Abstract
The advent of high-throughput technologies has provided exceptional assistance for lung scientists to discover novel genetic variants underlying the development and progression of complex lung diseases. However, the discovered variants thus far do not explain much of the estimated heritability of complex lung diseases. Here, we review the literature of successfully employed genome-wide association studies (GWAS) and identified the polymorphisms that reproducibly underpin the susceptibility to various non-cancerous complex lung diseases or affecting therapeutic responses. We also discuss the inherent limitations of GWAS approaches and how the utilization of next generation sequencing technologies (NGS) has furthered our understanding about the genetic determinants of these diseases. Next, we describe the contribution of the metagenomics in understanding the interactions of the airways microbiome with lung diseases. We then highlight the urgent need for new integrative genomics-phenomics methods to more effectively interrogate and understand multiple downstream ‘omics (e.g. chromatin modification patterns). Finally, we address the scarcity of genetics studies addressing underrepresented populations such as African Americans and Hispanics.
INTRODUCTION
High-throughput technologies have revolutionized our understanding of the molecular mechanisms underlying the development and progression of complex lung diseases. Importantly, genome-wide association studies (GWAS) have played a critical role as an advanced high-density genotyping approach that allows for characterizing the contribution of single-nucleotide polymorphisms (SNPs) scattered across the genome to the genetic susceptibility of individuals. Specifically, GWAS benefits from the variety of array platform technologies by them the genotyping of numerous common variants is possible. Until the advent of GWAS in 2007, the genetic research of complex inheritance was labor-intensive and biased, focusing on identifying genetic variants based on prior knowledge. GWAS offered a substantial higher rate of discovery and was not limited by the need for an a priori hypothesis or prior knowledge. However, the limited number of probes in GWAS platforms (e.g., 1 million probes), as compared to the more than 79,000,000 variants sites thus far identified by phase 3 release of 1000 Genomes Project (June 24, 2014), indicates a remaining bias. Importantly, the rapidly reducing cost of next generation DNA sequencing paves the way for a paradigm shift in discovery: personal polymorphisms inaccessible by biased methods.
Previous reviews in lung diseases focused primarily on GWAS publications including descriptions of disease-SNP associations observed in only one dataset. The present study focused on replicated GWAS discoveries as well as new discoveries in whole genome sequencing of patients with a variety of lung diseases. We summarize the contribution of GWAS in identifying polymorphisms that confer risk to the development of specific pulmonary disorders and the GWAS studies that may influence patient responses to therapy. In addition, we have included a summary of next generation sequencing (NGS) DNAseq findings benefiting from various NGS technologies. The advent of NGS has made unbiased sequencing of DNA faster and cheaper than that of Sanger sequencing method. We did not include the lung cancer polymorphisms, as the number of discoveries in that specialty would require an entire different review. Finally, we reflect on the potential considerations and avenues for future studies that would aim to catalog the genetic determinants of complex lung diseases.
RESULTS
GWAS and Complex Pulmonary Diseases
We divided the studies reviewed into GWAS and next generation sequencing (NGS) categories with GWAS studies subdivided into three sections: obstructive pulmonary diseases (asthma and COPD), restrictive pulmonary diseases, and miscellaneous lung diseases. We also provide a systems biology interpretation of discovered variants. The NHGRI GWAS catalog reports 207 lung disease-associated SNPs from 32 publications. Of these, 134 SNP-disease associations were reproducible in independent cohorts. However, we identified that only 61% of these SNPs were reported by the NHGRI with the correct identifier as originally published in the references. We also identified 13 reproducible lung disease SNPs in these NHGRI-reported publications that were missing in the NHGRI catalog. Here, we review the entirety of these and provide the rectified results in tables.
Obstructive Pulmonary Diseases
Asthma
Asthma is a common chronic inflammatory disorder of the airways, characterized by episodic and reversible airflow obstruction, airway hyperresponsiveness, and underlying inflammation.1 In the United States, children, women, racial and ethnic minorities, residents of inner cities, and economically disadvantaged populations have a disproportionately higher burden of asthma morbidity and mortality when compared to the general population.2–4 Among children younger than 18 years of age, asthma is the third major cause of the hospital admissions.5 Of note, the pattern of its prevalence is significantly different with respect to various ages, countries with different economic infrastructure, and degree of severity.5 Duffy et al. estimated that asthma heritability is about 60% 6, highlighting the underlying role of genetic determinants in asthma development.
Asthma susceptibility
Asthma genetic variants have been the most studied among pulmonary diseases. Moffatt et al.7 conducted the first asthma GWAS and uncovered the association of variants of ORMDL3, which down regulates the sphingolipid synthesis, with childhood-onset asthma (Table 1). Himes and colleagues subsequently performed a GWAS across asthma patients of various ethnicities8 and discovered novel PDE4D polymorphism in combined populations of Hispanics and of European descent but not in African American individuals (Table 1). The authors also corroborated the association of the ORMDL3 variants with asthma. DeWan and colleagues in a GWAS work found evidence in favor of the association of variants of PDE11A gene with childhood allergic asthma9. Interestingly, both PDE11A and PDE4D genes belong to the phosphodiesterase superfamily of genes, implying the potential underlying role of this superfamily in development of asthma.9
Table 1.
Asthma susceptibility polymorphisms. The entries of the table have been ordered based on the dates in which the studies were published from the bottom to the top.
| Study | Sub phenotype | Ancestry | Control + Case (Replicates included) | Genes | Variants | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Susceptibility | Progression | European | AFAM | Hispanic | Asian | Other | |||||
| Genome-wide association study and admixture mapping identify different asthma-associated loci in Latinos: the Genes-environments & Admixture in Latino Americans study. | ✓ | ✓ | 7761 | IKZF3, MUC21, MUC22, PBMUCL2 | rs907092, 6p21 | 15 | |||||
| Genome-wide association analysis identifies 11 risk variants associated with the asthma with hay fever phenotype. | ✓ | ✓ | 24109 | ZBTB10, CLEC16A | rs7009110, rs62026376 | 82 | |||||
| A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. | ✓ | ✓ | ✓ | ✓ | 24913 | CDHR3, GSDMB, IL33, RAD50, IL1RL1 | rs6967330, rs2305480, rs928413, rs6871536, rs1558641 | 18 | |||
| HLA-DQ strikes again: genome-wide association study further confirms HLA-DQ in the diagnosis of asthma among adults. | ✓ | ✓ | ✓ | 15054 | HLA-DQA1 | rs9272346 | 83 | ||||
| Genome-wide association studies of asthma indicate opposite immunopathogenesis direction from autoimmune diseases. | ✓ | ✓ | 37015 | IL1RL2, IL1R1, TNIP1 | rs3755285, rs12619383, rs1422673 | 19 | |||||
| Genome-wide association study to identify genetic determinants of severe asthma. | ✓ | ✓ | ✓ | 5855 | ORMDL3, IL18R1, IL1R1 | rs4794820, rs3771166, rs9807989, rs13035227 | 17 | ||||
| Genome-wide association study identifies PERLD1 as asthma candidate gene. | ✓ | ✓ | 2025 | PERLD1 | rs2941504 | 14 | |||||
| Identification of IL6R and chromosome 11q13.5 as risk loci for asthma. | ✓ | ✓ | 57800 | IL6R, LRRC32 | rs4129267, rs7130588 | 84 | |||||
| Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. | ✓ | ✓ | 35083 | LOC729675, TSLP, WDR36, NOTCH4, LOC338591, IKZF4 | rs7686660, rs1837253, rs404860, rs10508372, rs1701704 | 12 | |||||
| Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. | ✓ | ✓ | ✓ | ✓ | ✓ | 12561 | PYHIN1, IL1RL1, TSLP, IL33, GSDMB | rs1101999, rs3771180, rs1837253, rs2381416, rs11078927 | 11 | ||
| Genome-wide association study identifies HLA-DP as a susceptibility gene for pediatric asthma in Asian populations. | ✓ | ✓ | 6420 | HLA-DPA1, SLC30A8 | rs987870, rs3019885 | 13 | |||||
| Association between ORMDL3, IL1RL1 and a deletion on chromosome 17q21 with asthma risk in Australia. | ✓ | ✓ | 3436 | IL1RL1, ORMDL3 | rs10197862, rs6503525 | 20 | |||||
| PDE11A associations with asthma: results of a genome-wide association scan. | ✓ | ✓ | ✓ | ✓ | 607 | PDE11A | rs11684634 | 9 | |||
| A large-scale, consortium-based genomewide association study of asthma. | ✓ | ✓ | 30478 | IL18R1, HLA-DQ, IL33, SMAD3, GSDMB, GSDMA, IL2RB, HLA-DRB1, FCER1A, IL13, STAT6, IL4-R/IL21R | rs3771166, rs9273349, rs1342326, rs744910, rs2305480, rs3894194, rs2284033, rs9271300, rs2252226, rs20541, rs167769, rs1859308 | 10 | |||||
| Variants of DENND1B associated with asthma in children. | ✓ | ✓ | ✓ | ✓ | 8956 | DENND1B, CRB1 | rs2786098, rs1775456 | 16 | |||
| A pooling-based genome-wide analysis identifies new potential candidate genes for atopy in the European Community Respiratory Health Survey (ECRHS). | ✓ | ✓ | 876 | SGK493 | rs4952590 | 85 | |||||
| Genome-wide association analysis identifies PDE4D as an asthma-susceptibility gene. | ✓ | ✓ | ✓ | ✓ | 25360 | PDE4D | rs1588265, rs1544791 | 8 | |||
| Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. | ✓ | ✓ | 4557 | ORMDL3 | rs7216389 | 7 | |||||
In a seminal GWAS comprised of about 30,000 cases and controls, Moffatt et al. discovered novel predisposing asthma susceptibility variants (Table 1).10 The list contains interesting genes involved in T cell responses such as IL2RB and HLA-DQ. Interestingly, the authors found that later-onset asthma is more influenced by MHC region (HLA-DQ rs9273349) in contrast to childhood-onset asthma, which is primarily dominated by 17q region (GSDMA/B). Interestingly, their findings failed to corroborate the role of genetic determinants of lung function in predisposing the individuals to asthma. Afterward, Torgerson and colleagues11 conducted a large meta-analysis in 2011. Their work included admixed populations of African Americans, African Caribbeans and Hispanics. They revealed for the first time an association of four previously known loci, namely 17q, TSLP that enhances the T helper type 2 cell responses, IL1RL1, and IL33 in three distinctive ethnicities. Additionally, they discovered the association of novel variants of the PYHIN1 gene in African American asthmatic patients (Table 1). The pyrin domain of PYHIN1 is found in proteins that are part of the apoptotic and inflammatory signals and respond to interferon11.
The contribution of GWAS approaches to uncover the genetic determinants of asthma pathogenesis has not been limited only to cases of European ancestry. Other groups performed GWAS with focus on patients of Asian descent12–14. Two groups specifically identified susceptibility polymorphisms of PERLD1 and HLA-DPA1 genes in Chinese and Japanese peoples, respectively (Table 1). PERLD1 particularly has some effects on activation and proliferation of T-cells14. Hirota et al. also performed a GWAS in a cohort composed of more than 7,000 and 27,000 asthmatic and control adult Japanese individuals, respectively.12 The authors discovered five asthma susceptibility genomic regions, namely 4q31, 5q22, 6p21, 10p14, 12q13. They mapped these five regions onto LOC729675, TSLP- WDR36, NOTCH4, LOC338591, and IKZF4, respectively (Table 1). It is of interest that they replicated the correlation of the polymorphism of TSLP with asthma in an additional 499 non-Hispanic individuals of European descent. Furthermore, they corroborated three previously known polymorphisms of rs1063355 of HLA-DQ, rs11071559 of RORA, and rs744910 of SMAD3. Recently, Galanter and colleagues15 conducted a GWAS work that included only Hispanics, who represent an admixed population comprised of varying combinations of European, African and Native American ancestries. Through an admixture-mapping technique, they discovered a novel locus on 6p21 that includes mucin-producing MUC21, MUC22, and PBMUCL2 genes residing in the MHC region. They also corroborated the previously known asthma region 17q21 in which polymorphism of IKZF3 gene, which regulates the development and proliferation of B-lymphocyte, was particularly the most significant one (Table 1).
The majority of GWAS studies have primarily focused on asthma of mild to moderate severity. However, Sleiman et al. conducted a seminal GWAS in which the target population was composed of children with persistent asthma requiring daily inhaled glucocorticoid therapy.16 They identified a novel predisposing asthma locus on chromosome 1q31 that is mapped to the gene DENND1B, a modulator of the type 1 helper T-cell cytokine signaling pathway (Table 1). They also replicated their findings in a cohort comprised of children of African descent. Interestingly, they found that the allele of the discovered variant in the population of African ancestry is opposite to that of children of European descent. Another group also focused on individuals with severe asthma in their GWAS.17 They found the loci (2q12, 17q12–21) underlying the association with asthma severity and overlapped with the ones that had been already discovered in previous GWAS (Table 1). Bønnelykke and colleagues hypothesized that acute asthma exacerbation is potentially a distinctive clinical phenotype. Therefore, they identified asthma predisposing polymorphisms of five genes, namely CDHR3, IL1RL1, IL33, GSDMB, and RAD50 (Table 1).18 The CDHR3 gene that encodes cadherin-related family member 3, was a novel finding whereas the remainder were previously known to be associated with asthma. Of note, they showed that rs6967330 variant of CDHR3 correlates with higher risk of hospitalization in addition to severe exacerbations. The findings from another GWAS with focus on severe asthmatic patients are also interesting. Li et al. found the opposite effects of polymorphisms that correlate with asthma susceptibility and other autoimmune diseases19. For instance, they showed that asthma susceptibility variants of the IL13 and HLA-DRA genes are protective against developing psoriasis and ulcerative colitis, respectively.
Overall, the association of common variants of seven genes with asthma have been significantly reproduced in at least two well-replicated GWAS works, namely ORMDL3/GSDMB (17q12),7,10,11,17,18, 20 TSLP (5q22),11,12 IL18R1/ IL1R1/ IL1RL1 (2q12),10,11,17–21 and IL33 (9p24.1) (Tables 1 and 2).10,11,18
Table 2.
Asthma susceptibility polymorphisms discovered through endophenotypes. The entries of the table have been ordered based on the dates in which the studies were published from the bottom to the top.
| Study | Endophenotype | Ancestry | Control + Case (Replicates included) | Genes | Variants | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| European | AFAM | Hispanic | Asian | ||||||
| Genome-wide association study of lung function decline in adults with and without asthma. | Lung function decline | ✓ | 16136 | DLEU7 | rs9316500 | 23 | |||
| A large-scale, consortium-based genomewide association study of asthma. | IgE levels | ✓ | 30478 | IL18R1, HLA-DQ, IL33, SMAD3, GSDMB, GSDMA, IL2RB, HLA-DRB1, FCER1A, IL13, STAT6, IL4-R/IL21R | rs3771166, rs9273349, rs1342326, rs744910, rs2305480, rs3894194, rs2284033, rs9271300, rs2252226, rs20541, rs167769, rs1859308 | 10 | |||
| Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. | Eosinophil counts | ✓ | ✓ | 26722 | IL1RL1, IKZF2, GATA2, IL5, SH2B3 | rs1420101, rs12619285, rs4143832, rs9494145, rs3184504 | 21 | ||
| Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. | YKL-40 levels | ✓ | 1772 | CHI3L1 | rs4950928 | 22 | |||
Endophenotypes as Surrogates for Asthma Susceptibility
Specific GWAS works assessed the utilization of the endophenotypes (e.g., protein) as surrogates for asthma status. In pioneering work, Ober et al. conducted a GWAS to identify variants correlating with YKL-40 levels.22 They had previously shown that YKL-40 (a mediator of airway inflammation) level in blood correlates with the severity of asthma. Their effort uncovered that polymorphisms residing within gene CHI3L1, encoding YKL-40, are significantly associated with quantitative trait YKL-40 level, risk of asthma, and declined lung function (Table 2). Gudbjartsson and colleagues also considered the circulating eosinophil count as a quantitative trait, since eosinophils are the most observed cell type in the airways of asthmatic patients.21 They found several novel polymorphisms underlying asthma susceptibility in patients of European descent (Table 2). In a novel effort, Imboden and colleagues23 employed the rate of the lung function decline as an end point in their GWAS. While they did not find any significant polymorphism underlying asthma risk, they discovered that a variant of the gene DLEU7 correlates with FEV1 in non-asthmatic individuals (Table 2).
Increased circulating levels of Immunoglobulin E (IgE) are associated with the development of various inflammatory disorders such as asthma. Therefore, it is of clinical interest to assess whether there is an overlap between the polymorphisms underlying circulating IgE levels and those of asthma susceptibility. Moffatt et al. conducted a large meta-analysis on the individuals of European descent (Table 2).10 The authors identified a novel polymorphism of HLA-DRB1 in addition to confirming previously identified variants influencing IgE level. However, they noticed that the main genetic determinants of asthma susceptibility and those of IgE level were not dependent. Specifically, HLA-DRB1 and HLA-DQB1, that influence the increased IgE level and asthma susceptibility, respectively, are in poor linkage disequilibrium. Importantly, they also showed that controlling for IgE level did not affect the predisposing effect of HLA-DQB1 on asthma. The authors concluded that that increment of the IgE level is not probably a causal factor in asthma development but a secondary phenomenon.
Asthma and Response to Therapeutic Interventions
GWAS approaches have been successfully utilized to uncover variants influencing the response to medications in asthmatic patients. The prevalence of aspirin or non-steroidal anti-inflammatory drugs (NSAIDs) sensitivity, especially among patients with glucocorticoid-dependent asthma can be as high as 10–20%.24 Park and colleagues 24 conducted a GWAS and uncovered the association of the polymorphisms of the HLA-DPB1 gene with aspirin intolerance in Korean people (Table 3). Managing the signs and symptoms of asthmatic patients is challenging due to the heterogeneity of response to therapy among asthmatic patients.25 Among various medications, inhaled glucocorticoids are the most commonly prescribed therapy in clinical practice with variable treatment response.25 Several studies have employed a GWAS to identify polymorphisms underlying the response to glucocorticoids. Tantisira et al. showed that rs37972 that maps to the gene GLCCI1 significantly correlates with decreased responses to inhaled glucocorticoids.26 Other GWAS groups discovered a novel candidate gene T in asthmatic patients whose SNPs influence the response to inhaled corticosteroids27 with a 2–3-fold difference in FEV1 response. Wu and colleagues showed that inhaled corticosteroids modify the response to β2-agonist through ZNF432 polymorphisms28 with patients carrying two copies of the mutant allele in the presence of inhaled corticosteroids exhibiting a greater β2-agonist response.
Table 3.
Polymorphisms affecting the response to medications in asthmatic patients. The entries of the table have been ordered based on the dates in which the studies were published from the bottom to the top.
| Study | Medication Response | Ancestry | Control + Case (Replicates included) | Genes | Variants | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| European | AFAM | Hispanic | Asian | ||||||
| Inhaled corticosteroid treatment modulates ZNF432 gene variant’s effect on bronchodilator response in asthmatics. | Bronchodilator response | ✓ | 808 | ZNF432 | rs12460587, rs3450, rs3752120 | 28 | |||
| Genome-wide association study of aspirin-exacerbated respiratory disease in a Korean population. | Aspirin exacerbation | ✓ | 1940 | HLA-DPB1 | rs2281389, rs3117230 | 24 | |||
| Genome-wide association identifies the T gene as a novel asthma pharmacogenetic locus. | Glucocorticoid response | ✓ | 825 | T, AC007922.13-1 | rs3127412, rs6456042, rs9955411 | 27 | |||
| Genomewide association between GLCCI1 and response to glucocorticoid therapy in asthma. | Glucocorticoid response | ✓ | 2104 | GLCCI1 | rs37972 | 26 | |||
Chronic Obstructive Pulmonary Diseases (COPD)
The hallmark of COPD is chronic inflammation of the airways, emphysematous changes in the respiratory bronchioles and the acini leading to progressive obstruction of airflow, which is largely irreversible. COPD is associated with several comorbidities such as heart failure and diabetes.29 Importantly, the World Health Organization (WHO) estimates that COPD will be the third principal cause of death by the year 2030.30 While tobacco smoking is a well-known risk factor for COPD and its related pathogenesis,31,32 not all smokers contract COPD, implying the underlying role of genetic factors.33 GWAS has successfully contributed to the discovery of the genetic polymorphisms (Table 4), despite the fact that earlier studies suffer from the lack of replicate cohorts and limited number of sample size. For example, Siedlinski and colleagues identified several loci34 but failed to include a replication cohort. Cho et al. identified novel loci35 (FAM13A, RAB4B, CHRNA3/5, IREB2, HHIP) and recently focused on moderate to severe or severe COPD cases.36 They combined the data from COPDGene, ECLIPSE, NETT/NAS, Norway GenKOLS, and International COPD Genetics Network (ICGN) cohorts in a meta-analysis, that also includes African- American patients, to successfully confirm the underpinning roles of previously discovered genes CHRNA3 (a member of the nicotinic acetylcholine receptor family), FAM13A (a GTPase activator), and HHIP (a hedgehog family protein binding), and MMP12 (a member of matrix metalloproteinase family) (Table 4). The authors also found supporting evidence that the genes TGFB2, RIN3, and the locus 19q13 play important roles in COPD susceptibility. For example, they showed that TGFB2 is in strong linkage disequilibrium with rs6684205, which acts in pulmonary tissue as an expression quantitative trait locus for TGFB.36,37 Also, it is of interest that candidate COPD susceptibility genes, such as HHIP and FAM13A, overlap with genes that have a role in the normal function of lung (Table 6).38 It is of interest that Manichaikul et al. conducted a GWAS across different ethnic groups in which they considered percentage of emphysema as the outcome. They became able to identify two polymorphisms rs7957346 near to SNRPF and rs10947233 within PPT2 genes (Table 4) 39. However, they replicated the discovered SNPs only in patients of European descent.
Table 4.
COPD susceptibility polymorphisms. The entries of the table have been ordered based on the dates in which the studies were published from the bottom to the top.
| Study | Sub phenotype |
Ancestry | Control + Case (Replicates included) |
Genes | Variants | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Susceptibility | Progression | Endophenotype | Other/ Description | European | AFAM | Hispanic | Asian | Other | |||||
| Risk loci for chronic obstructive pulmonary disease: a genome-wide association study and meta-analysis. | ✓ | ✓ | ✓ | ✓ | 12337 | CHRNA3, FAM13A, HHIP, MMP12, RAB4B | rs12914385, rs4416442, rs13141641, rs626750, rs7937 | 36 | |||||
| Genome-wide study of percent emphysema on computed tomography in the general population. The Multi-Ethnic Study of Atherosclerosis Lung/SNP Health Association Resource Study. | ✓ | ✓ | Percentage of Emphysema | ✓ | ✓ | ✓ | ✓ | 7914 | SNRPF, PPT2 | rs7957346, rs10947233 | 39 | ||
| A genome-wide association study of COPD identifies a susceptibility locus on chromosome 19q13. | ✓ | ✓ | 8280 | FAM13A, RAB4B, EGLN2, CYP2A6, CHRNA3, CHRNA5, IREB2, HHIP | rs1964516, rs7937, rs11858836, rs13141641 | 35 | |||||||
| Genome-wide association analysis of body mass in chronic obstructive pulmonary disease. | ✓ | ✓ | 3452 | FTO | rs8050136 | 86 | |||||||
| Variants in FAM13A are associated with chronic obstructive pulmonary disease. | ✓ | ✓ | 9134 | FAM13A | rs7671167 | 87 | |||||||
| A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. | ✓ | ✓ | 5334 | CHRNA3, NP_001013641.2, HHIP | rs1051730, rs8034191, rs1828591, rs13118928 | 88 | |||||||
Table 6.
Polymorphisms underlying the normal lung function and susceptibility to miscellaneous pulmonary diseases (BPD: bronchopulmonary dysplasia, PVD: pulmonary vascular disease)
| Study | Disease/ Trait | Sub phenotype | Ancestry | Control + Case (Replicates included) |
Genes | Variants | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Susceptibility | Progression | Endophenotype | Other/ Description | European | AFAM | Hispanic | Asian | Other | ||||||
| Genome-wide association analysis identifies a susceptibility locus for pulmonary arterial hypertension. | PVD | ✓ | ✓ | 2150 | CBLN2 | rs2217560, rs9916909 | 57 | |||||||
| Identification of SPOCK2 as a susceptibility gene for bronchopulmonary dysplasia. | BPD | ✓ | ✓ | ✓ | 603 | SPOCK2 | rs1245560, rs1049269 | 52 | ||||||
| Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. | Normal Pulmonary Function | Pulmonary function | ✓ | 94612 | TNS1, FAM13A, GSTCD-NPNT, HHIP, HTR4, ADAM19, AGER, GPR126, PTCH1, TSHD4, MFAP2, TGFB2, HDAC4, RARB, MECOM, SPATA9, ZKSCAN3, ZNF323, NCR3, ARMC2, CDC123, C10orf11, LRP1, CCDC38, MMP15, CFDP1, KCNE2 | rs2571445, rs2869967, rs2045517, rs7671167, rs10516526, rs1032296, rs12504628, rs11100860, rs1980057, rs11168048, rs3995090, rs1985524, rs2277027, rs11134779, rs2070600, rs3817928, rs262129, rs16909859, rs16909898, rs12899618, rs8033889, rs2284746, rs993925, rs12477314, rs1529672, rs1344555, rs153916, rs6903823, rs2857595, rs2798641, rs7068966, rs11001819, rs11172113, rs1036429, rs12447804, rs2865531, rs9978142 | 38 | |||||||
Restrictive Pulmonary Disorders (Interstitial lung diseases)
Interstitial lung diseases (ILDs) are a heterogeneous group of pathologic conditions that manifest clinically and radiologically as diffuse parenchymal lung disease. The pathologic findings of ILDs include various stages and patterns of interstitial inflammation, interstitial fibrosis, and/or alveolar filling. When a cause is not clearly apparent, the term idiopathic interstitial pneumonia (IIP) is used. Physiologically, ILDs generally manifest as a restrictive ventilatory defect and a gas transfer defect.
Idiopathic Pulmonary Fibrosis (IPF)
Idiopathic pulmonary fibrosis (IPF) is the most common and the most severe form of IIP.40–41 Despite the dismal prognosis of IPF, a limited number of GWAS have been carried out, thus far, in order to uncover the polymorphisms underlying its course of development and progression. Our work and work by others have shown common genetic variants of TERT (a regulator of telomerase activity), TOLLIP (an activator of MYD88-dependent NF-κB in order to adjust the Toll-like receptor signaling and membrane trafficking), and MUC5B (an important gel-forming mucin in mucus), to be strongly associated with IPF susceptibility. Mushiroda et al. in a GWAS on a group of Japanese patients with sporadic IPF found an association between the common variants within the gene TERT and IPF (Table 5) 42. We performed a GWAS to discover novel polymorphisms underlying IPF susceptibility 43 (Table 5) and highlighted the importance of 11p15.5 in the development and progression of IPF. We identified a novel IPF susceptibility variant within TOLLIP and also confirmed the association of previously known MUC5B 44 with IPF. Of note, we discovered that the minor allele of the novel polymorphism of TOLLIP correlate with the patients’ odds of mortality.43 Parallel to our work, another group also confirmed the association of IPF with MUC5B at 11p15, TERT at 5p15, and the 3q26 region near TERC.45 They also identified other novel polymorphisms both at significant and suggestive level of statistical significance (Table 5).
Table 5.
Polymorphisms associated with pulmonary fibrosis and sarcoidosis susceptibility. The entries of the table have been ordered based on the dates in which the studies were published from the bottom to the top.
| Study | Disease | Sub phenotype | Ancestry | Control + Case (Replicates included) | Genes | Variants | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Susceptibility | Progression | European | AFAM | Hispanic | Asian | Other | ||||||
| Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. | Pulmonary Fibrosis | ✓ | ✓ | ✓ | 3341 | MUC5B, TOLLIP | rs35705950, rs5743890 | 43 | ||||
| Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. | ✓ | ✓ | 8610 | DSP, 7q22, 15q14–15, DPP9 | rs2076295, rs4727443, rs2034650, rs12610495 | 45 | ||||||
| A genome-wide association study identifies an association of a common variant in TERT with susceptibility to idiopathic pulmonary fibrosis. | ✓ | ✓ | 1711 | TERT | rs2736100 | 42 | ||||||
| Genome-wide association analysis reveals 12q13.3–q14.1 as new risk locus for sarcoidosis. | Sarcoidosis | ✓ | ✓ | 9275 | OS9 | rs1050045 | 89 | |||||
| A novel sarcoidosis risk locus for Europeans on chromosome 11q13.1. | ✓ | ✓ | 8878 | CCDC88B, KCNK4, PRDX5, PCLB3 | rs479777 | 48 | ||||||
| A genome-wide association study reveals evidence of association with sarcoidosis at 6p12.1. | ✓ | ✓ | 4138 | RAB23 | rs10484410 | 90 | ||||||
| Genome-wide association study identifies ANXA11 as a new susceptibility locus for sarcoidosis. | ✓ | ✓ | 4470 | ANXA11 | rs2789679, rs7091565 | 47 | ||||||
| Genome-wide association analysis in sarcoidosis and Crohn’s disease unravels a common susceptibility locus on 10p12.2. | ✓ | ✓ | 3582 | C10ORF67 | rs1398024 | 91 | ||||||
Granulomatous Disorders
An important subgroup of ILDs includes granulomatous disorders such as sarcoidosis. The pathologic hallmark of sarcoidosis is the presence of non-caseating epithelioid granulomas in the absence of a known etiology. Sarcoidoisis is a systemic disorder affecting various organs with a wide range of prognosis from self-limited disease to fatal outcome.46 The studies focusing on monozygotic twins, families, and various races have provided supporting evidence in favor of putative genetic loci predisposing individuals to sarcoidosis.46 Previous GWASs have successfully uncovered a limited number of non-HLA common susceptibility loci (Table 5). Among various genetic determinants that have been discovered thus far, two regions are of biological and clinical interest, namely 10q22.3 and 11q13.1. The former contains ANXA11, which is essential for cytokinesis and its product is also detected in the sera of patients with different autoimmune disorders, as a predisposing gene to sarcoidosis that has been replicated in patients of non-European descent, particularly African Americans.47 Other works had shown the association of the latter with other diseases including alopecia areata, Crohn’s disease, leprosy, psoriasis, and primary biliary cirrhosis.48 This region contains CCDC88B, KCNK4, PRDX5, PCLB3 genes with CCDC88B as the most promising putative sarcoidosis susceptibility gene (Table 5).48
Miscellaneous Pulmonary Diseases
GWAS has successfully interrogated various genomic loci in other pulmonary diseases as well, particularly in bronchopulmonary dysplasia (BPD) and pulmonary arterial hypertension (PAH).
BPD is a chronic form of neonatal respiratory disorders characterized by irreversible airflow obstruction,49 with a significant degree of morbidity.50 About one third of premature infants in neonatal care units develop BPD.50 Previous twin studies uncovered that the genetic factors strongly underpin the development of BPD.51 Despite the clinical importance of BPD, only a limited number of GWAS have identified putative susceptibility genetic loci.52,53 Hadchouel et al. successfully identified two BPD predisposing polymorphisms; rs1245560 and rs1049269 of SPOCK2 gene, which its expression level is positively correlated with alveolarization of normal rat lung (Table 6).52 Importantly, they showed that rs1245560 variant is associated with BPD susceptibility in both populations of European and African descents.
Pulmonary arterial hypertension (PAH) is a rare lung disorder. The pathological hallmark of PAH is the presence of intimal lesions that consist of eccentric thickening, fibrotic, plexiform, concentric, and dilation lesions in the vessels of the pulmonary arterial circulation. This translates into persistently high pulmonary artery pressures, progressive right heart failure, and a high mortality in untreated patients.54,55 Previous works had identified the association of several genotypes with PAH, such as BMPR2 (a member of trans-membrane serine/threonine kinases receptor family).56 Nevertheless, the finding that mutated BMPR2 accounts for approximately 80% and 15% of familial PAH and idiopathic PAH patients prompted Germain and colleagues to conduct a GWAS to discover novel susceptibility polymorphisms in these two PAH groups.56,57 They particularly controlled for BMPR2 mutation and found two novel SNPs, namely rs2217560 and rs9916909, which are in complete linkage disequilibrium. Accordingly, they identified the novel predisposing gene CBLN2 that resides upstream of the uncovered SNPs and encodes a protein, which binds an adhesion molecule in vascular smooth muscle cells (Table 6).
Systems biology implications of these polymorphisms
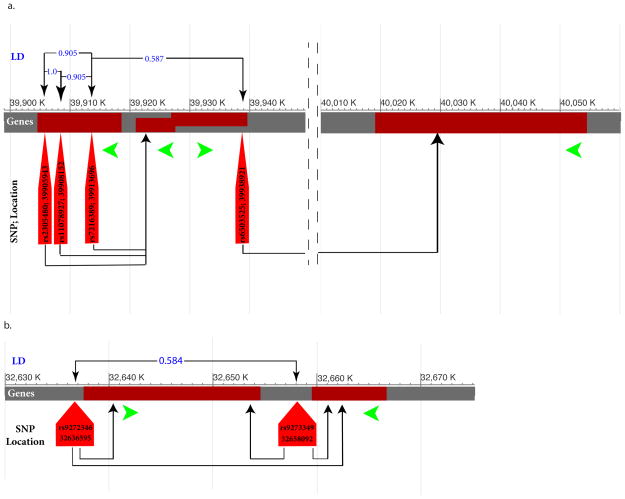
Using expression quantitative trait loci (eQTL), we identified two interesting findings about the mRNAs in cis and trans associations with SNPs reported in Tables 1–6 (National Center for Biotechnology Information eQTL Browser; http://ncbi.nlm.nih.gov/projects/gap/eqtl/index.cgi). There is a group of 4 asthma risk-associated SNPs in linkage disequilibrium with one another (Figure 1. panel a) located on the chromosome region 17q12 intragenic to two host genes GSDMB and LOC101928947. These four SNPs may be reporting a locus rather than a specific gene, as they adjust the expression values of either ORMDL3 or MED24 mRNAs (Figure 1. panel a). In addition, both asthma risk SNPs (Table 1) rs9272346 (2kb upstream of HLA-DQA1) and rs9273349 (2kb down-stream of HLA-DQB1) are in eQTL associations with HLA-DQA1 and HLA-DQB1 mRNAs. Given the transcription direction of these HLA genes, rs9273349 seems to be within a putative enhancer element, which needs to be experimentally validated. The latter 2 SNPs were also shown in many distinct GWAS that are associated with immune-related diseases (Figure 1. panel b; NCBI eQTL browser; celiac diseases, inflammatory bowel diseases, multiple sclerosis, nasal pharyngeal carcinoma, rheumatoid arthritis, schizophrenia, systemic lupus erythematous, type I diabetes mellitus). We also employed the Database for Annotation, Visualization and Integrated Discovery (DAVID v6.7; http://david.abcc.ncifcrf.gov/), in order to infer the functional relationship among the gene loci of SNPs reported in Tables 1–6. This analysis shows that the major biological processes to which the candidate lung diseases associated genes contribute are primarily related to various domains of immune response, such as cytokine receptor activity (Supplement Table 1).
Figure 1.
eQTL interaction diagram. The green arrows represent the direction of transcription of the genes. The upper part of each panel corresponds to the degree of linkage disequilibrium values between the SNPs (blue numbers). The arrows at the lower part of each panel show the direction of eQTL effects; the expression values of which genes are adjusted by which SNP (results from eQTL browser). a. The panel shows that three out four well-replicated asthma polymorphisms (LD > 0.9) reside within GSDMB genes while adjusting the expression value of the neighboring ORMDL3 gene. b. The panel represents how two other asthma susceptibility polymorphisms adjust the expression values of HLA-DQA1 and HLA-DQB1. rs9273349 is in an inter-genic region while rs9272346 resides 2 kb upstream of the HLA-DQA1.
Next generation sequencing technologies and complex pulmonary diseases
The underlying assumption of GWAS is the “common disease-common variant (CDCV) hypothesis”58 in which small number of alleles contribute to the risk of development or progression of a given disease and each allele has small to moderate effect size. Therefore, previous GWAS, thus far, has aimed to discover the association of genetic variants with MAF>5% and in some cases MAF>1% within the trait or disease of interest. However, it is now clear that GWAS discovered variants explain only a small amount of observed heritability of the related traits (missing heritability). In contrast to a CDCV hypothesis, the “common disease, rare variant (CDRV) hypothesis” states that genomic loci that confer risk of disease are catalogued with many rare and independent risk alleles with modest to high effect size that are scattered across the population.58 CDRV hypothesis implies the presence of an extensive degree of genetic heterogeneity in a putative risk gene or locus and also the lack of linkage disequilibrium among the variants, thereby debunking the inherent assumptions of genotyping-based methods but highlighting the importance of sequencing-based strategies. Several works have employed NGS methods in order to identify different lung disease predisposing genetic variants (Supplement Table 2). Others have utilized NGS techniques to profile the metagenome of airways and its interaction with distinct pulmonary ailments (Supplement Table 2).
Predisposing genetic variants to pulmonary diseases using DNA sequencing
DeWan and colleagues59 performed the first whole exome sequencing (WES) with focus on a family enriched with asthma. By re-sequencing the variants of three genes, PDE4DIP, CBLB, and KALRN, they showed that asthmatic members are heterozygote opposite to non-asthmatic ones. They did not find the significant enrichment of selective asthma related common variants in genes, ORMDL3, PDE11A, PDE4D, and RAD50, within affected members. They were unable to show that the asthmatic members have significantly higher burden of rare variants. Recently, Campbell et al.60 conducted a whole genome sequencing (WGS) on 16 individuals (8 asthmatic) from a Hutterite population in order to find novel asthma genetic variants that have not been uncovered in previous GWASs. However, none of their discovered variants met genome-wide significance threshold, specifically mutations within gene NEDD4L, which contributes to the ubiquitination of specific proteins for lysosomal degradation. They were unable to replicate their findings in other populations of Europeans, African Americans, and Hispanics. Wain et al. aimed at finding putative variants protecting smokers from lung function decline.61 They re-sequenced the exome of 100 smokers with uncompromised lung function, namely resistant smokers, and 396 individuals from two independent control groups. None of their detected signals survived multiple testing corrections. However, they reported rs10859974 as the strongest detected signal in CCDC38 gene, which has been known to be associated with lung function. While this finding is interesting, it demands to be replicated in additional independent cohorts.
The use of NGS methods has not been limited to only obstructive pulmonary diseases. Austin et al.62 utilized whole-exome sequencing (WES) to uncover novel hereditary PAH susceptibility variants in a family in which 4 and 8 members were affected and non-affected, respectively. By doing so, they were able to find mutated CAV1 as a new predisposing gene that is a modifier of TGF-β signaling at the plasma membrane. Interestingly, they noticed that some unaffected members also are carriers of the same mutation, indicating its incomplete penetrance. Of note, they controlled for the previously identified HPAH mutated genes, namely ALK1, ENG, or SMAD9. They replicated their finding in 260 unrelated patients of Caucasian descent with HPAH and IPAH. They also did not detect the mutated CAV1 in the genotype of 1000 ethnically matched healthy Caucasian. Ma and colleagues63 also employed WES and studied a family with several affected members. They also ruled out the presence of mutations in previously identified disease predisposing genes. Their effort gave rise to identifying new candidate predisposing gene, KCNK3 that is a potassium channel subfamily K. Importantly, they replicated their result in 92 unrelated patients with familial PAH and also 230 IPH. While the nuclear families were the target discovery set of the previous two studies, Perez et al. interrogated the genomes of 12 unrelated IPAH patients with WES. Consequently, they discovered TopBP1 as a novel IPAH susceptibility gene.64
Recently, some works have also employed WES techniques to uncover genetic determinants of acute respiratory distress syndrome (ARDS). Shortt et al.65 studied 96 patients with ARDS (70 Caucasian and 26 African Americans), and identified 76 novel ARDS susceptibility SNPs. They also selectively genotyped three of these SNPs, rs3848719 (ZNF335 gene that contributes in proliferation of neural progenitor cells and self-renewal), rs78142040 (ARSD gene that contributes to the development of cartilage and is a member of the sulfatase family), rs9605146 (in XKR3 gene that is a potential membrane transporter), in an additional 117 patients and found the correlation of rs3848719 and rs78142040 with APACHE II score quartile and 60-day mortality.
Metagenome and association of the microbiome with pulmonary diseases
Other works have utilized NGS techniques in order to examine the association of microbiome diversity in the respiratory tract with susceptibility to lung disease. In the first effort, Dannemiller et al.66 assessed the association of the various fungal taxa and their diversity in the house dust with childhood asthma in low-income Hispanic families. While positive association between various taxa with asthma was not identified, lower fungal diversity, particularly lower diversity within the genus Cryptococcus, was positively correlated with asthma susceptibility. Park and colleagues 67 studied the potential difference of the microbial community of upper respiratory tract of 18 asthmatics and 17 COPD patients compared to 12 healthy controls. Significant difference between the oropharynx microbiome of asthmatics and COPD patients was not discerned, although there was an abundance of Pseudomonas spp. from Proteobacteria and Lactobacillus spp. Garzoni and colleagues 68 examined the alteration of the composition of the microbiome of upper and lower respiratory tracts of 33 individuals with idiopathic interstitial pneumonia, sarcoidosis, pneumocystis pneumonia and healthy controls to determine disruption in respiratory tract microbiota and showed altered upper and lower airways microbiota in 23% patients. Han et al.69 employed the 454 pyrosequencing method and analyzed the degree of the impact of lung microbiome of 55 IPF patients on the progression of their disease and showed the potential association of a bacterial signature, members within the Staphylococcus and Streptococcus genera, with the patients’ odds of outcome, a correlation that remains significant after adjusting for genetic effects.
DISCUSSION
We have reviewed how GWAS has remarkably contributed to increasing our knowledge about the genetic determinants underlying the development and progression of distinctive pulmonary diseases and their responses to therapeutics. GWAS has helped researchers to discover the polymorphisms that make individuals prone to developing various complex lung diseases such as asthma, chronic obstructive pulmonary disease (COPD), and sarcoidosis. Despite all of the successes that GWAS have achieved thus far, the variants discovered with GWAS have yet to explain most of the estimated heritability of various complex diseases and traits,70,71 and our understanding about the pathogenesis of pulmonary diseases has remained incomplete.
While GWAS is a multiplexed interrogation of the genome, the downstream biological effect of many discovered susceptibility variants and the various ways they impact diseases pathogenesis remain unclear. It is common that researchers map the discovered intergenic variants to the closest neighboring gene. However, the recent discoveries from ENCyclopedia Of DNA Elements (ENCODE) Project72 have shown that variants residing in distal regulatory elements might affect the expression of not necessarily neighboring genes through various chromatin modification mechanisms.73 Future progress in utilizing new technologies, such as Hi-C, to explain various epigenomic mechanisms in the context of lung diseases will provide pulmonary scientists with a better explanation of the biological impacts of lung disease genetic variants. Integration of other various tiers of data, such as protein interaction 74 and expression quantitative trail loci data,75 is also important to increase the interpretability of GWAS results. Consequently, developing more sophisticated computational techniques/ heuristics is necessary. Much of the GWAS work has primarily focused on measuring the significant association of the variants individually with disease susceptibility, progression, or a certain outcome. Nevertheless, it has become exceedingly clear that the discovered lung disease susceptibility genetic variants, similar to other complex diseases, are not capable of explaining all of the measured heritability. Part of this missing heritability resides in the interaction between distinctive variants. For example, our work showed that IPF patients might have a higher rate of mortality despite inheriting the protective allele.43 Therefore, devising new innovative experiments to unravel the epistatic interaction is necessary. On the computational front, development of new methods to analyze the GWAS data is also needed, specifically the ones capable of detecting interaction effects effectively in a reasonable computational time.
Another obvious limitation of GWAS, thus far, is the lack of work with a focus on admixed populations, namely African Americans and Hispanics. Most of the GWASs have primarily targeted patients of European descent. For example, we found no GWAS for African American individuals with sarcoiodosis in our review. However, some work has reported the role of various ancestries in increment of disease susceptibility and response to therapeutics.76 For example, Rumpel et al. found evidence that asthmatic African Americans with the higher African ancestry experience higher rate of severe exacerbation.77 Another group also reported that Puerto Ricans have a higher asthma prevalence and its related morbidities compared to Mexican Americans,78,79 despite the fact that both groups are designated as Hispanics. On the other hand, Choudhry and colleagues found that socioeconomic status (SES) and ancestry significantly interact with each other among asthmatic Hispanic individuals.80 Particularly, they showed that at the lower spectrum of SES, the European ancestry correlates more with asthma risk, opposite to the other end of the SES in which African ancestry is more associated with the risk of asthma. Therefore, it is crucial to consider patients’ SES and ancestry in future GWAS. The underrepresented GWAS with focus on admixed populations could also be partly attributed to some limitations in the availability of genotyping technology. Most of the whole-genotyping platforms incorporate polymorphisms specific for subjects of European descent, thereby highlighting the urge for devising more comprehensive genotyping platforms.
Future GWASs should also focus on identifying genetic variants that distinguish various subtypes or clinical manifestations of pulmonary disorders in addition to asthma. For example, while our review identified GWAS results for interstitial pulmonary fibrosis, we found no work relating to non-specific interstitial pneumonia or differentiating between different subtypes of ILD. In addition, our review did not find GWASs identifying lung disease polymorphisms specific to males or females (gender specific polymorphisms). For instance, while there is a slightly higher prevalence of asthma attack among women (53.5%) 92, GWASs failed to identify gender specific asthma polymorphisms when looking for it 10,14,18. As another example, the male gender is one the risk factors for predicting the outcome of IPF 93, yet, Fingerlin et al. did not find any significant association of SNPs with gender 45. Additionally, while IPH is 1.7 times more common among women 55, Germain et al. did not observe any evidence in favor of existing gender specific polymorphism 57.
We also summarized the contribution of various NGS methods to uncover the role of rare genetic variants in making individuals prone to various lung diseases. We further explained how these techniques have been helpful for profiling the microbiome of the lung and its interaction with pulmonary diseases. NGS methods are capable of addressing how rare variants confer the disease risk. Of note, the NGS era indeed heralds the path to personalized medicine. The number of published works that have employed the novel and massively parallel WGS and WES technologies is still limited. However, a gradual drop of the cost during the course of the past few years, that is predicted to continue, will be providing the pulmonary scientist with a great opportunity to search for the rare variants that alter the risk to developing various lung diseases or response to various medications. It is easy to picture that pulmonologists will add the skill of how to interpret the results generated from the NGS methods in the near future to their knowledge repository, subsequently translating them to routine clinical practice.
In view of all these discoveries and the soon to be tsunami of personal polymorphisms, the new challenge is to associate clinically meaningful and actionable meaning with all these discoveries. As precision health requires understanding individuals, this may serve as an additional stimulus to interrogate the genetic make-ups of individuals with Hispanic and African American ancestries, as the studies with focus on these populations are lacking. Other strategies include more powerful study designs such as focusing on cases with more extreme and severe phenotypes, more sophisticated GWAS analysis technique and NGS analyzing methods, as well.
METHODS
We employed NHGRI GWAS catalogue81 to find the GWAS publications that are related to various primary non-cancerous lung diseases; we found 81 curated publications. Among them, we focused on publications with results of discovery cohorts that have been separately and significantly replicated in their replication cohorts. The tables comprise an exhaustive listing of reproducible variants reported in the studied manuscript. The authors might have mapped a certain polymorphism onto more than one gene. Therefore, the number of assigned genes might exceed the number of polymorphisms in several studies. We also used query terms of the various pulmonary diseases plus terms related to next generation/ whole exome/ whole genome sequencing in PubMed in order to find the publications where NGS have been utilized. We employed the original articles and the GeneCards (http://www.genecards.org/) in order to annotate the functions of the genes.
Supplementary Material
Table 1. Functional classification of polymorphisms underlying pulmonary disease/traits.
Table 2. Summary of the NGS findings.
Acknowledgments
This manuscript was funded in part by the University of Arizona Health Sciences Center, the University of Arizona Cancer Center (P30CA023074), and the University of Arizona Center for Biomedical Informatics and Biostatistics. We appreciate the contribution of Ms. Colleen Kenost. Dr. Joe G.N. Garcia is the founder, president, and majority shareholder of Aqualung Therapeutics, Corp. Dr. Yves A. Lussier is a member of the scientific advisory board of IPSEN corporation. All other authors do not have any financial or personal relationship with organizations that could potentially be perceived as influencing the described research. All authors have read the journal’s policy on disclosure of potential conflicts of interest.
Abbreviations
- GWAS
genome-wide association study
- NGS
next generation sequencing
- DNAseq
DNA sequencing
Footnotes
Conflict of interest
Dr. Joe G.N. Garciais the founder, president, and majority shareholder of Aqualung Therapeutics, Corp. Dr. Yves A. Lussier is a member of the scientific advisory board of IPSEN corporation. All other do not have any financial or personal relationship with organizations that could potentially be perceived as influencing the described research. All authors have read the journal’s policy on disclosure of potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma–Summary Report 2007. National Asthma Education and Prevention Program Expert Panel Report 3 - Guidelines for the Diagnosis and Management of Asthma, Summary Report 2007. 2007;120(5, Supplement 1):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Moorman JE, Rudd RA, Johnson CA, et al. National surveillance for asthma--United States, 1980–2004. Morbidity and mortality weekly report. Surveillance summaries. 2007;56(8):1–54. [PubMed] [Google Scholar]
- 3.Moorman JE, Mannino DM. Increasing U.S. asthma mortality rates: who is really dying? The Journal of asthma : official journal of the Association for the Care of Asthma. 2001;38(1):65–71. doi: 10.1081/jas-100000023. [DOI] [PubMed] [Google Scholar]
- 4.Moorman JE, Akinbami LJ, Bailey CM, et al. Vital & health statistics. Series 3, Analytical and epidemiological studies. 35. U.S. Dept. of Health and Human Services, Public Health Service, National Center for Health Statistics; 2012. National surveillance of asthma: United States, 2001–2010; pp. 1–67. [PubMed] [Google Scholar]
- 5.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355(21):2226–35. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 6.Duffy DL, Martin NG, Battistutta D, Hopper JL, Mathews JD. Genetics of asthma and hay fever in Australian twins. Am Rev Respir Dis. 1990;142(6_pt_1):1351–8. doi: 10.1164/ajrccm/142.6_Pt_1.1351. [DOI] [PubMed] [Google Scholar]
- 7.Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448(7152):470–3. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 8.Himes BE, Hunninghake GM, Baurley JW, Rafaels NM, Sleiman P, Strachan DP, et al. Genome-wide Association Analysis Identifies <i>PDE4D</i> as an Asthma-Susceptibility Gene. Am J Hum Genet. 2009;84(5):581–93. doi: 10.1016/j.ajhg.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeWan AT, Triche EW, Xu X, Hsu L-I, Zhao C, Belanger K, et al. PDE11A associations with asthma: results of a genome-wide association scan. J Allergy Clin Immunol. 2010;126(4):871–873. doi: 10.1016/j.jaci.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211–21. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Program CAM, Ober C, Nicolae DL MCAAS MCCAS, others. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011;43(9):887–92. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirota T, Takahashi A, Kubo M, Tsunoda T, Tomita K, Doi S, et al. Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. Nat Genet. 2011;43(9):893–6. doi: 10.1038/ng.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noguchi E, Sakamoto H, Hirota T, Ochiai K, Imoto Y, Sakashita M, et al. Genome-wide association study identifies HLA-DP as a susceptibility gene for pediatric asthma in Asian populations. PLoS Genet. 2011;7(7):e1002170. doi: 10.1371/journal.pgen.1002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anantharaman R, Andiappan AK, Nilkanth PP, Suri BK, Wang DY, Chew FT. Genome-wide association study identifies PERLD1 as asthma candidate gene. BMC Med Genet. 2011;12(1):170. doi: 10.1186/1471-2350-12-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galanter JM, Gignoux CR, Torgerson DG, Roth LA, Eng C, Oh SS, et al. Genome-wide association study and admixture mapping identify different asthma-associated loci in Latinos: The Genes-environments & Admixture in Latino Americans study. J Allergy Clin Immunol. 2014;134(2):295–305. doi: 10.1016/j.jaci.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sleiman PM, Flory J, Imielinski M, Bradfield JP, Annaiah K, Willis-Owen SA, et al. Variants of DENND1B associated with asthma in children. N Engl J Med. 2010;362(1):36–44. doi: 10.1056/NEJMoa0901867. [DOI] [PubMed] [Google Scholar]
- 17.Wan Y, Shrine N, Artigas MS, Wain L, Blakey J, Moffatt M, et al. Genome-wide association study to identify genetic determinants of severe asthma. Thorax. 2012;67(9):762–8. doi: 10.1136/thoraxjnl-2011-201262. [DOI] [PubMed] [Google Scholar]
- 18.Bønnelykke K, Sleiman P, Nielsen K, Kreiner-Møller E, Mercader JM, Belgrave D, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014;46(1):51–5. doi: 10.1038/ng.2830. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Ampleford EJ, Howard TD, Moore WC, Torgerson DG, Li H, et al. Genome-wide association studies of asthma indicate opposite immunopathogenesis direction from autoimmune diseases. J Allergy Clin Immunol. 2012;130(4):861–8. doi: 10.1016/j.jaci.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreira MA, McRae AF, Medland SE, Nyholt DR, Gordon SD, Wright MJ, et al. Association between ORMDL3, IL1RL1 and a deletion on chromosome 17q21 with asthma risk in Australia. Eur J Hum Genet. 2010;19(4):458–64. doi: 10.1038/ejhg.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41(3):342–7. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 22.Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358(16):1682–91. doi: 10.1056/NEJMoa0708801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imboden M, Bouzigon E, Curjuric I, Ramasamy A, Kumar A, Hancock DB, et al. Genome-wide association study of lung function decline in adults with and without asthma. J Allergy Clin Immunol. 2012;129(5):1218–28. doi: 10.1016/j.jaci.2012.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park BL, Kim T-H, Kim J-H, Bae JS, Pasaje CFA, Cheong HS, et al. Genome-wide association study of aspirin-exacerbated respiratory disease in a Korean population. Hum Genet. 2013;132(3):313–21. doi: 10.1007/s00439-012-1247-2. [DOI] [PubMed] [Google Scholar]
- 25.Drazen JM, Silverman EK, Lee TH. Heterogeneity of therapeutic responses in asthma. Br Med Bull. 2000;56(4):1054–70. doi: 10.1258/0007142001903535. [DOI] [PubMed] [Google Scholar]
- 26.Tantisira KG, Lasky-Su J, Harada M, Murphy A, Litonjua AA, Himes BE, et al. Genomewide association between GLCCI1 and response to glucocorticoid therapy in asthma. N Engl J Med. 2011;365(13):1173–83. doi: 10.1056/NEJMoa0911353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tantisira KG, Damask A, Szefler SJ, Schuemann B, Markezich A, Su J, et al. Genome-wide association identifies the T gene as a novel asthma pharmacogenetic locus. Am J Respir Crit Care Med. 2012;185(12):1286–91. doi: 10.1164/rccm.201111-2061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu AC, Himes BE, Lasky-Su J, Litonjua A, Peters SP, Lima J, et al. Inhaled corticosteroid treatment modulates <i>ZNF432</i> gene variant’s effect on bronchodilator response in asthmatics. J Allergy Clin Immunol. 2014;133(3):723–8. doi: 10.1016/j.jaci.2013.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnes P, Celli B. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–85. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 30.Berndt A, Leme AS, Shapiro SD. Emerging genetics of COPD. EMBO Mol Med. 2012;4(11):1144–55. doi: 10.1002/emmm.201100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. The lancet. 2009;374(9691):733–43. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 32.Shaykhiev R, Crystal RG. Early Events in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Smoking-induced Reprogramming of Airway Epithelial Basal Progenitor Cells. Ann Am Thorac Soc. 2014;11(Supplement 5):S252–S258. doi: 10.1513/AnnalsATS.201402-049AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Todd JL, Goldstein DB, Ge D, Christie J, Palmer SM. The state of genome-wide association studies in pulmonary disease: a new perspective. Am J Respir Crit Care Med. 2011;184(8):873–80. doi: 10.1164/rccm.201106-0971PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siedlinski M, Cho MH, Bakke P, Gulsvik A, Lomas DA, Anderson W, et al. Genome-wide association study of smoking behaviours in patients with COPD. Thorax. 2011;66(10):894–902. doi: 10.1136/thoraxjnl-2011-200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho MH, Castaldi PJ, Wan ES, Siedlinski M, Hersh CP, Demeo DL, et al. A genome-wide association study of COPD identifies a susceptibility locus on chromosome 19q13. Hum Mol Genet. 2012;21(4):947–57. doi: 10.1093/hmg/ddr524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho MH, McDonald M-LN, Zhou X, Mattheisen M, Castaldi PJ, Hersh CP, et al. Risk loci for chronic obstructive pulmonary disease: a genome-wide association study and meta-analysis. Lancet Respir Med. 2014;2(3):214–25. doi: 10.1016/S2213-2600(14)70002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hao K, Bossé Y, Nickle DC, Paré PD, Postma DS, Laviolette M, et al. Lung eQTLs to help reveal the molecular underpinnings of asthma. PLoS Genet. 2012;8(11):e1003029. doi: 10.1371/journal.pgen.1003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Artigas MS, Loth DW, Wain LV, Gharib SA, Obeidat M, Tang W, et al. Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet. 2011;43(11):1082–90. doi: 10.1038/ng.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manichaikul A, Hoffman EA, Smolonska J, Gao W, Cho MH, Baumhauer H, et al. Genome-wide study of percent emphysema on computed tomography in the general population. The Multi-Ethnic Study of Atherosclerosis Lung/SNP Health Association Resource Study. Am J Respir Crit Care Med. 2014;189(4):408–18. doi: 10.1164/rccm.201306-1061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–48. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zibrak JD, Price D. Interstitial lung disease: raising the index of suspicion in primary care. NPJ Prim Care Respir Med. 2014;24:14054. doi: 10.1038/npjpcrm.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mushiroda T, Wattanapokayakit S, Takahashi A, Nukiwa T, Kudoh S, Ogura T, et al. A genome-wide association study identifies an association of a common variant in TERT with susceptibility to idiopathic pulmonary fibrosis. J Med Genet. 2008;45(10):654–6. doi: 10.1136/jmg.2008.057356. [DOI] [PubMed] [Google Scholar]
- 43.Noth I, Zhang Y, Ma S-F, Flores C, Barber M, Huang Y, et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med. 2013;1(4):309–17. doi: 10.1016/S2213-2600(13)70045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Noth I, Garcia JG, Kaminski N. A variant in the promoter of MUC5B and idiopathic pulmonary fibrosis. N Engl J Med. 2011;364(16):1576–7. doi: 10.1056/NEJMc1013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fingerlin McLaughlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45(6):613–20. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spagnolo P, Grunewald J. Recent advances in the genetics of sarcoidosis. J Med Genet. 2013;50(5):290–7. doi: 10.1136/jmedgenet-2013-101532. [DOI] [PubMed] [Google Scholar]
- 47.Hofmann S, Franke A, Fischer A, Jacobs G, Nothnagel M, Gaede KI, et al. Genome-wide association study identifies ANXA11 as a new susceptibility locus for sarcoidosis. Nat Genet. 2008;40(9):1103–6. doi: 10.1038/ng.198. [DOI] [PubMed] [Google Scholar]
- 48.Fischer A, Schmid B, Ellinghaus D, Nothnagel M, Gaede KI, Schürmann M, et al. A novel sarcoidosis risk locus for Europeans on chromosome 11q13. 1. Am J Respir Crit Care Med. 2012;186(9):877–85. doi: 10.1164/rccm.201204-0708OC. [DOI] [PubMed] [Google Scholar]
- 49.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357(19):1946–55. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 50.Walsh MC, Szefler S, Davis J, Allen M, Van Marter L, Abman S, et al. Summary proceedings from the bronchopulmonary dysplasia group. Pediatrics. 2006;117(Supplement 1):S52–S56. doi: 10.1542/peds.2005-0620I. [DOI] [PubMed] [Google Scholar]
- 51.Lavoie PM, Pham C, Jang KL. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics. 2008;122(3):479–85. doi: 10.1542/peds.2007-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hadchouel A, Durrmeyer X, Bouzigon E, Incitti R, Huusko J, Jarreau P-H, et al. Identification of SPOCK2 as a susceptibility gene for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2011;184(10):1164–70. doi: 10.1164/rccm.201103-0548OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H, Julien KRS, Stevenson DK, Hoffmann TJ, Witte JS, Lazzeroni LC, et al. A genome-wide association study (GWAS) for bronchopulmonary dysplasia. Pediatrics. 2013;132(2):290–7. doi: 10.1542/peds.2013-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu D, Morrell NW. Genetics and the molecular pathogenesis of pulmonary arterial hypertension. Curr Hypertens Rep. 2013;15(6):632–7. doi: 10.1007/s11906-013-0393-9. [DOI] [PubMed] [Google Scholar]
- 55.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114(13):1417–31. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 56.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25_S) doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 57.Germain M, Eyries M, Montani D, Poirier O, Girerd B, Dorfmüller P, et al. Genome-wide association analysis identifies a susceptibility locus for pulmonary arterial hypertension. Nat Genet. 2013;45(5):518–21. doi: 10.1038/ng.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schork NJ, Murray SS, Frazer KA, Topol EJ. Common vs. rare allele hypotheses for complex diseases. Curr Opin Genet Dev. 2009;19(3):212–9. doi: 10.1016/j.gde.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeWan AT, Egan KB, Hellenbrand K, Sorrentino K, Pizzoferrato N, Walsh KM, et al. Whole-exome sequencing of a pedigree segregating asthma. BMC Med Genet. 2012;13(1):95. doi: 10.1186/1471-2350-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campbell CD, Mohajeri K, Malig M, Hormozdiari F, Nelson B, Du G, et al. Whole-genome sequencing of individuals from a founder population identifies candidate genes for asthma. PloS One. 2014;9(8):e104396. doi: 10.1371/journal.pone.0104396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wain LV, Sayers I, Artigas MS, Portelli MA, Zeggini E, Obeidat M, et al. Whole Exome Re-Sequencing Implicates CCDC38 and Cilia Structure and Function in Resistance to Smoking Related Airflow Obstruction. PLoS Genet. 2014;10(5):e1004314. doi: 10.1371/journal.pgen.1004314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Austin ED, Ma L, LeDuc C, Rosenzweig EB, Borczuk A, Phillips JA, et al. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet. 2012;5(3):336–43. doi: 10.1161/CIRCGENETICS.111.961888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma L, Roman-Campos D, Austin ED, Eyries M, Sampson KS, Soubrier F, et al. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med. 2013;369(4):351–61. doi: 10.1056/NEJMoa1211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Jesus Perez VA, Yuan K, Lyuksyutova MA, Dewey F, Orcholski ME, Shuffle EM, et al. Whole-Exome Sequencing Reveals TopBP1 as a Novel Gene in Idiopathic Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2014;189(10):1260–72. doi: 10.1164/rccm.201310-1749OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shortt K, Chaudhary S, Grigoryev D, Heruth DP, Venkitachalam L, Zhang LQ, et al. Identification of Novel Single Nucleotide Polymorphisms Associated with Acute Respiratory Distress Syndrome by Exome-Seq. PloS One. 2014;9(11):e111953. doi: 10.1371/journal.pone.0111953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dannemiller KC, Mendell MJ, Macher JM, Kumagai K, Bradman A, Holland N, et al. Next-generation DNA sequencing reveals that low fungal diversity in house dust is associated with childhood asthma development. Indoor Air. 2014;24(3):236–47. doi: 10.1111/ina.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park H, Shin JW, Park S-G, Kim W. Microbial Communities in the Upper Respiratory Tract of Patients with Asthma and Chronic Obstructive Pulmonary Disease. PloS One. 2014;9(10):e109710. doi: 10.1371/journal.pone.0109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garzoni C, Brugger SD, Qi W, Wasmer S, Cusini A, Dumont P, et al. Microbial communities in the respiratory tract of patients with interstitial lung disease. Thorax. 2013;68(12):1150–6. doi: 10.1136/thoraxjnl-2012-202917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han MK, Zhou Y, Murray S, Tayob N, Noth I, Lama VN, et al. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med. 2014;2(7):548–56. doi: 10.1016/S2213-2600(14)70069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maher B. The case of the missing heritability. Nature. 2008;456(7218):18–21. doi: 10.1038/456018a. [DOI] [PubMed] [Google Scholar]
- 72.Consortium EP, et al. The ENCODE (ENCyclopedia of DNA elements) project. Science. 2004;306(5696):636–40. doi: 10.1126/science.1105136. [DOI] [PubMed] [Google Scholar]
- 73.Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature. 2012;489(7414):109–13. doi: 10.1038/nature11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee Y, Li H, Li J, Rebman E, Achour I, Regan KE, et al. Network models of genome-wide association studies uncover the topological centrality of protein interactions in complex diseases. J Am Med Inform Assoc. 2013;20(4):619–29. doi: 10.1136/amiajnl-2012-001519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nicolae DL, Gamazon E, Zhang W, Duan S, Dolan ME, Cox NJ. Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet. 2010;6(4):e1000888. doi: 10.1371/journal.pgen.1000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ortega VE, Meyers DA. Pharmacogenetics: Implications of race and ethnicity on defining genetic profiles for personalized medicine. J Allergy Clin Immunol. 2014;133(1):16–26. doi: 10.1016/j.jaci.2013.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rumpel JA, Ahmedani BK, Peterson EL, Wells KE, Yang M, Levin AM, et al. Genetic ancestry and its association with asthma exacerbations among African American subjects with asthma. J Allergy Clin Immunol. 2012;130(6):1302–6. doi: 10.1016/j.jaci.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rose D, Mannino DM, Leaderer BP. Asthma prevalence among US adults, 1998–2000: role of Puerto Rican ethnicity and behavioral and geographic factors. Am J Public Health. 2006;96(5):880–8. doi: 10.2105/AJPH.2004.050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salari K, Burchard EG. Latino populations: a unique opportunity for epidemiological research of asthma. Paediatr Perinat Epidemiol. 2007;21(s3):15–22. doi: 10.1111/j.1365-3016.2007.00880.x. [DOI] [PubMed] [Google Scholar]
- 80.Choudhry S, Burchard EG, Borrell LN, Tang H, Gomez I, Naqvi M, et al. Ancestry–environment interactions and asthma risk among Puerto Ricans. Am J Respir Crit Care Med. 2006;174(10):1088–93. doi: 10.1164/rccm.200605-596OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A. 2009;106(23):9362–7. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferreira MA, Matheson MC, Tang CS, Granell R, Ang W, Hui J, et al. Genome-wide association analysis identifies 11 risk variants associated with the asthma with hay fever phenotype. J Allergy Clin Immunol. 2014;133(6):1564–71. doi: 10.1016/j.jaci.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lasky-Su J, Himes B, Raby B, Klanderman B, Sylvia JS, Lange C, et al. HLA-DQ strikes again: Genome-wide association study further confirms HLA-DQ in the diagnosis of asthma among adults. Clin Exp Allergy. 2012;42(12):1724–33. doi: 10.1111/cea.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferreira MA, Matheson MC, Duffy DL, Marks GB, Hui J, Le Souëf P, et al. Identification of IL6R and chromosome 11q13. 5 as risk loci for asthma. The Lancet. 2011;378(9795):1006–14. doi: 10.1016/S0140-6736(11)60874-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Castro-Giner F, Bustamante M, González JR, Kogevinas M, Jarvis D, Heinrich J, et al. A pooling-based genome-wide analysis identifies new potential candidate genes for atopy in the European Community Respiratory Health Survey (ECRHS) BMC Med Genet. 2009;10(1):128. doi: 10.1186/1471-2350-10-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wan ES, Cho MH, Boutaoui N, Klanderman BJ, Sylvia JS, Ziniti JP, et al. Genome-wide association analysis of body mass in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2011;45(2):304–10. doi: 10.1165/rcmb.2010-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cho MH, Boutaoui N, Klanderman BJ, Sylvia JS, Ziniti JP, Hersh CP, et al. Variants in FAM13A are associated with chronic obstructive pulmonary disease. Nat Genet. 2010;42(3):200–2. doi: 10.1038/ng.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pillai SG, Ge D, Zhu G, Kong X, Shianna KV, Need AC, et al. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet. 2009;5(3):e1000421. doi: 10.1371/journal.pgen.1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hofmann S, Fischer A, Nothnagel M, Jacobs G, Schmid B, Wittig M, et al. Genome-wide association analysis reveals 12q13. 3–q14. 1 as new risk locus for sarcoidosis. Eur Respir J. 2013;41(4):888–900. doi: 10.1183/09031936.00033812. [DOI] [PubMed] [Google Scholar]
- 90.Hofmann S, Fischer A, Till A, Müller-Quernheim J, Häsler R, Franke A, et al. A genome-wide association study reveals evidence of association with sarcoidosis at 6p12. 1. Eur Respir J. 2011;38(5):1127–35. doi: 10.1183/09031936.00001711. [DOI] [PubMed] [Google Scholar]
- 91.Franke A, Fischer A, Nothnagel M, Becker C, Grabe N, Till A, et al. Genome-wide association analysis in sarcoidosis and Crohn’s disease unravels a common susceptibility locus on 10p12. 2. Gastroenterology. 2008;135(4):1207–15. doi: 10.1053/j.gastro.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 92.Moorman JE, Person CJ, Zahran HS Centers for Disease Control and Prevention (CDC) Asthma Attacks Among Persons with Current Asthma—United States. 2013;62(Suppl 3):93–8. [PubMed] [Google Scholar]
- 93.Kolb M, Collard HR. Staging of idiopathic pulmonary fibrosis: past, present and future. 2014;23(132):220–4. doi: 10.1183/09059180.00002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Functional classification of polymorphisms underlying pulmonary disease/traits.
Table 2. Summary of the NGS findings.