Abstract
Adolescent development includes changes in the biological regulatory processes for the timing of sleep. Circadian rhythm changes and changes to the sleep-pressure system (sleep homeostasis) during adolescence both favor later timing of sleep. These changes, combined with prevailing social pressures, are responsible for most teens sleeping too late and too little; those who sleep least report consuming more caffeine. Although direct research findings are scarce, the likelihood of use and abuse of caffeine-laden products grows across the adolescent years due, in part, to excessive sleepiness.
Keywords: adolescent development, caffeine, circadian rhythms, sleep pressure, sleep
Introduction
Sleep phenomenology and sleep patterns change markedly in association with pubertal development and across the second decade of life. Parents often note a switch-like shift from an early-to-bed and early-to-rise fourth or fifth grader to a late-to-bed and impossible-to-wake middle-school/high-school student. Some attribute these changes to willful behavioral “acting out”; others sense a deeper change that feels less volitional and more a response to internal processes. Changes in sleep behavior often accompany deteriorating behavioral regulation, such as impulsivity and risk taking, irritability, negative thoughts and feelings, and fatigue. The changes in sleep behaviors across development can be attributed to two major factors: 1) environmental/psychosocial context and 2) bioregulatory processes. The goal of this article is to describe developmental changes in the bioregulatory processes that underlie sleep behavior and discuss how this biology interacts with the child's environment, leading to poor sleep and consequent behavioral dysregulation that may accompany exaggerated caffeine consumption.
Sleep Regulation
The biological regulation of sleep is controlled by two principal processes that function independent of one another and interact to influence the length and timing of sleep. This dual-process model of sleep regulation1 (Figure 1) is well known and has been examined across the second decade of life, providing insight into the organization of sleep behavior in adolescents. The circadian timing system (process C) is an intrinsic mechanism seated in the suprachiasmatic nucleus of the hypothalamus that regulates the daily timing of many physiological processes in addition to sleep. Process C includes a molecular clock mechanism that oscillates with a near 24-hour period (circa dies) and is responsive to light input for synchronization to the environment. It is important to understand that the response to light is phase dependent. That is, light signals that impinge on the clock in the evening and first part of the night tend to produce a phase delay (push timing, including sleep onset, later), whereas light signals in the late night and early morning tend to produce a phase advance (pull timing, including wake time, earlier). Under normal circumstances, these responses keep the clock set to a 24-hour day. The circadian timing system includes an underlying clock-dependent alerting feature that supports arousal late in our waking day; by the same token, the clock provides strong sleep pressure late in the night.
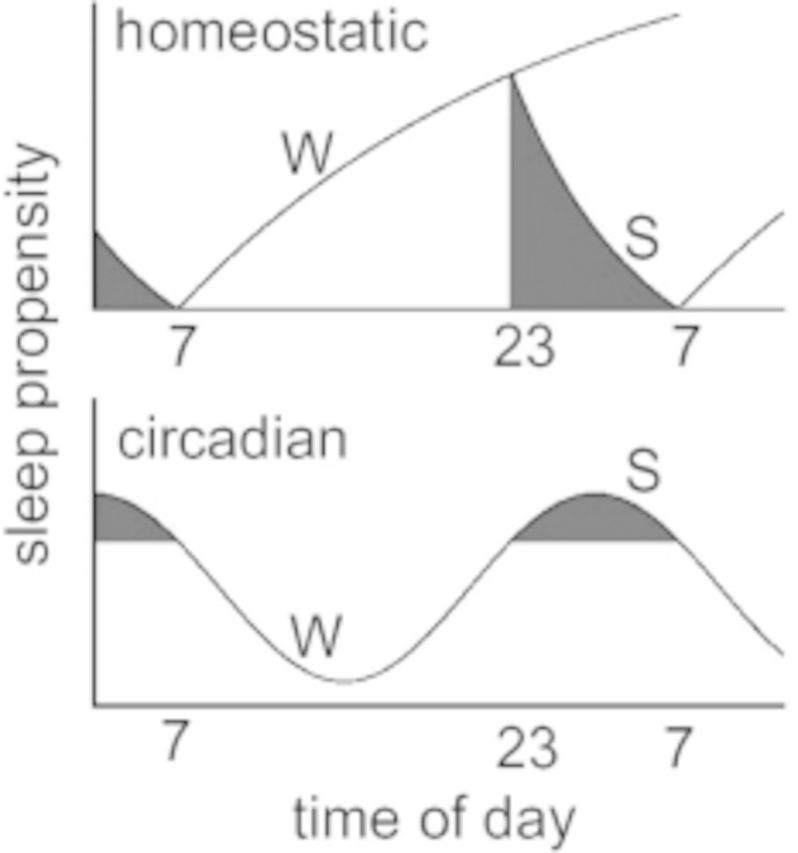
Schematic of the two-process model proposed by Borbély (1982).1 The top panel shows the dynamics of the homeostatic process. As shown, sleep pressure builds during waking (W) and dissipates during sleep (S). The circadian system is shown on the bottom panel and oscillates with a rhythm of approximately 24 h.
Interacting with the clock timing is process S, known as the sleep homeostatic system or in common parlance, the sleep pressure system. Though typically experienced as a daily function (since humans usually sleep once a day), this system is not timed to 24 hours, but relies on the length of the waking episodes to produce sleep pressure and the length of sleep to dissipate that sleep pressure. Hence, the longer humans extend their waking hours, the greater the buildup of sleep pressure; conversely, as people sleep, sleep pressure is expended and a recovery process occurs. Both sides of process S occur with nonlinear functions that can be modeled mathematically. Derived components of these functions include the decay-time constant, which estimates the rate of “recovery” across the night, and the rise-time constant, which estimates the rate of accumulation of sleep pressure across the day.
The ideal pattern of interaction between these regulatory systems is for a clock-dependent alerting function to offset the wake-dependent rise of sleep pressure in the later portion of a normal waking day. Conversely, a clock-dependent increase of sleep pressure late in the circadian night helps to support the continuation of sleep as sleep pressure diminishes during sleep. When the processes are aligned, sleeping and waking are well regulated. When the processes are misaligned, for example, with jet lag, neither sleeping nor waking is optimal. The ways in which these processes change across adolescence and how these changes may impact caffeine utilization are examined here with these concepts in mind.
Circadian timing system
As noted above, a principal feature of adolescent sleep is a marked trend for the timing of sleep to occur later in the day. Changes to the circadian timing system in the juvenile phase are not confined to humans. A review by Hagenauer et al.2 identified a nonhuman primate and several rodents that display similar features and provide evidence for some underlying mechanisms. In human adolescence, a delay is observed in the preferred timing of many activities,3 a profound delay in the timing of weekend sleep across the second decade,4 and a later timing of a biological marker of the internal circadian clock, the time at which melatonin secretion occurs.5 Melatonin is a hormone secreted by the pineal gland during the biological night and is under the regulation of the circadian timing mechanism. Because melatonin can be measured reliably in saliva samples, it is a relatively convenient way to assess the output of the central regulatory process. Golub et al.6 showed that activity offset (sleep start) moves later in female rhesus monkeys as they enter puberty, but this behavioral change is eliminated if puberty is inhibited by dietary zinc deprivation.
The question remains, however: What factors underlie these behavioral manifestations of a phase delay? One way to alter the circadian timing system is to modify light exposures, and human adolescents are champions at this. By staying up later, they expose the central clock to light at a time favoring phase delay; by the same token, adolescents shield themselves from morning, phase-advancing light with great effectiveness. Evidence from juvenile mice indicates that the sensitivity of the circadian timing system to the phase-shifting effects of light can change; thus, juvenile mice showed an exaggerated delay response to evening light relative to adult animals.7 Another mechanism that may affect timing of the circadian clock is the internal day length, i.e., the period of the intrinsic circadian rhythm. One study showed that period is longer in human adolescents than in adults.8 A longer intrinsic period (i.e., a longer internal day length) would facilitate staying up later in the evening. Synchronization of the internal rhythm to the environment is still possible because bright light in the morning can reset the rhythm each day, yet the longer intrinsic period supports a later bedtime. This complex process is explained in Hagenauer et al.2 and its implications are shown in Figure 2. Finally, another feature of the circadian timing system that may affect behavioral timing is the amplitude of the rhythm, specifically the amplitude of melatonin secretion. The circadian timing system provides an arousal signal in the late afternoon/early evening, and one function of melatonin is thought to be that of dampening this arousal. A recent paper showed that melatonin levels during the night decrease across puberty.9 Thus, if the amplitude of the circadian melatonin rhythm is lower, then the arousal signal may not be attenuated, thus interfering with falling asleep.
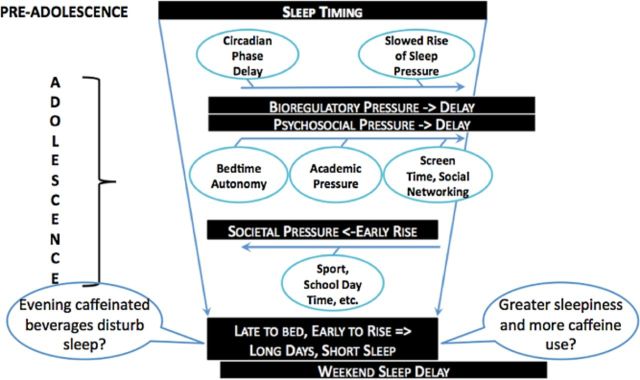
Changes in the timing and amount of sleep that occur during adolescence. The preadolescent sleep pattern is long and occurs at a relatively early time. Bioregulatory and psychosocial patterns push sleep to a later timing, though the need for sleep stays the same; therefore, an ideal circumstance would be sleeping the same amount as the preadolescent simply at a later time. Unfortunately for the typical adolescent, the school system mandates an earlier rise time on school days, leading to both later and shorter sleep. Weekend sleep patterns manifest the “social jet lag” resulting from later timing of sleep and significant lengthening of sleep in order to recover from week-day short sleep. The impact of caffeinated beverages on nocturnal sleep and the influence of nocturnal sleep on caffeinated beverage consumption represent gaps in research. Modified from Carskadon (2011).24
To summarize, observed phase is later in human, and some nonhuman, adolescents, as evidenced by the timing of sleep/activity behavior, chronotype, and later timing of melatonin secretion. Evidence also indicates that the sensitivity of the circadian timing system to light may differ in adolescence, favoring a greater delay response to evening light. In addition, the internal day length may be longer in adolescents than adults, thus contributing to the phase delay. Furthermore, adolescents experience lower amplitude of the daily rhythm of melatonin secretion, which may dampen the signal for sleep. The result of these changes is that late nights and late mornings are favored.
Sleep homeostatic system
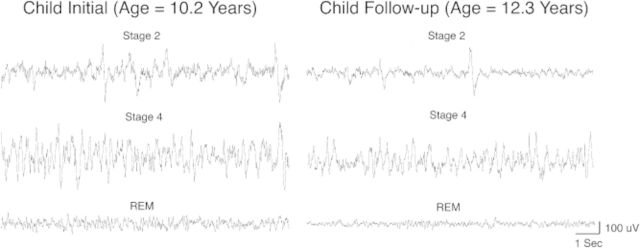
Most of what is known about developmental changes in the sleep homeostatic system comes from research in adolescent humans. This system is best examined through measurement of brain waves (EEG) during sleep, from which parameters of the homeostatic process can be derived. Of note, however, is that the phenomenology of the sleep EEG changes markedly across the second decade of life, when 40–50% of EEG amplitude is lost (Figure 3).11,12 This loss of EEG amplitude results from significant reorganization, indeed resculpting, of the central nervous system during adolescence.13,14 The two most prominent features of this developmental change are the diminution of cortical thickness and cortical synaptic density, and the increase in white matter and myelinated fiber tracts.13 The “pruning” of cortical synapses is thought to be the source of the change in EEG amplitude.15 These features of the adolescent central nervous system, however, do not necessarily impact sleep regulation. Indeed, studies examining the regulatory function show an interesting pattern whereby the recovery process (dissipation of sleep pressure across the night) does not change across adolescence16–18; however, the buildup of sleep pressure with waking shows a significant slowing during adolescent development.16 Another way to examine this process is to evaluate the speed of falling asleep as waking is extended, which was done in one study finding that early adolescents fall asleep significantly faster than more mature adolescents after about 14 to 16 hours of being awake.19
Developmental decline in sleep EEG amplitude that occurs during adolescence. Thirty seconds of EEG tracings in three different sleep stages are shown for a male who was initially recorded at the age of 10.2 years old and again a few years later at the age of 12.3 years. The diminished amplitude can be seen in all sleep stages, suggesting that this decline reflects a large-scale change in brain structure, namely the synaptic pruning that occurs during this developmental stage. Reproduced from Tarokh et al. (2011)10 with permission.
With regard to the homeostatic process then, current research indicates that sleep's dissipation (recovery) system is not noticeably modified across adolescent development.16–18 Hence, the need for sleep may stay at a similar level for early and late adolescents. On the other hand, evidence indicates that adolescent development is accompanied by a slowed buildup rate of sleep pressure across the waking hours.16 As shown by the findings of Taylor et al.,19 this slowing of sleep pressure accumulation appears to be permissive of or facilitate staying awake longer as children pass through adolescence. Thus, staying awake longer is easier, but the need for sleep is unchanged.
Adolescent Sleep Behavior and the Psychosocial Context
When adolescents are queried about their sleep schedules, their responses mirror the maturation of these bioregulatory mechanisms, particularly for weekend schedules. Thus, for example, the National Sleep Foundation's Sleep in America poll20 of sleep patterns for youngsters in grades 6 through 12 showed that the average reported weekend bedtime was 10:31 pm in grade six, 11:05 pm in grade 7, 11:26 pm in grade eight, 11:53 pm in grade 9, 12:03 am in grade 10, 12:25 am in grade 11, and 12:45 am in grade 12. Average rising times reported by students for weekend mornings were as early as 8:53 am in grade 6 and as late as 10:06 am in grade 11. A stark contrast is reported for sleep on school nights, however. Whereas reported bedtimes delay from an average of 9:24 pm in grade 6 to 11:02 pm in grade 12, the rising time does not get later; if anything, it occurs a bit earlier. Thus, average rising time is latest in grade 6 at 6:42 am and as early as 6:23 am in grades 10 and 11 and 6:31 am in grade 12. The amount of sleep acquired on school nights, therefore, dips markedly across this age range, from 8.4 hours a night in grade 6 students to 6.9 hours in grade 12. Across this range of adolescents, the amount of sleep reported on weekend nights averages about 1 to 1.5 hours longer, with sleep onset occurring between 1 hour and nearly 2 hours later. This phenomenon of short, early sleep on weekdays or schooldays and later, longer sleep on weekends has been called weekend sleep lag5 or social jet lag,21 with serious behavioral and health consequences.
Weekend lag is worst in adolescents living in communities in which the middle school and high school have an early first bell, because it is the school schedule that drives the sleep patterns of many adolescents. One study found that for students in grade 10 who had a first bell time of 7:20 am, the average amount of sleep measured with wrist-worn activity monitors was about 7 hours a night.5 Although sleep need is difficult to define, most scientists and clinicians agree that the optimal range for adolescents is 8.5 to 9.5 hours. Thus, most teens receive significantly less sleep than they need. In these youngsters, a measure of physiological sleepiness showed significant impairment, particularly in the morning. Furthermore, about 50% of these teens showed an abnormal pattern when falling asleep: not only did they fall asleep rapidly (within 2 min), they also experienced sleep-onset REM sleep episodes. This pattern is typical of a major sleep disorder, narcolepsy, and not of a healthy sleep pattern of teens. This excessive sleepiness and anomalous sleep-onset pattern was associated with teens getting too little sleep and waking up too early in their circadian cycle because they were phase delayed.
In summary, the negative consequences of the interactions of these sleep bioregulatory changes and adolescents’ lifestyles play out in many teenagers who manifest variable sleep timing, chronic insufficient sleep, excessive sleepiness, deficits in mood, learning, and impulse control, as well as illness.22 The proliferation of computer and smart phone use in the evening has exacerbated the sleep delay and sleep reduction in adolescents.23 Furthermore, it is known that the delayed and short sleep pattern is associated with increased caffeine use, as shown, for example, in the National Sleep Foundation poll, in which teens who reported sleeping less reported consuming more caffeinated beverages.20 Of interest, students who also reported falling asleep in school reported more caffeine use than those who did not fall asleep, indicating that the caffeine was not an effective countermeasure for excessive sleepiness.
Conclusion
Evidence from parental reports and adolescent self-reports, as well as from laboratory studies, converge to indicate that the sleep behaviors of many adolescents are not optimal (both in amount and timing) to support cognitive function, mental health, and physical well-being. These findings also provide strong evidence that adolescents are primed to consume caffeinated beverages at an increasing rate if public health interventions to moderate these extreme sleep patterns are not implemented. These concerns raise the importance of research to identify the interaction of caffeinated energy drink use with sleep and circadian timing in adolescence. Figure 2 illustrates the changes in sleep regulation and psychosocial environment affecting sleep behavior during adolescence and delineates some critical research questions regarding the interaction of these changes with caffeinated beverages. Current gaps in the science include answers to the following questions: What effect do caffeinated energy drinks have on nighttime sleep? Do caffeinated energy drinks affect circadian rhythms? What is the impact of such beverages on alertness, performance, attention, and learning acquisition in well-slept and insufficiently slept teens? Finally, do these beverages impair the sleep-related consolidation, stabilization, and strengthening of learning?
Acknowledgments
Funding
Dr. Carskadon's research has been supported by National Institutes of Health grants MH52415, MH01358, MH58879, MH076969, NR08381.
Declaration of interest
The authors have no relevant interests to declare.
References
- Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Hagenauer MH, Perryman JI, Lee TM, et al. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, et al. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–R1039. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Wolfson AR, Acebo C, et al. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21:871–881. [DOI] [PubMed] [Google Scholar]
- Golub MS, Takeuchi PT, Hoban-Higgins TM. Nutrition and circadian activity offset in adolescent rhesus monkeys. In: Carskadon MA, ed. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge: Cambridge University Press; 2002:50–68. [Google Scholar]
- Weinert D, Kompauerova V. Light induced phase and period responses of circadian activity rhythms in laboratory mice of different age. Zoology. 1998;101:45–52. [Google Scholar]
- Carskadon MA, Acebo C. Intrinsic circadian period in adolescents versus adults from forced desynchrony. Sleep. 2005;28(Suppl):A71. [Google Scholar]
- Crowley SJ, Acebo C, Carskadon MA. Human puberty: salivary melatonin profiles in constant conditions. Dev Psychobiol. 2012;54:468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Carskadon MA, Achermann P. Trait-like characteristics of the sleep EEG across adolescent development. J Neurosci. 2011;31:6371–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci U S A. 2009;106:5177–5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Carskadon MA. Developmental changes in the human sleep EEG during early adolescence. Sleep. 2010;33:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. [DOI] [PubMed] [Google Scholar]
- Buchmann A, Ringli M, Kurth S, et al. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb Cortex. 2011;21:607–615. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–1454. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Darchia N, Higgins LM, et al. Adolescent changes in homeostatic regulation of EEG activity in the delta and theta frequency bands during NREM sleep. Sleep. 2011;34:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Carskadon MA, Achermann P. Dissipation of sleep pressure is stable across adolescence. Neuroscience. 2012;216:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DJ, Jenni OG, Acebo C, et al. Sleep tendency during extended wakefulness: insights into adolescent sleep regulation and behavior. J Sleep Res. 2005;14:239–244. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation Sleep In America Poll Summary Findings . 2006. Available at: http://sleepfoundation.org/media-center/press-release/sleep-america-poll-summary-findings. Accessed August 28, 2014.
- Wittmann M, Dinich J, Merrow M, et al. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23:497–509. [DOI] [PubMed] [Google Scholar]
- Orzech KM, Acebo C, Seifer R, et al. Sleep patterns are associated with common illness in adolescents. J Sleep Res. 2014;23:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain N, Gradisar M. Electronic media use and sleep in school-aged children and adolescents: a review. Sleep Med. 2010;11:735–742. [DOI] [PubMed] [Google Scholar]
- Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. 2011;58:637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]