Abstract
Polychlorinated Biphenyls (PCBs) are industrial chemicals that have become a persistent threat to human health due to ongoing exposure. A subset of PCBs, known as dioxin-like PCBs, pose a special threat given their potent hepatic effects. Micronutrients, especially Cu, Zn and Se, homeostatic dysfunction is commonly seen after exposure to dioxin-like PCBs. This study investigates whether micronutrient alteration is the byproduct of the ongoing hepatotoxicity, marked by lipid accumulation, or a concurrent, yet independent event of hepatic damage. A time course study was carried out using male Sprague-Dawley rats with treatments of PCB126, the prototypical dioxin-like PCB, resulting in 6 different time points. Animals were fed a purified diet, based on AIN-93G, for three weeks to ensure micronutrient equilibration. A single IP injection of either tocopherol-stripped soy oil vehicle (5 mL/kg) or 5 umol/kg PCB126 dose in vehicle was given at various time points resulting in exposures of 9 hr, 18 hr, 36 hr, 3 days, 6 days, and 12 days. Mild hepatic vacuolar change was seen as early as 36 hours with drastic changes at the later time points, 6 and 12 days. Micronutrient alterations, specifically Cu, Zn, and Se, were not seen until after day 3 and only observed in the liver. No alterations were seen in the duodenum, suggesting that absorption and excretion may not be involved. Micronutrient alterations occur with ROS formation, lipid accumulation, and hepatomegaly. To probe the mechanistic underpinnings, alteration of gene expression of several copper chaperones was investigated; only metallothionein appeared elevated. These data suggest that the disruption in micronutrient status is a result of the hepatic injury elicited by PCB126 and is mediated in part by metallothionein.
Keywords: Micronutrients, Metals, Homeostasis, PCB126, Time Course, AhR
Graphical Abstract
1. Introduction
PCBs continue to be an enduring threat with an almost constant exposure whether through air or food (Ampleman et al. 2015; Hu et al. 2010). Given the recent elevation of PCBs to a group I human carcinogen by International Agency for Research on Cancer (IARC) and current research tying them to neurologic and metabolic disorders, a better understanding of their toxicity is needed (Baker et al. 2013; Grandjean and Landrigan 2006; Lauby-Secretan et al. 2013). A particularly persistent and toxic PCB conger, 3,3′,4,4′,5-pentachlorobiphenyl (PCB126), exerts adverse effects on many different systems from hepatic to immunological; including the homeostasis of micronutrients (Lai et al. 2010). PCB126, along with other dioxin-like PCBs, imparts its toxicity through activation of the aryl-hydrocarbon receptor (AhR) which in turn alters the expression of important xenobiotic metabolizing enzymes (CYPs) and also antioxidant proteins (Abel and Haarmann-Stemmann 2010). In addition, recent evidence suggests that supplementing the diet with certain micronutrients can actually mitigate some of the toxicity of PCB126, a finding that may propose a therapy for PCB exposure (Lai et al. 2012; Lai et al. 2013; Lai et al. 2011). So clearly, micronutrient status has some basis in PCB toxicity, but the extent and mechanism behind its role are unknown.
Micronutrient status is a crucial aspect of many different biological systems that is often overlooked in a diverse range of fields. It contributes to the redox status of the cell by modulating antioxidant enzymes or acting as prooxidants, e.g. copper’s fenton properties (Brewer 2008; Gadupudi and Chung 2011). In addition, gene expression can be altered by the lack of zinc in zinc-finger motifs in transcription factors (Cho et al. 2007). Various pathologies have been associated with changes in micronutrient status, including liver disease, Alzheimer’s disease, and glucose and insulin homeostasis (Adlard and Bush 2006; Fung et al. 2015; Mohammad et al. 2012). Whether the modifications to micronutrient homeostasis play an active role in disease progression or are just a symptom of it, need to be clarified. In any context, micronutrient status is fundamental for the normal function of the cell and the body as a whole.
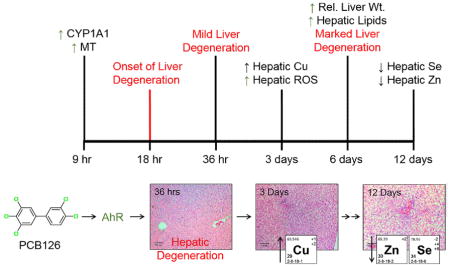
Dioxin-like compounds, in particular dioxin-like PCBs, cause disruptions in serum, hepatic, and renal micronutrients consisting of changes in tissue levels of copper, zinc, selenium and iron (Elsenhans et al. 1991; Wahba et al. 1988; Wahba et al. 1990). Yet a clear explanation of what is causing this disruption and how this contributes to the overall toxicity is still lacking. The current study aims to elucidate the progression of micronutrient alteration following an acute exposure to PCB126. With this timeline of metals disturbance, better insights into the mechanism behind this phenomenon can be obtained. Hepatocellular degeneration was observed as early as 36 hours following exposure to PCB126, whereas substantial micronutrient change, in particular copper, was not observe until day 3. This suggests that micronutrient alteration is mediated by PCB126 and is likely the result of hepatic injury.
2. Methods and Materials
2.1 Chemicals
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). PCB126 was synthesized by an improved Suzuki-coupling method of 3,4-dichlorophenyl boronic acid and 3,4,5-trichlorobenzene employing a palladium-catalyzed cross coupling reaction (Luthe et al. 2006). Crude product was purified on an aluminum oxide column and flash silica gel column chromatography followed by recrystallization from methanol. The final product purity was >99.8%, determined by GC/MS and the structure was confirmed by 13C NMR. Analytical standards, 2,4,6-trichlorobiphenyl (PCB30), 2,3,4′,5,6-pentachlorobiphenyl (PCB117), and 2,2′3,4,4′,5,6,6′-octachlorobiphenyl (PCB204), were purchased from AccuStandard (New Haven, CT). Caution: PCBs and their metabolites should be handled as hazardous compounds in accordance with NIH guidelines.
2.2 Animals
Experimental design and procedures were performed with the approval of the Institutional Animal Care and Use Committee of the University of Iowa. 4–5 week old male Sprague-Dawley rats were acquired from Harlan Sprague-Dawley (Indianapolis, IN). This age of animal was selected based on 1) their sensitivity/induction potential and 2) recent evidence of PCB-contaminated schools and the risk that poses to children (Grimm et al. 2015; Kato and Takanaka 1968). The 75–100 gram animals were housed one per cage in wire cages in a controlled environment maintained at 22°C with a 12 hour light-dark cycle. Animals were fed ad libitum a refined diet, AIN-93G, with a 10% fat content provided by tocopherol stripped soy oil purchased from Harlan Teklad (Madison WI), throughout the study. Since diets can vary widely in micronutrient content (Chen et al. 1990), rats were fed this refined diet for three weeks to allow for equilibration. Animals were then administered a single IP injection of vehicle (5 mL/kg body wt. of tocopherol stripped soy oil; Harlan Tekland, Madison, WI) or vehicle with 5 μmol/kg (1.63 mg/kg) dose of PCB126 at time points that result in exposures of 9 hr, 18 hr, 36 hr, 3 days, 6 days and 12 days (Reeves and DeMars 2006). To conserve the number of animals used in the study, three animals were used in each treatment group and only the 12 day vehicle control was used. The rats were then euthanized by carbon dioxide asphyxiation followed by cervical dislocation. Blood was collected via cardiac puncture and other organs were excised, weighed and processed for analysis.
2.3 Histology
Tissue sections from each animal were fixed in 10% neutral buffered formalin. Fixed tissues were routinely processed, embedded in paraffin and sectioned at 4 μm. Sections were further processed and stained with hematoxylin and eosin (H&E) followed by an analysis by a pathologist.
2.4 PCB126 and Lipid Analysis
2.4.1 Extraction of Tissues
Liver samples weighing between 0.5 g and 1 g were homogenized with diatomaceous earth and separated into two portions, one for PCB126 analysis and the other for total extractable lipids. The portion for PCB126 analysis was spiked with a recovery standard, PCB117, then extracted with hexane:acetone (1:1 v/v) using Accelerated Solvent Extractor (Dionex, Sunnyvale, CA) as described previously (Rignall et al. 2013). Cells containing only diatomaceous earth and florisil as well as the ongoing precision and recovery (OPR) standard (blanks spiked with all analytes) were extracted along with the hepatic samples. After extraction, the samples were spiked with internal standards, PCB30 and PCB204, then concentrated followed by a sulfur clean-up and a final treatment of sulfuric acid as described earlier (Rignall et al. 2013). The portion for total extractable lipids was extracted with chloroform:methanol (2:1 v/v) using Accelerated Solvent Extractor (Dionex, Sunnyvale, CA) as described (Bunaciu et al. 2007). Samples were concentrated and evaporated to dryness in pre-weighed vials. Vials were kept in a desiccator and weighed three times over the course of several days, to constant weight.
2.4.2 GC Analysis
An Agilent 6890N gas chromatograph equipped with a SPB-1 column (poly(dimethyl siloxane), 60 m, 0.25 mm ID, 0.25 μm film thickness; Supelco, St. Louis, MO) and 63Ni μ-electron capture detector was used for PCB126 quantification. 280°C and 300°C were used as the injector and detector temperatures, respectively. The temperature program was as follows: 80°C for 1 min, 15°C/min increase to 260°C, 1°C/min increase to 274°C, 15°C/min increase to 300°C. The level of PCB126 was calculated using PCB204 as a volume corrector and normalized to tissue weight.
2.4.3 Quality assurance/quality control
The recoveries for PCB117 were 91.5±8.3%. PCB126 concentrations were corrected by the recoveries of surrogate standards. OPR standard, which was run in parallel with hepatic samples, recovery was 92.1%
2.5 Micronutrient Analysis
Roughly 0.5 g of liver and PBS flushed duodenum were used to determine copper, zinc, iron and selenium content using a previously described method (Lai et al. 2010). Briefly, samples were acid (HNO3) digested by microwave-assisted closed vessel digestion prior to instrument measurement. Metal concentrations were quantitatively determined with an elemental mass spectrometer by Inductively Coupled Plasma – Mass Spectrometry (ICP-MS) using an Agilent 7500ce ICP-MS equipped with a CETAC AS520 auto sampler. Results were normalized to wet tissue weights.
2.6 Gene Expression Analysis
Gene expression of select proteins was determined by a two-step quantitative RT-PCR method using an Eppendorf Realplex Mastercycler. A Qiagen (Hilden, Germany) RNeasy Mini kit was used, following manufacturer’s instructions, to isolate total RNA from hepatic tissue. Using random primers from a High Capacity cDNA Reverse Transcription Kit from Applied Biosystems (Waltham, MA), the RNA was reverse transcribed into cDNA. The real time RT-PCR was carried out using 1–10 ng cDNA with 300 nM primers and Power SYBR green PCR master mix from Applied Biosystems. Reaction conditions were optimized for each primer set. Two technical replicates were used and averaged for further analysis. Actin or GAPDH were used as the reference gene and the 12 day vehicle treated animals served as the biological control in the Pfaffl method calculation for relative fold change. All primers used are listed in Supplemental Table 1 and were obtained from IDT (Coralville, IA) (Lai et al. 2013; Wang et al. Submitted).
2.7 ROS Determination
Hepatic ROS were determined using the OxiSelect InVitro ROS/RNS Assay Kit from Cell Biolabs (San Diego, CA) following the manufacturer’s instructions. Briefly, liver samples were weighed, homogenized in PBS, and diluted to a standard concentration. Samples was loaded into 96 well plate along with catalyst and DCFH dye. The reaction was incubated at room temperature for 45 minutes after which fluorescence was read at 480 nm excitation/530 nm emission. A DCF standard curve was used to adjust the raw fluorescence values then normalized to tissue weight.
2.8 Statistics
To access the progression of PCB126 toxicity, a one-way ANOVA was used along with a comparison to the day 12 vehicle control using a Dunnett’s Test. The PCB126 concentration data were log-transformed, then the one-way ANOVA and Dunnett’s test were conducted. Statistics were calculated in SAS 9.3 and significance of p-value<0.05 is notated by an *.
3. Results
3.1 Histological Examination
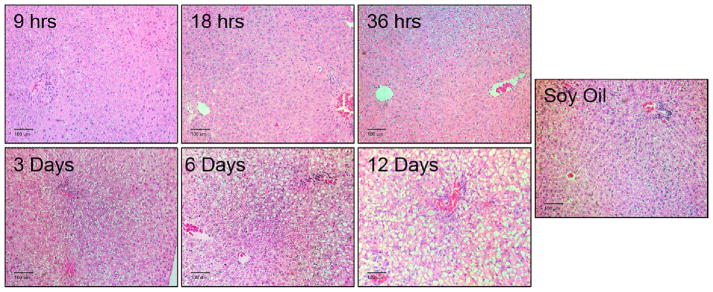
H&E sections of the liver were prepared and analyzed to determine the development of hepatotoxicity caused by PCB126. In the 18 and 36 hour exposure groups, mild vacuolar change and cytoplasmic clearing were observed (Fig. 1). At 6 and 12 days there was marked periportal to midzonal vacuolar change and cytoplasmic clearing. However, animals with the 12 day exposure showed diffused vacuolar changes in all zones of the liver.
Figure 1. Progression of PCB126-mediated histological changes in the liver.
Male SD rats were treated with 5 umol/kg PCB126, intraperionteally at various time points resulting in exposures of 9 hr, 18 hr, 3 days. 6 days, and 12 days. Sections of the liver were stained with H&E stain and analyzed by a pathologist. These are representative images taken from each group. Scale bar equals 100 um.
3.2 PCB126 Evaluation and Hallmarks of AhR activation
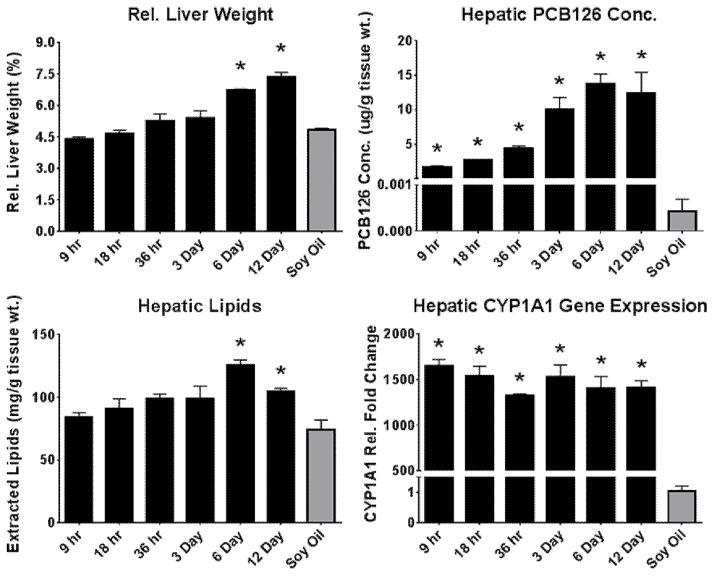
Analytical methods were used to establish the amount of PCB126 that accumulates in the liver after a single injection. Levels of PCB126 were elevated in the liver after 9 hours with substantial increases throughout the additional time of exposure (Fig. 2). The general increasing trend of hepatic PCB126 over time can also be seen with relative liver weight the increase of which is a hallmark of aryl-hydrocarbon receptor agonists. In accordance, significant increases in relative liver weight was observed at 6 and 12 days following treatment (Fig. 2). Although, PCB126 levels increase over time, the induction of CYP1A1, a well-established marker of AhR activation, was substantially increased at the first time point (9 hrs) and remained well over 1000-fold increased throughout the 12 day exposure period (Fig. 2). Lipid accumulation associated with the increased relative liver weight was also seen to gradually increase over the course of the exposure. The 6 and 12 day exposures showed significant levels of lipid accumulation (Fig. 2).
Figure 2. Assessment and confirmation of canonical AhR activation.
Traditional metrics of AhR activation were assessed to confirm PCB126-mediated AhR activation. (Bars are standard error, * represents p<0.05; one-way ANOVA, n=3)
3.3 Micronutrient Analysis
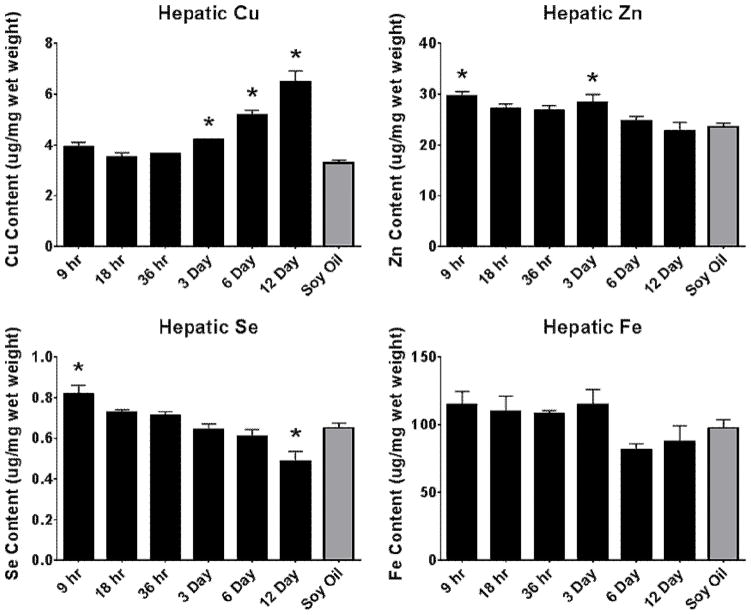
Micronutrient levels (Cu, Fe, Zn & Se) were determined in the liver to assess the progression of homeostatic disruption by PCB126 and are shown in Figure 3. Copper in the liver was significantly elevated at 3 days of exposure and increased over time, with levels that were doubled at 12 days. Alterations in iron levels were not seen in this study. Zinc was significantly increased, although slightly, at 9 hours, then decreased back to control levels at 18 hours. An increase was seen again at 3 days and decreased for the remaining experiment. Selenium was increased initially (9 hrs) then gradually decreased, similar to Zn, however levels dropped below those of the control animals at day 12.
Figure 3. Alterations in hepatic metal status over time following PCB126 exposure.
Hepatic micronutrient levels were determined to follow the progression of micronutrient change associated with PCB126. (Bars are standard error, * represents p<0.05; one-way ANOVA, n=3)
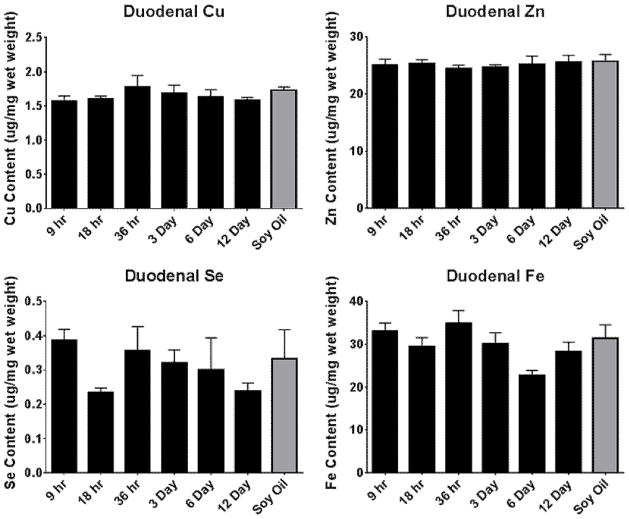
Duodenal levels of copper, iron, zinc, and selenium were measured to gain insight into modifications of dietary uptake and excretion of some these micronutrients. All micronutrients measured were unchanged in the duodenum, as seen in Figure 4. Interestingly, the levels of iron and selenium showed higher levels of variability than those of copper and zinc.
Figure 4. Alterations in duodenal metal status over time following PCB126 exposure.
Duodenal micronutrients were investigated to observe the role of excretion and absorption in PCB126-medaited micronutrient disruption. (Bars are standard error, * represents p<0.05; one-way ANOVA, n=3)
3.4 Gene Expression Analysis
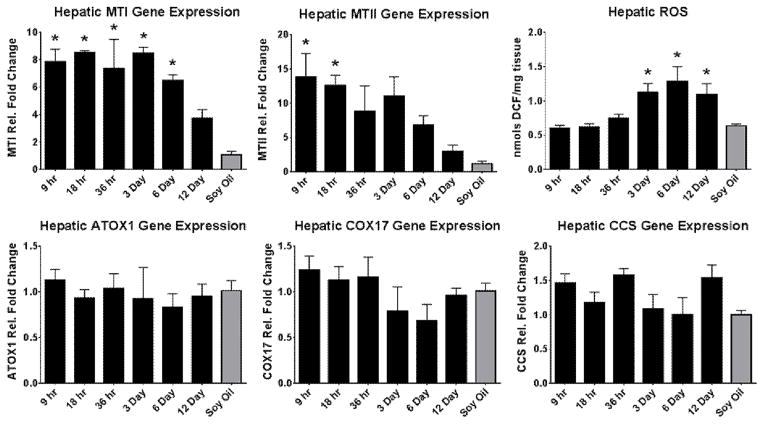
Several genes involved with metal homeostasis, in particular copper homeostasis, were investigated to potentially explain the shifts seen in micronutrient status. Figure 5 shows metallothionein I and II were highly induced at 9 hrs with a gradual reduction over the course of exposure. Interestingly, MTI maintained its induction until the 6 day time point however MTII was only significantly elevated at 9 and 18 hrs. ATOX1, COX17, and CCS are all intracellular copper chaperones that shuttle copper to the golgi apparatus, cytochrome c oxidase in the mitochondria, and Cu/ZnSOD in the cytoplasm, respectively (Prohaska 2008). The hepatic gene expression of all three were unchanged throughout all time points, suggesting that the additional copper may be binding to the increased metallothionein.
Figure 5. Hepatic copper chaperone gene expression and ROS changes with PCB126.
The mechanism of alteration was investigated with the gene expression of proteins involved in copper trafficking. Actin or GAPDH was used as the reference gene. (Bars are standard error, * represents p<0.05; one-way ANOVA, n=3)
3.5 ROS Determination
Reactive oxygen species were investigated to assess the progression in the amounts of ROS upon exposure to PCB126 over time. Figure 5 shows total ROS was increased after longer exposure to PCB126. At 3 days, the ROS level was significantly elevated and remained elevated for the remaining exposures with a further increase at 6 days followed by a slight decrease, although still elevated from control, on day 12.
4. Discussion
The fundamental basis for TCDD associated disruption of hepatic micronutrient levels, specifically alterations in copper, zinc, selenium and iron, has been unknown since its discovery some 30 years ago. Hypotheses have been presented but none completely address the complex and multi-faceted nature of the phenomenon (Elsenhans et al. 1991; Twaroski et al. 2001; Wahba et al. 1988). In addition, it is still unclear whether the disruption is the result of hepatic degeneration or partially the cause, and the extent of the toxicity. The present research has the goal of addressing these questions with an animal study that followed the progression of hepatotoxicity associated with a dioxin-like PCB, PCB126.
In these investigations it is important to consider the effect of food consumption and body weight alterations especially since PCB126, like TCDD, is known to cause hypophagia (Potter et al. 1986). Early investigations by Elsenhans et. al. (1991) showed that some micronutrient disruption caused by TCDD was independent of the decrease in food intake by using a pairwise feeding model. Similar results were seen here as no statistical change was seen in body weight or feed efficiency following exposure; suggesting that the dose used was below that which caused reduced food intake (see Supplemental Figures 1 & 2). This posits that the effects seen in this study are that of PCB126 and not that of any secondary effects caused by the hypophagia.
As seen in Figure 1, the development of mild hepatocyte vacuolation was observed as early as 36 hours and continued in severity at the later time points which agrees with previous studies (Robertson et al. 1991). More pronounced vacuolation was seen at 3 days, which corresponds to increases seen in copper levels and ROS formation. This would suggest that the hepatic injury may be contributing to the changes in copper status. Increased copper has been seen in other hepatic injuries including carbon tetrachloride induced cirrhosis, hepatitis C, and alcoholic liver disease, although these are more traditionally chronic injuries (Dashti et al. 1992; Hatano et al. 2000; Rodriguez-Moreno et al. 1997).
As is classically seen with AhR agonist exposure, clear hepatomegaly and CYP1A1 induction were seen. Both relative (Fig. 2) and absolute liver weights (data not shown) increased over the course of the exposure rising to statistical significance after the 6th day. This increase in liver weight is most likely associated with hepatocellular hypertrophy similar to what has previously been suggested (Ovando et al. 2010). As seen in other studies, CYP1A1 was induced to a maximal level within hours of exposure thus confirming the early and prolonged activation of the AhR, and the resulting expression of the protein (Wang et al. 2011).
Among the hepatic micronutrient content examined, a pronounced increase in hepatic copper levels was observed, rising in a stepwise fashion with time (Fig. 3). Although previous studies have seen a decrease in hepatic zinc, this study shows an initial increase in Zn levels followed by a return to control levels (Lai et al. 2010; Lai et al. 2013). Selenium showed a decrease over time with a slight increase initially. Although, Zn and Se were both increased at 9 hrs, the increase was minor. This may be a result of stress response from injection, as both metals have been associated with the acute phase response (Liuzzim et al. 2005; Stoedter et al. 2010). Micronutrient status in the duodenum was unaffected by PCB126, similar to previous investigations using TCDD (Elsenhans et al. 1991). The relatively static levels of duodenal metals overtime diminishes the likelihood that dietary absorbance is the explanation of PCB126 induced micronutrient alterations, additional studies maybe warranted. Inhibition of biliary excretion has been seen with AhR agonists, although this potentially explains the increase in copper, it fails to explain decreases in other biliarially excreted metals in particular manganese (not investigated here) (Yang et al. 1983).
The gene expression of several copper chaperones was investigated to possibly explain the increase in hepatic copper (Prohaska 2008). The expression of the highly inducible metallothionein proteins was greatly increased initially (9–14 fold) but gradually decreased over the course of the experiment. Previous studied have demonstrated a 4-fold increase in metallothionein at 14 days PCB126 exposure, it is likely with larger group sizes this increase would be significant (Lai et al. 2013; Wang et al. Submitted). That said, a single metallothionein protein can bind up to 13 copper atoms; thus a small increase in metallothionein could account for a major increase in copper content. Previous studies have connected the induction of metallothionein and the AhR via the glucocorticoid receptor and that is likely occurring here (Sato et al. 2013). Other copper chaperones were investigated but none showed any significant change.
The alteration of hepatic micronutrient status caused by PCB126 appears to develop over several days consistent with the histopathological changes. Hours after exposure, CYP1A1 and metallothionein expression were increased (9 hrs); this was followed by mild hepatocyte vacuolation (36 hrs) and later increases in hepatic copper and ROS (3 days). After 6 days, hepatic lipids were elevated and hepatomegaly was observed. In particular, the main alteration in copper status (3 days) occurred after the initial vacuolar changes in the hepatocytes (36 hrs). This suggests that hepatocyte liver injury cause alterations in copper status. The involvement of this process in the alteration of other micronutrients, in particular selenium, remains unknown. In addition, the mechanism by which copper is increased is unknown. Although unbound copper is very unlikely, given its reactivity, whether the copper is bound to the increased metallothionein is uncertain however it appears likely. This study suggests the hepatic degeneration causes the micronutrient disruption, and that changes in these important elements can further potentiate the liver injury; however, this explanation fails to address the precise mechanism involved. Further investigations looking at the involvement of metallothionein in the PCB126 induced hepatic micronutrient alterations could potentially provide additional insight. The possibility that micronutrient supplementation may mediate some aspect of PCB126-induced hepatic injury needs to be investigated. Similar mitigation strategies have been seen with zinc therapy in alcoholic liver disease (Kang et al. 2009).
Supplementary Material
Acknowledgments
This research which forms a portion of the dissertation work of W. Klaren, was supported by funding from NIH (P42 ES013661). The opinions expressed are those of the authors and not of any granting agency. The authors thank Dr. Hans Lehmler and Dr. Iza Korwel for assistance in PCB126 quantification and lipid analysis, as well as Dr. Gregor Luthe for the synthesis of PCB126. In addition, the authors thank Ms. Susanne Flor and all the lab members for their assistance with laboratory infrastructure and help with the animal studies. Finally, W. Klaren graciously recognizes support from the Iowa Superfund Research Program (P42 ES013661) Training Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel J, Haarmann-Stemmann T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol Chem. 2010;391:1235–1248. doi: 10.1515/BC.2010.128. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Bush Al. Metals and Alzheimer’s Disease. J Alzheimers Dis. 2006;10:145–163. doi: 10.3233/jad-2006-102-303. [DOI] [PubMed] [Google Scholar]
- Ampleman MD, Martinez A, DeWall J, Rawn DF, Hornbuckle KC, Thorne PS. Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environ Sci Technol. 2015;49:1156–1164. doi: 10.1021/es5048039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NA, Karounos M, English V, Fang J, Wei Y, Stromberg A, Sunkara M, Morris AJ, Swanson HI, Cassis LA. Coplanar polychlorinated biphenyls impair glucose homeostasis in lean C57BL/6 mice and mitigate beneficial effects of weight loss on glucose homeostasis in obese mice. Environ Health Perspect. 2013;121:105–110. doi: 10.1289/ehp.1205421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ. The risks of free copper in the body and the development of useful anticopper drugs. Curr Opin Clin Nutr Metab Care. 2008;11:727–732. doi: 10.1097/MCO.0b013e328314b678. [DOI] [PubMed] [Google Scholar]
- Bunaciu RP, Tharappel JC, Lehmler HJ, Kania-Korwel I, Robertson LW, Srinivasan C, Spear BT, Glauert HP. The effect of dietary glycine on the hepatic tumor promoting activity of polychlorinated biphenyls (PCBs) in rats. Toxicology. 2007;239:147–155. doi: 10.1016/j.tox.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Borges T, Glauert HP, Knight SAB, Sunde RA, Schramm H, Oesch F, Chow CK, Robertson LW. Modulation of selenium-dependent glutathione peroxidase by perfluorodecanoic acid in rats: effect of dietary selenium. J Nutr. 1990;120:298–304. doi: 10.1093/jn/120.3.298. [DOI] [PubMed] [Google Scholar]
- Cho YE, Lomeda RA, Ryu SH, Lee JH, Beattie JH, Kwun IS. Cellular Zn depletion by metal ion chelators (TPEN, DTPA and chelex resin) and its application to osteoblastic MC3T3-E1 cells. Nutr Res Pract. 2007;1:29–35. doi: 10.4162/nrp.2007.1.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti HM, al-Sayer H, Behbehani A, Madda J, Christenson JT. Liver cirrhosis induced by carbon tetrachloride and the effect of superoxide dismutase and xanthine oxidase inhibtor treatment. J R Cool Surg Edinb. 1992;37:23–28. [PubMed] [Google Scholar]
- Elsenhans B, Forth W, Richter E. Increased copper concentrations in rat tissues after acute intoxication with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Arch Toxicol. 1991;65:429–432. doi: 10.1007/BF02284268. [DOI] [PubMed] [Google Scholar]
- Fung EB, Gildengorin G, Talwar S, Hagar L, Lal A. Zinc status affects glucose homeostasis and insulin secretion in patients with thalassemia. Nutrients. 2015;7:4296–4307. doi: 10.3390/nu7064296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadupudi GS, Chung KT. Comparative genotoxicity of 3-hydroxyanthranilic acid and anthranilic acid in the presence of a metal cofactor Cu (II) in vitro. Mutat Res. 2011;726:200–208. doi: 10.1016/j.mrgentox.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Grimm FA, Hu D, Kania-Korwel I, Lehmler HJ, Ludewig G, Hornbuckle KC, Duffel MW, Bergman A, Robertson LW. Metabolism and metabolites of polychlorinated biphenyls. Critical reviews in toxicology. 2015;45:245–272. doi: 10.3109/10408444.2014.999365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano R, Ebara M, Fukuda H, Yoshikawa M, Sugiura N, Kondo T, Yukawa M, Saisho H. Accumulation of copper in the liver of hepatic injury in chronic hepatitis C. Journal of Gastroenterology and Hepatology. 2000;15:786–791. doi: 10.1046/j.1440-1746.2000.02199.x. [DOI] [PubMed] [Google Scholar]
- Hu D, Lehmler HJ, Martinez A, Wang K, Hornbuckle KC. Atmospheric PCB congeners across Chicago. Atmos Environ (1994) 2010;44:1550–1557. doi: 10.1016/j.atmosenv.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang X, Zhong W, Liu J, Song Z, McClain CJ, Kang YJ, Zhou Z. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4alpha and peroxisome proliferator-activated receptor-alpha. Hepatology. 2009;50:1241–1250. doi: 10.1002/hep.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato R, Takanaka A. Effect of Phenobarbital on Electron Transport System, Oxidation and Reduction of Drugs in Liver Microsomes of Rats of Different Ages. J Biochem. 1968;63:406–408. [PubMed] [Google Scholar]
- Lai I, Chai Y, Simmons D, Luthe G, Coleman MC, Spitz D, Haschek WM, Ludewig G, Robertson LW. Acute toxicity of 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) in male Sprague-Dawley rats: effects on hepatic oxidative stress, glutathione and metals status. Environ Int. 2010;36:918–923. doi: 10.1016/j.envint.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai I, Dhakal K, Gapdupudi G, Miao L, Ludewig G, Robertson LW, Olivier A. N-acetylcystiene (NAC) diminishes the severity of PCB126-induced fatty liver in male rodents. Toxicology. 2012;302:25–33. doi: 10.1016/j.tox.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai I, Klaren WD, Li M, Wels B, Simmons D, Olivier AK, Haschek WM, Wang K, Ludewig G, Robertson LW. Does Dietary Copper Supplementation Enhance or Diminish PCB126 Toxicity in the Rodent Liver? Chem Res Toxicol. 2013;26:634–644. doi: 10.1021/tx400049s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai IK, Chai Y, Simmons D, Watson WH, Tan R, Haschek WM, Wang K, Wang B, Ludewig G, Robertson LW. Dietary Selenium as a Modulator of PCB 126-Induced Hepatotoxicity in Male Sprague-Dawley Rats. Toxicol Sci. 2011;124:202–214. doi: 10.1093/toxsci/kfr215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauby-Secretan B, Loomis D, Grosse Y, Ghissassi FE, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K International Agency for Research on Cancer Monograph Working Group Iarc LF. Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 2013;14:287–288. doi: 10.1016/S1470-2045(13)70104-9. [DOI] [PubMed] [Google Scholar]
- Liuzzim JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson TB, Ganz T, Cousins RJ. Interleuin-6 regulates the zinc transporter Zip14 in liver and contributes to teh hypozincemia of the acute-phase response. Proc Natl Acad Sci. 2005;102:6843–6848. doi: 10.1073/pnas.0502257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthe GM, Schut BG, Aaseng JE. Monofluorinated analogues of polychlorinated biphenyls (F-PCBs): Synthesis using the Suzuki-coupling, characterization, specific properties and intended use. Chemosphere. 2006;77:1242–1248. doi: 10.1016/j.chemosphere.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Mohammad MK, Zhou Z, Cave M, Barve A, McClain CJ. Zinc and liver disease. Nutr Clin Pract. 2012;27:8–20. doi: 10.1177/0884533611433534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovando BJ, Ellison CA, Vezina CM, Olson JR. Toxicogenomic analysis of exposure to TCDD, PCB126 and PCB153: identification of genomic biomarkers of exposure to AhR ligands. Bmc Genomics. 2010:11. doi: 10.1186/1471-2164-11-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter CL, Menahan LA, Peterson RE. Relationship of Alterations in Energy Metabolism to Hypophagia in Rats Treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam Appl Toxicol. 1986;6:89–97. doi: 10.1016/0272-0590(86)90267-8. [DOI] [PubMed] [Google Scholar]
- Prohaska JR. Role of copper transporters in copper homeostasis. Am J Clin Nutr. 2008;88:826S–829S. doi: 10.1093/ajcn/88.3.826S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves P, DeMars L. Signs of iron deficiency in copper-deficient rats are not affected by iron supplements administered by diet or by injection. J Nutr Biochem. 2006;17:635–642. doi: 10.1016/j.jnutbio.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Rignall B, Grote K, Gavrilov A, Weimer M, Kopp-Schneider A, Krause E, Appel KE, Buchmann A, Robertson LW, Lehmler HJ, Kania-Korwel I, Chahoud I, Schwarz M. Biological and tumor-promoting effects of dioxin-like and non-dioxin-like polychlorinated biphenyls in mouse liver after single or combined treatment. Toxicol Sci. 2013;133:29–41. doi: 10.1093/toxsci/kft034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LW, Silberhorn EM, Glauert HP, Schwarz M, Buchmann A. Do Structure-Activity Relationships for the Acute Toxcity of PCBs and PBBs also apply for Induction of Hepatocellular Carcinoma. Environ Toxicol Chem. 1991;10:715–726. [Google Scholar]
- Rodriguez-Moreno F, Gonzalez-Reimers E, Santolaria-Fernandez F, Galindo-Martin L, Hernandez-Torres O, Batista-Lopez N, Molina-Perez M. Zinc, Copper, Manganese, and Iron in Chronic Alcholic Liver Disease. Alcohol. 1997;14:39–44. doi: 10.1016/s0741-8329(96)00103-6. [DOI] [PubMed] [Google Scholar]
- Sato S, Shirakawa H, Tomita S, Tohkin M, Gonzalez FJ, Komai M. The aryl hydrocarbon receptor and glucocorticoid receptor interact to activate human metallothionein 2A. Toxicology and applied pharmacology. 2013;273:90–99. doi: 10.1016/j.taap.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoedter M, Renko K, Hog A, Schomburg L. Selenium controls the sex-specific immune response and selenoprotein expression during the acute-phase response in mice. The Biochemical journal. 2010;429:43–51. doi: 10.1042/BJ20091868. [DOI] [PubMed] [Google Scholar]
- Twaroski TP, O’Brien ML, Robertson LW. Effects of selected polychlorinated biphenyl (PCB) congeners on hepatic glutathione, glutathione-related enzymes, and selenium status: implications for oxidative stress. Biochem Pharmacol. 2001;62:273–281. doi: 10.1016/s0006-2952(01)00668-2. [DOI] [PubMed] [Google Scholar]
- Wahba ZZ, al-Bayati ZA, Stohs SJ. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the hepatic distribution of iron, copper, zinc, and magnesium in rats. J Biochem Toxicol. 1988;3:121–129. doi: 10.1002/jbt.2570030206. [DOI] [PubMed] [Google Scholar]
- Wahba ZZ, Murray WJ, Stohs SJ. Altered hepatic iron distribution and release in rats after exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Bull Environ Contam Toxicol. 1990;45:436–445. doi: 10.1007/BF01701169. [DOI] [PubMed] [Google Scholar]
- Wang B, Robertson LW, Wang K, Ludewig G. Species difference in the regulation of cytochrome P450 2S1: a lack of induction in rats by the aryl hydrocarbon receptor agonist PCB126. Xenobiotica. 2011;41:1031–1043. doi: 10.3109/00498254.2011.603763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Simmons D, Klaren W, Olivier A, Wang K, Robertson LW, Ludewig G. Dietary manganese modulates PCB126 toxicity, metal status, and MnSOD in the rat. doi: 10.1093/toxsci/kfv312. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang KH, Yoo BS, Choe SY. Effects of Halogenated dibenzo-p-dioxins on plasma disappearance and biliary excretion of ouabain in rats. Toxicol Lett. 1983;15:259–264. doi: 10.1016/0378-4274(83)90225-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.