Abstract
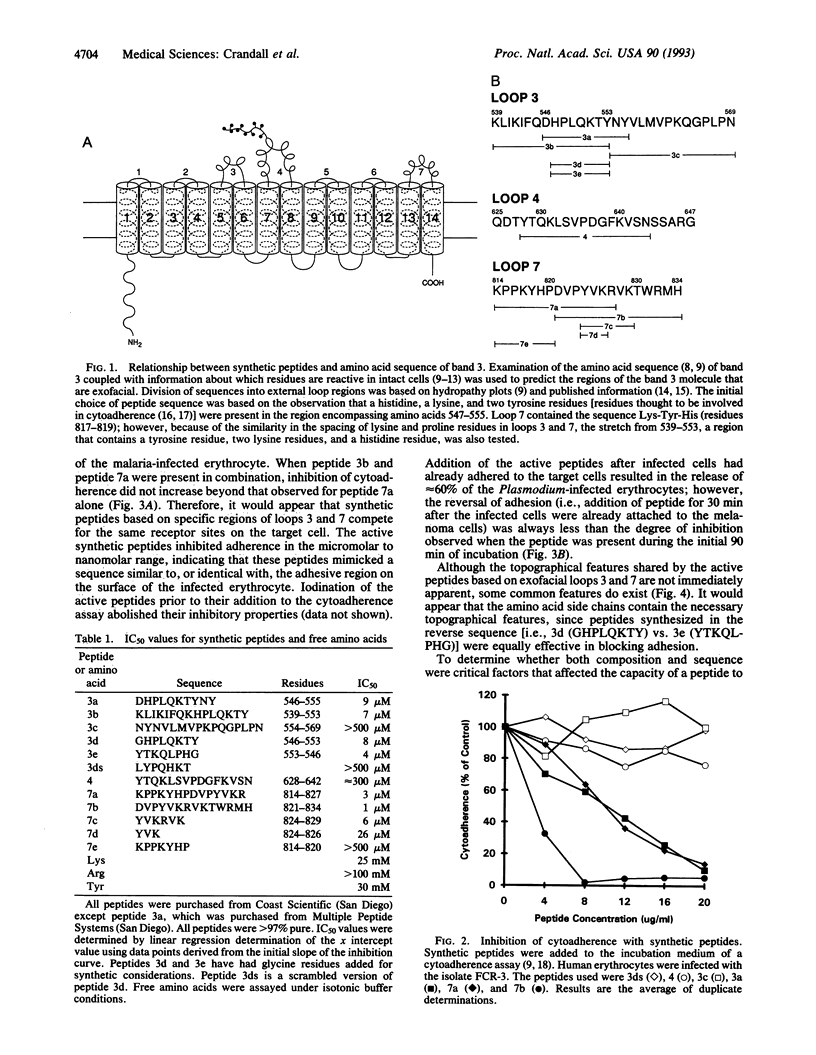
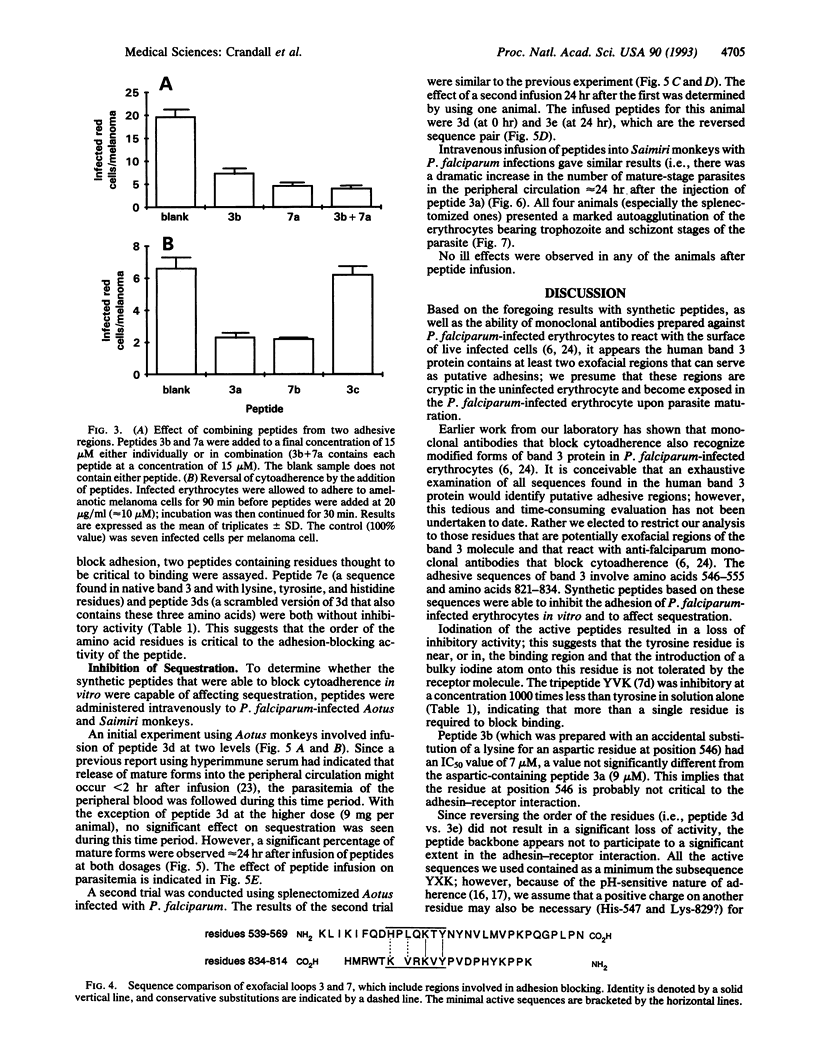
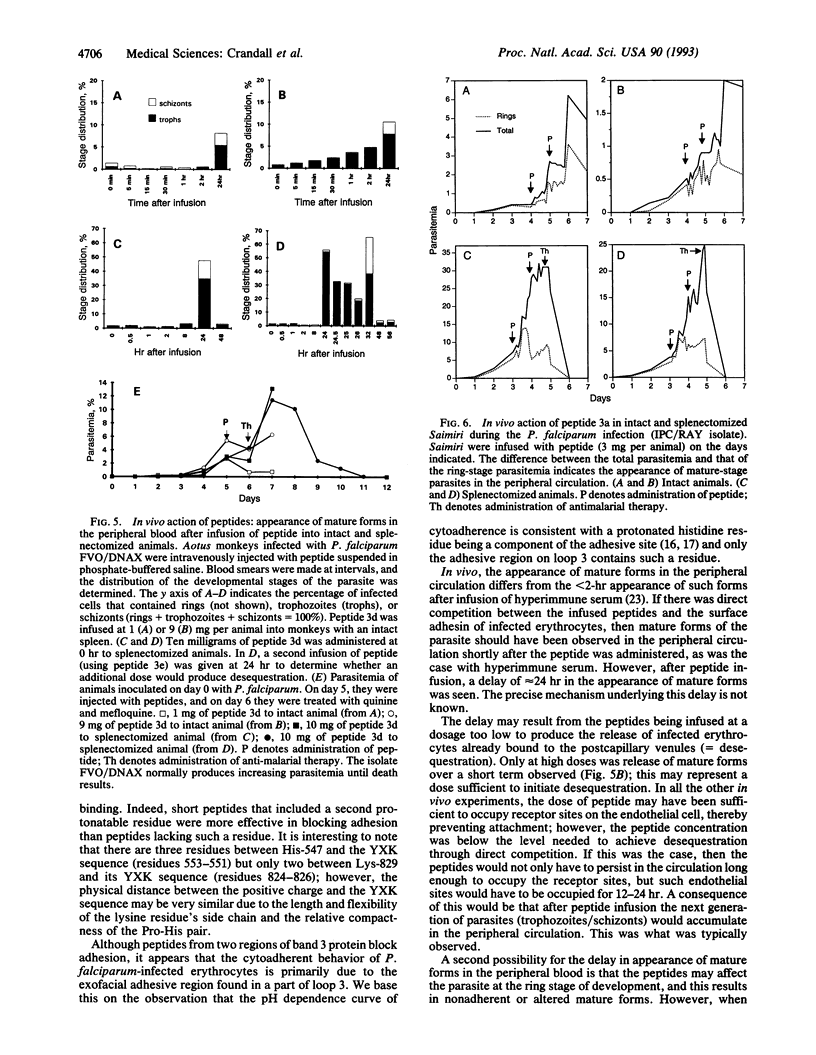
Synthetic peptides patterned on the amino acid sequences found in two exofacial regions of band 3 protein (residues 824-829 of loop 7 and residues 547-553 of loop 3) blocked, in a dose-dependent fashion, the in vitro adherence of Plasmodium falciparum-infected erythrocytes to C32 amelanotic melanoma cells. Intravenous infusion of these synthetic peptides into Aotus and Saimiri monkeys infected with sequestering isolates of P. falciparum resulted in the appearance of mature forms of the parasite in the peripheral circulation. The finding that the peptides were effective as adhesion blockers in the micromolar range suggests that cerebral malaria could be managed through antiadhesion therapy.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Iseki M., Barnwell J. W., Taylor D., Oo M. M., Howard R. J. The pathology of human cerebral malaria. Am J Trop Med Hyg. 1990 Aug;43(2 Pt 2):30–37. doi: 10.4269/ajtmh.1990.43.30. [DOI] [PubMed] [Google Scholar]
- Berendt A. R., Simmons D. L., Tansey J., Newbold C. I., Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989 Sep 7;341(6237):57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Collins W. E., Stanfill P. G., Richardson B. B., Smith C. S. Transmission of Plasmodium fragile to Aotus trivirgatus monkeys. J Parasitol. 1974 Aug;60(4):719–720. [PubMed] [Google Scholar]
- Crandall I., Sherman I. W. Plasmodium falciparum (human malaria)-induced modifications in human erythrocyte band 3 protein. Parasitology. 1991 Jun;102(Pt 3):335–340. doi: 10.1017/s0031182000064271. [DOI] [PubMed] [Google Scholar]
- Crandall I., Smith H., Sherman I. W. Plasmodium falciparum: the effect of pH and Ca2+ concentration on the in vitro cytoadherence of infected erythrocytes to amelanotic melanoma cells. Exp Parasitol. 1991 Oct;73(3):362–368. doi: 10.1016/0014-4894(91)90108-9. [DOI] [PubMed] [Google Scholar]
- David P. H., Hommel M., Miller L. H., Udeinya I. J., Oligino L. D. Parasite sequestration in Plasmodium falciparum malaria: spleen and antibody modulation of cytoadherence of infected erythrocytes. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5075–5079. doi: 10.1073/pnas.80.16.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H., Perraut R., Garraud O., Poingt J. P., Gysin J. Functional characterization of the antibody-mediated protection against blood stages of Plasmodium falciparum in the monkey Saimiri sciureus. Eur J Immunol. 1990 Oct;20(10):2317–2323. doi: 10.1002/eji.1830201022. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Handunnetti S. M., Hasler T., Gilladoga A., de Aguiar J. C., Pasloske B. L., Morehead K., Albrecht G. R., van Schravendijk M. R. Surface molecules on Plasmodium falciparum-infected erythrocytes involved in adherence. Am J Trop Med Hyg. 1990 Aug;43(2 Pt 2):15–29. doi: 10.4269/ajtmh.1990.43.15. [DOI] [PubMed] [Google Scholar]
- Jennings M. L., Passow H. Anion transport across the erythrocyte membrane, in situ proteolysis of band 3 protein, and cross-linking of proteolytic fragments by 4,4'-diisothiocyano dihydrostilbene-2,2'-disulfonate. Biochim Biophys Acta. 1979 Jul 5;554(2):498–519. doi: 10.1016/0005-2736(79)90387-0. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Localization of senescent cell antigen on band 3. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5753–5757. doi: 10.1073/pnas.81.18.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M., Marchalonis J. J., Hughes J., Watanabe K., Schluter S. F. Definition of a physiologic aging autoantigen by using synthetic peptides of membrane protein band 3: localization of the active antigenic sites. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5734–5738. doi: 10.1073/pnas.87.15.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Kopito R. R., Lodish H. F. Cloning and characterization of band 3, the human erythrocyte anion-exchange protein (AE1). Proc Natl Acad Sci U S A. 1989 Dec;86(23):9089–9093. doi: 10.1073/pnas.86.23.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz S., Marchesi V. T. The carboxyl-terminal domain of human erythrocyte band 3. Description, isolation, and location in the bilayer. J Biol Chem. 1981 Jun 25;256(12):6463–6468. [PubMed] [Google Scholar]
- Marsh K., Marsh V. M., Brown J., Whittle H. C., Greenwood B. M. Plasmodium falciparum: the behavior of clinical isolates in an in vitro model of infected red blood cell sequestration. Exp Parasitol. 1988 Apr;65(2):202–208. doi: 10.1016/0014-4894(88)90123-3. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Klotz F. W., Tandon N. N., Jamieson G. A. Sequestrin, a CD36 recognition protein on Plasmodium falciparum malaria-infected erythrocytes identified by anti-idiotype antibodies. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3175–3179. doi: 10.1073/pnas.88.8.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raida M., Wendel J., Kojro E., Fahrenholz F., Fasold H., Legrum B., Passow H. Major proteolytic fragments of the murine band 3 protein as obtained after in situ proteolysis. Biochim Biophys Acta. 1989 Apr 28;980(3):291–298. doi: 10.1016/0005-2736(89)90315-5. [DOI] [PubMed] [Google Scholar]
- Roberts D. D., Sherwood J. A., Spitalnik S. L., Panton L. J., Howard R. J., Dixit V. M., Frazier W. A., Miller L. H., Ginsburg V. Thrombospondin binds falciparum malaria parasitized erythrocytes and may mediate cytoadherence. Nature. 1985 Nov 7;318(6041):64–66. doi: 10.1038/318064a0. [DOI] [PubMed] [Google Scholar]
- Sherman I. W., Valdez E. In vitro cytoadherence of Plasmodium falciparum-infected erythrocytes to melanoma cells: factors affecting adhesion. Parasitology. 1989 Jun;98(Pt 3):359–369. doi: 10.1017/s0031182000061436. [DOI] [PubMed] [Google Scholar]
- Tanner M. J., Martin P. G., High S. The complete amino acid sequence of the human erythrocyte membrane anion-transport protein deduced from the cDNA sequence. Biochem J. 1988 Dec 15;256(3):703–712. doi: 10.1042/bj2560703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Udeinya I. J., Schmidt J. A., Aikawa M., Miller L. H., Green I. Falciparum malaria-infected erythrocytes specifically bind to cultured human endothelial cells. Science. 1981 Jul 31;213(4507):555–557. doi: 10.1126/science.7017935. [DOI] [PubMed] [Google Scholar]
- Winograd E., Greenan J. R., Sherman I. W. Expression of senescent antigen on erythrocytes infected with a knobby variant of the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1931–1935. doi: 10.1073/pnas.84.7.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winograd E., Sherman I. W. Characterization of a modified red cell membrane protein expressed on erythrocytes infected with the human malaria parasite Plasmodium falciparum: possible role as a cytoadherent mediating protein. J Cell Biol. 1989 Jan;108(1):23–30. doi: 10.1083/jcb.108.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]



