Abstract
Infections with Opisthorchis viverrini, Clonorchis sinensis and Schistosoma haematobium are classified as Group 1 biological carcinogens: definitive causes of cancer. These worms are metazoan eukaryotes, unlike the other Group 1 carcinogens including human papilloma virus, hepatitis C virus, and Helicobacter pylori. By contrast, infections with phylogenetic relatives of these helminths, also trematodes of the phylum Platyhelminthes and major human pathogens, are not carcinogenic. These inconsistencies prompt several questions, including how might these infections cause cancer? And why is infection with only a few helminth species carcinogenic? Here we present an interpretation of mechanisms contributing to the carcinogenicity of these helminth infections, including roles for catechol estrogen- and oxysterol-metabolites of parasite origin as initiators of carcinogenesis.
Keywords: Infection-related cancer, helminth parasites, opisthorchiasis, urogenital schistosomiasis, cholangiocarcinoma, squamous cell cancer of the bladder, carcinogenesis, catechol estrogen quinone, oxysterol, depurinating DNA adduct
Infection with some helminth pathogens represents a biological carcinogen
More than 20% of cancers in the developing world are caused by infections. Whereas a dozen or so pathogens including human papilloma virus, Epstein-Barr virus and Helicobacter pylori are widely known as biological carcinogens, less appreciated is that infection with helminths (see Glossary) can cause malignancy [1–3]. The International Agency for Research on Cancer (IARC) recognizes as definitive causes of cancer, infection with the fish-borne trematodes (FZT) Opisthorchis viverrini and Clonorchis sinensis and the blood fluke Schistosoma haematobium. In addition to directly damaging development, health and prosperity of infected populations, infection with these parasites leads to cholangiocarcinoma (CCA) (bile duct cancer) and squamous cell carcinoma (SCC) of the urinary bladder, respectively [1]. Here we outline understanding of the mechanism of these helminth infection induced cancers. We outline recent findings based on which we hypothesize that reactive metabolites of oxysterol-like and estrogen-like precursors of helminth origin represent genotoxins that mutate genes of epithelial cells lining the biliary tract and urinary blabber. We speculate that these novel, helminth-derived metabolites initiate bile duct and squamous cell carcinoma of the bladder during opisthorchiasis and urogenital schistosomiasis.
Liver fluke infection-induced cholangiocarcinoma
The liver fluke O. viverrini is endemic in Thailand, Lao PDR, Vietnam and Cambodia, whereas C. sinensis is endemic in Vietnam, China, South Korea, North Korea and far-eastern Russia [1, 4–6]. Clonorchiasis, opisthorchiasis and related infections remain as substantial public health burdens in East Asia, Eurasia and central Europe where >40 million people are infected [1]. These diseases are spreading [7]. Opisthorchiasis results from ingestion of the metacercarial stage of O. viverrini encysted in undercooked, fresh water cyprinoid fish. Related opisthorchiid flukes, C. sinensis and Opisthorchis felineus, which like O. viverrini also are transmitted through consumption of undercooked cyprinoid fish, cause similar diseases [4, 8]. Following ingestion, the immature worms migrate from the duodenum into the biliary tract, where they mature into the adult worms within the lumen of the bile ducts and gall bladder. Parasite eggs are shed into the bile and pass out to the environment in the fecal stream. Freshwater snails ingest the eggs, after which the trematode transforms into the sporocysts, which in turn release aquatic cercariae that penetrate the flesh of fishes that are the second intermediate hosts in the fluke’s developmental cycle (Fig. 1).
Figure 1. Developmental cycle of the fish-borne liver fluke Opisthorchis viverrini.
Liver flukes are obligate parasites with a complex developmental cycle that includes three hosts, an aquatic snail and a fresh-water fish as intermediate hosts and the definitive human (or other fish-eating mammal) host. In the human, the hermaphroditic worms reside within the lumen of bile ducts in the liver, and reproduce sexually. Eggs released by the liver flukes pass with bile into the small intestine, from where they exit with the feces. Snails of the genus Bithynia ingest the eggs in situations where human waste from infected people is deposited into the freshwater environment. The parasite egg hatches in the digestive tract of the snail, the larval miracidium migrates to the hepatopancreas of the snail. Asexual reproduction through sporocyst and rediae stages follows, culminating in release from the snail of a motile aquatic larva termed a cercariae. The cercaria enters the water and seeks out a suitable species of fish of the family Cyprinidae where it encysts on the skin or muscles of the fish.
The activities of the parasite abrade the epithelial lining of the bile duct, leading to cholangitis, obstructive jaundice, hepatomegaly, biliary periductal fibrosis, cholecystitis and cholelithiasis [9, 10]. Of greater concern, chronic opisthorchiasis frequently leads to a major sub-type of liver cancer, cholangiocarcinoma (CCA) [1, 2]. CCA is an adenocarcinoma with a dismal prognosis. These tumors slowly spread along bile ducts with periductal and mass forming extensions. Prognosis is poor owing to the silent clinical character, difficulty in early diagnosis, and limited options for therapy in resource poor settings [11]. Surgical management is potentially curative, but is restricted to early-stage disease [12]. In Thailand and elsewhere in East Asia, infection with fish-borne liver flukes is the dominant risk factor for CCA. However, CCA has a worldwide distribution where risks factors include primary sclerosing cholangitis, choledochal cysts, hepatolithiasis and congenital bile duct anomalies [13]. All these factors cause chronic injury and inflammation of the biliary ducts, as does infection with O. viverrini or C. sinensis [6, 9, 14–17] (Table S1).
Urogenital schistosomiasis-induced squamous cell carcinoma
Three major species of schistosomes are the agents of human schistosomiasis - Schistosoma japonicum and S. mansoni cause intestinal schistosomiasis in East Asia, Africa, South America and the Caribbean while S. haematobium, which occurs through Africa and the Middle East, causes urogenital schistosomiasis (UGS). Historically considered restricted to the tropics and sub-tropics, suitable habitats for transmission of schistosomiasis have recently expanded into Western Europe [18]. Only S. haematobium has been classified as a Group 1 carcinogen [1]. Of the more 110 million cases of UGS in sub-Saharan Africa, 70 million are associated with hematuria, 18 million with major bladder wall pathology, and 10 million with hydronephrosis leading to kidney damage [19, 20]. The continuous deposition of eggs of S. haematobium in the bladder can lead to squamous cell carcinoma (SSC) [21, 22].
SCC is a poorly differentiated neuroendocrine neoplasm and the common form of bladder cancer where UGS is prevalent [23–25]. In contrast, urothelial cell carcinoma (UCC) that arises from the transitional epithelium lining of the bladder represents most bladder cancers in developing countries and regions not endemic for UGS. Schistosome eggs trapped in the bladder wall release metabolites, presumably to facilitate egress of these eggs to the urine and hence to the external environment. Nonetheless, the phenomenon leads to hematuria and to chronic inflammation, in turn increasing risk of urothelial hyperplasia, dysplasia and SCC [25]. UGS is a chronic infection, the adult, egg-producing schistosomes live for many years, re-infections frequently occur, and UGS induced-SCC often appears by the mid-decades of life [23].
Risk factors leading to CCA and SCC
Carcinogenesis is a complex process in which normal cell growth is modified as a result of the interaction of multiple factors. Current understanding of the mechanisms of carcinogenesis during infection with these neglected tropical diseases that lead to malignancy has been reviewed [1, 5, 6, 25–28]. In regions of high prevalence of opisthorchiasis, the risk factors for CCA are chronic inflammation and concomitant injury of the biliary epithelium as the consequence of persistent parasitism by the flukes [6, 9, 14–17]. Other factors likely are involved, including spillover effects from local and systemic chronic inflammation (ROS/ RNS, interleukin-6, etc.) directed against the worms, liver fluke-associated oxysterols that damage DNA [29, 30], secretion by the parasites of mitogens such as granulin, thioredoxin and other mediators conducive to establishment and maintenance of a tumorigenic microenvironment [6, 31], dietary nitrosamines, and interactions or changes in the gastro-intestinal and biliary microbiota. Other risk factors for CCA have been documented, including primary sclerosing cholangitis [12], inflammatory bowel disease [32], metabolic syndromes [33], hepatitis virus [34] and infection with species of Helicobacter [35]. The latter has attracted increasing interest [36–38], and the presence of H. pylori in the gut epithelium of O. viverrini supports the hypothesis that this liver fluke may be a reservoir of this carcinogenic bacterium. During establishment of infection, juvenile O. viverrini may vector species of Helicobacter, H. pylori and H. bilis, and other prokaryotes from the gastrointestinal tract or even from the external environment into the biliary tree [39]. These co-infections may be integral in the pathogenesis of liver fluke-induced hepatobiliary diseases including CCA [39](Fig. 2; Table S1).
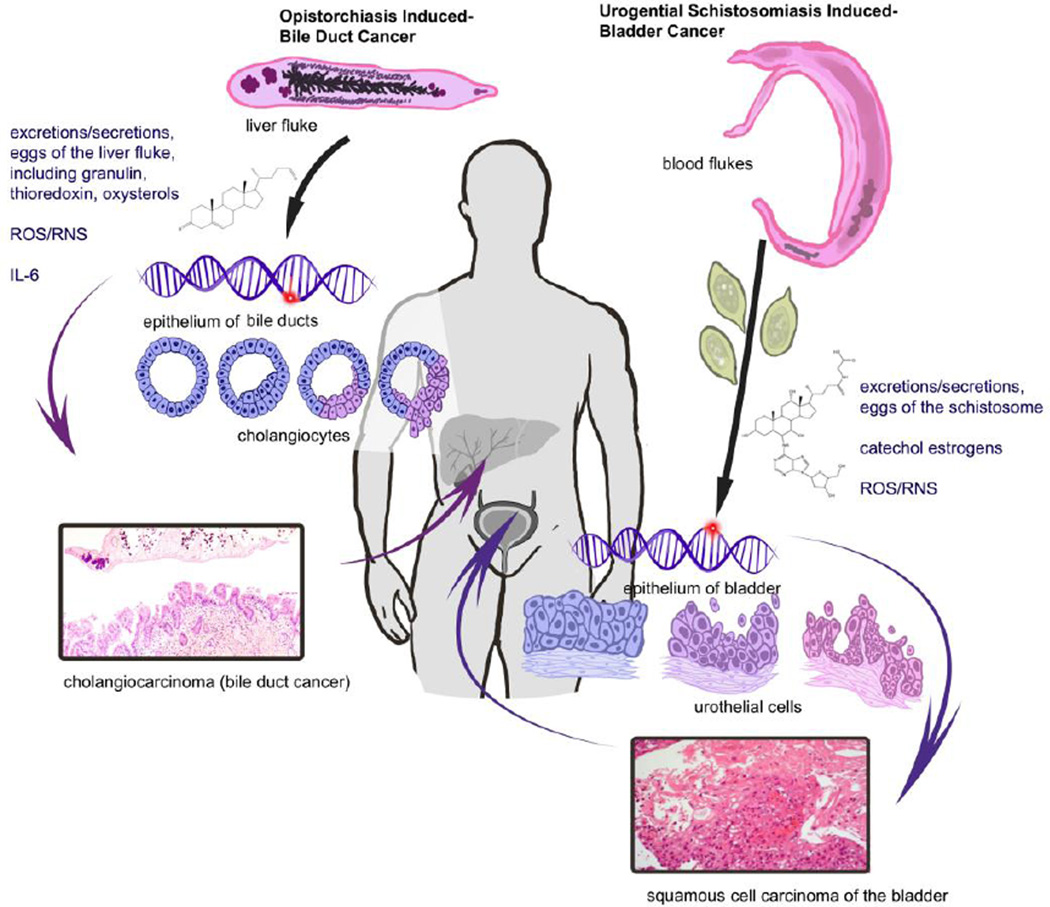
Figure 2.
Schematic representation of cancers of the biliary and bladder/urogenital tract resulting from infection with the liver fluke Opisthorchis viverrini and the blood fluke Schistosoma haematobium, respectively. The adult stage fluke of Opisthorchis viverrini, and small bile ducts lined with cholangiocytes are depicted on the left side of the diagram. This epithelium displays dysplasia during chronic opisthorchiasis and progression to frank malignancy. The right side of the illustration depicts the adult blood flukes and eggs of Schistosoma haematobium, and the urothelium (epithelium) lining the urinary bladder. Squamous cell carcinoma of the bladder arises from urothelial cells transformed during chronic urogenital schistosomiasis.
Multistep and multifactorial models for UGS-induced bladder cancer have been proposed [25]. Some models attribute the initiation of carcinogenesis to low doses of nitrosamines or other environmental carcinogens, with the infection supplying the proliferative stimulus to drive the expansion of clones of initiated cells. Although studies on UGS in laboratory animals are challenging, early findings supported this model [40]. In particular, whereas proliferative and inflammatory changes occurred in baboons without exposure to carcinogen, treatment with nitrosamines was necessary before appearance of neoplasia. Low doses of nitrosamine alone failed to produce changes in the tissue. Yet the mechanism by which the schistosome infection contributes to carcinogenesis remains unresolved, along with whether the inflammation itself is critical or whether UGS influences cell proliferation [41]. Exposure of Chinese hamster ovary (CHO) cells to secretions and lysates of eggs and other stages of S. haematobium stimulates cellular proliferation, migration and invasion, inhibits apoptosis, up-regulates expression of Bcl-2, and facilitates loss of p27 [42–48] - processes that are hallmarks of tumorigenesis and cancer cell survival [49]. Also, intravesical administration to mice of S. haematobium extracts induces urothelial dysplasia, a non-invasive malignant flat lesion [45]. These reports indicate that infection with S. haematobium induces malignization of the urothelium in the absence of co-factors such as nitrosamines. The risk of SCC during UGS appears to be promoted by concurrent risks for bladder cancer at large, including exposure to industrial and agricultural dyes, tobacco smoke, and vitamin A deficiency [23, 25]. In addition to these other potential risks, we have hypothesized including roles for catechol estrogen- and oxysterol-metabolites of parasite origin as initiators of carcinogenesis [50].
Several mechanisms explain the role of estrogens in disease. The better-known hypothesis is that the estrogen receptor mediates cell proliferation, increasing errors in DNA replication [48, 51, 52]. Another interpretation postulates that electrophilic metabolites of estrogen react covalently with DNA bases by redox cycling or by forming an abasic site. Subsequent error-prone repair of the modified DNA generates oncogenic mutations [51, 53–55]. Methylation of catechol estrogens by catechol-O-methyltransferase, conjugation of the catechol estrogen quinones with glutathione, and enzymatic reduction to reform catechol estrogens are processes that prevent accumulation of the highly reactive metabolites. However, if the latter protective processes are insufficient, catechol-estrogen quinones accumulate, potentially damaging DNA either by oxidation or depurination, and release of catechol-estrogen modified purines [51, 55, 56] (Fig. 3).
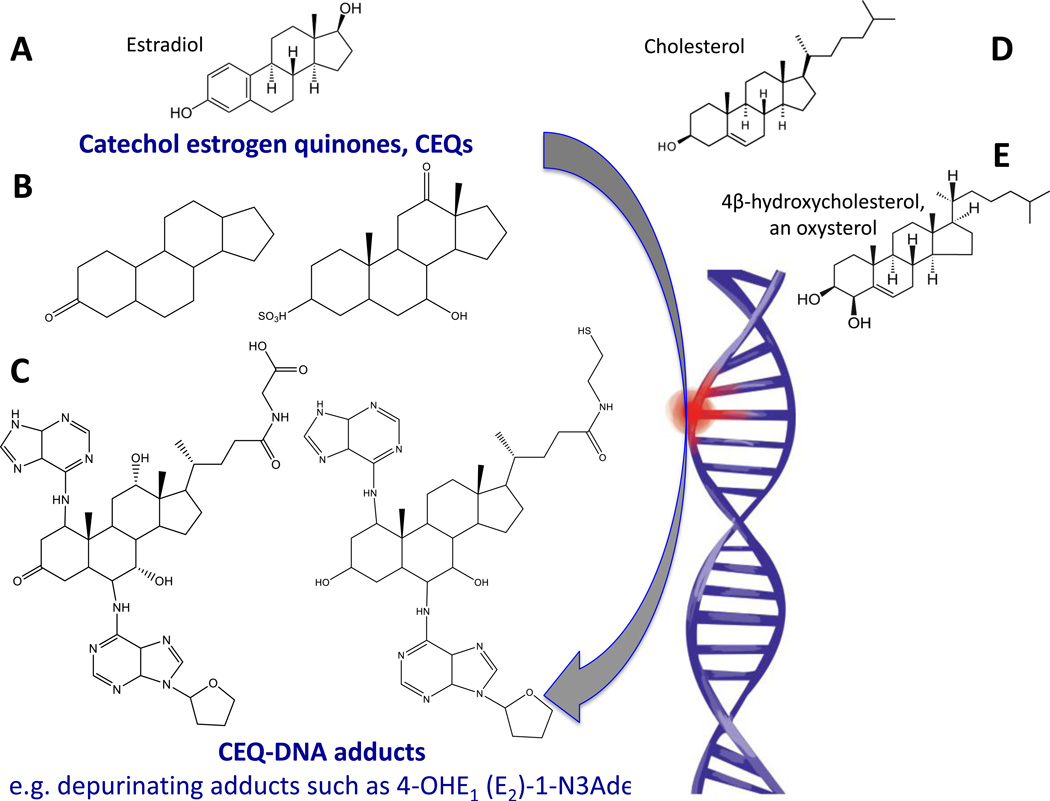
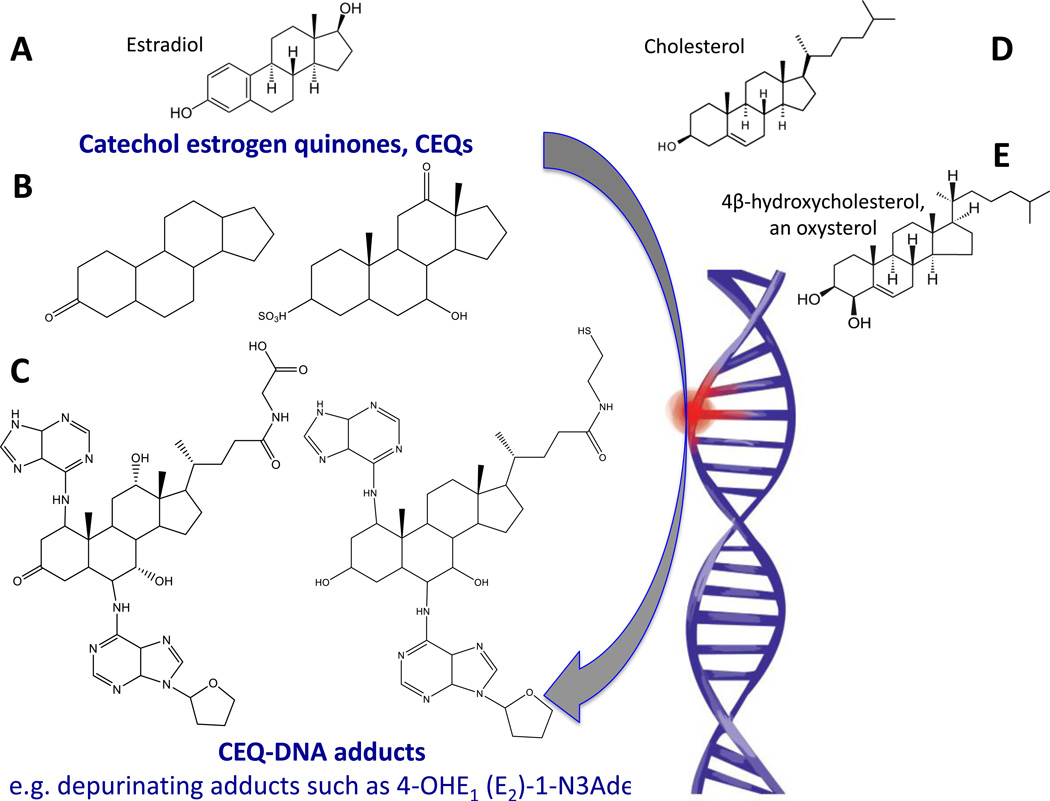
Figure 3.
Schematic representation to explain the appearance DNA adducts recovered from schistosomes or liver flukes and helminth-infected people. In steroidogenesis, cholesterol is the protypical animal setrol precursor for estradiol, the primary female sex hormone. Also, oxysterols are oxidation products of cholesterol. Structures A, D and E depict estradiol (A), cholesterol (D), and 4β-hydroxycholesterol, an oxysterol (E). Examples of potentially reactive catechol estrogen quinones are shown in B. Structure C presents examples of catechol estrogen quinone-DNA adducts from urine during urogenital schistosomiasis [48, 59]. Hydroxylation of estrogens forms the 2- and 4-catechol estrogens, which can be further oxidized to semiquinones and quinones, including the catechol estrogen-3,4-quinones, the major carcinogenic metabolites of estrogens [53]. These kinds of electrophiles can react with DNA to produce depurinating adducts such as those shown n panel E that, in turn, can lead to mutations and cancer initiation [53].
Catechol estrogens and oxysterols from helminth parasites as initiators of cancer
Onset of malignancy involves distinct initial and sequential effects on the target cells: initiation, promotion, and progression. The first involves damage to DNA, leading to mutation in a somatic cell. An essential feature of promotion is the induction of proliferation in the initiated cells by agents that are not necessarily carcinogenic. Both liver fluke induced-CCA and UGS-SCC proceed through initiation, promotion and progression, and otherwise display the ‘hallmarks of cancer’ [23, 25, 57]. Cholesterol, oxysterols and estrogens are closely related steroids [58]. Oxysterols and catechol-estrogens occur in both O. viverrini and S. haematobium [29, 30, 47, 59]. Formation of these metabolites might relate to the physiology of the worms and/or parasite-host interactions implicated in metabolic pathways of steroid hormones and bile acids. Oxysterols and/or catechol estrogens of trematode origin and/or precursors modified as the consequence of opisthorchiasis or UGS [29, 30, 48, 59, 60] are candidate initiators given these kinds of metabolites mutate genes in other settings [61] (Table 1; below). Further, given that initiators of cancer and/or promoters may need to be metabolized for activation (below), the initiators and/or cancer promoting factors may be specific for organ systems, tissues and/or epithelia [1, 62].
Elevation in levels of the steroid hormone estradiol in sera but not luteinizing hormone or testosterone occurs during UGS [43]. Schistosomes produce estrogen-related metabolites which might contribute to the elevated estradiol levels [44]. The schistosome genome encodes an orthologue of estradiol 17β dehydrogenase that participates in the synthesis pathway of estradiol [63], and it has been suggested that species-specific spatial and temporal expression associated with the synthesis of estradiol-like metabolites of S. haematobium and the parasite eggs in the bladder wall may contribute to carcinogenesis [63]. Estradiol-related metabolites including catechol estrogen quinones (CEQ) and CEQ-DNA adducts have been characterized from sera of UGS cases and, revealingly, within the schistosomes including the eggs [48, 59, 64]. Catechol estrogens are formed by hydroxylation on the steroid aromatic ring A. Mass spectrometric analysis of urine during UGS revealed that hydroxylation of both C-2 and C-3 on a steroid ring occurs with subsequent oxidation to an estradiol-2, 3-quinone [48, 50, 59, 64]. Estrogenic metabolites of schistosome origin passed in urine during UGS appear to arise by reaction of quinones of catechol estrogens with chromosomal DNA [42, 48, 59], which supports a role for these reactive metabolites of estrogens as the mutagens that initiate UGS-induced SCC [50](Fig. 3).
Notably, metabolites of estrogen including catechol-estrogens and also oxysterols have been characterized in O. viverrini liver flukes from experimentally infected hamsters using by liquid chromatography-mass spectrometry Many of the structures are ramified at C-17, i.e. they display discrete and variable chains linked to carbon 17 of the steroid ring [30](Fig. 3; and below). As with catechol-estrogen quinones, oxysterols are potentially mutagenic and genotoxic, and also display pro-oxidative and pro-inflammation activity that promotes carcinogenesis [65, 66]. Oxysterols are products of oxidation of cholesterol by either P450 enzymes or non-enzymatic processes. Effects of individual oxysterol species can be anticipated to be structure-dependent, with the metabolic conversions releasing biologically active and inactive forms. Draft genomes and transcriptomes of C. sinensis and O. viverrini are available [67, 68]. Although these parasites cannot synthesize cholesterol de novo, relying on the host for this key nutrient, the liver flukes evolved metabolic pathways highly adapted to a lipid-rich diet from bile and/or cholangiocytes. These pathways exhibit the capacity to liberate reactive steroid metabolites. Indeed, studies in hamsters revealed that the oxysterols, cholestan-3β, 5α, 6β-triol and 3 keto-cholest-4-ene, occur at elevated levels in the livers with O. viverrini-induced cholangiocarcinoma, and induce DNA adduct formation in cholangiocytes in vitro [29, 65]. Carcinogenesis-associated steroids occur in the adult developmental stage of O. viverrini within the biliary tract of the host [30]. As with UGS, we hypothesize a role for these reactive metabolites of cholesterol and estrogen as the mutagens that contribute to liver-fluke infection induced CCA (Fig. 3).
Organotropism and cancer
Why some trematodes are carcinogenic and some are not and the explanations of why that is are intriguing questions, for which firm answers have yet to be established (Outstanding Questions). Organ site preference by the helminths and their eggs may be part of it. Other FZT (Glossary), close relatives of O. viverrini and C. sinensis including O. felineus and species of Metorchis, also reside in the human biliary tree [7]. Whereas there is insufficient evidence to date to link these latter FZT to malignancy, some also are categorized as potential biological carcinogens [1]. By contrast, close phylogenetic relatives including the minute intestinal flukes such as Haplorchis taichui, which reside in the human intestines, do not cause cancer [69, 70]. Similarly, infection with the better-known liver flukes Fasciola hepatica and F. gigantica does not appear to cause liver cancer [71]. Notably, the hepato-intestinal schistosomes of humans, S. japonicum and S. mansoni, do not cause cancer. Accordingly, the preference of O. viverrini for residence in the bile ducts and of S. haematobium for the bladder, and of related flukes for other organ systems, may underlie the fluke species-specific carcinogenicity (Fig. 2). In turn, this may (at least partly) explain the general absence of carcinogenicity in closely related trematode pathogens including species of Schistosoma and Fasciola.
Mutational landscape of genomes of helminth infection-induced cancers
Information is accumulating on the genetic and cytogenetic profiles of both CCA and SCC of the bladder. The mutation profiles of opisthorchiasis-induced and non-opisthorchiasis-related CCA are discrete. A series of highly recurrent mutations occur in genes including those encoding TP53, KRAS, SMAD4, BRAF, MLL3, ARID1A, PBRM1 and BAP1, involved in cell cycle control, cell signaling pathways and chromatin dynamics [60]. Intrahepatic O. viverrini-induced and other CCA display marked differences in mutation patterns: BAP1, IDH1 and IDH2 (isocitrate dehydrogenase 1 and 2 genes) are more frequently mutated in non-O. viverrini CCA, whereas mutations of TP53 show the reciprocal pattern. Functional studies demonstrated tumor suppressive functions for BAP1 and ARID1A, establishing the role of chromatin modulators in CCA pathogenesis. Accordingly, different causative etiologies induce distinct somatic alterations, even within the same tumor type [72–74]. Liver fluke infection-induced CCA also has altered DNA methylation and transcriptional profiles associated with xenobiotic metabolism and pro-inflammatory responses in comparison to non-liver fluke induced CAA and to healthy tissues, indicative of distinctive pathobiology in O. viverrini-induced bile duct cancer [60, 74, 75]. These mutational profiles have implications for diagnosis, therapy and public health interventions.
In like fashion, substantial details also have emerged from whole exome sequences of urothelial carcinomas (UCC) of the bladder [76]. Recurrent mutations occur in more than 30 genes, including genes involved in cell-cycle regulation, chromatin regulation, and kinase signaling pathways such as MLL2, CDKN1A, ERCC2, STAG2 and RXRA [77]. However, much less is known on the mutational landscape of UGS-induced SCC [1, 23], for which similar studies have yet to be reported.
Concluding remarks
Multiple factors likely contribute the carcinogenesis of these helminth-induced cancers. As sterol derivatives, catechol estrogens and oxysterols share a core cyclopentanophenanthrene-4-ring, and the ability to oxidize DNA and other macromolecules. Elevated levels of these metabolites occur in the helminths themselves and in the infected people; and infected cases excrete metabolites of DNA-estrogen adducts and oxysterol-adducts [29, 59, 65]. Moreover, elevated levels of oxidized DNA (8-nitroguanine and 8-oxo-2'-deoxyguanosine) during chronic opisthorchiasis and UGS perhaps reflect carcinogenic process initiated by catechol estrogens and oxysterols [44, 50, 54, 55, 59, 61]. The roles of these and other novel trematode metabolites in CCA and SCC (Figs. 2, 3; Table S1) remain to be examined in depth. How and why these specific helminth pathogens induce cancer is worthy of deeper investigation, not the least because these phenomena offer the promise of novel interventions including vaccines against the parasites (Outstanding Questions). Notwithstanding that these helminth infection-induced cancers are probably preventable by behavioral changes, it also is worthwhile noting that vaccines that could block infection with these carcinogenic helminths would, in turn, represent anti-cancer vaccines, in the same fashion that vaccination against papilloma viruses blocks cervical cancer [30, 48, 78].
Supplementary Material
Acknowledgements
The authors thank our colleagues at the Pita Public Clinic, the urological services of Américo Boavida Hospital and Clínica Sagrada Esperança, Faculty of Medicine of Agostinho Neto University (Angola), the Instituto Português de Oncologia, Porto, and University of Porto. We thank Meredith E. Brindley for expert artwork, Victoria Mann for proofreading, and the editors and reviewers at Trends in Cancer for advice to improve this article. We acknowledge support from the Fundação para a Ciência e Tecnologia (FCT, Portugal) and the FEDER (European Union). PJB and BS also were supported by from awards R01CA155297 and R01CA164719 from the National Cancer Institute (NCI) and P50AI098639 from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI, NIAID or NIH.
Glossary
- Blood flukes
The schistosomes; trematodes that live and reproduce in the human circulatory system. The three major species parasitizing humans are Schistosoma haematobium, S. japonicum, and S. mansoni. These infections cause major neglected tropical diseases.
- Catechol estrogen quinones
electrophilic metabolites of estrogen that can react with DNA covalently to form depurinating adducts.
- Cholangiocarcinoma (CCA)
Bile duct cancer; an adenocarcinoma arising from the epithelium of the bile ducts. Prevalence of CCA is notably elevated in regions endemic for fish-borne liver fluke infections.
- Cholangiocyte
Epithelial cells that line the biliary tract.
- Fish-borne trematodes (FZT)
Fish-borne trematodes including the families Opisthorchiidae and Heterophyidae.
- Helminth parasites
Parasitic worms. Metazoan parasites from the phyla Platyhelminthes (flatworms) and Nematoda (roundworms). Helminth infections have been termed the great neglected tropical diseases of humanity.
- Liver flukes
Trematodes that reside and reproduce within the liver, including the biliary tree; species known to parasitize humans include Opisthorchis viverrini, O. felineus, Clonorchis sinensis, Fasciola gigantica, F. hepatica, and Dicrocoelium dendriticum.
- Oxysterols
oxidized derivatives of cholesterol
- Squamous cell carcinoma (SCC) of the urinary bladder
A malignancy arising from the urothelium of the bladder. A common form of bladder cancer in regions where urogenital schistosomiasis is prevalent.
- Trematoda
A class of the phylum Platyhelminthes; the flukes. All trematodes are parasites.
- Urogenital schistosomiasis (UGS)
Infection with Schistosoma haematobium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.IARC. IARC monographs on the evaluation of carcinogenic risks to humans. Pt B. Vol. 100. World Health Organization, International Agency for Research on Cancer; 2012. Biological agents. Volume 100 B. A review of human carcinogens; pp. 1–441. [Google Scholar]
- 2.de Martel C, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. The lancet oncology. 2012;13(6):607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 3.Pagano JS, et al. Infectious agents and cancer: criteria for a causal relation. Semin Cancer Biol. 2004;14(6):453–471. doi: 10.1016/j.semcancer.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Petney TN, et al. The zoonotic, fish-borne liver flukes Clonorchis sinensis, Opisthorchis felineus and Opisthorchis viverrini. Int J Parasitol. 2013;43(12–13):1031–1046. doi: 10.1016/j.ijpara.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Sithithaworn P, et al. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitology international. 2012;61(1):10–16. doi: 10.1016/j.parint.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sripa B, et al. The tumorigenic liver fluke Opisthorchis viverrini--multiple pathways to cancer. Trends in parasitology. 2012;28(10):395–407. doi: 10.1016/j.pt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mordvinov VA, et al. Opisthorchis felineus and Metorchis bilis are the main agents of liver fluke infection of humans in Russia. Parasitol Int. 2012;61(1):25–31. doi: 10.1016/j.parint.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 8.Ogorodova LM, et al. Opisthorchiasis: an overlooked danger. PLoS Negl Trop Dis. 2015;9(4):e0003563. doi: 10.1371/journal.pntd.0003563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blechacz B, et al. Clinical diagnosis and staging of cholangiocarcinoma. Nature reviews. Gastroenterology & hepatology. 2011;8(9):512–522. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mairiang E, et al. Ultrasonography assessment of hepatobiliary abnormalities in 3359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand, in Parasitology international. 2012:208–211. doi: 10.1016/j.parint.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thunyaharn N, et al. Survival of cholangiocarcinoma patients in northeastern Thailand after supportive treatment. Asian Pac J Cancer Prev. 2013;14(11):7029–7032. doi: 10.7314/apjcp.2012.14.11.7029. [DOI] [PubMed] [Google Scholar]
- 12.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torre LA, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 14.Johnson C, et al. Interleukin-6 and its receptor, key p layers in hepatobiliary inflammation and cancer. Translational gastrointestinal cancer. 2012;1(1):58–70. doi: 10.3978/j.issn.2224-4778.2011.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Hara SP, et al. The dynamic biliary epithelia: Molecules, pathways, and disease. Journal of hepatology. 2013;58(3):575–582. doi: 10.1016/j.jhep.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11(1):13–21 e1. doi: 10.1016/j.cgh.2012.09.009. quiz e3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sripa B, et al. Advanced periductal fibrosis from infection with the carcinogenic human liver fluke Opisthorchis viverrini correlates with elevated levels of interleukin-6. Hepatology. 2009;50(4):1273–1281. doi: 10.1002/hep.23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holtfreter MC, et al. Schistosoma haematobium infections acquired in Corsica, France, August 2013. Euro Surveill. 2014;19(22) doi: 10.2807/1560-7917.es2014.19.22.20821. [DOI] [PubMed] [Google Scholar]
- 19.van der Werf MJ, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta tropica. 2003;86(2–3):125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 20.King CH. Parasites and poverty: the case of schistosomiasis. Acta tropica. 2010;113(2):95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodder SL, et al. Predisposition to urinary tract epithelial metaplasia in Schistosoma haematobium infection. The American journal of tropical medicine and hygiene. 2000;63(3–4):133–138. doi: 10.4269/ajtmh.2000.63.133. [DOI] [PubMed] [Google Scholar]
- 22.Parkin DM. The global health burden of infection-associated cancers in the year 2002. International journal of cancer. Journal international du cancer. 2006;118(12):3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 23.Mostafa MH, Sheweita SA, O'Connor PJ. Relationship between schistosomiasis and bladder cancer. Clinical microbiology reviews. 1999;12(1):97–111. doi: 10.1128/cmr.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong X, et al. Hypermethylation of genes detected in urine from Ghanaian adults with bladder pathology associated with Schistosoma haematobium infection. PLoS One. 2013;8(3):e59089. doi: 10.1371/journal.pone.0059089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honeycutt J, et al. Controversies and challenges in research on urogenital schistosomiasis-associated bladder cancer. Trends in parasitology. 2014;30(7):324–332. doi: 10.1016/j.pt.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oikonomopoulou K, et al. The bifacial role of helminths in cancer: involvement of immune and non-immune mechanisms. Crit Rev Clin Lab Sci. 2014;51(3):138–148. doi: 10.3109/10408363.2014.886180. [DOI] [PubMed] [Google Scholar]
- 27.Vennervald BJ, Polman K. Helminths and malignancy. Parasite Immunol. 2009;31(11):686–696. doi: 10.1111/j.1365-3024.2009.01163.x. [DOI] [PubMed] [Google Scholar]
- 28.Fried B, Reddy A, Mayer D. Helminths in human carcinogenesis. Cancer Lett. 2011;305(2):239–249. doi: 10.1016/j.canlet.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Jusakul A, et al. Liver fluke-induced hepatic oxysterols stimulate DNA damage and apoptosis in cultured human cholangiocytes. Mutat Res. 2012;731(1–2):48–57. doi: 10.1016/j.mrfmmm.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Vale N, et al. Carcinogenic liver fluke Opisthorchis viverrini oxysterols detected by LC-MS/MS survey of soluble fraction parasite extract. Parasitology international. 2013;62(6):535–542. doi: 10.1016/j.parint.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satarug S, et al. Relationships between the synthesis of N-nitrosodimethylamine and immune responses to chronic infection with the carcinogenic parasite, Opisthorchis viverrini, in men. Carcinogenesis. 1998;19(3):485–491. doi: 10.1093/carcin/19.3.485. [DOI] [PubMed] [Google Scholar]
- 32.Huai JP, et al. Inflammatory bowel disease and risk of cholangiocarcinoma: e vidence from a meta-analysis of population-based studies. Asian Pac J Cancer Prev. 2014;15(8):3477–3482. doi: 10.7314/apjcp.2014.15.8.3477. [DOI] [PubMed] [Google Scholar]
- 33.Wu Q, et al. The metabolic syndrome and risk factors for biliary tract cancer: a case521 control study in China. Asian Pac J Cancer Prev. 2012;13(5):1963–1969. doi: 10.7314/apjcp.2012.13.5.1963. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto K, et al. Hepatitis B and C virus infection is a risk factor for the development of cholangiocarcinoma. Intern Med. 2014;53(7):651–654. doi: 10.2169/internalmedicine.53.1410. [DOI] [PubMed] [Google Scholar]
- 35.Zhou D, et al. Infections of Helicobacter spp. in the biliary system are associated with biliary tract cancer: a meta-analysis. Eur J Gastroenterol Hepatol. 2013;25(4):447–454. doi: 10.1097/MEG.0b013e32835c0362. [DOI] [PubMed] [Google Scholar]
- 36.Mateos-Munoz B, et al. Enterohepatic Helicobacter other than Helicobacter pylori. Rev Esp Enferm Dig. 2013;105(8):477–484. doi: 10.4321/s1130-01082013000800006. [DOI] [PubMed] [Google Scholar]
- 37.Murphy G, et al. Association of seropositivity to Helicobacter species and biliary tract cancer in the ATBC study. Hepatology. 2014;60(6):1963–1971. doi: 10.1002/hep.27193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deenonpoe R, et al. The carcinogenic liver fluke Opisthorchis viverrini is a reservoir for species of Helicobacter. Asian Pac J Cancer Prev. 2015;16(5):1751–1758. doi: 10.7314/apjcp.2015.16.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plieskatt JL, et al. Infection with the carcinogenic liver fluke Opisthorchis viverrini modifies intestinal and biliary microbiome. FASEB J. 2013;27(11):4572–4584. doi: 10.1096/fj.13-232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hicks RM, James C, Webbe G. Effect of Schistosoma haematobium and N-butyl-N-(4-hydroxybutyl)nitrosamine on the development of urothelial neoplasia in the baboon. Br J Cancer. 1980;42(5):730–755. doi: 10.1038/bjc.1980.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosin MP, Anwar WA, Ward AJ. Inflammation, chromosomal instability, and cancer: the schistosomiasis model. Cancer Res. 1994;54(7 Suppl):1929s–1933s. [PubMed] [Google Scholar]
- 42.Botelho M, et al. Schistosoma haematobium total antigen induces increased proliferation, migration and invasion, and decreases apoptosis of normal epithelial cells. International journal for parasitology. 2009;39(10):1083–1091. doi: 10.1016/j.ijpara.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 43.Botelho MC, et al. Schistosoma haematobium and Schistosomiasis mansoni: production of an estradiol-related compound detected by ELISA. Experimental parasitology. 2009;122(3):250–253. doi: 10.1016/j.exppara.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Botelho MC, et al. Targeting molecular signaling pathways of Schistosoma haemotobium infection in bladder cancer. Virulence. 2011;2(4):267–279. doi: 10.4161/viru.2.4.16734. [DOI] [PubMed] [Google Scholar]
- 45.Botelho MC, et al. Urothelial dysplasia and inflammation induced by Schistosoma haematobium total antigen instillation in mice normal urothelium. Urol Oncol. 2011;29(6):809–814. doi: 10.1016/j.urolonc.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Botelho MC, et al. Urothelial dysplasia and inflammation induced by Schistosoma haematobium total antigen instillation in mice normal urothelium. Urologic oncology. 2009 doi: 10.1016/j.urolonc.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 47.Botelho MC, et al. Schistosoma haematobium: identification of new estrogenic molecules with estradiol antagonistic activity and ability to inactivate estrogen receptor in mammalian cells. Experimental parasitology. 2010;126(4):526–535. doi: 10.1016/j.exppara.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 48.Botelho MC, et al. Tumour-like phenotypes in urothelial cells after exposure to antigens from eggs of Schistosoma haematobium: an oestrogen-DNA adducts mediated pathway? International journal for parasitology. 2013;43(1):17–26. doi: 10.1016/j.ijpara.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 49.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 50.Correia da Costa JM, et al. Schistosome and liver fluke derived catechol-estrogens and helminth associated cancers. Front Genet. 2014;5:444. doi: 10.3389/fgene.2014.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. The New England journal of medicine. 2006;354(3):270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 52.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344(4):276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 53.Cavalieri E, Rogan E. The molecular etiology and prevention of estrogen-initiated cancers: Ockham's Razor: Pluralitas non est ponenda sine necessitate. Plurality should not be posited without necessity. Mol Aspects Med. 2014;36:1–55. doi: 10.1016/j.mam.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cavalieri EL, Rogan EG. Unbalanced metabolism of endogenous estrogens in the etiology and prevention of human cancer. The Journal of steroid biochemistry and molecular biology. 2011;125(3–5):169–180. doi: 10.1016/j.jsbmb.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cavalieri EL, et al. Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(20):10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liehr JG, et al. Carcinogenicity of catechol estrogens in Syrian hamsters. J Steroid Biochem. 1986;24(1):353–356. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- 57.Mesri EA, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host Microbe. 2014;15(3):266–282. doi: 10.1016/j.chom.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shackleton CH. Role of a disordered steroid metabolome in the elucidation of sterol and steroid biosynthesis. Lipids. 2012;47(1):1–12. doi: 10.1007/s11745-011-3605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gouveia MJ, et al. Estrogen-like metabolites and DNA-adducts in urogenital schistosomiasis-associated bladder cancer. Cancer Lett. 2015;359(2):226–232. doi: 10.1016/j.canlet.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 60.Jusakul A, Kongpetch S, Teh BT. Genetics of Opisthorchis viverrini-related cholangiocarcinoma. Curr Opin Gastroenterol. 2015;31(3):258–263. doi: 10.1097/MOG.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 61.Cavalieri E, Rogan E. The molecular etiology and prevention of estrogen-initiated cancers: Ockham's Razor: Pluralitas non est ponenda sine necessitate. Plurality should not be posited without necessity. Molecular aspects of medicine. 2014;36:1–55. doi: 10.1016/j.mam.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pitot HC, Goldsworthy T, Moran S. The natural history of carcinogenesis: implications of experimental carcinogenesis in the genesis of human cancer. J Supramol Struct Cell Biochem. 1981;17(2):133–146. doi: 10.1002/jsscb.380170204. [DOI] [PubMed] [Google Scholar]
- 63.Young ND, et al. Whole-genome sequence of Schistosoma haematobium. Nat Genet. 2012;44(2):221–225. doi: 10.1038/ng.1065. [DOI] [PubMed] [Google Scholar]
- 64.Gouveia MJ, et al. Mass spectrometry techniques in the survey of steroid metabolites as potential disease biomarkers: a review. Metabolism: clinical and experimental. 2013;62(9):1206–1217. doi: 10.1016/j.metabol.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuver R. Mechanisms of oxysterol-induced disease: insights from the biliary system. Clin Lipidol. 2012;7(5):537–548. doi: 10.2217/clp.12.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jusakul A, et al. Mechanisms of oxysterol-induced carcinogenesis. Lipids Health Dis. 2011;10:44. doi: 10.1186/1476-511X-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang Y, et al. The carcinogenic liver fluke, Clonorchis sinensis: new assembly, reannotation and analysis of the genome and characterization of tissue transcriptomes. PLoS One. 2013;8(1):e54732. doi: 10.1371/journal.pone.0054732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Young ND, et al. The Opisthorchis viverrini genome provides insights into life in the bile duct. Nat Commun. 2014;5:4378. doi: 10.1038/ncomms5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sripa B, et al. Food-borne trematodiases in Southeast Asia epidemiology, pathology, clinical manifestation and control. Adv Parasitol. 2010;72:305–350. doi: 10.1016/S0065-308X(10)72011-X. [DOI] [PubMed] [Google Scholar]
- 70.Sohn WM, et al. Prevalence of Haplorchis taichui among humans and fish in Luang Prabang Province, Lao PDR. Acta Trop. 2014;136:74–80. doi: 10.1016/j.actatropica.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 71.Mas-Coma S, Valero MA, Bargues MD. Fascioliasis. Adv Exp Med Biol. 2014;766:77–114. doi: 10.1007/978-1-4939-0915-5_4. [DOI] [PubMed] [Google Scholar]
- 72.Jiao Y, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45(12):1470–1473. doi: 10.1038/ng.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kongpetch S, et al. Pathogenesis of cholangiocarcinoma: From genetics to signalling pathways. Best Pract Res Clin Gastroenterol. 2015;29(2):233–244. doi: 10.1016/j.bpg.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 74.Chan-On W, et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat Genet. 2013;45(12):1474–1478. doi: 10.1038/ng.2806. [DOI] [PubMed] [Google Scholar]
- 75.Andresen K, et al. Four DNA methylation biomarkers in biliary brush samples accurately identify the presence of cholangiocarcinoma. Hepatology. 2015;61(5):1651–1659. doi: 10.1002/hep.27707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo G, et al. Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation. Nat Genet. 2013;45(12):1459–1463. doi: 10.1038/ng.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cancer Genome Atlas Research, N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cuzick J. Gardasil 9 joins the fight against cervix cancer. Expert Rev Vaccines. 2015;14(8):1047–1049. doi: 10.1586/14760584.2015.1051470. [DOI] [PubMed] [Google Scholar]
- 79.Smout MJ, et al. A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini, promotes proliferation of host cells. PLoS Pathog. 2009;5(10):e1000611. doi: 10.1371/journal.ppat.1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mulvenna J, et al. The secreted and surface proteomes of the adult stage of the carcinogenic human liver fluke Opisthorchis viverrini. Proteomics. 2010;10(5):1063–1078. doi: 10.1002/pmic.200900393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matchimakul P, et al. Apoptosis of cholangiocytes modulated by thioredoxin of carcinogenic liver fluke. Int J Biochem Cell Biol. 2015 doi: 10.1016/j.biocel.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hsieh YJ, Fu CL, Hsieh MH. Helminth-induced interleukin-4 abrogates invariant natural killer T cell activation-associated clearance of bacterial infection. Infect Immun. 2014;82(5):2087–2097. doi: 10.1128/IAI.01578-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Antony JS, et al. Correlation of Interleukin-6 levels and lectins during Schistosoma haematobium infection. Cytokine. 2015 doi: 10.1016/j.cyto.2015.04.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.