Abstract
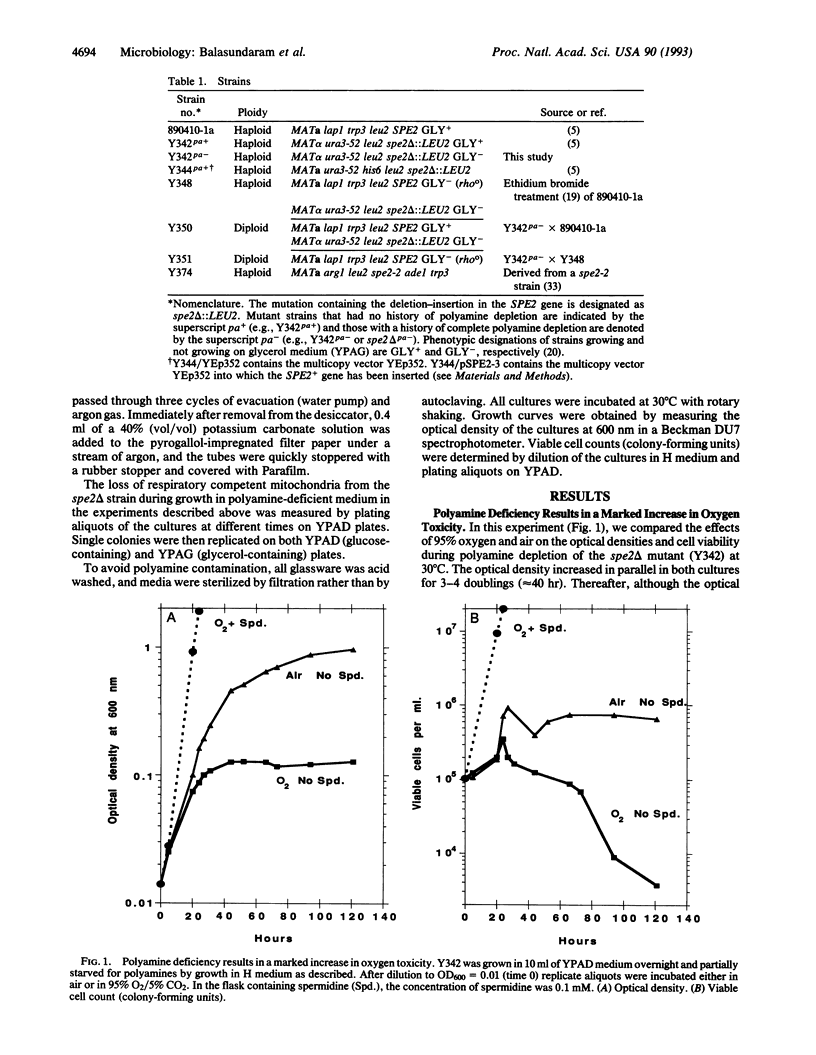
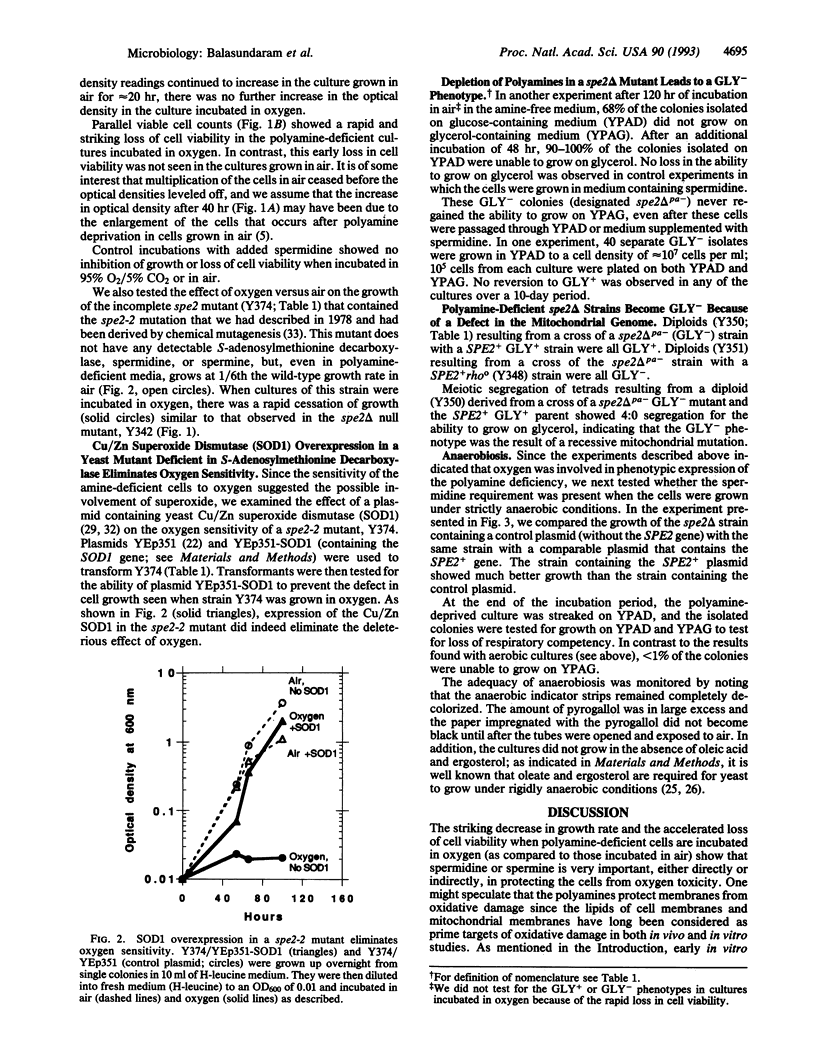
When a mutant of Saccharomyces cerevisiae (spe2 delta) that cannot make spermidine or spermine was incubated in a polyamine-deficient medium in oxygen, there was a rapid cessation of cell growth and associated cell death. In contrast, when the mutant cells were incubated in the polyamine-deficient medium in air or anaerobically, the culture stopped growing more gradually, and there was no significant loss of cell viability. We also found that the polyamine-deficient cells grown in air, but not those grown anaerobically, showed a permanent loss of functional mitochondria ("respiratory competency"), as evidenced by their inability to grow on glycerol as the sole carbon source. These data support the postulation that polyamines act, in part, by protecting cell components from damage resulting from oxidation. However, since the mutant cells still required spermidine or spermine for growth when incubated under strictly anaerobic conditions, polyamines must also have other essential functions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balasundaram D., Tabor C. W., Tabor H. Spermidine or spermine is essential for the aerobic growth of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5872–5876. doi: 10.1073/pnas.88.13.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham-McDonogh O., Gralla E. B., Valentine J. S. The copper, zinc-superoxide dismutase gene of Saccharomyces cerevisiae: cloning, sequencing, and biological activity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4789–4793. doi: 10.1073/pnas.85.13.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biliński T., Krawiec Z., Liczmański A., Litwińska J. Is hydroxyl radical generated by the Fenton reaction in vivo? Biochem Biophys Res Commun. 1985 Jul 31;130(2):533–539. doi: 10.1016/0006-291x(85)90449-8. [DOI] [PubMed] [Google Scholar]
- Bloch K. E. Sterol structure and membrane function. CRC Crit Rev Biochem. 1983;14(1):47–92. doi: 10.3109/10409238309102790. [DOI] [PubMed] [Google Scholar]
- Cabrini L., Tadolini B., Landi L., Fiorentini D., Sechi A. M. The influence of polyunsaturated fatty acids on spermine inhibition of lipoperoxidation. Studies on liposomes prepared with microsomal and mitochondrial phospholipids of sea bass (Dicentrarchus labrax L.) and rat liver. Comp Biochem Physiol B. 1989;93(3):647–651. doi: 10.1016/0305-0491(89)90390-8. [DOI] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Isolation and characterization of Saccharomyces cerevisiae mutants deficient in S-adenosylmethionine decarboxylase, spermidine, and spermine. J Bacteriol. 1978 Apr;134(1):208–213. doi: 10.1128/jb.134.1.208-213.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Regulatory mutations affecting ornithine decarboxylase activity in Saccharomyces cerevisiae. J Bacteriol. 1980 Jun;142(3):791–799. doi: 10.1128/jb.142.3.791-799.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Morris D. R., Coffino P. Sequestered end products and enzyme regulation: the case of ornithine decarboxylase. Microbiol Rev. 1992 Jun;56(2):280–290. doi: 10.1128/mr.56.2.280-290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem. 1989 May 15;264(14):7761–7764. [PubMed] [Google Scholar]
- Goldring E. S., Grossman L. I., Krupnick D., Cryer D. R., Marmur J. The petite mutation in yeast. Loss of mitochondrial deoxyribonucleic acid during induction of petites with ethidium bromide. J Mol Biol. 1970 Sep 14;52(2):323–335. doi: 10.1016/0022-2836(70)90033-1. [DOI] [PubMed] [Google Scholar]
- Gralla E. B., Valentine J. S. Null mutants of Saccharomyces cerevisiae Cu,Zn superoxide dismutase: characterization and spontaneous mutation rates. J Bacteriol. 1991 Sep;173(18):5918–5920. doi: 10.1128/jb.173.18.5918-5920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Hosaka K., Yamashita S. Induction of choline transport and its role in the stimulation of the incorporation of choline into phosphatidylcholine by polyamines in a polyamine auxotroph of Saccharomyces cerevisiae. Eur J Biochem. 1981 May;116(1):1–6. doi: 10.1111/j.1432-1033.1981.tb05292.x. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi K., Taneja S. K., Liu T. Y., Tabor C. W., Tabor H. Spermidine biosynthesis in Saccharomyces cerevisiae. Biosynthesis and processing of a proenzyme form of S-adenosylmethionine decarboxylase. J Biol Chem. 1990 Dec 25;265(36):22321–22328. [PubMed] [Google Scholar]
- Khan A. U., Di Mascio P., Medeiros M. H., Wilson T. Spermine and spermidine protection of plasmid DNA against single-strand breaks induced by singlet oxygen. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11428–11430. doi: 10.1073/pnas.89.23.11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. U., Mei Y. H., Wilson T. A proposed function for spermine and spermidine: protection of replicating DNA against damage by singlet oxygen. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11426–11427. doi: 10.1073/pnas.89.23.11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Effects of superoxide on the erythrocyte membrane. J Biol Chem. 1978 Mar 25;253(6):1838–1845. [PubMed] [Google Scholar]
- Minton K. W., Tabor H., Tabor C. W. Paraquat toxicity is increased in Escherichia coli defective in the synthesis of polyamines. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2851–2855. doi: 10.1073/pnas.87.7.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miret J. J., Solari A. J., Barderi P. A., Goldemberg S. H. Polyamines and cell wall organization in Saccharomyces cerevisiae. Yeast. 1992 Dec;8(12):1033–1041. doi: 10.1002/yea.320081206. [DOI] [PubMed] [Google Scholar]
- Pitkin J., Davis R. H. The genetics of polyamine synthesis in Neurospora crassa. Arch Biochem Biophys. 1990 May 1;278(2):386–391. doi: 10.1016/0003-9861(90)90275-4. [DOI] [PubMed] [Google Scholar]
- Schuber F. Influence of polyamines on membrane functions. Biochem J. 1989 May 15;260(1):1–10. doi: 10.1042/bj2600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tadolini B. Polyamine inhibition of lipoperoxidation. The influence of polyamines on iron oxidation in the presence of compounds mimicking phospholipid polar heads. Biochem J. 1988 Jan 1;249(1):33–36. doi: 10.1042/bj2490033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney P. A., Morris D. R. Polyamine auxotrophs of Saccharomyces cerevisiae. J Bacteriol. 1978 Apr;134(1):214–220. doi: 10.1128/jb.134.1.214-220.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon A. P., Pesold-Hurt B., Schatz G. A yeast mutant lacking mitochondrial manganese-superoxide dismutase is hypersensitive to oxygen. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3820–3824. doi: 10.1073/pnas.83.11.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]


