 | ND | 1.54 | 10000 |
| Ro15-1788 | 0.8 | 0.9 | 1.05 | ND | 0.6 | 148 |
| WYS10 C14H9F3N2O2 | 0.88 | 36 | 25.6 | ND | 548.7 | 15.3 |
| WY-B-15 | 0.92 | 0.83 | 0.58 | 2080 | 4.42 | 646 |
| WY-A-99-2 (WYS8) | 0.972 | 111 | 102 | 2000 | 208 | 1980 |
| XHE-III-06a | 1 | 2 | 1 | 5 | 1.8 | 37 |
| Xli366 C22H21N3O2 | 1 | ND | ND | ND | ND | ND |
| JYI-59 (C22H13N3O2F4) | 1.08 | 2.6 | 11.82 | ND | 11.5 | 5000 |
| WYSC1 C16H16N2O2 | 1.094 | 5.44 | 12.3 | ND | 69.8 | 21.2 |
| MLT-I-70 | 1.1 | 1.2 | 1.1 | ND | 40.3 | 1000 |
| SVO-8-30 | 1.1 | 5.3 | 5.3 | 2.8 | 0.6 | 15 |
| BCCE | 1.2 | 4.9 | 5.7 | ND | 26.8 | 2700 |
| XHE-III-04 | 1.2 | 2 | 1.1 | 219 | 0.4 | 500 |
| XLi350 C17H11ClN2O | 1.224 | 1.188 | ND | ND | 2.9 | ND |
| XHE-III-49 | 1.3 | 5.5 | 4.2 | 38.7 | 11.3 | 85.1 |
| PWZ-009A1 | 1.34 | 1.31 | 1.26 | ND | 0.84 | 2.03 |
| DM-239 | 1.5 | ND | 0.53 | ND | 0.14 | 6.89 |
| XLi351 C21H21ClN2OSi | 1.507 | 0.967 | ND | ND | 1.985 | ND |
| XLi352 C18H13ClN2O | 1.56 | 0.991 | ND | ND | 1.957 | ND |
| TG-4-39 | 1.6 | 34 | 24 | 5.6 | 1.4 | 23 |
| TG-II-82 | 1.6 | 2.9 | 2.8 | ND | 1 | 1000 |
| CM-A87 | 1.62 | 4.54 | 14.73 | 1000 | 4.61 | 1000 |
| QH-II-082 | 1.7 | 1.8 | 1.6 | ND | 6.1 | 100 |
| JYI-49 (C20H12N3O2F4Br) | 1.87 | 2.38 | ND | ND | 6.7 | 3390 |
| LJD-III-15E | 1.93 | 14 | 19 | ND | 70.8 | 1000 |
| SPH-38 | 2 | 5.4 | 10.8 | ND | 18.5 | 3000 |
| XHE-I-093 | 2 | 7.1 | 8.9 | 1107 | 20 | 1162 |
| MSA-IV-35 | 2.1 | 16 | 21 | ND | 995 | 3000 |
| JYI-19 (C23H23N3O3S) | 2.176 | 205 | ND | ND | 34 | 12.7 |
| FLUNITRAZEPAM | 2.2 | 2.5 | 4.5 | ND | 2.1 | 2000 |
| YCT-5 | 2.2 | 11.46 | 16.3 | ND | 200 | 10000 |
| TJH-IV-51 | 2.39 | 17.4 | 14.5 | ND | 316 | 10000 |
| WYS13 C20H18N2O3 | 2.442 | 13 | 27.5 | ND | 163 | 5000 |
| YT-III-25 | 2.531 | 5.786 | 5.691 | ND | 0.095 | ND |
| XHE-III-14 | 2.6 | 10 | 13 | 2 | 7 | |
| WYS9 C16H15IN2O2 | 2.72 | 22.2 | 23.1 | ND | 562 | 122 |
| JYI-47 | 2.759 | 2.282 | 0.511 | ND | 0.427 | ND |
| CM-A82a | 2.78 | 8.93 | 24.51 | 1000 | 7.49 | 1000 |
| TG-4-29 | 2.8 | 3.9 | 2.7 | 2.1 | 0.18 | 3.9 |
| XLi268 C17H13BrN4 | 2.8145 | 0.6862 | ND | ND | 0.6243 | ND |
| JYI-54 (C24H15N3O3F4) | 2.89 | 172 | 6.7 | ND | 57 | 1890 |
| MMB-II-74 | 3 | 24.5 | 41.7 | 500 | 125.7 | 1000 |
| MMB-III-016 | 3 | 1.97 | 2 | 1074 | 0.26 | 211 |
| MMB-III-16 | 3 | 1.97 | 2 | 1074 | 0.26 | 211 |
| QH-II-080b | 3 | 3.7 | 4.7 | ND | 24 | 1000 |
| YCT-7A | 3 | 23.8 | 30.5 | ND | 240 | 10000 |
| JYI-32 (C20H15N3O2BrF) | 3.07 | 4.96 | ND | ND | 2.92 | 52.24 |
| Ro15-4513 | 3.3 | 2.6 | 2.5 | ND | 0.26 | 3.8 |
| XHE-II-017 | 3.3 | 10 | 7 | 258 | 17 | 294 |
| XLi-JY-DMH ANX3 | 3.3 | 0.58 | 1.9 | ND | 4.4 | 5000 |
| MLT-II-18 | 3.4 | 11.7 | 11 | ND | 225 | 10000 |
| TJH-V-88 | 3.41 | 30 | ND | 140.9 | 10000 | |
| XLI-2TC | 3.442 | 1.673 | 44.08 | ND | 1.121 | |
| WYS15 C22H20N2O2 | 3.63 | 2.02 | 44.3 | ND | 76.5 | 5000 |
| CM-A57 | 3.7 | 27 | 40 | ND | 254 | 1000 |
| XHE-II-006b | 3.7 | 15 | 12 | 1897 | 144 | 1000 |
| JYI-60 | 3.73 | 1.635 | 4.3 | ND | 1.7 | 5000 |
| RY-008 | 3.75 | 7.2 | 4.14 | ND | 1.11 | 44.3 |
| MLT-II-18 | 3.9 | 12.2 | 24.4 | ND | 210 | 10000 |
| OMB-18 | 3.9 | 1.2 | 3.4 | 1733 | 0.8 | 5 |
| WY-B-09-1 | 3.99 | 8 | 32 | 1000 | 461 | 2000 |
| SHU-1-19 | 4 | 12 | 7 | 48 | 14 | 84 |
| ZK 93423 | 4.1 | 4.2 | 6 | ND | 4.5 | 1000 |
| WY-B-23-2 (WYS11) | 4.2 | 37.7 | 39 | 2000 | 176 | 69.4 |
| WY-B-23-2 (WYS11) | 4.2 | 37.7 | 73 | ND | 176 | 69.4 |
| WY-B-99-1 | 4.4 | 4.5 | 5.58 | 2000 | 47 | 2000 |
| WY-B-26-2 | 4.45 | 44.57 | 42.66 | 2000 | 124 | 2000 |
| XHE-II-006a | 4.7 | 4.4 | 20 | 1876 | 89 | 3531 |
| CM-B01 | 4.8 | 31 | 34 | 1000 | 286 | 1000 |
| PWZ-085 | 4.86 | 13 | 8.5 | ND | 0.55 | 40 |
| MLT-II-16 | 5.05 | 10.41 | 18.4 | ND | 260 | 10000 |
| 3 PBC | 5.3 | 52.3 | 68.8 | ND | 591 | 1000 |
| MA-3-PROPOXYL | 5.3 | 52.3 | 68.8 | ND | 591 | 1000 |
| TJH-IV-43 | 5.42 | 30.19 | 48.9 | ND | 475 | 10000 |
| DMCM | 5.69 | 8.29 | 4 | ND | 1.04 | 134 |
| DM-139 | 5.8 | ND | 169 | ND | 9.25 | 325 |
| XHE-II-073A (R ENRICHED) | 5.9 | 11 | 10 | 15 | 1.18 | 140 |
| MSR-I-032 | 6.2 | 18.7 | 4 | ND | 3.3 | 74.9 |
| JYI-70 (C19H13N4F) | 6.3 | 2.1 | ND | ND | 0.56 | 5000 |
| XLi343 C20H19ClN2OSi | 6.375 | 17.71 | ND | ND | 150.5 | ND |
| 3 EBC | 6.43 | 25.1 | 28.2 | ND | 826 | 1000 |
| DM-146 | 6.44 | ND | 148 | ND | 4.23 | 247 |
| DM-215 | 6.74 | ND | 7.42 | ND | 0.293 | 8.28 |
| ZG69A | 6.8 | 16.3 | 9.2 | ND | 0.85 | 54.6 |
| ZG-69a (Ro15-1310) | 6.8 | 16.3 | 9.2 | ND | 0.85 | 54.6 |
| WY-B-14 (WYS7) | 6.84 | 30 | 36 | 2000 | 108 | 1000 |
| YT-II | 6.932 | 0.8712 | 3.518 | ND | 5.119 | ND |
| SVO-8-67 | 7 | 41 | 26 | 15 | 2.3 | 191 |
| MLT-II-34 | 7.04 | 15.95 | 22.3 | ND | 158 | 1000 |
| SPH-195 | 7.2 | 168.5 | 283.5 | ND | 271 | 10000 |
| XHE-I-065 | 7.2 | 17 | 18 | 500 | 57 | 500 |
| ZG-234 | 7.25 | 22.14 | 9.84 | ND | 0.3 | 5.25 |
| SH-I-04 | 7.3 | 6.136 | 5.1 | ND | 7.664 | ND |
| XHE-I-038 | 7.3 | 5 | 34 | ND | 132 | 1000 |
| XHE-III-13 | 7.3 | ND | 7.1 | 880 | 1.6 | 311 |
| WY-B-25 | 7.6 | 40 | 66 | 2000 | 263 | 2000 |
| CM-A49 (R) | 7.7 | 32.5 | 43 | ND | 69 | 1000 |
| SVO-8-14 | 8 | 25 | 8 | 6.9 | 0.9 | 14 |
| TG-4-29 | 8.3 | 10.2 | 6.9 | ND | 0.4 | 7.61 |
| XHE-II-002 | 8.3 | 18 | 13 | 3.9 | 1.5 | 11 |
| WY-B-14 (WYS7) | 8.5 | 165 | 245 | ND | 1786 | 5000 |
| XHE-II-011 | 9 | 60 | 39 | 3233 | 90 | 1000 |
| WY-B-27-2 | 9.19 | 111 | 72 | 2000 | 449 | 2000 |
| QH-II-063 | 9.4 | 9.3 | 31 | ND | 7.7 | 3000 |
| JC184 C13H9BrN2OS | 9.606 | 10.5 | ND | ND | 6.709 | ND |
| ZG-208 | 9.7 | 11.2 | 10.9 | ND | 0.38 | 4.6 |
| RY-I-31 | 10 | 45 | 19 | ND | 6 | 1000 |
| WY-B-23-1 | 10 | 33 | 43 | 1000 | 189 | 2000 |
| RY-098 | 10.1 | 22.2 | 16.5 | ND | 1.68 | 100 |
| Hz148 C18H15N3 | 10.98 | 5000 | ND | ND | 256 | 5000 |
| SVO-8-20 | 11 | 40 | 28 | 19 | 8.6 | 138 |
| XHE-II-073B (S-ENRICHED) | 11 | 17 | 12 | 33 | 2.1 | 269 |
| SH-I-085 | 11.08 | 4.866 | 13.75 | ND | 0.24 | ND |
| PWZ-096 | 11.1 | 36 | 16.9 | ND | 1.07 | 51.5 |
| ZG-168 | 11.2 | 10.7 | 9.2 | ND | 0.47 | 9.4 |
| CM-A77 | 11.51 | 51.9 | 105.16 | 1000 | 42.62 | 1000 |
| WY-B-20 | 12 | 39 | 47 | 2000 | 122 | 3000 |
| ABECARNIL | 12.4 | 15.3 | 7.5 | ND | 6 | 1000 |
| SH-I-89S | 12.78 | 8.562 | 8.145 | ND | 3.23 | ND |
| ZG-213 | 12.8 | 49.8 | 30.2 | ND | 3.5 | 22.5 |
| EDC-I-071 | 12.9 | 83.1 | ND | ND | 314 | 5000 |
| MMB-III-14 | 13 | 13 | 6.9 | 333 | 1.1 | 333 |
| DM-173 | 13.1 | ND | 38.1 | ND | 0.78 | 118 |
| XLI-348 | 13.56 | 11.17 | 1.578 | ND | 82.05 | ND |
| EDC-I-093 | 13.6 | 423 | ND | ND | 2912 | 5000 |
| diazepam | 14 | 20 | 15 | ND | 11 | ND |
| XLi223 C22H20BrN3O2 | 14 | 8.7 | 18 | 1000 | 10 | 2000 |
| WYSC2 C15H11F3N2O2 | 14.14 | 113 | 170 | ND | 518 | 61.2 |
| SH-I-030 | 14.42 | 11.04 | 19.09 | ND | 1.89 | ND |
| CM-A100 | 14.49 | 44.91 | 123.8 | 1000 | 65.31 | 1000 |
| RY-033 | 14.8 | 56 | 25.3 | ND | 1.72 | 22.9 |
| HJ-I-037 | 15.07 | 8.127 | 28.29 | ND | 0.818 | ND |
| YT-6 | 15.31 | 87.8 | 60.49 | ND | 1.039 | ND |
| EDC-II-044 | 15.4 | ND | 293 | ND | 323 | 1000 |
| CM-A58 | 16 | 120 | 184 | ND | 1000 | 1012 |
| QH-II-067a | 16 | 31 | 52 | ND | 199 | 3000 |
| CD-214 | 16.4 | 48.2 | 42.5 | ND | 9.8 | 168 |
| JYI-06 (C23H23N3O4) | 16.5 | 5.48 | 5000 | ND | 12.6 | 5000 |
| CM-A50 (S) | 17 | 59 | 88 | ND | 144 | 1000 |
| RY-061 | 17 | 13 | 6.7 | ND | 0.3 | 31 |
| ZG-224 | 17.1 | 33.7 | 50 | ND | 2.5 | 30.7 |
| ZG-63A | 17.3 | 21.6 | 29.1 | ND | 0.65 | 4 |
| DM-II-30 (C20H13N3O2BrF3) | 17.6 | 13.4 | 28.51 | ND | 7.8 | 5000 |
| CM-A64 | 18 | 60 | 116 | ND | 216 | 1000 |
| RY-071 | 19 | 56 | 91 | ND | 7.2 | 266 |
| WZ-113 | 19.2 | 13.2 | 13.4 | ND | 11.5 | 300 |
| YT-III-23 | 19.83 | 23.65 | 19.87 | ND | 1.105 | ND |
| CM-E09b | 20 | 22 | 19 | 55 | 0.45 | 69 |
| MMB-II-90 | 20 | 24 | 5.7 | 9 | 0.25 | 36 |
aAffinity of compounds at GABAA/BzR recombinant subtypes was measured by competition for [3H]flunitrazepam or [3H] Ro15-4513 binding to HEK cell membranes expressing human receptors of compositions α1β3γ2, α2β3γ2, α3β3γ2, α4β3γ2, α5β3γ2, and α6β3γ2 [139]. Data represent the average of at least three determinations with a SEM of ±5%. The structures of these ligands are in the Ph.D. thesis of Clayton (2011) [22] and Supporting Information.
Table 6.
Ligands with potent affinity for α2; ligands bound with K i values <20 nM at this subtype. The structures of these ligands are in the Ph.D. thesis of Clayton (2011) [22].
| Cook codea | α1 | α2 | α3 | α4 | α5 | α6 |
|---|---|---|---|---|---|---|
| QH-II-092 | 0.07 | 0.03 | 0.04 | ND | 0.17 | ND |
| SH-TSC-2 (BCCT) | 0.03 | 0.0419 | 0.035 | ND | 69.32 | ND |
| QH-II-085 | 0.08 | 0.06 | 0.02 | ND | 0.08 | ND |
| XLI-286 | 0.051 | 0.064 | 0.118 | ND | 0.684 | ND |
| JYI-57 | 0.076 | 0.076 | 0.131 | ND | 0.036 | ND |
| QH-II-090 (CGS-8216) | 0.05 | 0.08 | 0.12 | ND | 0.25 | 17 |
| QH-II-077 | 0.06 | 0.08 | 0.05 | ND | 0.12 | 4 |
| PWZ-007A | 0.11 | 0.1 | 0.09 | ND | 0.2 | 10 |
| JYI-42 | 0.257 | 0.146 | 0.278 | ND | 0.256 | ND |
| PWZ-0071 | 0.23 | 0.17 | 0.12 | ND | 0.44 | 17.31 |
| SH-I-048A | 0.774 | 0.1723 | 0.383 | ND | 0.11 | ND |
| XHE-II-024 | 0.09 | 0.18 | 0.32 | 14 | 0.24 | 11 |
| QH-II-075 | 0.18 | 0.21 | 0.25 | ND | 1.3 | 40 |
| XLi-JY-DMH ANX3 | 3.3 | 0.58 | 1.9 | ND | 4.4 | 5000 |
| alprazolam | 0.8 | 0.59 | 1.43 | ND | 1.54 | 10000 |
| YT-5 | 0.421 | 0.6034 | 36.06 | ND | 1.695 | ND |
| BRETAZENIL | 0.35 | 0.64 | 0.2 | ND | 0.5 | 12.7 |
| XLi268 C17H13BrN4 | 2.8145 | 0.6862 | ND | ND | 0.6243 | ND |
| WY-B-15 | 0.92 | 0.83 | 0.58 | 2080 | 4.42 | 646 |
| YT-II | 6.932 | 0.8712 | 3.518 | ND | 5.119 | |
| Ro15-1788 | 0.8 | 0.9 | 1.05 | ND | 0.6 | 148 |
| XLi351 C21H21ClN2OSi | 1.507 | 0.967 | ND | ND | 1.985 | ND |
| WY-TSC-4 (WYS8) | 0.007 | 0.99 | 1.63 | ND | 51.04 | ND |
| XLi352 C18H13ClN2O | 1.56 | 0.991 | ND | ND | 1.957 | ND |
| DM-II-90 (C17H12N4BrCl) | 0.505 | 1 | 0.63 | ND | 0.37 | 5000 |
| JYI-64 (C17H12N4FBr) | 0.305 | 1.111 | 0.62 | ND | 0.87 | 5000 |
| XLi350 C17H11ClN2O | 1.224 | 1.188 | ND | ND | 2.9 | ND |
| SPH-121 | 0.14 | 1.19 | 1.72 | ND | 4 | 479 |
| MLT-I-70 | 1.1 | 1.2 | 1.1 | ND | 40.3 | 1000 |
| OMB-18 | 3.9 | 1.2 | 3.4 | 1733 | 0.8 | 5 |
| 6-PBC | 0.49 | 1.21 | 2.2 | ND | 2.39 | 1343 |
| YT-III-271 | 32.54 | 1.26 | 2.35 | ND | 103 | ND |
| PWZ-009A1 | 1.34 | 1.31 | 1.26 | ND | 0.84 | 2.03 |
| DM-II-72 (C15H10N20BrCl) | 5000 | 1.37 | ND | ND | 2.02 | 5000 |
| JYI-60 (C17H11N2OF) | 3.73 | 1.635 | 4.3 | ND | 1.7 | 5000 |
| XLI-2TC | 3.442 | 1.673 | 44.08 | ND | 1.121 | ND |
| QH-II-082 | 1.7 | 1.8 | 1.6 | ND | 6.1 | 100 |
| TC-YT-II-76 | 101.1 | 1.897 | 5.816 | ND | 11.99 | ND |
| MMB-III-016 | 3 | 1.97 | 2 | 1074 | 0.26 | 211 |
| MMB-III-16 | 3 | 1.97 | 2 | 1074 | 0.26 | 211 |
| XHE-III-06a | 1 | 2 | 1 | 5 | 1.8 | 37 |
| XHE-III-04 | 1.2 | 2 | 1.1 | 219 | 0.4 | 500 |
| WYS15 C22H20N2O2 | 3.63 | 2.02 | 44.3 | ND | 76.5 | 5000 |
| FG8205 | 0.4 | 2.08 | 1.16 | ND | 1.54 | 227 |
| JYI-70 (C19H13N4F) | 6.3 | 2.1 | ND | ND | 0.56 | 5000 |
| JYI-47 | 2.759 | 2.282 | 0.511 | ND | 0.427 | ND |
| JYI-49 (C20H12N3O2F4Br) | 1.87 | 2.38 | ND | ND | 6.7 | 3390 |
| FLUNITRAZEPAM | 2.2 | 2.5 | 4.5 | ND | 2.1 | 2000 |
| JYI-59 (C22H13N3O2F4) | 1.08 | 2.6 | 11.82 | ND | 11.5 | 5000 |
| Ro15-4513 | 3.3 | 2.6 | 2.5 | ND | 0.26 | 3.8 |
| SPH-165 | 0.63 | 2.79 | 4.85 | ND | 10.4 | 1150 |
| YT-II-76 | 95.34 | 2.797 | 0.056 | ND | 0.04 | ND |
| TG-II-82 | 1.6 | 2.9 | 2.8 | ND | 1 | 1000 |
| QH-II-080b | 3 | 3.7 | 4.7 | ND | 24 | 1000 |
| TG-4-29 | 2.8 | 3.9 | 2.7 | 2.1 | 0.18 | 3.9 |
| PS-1-34B C20H17N4BrO | ND | 4.198 | 3.928 | ND | ND | ND |
| ZK 93423 | 4.1 | 4.2 | 6 | ND | 4.5 | 1000 |
| XHE-II-006a | 4.7 | 4.4 | 20 | 1876 | 89 | 3531 |
| WY-B-99-1 | 4.4 | 4.5 | 5.58 | 2000 | 47 | 2000 |
| CM-A87 | 1.62 | 4.54 | 14.73 | 1000 | 4.61 | 1000 |
| OMB-19 | 22 | 4.6 | 20 | 3333 | 3.5 | 40 |
| SH-I-085 | 11.08 | 4.866 | 13.75 | ND | 0.24 | ND |
| BCCE | 1.2 | 4.9 | 5.7 | ND | 26.8 | 2700 |
| JYI-32 (C20H15N3O2BrF) | 3.07 | 4.96 | ND | ND | 2.92 | 52.24 |
| XHE-I-038 | 7.3 | 5 | 34 | ND | 132 | 1000 |
| SVO-8-30 | 1.1 | 5.3 | 5.3 | 2.8 | 0.6 | 15 |
| SPH-38 | 2 | 5.4 | 10.8 | ND | 18.5 | 3000 |
| WYSC1 C16H16N2O2 | 1.094 | 5.44 | 12.3 | ND | 69.8 | 21.2 |
| JYI-06 (C23H23N3O4) | 16.5 | 5.48 | 5000 | ND | 12.6 | 5000 |
| XHE-III-49 | 1.3 | 5.5 | 4.2 | 38.7 | 11.3 | 85.1 |
| YT-III-25 | 2.531 | 5.786 | 5.691 | ND | 0.095 | ND |
| SH-I-04 | 7.3 | 6.136 | 5.1 | ND | 7.664 | ND |
| XHE-I-093 | 2 | 7.1 | 8.9 | 1107 | 20 | 1162 |
| RY-008 | 3.75 | 7.2 | 4.14 | ND | 1.11 | 44.3 |
| DMH-D-053 (C43H30N6O4) | 236 | 7.4 | 272 | 5000 | 194.2 | 5000 |
| WY-B-09-1 | 3.99 | 8 | 32 | 1000 | 461 | 2000 |
| HJ-I-037 | 15.07 | 8.127 | 28.29 | ND | 0.818 | ND |
| DMCM | 5.69 | 8.29 | 4 | ND | 1.04 | 134 |
| SH-I-89S | 12.78 | 8.562 | 8.145 | ND | 3.23 | ND |
| XLi223 C22H20BrN3O2 | 14 | 8.7 | 18 | 1000 | 10 | 2000 |
| CM-A82a | 2.78 | 8.93 | 24.51 | 1000 | 7.49 | 1000 |
| QH-II-063 | 9.4 | 9.3 | 31 | ND | 7.7 | 3000 |
| 9.4 | 9.3 | 31 | ND | 7.7 | 3000 | |
| XHE-II-017 | 3.3 | 10 | 7 | 258 | 17 | 294 |
| TG-4-29 | 8.3 | 10.2 | 6.9 | ND | 0.4 | 7.61 |
| MLT-II-16 | 5.05 | 10.41 | 18.4 | ND | 260 | 10000 |
| JC184 C13H9BrN2OS | 9.606 | 10.5 | ND | ND | 6.709 | ND |
| ZG-168 | 11.2 | 10.7 | 9.2 | ND | 0.47 | 9.4 |
| XHE-II-073A (R ENRICHED) | 5.9 | 11 | 10 | 15 | 1.18 | 140 |
| XLI-8TC | 21.52 | 11.01 | 2.155 | ND | 4.059 | ND |
| SH-I-030 | 14.42 | 11.04 | 19.09 | ND | 1.89 | ND |
| XLI-348 | 13.56 | 11.17 | 1.578 | ND | 82.05 | ND |
| ZG-208 | 9.7 | 11.2 | 10.9 | ND | 0.38 | 4.6 |
| YT-TC-3 | 141.4 | 11.43 | 118.1 | ND | 29.22 | ND |
| YCT-5 | 2.2 | 11.46 | 16.3 | ND | 200 | 10000 |
| MLT-II-18 | 3.4 | 11.7 | 11 | ND | 225 | 10000 |
| XHE-II-O53-ACID | 50.35 | 11.8 | 44 | ND | 5.9 | 5000 |
| SHU-1-19 | 4 | 12 | 7 | 48 | 14 | 84 |
| RY-067 | 21 | 12 | 10 | ND | 0.37 | 42 |
| DM-III-01 (C18H12N3O2Br) | 5000 | 12 | ND | ND | 4.73 | 5000 |
| MLT-II-18 | 3.9 | 12.2 | 24.4 | ND | 210 | 10000 |
| SH-053-2′F | 21.99 | 12.34 | 34.9 | ND | 0.671 | ND |
| WYS13 C20H18N2O3 | 2.442 | 13 | 27.5 | ND | 163 | 5000 |
| PWZ-085 | 4.86 | 13 | 8.5 | ND | 0.55 | 40 |
| MMB-III-14 | 13 | 13 | 6.9 | 333 | 1.1 | 333 |
| RY-061 | 17 | 13 | 6.7 | ND | 0.3 | 31 |
| WZ-113 | 19.2 | 13.2 | 13.4 | ND | 11.5 | 300 |
| YT-II-83 | 32.74 | 13.22 | 24.1 | ND | 3.548 | ND |
| DM-II-30 (C20H13N3O2BrF3) | 17.6 | 13.4 | 28.51 | ND | 7.8 | 5000 |
| LJD-III-15E | 1.93 | 14 | 19 | ND | 70.8 | 1000 |
| YT-III-272 | 295.9 | 14.98 | 10.77 | ND | 103.3 | ND |
| BCCt | 0.72 | 15 | 18.9 | ND | 110.8 | 5000 |
| XHE-II-006b | 3.7 | 15 | 12 | 1897 | 144 | 1000 |
| ABECARNIL | 12.4 | 15.3 | 7.5 | ND | 6 | 1000 |
| MLT-II-34 | 7.04 | 15.95 | 22.3 | ND | 158 | 1000 |
| MSA-IV-35 | 2.1 | 16 | 21 | ND | 995 | 3000 |
| JYI-04 (C21H23N3O3) | 28.3 | 16 | ND | ND | 0.51 | 1.57 |
| PS-1-35 C23H22N5OBr | ND | 16.03 | 24.41 | ND | ND | ND |
| ZG69A | 6.8 | 16.3 | 9.2 | ND | 0.85 | 54.6 |
| ZG-69a (Ro15-1310) | 6.8 | 16.3 | 9.2 | ND | 0.85 | 54.6 |
| YT-III-42 | 382.9 | 16.83 | 44.04 | ND | 9.77 | ND |
| XHE-I-065 | 7.2 | 17 | 18 | 500 | 57 | 500 |
| XHE-II-073B (S-ENRICHED) | 11 | 17 | 12 | 33 | 2.1 | 269 |
| TJH-IV-51 | 2.39 | 17.4 | 14.5 | ND | 316 | 10000 |
| SH-I-047 | 1710 | 17.52 | 1222 | ND | 1519 | ND |
| XLi343 C20H19ClN2OSi | 6.375 | 17.71 | ND | ND | 150.5 | ND |
| XHE-II-002 | 8.3 | 18 | 13 | 3.9 | 1.5 | 11 |
| YT-III-38 | 1461 | 18.21 | 14.63 | ND | 3999 | |
| JYI-72 (C22H21N4SiF) | 48.5 | 18.5 | ND | ND | 11.5 | 5000 |
| MSR-I-032 | 6.2 | 18.7 | 4 | ND | 3.3 | 74.9 |
| JC208 C15H10N2OS | 22.42 | 18.89 | ND | ND | 5.039 | ND |
| diazepam | 14 | 20 | 15 | ND | 11 | ND |
aAffinity of compounds at GABAA/BzR recombinant subtypes was measured by competition for [3H]flunitrazepam or [3H] Ro15-4513 binding to HEK cell membranes expressing human receptors of compositions α1β3γ2, α2β3γ2, α3β3γ2, α4β3γ2, α5β3γ2, and α6β3γ2 [22, 139]. Data represent the average of at least three determinations with a SEM of ±5%. ND: not determined.
Table 10.
Ligands with potent affinity for α6; ligands bound with K i values <20 nM at this subtype. The structures of these ligands are in the Ph.D. thesis of Clayton (2011) [22].
| Cook codea | α1 | α2 | α3 | α4 | α5 | α6 |
|---|---|---|---|---|---|---|
| CM-D45 C19H21N3O4 | 90.5 | 65.5 | 30.3 | 0.15 | 1.65 | 0.23 |
| CM-D44 | 34.3 | 56.3 | 20.7 | 0.33 | 0.57 | 0.92 |
| JYI-04 (C21H23N3O3) | 28.3 | 16 | ND | ND | 0.51 | 1.57 |
| PZII-028 | 0.2 | ND | 0.2 | ND | 0.32 | 1.9 |
| PWZ-009A1 | 1.34 | 1.31 | 1.26 | ND | 0.84 | 2.03 |
| JYI-01 (C19H20N3O3Br) | 59.2 | 159 | 96 | ND | 10.6 | 2.88 |
| JYI-03 (C21H21N3O3) | 185.4 | 107 | ND | ND | 0.954 | 3.34 |
| Ro15-4513 | 3.3 | 2.6 | 2.5 | ND | 0.26 | 3.8 |
| TG-4-29 | 2.8 | 3.9 | 2.7 | 2.1 | 0.18 | 3.9 |
| JYI-11 (C22H22N3O3F3Si) | 5000 | 5000 | ND | ND | 648 | 3.97 |
| QH-II-077 | 0.06 | 0.08 | 0.05 | ND | 0.12 | 4 |
| ZG-63A | 17.3 | 21.6 | 29.1 | ND | 0.65 | 4 |
| ZG-208 | 9.7 | 11.2 | 10.9 | ND | 0.38 | 4.6 |
| OMB-18 | 3.9 | 1.2 | 3.4 | 1733 | 0.8 | 5 |
| RY-024 C19H19N3O3 | 26.9 | 26.3 | 18.7 | ND | 0.4 | 5.1 |
| ZG-234 | 7.25 | 22.14 | 9.84 | ND | 0.3 | 5.25 |
| XHE-III-74 | 77 | 105 | 38 | 0.42 | 2.2 | 5.8 |
| JYI-12 (C19H16N3O3F3) | 91 | 39 | ND | ND | 4.5 | 6.8 |
| DM-239 | 1.5 | ND | 0.53 | ND | 0.14 | 6.89 |
| XHE-III-14 | 2.6 | ND | 10 | 13 | 2 | 7 |
| TG-4-29 | 8.3 | 10.2 | 6.9 | ND | 0.4 | 7.61 |
| DM-215 | 6.74 | ND | 7.42 | ND | 0.293 | 8.28 |
| JYI-13 (C21H16N3O4F3) | 5000 | 63.7 | ND | ND | 16 | 8.38 |
| CGS9895 | 0.21 | ND | ND | ND | ND | 9.3 |
| ZG-168 | 11.2 | 10.7 | 9.2 | ND | 0.47 | 9.4 |
| PWZ-007A | 0.11 | 0.1 | 0.09 | ND | 0.2 | 10 |
| PZII-029 | 0.34 | ND | 0.79 | ND | 0.52 | 10 |
| XHE-II-024 | 0.09 | 0.18 | 0.32 | 14 | 0.24 | 11 |
| XHE-II-002 | 8.3 | 18 | 13 | 3.9 | 1.5 | 11 |
| BRETAZENIL | 0.35 | 0.64 | 0.2 | ND | 0.5 | 12.7 |
| JYI-19 (C23H23N3O3S) | 2.176 | 205 | ND | ND | 34 | 12.7 |
| SVO-8-14 | 8 | 25 | 8 | 6.9 | 0.9 | 14 |
| SVO-8-30 | 1.1 | 5.3 | 5.3 | 2.8 | 0.6 | 15 |
| WYS10 C14H9F3N2O2 | 0.88 | 36 | 25.6 | ND | 548.7 | 15.3 |
| QH-II-090 (CGS-8216) | 0.05 | 0.08 | 0.12 | ND | 0.25 | 17 |
| PWZ-0071 | 0.23 | 0.17 | 0.12 | 0.44 | 17.31 |
aThe affinity of compounds at GABAA/BzR recombinant subtypes was measured by competition for [3H]flunitrazepam binding to HEK cell membranes expressing human receptors of compositions α1β3γ2, α2β3γ2, α3β3γ2, α4β3γ2, α5β3γ2, and α6β3γ2 [139]. Data represent the average of at least three determinations with a SEM of ±5%. ND: not determined.
12. The α1β3γ2 Receptor Subtype
The focus of this research was aimed at diazepam sensitive receptors; additional features to the α4β3γ2 and α6β3γ2 receptors were not identified (see Table 5, Figures 34 and 35). The major new feature identified for the α5β3γ2 receptor was a new L 4 pocket. This new lipophilic pocket was identified with SH-053-R-CH3 (15) and SH-053-S-CH3 (16) chiral enantionmers as well as the 2′F analogs [74, 135, 136].
Figure 35.
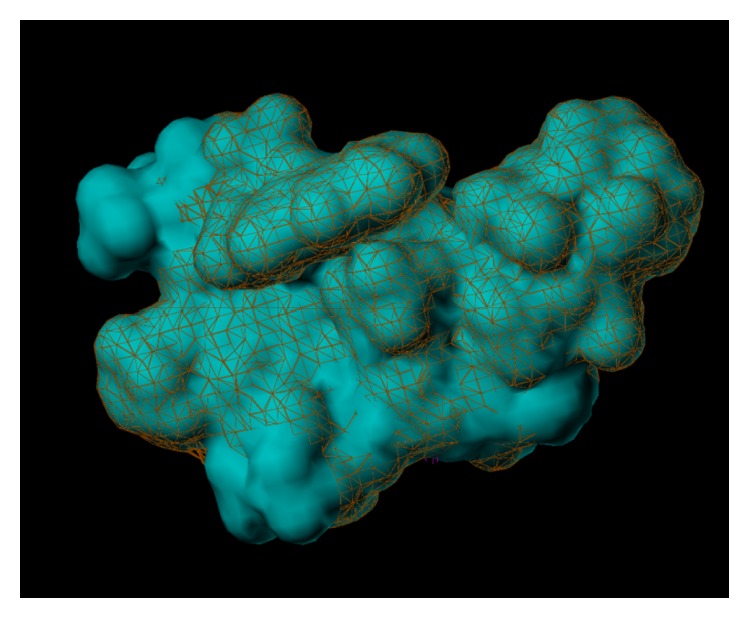
Updated α1β3γ2 subtype (blue solid) overlaid with the previous model (red wire). Overlap identified where wire and solid overlap.
13. The α2β3γ2 Receptor Subtype
See Table 6 and Figures 36 and 37.
Figure 36.
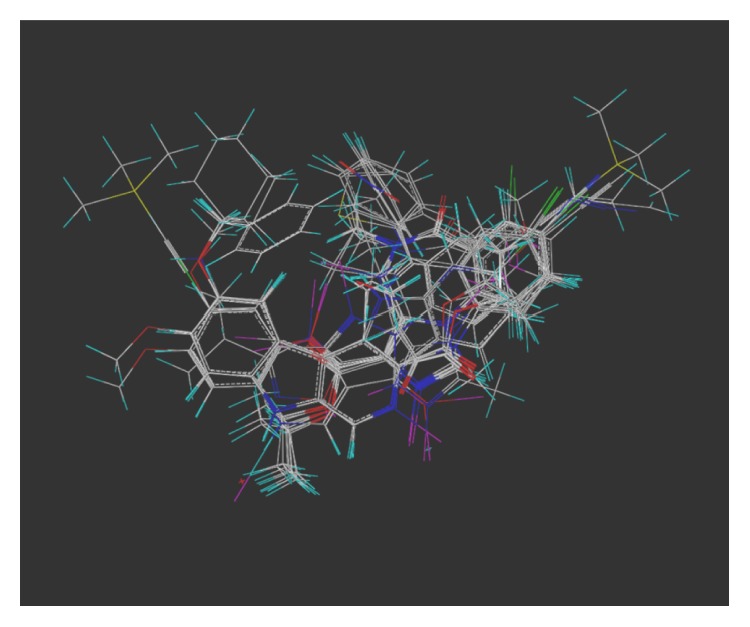
Overlay of compounds selective for α2β3γ2 subtype.
Figure 37.
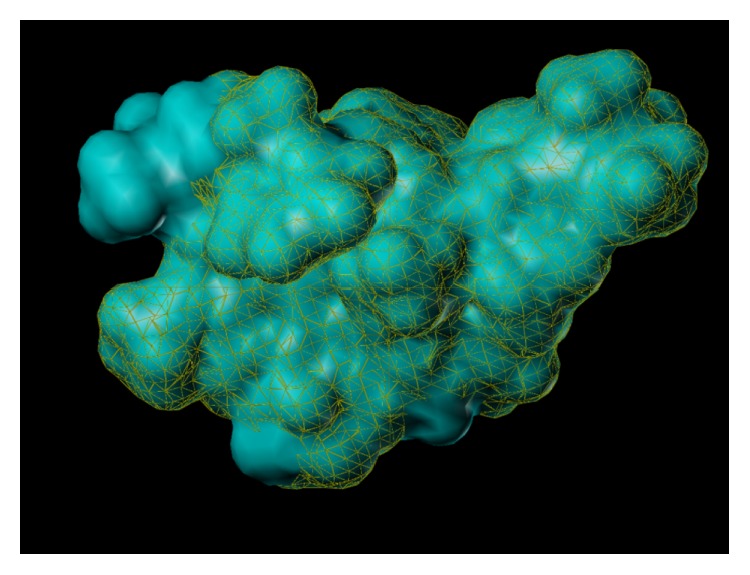
Updated α2β3γ2 subtype (solid) overlaid with the previous model (red wire). Overlap identified where wire and solid overlap.
14. The α3β3γ2 Receptor Subtype
See Table 7 and Figures 38 and 39.
Table 7.
Ligands with potent affinity for α3; ligands bound with K i values <20 nM at this subtype. The structures of these ligands are in the Ph.D. thesis of Clayton (2011) [22].
| Cook codea | α1 | α2 | α3 | α4 | α5 | α6 |
|---|---|---|---|---|---|---|
| QH-II-085 | 0.08 | 0.06 | 0.02 | ND | 0.08 | ND |
| SH-TSC-2 (BCCT) | 0.03 | 0.0419 | 0.035 | ND | 69.32 | ND |
| QH-II-092 | 0.07 | 0.03 | 0.04 | ND | 0.17 | ND |
| QH-II-077 | 0.06 | 0.08 | 0.05 | ND | 0.12 | 4 |
| YT-II-76 | 95.34 | 2.797 | 0.056 | ND | 0.04 | ND |
| PWZ-007A | 0.11 | 0.1 | 0.09 | ND | 0.2 | 10 |
| XLI-286 | 0.051 | 0.064 | 0.118 | ND | 0.684 | ND |
| QH-II-090 (CGS-8216) | 0.05 | 0.08 | 0.12 | ND | 0.25 | 17 |
| PWZ-0071 | 0.23 | 0.17 | 0.12 | ND | 0.44 | 17.31 |
| JYI-57 | 0.076 | 0.076 | 0.131 | ND | 0.036 | ND |
| BRETAZENIL | 0.35 | 0.64 | 0.2 | ND | 0.5 | 12.7 |
| PZII-028 | 0.2 | ND | 0.2 | ND | 0.32 | 1.9 |
| QH-II-075 | 0.18 | 0.21 | 0.25 | ND | 1.3 | 40 |
| JYI-42 | 0.257 | 0.146 | 0.278 | ND | 0.256 | ND |
| XHE-II-024 | 0.09 | 0.18 | 0.32 | 14 | 0.24 | 11 |
| SH-I-048A | 0.774 | 0.1723 | 0.383 | ND | 0.11 | ND |
| JYI-55 | 41.39 | ND | 0.504 | ND | 24.75 | ND |
| JYI-47 | 2.759 | 2.282 | 0.511 | ND | 0.427 | ND |
| DM-239 | 1.5 | ND | 0.53 | ND | 0.14 | 6.89 |
| WY-B-15 | 0.92 | 0.83 | 0.58 | 2080 | 4.42 | 646 |
| JYI-64 (C17H12N4FBr) | 0.305 | 1.111 | 0.62 | ND | 0.87 | 5000 |
| DM-II-90 (C17H12N4BrCl) | 0.505 | 1 | 0.63 | ND | 0.37 | 5000 |
| QH-146 | 0.49 | ND | 0.76 | ND | 7.7 | 10000 |
| PZII-029 | 0.34 | ND | 0.79 | ND | 0.52 | 10 |
| WYS19 C26H32N2O4Si | ND | ND | 0.89 | ND | ND | ND |
| XHE-III-06a | 1 | 2 | 1 | 5 | 1.8 | 37 |
| Ro15-1788 | 0.8 | 0.9 | 1.05 | ND | 0.6 | 148 |
| MLT-I-70 | 1.1 | 1.2 | 1.1 | ND | 40.3 | 1000 |
| XHE-III-04 | 1.2 | 2 | 1.1 | 219 | 0.4 | 500 |
| FG8205 | 0.4 | 2.08 | 1.16 | ND | 1.54 | 227 |
| PWZ-009A1 | 1.34 | 1.31 | 1.26 | ND | 0.84 | 2.03 |
| alprazolam | 0.8 | 0.59 | 1.43 | ND | 1.54 | 10000 |
| XLI-348 | 13.56 | 11.17 | 1.578 | ND | 82.05 | ND |
| QH-II-082 | 1.7 | 1.8 | 1.6 | ND | 6.1 | 100 |
| WY-TSC-4 (WYS8) | 0.007 | 0.99 | 1.63 | ND | 51.04 | ND |
| SPH-121 | 0.14 | 1.19 | 1.72 | ND | 4 | 479 |
| XLi-JY-DMH ANX3 | 3.3 | 0.58 | 1.9 | ND | 4.4 | 5000 |
| MMB-III-016 | 3 | 1.97 | 2 | 1074 | 0.26 | 211 |
| MMB-III-16 | 3 | 1.97 | 2 | 1074 | 0.26 | 211 |
| XLI-8TC | 21.52 | 11.01 | 2.155 | ND | 4.059 | ND |
| 6-PBC | 0.49 | 1.21 | 2.2 | ND | 2.39 | 1343 |
| YT-III-271 | 32.54 | 1.26 | 2.35 | ND | 103 | ND |
| Ro15-4513 | 3.3 | 2.6 | 2.5 | ND | 0.26 | 3.8 |
| TG-4-29 | 2.8 | 3.9 | 2.7 | 2.1 | 0.18 | 3.9 |
| TG-II-82 | 1.6 | 2.9 | 2.8 | ND | 1 | 1000 |
| OMB-18 | 3.9 | 1.2 | 3.4 | 1733 | 0.8 | 5 |
| YT-II | 6.932 | 0.8712 | 3.518 | ND | 5.119 | ND |
| PS-1-34B C20H17N4BrO | ND | 4.198 | 3.928 | ND | ND | ND |
| DMCM | 5.69 | 8.29 | 4 | ND | 1.04 | 134 |
| MSR-I-032 | 6.2 | 18.7 | 4 | ND | 3.3 | 74.9 |
| RY-008 | 3.75 | 7.2 | 4.14 | ND | 1.11 | 44.3 |
| XHE-III-49 | 1.3 | 5.5 | 4.2 | 38.7 | 11.3 | 85.1 |
| JYI-60 (C17H11N2OF) | 3.73 | 1.635 | 4.3 | ND | 1.7 | 5000 |
| FLUNITRAZEPAM | 2.2 | 2.5 | 4.5 | ND | 2.1 | 2000 |
| XLI-317 | 60.24 | 24.05 | 4.562 | ND | 0.295 | ND |
| QH-II-080b | 3 | 3.7 | 4.7 | ND | 24 | 1000 |
| SPH-165 | 0.63 | 2.79 | 4.85 | ND | 10.4 | 1150 |
| SH-I-04 | 7.3 | 6.136 | 5.1 | ND | 7.664 | ND |
| SVO-8-30 | 1.1 | 5.3 | 5.3 | 2.8 | 0.6 | 15 |
| WY-B-99-1 | 4.4 | 4.5 | 5.58 | 2000 | 47 | 2000 |
| YT-III-25 | 2.531 | 5.786 | 5.691 | ND | 0.095 | ND |
| BCCE | 1.2 | 4.9 | 5.7 | ND | 26.8 | 2700 |
| MMB-II-90 | 20 | 24 | 5.7 | 9 | 0.25 | 36 |
| TC-YT-II-76 | 101.1 | 1.897 | 5.816 | ND | 11.99 | ND |
| ZK 93423 | 4.1 | 4.2 | 6 | ND | 4.5 | 1000 |
| RY-061 | 17 | 13 | 6.7 | ND | 0.3 | 31 |
| JYI-54 (C24H15N3O3F4) | 2.89 | 172 | 6.7 | ND | 57 | 1890 |
| TG-4-29 | 8.3 | 10.2 | 6.9 | ND | 0.4 | 7.61 |
| MMB-III-14 | 13 | 13 | 6.9 | 333 | 1.1 | 333 |
| XHE-II-017 | 3.3 | 10 | 7 | 258 | 17 | 294 |
| SHU-1-19 | 4 | 12 | 7 | 48 | 14 | 84 |
| XHE-III-13 | 7.3 | ND | 7.1 | 880 | 1.6 | 311 |
| DM-215 | 6.74 | ND | 7.42 | ND | 0.293 | 8.28 |
| ABECARNIL | 12.4 | 15.3 | 7.5 | ND | 6 | 1000 |
| SVO-8-14 | 8 | 25 | 8 | 6.9 | 0.9 | 14 |
| XHE-III-24 | 0.25 | ND | 8 | 222 | 10 | 328 |
| SH-I-89S | 12.78 | 8.562 | 8.145 | ND | 3.23 | ND |
| PWZ-085 | 4.86 | 13 | 8.5 | ND | 0.55 | 40 |
| XHE-I-093 | 2 | 7.1 | 8.9 | 1107 | 20 | 1162 |
| ZG-168 | 11.2 | 10.7 | 9.2 | ND | 0.47 | 9.4 |
| ZG69A | 6.8 | 16.3 | 9.2 | ND | 0.85 | 54.6 |
| ZG-69a (Ro15-1310) | 6.8 | 16.3 | 9.2 | ND | 0.85 | 54.6 |
| ZG-234 | 7.25 | 22.14 | 9.84 | ND | 0.3 | 5.25 |
| XHE-II-073A (R ENRICHED) | 5.9 | 11 | 10 | 15 | 1.18 | 140 |
| RY-067 | 21 | 12 | 10 | ND | 0.37 | 42 |
| XHE-III-14 | 2.6 | ND | 10 | 13 | 2 | 7 |
| YT-III-272 | 295.9 | 14.98 | 10.77 | ND | 103.3 | ND |
| SPH-38 | 2 | 5.4 | 10.8 | ND | 18.5 | 3000 |
| ZG-208 | 9.7 | 11.2 | 10.9 | ND | 0.38 | 4.6 |
| MLT-II-18 | 3.4 | 11.7 | 11 | ND | 225 | 10000 |
| DM-II-33 (C20H13N3O2BrCl3) | 88.6 | 85 | 11.6 | ND | 26.2 | 5000 |
| JYI-59 (C22H13N3O2F4) | 1.08 | 2.6 | 11.82 | ND | 11.5 | 5000 |
| XHE-II-006b | 3.7 | 15 | 12 | 1897 | 144 | 1000 |
| XHE-II-073B (S-ENRICHED) | 11 | 17 | 12 | 33 | 2.1 | 269 |
| CM-B44 (ss) | 32 | 43 | 12 | 379 | 4.3 | 485 |
| WYSC1 C16H16N2O2 | 1.094 | 5.44 | 12.3 | ND | 69.8 | 21.2 |
| JYI-48 | 75.59 | 90.68 | 12.78 | ND | 31.28 | ND |
| XHE-II-002 | 8.3 | 18 | 13 | 3.9 | 1.5 | 11 |
| RY-076 | 26 | 27 | 13 | ND | 0.7 | 22 |
| WZ-113 | 19.2 | 13.2 | 13.4 | ND | 11.5 | 300 |
| SH-I-085 | 11.08 | 4.866 | 13.75 | ND | 0.24 | ND |
| CM-E10 | 23 | 26 | 14 | 215 | 0.51 | 96 |
| TJH-IV-51 | 2.39 | 17.4 | 14.5 | ND | 316 | 10000 |
| YT-III-38 | 1461 | 18.21 | 14.63 | ND | 3999 | ND |
| CM-A87 | 1.62 | 4.54 | 14.73 | 1000 | 4.61 | 1000 |
| diazepam | 14 | 20 | 15 | ND | 11 | ND |
| RY-053 | 49 | 29 | 15 | ND | 1 | 46 |
| YCT-5 | 2.2 | 11.46 | 16.3 | ND | 200 | 10000 |
| RY-098 | 10.1 | 22.2 | 16.5 | ND | 1.68 | 100 |
| PWZ-096 | 11.1 | 36 | 16.9 | ND | 1.07 | 51.5 |
| XLi223 C22H20BrN3O2 | 14 | 8.7 | 18 | 1000 | 10 | 2000 |
| XHE-I-065 | 7.2 | 17 | 18 | 500 | 57 | 500 |
| SH-I-02B | 29.82 | 1315 | 18 | ND | 74.05 | ND |
| MLT-II-16 | 5.05 | 10.41 | 18.4 | ND | 260 | 10000 |
| RY-024 C19H19N3O3 | 26.9 | 26.3 | 18.7 | ND | 0.4 | 5.1 |
| BCCt | 0.72 | 15 | 18.9 | ND | 110.8 | 5000 |
| LJD-III-15E | 1.93 | 14 | 19 | ND | 70.8 | 1000 |
| CM-E09b | 20 | 22 | 19 | 55 | 0.45 | 69 |
| RY-I-31 | 10 | 45 | 19 | ND | 6 | 1000 |
| SH-I-030 | 14.42 | 11.04 | 19.09 | ND | 1.89 | ND |
| YT-III-23 | 19.83 | 23.65 | 19.87 | ND | 1.105 | ND |
| XHE-II-006a | 4.7 | 4.4 | 20 | 1876 | 89 | 3531 |
| OMB-19 | 22 | 4.6 | 20 | 3333 | 3.5 | 40 |
| XHE-III-06b | 32 | 33 | 20 | 299 | 28.6 | 740 |
aAffinity of compounds at GABAA/BzR recombinant subtypes was measured by competition for [3H]flunitrazepam or [3H] Ro15-4513 binding to HEK cell membranes expressing human receptors of compositions α1β3γ2, α2β3γ2, α3β3γ2, α4β3γ2, α5β3γ2, and α6β3γ2 [22, 139]. Data represent the average of at least three determinations with a SEM of ±5%. ND: not determined.
Figure 38.
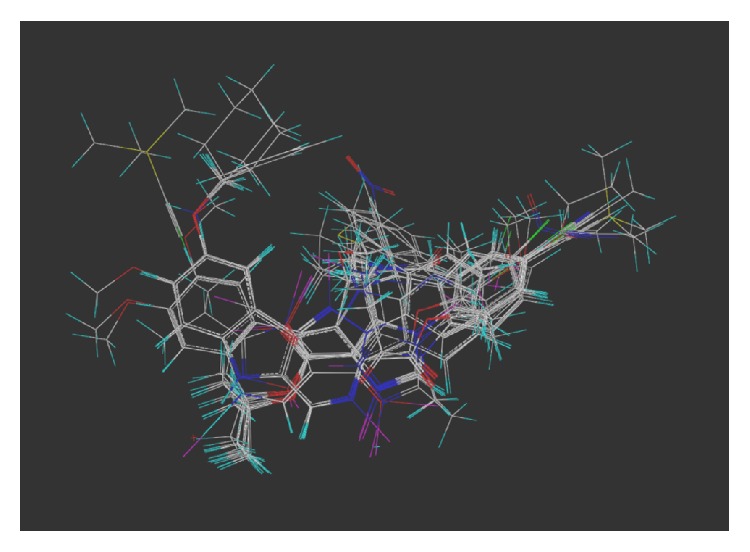
Overlay of compounds selective for α3β3γ2 subtype.
Figure 39.
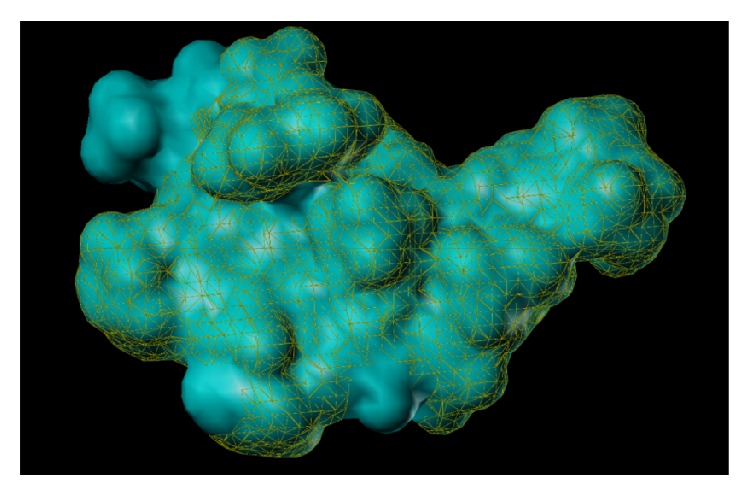
Updated α3β3γ2 subtype (blue solid) overlaid with the previous model (red wire). Overlap identified where wire and solid overlap.
15. The α4β3γ2 Receptor Subtype
See Table 8 and Figures 40 and 41.
Table 8.
Ligands with potent affinity for α4; ligands bound with K i values <20 nM at this subtype. The structures of these ligands are in the Ph.D. thesis of Clayton (2011) [22].
| Cook codea | α1 | α2 | α3 | α4 | α5 | α6 |
|---|---|---|---|---|---|---|
| CM-D45 C19H21N3O4 | 90.5 | 65.5 | 30.3 | 0.15 | 1.65 | 0.23 |
| CM-D44 | 34.3 | 56.3 | 20.7 | 0.33 | 0.57 | 0.92 |
| XHE-III-74 | 77 | 105 | 38 | 0.42 | 2.2 | 5.8 |
| TG-4-29 | 2.8 | 3.9 | 2.7 | 2.1 | 0.18 | 3.9 |
| SVO-8-30 | 1.1 | 5.3 | 5.3 | 2.8 | 0.6 | 15 |
| XHE-II-002 | 8.3 | 18 | 13 | 3.9 | 1.5 | 11 |
| XHE-III-06a | 1 | 2 | 1 | 5 | 1.8 | 37 |
| RY-080 C17H15N3O3 | 28.4 | 21.4 | 25.8 | 5.3 | 0.49 | 28.8 |
| TG-4-39 | 1.6 | 34 | 24 | 5.6 | 1.4 | 23 |
| SVO-8-14 | 8 | 25 | 8 | 6.9 | 0.9 | 14 |
| RY-023 C22H27N3O3Si | 197 | 142.6 | 255 | 7.8 | 2.61 | 58.6 |
| MMB-II-90 | 20 | 24 | 5.7 | 9 | 0.25 | 36 |
| XHE-III-14 | 2.6 | 10 | 13 | 2 | 7 | |
| XHE-II-024 | 0.09 | 0.18 | 0.32 | 14 | 0.24 | 11 |
| XHE-II-073A (R ENRICHED) | 5.9 | 11 | 10 | 15 | 1.18 | 140 |
| SVO-8-67 | 7 | 41 | 26 | 15 | 2.3 | 191 |
| CM-B31i (ss) | 90 | 184 | 78 | 18 | 4.9 | 121 |
| SVO-8-20 | 11 | 40 | 28 | 19 | 8.6 | 138 |
aAffinity of compounds at GABAA/BzR recombinant subtypes was measured by competition for [3H]flunitrazepam or [3H] Ro15-4513 binding to HEK cell membranes expressing human receptors of compositions α1β3γ2, α2β3γ2, α3β3γ2, α4β3γ2, α5β3γ2, and α6β3γ2 [22, 139]. Data represent the average of at least three determinations with a SEM of ±5%. ND: not determined.
Figure 40.
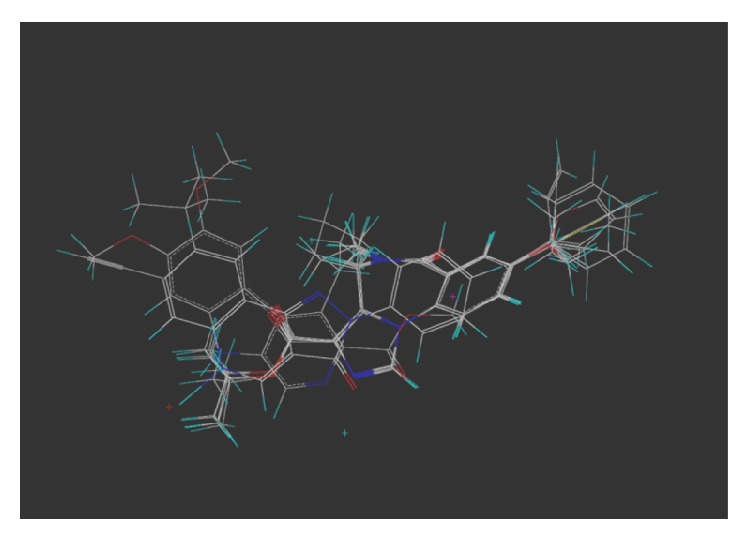
Overlay of selected compounds selective for α4β3γ2 subtype.
Figure 41.

Updated α4β3γ2 subtype (blue solid) overlaid with the previous model (yellow wire). Overlap identified where wire and solid overlap.
16. The α5β3γ2 Receptor Subtype
The multiple volume contours displayed in Figures 34–47 were created using the mvolume function (multiple volume contour function) in Sybyl and compounds with binding affinity at the receptor less than or equal to 20 nM. To create the overlays, first, the display (dsp) and contour (cnt) files were created for the α5β3γ2 receptor subtype and the α1β3γ2 receptor subtype by overlaying the compounds for each of these receptors (see Table 9 and Figures 42 –45). Using the mvolume function, a logical expression was entered to create the surfaces making up the union as well as the included volume for each receptor subtype itself. It is clear from the included volume overlay that the L 2 pocket is deeper for the α5 subtype, as determined previously [13, 21–23, 110, 119]. The new L 4 pocket can be distinguished as the new yellow region of the α5β3γ2 subtype which is due to recently designed R-isomers by Huang [135], Poe and Li.
Figure 47.
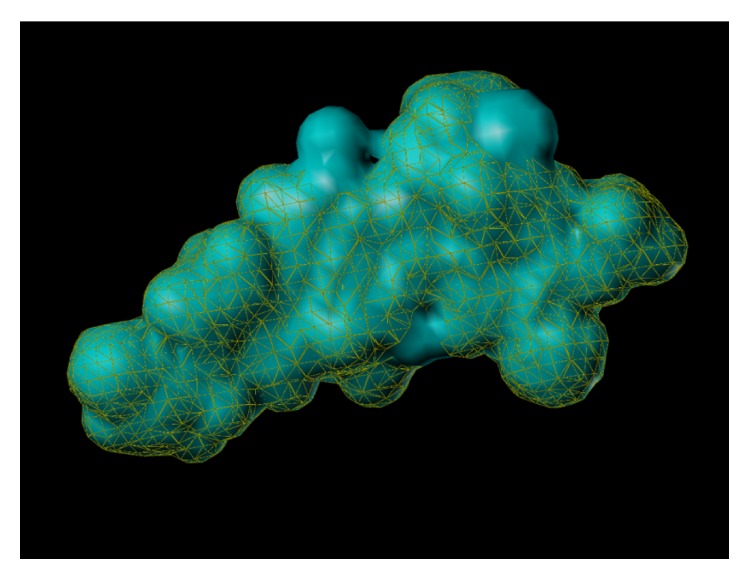
Updated α6β3γ2 subtype (blue solid) overlaid with the previous model (yellow wire). Overlap identified where wire and solid overlap.
Table 9.
Ligands with potent affinity for α5; ligands bound with K i values <20 nM at this subtype. The structures of these ligands are in the Ph.D. thesis of Clayton (2011) [22].
| Cook codea | α1 | α2 | α3 | α4 | α5 | α6 |
|---|---|---|---|---|---|---|
| JYI-57 | 0.076 | 0.076 | 0.131 | ND | 0.036 | ND |
| YT-II-76 | 95.34 | 2.797 | 0.056 | ND | 0.04 | ND |
| QH-II-085 | 0.08 | 0.06 | 0.02 | ND | 0.08 | ND |
| YT-III-25 | 2.531 | 5.786 | 5.691 | ND | 0.095 | ND |
| SH-I-048A | 0.774 | 0.1723 | 0.383 | ND | 0.11 | ND |
| QH-II-077 | 0.06 | 0.08 | 0.05 | ND | 0.12 | 4 |
| DM-239 | 1.5 | ND | 0.53 | ND | 0.14 | 6.89 |
| QH-II-092 | 0.07 | 0.03 | 0.04 | ND | 0.17 | ND |
| TG-4-29 | 2.8 | 3.9 | 2.7 | 2.1 | 0.18 | 3.9 |
| SH-I-75 | 1487 | 989.9 | 773 | ND | 0.1825 | ND |
| PWZ-007A | 0.11 | 0.1 | 0.09 | ND | 0.2 | 10 |
| XHE-II-024 | 0.09 | 0.18 | 0.32 | 14 | 0.24 | 11 |
| SH-I-085 | 11.08 | 4.866 | 13.75 | ND | 0.24 | ND |
| MMB-II-90 | 20 | 24 | 5.7 | 9 | 0.25 | 36 |
| QH-II-090 (CGS-8216) | 0.05 | 0.08 | 0.12 | ND | 0.25 | 17 |
| JYI-42 | 0.257 | 0.146 | 0.278 | ND | 0.256 | ND |
| MMB-III-016 | 3 | 1.97 | 2 | 1074 | 0.26 | 211 |
| MMB-III-16 | 3 | 1.97 | 2 | 1074 | 0.26 | 211 |
| Ro15-4513 | 3.3 | 2.6 | 2.5 | ND | 0.26 | 3.8 |
| DM-215 | 6.74 | ND | 7.42 | ND | 0.293 | 8.28 |
| XLI-317 | 60.24 | 24.05 | 4.562 | ND | 0.295 | ND |
| RY-061 | 17 | 13 | 6.7 | ND | 0.3 | 31 |
| ZG-234 | 7.25 | 22.14 | 9.84 | ND | 0.3 | 5.25 |
| PZII-028 | 0.2 | ND | 0.2 | ND | 0.32 | 1.9 |
| RY-067 | 21 | 12 | 10 | ND | 0.37 | 42 |
| DM-II-90 (C17H12N4BrCl) | 0.505 | 1 | 0.63 | ND | 0.37 | 5000 |
| ZG-208 | 9.7 | 11.2 | 10.9 | ND | 0.38 | 4.6 |
| XHE-III-04 | 1.2 | 2 | 1.1 | 219 | 0.4 | 500 |
| TG-4-29 | 8.3 | 10.2 | 6.9 | ND | 0.4 | 7.61 |
| RY-024 C19H19N3O3 | 26.9 | 26.3 | 18.7 | ND | 0.4 | 5.1 |
| JYI-47 | 2.759 | 2.282 | 0.511 | ND | 0.427 | ND |
| PWZ-0071 | 0.23 | 0.17 | 0.12 | ND | 0.44 | 17.31 |
| CM-E09b | 20 | 22 | 19 | 55 | 0.45 | 69 |
| ZG-168 | 11.2 | 10.7 | 9.2 | ND | 0.47 | 9.4 |
| RY-080 C17H15N3O3 | 28.4 | 21.4 | 25.8 | 5.3 | 0.49 | 28.8 |
| BRETAZENIL | 0.35 | 0.64 | 0.2 | ND | 0.5 | 12.7 |
| CM-E10 | 23 | 26 | 14 | 215 | 0.51 | 96 |
| JYI-04 (C21H23N3O3) | 28.3 | 16 | ND | ND | 0.51 | 1.57 |
| PZII-029 | 0.34 | ND | 0.79 | ND | 0.52 | 10 |
| PWZ-085 | 4.86 | 13 | 8.5 | ND | 0.55 | 40 |
| JYI-70 (C19H13N4F) | 6.3 | 2.1 | ND | ND | 0.56 | 5000 |
| CM-D44 | 34.3 | 56.3 | 20.7 | 0.33 | 0.57 | 0.92 |
| SVO-8-30 | 1.1 | 5.3 | 5.3 | 2.8 | 0.6 | 15 |
| Ro15-1788 | 0.8 | 0.9 | 1.05 | ND | 0.6 | 148 |
| XLi268 C17H13BrN4 | 2.8145 | 0.6862 | ND | ND | 0.6243 | ND |
| ZG-63A | 17.3 | 21.6 | 29.1 | ND | 0.65 | 4 |
| SH-053-2′F | 21.99 | 12.34 | 34.9 | ND | 0.671 | ND |
| XLI-286 | 0.051 | 0.064 | 0.118 | ND | 0.684 | ND |
| SH-I-S66 | 22.93 | 30.36 | 55.26 | ND | 0.69 | ND |
| RY-076 | 26 | 27 | 13 | ND | 0.7 | 22 |
| DM-173 | 13.1 | ND | 38.1 | ND | 0.78 | 118 |
| OMB-18 | 3.9 | 1.2 | 3.4 | 1733 | 0.8 | 5 |
| HJ-I-037 | 15.07 | 8.127 | 28.29 | ND | 0.818 | ND |
| PWZ-009A1 | 1.34 | 1.31 | 1.26 | ND | 0.84 | 2.03 |
| ZG69A | 6.8 | 16.3 | 9.2 | ND | 0.85 | 54.6 |
| ZG-69a (Ro15-1310) | 6.8 | 16.3 | 9.2 | ND | 0.85 | 54.6 |
| JYI-64 (C17H12N4FBr) | 0.305 | 1.111 | 0.62 | ND | 0.87 | 5000 |
| SVO-8-14 | 8 | 25 | 8 | 6.9 | 0.9 | 14 |
| JYI-03 (C21H21N3O3) | 185.4 | 107 | ND | ND | 0.954 | 3.34 |
| TG-II-82 | 1.6 | 2.9 | 2.8 | ND | 1 | 1000 |
| RY-053 | 49 | 29 | 15 | ND | 1 | 46 |
| YT-6 | 15.31 | 87.8 | 60.49 | ND | 1.039 | ND |
| DMCM | 5.69 | 8.29 | 4 | ND | 1.04 | 134 |
| PWZ-096 | 11.1 | 36 | 16.9 | ND | 1.07 | 51.5 |
| MMB-III-14 | 13 | 13 | 6.9 | 333 | 1.1 | 333 |
| YT-III-23 | 19.83 | 23.65 | 19.87 | ND | 1.105 | ND |
| RY-008 | 3.75 | 7.2 | 4.14 | ND | 1.11 | 44.3 |
| XLI-2TC | 3.442 | 1.673 | 44.08 | ND | 1.121 | ND |
| XHE-II-073A (R ENRICHED) | 5.9 | 11 | 10 | 15 | 1.18 | 140 |
| QH-II-075 | 0.18 | 0.21 | 0.25 | ND | 1.3 | 40 |
| RY-054 | 59 | 44 | 27 | ND | 1.3 | 126 |
| TG-4-39 | 1.6 | 34 | 24 | 5.6 | 1.4 | 23 |
| XHE-II-002 | 8.3 | 18 | 13 | 3.9 | 1.5 | 11 |
| RY-031 (RY-10) | 20.4 | 27 | 26.1 | ND | 1.5 | 176 |
| FG8205 | 0.4 | 2.08 | 1.16 | ND | 1.54 | 227 |
| alprazolam | 0.8 | 0.59 | 1.43 | ND | 1.54 | 1000 |
| XHE-III-13 | 7.3 | ND | 7.1 | 880 | 1.6 | 311 |
| CM-D45 C19H21N3O4 | 90.5 | 65.5 | 30.3 | 0.15 | 1.65 | 0.23 |
| RY-098 | 10.1 | 22.2 | 16.5 | ND | 1.68 | 100 |
| YT-5 | 0.421 | 0.6034 | 36.06 | ND | 1.695 | ND |
| JYI-60 (C17H11N2OF) | 3.73 | 1.635 | 4.3 | ND | 1.7 | 5000 |
| RY-033 | 14.8 | 56 | 25.3 | ND | 1.72 | 22.9 |
| XHE-III-06a | 1 | 2 | 1 | 5 | 1.8 | 37 |
| SH-I-030 | 14.42 | 11.04 | 19.09 | ND | 1.89 | ND |
| XLi352 C18H13ClN2O | 1.56 | 0.991 | ND | ND | 1.957 | ND |
| XLi351 C21H21ClN2OSi | 1.507 | 0.967 | ND | ND | 1.985 | ND |
| XHE-III-14 | 2.6 | ND | 10 | 13 | 2 | 7 |
| DM-II-72 (C15H10N20BrCl) | 5000 | 1.37 | ND | ND | 2.02 | 5000 |
| XHE-II-073B (S-ENRICHED) | 11 | 17 | 12 | 33 | 2.1 | 269 |
| FLUNITRAZEPAM | 2.2 | 2.5 | 4.5 | ND | 2.1 | 2000 |
| XHE-III-74 | 77 | 105 | 38 | 0.42 | 2.2 | 5.8 |
| SVO-8-67 | 7 | 41 | 26 | 15 | 2.3 | 191 |
| 6-PBC | 0.49 | 1.21 | 2.2 | ND | 2.39 | 1343 |
| RY-058 | 86 | 40 | 85 | ND | 2.4 | 150 |
| ZG-224 | 17.1 | 33.7 | 50 | ND | 2.5 | 31.7 |
| RY-066 | 83 | 60 | 48 | ND | 2.6 | 180 |
| RY-023 C22H27N3O3Si | 197 | 142.6 | 255 | 7.8 | 2.61 | 58.6 |
| XLi350 C17H11ClN2O | 1.224 | 1.188 | ND | ND | 2.9 | ND |
| JYI-32 (C20H15N3O2BrF) | 3.07 | 4.96 | ND | ND | 2.92 | 52.24 |
| SH-I-89S | 12.78 | 8.562 | 8.145 | ND | 3.23 | ND |
| MSR-I-032 | 6.2 | 18.7 | 4 | ND | 3.3 | 74.9 |
| OMB-19 | 22 | 4.6 | 20 | 3333 | 3.5 | 40 |
| ZG-213 | 12.8 | 49.8 | 30.2 | ND | 3.5 | 22.5 |
| YT-II-83 | 32.74 | 13.22 | 24.1 | ND | 3.548 | ND |
| RY-059 | 89 | 70 | 91 | ND | 3.7 | 301 |
| SPH-121 | 0.14 | 1.19 | 1.72 | ND | 4 | 479 |
| RY-047 | 200 | 124 | 79 | ND | 4 | 340 |
| XLI-8TC | 21.52 | 11.01 | 2.155 | ND | 4.059 | ND |
| YT-I-38 | 945.9 | 326.8 | 245.9 | ND | 4.07 | ND |
| DM-146 | 6.44 | ND | 148 | ND | 4.23 | 247 |
| CM-B44 (ss) | 32 | 43 | 12 | 379 | 4.3 | 485 |
| CM-B47 | 32 | 63 | 34 | 2007 | 4.4 | 717 |
| XLi-JY-DMH ANX3 | 3.3 | 0.58 | 1.9 | ND | 4.4 | 5000 |
| WY-B-15 | 0.92 | 0.83 | 0.58 | 2080 | 4.42 | 646 |
| ZK 93423 | 4.1 | 4.2 | 6 | ND | 4.5 | 1000 |
| JYI-12 (C19H16N3O3F3) | 91 | 39 | ND | ND | 4.5 | 6.8 |
| CM-A87 | 1.62 | 4.54 | 14.73 | 1000 | 4.61 | 1000 |
| DM-III-01 (C18H12N3O2Br) | 5000 | 12 | ND | ND | 4.73 | 5000 |
| RY-057 | 73 | 85 | 97 | ND | 4.8 | 333 |
| JYI-15 (C19H14N3O3F3) | 205 | 812 | ND | ND | 4.8 | 22 |
| CM-B31i (ss) | 90 | 184 | 78 | 18 | 4.9 | 121 |
| RY-079 | 121.1 | 141.9 | 198.4 | 159 | 5 | 113.7 |
| JC208 C15H10N2OS | 22.42 | 18.89 | ND | ND | 5.039 | ND |
| YT-II | 6.932 | 0.8712 | 3.518 | ND | 5.119 | ND |
| XLi270 C19H14N4 | 36.39 | 25.81 | ND | ND | 5.291 | ND |
| XHE-I-051 | 35 | 39 | 42 | ND | 5.3 | 979 |
| MMB-II-87 | 200 | 333 | 107 | 109 | 5.4 | 333 |
| XLI-210 | 231 | 661 | 2666 | ND | 5.4 | 54.22 |
| XHE-II-O53-ACID | 50.35 | 11.8 | 44 | ND | 5.9 | 5000 |
| ABECARNIL | 12.4 | 15.3 | 7.5 | ND | 6 | 1000 |
| RY-I-31 | 10 | 45 | 19 | ND | 6 | 1000 |
| QH-II-082 | 1.7 | 1.8 | 1.6 | ND | 6.1 | 100 |
| SH-TSC-1 (PWZ-029) | 362.4 | 180.3 | 328.2 | ND | 6.185 | ND |
| XHE-II-065 | 1000 | 409 | 216 | 37 | 6.4 | 175 |
| JYI-49 (C20H12N3O2F4Br) | 1.87 | 2.38 | ND | ND | 6.7 | 3390 |
| JC184 C13H9BrN2OS | 9.606 | 10.5 | ND | ND | 6.709 | ND |
| QH-II-066 | 76.3 | 42.1 | 47.4 | 2000 | 6.8 | 3000 |
| XLI-381 | 619.9 | 285.6 | 3639 | ND | 7.051 | ND |
| RY-071 | 19 | 56 | 91 | ND | 7.2 | 266 |
| RY-I-28 | 283 | 318 | 102 | ND | 7.2 | 61 |
| CM-A82a | 2.78 | 8.93 | 24.51 | 1000 | 7.49 | 1000 |
| YT-III-31 | 36.39 | 67.85 | 129.7 | ND | 7.59 | ND |
| SH-I-04 | 7.3 | 6.136 | 5.1 | ND | 7.664 | ND |
| QH-146 | 0.49 | ND | 0.76 | ND | 7.7 | 1000 |
| QH-II-063 | 9.4 | 9.3 | 31 | ND | 7.7 | 3000 |
| JC221 ANX1 | 106.175 | 49.405 | 182 | ND | 7.7495 | 362 |
| DM-II-30 (C20H13N3O2BrF3) | 17.6 | 13.4 | 28.51 | ND | 7.8 | 5000 |
| SH-TS-CH3 | 107.2 | 50.09 | 20.95 | ND | 8.068 | ND |
| RY-073 | 156 | 88 | 122 | ND | 8.5 | 267 |
| SVO-8-20 | 11 | 40 | 28 | 19 | 8.6 | 138 |
| SHU-221-1 | 66 | 41 | 43 | 3000 | 9 | 3000 |
| YT-III-231 | 51.09 | 61.46 | 26.34 | ND | 9.124 | ND |
| CM-E09a | 176 | 192 | 122 | 490 | 9.2 | 718 |
| DM-139 | 5.8 | ND | 169 | ND | 9.25 | 325 |
| YT-III-42 | 382.9 | 16.83 | 44.04 | ND | 9.77 | ND |
| CD-214 | 16.4 | 48.2 | 42.5 | ND | 9.8 | 168 |
| XHE-III-24 | 0.25 | ND | 8 | 222 | 10 | 328 |
| XLi223 C22H20BrN3O2 | 14 | 8.7 | 18 | 1000 | 10 | 2000 |
| SPH-165 | 0.63 | 2.79 | 4.85 | ND | 10.4 | 1150 |
| JYI-01 (C19H20N3O3Br) | 59.2 | 159 | 96 | ND | 10.6 | 2.88 |
| diazepam | 14 | 20 | 15 | ND | 11 | ND |
| XHE-III-49 | 1.3 | 5.5 | 4.2 | 38.7 | 11.3 | 85.1 |
| WZ-113 | 19.2 | 13.2 | 13.4 | ND | 11.5 | 300 |
| JYI-59 (C22H13N3O2F4) | 1.08 | 2.6 | 11.82 | ND | 11.5 | 5000 |
| JYI-72 (C22H21N4SiF) | 48.5 | 18.5 | ND | ND | 11.5 | 5000 |
| TC-YT-II-76 | 101.1 | 1.897 | 5.816 | ND | 11.99 | ND |
| JYI-10 (C17H13N3O3F3Br) | 5000 | 368 | ND | ND | 12.3 | 23 |
| WZ-069 | 40 | 30.5 | 38.5 | ND | 12.6 | 1000 |
| JYI-06 (C23H23N3O4) | 16.5 | 5.48 | 5000 | ND | 12.6 | 5000 |
| RY-072 | 220 | 150 | 184 | ND | 12.7 | 361 |
| JYI-14 (C17H14N3O3F3) | 32 | 25 | ND | ND | 13 | 565 |
| XHE-II-053 | 287 | 45 | 96 | 1504 | 13.8 | 1000 |
| Xli-347 C34H28N6O7 | 828.05 | 690.2 | ND | ND | 13.87 | ND |
| SHU-1-19 | 4 | 12 | 7 | 48 | 14 | 84 |
| CM-C28 (SR) | 176 | 752 | 244 | 290 | 14 | 141 |
| CM-E11 | 333 | 308 | 161 | 394 | 14 | 750 |
| XHE-II-012 | 49 | 24 | 31 | 1042 | 14 | 2038 |
| MMB-III-018 | 117 | 140 | 78 | 3500 | 14 | 976 |
| MMB-III-18 | 117 | 140 | 78 | 3500 | 14 | 976 |
| CM-B31c (ss) | 118 | 319 | 173 | 37 | 15 | 137 |
| CM-B45 | 230 | 557 | 336 | 265 | 15 | 230 |
| XLI-093 | 1000 | 1000 | 858 | 1550 | 15 | 2000 |
| DM-II-20 (C22H14N3O2F3) | 54.3 | 27.14 | 35.68 | ND | 15.35 | 5000 |
| XLi269 C22H22N4Si | 221.8 | 154.2 | ND | ND | 15.51 | ND |
| SH-O53-S-CH3-2′F | 350 | 141 | 1237 | ND | 16 | 5000 |
| JYI-13 (C21H16N3O4F3) | 5000 | 63.7 | ND | ND | 16 | 8.38 |
| CM-B34 | 472 | 451 | 223 | 114 | 17 | 175 |
| XHE-II-017 | 3.3 | 10 | 7 | 258 | 17 | 294 |
| JC222 C16H12N2OS | 86.7 | 45.11 | ND | ND | 17.63 | ND |
| SPH-38 | 2 | 5.4 | 10.8 | ND | 18.5 | 3000 |
| WZ-070 | 72.7 | 30.7 | 53.2 | ND | 18.6 | 300 |
| RY-069 | 692 | 622 | 506 | ND | 19 | 1000 |
| SH-053-2′F-S-CH3 | 468.2 | 33.27 | 291.5 | ND | 19.2 | ND |
| XHE-I-093 | 2 | 7.1 | 8.9 | 1107 | 20 | 1162 |
aAffinity of compounds at GABAA/BzR recombinant subtypes was measured by competition for [3H]flunitrazepam or [3H] Ro15-4513 binding to HEK cell membranes expressing human receptors of compositions α1β3γ2, α2β3γ2, α3β3γ2, α4β3γ2, α5β3γ2, and α6β3γ2 [22, 139]. Data represent the average of at least three determinations with a SEM of ±5%. ND: not determined.
Figure 42.

Overlay of selected compounds selective for α5β3γ2 subtype.
Figure 43.

Updated α5β3γ2 subtype (blue solid) overlaid with the previous model (yellow wire). Overlap identified where wire and solid overlap.
Figure 44.

Overlay of the α5β3γ2 receptor (yellow) subtype with the α1β3γ2 receptor (magenta) subtype. Orange surfaces indicate overlapping regions.
Figure 45.
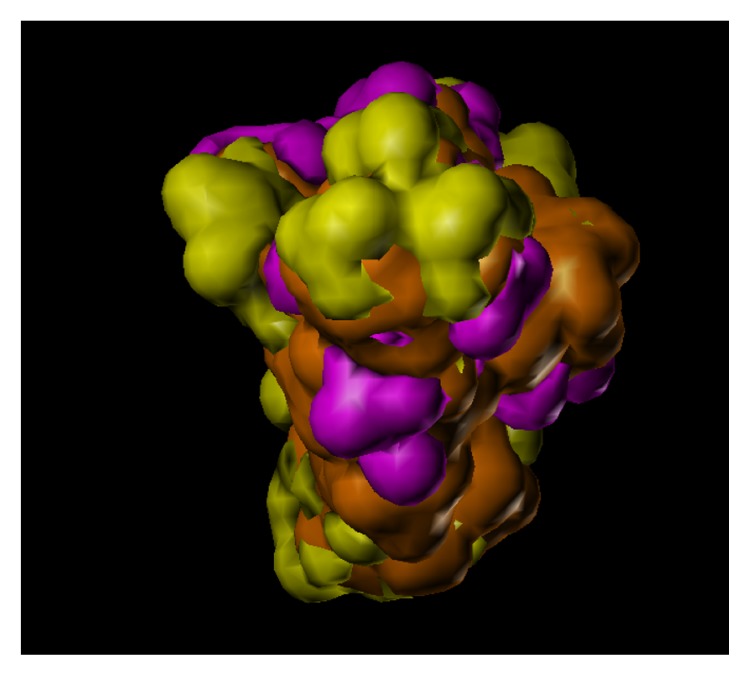
Overlay of the α5β3γ2 receptor (yellow) subtype with the α1β3γ2 receptor (magenta) subtype (Figure 44 rotated 90°). Orange surfaces indicate overlapping regions.
17. The α6β3γ2 Receptor Subtype
See Table 10 and Figures 46 and 47.
Figure 46.

Overlay of selected compounds selective for α6β3γ2 subtype.
18. Updates to the Previous Model
In addition to the newly discovered L 4 pocket, the updated library of binding affinity led to two specific updates in the previous model (Figure 48).
Figure 48.
The previous benzodiazepine subtype selective receptor pharmacophore models [23]. (1) The L 2 region in the α5 subtype is larger than the α1 subtypes. This is a key result. It is the principle difference between α5 subtypes compared to α2 and α3 subtypes, but especially in regard to α1 subtypes (L 2 smaller in α1). (2) The L 3 region is larger in the α5 subtype as compared to the α1, α2, α3, α4, and α6 BzR sites. R analogs of benzodiazepines with pendant phenyls had increased affinity to α5 supporting the larger L 3 pocket in this receptor subtype, while S isomers bound to α2, α3, and α5 subtypes because of different conformational constraints.
19. QSAR
A nontraditional quantitative structure activity relationship (QSAR) approach was executed to observe steric and electrostatic preferences for each receptor subtype. A subset of the compounds used in each subtype pharmacophore/receptor model were chosen with a good cross section of scaffold variety. The compounds used in the COMFA maps are the imidazobenzodiazepines published previously [110, 137] and additionally alternative scaffolds which bound with <20 nM at the respective subtype [22].
The interest here was in creation of steric and electrostatic maps of the comparative molecular field analyses (COMFA) created from molecular spreadsheets. A variety of compounds selective for each subtype were selected and placed into a dataset used to build the CoMFA models. Activities (K i values) were converted to logarithmic units for this study. A CoMFA descriptor set was created based on the –log (K i) of over 70 structures. The goal was to derive an alternative three-dimensional shape of the receptor using biological activity of the most selective compounds. Structures were determined by crystal structure where available or by calculation. Charges were provided based on the Gasteiger-Huckel model. Conformations were kept consistent based on previous studies of low energy conformations [110]. It should be noted that this was not a traditional QSAR study as nonselective compounds were excluded. Therefore, K i values did not cross 3 log units. This was acceptable since the goal was not to create a predictive QSAR predictive algorithm, rather a map of the receptor based on sterics and electrostatics. Hydrogen acceptor radii were set to 3.0 and the hydrogen donor radii were set to 2.6 based on recommendations from Certara (Tripos). Analyses were executed using PLS (partial least squares). The details of modeling will be further discussed in the SI.
For each of the following QSAR models (Figures 49 –64), green areas represent desirable steric bulk and yellow represents undesirable steric bulk. Positive electrostatic contributions are represented by blue and negative electrostatic contributions are represented by red.
Figure 49.

Steric (left) and electrostatic maps of the α1β3γ2 receptor subtype shown in the transparent mode as seen from the classic perspective.
Figure 50.

Steric (left) and electrostatic maps of the α1β3γ2 receptor subtype shown in the transparent mode as seen from the classic perspective (Figure 45) rotated 90°.
Figure 51.

Steric (left) and electrostatic maps of the α1β3γ2 receptor subtype shown in line mode as seen from the classic perspective.
Figure 52.

Steric (left) and electrostatic maps of the α1β3γ2 receptor subtype shown in line mode as seen from the classic perspective rotated 90°.
Figure 53.

Steric (left) and electrostatic maps of the α2β3γ2 receptor subtype shown in the transparent mode as seen from the classic perspective.
Figure 54.
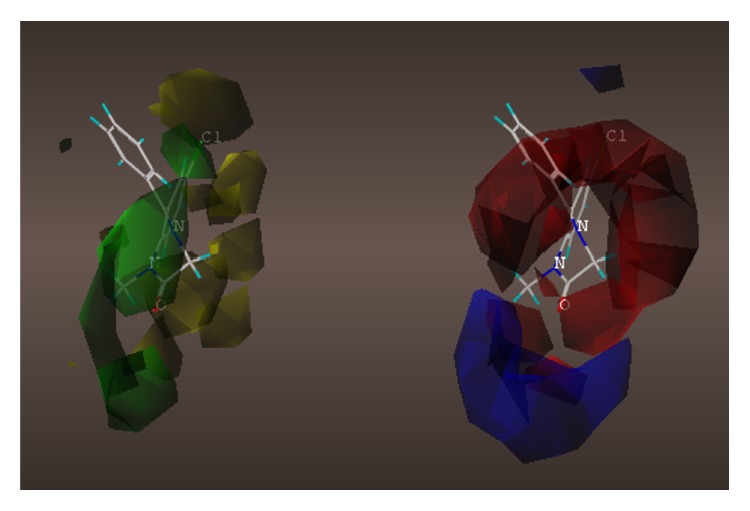
Steric (left) and electrostatic maps of the α2β3γ2 receptor subtype shown in the transparent mode as seen from the classic perspective rotated 90°.
Figure 55.

Steric (left) and electrostatic maps of the α2β3γ2 receptor subtype shown in line mode as seen from the classic perspective.
Figure 56.
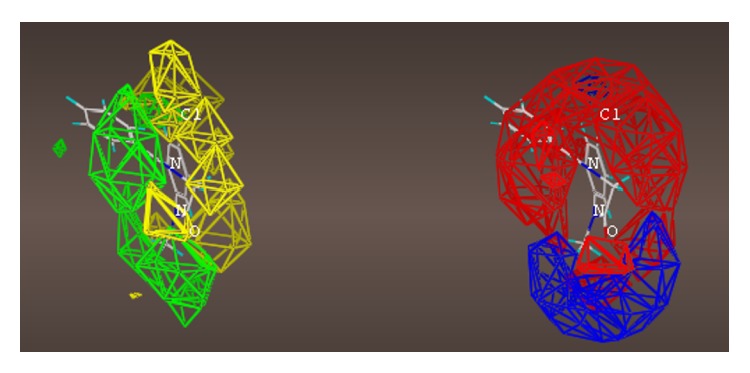
Steric (left) and electrostatic maps of the α2β3γ2 receptor subtype shown in line mode as seen from the classic perspective rotated 90°.
Figure 57.
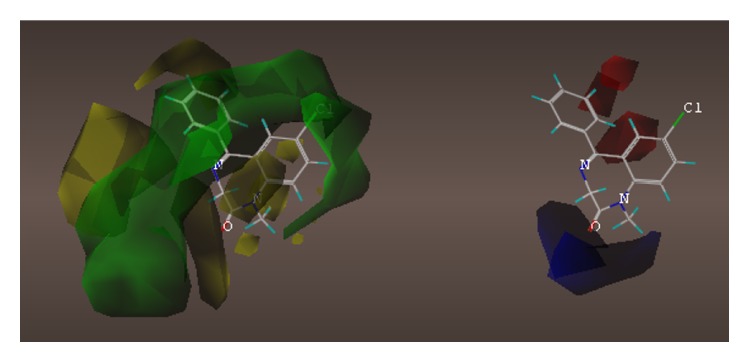
Steric (left) and electrostatic maps of the α3β3γ2 receptor subtype shown in the transparent mode as seen from the classic perspective.
Figure 58.

Steric (left) and electrostatic maps of the α3β3γ2 receptor subtype shown in the transparent mode as seen from the classic perspective rotated 90°.
Figure 59.
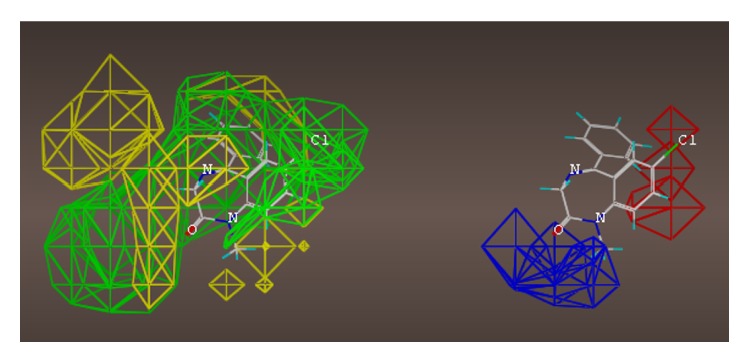
Steric (left) and electrostatic maps of the α3β3γ2 receptor subtype shown in line mode as seen from the classic perspective.
Figure 60.
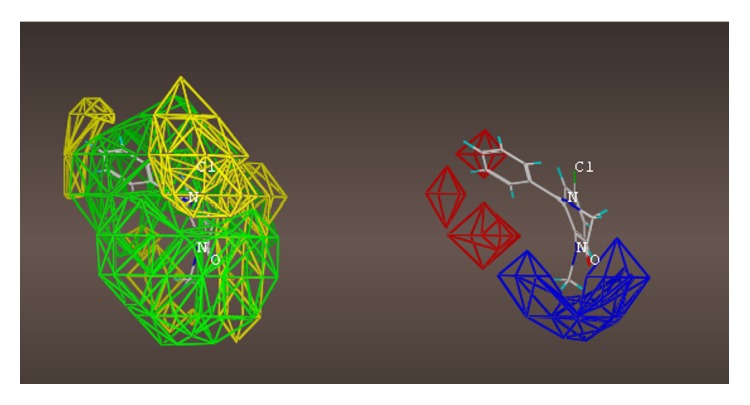
Steric (left) and electrostatic maps of the α3β3γ2 receptor subtype shown in line mode as seen from the classic perspective rotated 90°.
Figure 61.
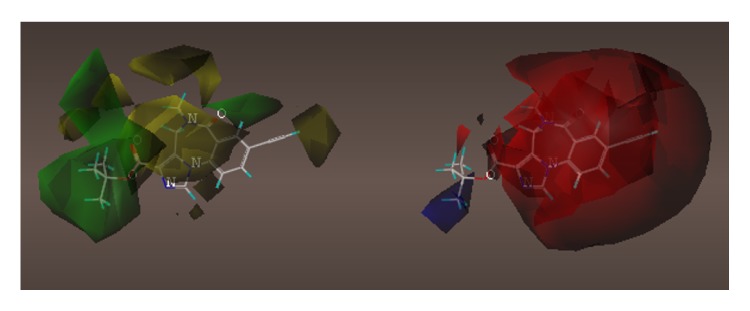
Steric (left) and electrostatic maps of the α5β3γ2 receptor subtype shown in the transparent mode as seen from the classic perspective.
Figure 62.

Steric (left) and electrostatic maps of the α5β3γ2 receptor subtype shown in the transparent mode as seen from the classic perspective rotated 90°.
Figure 63.
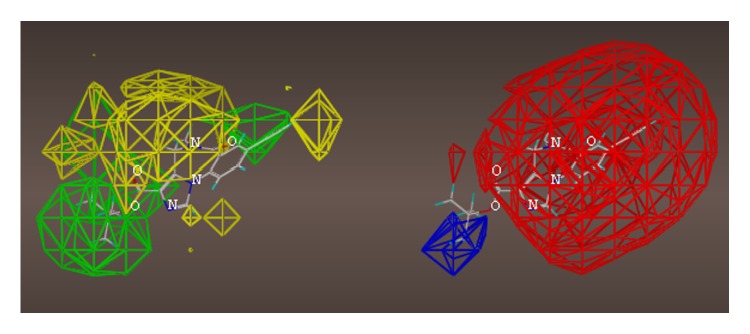
Steric (left) and electrostatic maps of the α5β3γ2 receptor subtype shown in line mode as seen from the classic perspective.
Figure 64.
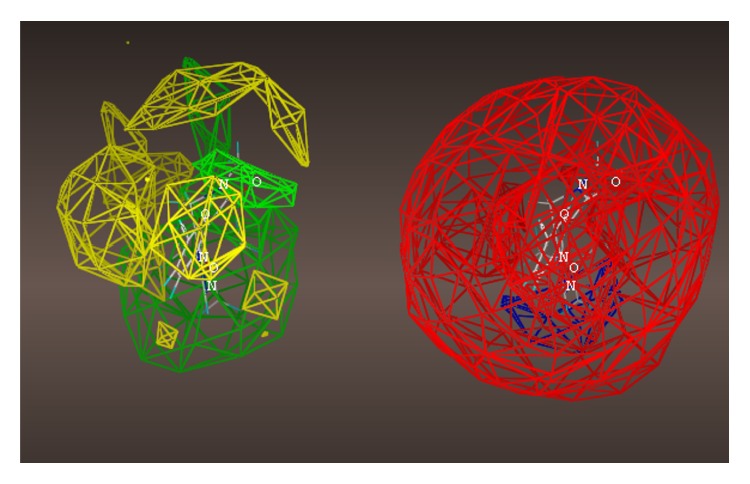
Steric (left) and electrostatic maps of the α5β3γ2 receptor subtype shown in line mode as seen from the classic perspective rotated 90°.
20. The α1β3γ2 Receptor Subtype
21. The α2β3γ2 Receptor Subtype
22. The α3β3γ2 Receptor Subtype
23. The α5β3γ2 Receptor Subtype
From the CoMFA maps several observations (Figure 65) can be made. The yellow steric regions near L 3 in the α5β3γ2 map are unique. This illustrated that, in general, benzodiazepines lacking a pendant phenyl are more suited to targeting the α5 subtype. The L Di region of the α1 subtype is most tolerable for compounds with steric interactions in this location while the α3 subtype receptor compounds prefer no steric interaction in this location. Negative electrostatics are most preferred by the L 3 pocket of the α2 and α5 receptors. In general, the α1 subtype receptor prefers molecules without a dipole. It should be noted that none of the analogs are ionic in nature and the charges for this model were provided by the Gasteiger-Huckel model. For this reason more emphasis is placed on the steric relationships which exclude interactions in the pharmacophores. In the future a QSAR study which includes nonbinding benzodiazepines in the data set along with activity data will permit the creation of a predictive algorithm which will be very useful in lead targeting (see Figures 61–65).
Figure 65.
Clockwise from the top left, line maps of the α1β3γ2, α2β3γ3, α3β3γ2, and α5β3γ2 CoMFA.
24. Conclusion
Benzodiazepines, β-carbolines, and other classes of compounds readily target the GABAA receptors. The difficulty is finding subtype selective ligands, since there is no crystal structure of the Bz/GABAA-ergic site itself, just one composed of five beta-subunits which has no Bz site to date. The α5-BzR/GABAA subunit has recently been shown to be important in the search to treat numerous cognition-based illnesses including Alzheimer's, schizophrenia, bipolar, and depression, as well as more recently a bronchodilator, potentially important in the treatment of asthma. As an inverse agonist, PWZ-029 was able to counteract the memory-impairing effects of scopolamine, a muscarinic antagonist, in both object recognition tests and object retrieval tests in rodents, and was active in primates, as well as samaritan Alzheimer's rats. The implications of these tests point to a use as a possible treatment for Alzheimer's disease. The docking of PWZ-029 in the α5γ2 GABAAR-subunit details the interactions between the pharmacophore/receptor model binding site and this important negative allosteric modulator. Furthermore, α5-BzR/GABAA positive allosteric modulator, SH-053-2′F-R-CH3, was shown to reverse deleterious effects in the MAM-model of schizophrenia. The recent discovery of α5-GABAAR in airway smooth muscle by Emala et al. has also lead to the testing of SH-053-2′F-R-CH3 as a bronchodilator. This SH-053-2′F-R-CH3 was found to be effective in relaxing preconstricted airway smooth muscle, as well as attenuating calcium-ion entry through the plasma membrane. In addition, XLi-093 (an α5 receptor antagonist), a potently binding α5-subtype selective bivalent ligand, has been shown to inhibit the α5-cognition deficits effected by diazepam and is a very good α5 benzodiazepine receptor site antagonist. It has also been shown to reverse the effects of α5 PAMs and NAMs in both rodent and primate models. These findings led to the exploration of the α5-binding pocket in the Milwaukee-based pharmacophore.
New features have been introduced to the unified pharmacophore/receptor model based on many substance classes that act at the diazepam sensitive and diazepam insensitive BzR binding sites of GABAA receptors. The major new feature identified for the α5β3γ2 receptor was a new L 4 pocket which was found by using pendant 6-phenyl benzodiazepines with a R-CH3 at the prochiral center at C4. Further enhancement of potency was achieved by addition of 2′-F or 2′-N substituent in the pendant phenyl ring at C-6. While these changes have led to enhanced subtype selective ligands, the overall development guided by this pharmacophore model described here has led to new agents with varying, fascinating pharmacological profiles, ranging from use in cognition-based diseases such as Alzheimer's and schizophrenia, to use as a bronchodilator. This research on updating the Milwaukee-based pharmacophore/receptor model can be used in the rational design for improving the selectivity of α5 ligands. As the library of compounds increases, the data which follows can then be further evaluated and can lead to more insight to the identification of the possible roles each individual residue may have with the binding pocket.
The X-ray structure determination of the α5β3γ2 GABA(A) receptor is eagerly awaited, while that with five β3-subunits has been reported recently (Miller and Aricescu, Nature 2014). It is hoped that the proposed orientation may be used by others to gain additional insight into the potential mechanisms underlying binding and modulation at the Bz site, all of which will lead to a better understanding of the structure and function of GABA(A) receptors, ultimately targeted toward treatment of diseases.
25. Synthesis of Ligands with α5 BzR Subtype Selectivity
Briefly, bromoacetyl bromide was added to 2-aminobenzophenone 44, followed by treatment with methanol, which had been saturated with ammonia (g) under the cooling of an ice-water bath. The benzodiazepine, 45, was brominated to provide 46 and then reacted with ethyl isocyanoacetate to generate the imidazobenzodiazepine, 47. A much better one-pot process has now been devised using KtBuO at −30°C [140]. The bromide 48 was subjected to a Stille-type coupling to give DM-I-81 (9) [126]. This route (Scheme 1) can be executed on several hundred gram scales.
Scheme 1.
Synthesis of 8-substituted imidazobenzodiazepines following chemistry earlier developed by Sternbach, Fryer et al. Reagents and Conditions. (a) Bromoacetyl bromide, sodium bicarbonate, and chloroform; (b) ammonia (anhydrous), methanol, and reflux; (c) bromine, sulfuric acid, and acetic acid; (d) sodium hydride, diethyl chlorophosphate, and tetrahydrofuran; (e) sodium hydride, ethyl isocyanoacetate, and tetrahydrofuran, −30°C to r.t.; (f) tributyl(phenyl)stannane, Pd(PPh3)4.
The benzodiazepine monomers were prepared by the method of Fryer and Gu [89, 141]. The isatoic anhydride was heated with sarcosine in dimethyl sulfoxide to provide amide 49. Bromination of 49 in a mixture of acetic acid, bromine, and sodium acetate afforded the corresponding monosubstituted bromide 50 in good yield. Deprotonation of 50 with lithium diisopropyl amide (LDA) in THF was followed by treatment with diethyl chlorophosphate to provide the intermediate enol phosphate. The enol phosphate was stirred with a solution of ethyl isocyanoacetate and LDA to yield the imidazo congener. Again, a better one-pot procedure has been developed using KtBuO at −30°C in place of LDA at 0°C. A Heck type coupling reaction was employed with the bromide 51 with bis(acetate)bis(triphenylphosphine)palladium(II) to provide the TMS-acetylene 52. Treatment of 52 with Bu4NF removed the trimethylsilyl group. Hydrolysis of the ester function of 53 provided the acid 54 in excellent yield and this material was dried scrupulously and subjected to a standard CDI-mediated coupling reaction to furnish bivalent ligand XLi-093 (4). The imidazobenzodiazepine diethyl diester XLi-356 (10) was obtained from XLi-093 (Scheme 2) in high yield via catalytic hydrogenation (Pd/C, H2).
Scheme 2.
Synthesis of 8-substituted imidazobenzodiazepine bivalent ligands. Reagents and Conditions. (a) DMSO, 180°C, 90%; (b) bromine, sodium acetate, and acetic acid, r.t., 80%; (c) LDA, THF, and diethyl chlorophosphate, 0°C; (d) LDA, THF, and ethyl isocyanoacetate; (e) trimethylsilyl acetylene, Pd(OAc)2(PPh3)2, triethylamine, acetonitrile, and reflux, 80%; (f) tetrabutylammonium fluoride, THF, and H2O, r.t., 88%; (g) 2N NaOH and ethanol, 70°C, 90%; (h) CDI, DMF, HO(CH2)3OH, and DBU, 60%; (i) Pd/C, H2, ethanol, and DCM, 90%.
26. Synthesis of Bivalents
Inverse agonist 53 was synthesized via the reported procedure. Hydrolysis of the ester function of 53 provided the acid 54 in excellent yield. This material was dried scrupulously and was subjected to a standard CDI-mediated coupling reaction to furnish bivalent ligands 4, 55, and 56 in 60% yield (Scheme 3) [13].
Scheme 3.
Synthesis of bivalent analogs of XLi-093 (4). Reagents and Conditions. (a) 2 M NaOH, EtOH, 70°C; (b) 10% aq HCl; (c) CDI, DMF; (d) diol, DBU.
The acid 57, obtained from the ester 47, which was available from the literature [13], was stirred with CDI in DMF, followed by stirring with the required diol and DBU to provide bromide substituted dimers 58 or 59, respectively. They were converted into the trimethylsilylacetylenyl 60 or 61, respectively, under standard conditions (Pd-mediated, Heck-type coupling) [142]. The bisacetylene 62 or 63 (individually) was easily obtained by treatment of the trimethylsilyl ligand 60 or 61 with fluoride anion, as shown in Scheme 4.
Scheme 4.
Synthesis of bivalent analogues of DMH-D-053 (63). Reagents and Conditions. (a) 2 N NaOH, EtOH, and reflux; (b) 10% aq. HCl; (c) CDI, DMF; (d) diol, DBU; (e) trimethylsilylacetylene, Pd(OAc)2(PPH3)2, Et3N, CH3CN, and reflux; (f) TBAF∗0.5H2O, THF, −78°C.
27. Materials, Methods, and Experimental
27.1. Materials and General Instrumentation
Chemicals were purchased from Aldrich Chemical Co. or Tokyo Chemical Industries and were used without further purification except where otherwise noted. Anhydrous THF was distilled from sodium/benzophenone ketyl. TLC analyses were carried out on Merck Kieselgel 60 F254, and flash column chromatography was performed on silica gel 60b purchased from E. M. Laboratories. Melting points were taken on a Thomas-Hoover melting point apparatus or an Electrothermal Model IA8100 digital melting point apparatus and are reported uncorrected. NMR spectra were recorded on a Bruker 300 or 500 MHz multiple-probe spectrometer. Infrared spectra were recorded on a Nicolet DX FTIR BX V5.07 spectrometer or a Mattson Polaris IR-10400 instrument. Low-resolution mass spectral data (EI/CI) were obtained on a Hewlett-Packard 5985B GC-mass spectrometer, while high resolution mass spectral data were taken on a VG autospectrometer (Double Focusing High Resolution GC/Mass Spectrometer, UK). Microanalyses were performed on a CE Elantech EA1110 elemental analyzer. Methods of specific experiments can be found in corresponding cited works.
27.2. Competition Binding Assays
Competition binding assays were performed in a total volume of 0.5 mL of a 50 mM Tris-acetate at 4° degree centigrade for 1 hour using [3H]flunitrazepam as the radioligand. For these binding assays, 20–50 mg of membrane protein harvested with hypotonic buffer (50 mM Tris-acetate pH 7.4 at 4 degree) was incubated with the radiolabel as previously described [139, 143]. Nonspecific binding was defined as radioactivity bound in the presence of 100 μM diazepam and represented less than 20% of total binding. Membranes were harvested with a Brandel cell harvester followed by three ice-cold washes onto polyethyleneimine-pretreated (0.3%) Whatman GF/C filters. Filters were dried overnight and then soaked in Ecoscint A liquid scintillation cocktail (National Diagnostics; Atlanta, GA). Bound radioactivity was quantified by liquid scintillation counting. Membrane protein concentrations were determined using an assay kit from Bio-Rad (Hercules, CA) with bovine serum albumin as the standard.
27.3. Radioligand Binding Assays (Drs. McKernan and Atack) [12]
In brief, the affinity of compounds for human recombinant GABA(A) receptors was measured by competition binding using 0.5 nM [3H]flunitrazepam. Transfected HEK cells (beta2 gamma2 and desired alpha subtype) were harvested into phosphate-buffered saline, centrifuged at 3,000 g, and stored at −70°C until required. On the day of the assay, pellets were thawed and resuspended in sufficient volume of 50 mM Tris/acetate (pH 7.4 at 4°C) to give a total binding of approximately 1500–2000 dpm. Nonspecific binding was defined in the presence of 100 mM (final concentration) diazepam. Test compounds were dissolved in DMSO at a concentration of 10 mM and diluted in assay buffer to give an appropriate concentration range in the assay, such that the final DMSO concentration in the assay was always less than 1%. Total assay volume was 0.5 mL and assays were carried out in 96-well plates and incubation time started by the addition of 0.1 mL of resuspended cell membranes. Following incubation for 1 hour at 4°C, assays were terminated by filtration through GF/B filters, washed with 10 mL ice cold buffer, dried, and then counted using a liquid scintillation counter. The percentage of inhibition of [3H]flunitrazepam binding, the IC50, and the K i values were calculated using the Activity Base Software Package (ID Business Solutions, Guildford, UK) according to the Cheng-Prusoff equation [143]. We have previously reported the synthesis of the following.
1,3-Bis(8-acetyleno-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5a][1,4]benzodiazepine-3-carboxy) propyl diester 4 (XLi-093) (Procedure A), experimental details previously reported [17].
1,5-Bis(8-acetyleno-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5a][1,4]benzodiazepine-3-carboxy) pentyl diester 56 (XLi-210), experimental details previously reported [17].
1,3-Bis(8-ethyl-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5a][1,4]benzodiazepine-3-carboxy) propyl diester 10 (Xli-356), experimental previously published [144].
Bis(8-acetyleno-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5a][1,4]benzodiazepine-3-carboxy) dimethyl glycol diester 55 (Xli-374), experimental details previously reported [17].
8-Bromo-6-phenyl-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxylic acid 57, experimental details previously reported [17].
1,3-Bis(8-bromo-6-phenyl-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxy) propyl diester 59 (DMH-D-070) (Procedure B), experimental details previously reported [17].
1,3-Bis(8-trimethylsilylacetylenyl-6-phenyl-4H-benzo[f]imidazo[1,5-a][1,4]-diazepine-3-carboxy) propyl diester 61 (DMH-D-048) (Procedure C), experimental details previously reported [17].
1,3-Bis(8-acetylenyl-6-phenyl-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxy) propyl diester 63 (DMH-D-053): experimental details previously reported [17].
Bis(8-bromo-6-phenyl-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxy) diethylene glycol diester 58 (DM-III-93), experimental details previously reported [17].
Bis(8-trimethylsilylacetylenyl-6-phenyl-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxy) diethylene glycol diester 60 (DM-III-94), experimental details previously reported [17].
Bis(8-acetylenyl-6-phenyl-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxy) diethylene glycol diester 62 (DM-III-96), experimental details previously reported [17].
Supplementary Material
The supporting information contains details on the construction of the Unified Pharmacophore/Receptor Model. In addition, the crystallographic data (excluding structure factors) for the structures in this report have been deposited with the Cambridge Crysallographic Data Centre as supplementary publication numbers 687205 (DMH-D-053), 222395 (XLi-093), and 222396 (DM-II-96). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK, (fax +44-(0)1223-336033 or email: deposit@ccdc.cam.ac.uk). Structures of all compounds found within Tables 5-9 are also contained within the supporting information under Appendix III.
Acknowledgments
The authors gratefully acknowledge the work of Dr. Ruth McKernan and Dr. Bryan Roth for receptor binding. This was supported by NS-076517, MH-096463, NIA AG-039511, and AG-048446. The authors acknowledge support from the Milwaukee Institute for Drug Design.
Abbreviations
- APD:
Antipsychotic drug
- ASM:
Airway smooth muscle
- BS:
Binding site
- BZD, Bz:
Benzodiazepine
- BzR:
Benzodiazepine receptor
- DA:
Dopamine
- DAPI:
4′,6-Diamidino-2-phenylindole
- GABA:
Gamma amino butyric acid
- GABAA:
Gamma amino butyric acid A
- GABAAR:
Gamma amino butyric acid A receptor
- HAL:
Haloperidol
- HEK:
Human embryonic kidney
- HPC:
Hippocampal
- LTK:
Leukocyte tyrosine kinase
- MAM:
Methylazoxymethanol
- NAM:
Negative allosteric modulator
- QSAR:
Quantitative structure-activity relationship
- PAM:
Positive allosteric modulator
- PV:
Parvalbumin
- SAL:
Saline
- SH-053:
SH-053-2′F-R-CH3
- SMA:
Smooth muscle actin
- TTX:
Tetrodotoxin
- VTA:
Ventral tegmental area.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Terry Clayton and Michael M. Poe contributed equally to this work.
References
- 1.Miller P. S., Aricescu A. R. Crystal structure of a human GABAA receptor. Nature. 2014;512(7514):270–275. doi: 10.1038/nature13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backus K. H., Arigoni M., Drescher U., et al. Stoichiometry of a recombinant GABAA receptor deduced from mutation-induced rectification. Neuroreport. 1993;5(3):285–288. doi: 10.1097/00001756-199312000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Möhler H. Brain disorders and novel therapeutics. Chimia. 2004;58(10):718–720. doi: 10.2533/000942904777677461. [DOI] [Google Scholar]
- 4.Möhler H., Fritschy J.-M., Crestani F., Hensch T., Rudolph U. Specific GABAA circuits in brain development and therapy. Biochemical Pharmacology. 2004;68(8):1685–1690. doi: 10.1016/j.bcp.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Rudolph U., Möhler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annual Review of Pharmacology and Toxicology. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- 6.Bailey D. J., Tetzlaff J. E., Cook J. M., He X., Helmstetter F. J. Effects of hippocampal injections of a novel ligand selective for the α5β2γ2 subunits of the GABA/benzodiazepine receptor on Pavlovian conditioning. Neurobiology of Learning and Memory. 2002;78(1):1–10. doi: 10.1006/nlme.2001.4050. [DOI] [PubMed] [Google Scholar]
- 7.DeLorey T. M., Lin R. C., McBrady B., et al. Influence of benzodiazepine binding site ligands on fear-conditioned contextual memory. European Journal of Pharmacology. 2001;426(1-2):45–54. doi: 10.1016/s0014-2999(01)01199-2. [DOI] [PubMed] [Google Scholar]
- 8.Chambers M. S., Atack J. R., Bromidge F. A., et al. 6,7-Dihydro-2-benzothiophen-4(5H)-ones: a novel class of GABA-A α5 receptor inverse agonists. Journal of Medicinal Chemistry. 2002;45(6):1176–1179. doi: 10.1021/jm010471b. [DOI] [PubMed] [Google Scholar]
- 9.Chambers M. S., Atack J. R., Broughton H. B., et al. Identification of a novel, selective GABAa α5 receptor inverse agonist which enhances cognition. Journal of Medicinal Chemistry. 2003;46(11):2227–2240. doi: 10.1021/jm020582q. [DOI] [PubMed] [Google Scholar]
- 10.Sur C., Quirk K., Dewar D., Atack J., Mckernan R. Rat and human hippocampal alpha 5 subunit-containing gamma-aminobutyric acid(A) receptors have alpha 5 beta 3 gamma 2 pharmacological characteristics. Molecular Pharmacology. 1998;54(5):928–933. doi: 10.1124/mol.54.5.928. [DOI] [PubMed] [Google Scholar]
- 11.Sarter M. Taking stock of cognition enhancers. Trends in Pharmacological Sciences. 1991;12:456–461. doi: 10.1016/0165-6147(91)90636-7. [DOI] [PubMed] [Google Scholar]
- 12.Atack J. R., Alder L., Cook S. M., Smith A. J., McKernan R. M. In vivo labelling of α5 subunit-containing GABAA receptors using the selective radioligand [3H]L-655,708. Neuropharmacology. 2005;49(2):220–229. doi: 10.1016/j.neuropharm.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Li X. Y., Cao H., Zhang C. C., et al. Synthesis, in vitro affinity, and efficacy of a bis 8-ethynyl-4H-imidazo[1,5α]-[1,4]benzodiazepine analogue, the first bivalent α5 subtype selective BzR/GABAA antagonist. Journal of Medicinal Chemistry. 2003;46(26):5567–5570. doi: 10.1021/jm034164c. [DOI] [PubMed] [Google Scholar]
- 14.Li X. Synthesis of selective ligands for GABAA/benzodiazepine receptors [Ph.D. thesis] Milwaukee, Wis, USA: University of Wisconsin-Milwaukee; 2004. [Google Scholar]
- 15.Abadi A. H., Lankow S., Hoefgen B., Decker M., Kassack M. U., Lehmann J. Dopamine/serotonin receptor ligands, part III: synthesis and biological activities of 7,7′-alkylene-bis-6,7,8,9,14,15-hexahydro-5H-benz[d]indolo[2,3-g]azecines—application of the bivalent ligand approach to a novel type of dopamine receptor antagonist. Archiv der Pharmazie. 2002;335(8):367–373. doi: 10.1002/1521-4184(200211)335:8<367::AID-ARDP367>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 16.Yin W., Rivas F., Furtmueller R., et al. Synthesis, in-vitro affinity and efficacy of the first bivalent alpha 5 subtype selective BzR/GABA(A) antagonist. Proceedings of the 2004 Neuroscience Meeting; 2004; San Diego, Calif, USA. [Google Scholar]
- 17.Han D., Holger Försterling F., Li X., et al. A study of the structure-activity relationship of GABAA-benzodiazepine receptor bivalent ligands by conformational analysis with low temperature NMR and X-ray analysis. Bioorganic and Medicinal Chemistry. 2008;16(19):8853–8862. doi: 10.1016/j.bmc.2008.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han D. M., Försterling F. H., Li X. Y., Deschamps J. R., Cao H., Cook J. M. Determination of the stable conformation of GABAA-benzodiazepine receptor bivalent ligands by low temperature NMR and X-ray analysis. Bioorganic & Medicinal Chemistry Letters. 2004;14(6):1465–1469. doi: 10.1016/j.bmcl.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Zhang C. C. Structure Activity Relationships and Cytotoxic Activity of Analogs of Tryprostatin A and B. Preparation of Irreversible Inhibitors for Studies of Mechanism and Action. II. Pharmacophore Receptor Models of GABA(A)/BzR. Milwaukee, Wis, USA: University of Wisconsin-Milwaukee; 2004. [Google Scholar]
- 20.Liu R. Y., Hu R. J., Zhang P. W., Skolnick P., Cook J. M. Synthesis and pharmacological properties of novel 8-substituted imidazobenzodiazepines: high-affinity, selective probes for α5-containing GABAA receptors. Journal of Medicinal Chemistry. 1996;39(9):1928–1934. doi: 10.1021/jm950887n. [DOI] [PubMed] [Google Scholar]
- 21.Huang Q., He X. H., Ma C. R., et al. Pharmacophore/receptor models for GABAA/BzR subtypes (α1β3γ2, α5β3γ2, and α6β3γ2) via a comprehensive ligand-mapping approach. Journal of Medicinal Chemistry. 2000;43(1):71–95. doi: 10.1021/jm990341r. [DOI] [PubMed] [Google Scholar]
- 22.Clayton T. Part I. Unified pharmacophore protein models of the benzodiazepine receptor subtypes. Part II. Subtype selective ligands for alpha5 Gaba(A) /BZ receptors [Ph.D. thesis] Milwaukee, Wis, USA: University of Wisconsin-Milwaukee; 2011. [Google Scholar]
- 23.Clayton T., Chen J. L., Ernst M., et al. An updated unified pharmacophore model of the benzodiazepine binding site on γ-aminobutyric acida receptors: correlation with comparative models. Current Medicinal Chemistry. 2007;14(26):2755–2775. doi: 10.2174/092986707782360097. [DOI] [PubMed] [Google Scholar]
- 24.Savić M. M., Clayton T., Furtmüller R., et al. PWZ-029, a compound with moderate inverse agonist functional selectivity at GABA-A receptors containing alpha5 subunits, improves passive, but not active, avoidance learning in rats. Brain Research. 2008;1208:150–159. doi: 10.1016/j.brainres.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milić M., Timić T., Joksimović S., et al. PWZ-029, an inverse agonist selective for α5 GABAA receptors, improves object recognition, but not water-maze memory in normal and scopolamine-treated rats. Behavioural Brain Research. 2013;241(1):206–213. doi: 10.1016/j.bbr.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowlett J. K., Moran C. A., Clayton T., Rallapalli S., Roth B., Cook J. M. PWZ-029, An Inverse Agonist Selective for α5 Subunit Containing GABA(A) Receptors, Enhances Performance on an Executive Function Task in Monkeys. Rome, Italy: European Behavioral Pharmacology Society; 2009. [Google Scholar]
- 27.Benes F. M., Lim B., Matzilevich D., Walsh J. P., Subburaju S., Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(24):10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rimol L. M., Hartberg C. B., Nesvåg R., et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biological Psychiatry. 2010;68(1):41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 29.Pantelis C., Velakoulis D., McGorry P. D., et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. The Lancet. 2003;361(9354):281–288. doi: 10.1016/s0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 30.Schobel S. A., Kelly M. A., Corcoran C. M., et al. Anterior hippocampal and orbitofrontal cortical structural brain abnormalities in association with cognitive deficits in schizophrenia. Schizophrenia Research. 2009;114(1–3):110–118. doi: 10.1016/j.schres.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schobel S. A., Lewandowski N. M., Corcoran C. M., et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Archives of General Psychiatry. 2009;66(9):938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss A. P., Goff D., Schacter D. L., et al. Fronto-hippocampal function during temporal context monitoring in schizophrenia. Biological Psychiatry. 2006;60(11):1268–1277. doi: 10.1016/j.biopsych.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 33.Wolf R. C., Höse A., Frasch K., Walter H., Vasic N. Volumetric abnormalities associated with cognitive deficits in patients with schizophrenia. European Psychiatry. 2008;23(8):541–548. doi: 10.1016/j.eurpsy.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Moore H., Jentsch J. D., Ghajarnia M., Geyer M. A., Grace A. A. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biological Psychiatry. 2006;60(3):253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flagstad P., Mørk A., Glenthøj B. Y., Van Beek J., Michael-Titus A. T., Didriksen M. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology. 2004;29(11):2052–2064. doi: 10.1038/sj.npp.1300516. [DOI] [PubMed] [Google Scholar]
- 36.Lodge D. J., Grace A. A. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. The Journal of Neuroscience. 2007;27(42):11424–11430. doi: 10.1523/jneurosci.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lodge D. J., Behrens M. M., Grace A. A. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. The Journal of Neuroscience. 2009;29(8):2344–2354. doi: 10.1523/jneurosci.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gill K. M., Lodge D. J., Cook J. M., Aras S., Grace A. A. A novel α5GABAAR-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36(9):1903–1911. doi: 10.1038/npp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang W., Koehler K. F., Zhang P., Cook J. M. Development of a comprehensive pharmacophore model for the benzodiazepine receptor. Drug Design and Discovery. 1995;12(3):193–248. [PubMed] [Google Scholar]
- 40.Zhang W., Diaz-Arauzo H., Allen M. S., Koehler K. F., Cook J. M. Chemical and computer assisted development of the inclusive pharmacophore of benzodiazepine receptors. In: Choudhary M. I., editor. Studies in Medicinal Chemistry. Harwood Academic Publishers; 1996. p. p. 303. [Google Scholar]
- 41.Zhang P. W., Zhang W. J., Liu R. Y., Harris B., Skolnick P., Cook J. M. Synthesis and SAR study of novel imidazobenzodiazepines at ‘diazepam-insensitive’ benzodiazepine receptors. Journal of Medicinal Chemistry. 1995;38(10):1679–1688. doi: 10.1021/jm00010a013. [DOI] [PubMed] [Google Scholar]
- 42.Huang Q., Cox E. D., Gan T., et al. Studies of molecular pharmacophore/receptor models for GABA(A)/benzodiazepine receptor subtypes: binding affinities of substituted β-carbolines at recombinant α1β3γ2 subtypes and quantitative structure-activity relationship studies via a comparative molecular field analysis. Drug Design and Discovery. 1999;16(1):55–76. [PubMed] [Google Scholar]
- 43.Harris D., Clayton T., Cook J., et al. Selective influence on contextual memory: physiochemical properties associated with selectivity of benzodiazepine ligands at GABAA receptors containing the α5 subunit. Journal of Medicinal Chemistry. 2008;51(13):3788–3803. doi: 10.1021/jm701433b. [DOI] [PubMed] [Google Scholar]
- 44.Rüedi-Bettschen D., Rowlett J. K., Rallapalli S., Clayton T., Cook J. M., Platt D. M. Modulation of α5 subunit-containing GABAA receptors alters alcohol drinking by rhesus monkeys. Alcoholism: Clinical and Experimental Research. 2013;37(4):624–634. doi: 10.1111/acer.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Savić M. M., Milinković M. M., Rallapalli S., et al. The differential role of alpha1- and alpha5-containing GABAA receptors in mediating diazepam effects on spontaneous locomotor activity and water-maze learning and memory in rats. International Journal of Neuropsychopharmacology. 2009;12(9):1179–1193. doi: 10.1017/s1461145709000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X. Y., Ma C. R., He X. H., et al. Studies in search of diazepam-insensitive subtype selective agents for GABAA/Bz receptors. Medicinal Chemistry Research. 2003;11(9):504–537. [Google Scholar]
- 47.Duggan L., Fenton M., Rathbone J., Dardennes R., El-Dosoky A., Indran S. Olanzapine for schizophrenia. Cochrane Database of Systematic Reviews. 2005;(2) doi: 10.1002/14651858.CD001359.pub2.CD001359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lieberman J. A., Stroup T. S., McEvoy J. P., et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. The New England Journal of Medicine. 2005;353(12):1209–1223. doi: 10.1056/nejmoa051688. [DOI] [PubMed] [Google Scholar]
- 49.Komossa K., Rummel-Kluge C., Hunger H., et al. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database of Systematic Reviews. 2010;(3) doi: 10.1002/14651858.CD006654.pub2.CD006654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komossa K., Rummel-Kluge C., Hunger H., et al. Zotepine versus other atypical antipsychotics for schizophrenia. Cochrane Database of Systematic Reviews. 2010;(1):p. CD006628. doi: 10.1002/14651858.CD006628.pub2. [DOI] [PubMed] [Google Scholar]
- 51.Komossa K., Rummel-Kluge C., Hunger H., et al. Amisulpride versus other atypical antipsychotics for schizophrenia. Cochrane Database of Systematic Reviews. 2010;(1):p. CD006624. doi: 10.1002/14651858.CD006624.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Komossa K., Rummel-Kluge C., Schmid F., et al. Quetiapine versus other atypical antipsychotics for schizophrenia. Cochrane Database of Systematic Reviews. 2010;(1) doi: 10.1002/14651858.CD006625.pub2.CD006625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sur C., Fresu L., Howell O., McKernan R. M., Atack J. R. Autoradiographic localization of α5 subunit-containing GABA(A) receptors in rat brain. Brain Research. 1999;822(1-2):265–270. doi: 10.1016/S0006-8993(99)01152-X. [DOI] [PubMed] [Google Scholar]
- 54.June H. L., Harvey S. C., Foster K. L., et al. GABAA receptors containing α5 subunits in the CA1 and CA3 hippocampal fields regulate ethanol-motivated behaviors: an extended ethanol reward circuitry. The Journal of Neuroscience. 2001;21(6):2166–2177. doi: 10.1523/JNEUROSCI.21-06-02166.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lingford-Hughes A., Hume S. P., Feeney A., et al. Imaging the GABA-benzodiazepine receptor subtype containing the alpha5-subunit in vivo with [11C]Ro15 4513 positron emission tomography. Journal of Cerebral Blood Flow and Metabolism. 2002;22(7):878–889. doi: 10.1097/00004647-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 56.Hutcheon B., Fritschy J. M., Poulter M. O. Organization of GABAA receptor alpha-subunit clustering in the developing rat neocortex and hippocampus. European Journal of Neuroscience. 2004;19(9):2475–2487. doi: 10.1111/j.0953-816x.2004.03349.x. [DOI] [PubMed] [Google Scholar]
- 57.Ramos B., Lopez-Tellez J. F., Vela J., et al. Expression of α5 GABAA receptor subunit in developing rat hippocampus. Developmental Brain Research. 2004;151(1-2):87–98. doi: 10.1016/j.devbrainres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 58.Towers S. K., Gloveli T., Traub R. D., et al. Alpha5 subunit-containing GABAA receptors affect the dynamic range of mouse hippocampal kainate-induced gamma frequency oscillations in vitro. Journal of Physiology. 2004;559(3):721–728. doi: 10.1113/jphysiol.2004.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heldt S. A., Ressler K. J. Forebrain and midbrain distribution of major benzodiazepine-sensitive GABAA receptor subunits in the adult C57 mouse as assessed with in situ hybridization. Neuroscience. 2007;150(2):370–385. doi: 10.1016/j.neuroscience.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Papatheodoropoulos C., Koniaris E. α5GABAA receptors regulate hippocampal sharp wave-ripple activity in vitro. Neuropharmacology. 2011;60(4):662–673. doi: 10.1016/j.neuropharm.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 61.Gill K. M., Cook J. M., Poe M. M., Grace A. A. Prior antipsychotic drug treatment prevents response to novel antipsychotic agent in the methylazoxymethanol acetate model of schizophrenia. Schizophrenia Bulletin. 2014;40(2):341–350. doi: 10.1093/schbul/sbt236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaczorowski C. C., Disterhoft J. F. Memory deficits are associated with impaired ability to modulate neuronal excitability in middle-aged mice. Learning and Memory. 2009;16(6):362–366. doi: 10.1101/lm.1365609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaczorowski C. C., Sametsky E., Shah S., Vassar R., Disterhoft J. F. Mechanisms underlying basal and learning-related intrinsic excitability in a mouse model of Alzheimer's disease. Neurobiology of Aging. 2011;32(8):1452–1465. doi: 10.1016/j.neurobiolaging.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaczorowski C. C., Davis S. J., Moyer J. R., Jr. Aging redistributes medial prefrontal neuronal excitability and impedes extinction of trace fear conditioning. Neurobiology of Aging. 2012;33(8):1744–1757. doi: 10.1016/j.neurobiolaging.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 65.Rallapalli S. I. Synthesis of Agents to Enhance Cognition. II. Synthesis of Indole Alkaloids. University of Wisconsin-Milwaukee; 2014. [Google Scholar]
- 66.Barnes P. J. Biochemistry of asthma. Trends in Biochemical Sciences. 1991;16:365–369. doi: 10.1016/0968-0004(91)90152-l. [DOI] [PubMed] [Google Scholar]
- 67.Cockcroft D. W. Clinical concerns with inhaled β2-agonists: adult asthma. Clinical Reviews in Allergy and Immunology. 2006;31(2-3):197–208. doi: 10.1385/criai:31:2:197. [DOI] [PubMed] [Google Scholar]
- 68.Morton R. W., Everard M. L., Elphick H. E. Adherence in childhood asthma: the elephant in the room. Archives of Disease in Childhood. 2014;99(10):949–953. doi: 10.1136/archdischild-2014-. [DOI] [PubMed] [Google Scholar]
- 69.Jentzsch N. S., Camargos P., Sarinho E. S. C., Bousquet J. Adherence rate to beclomethasone dipropionate and the level of asthma control. Respiratory Medicine. 2012;106(3):338–343. doi: 10.1016/j.rmed.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Chu E. K., Drazen J. M. Asthma: one hundred years of treatment and onward. The American Journal of Respiratory and Critical Care Medicine. 2005;171(11):1202–1208. doi: 10.1164/rccm.200502-257oe. [DOI] [PubMed] [Google Scholar]
- 71.Gallos G., Gleason N. R., Zhang Y., et al. Activation of endogenous GABAA channels on airway smooth muscle potentiates isoproterenol-mediated relaxation. The American Journal of Physiology—Lung Cellular and Molecular Physiology. 2008;295(6):L1040–L1047. doi: 10.1152/ajplung.90330.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mizuta K., Xu D., Pan Y., et al. GABAA receptors are expressed and facilitate relaxation in airway smooth muscle. The American Journal of Physiology—Lung Cellular and Molecular Physiology. 2008;294(6):L1206–L1216. doi: 10.1152/ajplung.00287.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Savić M. M., Clayton T., Furtmüller R., et al. PWZ-029, a compound with moderate inverse agonist functional selectivity at GABAA receptors containing α5 subunits, improves passive, but not active, avoidance learning in rats. Brain Research. 2008;1208:150–159. doi: 10.1016/j.brainres.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Savić M. M., Huang S., Furtmüller R., et al. Are GABAA receptors containing alpha5 subunits contributing to the sedative properties of benzodiazepine site agonists? Neuropsychopharmacology. 2008;33(2):332–339. doi: 10.1038/sj.npp.1301403. [DOI] [PubMed] [Google Scholar]
- 75.Gallos G., Yocum G. T., Siviski M. E., et al. Selective targeting of the α5 subunit of GABAA receptors relaxes airway smooth muscle and inhibits cellular calcium handling. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2015;308(9):L931–L942. doi: 10.1152/ajplung.00107.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Allen M. S., Tan Y.-C., Trudell M. L., et al. Synthetic and computer-assisted analyses of the pharmacophore for the benzodiazepine receptor inverse agonist site. Journal of Medicinal Chemistry. 1990;33(9):2343–2357. doi: 10.1021/jm00171a007. [DOI] [PubMed] [Google Scholar]
- 77.Allen M. S., Hagen T. J., Trudell M. L., Codding P. W., Skolnick P., Cook J. M. Synthesis of novel 3-substituted β-carbolines as benzodiazepine receptor ligands: probing the benzodiazepine receptor pharmacophore. Journal of Medicinal Chemistry. 1988;31(9):1854–1861. doi: 10.1021/jm00117a029. [DOI] [PubMed] [Google Scholar]
- 78.Diaz-Arauzo H., Evoniuk G. E., Skolnick P., Cook J. M. The agonist pharmacophore of the benzodiazepine receptor. Synthesis of a selective anticonvulsant/anxiolytic. Journal of Medicinal Chemistry. 1991;34(5):1754–1756. doi: 10.1021/jm00109a035. [DOI] [PubMed] [Google Scholar]
- 79.Diaz-Arauzo H., Koehler K. F., Hagen T. J., Cook J. M. Synthetic and computer assisted analysis of the pharmacophore for agonists at benzodiazepine receptors. Life Sciences. 1991;49(3):207–216. doi: 10.1016/0024-3205(91)90005-v. [DOI] [PubMed] [Google Scholar]
- 80.Zhang W. J., Koehler K. F., Harris B., Skolnick P., Cook J. M. Synthesis of benzo-fused benzodiazepines employed as probes of the agonist pharmacophore of benzodiazepine receptors. Journal of Medicinal Chemistry. 1994;37(6):745–757. doi: 10.1021/jm00032a007. [DOI] [PubMed] [Google Scholar]
- 81.Trudell M. L., Lifer S. L., Tan Y.-C., et al. Synthesis of substituted 7,12-dihydropyrido[3,2-b:5,4-b′]diindoles: rigid planar benzodiazepine receptor ligands with inverse agonist/antagonist properties. Journal of Medicinal Chemistry. 1990;33(9):2412–2420. doi: 10.1021/jm00171a015. [DOI] [PubMed] [Google Scholar]
- 82.Trudell M. L., Basile A. S., Shannon H. E., Skolnick P., Cook J. M. Synthesis of 7,12-dihydropyrido[3,4-b:5,4-b′]diindoles. A novel class of rigid, planar benzodiazepine receptor ligands. Journal of Medicinal Chemistry. 1987;30(3):456–458. doi: 10.1021/jm00386a003. [DOI] [PubMed] [Google Scholar]
- 83.Frisch M. J., Trucks G. W., Head-Gordon M., et al. Gaussian 92. Pittsburgh, Pa, USA: Gaussian; 1992. http://www.lct.jussieu.fr/manuels/Gaussian98/00000119.htm. [Google Scholar]
- 84.Trudell M. L. I. The synthesis and study of the pharmacologic activity of 7,12 dihydropyrido[3,2 b:5,4 b′]diindoles. A novel class of rigid, planar benzodiazepine receptor ligands. II. The total synthesis of the indole alkaloid, (±) suaveoline [Ph.D. thesis] Milwaukee, Wis, USA: University of Wisconsin-Milwaukee; 1989. [Google Scholar]
- 85.Yin W., Majumder S., Clayton T., et al. Design, synthesis, and subtype selectivity of 3,6-disubstituted β-carbolines at Bz/GABA(A)ergic receptors. SAR and studies directed toward agents for treatment of alcohol abuse. Bioorganic and Medicinal Chemistry. 2010;18(21):7548–7564. doi: 10.1016/j.bmc.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Han D., Forsterling F. H., Li X., Deschamps J., Cao H., Cook J. M. Study of the structure activity relationships of GABAA-benzodiazepine receptor ligands by low termperature NMR spectroscopy and X-ray analysis. Proceedings of the 227th ACS National Meeting; March-April 2004; Anaheim, Calif, USA. [Google Scholar]
- 87.Fischer B. D., Licata S. C., Zhou H., et al. Anxiolytic-like effects of 8-acetylene imidazobenzodiazepines in a rhesus monkey conflict procedure. Neuropharmacology. 2010;59(7-8):612–618. doi: 10.1016/j.neuropharm.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haefely W., Kyburz E., Gerecke M., Mohler H. Advances in Drug Research. Vol. 99. New York, NY, USA: Academic Press; 1985. Recent advances in the molecular pharmacology of benzodiazepine receptors and in the structure—activity relationships of their agonist and antagonists; pp. 165–322. [Google Scholar]
- 89.Fryer R. I., Gu Z.-Q., Wang C.-G. Synthesis of novel, substituted 4H-imidazo[1,5-a][1,4]benzodiazepines. Journal of Heterocyclic Chemistry. 1991;28(7):1661–1669. doi: 10.1002/jhet.5570280704. [DOI] [Google Scholar]
- 90.Fryer R. I., Zhang P., Rios R., Gu Z.-Q., Basile A. S., Skolnick P. Structure-activity relationship studies at the benzodiazepine receptor (Bzr)—a comparison of the substituent effects of pyrazoloquinolinone analogs. Journal of Medicinal Chemistry. 1993;36(11):1669–1673. doi: 10.1021/jm00063a017. [DOI] [PubMed] [Google Scholar]
- 91.Rivas F. M., Edwankar C. R., Cook J. M., et al. Antiseizure activity of novel γ-aminobutyric acid (A) receptor subtype-selective benzodiazepine analogues in mice and rat models. Journal of Medicinal Chemistry. 2009;52(7):1795–1798. doi: 10.1021/jm801652d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cook J. M., Han D., He X., et al. Anxiolytic agents with reduced sedative and ataxic effects. 7119196 B2, 2006.
- 93.Cook J. M., Zhao H., Huang S., Sarma P. S., Zhang C. C. Stereospecific anxiolytic and anticonvulsant agents with reduced muscle-relaxant, sedative-hypnotic and ataxic effects. US Patent 2006004945, 2007.
- 94.Fryer R. I. Comprehensive Medicinal Chemistry. Vol. 99. Oxford, UK: Pergamon Press; 1989. [Google Scholar]
- 95.Villar H. O., Davies M. F., Loew G. H., Maguire P. A. Molecular models for recognition and activation at the benzodiazepine receptor: a review. Life Sciences. 1991;48(7):593–602. doi: 10.1016/0024-3205(91)90533-h. [DOI] [PubMed] [Google Scholar]
- 96.Villar H. O., Uyeno E. T., Toll L., Polgar W., Davies M. F., Loew G. H. Molecular determinants of benzodiazepine receptor affinities and anticonvulsant activities. Molecular Pharmacology. 1989;36(4):589–600. [PubMed] [Google Scholar]
- 97.Crippen G. M. Distance geometry analysis of the benzodiazepine binding site. Molecular Pharmacology. 1982;22(1):11–19. [PubMed] [Google Scholar]
- 98.Ghose A. K., Crippen G. M. Modeling the benzodiazepine receptor binding site by the general three-dimensional structure-directed quantitative structure-activity relationship method REMOTEDISC. Molecular Pharmacology. 1990;37(5):725–734. [PubMed] [Google Scholar]
- 99.Codding P. W., Muir A. K. S. Molecular structure of Ro15-1788 and a model for the binding of benzodiazepine receptor ligands. Structural identification of common features in antagonists. Molecular Pharmacology. 1985;28(2):178–184. [PubMed] [Google Scholar]
- 100.Muir A. K. S., Codding P. W. Structure-activity studies of β-carbolines. 3. Crystal and molecular structures of methyl β-carboline-3-carboxylate. Canadian Journal of Chemistry. 1985;63(10):2752–2756. doi: 10.1139/v85-458. [DOI] [Google Scholar]
- 101.Codding M. G., Roszak A. W., Szkaradzinska M. B., Cook J. M., Aha L. J. Modeling of the Benzodiazepine Receptor Using Structural and Theoretical Characterization of Novel Beta-Carbolines. Amsterdam, The Netherlands: Elsevier Science; 1989. [Google Scholar]
- 102.Ferretti V., Gilli P., Borea P. A. Structural features controlling the binding of β-carbolines to the benzodiazepine receptor. Acta Crystallographica Section B: Structural Science. 2004;60(4):481–489. doi: 10.1107/s0108768104013564. [DOI] [PubMed] [Google Scholar]
- 103.Borea P. A., Gilli G., Bertolasi V., Ferretti V. Stereochemical features controlling binding and intrinsic activity properties of benzodiazepine-receptor ligands. Molecular Pharmacology. 1987;31(4):334–344. [PubMed] [Google Scholar]
- 104.Bertolasi V., Feretti V., Gilli G., Borea P. A. Stereochemistry of benzodiazepine-receptor ligands.1. Structure of methyl beta-carboline-3-carboxylate (beta-CCM), C13H10N2O2 . Acta Crystallographica Section C: Crystal Structure Communications. 1984;40:p. 1981. [Google Scholar]
- 105.Ferretti V., Bertolasi V., Gilli G., Borea P. A. Structures of two 2-arylpyrazolo[4,3-c]quinolin-3-ones: CGS8216, C16H11N3O, and CGS9896, C16H10ClN3O. Acta Crystallographica Section C: Crystal Structure Communications. 1985;41(1):107–110. doi: 10.1107/s0108270185002943. [DOI] [Google Scholar]
- 106.Tebib S., Bourguignon J.-J., Wermuth C.-G. The active analog approach applied to the pharmacophore identification of benzodiazepine receptor ligands. Journal of Computer-Aided Molecular Design. 1987;1(2):153–170. doi: 10.1007/BF01676959. [DOI] [PubMed] [Google Scholar]
- 107.Gardner C. R. A review of recently-developed ligands for neuronal benzodiazepine receptors and their pharmacological activities. Progress in Neuropsychopharmacology and Biological Psychiatry. 1992;16(6):755–781. doi: 10.1016/0278-5846(92)90099-Z. [DOI] [PubMed] [Google Scholar]
- 108.Allen M. S., LaLoggia A. J., Dorn L. J., et al. Predictive binding of β-carboline inverse agonists and antagonists via the CoMFA/GOLPE approach. Journal of Medicinal Chemistry. 1992;35(22):4001–4010. doi: 10.1021/jm00100a004. [DOI] [PubMed] [Google Scholar]
- 109.Huang Q., Cox E., Gan T., et al. Studies of molecular pharmacophore/receptor models for GABAA/benzodiazepine receptor subtypes: binding affinities of substituted β-carbolines at recombinant alpha x beta 3 gamma 2 subtypes and quantitative structure-activity relationship studies via a comparative molecular field analysis. Drug Design and Discovery. 1999;16(1):55–76. [PubMed] [Google Scholar]
- 110.He X., Huang Q., Ma C., Yu S., McKernan R., Cook J. M. Pharmacophore/receptor models for GABA(A)/BzR α2β3γ2, α3β3γ2 and α4β3γ2 recombinant subtypes. Included volume analysis and comparison to α1β3γ2, α5β3γ2 and α6β3γ2 subtypes. Drug Design and Discovery. 2000;17(2):131–171. [PubMed] [Google Scholar]
- 111.Cox E. D., Diaz-Arauzo H., Huang Q., et al. Synthesis and evaluation of analogues of the partial agonist 6- (propyloxy)-4-(methoxymethyl)-β-carboline-3-carboxylic acid ethyl ester (6- PBC) and the full agonist 6-(benzyloxy)-4-(methoxymethyl)-β-carboline-3-carboxylic acid ethyl ester (Zk 93423) at wild type and recombinant GABA(A) receptors. Journal of Medicinal Chemistry. 1998;41(14):2537–2552. doi: 10.1021/jm970460b. [DOI] [PubMed] [Google Scholar]
- 112.Martin M. J., Trudell M. L., Araúzo H. D., et al. Molecular yardsticks—rigid probes to define the spatial dimensions of the benzodiazepine receptor binding site. Journal of Medicinal Chemistry. 1992;35(22):4105–4117. doi: 10.1021/jm00100a017. [DOI] [PubMed] [Google Scholar]
- 113.Naryanan K., Cook J. M. Probing the dimensions of the benzodiazepine receptor inverse agonist site. Heterocycles. 1990;31(2):203–209. doi: 10.3987/COM-89-5248. [DOI] [Google Scholar]
- 114.Hollinshead S. P., Trudell M. L., Skolnick P., Cook J. M. Structural requirements for agonist actions at the benzodiazepine receptor: studies with analogues of 6-(benzyloxy)-4-(methoxymethyl)-beta-carboline-3-carboxylic acid ethyl ester. Journal of Medicinal Chemistry. 1990;33(3):1062–1069. doi: 10.1021/jm00165a028. [DOI] [PubMed] [Google Scholar]
- 115.Cook J. M., Diaz-Arauzo H., Allen M. S. Inverse agonists: probes to study the structure, topology and function of the benzodiazepine receptor. In: Harris L. S., editor. Proceedings of the 51st Annual Scientific Meeting; 1991; The College on Problems of Drug Dependence; pp. 133–139. [PubMed] [Google Scholar]
- 116.Trullas R., Ginter H., Jackson B., et al. 3-Ethoxy-beta-carboline: a high affinity benzodiazepine receptor ligand with partial inverse agonist properties. Life Sciences. 1988;43(15):1189–1197. doi: 10.1016/0024-3205(88)90208-1. [DOI] [PubMed] [Google Scholar]
- 117.Cain M., Weber R. W., Guzman F., et al. β-Carbolines: synthesis and neurochemical and pharmacological actions on brain benzodiazepine receptors. Journal of Medicinal Chemistry. 1982;25(9):1081–1091. doi: 10.1021/jm00351a015. [DOI] [PubMed] [Google Scholar]
- 118.He X. H., Zhang C. C., Cook J. M. Model of the BzR binding site: correlation of data from site-directed mutagenesis and the pharmacophore/receptor model. Medicinal Chemistry Research. 2001;10(5):269–308. [Google Scholar]
- 119.Huang Q., Liu R. Y., Zhang P. W., et al. Predictive models for GABAA/benzodiazepine receptor subtypes: studies of quantitative structure-activity relationships for imidazobenzodiazepines at five recombinant GABAA/benzodiazepine receptor subtypes [αxβ3γ2 (x = 1−3, 5, and 6)] via comparative molecular field analysis. Journal of Medicinal Chemistry. 1998;41(21):4130–4142. doi: 10.1021/jm980317y. [DOI] [PubMed] [Google Scholar]
- 120.Liu R. Y., Zhang P. W., Gan T., McKernan R. M., Cook J. M. Evidence for the conservation of conformational topography at five major GABA(A)/benzodiazepine receptor subsites. Potent affinities of the (S)-enantiomers of framework-constrained 4,5-substituted pyrroloimidazo-benzodiazepines. Medicinal Chemistry Research. 1997;7(1):25–35. [Google Scholar]
- 121.Huang Q., Zhang W., Liu R., McKernan R. M., Cook J. M. Benzo-fused benzodiazepines employed as topological probes for the study of benzodiazepine receptor subtypes. Medicinal Chemistry Research. 1996;6(6):384–391. [Google Scholar]
- 122.Yu S., He X. H., Ma C. R., McKernan R., Cook J. M. Studies in search of α2 selective ligands for GABAA/BzR receptor subtypes. Part I. Evidence for the conservation of pharmacophoric descriptors for DS subtypes. Medicinal Chemistry Research. 1999;9(3):186–202. [Google Scholar]
- 123.Arbilla S., Depoortere H., George P., Langer S. Z. Pharmacological profile of the imidazopyridine zolpidem at benzodiazepine receptors and electrocorticogram in rats. Naunyn-Schmiedeberg's Archives of Pharmacology. 1985;330(3):248–251. doi: 10.1007/bf00572441. [DOI] [PubMed] [Google Scholar]
- 124.Wong G., Koehler K. F., Skolnick P., et al. Synthetic and computer-assisted analysis of the structural requirements for selective, high-affinity ligand binding to diazepam-insensitive benzodiazepine receptors. Journal of Medicinal Chemistry. 1993;36(13):1820–1830. doi: 10.1021/jm00065a004. [DOI] [PubMed] [Google Scholar]
- 125.Camerman A., Camerman N. Stereochemical basis of anticonvulsant drug action. 2. Molecular structure of diazepam. Journal of the American Chemical Society. 1972;94(1):268–272. doi: 10.1021/ja00756a047. [DOI] [PubMed] [Google Scholar]
- 126.Hempel A., Camerman N., Camerman A. Benzodiazepine stereochemistry: crystal structures of the diazepam antagonist Ro 15-1788 and the anomalous benzodiazepine Ro 5-4864. Canadian Journal of Chemistry. 1987;65(7):1608–1612. doi: 10.1139/v87-269. [DOI] [Google Scholar]
- 127.Neidle S., Webster G. D., Jones G. B., Thurston D. E. Structures of two DNA minor-groove binders, based on pyrrolo[2,1-c][1,4]-benzodiazepines. Acta Crystallographica Section C: Crystal Structure Communications. 1991;47(12):2678–2680. doi: 10.1107/S0108270191005966. [DOI] [Google Scholar]
- 128.Halgren T. A. Merck molecular force field. V. Extension of MMFF94 using experimental data, additional computational data, and empirical rules. Journal of Computational Chemistry. 1996;17(5-6):616–641. [Google Scholar]
- 129.Halgren T. A. Merck molecular force field. III. Molecular geometries and vibrational frequencies for MMFF94. Journal of Computational Chemistry. 1996;17(5-6):553–586. doi: 10.1002/(SICI)1096-987X(199604)17:5/6<553::AID-JCC3>3.0.CO;2-T. [DOI] [Google Scholar]
- 130.Halgren T. A. Merck molecular force field. II. MMFF94 van der Waals and electrostatic parameters for intermolecular interactions. Journal of Computational Chemistry. 1996;17(5-6):520–552. doi: 10.1002/(SICI)1096-987X(199604)17:5/6<520::AID-JCC2>3.0.CO;2-W. [DOI] [Google Scholar]
- 131.Halgren T. A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. Journal of Computational Chemistry. 1996;17(5-6):490–519. doi: 10.1002/(SICI)1096-987X(199604)17:5/6<490::AID-JCC1>3.0.CO;2-P. [DOI] [Google Scholar]
- 132.Halgren T. A., Nachbar R. B. Merck molecular force field. IV. Conformational energies and geometries for MMFF94. Journal of Computational Chemistry. 1996;17(5-6):587–615. [Google Scholar]
- 133.June H. L., Harvey S. C., Foster K. L., et al. GABAAreceptors containing α5 subunits in the CA1 and CA3 hippocampal fields regulate ethanol-motivated behaviors: an extended ethanol reward circuitry. Journal of Neuroscience. 2001;21(6):2166–2177. doi: 10.1523/JNEUROSCI.21-06-02166.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kaminski B. J., Van Linn M. L., Cook J. M., Yin W., Weerts E. M. Effects of the benzodiazepine GABAA α1-preferring ligand, 3-propoxy-β-carboline hydrochloride (3-PBC), on alcohol seeking and self-administration in baboons. Psychopharmacology. 2013;227(1):127–136. doi: 10.1007/s00213-012-2946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang S. Synthesis of Optically Active Subtype Selective Benzodiazepine Receptor Ligands. Milwaukee, Wis, USA: University of Wisconsin; 2007. [Google Scholar]
- 136.Savić M. M., Majumder S., Huang S., et al. Novel positive allosteric modulators of GABAA receptors: do subtle differences in activity at α1 plus α5 versus α2 plus α3 subunits account for dissimilarities in behavioral effects in rats? Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34(2):376–386. doi: 10.1016/j.pnpbp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ernst M., Brauchart D., Boresch S., Sieghart W. Comparative modeling of GABAA receptors: limits, insights, future developments. Neuroscience. 2003;119(4):933–943. doi: 10.1016/s0306-4522(03)00288-4. [DOI] [PubMed] [Google Scholar]
- 138.Roth B. L. Contract #. HHSN-271-2013-00017-C (NIMH PDSP) The NIMH PDSP; 2013. Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program. https://pdspdb.unc.edu/pdspWeb/ [Google Scholar]
- 139.Choudhary M. S., Craigo S., Roth B. L. Identification of receptor domains that modify ligand binding to 5-hydroxytryptamine2 and 5-hydroxytryptamine1c serotonin receptors. Molecular Pharmacology. 1992;42(4):627–633. [PubMed] [Google Scholar]
- 140.Yang J., Teng Y., Ara S., Rallapalli S., Cook J. M. An improved process for the synthesis of 4H-imidazo[1,5-a][1,4]benzodiazepines. Synthesis. 2009;(6):1036–1040. doi: 10.1055/s-0028-1083358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gu Z.-Q., Wong G., Dominguez C., De Costa B. R., Rice K. C., Skolnick P. Synthesis and evaluation of imidazo[1,5-a][1,4]benzodiazepine esters with high affinities and selectivities at ‘diazepam-insensitive’ benzodiazepine receptors. Journal of Medicinal Chemistry. 1993;36(8):1001–1006. doi: 10.1021/jm00060a007. [DOI] [PubMed] [Google Scholar]
- 142.Buckingham J. Dictionary of Organic Compounds. Vol. 2. New York, NY, USA: Chapman & Hall; 1982. [Google Scholar]
- 143.Yung-Chi C., Prusoff W. H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochemical Pharmacology. 1973;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 144.Cook J. M., Clayton T., Johnson Y. T., Rallapalli S., Han D. GABAergic agents to treat memory deficits. US Patent 2010/0130479 A1, 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supporting information contains details on the construction of the Unified Pharmacophore/Receptor Model. In addition, the crystallographic data (excluding structure factors) for the structures in this report have been deposited with the Cambridge Crysallographic Data Centre as supplementary publication numbers 687205 (DMH-D-053), 222395 (XLi-093), and 222396 (DM-II-96). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK, (fax +44-(0)1223-336033 or email: deposit@ccdc.cam.ac.uk). Structures of all compounds found within Tables 5-9 are also contained within the supporting information under Appendix III.