Abstract
AMP-activated protein kinase (AMPK), an important downstream effector of the tumor suppressor liver kinase 1 (LKB1) and pharmacologic target of metformin, is well known to exert a preventive and inhibitory effect on tumorigenesis; however, its role in cancer progression and metastasis has not been well characterized. The present study investigates the potential roles of AMPK in inhibiting cancer-cell migration and epithelial-to-mesenchymal transition (EMT) by regulating the canonical transforming growth factor β (TGF-β) signaling pathway, an important promoting factor for cancer progression. Our results showed that activation of AMPK by metformin inhibited TGF-β–induced Smad2/3 phosphorylation in cancer cells in a dose-dependent manner. The effect of metformin is dependent on the presence of LKB1. A similar effect was obtained by expressing a constitutive active mutant of AMPKα1 subunit, whereas the expression of a dominant negative mutant of AMPKα1 or ablation of AMPKα subunits greatly enhanced TGF-β stimulation of Smad2/3 phosphorylation. As a consequence, expression of genes downstream of Smad2/3, including plasminogen activator inhibitor-1, fibronectin, and connective tissue growth factor, was suppressed by metformin in a LKB1-dependent fashion. In addition, metformin blocked TGF-β–induced inteleukin-6 expression through both LKB1-dependent and -independent mechanisms. Our results also indicate that activation of LKB1/AMPK inhibits TGF-β–stimulated cancer cell migration. Finally, TGF-β induction of EMT was inhibited by phenformin and enhanced by knockdown of LKB1 expression with shRNA. Together, our data suggest that AMPK could be a drug target for controlling cancer progression and metastasis.
Introduction
AMP-activated protein kinase (AMPK) acts downstream of the tumor suppressor liver kinase 1 (LKB1) to regulate the metabolism and growth of cancer cells (Luo et al., 2005, 2010; Shackelford and Shawe, 2009). Loss-of-function mutation of LKB1 accounts for the development and pathogenesis of Peutz-Jeghers syndrome, an autosomal dominant genetic disorder, featured by multiple hamartomatous polyps in the gastrointestinal tract (Alessi et al., 2006). Somatic mutations of the lkb1 gene have been found in approximately 34% of lung adenocarcinomas, 19% of squamous cell carcinomas, 20% cervical carcinomas, and sporadically in other cancers (Luo et al., 2010). In addition, ablation of LKB1 on the context of the oncogenic vras transgene promotes cancer metastasis in animals (Carretero et al., 2010). Evidence has shown that tumor with a hypomorphic mutation of LKB1 becomes resistant to the AMPK activator metformin, supporting the notion that the tumor-suppressive function of LKB1 is mediated by AMPK (Huang et al., 2008). Consistent with this notion, a retrospective investigation has reported that the incidence of cancer is significantly reduced in patients with type 2 diabetes taking metformin (Evans et al., 2005). Furthermore, clinical studies have demonstrated that AMPK activity is reduced in advanced breast cancer (Shen et al., 2002; Hadad et al., 2009). Intriguingly, it has been reported that breast cancer patients concurrently having type 2 diabetes with metformin as a neoadjuvant exhibit a higher pathologic complete response rate than those with other glucose-lowering medicine and that the latter have a trend to distant metastasis (Jiralerspong et al., 2009; Bayraktar et al., 2012).
Transforming growth factor-β (TGF-β), which inhibits tumor growth in early stages of tumorigenesis, promotes tumor progression in late stages (Drabsch and ten Dijke, 2012). The mechanism of promoting cancer progression by TGF-β is rather complex. First, TGF-β plays an important role in regulating epithelial to mesenchymal transition (EMT), a critical step for cancer stem cell formation and cancer metastasis (Singh and Settleman, 2010) in which TGF-β downregulates claudins, occludins, and ZO1, followed by degradation of tight junction (Moustakas and Heldin, 2007). Phosphorylated R-SMADS associated with EMT-related transcription factors such as transcription repressors for E-cadherin, high-mobility group AT-hook 2, and zinc finger E-box binding homeobox 1 upregulate EMT modulators (Xu et al., 2009; Fuxe et al., 2010). In addition, TGF-β regulates many genes involved in metastasis. In estrogen receptor-negative primary breast tumors, for instance, TGF-β stimulates angiopoietin-like 4, which is associated with metastasis specifically to the lung (Padua et al., 2008). The TGF-β/SMAD signaling pathway also promotes osteoclastogenesis and bone metastasis by inducing pro-osteolytic factors, such as parathyroid hormone–related protein, interleukin 11, chemokine receptor 4, and connective tissue growth factor (CTGF) (Guise et al., 1996; Yin et al., 1999; Muller et al., 2001; Kang et al., 2003).
Recent studies have shown that AMPK inhibits the TGF-β signaling pathway (Mishra et al., 2008; Fisslthaler and Fleming, 2009; Cufi et al., 2010; Xiao et al., 2010; Lim et al., 2012). In addition, AMPK inhibits cancer cell migration and EMT via both TGF-β–dependent and –independent mechanisms (Cufi et al., 2010; Xiao et al., 2010; Lim et al., 2012; Chou et al., 2014; Wang et al., 2014; Zhang et al., 2014; Thakur et al., 2015; Yan et al., 2015). With regard to AMPK regulation of the TGF-β signaling pathway, the mechanism is not clear. In the present study, we interrogated the action of AMPK on the canonical TGF-β signaling pathway in cancer cells, as well as its impact on malignant behavior and EMT. Our results showed that AMPK activation inhibited Smad2/3 phosphorylation, as well as the expression of target genes involved in EMT and metastasis. Accordingly, AMPK activation also suppressed tumor cell migration and EMT. These findings thus indicate that AMPK can be a therapeutic target for cancer metastasis.
Materials and Methods
Materials.
Metformin, phenformin, doxycycline, and 5-amino-4-imidazolecarboxamide riboside (AICAR) were purchased from Sigma-Aldrich (St. Louis, MO). A769962 was from LC Laboratories (Woburn, MA). Human TGF-β1 and antibodies against phospho-smad2/3 (Ser423/425), total smad3, phospho-AMPKα (T172), total AMPKα, and EMT markers were from Cell Signaling Technology, Inc. (Danvers, MA). Monoclonal antibodies against LKB1 and β-actin were from EMD Millipore Corporation (Billerica, MA). Lentiviral construct for LKB1 ShRNA was a gift from Dr. Bin Zheng of Harvard Medical School. Luciferase reporter plasmid for Smad2/3 binding elements was a gift from Dr. Ye-Guang Chen of Tsinghua University.
Cell Culture.
Lung adenocarcinoma A549 cells and mouse embryonic fibroblast (MEF) cells were cultured in 10% fetal bovine serum (FBS)-Dulbecco’s modified Eagle’s medium (DMEM) and human bronchial epithelial BEAS-2B cells in bronchial epithelial cell growth medium (BEGM) (Lonza, Allendale, NJ). Prostate cancer C4-2 cells were cultured in 10% FBS-RPMI 1640 at 37°C and 5% CO2. BEAS-2B cells stably expressing LKB1 shRNA or GFP shRNA were established by infecting lentivirus and selected with puromycin. A549 cells stably expressing LKB1 and C4-2 cells stably expressing a dominant negative mutant of AMPKα1 were established as described previously (Zhou et al., 2009).
Western Blot.
Cell extracts were prepared in lysis buffer (25 mM Tris-HCl, pH 7.8, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, and 25 mM β-glycerol-phosphate, 1 mM DTT, 1% NP-40, and protease inhibitors). Cell debris was removed by centrifugation at 14,000g at 4°C for 15 minutes. Protein concentrations were determined using Bio-Rad Protein Assay kit. Protein samples (25 μg) were subjected to SDS-PAGE and transferred to PVDF membranes (EMD Millipore). The membranes were sequentially blotted with the first and second antibodies, and signals were visualized by the enhanced chemiluminescence (ECL) method (Luo et al., 2013).
Real-Time Polymerase Chain Reaction.
The expression of mRNA was examined by real-time polymerase chain reaction (PCR) with the ABI Step One Plus PCR System using SYBRGREEN PCR Master Mix 2×reagent in 20 μl of reaction volume according to protocol provided by manufacture (Applied Biosystems, Foster City, CA). Primer sequences are listed in Table 1. Each sample was amplified in triplicates and normalized with the expression level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Results were evaluated by the comparative threshold cycle value method (2−ΔΔ Ct) for relative quantification of gene expression (Zhou et al., 2009).
TABLE 1.
Sequences of primers for real time PCR
| qPCR primers | Sequence (5′->3′) |
|---|---|
| PAI-1 | |
| Forward primer | GGGCCATGGAACAAGGATGA |
| Reverse primer | CTCCTTTCCCAAGCAAGTTG |
| IL-6 | |
| Forward primer | GGT ACA TCC TCG ACG GCA TCT |
| Reverse primer | GTG CCT CTT TGC TGC TTT CAC |
| FN | |
| Forward primer | CAG GAT CAC TTA CGG AGA AAC AG |
| Reverse primer | GCC AGT GAC AGC ATA CAC AGT G |
| CTGF | |
| Forward primer | GCTGGAGAAGCAGAGTCGTC |
| Reverse primer | CCACAGAACTTAGCCCGGTA |
| GAPDH | |
| Forward primer | CAGGGCTGCTTTTAACTCTGGT |
| Reverse primer | GATTTTGGAGGGATCTCGCT |
Wound-Healing Assay.
Cells were seeded in six-well plates, and the wells were marked with a straight black line on the bottom. When cell density reached confluence, cells were starved in 0.1% FBS for 8 hours before three scratches across the black line were made in each well with a 200-μl pipette tip. Loose cells were washed with medium. TGF-β1 (5 ng/ml) and/or metformin (10 mM) in fresh medium (0.1% FBS) or fresh medium alone were added to the wells, and the cells were incubated for a given time (indicated in figure legends). Microphotographs were taken on an inverted microscope immediately at scratch (0 hour) and the endpoints of the experiment. Images were aligned using the orientation line to ensure that the identical spots were followed over time. Experiments were conducted in triplicate.
Boyden Chamber Migration Assay.
Cell migration was measured using transwell chambers with 8-μm pore-size membranes (6.5-mm diameter inserted into 24-well plates) according to the protocol provided by the manufacturer (Corning Inc., Corning, NY). The lower chamber was filled with 600 μl of DMEM containing 10% FBS. Cells (1 × 105) suspended in 100 μl of serum-free DMEM were added and evenly distributed onto the upper chamber. After 16 hours of culture, cells remaining on the upper surface of the filters were removed with cotton-tipped applicators, and those on the lower surface were fixed with 100% methanol and stained with crystal violet. The membranes containing stained cells were cut out and mounted on a glass slide. Microphotographs were taken under fluorescent microscope and cell numbers counted. The average number of cells from triplicates represents the number of migrated cells.
Adenovirus Preparation.
cDNA encoding the constitutively active mutant of human AMPKα1 subunit was made by deleting the segment of wildtype cDNA encoding amino acids 313 to 390, tagged with flag epitope, and subcloned to pAdTrack-CMV vector (He et al., 1998). Recombinant adenovirus was prepared and purified using AdEasy Adenoviral Vector System kit and AdEasy virus purification kit (Agilent Technologies, Santa Clara, CA).
Statistical Analysis.
Significance between groups was tested using two-tailed Student’s t test.
Results
AMPK Inhibits Phosphorylation and Transcriptional Activity of Smad2/3.
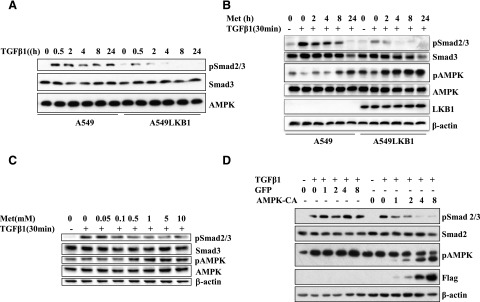
The effects of AMPK activation on the canonical TGF-β signaling pathway were first studied using A549 cells, a human lung adenocarcinoma cell line with a loss-of-function mutation of LKB1, and A549-LKB1 cells in which LKB1 was stably expressed. The cells were treated with TGF-β1 for different periods, and the levels of phosphorylation of Smad2/3 and AMPK were examined. As shown in Fig. 1A, whereas TGF-β1 did not affect Smad2/3 or AMPK expression, it significantly stimulated Smad2/3 phosphorylation in A549 cells. In contrast, the induction of Smad2/3 phosphorylation was barely detectable in A549-LKB1 cells. In A549 cells, metformin failed to activate AMPK, whereas TGF-β potently induced Smad2/3 phosphorylation (Fig. 1B). Ectopic expression of LKB1 (labeled A549-LKB1 cells) restored AMPK activation by metformin, concomitantly with a marked inhibition of TGF-β signaling (Fig. 1B). Inhibition of Smad2/3 phosphorylation by metformin was dose-dependent, as shown in A549-LKB1 cells, where the inhibition of Smad2/3 phosphorylation paralleled the degree of AMPK activation by metformin (Fig. 1C).
Fig. 1.
LKB1 mediates the effect of metformin on Smad2/3 phosphorylation. (A) Time course of TGF-β–induced phosphorylation of Smad2/3. A549 and A549-LKB1 cells were incubated with TGF-β1 (5 ng/ml) for different times. (B) Time-course effect of metformin on TGF-β signaling. The cells in (A) were treated with metformin (10 mM) for different times, followed by TGF-β1 for 30 minutes. (C) Dose effect of metformin on TGF-β signaling. A549-LKB1 cells were treated with metformin at different doses for 2 hours, followed by TGF-β1 for 30 minutes. (D) Effect of active AMPK mutant on TGF-β signaling. A549 cells were infected with different volumes (μl) of adenovirus encoding an active mutant of AMPKα1 subunit tagged with flag epitope (AMPK-CA) or GFP as a control for 2 days and then treated with TGF-β1 for 30 minutes. Equal amounts of cell extracts (25 μg) for all panels were subjected to SDS-PAGE and immunoblots with antibodies as indicated.
To ascertain whether the effect of metformin was mediated by AMPK, we used a fag-tagged active mutant of AMPKα1 subunit where the autoinhibitory domain encompassing amino acids 313–390 was deleted (Crute et al., 1998). Expression of active AMPKα1 subunit in A549 cells via adenoviral vector inhibited TGF-β1-induced Smad2/3 phosphorylation in a dose-dependent fashion, whereas no effect was observed with adenovirus encoding GFP (Fig. 1D).
We next examined the effects of AMPK on TGF-β1 signaling in C4-2 cells, a prostate cancer cell line, and MEF cells with deletion of both AMPKα1 and α2 alleles (Fig. 2). When treated with different doses of metformin, the level of AMPK activation in C4-2 cells was inversely correlated with the levels of TGF-β1–induced Smad2/3 phosphorylation (Fig. 2A). The influence of AMPK on TGF-β1 signaling was further investigated in C4-2 cells by expressing a dominant negative mutant of AMPK α1 (DN-AMPK). Stable expression of DN-AMPK was made by lentiviral vector, and the expression was under the control of the tet-off system (Zhou et al., 2009). When DN-AMPK was expressed in the absence of doxycycline, TGF-β1 significantly induced Smad2/3 phosphorylation (Fig. 2B). The addition of doxycycline, which abrogated DN-AMPK expression and allowed endogenous wild-type AMPK to function, blocked the TGF-β1–induced Smad2/3 phosphorylation (Fig. 2B). Apparently, although phosphosignals of AMPK in the presence or absence of doxycycline appeared to be similar, the ratio of p-AMPK to total AMPK, which included DN-AMPK, was much greater after doxycycline treatment, suggesting that the dominant negative AMPK was not phosphorylated, but it effectively inhibited the function of endogenous AMPK. Finally, we compared the ability of TGF-β1 to stimulate Smad2/3 phosphorylation in MEF cells with and without AMPK. TGF-β–induced Smad2/3 phosphorylation was significantly higher in the cells with no AMPK (Fig. 2C). These results provide evidence that TGF-β signaling is regulated by AMPK.
Fig. 2.
AMPK inhibits Smad2/3 phosphorylation by TGF-β. (A) Dose-dependent inhibition of TGF-β signaling by metformin. C4-2 cells were treated with metformin at different doses, followed by TGF-β1 for 30 minutes. (B) Effect of dominant negative mutant of AMPK on TGF-β signaling. C4-2 cells bearing flag-tagged dominant negative mutant of AMPKα1 under the control of Tet-off system were treated with or without doxycycline (2 μg/ml) for 2 days and then with TGF-β1 for indicated periods. (C) Effect of AMPK ablation on TGF-β signaling. MEF cells, wild-type, and α1α2 knockout were treated with TGFβ1 for different times. Equal amounts of cell extracts (25 μg) were blotted with antibodies as indicated.
AMPK Inhibits Expression of TGF-β Downstream Effectors.
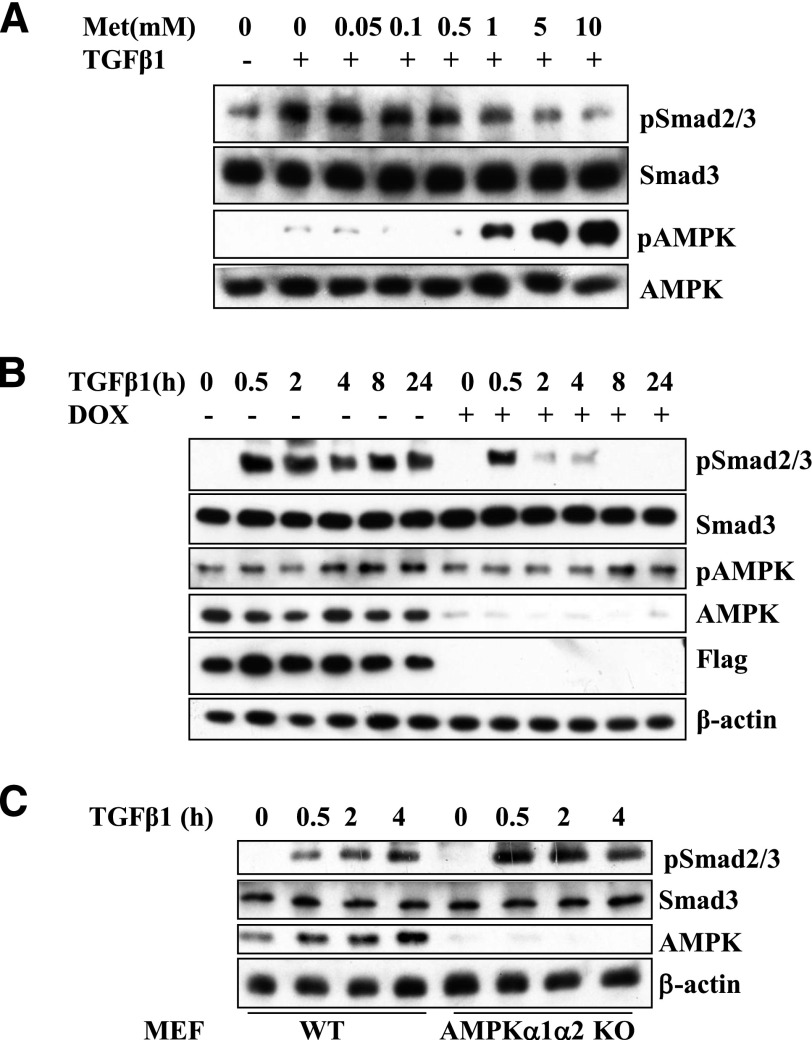
To examine how the expression of genes downstream of Smad2/3 is influenced by AMPK activators in the A549 cells, we performed quantitative PCR to analyze the expression of plasminogen activator inhibitor-1(PAI-1) (Lund et al., 1987), fibronectin (FN) (Ignotz and Massague 1986), CTGF (Igarashi et al., 1993), and interleukin-6 (IL-6) (Elias et al., 1991) (primer sequences are listed in Table 1). A549 and A549-LKB1 cells were treated with metformin with or without TGF-β1 for 8 hours, and the expression of a given gene was determined by real-time qPCR. As shown in Fig. 3, TGF-β1 remarkably upregulated transcription of all four target genes in A549 cells, and metformin did not show significant inhibition of PAI-1, FN, and CTGF. Ectopic expression of LKB1 inhibited TGF-β–induced expression in all four genes (Fig. 3). Remarkably, metformin further suppressed the TGF-β–induced expression in all four genes in A549-LKB1 cells (Fig. 3). These results clearly demonstrate that metformin suppresses gene expression of these TGF-β targets through a LKB1/AMPK-dependent mechanism. Interestingly, metformin was able to inhibit TGF-β-induced IL-6 expression in A549 cells, suggesting that metformin regulates TGF-β–induced expression of IL-6 through both LKB1-dependent and -independent mechanisms.
Fig. 3.
AMPK activation suppresses expression of TGF-β target genes. A549 and A549-LKB1 cells were treated with metformin and/or TGF-β1 for 8 hours. Total RNA was prepared and quantitative real-time PCR conducted on (A) PAI-1, (B) FN, (C) CTGF, and (D) IL-6. The results were normalized with values of GAPDH and expressed as -fold of A549 control (mean ± S.D., n = 3). Significance was tested by Student’s t test (*P > 0.05; **P < 0.05).
AMPK Inhibits Cell Migration in Response to TGF-β.
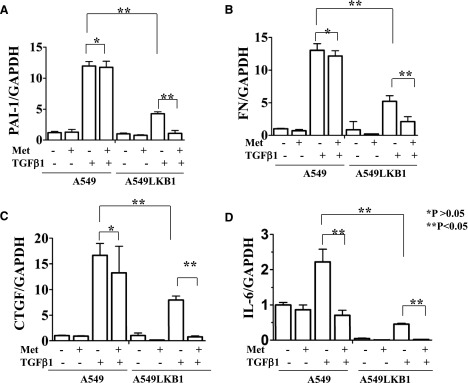
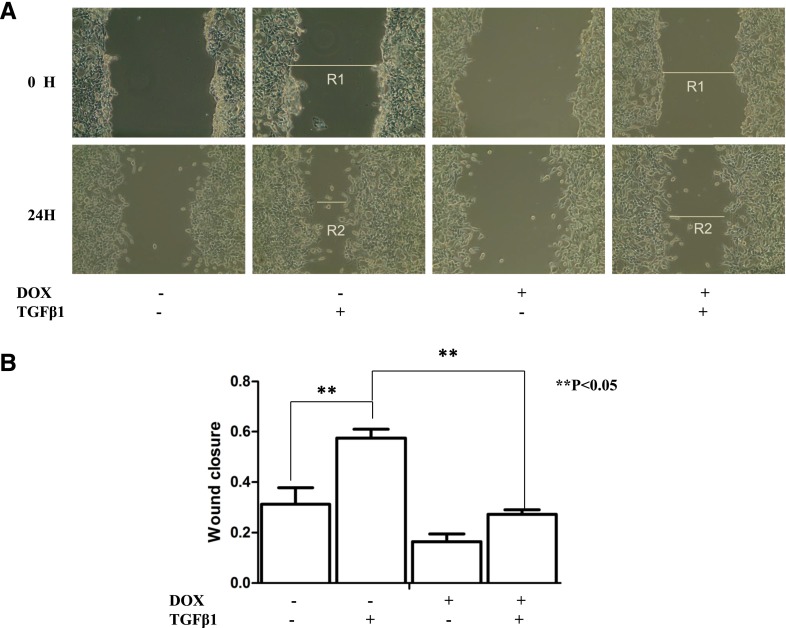
To examine the effect of metformin on cell mobility in response to TGF-β, we first conducted wound-healing analysis in which cells were serum starved for 8 hours and plates scratched to form cell-free paths. Remaining cells were then incubated in medium for 24 hours, with or without metformin, and with or without TGF-β1. As shown in Fig. 4, A and B, in response to TGF-β1, A549 cells migrated faster than A549-LKB1 cells, and metformin elicited greater inhibition of TGF-β1–triggered closure of scratch in A549-LKB1 cells than in A549 cells. Next, we carried out cell-migration assays using Boyden chambers. Migration of both A549 and A549-LKB1 cells was stimulated by TGF-β1, which was suppressed by metformin or A769962 (Fig. 4C). The inhibitory effect of AMPK activators appeared greater in A549-LKB1 cells.
Fig. 4.
AMPK activation inhibits migration of A549 cancer cells Assays were performed according to Materials and Methods. (A) Wound-healing assay. Confluent A549 and A549-LKB1 cells were starved for 8 hours, scratched, and grown in the presence of metformin (10 mM) and/or TGF-β1 (5 ng/ml) as indicated. Photos were taken at the beginning (0 H) and end of experiments (24 H). Wound closure was calculated by the equation (R1−R2)/R1 as indicated. The graph represents averages of three independent experiments (mean ± S.D., n = 3). *P > 0.05; **P < 0.05. (B) Migration on transwells. The cells were loaded into membranes of chambers and treated with or without metformin or A769962 (1 μM) ± TGF-β1, as indicated, and migration was terminated after 20 hours. The number of cells migrated to the lower side of the membranes was counted and expressed as the percentage of control cells (mean ± S.D., n = 3). Each assay was repeated at least four times. Significance (P < 0.05) has been found between (1) control and TGF-β, and (2) TGF-β and TGF-β + metformin or TGF-β + A769962 in A549 and A549-LKB1 cells, respectively.
The wound-healing assay was repeated with C4-2 cells expressing DN-AMPK. Cell migration was faster under basal and TGF-β1–treated conditions in the absence of doxycycline than in the presence of doxycycline (Fig. 5). Hence, LKB1/AMPK counteracted the stimulatory effect of TGF-β on migration of the cancer cells.
Fig. 5.
AMPK inhibits TGF-β enhancement of C4-2 cancer cell migration (A) Wound-healing assays. C4-2 cells expressing the dominant negative mutant of AMPK were treated with or without doxycycline for 2 days, as described in Fig. 2B. Wound-healing assay conducted, and photos were taken at the beginning (0 H) and end of experiments (24 H). (B) The graph represents averages of a triplicate experiment (mean ± S.D., n = 3). Significance was tested with Student’s t test (**P < 0.05).
AMPK Inhibits TGF-β–Induced Epithelial Mesenchymal Transition.
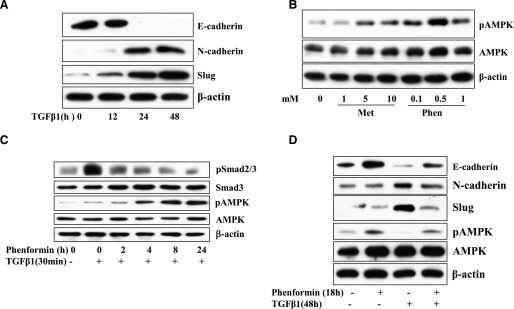
TGF-β is known to play an important role in EMT, a critical step for cancer metastasis as well as fibrosis. To explore the potential inhibitory effect of LKB1/AMPK on TGF-β–induced EMT, we used BEAS-2B cells, a human bronchial epithelial cell line transformed with adenovirus-12 and SV40 hybrid virus, which are not tumorigenic. E-cadherin was downregulated, whereas N-cadherin and Slug were upregulated in BEAS-2B cells after prolonged incubation with TGF-β (Fig. 6A). Since BEAS-2B cells require special culture medium and are sensitive to the addition of metformin in large volume during chronic incubation, we decided to use phenformin instead. The latter activates AMPK by increasing intracellular AMP, as does metformin, but with more potency. In fact, our data indicate that phenformin is at least 10 times more potent in activation of AMPK than metformin is (Fig. 6B). Smad2/3 phosphorylation was found to be progressively inhibited, which paralleled the activation of AMPK after cells were incubated with phenformin for a given period (Fig. 6C). Then we treated the cells with TGF-β1 for 48 hours and added phenformin in the last 18 hours. Phenformin restored the expression of E-cadherin and suppressed the TGF-β–induced expression of N-cadherin and Slug and thus effectively blocked the ability of TGF-β to induce EMT (Fig. 6D).
Fig. 6.
Regulation of EMT marker expression by phenformin and TGF-β. (A) TGF-β regulation of EMT markers. BEAS-2B cells were treated with TGF-β1 for different times. (B) Dose-dependent activation of AMPK by biguanides. BEAS-2B cells were treated with metformin or phenformin at different doses, as indicated, to assess the efficiency of AMPK activation. (C) Inhibition of TGF-β signaling by phenformin. The cells were treated with phenformin (1 mM) for indicated times and then with TGF-β (5 ng/ml) for 30 minutes. (D) AMPK inhibition of TGF-β–induced expression of EMT markers. The cells were treated with TGF-β1 for 48 hours, and phenformin was added in the last 18 hours. Cell extracts (25 μg) were blotted with antibodies as indicated.
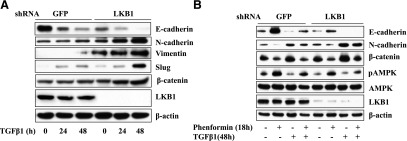
To ascertain that the effect of phenformin is mediated by LKB1/AMPK, the expression of LKB1 was knocked down in the cells stably expressing LKB1 shRNA. The cells stably expressing GFP shRNA were used as control. Our results showed that expression of E-cadherin was significantly reduced by LKB1 shRNA (Fig. 7A). In constast, the TGF-β1–induced expression of N-cadherin, vimentin, Slug, and β-catenin was markedly enhanced (Fig. 7A), and the efficacy of phenformin in inhibiting TGF-β–induced changes of EMT markers was markedly suppressed (Fig. 7B). Furthermore, the expression levels of these EMT markers correlated with degrees of AMPK activation, which strongly suggests that the effect of phenformin is mediated by AMPK.
Fig. 7.
LKB1 inhibits the regulatory effect of TGF-β on EMT marker expression (A). Effect of silencing LKB1 on TGF-β–induced changes of EMT markers. BEAS-2B cells containing shRNA for LKB1 or GFP as a control were treated with TGF-β1 for different times. (B) Silencing LKB1 offsets the inhibitory effect of phenformin on TGF-β–induced changes of EMT markers. The cells were treated with TGF-β1 for 48 hours, and phenformin was added in the last 18 hours. The cell extracts (25 μg) were blotted with antibodies as indicated.
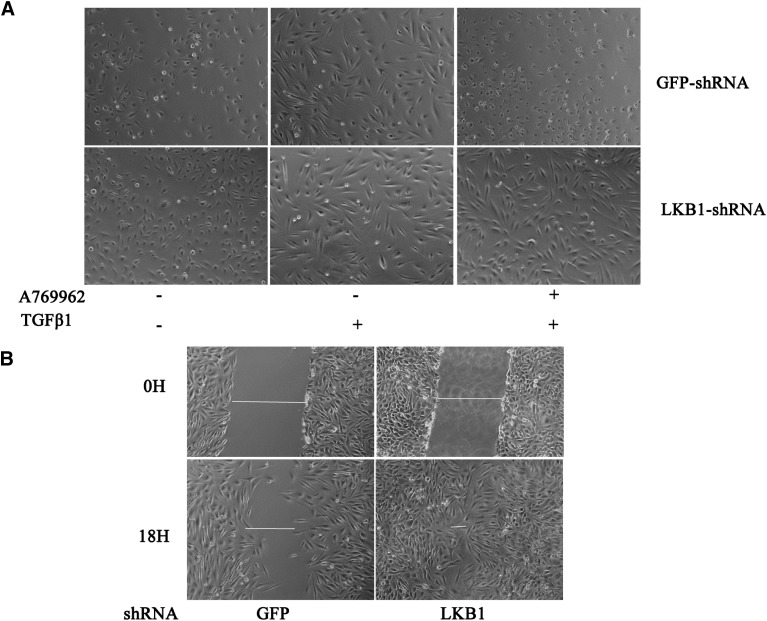
Morphologically, the TGF-β–induced EMT change is less affected by A769962 in the cells with LKB1 knockdown than in the cells with GFP shRNA. When treated with TGF-β, most of the cells containing GFP shRNA remained epithelium shaped in the presence of A769962 (Fig. 8A) or detached. Cell detachment, a sign of cell death, was more severe upon treatment with phenformin or metformin (data not shown). In contrast, the cells bearing LKB1 shRNA displayed a mesenchymal shape, which was not altered by A769962. These results indicate that AMPK activation prevents TGF-β–induced EMT or even induces cell apoptosis. In addition, we found that with LKB1 knockdown, BEAS-2B cells migrated faster than did control cells on wound healing (Fig. 8B), an indication of mesenchymal transition.
Fig. 8.
Knockdown of LKB1 enhances migration of epithelial cells and confers resistance to phenformin-induced inhibition of EMT behaviors of the BEAS-2B cells with LKB1 shRNA and GFP shRNA, the effects of which were compared. (A) Knockdown of LKB1 changes morphology of the cells toward mesenchymal-like shape. The cells were treated with or without TGF-β1 for 30 hours, and cell culture was continued in the presence of TGF-β1± A769962 (1 μM) for additional 18 hours. (B) Wound-healing assay showed that knockdown of LKB1 enabled the cells to migrate faster. Photos were taken under phase-contrast microscope.
Discussion
AMPK is a well-received drug target for type 2 diabetes and other metabolic disorders inasmuch as its activation enhances insulin sensitivity, lowers blood glucose levels, and improves lipid profiles. In the last decade, mounting evidence has indicated that AMPK is implicated in control of tumor cell growth and tumorigenesis; however, less is known about its effect on cancer progression and metastasis. In present study, we attempted to explore the effect of AMPK on the canonical TGF-β signaling pathway, which is often activated in the advanced stage of cancer and plays an important role in EMT and cancer metastasis. Our results showed that AMPK activators, including metformin, phenformin, or A769962, suppressed Smad2/3 phosphorylation and the expression of target genes, including PAI-1,CTGF, FN, and IL-6 in response to TGF-β, concurrently with a decrease in the ability of cancer cells to migrate on wound-healing and transwell assays. Furthermore, we found that AMPK activation counteracted the stimulatory effect of TGF-β on expression of EMT markers and morphologic changes of BEAS-2B cells. All these effects of metformin were dependent on functional LKB1, although the inhibitory effect of metform on TGF-β-induced IL-6 expression can also be mediated by a putative LKB1-independent pathway. Consistently, expression of dominant negative mutant of AMPK or deletion of AMPK α subunits augmented TGF-β-stimulated phosphorylation of Smad2/3, whereas expression of the active mutant of AMPK diminished the effect of TGF-β. Therefore, our results indicate that AMPK activation inhibits TGF-β–modulated EMT and cancer metastasis.
AMPK consists of three subunits (α, β, and γ) and is activated by both allosteric activators and phosphorylation. A physiologic allosteric activator is 5′-AMP, which is elevated under stresses such as hypoxia, ischemia, and glucose starvation. Other allosteric activators include AICAR (a cell-permeable molecule that is phosphorylated by nucleoside kinase and converted to 5-amino-4-imidazolecarboxamide ribotide, an analog of AMP), as well as salicylate and A769662, both of which directly bind to the autoinhibitory domain of AMPK (Hawley et al., 2012). Metformin, a clinically used antidiabetic drug, and phenformin, which had been in clinic use but was withdrawn because of toxicity, belong to the biguanides family; these agents activate AMPK by inhibiting mitochondrial respiratory chain and thus increasing intracellular AMP. Steady-state concentration of metformin in plasma of type 2 diabetes is in the range of 20–40 µM and can reach 50 µM in portal vein under oral administration at regularly prescribed doses (Owen et al., 2000); He et al., 2015). Metformin and phenformin are positively changed and require transporters to enter cells and mitochondria. The expression level of transporters varies on cells (Viollet et al., 2012). Phenformin is relatively hydrophobic and easy to be delivered to cells. Thus, for most in vitro studies, metformin is used in the range of 1–10 mM, compared with 100 μM–1 mM of phenformin to reach optimal doses in mitochondria (Zhou et al., 2001; Janzer et al., 2014).
Binding of AMP to AMPK γ subunit facilitates phosphorylation of T172 on the activation loop of the α catalytic subunit and also protects the phosphorylated T172 against dephosphorylation by phosphatases. Several protein kinases have been reported to phosphorylate T172, including LKB1 and calmodulin-dependent protein kinase kinase β (CamKKβ). Whereas CamKKβ is activated by Ca2+, LKB1 is constitutively active. Maximal activation of AMPK is achieved by both AMP binding and phosphorylation of T172 (Hardie 2011). In addition to AMPK, LKB1 regulates 13 other protein kinases, and some of the tumor-suppressive functions of LKB1 are mediated by these other AMPK-related kinases (Luo et al., 2010). One way to distinguish them from AMPK is the fact that the activation of the latter depends on both AMP and LKB1, whereas others are independent of AMP.
Recent studies have illustrated an inhibitory effect of AMPK on the canonical TGF-β signaling pathway, but data published are not consistent with regard to the underlying mechanisms. Mishra et al. (2008) have shown that AMPK suppresses transdifferentiation of myofibroblast induced by TGF-β, an event that is not induced by phosphorylation of Smad2/3 but rather through inhibition of a downstream event. A later study has reported that AMPK could induce degradation of the transcription coactivator p300, reduce acetylation of Smad3, and disrupt TGF-β–elicited interaction between p300 and Smad3 but with no effect on Smad phosphorylation and nuclear translocation (Lim et al., 2012). Another mechansim involves metformin-induced inhibition of smad2/3 phosphorylation (Park et al., 2014). The differences among these studies might reflect the cell context. Our present study provides solid evidence that AMPK attenuates the canonical TGF-β signaling pathway by inhibiting Smad2/3 phosphorylation and transcriptional events. We have tested both cancer cells and precancerous cells with consistent results that lead to the same conclusion.
Intriguingly, our data suggest that AMPK could attenuate the prometastatic effect of TGF-β through regulation of transcriptional targets of Smad2/3. Our previous study has found that AMPK activation downregulates fibronectin in prostate cancer cells (Zhou et al., 2009). In the present study, using a lung adenocarcinoma cell line, our results underpinned the negative effect of AMPK on fibronectin. Furthermore, our data demonstrate that AMPK activation can offset TGF-β in the regulation of CTGF and IL-6, two important molecules in cancer metastasis and EMT (Kang et al., 2003; Chu et al., 2008; Sullivan et al., 2009; Zhao et al., 2014). Interestingly, we found that IL-6 is regulated via both LKB1/AMPK dependent and independent mechanisms. It has been reported that metformin can regulate events independent of AMPK. For instance, metformin was found to suppress SNARK, an AMPK-related kinase that can enhance TGF-β signaling (Goto et al., 2013). It will be interesting to test whether SNARK is an additional target of metformin in our setting.
AMPK has been recently documented to be involved in EMT (Cufi et al., 2010; Vazquez-Martin et al., 2010; Wang et al., 2010, 2014; Lee et al., 2013; Chou et al., 2014; Zhang et al., 2014). Its effect was first described in tissue fibrosis, where opposite effects were reported (Wang et al., 2010; Lee et al., 2013). Several studies have shown that AMPK inhibits cancer EMT, which could occur through different mechanisms (Chou et al., 2014; Zhang et al., 2014; Zhao et al., 2014). Our study using BEAS-2B cells clearly demonstrates that inhibition of TGF-β action is one of the mechanisms.
In summary, we have shown that AMPK activation inhibits TGF-β-induced phosphorylation and transcriptional activity of Smad2/3. As such, AMPK attenuates cancer cell migration and invasion. In addition, our results have revealed that AMPK inhibits TGF-β–evoked EMT. Therefore, our present study delineates the role of AMPK in tumor cell migration, progression, and EMT and strongly supports the notion that AMPK could serve as a preventive and therapeutic target for cancer metastasis. This study may help to tune the focus of current clinical trials with metformin to cancer metastasis (He et al., 2015).
Acknowledgments
The authors thank Dr. Bin Zheng for LKB1 shRNA and GFP shRNA lentiviral constructs and Dr. Benoit Viollet for α1α2 knockout MEF cells.
Abbreviations
- AICAR
5-amino-4-imidazolecarboxamide riboside
- AMPK
5′-AMP-activated protein kinase
- BEAS
bronchial epithelial 2B cell line
- CTGF
connective tissue growth factor
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- FN
fibronectin
- IL-6
interleukin-6
- LKB1
liver kinase 1
- MEF
mouse embryonic fibroblast
- PAI-1
plasminogen activator inhibitor-1
- PCR
polymerase chain reaction
- TGF-β
transforming growth factor-β
Authorship Contributions
Participated in research design: Lin, He, Huang, Luo.
Conducted experiments: Lin, Li, He, Ying, Sunkara, Luo
Performed data analysis: Lin, Huang, Lv, Luo.
Writing and contributed to writing of the manuscript: Luo, Huang.
Footnotes
This work was supported by the National Nature Science Foundation of China (Grants 81171952, 81272926, 81460374, 31460304) and in part by the National Institutes of Health (Grant R21EY024388). H. Lin and H. He were supported by scholarships for postgraduate study from Nanchang University.
References
- Alessi DR, Sakamoto K, Bayascas JR. (2006) LKB1-dependent signaling pathways. Annu Rev Biochem 75:137–163. [DOI] [PubMed] [Google Scholar]
- Bayraktar S, Hernadez-Aya LF, Lei X, Meric-Bernstam F, Litton JK, Hsu L, Hortobagyi GN, Gonzalez-Angulo AM. (2012) Effect of metformin on survival outcomes in diabetic patients with triple receptor-negative breast cancer. Cancer 118:1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretero J, Shimamura T, Rikova K, Jackson AL, Wilkerson MD, Borgman CL, Buttarazzi MS, Sanofsky BA, McNamara KL, Brandstetter KA, et al. (2010) Integrative genomic and proteomic analyses identify targets for Lkb1-deficient metastatic lung tumors. Cancer Cell 17:547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CC, Lee KH, Lai IL, Wang D, Mo X, Kulp SK, Shapiro CL, Chen CS. (2014) AMPK reverses the mesenchymal phenotype of cancer cells by targeting the Akt-MDM2-Foxo3a signaling axis. Cancer Res 74:4783–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CY, Chang CC, Prakash E, Kuo ML. (2008) Connective tissue growth factor (CTGF) and cancer progression. J Biomed Sci 15:675–685. [DOI] [PubMed] [Google Scholar]
- Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. (1998) Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem 273:35347–35354. [DOI] [PubMed] [Google Scholar]
- Cufí S, Vazquez-Martin A, Oliveras-Ferraros C, Martin-Castillo B, Joven J, Menendez JA. (2010) Metformin against TGFβ-induced epithelial-to-mesenchymal transition (EMT): from cancer stem cells to aging-associated fibrosis. Cell Cycle 9:4461–4468. [DOI] [PubMed] [Google Scholar]
- Drabsch Y, ten Dijke P. (2012) TGF-β signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev 31:553–568. [DOI] [PubMed] [Google Scholar]
- Elias JA, Lentz V, Cummings PJ. (1991) Transforming growth factor-beta regulation of IL-6 production by unstimulated and IL-1-stimulated human fibroblasts. J Immunol 146:3437–3443. [PubMed] [Google Scholar]
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. (2005) Metformin and reduced risk of cancer in diabetic patients. The BMJ 330:1304–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisslthaler B, Fleming I. (2009) Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res 105:114–127. [DOI] [PubMed] [Google Scholar]
- Fuxe J, Vincent T, Garcia de Herreros A. (2010) Transcriptional crosstalk between TGF-β and stem cell pathways in tumor cell invasion: role of EMT promoting Smad complexes. Cell Cycle 9:2363–2374. [DOI] [PubMed] [Google Scholar]
- Goto K, Lin W, Zhang L, Jilg N, Shao RX, Schaefer EA, Zhao H, Fusco DN, Peng LF, Kato N, et al. (2013) The AMPK-related kinase SNARK regulates hepatitis C virus replication and pathogenesis through enhancement of TGF-β signaling. J Hepatol 59:942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, Yoneda T, Mundy GR. (1996) Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest 98:1544–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadad SM, Baker L, Quinlan PR, Robertson KE, Bray SE, Thomson G, Kellock D, Jordan LB, Purdie CA, Hardie DG, et al. (2009) Histological evaluation of AMPK signalling in primary breast cancer. BMC Cancer 9:307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. (2011) AMP-activated protein kinase: a cellular energy sensor with a key role in metabolic disorders and in cancer. Biochem Soc Trans 39:1–13. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green KA, Mustard KJ, et al. (2012) The ancient drug salicylate directly activates AMP-activated protein kinase. Science 336:918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Ke R, Lin H, Ying Y, Liu D, Luo Z. (2015) Metformin, an old drug, brings a new era to cancer therapy. Cancer J 21:70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. (1998) A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 95:2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Wullschleger S, Shpiro N, McGuire VA, Sakamoto K, Woods YL, McBurnie W, Fleming S, Alessi DR. (2008) Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J 412:211–221. [DOI] [PubMed] [Google Scholar]
- Igarashi A, Okochi H, Bradham DM, Grotendorst GR. (1993) Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell 4:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz RA, Massagué J. (1986) Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem 261:4337–4345. [PubMed] [Google Scholar]
- Janzer A, German NJ, Gonzalez-Herrera KN, Asara JM, Haigis MC, Struhl K. (2014) Metformin and phenformin deplete tricarboxylic acid cycle and glycolytic intermediates during cell transformation and NTPs in cancer stem cells. Proc Natl Acad Sci USA 111:10574–10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, Hsu L, Hung MC, Hortobagyi GN, Gonzalez-Angulo AM. (2009) Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 27:3297–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C, Guise TA, Massagué J. (2003) A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3:537–549. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim JH, Kim JS, Chang JW, Kim SB, Park JS, Lee SK. (2013) AMP-activated protein kinase inhibits TGF-β-, angiotensin II-, aldosterone-, high glucose-, and albumin-induced epithelial-mesenchymal transition. Am J Physiol Renal Physiol 304:F686–F697. [DOI] [PubMed] [Google Scholar]
- Lim JY, Oh MA, Kim WH, Sohn HY, Park SI. (2012) AMP-activated protein kinase inhibits TGF-β-induced fibrogenic responses of hepatic stellate cells by targeting transcriptional coactivator p300. J Cell Physiol 227:1081–1089. [DOI] [PubMed] [Google Scholar]
- Lund LR, Riccio A, Andreasen PA, Nielsen LS, Kristensen P, Laiho M, Saksela O, Blasi F, Danø K. (1987) Transforming growth factor-beta is a strong and fast acting positive regulator of the level of type-1 plasminogen activator inhibitor mRNA in WI-38 human lung fibroblasts. EMBO J 6:1281–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Huang W, Tao R, Hu N, Xiao ZX, Luo Z. (2013) ATM and LKB1 dependent activation of AMPK sensitizes cancer cells to etoposide-induced apoptosis. Cancer Lett 328:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Saha AK, Xiang X, Ruderman NB. (2005) AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci 26:69–76. [DOI] [PubMed] [Google Scholar]
- Luo Z, Zang M, Guo W. (2010) AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol 6:457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R, Cool BL, Laderoute KR, Foretz M, Viollet B, Simonson MS. (2008) AMP-activated protein kinase inhibits transforming growth factor-beta-induced Smad3-dependent transcription and myofibroblast transdifferentiation. J Biol Chem 283:10461–10469. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. (2007) Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci 98:1512–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410:50–56. [DOI] [PubMed] [Google Scholar]
- Owen MR, Doran E, Halestrap AP. (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348:607–614. [PMC free article] [PubMed] [Google Scholar]
- Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massagué J. (2008) TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133:66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Um JY, Hong SM, Cho JS, Lee SH, Lee SH, Lee HM. (2014) Metformin reduces TGF-β1-induced extracellular matrix production in nasal polyp-derived fibroblasts. Otolaryngol Head Neck Surg 150:148–153. [DOI] [PubMed] [Google Scholar]
- Shackelford DB, Shaw RJ. (2009) The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer 9:563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Wen XF, Lan F, Shen ZZ, Shao ZM. (2002) The tumor suppressor gene LKB1 is associated with prognosis in human breast carcinoma. Clin Cancer Res 8:2085–2090. [PubMed] [Google Scholar]
- Singh A, Settleman J. (2010) EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29:4741–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM. (2009) Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene 28:2940–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur S, Viswanadhapalli S, Kopp JB, Shi Q, Barnes JL, Block K, Gorin Y, Abboud HE. (2015) Activation of AMP-activated protein kinase prevents TGF-beta1-induced epithelial-mesenchymal transition and myofibroblast activation. Am J Pathol 185:2168–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Martin A, Oliveras-Ferraros C, Cufí S, Del Barco S, Martin-Castillo B, Menendez JA. (2010) Metformin regulates breast cancer stem cell ontogeny by transcriptional regulation of the epithelial-mesenchymal transition (EMT) status. Cell Cycle 9:3807–3814. [PubMed] [Google Scholar]
- Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. (2012) Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 122:253–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pan X, Song J. (2010) AMP-activated protein kinase is required for induction of apoptosis and epithelial-to-mesenchymal transition. Cell Signal 22:1790–1797. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yao B, Wang Y, Zhang M, Fu S, Gao H, Peng R, Zhang L, Tang J. (2014) Increased FoxM1 expression is a target for metformin in the suppression of EMT in prostate cancer. Int J Mol Med 33:1514–1522. [DOI] [PubMed] [Google Scholar]
- Xiao H, Ma X, Feng W, Fu Y, Lu Z, Xu M, Shen Q, Zhu Y, Zhang Y. (2010) Metformin attenuates cardiac fibrosis by inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc Res 87:504–513. [DOI] [PubMed] [Google Scholar]
- Xu J, Lamouille S, Derynck R. (2009) TGF-beta-induced epithelial to mesenchymal transition. Cell Res 19:156–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Tsukamoto O, Nakano A, Kato H, Kioka H, Ito N, Higo S, Yamazaki S, Shintani Y, Matsuoka K, et al. (2015) Augmented AMPK activity inhibits cell migration by phosphorylating the novel substrate Pdlim5. Nat Commun 6:6137–6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massagué J, Mundy GR, Guise TA. (1999) TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest 103:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Shen C, Wang L, Ma Q, Xia P, Qi M, Yang M, Han B. (2014) Metformin inhibits epithelial-mesenchymal transition in prostate cancer cells: involvement of the tumor suppressor miR30a and its target gene SOX4. Biochem Biophys Res Commun 452:746–752. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Cheng X, Wang Y, Han R, Li L, Xiang T, He L, Long H, Zhu B, He Y. (2014) Metformin inhibits the IL-6-induced epithelial-mesenchymal transition and lung adenocarcinoma growth and metastasis. PLoS One 9:e95884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Huang W, Tao R, Ibaragi S, Lan F, Ido Y, Wu X, Alekseyev YO, Lenburg ME, Hu GF, et al. (2009) Inactivation of AMPK alters gene expression and promotes growth of prostate cancer cells. Oncogene 28:1993–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]