Abstract
14-3-3s are a class of conserved regulatory proteins ubiquitously found in eukaryotes, which play important roles in a variety of cellular processes including response to diverse stresses. Although much has been learned about 14-3-3s in several plant species, it remains unknown in common bean. In this study, 9 common bean 14-3-3s (PvGF14s) were identified by exhaustive data mining against the publicly available common bean genomic database. A phylogenetic analysis revealed that each predicted PvGF14 was clustered with two GmSGF14 paralogs from soybean. Both epsilon-like and non-epsilon classes of PvGF14s were found in common bean, and the PvGF14s belonging to each class exhibited similar gene structure. Among 9 PvGF14s, only 8 are transcribed in common bean. Expression patterns of PvGF14s varied depending on tissue type, developmental stage and exposure of plants to stress. A protein-protein interaction study revealed that PvGF14a forms dimer with itself and with other PvGF14 isoforms. This study provides a first comprehensive look at common bean 14-3-3 proteins, a family of proteins with diverse functions in many cellular processes, especially in response to stresses.
Introduction
14-3-3 proteins are a group of conserved regulatory molecules that ubiquitously exist in all eukaryotes. Generally, 14-3-3 proteins act as homo- or heterodimers to function through their ability to bind with their phosphorylated protein clients. This process results in alteration in stability, activity, intracellular localization or interaction capability of their client proteins [1–3]. It has been demonstrated that 14-3-3 proteins are able to recognize highly conserved binding motif within their client protein. So far, three canonical motifs have been defined for 14-3-3 binding such as (R/K)SX(S/T)PXP, (R/R)XΦX(S/T)PXP and (S/T)PX1-2-COOH [4], where X,Φ and (S/T)P indicate any amino acid, aromatic/aliphatic amino acid, and serine/threonine that could be potentially phosphorylated, respectively. Nevertheless, 14-3-3s can also bind some protein clients by means of noncanonical or phosphorylation-independent motifs such as WLDLE and GHSL [5,6].
Plant 14-3-3 proteins were identified concurrently from Arabidopsis thaliana, Hordeumvulgare, Spinaceaoleraceaand Oenotherahookeri [7–9]. Since then, many 14-3-3s have been isolated and characterized in several other plant species [10–17]. To date, many efforts have been made to elucidate the roles of 14-3-3s in plant development and response to abiotic stresses [18–23]. Over-expression or silencing of 14-3-3s influenced stress tolerance in plants. For example, over-expression of Arabidopsis AtGF14λ increased drought tolerance in cotton [24], whereas silencing of AtGF14μ in Arabidopsis promoted drought tolerance [25]. Similarly, over-expression of TOMATO 14-3-3 PROTEIN 4 (TFT4) in Arabidopsis increased alkaline stress response [26], while the knock-out of RCI1A/AtGF14ψ enhanced the constitutive freezing tolerance [27]. Additionally, 14-3-3s themselves can be affected by abiotic stresses. For instance, transcriptional accumulations of 14-3-3s were altered by cold, heat, drought, salinity and nutrition deficiency [27–31]. 14-3-3s also interact with components of stress signaling pathways such as ABA-responsive element binding factors, involved in ABA-dependent signaling pathway under salinity stresses [32], H+-ATPase, creating gradient for stomatal opening [33], SALT OVERLY SENSITIVE 2 (SOS2) that mediates intracellular sodium ion homeostasis and salt tolerance [34].
Compared to other organisms, plants contain a large number of 14-3-3 isoforms. For example, there are 13 14-3-3 protein isoforms in Arabidopsis [35], 8 in rice [16], 16 in soybean [14], 8 in foxtail millet [36] and 10 in rubber [15]. These isoforms are encoded by multi-gene family with small difference in sequence. However, emerging evidences indicated that 14-3-3s exert their regulatory functions in an isoform-specific manner. It has been demonstrated that 14-3-3 isoforms displayed differential subcellular localization, distinct tissue-specific and/or inducible expression [14,15,26,37,38], which implied their specific interactions with cellular clients during developmental processes or in response to diverse stresses. For instance, soybean 14-3-3 isoforms showed different binding affinity to GmMYB176 (an isoflavonoid regulator) [19], while rice 14-3-3 isoforms displayed differential binding specificity towards ACC synthase [39]. Evidently, 14-3-3 isoforms play important roles in determining complexity and specificity of biological functions in plants. Thus, addressing the implications of 14-3-3 family diversity becomes an important step towards elucidating their roles in plant developmental processes and/or resistance to stresses.
Common bean (Phaseolus vulgaris L.) is one of the most important crop legumes worldwide. It is a diploid species with 11 chromosomes (2n = 2x = 22) [40], and a genome size of 473 Mb [41]. Although much has been learned about 14-3-3s in several plant species, no 14-3-3 has been identified in common bean. Availability of the whole genome sequence of common bean facilitates to systematically analyze gene family members and their possible roles in common bean. In this study, data mining was conducted against publicly available common bean genomic database, and a total of 9 14-3-3s (PvGF14s) were identified. The PvGF14 isoforms showed high sequence conservation with SGF14s from soybean. Furthermore, PvGF14s displayed tissue-specific expression patterns, and their transcriptional activities were altered when subjected to cold, drought and salinity stress. These findings provide a foundation for elucidating the roles of PvGF14s in common bean during development or in response to abiotic stress.
Materials and Methods
Plant materials and treatments
Common bean (Phaseolus vulgaris L.) cv Dongbeixiaoyoudou is a local cultivar in the northeast of China. Plants (Dongbeixiaoyoudou) were grown at experimental station in Jilin University (Changchun, Jilin Province, China), in 2013, and seeds were collected for the following experiments.
Seeds of common bean cultivar "Dongbeixiaoyoudou" were surfacesterilized by using 10% (w/v) sodium hypochlorite for 20 min, and then washed thoroughly with sterile distilled water. These sterilized seeds were allowed to germinate in 150mm diameter plate with wet filter paper under sterile conditions. Subsequently, six well-germinated seeds were chosen and sown on each pot filled with 65g vermiculite. All the seedlings were grown under a 14 h light and 10 h dark photoperiod at 25°C (light) and 20°C (dark) in a chamber and regularly watered with Hoagland liquid medium. Ten-day-old seedlings were subjected to the following treatments and six pots of seedlings were used for each treatment: (1) For cold stress, seedlings were transferred to 4°C and samples were collected at 0, 1, 3, 6, 12 and 24h after cold treatment; (2)For drought stress, water supply was withheld and samples were collected at 0, 1, 3, 5, 7 and 9 days of water stress; (3) For salinity stress, 200 mM NaCl solution was applied to seedlings and samples were collected at 0, 3, 6, 12, 33, 48 and 72h after salt treatment. The above-ground parts were collected and frozen in liquid nitrogen and stored at -80°C.
Identification of PvGF14s in common bean
The known 14-3-3 protein sequences from soybean, Arabidopsis and rice were obtained from NCBI database, which were used as queries to conduct BLAST search against the public genomic database (http://phytozome.jgi.doe.gov/pz/portal.html). The accession numbers of 14-3-3 proteins from Arabidopsis, soybean and rice were listed in S1 Table. Additionally, to identify all PvGF14s, a key word search using the word "14-3-3" was conducted against the common bean whole genome database (http://phytozome.jgi.doe.gov/pz/portal.html#!search?show=KEYWORD&method=Org_Pvulgaris). All the putative PvGF14s were searched for 14-3-3-specific domain and signature using PROSITE (http://prosite.expasy.org/), Pfam (http://pfam.xfam.org/search) and SMART (http://smart.embl-heidelberg.de/) programs. Their sub-cellular localization was predicted using PSORT algorithms with default parameters (http://www.psort.org/).
Multiple sequence alignment, phylogenetic tree construction and gene structure
Multiple sequence alignment of all putative PvGF14 proteins were performed by Clustal X, and phylogenetic trees were constructed by the Neighbor-joining (NJ) method using MEGA5 software [42]. Bootstrap values were calculated using 1000 replicates. Gene structures of PvGF14s were built using SIM4 (http://pbil.univ-lyon1.fr/members/duret/cours/inserm210604/exercise4/sim4.html).
Calculation of Ka/Ks values
The DnaSP program version 5.10.1 was used to calculate the ratios of non-synonymous (Ka) versus synonymous (Ks) substitution rate (Ka/Ks) for orthologous gene pairs of 14-3-3s [43]. Generally, Ka/Ks = 1 refers to neutral selection, Ka/Ks >1 refers to positive selection to accelerate evolution, and Ka/Ks <1 refers to purifying selection during evolution [44].
Chromosomal localization and gene duplication
To determine the location of putative PvGF14s in common bean chromosomes, coordinate of individual gene and chromosome length were obtained from Phytozome database. PvGF14s in duplicated genomic regions and Ka/Ks values for each duplicated PvGF14 were retrieved from batch download option of Plant Genome Duplication Database (http://chibba.agtec.uga.edu/). Tandem duplications were defined as two paralogs separated by less than five genes in the same chromosome [45], while segmental duplications referred to those homologous genes distributed on duplicated chromosomal blocks from the same genome lineage.
Expression analysis of PvGF14s
Total RNA was isolated from common bean tissues using RNAprep Pure Plant Kit (Tiangen Inc, China), according to the manufacture’s instruction followed by RNase-Free DNase I (NEB Inc, New England) treatment to remove DNA. Total RNA (2μg) was used to synthesize first-strand cDNA using pRimeScript RT reagent kit with gDNA Eraser (Takara Inc, Japan). RT-PCR was performed by using the PvGF14 gene-specific primers (S2 Table). qPCR was conducted using ABI7500 real-time PCR detection system and SYBR Premix Ex Taq (TakaraInc, Japan). Data were analyzed by ABI7500 software v.2.0.6, using ACTIN11 as the internal reference. The expression levels of the controls for each type of stress treatments were set as 1, and relative expression level of each PvGF14 for each treatment was normalized accordingly. The primer sequences used in the study are listed in S3 Table. Statistical significance of the data was analyzed by one-way ANOVA with LSD test, and p value < 0.05 was considered to be statistically significant.
To analyze the expressions of PvGF14s in different tissues, fragments per kilobase of transcript per million mapped reads (FPKM) values for each PvGF14 were extracted from Phytozome database by tracking common bean gene-level expression (http://www.phytozome.net). The heatmap for PvGF14genes was generated in R using the heatmap.2 function from the gplots CRAN library (http://CRAN.R-project.org/package=gplots). To confirm the RNA-seq data from the public database, RT-PCR was performed by using the PvGF14 gene-specific primers (S3 Table). For promoter analysis, 1500 bp upstream region of the transcriptional start site of each PvGF14 gene were analyzed in PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
Yeast two-hybrid assay
To conduct yeast two-hybrid (Y2H) assay, the gateway-compatible vectors pGBKT7-DEST and pGADT7-DEST were utilized to prepare the bait and the prey, respectively [46]. Full-length PvGF14a cDNA was cloned into pGBKT7-DEST as a bait and self-activation was checked. The full-length cDNAs of all PvGF14s except PvGF14q were cloned separately into the pGADT7-DEST vector as preys. The bait vector and each of the prey vectors containing PvGF14 gene were co-transformed into yeast strain AH109, and grown on synthetic defined (SD)/-Leu/-Trp selective agar medium. Selected individual yeast transformants were grown on liquid medium, and 5 μL of yeast suspension culture with a series of 10X dilutions was spotted onto SD/-Leu/-Trp and SD/-Ade/-His/-Leu/-Trp plates, and grown for 5 days at 30°C. Empty vectors were co-transformed as negative controls.
Results
Identification of common bean 14-3-3 gene family
To identify 14-3-3 protein genes in common bean, 14-3-3 protein sequences from soybean, Arabidopsis and rice were used as queries to conduct BLAST search against the common bean genomic database (http://www.phytozome.net/). In addition, a key word search using the word ‘14-3-3’ was also conducted against the above database. This process identified a total of 9 putative 14-3-3 genes (PvGF14a-PvGF14e, PvGF14g, PvGF14h, PvGF14n and PvGF14q), which were named in term of 14-3-3 nomenclature in soybean. Table 1 provides detail information on all putative PvGF14s. The deduced 14-3-3 proteins contain 248 to 263 amino acids residues with the calculated molecular weights from 28.19 to 30.21kDa, and the estimated isoelectric points from 4.56 to 4.85. As shown in Fig 1, all the predicted PvGF14s contain a 14-3-3 conserved domain featured by one or two 14-3-3 protein signatures, and they were predicted to localize in cytoplasm, peroxisome, chloroplast and/or mitochondria (Table 1).
Table 1. Characteristics of PvGF14s genes in common bean.
| Gene name | Locus name | Chromosomal location a | Protein | Predicted subcellularlocation b | CorrespondingEST c | ||
|---|---|---|---|---|---|---|---|
| aa | Mw(kDa) | pI | |||||
| PvGF14n | Phvul.005G066600 | Chr05:10288469–10295349 (+strand) | 263 | 30.21 | 4.75 | Cyto, chl, per | CV539470 |
| PvGF14d | Phvul.005G095500 | Chr05:28501915–28505383 (+strand) | 261 | 29.56 | 4.71 | Cyto, chl, per | GW892341 |
| PvGF14c | Phvul.002G238300 | Chr02:40393090..40396629 (-strand) | 258 | 29.25 | 4.77 | Cyto, chl, per | CV530328 |
| PvGF14q | Phvul.002g102500 | Chr02:20470454..20474678 (-strand) | 258 | 29.45 | 4.75 | Cyto, mito, per | none |
| PvGF14e | Phvul.003G043200 | Chr03:4827912..4831544 (-strand) | 259 | 29.51 | 4.83 | Cyto, chl, per | HS103842 |
| PvGF14b | Phvul.009G143400 | Chr09:20974074..20976460 (-strand) | 248 | 28.19 | 4.85 | Cyto, chl, per | FE704892 |
| PvGF14a | Phvul.008G004300 | Chr08:482680..485463 (-strand) | 256 | 28.98 | 4.59 | Cyto, chl, per | CV530850 |
| PvGF14g | Phvul.008G162500 | Chr08:41741118..41743699 (+strand) | 261 | 29.30 | 4.57 | Cyto, chl, per | GW901700 |
| PvGF14h | Phvul.009G032500 | Chr09:7120999..7123241 (+strand) | 259 | 29.16 | 4.56 | Cyto, chl, per | GW904281 |
a Chromosomal location indicates the position of each gene in chromosome
bcyto, chl, mito and per refer to cytoplasm, chloroplast, mitochondrial and peroxisome, respectively
cEST (Expressed Sequence Tags) accession with the highest homology to corresponding PvGF14 gene; aa, amino acid; pI, isoelectric point; Mw, molecular weight.
Fig 1. Sequence alignment of candidate PvGF14 proteins.
Identical amino acid residues are shown in black. The α-helixs of PvGF14s are shown as a line and 14-3-3 signatures of PvGF14s are shown in rectangular boxes.
An alignment of deduced amino acid sequences of PvGF14s with each other indicated that the isoforms exhibit high sequence conservation with the identity ranging from 63.0% to 94.2% at amino acid level (Fig 1 and S4 Table). The sequence diversification mainly occurred at the N-terminal and the C-terminal regions, suggesting that those regions are possibly responsible for isoform specificity [47]. Additionally, sequence conservation at amino acid level (57.6% to 96.9%) was also observed between soybean and common bean 14-3-3 proteins. Each PvGF14 showed more than 92% sequence identity with its orthologs in soybean (S5 Table).
Evolutionary relationship and gene structure of 14-3-3 gene family in common bean
To examine the evolutionary relationship of 14-3-3 proteins from common bean and other plant species (soybean, Arabidopsis and rice), a phylogenetic analysis was conducted at both nucleotide and protein levels. In both the cases, trees with similar topologies were obtained (Fig 2). PvGF14n, PvGF14d, PvGF14c, PvGF14e and PvGF14q were clustered into the epsilon-like class, while PvGF14a, PvGF14g, PvGF14h and PvGF14b were grouped together with non-epsilon isoforms of 14-3-3 proteins from Arabidopsis, rice and soybean. Each PvGF14 was clustered together with two GmSGF14 orthologs (Fig 2 and S5 Table). The analysis revealed that PvGF14s are evolutionarily closer to soybean 14-3-3s compared to Arabidopsis and rice, which is consistent with the species evolutionary history [40]. The 14-3-3s from Arabidopsis and rice formed separate clades or branches in the phylogenetic tree such as AtGF14pi, AtGF14epsilon, AtGF14omicron and OsGF14h (Fig 2), suggesting that these epsilon-like 14-3-3 genes were lost in common bean and soybean during evolution or evolved a new function.
Fig 2. Phylogenetic analysis of PvGF14s and other GF14s from different species.
Phylogenetic trees were calculated based on CDS matrix (A) and protein matrix (B) from common bean (PvGF14), soybean (GmSGF14), Arabidopsis (AtGF14) and rice (OsG14F), and the tree was classified into epsilon and non-epsilon groups. Each PvGF14/PvGF14 is indicated by a star.
To better understand the evolutionary relationship between 14-3-3s, the ratios of Ka/Ks for 14-3-3 pairs from common bean, soybean, Arabidopsis and rice were estimated (S6 Table). As a result, the Ka/Ks values ranged from 0 to 0.347 with an average of 0.083.All the 14-3-3s appear to be under purifying selection during evolution, as their Ka/Ks ratios were estimated <1. Since each of PvGF14s and its two closely-related orthologs in soybean were clustered into same discrete clade in the phylogenetic tree, the Ka/Ks ratios were further observed. The Ka/Ks ratios for the closest ortholog pairs varied from 0 to 0.153 with an average of 0.060, suggesting that the ortholog pairs among legumes tend to have less evolutionary diversification.
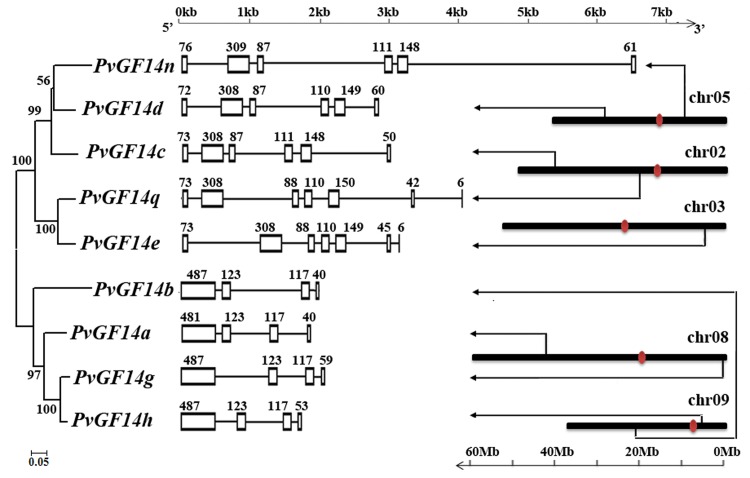
To investigate the exon-intron organization in PvGF14s, gene structures were mapped on the basis of the genomic and coding region sequences. Common bean 14-3-3gene structure comprised of 4 exons in non-epsilon class, and 6–7 exons in epsilon-like class (Fig 3). The PvGF14s belonging to the same class contained similar size of exons, such as PvGF14q and PvGF14e, PvGF14n and PvGF14d, PvGF14g and PvGF14h (Figs 2 and 3). Evidently, conserved gene structure of PvGF14s strongly supports the reliability of phylogenetic tree.
Fig 3. Gene structures and chromosomal localization of PvGF14s in common bean.
The left panel is the phylogenetic tree of PvGF14s; the middle panel is the intron-exon structures where the exons are shown by rectangular, and the introns are represented by thin lines; the right panel displays the chromosomal localization of PvGF14s.
Chromosomal distribution and duplications of 14-3-3s in common bean
The nine PvGF14s are located on five different chromosomes (chromosomes 2, 3, 5, 8 and 9) in common bean. Each chromosome contains two PvGF14s except chromosome 3 (Fig 3). Gene family can arise from the segmental duplication or tandem amplification of chromosomal regions [48]. Generally, tandem amplification was defined as two paralogs separated by less than five genes in the same chromosome. The PvGF14s in the same chromosome were distributed far from each other (Table 1 and Fig 3), suggesting that common bean 14-3-3 gene family was likely derived from segmental duplication rather than tandem amplification of chromosomal regions. Furthermore, we investigated whether traceable genome duplications contributeto the expansion of the 14-3-3gene familyin common bean. The results revealed that the sets of PvGF14s (PvGF14c and PvGF14d, PvGF14q and PvGF14e) were mapped on the duplicated block120 and block93, respectively, suggesting that these two pairs were possibly derived from segmental duplication events during the evolutionary process. No traceable duplication event was observed for other PvGF14s. To investigate the selective evolutionary pressure on PvGF14 gene divergence after duplication, the non-synonymous/synonymous substitution ratio (Ka/Ks) was retrieved for the two duplication pairs of 14-3-3 genes. Consequently, Ka/Ks value of the gene duplication pairs, PvGF14c and PvGF14d as well as PvGF14q and PvGF14e were 0.058 and 0.073, respectively, suggesting that these genes possibly have undergone a purifying selection with limited functional divergence after duplication.
Expression analysis of PvGF14s in common bean tissues
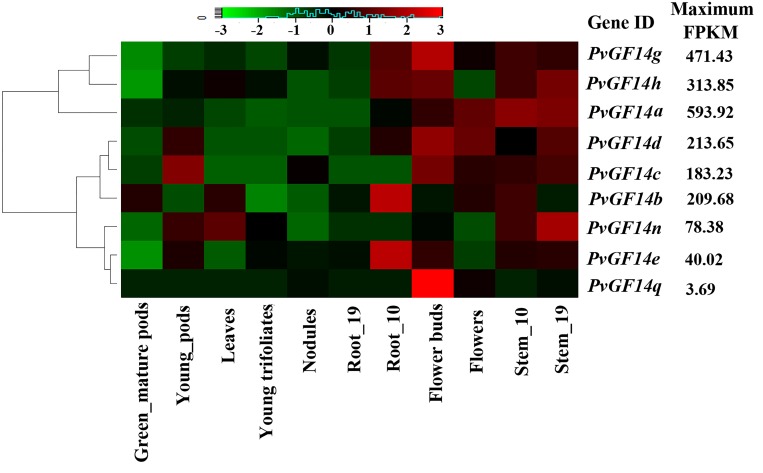
To investigate expression patterns of PvGF14s, we utilized the publicly available genome-wide transcript profiling data of common bean tissues from Phytozome database (http://www.phytozome.net), which contains RNAseq reads from vegetative tissues (trifoliates, nodule, root, stem, leaf) and productive tissues (flower bud, flower, pod). All the common bean 14-3-3 genes showed tissue-specific expression patterns (S7 Table). Over all, PvGF14 transcripts can be categorized into 3 groups based on their expression patterns (Fig 4). Group 1 comprised of PvGF14g, PvGF14h and PvGF14a, which were mainly expressed in stems, flower buds and/or root_10. Group 2 contained PvGF14d, PvGF14c and PvGF14b with high expression in flowers, flower buds, stems and/or pods, while group 3 consisted of PvGF14n, PvGF14e and PvGF14q with transcript abundance either in stems or roots or flower buds. Also, four representative PvGF14s (PvGF14c and PvGF14d from epsilon-like class, PvGF14a and PvGF14b from non-epsilon class), were chosen to confirm the publicly available transcript profiling data using RT-PCR approach, and similar expression patterns were observed in stem, leaf, flower and pod of common bean (S1 Fig).
Fig 4. Expression analysis of PvGF14 genes in various tissues.
The transcriptome data of common bean across different tissues and developmental stages were extracted from the publicly-available Phytozome database (http://www.phytozome.net) for heatmap generation. The color scale above the heat map indicates gene expression levels, low transcript abundance indicated by green color and high transcript abundance indicated by red color. Maximum FPKM value for each PvGF14 is shown.
The maximum fragments per kilo base of transcript per million mapped reads (FPKM) for PvGF14q was low (3.69) compared to the reads for other PvGF14s (40.02 to 593.92). Additionally, blast search against Expressed Sequence Tags (ESTs) in NCBI database did not find any EST corresponding to PvGF14q (Table 1). Our attempts to amplify PvGF14s using cDNA synthesized from RNA isolated from several different tissues of common bean yielded successful results for all the PvGF14s except PvGF14q. These results indicated that common bean contain only 8 putative 14-3-3 genes that are transcribed.
Effect of abiotic stresses on the expression of PvGF14s
Several studies have documented a role of plant 14-3-3 proteins in abiotic stress response [24–31]. To investigate if PvGF14s also have similar roles in common bean, we examined the expression patterns of PvGF14s in response to cold, drought and salinity stress.
Ten-day-old common bean seedlings were exposed to cold stress at 4°C for 0, 1, 3, 6, 12 or 24h, and expression of PvGF14s were monitored. The results revealed that cold stress altered the expressions of PvGF14s that could be grouped into 2 categories. As indicated in Fig 5A, category 1 contained genes that showed gradual increase in transcript accumulation as the stress prolonged. For example,PvGF14n, PvGF14d, PvGF14e, PvGF14g and PvGF14h transcript levels increased to 1.9, 2.1, 2.6, 2.8 and 1.8 fold, respectively as compared to control. All these five gene family members were expressed to their highest level either at 12 or 24h after cold stress. The category 2 comprised the genes (PvGF14a, PvGF14b andPvGF14c) whose transcript levels increased with cold stress treatment followed by gradual decrease as the stress continued. PvGF14c and PvGF14a transcripts reached to their maximum level at 12h cold stress with 3.58 and 1.85 fold increase, respectively as compared to control, while PvGF14b was expressed to the highest level at 3h with 3.73 fold increase as compared to control.
Fig 5. Expression analysis of PvGF14 genes in response to abiotic stresses.
Ten-day-old common bean seedlings were exposed to stress treatment as indicated below. Gene expression analysis was conducted by qRT-PCR using gene specific primers. (A) gene expression pattern of PvGF14s in seedlings exposed to cold stress for 0, 1, 3,6, 12 and 24h. (B) gene expression pattern of PvGF14s in seedlings exposed to drought stress for 0, 1, 3, 5, 7 and 9d. (C)gene expression pattern of PvGF14s in seedlings exposed to salinity stress for 0, 3, 6, 12, 33, 48 and 72h. Error bars indicate SE of two biological and three technical replicates. Values were normalized against the ACTIN11 gene. Significant differences are denoted by asterisks: * p<0.05,**p< 0.01.
When common bean seedlings were exposed to drought stress, the expression of PvGF14e, PvGF14gandPvGF14h was decreased to 5.3, 13.0 and 2.9 fold on 9, 7 and 5 days after treatment, respectively (Fig 5B). On the contrary, a distinct increase in transcript accumulation on 9 days of drought treatment was observed for PvGF14n and PvGF14c with 2.82 and 1.86 fold changes, respectively. A dramatic increase in PvGF14a transcript (5.32 fold) as compared to control was observed on 5 days of drought treatment.
When young seedlings were subjected to salt stress, the expressions of PvGF14n, PvGF14e, PvGF14g and PvGF14h were gradually decreased as the salt stress duration increased, and their maximum fold changes were up to 3.54, 1.97, 1.83 and 1.67, respectively (Fig 5C). On the contrary, the expressions of PvGF14d, PvGF14b and PvGF14a were pronounced at one or more stress time points by 1.54, 1.42 and 1.47 fold, respectively as compared to non-salinity control. PvGF14q transcript was not detected for any of the stress treatment used in this study.
To better understand the role of PvGF14s in abiotic stress response, promoter analysis was also conducted. It was predicted that promoter regions of PvGF14e and PvGF14g contain LTR cis-acting element involved in low-temperature responsiveness, supporting 2.6 and 2.8fold increase in the expression of PvGF14e and PvGF14g under cold stress. PvGF14q, PvGF14n and PvGF14d contain ABRE cis-acting element in their promoter, which is implicated in ABA response. HSE, a cis-acting element related to heat stress response, was found in the promoters of PvGF14q, PvGF14c, PvGF14n, PvGF14g and PvGF14h. All the 14-3-3 genes except PvGF14c contain TC-rich repeat, a cis-acting element involved in defense and stress response. In addition, a MYB binding site associated with drought-inducibility, was predicted in the promoter region of all the PvGF14s. The prediction of promoter elements provided some clues for the response of PvGF14s to various abiotic stresses.
PvGF14 proteins form dimers
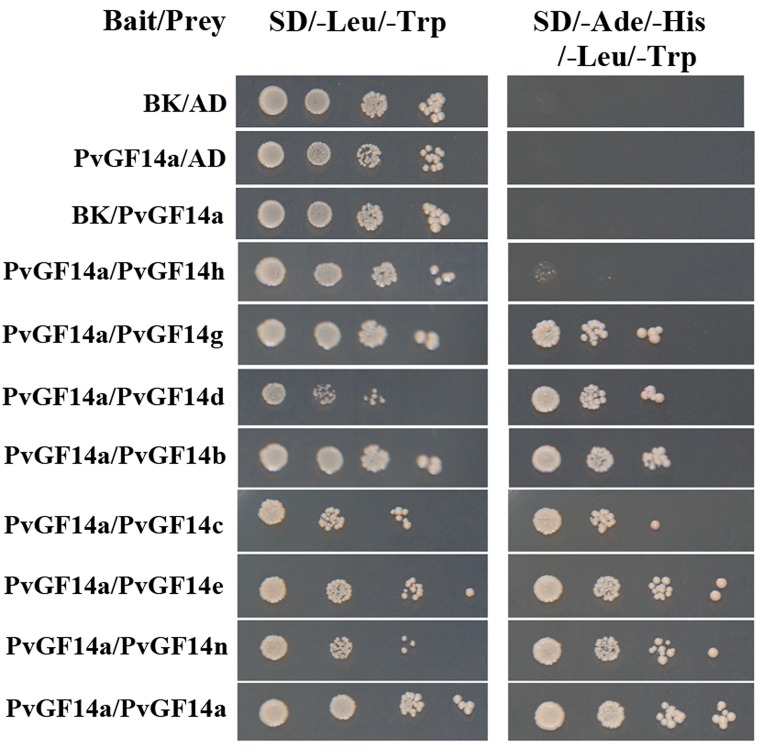
It has been established that 14-3-3 isoforms function as homo- or heterodimers creating 14-3-3 isoform specificity [3,49]. To investigate whether PvGF14s also form dimers, we chose PvGF14a as a representative and investigated its ability to form dimer with itself and with other PvGF14 isoforms. PvGF14awas chosen because it not only showed an increased transcript abundance in response to cold, drought and salinity stress (Fig 5), but also exhibited the highest FPKM value among the PvGF14s (Fig 4). As shown in Fig 6, co-transformed yeast colonies containing PvGF14a (bait) and other PvGF14s (prey) grew on SD/-Ade/-His/-Leu/-Trp, while yeast colony in three negative controls including bait/prey empty vectors, PvGF14a/empty prey vector and empty bait vector/PvGF14a did not grow on SD/-Ade/-His/-Leu/-Trp, indicating that PvGF14a interacts with each of all the examined PvGF14s in yeast cell, including itself. This result was consistent with previous report that 14-3-3ω in Arabidopsis could form homodimers as well as heterodimers with multiple isoforms [50]. Although the interaction activity was not quantified in the study, a weak interaction was observed between PvGF14a and PvGF14h, supporting the fact that preferences for certain dimer combinations exist among 14-3-3 isoforms [3].
Fig 6. Interaction between PvGF14a and other PvGFs in yeast two-hybrid assay.
Yeast cells were co-transformed with combination of DNA-binding domain (BK, Bait) and activation domain (AD, Prey) fused constructs as indicated. Yeast suspension culture (5μL) with a series of 10X dilutions was spotted onto synthetic defined (SD) selection plates. Growth on SD without leucine and tryptophan (SD/-Leu/-Trp) showed the presence of both the vectors, while growth on SD without leucine, tryptophan, adenine and histidine (SD/-Ade/-His/-Leu/-Trp) indicated interaction between bait and prey.
Discussion
14-3-3 proteins are implicated in a wide range of cellular and physiological processes in plants and other eukaryotes. The functional diversity and specificity of 14-3-3 isoforms have been studied in great detail [2,51]. Even though a large number of 14-3-3s have been identified in several plant species, additional 14-3-3s are expected to be identified and characterized in more plant species, especially those with economic importance for elucidating their roles in developmental processes or stresses. In this study, we identified 9 14-3-3 isoforms (PvGF14s) in common bean. Similar to soybean and other plant species, they are grouped into epsilon and non-epsilon groups with distinct intron-exon structures (Figs 2 and 3). Phylogenetic analysis, Ka/Ks ratios and sequence identity suggested that PvGF14s share closer evolutionary relationship with GmSGF14s from soybean and that the 14-3-3s from these two plant species may share similar function. They not only formed a discrete clade in the phylogenetic tree (such as PvGF14g, GmSGF14g and GmSGF14k), but also showed low Ka/Ks ratios (0–0.153) and above 92% sequence identity (Fig 2, S5 and S6 Tables). This result was not surprising since both soybean and common bean belong to the leguminosae family and had undergone whole-genome duplication event ~56.5 million years ago [52]. Intriguingly, the number of 14-3-3s identified in common bean is only half of the ones in soybean, and each PvGF14 corresponds to two SGF14s orthologs in soybean with sequence identity above 92% (S5 Table). These observations are consistent with the evolutionary history that common bean and soybean diverged ~19.2 million years ago, and soybean subsequently experienced another whole-genome duplication event independently of common bean [53]. The evolutionary relationship and sequence identity together with genome evolution suggest that each PvGF14 possibly share similar function with soybean 14-3-3s belonging to the same clade. Additionally, phylogenetic analysis indicated that PvGF14q was clustered together with GmSGF14q and GmSGF14r (Fig 2), which are not transcribed in soybean [14]. Our attempts to amplify PvGF14q from different tissues of common bean under normal and stress conditions failed to detect any transcript, suggesting that PvGF14q is possibly a pseudogene or transcribed at specific developmental processes or under special conditions. In this study, 8PvGF14s were successfully cloned, and their transcribed sequences were identical to the prediction obtained from Phytozome database, thus verifying the gene organizations of these 8PvGF14s (Fig 3). Furthermore, our yeast two-hybrid assay also indicated that these 8 PvGF14s form active proteins with functional protein-protein interaction domains (Fig 6).
Stress induced 14-3-3 isoforms have been reported in many plant species such as Arabidopsis, rice, tomato, maize, cotton and Physcomitrella patens [26,54–60]. Over-expression of 14-3-3 isoforms can increase or reduce stress tolerance in cotton, Arabidopsis, maize and rice [24,25,55], indicating that 14-3-3 family plays regulatory roles in response to stress. The presence of stress-responsive elements in the promoter regions of PvGF14s pointed out their possible roles in response to cold, drought and salinity stress. Furthermore, the altered expression patterns of PvGF14 genes in response to cold, drought and salinity (Fig 5) suggested their prominent roles under these stresses. Some common bean PvGF14s displayed similar expression pattern to their homologs in other plant species under cold, drought and salinity stress. For example, RCI1A/RCI1/14-3-3ψ and RCI1B/RCI2/14-3-3λ in Arabidopsis are most closely related to PvGF14a and PvGF14b within the phylogenetic tree (Fig 2). RCI1A and RCI1B are two cold-inducible genes that are involved in freezing tolerance and cold acclimation in Arabidopsis [27,30]. The expression of PvGF14a and PvGF14b were elevated 1.85 and 3.73 fold, respectively by cold stress (Fig 5A), implying that PvGF14a and PvGF14b possibly function as a modulator of cold-induced signaling pathways. Similar to TFT1 and TFT4 in tomato that were up-regulated by salt treatments [29], we found that transcript accumulation of PvGF14b (homolog of TFT1 and TFT4 in common bean) increased by 1.42 fold under salt stress (Fig 5C). Likewise, the expression of ZmGF14-6 in maize was down-regulated by drought stress [55], and transcript level of its closely-related homolog in common bean (PvGF14h) decreased by 2.9 fold under drought (Figs 2 and 5B). These similarities in the expression patterns suggest that they might perform similar functions as their homologs in other plant species.
It has been well accepted that plant responses and signaling pathways activated by stresses are largely overlapping. Sun et al. (2011) reported that CGF14-4 was more sensitive to both drought and salinity stress, while other 14-3-3s in cotton responded only to either drought or salinity stress [54]. The evidence from tomato indicated that TFT7, a tomato 14-3-3 gene, mediates crosstalk between salt stress and potassium and iron-deficiency signaling pathways in roots [56]. In the study, PvGF14n, PvGF14c and PvGF14a were up-regulated (1.9–5.3 fold) after exposure to cold and drought stress, while PvGF14e, PvGF14g and PvGF14h were down-regulated (1.7–13.0 fold) by both salt and drought stress (Fig 5), suggesting that these 14-3-3 genes may play a role in crosstalk between drought and salinity or cold stress signaling pathways. However, some PvGF14s can differentially respond to these abiotic stresses. For example, the expressions of PvGF14e and PvGF14h were increased by cold stress and decreased by salinity and drought stress (Fig 5), consistent with the previous report that ZmGF14-6 was activated in response to salinity and depressed by drought stress [55]. These observations suggested that PvGF14s might perform functions in a stress-specific manner.
Spatial-temporal or specific expression of 14-3-3 isoforms is a crucial determinant of isoform specificity [14,29,30,61,62]. The differential expression pattern of PvGF14s in various tissues indicated their organ-specific functions (Fig 4). When exposed to cold, drought and salinity stress, expression pattern of 14-3-3 isoforms differed among the same group, and even different expression change occurred among the most closely relevant 14-3-3 isoforms (Fig 5). Thus, the organ-specific and stress-specific properties of PvGF14s provided important clues for elucidating their functions and isoform specificity. It has been proposed that members of a certain evolutionary branch have potential to share similar interactions and functions [3]. PvGF14h and PvGF14g are the closest isoform pair in the phylogenetic tree (Fig 3), and they also showed 94.2% sequence identity (S4 Table). Noticeably, similar expression patterns were observed for PvGF14h and PvGF14g in response to cold, drought and salinity stresses (Fig 5) and indifferent tissues during the development (Fig 4). The observations suggest that PvGF14h and PvGF14g perform similar cellular functions during developmental processes or in response to various stresses.
In conclusion, this study presents a comprehensive classification of common bean 14-3-3s. Although there are 9 predicted PvGF14s in common bean, only 8 are transcribed. The detail characterization of PvGF14s in terms of their transcript accumulation in different tissues during the development and in response to a variety of abiotic stresses provides strong evidence for isoform specificity in common bean. This research outcome adds new members into plant 14-3-3 family, and also strengthens the link between 14-3-3s and stress response. Future study will aim at investigating the effect of each PvGF14 gene on stress tolerance, identifying their potential clients and functional interaction, and 14-3-3 isoform combination in response to specific stress.
Supporting Information
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We thank Yingqiao Luan for the help with preparing the Fig 4, and the anonymous reviewers and the academic editor for valuable suggestions that have improved the work.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was financially supported by the National Natural Science Foundation of China (No. 31300253), http://www.nsfc.gov.cn/.
References
- 1. Gokirmak T, Paul AL, Ferl RJ. Plant phosphopeptide-binding proteins as signaling mediators. Current opinion in plant biology. 2010;13:527–32. 10.1016/j.pbi.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 2. Jaspert N, Throm C, Oecking C. Arabidopsis 14-3-3 proteins: fascinating and less fascinating aspects. Frontiers in plant science. 2011;2:96 10.3389/fpls.2011.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paul AL, Denison FC, Schultz ER, Zupanska AK, Ferl RJ. 14-3-3 phosphoprotein interaction networks—does isoform diversity present functional interaction specification? Frontiers in plant science. 2012;3:190 10.3389/fpls.2012.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ganguly S, Weller JL, Ho A, Chemineau P, Malpaux B, Klein DC. Melatonin synthesis: 14-3-3-dependent activation and inhibition of arylalkylamine N-acetyltransferase mediated by phosphoserine-205. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1222–7. 10.1073/pnas.0406871102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andrews RK, Harris SJ, McNally T, Berndt MC. Binding of purified 14-3-3 zeta signaling protein to discrete amino acid sequences within the cytoplasmic domain of the platelet membrane glycoprotein Ib-IX-V complex. Biochemistry. 1998;37:638–47. 10.1021/bi970893g [DOI] [PubMed] [Google Scholar]
- 6. Petosa C, Masters SC, Bankston LA, Pohl J, Wang B, Fu H, et al. 14-3-3zeta binds a phosphorylated Raf peptide and an unphosphorylated peptide via its conserved amphipathic groove. The Journal of biological chemistry. 1998;273:16305–10. 10.1074/jbc.273.26.16305 [DOI] [PubMed] [Google Scholar]
- 7. Brandt J, Thordal-Christensen H, Vad K, Gregersen PL, Collinge DB. A pathogen-induced gene of barley encodes a protein showing high similarity to a protein kinase regulator. The Plant journal. 1992;2:815–20. 10.1046/j.1365-313X.1992.t01-18-00999.x [DOI] [PubMed] [Google Scholar]
- 8. Hirsch S, Aitken A, Bertsch U, Soll J. A plant homologue to mammalian brain 14-3-3 protein and protein kinase C inhibitor. FEBS letters. 1992;296:222–4. 10.1016/0014-5793(92)80384-S [DOI] [PubMed] [Google Scholar]
- 9. Lu G, DeLisle AJ, de Vetten NC, Ferl RJ. Brain proteins in plants: an Arabidopsis homolog to neurotransmitter pathway activators is part of a DNA binding complex. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:11490–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Q, Kan Q, Wang P, Yu W, Yu Y, Zhao Y, et al. Phosphorylation and Interaction with the 14-3-3 protein of the plasma membrane H+-ATPase are involved in the regulation of magnesium-mediated increases in aluminum-induced citrate exudation in broad bean (Vicia faba. L). Plant & cell physiology. 2015;56(6):1144–53. [DOI] [PubMed] [Google Scholar]
- 11. de Vetten NC, Ferl RJ. Two genes encoding GF14 (14-3-3) proteins in Zea mays. Structure, expression, and potential regulation by the G-box binding complex. Plant physiology. 1994;106:1593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laughner B, Lawrence SD, Ferl RJ. Two tomato fruit homologs of 14-3-3 mammalian brain proteins. Plant physiology. 1994;105:1457–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li MY, Xu BY, Liu JH, Yang XL, Zhang JB, Jia CH, et al. Identification and expression analysis of four 14-3-3 genes during fruit ripening in banana (Musa acuminata L. AAA group, cv. Brazilian). Plant cell reports. 2012;31:369–78. 10.1007/s00299-011-1172-1 [DOI] [PubMed] [Google Scholar]
- 14. Li X, Dhaubhadel S. Soybean 14-3-3 gene family: identification and molecular characterization. Planta. 2011;233:569–82. 10.1007/s00425-010-1315-6 [DOI] [PubMed] [Google Scholar]
- 15. Yang ZP, Li HL, Guo D, Tang X, Peng SQ. Identification and characterization of the 14-3-3 gene family in Hevea brasiliensis . Plant physiology and biochemistry. 2014;80:121–7. 10.1016/j.plaphy.2014.03.034 [DOI] [PubMed] [Google Scholar]
- 16. Yao Y, Du Y, Jiang L, Liu JY. Molecular analysis and expression patterns of the 14-3-3 gene family from Oryza sativa . Journal of biochemistry and molecular biology. 2007;40:349–57. [DOI] [PubMed] [Google Scholar]
- 17. Tian F, Wang T, Xie Y, Zhang J, Hu J. Genome-wide identification, classification, and expression analysis of 14-3-3 gene family in Populus . PloS one. 2015;10:e0123225 10.1371/journal.pone.0123225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sehnke PC, DeLille JM, Ferl RJ. Consummating signal transduction: the role of 14-3-3 proteins in the completion of signal-induced transitions in protein activity. The Plant cell. 2002;14 Suppl:S339–54. 10.1105/tpc.010430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li X, Chen L, Dhaubhadel S. 14-3-3 proteins regulate the intracellular localization of the transcriptional activator GmMYB176 and affect isoflavonoid synthesis in soybean. The Plant journal. 2012;71:239–50. 10.1111/j.1365-313X.2012.04986.x [DOI] [PubMed] [Google Scholar]
- 20. Taoka K, Ohki I, Tsuji H, Furuita K, Hayashi K, Yanase T, et al. 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature. 2011;476:332–5. 10.1038/nature10272 [DOI] [PubMed] [Google Scholar]
- 21. Schoonheim PJ, Sinnige MP, Casaretto JA, Veiga H, Bunney TD, Quatrano RS, et al. 14-3-3 adaptor proteins are intermediates in ABA signal transduction during barley seed germination. The Plant journal. 2007;49:289–301. 10.1111/j.1365-313X.2006.02955.x [DOI] [PubMed] [Google Scholar]
- 22. Swatek KN, Graham K, Agrawal GK, Thelen JJ. The 14-3-3 isoforms chi and epsilon differentially bind client proteins from developing Arabidopsis seed. Journal of proteome research. 2011;10:4076–87. 10.1021/pr200263m [DOI] [PubMed] [Google Scholar]
- 23. Denison FC, Paul AL, Zupanska AK, Ferl FJ. 14-3-3 proteins in plant physiology.Seminars in Cell & Developmental Biology. 2011;22(7):720–7. [DOI] [PubMed] [Google Scholar]
- 24. Yan J, He C, Wang J, Mao Z, Holaday SA, Allen RD, et al. Overexpression of the Arabidopsis 14-3-3 protein GF14 lambda in cotton leads to a "stay-green" phenotype and improves stress tolerance under moderate drought conditions. Plant & cell physiology. 2004;45:1007–14. [DOI] [PubMed] [Google Scholar]
- 25. Sun X, Luo X, Sun M, Chen C, Ding X, Wang X, et al. A Glycine soja 14-3-3 protein GsGF14o participates in stomatal and root hair development and drought tolerance in Arabidopsis thaliana . Plant & cell physiology. 2014;55:99–118. [DOI] [PubMed] [Google Scholar]
- 26. Xu W, Jia L, Shi W, Baluska F, Kronzucker HJ, Liang J, et al. The Tomato 14-3-3 protein TFT4 modulates H+ efflux, basipetal auxin transport, and the PKS5-J3 pathway in the root growth response to alkaline stress. Plant physiology. 2013;163:1817–28. 10.1104/pp.113.224758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Catala R, Lopez-Cobollo R, Mar Castellano M, Angosto T, Alonso JM, Ecker JR, et al. The Arabidopsis 14-3-3 protein RARE COLD INDUCIBLE 1A links low-temperature response and ethylene biosynthesis to regulate freezing tolerance and cold acclimation. The Plant cell. 2014;26:3326–42. 10.1105/tpc.114.127605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dobrá J, Černý M, Štorchová H, Dobrev P, Skalák J, Jedelský PL, et al. The impact of heat stress targeting on the hormonal and transcriptomic response in Arabidopsis.Plant science. 2015;231:52–61. 10.1016/j.plantsci.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 29. Xu WF, Shi WM. Expression profiling of the 14-3-3 gene family in response to salt stress and potassium and iron deficiencies in young tomato (Solanum lycopersicum) roots: analysis by real-time RT-PCR. Annals of botany. 2006;98:965–74. 10.1093/aob/mcl189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jarillo JA, Capel J, Leyva A, Martinez-Zapater JM, Salinas J. Two related low-temperature-inducible genes of Arabidopsis encode proteins showing high homology to 14-3-3 proteins, a family of putative kinase regulators. Plant molecular biology. 1994;25:693–704. [DOI] [PubMed] [Google Scholar]
- 31. Porcel R, Aroca R, Cano C, Bago A, Ruiz-Lozano JM. Identification of a gene from the arbuscular mycorrhizal fungus Glomus intraradices encoding for a 14-3-3 protein that is up-regulated by drought stress during the AM symbiosis. Microbial ecology. 2006;52:575–82. 10.1007/s00248-006-9015-2 [DOI] [PubMed] [Google Scholar]
- 32. Vysotskii DA, de Vries-van Leeuwen IJ, Souer E, Babakov AV, de Boer AH. ABF transcription factors of Thellungiella salsuginea: Structure, expression profiles and interaction with 14-3-3 regulatory proteins.Plant Signal& Behavior. 2013;8(1):e22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kinoshita T, Shimazaki K. Biochemical evidence for the requirement of 14-3-3 protein binding in activation of the guard-cell plasma membrane H+-ATPase by blue light. Plant and Cell Physiology. 2002;43:1359–65. 10.1093/Pcp/Pcf167 [DOI] [PubMed] [Google Scholar]
- 34. Zhou H, Lin H, Chen S, Becker K, Yang Y, Zhao J, et al. Inhibition of the Arabidopsis salt overly sensitive pathway by 14-3-3 proteins. The Plant cell. 2014;26:1166–82. 10.1105/tpc.113.117069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenquist M, Alsterfjord M, Larsson C, Sommarin M. Data mining the Arabidopsis genome reveals fifteen 14-3-3 genes. Expression is demonstrated for two out of five novel genes. Plant physiology. 2001;127:142–9. 10.1104/pp.127.1.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kumar K, Muthamilarasan M, Bonthala VS, Roy R, Prasad M. Unraveling 14-3-3 proteins in C4 panicoids with emphasis on model plant Setaria italica reveals phosphorylation-dependent subcellular localization of RS splicing factor. PloS one. 2015;10:e0123236 10.1371/journal.pone.0123236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paul AL, Sehnke PC, Ferl RJ. Isoform-specific subcellular localization among 14-3-3 proteins in Arabidopsis seems to be driven by client interactions. Molecular biology of the cell. 2005;16:1735–43. 10.1091/mbc.E04-09-0839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alsterfjord M, Sehnke PC, Arkell A, Larsson H, Svennelid F, Rosenquist M, et al. Plasma membrane H(+)-ATPase and 14-3-3 isoforms of Arabidopsis leaves: evidence for isoform specificity in the 14-3-3/H(+)-ATPase interaction. Plant & cell physiology. 2004;45:1202–10. [DOI] [PubMed] [Google Scholar]
- 39. Yao Y, Du Y, Jiang L, Liu JY. Interaction between ACC synthase 1 and 14-3-3 proteins in rice: a new insight. Biochemistry (Mosc). 2007;72:1003–7. [DOI] [PubMed] [Google Scholar]
- 40. Pedrosa A, Vallejos CE, Bachmair A, Schweizer D. Integration of common bean (Phaseolus vulgaris L.) linkage and chromosomal maps. Theoretical and applied genetics. 2003;106:205–12. 10.1007/s00122-002-1138-3 [DOI] [PubMed] [Google Scholar]
- 41. Schmutz J, McClean PE, Mamidi S, Wu GA, Cannon SB, Grimwood J, et al. A reference genome for common bean and genome-wide analysis of dual domestications. Nature genetics. 2014;46:707–13. 10.1038/ng.3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular biology and evolution. 2011;28:2731–9. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–2. 10.1093/bioinformatics/btp187 [DOI] [PubMed] [Google Scholar]
- 44. Yang XH, Tuskan GA, Cheng ZM. Divergence of the Dof gene families in poplar, Arabidopsis, and rice suggests multiple modes of gene evolution after duplication. Plant physiology. 2006;142:820–30. 10.1104/pp.106.083642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yuan SX, Xu B, Zhang J, Xie ZN, Cheng Q, Yang ZM, et al. Comprehensive analysis of CCCH-type zinc finger family genes facilitates functional gene discovery and reflects recent allopolyploidization event in tetraploid switchgrass. BMC Genomics. 2015;16:129 10.1186/S12864-015-1328-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lu Q, Tang XR, Tian G, Wang F, Liu KD, Nguyen V, et al. Arabidopsis homolog of the yeast TREX-2 mRNA export complex: components and anchoring nucleoporin. Plant Journal. 2010;61:259–70. 10.1111/j.1365-313X.2009.04048.x [DOI] [PubMed] [Google Scholar]
- 47. Ferl RJ, Manak MS, Reyes MF. The 14-3-3s. Genome biology. 2002;3:REVIEWS3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leister D. Tandem and segmental gene duplication and recombination in the evolution of plant disease resistance genes. Trends in genetics. 2004;20:116–22. 10.1016/j.tig.2004.01.007 [DOI] [PubMed] [Google Scholar]
- 49. Zhang ZT, Zhou Y, Li Y, Shao SQ, Li BY, Shi HY, et al. Interactome analysis of the six cotton 14-3-3s that are preferentially expressed in fibres and involved in cell elongation. Journal of experimental botany. 2010;61:3331–44. 10.1093/jxb/erq155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chang IF, Curran A, Woolsey R, Quilici D, Cushman JC, Mittler R, et al. Proteomic profiling of tandem affinity purified 14-3-3 protein complexes in Arabidopsis thaliana . Proteomics. 2009;9:2967–85. 10.1002/pmic.200800445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sehnke PC, Rosenquist M, Alsterfjord M, DeLille J, Sommarin M, Larsson C, et al. Evolution and isoform specificity of plant 14-3-3 proteins. Plant molecular biology. 2002;50:1011–8. [DOI] [PubMed] [Google Scholar]
- 52. Lavin M, Herendeen PS, Wojciechowski MF. Evolutionary rates analysis of Leguminosae implicates a rapid diversification of lineages during the tertiary. Systematic biology. 2005;54:575–94. 10.1080/10635150590947131 [DOI] [PubMed] [Google Scholar]
- 53. Schmutz J, Cannon SB, Schlueter J, Ma JX, Mitros T, Nelson W, et al. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463(7278):178–83. 10.1038/Nature08957 [DOI] [PubMed] [Google Scholar]
- 54. Sun G, Xie F, Zhang B. Transcriptome-wide identification and stress properties of the 14-3-3 gene family in cotton (Gossypium hirsutum L.). Functional & integrative genomics. 2011;11:627–36. [DOI] [PubMed] [Google Scholar]
- 55. Campo S, Peris-Peris C, Montesinos L, Penas G, Messeguer J, San Segundo B. Expression of the maize ZmGF14-6 gene in rice confers tolerance to drought stress while enhancing susceptibility to pathogen infection. Journal of experimental botany. 2012;63:983–99. 10.1093/jxb/err328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xu W, Shi W, Jia L, Liang J, Zhang J. TFT6 and TFT7, two different members of tomato 14-3-3 gene family, play distinct roles in plant adaption to low phosphorus stress. Plant, cell & environment. 2012;35:1393–406. [DOI] [PubMed] [Google Scholar]
- 57. Shanko AV, Mesenko MM, Klychnikov OI, Nosov AV, Ivanov VB. Proton pumping in growing part of maize root: its correlation with 14-3-3 protein content and changes in response to osmotic stress. Biochemistry Biokhimiia. 2003;68:1320–6. [DOI] [PubMed] [Google Scholar]
- 58. Ho SL, Huang LF, Lu CA, He SL, Wang CC, Yu SP, et al. Sugar starvation- and GA-inducible calcium-dependent protein kinase 1 feedback regulates GA biosynthesis and activates a 14-3-3 protein to confer drought tolerance in rice seedlings. Plant molecular biology. 2013;81:347–61. 10.1007/s11103-012-0006-z [DOI] [PubMed] [Google Scholar]
- 59. Chen F, Li Q, Sun L, He Z. The rice 14-3-3 gene family and its involvement in responses to biotic and abiotic stress. DNA research: an international journal for rapid publication of reports on genes and genomes. 2006;13:53–63. 10.1093/dnares/dsl001 [DOI] [PubMed] [Google Scholar]
- 60. Wang X, Yang P, Zhang X, Xu Y, Kuang T, Shen S, et al. Proteomic analysis of the cold stress response in the moss, Physcomitrella patens . Proteomics. 2009;9:4529–38. 10.1002/pmic.200900062 [DOI] [PubMed] [Google Scholar]
- 61. van Kleeff PJ, Jaspert N, Li KW, Rauch S, Oecking C, de Boer AH. Higher order Arabidopsis 14-3-3 mutants show 14-3-3 involvement in primary root growth both under control and abiotic stress conditions. Journal of experimental botany. 2014;65:5877–88. 10.1093/jxb/eru338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Aksamit A, Korobczak A, Skala J, Lukaszewicz M, Szopa J. The 14-3-3 gene expression specificity in response to stress is promoter-dependent. Plant & cell physiology. 2005;46:1635–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.