Highlights
-
•
Voltage-gated calcium channel classification—genes and proteins.
-
•
Genetic analysis of neuropsychiatric syndromes.
-
•
Calcium channel genes identified from GWA studies of psychiatric disorders.
-
•
Rare mutations in calcium channel genes in psychiatric disorders.
-
•
Pathophysiological sequelae of CACNA1C mutations and polymorphisms.
-
•
Monogenic disorders resulting from harmful mutations in other voltage-gated calcium channel genes.
-
•
Changes in calcium channel gene expression in disease.
-
•
Involvement of voltage-gated calcium channels in early brain development.
Keywords: Calcium channel, Neuropsychiatric disorder, Polygenic disorder, Mutation, Single nucleotide polymorphism
Abbreviations: DISC1, Disrupted in Schizophrenia 1; fMRI, functional magnetic resonance imaging; FMRP, Fragile X mental retardation protein; GWAS, genome wide association study; SNP, single nucleotide polymorphism
Abstract
This review summarises genetic studies in which calcium channel genes have been connected to the spectrum of neuropsychiatric syndromes, from bipolar disorder and schizophrenia to autism spectrum disorders and intellectual impairment. Among many other genes, striking numbers of the calcium channel gene superfamily have been implicated in the aetiology of these diseases by various DNA analysis techniques. We will discuss how these relate to the known monogenic disorders associated with point mutations in calcium channels. We will then examine the functional evidence for a causative link between these mutations or single nucleotide polymorphisms and the disease processes. A major challenge for the future will be to translate the expanding psychiatric genetic findings into altered physiological function, involvement in the wider pathology of the diseases, and what potential that provides for personalised and stratified treatment options for patients.
1. Introduction
Excitable cells can be defined as those that are able to fire an action potential in response to depolarization, or more loosely, they contain functional voltage-gated ion channels. Neurons and muscle cells are conventionally excitable, but many other cell types show oscillatory changes in voltage, dependent on calcium entry (Hu et al., 2012). Free intracellular Ca2+ is normally controlled at a low level in the cytoplasm (10–100 nM), by plasma membrane and endoplasmic/sarcoplasmic reticulum pumps and exchangers, as well as by mitochondrial sequestering. Voltage-gated calcium channels react to membrane potential depolarization by opening to allow calcium ions to flow down their electrochemical gradient. Ca2+ entry, particularly but not exclusively through voltage-gated calcium channels, provides an elevation of calcium ions to drive many processes. These include hormone secretion, neurotransmitter release, calcium-dependent gene transcription, and also spontaneous pacemaker activity in some neurons, muscles, and secretory cells (Mangoni et al., 2003, Putzier et al., 2009, Guzman et al., 2009, Hu et al., 2012, Striessnig et al., 2015). Ca2+ activates numerous calcium-dependent enzymes, including kinases, phosphatases, and proteases, and also activates ion channels, including Ca2+-activated K+ channels. It therefore has multiple effects on cell function depending crucially on where Ca2+ entry occurs (Catterall et al., 2013, Cohen et al., 2015). In skeletal muscle, the voltage-gated calcium channels link depolarization to release of internal Ca2+ from sarcoplasmic reticulum in the process of excitation-contraction coupling (Bannister and Beam, 2013), and in cardiac and smooth muscle, the Ca2+ entry through voltage-gated calcium channels mediates Ca2+-induced Ca2+ release (Fabiato, 1985, Valdeolmillos et al., 1989).
Because of the key roles of voltage-gated calcium channels in processes that are so critical for cellular function, it is essential that they are inserted into the plasma membrane in a regulated manner. Muscles, secretory cells, and neurons all have specialized regions of their plasma membrane where voltage-gated calcium channels are found to be clustered in an ordered manner. The distinctive biophysical properties that are intrinsic to the different subtypes of pore-forming calcium channel subunits, as well as their regulation by auxiliary subunits and other binding proteins, ensure that the functions of voltage-gated calcium channels are customized for the different roles that they fulfil in particular subcellular locations and cells in which they occur. It also goes some way to explaining why there are so many different diseases associated, either directly or indirectly, with voltage-gated calcium channel dysfunction.
There is currently a great deal of interest in voltage-gated calcium channels, with regard to their involvement in the genesis of a spectrum of psychiatric disorders; particularly autism spectrum disorder, schizophrenia, and bipolar disorder, and this review aims to bring together both the genetic and the available functional information.
2. Voltage-gated Calcium Channel Classification—Genes and Proteins
2.1. Calcium Channel Subtypes
In order to understand how voltage-gated calcium channels may relate to neuropsychiatric diseases, it is important to be aware of the classification of these channels, their different properties and functions, as well as their distribution and interaction with other proteins, which are summarized in this section.
The first clear evidence for more than one category of voltage-gated calcium channel was that currents underlying the calcium conductances in several cell types had distinct high- and low-voltage-activated components, which also had distinctive kinetic properties (Carbone and Lux, 1984, Nilius et al., 1985). Pharmacological tools were essential to further define these components of the calcium currents found in different neurons and muscle cells. The development of calcium channel blockers then allowed particular subtypes of voltage-gated calcium channel to be more clearly defined. The first organic blockers to be characterised were those that selectively targeted a subclass of high-voltage activated channels, prevalent in cardiac and smooth muscle. These drugs included verapamil, diltiazem, and 1,4-dihydropyridines (Fleckenstein, 1983, Triggle, 1987). The channels that were sensitive to these drugs were termed L-type, particularly because the single-channel openings were of large conductance (Nowycky et al., 1985a).
Following the discovery of a number of cone shell and spider peptide toxins that selectively blocked different voltage-gated calcium channel components, further subtypes of voltage-gated calcium channel could be delineated on both physiological and pharmacological bases. In addition to the L-type channels, these were named the N-type, P/Q-type, and R-type for the high-voltage activated calcium channels, whereas the low-voltage activated channels were termed T-type (Nowycky et al., 1985b, Fox et al., 1987a, Fox et al., 1987b, Mintz et al., 1992b, Zhang et al., 1993; for review, see Dolphin, 2006).
2.2. Calcium Channel Subunit Structure
Voltage-gated calcium channels can consist of maximally four different subunits, the pore-forming α1 subunit, as well as the auxiliary α2δ and β (and in some cases γ) subunits. The α1 subunits principally determine the kinetics, voltage dependence, single-channel conductance, and pharmacology of the voltage-gated calcium channels, although many of these properties can be modulated by the β and α2δ auxiliary subunits, which also have significant roles in voltage-gated calcium channel trafficking.
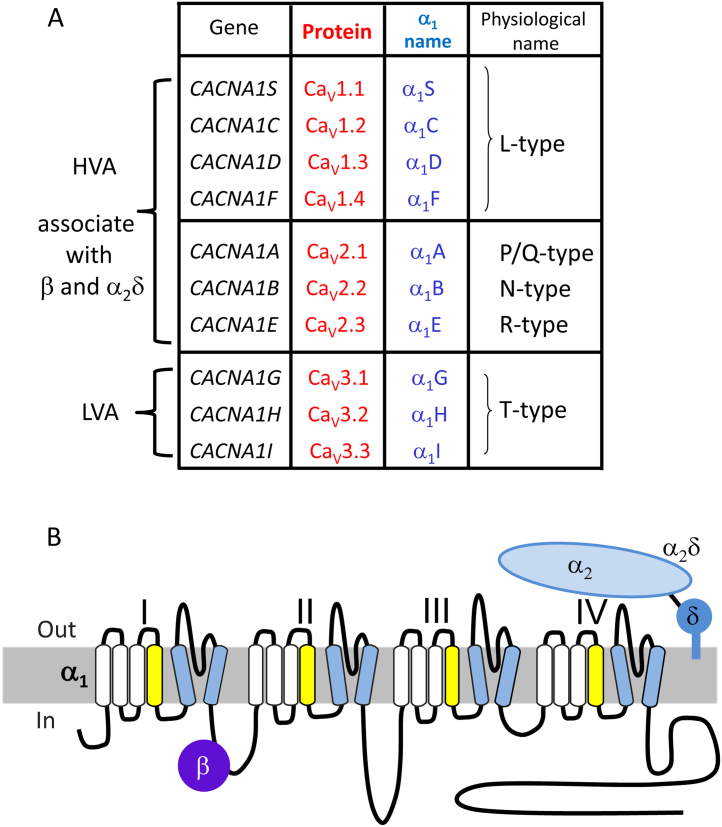
Functional voltage-gated calcium channels are formed from one of 10 different mammalian calcium channel α1 subunit gene products, encoded by the CACNA1 genes. The nomenclature used for the channel and gene names is described here (Catterall et al., 2003) and is summarized in Fig. 1A. In the case of the CaV1.1–CaV1.4 channels (known as L-type channels), these are encoded by CACNA1S, C, D, and F, respectively. The CaV2.1–CaV2.3 channels (termed P/Q-, N-, and R-type channels) are encoded by CACNA1A, B, and E, respectively. Both the CaV1 and CaV2 channels form an α1/β/α2δ complex, co-assembling with one of four α2δ subunits (encoded by CACNA2D1–4) and one of four β subunits (encoded by CACNB1–4) (Fig. 1B). For the CaV3 channels (encoded by CACNA1G, H, and I), the α1 subunits can form functional channels alone, but may also associate with other proteins.
Fig. 1.
Calcium channel α1, β, and α2δ subunits, and their topology. (A) Nomenclature of calcium channel subunits, including gene name, initial names of cloned α1 subunits, rationalised protein names (CaV nomenclature), and names used in physiological discovery of the channels. HVA, LVA = classical definition of channels as high- or low-voltage-activated. (B) Calcium channel α1, β, and α2δ subunit topology. The α1 subunit has 24 transmembrane segments, comprising four homologous domains, labelled I–IV. Each domain has six transmembrane segments (S1–S6), including the S4 voltage sensor (yellow), and the S5–S6 pore-forming segments (blue).
All of the subunit transcripts may exhibit a number of variants, as a result of alternative splicing events. The different channel isoforms and possible combinations make for an enormous potential diversity in the properties and function of the calcium channel complexes.
The auxiliary α2δ and β subunits also have major roles in trafficking the CaV1 and CaV2 channels to the plasma membrane and to specific domains of polarized cells, including neurons (Dolphin, 2012, D’Arco et al., 2015). From purification studies, the L-, P/Q-, and N-type calcium channels were all found to be associated with auxiliary β and α2δ subunits (Tanabe et al., 1987, Witcher et al., 1993, Liu et al., 1996). However, the association of α2δ subunits with the calcium channel complexes was found to be relatively weak and easily disrupted (Takahashi et al., 1987, Müller et al., 2010), compared to the more robust interaction of the β subunits, which show a low nM affinity for interaction with the I–II linker of CaV1 and CaV2 channels (Pragnell et al., 1994, Canti et al., 2001). Despite this difference, both the β and α2δ subunits markedly enhance the expression and function of these channels.
Skeletal muscle calcium channels are also found to be associated with a γ subunit (termed γ1, encoded by CACNG1) (Jay et al., 1990), but other so-called γ subunits (encoded by CACNG2–8), do not appear to form part of cardiac (Walsh et al., 2009) or neuronal (Moss et al., 2002, Müller et al., 2010) calcium channel complexes, and are better considered as transmembrane AMPA-glutamate receptor modifying proteins.
2.3. Voltage-gated Calcium Channel Distribution
CaV1.1 is the skeletal muscle isoform of this family and shows very low expression in brain. In contrast, CaV1.2 (α1C) encoded by CACNA1C is the predominant calcium channel in ventricular cardiac muscle and is also present in smooth muscle, many secretory cells, and throughout the brain (Striessnig et al., 2014). CaV1.3 (α1D) encoded by CACNA1D has a more restricted distribution than CaV1.2, being particularly important in sinoatrial node in the heart and in inner hair cells of the ear (Platzer et al., 2000, Mangoni et al., 2003, Baig et al., 2011), although it is also present in the brain. The differential distribution and function(s) of CaV1.2 and CaV1.3 in various brain regions is discussed in detail in a recent review (Zamponi et al., 2015). CaV1.3 is activated at lower voltage thresholds than CaV1.2 (Koschak et al., 2001, Helton et al., 2005). CaV1.4 (α1F), encoded by CACNA1F, shows very restricted expression, mainly in the retina (Mansergh et al., 2005). The L-type channels, CaV1.2 and CaV1.3, both have a postsynaptic role to play in dendritic signalling, and in signalling to the nucleus in a process termed excitation–transcription coupling (Wheeler et al., 2012, Striessnig et al., 2014).
Members of the CaV2 class of channels have a predominantly neuronal distribution. CaV2.1 (α1A) is the molecular equivalent of P/Q-type calcium channels and is encoded by CACNA1A (Stea et al., 1994). CaV2.1 channels are present throughout the brain and particularly prevalent in cerebellum (Ophoff et al., 1996). They are the predominant calcium channels involved in neurotransmission in most mature central presynaptic terminals investigated (Westenbroek et al., 1995, Iwasaki et al., 2000, Ishikawa et al., 2005, Nakamura et al., 2015) and also make up a substantial proportion of the calcium current recorded in many neuronal cell bodies, particularly Purkinje neurons (Mintz et al., 1992a, Westenbroek et al., 1995). CaV2.2, or α1B, is the molecular counterpart of the N-type calcium channels and is encoded by CACNA1B (Williams et al., 1992). It is widely distributed throughout the central (Westenbroek et al., 1992) and peripheral nervous systems (Lipscombe et al., 1988, Boland et al., 1994, Wheeler et al., 1994) and is particularly important for neurotransmission early in development and also in the mature peripheral nervous system, including nociceptive pathways (Chaplan et al., 1994, Bowersox et al., 1996, Iwasaki et al., 2000). CaV2.3 (α1E) is encoded by CACNA1E (Soong et al., 1993). It was originally described as a low-voltage activated channel, but is now understood to correspond, at least in part, to the residual R-type calcium current, present after pharmacological block of N-type, P/Q-type, and L-type channels (Zhang et al., 1993, Tottene et al., 2000, Wilson et al., 2000). CaV2.3 is widely distributed in many brain regions including the hippocampus and is present both pre- and post-synaptically (Parajuli et al., 2012).
In neurons, the CaV2 channels, particularly CaV2.1 and CaV2.2 (P/Q- and N-type calcium channels), are essential in most synapses for supplying the Ca2+ that mediates presynaptic transmitter release (Takahashi and Momiyama, 1993, Wu et al., 1999, Cao and Tsien, 2010) and there is a developmental switch towards greater reliance on CaV2.1 channels at many synapses in mature animals (Iwasaki et al., 2000). CaV2.3 has been found to be differentially important for triggering spontaneous release of glutamate (Ermolyuk et al., 2013), although there are caveats to the use of the blocker SNX-482 to delineate the physiological roles of CaV2.3 (Kimm and Bean, 2014).
The CaV3 group of channels (α1G, α1H, and α1I), encoded by CACNA1G, H, and I (Cribbs et al., 1998, Perez-Reyes, 1998) are the molecular counterparts of the T-type calcium channels and are more divergent with respect to their sequence from the high-voltage activated channels (Perez-Reyes et al., 2009). CaV3 channels are widely distributed in excitable cells. In the brain, they are present in most neurons and are particularly prevalent in the thalamus (Perez-Reyes, 2003). CaV3 channels (and also CaV1.3 which is relatively low-voltage activated) have important roles in neuronal excitability and pacemaker activity (Perez-Reyes, 2003, Guzman et al., 2009, Putzier et al., 2009). As well as having a postsynaptic distribution and function, at some synapses they also have a presynaptic function to modulate, and in some cases directly mediate, transmitter release (Huang et al., 2011, Carbone et al., 2014).
2.4. Voltage-gated Calcium Channel Pharmacology
CaV1 channels are inhibited by a number of L-type calcium channel blockers, many of which are in clinical use, particularly for hypertension. The most widely used are dihydropyridines, such as nifedipine. Other drugs that block L-type calcium channels, including verapamil and diltiazem, interact with overlapping high-affinity drug-binding sites on these channels (Striessnig et al., 1990, Striessnig et al., 1991). These drugs inhibit CaV1.2 more effectively than CaV1.3 or CaV1.4, partly because the drugs have a much higher affinity for the inactivated state of the channels, and CaV1.2 shows more inactivation than CaV1.3 or CaV1.4 (for review, see Zamponi et al., 2015). Furthermore, because vascular smooth muscle generally sits at a more depolarized membrane potential than most neurons or cardiac ventricular muscle, CaV1.2 in these cells is preferentially targeted by the voltage-dependent dihydropyridine antagonists (Bean et al., 1986). An additional nuance is that although these drugs can bind to the skeletal muscle calcium channel CaV1.1, the primary essential voltage-sensor function of this channel is not significantly inhibited by these calcium channel blockers.
Members of the CaV2 class of channels are not blocked by low concentrations of dihydropyridine antagonists. CaV2.1 can be blocked by certain peptide toxins such as ω-agatoxin IVA (Mintz et al., 1992b). CaV2.2 is irreversibly inhibited by the peptide toxin, ω-conotoxin GVIA (Reynolds et al., 1986), and reversibly blocked by other related ω-conotoxins (McDonough et al., 1996), one of which (ω-conotoxin MVIIC) is licensed for use in certain chronic pain conditions (Staats et al., 2004). R-type calcium current is defined as that which is present after pharmacological block of N-type channels with ω-conotoxin GVIA, block of P/Q-type channels with ω-agatoxin IVA, and block of L-type channels with a selective dihydropyridine antagonist (Zhang et al., 1993). By this process of elimination, it is thought to be the molecular counterpart of CaV2.3 channels. R-type current can be blocked by a peptide toxin SNX 482 (Newcomb et al., 1998, Bourinet et al., 2001); however this toxin also inhibits L-type channels (Bourinet et al., 2001) and some K+ currents (Kimm and Bean, 2014), and thus cannot readily be used to define the role of CaV2.3 channels physiologically.
There are a number of pharmacological agents that target CaV3 channels, including mibefradil (Mishra and Hermsmeyer, 1994, Ertel and Clozel, 1997), although this compound is poorly selective and also targets other calcium channels (Bezprozvanny and Tsien, 1995). In contrast, TTA-A2 and TTA-P2 are selective CaV3 blockers (Choe et al., 2011, Francois et al., 2013), which are of use in defining the physiological roles of T-type channels. Z944 is also a selective T-type channel blocker (Tringham et al., 2012), with therapeutic potential (Lee, 2014).
3. Genetic Analysis of Neuropsychiatric Syndromes
Genetic analysis of disorders that do not show clear Mendelian inheritance can involve a number of different strategies. Historically, the candidate gene strategy for schizophrenia was often centred on the hypothesis that monoaminergic systems were disrupted, based on the ability of drugs (for example, chronic amphetamine), to mimic some of the symptoms of schizophrenia, and the fact that the mainstay of drug treatment of schizophrenia remains the dopamine D2 receptor antagonists. However, these approaches did not lead to useful identification of genes disrupted in these pathways, in part because, by current standards, the early candidate gene studies used very small samples, therefore they did not have adequate statistical power to detect true risk alleles even if they existed in the candidate genes tested (Mitchell, 2011). Nevertheless, for a few of these candidate genes (notably the gene encoding the D2 dopamine receptor, DRD2), an association has indeed now been established from large genome-wide association studies (GWAS) (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014).
A second linkage analysis approach is aimed at identification of the genes mutated in rare families in which the disorder appears to be inherited. For example, the gene Disrupted in Schizophrenia 1 (DISC1) was identified in this way, by virtue of its association with a chromosomal translocation breakpoint (Millar et al., 2000). However, this type of study has had limited success with respect to understanding the aetiology of common psychiatric disorders, because of their inherently complex polygenic nature. In large resequencing studies, the hypothesis that genes, such as DISC1, contain uncommon allelic variants that are of relevance to schizophrenia has not been borne out (Crowley et al., 2013). Nevertheless, meta-analyses have shown some consistency across linkage studies (Lewis et al., 2003).
Thus, aided by the rapid advances in high-throughput genotyping and sequencing technologies, other unbiased techniques are now widely employed. These include GWAS, which examine the frequencies of common genetic variants represented by single nucleotide polymorphisms (SNPs), across a large number of subjects with the disorder in question, compared to a set of matched controls. SNPs are defined as point mutations occurring with a frequency of greater than 1 in 100, and subjects can therefore be either heterozygous or homozygous for these alleles. The definition of an allele is a particular sequence at any given locus in the genome where more than one sequence is present in the population. SNPs are found throughout the genome and most are inevitably in non-coding regions. Furthermore, it is not usually known whether the implicated SNP actually represents the disease-associated alteration, or whether it is in linkage disequilibrium with the variant causing the functional effect. The effect size of individual SNPs associated with common psychiatric disorders is generally small (odds ratio <1.2). However, studies have now shown that collectively, common alleles can account for a large fraction of the genetic liability to develop psychiatric disorders (Purcell et al., 2009). For example, one comprehensive GWAS calculated that common SNPs can collectively account for at least 32% of the variance in liability to develop schizophrenia in the population studied (Ripke et al., 2013). GWAS have identified a number of associations, surpassing genome-wide levels of significance, in calcium channel genes for these diseases, as described below.
Evidence that rare mutations in calcium channel genes also have an important role in the development of neuropsychiatric disorders has primarily come from whole exome sequencing studies. Whole exome sequencing is best suited for discovering rare coding mutations, such as single nucleotide variants, which can either be synonymous (not change the amino acid sequence) or non-synonymous, and either change amino acid residues (missense) or produce premature truncations by mutation to a stop codon. They may also identify deletion or insertion of one or more base pairs (indels), which cause truncation by changing the coding frame, usually resulting in introduction of a premature stop codon preceded by one or more aberrant residues. These studies can be performed in a number of different ways, either de novo mutations are identified in cases with the disorder and compared with those found in matched controls; or rare/de novo mutations in affected subjects are compared to their own unaffected parents and sibling(s) (trio/quartet studies) to identify variants associated with the condition. Since it has become clear that all humans carry a large number of rare, potentially deleterious mutations (Keinan and Clark, 2012, Gao et al., 2015, Sulem et al., 2015), these types of systematic controls are important, if one is to infer a causative role for such mutations in the disorder under examination. It should also be noted that gain-of-function mutations are harder to predict than loss of function mutations and therefore could be missed by any study (Flanagan et al., 2010).
Studies investigating rare copy number variations (these are >1 kb in size and consist of large deletions, duplications, or other more complex rearrangements) have also identified calcium channel genes to be associated with some of these disorders (see below). It is likely that the functional consequence of whole gene deletion or duplication is altered gene dosage (Lee and Scherer, 2010). The functional consequences of copy number variations that partially intersect a gene are more difficult to interpret, but they could have similar effects to loss of function single nucleotide variants/indels.
Given the evidence that the spectrum of psychiatric disorders under discussion in this review all involve synaptic dysfunction (Fatemi and Folsom, 2009), and a large number of synaptic proteins harbour disease-associated SNPs, deleterious mutations, and copy number variations (Ting et al., 2012, Malhotra and Sebat, 2012), it is perhaps not surprising that voltage-gated calcium channels are one of the groups of synapse-associated proteins observed to be involved in these disorders, from many studies. This will be explored in Sections 4, 5.
4. Calcium Channel Genes Identified from GWA Studies of Psychiatric Disorders
SNPs in calcium channel genes have been identified as risk alleles in a spectrum of psychiatric disorders (Cross-disorder group of Psychiatric Genomics Consortium, 2013, Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). As described above, SNPs are point mutations occurring with a frequency >0.01. Although the identified SNP does not necessarily directly cause the biological effect, it must be in linkage disequilibrium with the causative mutation; thus the position of the associated SNP is of functional importance. Most of the associated SNPs are in introns or intergenic regions, where they may be involved in cis-regulatory elements including promoter and enhancer regions or regulation of alternative splice variant expression. cis-elements are situated within or near to the regulated gene and contain binding sites for regulatory factors required for tissue-specific and temporal expression of genes.
The common allele associations identified in calcium channel genes, particularly those found in CACNA1C (SNP rs1006737 and other SNPs in linkage disequilibrium with this SNP), have been reproduced in multiple studies to be associated with psychiatric disorders, including schizophrenia (http://bdgene.psych.ac.cn/geneDetail.do?name=CACNA1C). For example, in a recent study, SNP rs4765905 in CACNA1C showed an association with schizophrenia with an odds ratio of 1.0944 and P = 1.2e-8 (Hamshere et al., 2013). Many of these SNPs are quite common in the population, for example, at rs1006737 the SNP can be either G or A, with A being the risk variant and occurring in the general population with a frequency of about 0.33. In the most recent study, in which 108 SNPs were identified as risk factors in schizophrenia (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014), three calcium channel gene SNPs were identified as risk factors with genome-wide levels of significance. For CACNA1C (SNP rs2007044), the odds ratio for schizophrenia was 0.912 for the common allele (P = 3.22e-18). For CACNB2 (SNP rs7893279), the odds ratio was 1.125 (P = 1.97e-12), and for CACNA1I (Chr22_39987017_D) the odds ratio was 0.930 (P = 4.725e-11). SNPs in several of these genes, particularly CACNA1C, had also been identified previously, and subsequently confirmed with greater power, in studies of people with bipolar disorder (Ferreira et al., 2008, GWAS Consortium, 2011, Lee et al., 2011, Ripke et al., 2013, Cross-disorder group of Psychiatric Genomics Consortium, 2013, Green et al., 2013, Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014, Nurnberger et al., 2014). It is important to note that some identified SNPs are associated with a specific susceptibility to either bipolar disorder or schizophrenia, whereas for two of the genes, CACNA1C and CACNB2, the SNPs were found to confer susceptibility to schizophrenia, bipolar disorder, and also to major depressive disorder (Green et al., 2010, Cross-disorder group of Psychiatric Genomics Consortium, 2013). Indeed SNPs in multiple voltage-gated calcium channel subunit genes were associated with disease in this five disorder meta-analysis, including the α1 subunit genes CACNA1C, CACNA1D, CACNA1E, CACNA1S, the α2δ subunit genes CACNA2D2 and CACNA2D4, and the β subunit gene CACNB2 (Cross-disorder group of Psychiatric Genomics Consortium, 2013).
SNPs in other calcium channel genes have been found to be associated with autism spectrum disorder, in particular, the T-type calcium channel genes CACNA1G (Strom et al., 2010, Lu et al., 2012), and CACNA1I (Lu et al., 2012). In the latter study, 2781 parent/affected child trios were examined, and associations were considered significant if they survived correction for the 10 genes examined (Lu et al., 2012). Furthermore, although it is not a psychiatric disorder, variations in central pain perception and processing have been associated with an SNP in CACNA2D3 (Neely et al., 2010).
5. Rare Mutations in Calcium Channel Genes in Psychiatric Disorders
It has recently been demonstrated that healthy individuals exhibit multiple rare de novo or inherited neutral and disruptive mutations in coding sequences (Keinan and Clark, 2012, MacArthur et al., 2012). This was well illustrated by an ion channel exome sequencing study of subjects with epilepsy, compared to controls which did not find an excess of rare potentially causative mutations in the affected individuals (Klassen et al., 2011).
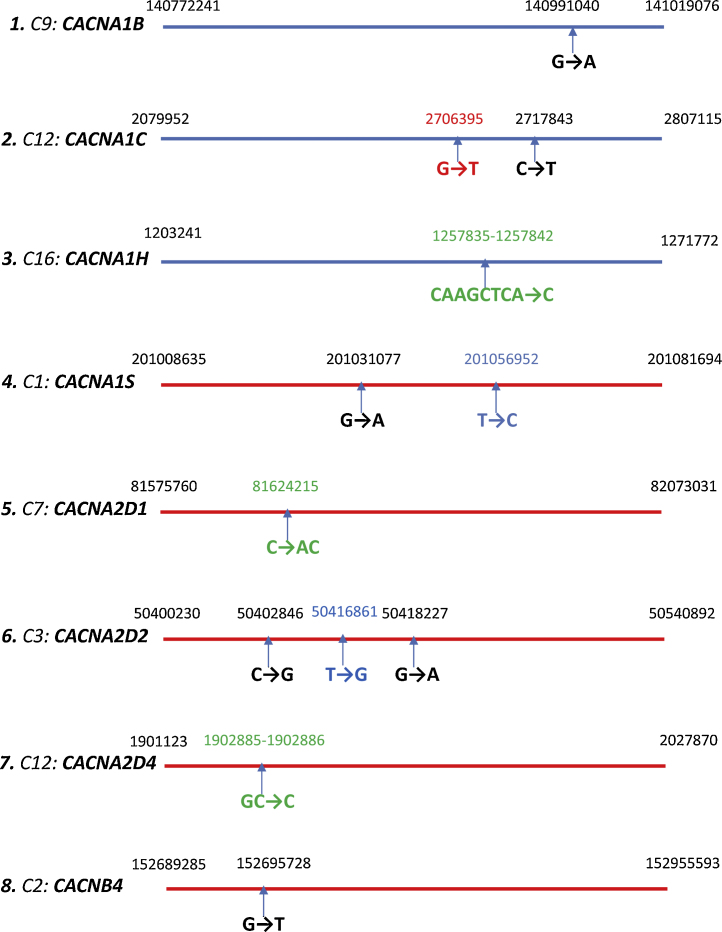
In the largest whole exome sequencing study of schizophrenia to date, rare (less than 1 in 10,000), disruptive alleles were significantly enriched in cases, among ∼2500 genes previously implicated in schizophrenia. At the level of gene sets, disruptive alleles were enriched in voltage-gated calcium channels, with the strongest signal found for ultra-rare alleles observed only once in the sample (12 in cases, one in controls; P = 2 × 10−3, odds ratio = 8.4). Strikingly, in this study, the voltage-gated calcium channel gene subset was highly represented by CACNA1B, CACNA1C, CACNA1H, CACNA1S, CACNB4, and also CACNA2D1, CACNA2D2, and CACNA2D4 (Purcell et al., 2014). Two mutations in CACNA1C were identified; this was one of the calcium channel genes previously implicated in several studies by GWAS of both bipolar disorder and schizophrenia (see section above). Both these mutations are truncating and predicted to cause loss of function. We show, in Fig. 2, the positions of a number of the mutations in calcium channel genes, in CACNA1B, CACNA1C, CACNA1H, CACNA1S; CACNA2D1, CACNA2D2, CACNA2D4, and CACNB4, described in Purcell et al. (2014). All these mutations involve either frameshift, mutation to a stop codon, or splice site mutations, and from their position all are likely to cause partial or complete loss of function (Fig. 2). Given their rarity, individuals with these mutations are likely to be heterozygous and therefore carry one non-mutated allele.
Fig. 2.
-
1)CACNA1B: G→A will convert a tryptophan residue into a stop codon, truncating the protein.
-
2)CACNA1C: G→T is a splice acceptor mutation, whereas C→T will convert a glutamine residue into a stop codon, truncating the protein.
-
3)CACNA1H: CAAGCTCA→C is a 7 nucleotide deletion, causing a frameshift leading to addition of 7 different amino acids followed by a stop codon—the original protein is 2347aa, this truncated protein is 1050aa. A deletion at position 1260920, TC→T, causes a frameshift leading to a truncation (15 missense aa before the stop codon-truncated protein is 1406aa, the WT protein is 2347aa).
-
4)CACNA1S: G→A will convert a tryptophan residue into a stop codon, truncating the protein. T→C is a splice donor mutation.
-
5)CACNA2D1: C→AC is an insertion, disrupting the amino acid code thereafter due to frameshift. It will cause truncation of the protein.
-
6)CACNA2D2: C→G will convert a tyrosine residue into a stop codon, truncating the protein. T→G is a splice donor mutation. G→A will convert a tryptophan residue into a stop codon, truncating the protein.
-
7)CACNA2D4: GC→C single nucleotide deletion causes a frameshift, changing the following 21 amino acids and adding an additional 28 amino acids before a stop codon occurs. The original protein is 1137aa, this predicted mutated protein is 1165aa.
-
8)CACNB4: G→T will convert a glutamic acid residue into a stop codon, truncating the protein. NCBI reference numbers for the calcium channels sequences used: CACNA1B: NM_000718; CACNA1C: NM_000719; CACNA1D: NM_000720; CACNA1E: NM_000721; CACNA1F: NM_001256789; CACNA1H: NM_001005407; CACNA1S: NM_000069; CACNA2D1: NM_000722; CACNA2D2: NM_001005505; CACNA2D4: NM_172364; CACNB2: NM_201597; CACNB3: NM_000725; CACNB4: NM_000726.
A recent study has highlighted the importance of accurate prediction of whether transcripts containing a premature stop codon will trigger nonsense-mediated mRNA decay (Rivas et al., 2015). In the case of the truncated α1 subunits, if the mRNA was not completely degraded by nonsense-mediated decay, and if a partial protein were translated, it could potentially behave in a dominant-negative manner, as has been shown for truncated CaV2.1, CaV2.2, and CaV3 channels (Raghib et al., 2001, Page et al., 2004, Page et al., 2010, Mezghrani et al., 2008), and this may potentially occur for CaV2.1 in the monogenic disorder episodic ataxia 2 (Jouvenceau et al., 2001, Page et al., 2004, Mezghrani et al., 2008). It should also be noted that CACNA2D4, encoding α2δ-4, implicated in Purcell et al. (2014) (Fig. 2) is primarily expressed in the retina (Lee et al., 2015).
Although not directly related to calcium channels, it is of interest that in another whole exome sequencing study in schizophrenia (Fromer et al., 2014), disruptive mutations were identified in members of the postsynaptic density gene set, including the gene for a calcium activated protease (CAPN5). This gene encodes calpain, which can be activated in dendrites by voltage-gated calcium channels (Kanamori et al., 2013).
Exome sequencing in individuals with autism spectrum disorder also identified an excess of de novo deleterious mutations (nonsense, splice site, and frame shifts) in cases, compared to unaffected siblings (Iossifov et al., 2012). In a recent analysis of de novo, inherited and case–control mutations identified from exome sequence of 3871 autism cases and 9937 ancestry-matched or parental controls, CACNA2D3 was implicated as a risk gene following the identification of two de novo loss of function mutations in cases and none in controls (false discovery rate <0.05)(De Rubeis et al., 2014). Interestingly, in a previous study of 343 families, each with a single child on the autism spectrum, and at least one unaffected sibling, a single de novo splice site mutation was found in CACNA2D3, one of many gene-disrupting mutations identified (Iossifov et al., 2012). A copy number variation study in autism also revealed a deletion in CACNA2D3 (Girirajan et al., 2013). CACNA2D3 encodes α2δ-3, which is widely expressed in the brain and involved in neurotransmitter release (Hoppa et al., 2012) and synaptic function (Pirone et al., 2014).
In the same large exome sequencing study described above that identified CACNA2D3, five mutations were also identified in CACNA1D (De Rubeis et al., 2014); these included G407R (in exon 8a) and A749G, both of which were described previously (Iossifov et al., 2012, O’Roak et al., 2012) and subsequently shown to cause a gain of function (Pinggera et al., 2015). Other mutations identified were A59V, which maps to an N-terminal sequence involved in Ca2+-dependent inactivation (Dick et al., 2008), and two C-terminal mutations (S1977L and R2021H), which are in a C-terminal proline-rich domain, involved in interaction with SHANK3 (Zhang et al., 2005).
Furthermore, in a whole genome sequencing study of autism spectrum disorder, a rare missense mutation in CACNA1C (R1522Q) in the proximal C-terminus was identified in a proband with autism but it was also found in an unaffected sibling, and also their father (who had the cardiac disorder, Wolff Parkinson White syndrome) (Jiang et al., 2013). In this family, it was therefore unclear whether the mutation in CACNA1C was causative of autism. In another study, three rare missense variants in the coding region of CACNB2 (G167S, S197F, and F240L) were identified in autism spectrum disorder probands, and absent from controls, following exome sequencing of this gene for 259 controls and 155 probands (Breitenkamp et al., 2014). However, the variants showed incomplete segregation with the disorder in the families affected. Nevertheless, all three variants showed altered time-dependent inactivation of Ca2+ currents, although two mutations were associated with significantly slower inactivation, whereas the third mutation (F240L) showed increased inactivation (Breitenkamp et al., 2014). More recently, in an exome sequencing study of autism risk genes, investigating families where two siblings were autistic, a mutation in CACNB2 (V2D) was identified as one of relatively few genes that showed mutations in both affected siblings (Yuen et al., 2015). The functional relevance of this mutation has not yet been studied.
It has now been shown that there is an increased burden of microdeletions and duplications (all included in the term copy number variation) in schizophrenia but not in bipolar disorder (International Schizophrenia Consortium, 2008, Green et al., 2015). These copy number variations often involve multiple genes, so that it is difficult to identify with certainty the causative genes (for review, see Doherty et al., 2012). For example, copy number variation in CACNA1B has been observed in subjects with disruption of the subtelomeric 9q34 region, but the rearrangements usually also involve another gene, EHMT1, and the phenotype observed is variable. In some cases, however, there was monogenic duplication of the CACNA1B gene, with a phenotype including Autism Spectrum Disorder (Yatsenko et al., 2012).
Other presynaptic markers have also been found to be implicated in this spectrum of disorders, including the synaptic scaffolding protein neurexin-1 encoded by NRXN1 (Doherty et al., 2012). Neurexins are key molecules involved in synaptic function. The α-neurexins have been found to organize active zones, by promoting the functional coupling of voltage-gated Ca2+ channels to presynaptic machinery (Missler et al., 2003). Neurexins are also selectively trafficked to presynaptic terminals in transport vesicles, together with other presynaptic proteins including calcium channels (Fairless et al., 2008). Recently, rare exonic micro-deletions and other mutations in NRXN1 have been linked with schizophrenia (Walsh et al., 2008, Rees et al., 2014) and autism (Szatmari et al., 2007, Iossifov et al., 2012, De Rubeis et al., 2014); and early GWA studies provided some evidence for common schizophrenia risk alleles existing in this gene (O’Dushlaine et al., 2011). Although NRXN1 was not one of the 108 genes identified from the recent schizophrenia GWA study (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014), it is of interest that this study did implicate NLGN4, encoding a neuroligin, which is a postsynaptic binding partner of neurexins.
For autism spectrum disorder, data relating to copy number variations and deleterious single nucleotide variants have recently been combined to show convergence on a few pathways, involving chromatin modification/transcription regulation, MAP kinase/cellular signalling, and neuronal development/axon guidance. CACNA1C, CACNA1B, and CACNA1F were included in this analysis in the latter two gene networks (Pinto et al., 2014).
6. Pathophysiological Sequelae of CACNA1C Mutations and Polymorphisms
In investigating whether SNPs, rare single nucleotide variants and other genetic disruptions are associated with gain or loss of function of the implicated channel subunits, it depends on the way in which these terms are defined. Channel truncation or disruption of mRNA expression resulting from the very rare deleterious mutations described above are likely to result in loss of channel function and may even be dominant-negative, if a channel fragment is produced (Table 1). In contrast, some point mutations may increase calcium currents or reduce inactivation, which could be seen crudely as gain of function, although since tight control must be exerted over intracellular Ca2+ levels, loss of channel inactivation is also a deleterious event in neurons and other cell types (Table 1).
Table 1.
Evidence for change in calcium channel function in psychiatric disorders linked with calcium channel genes.
| Disorder | Gene | Type of mutation | Gain/loss of function | Effects on calcium channel function | Reference |
|---|---|---|---|---|---|
| Schizophrenia | CACNA1B, CACNA1C, CACNA1H, CACNA1S, CACNB4, CACNA2D1,2,4 | Rare germline mutations in coding region or splice sites, mainly causing truncation (heterozygous) | Loss likely in most cases | Truncated protein/also nonsense-mediated mRNA degradation (see Fig. 2) | (Purcell et al., 2014). |
| Schizophrenia bipolar disorder autism | CACNA1C | Intronic SNPs (esp. rs1006737) subjects can be hetero- or homozygous for risk allele, A in rs1006737) | Gain | Increased mRNA expression in brain and induced neurons | (Bigos et al., 2010, Yoshimizu et al., 2015) |
| Partial loss | Reduced transcription because of reduced interaction with promoter | (Roussos et al., 2014) | |||
| Partial loss | rs1006737 and rs1024582 decreased gene expression in post-mortem cerebellum | (Gershon et al., 2014) | |||
| Timothy syndrome (includes autism) | CACNAIC | Rare germline missense point mutations | Gain | Loss of inactivation | (Splawski et al., 2004) |
| Autism | CACNA2D3 | Rare germline mutations; truncating, splice site, deletion (heterozygous) | Complete loss | Truncated protein or nonsense-mediated mRNA degradation | (De Rubeis et al., 2014, Iossifov et al., 2012, Girirajan et al., 2013) |
| CACNB2 | Rare germline point mutations in coding sequence (heterozygous but incomplete segregation with disease) | Gain and loss | Inconsistent effects on channel function | (Breitenkamp et al., 2014) | |
| CACNA1H | Rare germline point mutations in coding region (did not segregate completely with disease) | Loss | Reduced currents | (Splawski et al., 2006) | |
| CACNA1D | Rare germline point mutations in coding region | Gain | Increased currents or loss of inactivation | (Pinggera et al., 2015) | |
| Intellectual disability/hyper-aldosteronism | CACNA1D | Rare germline point mutations in coding region | Gain | Loss of inactivation or hyperpolarization of window current | (Scholl et al., 2013) |
| Intellectual disability/epilepsy | CACNA2D2 | Rare recessive point mutation | Complete loss | Loss of function of α2δ-2 to increase calcium currents | (Pippucci et al., 2013, Edvardson et al., 2013) |
| Fragile X syndrome (cognitive impairment, autism) | FMR1 | CAG repeat expansion | Loss of FMRP protein | Gain of CaV2 calcium channel function | (Ferron et al., 2014) |
| CACNA1A, B, E, G, I and CACNB1, 3 mRNAs are FMRP targets, loss of FMRP will upregulate expression | (Darnell et al., 2011) |
Furthermore, a recent study identified a group of 19 genes implicated in schizophrenia from their previous GWAS results and showed their brain mRNA expression was correlated and further pointed out that these gene products either interact with each other or with other schizophrenia-associated gene products. Their data support a prominent role for calcium channels and associated calcium signaling pathways in the pathogenesis of schizophrenia (Hertzberg et al., 2015).
6.1. CACNA1C Polymorphisms
Studies on the neuropsychiatric phenotype of Timothy syndrome, resulting from CaV1.2 gain of function mutations (see Section 7.3), suggest that Cav1.2 dysfunction may operate as a risk factor in these disorders more generally. In the GWA studies described above, the association signal identified in the CACNA1C gene is in a large intron between exons 3 and 4, which contains several SNPs in linkage disequilibrium, that have been implicated in this neuropsychiatric disease spectrum (Ripke et al., 2013). The implicated SNPs include rs1006737 (Hamshere et al., 2013) and rs2007044 (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). Recent studies on the pathophysiological consequences of these intronic risk alleles are divergent. It has been found that there is a specific interaction of an enhancer region, situated in the intron containing this SNP, with the proximal CACNA1C gene promoter, which results in the risk variant SNP being associated with reduced CACNA1C gene expression (Roussos et al., 2014). Another recent study also reported that the CACNA1C risk alleles rs1006737 and rs1024582 are associated with decreased gene expression in post-mortem cerebellum, but not in parietal cortex (Gershon et al., 2014). In contrast, in another study of post-mortem human brain samples from dorsolateral prefrontal cortex of control subjects, the homozygous rs1006737 risk allele (AA) was associated with a small increase in CaV1.2 mRNA (Bigos et al., 2010). Furthermore, in a study comparing induced neurons derived from skin fibroblasts, from subjects with either AA, AG, or GG containing alleles, the presence of the homozygous rs1006737 risk allele AA also resulted in increased CACNA1C mRNA compared to those with AG or GG. In parallel, these neurons exhibited increased calcium current density for 10/11 risk allele lines analysed (Yoshimizu et al., 2015). However, in one of the cell lines analysed, homozygous for the risk genotype, there was lower CACNA1C mRNA levels and lower calcium current density, and thus the pattern is not completely penetrant (Yoshimizu et al., 2015).
Human functional studies, examining heterozygous and homozygous individuals harbouring the risk allele for SNP rs1006737, have identified alterations in brain function, using functional magnetic resonance imaging (fMRI), associated with working memory. However, these investigations are limited by examining small numbers of subjects, and some contradictory results are reported between the studies. Interestingly, in one study, healthy carriers of the CACNA1C risk variant rs1006737 were found to have a reduction of bilateral hippocampal activation during episodic memory recall, and several other alterations (Erk et al., 2010). The rs1006737 risk allele was also associated in a dose-dependent manner with blunted reward responsiveness (Lancaster et al., 2014). Another recent study compared the brain activity of healthy rs1006736 risk allele carriers with matched controls (Paulus et al., 2014). Here, a significant decrease in activity in the dorsolateral prefrontal cortex was observed, as well as a decrease the connectivity with the medial temporal lobe during working memory tasks, in those homozygous for the risk allele (Paulus et al., 2014), which was the opposite result compared to a previous study (Bigos et al., 2010). Although these two studies appear to be confounding, the data are actually consistent with another study that identified both increases and decreases in the capacity of working memory in bipolar and schizophrenic rs1006737 carriers, respectively (Zhang et al., 2012). It is also consistent with studies that identified both increased and reduced expression of CACNA1C in the presence of the rs1006737 risk allele (Bigos et al., 2010, Roussos et al., 2014, Gershon et al., 2014) (Table 1).
A number of other behavioural and imaging studies have also demonstrated alterations in individuals carrying the CACNA1C rs1006737 risk allele, although again these are not always consistent. An fMRI study of emotional processing in the amygdala in bipolar, schizophrenic, and healthy control carriers of the rs1006737 risk allele (AA/AG) found that the presence of the risk allele affected amygdala activity during emotional processing across all diagnostic groups (Tesli et al., 2013). Another study showed a significant association for alleles in intron 3 of CACNA1C (both rs1006737 and the related SNP rs7959938) with increased brainstem volume (Franke et al., 2010). However, an effect of the rs1006737 risk allele on global grey matter volume which had been identified previously in healthy individuals (Kempton et al., 2009) could not be confirmed in this study (Franke et al., 2010).
7. Monogenic Disorders Resulting from Harmful Mutations in Other Voltage-gated Calcium Channel Genes
7.1. CACNA1A
It is notable that this gene has not been implicated in the spectrum of neuropsychiatric disorders discussed above. It encodes the mainly presynaptic P/Q-type calcium channel (CaV2.1), which is primarily essential for neurotransmitter release at mature excitatory and inhibitory synapses and is also extremely important in cerebellar function, being strongly expressed in Purkinje neurons. Multiple dominant gain and loss of function mutations in CACNA1A have been described in patients, resulting in a spectrum of disorders: in particular, familial hemiplegic migraine (FHM)-1, Episodic ataxia (EA)-2, spinocerebellar ataxia (SCA)-6, and epilepsy. These diseases and their molecular basis have been described in extensive recent reviews (Pietrobon, 2010, Pietrobon and Moskowitz, 2013) and will not be covered in detail here. In summary, mutations in CaV2.1 causing FHM1 are thought to result in gain of function changes in CaV2.1 expression resulting in increased excitatory synaptic function (Tottene et al., 2009) and increased basal Ca2+ (Di Guilmi et al., 2014). In contrast, the mainly truncating mutations that result in EA2 are due to loss of function, with the possibility of dominant-negative action of the mutant gene product (Jouvenceau et al., 2001, Page et al., 2004, Mezghrani et al., 2008). The mechanism of SCA6, which results from an increased CAG repeat of one C-terminal CaV2.1 splice variant, is still unclear (Riess et al., 1997). The question remains as to whether the pathology is due to altered CaV2.1 function or to the expanded (21–30) CAG repeat region, which is nevertheless a relatively low number of repeats that would not be deleterious in other CAG expansion diseases (for discussion, see Pietrobon, 2010).
7.2. CACNA1B
Until recently, no mutations in CACNA1B, which encodes the mainly presynaptic neuronal N-type calcium channel CaV2.2, had been described in patients. Recently, one inherited gain of function mutation has now been described, in the outer mouth of the channel pore, which is found to be linked to a myoclonus-dystonia syndrome (Groen et al., 2015), although this finding has been disputed (Mencacci et al., 2015).
7.3. CACNA1C: Timothy Syndrome
This is an autosomal dominant genetic disorder affecting multiple organs, but in particular displaying cardiac defects. It is characterized primarily by prolonged QT syndrome, syndactyly, and craniofacial abnormalities, although patients also exhibit cognitive impairment, major developmental delays, and autism-like behaviours (Splawski et al., 2004). The predominant genetic causes are de novo missense mutations, particularly in mutually exclusive exons 8 or 8a of CACNA1C, which encodes residues at the base of domain I S6, associated with the activation gate. These mutations are described as gain of function (Table 1), as they involve loss of channel inactivation, which results in elevated Ca2+ entry (Splawski et al., 2004, Barrett and Tsien, 2008, Ramachandran et al., 2013). In cardiomyocytes, the increased Ca2+ entry gives rise to delayed repolarization of the action potential and ventricular arrhythmias, which are the main cause of death.
The Timothy syndrome mutations are subdivided into TS1 mutations (G406R in exon 8a) and TS2 mutations (G406R or G402S in exon 8). Tissue-specific differences in the expression of these two mutually exclusive exons result in patients with a mutation in exon 8 showing some variation in symptoms from those with a mutation in exon 8a. It might be expected that calcium channel blocking drugs would be of use in the treatment of Timothy syndrome, but the gain of function mutations are in many cases less sensitive to block, particularly by dihydropyridine antagonists, because of the loss of inactivation (Splawski et al., 2004).
Recently, quite a number of other rare gain of function Timothy syndrome mutations have been observed outside I S6. For example, a mutation in exon 27 (I1166T), which is predicted to be at the base of domain III S6, results in an increased window current (Boczek et al., 2015). Also a patient with an A1473G mutation, predicted to be in domain IV S6, had a complex phenotype including seizures (Gillis et al., 2012), and G1911R, in the C-terminus, leads to a multi-organ syndrome including seizures and developmental delay (Hennessey et al., 2014).
Of relevance to the GWA studies described above, a patient with a G402S mutation in exon 8, for which he is a mosaic, developed bipolar disorder when aged 22 (Gershon et al., 2014). Relevant to this, heterozygous knock-in mice, expressing a human TS2 mutation (G406R) at a low level, show behavioural changes consistent with autistic-like behaviour (Bader et al., 2011). Furthermore, neurons derived from induced pluripotent stem cells from patients with the G406R mutation show multiple changes in gene expression, probably secondary to altered CaV1.2 function (Pasca et al., 2011) and also exhibit activity-dependent dendrite retraction (Krey et al., 2013).
7.4. CACNA1D
The CaV1.3 channels encoded by CACNA1D are L-type calcium channels which are important for pacemaker activity in the sinoatrial node and brain, as well as in hearing, as identified by a rare recessive human loss of function mutation, which gives rise to sino-atrial node dysfunction and deafness (SANDD) syndrome (Baig et al., 2011). Somatic gain of function mutations have been identified in CACNA1D in aldosterone producing adenomas in the adrenal zona glomerulosa (Azizan et al., 2013, Scholl et al., 2013). These mutations are concentrated in the pore region, the activation gate, and the voltage sensors and include mutations in exon 8a, in a striking parallel to those in Timothy syndrome. Furthermore, germline gain of function mutations were also found, identical to two of the somatic mutations (G403D and I770M), in patients with juvenile hypertension resulting from hyperaldosteronism (Scholl et al., 2013). These patients also displayed a complex neurological syndrome including cerebral palsy.
It is interesting that two of the mutations described above to be associated with autism spectrum disorder (G407R and A749G) (Iossifov et al., 2012, O’Roak et al., 2012, De Rubeis et al., 2014) were located near to the residues mutated in patients with primary aldosteronism and neurological deficits. In the brain, L-type calcium channels, including CaV1.3, play a role in the pacemaker activity in brain dopaminergic neurons (Guzman et al., 2009, Liu et al., 2014), and CaV1.3 is also involved in synaptic pruning during development (Hirtz et al., 2012), two processes whose disruption might be likely to be implicated in psychiatric disorders (for review, see Striessnig et al., 2014). It is likely that the CACNA1D gain of function mutations resulting in autism may have a milder effect on channel function, whereas severe gain of function mutations result in more extensive disability and hyperaldosteronism (Scholl et al., 2013, Pinggera et al., 2015) (Table 1).
7.5. CACNA1H
Rare missense mutations in CACNA1H were found in 6 of 461 individuals with autism spectrum disorder. R212C and R902W are in domain I and domain II voltage sensors, W962C is in domain II pore, and A1874V is in the proximal C terminus. However, some of the mutations were also present in unaffected family members, indicating that the mutations are not fully penetrant. However, all these mutations reduced CaV3.2 function in an expression system (Splawski et al., 2006). It should also be noted that gain of function mutations in CaV3.2 have been linked to childhood absence epilepsy (Chen et al., 2003, Khosravani et al., 2004) and also to hypertension associated with hyperaldosteronism (Scholl et al., 2015).
7.6. CACNA2D1
The α2δ-1 protein, encoded by CACNA2D1, is highly expressed in skeletal, cardiac, and smooth muscle, as well as in the brain (Ellis et al., 1988, Jay et al., 1991, Klugbauer et al., 1999). Mutations in CACNA2D1 have been found to be associated with cardiac dysfunction, including Brugada (Burashnikov et al., 2010) and short QT (Templin et al., 2011, Bourdin et al., 2015) syndromes. However, no central phenotypes have been identified in humans, possibly because most neurons contain more than one subtype of α2δ subunit and these proteins may have a partially interchangeable function. Nevertheless, in homozygous cacna2d1 knockout mice, a mechanosensory and pain phenotype (Patel et al., 2013), as well as abnormal cardiac function (Fuller-Bicer et al., 2009) have been documented.
7.7. CACNA2D2
Two human family pedigrees with recessive mutations in CACNA2D2, encoding α2δ-2, causing infantile epileptic encephalopathy have been found (Pippucci et al., 2013, Edvardson et al., 2013). The carriers had no phenotype, in agreement with the lack of phenotype in heterozygote mice lacking cacna2d2 expression, despite the homozygous knockout mice having a major ataxic and epileptic phenotype (Barclay et al., 2001, Brill et al., 2004).
7.8. CACNB4
β4, encoded by CACNB4, is widely expressed in the brain and is one of the main β subunits associated with neuronal calcium channels (for review, see Dolphin, 2003). Its involvement in neurological disease was first suggested by studies on the lethargic mouse strain (Burgess et al., 1997). Furthermore, a truncating mutation (loss of 38 C-terminal residues of β4) has been found in patients with generalised epilepsy and ataxia (Escayg et al., 2000). A particular β4 splice variant is also localised in the nucleus (Hibino et al., 2003) and it may therefore have additional functions on gene expression (Etemad et al., 2014).
8. Changes in Calcium Channel Gene Expression in Disease
A number of studies have been performed to examine changes in gene expression of ion channels, including calcium channel subunits, in post-mortem brains from patients with neuropsychiatric diseases (Iwamoto et al., 2004, Smolin et al., 2012). However, there are many confounding factors that affect such studies, including the fact that patients have been exposed to a variety of drugs, making it difficult to reach firm conclusions. In one study, expression of CACNA1A mRNA measured by microarray assay was down-regulated in post-mortem brains of 11 patients with bipolar disorder, although this was not confirmed by RT-PCR studies (Iwamoto et al., 2004). In another study, changes in expression of some calcium channel β subunit mRNAs were observed in some brain regions, in 14 patients each with bipolar disorder, schizophrenia, or major depression (Smolin et al., 2012), but again, confounding factors age, gender, post-mortem interval, and drug treatment make firm conclusions difficult. There are, however, several other neuropsychiatric disorders in which gene expression changes do occur, as described below.
8.1. Fragile X Syndrome
Fragile X syndrome is the most common inherited form of intellectual disability. Fragile X syndrome has a prevalence of 1 in 2500–4000 males and 1 in 6000–8000 females. People with Fragile X syndrome show mild to moderate cognitive dysfunction, frequently associated with autistic spectrum disorders (Bhakar et al., 2012). In addition, Fragile X syndrome patients often display peripheral autonomic and sensory symptoms, including heightened tactile sensitivity and gastrointestinal motility changes (Boyle and Kaufmann, 2010). Fragile X syndrome results from the partial or complete loss of Fragile X mental retardation protein (FMRP) expression and function. FMRP is present both in the nucleus and the cytoplasm and is a component of cytoplasmic RNA granules, where it serves both to traffic-specific mRNAs to sites of translation and to stall their translation (Bassell and Warren, 2008, Darnell et al., 2011). Loss of FMRP in fmr1 knockout mice results in dysregulation of mRNA translation (Bassell and Warren, 2008) and an alteration of synapse number and shape (Antar et al., 2006). Research has concentrated particularly on the dendritic/postsynaptic role of FMRP (Ronesi and Huber, 2008, Krueger and Bear, 2011). Loss of FMRP results in excessive and unregulated dendritic mRNA translation (Antar et al., 2004, Bassell and Warren, 2008). However, there is now increasing evidence for an additional presynaptic role of FMRP. Loss of presynaptic FMRP reduces functional synapse formation (Hanson and Madison, 2007), decreases the size of the presynaptic active zone and synaptic vesicle number, and affects presynaptic protein levels (Klemmer et al., 2011). Granules containing FMRP have been identified in central presynaptic terminals and axons, being particularly prevalent during synapse maturation (Christie et al., 2009). Recent studies also describe a role for FMRP in local protein synthesis in peripheral sensory axons (Price et al., 2006). Although fmr1 knockout mice exhibit normal acute nociceptive responses, they show alterations in chronic responses, both peripherally and centrally (Price et al., 2007). As described above, heightened tactile sensitivity and self-injurious behaviour is observed in some Fragile X syndrome patients and this could be related to dysregulation of nocifensive behaviour (Price et al., 2007).
Several calcium channel mRNAs have been identified as FMRP targets, suggesting they would be upregulated as a result of loss of FMRP, including CACNB3 and CACNA1C (Darnell et al., 2001, Brown et al., 2001), and more recently a larger array of calcium channel genes including CACNA1A, CACNA1B, CACNA1E, CACNA1G, CACNA1I, CACNB1, and CACNB3 (Darnell et al., 2011). In addition to its role as an RNA binding protein, FMRP has also been shown to associate with ion channels. FMRP has been shown to interact with, and modify the activation of, both sodium-activated K+ channels (Brown et al., 2010) and calcium-activated K+ channels (Deng et al., 2013). It has also recently been shown that FMRP interacts with voltage-gated calcium channels and modulates presynaptic neurotransmitter release (Ferron et al., 2014). Furthermore, it is relevant to mention here that rare alleles in a number of FMRP targets have been associated with schizophrenia across multiple studies (Fromer et al., 2014, Purcell et al., 2014).
8.2. Neuropathic Pain
While changes in calcium channel gene expression have not been widely reported in neurological and psychiatric diseases, in neuropathic pain this phenomenon is widely observed following damage to peripheral nerves, which have the capacity for regeneration. Peripheral sensory nerve damage has as one of its sequelae the change in transcription of many genes, which may be either up- or down-regulated (Newton et al., 2001, Wang et al., 2002, Xiao et al., 2002, Davis-Taber and Scott, 2006). These gene expression changes have a major role in the development and maintenance of chronic pain that long outlasts the injury. One of the many molecular consequences of experimental peripheral nerve injury is an increase in the level of α2δ-1 mRNA in damaged sensory dorsal root ganglion neurons, shown by in situ hybridization (Newton et al., 2001), microarray analysis (Wang et al., 2002, Xiao et al., 2002, Davis-Taber and Scott, 2006), PCR (Luo et al., 2001), and quantitative PCR (Bauer et al., 2009). There is also an alteration in expression of α2δ-1 splice variants, with concomitant pharmacological consequences (Lana et al., 2014). In contrast to α2δ-1, the mRNA for α2δ-2 and α2δ-3 has been shown to be down-regulated in rat dorsal root ganglion neurons following nerve injury (Bauer et al., 2009).
Sensory nerve damage may occur due to direct physical trauma, or it may be a result of poorly regulated plasma glucose in diabetes, herpes virus infection, certain chemotherapeutic drugs, and other causes. The injury-induced increase in α2δ-1 protein occurs in dorsal root ganglion somata and in their axons and central terminals in the spinal cord, as determined by Western blot (Luo et al., 2001) and immunohistochemistry (Bauer et al., 2009). In contrast, mRNA and protein for the main calcium channel α1 subunit involved in pain transmission (CaV2.2) is not up-regulated following sensory nerve damage (Wang et al., 2002, Li et al., 2006), although there is a change in splicing (Altier et al., 2007). This leads to the possibility that up-regulated α2δ-1 enhances CaV2.2 trafficking and presynaptic function, although it may also have other functions, separate from its role as a calcium channel subunit (Eroglu et al., 2009). There is also no observed up-regulation of β subunits reported in microarray (Wang et al., 2002, Xiao et al., 2002, Davis-Taber and Scott, 2006) or other studies (Lana et al., 2014). In contrast, CACNA1D expression has been shown to be down-regulated two-fold in small dorsal root ganglions (Davis-Taber and Scott, 2006).
9. Involvement of Voltage-gated Calcium Channels in Early Brain Development
Although beyond the scope of this review, the role of neuronal calcium spikes and waves during brain development and in the establishment of neuronal circuitry is the subject of extensive research (Gu et al., 1994, Nishiyama et al., 2011, Rosenberg and Spitzer, 2011, Lepski et al., 2013). Calcium spikes are found in neurons at the earliest stages of development (Gu and Spitzer, 1993, Desarmenien et al., 1993, Hirtz et al., 2012). These spikes involve activity that is both spontaneous and also dependent on networks. In one study, L-type calcium channels were found to be involved in thalamocortical axon outgrowth (Mire et al., 2012). Furthermore, it has also been observed that calcium spike activity mediates dopaminergic and GABAergic specification within the ventral suprachiasmatic nucleus of Xenopus laevis. This specification is seen to be activity-dependent and is prevented by inhibiting calcium transients (Marek et al., 2010, Lewis et al., 2014). Thus, it is not surprising that subtle alterations in calcium channel function can alter neuronal connectivity, influencing cognitive functions later in life. For example, as reviewed recently (Lewis, 2011), GABAergic neurotransmission in the dorsolateral prefrontal cortex of subjects with schizophrenia has been found to be reduced in a number of studies. The GABAergic neurons in question are chandelier neurons which synapse onto the axon initial segment of pyramidal neurons. How these neurons become dysfunctional in schizophrenia remains to be determined, but disruption of specific genes can have a defined effect, for example, on this subtype of cortical interneuron, which can subsequently disrupt network activity and cortical integration (Del Pino et al., 2013). Furthermore, it has been shown in Drosophila melanogaster that dendritic pruning during development occurs in a sequence of events involving calcium transients, mediated by both CaV1 and CaV2 calcium channels, which activate a calcium-activated protease (calpain) pathway (Kanamori et al., 2013). Notably, members of these three gene families (CACNA1C, CACNA1D, CAPN5) are implicated in schizophrenia from the recent GWAS and whole exome sequencing investigations described in Sections 5 and 6.
10. Conclusions and Perspectives
The many genome-wide investigations carried out in the last few years have emphasized the massive polygenicity in populations, where thousands of coding and non-coding loci are polymorphic, some of which represent risk alleles for a variety of disorders, and many of which are pleiotropic, spanning a number of disorders including those reviewed here (Lee et al., 2013). Schizophrenia and other psychiatric conditions are found to have a high heritability (Owen, 2014). A large number of studies now indicate that the genetic component of psychiatric disease is a result of a combination of multiple fairly common alleles, represented by SNPs, each with a small effect, together with a few very rare alleles, represented by deleterious mutations and copy number variations, which might produce a relatively large increased risk in a very small subset of patients with the disorder (Malhotra and Sebat, 2012). Thus, the genetic risk of complex phenotypes, represented by the neuropsychiatric disorders described here, is conferred by a large number of both rare and common alleles distributed across the genome. The sum of these genetic risks will interact with the many environmental risk factors that have been identified to be associated with these diseases, including cannabis consumption in the case of schizophrenia (Di Forti et al., 2014).
The voltage-gated calcium channel genes discussed in this review, which are implicated in the aetiology of a spectrum of psychiatric syndromes from bipolar disorder through schizophrenia to autistic spectrum disorder, are one piece of a jigsaw puzzle in which synaptic proteins are strongly represented. Common and rare mutations in numerous receptors and scaffolding proteins that mediate mainly postsynaptic functions have been associated with these diseases in multiple studies (Doherty et al., 2012, Iossifov et al., 2014). However, although the rare variant associations identified from exome sequencing studies largely involve loss of function mutations, it is much less clear whether common alleles associated with particular or multiple neuropsychiatric disorders, identified in the GWA studies described here, result in an up- or down-regulation of expression of the gene in question (Table 1). Whether the presence of particular SNPs alter the relative expression of channel splice variants, which may have substantially different properties, is another possibility that has not been explored.
10.1. Relative Roles of Pre- and Post-synaptic Calcium Channel Dysfunction
It should be noted that most of the calcium channel genes implicated in these studies do not have a primarily presynaptic function; for example, the L-type channel CaV1.2 (encoded by CACNA1C), which is strongly implicated as a risk gene across the spectrum of psychiatric disorders discussed here, is not involved in presynaptic fast transmitter release. These channels mainly play a postsynaptic modulatory role, being located on cell bodies, as well as on dendritic spines and shafts (Hell et al., 1993, Di Biase et al., 2011, Hall et al., 2013). Here, they modulate dendritic processing (see, for example, Moosmang et al., 2005) and have a long-range role in coupling neuronal activity to gene transcription (Wheeler et al., 2012, Ma et al., 2013). It is of interest that distinct roles for CaV1.2 and CaV1.3 were also found in several forms of memory (Moosmang et al., 2005, Busquet et al., 2008, McKinney et al., 2009), and in anxiety and depression-like behaviours in mice (Dao et al., 2010, Lee et al., 2012). It is also important to realise that some of the calcium channel genes implicated in these disorders are expressed at extremely low levels in the brain, particularly CaV1.1 (encoded by CACNA1S) and CaV1.4 (encoded by CACNA1F) (Sinnegger-Brauns et al., 2009). It is of course possible that these transcripts have a significant but transient role during development and this could also be a fruitful area for research.
Regarding the involvement of presynaptic calcium channels, although many calcium channel subunits have been associated with the neuropsychiatric spectrum of disorders discussed in this review, there is very little evidence for involvement of CACNA1A, encoding CaV2.1, which is the key presynaptic channel involved in neurotransmitter release at mature central synapses. In contrast, alterations in CACNA1B, encoding CaV2.2, have been identified from a number of GWA and whole exome sequencing studies. CaV2.2 is more important for synaptic transmission early in development, at least in rodents (Iwasaki et al., 2000). If the same is true in humans, this may point to a neurodevelopmental role for the changes in the CaV2.2 gene, observed in the neuropsychiatric studies. In contrast, there are numerous Mendelian disorders involving CaV2.1, as described above, whereas only one family has been found possibly to have a disease-associated germline point mutation in CaV2.2 (Groen et al., 2015), although this has been disputed (Mencacci et al., 2015).
A well-established pathway for modulation of presynaptic calcium channels occurs via presynaptic G protein coupled receptors, with dopamine receptors being particularly relevant to psychotic disorders. It has recently been found that common alleles in the gene encoding the dopamine D2 receptor, DRD2, which is the primary target of the D2 antagonist antipsychotic drugs, have been associated with schizophrenia by GWAS (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). One of the primary effects of dopamine acting on this receptor (which is coupled to heterotrimeric G proteins of the Gi/Go class) is presynaptic inhibition by activation of G protein activated inwardly rectifying K+ channels (Liu et al., 1999), and inhibition of CaV2 calcium channels (particularly CaV2.2, but also CaV2.1 and CaV2.3) (Yan et al., 1997, Meir et al., 2000, Leroy et al., 2005). Block of D2 receptors by antipsychotic drugs would have among their effects the relief of presynaptic inhibition mediated by endogenous dopamine via these pathways. It is also worth mentioning here that the cannabinoid CB1 receptor is in the same Gi/o coupled receptor superfamily as the dopamine D2 receptor, and its activation can also mediate presynaptic inhibition (Brown et al., 2003).
The other calcium channel subunits implicated in the GWA and whole exome sequencing studies described here (different β and α2δ subunits) are auxiliary and will affect both CaV1 and CaV2 families of channels. Many of the considerations described above point to the dysfunctional involvement of these channels either neurodevelopmentally, or that the disruption mainly involves postsynaptic dendritic integration. This is also consistent with these disorders as neurodevelopmental, particularly in terms of gene expression, as this can alter the balance of neurons containing particular neurotransmitters, and the synaptic contacts which they form.
As we have attempted to summarise in Table 1, we have not been able to discern a clear consensus concerning gain or loss of function of the calcium channel gene products associated with the various disorders which form the basis of this review, but since intracellular Ca2+ is so important for cellular signalling processes, and its intracellular levels are so tightly regulated in neurons, and indeed in all cells, it is highly likely that dysregulation of these calcium channels in either direction will cause disruption of neural developmental pathways. A major challenge for the future is to translate the psychiatric genetic findings reviewed here into altered developmental and physiological function, involvement in pathology, and potential for personalised and stratified treatments for patients.
Acknowledgements
This review stemmed from a summer vacation internship and a literature project undertaken by Samuel Heyes while a Neuroscience BSc undergraduate at University College London. This work was supported in part by a Wellcome Trust senior Investigator award [Grant 098360/Z/12/Z] to A.C.D. A.C.D. wrote most of the text, including information and text from S.H. and E.R. W.S.P. and E.R. analysed and checked published datasets and extensively corrected the manuscript. L.F. wrote one section of the manuscript. S.D. and M.J.O. reviewed and revised the manuscript.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.pneurobio.2015.09.002.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Altier C., Dale C.S., Kisilevsky A.E., Chapman K., Castiglioni A.J., Matthews E.A., Evans R.M., Dickenson A.H., Lipscombe D., Vergnolle N., Zamponi G.W. Differential role of N-type calcium channel splice isoforms in pain. J. Neurosci. 2007;27:6363–6373. doi: 10.1523/JNEUROSCI.0307-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antar L.N., Afroz R., Dictenberg J.B., Carroll R.C., Bassell G.J. Metabotropic glutamate receptor activation regulates fragile × mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses. J. Neurosci. 2004;24:2648–2655. doi: 10.1523/JNEUROSCI.0099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antar L.N., Li C., Zhang H., Carroll R.C., Bassell G.J. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol. Cell Neurosci. 2006;32:37–48. doi: 10.1016/j.mcn.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Azizan E.A., Poulsen H., Tuluc P., Zhou J., Clausen M.V., Lieb A., Maniero C., Garg S., Bochukova E.G., Zhao W., Shaikh L.H., Brighton C.A., Teo A.E., Davenport A.P., Dekkers T., Tops B., Kusters B., Ceral J., Yeo G.S., Neogi S.G., McFarlane I., Rosenfeld N., Marass F., Hadfield J., Margas W., Chaggar K., Solar M., Deinum J., Dolphin A.C., Farooqi I.S., Striessnig J., Nissen P., Brown M.J. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat. Genet. 2013;45:1055–1060. doi: 10.1038/ng.2716. [DOI] [PubMed] [Google Scholar]
- Bader P.L., Faizi M., Kim L.H., Owen S.F., Tadross M.R., Alfa R.W., Bett G.C., Tsien R.W., Rasmusson R.L., Shamloo M. Mouse model of Timothy syndrome recapitulates triad of autistic traits. Proc. Natl. Acad. Sci. USA. 2011;108:15432–15437. doi: 10.1073/pnas.1112667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig S.M., Koschak A., Lieb A., Gebhart M., Dafinger C., Nurnberg G., Ali A., Ahmad I., Sinnegger-Brauns M.J., Brandt N., Engel J., Mangoni M.E., Farooq M., Khan H.U., Nurnberg P., Striessnig J., Bolz H.J. Loss of Ca(v)1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat. Neurosci. 2011;14:77–84. doi: 10.1038/nn.2694. [DOI] [PubMed] [Google Scholar]
- Bannister R.A., Beam K.G. Ca(V)1.1: The atypical prototypical voltage-gated Ca(2)(+) channel. Biochim. Biophys. Acta. 2013;1828:1587–1597. doi: 10.1016/j.bbamem.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay J., Balaguero N., Mione M., Ackerman S.L., Letts V.A., Brodbeck J., Canti C., Meir A., Page K.M., Kusumi K., PerezReyes E., Lander E.S., Frankel W.N., Gardiner R.M., Dolphin A.C., Rees M. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J. Neurosci. 2001;21:6095–6104. doi: 10.1523/JNEUROSCI.21-16-06095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett C.F., Tsien R.W. The Timothy syndrome mutation differentially affects voltage- and calcium-dependent inactivation of CaV1.2 L-type calcium channels. Proc. Natl. Acad. Sci. USA. 2008;105:2157–2162. doi: 10.1073/pnas.0710501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell G.J., Warren S.T. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C.S., Nieto-Rostro M., Rahman W., Tran-Van-Minh A., Ferron L., Douglas L., Kadurin I., Sri Ranjan Y., Fernandez-Alacid L., Millar N.S., Dickenson A.H., Lujan R., Dolphin A.C. The increased trafficking of the calcium channel subunit α2δ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2δ ligand pregabalin. J. Neurosci. 2009;29:4076–4088. doi: 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B.P., Sturek M., Puga A., Hermsmeyer K. Calcium channels in muscle cells isolated from rat mesenteric arteries: modulation by dihydropyridine drugs. Circ. Res. 1986;59:229–235. doi: 10.1161/01.res.59.2.229. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny L., Tsien R.W. Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967) Mol. Pharmacol. 1995;48:540–549. [PubMed] [Google Scholar]
- Bhakar A.L., Dolen G., Bear M.F. The pathophysiology of fragile X (and what it teaches us about synapses) Annu. Rev. Neurosci. 2012;35:417–443. doi: 10.1146/annurev-neuro-060909-153138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigos K.L., Mattay V.S., Callicott J.H., Straub R.E., Vakkalanka R., Kolachana B., Hyde T.M., Lipska B.K., Kleinman J.E., Weinberger D.R. Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch. Gen. Psychiatry. 2010;67:939–945. doi: 10.1001/archgenpsychiatry.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boczek N.J., Miller E.M., Ye D., Nesterenko V.V., Tester D.J., Antzelevitch C., Czosek R.J., Ackerman M.J., Ware S.M. Novel Timothy syndrome mutation leading to increase in CACNA1C window current. Heart Rhythm. 2015;12:211–219. doi: 10.1016/j.hrthm.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland L.M., Morrill J.A., Bean B.P. Omega-Conotoxin block of N-type calcium channels in frog and rat sympathetic neurons. J. Neurosci. 1994;14:5011–5027. doi: 10.1523/JNEUROSCI.14-08-05011.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdin B., Shakeri B., Tetreault M.P., Sauve R., Lesage S., Parent L. Functional characterization of CaValpha2delta mutations associated with sudden cardiac death. J. Biol. Chem. 2015;290:2854–2869. doi: 10.1074/jbc.M114.597930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourinet E., Stotz S.C., Spaetgens R.L., Dayanithi G., Lemos J., Nargeot J., Zamponi G.W. Interaction of SNX482 with domains III and IV inhibits activation gating of α1E (CaV2.3) calcium channels. Biophys. J. 2001;81:79–88. doi: 10.1016/S0006-3495(01)75681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowersox S.S., Gadbois T., Singh T., Pettus M., Wang Y.X., Luther R.R. Selective N-type neuronal voltage-sensitive calcium channel blocker, SNX-111, produces spinal antinociception in rat models of acute, persistent and neuropathic pain. J. Pharmacol. Exp. Ther. 1996;279:1243–1249. [PubMed] [Google Scholar]
- Boyle L., Kaufmann W.E. The behavioral phenotype of FMR1 mutations. Am. J. Med. Genet. C Semin. Med. Genet. 2010;154C:469–476. doi: 10.1002/ajmg.c.30277. [DOI] [PubMed] [Google Scholar]
- Breitenkamp A.F., Matthes J., Nass R.D., Sinzig J., Lehmkuhl G., Nurnberg P., Herzig S. Rare mutations of CACNB2 found in autism spectrum disease-affected families alter calcium channel function. PLoS ONE. 2014;9:e95579. doi: 10.1371/journal.pone.0095579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill J., Klocke R., Paul D., Boison D., Gouder N., Klugbauer N., Hofmann F., Becker C.M., Becker K. entla, a novel epileptic and ataxic Cacna2d2 mutant of the mouse. J. Biol. Chem. 2004;279:7322–7330. doi: 10.1074/jbc.M308778200. [DOI] [PubMed] [Google Scholar]
- Brown M.R., Kronengold J., Gazula V.R., Chen Y., Strumbos J.G., Sigworth F.J., Navaratnam D., Kaczmarek L.K. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nat. Neurosci. 2010;13:819–821. doi: 10.1038/nn.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T.M., Brotchie J.M., Fitzjohn S.M. Cannabinoids decrease corticostriatal synaptic transmission via an effect on glutamate uptake. J. Neurosci. 2003;23:11073–11077. doi: 10.1523/JNEUROSCI.23-35-11073.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V., Jin P., Ceman S., Darnell J.C., O’Donnell W.T., Tenenbaum S.A., Jin X., Feng Y., Wilkinson K.D., Keene J.D., Darnell R.B., Warren S.T. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Burashnikov E., Pfeiffer R., Barajas-Martinez H., Delpon E., Hu D., Desai M., Borggrefe M., Haissaguerre M., Kanter R., Pollevick G.D., Guerchicoff A., Laino R., Marieb M., Nademanee K., Nam G.B., Robles R., Schimpf R., Stapleton D.D., Viskin S., Winters S., Wolpert C., Zimmern S., Veltmann C., Antzelevitch C. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm. 2010;7:1872–1882. doi: 10.1016/j.hrthm.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess D.L., Jones J.M., Meisler M.H., Noebels J.L. Mutation of the Ca2+ channel beta subunit gene Cchb4 is associated with ataxia and seizures in the lethargic (lh) mouse. Cell. 1997;88:385–392. doi: 10.1016/s0092-8674(00)81877-2. [DOI] [PubMed] [Google Scholar]
- Busquet P., Hetzenauer A., Sinnegger-Brauns M.J., Striessnig J., Singewald N. Role of L-type Ca2+ channel isoforms in the extinction of conditioned fear. Learn. Mem. 2008;15:378–386. doi: 10.1101/lm.886208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canti C., Davies A., Berrow N.S., Butcher A.J., Page K.M., Dolphin A.C. Evidence for two concentration-dependent processes for β subunit effects on α1B calcium channels. Biophys. J. 2001;81:1439–1451. doi: 10.1016/S0006-3495(01)75799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.Q., Tsien R.W. Different relationship of N- and P/Q-type Ca2+ channels to channel-interacting slots in controlling neurotransmission at cultured hippocampal synapses. J. Neurosci. 2010;30:4536–4546. doi: 10.1523/JNEUROSCI.5161-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Calorio C., Vandael D.H. T-type channel-mediated neurotransmitter release. Pflugers Arch. 2014;466:677–687. doi: 10.1007/s00424-014-1489-z. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H.D. A low voltage-activated fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984;310:501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Catterall W.A., Leal K., Nanou E. Calcium channels and short-term synaptic plasticity. J. Biol. Chem. 2013;288:10742–10749. doi: 10.1074/jbc.R112.411645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W.A., Striessnig J., Snutch T.P., Perez-Reyes E. International Union of Pharmacology. XL. Compendium of voltage-gated ion channels: calcium channels. Pharmacol. Rev. 2003;55:579–581. doi: 10.1124/pr.55.4.8. [DOI] [PubMed] [Google Scholar]
- Chaplan S.R., Pogrel J.W., Yaksh T.L. Role of voltage-dependent calcium channel subtypes in experimental tactile allodynia. J. Pharmacol. Exp. Ther. 1994;269:1117–1123. [PubMed] [Google Scholar]
- Chen Y., Lu J., Pan H., Zhang Y., Wu H., Xu K., Liu X., Jiang Y., Bao X., Yao Z., Ding K., Lo W.H., Qiang B., Chan P., Shen Y., Wu X. Association between genetic variation of CACNA1H and childhood absence epilepsy. Ann. Neurol. 2003;54:239–243. doi: 10.1002/ana.10607. [DOI] [PubMed] [Google Scholar]
- Choe W., Messinger R.B., Leach E., Eckle V.S., Obradovic A., Salajegheh R., Jevtovic-Todorovic V., Todorovic S.M. TTA-P2 is a potent and selective blocker of T-type calcium channels in rat sensory neurons and a novel antinociceptive agent. Mol. Pharmacol. 2011;80:900–910. doi: 10.1124/mol.111.073205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie S.B., Akins M.R., Schwob J.E., Fallon J.R. The FXG: a presynaptic fragile X granule expressed in a subset of developing brain circuits. J. Neurosci. 2009;29:1514–1524. doi: 10.1523/JNEUROSCI.3937-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S.M., Li B., Tsien R.W., Ma H. Evolutionary and functional perspectives on signaling from neuronal surface to nucleus. Biochem. Biophys. Res. Commun. 2015;460:88–99. doi: 10.1016/j.bbrc.2015.02.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs L.L., Lee J.-H., Yang J., Satin J., Zhang Y., Daud A., Barclay J., Williamson M.P., Fox M., Rees M., Perez-Reyes E. Cloning and characterization of α1H from human heart, a member of the T type Ca2+ channel gene family. Circ. Res. 1998;83:103–109. doi: 10.1161/01.res.83.1.103. [DOI] [PubMed] [Google Scholar]
- Cross-disorder group of Psychiatric Genomics Consortium Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley J.J., Hilliard C.E., Kim Y., Morgan M.B., Lewis L.R., Muzny D.M., Hawes A.C., Sabo A., Wheeler D.A., Lieberman J.A., Sullivan P.F., Gibbs R.A. Deep resequencing and association analysis of schizophrenia candidate genes. Mol. Psychiatry. 2013;18:138–140. doi: 10.1038/mp.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao D.T., Mahon P.B., Cai X., Kovacsics C.E., Blackwell R.A., Arad M., Shi J., Zandi P.P., O’Donnell P., Knowles J.A., Weissman M.M., Coryell W., Scheftner W.A., Lawson W.B., Levinson D.F., Thompson S.M., Potash J.B., Gould T.D. Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol. Psychiatry. 2010;68:801–810. doi: 10.1016/j.biopsych.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arco M., Margas W., Cassidy J.S., Dolphin A.C. The upregulation of 2.-1 subunit modulates activity-dependent Ca2+ signals in sensory neurons. J. Neurosci. 2015;35:5935–5940. doi: 10.1523/JNEUROSCI.3997-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J.C., Jensen K.B., Jin P., Brown V., Warren S.T., Darnell R.B. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–499. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Darnell J.C., Van Driesche S.J., Zhang C., Hung K.Y., Mele A., Fraser C.E., Stone E.F., Chen C., Fak J.J., Chi S.W., Licatalosi D.D., Richter J.D., Darnell R.B. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Taber R.A., Scott V.E. Transcriptional profiling of dorsal root gangliain a neuropathic pain model using microarrayand laser capture microdissection. Drug Dev. Res. 2006;67:308–330. [Google Scholar]
- Del Pino I., Garcia-Frigola C., Dehorter N., Brotons-Mas J.R., Alvarez-Salvado E., Martinez de L.M., Ciceri G., Gabaldon M.V., Moratal D., Dierssen M., Canals S., Marin O., Rico B. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79:1152–1168. doi: 10.1016/j.neuron.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Deng P.Y., Rotman Z., Blundon J.A., Cho Y., Cui J., Cavalli V., Zakharenko S.S., Klyachko V.A. FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron. 2013;77:696–711. doi: 10.1016/j.neuron.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rubeis S., He X., Goldberg A.P., Poultney C.S., Samocha K., Cicek A.E., Kou Y., Liu L., Fromer M., Walker S., Singh T., Klei L., Kosmicki J., Shih-Chen F., Aleksic B., Biscaldi M., Bolton P.F., Brownfeld J.M., Cai J., Campbell N.G., Carracedo A., Chahrour M.H., Chiocchetti A.G., Coon H., Crawford E.L., Curran S.R., Dawson G., Duketis E., Fernandez B.A., Gallagher L., Geller E., Guter S.J., Hill R.S., Ionita-Laza J., Jimenz G.P., Kilpinen H., Klauck S.M., Kolevzon A., Lee I., Lei I., Lei J., Lehtimaki T., Lin C.F., Ma’ayan A., Marshall C.R., McInnes A.L., Neale B., Owen M.J., Ozaki N., Parellada M., Parr J.R., Purcell S., Puura K., Rajagopalan D., Rehnstrom K., Reichenberg A., Sabo A., Sachse M., Sanders S.J., Schafer C., Schulte-Ruther M., Skuse D., Stevens C., Szatmari P., Tammimies K., Valladares O., Voran A., Li-San W., Weiss L.A., Willsey A.J., Yu T.W., Yuen R.K., Cook E.H., Freitag C.M., Gill M., Hultman C.M., Lehner T., Palotie A., Schellenberg G.D., Sklar P., State M.W., Sutcliffe J.S., Walsh C.A., Scherer S.W., Zwick M.E., Barett J.C., Cutler D.J., Roeder K., Devlin B., Daly M.J., Buxbaum J.D. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desarmenien M.G., Clendening B., Spitzer N.C. In vivo development of voltage-dependent ionic currents in embryonic Xenopus spinal neurons. J. Neurosci. 1993;13:2575–2581. doi: 10.1523/JNEUROSCI.13-06-02575.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Biase V., Tuluc P., Campiglio M., Obermair G.J., Heine M., Flucher B.E. Surface traffic of dendritic CaV1.2 calcium channels in hippocampal neurons. J. Neurosci. 2011;31:13682–13694. doi: 10.1523/JNEUROSCI.2300-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick I.E., Tadross M.R., Liang H., Tay L.H., Yang W., Yue D.T. A modular switch for spatial Ca2+ selectivity in the calmodulin regulation of CaV channels. Nature. 2008;451:830–834. doi: 10.1038/nature06529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Forti M., Sallis H., Allegri F., Trotta A., Ferraro L., Stilo S.A., Marconi A., La C.C., Reis M.T., Pariante C., Dazzan P., Mondelli V., Paparelli A., Kolliakou A., Prata D., Gaughran F., David A.S., Morgan C., Stahl D., Khondoker M., Maccabe J.H., Murray R.M. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr. Bull. 2014;40:1509–1517. doi: 10.1093/schbul/sbt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Guilmi M.N., Wang T., Inchauspe C.G., Forsythe I.D., Ferrari M.D., Van den Maagdenberg A.M., Borst J.G., Uchitel O.D. Synaptic gain-of-function effects of mutant Cav2.1 channels in a mouse model of familial hemiplegic migraine are due to increased basal [Ca2+]i. J. Neurosci. 2014;34:7047–7058. doi: 10.1523/JNEUROSCI.2526-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty J.L., O’Donovan M.C., Owen M.J. Recent genomic advances in schizophrenia. Clin. Genet. 2012;81:103–109. doi: 10.1111/j.1399-0004.2011.01773.x. [DOI] [PubMed] [Google Scholar]
- Dolphin A.C. β subunits of voltage-gated calcium channels. J. Bioeng. Biomemb. 2003;35:599–620. doi: 10.1023/b:jobb.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- Dolphin A.C. A short history of voltage-gated calcium channels. Br. J Pharmacol. 2006;147(Suppl 1):S56–S62. doi: 10.1038/sj.bjp.0706442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A.C. Calcium channel auxiliary alpha(2)delta and beta subunits: trafficking and one step beyond. Nat. Rev. Neurosci. 2012;13:542–555. doi: 10.1038/nrn3311. [DOI] [PubMed] [Google Scholar]
- Edvardson S., Oz S., Abulhijaa F.A., Taher F.B., Shaag A., Zenvirt S., Dascal N., Elpeleg O. Early infantile epileptic encephalopathy associated with a high voltage gated calcium channelopathy. J. Med. Genet. 2013;50:118–123. doi: 10.1136/jmedgenet-2012-101223. [DOI] [PubMed] [Google Scholar]
- Ellis S.B., Williams M.E., Ways N.R., Brenner R., Sharp A.H., Leung A.T., Campbell K.P., McKenna E., Koch W.J., Hui A., Schwartz A., Harpold M.M. Sequence and expression of mRNAs encoding the α1 and α2 subunits of a DHP-sensitive calcium channel. Science. 1988;241:1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- Erk S., Meyer-Lindenberg A., Schnell K., Opitz von B.C., Esslinger C., Kirsch P., Grimm O., Arnold C., Haddad L., Witt S.H., Cichon S., Nothen M.M., Rietschel M., Walter H. Brain function in carriers of a genome-wide supported bipolar disorder variant. Arch. Gen. Psychiatry. 2010;67:803–811. doi: 10.1001/archgenpsychiatry.2010.94. [DOI] [PubMed] [Google Scholar]
- Ermolyuk Y.S., Alder F.G., Surges R., Pavlov I.Y., Timofeeva Y., Kullmann D.M., Volynski K.E. Differential triggering of spontaneous glutamate release by P/Q-, N- and R-type Ca2+ channels. Nat. Neurosci. 2013;16:1754–1763. doi: 10.1038/nn.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C., Allen N.J., Susman M.W., O’Rourke N.A., Park C.Y., Ozkan E., Chakraborty C., Mulinyawe S.B., Annis D.S., Huberman A.D., Green E.M., LAWLER J., Dolmetsch R., Garcia K.C., Smith S.J., Luo Z.D., Rosenthal A., Mosher D.F., Barres B.A. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel S.I., Clozel J.P. Mibefradil (Ro 40-5967): the first selective T-type Ca2+ channel blocker. Expert. Opin. Investig. Drugs. 1997;6:569–582. doi: 10.1517/13543784.6.5.569. [DOI] [PubMed] [Google Scholar]
- Escayg A., De Waard M., Lee D.D., Bichet D., Wolf P., Mayer T., Johnston J., Baloh R., Sander T., Meisler M.H. Coding and noncoding variation of the human calcium-channel β4-subunit gene CACNB4 in patients with idiopathic generalized epilepsy and episodic ataxia. Am. J. Hum. Genet. 2000;66:1531–1539. doi: 10.1086/302909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemad S., Obermair G.J., Bindreither D., Benedetti A., Stanika R., Di B.V., Burtscher V., Koschak A., Kofler R., Geley S., Wille A., Lusser A., Flockerzi V., Flucher B.E. Differential neuronal targeting of a new and two known calcium channel beta4 subunit splice variants correlates with their regulation of gene expression. J. Neurosci. 2014;34:1446–1461. doi: 10.1523/JNEUROSCI.3935-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J. Gen. Physiol. 1985;85:247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairless R., Masius H., Rohlmann A., Heupel K., Ahmad M., Reissner C., Dresbach T., Missler M. Polarized targeting of neurexins to synapses is regulated by their C-terminal sequences. J. Neurosci. 2008;28:12969–12981. doi: 10.1523/JNEUROSCI.5294-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi S.H., Folsom T.D. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr. Bull. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M.A., O’Donovan M.C., Meng Y.A., Jones I.R., Ruderfer D.M., Jones L., Fan J., Kirov G., Perlis R.H., Green E.K., Smoller J.W., Grozeva D., Stone J., Nikolov I., Chambert K., Hamshere M.L., Nimgaonkar V.L., Moskvina V., Thase M.E., Caesar S., Sachs G.S., Franklin J., Gordon-Smith K., Ardlie K.G., Gabriel S.B., Fraser C., Blumenstiel B., Defelice M., Breen G., Gill M., Morris D.W., Elkin A., Muir W.J., McGhee K.A., Williamson R., MacIntyre D.J., MacLean A.W., St C.D., Robinson M., Van B.M., Pereira A.C., Kandaswamy R., McQuillin A., Collier D.A., Bass N.J., Young A.H., Lawrence J., Ferrier I.N., Anjorin A., Farmer A., Curtis D., Scolnick E.M., McGuffin P., Daly M.J., Corvin A.P., Holmans P.A., Blackwood D.H., Gurling H.M., Owen M.J., Purcell S.M., Sklar P., Craddock N. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat. Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron L., Nieto-Rostro M., Cassidy J.S., Dolphin A.C. Fragile X mental retardation protein controls synaptic vesicle exocytosis by modulating N-type calcium channel density. Nat. Commun. 2014;5:3628. doi: 10.1038/ncomms4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan S.E., Patch A.M., Ellard S. Using SIFT and PolyPhen to predict loss-of-function and gain-of-function mutations. Genet. Test. Mol. Biomarkers. 2010;14:533–537. doi: 10.1089/gtmb.2010.0036. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A. History of calcium antagonists. Circ. Res. 1983;52:I3–I16. [PubMed] [Google Scholar]
- Fox A.P., Nowycky M.C., Tsien R.W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J. Physiol. (Lond.) 1987;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A.P., Nowycky M.C., Tsien R.W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J. Physiol. (Lond.) 1987;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois A., Kerckhove N., Meleine M., Alloui A., Barrere C., Gelot A., Uebele V.N., Renger J.J., Eschalier A., Ardid D., Bourinet E. State-dependent properties of a new T-type calcium channel blocker enhance Ca(V)3.2 selectivity and support analgesic effects. Pain. 2013;154:283–293. doi: 10.1016/j.pain.2012.10.023. [DOI] [PubMed] [Google Scholar]
- Franke B., Vasquez A.A., Veltman J.A., Brunner H.G., Rijpkema M., Fernandez G. Genetic variation in CACNA1C, a gene associated with bipolar disorder, influences brainstem rather than gray matter volume in healthy individuals. Biol. Psychiatry. 2010;68:586–588. doi: 10.1016/j.biopsych.2010.05.037. [DOI] [PubMed] [Google Scholar]
- Fromer M., Pocklington A.J., Kavanagh D.H., Williams H.J., Dwyer S., Gormley P., Georgieva L., Rees E., Palta P., Ruderfer D.M., Carrera N., Humphreys I., Johnson J.S., Roussos P., Barker D.D., Banks E., Milanova V., Grant S.G., Hannon E., Rose S.A., Chambert K., Mahajan M., Scolnick E.M., Moran J.L., Kirov G., Palotie A., McCarroll S.A., Holmans P., Sklar P., Owen M.J., Purcell S.M., O’Donovan M.C. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Bicer G.A., Varadi G., Koch S.E., Ishii M., Bodi I., Kadeer N., Muth J.N., Mikala G., Petrashevskaya N.N., Jordan M.A., Zhang S.P., Qin N., Flores C.M., Isaacsohn I., Varadi M., Mori Y., Jones W.K., Schwartz A. Targeted disruption of the voltage-dependent Ca2+ channel {alpha}2/{delta}-1 subunit. Am. J. Physiol Heart Circ. Physiol. 2009;297:H117–H124. doi: 10.1152/ajpheart.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Waggoner D., Stephens M., Ober C., Przeworski M. An estimate of the average number of recessive lethal mutations carried by humans. Genetics. 2015;199:1243–1254. doi: 10.1534/genetics.114.173351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon E.S., Grennan K., Busnello J., Badner J.A., Ovsiew F., Memon S., Alliey-Rodriguez N., Cooper J., Romanos B., Liu C. A rare mutation of CACNA1C in a patient with bipolar disorder, and decreased gene expression associated with a bipolar-associated common SNP of CACNA1C in brain. Mol. Psychiatry. 2014;19:890–894. doi: 10.1038/mp.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis J., Burashnikov E., Antzelevitch C., Blaser S., Gross G., Turner L., Babul-Hirji R., Chitayat D. Long QT, syndactyly, joint contractures, stroke and novel CACNA1C mutation: expanding the spectrum of Timothy syndrome. Am. J. Med. Genet. A. 2012;158A:182–187. doi: 10.1002/ajmg.a.34355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girirajan S., Dennis M.Y., Baker C., Malig M., Coe B.P., Campbell C.D., Mark K., Vu T.H., Alkan C., Cheng Z., Biesecker L.G., Bernier R., Eichler E.E. Refinement and discovery of new hotspots of copy-number variation associated with autism spectrum disorder. Am. J. Hum. Genet. 2013;92:221–237. doi: 10.1016/j.ajhg.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E.K., Grozeva D., Jones I., Jones L., Kirov G., Caesar S., Gordon-Smith K., Fraser C., Forty L., Russell E., Hamshere M.L., Moskvina V., Nikolov I., Farmer A., McGuffin P., Holmans P.A., Owen M.J., O’Donovan M.C., Craddock N. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol. Psychiatry. 2010;15:1016–1022. doi: 10.1038/mp.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E.K., Hamshere M., Forty L., Gordon-Smith K., Fraser C., Russell E., Grozeva D., Kirov G., Holmans P., Moran J.L., Purcell S., Sklar P., Owen M.J., O’Donovan M.C., Jones L., Jones I.R., Craddock N. Replication of bipolar disorder susceptibility alleles and identification of two novel genome-wide significant associations in a new bipolar disorder case-control sample. Mol. Psychiatry. 2013;18:1302–1307. doi: 10.1038/mp.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E.K., Rees E., Walters J.T., Smith K.G., Forty L., Grozeva D., Moran J.L., Sklar P., Ripke S., Chambert K.D., Genovese G., McCarroll S.A., Jones I., Jones L., Owen M.J., O’Donovan M.C., Craddock N., Kirov G. Copy number variation in bipolar disorder. Mol. Psychiatry. 2015 doi: 10.1038/mp.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen J.L., Andrade A., Ritz K., Jalalzadeh H., Haagmans M., Bradley T.E., Jongejan A., Verbeek D.S., Nurnberg P., Denome S., Hennekam R.C., Lipscombe D., Baas F., Tijssen M.A. CACNA1B mutation is linked to unique myoclonus-dystonia syndrome. Hum. Mol. Genet. 2015;24:987–993. doi: 10.1093/hmg/ddu513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X., Olson E.C., Spitzer N.C. Spontaneous neuronal calcium spikes and waves during early differentiation. J. Neurosci. 1994;14:6325–6335. doi: 10.1523/JNEUROSCI.14-11-06325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X., Spitzer N.C. Low-threshold Ca2+ current and its role in spontaneous elevations of intracellular Ca2+ in developing Xenopus neurons. J. Neurosci. 1993;13:4936–4948. doi: 10.1523/JNEUROSCI.13-11-04936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman J.N., Sanchez-Padilla J., Chan C.S., Surmeier D.J. Robust pacemaking in substantia nigra dopaminergic neurons. J. Neurosci. 2009;29:11011–11019. doi: 10.1523/JNEUROSCI.2519-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D.D., Dai S., Tseng P.Y., Malik Z., Nguyen M., Matt L., Schnizler K., Shephard A., Mohapatra D.P., Tsuruta F., Dolmetsch R.E., Christel C.J., Lee A., Burette A., Weinberg R.J., Hell J.W. Competition between alpha-actinin and Ca(2)(+)-calmodulin controls surface retention of the L-type Ca(2)(+) channel Ca(V)1.2. Neuron. 2013;78:483–497. doi: 10.1016/j.neuron.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamshere M.L., Walters J.T., Smith R., Richards A.L., Green E., Grozeva D., Jones I., Forty L., Jones L., Gordon-Smith K., Riley B., O’Neill F.A., Kendler K.S., Sklar P., Purcell S., Kranz J., Morris D., Gill M., Holmans P., Craddock N., Corvin A., Owen M.J., O’Donovan M.C. Genome-wide significant associations in schizophrenia to ITIH3/4, CACNA1C and SDCCAG8, and extensive replication of associations reported by the Schizophrenia PGC. Mol. Psychiatry. 2013;18:708–712. doi: 10.1038/mp.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.E., Madison D.V. Presynaptic FMR1 genotype influences the degree of synaptic connectivity in a mosaic mouse model of fragile X syndrome. J. Neurosci. 2007;27:4014–4018. doi: 10.1523/JNEUROSCI.4717-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell J.W., Westenbroek R.E., Warner C., Ahlijanian M.K., Prystay W., Gilbert M.M., Snutch T.P., Catterall W.A. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel α1 subunits. J. Cell. Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton T.D., Xu W., Lipscombe D. Neuronal L-type calcium channels open quickly and are inhibited slowly. J. Neurosci. 2005;25:10247–10251. doi: 10.1523/JNEUROSCI.1089-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessey J.A., Boczek N.J., Jiang Y.H., Miller J.D., Patrick W., Pfeiffer R., Sutphin B.S., Tester D.J., Barajas-Martinez H., Ackerman M.J., Antzelevitch C., Kanter R., Pitt G.S. A CACNA1C variant associated with reduced voltage-dependent inactivation, increased CaV1.2 channel window current, and arrhythmogenesis. PLoS ONE. 2014;9:e106982. doi: 10.1371/journal.pone.0106982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg L., Katsel P., Roussos P., Haroutunian V., Domany E. Integration of gene expression and GWAS results supports involvement of calcium signaling in Schizophrenia. Schizophr. Res. 2015;164:92–99. doi: 10.1016/j.schres.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Hibino H., Pironkova R., Onwumere O., Rousset M., Charnet P., Hudspeth A.J., Lesage F. Direct interaction with a nuclear protein and regulation of gene silencing by a variant of the Ca2+ channel β4 subunit. Proc. Natl. Acad. Sci. USA. 2003;100:307–312. doi: 10.1073/pnas.0136791100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirtz J.J., Braun N., Griesemer D., Hannes C., Janz K., Lohrke S., Muller B., Friauf E. Synaptic refinement of an inhibitory topographic map in the auditory brainstem requires functional Cav1.3 calcium channels. J. Neurosci. 2012;32:14602–14616. doi: 10.1523/JNEUROSCI.0765-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppa M., Lana B., Margas W., Dolphin A.C., Ryan T.A. α2δ couples calcium channels to neurotransmitter release sites to control release probability. Nature. 2012;486:122–125. doi: 10.1038/nature11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C., Rusin C.G., Tan Z., Guagliardo N.A., Barrett P.Q. Zona glomerulosa cells of the mouse adrenal cortex are intrinsic electrical oscillators. J. Clin. Invest. 2012;122:2046–2053. doi: 10.1172/JCI61996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Lujan R., Kadurin I., Uebele V.N., Renger J.J., Dolphin A.C., Shah M.M. Presynaptic HCN1 channels regulate Ca(V)3.2 activity and neurotransmission at select cortical synapses. Nat. Neurosci. 2011;14:478–486. doi: 10.1038/nn.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Schizophrenia Consortium Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I., O’Roak B.J., Sanders S.J., Ronemus M., Krumm N., Levy D., Stessman H.A., Witherspoon K.T., Vives L., Patterson K.E., Smith J.D., Paeper B., Nickerson D.A., Dea J., Dong S., Gonzalez L.E., Mandell J.D., Mane S.M., Murtha M.T., Sullivan C.A., Walker M.F., Waqar Z., Wei L., Willsey A.J., Yamrom B., Lee Y.H., Grabowska E., Dalkic E., Wang Z., Marks S., Andrews P., Leotta A., Kendall J., Hakker I., Rosenbaum J., Ma B., Rodgers L., Troge J., Narzisi G., Yoon S., Schatz M.C., Ye K., McCombie W.R., Shendure J., Eichler E.E., State M.W., Wigler M. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I., Ronemus M., Levy D., Wang Z., Hakker I., Rosenbaum J., Yamrom B., Lee Y.H., Narzisi G., Leotta A., Kendall J., Grabowska E., Ma B., Marks S., Rodgers L., Stepansky A., Troge J., Andrews P., Bekritsky M., Pradhan K., Ghiban E., Kramer M., Parla J., Demeter R., Fulton L.L., Fulton R.S., Magrini V.J., Ye K., Darnell J.C., Darnell R.B., Mardis E.R., Wilson R.K., Schatz M.C., McCombie W.R., Wigler M. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74:285–299. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Kaneko M., Shin H.S., Takahashi T. Presynaptic N-type and P/Q-type Ca2+ channels mediating synaptic transmission at the calyx of Held of mice. J. Physiol. 2005;568:199–209. doi: 10.1113/jphysiol.2005.089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K., Kakiuchi C., Bundo M., Ikeda K., Kato T. Molecular characterization of bipolar disorder by comparing gene expression profiles of postmortem brains of major mental disorders. Mol. Psychiatry. 2004;9:406–416. doi: 10.1038/sj.mp.4001437. [DOI] [PubMed] [Google Scholar]
- Iwasaki S., Momiyama A., Uchitel O.D., Takahashi T. Developmental changes in calcium channel types mediating central synaptic transmission. J. Neurosci. 2000;20:59–65. doi: 10.1523/JNEUROSCI.20-01-00059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay S.D., Ellis S.B., McCue A.F., Williams M.E., Vedvick T.S., Harpold M.M., Campbell K.P. Primary structure of the gamma subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1990;248:490–492. doi: 10.1126/science.2158672. [DOI] [PubMed] [Google Scholar]
- Jay S.D., Sharp A.H., Kahl S.D., Vedvick T.S., Harpold M.M., Campbell K.P. Structural characterization of the dihydropyridine-sensitive calcium channel α2-subunit and the associated δ peptides. J. Biol. Chem. 1991;266:3287–3293. [PubMed] [Google Scholar]
- Jiang Y.H., Yuen R.K., Jin X., Wang M., Chen N., Wu X., Ju J., Mei J., Shi Y., He M., Wang G., Liang J., Wang Z., Cao D., Carter M.T., Chrysler C., Drmic I.E., Howe J.L., Lau L., Marshall C.R., Merico D., Nalpathamkalam T., Thiruvahindrapuram B., Thompson A., Uddin M., Walker S., Luo J., Anagnostou E., Zwaigenbaum L., Ring R.H., Wang J., Lajonchere C., Wang J., Shih A., Szatmari P., Yang H., Dawson G., Li Y., Scherer S.W. Detection of clinically relevant genetic variants in autism spectrum disorder by whole-genome sequencing. Am. J. Hum. Genet. 2013;93:249–263. doi: 10.1016/j.ajhg.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvenceau A., Eunson L.H., Spauschus A., Ramesh V., Zuberi S.M., Kullmann D.M., Hanna M.G. Human epilepsy associated with dysfunction of the brain P/Q-type calcium channel. Lancet. 2001;358:801–807. doi: 10.1016/S0140-6736(01)05971-2. [DOI] [PubMed] [Google Scholar]
- Kanamori T., Kanai M.I., Dairyo Y., Yasunaga K., Morikawa R.K., Emoto K. Compartmentalized calcium transients trigger dendrite pruning in Drosophila sensory neurons. Science. 2013;340:1475–1478. doi: 10.1126/science.1234879. [DOI] [PubMed] [Google Scholar]
- Keinan A., Clark A.G. Recent explosive human population growth has resulted in an excess of rare genetic variants. Science. 2012;336:740–743. doi: 10.1126/science.1217283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton M.J., Ruberto G., Vassos E., Tatarelli R., Girardi P., Collier D., Frangou S. Effects of the CACNA1C risk allele for bipolar disorder on cerebral gray matter volume in healthy individuals. Am. J. Psychiatry. 2009;166:1413–1414. doi: 10.1176/appi.ajp.2009.09050680. [DOI] [PubMed] [Google Scholar]
- Khosravani H., Altier C., Simms B., Hamming K.S., Snutch T.P., Mezeyova J., Mcrory J.E., Zamponi G.W. Gating effects of mutations in the Cav3.2 T-type calcium channel associated with childhood absence epilepsy. J. Biol. Chem. 2004;279:9681–9684. doi: 10.1074/jbc.C400006200. [DOI] [PubMed] [Google Scholar]
- Kimm T., Bean B.P. Inhibition of A-type potassium current by the peptide toxin SNX-482. J. Neurosci. 2014;34:9182–9189. doi: 10.1523/JNEUROSCI.0339-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen T., Davis C., Goldman A., Burgess D., Chen T., Wheeler D., McPherson J., Bourquin T., Lewis L., Villasana D., Morgan M., Muzny D., Gibbs R., Noebels J. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell. 2011;145:1036–1048. doi: 10.1016/j.cell.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemmer P., Meredith R.M., Holmgren C.D., Klychnikov O.I., Stahl-Zeng J., Loos M., Van der Schors R.C., Wortel J., Spijker S., Rotaru D.C., Mansvelder H.D., Smit A.B., Li K.W. Proteomics, ultrastructure and physiology of hippocampal synapses in a Fragile X Syndrome mouse model reveals pre-synaptic phenotype. J. Biol. Chem. 2011;286:25495–25504. doi: 10.1074/jbc.M110.210260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugbauer N., Lacinova L., Marais E., Hobom M., Hofmann F. Molecular diversity of the calcium channel α2-δ subunit. J. Neurosci. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschak A., Reimer D., Huber I., Grabner M., Glossmann H., Engel J., Striessnig J. alpha 1D (Cav1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J. Biol. Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- Krey J.F., Pasca S.P., Shcheglovitov A., Yazawa M., Schwemberger R., Rasmusson R., Dolmetsch R.E. Timothy syndrome is associated with activity-dependent dendritic retraction in rodent and human neurons. Nat. Neurosci. 2013;16:201–209. doi: 10.1038/nn.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger D.D., Bear M.F. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu. Rev. Med. 2011;62:411–429. doi: 10.1146/annurev-med-061109-134644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lana B., Schlick B., Martin S., Pratt W.S., Page K.M., Goncalves L., Rahman W., Dickenson A.H., Bauer C.S., Dolphin A.C. Differential up-regulation in DRG neurons of an alphadelta-1 splice variant with a lower affinity for gabapentin after peripheral sensory nerve injury. Pain. 2014;155:522–533. doi: 10.1016/j.pain.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster T.M., Heerey E.A., Mantripragada K., Linden D.E. CACNA1C risk variant affects reward responsiveness in healthy individuals. Transl. Psychiatry. 2014;4:e461. doi: 10.1038/tp.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Wang S., Williams B., Hagen J., Scheetz T.E., Haeseleer F. Characterization of Cav1.4 Complexes (alpha11.4, beta2, and alpha2delta4) in HEK293T Cells and in the retina. J. Biol. Chem. 2015;290:1505–1521. doi: 10.1074/jbc.M114.607465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.S., Ra S., Rajadhyaksha A.M., Britt J.K., De Jesus-Cortes H., Gonzales K.L., Lee A., Moosmang S., Hofmann F., Pieper A.A., Rajadhyaksha A.M. Forebrain elimination of cacna1c mediates anxiety-like behavior in mice. Mol. Psychiatry. 2012;17:1054–1055. doi: 10.1038/mp.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Scherer S.W. The clinical context of copy number variation in the human genome. Expert. Rev. Mol. Med. 2010;12:e8. doi: 10.1017/S1462399410001390. [DOI] [PubMed] [Google Scholar]
- Lee M. Z944: a first in class T-type calcium channel modulator for the treatment of pain. J. Peripher. Nerv. Syst. 2014;19(Suppl 2):S11–S12. doi: 10.1111/jns.12080_2. [DOI] [PubMed] [Google Scholar]
- Lee M.T., Chen C.H., Lee C.S., Chen C.C., Chong M.Y., Ouyang W.C., Chiu N.Y., Chuo L.J., Chen C.Y., Tan H.K., Lane H.Y., Chang T.J., Lin C.H., Jou S.H., Hou Y.M., Feng J., Lai T.J., Tung C.L., Chen T.J., Chang C.J., Lung F.W., Chen C.K., Shiah I.S., Liu C.Y., Teng P.R., Chen K.H., Shen L.J., Cheng C.S., Chang T.P., Li C.F., Chou C.H., Chen C.Y., Wang K.H., Fann C.S., Wu J.Y., Chen Y.T., Cheng A.T. Genome-wide association study of bipolar I disorder in the Han Chinese population. Mol. Psychiatry. 2011;16:548–556. doi: 10.1038/mp.2010.43. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Ripke S., Neale B.M., Faraone S.V., Purcell S.M., Perlis R.H., Mowry B.J., Thapar A., Goddard M.E., Witte J.S., Absher D., Agartz I., Akil H., Amin F., Andreassen O.A., Anjorin A., Anney R., Anttila V., Arking D.E., Asherson P., Azevedo M.H., Backlund L., Badner J.A., Bailey A.J., Banaschewski T., Barchas J.D., Barnes M.R., Barrett T.B., Bass N., Battaglia A., Bauer M., Bayes M., Bellivier F., Bergen S.E., Berrettini W., Betancur C., Bettecken T., Biederman J., Binder E.B., Black D.W., Blackwood D.H., Bloss C.S., Boehnke M., Boomsma D.I., Breen G., Breuer R., Bruggeman R., Cormican P., Buccola N.G., Buitelaar J.K., Bunney W.E., Buxbaum J.D., Byerley W.F., Byrne E.M., Caesar S., Cahn W., Cantor R.M., Casas M., Chakravarti A., Chambert K., Choudhury K., Cichon S., Cloninger C.R., Collier D.A., Cook E.H., Coon H., Cormand B., Corvin A., Coryell W.H., Craig D.W., Craig I.W., Crosbie J., Cuccaro M.L., Curtis D., Czamara D., Datta S., Dawson G., Day R., De Geus E.J., Degenhardt F., Djurovic S., Donohoe G.J., Doyle A.E., Duan J., Dudbridge F., Duketis E., Ebstein R.P., Edenberg H.J., Elia J., Ennis S., Etain B., Fanous A., Farmer A.E., Ferrier I.N., Flickinger M., Fombonne E., Foroud T., Frank J., Franke B., Fraser C., Freedman R., Freimer N.B., Freitag C.M., Friedl M., Frisen L., Gallagher L., Gejman P.V., Georgieva L., Gershon E.S., Geschwind D.H., Giegling I., Gill M., Gordon S.D., Gordon-Smith K., Green E.K., Greenwood T.A., Grice D.E., Gross M., Grozeva D., Guan W., Gurling H., De H.L., Haines J.L., Hakonarson H., Hallmayer J., Hamilton S.P., Hamshere M.L., Hansen T.F., Hartmann A.M., Hautzinger M., Heath A.C., Henders A.K., Herms S., Hickie I.B., Hipolito M., Hoefels S., Holmans P.A., Holsboer F., Hoogendijk W.J., Hottenga J.J., Hultman C.M., Hus V., Ingason A., Ising M., Jamain S., Jones E.G., Jones I., Jones L., Tzeng J.Y., Kahler A.K., Kahn R.S., Kandaswamy R., Keller M.C., Kennedy J.L., Kenny E., Kent L., Kim Y., Kirov G.K., Klauck S.M., Klei L., Knowles J.A., Kohli M.A., Koller D.L., Konte B., Korszun A., Krabbendam L., Krasucki R., Kuntsi J., Kwan P., Landen M., Langstrom N., Lathrop M., Lawrence J., Lawson W.B., Leboyer M., Ledbetter D.H., Lee P.H., Lencz T., Lesch K.P., Levinson D.F., Lewis C.M., Li J., Lichtenstein P., Lieberman J.A., Lin D.Y., Linszen D.H., Liu C., Lohoff F.W., Loo S.K., Lord C., Lowe J.K., Lucae S., MacIntyre D.J., Madden P.A., Maestrini E., Magnusson P.K., Mahon P.B., Maier W., Malhotra A.K., Mane S.M., Martin C.L., Martin N.G., Mattheisen M., Matthews K., Mattingsdal M., McCarroll S.A., McGhee K.A., McGough J.J., McGrath P.J., McGuffin P., McInnis M.G., McIntosh A., McKinney R., McLean A.W., McMahon F.J., McMahon W.M., McQuillin A., Medeiros H., Medland S.E., Meier S., Melle I., Meng F., Meyer J., Middeldorp C.M., Middleton L., Milanova V., Miranda A., Monaco A.P., Montgomery G.W., Moran J.L., Moreno-De-Luca D., Morken G., Morris D.W., Morrow E.M., Moskvina V., Muglia P., Muhleisen T.W., Muir W.J., Muller-Myhsok B., Murtha M., MYERS R.M., Myin-Germeys I., Neale M.C., Nelson S.F., Nievergelt C.M., Nikolov I., Nimgaonkar V., Nolen W.A., Nothen M.M., Nurnberger J.I., Nwulia E.A., Nyholt D.R., O’Dushlaine C., Oades R.D., Olincy A. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet. 2013;45:984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepski G., Jannes C.E., Nikkhah G., Bischofberger J. cAMP promotes the differentiation of neural progenitor cells in vitro via modulation of voltage-gated calcium channels. Front Cell Neurosci. 2013;7:155. doi: 10.3389/fncel.2013.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy J., Richards M.S., Butcher A.J., Nieto-Rostro M., Pratt W.S., Davies A., Dolphin A.C. Interaction via a key tryptophan in the I-II linker of N-type calcium channels is required for beta1 but not for palmitoylated beta2, implicating an additional binding site in the regulation of channel voltage-dependent properties. J. Neurosci. 2005;25:6984–6996. doi: 10.1523/JNEUROSCI.1137-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B.B., Miller L.E., Herbst W.A., Saha M.S. The role of voltage-gated calcium channels in neurotransmitter phenotype specification: coexpression and functional analysis in Xenopus laevis. J. Comp. Neurol. 2014;522:2518–2531. doi: 10.1002/cne.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C.M., Levinson D.F., Wise L.H., DeLisi L.E., Straub R.E., Hovatta I., Williams N.M., Schwab S.G., Pulver A.E., Faraone S.V., Brzustowicz L.M., Kaufmann C.A., Garver D.L., Gurling H.M., Lindholm E., Coon H., Moises H.W., Byerley W., Shaw S.H., Mesen A., Sherrington R., O’Neill F.A., Walsh D., Kendler K.S., Ekelund J., Paunio T., Lonnqvist J., Peltonen L., O’Donovan M.C., Owen M.J., Wildenauer D.B., Maier W., Nestadt G., Blouin J.L., Antonarakis S.E., Mowry B.J., Silverman J.M., Crowe R.R., Cloninger C.R., Tsuang M.T., Malaspina D., Harkavy-Friedman J.M., Svrakic D.M., Bassett A.S., Holcomb J., Kalsi G., McQuillin A., Brynjolfson J., Sigmundsson T., Petursson H., Jazin E., Zoega T., Helgason T. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am. J. Hum. Genet. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.A. The chandelier neuron in schizophrenia. Dev. Neurobiol. 2011;71:118–127. doi: 10.1002/dneu.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.Y., Zhang X.L., Matthews E.A., Li K.W., Kurwa A., Boroujerdi A., Gross J., Gold M.S., Dickenson A.H., Feng G., Luo Z.D. Calcium channel alpha(2)delta(1) subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125:20–34. doi: 10.1016/j.pain.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D., Madison D.V., Poenie M., Reuter H., Tsien R.Y., Tsien R.W. Spatial distribution of calcium channels and cytosolic calcium transients in growth cones and cell bodies of sympathetic neurons. Proc. Natl. Acad. Sci. USA. 1988;85:2398–2402. doi: 10.1073/pnas.85.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., De Waard M., Scott V.E.S., Gurnett C.A., Lennon V.A., Campbell K.P. Identification of three subunits of the high affinity w-conotoxin MVIIC-sensitive Ca2+ channel. J. Biol. Chem. 1996;271:13804–13810. [PubMed] [Google Scholar]
- Liu L.X., Burgess L.H., Gonzalez A.M., Sibley D.R., Chiodo L.A. D2S, D2L, D3, and D4 dopamine receptors couple to a voltage-dependent potassium current in N18TG2 x mesencephalon hybrid cell (MES-23.5) via distinct G proteins. Synapse. 1999;31:108–118. doi: 10.1002/(SICI)1098-2396(199902)31:2<108::AID-SYN3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Liu Y., Harding M., Pittman A., Dore J., Striessnig J., Rajadhyaksha A., Chen X. Cav1.2 and Cav1.3 L-type calcium channels regulate dopaminergic firing activity in the mouse ventral tegmental area. J. Neurophysiol. 2014;112:1119–1130. doi: 10.1152/jn.00757.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A.T., Dai X., Martinez-Agosto J.A., Cantor R.M. Support for calcium channel gene defects in autism spectrum disorders. Mol. Autism. 2012;3:18. doi: 10.1186/2040-2392-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z.D., Chaplan S.R., Higuera E.S., Sorkin L.S., Stauderman K.A., Williams M.E., Yaksh T.L. Upregulation of dorsal root ganglion α2δ calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J. Neurosci. 2001;21:1868–1875. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Cohen S., Li B., Tsien R.W. Exploring the dominant role of Cav1 channels in signalling to the nucleus. Biosci. Rep. 2013;33:97–101. doi: 10.1042/BSR20120099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur D.G., Balasubramanian S., Frankish A., Huang N., Morris J., Walter K., Jostins L., Habegger L., Pickrell J.K., Montgomery S.B., Albers C.A., Zhang Z.D., Conrad D.F., Lunter G., Zheng H., Ayub Q., DePristo M.A., Banks E., Hu M., Handsaker R.E., Rosenfeld J.A., Fromer M., Jin M., Mu X.J., Khurana E., Ye K., Kay M., Saunders G.I., Suner M.M., Hunt T., Barnes I.H., Amid C., Carvalho-Silva D.R., Bignell A.H., Snow C., Yngvadottir B., Bumpstead S., Cooper D.N., Xue Y., Romero I.G., Wang J., Li Y., Gibbs R.A., McCarroll S.A., Dermitzakis E.T., Pritchard J.K., Barrett J.C., Harrow J., Hurles M.E., Gerstein M.B., Tyler-Smith C. A systematic survey of loss-of-function variants in human protein-coding genes. Science. 2012;335:823–828. doi: 10.1126/science.1215040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra D., Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148:1223–1241. doi: 10.1016/j.cell.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangoni M.E., Couette B., Bourinet E., Platzer J., Reimer D., Striessnig J., Nargeot J. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc. Natl. Acad. Sci. USA. 2003;100:5543–5548. doi: 10.1073/pnas.0935295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansergh F., Orton N.C., Vessey J.P., Lalonde M.R., Stell W.K., Tremblay F., Barnes S., Rancourt D.E., Bech-Hansen N.T. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum. Mol. Genet. 2005;14:3035–3046. doi: 10.1093/hmg/ddi336. [DOI] [PubMed] [Google Scholar]
- Marek K.W., Kurtz L.M., Spitzer N.C. cJun integrates calcium activity and tlx3 expression to regulate neurotransmitter specification. Nat. Neurosci. 2010;13:944–950. doi: 10.1038/nn.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough S.I., Swartz K.J., Mintz I.M., Boland L.M., Bean B.P. Inhibition of calcium channels in rat central and peripheral neurons by omega-conotoxin MVIIC. J. Neurosci. 1996;16:2612–2623. doi: 10.1523/JNEUROSCI.16-08-02612.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney B.C., Sze W., Lee B., Murphy G.G. Impaired long-term potentiation and enhanced neuronal excitability in the amygdala of Ca(V)1.3 knockout mice. Neurobiol. Learn. Mem. 2009;92:519–528. doi: 10.1016/j.nlm.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir A., Bell D.C., Stephens G.J., Page K.M., Dolphin A.C. Calcium channel β subunit promotes voltage-dependent modulation of α1B by Gβγ. Biophys. J. 2000;79:731–746. doi: 10.1016/S0006-3495(00)76331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencacci N.E., R'bibo L., Bandres-Ciga S., Carecchio M., Zorzi G., Nardocci N., Garavaglia B., Batla A., Bhatia K.P., Pittman A.M., Hardy J., Weissbach A., Klein C., Gasser T., Lohmann E., Wood N.W. The CACNA1B R1389H variant is not associated with myoclonus-dystonia in a large European multicentric cohort. Hum. Mol. Genet. 2015;24:5326–5329. doi: 10.1093/hmg/ddv255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezghrani A., Monteil A., Watschinger K., Sinnegger-Brauns M.J., Barrere C., Bourinet E., Nargeot J., Striessnig J., Lory P. A destructive interaction mechanism accounts for dominant-negative effects of misfolded mutants of voltage-gated calcium channels. J. Neurosci. 2008;28:4501–4511. doi: 10.1523/JNEUROSCI.2844-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J.K., Wilson-Annan J.C., Anderson S., Christie S., Taylor M.S., Semple C.A., Devon R.S., St Clair D.M., Muir W.J., Blackwood D.H., Porteous D.J. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum. Mol. Genet. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- Mintz I.M., Adams M.E., Bean B.P. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Mintz I.M., Venema V.J., Swiderek K.M., Lee T.D., Bean B.P., Adams M.E. P-type calcium channels blocked by the spider toxin w-Aga-IVA. Nature. 1992;355:827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- Mire E., Mezzera C., Leyva-Diaz E., Paternain A.V., Squarzoni P., Bluy L., Castillo-Paterna M., Lopez M.J., Peregrin S., Tessier-Lavigne M., Garel S., Galceran J., Lerma J., Lopez-Bendito G. Spontaneous activity regulates Robo1 transcription to mediate a switch in thalamocortical axon growth. Nat. Neurosci. 2012;15:1134–1143. doi: 10.1038/nn.3160. [DOI] [PubMed] [Google Scholar]
- Mishra S.K., Hermsmeyer K. Selective inhibition of T-type Ca2+ channels by Ro 40-5967. Circ. Res. 1994;75:144–148. doi: 10.1161/01.res.75.1.144. [DOI] [PubMed] [Google Scholar]
- Missler M., Zhang W., Rohlmann A., Kattenstroth G., Hammer R.E., Gottmann K., Sudhof T.C. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;424:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Mitchell K.J. The genetics of neurodevelopmental disease. Curr. Opin. Neurobiol. 2011;21:197–203. doi: 10.1016/j.conb.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Moosmang S., Haider N., Klugbauer N., Adelsberger H., Langwieser N., Muller J., Stiess M., Marais E., Schulla V., Lacinova L., Goebbels S., Nave K.A., Storm D.R., Hofmann F., Kleppisch T. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J. Neurosci. 2005;25:9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss F.J., Viard P., Davies A., Bertaso F., Page K.M., Graham A., Canti C., Plumpton M., Plumpton C., Clare J.J., Dolphin A.C. The novel product of a five-exon stargazin-related gene abolishes CaV2.2 calcium channel expression. EMBO J. 2002;21:1514–1523. doi: 10.1093/emboj/21.7.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C.S., Haupt A., Bildl W., Schindler J., Knaus H.G., Meissner M., Rammner B., Striessnig J., Flockerzi V., Fakler B., Schulte U. Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. PNAS. 2010;107:14950–14957. doi: 10.1073/pnas.1005940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Harada H., Kamasawa N., Matsui K., Rothman J.S., Shigemoto R., Silver R.A., DiGregorio D.A., Takahashi T. Nanoscale distribution of presynaptic Ca(2+) channels and its impact on vesicular release during development. Neuron. 2015;85:145–158. doi: 10.1016/j.neuron.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely G.G., Hess A., Costigan M., Keene A.C., Goulas S., Langeslag M., Griffin R.S., Belfer I., Dai F., Smith S.B., Diatchenko L., Gupta V., Xia C.P., Amann S., Kreitz S., Heindl-Erdmann C., Wolz S., Ly C.V., Arora S., Sarangi R., Dan D., Novatchkova M., Rosenzweig M., Gibson D.G., Truong D., Schramek D., Zoranovic T., Cronin S.J., Angjeli B., Brune K., Dietzl G., Maixner W., Meixner A., Thomas W., Pospisilik J.A., Alenius M., Kress M., Subramaniam S., Garrity P.A., Bellen H.J., Woolf C.J., Penninger J.M. A genome-wide Drosophila screen for heat nociception identifies alpha2delta3 as an evolutionarily conserved pain gene. Cell. 2010;143:628–638. doi: 10.1016/j.cell.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb R., Szoke B., Palma A., Wang G., Chen X.H., Hopkins W., Cong R., Miller J., Urge L., Tarczy-Hornoch K., Loo J.A., Dooley D.J., Nadasdi L., Tsien R.W., Lemos J., Miljanich G. Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry. 1998;37:15353–15362. doi: 10.1021/bi981255g. [DOI] [PubMed] [Google Scholar]
- Newton R.A., Bingham S., Case P.C., Sanger G.J., Lawson S.N. Dorsal root ganglion neurons show increased expression of the calcium channel alpha2delta-1 subunit following partial sciatic nerve injury. Brain Res. Mol. Brain Res. 2001;95:1–8. doi: 10.1016/s0169-328x(01)00188-7. [DOI] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J.B., Tsien R.W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985;316:443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Nishiyama M., Togashi K., von Schimmelmann M.J., Lim C.S., Maeda S., Yamashita N., Goshima Y., Ishii S., Hong K. Semaphorin 3A induces CaV2.3 channel-dependent conversion of axons to dendrites. Nat. Cell Biol. 2011;13:676–685. doi: 10.1038/ncb2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M.C., Fox A.P., Tsien R.W. Long-opening mode of gating of neuronal calcium channels and its promotion by the dihydropyridine calcium agonist Bay K 8644. Proc. Natl. Acad. Sci. USA. 1985;82:2178–2182. doi: 10.1073/pnas.82.7.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M.C., Fox A.P., Tsien R.W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316:440–446. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Nurnberger J.I., Jr., Koller D.L., Jung J., Edenberg H.J., Foroud T., Guella I., Vawter M.P., Kelsoe J.R. Identification of pathways for bipolar disorder: a meta-analysis. JAMA Psychiatry. 2014;71:657–664. doi: 10.1001/jamapsychiatry.2014.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dushlaine C., Kenny E., Heron E., Donohoe G., Gill M., Morris D., Corvin A. Molecular pathways involved in neuronal cell adhesion and membrane scaffolding contribute to schizophrenia and bipolar disorder susceptibility. Mol. Psychiatry. 2011;16:286–292. doi: 10.1038/mp.2010.7. [DOI] [PubMed] [Google Scholar]
- O’Roak B.J., Vives L., Girirajan S., Karakoc E., Krumm N., Coe B.P., Levy R., Ko A., Lee C., Smith J.D., Turner E.H., Stanaway I.B., Vernot B., Malig M., Baker C., Reilly B., Akey J.M., Borenstein E., Rieder M.J., Nickerson D.A., Bernier R., Shendure J., Eichler E.E. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophoff R.A., Terwindt G.M., Vergouwe M.N., van Eijk R., Oefner P.J., Hoffman S.M., Lamerdin J.E., Mohrenweiser H.W., Bulman D.E., Ferrari M., Haan J., Lindhout D., van Ommen G.J., Hofker M.H., Ferrari M.D., Frants R.R. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- Owen M.J. New approaches to psychiatric diagnostic classification. Neuron. 2014;84:564–571. doi: 10.1016/j.neuron.2014.10.028. [DOI] [PubMed] [Google Scholar]
- Page K.M., Heblich F., Davies A., Butcher A.J., Leroy J., Bertaso F., Pratt W.S., Dolphin A.C. Dominant-negative calcium channel suppression by truncated constructs involves a kinase implicated in the unfolded protein response. J. Neurosci. 2004;24:5400–5409. doi: 10.1523/JNEUROSCI.0553-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page K.M., Heblich F., Margas W., Pratt W.S., Chaggar K., Sandhu K., Davies A., Dolphin A.C. The N-terminus is key to dominant-negative suppression of Cav2 channels: implications for episodic ataxia-2. J. Biol. Chem. 2010;285:835–844. doi: 10.1074/jbc.M109.065045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parajuli L.K., Nakajima C., Kulik A., Matsui K., Schneider T., Shigemoto R., Fukazawa Y. Quantitative regional and ultrastructural localization of the Ca(v)2.3 subunit of R-type calcium channel in mouse brain. J. Neurosci. 2012;32:13555–13567. doi: 10.1523/JNEUROSCI.1142-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca S.P., Portmann T., Voineagu I., Yazawa M., Shcheglovitov A., Pasca A.M., Cord B., Palmer T.D., Chikahisa S., Nishino S., Bernstein J.A., Hallmayer J., Geschwind D.H., Dolmetsch R.E. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat. Med. 2011;17:1657–1662. doi: 10.1038/nm.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Bauer C.S., Nieto-Rostro M., Margas W., Ferron L., Chaggar K., Crews K., Ramirez J.D., Bennett D.L., Schwartz A., Dickenson A.H., Dolphin A.C. α2δ − 1 gene deletion affects somatosensory neuron function and delays mechanical hypersensitivity in response to peripheral nerve damage. J. Neurosci. 2013;33:16412–16426. doi: 10.1523/JNEUROSCI.1026-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus F.M., Bedenbender J., Krach S., Pyka M., Krug A., Sommer J., Mette M., Nothen M.M., Witt S.H., Rietschel M., Kircher T., Jansen A. Association of rs1006737 in CACNA1C with alterations in prefrontal activation and fronto-hippocampal connectivity. Hum. Brain Mapp. 2014;35:1190–1200. doi: 10.1002/hbm.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular characterization of a novel family of low voltage-activated T-type, calcium channels. J. Bioenerg. Biomembr. 1998;30:313–318. doi: 10.1023/a:1021981420839. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol. Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E., Van Deusen A.L., Vitko I. Molecular pharmacology of human Cav3.2 T-type Ca2+ channels: block by antihypertensives, antiarrhythmics, and their analogs. J. Pharmacol. Exp. Ther. 2009;328:621–627. doi: 10.1124/jpet.108.145672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobon D. CaV2.1 channelopathies. Pflugers Arch. 2010;460:375–393. doi: 10.1007/s00424-010-0802-8. [DOI] [PubMed] [Google Scholar]
- Pietrobon D., Moskowitz M.A. Pathophysiology of migraine. Annu. Rev. Physiol. 2013;75:365–391. doi: 10.1146/annurev-physiol-030212-183717. [DOI] [PubMed] [Google Scholar]
- Pinggera A., Lieb A., Benedetti B., Lampert M., Monteleone S., Liedl K.R., Tuluc P., Striessnig J. CACNA1D de novo mutations in autism spectrum disorders activate Cav1.3 L-type calcium channels. Biol. Psychiatry. 2015;77:816–822. doi: 10.1016/j.biopsych.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D., Delaby E., Merico D., Barbosa M., Merikangas A., Klei L., Thiruvahindrapuram B., Xu X., Ziman R., Wang Z., Vorstman J.A., Thompson A., Regan R., Pilorge M., Pellecchia G., Pagnamenta A.T., Oliveira B., Marshall C.R., Magalhaes T.R., Lowe J.K., Howe J.L., Griswold A.J., Gilbert J., Duketis E., Dombroski B.A., De Jonge M.V., Cuccaro M., Crawford E.L., Correia C.T., Conroy J., Conceicao I.C., Chiocchetti A.G., Casey J.P., Cai G., Cabrol C., Bolshakova N., Bacchelli E., Anney R., Gallinger S., Cotterchio M., Casey G., Zwaigenbaum L., Wittemeyer K., Wing K., Wallace S., Van E.H., Tryfon A., Thomson S., Soorya L., Roge B., Roberts W., Poustka F., Mouga S., Minshew N., McInnes L.A., McGrew S.G., Lord C., Leboyer M., Le Couteur A.S., Kolevzon A., Jimenez G.P., Jacob S., Holt R., Guter S., Green J., Green A., Gillberg C., Fernandez B.A., Duque F., Delorme R., Dawson G., Chaste P., Cafe C., Brennan S., Bourgeron T., Bolton P.F., Bolte S., Bernier R., Baird G., Bailey A.J., Anagnostou E., Almeida J., Wijsman E.M., Vieland V.J., Vicente A.M., Schellenberg G.D., Pericak-Vance M., Paterson A.D., Parr J.R., Oliveira G., Nurnberger J.I., Monaco A.P., Maestrini E., Klauck S.M., Hakonarson H., Haines J.L., Geschwind D.H., Freitag C.M., Folstein S.E., Ennis S., Coon H., Battaglia A., Szatmari P., Sutcliffe J.S., Hallmayer J., Gill M., Cook E.H., Buxbaum J.D., Devlin B., Gallagher L., Betancur C., Scherer S.W. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am. J. Hum. Genet. 2014;94:677–694. doi: 10.1016/j.ajhg.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippucci T., Parmeggiani A., Palombo F., Maresca A., Angius A., Crisponi L., Cucca F., Liguori R., Valentino M.L., Seri M., Carelli V. A novel null homozygous mutation confirms CACNA2D2 as a gene mutated in epileptic encephalopathy. PLoS ONE. 2013;8:e82154. doi: 10.1371/journal.pone.0082154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirone A., Kurt S., Zuccotti A., Ruttiger L., Pilz P., Brown D.H., Franz C., Schweizer M., Rust M.B., Rubsamen R., Friauf E., Knipper M., Engel J. alpha2delta3 is essential for normal structure and function of auditory nerve synapses and is a novel candidate for auditory processing disorders. J. Neurosci. 2014;34:434–445. doi: 10.1523/JNEUROSCI.3085-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzer J., Engel J., Schrott-Fischer A., Stephan K., Bova S., Chen H., Zheng H., Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Pragnell M., De Waard M., Mori Y., Tanabe T., Snutch T.P., Campbell K.P. Calcium channel β-subunit binds to a conserved motif in the I-II cytoplasmic linker of the α1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- Price T.J., Flores C.M., Cervero F., Hargreaves K.M. The RNA binding and transport proteins staufen and fragile X mental retardation protein are expressed by rat primary afferent neurons and localize to peripheral and central axons. Neuroscience. 2006;141:2107–2116. doi: 10.1016/j.neuroscience.2006.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price T.J., Rashid M.H., Millecamps M., Sanoja R., Entrena J.M., Cervero F. Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR. J. Neurosci. 2007;27:13958–13967. doi: 10.1523/JNEUROSCI.4383-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S.M., Moran J.L., Fromer M., Ruderfer D., Solovieff N., Roussos P., O’Dushlaine C., Chambert K., Bergen S.E., Kahler A., Duncan L., Stahl E., Genovese G., Fernandez E., Collins M.O., Komiyama N.H., Choudhary J.S., Magnusson P.K., Banks E., Shakir K., Garimella K., Fennell T., DePristo M., Grant S.G., Haggarty S.J., Gabriel S., Scolnick E.M., Lander E.S., Hultman C.M., Sullivan P.F., McCarroll S.A., Sklar P. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–190. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S.M., Wray N.R., Stone J.L., Visscher P.M., O’Donovan M.C., Sullivan P.F., Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzier I., Kullmann P.H., Horn J.P., Levitan E.S. Cav1.3 channel voltage dependence, not Ca2+ selectivity, drives pacemaker activity and amplifies bursts in nigral dopamine neurons. J. Neurosci. 2009;29:15414–15419. doi: 10.1523/JNEUROSCI.4742-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghib A., Bertaso F., Davies A., Page K.M., Meir A., Bogdanov Y., Dolphin A.C. Dominant-negative synthesis suppression of voltage-gated calcium channel Cav2.2 induced by truncated constructs. J. Neurosci. 2001;21:8495–8504. doi: 10.1523/JNEUROSCI.21-21-08495.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran K.V., Hennessey J.A., Barnett A.S., Yin X., Stadt H.A., Foster E., Shah R.A., Yazawa M., Dolmetsch R.E., Kirby M.L., Pitt G.S. Calcium influx through L-type CaV1.2 Ca2+ channels regulates mandibular development. J. Clin. Invest. 2013;123:1638–1646. doi: 10.1172/JCI66903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees E., Walters J.T., Georgieva L., Isles A.R., Chambert K.D., Richards A.L., Mahoney-Davies G., Legge S.E., Moran J.L., McCarroll S.A., O’Donovan M.C., Owen M.J., Kirov G. Analysis of copy number variations at 15 schizophrenia-associated loci. Br. J. Psychiatry. 2014;204:108–114. doi: 10.1192/bjp.bp.113.131052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds I.J., Wagner J.A., Snyder S.H., Thayer S.A., Olivera B.M., Miller R.J. Brain voltage-sensitive calcium channel subtypes differentiated by omega-conotoxin fraction GVIA. Proc. Natl. Acad. Sci. USA. 1986;83:8804–8807. doi: 10.1073/pnas.83.22.8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riess O., Schöls L., Böttger H., Nolte D., Vieira-Saecker A.M.M., Schimming C., Kreuz F., Macek M., Jr., Krebsová A., Macek M., Sr., Klockgether T., Zühlke C., Laccone F.A. SCA6 is caused by moderate CAG expansion in the α1A-voltage-dependent calcium channel gene. Hum. Mol. Genet. 1997;6:1289–1293. doi: 10.1093/hmg/6.8.1289. [DOI] [PubMed] [Google Scholar]
- Ripke S., O’Dushlaine C., Chambert K., Moran J.L., Kahler A.K., Akterin S., Bergen S.E., Collins A.L., Crowley J.J., Fromer M., Kim Y., Lee S.H., Magnusson P.K., Sanchez N., Stahl E.A., Williams S., Wray N.R., Xia K., Bettella F., Borglum A.D., Bulik-Sullivan B.K., Cormican P., Craddock N., de L.C., Durmishi N., Gill M., Golimbet V., Hamshere M.L., Holmans P., Hougaard D.M., Kendler K.S., Lin K., Morris D.W., Mors O., Mortensen P.B., Neale B.M., O’Neill F.A., Owen M.J., Milovancevic M.P., Posthuma D., Powell J., Richards A.L., Riley B.P., Ruderfer D., Rujescu D., Sigurdsson E., Silagadze T., Smit A.B., Stefansson H., Steinberg S., Suvisaari J., Tosato S., Verhage M., Walters J.T., Levinson D.F., Gejman P.V., Kendler K.S., Laurent C., Mowry B.J., O’Donovan M.C., Owen M.J., Pulver A.E., Riley B.P., Schwab S.G., Wildenauer D.B., Dudbridge F., Holmans P., Shi J., Albus M., Alexander M., Campion D., Cohen D., Dikeos D., Duan J., Eichhammer P., Godard S., Hansen M., Lerer F.B., Liang K.Y., Maier W., Mallet J., Nertney D.A., Nestadt G., Norton N., O’Neill F.A., Papadimitriou G.N., Ribble R., Sanders A.R., Silverman J.M., Walsh D., Williams N.M., Wormley B., Arranz M.J., Bakker S., Bender S., Bramon E., Collier D., Crespo-Facorro B., Hall J., Iyegbe C., Jablensky A., Kahn R.S., Kalaydjieva L., Lawrie S., Lewis C.M., Lin K., Linszen D.H., Mata I., McIntosh A., Murray R.M., Ophoff R.A., Powell J., Rujescu D., Van O.J., Walshe M., Weisbrod M., Wiersma D., Donnelly P., Barroso I., Blackwell J.M., Bramon E., Brown M.A., Casas J.P., Corvin A.P., Deloukas P., Duncanson A., Jankowski J., Markus H.S., Mathew C.G., Palmer C.N., Plomin R., Rautanen A., Sawcer S.J., Trembath R.C., Viswanathan A.C., Wood N.W., Spencer C.C., Band G., Bellenguez C., Freeman C., Hellenthal G., Giannoulatou E., Pirinen M., Pearson R.D., Strange A., Su Z., Vukcevic D., Donnelly P., Langford C., Hunt S.E., Edkins S., Gwilliam R., Blackburn H., Bumpstead S.J., Dronov S., Gillman M., Gray E., Hammond N., Jayakumar A., McCann O.T., Liddle J., Potter S.C., Ravindrarajah R., Ricketts M., Tashakkori-Ghanbaria A., Waller M.J., Weston P., Widaa S., Whittaker P., Barroso I., Deloukas P., Mathew C.G., Blackwell J.M., Brown M.A., Corvin A.P., McCarthy M.I., Spencer C.C., Bramon E., Corvin A.P., O’Donovan M.C., Stefansson K., Scolnick E., Purcell S., McCarroll S.A., Sklar P., Hultman C.M., Sullivan P.F. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat. Genet. 2013;45:1150–1159. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas M.A., Pirinen M., Conrad D.F., Lek M., Tsang E.K., Karczewski K.J., Maller J.B., Kukurba K.R., DeLuca D.S., Fromer M., Ferreira P.G., Smith K.S., Zhang R., Zhao F., Banks E., Poplin R., Ruderfer D.M., Purcell S.M., Tukiainen T., Minikel E.V., Stenson P.D., Cooper D.N., Huang K.H., Sullivan T.J., Nedzel J., Bustamante C.D., Li J.B., Daly M.J., Guigo R., Donnelly P., Ardlie K., Sammeth M., Dermitzakis E.T., McCarthy M.I., Montgomery S.B., Lappalainen T., MacArthur D.G. Effect of predicted protein-truncating genetic variants on the human transcriptome. Science. 2015;348:666–669. doi: 10.1126/science.1261877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi J.A., Huber K.M. Metabotropic glutamate receptors and fragile x mental retardation protein: partners in translational regulation at the synapse. Sci. Signal. 2008;1:e6. doi: 10.1126/stke.15pe6. [DOI] [PubMed] [Google Scholar]
- Rosenberg S.S., Spitzer N.C. Calcium signaling in neuronal development. Cold Spring Harb. Perspect. Biol. 2011;3:a004259. doi: 10.1101/cshperspect.a004259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos P., Mitchell A.C., Voloudakis G., Fullard J.F., Pothula V.M., Tsang J., Stahl E.A., Georgakopoulos A., Ruderfer D.M., Charney A., Okada Y., Siminovitch K.A., Worthington J., Padyukov L., Klareskog L., Gregersen P.K., Plenge R.M., Raychaudhuri S., Fromer M., Purcell S.M., Brennand K.J., Robakis N.K., Schadt E.E., Akbarian S., Sklar P. A role for noncoding variation in schizophrenia. Cell Rep. 2014;9:1417–1429. doi: 10.1016/j.celrep.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium Genome-wide association study identifies five new schizophrenia loci. Nat. Genet. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl U.I., Goh G., Stolting G., de Oliveira R.C., Choi M., Overton J.D., Fonseca A.L., Korah R., Starker L.F., Kunstman J.W., Prasad M.L., Hartung E.A., Mauras N., Benson M.R., Brady T., Shapiro J.R., Loring E., Nelson-Williams C., Libutti S.K., Mane S., Hellman P., Westin G., Akerstrom G., Bjorklund P., Carling T., Fahlke C., Hidalgo P., Lifton R.P. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat. Genet. 2013;45:1050–1054. doi: 10.1038/ng.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl U.I., Stolting G., Nelson-Williams C., Vichot A.A., Choi M., Loring E., Prasad M.L., Goh G., Carling T., Juhlin C.C., Quack I., Rump L.C., Thiel A., Lande M., Frazier B.G., Rasoulpour M., Bowlin D.L., Sethna C.B., Trachtman H., Fahlke C., Lifton R.P. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife. 2015;4:e06315. doi: 10.7554/eLife.06315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnegger-Brauns M.J., Huber I.G., Koschak A., Wild C., Obermair G.J., Einzinger U., Hoda J.C., Sartori S.B., Striessnig J. Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol. Pharmacol. 2009;75:407–414. doi: 10.1124/mol.108.049981. [DOI] [PubMed] [Google Scholar]
- Smolin B., Karry R., Gal-Ben-Ari S., Ben-Shachar D. Differential expression of genes encoding neuronal ion-channel subunits in major depression, bipolar disorder and schizophrenia: implications for pathophysiology. Int. J. Neuropsychopharmacol. 2012;15:869–882. doi: 10.1017/S1461145711001428. [DOI] [PubMed] [Google Scholar]
- Soong T.W., Stea A., Hodson C.D., Dubel S.J., Vincent S.R., Snutch T.P. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science. 1993;260:1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- Splawski I., Timothy K.W., Sharpe L.M., Decher N., Kumar P., Bloise R., Napolitano C., Schwartz P.J., Joseph R.M., Condouris K., Tager-Flusberg H., Priori S.G., Sanguinetti M.C., Keating M.T. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Splawski I., Yoo D.S., Stotz S.C., Cherry A., Clapham D.E., Keating M.T. CACNA1H mutations in autism spectrum disorders. J. Biol. Chem. 2006;281:22085–22091. doi: 10.1074/jbc.M603316200. [DOI] [PubMed] [Google Scholar]
- Staats P.S., Yearwood T., Charapata S.G., Presley R.W., Wallace M.S., Byas-Smith M., Fisher R., Bryce D.A., Mangieri E.A., Luther R.R., Mayo M., McGuire D., Ellis D. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. 2004;291:63–70. doi: 10.1001/jama.291.1.63. [DOI] [PubMed] [Google Scholar]
- Stea A., Tomlinson W.J., Soong T.W., Bourinet E., Dubel S.J., Vincent S.R., Snutch T.P. Localization and functional properties of a rat brain α1A calcium channel reflect similarities to neuronal Q- and P-type channels. Proc. Natl. Acad. Sci. USA. 1994;91:10576–10580. doi: 10.1073/pnas.91.22.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striessnig J., Glossmann H., Catterall W.A. Identification of a phenylalkylamine binding region within the α1 subunit of skeletal muscle Ca2+ channels. Proc. Natl. Acad. Sci. USA. 1990;87:9108–9112. doi: 10.1073/pnas.87.23.9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striessnig J., Murphy B.J., Catterall W.A. Dihydropyridine receptor of L-type Ca2+ channels: identification of binding domains for [3H](+)-PN200-110 and [3H]azidopine within the α1 subunit. Proc. Natl. Acad. Sci. USA. 1991;88:10769–10773. doi: 10.1073/pnas.88.23.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striessnig J., Ortner N.J., Pinggera A. Pharmacology of L-type calcium channels: novel drugs for old targets? Curr. Mol. Pharmacol. 2015;67:821–870. doi: 10.2174/1874467208666150507105845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striessnig J., Pinggera A., Kaur G., Bock G., Tuluc P. L-type Ca channels in heart and brain. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2014;3:15–38. doi: 10.1002/wmts.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom S.P., Stone J.L., Ten Bosch J.R., Merriman B., Cantor R.M., Geschwind D.H., Nelson S.F. High-density SNP association study of the 17q21 chromosomal region linked to autism identifies CACNA1G as a novel candidate gene. Mol. Psychiatry. 2010;15:996–1005. doi: 10.1038/mp.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulem P., Helgason H., Oddson A., Stefansson H., Gudjonsson S.A., Zink F., Hjartarson E., Sigurdsson G.T., Jonasdottir A., Jonasdottir A., Sigurdsson A., Magnusson O.T., Kong A., Helgason A., Holm H., Thorsteinsdottir U., Masson G., Gudbjartsson D.F., Stefansson K. Identification of a large set of rare complete human knockouts. Nat. Genet. 2015;47:448–452. doi: 10.1038/ng.3243. [DOI] [PubMed] [Google Scholar]
- Szatmari P., Paterson A.D., Zwaigenbaum L., Roberts W., Brian J., Liu X.Q., Vincent J.B., Skaug J.L., Thompson A.P., Senman L., Feuk L., Qian C., Bryson S.E., Jones M.B., Marshall C.R., Scherer S.W., Vieland V.J., Bartlett C., Mangin L.V., Goedken R., Segre A., Pericak-Vance M.A., Cuccaro M.L., Gilbert J.R., Wright H.H., Abramson R.K., Betancur C., Bourgeron T., Gillberg C., Leboyer M., Buxbaum J.D., Davis K.L., Hollander E., Silverman J.M., Hallmayer J., Lotspeich L., Sutcliffe J.S., Haines J.L., Folstein S.E., Piven J., Wassink T.H., Sheffield V., Geschwind D.H., Bucan M., Brown W.T., Cantor R.M., Constantino J.N., Gilliam T.C., Herbert M., Lajonchere C., Ledbetter D.H., Lese-Martin C., Miller J., Nelson S., Samango-Sprouse C.A., Spence S., State M., Tanzi R.E., Coon H., Dawson G., Devlin B., Estes A., Flodman P., Klei L., McMahon W.M., Minshew N., Munson J., Korvatska E., Rodier P.M., Schellenberg G.D., Smith M., Spence M.A., Stodgell C., Tepper P.G., Wijsman E.M., Yu C.E., Roge B., Mantoulan C., Wittemeyer K., Poustka A., Felder B., Klauck S.M., Schuster C., Poustka F., Bolte S., Feineis-Matthews S., Herbrecht E., Schmotzer G., Tsiantis J., Papanikolaou K., Maestrini E., Bacchelli E., Blasi F., Carone S., Toma C., Van E.H., de J.M., Kemner C., Koop F., Langemeijer M., Hijmans C., Staal W.G., Baird G., Bolton P.F., Rutter M.L., Weisblatt E., Green J., Aldred C., Wilkinson J.A., Pickles A., Le C.A., Berney T., McConachie H., Bailey A.J., Francis K., Honeyman G., Hutchinson A., Parr J.R., Wallace S., Monaco A.P., Barnby G., Kobayashi K., Lamb J.A., Sousa I., Sykes N., Cook E.H., Guter S.J., Leventhal B.L., Salt J., Lord C., Corsello C., Hus V., Weeks D.E., Volkmar F., Tauber M., Fombonne E., Shih A., Meyer K.J. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat. Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Seagar M.J., Jones J.F., Reber B.F., Catterall W.A. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc. Natl. Acad. Sci. USA. 1987;84:5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987;328:313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Templin C., Ghadri J.R., Rougier J.S., Baumer A., Kaplan V., Albesa M., Sticht H., Rauch A., Puleo C., Hu D., Barajas-Martinez H., Antzelevitch C., Luscher T.F., Abriel H., Duru F. Identification of a novel loss-of-function calcium channel gene mutation in short QT syndrome (SQTS6) Eur. Heart J. 2011;32:1077–1088. doi: 10.1093/eurheartj/ehr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesli M., Skatun K.C., Ousdal O.T., Brown A.A., Thoresen C., Agartz I., Melle I., Djurovic S., Jensen J., Andreassen O.A. CACNA1C risk variant and amygdala activity in bipolar disorder, schizophrenia and healthy controls. PLoS ONE. 2013;8:e56970. doi: 10.1371/journal.pone.0056970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting J.T., Peca J., Feng G. Functional consequences of mutations in postsynaptic scaffolding proteins and relevance to psychiatric disorders. Annu. Rev. Neurosci. 2012;35:49–71. doi: 10.1146/annurev-neuro-062111-150442. [DOI] [PubMed] [Google Scholar]
- Tottene A., Conti R., Fabbro A., Vecchia D., Shapovalova M., Santello M., Van den Maagdenberg A.M., Ferrari M.D., Pietrobon D. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Ca(v)2.1 knockin migraine mice. Neuron. 2009;61:762–773. doi: 10.1016/j.neuron.2009.01.027. [DOI] [PubMed] [Google Scholar]
- Tottene A., Volsen S., Pietrobon D. alpha(1E) subunits form the pore of three cerebellar R-type calcium channels with different pharmacological and permeation properties. J. Neurosci. 2000;20:171–178. doi: 10.1523/JNEUROSCI.20-01-00171.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggle D.J. Calcium channel ligands. Ann. Rev. Pharmacol. Toxicol. 1987;27:347–369. doi: 10.1146/annurev.pa.27.040187.002023. [DOI] [PubMed] [Google Scholar]
- Tringham E., Powell K.L., Cain S.M., Kuplast K., Mezeyova J., Weerapura M., Eduljee C., Jiang X., Smith P., Morrison J.L., Jones N.C., Braine E., Rind G., Fee-Maki M., Parker D., Pajouhesh H., Parmar M., O’Brien T.J., Snutch T.P. T-type calcium channel blockers that attenuate thalamic burst firing and suppress absence seizures. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003120. 121ra19. [DOI] [PubMed] [Google Scholar]
- Valdeolmillos M., O’Neill S.C., Smith G.L., Eisner D.A. Calcium-induced calcium release activates contraction in intact cardiac cells. Pflügers Arch. 1989;413:676–678. doi: 10.1007/BF00581820. [DOI] [PubMed] [Google Scholar]
- Walsh C.P., Davies A., Butcher A.J., Dolphin A.C., Kitmitto A. 3D structure of CaV3.1—comparison with the cardiac L-type voltage-gated calcium channel monomer architecture. J. Biol. Chem. 2009;284:22310–22321. doi: 10.1074/jbc.M109.017152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T., McClellan J.M., McCarthy S.E., Addington A.M., Pierce S.B., Cooper G.M., Nord A.S., Kusenda M., Malhotra D., Bhandari A., Stray S.M., Rippey C.F., Roccanova P., Makarov V., Lakshmi B., Findling R.L., Sikich L., Stromberg T., Merriman B., Gogtay N., Butler P., Eckstrand K., Noory L., Gochman P., Long R., Chen Z., Davis S., Baker C., Eichler E.E., Meltzer P.S., Nelson S.F., Singleton A.B., Lee M.K., Rapoport J.L., King M.C., Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Wang H., Sun H., Della P.K., Benz R.J., Xu J., Gerhold D.L., Holder D.J., Koblan K.S. Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience. 2002;114:529–546. doi: 10.1016/s0306-4522(02)00341-x. [DOI] [PubMed] [Google Scholar]
- Westenbroek R.E., Hell J.W., Warner C., Dubel S.J., Snutch T.P., Catterall W.A. Biochemical properties and subcellular distribution of an N-type calcium channel α1 subunit. Neuron. 1992;9:1099–1115. doi: 10.1016/0896-6273(92)90069-p. [DOI] [PubMed] [Google Scholar]
- Westenbroek R.E., Sakurai T., Elliott E.M., Hell J.W., Starr T.V.B., Snutch T.P., Catterall W.A. Immunochemical identification and subcellular distribution of the a1A subunits of brain calcium channels. J. Neurosci. 1995;15:6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D.B., Randall A., Tsien R.W. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- Wheeler D.G., Groth R.D., Ma H., Barrett C.F., Owen S.F., Safa P., Tsien R.W. Ca(V)1 and Ca(V)2 channels engage distinct modes of Ca(2+) signaling to control CREB-dependent gene expression. Cell. 2012;149:1112–1124. doi: 10.1016/j.cell.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.E., Brust P.F., Feldman D.H., Patthi S., Simerson S., Maroufi A., McCue A.F., Veliçelebi G., Ellis S.B., Harpold M.M. Structure and functional expression of an w-conotoxin-sensitive human N-type calcium channel. Science. 1992;257:389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- Wilson S.M., Toth P.T., Oh S.B., Gillard S.E., Volsen S., Ren D., Philipson L.H., Fletcher C.F., Tessarollo L., Copeland N.G., Jenkins N.A., Miller R.J. The status of voltage-dependent calcium channels in α1E knockout mice. J. Neurosci. 2000;20:8566–8571. doi: 10.1523/JNEUROSCI.20-23-08566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witcher D.R., De Waard M., Sakamoto J., Franzini-Armstrong C., Pragnell M., Kahl S.D., Campbell K.P. Subunit identification and reconstitution of the N-type Ca2+ channel complex purified from brain. Science. 1993;261:486–489. doi: 10.1126/science.8392754. [DOI] [PubMed] [Google Scholar]
- Wu L.G., Westenbroek R.E., Borst J.G.G., Catterall W.A., Sakmann B. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J. Neurosci. 1999;19:726–736. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H.S., Huang Q.H., Zhang F.X., Bao L., Lu Y.J., Guo C., Yang L., Huang W.J., Fu G., Xu S.H., Cheng X.P., Yan Q., Zhu Z.D., Zhang X., Chen Z., Han Z.G., Zhang X. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc. Natl. Acad. Sci. USA. 2002;99:8360–8365. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Song W.J., Surmeier J. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J. Neurophysiol. 1997;77:1003–1015. doi: 10.1152/jn.1997.77.2.1003. [DOI] [PubMed] [Google Scholar]
- Yatsenko S.A., Hixson P., Roney E.K., Scott D.A., Schaaf C.P., Ng Y.T., Palmer R., Fisher R.B., Patel A., Cheung S.W., Lupski J.R. Human subtelomeric copy number gains suggest a DNA replication mechanism for formation: beyond breakage-fusion-bridge for telomere stabilization. Hum. Genet. 2012;131:1895–1910. doi: 10.1007/s00439-012-1216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimizu T., Pan J.Q., Mungenast A.E., Madison J.M., Su S., Ketterman J., Ongur D., McPhie D., Cohen B., Perlis R., Tsai L.H. Functional implications of a psychiatric risk variant within CACNA1C in induced human neurons. Mol. Psychiatry. 2015;20:162–169. doi: 10.1038/mp.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen R.K., Thiruvahindrapuram B., Merico D., Walker S., Tammimies K., Hoang N., Chrysler C., Nalpathamkalam T., Pellecchia G., Liu Y., Gazzellone M.J., D’Abate L., Deneault E., Howe J.L., Liu R.S., Thompson A., Zarrei M., Uddin M., Marshall C.R., Ring R.H., Zwaigenbaum L., Ray P.N., Weksberg R., Carter M.T., Fernandez B.A., Roberts W., Szatmari P., Scherer S.W. Whole-genome sequencing of quartet families with autism spectrum disorder. Nat. Med. 2015;21:185–191. doi: 10.1038/nm.3792. [DOI] [PubMed] [Google Scholar]
- Zamponi G.W., Striessnig J., Koschak A., Dolphin A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 2015;67:821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Maximov A., Fu Y., Xu F., Tang T.S., Tkatch T., Surmeier D.J., Bezprozvanny I. Association of CaV1.3 L-type calcium channels with Shank. J. Neurosci. 2005;25:1037–1049. doi: 10.1523/JNEUROSCI.4554-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-F., Randall A.D., Ellinor P.T., Horne W.A., Sather W.A., Tanabe T., Schwarz T.L., Tsien R.W. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Shen Q., Xu Z., Chen M., Cheng L., Zhai J., Gu H., Bao X., Chen X., Wang K., Deng X., Ji F., Liu C., Li J., Dong Q., Chen C. The effects of CACNA1C gene polymorphism on spatial working memory in both healthy controls and patients with schizophrenia or bipolar disorder. Neuropsychopharmacology. 2012;37:677–684. doi: 10.1038/npp.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.