Abstract
Carbohydrate-responsive element–binding protein (ChREBP) is a glucose-sensing transcription factor required for glucose-stimulated proliferation of pancreatic β-cells in rodents and humans. The full-length isoform (ChREBPα) has a low glucose inhibitory domain (LID) that restrains the transactivation domain when glucose catabolism is minimal. A novel isoform of ChREBP (ChREBPβ) was recently described that lacks the LID domain and is therefore constitutively and more potently active. ChREBPβ has not been described in β-cells nor has its role in glucose-stimulated proliferation been determined. We found that ChREBPβ is highly expressed in response to glucose, particularly with prolonged culture in hyperglycemic conditions. In addition, small interfering RNAs that knocked down ChREBPβ transcripts without affecting ChREBPα expression or activity decreased glucose-stimulated expression of carbohydrate response element–containing genes and glucose-stimulated proliferation in INS-1 cells and in isolated rat islets. Quantitative chromatin immunoprecipitation, electrophoretic mobility shift assays, and luciferase reporter assays were used to demonstrate that ChREBP binds to a newly identified powerful carbohydrate response element in β-cells and hepatocytes, distinct from that in differentiated 3T3-L1 adipocytes. We conclude that ChREBPβ contributes to glucose-stimulated gene expression and proliferation in β-cells, with recruitment of ChREBPα to tissue-specific elements of the ChREBPβ isoform promoter.
Introduction
Carbohydrate-responsive element–binding protein (ChREBP; gene name, MLXIPL) is a nutrient-sensing transcription factor that is activated by products of glucose catabolism (1). ChREBP is expressed in numerous tissues, and although it is clearly involved in lipogenesis in the liver and in adipocytes (2,3), its role in other tissues is less well understood. Changes in blood glucose levels are sensed in pancreatic β-cells by corresponding changes in the rate of cellular glucose metabolism, which drives the secretion of insulin to maintain glucose homeostasis (4). In addition, circulating glucose is a systemic regulator of β-cell mass, which expands in response to persistent hyperglycemia and increased “workload.” This proliferative process also requires β-cell–specific glucose catabolism (5). We recently found that ChREBP is expressed in pancreatic β-cells at levels comparable to the liver in rodents and humans and that ChREBP is required for glucose-stimulated β-cell proliferation (6).
ChREBP is a large transcription factor with an N-terminal glucose–sensing domain comprising conserved Mondo regions that can be broadly described as a low glucose inhibitory, or LID domain, that folds over and represses a glucose response activation conserved element (GRACE) domain (7,8). Glucose metabolism leads to molecular events, including nuclear localization, binding to carbohydrate response elements (ChoREs), and conformational changes in the LID and GRACE domains that allow interaction with coactivators and transactivation of glucose responsive genes. ChREBPα, the full-length form of ChREBP, is transcriptionally inactive in low glucose and exquisitely sensitive to increased glucose metabolism, making this factor a transcriptional sensor of glucose uptake and utilization (1).
Herman et al. (2) recently described ChREBPβ, a novel isoform of ChREBP produced from a newly identified exon (ChREBP exon 1b) transcription start site and promoter, resulting in an alternatively spliced mRNA with the first translational start site located in exon 4 rather than exon 1a. The resultant truncated ChREBPβ lacks the LID domain (also containing the nuclear export signals), generating a constitutively active, constitutively nuclear, and transcriptionally potent transcription factor. The expression of ChREBPβ is driven by a ChoRE, creating a feed-forward amplification of the glucose signal wherein activation of ChREBPα leads to the production of the more potent and constitutively active isoform. Expression of ChREBPβ correlates positively with adipocyte lipogenesis and insulin sensitivity. By contrast, its expression in liver correlates with hepatic insulin resistance and steatosis (2,9,10). Given that ChREBP is necessary for glucose-stimulated β-cell proliferation (6), here we sought to determine the role of ChREBPβ in β-cell gene expression and proliferation. We found that ChREBPβ is present in primary islet cells under basal conditions at much lower levels than ChREBPα but, nonetheless, contributes significantly to glucose-stimulated gene expression and β-cell proliferation and does so through recruitment to novel and powerful tissue-specific genomic elements.
Research Design and Methods
Cell Culture
INS-1–derived 832/13 rat insulinoma cells (provided by Dr. Christopher Newgard, Sarah W. Stedman Nutrition and Metabolism Center, Duke University, Durham, NC) were maintained as described previously (11). Murine 3T3-L1 preadipocytes were grown in DMEM containing 10% FBS. Cells were induced to differentiate using a standard induction protocol (12).
Isolation of Rodent Islets and Liver
Islets were isolated, dispersed, and cultured from Wistar rats (80–87 days old; Charles River Laboratories, Wilmington, MA), as described previously (13,14). Mouse liver was isolated, flash frozen, and powdered, as previously described (15). These animal studies were performed in compliance with and with the approval of the University of Pittsburgh and the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committees.
Human Islets
Human cadaveric islets were obtained through National Institute of Diabetes and Digestive and Kidney Diseases–supported Integrated Islet Distribution Program (https://iidp.coh.org). The donors were a mean age of 38, and average purity was 86%. Islets were cultured and dispersed by trypsinization, as described previously (16).
RNA Isolation and Quantitative RT-PCR Analysis
RNA (0.5–1 μg) was used for reverse transcription, and RT-PCR analysis was conducted using The iTaq Universal SYBR Green Supermix (Bio-Rad, Cat. #172-5124) on the 7500 Applied Biosystems Real-Time System. Primers are listed in Supplementary Table 1. The efficiency of primer pairs in the PCR was determined as described (17). RNA from human and rat islet cells was extracted using the RNeasy microRNA isolation kit (Qiagen, Cat. #74004).
Small Interfering RNA Duplex-Mediated Gene Suppression
Two small interfering (si)RNAs (Thermo Scientific) were designed to target the rat ChREBPβ coding region. siRNAs were transfected into 832/13 for 72 h in the presence of lipofectamine RNAiMAX (Life Technologies, Cat. #13778150) by reverse transfection. The sequences were: 01, 5′-CGAGGUCCCAGGAUCCAGUUU-3′, and 02, 5′-GUCCCAGGAUCCAGUCCGAUU-3′. The cells were treated with 2 mmol/L or 20 mmol/L glucose for 16 h, then RNA was extracted, and RT-PCR was performed. A duplex with no known sequence homology or biological effect (siControl) was used as a control (Cat. #D-001810–01).
The Accell siRNA treatment was as previously described (6). After incubation for 4 days, cells were fixed with 2% paraformaldehyde and stained with antibodies specific for Ki67 and insulin. Accell siRNA sequences were: 01, 5′-CGAGGUCCCAGGAUCCAGUUU-3′, 5′ACUGGAUCCUGGGACCUCGUU3′; 02, 5′GUCCCAGGAUCCAGUCCGAUU3′, 5′UCGGACUGGAUCCUGGGACUU3′.
Cell Proliferation Assay
INS-1–derived 832/13 cells grown in Nunc Lab-Tek II Chamber Slide System (Thermo Fisher, Cat. #154534) were transfected with ChREBPβ siRNA for 48 h. Cells were then cultured in 2 mmol/L or 20 mmol/L glucose for 16 h, and 100 μmol/L BrdU was added for 30 min. Cells were fixed with 2% paraformaldehyde, and treated with 1 N HCl for 30 min before treatment with 4% normal goat serum, 1% BSA, and 0.5% Triton for 2 h. Cells were incubated overnight with a BrdU antibody (Abcam, Cat. #ab6326) at 1:200. After washing with PBS twice, Alexa 594 anti-rat (1:10,000) was used as the second antibody. The slides were mounted with mounting medium containing DAPI (H-1500; Vector Laboratories, Inc.). To determine proliferation in dispersed rat islets, cells were stained for Ki67 using rabbit anti-Ki67 (Thermo Fisher Scientific, Cat. #9106). All images were acquired using a Zeiss Axioplan 2 at the Microscopy Shared Resource Facility of Icahn School of Medicine at Mount Sinai.
Electrophoretic Mobility Shift Assay
HeLa cells were transfected using Lipofectamine 2000 (Life Technologies) to overexpress Flag-tagged ChREBP and hemagglutinin (HA)-tagged Mlx, the obligate heterodimer binding partner for ChREBP (provided by Dr. Towle, University of Minnesota) (18), or HeLa cells transfected with empty vector control. Nuclear extracts were obtained using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific). Nuclear extracts (15 μg) were added to the electrophoretic mobility shift assay (EMSA) reaction buffer (20 mmol/L HEPES [pH 7.9], 5 mmol/L MgCl2, 0.5 mmol/L EDTA, 50 mmol/L KCl, 1 mmol/L dithiothreitol, 6.25% glycerol, 1 μg BSA, 2 μg salmon sperm DNA, 2 μg poly[deoxyinosinic-deoxycytidylic]). The E-box/ChoRE oligonucleotides were fluorescently labeled with 5′IRD700 (Integrated DNA Technologies), and these oligos or unlabeled oligos (“unlabeled probes”) were annealed for 5 min at 95°C. For antibody displacement experiments, nuclear extracts (15 μg) were preincubated with anti-Flag, anti-HA, or IgG antibodies (1:500) for 10 min at room temperature before the addition of labeled DNA probe. Oligonucleotide sequences can be found in Supplementary Table 2. Samples were loaded onto 4.5% TBE gels (Life Technologies) and scanned using the LI-COR laser-based image detection method.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) assays were performed as previously described (14,19). Cross-linked 832/13 or NIH 3T3-L1 chromatin (1 mg) was incubated overnight (18 h) at 4°C with 3 μg rabbit anti-ChREBP (ab157153; Abcam) antibody, anti-Myc antibody (N-262, sc-764; Santa Cruz Biotechnology), or normal rabbit IgG (Santa Cruz Biotechnology). Immune complexes were captured with 60 μL 50% protein A-Sepharose agarose slurry (Millipore #16-157). DNA-protein crosslinking was reversed by adding 50% Chelex beads and boiling 10 min. Quantitative PCR using SYBR Green was performed in 5 µL supernatant containing immunoprecipitated DNA fragments, as previously described (14,19).
For ChIP from frozen liver tissue, liver tissue was finely powdered and fixed with 1% formaldehyde. The powder was homogenized with a Dounce homogenizer with tight (A) pestle in a hypotonic solution (10 mmol/L HEPES [pH 7.9], 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.2% Nonidet P-40, 0.2 mmol/L sodium orthovanadate, 0.15 mmol/L spermine, 0.5 mmol/L spermidine, 1 mmol/L EDTA, 5% sucrose, 1 mmol/L dithiothreitol, and protease inhibitors) and layered onto a sucrose cushion buffer (10 mmol/L Tris/HCl [pH 7.5], 15 mmol/L NaCl, 60 mmol/L KCl, 0.15 mmol/L spermine, 0.5 mmol/L spermidine, 1 mmol/L EDTA, 10% sucrose, and protease inhibitors). Sucrose gradient centrifugation was performed for 1 h at 30,000 rpm. The pellet was washed once with ice-cold PBS and resuspended in SDS lysis buffer. The sample was then sonicated with a Bioruptor and processed as described above.
For rat islets, we used a micro-ChIP assay. Briefly, rat islets cultured with 20 mmol/L glucose culture medium for 2 days were crosslinked with 1% formaldehyde, followed by neutralization with 125 mmol/L glycine. After fixation, a ChIP assay was performed using the Thermo Scientific Pierce Magnetic ChIP Kit (Cat. #26157), according to the manufacturers’ instructions. Antibodies to ChREBP (Thermo Scientific, Cat. #PA5-22924) and IgG control antibody (Santa Cruz Biotechnology, Cat. #2027) were used to immunoprecipitate chromatin-bound supernatants. The PCR primers for amplification of ChIP products are listed in Supplementary Table 1.
Luciferase Assay
832/13 cells were transfected with ChREBPβ promoter luciferase wild-type, ChoRE, or E-box mutation vectors (2) by Lipofectamine 2000 (Life Technologies, Cat. #11668-019). Cells were harvested after 16 h, and luciferase activity was measured using the Luciferase Reporter Assay System (Promega, Cat. #E1500) on a MicroBeta2 LumiJET Microplate Counter (PerkinElmer). Firefly luciferase activity was normalized to protein concentration.
Statistical Analysis
Results are represented as means with SEM. Comparisons between two means were conducted using the unpaired Student t test. Comparisons between multiple groups were conducted using two-way ANOVA with the Tukey post hoc test. Statistical significance was set at P < 0.05.
Results
ChREBPβ Is Expressed in Rat and Human β-Cells
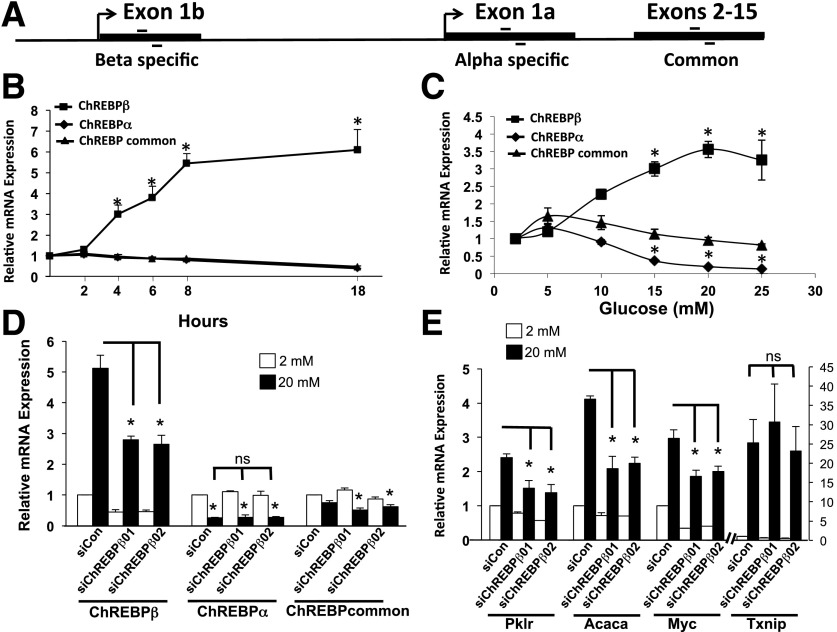
The ChREBPβ isoform was originally identified in adipose tissue and is also expressed in the liver (2). However, whether ChREBPβ is expressed or serves important functions in pancreatic β-cells has not been addressed. RT-PCR experiments were performed using oligonucleotide primers designed for ChREBP-specific sequences derived from exon 1b, exon 1a, or primers that recognize both (Fig. 1A and Supplementary Table 1). In INS-1–derived 832/13 cells, we found robust induction of ChREBPβ mRNA in response to glucose in a time- and dose-dependent manner (Fig. 1B and C). Using the ΔΔCT method and adjusting to primer efficiencies and normalizing to β-actin, we found that the expression of ChREBPβ was approximately eightfold lower than ChREBPα in low glucose (see Fig. 5 below). But after glucose treatment, ChREBPβ mRNA increased fourfold by 6 h, reaching sixfold by 24 h, a level that was greater than ChREBPα after the glucose treatment. As expected, the induction of ChREBPβ mRNA by glucose was consistent with its being regulated by glucokinase, because the half-maximal dose was near the S0.5 of glucokinase (20). Thus, the rate of glucose metabolism through glucokinase correlated with the stimulation of ChREBPβ mRNA. Remarkably, we found that glucose inhibited ChREBPα-specific transcripts, suggesting that transcription of exon 1b excludes the use of exon 1a in these cells (Fig. 1B–D).
Figure 1.
ChREBPβ is expressed in β-cells and is responsive to glucose. A: Diagram of the primers used in RT-PCR assays. Primers were designed to be specific for exon 1b, exon 1a, or for a region that is common to both isoforms. B: Time course of ChREBPβ expression in response to 20 mmol/L glucose. 832/13 INS-1–derived rat insulinoma cells were cultured in media containing 2 or 20 mmol/L glucose for the indicated times, and RT-PCR was performed using primers specific for the three forms of ChREBP or for β-actin as a control. C: Dose response of ChREBPβ expression. 832/13 cells were cultured in the indicated concentrations of glucose for 18 h, and RT-PCR was performed as in B. D and E: 832/13 cells were cultured in the indicated concentrations of glucose for 18 h and treated with a scrambled control siRNA (siCon) or with siRNAs directed against ChREBPβ (siChREBP01 and -02). RT-PCRs were performed using primers for the indicated genes. Data are presented as the fold change relative to starting time point (B), or the lowest concentration (C), or to the low glucose scramble control (D and E) for each primer pair after normalizing to β-actin using the ΔΔCT method. All experiments were performed at least three times. Error bars represent the SEM. *P < 0.05; ns, not significant.
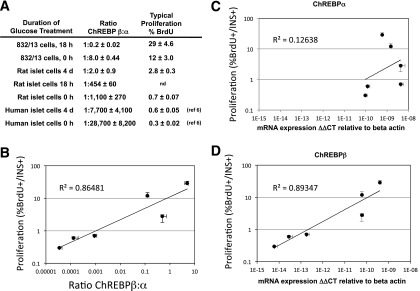
Figure 5.
Correlation of proliferation and ChREBPβ expression of pancreatic β-cells. A: The ratios of ChREBPβ to ChREBPα are displayed in relation to percent BrdU incorporation for each of the indicated model systems after expression for the indicated times in 15 or 20 mmol/L glucose was measured in absolute terms, using the ΔΔCT method relative to β-actin and adjusting for primer efficiency. Data are listed in the order of highest to lowest BrdU incorporation, corresponding to highest to lowest ChREBPβ-to-ChREBPα ratio. nd, not determined. B: Data from primary rodent and human cells from A were plotted on a double log scale and fitted with a power least squares fit and R2 value, calculated in Excel. We note that inclusion of data from INS-1–derived insulinoma cells decreased the R2 value to 0.84. Data from ChREBPα (C) and ChREBPβ (D) and their relation to proliferation are presented separately (n = 3–5). The error bars represent the SEM.
We knocked down ChREBPβ to determine its role in β-cells. To minimize off-target effects, two siRNAs were found to decrease ChREBPβ mRNA by ∼50%, both in low and high glucose concentrations, without affecting ChREBPα mRNA levels (Fig. 1D). The abundance of amplicons derived from primers that recognize both isoforms (ChREBP-common) remained relatively stable, with small but significant changes observed after treatment with the siRNAs and glucose, reflecting diminished ChREBPβ in these cells (Fig. 1D). Note that the four- to sixfold increase of ChREBPβ in response to glucose is accompanied by proportionately smaller changes in ChREBP-common, reflecting the small fraction of ChREBPβ relative to total ChREBP mRNA (see Fig. 5 below). Treatment with these siRNAs led to decreased expression of direct ChREBP target genes Pklr and Acaca and the indirect target gene Myc but had no significant effect on Txnip mRNA levels (Fig. 1E). Because Txnip gene expression is induced by glucose through the binding of ChREBP (21), these results suggest that ChREBPβ and ChREBPα may have different gene targets or that the glucose-mediated induction of Txnip mRNA is driven by other factors, such as MondoA (22).
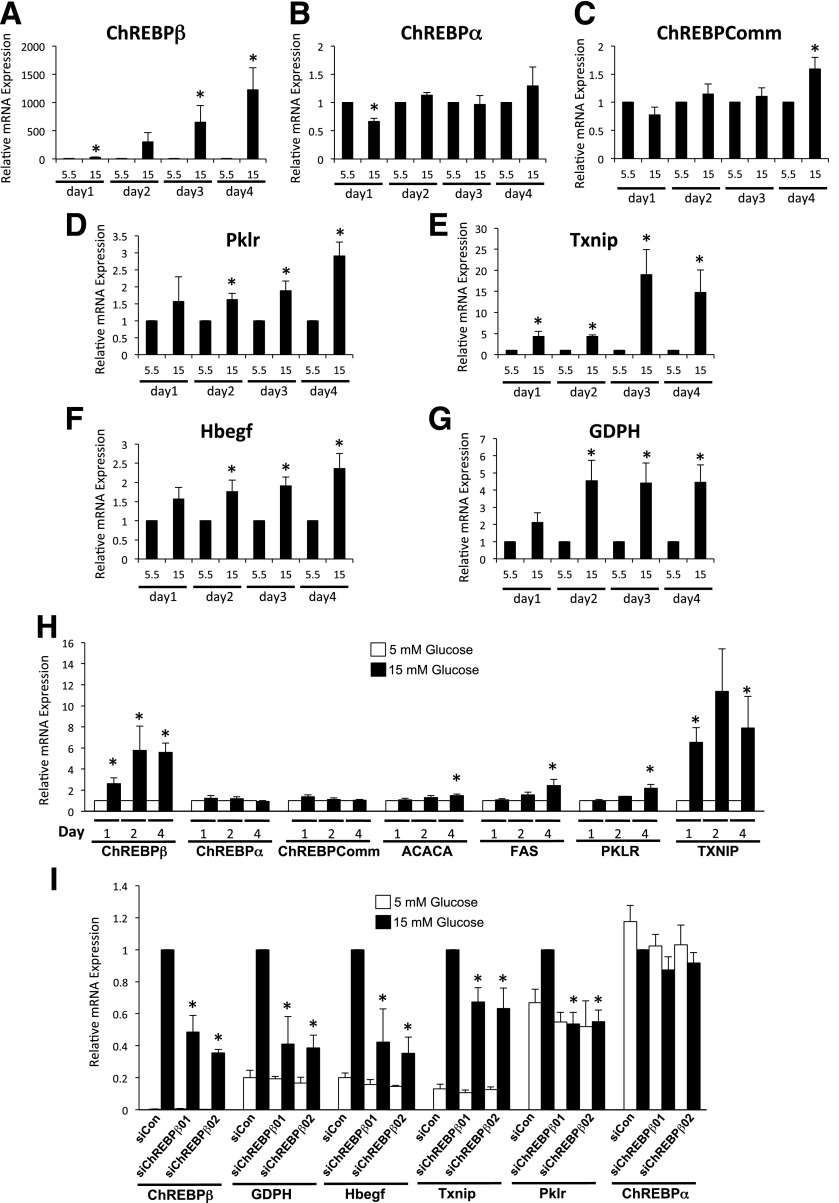
In isolated rat islet cells, glucose stimulated the expression of several ChoRE-containing glucose responsive genes, ranging from 2-fold to 20-fold for Hbegf, Pklr, GDPH, and Txnip (Fig. 2A–G) (14). By contrast, ChREBPβ mRNA was increased ∼2-fold after 1 day and then increased exponentially to more than 1,000-fold by day 4. Note that the abundance of ChREBPβ was ∼1,000-fold lower than ChREBPα in low glucose (see Fig. 5 below). ChREBPα did not increase significantly over the same time frame, and the ChREBP-common amplicon increased significantly, but less than twofold, only on day 4 (Fig. 2A–C). Thus, ChREBPβ is capable of enormous amplification if given enough time in hyperglycemic conditions. In addition, we found that a 2- to 4-day incubation with 15 mmol/L glucose resulted in a fivefold increase in ChREBPβ in isolated human islet cells, correlating with a robust increase in Txnip, and small, but significant increases in the other ChoRE-containing genes tested (Fig. 2H). Importantly, we found that inhibition of ChREBPβ using two individual siRNAs that did not significantly affect ChREBPα led to a significant decrease in ChoRE-containing glucose-responsive genes in dispersed rat islet cells (Fig. 2I). Note that Txnip expression was significantly reduced by the siRNAs, highlighting a difference between rat islet and 832/13 cells and that ChREBPβ is important for at least part of the glucose-mediated induction of Txnip in primary cells. Thus, the induction of ChREBPβ is essential for the glucose-mediated stimulation of glucose-responsive genes in primary rat islet cells.
Figure 2.
Glucose induces ChREBPβ in primary rat and human islet cells. A–G: Dispersed rat islet cells were incubated in media containing 5.5 or 15 mmol/L glucose for 1 to 4 days, and the expression of the indicated genes were determined and shown as fold change relative to each day after normalizing to β-actin using the ΔΔCT method. H: Isolated human islet cells were cultured in media containing 5.5 or 15 mmol/L glucose for 1, 2, or 4 days. Total RNA was isolated and subjected to RT-PCR using primers specific for the indicated genes. The data are expressed as a fold change from 5.5 mmol/L glucose. I: Rat islets were isolated, dispersed, and incubated with lipid-conjugated Accell siRNA for 4 days. Total RNA was isolated and subjected to RT-PCR. Data are presented as relative to the scramble control (SiCon) 15 mmol/L treatment, after normalization to β-actin using the ΔΔCT method. Error bars are the SEM (n = 3–4 for rat islets, n = 5–9 for human islets). ChREBPComm, ChREBP-common; siChREBPβ01 and -02, siRNAs directed against ChREBPβ. *P < 0.05.
ChREBP Binds to Elements in the ChREBPβ Regulatory Region in a Tissue-Specific Manner
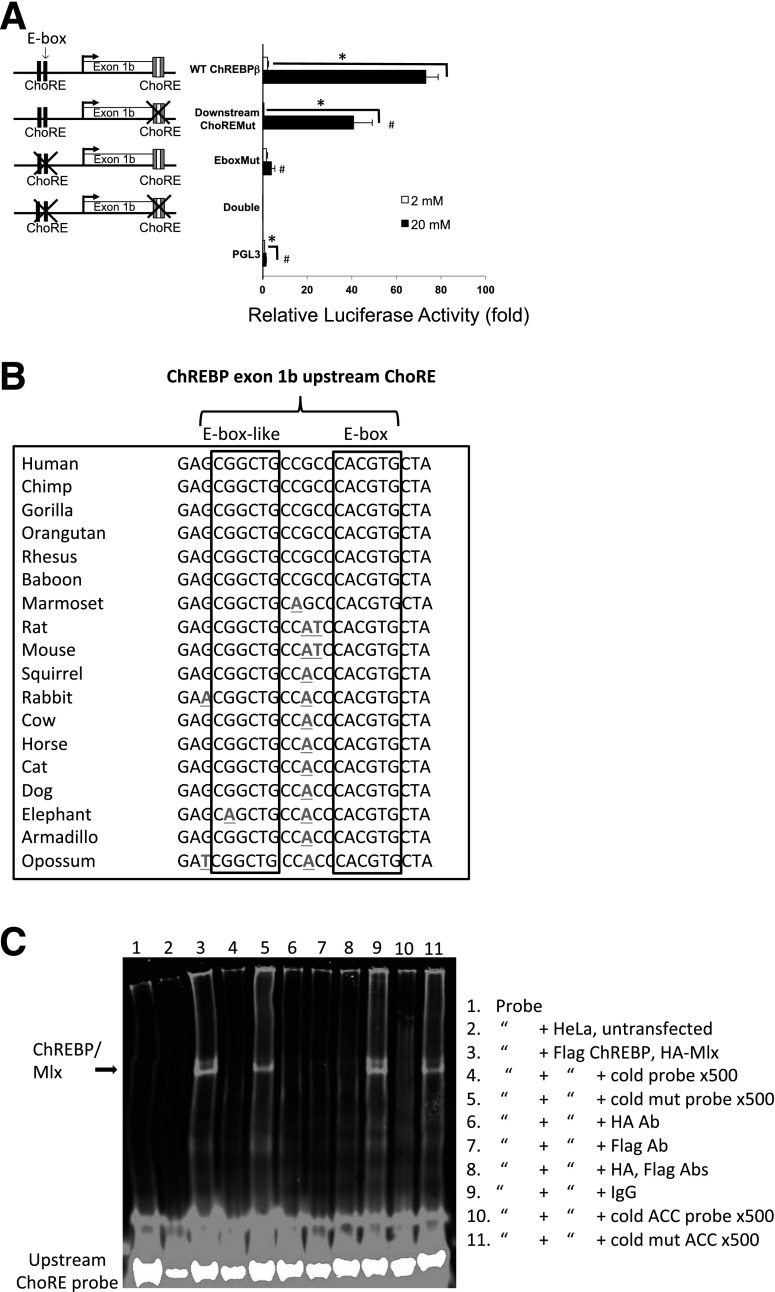
Herman et al. (2) described a ChoRE responsible for the glucose-mediated induction of ChREBPβ at the 5′ end of exon 1b. The study also described an E-box responsible for ∼50% of the glucose response located ∼100 bp upstream of the start site (Fig. 3). We tested whether the same arrangement of elements is used for the glucose-mediated expression of ChREBPβ in pancreatic β-cells. First, we determined the relative importance of the E-box and ChoRE motifs for glucose-responsive gene expression in INS-1–derived 832/13 cells using promoter-reporter constructs (Fig. 3A). Mutation of the upstream E-box completely blocked the very robust 80-fold transcriptional glucose response, as did mutating both the E-box and downstream ChoRE elements, whereas mutation of the downstream ChoRE decreased luciferase activity by ∼50%. This result suggested that the E-box motif is essential for ChREBPβ mRNA expression in β-cells and that this element may be a ChoRE. To test this idea, we examined the sequence surrounding the E-box and found an E-box–like element 5 bp upstream that is highly conserved in mammals (Fig. 3B), an arrangement consistent with known ChoREs but one that is unique due to the guanine in the third position (23–25).
Figure 3.
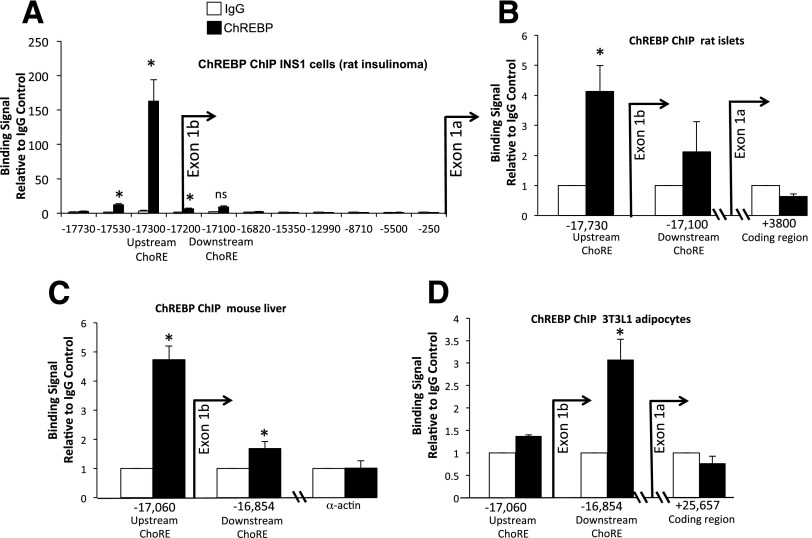
ChREBP binds to tissue-specific ChoREs of exon 1b. A: Luciferase assays identify the exon 1b E-box as a functional ChoRE. A total of 832 cells were transfected with luciferase reporter plasmids driven by exon 1b and upstream sequences containing wild-type (WT) or mutant versions of an upstream E-box element and a previously defined downstream ChoRE, as indicated, or an empty vector control (PGL3). Cells were treated for 18 h with 2 or 20 mmol/L glucose, and luciferase activity was measured from cell lysates. Results shown are relative fold luciferase activity, normalized to protein (n = 3). Error bars are SEM. *P < 0.05 when comparing 20 vs. 2 mmol/L glucose; #P < 0.05 when compared with the WT ChREBPβ promoter construct. B: Conservation of flanking sequence of the upstream E-box of ChREBP exon 1b were aligned from data obtained from the University of California, Santa Cruz genome browser (http://genome.ucsc.edu/). Sequence deviation from human is denoted with bolding and underlining. Together, the conserved sequences are consistent with a consensus ChoRE, with two E-box–like elements separated by 5 bp. C: EMSA demonstrates ChREBP binding to the newly identified upstream ChoRE. HeLa cells were transfected with plasmids expressing Flag-tagged ChREBP and HA-tagged Mlx (the obligate heterodimer binding partner of ChREBP), and cell lysates were used for gel shift assays using the upstream ChoRE and flanking sequences as fluorescently labeled double-stranded probes with the indicated incubation parameters. The gel was visualized with a LI-COR Odyssey System and is representative of three independent experiments with essentially identical results. Abs, antibodies; ACC, Acaca ChoRE.
To confirm that ChREBP binds to this element, an EMSA was performed using extracts from HeLa cells transfected with vectors expressing Flag-tagged ChREBP and HA-tagged Mlx (the obligate heterodimer binding partner of ChREBP), together with a fluorescently labeled double-stranded probe containing the upstream motif. A band representing ChREBP/Mlx was competed by a 500-fold molar excess of unlabeled probe but not a mutant probe with the same sequence of the mutant used in Fig. 3A. Further, antibodies against Flag or HA, but not an IgG control, abrogated the shifted band. Finally, the band was competed by an unlabeled probe with a consensus ChoRE from the Acaca gene promoter but not with a mutant version of the Acaca ChoRE (probe sequences in Supplementary Table 2).
We conclude that the upstream element is an authentic ChoRE and will hereafter refer to this element as the upstream ChoRE motif. To demonstrate binding of ChREBP to the upstream ChoRE motif in cells, Fig. 4 shows a ChIP assay from INS-1–derived 832/13 cells cultured in low or high glucose (2 or 20 mmol/L, respectively) using antibodies directed against ChREBP or IgG as a negative control. The signal for bound protein was determined by quantitative PCR using primers for sites near the transcription start site of exon 1b, and at intervals 5′ of exon 1b to the start site of exon 1a. In response to glucose, we found robust recruitment of ChREBP to exon 1b, with the location of the greatest signal corresponding to a region near the upstream ChoRE motif. By contrast, the region with the previously defined ChoRE at the 3′ end of exon 1b (2) did not bind ChREBP significantly in these cells in response to glucose. It is possible that different tissues use different regulatory elements to promote the glucose response. To test this, we performed ChIP experiments with rat islets, mouse liver tissue, and 3T3-L1 cells after differentiation into adipocytes (Fig. 4B–D). We found that ChREBP binds to regions containing both the upstream ChoRE motif and the exon 1b ChoRE in mouse liver and rat islets, with a clear preference for the upstream ChoRE in these tissues. By contrast, ChREBP recruitment is restricted to a region near the exon 1b ChoRE in 3T3-L1 cells, confirming the previous report (2). Thus, in supporting glucose-stimulated transcription, both elements remain important for a complete response to glucose, and ChREBP binds differentially to these two elements in a tissue-specific manner.
Figure 4.
ChIP assays reveal tissue-specific recruitment of ChREBP to exon 1b ChoREs. A: INS-1–derived 832/13 cells were treated with 2 or 20 mmol/L glucose for 18 h, and cells were fixed with formaldehyde and subjected to a ChIP assay using antibodies directed against ChREBP or an IgG control. The results are presented as fold enrichment over the control IgG signal. Numerous primer pairs were chosen to scan regions of the ChREBP gene upstream of exons 1a and 1b as indicated. The results are from four to seven independent experiments. Chromatin isolated from rat islets cultured for 4 days in 15 mmol/L glucose (B) or from frozen mouse liver from ad libitum–fed mice (C) was sheared and subjected to a ChIP assay using antibodies against ChREBP or IgG as the control. Results relative to the IgG control and are from three mice and four rats. D: NIH 3T3-L1 cells were differentiated into mature adipocytes, cultured for 18 h in 20 mmol/L glucose, and processed for a quantitative ChIP assay. Results of three to four independent experiments are expressed as the signal from an antibody directed against ChREBP relative to the IgG control. Error bars are the SEM. *P < 0.05; ns, not significant.
ChREBPβ Is Required for Glucose-Stimulated Proliferation in INS-1–Derived 832/13 Cells
A striking observation was that the relative abundance of ChREBPβ in response to increased glucose changes over time when compared with ChREBPα, particularly in primary tissues. After adjusting for primer efficiencies, we found that ChREBPβ is expressed at much lower levels than ChREBPα, and when cultured in high glucose concentrations, the abundance of ChREBPβ increases dramatically in the rodent models, but the abundance of ChREBPα does not change very much. Thus, the ratios of the absolute abundance of ChREBPβ to ChREBPα increases with increasing time in culture supplemented with high concentrations of glucose (15 to 20 mmol/L). For instance, in INS-1–derived 832/13 cells, the β-to-α ratio was 1:8 in low glucose and rapidly increased to 1:0.2 after 18 h. In isolated rat islet cells, the ratio of the β to α isoform was 1:1,100 under basal conditions (5.5 mmol/L), which increased to 1:454 by 18 h and reached 1:2 by 4 days. Furthermore, in human islet cells, ChREBPβ was expressed at a ratio of 1:28,700 compared with ChREBPα, which increased to 1:7,700 after 4 days in culture (Fig. 5A). Interestingly, we found a strong correlation between the ratio of the ChREBP isoforms and the proliferative capacity of the cell systems examined, particularly in the primary cells (Fig. 5B). When we plotted the relationship between each isoform of ChREBP independently, we found that ChREBPβ expression correlated with proliferation much better than ChREBPα (Fig. 5C and D).
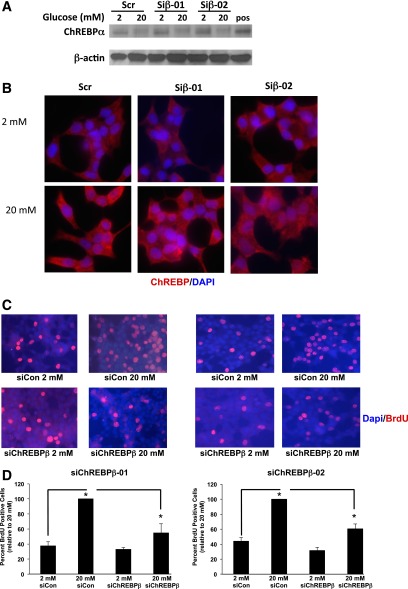
We recently found that ChREBP is required for glucose-stimulated β-cell proliferation in mice, rats, and humans (6). These observations were made before the discovery of the β isoform of ChREBP and used tools that did not distinguish between the two isoforms. Indeed, because ChREBPα is required for the induction of ChREBPβ, depletion of ChREBPα automatically decreases the abundance of ChREBPβ (we confirmed this in Supplementary Fig. 1). In Fig. 6, we used siRNAs to deplete ChREBPβ mRNA to test if ChREBPβ is necessary for glucose-stimulated β-cell proliferation. As shown in Fig. 1, two siRNAs were found that decreased ChREBPβ mRNA ∼50% in INS-1-derived 832/13 cells but had no effect on ChREBPα mRNA levels. In addition, these siRNAs had no effect on the abundance or glucose-stimulated translocation of the full-length ChREBPα protein (Fig. 6A and B). Note that we were unable to measure endogenous ChREBPβ protein because measurement of the α isoform is just detectable by Western blot and the ChREBPβ isoform is expressed at ∼2- to 10-fold lower levels (Fig. 5). Depletion of ChREBPβ mRNA resulted in a significant 40% decrease in glucose-stimulated proliferation in 832/13 cells as determined by BrdU incorporation (Fig. 6C and D), demonstrating that a complete glucose response requires the expression of ChREBPβ in these cells.
Figure 6.
ChREBPβ depletion attenuates glucose-stimulated β-cell proliferation in INS-1–derived 832/13 cells. A: INS-1–derived 832/13 cells were treated with control siRNAs (siCon) or siRNAs targeted against ChREBPβ mRNA (Siβ-01 and -02) and 24 h later cultured for 16 h in 2 or 20 mmol/L glucose. Immunoblotting was performed using antibodies against ChREBP (ChREBPα is visualized) and β-actin (note that these siRNAs decreased ChREBPβ mRNA but had no effect on ChREBPα, Fig. 1). pos, positive control. B: After 48 h of the siRNA treatment, cells were treated for 2 h with 2 or 20 mmol/L glucose and fixed and stained with an antibody recognizing ChREBPα. C: After 48 h, cells were cultured in 2 or 20 mmol/L glucose for 16 h, and BrdU was added 30 min before fixation and staining for BrdU (red) and DAPI (blue). D: Quantification of the results in C, wherein at least 1,000 cells were counted. Results are from four independent experiments. Error bars are the SEM. siChREBPβ, siRNAs directed against ChREBPβ. *P < 0.05.
ChREBPβ Is Required for Glucose-Stimulated Proliferation in Primary Rat β-Cells
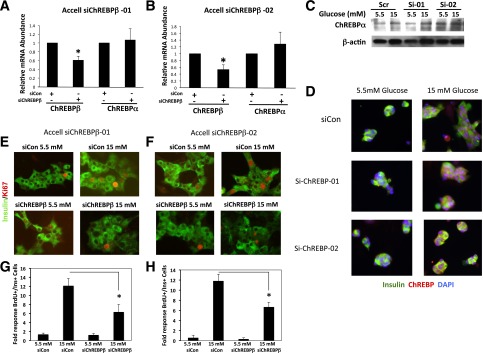
We used freshly isolated rat islets to test the importance of ChREBPβ in glucose-stimulated β-cell proliferation in primary cells. We used Accell siRNA, a lipid-conjugated siRNA that is less toxic and provides a convenient way to deplete primary cells of specific mRNAs (6,26). Accell siRNA decreased ChREBPβ mRNA by ∼40–50% in isolated rat islet cells without affecting ChREBPα mRNA levels (Fig. 7A and B). Importantly, depletion of ChREBPβ mRNA had no effect on the abundance or translocation of the full-length ChREBPα as determined by immunoblotting and confocal microscopy (Fig. 7C and D). Finally, depletion of ChREBPβ mRNA by two different Accell siRNAs to diminish the possibility of off-target effects led to a 50% decrease in glucose-stimulated β-cell proliferation as measured by Ki67 staining of insulin-positive cells (Fig. 7E–H). Taken together, these observations suggest that expression of ChREBPβ is required for a complete proliferative response to glucose in β-cells.
Figure 7.
Depletion of ChREBPβ attenuates glucose-stimulated β-cell proliferation in isolated rat islet cells. A and B: Isolated rat islet cells were treated with lipid-conjugated (Accell) siRNAs and cultured in 5.5 or 15 mmol/L glucose for 96 h. Total RNA was collected and subjected to RT-PCR using primers specific for ChREBPβ or ChREBPα mRNAs. C: Protein extracts were subjected to immunoblotting using an antibody against ChREBPα and β-actin. D: To determine the effects of the siRNAs on ChREBP translocation, cells were treated with control or Accell siRNA against ChREBPβ cultured in 5.5 or 15 mmol/L glucose for 32 h and were fixed and stained for insulin, ChREBP, and DNA (DAPI). E: Isolated rat islet cells were treated with Accell siRNAs and cultured in 5.5 or 15 mmol/L glucose for 96 h and fixed and stained with Ki67 and insulin. F: Quantification of the results in E, wherein at least 1,000 insulin-positive cells were counted. Results are from four different rat islet isolations. siChREBPβ-01 and -02, siRNAs directed against ChREBPβ; siCon, scramble control siRNA. Error bars are the SEM. *P < 0.05.
Discussion
ChREBPβ is a newly identified isoform of ChREBP, the glucose-sensing transcription factor that is generated by a feed-forward loop and the expression of which in adipocytes and hepatocytes correlates with alterations in insulin sensitivity (2,9,10). Exploration of the role of ChREBPβ in glucose-stimulated gene expression and proliferation in β-cells led to a number of novel observations: 1) ChREBPβ is expressed in β-cells and is highly responsive to glucose; 2) depletion of ChREBPβ results in diminished expression of glucose-responsive genes, with Txnip being an exception; 3) ChREBPβ is required for a complete proliferative response to glucose and has a surprisingly strong control strength relative to the full-length glucose-responsive isoform; and 4) ChREBP is recruited to a newly described ChREBPβ promoter ChoRE in β-cells and liver and, therefore, binds to the regulatory regions of ChREBP exon 1b in a tissue-specific manner.
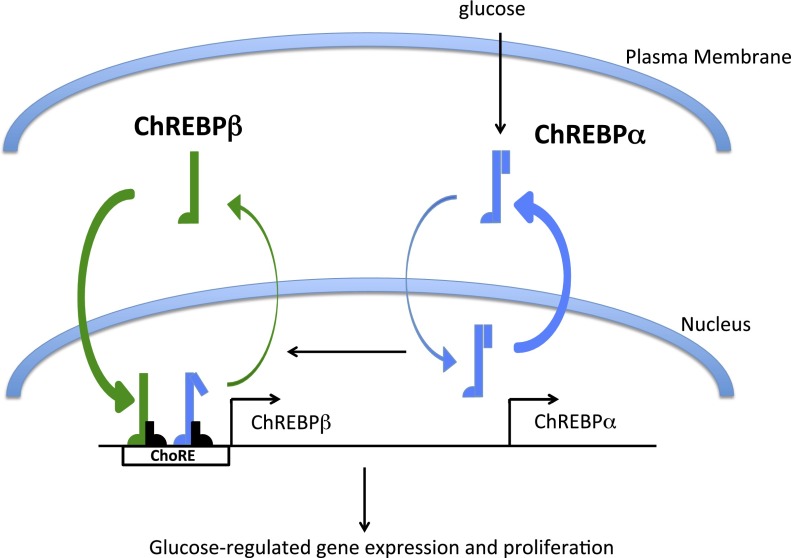
Glucose is a natural β-cell mitogen, and much attention has been focused on understanding the basic mechanisms by which glucose drives β-cell proliferation (5,27–35). We recently demonstrated that ChREBP is required for glucose-stimulated β-cell proliferation in mouse, rat, and human β-cells (6). Islet cells isolated from ChREBP global knockout mice failed to proliferate in response to glucose. Further, siRNAs targeting the coding region of ChREBP attenuated glucose-stimulated β-cell proliferation in islet cells isolated from rat and human islets. These observations were made before the description of ChREBPβ, a new isoform of ChREBP that is formed by alternative promoter usage and splicing (2). Because ChREBPβ expression is stimulated by glucose through a ChoRE that recruits both isoforms, the induction of ChREBPβ is initially mediated by glucose-activated ChREBPα, and once ChREBPβ protein levels increase, the truncated ChREBPβ, missing its low glucose inhibitory domain, is constitutively active and may contribute to its own production as long as the glucose signal is present through a feed-forward loop. We found that targeting ChREBP exon 1a with siRNA led to a decrease in glucose-stimulated ChREBPβ mRNA, confirming this arrangement (Supplementary Fig. 1). The feed-forward amplification was most clear in dispersed rat islet cells cultured for up to 4 days in high glucose, where we saw a 27-fold increase in ChREBPβ after 1 day and a 1,000-fold increase after 4 days (Fig. 2). This was made possible by the newly discovered β-cell–specific ChoRE, upstream of exon 1b, which is very strong, with an 80-fold response versus a 3- to 4-fold response of the Pklr ChoRE (14,19,36). We propose a model (Fig. 8) where a small proportion of ChREBPα, which is primarily cytoplasmic in β-cells in low glucose and only partially nuclear in high glucose (37–40), translocates to the nucleus in response to glucose and binds to a ChoRE located upstream of the ChREBP exon 1b transcription start site, driving the expression of ChREBPβ. ChREBPβ is constitutively nuclear and is more transcriptionally potent than ChREBPα (2). Thus, continued glucose metabolism drives a transcriptional amplification of the glucose signal. Our study suggests this feed-forward amplification is required for glucose-stimulated β-cell proliferation. We propose that in a physiological setting, a measured, intermittent, and somewhat prolonged glucose signal, as might happen as an animal becomes increasingly insulin resistant on a high-fat diet, is necessary for β-cell expansion. However, if hyperglycemia persists beyond a critical threshold, the signal becomes glucotoxic and can lead to apoptosis, as demonstrated by overexpression of a truncated and constitutively active form functionally identical to ChREBPβ in INS-1 cells and in mice (41).
Figure 8.
A model of ChREBPβ-mediated glucose-stimulated β-cell proliferation. ChREBPα is mostly cytoplasmic in β-cells, and only a small percentage actually enters the nucleus in response to increased glucose metabolism (37,39). ChREBPβ is a target gene of ChREBPα and is constitutively nuclear and more transcriptionally potent than ChREBPα (2). Thus, glucose drives a feed-forward amplification signal that continues as long as glucose metabolism remains elevated. We propose that elevation of glucose metabolism for a period of time is required for glucose-stimulated proliferation but that hyperglycemia for too long results in ChREBPβ-contributed glucose toxicity.
A limitation of the current study was our inability to test the role of ChREBPβ in human β-cells for two reasons. Firstly, for technical reasons, we were unable to find an siRNA that would effectively knock down human ChREBPβ; exon 1b for ChREBP is very short (100 bp), and there is only a short stretch that might accommodate repression by siRNA. Secondly, the expression level of ChREBPβ in human islet cells is extremely low—only 1:10,000 of the ChREBPα isoform. This may be partly due to the relatively lower percentage of β-cells in human islets (30–50%) compared with rodent islets (90%), but clearly less ChREBPβ is expressed in human β-cells than in rodent β-cells. Possible implications of this observation include: 1) ChREBPβ plays a less important role in human than in rodent β-cell biology; 2) ChREBPβ, by promoting lipogenesis and lipotoxicity (42), may be highly toxic to human β-cells and so its expression is highly repressed; or 3) alternatively, ChREBPβ expression, in a moderate and temporally controlled manner, may potentiate glucose-stimulated β-cell proliferation in human β-cells, and measuring ChREBPβ in human β-cells is difficult simply because so few human β-cell actually proliferate. Experiments are in progress to test these possibilities.
In summary, we found that ChREBPβ expression is strongly induced by glucose in β-cells. ChREBPβ binds to elements in the regulatory region of exon 1b in a tissue-specific manner, binding to different ChoREs in different proportions in liver, β-cells, and adipose cells. Finally, we found that CREBPβ is required for a complete glucose-stimulated β-cell proliferative response in 832/13 cells and in cultured rat β-cells. Clearly, much more needs to be learned about ChREBPβ and its relationship to β-cell lipotoxicity and proliferation.
Supplementary Material
Article Information
Acknowledgments. Human islets were provided from the National Institute of Diabetes and Digestive and Kidney Diseases–funded Integrated Islet Distribution Program. The authors thank Drs. Adolfo Garcia-Ocaña, Rupangi Vasavada, Nathalie Fiaschi-Taesch, and Andrew F. Stewart of the Diabetes, Obesity and Metabolism Institute of the Icahn School of Medicine at Mount Sinai for helpful discussions.
Funding. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants R01-DK-100425 (M.A.H.) and R01-DK-65149 (D.K.S.), by American Diabetes Association grant 7-11-BS-128 (D.K.S.), and by JDRF (Strategic Research Agreement 17-2011-598; D.K.S).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. P.Z. designed and performed most of the experiments and contributed to the writing of the manuscript. A.K. and L.S.K. researched data and contributed to the writing and editing of the manuscript. L.L. and M.P. researched data. M.A.H. contributed to the discussion and editing of the manuscript. D.K.S. conceived of the study and wrote the manuscript. D.K.S. is the guarantor of this work, and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. A portion of this study was presented at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-0239/-/DC1.
References
- 1.Postic C, Dentin R, Denechaud PD, Girard J. ChREBP, a transcriptional regulator of glucose and lipid metabolism. Annu Rev Nutr 2007;27:179–192 [DOI] [PubMed] [Google Scholar]
- 2.Herman MA, Peroni OD, Villoria J, et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 2012;484:333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci U S A 2004;101:7281–7286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newgard CB, McGarry JD. Metabolic coupling factors in pancreatic beta-cell signal transduction. Annu Rev Biochem 1995;64:689–719 [DOI] [PubMed] [Google Scholar]
- 5.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab 2011;13:440–449 [DOI] [PubMed] [Google Scholar]
- 6.Metukuri MR, Zhang P, Basantani MK, et al. ChREBP mediates glucose-stimulated pancreatic β-cell proliferation. Diabetes 2012;61:2004–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies MN, O’Callaghan BL, Towle HC. Activation and repression of glucose-stimulated ChREBP requires the concerted action of multiple domains within the MondoA conserved region. Am J Physiol Endocrinol Metab 2010;299:E665–E674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li MV, Chang B, Imamura M, Poungvarin N, Chan L. Glucose-dependent transcriptional regulation by an evolutionarily conserved glucose-sensing module. Diabetes 2006;55:1179–1189 [DOI] [PubMed] [Google Scholar]
- 9.Eissing L, Scherer T, Tödter K, et al. De novo lipogenesis in human fat and liver is linked to ChREBP-β and metabolic health. Nat Commun 2013;4:1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kursawe R, Caprio S, Giannini C, et al. Decreased transcription of ChREBP-α/β isoforms in abdominal subcutaneous adipose tissue of obese adolescents with prediabetes or early type 2 diabetes: associations with insulin resistance and hyperglycemia. Diabetes 2013;62:837–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedersen KB, Zhang P, Doumen C, et al. The promoter for the gene encoding the catalytic subunit of rat glucose-6-phosphatase contains two distinct glucose-responsive regions. Am J Physiol Endocrinol Metab 2007;292:E788–E801 [DOI] [PubMed] [Google Scholar]
- 12.Richard AJ, Amini ZJ, Ribnicky DM, Stephens JM. St. John's Wort inhibits insulin signaling in murine and human adipocytes. Biochim Biophys Acta 2012;1822:557–563 [DOI] [PMC free article] [PubMed]
- 13.Ricordi C, Rastellini C. Methods in pancreatic islet isolation. In Methods in Cell Transplantation. Ricordi C, Ed. Austin, TX, R.G. Landes, 1995, p. 433–438 [Google Scholar]
- 14.Zhang P, Metukuri MR, Bindom SM, Prochownik EV, O’Doherty RM, Scott DK. c-Myc is required for the CHREBP-dependent activation of glucose-responsive genes. Mol Endocrinol 2010;24:1274–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Doherty RM, Jensen PB, Anderson P, et al. Activation of direct and indirect pathways of glycogen synthesis by hepatic overexpression of protein targeting to glycogen. J Clin Invest 2000;105:479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiaschi-Taesch NM, Salim F, Kleinberger J, et al. Induction of human beta-cell proliferation and engraftment using a single G1/S regulatory molecule, cdk6. Diabetes 2010;59:1926–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoeckman AK, Ma L, Towle HC. Mlx is the functional heteromeric partner of the carbohydrate response element-binding protein in glucose regulation of lipogenic enzyme genes. J Biol Chem 2004;279:15662–15669 [DOI] [PubMed] [Google Scholar]
- 19.Collier JJ, Zhang P, Pedersen KB, Burke SJ, Haycock JW, Scott DK. c-Myc and ChREBP regulate glucose-mediated expression of the L-type pyruvate kinase gene in INS-1-derived 832/13 cells. Am J Physiol Endocrinol Metab 2007;293:E48–E56 [DOI] [PubMed] [Google Scholar]
- 20.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 2000;49:424–430 [DOI] [PubMed] [Google Scholar]
- 21.Cha-Molstad H, Saxena G, Chen J, Shalev A. Glucose-stimulated expression of Txnip is mediated by carbohydrate response element-binding protein, p300, and histone H4 acetylation in pancreatic beta cells. J Biol Chem 2009;284:16898–16905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoltzman CA, Kaadige MR, Peterson CW, Ayer DE. MondoA senses non-glucose sugars: regulation of thioredoxin-interacting protein (TXNIP) and the hexose transport curb. J Biol Chem 2011;286:38027–38034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shih HM, Liu Z, Towle HC. Two CACGTG motifs with proper spacing dictate the carbohydrate regulation of hepatic gene transcription. J Biol Chem 1995;270:21991–21997 [DOI] [PubMed] [Google Scholar]
- 24.Herman MA, Kahn BB. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J Clin Invest 2006;116:1767–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong YS, Kim D, Lee YS, et al. Integrated expression profiling and genome-wide analysis of ChREBP targets reveals the dual role for ChREBP in glucose-regulated gene expression. PLoS One 2011;6:e22544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deering TG, Ogihara T, Trace AP, Maier B, Mirmira RG. Methyltransferase Set7/9 maintains transcription and euchromatin structure at islet-enriched genes. Diabetes 2009;58:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonso LC, Yokoe T, Zhang P, et al. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes 2007;56:1792–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assmann A, Ueki K, Winnay JN, Kadowaki T, Kulkarni RN. Glucose effects on beta-cell growth and survival require activation of insulin receptors and insulin receptor substrate 2. Mol Cell Biol 2009;29:3219–3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes 1989;38:49–53 [DOI] [PubMed] [Google Scholar]
- 30.Del Zotto H, Gómez Dumm CL, Drago S, Fortino A, Luna GC, Gagliardino JJ. Mechanisms involved in the beta-cell mass increase induced by chronic sucrose feeding to normal rats. J Endocrinol 2002;174:225–231 [DOI] [PubMed] [Google Scholar]
- 31.Jetton TL, Everill B, Lausier J, et al. Enhanced beta-cell mass without increased proliferation following chronic mild glucose infusion. Am J Physiol Endocrinol Metab 2008;294:E679–E687 [DOI] [PubMed] [Google Scholar]
- 32.Levitt HE, Cyphert TJ, Pascoe JL, et al. Glucose stimulates human beta cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia 2011;54:572–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu YQ, Han J, Epstein PN, Long YS. Enhanced rat beta-cell proliferation in 60% pancreatectomized islets by increased glucose metabolic flux through pyruvate carboxylase pathway. Am J Physiol Endocrinol Metab 2005;288:E471–E478 [DOI] [PubMed] [Google Scholar]
- 34.Liu YQ, Montanya E, Leahy JL. Increased islet DNA synthesis and glucose-derived lipid and amino acid production in association with beta-cell hyperproliferation in normoglycaemic 60 % pancreatectomy rats. Diabetologia 2001;44:1026–1033 [DOI] [PubMed] [Google Scholar]
- 35.Salpeter SJ, Klochendler A, Weinberg-Corem N, et al. Glucose regulates cyclin D2 expression in quiescent and replicating pancreatic β-cells through glycolysis and calcium channels. Endocrinology 2011;152:2589–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burke SJ, Collier JJ, Scott DK. cAMP opposes the glucose-mediated induction of the L-PK gene by preventing the recruitment of a complex containing ChREBP, HNF4alpha, and CBP. FASEB J 2009;23:2855–2865 [DOI] [PubMed] [Google Scholar]
- 37.Davies MN, O’Callaghan BL, Towle HC. Glucose activates ChREBP by increasing its rate of nuclear entry and relieving repression of its transcriptional activity. J Biol Chem 2008;283:24029–24038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J, Jing G, Xu G, Shalev A. Thioredoxin-interacting protein stimulates its own expression via a positive feedback loop. Mol Endocrinol 2014;28:674–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li MV, Chen W, Poungvarin N, Imamura M, Chan L. Glucose-mediated transactivation of carbohydrate response element-binding protein requires cooperative actions from Mondo conserved regions and essential trans-acting factor 14-3-3. Mol Endocrinol 2008;22:1658–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noordeen NA, Meur G, Rutter GA, Leclerc I. Glucose-induced nuclear shuttling of ChREBP is mediated by sorcin and Ca(2+) ions in pancreatic β-cells. Diabetes 2012;61:574–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poungvarin N, Lee JK, Yechoor VK, et al. Carbohydrate response element-binding protein (ChREBP) plays a pivotal role in beta cell glucotoxicity. Diabetologia 2012;55:1783–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.da Silva Xavier G, Rutter GA, Diraison F, Andreolas C, Leclerc I. ChREBP binding to fatty acid synthase and L-type pyruvate kinase genes is stimulated by glucose in pancreatic beta-cells. J Lipid Res 2006;47:2482–2491 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.