Abstract
Drug treatment of neonates and infants and its long-term consequences on drug responses have emerged in recent years as a major challenge for health care professionals. In the current study, we use phenobarbital as a model drug and mouse as an in vivo model to demonstrate that the dose of phenobarbital and age of treatment are two key factors for the persistent induction of gene expression and consequential increases of enzyme activities of Cyp2b, Cyp2c, and Cyp3a in adult livers. We show that phenobarbital treatment at early life of day 5 after birth with a low dose (<100 mg/kg) does not change expression and enzyme activities of Cyp2b, Cyp2c, and Cyp3a in adult mouse liver, whereas phenobarbital treatment with a high dose (>200 mg/kg) significantly increases expression and enzyme activities of these P450s in adult liver. We also demonstrate that phenobarbital treatment before day 10 after birth, but not at later ages, significantly increases mRNAs, proteins, and enzyme activities of the tested P450s. Such persistent induction of P450 gene expression and enzyme activities in adult livers by phenobarbital treatment only occurs within a sensitive age window early in life. The persistent induction in gene expression and enzyme activities is higher in female mice than in male mice for Cyp2b10 but not for Cyp2c29 and Cyp3a11. These results will stimulate studies to evaluate the long-term impacts of drug treatment with different doses at neonatal and infant ages on drug metabolism, therapeutic efficacy, and drug-induced toxicity throughout the rest of life.
Introduction
The cytochrome P450 (P450) enzymes are a large and diverse group of enzymes that account for ∼70–80% of the total drug metabolism reactions in humans (Blanchard and Smoliga, 2015). Metabolism rates mediated by P450 enzymes can influence drug responses either by affecting therapeutic efficacy (too little drug or metabolites on target organs and cells) or by causing toxic reactions (too much drug or metabolites in the body) (Renaud et al., 2011). Induction of gene expression and further increase of enzyme activities of some key drug metabolizing P450 genes by drugs has been characterized as a key factor resulting in variability in drug responses (Lin and Lu, 2001). Some drugs can serve as ligands to activate nuclear receptors and induce the expression of P450 genes in human hepatocytes (Handschin and Meyer, 2003). For example, constitutive androstane receptor (CAR) can be activated by phenobarbital (Gervot et al., 1999; Sueyoshi et al., 1999) and phenytoin (Wang et al., 2004), pregnane X receptor by rifampicin (Goodwin et al., 1999, 2001; Gorski et al., 2003; Chen et al., 2004) and hyperforin (Chen et al., 2004), glucocorticoid receptor (GR) by dexamethasone (Onica et al., 2008), aryl hydrocarbon receptor by omeprazole (Gerbal-Chaloin et al., 2006), and peroxisome proliferator-activated receptor γ by ibuprofen (Rekka et al., 1994).
Millions of neonates and infants are treated with drugs for various disease conditions. Some widely used drugs for neonates and infants have the ability to activate nuclear receptors, including phenobarbital, phenytoin, rifampicin, dexamethasone, omeprazole, and ibuprofen. However, in medicine, the current practice is to not consider whether drug treatment at the neonatal or infant ages can result in any long-term effect on P450 expression throughout the rest of their lives.
Scientists have tried to address this question in animal models in the past 20 years. Using phenobarbital as a testing drug, Dr. Shapiro’s laboratory examined the short- and long-term effects on P450 expression and enzyme activities after neonatal treatment in rats (Agrawal et al., 1995; Agrawal and Shapiro, 1996a, 2000). Different ages of rats were treated with 40 mg/kg phenobarbital for the first 7 days after birth, and mRNA, protein, and enzyme activity levels of P450s, such as CYP2C6, 2C12, 3A1, and 3A2, rose precipitously at 4 and 8 days of age but rapidly declined to preinduction levels at adult ages after withdrawal of the treatment. In these studies, neonatal administration of phenobarbital appeared to have no long-term consequence on the hepatic basal expression of P450s in either adult male or female rats. On the basis of these reports, phenobarbital administration initiates only an immediate transient induction; however, we demonstrate that permanent induction of P450s depends on the dose of phenobarbital treatment as well as the timing of the dose. The doses used in Dr. Shapiro’s studies were insufficient to observe this effect.
Phenobarbital is used as an antiepileptic drug to manage seizures in neonates and infants (Bartha et al., 2007; van Rooij et al., 2013). Neonatal seizure is a common problem affecting 1 to 4 infants for every 1000 births in North America (Lanska et al., 1995; Ronen et al., 1999). The Food and Drug Administration has a dose guidance to manage seizure with phenobarbital in neonates and infants. To quickly produce blood levels to approximate 20 μg/ml for optimal anticonvulsant effects, a much higher loading dose between 15 and 20 mg/kg is given by intravenous injection before a lower maintenance dose between 3 and 6 mg/kg/day is applied (Carmo and Barr, 2005; Lee et al., 2012). This has promoted us to reevaluate Dr. Shapiro’s original studies in the context of a phenobarbital dosing regimen that is consistent with its clinical use in early life. We show here that the dose of phenobarbital and age of treatment at early life are the two key factors for the persistent induction of P450 expression and activities in adult mouse liver.
Materials and Methods
Chemicals.
Phenobarbital, pentoxyresorfin, midazolam, paclitaxel, phosphate-buffered saline, NADPH, potassium phosphate, magnesium chloride, formic acid, and acetonitrile were purchased from Sigma (Sigma-Aldrich, St. Louis, MO). TRIzol reagent was obtained from Life Technologies (Life Technologies, Guilford, CT).
Animals.
Eight-week-old C57BL/6 mouse breeding pairs were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were housed according to the animal care guidelines provided by the American Association for Animal Laboratory Sciences and were bred under standard conditions in the Laboratory Animal Resources Facility at the University of Connecticut. The use of these mice was approved by the Institutional Animal Care and Use Committee, Office of Research Compliance. A total of 160 mice from 40–42 different litters were assigned randomly to control and phenobarbital treatment groups per sex. In a study with phenobarbital treatment at different doses, C57BL/6 male and female mice on day 5 after birth (n = 5–8 mice per group) were intraperitoneally injected with different doses of phenobarbital (20, 60, 100, 140, 200, and 250 mg/kg) dissolved in saline or saline alone (control). In a study with phenobarbital treatment at different ages, C57BL/6 male and female mice (n = 5–8) were treated with a fixed dose of phenobarbital at 200 mg/kg at different ages (day 5, 10, 15, 20, and 25 after birth). In a study with phenobarbital treatment at multiple ages, male mice (n = 5–8) were injected with phenobarbital at 200 mg/kg at days 5, 15, and 25 after birth. At day 60 after birth, all mice were killed and liver tissues were harvested for measurement of mRNAs, proteins, and in vitro enzyme activities of Cyp2b, Cyp2c, and Cyp3a.
Quantitative Real-time Polymerase Chain Reaction.
Total RNAs were isolated from same sections of livers without gallbladders at day 60 after various treatments of phenobarbital using TRIzol reagent (Life Technologies) according to the manufacturer's protocol. RNA concentrations were quantified using a Nano Drop spectrophotometer (Nano Drop Technologies, Wilmington, DE) at a wavelength of 260 nm. The integrity of the total RNAs was evaluated on an Agilent 2200 Tape Station (Agilent Technologies, Santa Clara, CA). Gene expression at the mRNA level of the Cyp2b10, Cyp2c29, Cyp3a11, and Gapdh genes was determined by TaqMan assay according to the manufacturer's protocol (Life Technologies). Data were analyzed by the delta delta Ct (ΔΔCt) method for relative quantifications. The values for all P450s genes were analyzed against Gapdh values.
Western Blot Analysis.
Expression of Cyp2b10 and Cyp3a11 at the protein level in three random selected male mouse livers was determined by Western blot. Total cellular proteins were isolated from the same sections of the mouse liver tissues based on the manufacture protocol (Life Technologies). Protein concentration was determined by a Qubit Protein Assay Kit (Life Technologies). The extracted proteins (40 μg/ml) were separated on a 12% SDS-PAGE gel using the Bio-Rad electrophoresis system and electro-blotted onto a PVDF membrane by the Bio-Rad transfer system (Bio-Rad, Hercules, CA). The membranes were blocked with 5% nonfat milk at room temperature for 1 hour followed by incubation with a primary antibody against Cyp3a11 (1:1000) or Cyp2b10 (1:1500) (Millipore Corporation, Bedford, MA). Gapdh antibody (Sigma-Aldrich) was used as a loading control for normalization (1:3000). Immunoreactive bands were detected by chemiluminescence using corresponding horseradish peroxidase-conjugated secondary antibodies (1:3000) (Sigma-Aldrich). The Western blot signals were quantified using Image J software (http://imagej.nih.gov/ij/index.html).
Enzyme Activities of P450s.
The enzyme activities of Cyp2b, Cyp2c, and Cyp3a in mouse microsomes were determined by ultra-performance liquid chromatography-quadrapole time of flight mass spectrometry (UPLC-QTOFMS) according to the previously established methods (Gustafson et al., 2005; Ma et al., 2007; Chang et al., 2008; Lam et al., 2010). The liver tissues were homogenized in ice-cold buffer (150 mM KCl and 250 mM sucrose in 100 mM phosphate buffer, pH 7.4). The homogenized tissues were centrifuged at 10,000 g for 25 minutes at 4°C. The resulting supernatant was further centrifuged at 100,000 g for 1 hour at 4°C. The pellets were washed once and resuspended in a buffer containing 100 mM phosphate and 20% glycerol. Protein concentrations were determined by a Modified Lowry Protein Assay Kit (Thermo Fisher Scientific Inc., Waltham, MA). Pentoxyresorufin, paclitaxel, and midazolam were used as the probe substrates in the reactions of pentoxyresorufin O-dealkylation, paclitaxel 6-hydroxylation, and midazolam 1′-hydroxylation to detect the enzyme activities of Cyp2b (Lam et al., 2010), Cyp2c (Gustafson et al., 2005), and Cyp3a (Lam et al., 2010), respectively. The detected metabolites were resorufin, 6-hydroxypaclitaxel, and 1′-hydroxymidazolam. In brief, incubations were carried out in 1 × phosphate-buffered saline (pH 7.4), containing 50 μg of mouse liver microsomal proteins and 30 μM substrates in a final volume of 95 μl. The reactions were initiated by adding 5 μl of 20 mM NADPH and continued for 30 minutes for pentoxyresorufin and paclitaxel or 10 minutes for midazolam. Incubations were terminated by adding 100 μl of ice-cold acetonitrile. The samples were vortexed for 30 seconds and centrifuged at 15,000 rpm for 10 minutes. A 1.0-μl aliquot of supernatant was injected into the Synapt G2-S QTOFMS system (Waters, Milford, MA) for metabolite analysis. Chromatographic separation of the metabolites was performed on an Acquity UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm, Waters). Mobile phase A consisted of 0.1% formic acid in water and mobile phase B (MPB) of 0.1% formic acid in acetonitrile. The gradient for aqueous extraction began at 5% MPB and held for 0.5 minute, followed by a 5-minute linear gradient to 95% MPB, held for 2 minutes, and decreased to 5% MPB for column equilibration. The flow rate of mobile phase was 0.50 ml/min, and the column temperature was maintained at 50°C. The G2-S QTOFMS system was operated in a resolution mode (resolution ∼20,000) with electrospray ionization. The source and desolvation temperatures were set at 150 and 500°C, respectively. Nitrogen was applied as the cone gas (50 l/hour) and desolvation gas (800 l/hour). The capillary and cone voltages were set at 0.8 kV and 40 V. The data were acquired in positive ionization mode. QTOFMS was calibrated with sodium formate and monitored by the intermittent injection of lock mass leucine enkephalin (m/z = 556.2771) in real time. Quanlynx software (Waters) was used for the quantification of the concentrations of metabolites.
Statistical Analysis.
Data are presented as the mean ± S.D. Statistical analyses were performed using IBM SPSS 20 (IBM, Armonk, NY). Comparison between groups was performed using one-way analysis of variance, followed by the Scheffe’s procedure for the post hoc test. P < 0.05 was considered to be statistically significant.
Results
Dose of Phenobarbital Treatment at Early Life Is a Key Factor for the Persistent Induction of Gene Expression and Enzyme Activities of Cyp2b, Cyp2c, and Cyp3a in Adult Mouse Liver.
Mouse was selected in this study rather than rat because of our prior studies of P450 ontogeny in mouse liver (Hart et al., 2009; Peng et al., 2012). Human subjects were not included in this study because direct work on human subjects would require tracking phenobarbital-treated infants into adulthood for at least 15–20 years, which would be cost prohibitive and difficult to control factors outside of phenobarbital treatment.
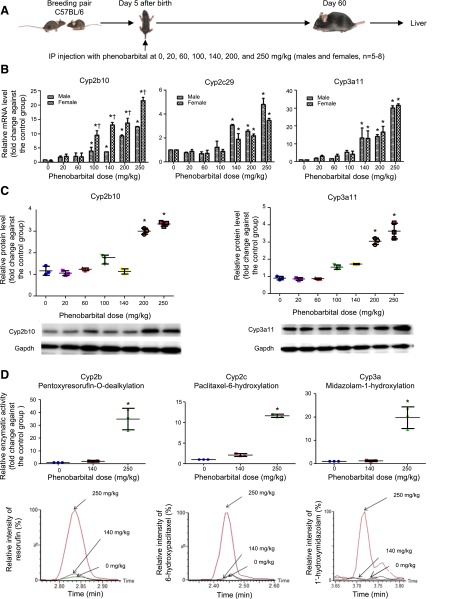
Mice at day 5 after birth were intraperitoneally injected with a single dose of either saline (control) or phenobarbital from 20 to 250 mg/kg in saline. At day 60 after birth, the mice were killed and livers were harvested for determination of mRNAs, proteins, and in vitro enzyme activities of Cyp2b, Cyp2c, and Cyp3a. Phenobarbital treatment at early life resulted in statistically significant increases of mRNA levels in both male and female mouse livers only in the groups with a dose higher than 100 mg/kg for Cyp2b10 and 140 mg/kg for Cyp2c29 and Cyp3a11 (Fig. 1A). For example, the average mRNA levels of Cyp2b10, Cyp2c29, and Cyp3a11 in the 250 mg/kg group of male and female mice together increased 20-, 4-, and 30-fold, respectively, in comparison with the control group. A statistically significant higher mRNA level was found in female mice than in male mice for Cyp2b10, but not for Cyp2c29 and Cyp3a11 in all groups with a dose higher than 100 mg/kg.
Fig. 1.
Alterations of mRNAs, proteins, and enzyme activities of Cyp2b, Cyp2c, and Cyp3a in adult mouse livers due to phenobarbital treatment at early life with different doses. (A) A diagram showing experimental design for neonatal phenobarbital treatment with different doses. (B) Quantitative PCR analysis of mRNA expression of Cyp2b10, Cyp2c29, and Cyp3a11 in adult male and female mice livers (n = 5–8). (C) Western blot analysis for protein expression of Cyp2b10 and Cyp3a11 in three randomly selected adult male livers. A representative Western blot image including one sample from each group is shown for each P450 protein. (D) Quantitative analysis of in vitro enzyme activities for Cyp2b, Cyp2c, and Cyp3a in three randomly selected adult male livers in the control or phenobarbital-treated groups at 140 and 250 mg/kg determined by UPLC-QTOFMS. A representative QTOFMS chromatogram image from each group is shown for each P450 enzyme. Values are represented as mean ± S.D. *P < 0.05 in a phenobarbital-treated group compared with the control group of same sex. †P < 0.05 in a female group compared with its related male group.
Next, we measured gene expression of Cyp2b10 and Cyp3a11 at the protein level by Western blot in three random selected male mouse livers from each group. We did not include Cyp2c29, because no commercial antibody is available to detect this protein. Again, we found that phenobarbital treatment at early life resulted in significant increases of Cyp2b10 and Cyp3a11 proteins only in the high dose groups (200 and 250 mg/kg) (Fig. 1B). The average protein levels for Cyp2b10 and Cyp3a11 in the 250 mg/kg group increased approximately 3.2- and 3.5-fold, respectively, after normalization with Gapdh protein.
The in vitro enzyme activities of Cyp2b, Cyp2c, and Cyp3a were determined by UPLC-QTOFMS using the rates of pentoxyresorufin O-dealkylation (Lam et al., 2010), paclitaxel 6-hydroxylation (Gustafson et al., 2005), and midazolam 1′-hydroxylation (Lam et al., 2010), respectively, in liver microsome samples from three randomly selected male mice from the control and phenobarbital- treated groups at 140 and 250 mg/kg (Fig. 1C). The average enzyme activity of Cyp2b, Cyp2c, or Cyp3a increased 35-, 12-, and 20-fold, respectively, in the phenobarbital-treated group at 250 mg/kg compared with the control group. No differences in enzyme activities were observed between the phenobarbital-treated group at 140 mg/kg and the control group.
These findings indicate that phenobarbital treatment at a dose level over a certain threshold (>200 mg/kg) at early life can significantly increase expression of the P450 genes, resulting in the increase of P450 enzyme activities in adult livers. The dose of phenobarbital is a key factor for the persistent induction of P450 expression and increase of enzyme activities in adult mouse liver.
The Age at Which Mice Are Treated with Phenobarbital Is Another Key Factor for the Persistent Induction of Gene Expression and Enzyme Activities of Cyp2b, Cyp2c, and Cyp3a in Adult Mouse Liver.
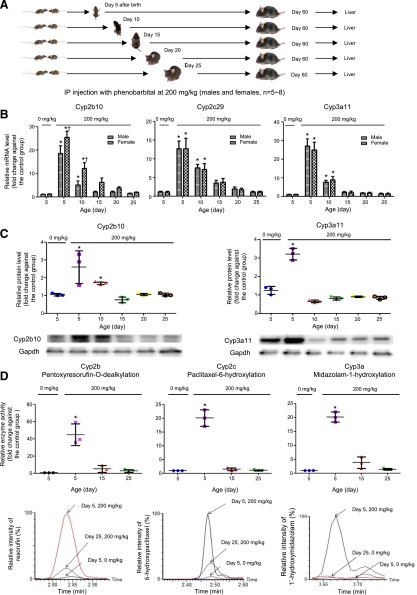
We then asked a further question, “Is there a sensitive age window for phenobarbital treatment that can result in the persistent induction of the P450 gene expression and enzyme activities in adult liver?” Mice were intraperitoneally injected with a single dose of 200 mg/kg phenobarbital at days 5, 10, 15, 20, or 25 after birth. At day 60, the mice were killed and livers were harvested for determination of mRNAs, proteins, and in vitro enzyme activities of Cyp2b, Cyp2c, and Cyp3a. The ages were selected based on our previous findings in P450 ontogeny during postnatal liver maturation in mice (Hart et al., 2009; Peng et al., 2012), and this time period is the most rapidly growing stage of postnatal liver maturation from a hematopoietic organ to a metabolic organ (Kinoshita and Miyajima, 2002).
Figure 2A shows again that phenobarbital treatment with 200 mg/kg at day 5 after birth resulted in significant increases of mRNAs of Cyp2b10, Cyp2c29, and Cyp3a11 with an average fold change of 12, 8, and 20, respectively, in the adult livers for males and females together. A higher induction of mRNA expression in females than in males was observed in Cyp2b10 but not in Cyp2c29 and Cyp3a11 in this group. Gene expression of Cyp2b10, Cyp2c29, and Cyp3a11 was also significantly higher in the phenobarbital treatment group at day 10 compared with the control group, but the fold changes are smaller than the phenobarbital treatment group at day 5. No differences were found in the phenobarbital treatment groups at day 15, 20, or 25 compared with the control group.
Fig. 2.
Alterations of mRNAs, proteins, and enzyme activities of Cyp2b, Cyp2c, and Cyp3a in adult mouse livers due to phenobarbital treatment at different ages. (A) A diagram showing experimental design for phenobarbital treatment at different ages. (B) Quantitative PCR analysis of mRNA expression of Cyp2b10, Cyp2c29, and Cyp3a11 in adult male and female mice livers (n = 5–8). (C) Western blot analysis for protein expression of Cyp2b10 and Cyp3a11 in three randomly selected adult male livers. A representative Western blot image including one sample from each group is shown for each P450 protein. (D) Quantitative analysis of enzyme activities for Cyp2b, Cyp2c, and Cyp3a in three random selected adult male livers from the control or phenobarbital-treated groups at days 5, 15, and 25 determined by UPLC-QTOFMS. A representative QTOFMS chromatogram image from each group is shown for each P450 enzyme. Values are represented as mean ± S.D. *P < 0.05 in a phenobarbital-treated group compared with the control group of same sex. †P < 0.05 in a female group compared with its related male group.
Results for gene expression of Cyp3a11 and Cyp2b10 at the protein level in three random selected male livers (Fig. 2B) and in vitro enzyme activities of Cyp2b, Cyp2c, and Cyp3a in three random selected male liver microsomal samples (Fig. 2C) are consistent with the findings at the mRNA level, indicating that the persistent induction of P450 gene expression and enzyme activities in adult livers by phenobarbital treatment can only occur at early life before day 15 after birth. There is a sensitive age window for phenobarbital treatment that can result in the persistent induction of the P450 gene expression and enzyme activities in adult liver.
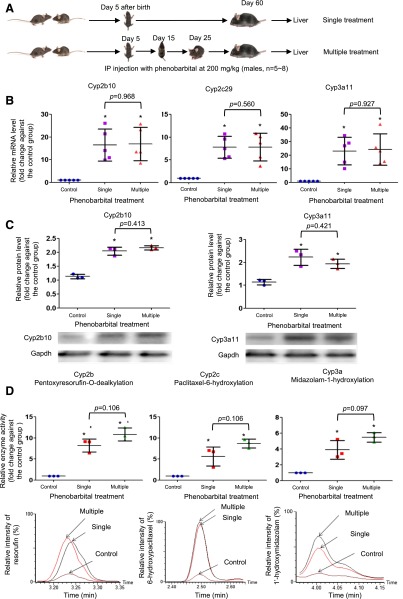
The sensitive age window is further proven by an experiment with multiple treatments of phenobarbital at different ages. Male mice were repeatedly intraperitoneally injected with 200 mg/kg phenobarbital at days 5, 10, and 15 after birth and were compared with mice intraperitoneally injected only with a single dose of 200 mg/kg phenobarbital at day 5. At day 60 after birth, the mice were killed and livers were harvested for determination of mRNAs, proteins, and enzyme activities. Both groups with single treatment of phenobarbital at infant age and multiple treatments from infant to adolescence ages had significant increases of mRNAs, proteins, and enzyme activities of the examined P450 genes in adult livers compared with the control group (Fig. 3, A–C). However, no statistical differences were observed between the single and multiple treatment groups. These results suggest that phenobarbital treatment at later ages (days 10 and 15) does not further enhance the increases in gene expression and enzyme activities of P450s in adult livers. The above experiments demonstrate that the age of treatment is another key factor for the persistent induction of P450 expression and enzyme activities in adult mouse liver.
Fig. 3.
Alterations of mRNAs, proteins, and enzyme activities of Cyp2b, Cyp2c, and Cyp3a in adult mouse livers due to phenobarbital treatment with single or multiple doses at different ages. (A) A diagram showing phenobarbital treatment with single dose at day 5 and multiple doses at days 5, 15, and 25. (B) Quantitative PCR analysis of mRNA expression of Cyp2b10, Cyp2c29, and Cyp3a11 in the adult male mice livers (n = 5). (C) Western blot analysis for protein expression of Cyp2b10 and Cyp3a11 in three randomly selected adult male livers. A representative Western blot image including one sample from each group is shown for each P450 protein. (D) Quantitative analysis of enzyme activities for Cyp2b, Cyp2c, and Cyp3a in three randomly selected adult male livers from control, phenobarbital single, or multiple treatment groups determined by UPLC-QTOFMS. A representative QTOFMS chromatogram image from each group is shown for each P450 enzyme. Values are represented as mean ± S.D. *P < 0.05 in a phenobarbital treated group compared with the control group of same sex.
Discussion
In the current study, we demonstrate that the dose of phenobarbital and age of treatment are the two key factors for the persistent induction of gene expression and increases of enzyme activities of Cyp2b, Cyp2c, and Cyp3a in adult mouse livers. When mice at an age within the sensitive window at early life (younger than day 15 after birth) are treated with phenobarbital at a dose level higher than a certain threshold (>200 mg/kg), induction of gene expression and consequent increases in enzyme activities of the examined P450s are observed in adult mouse livers. Dr. Shapiro’s laboratory observes that induction in gene expression and increases in enzyme activities of P450s due to phenobarbital treatment at neonatal and infant ages are only short term. Inducibility is heightened in the long term, but induced basal levels do not persist in rat adult livers (Agrawal and Shapiro, 1996b, 2000, 2005). The dose of phenobarbital used in their studies is 40 mg/kg/day for rats, which is equivalent to 80 mg/kg/day for mice based on a dose conversion formula using the body surface area (BSA) normalization method (Reagan-Shaw et al., 2008). Not surprisingly, this dose is not high enough to induce expression of the P450 genes in adult liver. On the basis of the simple BSA conversion method, a dose of phenobarbital between 200 and 250 mg/kg in mice, which has the ability to persistently induce the P450 gene expression, can be converted to a human equivalent dose between 15 and 20 mg/kg. This dose is equivalent to a loading dose of phenobarbital used to quickly produce blood levels to approximately 20 μg/ml for optimal anticonvulsant effects in the Food and Drug Administration's dose guidance for treatment of neonatal seizure by phenobarbital (Carmo and Barr, 2005; Lee et al., 2012). However, in a clinical application, using the BSA conversion from an animal species to human is inappropriate in many cases. A more accurate conversion based on physiologically pharmacokinetic modeling (Blanchard and Smoliga, 2015) should be applied, but such data are not available for pharmacokinetic modeling of phenobarbital between neonatal mice and humans. Although exposure assessment between species would be aided by assessing phenobarbital in blood, it is not feasible to collect a sufficient amount of blood from mice at 5 days after birth for pharmacokinetic studies. Considering that phenobarbital treatment at early life with a dose level higher than a threshold may have the potential to persistently induce P450 gene expression throughout the rest of life for humans, the long-term impacts on drug metabolism, therapeutic efficacy, and drug-induced toxicity need to be reevaluated for phenobarbital treatment at neonatal and infant ages.
We found there is a discrepancy in the magnitudes of induction at levels of mRNAs, proteins, and enzyme activities. For example, neonatal treatment of phenobarbital at 250 mg/kg led to 12-, 3.5-, and 35-fold induction in Cyp2b10 mRNA, protein, and enzyme activity in male mice, respectively. In many cases, the correlation between mRNA and protein abundances in the cell is notoriously poor, because transcription and translation are far from having a linear and simple relationship (Maier et al., 2009). The parameters, which may influence mRNA-protein correlation, include RNA secondary structure for translation efficiency, translational modulating proteins, ribosome occupancy, protein half-lives, and experimental error and noise (Vogel and Marcotte, 2012). It is unclear what parameters in the current study resulted in the discrepancy in the fold induction of the P450s at mRNA, protein, and enzyme activity levels, but an increased trend exists at all levels of gene expression and enzyme function.
Gender differences in P450 expression may help to explain sex-dependent variations in drug response and to provide a useful tool to investigate mechanisms of toxicity (Tanaka, 1999; Gandhi et al., 2004). Sex-specific expression of P450s is commonly observed in mice. A previous study has identified 29 mouse P450 isoforms with considerable sex-predominant expression at basal levels (Renaud et al., 2011). For example, Cyp2a4, Cyp2b10, and Cyp2c40 were highly expressed in females, whereas Cyp2d9, Cyp2u1, and Cyp4a12a/4a12b were predominant in males. No sex differences in liver were observed for basal expression of Cyp2c29 and Cyp3a11. In the current study, we found that neonatal administration of phenobarbital at a high dose range (100–250 mg/kg) resulted in significantly higher fold induction of Cyp2b10 mRNA expression in female mice than in male mice, but fold induction for Cyp2c29 and Cyp3a11 was similar between males and females.
The mechanism of how phenobarbital treatment at early life influences P450 expression in adult liver needs to be investigated. Many well characterized P450s have been found to be enriched in adult liver compared with fetal and neonatal liver in both humans (de Wildt et al., 1999; Koukouritaki et al., 2004) and mice (Hart et al., 2009; Peng et al., 2012). We demonstrated that the ontogenic patterns of Cyp3a genes in mouse liver are associated with dynamic alterations of chromatin epigenetic signatures, such as histone methylation, within the Cyp3a locus (Li et al., 2009). If phenobarbital has the ability to alter the epigenetic signature associated with the P450 genes during liver maturation, the drug may have ability to permanently induce P450 expression throughout the rest of life. Indeed, Chen et al. (2012) recently demonstrated that neonatal exposure at 3 days after birth to 1,4-bis[2-(3,5-dichloropyridyloxy)]–benzene, a CAR-specific ligand, at 3 mg/kg results in the permanent induction of P450 gene expression in adult mouse liver by alterations of epigenetic memory, such as histone modifications. Neonatal activation of CAR leads to a permanent increase of histone 3 lysine 4 mono-, di-, and trimethylation and decrease of H3K9 trimethylation within the Cyp2b10 locus. Transcriptional coactivator ASC-2 and histone demethylase JMJD2d participate in this CAR-dependent alteration of epigenetic memory. Phenobarbital is a well-known CAR activator (Honkakoski et al., 1998; Sueyoshi et al., 1999). Activation of CAR and alteration of epigenetic memory may be involved in the permanent induction of P450 expression in adult mouse liver by neonatal treatment to phenobarbital, but the alteration of epigenetic memory by activated CAR may be dependent on the dose of phenobarbital and the age of treatment at early life.
In the current study, we demonstrate that phenobarbital treatment at early life, the neonatal and infant ages, has the maximal influence on P450 induction in adult liver but much less at later developmental ages, indicating that there is a sensitive time window for the persistent induction of P450s in adult liver by phenobarbital treatment. As we demonstrated previously, the expression of most P450s markedly changes during early life (Peng et al., 2012), which is also the most rapidly growing stage in postnatal liver maturation. In this period, cell proliferation in mouse liver is high and then the hepatic architecture begins to resemble that of adult liver. This period of time may be critical to establish epigenetic memory and the ontogenic transcriptome profile in liver (Apte et al., 2007). Any treatment with compounds, which have the ability to alter epigenetic memory at early life as the liver is maturing, may influence the transcriptional regulation of important drug metabolizing enzymes in liver and impact drug metabolism in later life. However, molecular mechanisms involved in these processes need to be investigated in future studies.
Many drugs used in neonates and infants have the ability to activate nuclear receptors and further induce expression of P450s, such as activation of CAR by phenytoin (Wang et al., 2004), pregnane X receptor by rifampicin (Goodwin et al., 2001), GR by dexamethasone (Onica et al., 2008), and aryl hydrocarbon receptor by omeprazole (Gerbal-Chaloin et al., 2006). Considering that millions of neonates and infants have been treated with these drugs, there may be a general phenomenon that neonatal drug treatment can result in the persistent elevation of P450 activities in adult liver. The current study provides evidence to support the persistent activation of P450s in adult liver by neonatal exposure to phenobarbital. A previous study also shows that neonatal exposure to dexamethasone, which can activate GR, has long-term consequences on induction of expression of P450 enzymes involved in bile acid metabolism in adult life (Liu et al., 2008). These studies may stimulate research to systematically evaluate the short- and long-term impacts on gene expression and enzyme activities of P450s in liver after drug treatment at early life. It has been widely accepted that increases in drug metabolism can decrease therapeutic efficacy and increase drug-induced toxicity (Kir et al., 2011; Madian et al., 2012). The current study may stimulate further studies to examine the potential long-term impacts on therapeutic efficacy and drug-induced toxicity in adult life caused by drug treatment at early life.
Abbreviations
- BSA
body surface area
- CAR
constitutive androstane receptor
- GR
glucocorticoid receptor
- MPB
mobile phase B
- P450
cytochrome P450
Authorship Contributions
Participated in research design: Tien, Ma, and Zhong.
Conducted experiments: Tien, Liu, Pope, and Wang.
Performed data analysis: Tien, Ma, and Zhong.
Wrote or contributed to the writing of the manuscript: Tien, Liu, Pope, Wang, Ma, and Zhong.
Footnotes
This study was supported by the National Institutes of Health National Institute for Environmental Health Science [Grant R01ES-019487] (to X.B.Z), National Institute of General Medical Sciences [Grant R01GM-087376] (to X.B.Z), and National Institute for Diabetes and Digestive and Kidney Diseases [Grant R01DK090305] (to X.M).
References
- Agrawal AK, Pampori NA, Shapiro BH. (1995) Neonatal phenobarbital-induced defects in age- and sex-specific growth hormone profiles regulating monooxygenases. Am J Physiol 268:E439–E445. [DOI] [PubMed] [Google Scholar]
- Agrawal AK, Shapiro BH. (1996a) Imprinted overinduction of hepatic CYP2B1 and 2B2 in adult rats neonatally exposed to phenobarbital. J Pharmacol Exp Ther 279:991–999. [PubMed] [Google Scholar]
- Agrawal AK, Shapiro BH. (1996b) Phenobarbital induction of hepatic CYP2B1 and CYP2B2: pretranscriptional and post-transcriptional effects of gender, adult age, and phenobarbital dose. Mol Pharmacol 49:523–531. [PubMed] [Google Scholar]
- Agrawal AK, Shapiro BH. (2000) Latent overexpression of hepatic CYP2C7 in adult male and female rats neonatally exposed to phenobarbital: a developmental profile of gender-dependent P450s. J Pharmacol Exp Ther 293:1027–1033. [PubMed] [Google Scholar]
- Agrawal AK, Shapiro BH. (2005) Neonatal phenobarbital imprints overexpression of cytochromes P450 with associated increase in tumorigenesis and reduced life span. FASEB J 19:470–472. [DOI] [PubMed] [Google Scholar]
- Apte U, Zeng G, Thompson MD, Muller P, Micsenyi A, Cieply B, Kaestner KH, Monga SP. (2007) beta-Catenin is critical for early postnatal liver growth. Am J Physiol Gastrointest Liver Physiol 292:G1578–G1585. [DOI] [PubMed] [Google Scholar]
- Bartha AI, Shen J, Katz KH, Mischel RE, Yap KR, Ivacko JA, Andrews EM, Ferriero DM, Ment LR, Silverstein FS. (2007) Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol 37:85–90. [DOI] [PubMed] [Google Scholar]
- Blanchard OL, Smoliga JM. (2015) Translating dosages from animal models to human clinical trials--revisiting body surface area scaling. FASEB J 29:1629–1634. [DOI] [PubMed] [Google Scholar]
- Carmo KB, Barr P. (2005) Drug treatment of neonatal seizures by neonatologists and paediatric neurologists. J Paediatr Child Health 41:313–316. [DOI] [PubMed] [Google Scholar]
- Chang SY, Li W, Traeger SC, Wang B, Cui D, Zhang H, Wen B, Rodrigues AD. (2008) Confirmation that cytochrome P450 2C8 (CYP2C8) plays a minor role in (S)-(+)- and (R)-(-)-ibuprofen hydroxylation in vitro. Drug Metab Dispos 36:2513–2522. [DOI] [PubMed] [Google Scholar]
- Chen WD, Fu X, Dong B, Wang YD, Shiah S, Moore DD, Huang W. (2012) Neonatal activation of the nuclear receptor CAR results in epigenetic memory and permanent change of drug metabolism in mouse liver. Hepatology 56:1499–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ferguson SS, Negishi M, Goldstein JA. (2004) Induction of human CYP2C9 by rifampicin, hyperforin, and phenobarbital is mediated by the pregnane X receptor. J Pharmacol Exp Ther 308:495–501. [DOI] [PubMed] [Google Scholar]
- de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. (1999) Cytochrome P450 3A: ontogeny and drug disposition. Clin Pharmacokinet 37:485–505. [DOI] [PubMed] [Google Scholar]
- Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. (2004) Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol 44:499–523. [DOI] [PubMed] [Google Scholar]
- Gerbal-Chaloin S, Pichard-Garcia L, Fabre JM, Sa-Cunha A, Poellinger L, Maurel P, Daujat-Chavanieu M. (2006) Role of CYP3A4 in the regulation of the aryl hydrocarbon receptor by omeprazole sulphide. Cell Signal 18:740–750. [DOI] [PubMed] [Google Scholar]
- Gervot L, Rochat B, Gautier JC, Bohnenstengel F, Kroemer H, de Berardinis V, Martin H, Beaune P, de Waziers I. (1999) Human CYP2B6: expression, inducibility and catalytic activities. Pharmacogenetics 9:295–306. [PubMed] [Google Scholar]
- Goodwin B, Hodgson E, Liddle C. (1999) The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol 56:1329–1339. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Moore LB, Stoltz CM, McKee DD, Kliewer SA. (2001) Regulation of the human CYP2B6 gene by the nuclear pregnane X receptor. Mol Pharmacol 60:427–431. [PubMed] [Google Scholar]
- Gorski JC, Vannaprasaht S, Hamman MA, Ambrosius WT, Bruce MA, Haehner-Daniels B, Hall SD. (2003) The effect of age, sex, and rifampin administration on intestinal and hepatic cytochrome P450 3A activity. Clin Pharmacol Ther 74:275–287. [DOI] [PubMed] [Google Scholar]
- Gustafson DL, Long ME, Bradshaw EL, Merz AL, Kerzic PJ. (2005) P450 induction alters paclitaxel pharmacokinetics and tissue distribution with multiple dosing. Cancer Chemother Pharmacol 56:248–254. [DOI] [PubMed] [Google Scholar]
- Handschin C, Meyer UA. (2003) Induction of drug metabolism: the role of nuclear receptors. Pharmacol Rev 55:649–673. [DOI] [PubMed] [Google Scholar]
- Hart SN, Cui Y, Klaassen CD, Zhong XB. (2009) Three patterns of cytochrome P450 gene expression during liver maturation in mice. Drug Metab Dispos 37:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkakoski P, Zelko I, Sueyoshi T, Negishi M. (1998) The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol 18:5652–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Miyajima A. (2002) Cytokine regulation of liver development. Biochim Biophys Acta 1592:303–312. [DOI] [PubMed] [Google Scholar]
- Kir S, Kliewer SA, Mangelsdorf DJ. (2011) Roles of FGF19 in liver metabolism. Cold Spring Harb Symp Quant Biol 76:139–144. [DOI] [PubMed] [Google Scholar]
- Koukouritaki SB, Manro JR, Marsh SA, Stevens JC, Rettie AE, McCarver DG, Hines RN. (2004) Developmental expression of human hepatic CYP2C9 and CYP2C19. J Pharmacol Exp Ther 308:965–974. [DOI] [PubMed] [Google Scholar]
- Lam JL, Jiang Y, Zhang T, Zhang EY, Smith BJ. (2010) Expression and functional analysis of hepatic cytochromes P450, nuclear receptors, and membrane transporters in 10- and 25-week-old db/db mice. Drug Metab Dispos 38:2252–2258. [DOI] [PubMed] [Google Scholar]
- Lanska MJ, Lanska DJ, Baumann RJ, Kryscio RJ. (1995) A population-based study of neonatal seizures in Fayette County, Kentucky. Neurology 45:724–732. [DOI] [PubMed] [Google Scholar]
- Lee SM, Chung JY, Lee YM, Park MS, Namgung R, Park KI, Lee C. (2012) Effects of cytochrome P450 (CYP)2C19 polymorphisms on pharmacokinetics of phenobarbital in neonates and infants with seizures. Arch Dis Child 97:569–572. [DOI] [PubMed] [Google Scholar]
- Li Y, Cui Y, Hart SN, Klaassen CD, Zhong XB. (2009) Dynamic patterns of histone methylation are associated with ontogenic expression of the Cyp3a genes during mouse liver maturation. Mol Pharmacol 75:1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Lu AY. (2001) Interindividual variability in inhibition and induction of cytochrome P450 enzymes. Annu Rev Pharmacol Toxicol 41:535–567. [DOI] [PubMed] [Google Scholar]
- Liu Y, Havinga R, Van Der Leij FR, Boverhof R, Sauer PJ, Kuipers F, Stellaard F. (2008) Dexamethasone exposure of neonatal rats modulates biliary lipid secretion and hepatic expression of genes controlling bile acid metabolism in adulthood without interfering with primary bile acid kinetics. Pediatr Res 63:375–381. [DOI] [PubMed] [Google Scholar]
- Ma X, Shah Y, Cheung C, Guo GL, Feigenbaum L, Krausz KW, Idle JR, Gonzalez FJ. (2007) The PREgnane X receptor gene-humanized mouse: a model for investigating drug-drug interactions mediated by cytochromes P450 3A. Drug Metab Dispos 35:194–200. [DOI] [PubMed] [Google Scholar]
- Madian AG, Wheeler HE, Jones RB, Dolan ME. (2012) Relating human genetic variation to variation in drug responses. Trends Genet 28:487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier T, Güell M, Serrano L. (2009) Correlation of mRNA and protein in complex biological samples. FEBS Lett 583:3966–3973. [DOI] [PubMed] [Google Scholar]
- Onica T, Nichols K, Larin M, Ng L, Maslen A, Dvorak Z, Pascussi JM, Vilarem MJ, Maurel P, Kirby GM. (2008) Dexamethasone-mediated up-regulation of human CYP2A6 involves the glucocorticoid receptor and increased binding of hepatic nuclear factor 4 alpha to the proximal promoter. Mol Pharmacol 73:451–460. [DOI] [PubMed] [Google Scholar]
- Peng L, Yoo B, Gunewardena SS, Lu H, Klaassen CD, Zhong XB. (2012) RNA sequencing reveals dynamic changes of mRNA abundance of cytochromes P450 and their alternative transcripts during mouse liver development. Drug Metab Dispos 40:1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. (2008) Dose translation from animal to human studies revisited. FASEB J 22:659–661. [DOI] [PubMed] [Google Scholar]
- Rekka E, Ayalogu EO, Lewis DF, Gibson GG, Ioannides C. (1994) Induction of hepatic microsomal CYP4A activity and of peroxisomal beta-oxidation by two non-steroidal anti-inflammatory drugs. Arch Toxicol 68:73–78. [DOI] [PubMed] [Google Scholar]
- Renaud HJ, Cui JY, Khan M, Klaassen CD. (2011) Tissue distribution and gender-divergent expression of 78 cytochrome P450 mRNAs in mice. Toxicol Sci 124:261–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen GM, Penney S, Andrews W. (1999) The epidemiology of clinical neonatal seizures in Newfoundland: a population-based study. J Pediatr 134:71–75. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. (1999) The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J Biol Chem 274:6043–6046. [DOI] [PubMed] [Google Scholar]
- Tanaka E. (1999) Gender-related differences in pharmacokinetics and their clinical significance. J Clin Pharm Ther 24:339–346. [DOI] [PubMed] [Google Scholar]
- van Rooij LG, Hellström-Westas L, de Vries LS. (2013) Treatment of neonatal seizures. Semin Fetal Neonatal Med 18:209–215. [DOI] [PubMed] [Google Scholar]
- Vogel C, Marcotte EM. (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Faucette S, Moore R, Sueyoshi T, Negishi M, LeCluyse E. (2004) Human constitutive androstane receptor mediates induction of CYP2B6 gene expression by phenytoin. J Biol Chem 279:29295–29301. [DOI] [PubMed] [Google Scholar]