Abstract
The African spiny mouse (Acomys spp.) can heal full thickness excisional skin wounds in a scar-free manner with regeneration of all dermal components including hair and associated structures. Comparing Acomys scar-free healing from Mus scarring identifies gene expression differences that discriminate these processes. We have performed an extensive comparison of gene expression profiles in response to 8mm full-thickness excisional wounds at days 3, 5, 7 and 14 post-wounding between Acomys and Mus to characterize differences in wound healing, and identify mechanisms involved in scar-free healing. We also identify similarities with scar-free healing observed in fetal wounds. While wounding in Mus elicits a strong inflammatory response, wounding in Acomys produces a moderated immune response and little to no increase in expression for most cytokines and chemokines assayed. We also identified differences in the ECM profiles of the Acomys wounds, which appear to have a collagen profile more similar to fetal wounds, with larger increases in expression of collagen types III and V. In contrast, Mus wounds have very high levels of collagen XII. This data suggests that an overall lack of induction of cytokines and chemokines, coupled with an ECM profile more similar to fetal wounds, may underlie scar-free wound healing in Acomys skin. These data identify candidate genes for further testing in order to elucidate the causal mechanisms of scar-free healing.
Introduction
Wound healing is a dynamic and highly coordinated series of complex events, that has been described extensively [1]. In order to attain tissue integrity following wounding in adult mammalian tissue, the healing process occurs in three overlapping phases: inflammation, tissue formation and tissue remodeling. Immediately following wounding, hemostasis occurs in the presence of aggregated platelets. During the inflammatory phase first neutrophils, and subsequently monocytes, infiltrate the wound and eliminate tissue debris and contaminating bacteria through phagocytosis. Granulation tissue is formed during the tissue formation phase of wound healing. This is characterized as a loose matrix of fibronectin and immature collagen fibers supporting migration of proliferative fibroblasts and vascularization of the wound bed. This newly formed tissue is then covered by a new wound epidermis formed by migration of cells from the wound edge, and results in the restoration of tissue continuity of the wound. In the final phase of wound healing the granulation tissue is remodeled, which results in an altered collagen profile and reduced vascularity. The end result of this series of events is scar tissue comprised of non-functional dermal tissue covered by a smooth, hairless epidermis.
In contrast, wounding of fetal mammalian tissue, up to the middle of the third trimester, results in scar free healing. This phenotypic difference in wound healing outcomes has lead to numerous studies comparing fetal and adult wound healing in order to determine what is responsible for the improved outcome (reviewed in [2]). These studies have been highly informative and have shown differences in several processes involved in wound healing between adult and fetal tissue; fetal wounds show a blunted inflammatory response, reduced fibrosis and vascularization, and a different extracellular matrix (ECM) profile.
A consistently observed characteristic associated with fetal skin wounding is a substantially blunted inflammatory immune response relative to that initiated from adult wounding [3–5]. This is due in part to reduced levels of Pdgfa, Tgf-β1 and Tgf-β2 [6]. The number of immune cells present in fetal wounds is also decreased, with fetal wounds having fewer macrophages that are present for a shorter duration [7]. Fetal wounds also contain fewer neutrophils and they demonstrate reduced phagocytic activity [8, 9]. The reduced presence of immune cells results in reduced levels of inflammatory cytokines and growth factors such as Tgf-β1 [10].
There are also differences in the ECM composition between fetal and adult wounds. The ECM has been shown to regulate cytokines and growth factors and to promote cell migration through the wound, and as such it is considered an important component of wound healing [11]. The fetal ECM has a higher ratio of collagen III:collagen I and higher levels of collagen V as well [12–14]. In addition, fetal wounds have increased levels of matrix metalloproteinases (MMPs) and lower levels of tissue inhibitors of matrix metalloproteinases (TIMPs), with these ratios reversed in adult wounds [15–17], suggesting that there is more active degradation and remodeling of wound tissue in fetal wounds.
Despite the extensive data comparing fetal scar-free and adult scarring mechanisms, very little has successfully been translated into improved outcomes following wounding in adult tissue. This is in part due to the inherent differences between fetal and adult tissues. Furthermore, our ability to compare scar-free healing vs scarring in two adult tissues has, until recently, been limited to contrasting wound repair between evolutionarily distant vertebrates. Although Urodeles have a remarkable capacity for regeneration [18, 19], it is difficult to translate findings from such disparate groups into improved clinical outcomes.
We have recently shown that the African spiny mouse, a member of the Muridae approximately 20MY diverged from Mus [20], can heal full thickness excisional skin wounds in a scar-free manner with regeneration of all dermal components including regeneration of hair and associated structures [21]. This discovery enables comparison of scar-free healing vs scarring in two closely related adult mammalian species.
In order to examine differences in wound healing between Acomys and Mus and to identify genes driving the mechanism of scar-free healing, we have performed an extensive comparison of gene expression profiles for 8mm full-thickness excisional wounds at days 3, 5, 7 and 14 post-wounding. Additionally, we asked whether scar-free healing in Acomys shares any similarities with scar-free healing described above for fetal tissues. Here we present evidence of expression differences in genes participating in several aspects of wound healing between Mus and Acomys, especially in the inflammatory pathway and the digestion and deposition of the ECM. While wounding in Mus elicits a strong inflammatory response, the response in Acomys wounds is substantially muted and exhibits little to no increase in expression for most cytokines and chemokines assayed. We also show that the ECM profiles of the Acomys wounds indicate large increases in expression of collagen types III and V, and little collagen XII relative to Mus wounds, indicating they are more similar to fetal rather than adult wounds. This data suggests that an overall lack of induction of cytokines and chemokines, coupled with an ECM profile more similar to fetal wounds, may be responsible for this remarkable scar-free wound healing observed in Acomys skin.
Methods
Animals
All experiments were performed following guidelines of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Florida (Protocol Numbers: 201203505 (Mus) and 201207707 (Acomys)), and all animals were housed under the care of the University of Florida’s Animal Care Services. All surgeries were performed under isoflurane inhalational anesthesia and all efforts were made to minimize suffering. CD-1 outbred mice (Charles River Laboratories) were used as Mus controls for all experiments. Animals were between 6 months to 1 year old at time of experiments; One to two 8-mm biopsy punches were excised from the dorsal skin of anaesthetized, shaved animals. Excised skin was immediately placed in RNALater (Qiagen Cat. 76104) at room temperature for 24 hours and then stored at -80°C. Wounds were excised at appropriate time points (3, 5, 7 or 14 days post wounding), excluding surrounding normal skin, and stored as before for normal skin.
RNA Extraction
Tissue samples were removed from -80°C and thawed at 4°C for 24 hours before processing. Total RNA was extracted using RNeasy Fibrous Tissue Mini Kit (Qiagen Cat. 74704) following the manufactures recommended protocol, with tissue homogenization being performed using a rotor stator type tissue homogenizer (ProScientific Bio-Gen PRO200 Homogenizer; Multi-Gen 7XL Generator Probes) in RLT Buffer (Qiagen Cat. 74704). RNA quality was assayed using an Agilent 2200 TapeStation (Agilent, Andover, MA). All samples had a RIN score ≥ 6.0.
RT-qPCR analysis
cDNA was generated from 500ng of RNA using iScriptTM cDNA Synthesis Kit (Bio-Rad cat. no. 170–8891) following manufacturer’s protocol. Real-time PCR was performed using SsoFastTM EvaGreen® Supermix (Bio-Rad cat. no. 172–5200) following manufacturer’s protocol on a Bio-Rad CFX-96 Real-Time PCR Detection System. Fold change of expression was calculated using the ΔΔCt relative expression method [22]. Change in expression was expressed as normal skin versus wounded skin. Expression values for RT-PCR arrays (RT2 Qiagen cat. no. PAMM-121Z) were calculated using Hsp90ab1 (Heat shock protein 90 alpha, class B member 1) and Gusb (Glucuronidase, beta) as reference genes.
Microarray analysis
100ng of total RNA was processed for microarray analysis using the GeneChip® WT PLUS Reagent Kit (Affymetrix, Santa Clara, CA) following manufacturer’s recommended protocols. cDNA (5.5ug) was fragmented, terminally labeled and hybridized to Affymetrix GeneChip® Mouse Gene 2.0 ST Array (MoGene 2.0) for 16 h at 45°C and washed following Affymetrix fluidics protocols FS450_001. Microarrays were normalized using the robust multiarray average (RMA) method as implemented in Partek Genomics Suite 6.6 (Partek Incorporated, St Louis MO). Only annotated probe sets were used in the subsequent analysis. BRB-ArrayTools (version 4.3.0 Stable Release, developed by Richard Simon & BRB-ArrayTools Development Team (http://linus.nci.nih.gov/BRB-ArrayTools.html)) was utilized to identify significant genes (p<0.001) using the class prediction tool. Leave-one-out-cross-validation studies and Monte Carlo simulations were performed using BRB-Array Tools. Venn diagrams were generated using the R package VennDiagram (http://cran.r-project.org/web/packages/VennDiagram/index.html).
Pathway analysis was performed using WEB-based Gene Set Analysis Toolkit (WebGestalt) [23, 24]. Gene Ontology enrichment analysis summarization was performed using REViGO [25]. Semantic similarity-based scatterplots were generated using REViGO and modified in R using the REViGO generated R script.
Data availability
The microarray dataset discussed in this publication has been deposited in NCBI’s Gene Expression Omnibus and is accessible through GEO Series accession number GSE74387. (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE74387)
Results
Pathway focused RT-PCR arrays
In order to elucidate the observed differences in wound healing between Mus and Acomys we examined the expression profile of full-thickness excisional wounds at 3 and 5 days post-wounding (compared to normal skin) using pathway-focused RT-PCR arrays with 84 genes targeted for involvement in wound healing (RT2 Qiagen cat. no. PAMM-121Z). In Mus there were 21 genes up and 1 gene downregulated at day 3 post-wounding, and 24 up and 3 downregulated genes at day 5 post wounding, as compared to normal skin. In Acomys, there were 14 genes up and 1 gene downregulated at day 3, and 14 up and 2 downregulated at day 5 post wounding compared to normal skin (S1 Table) (p-values ≤ 0.01). At both day 3 and 5 in the Mus, the top upregulated genes are those involved in the inflammatory pathway, as well as neutrophil and macrophage activation and migration, with a substantial increase in c-x-c motif chemokines, interleukins and growth factors (Table 1). In comparison, the top upregulated genes in the Acomys, at both days 3 and 5, are those involved in tissue remodeling, including extracellular matrix degradation and deposition, with the inflammatory response being quite blunted (Table 1).
Table 1. Differentially expressed genes in day 3 and 5 wounds.
Differentially expressed genes in day 3 and 5 wounds, compared to normal skin, within each species. Bold entries are those with a p-value ≤0.01.
| Mus musculus | Acomys cahirinus | ||||
|---|---|---|---|---|---|
| Gene | Day 3 vs 0 | Day 5 vs 0 | Gene | Day 3 vs 0 | Day 5 vs 0 |
| Cxcl3 | 47963.253 | 109235.609 | Ptgs2 | 433.720 | 902.276 |
| Csf3 | 8369.125 | 9126.401 | Csf3 | 181.376 | 294.590 |
| Cxcl5 | 4067.714 | 4464.773 | Mmp9 | 90.509 | 65.085 |
| Il1b | 358.635 | 220.279 | Tnf | 54.254 | 31.358 |
| Serpine1 | 233.382 | 275.948 | Actc1 | 26.960 | 5.313 |
| Cxcl1 | 151.834 | 239.431 | Mmp2 | 7.223 | 7.881 |
| Ptgs2 | 70.466 | 67.940 | Tgfb1 | 6.862 | 8.716 |
| Timp1 | 68.761 | 56.027 | Wisp1 | 6.127 | 9.138 |
| Plaur | 48.848 | 62.479 | Vegfa | 5.741 | 7.545 |
| Tnf | 27.552 | 29.862 | Col5a3 | 4.811 | 6.196 |
| Col5a3 | 16.050 | 17.202 | Itgb3 | 4.363 | 9.454 |
| Mmp9 | 13.534 | 14.893 | Itgb1 | 3.700 | 3.875 |
| Csf2 | 13.388 | 34.471 | Col5a1 | 3.623 | 8.626 |
| Wisp1 | 10.296 | 15.814 | Col5a2 | 3.489 | 7.405 |
| Ccl7 | 7.142 | 11.577 | Ctsg | 2.321 | N/A |
| Il10 | 6.664 | 6.198 | Il1b | 2.231 | 17.350 |
| Col14a1 | 4.364 | 7.799 | Fgf2 | 2.126 | 4.205 |
| Vegfa | 4.285 | 6.506 | Plat | 1.927 | 3.433 |
| Hgf | 3.851 | 5.408 | Cxcl3 | 1.775 | 3.308 |
| Plau | 3.740 | 3.786 | Mif | 1.566 | 1.807 |
| Plat | 3.648 | 4.283 | Col4a3 | 1.561 | -12.627 |
| Itgb3 | 3.250 | 4.290 | Col14a1 | 1.552 | 4.846 |
| Mif | 3.030 | 3.755 | Col3a1 | 1.528 | 3.313 |
| Tgfb1 | 2.937 | 3.545 | Timp1 | 1.294 | 3.095 |
| Actc1 | 2.408 | 12.429 | Plaur | -1.337 | N/A |
| Col5a1 | 2.088 | 2.805 | Itgb6 | -1.494 | -5.143 |
| Itgb1 | 2.062 | 2.273 | Csf2 | -2.768 | -1.273 |
| Fgf7 | 1.901 | 2.918 | Il4 | -6.935 | -5.590 |
| Col5a2 | 1.671 | 2.118 | Ccl7 | N/A | N/A |
| Il4 | 1.208 | -1.557 | Cxcl1 | N/A | N/A |
| Mmp2 | 1.158 | 1.743 | Cxcl5 | N/A | N/A |
| Ifng | -1.180 | -2.665 | Fgf7 | N/A | N/A |
| Col3a1 | -2.144 | -1.551 | Hgf | N/A | N/A |
| Itgb6 | -2.272 | -1.449 | Ifng | N/A | N/A |
| Fgf2 | -2.445 | -2.325 | Il10 | N/A | N/A |
| Ctsg | -8.594 | -2.514 | Plau | N/A | N/A |
| Col4a3 | -10.323 | -5.921 | Serpine1 | N/A | N/A |
Examining expression differences in genes known to participate in the inflammation pathway and the extra-cellular matrix during cutaneous wound healing
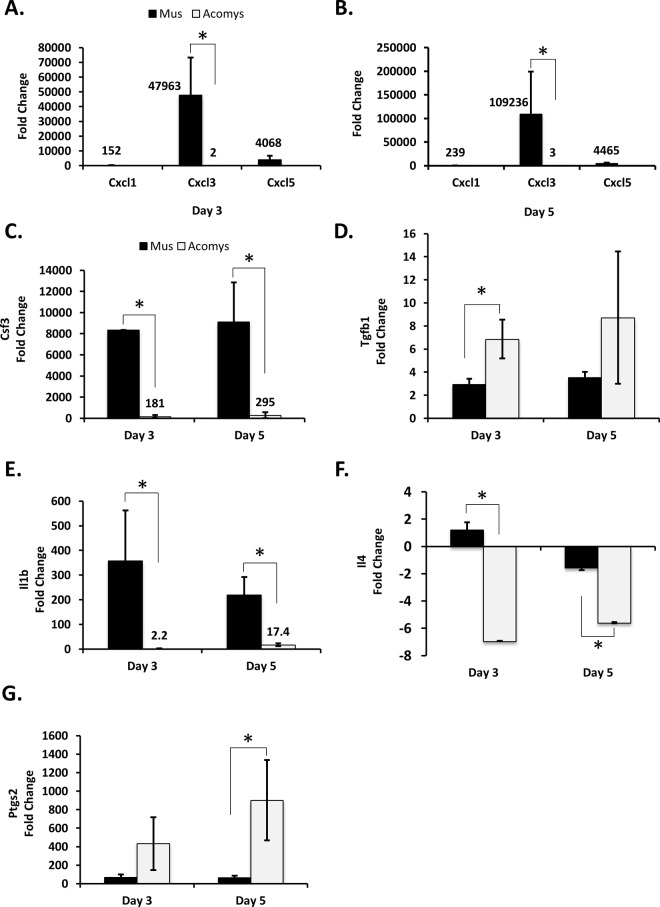
The c-x-c motif family of inflammatory chemokines acts by attracting immune cells to the wound site. In Mus wounds Cxcl1, Cxcl3 and Cxcl5 were all significantly upregulated to high degree (from >150 for Cxcl1 and >4000-fold for Cxcl3 and Cxcl5). In contrast, the increase in Cxcl3 was relatively modest in Acomys, with only a 2 to 3 fold increase in day 3 and 5 wounds compared to normal skin (Fig 1A and 1B). There were no detectable transcripts for Cxcl1, and very low levels of transcripts detected for Cxcl5 in Acomys (Fig 1A and 1B).
Fig 1. Immune response appears blunted in Acomys following wounding.
RT-qPCR analysis of day 3 and 5 wounds, compared to normal skin. Data are presented as mean ± SEM. * p ≤ 0.01; n = 3. Mus is represented by black bars and Acomys is represented by white bars. The Cxcl cytokines, Cxcl1, Cxcl3 and Cxcl5, were analyzed for day 3 (A) and day 5 (B) wounds. The remaining genes analyzed were: (C) Csf3, (D) Tgf-β1(E) Il1β, (F) Il4, and (G) Ptgs2.
The glycoprotein granulocyte colony stimulating factor 3 (Csf 3) stimulates the production of granulocytes [26], promotes the survival of mature neutrophils [27] and is often used to treat neutropenia [28]. Csf3 was induced to very high levels of expression in day 3 and 5 wounds in Mus. In contrast, the levels of Csf3 in Acomys, while substantially upregulated (~200 to 300 fold) represent approximately 2% of the levels observed in Mus (Fig 1C).
Tgf-β1(Transforming growth factor beta 1), thought to play a role in nearly all stages of wound healing, was ~3 fold upregulated in Mus in day 3 and 5 wounds compared to normal skin. Expression levels of Tgf-β1 in Acomys were approximately twice that observed in Mus, with a 7-fold increase in expression observed in wounds vs. unwounded skin (Fig 1D).
The interleukin family of secreted cytokines facilitates intercellular communication between immune cells. The pro-inflammatory cytokine Il1β, can act pleiotropically to induce a diverse range of effects, such as proliferation and differentiation, lymphocyte activation, angiogenesis and leukocyte attraction [29]. Interleukin 4 (Il4) is another pleiotropic cytokine that has been shown to stimulate production of components of the ECM [30–32]. The levels of Interleukins 1β and 4 mRNA were significantly higher in Mus than in Acomys (Fig 1E and 1F). The expression of Il1β was expressed ~350 fold higher and ~220 fold higher in Mus day 3 and day 5 wounds relative to unwounded skin, respectively. In Acomys the expression of Il1β is also elevated in wounded vs. non-wounded skin. However, the magnitude of the elevation in expression of Il1β is greatly reduced in Acomys relative to Mus with Acomys day 3 and day 5 wounds exhibiting a 2.2 and 17.4 fold, increase in expression relative to unwounded skin, respectively. (Fig 1E.) There was essentially no change observed in Il4 expression in Mus wounds relative to unwounded skin (<2-fold), while in Acomys, Il4 expression was reduced by 7 and 6.6 fold in day 3 and 5 wounds relative to unwounded skin, respectively (Fig 1F.)
Prostaglandin-endoperoxide synthase 2 (Ptgs2, also known as cyclooxygenase-2 (Cox-2) is an immediate early response gene that is upregulated immediately after wounding [33], and has been shown to impair wound healing and to promote scar formation [34, 35]. Interestingly, the levels of Ptgs2 mRNA were significantly higher in Acomys than those observed in Mus (Fig 1G), with an increase of ~ 70 fold in Mus and over 400 fold in Acomys observed in both day 3 and 5 wounds.
Extracellular matrix
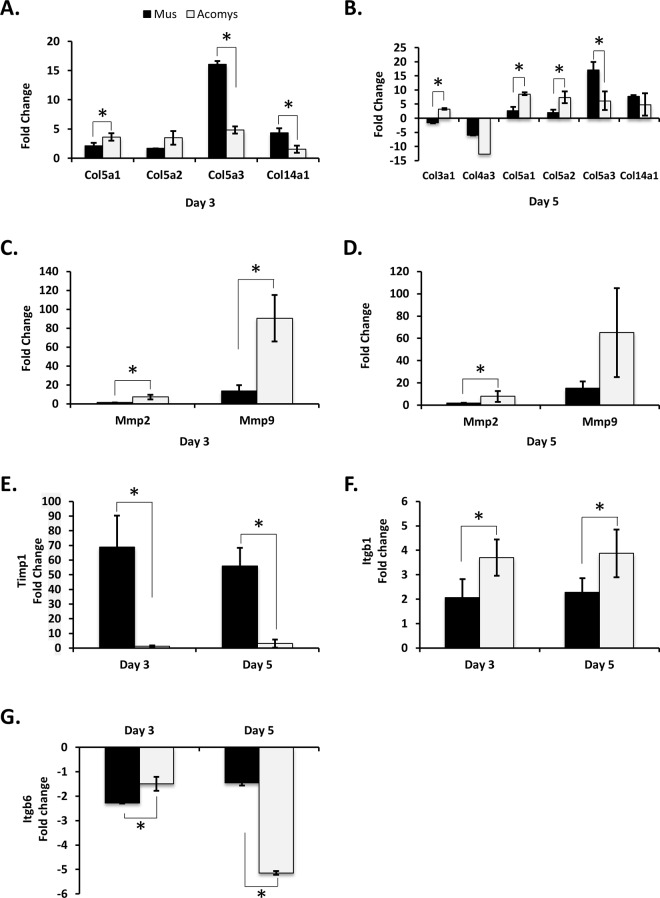
Several genes associated with the organization of the extracellular matrix showed significant differences in mRNA levels between Mus and Acomys wounds (Fig 2). At days 3 and 5 post wounding, there appears to be a different collagen expression profile between Mus and Acomys (Fig 2A and 2B). In day 3 wounds Col5a2 was expressed at significantly higher levels in Acomys, compared to unwounded skin, while Col5a3 and Col14a1 were significantly higher in Mus, compared to unwounded skin (Fig 2A). At 5 days post wounding, both Col5a1 and Col5a2 showed significantly higher increases in expression, as compared to unwounded skin, in Acomys, while Col5a3 was higher in Mus. Col4a3 was downregulated in Mus and Acomys in day 5 wounds, although the difference between the species was not significant (Fig 2B). In addition to components of the ECM, there were also genes involved in ECM degradation which showed differences in their gene expression profiles between Mus and Acomys (Fig 2C–2E). The matrix metalloproteinases Mmp2 and Mmp9 were both significantly upregulated at days 3 and 5 post wounding in the Acomys (Fig 2C and 2D). Mmp2 was upregulated ~ 7.5 fold in both day 3 and 5 wounds in Acomys, with no significant changes observed in Mus. Mmp9 was upregulated ~90 fold at day 3, and ~65 fold at day 5 post wounding in Acomys. In Mus, Mm9 was upregulated ~13 and 15 fold in day 3 and 5 wounds, respectively (Fig 2C and 2D). Although Mmp9 was upregulated roughly 15 fold in Mus at days 3 and 5, an inhibitor of metalloproteinases, Timp1, was upregulated greater than ~50 fold in Mus, with no significant increase observed for Timp1 in Acomys (Fig 2E). Taken together, this suggests that ECM degradation is more active in Acomys wound healing than in Mus.
Fig 2. Differences in ECM composition, matrix digestion and cell motility between Acomys and Mus.
RT-qPCR analysis was performed for the following genes: Collagens were analyzed for day 3 (A) and day 5 (B) wounds. Mmp2 and 9 were analyzed for day 3 (C) and day 5 (D) wounds, Timp1 (E), Itgβ1 (F), and Itgβ6 (G). All labels, symbols and calculations are as those described in Fig 1.
The Integrin family of proteins plays a key role in cellular binding to the extracellular matrix, as well as relaying information regarding the ECM composition to the cell, and is therefore important in wound healing. For most of the integrins analyzed, there was either no appreciable change in expression in the wounds as compared to normal skin, or there was no detectable level of transcription in at least one time point in the Acomys (Table 1). However, integrin β1 (Itgβ1) was significantly elevated in day 3 and 5 wounds for both Mus and Acomys. In Mus Itgβ1 was upregulated 2.1 fold in day 3 and 2.3 fold in day 5 wounds, while in Acomys, Itgβ1 was upregulated 3.7 fold in day 3 and 3.9 fold in day 5 wounds (Fig 2F). Integrin β6 (Itgβ6), which also acts as a potent activator of transforming growth factor (Tgf)-beta [36, 37], mRNA levels are lower in both day 3 and 5 wounds for both Mus and Acomys. In Mus, Itgβ6 is downregulated -2.3 fold in day 3 and -1.5 fold in day 5 wounds. In Acomys, Itgβ6 is downregulated -1.5 fold and -5.1 fold in day 3 and 5 wounds, respectively (Fig 2G). These data suggest that the levels of Itgβ6 are increasing in Mus and decreasing in Acomys from days 3 to 5 post wounding, with no change in expression for either species in day 7 and 14 wounds (by microarray analysis).
Microarray analysis
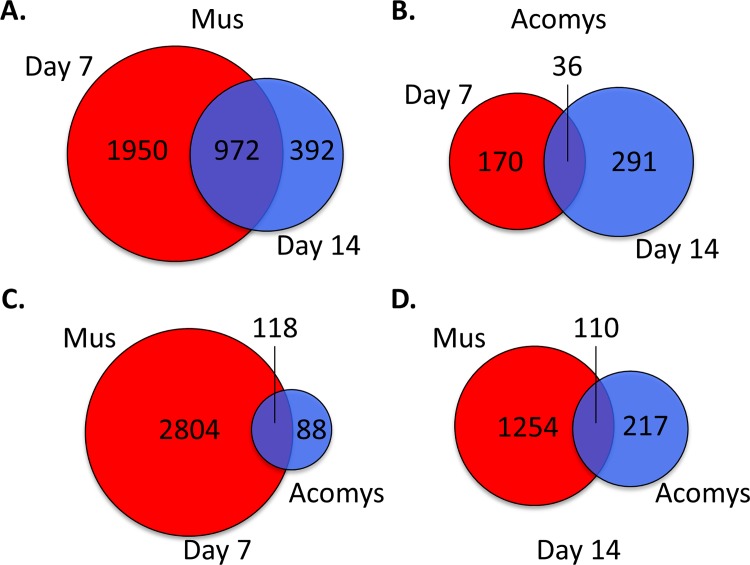
To examine the mechanisms involved in wound healing, genome wide gene expression analysis of full-depth excisional skin wounds at two additional time points, day 7 and day 14 after wounding, in both Mus and Acomys were evaluated with Affymetrix GeneChip® microarrays. Changes in gene expression levels were considered significant if the fold change in expression was at least 1.4 fold, in either direction, and with a p-value of significance of ≤ 0.001. At day 7 after wounding there were a total of 2922 genes exhibiting differential expression relative to normal skin in Mus (S2 Table). There were 1872 genes that were upregulated and 1050 genes downregulated. At day 14 there were 1364 genes showing statistically significant changes in gene expression compared to normal skin. Of these, 795 were upregulated and 569 were downregulated. Comparing genes with altered expression at both days 7 and 14 post wounding, we find that there were 1950 unique to day 7, 392 unique to day 14, and 972 common to both (Fig 3A).
Fig 3. Venn diagram of differentially expressed genes in day 7 and 14 wounds, compared to normal skin.
Visual representation of the number of unique differentially genes at days 7 and 14 and those common to both days for (A) Mus and (B) Acomys. Unique and common differentially expressed genes between Acomys and Mus for (C) day 7 and (D) day 14. For (A) and (B), day 7 genes are in red, day 14 genes in blue and common genes are the overlap. For (C) and (D) Mus genes are in red and Acomys genes are in blue and common genes are the overlap.
In Acomys there were a total of 206 genes showing differential expression at day 7 after wounding compared to normal skin (S2 Table). Of these, 176 were upregulated and 30 were downregulated. At day 14 there were 327 genes with altered expression compared to normal skin, with 159 genes upregulated and 168 downregulated. Comparing days 7 and 14, there were 170 genes whose change in expression was unique to day 7, 291 unique to day 14, and 30 common to both days 7 and 14 (Fig 3B).
At 7 days post wounding in Mus, there was considerable upregulation of genes associated with the inflammatory response, in agreement with our observations by RT-PCR at days 3 and 5. There were a wide array of pro inflammatory genes, including most chemokines and cytokines, whose expression in the wound were increased from ~3 to 90-fold, as well as most interleukins, with interleukin 1 beta (Il1β) showing the largest increase at ~145-fold (S2 Table).
While most members of the collagen family were moderately upregulated, as would be expected in a healing wound, collagen 12a1 (Col12a1) was increased by nearly 30 fold in Mus (this increased expression persisted in the day 14 post wounding samples as well). This was unexpected since there is a lack of evidence in the literature for the role of Col12a1 in dermal wound healing. We confirmed the high levels of Col12a1 in Mus and lack of Col12a1 in Acomys by immunofluorescence using 2 different antibodies (Brant et al. manuscript under review).
In stark contrast to the high degree of response observed in Mus, the overall response to wounding in Acomys appears to be reduced, not only in the number of genes affected, but in the magnitude of change as well (from +63 to -2.3 fold). The genes with the largest increase in expression were not those associated with the inflammatory pathways, as observed in Mus, but were instead genes associated with the organization of the extracellular-matrix, such as collagens and platelet-derived growth factor binding proteins, as well as peptidases and matrix metalloproteinases (S2 Table).
We recognize that cross-species hybridization could possibly explain the decrease in the number of genes with a change in expression; we therefore performed an analysis of those genes whose change in expression was unique in Acomys. That is, these are genes with no detectable level of expression, or no observable change in expression in Mus wounds, whose expression is significantly altered in the wound in Acomys. Additionally, we also analyzed those genes whose changes in expression were unique to Mus. When comparing genes with altered expression at day 7 versus normal skin for both Mus and Acomys, there are 2804 genes unique to Mus and only 88 genes unique to Acomys, with 118 genes in common (Fig 3C).
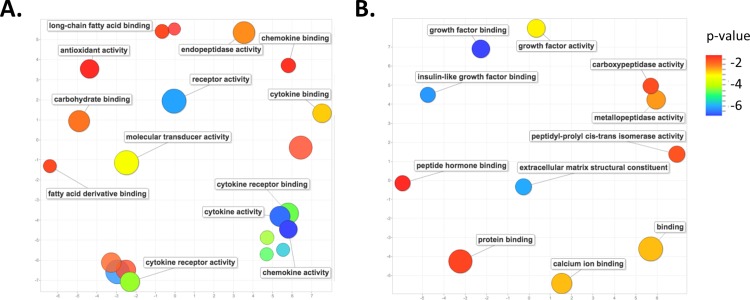
Those genes whose altered expression is unique to either Mus (2804) or Acomys (88) could provide insight into the observed difference in wound healing, i.e. scaring in Mus versus scar-free wound healing in Acomys. We performed an analysis of gene ontology (GO) terms associated with these unique genes. At day 7 post wounding in Mus there was a marked enrichment for GO terms associated with the inflammatory pathway, including cytokine receptor activity, chemokine and cytokine activity, cytokine receptor binding, cytokine and chemokine binding, fatty acid and carbohydrate binding, as well as endopeptidase and antioxidant activity (Fig 4A). A pathway enrichment analysis identified the top enriched pathways for genes unique to day 7 Mus wounds to be, as expected, those involved in the inflammatory pathways, including cytokine-cytokine receptor interaction, chemokine signaling pathway, Leishmanias, osteoclast differentiation, toll-like receptor signaling pathway and cell adhesion molecules (S3 Table).
Fig 4. GO term enrichment analysis for differentially expressed genes unique to each species at day 7 post-wounding.
Scatter plot representing the summarized GO term analysis of differentially expressed genes at day 7 post wounding in (A) Mus and (B) Acomys. Semantically similar GO terms are plotted near to each other on the (unit-less) X-Y axes, such that functionally similar terms are located nearby, and more unrelated terms are further apart in space. The size of the individual plots represents the frequency of the GO term in the database so that larger bubbles represent more general terms and smaller bubbles are more specific terms. The color indicates the p-value of enrichment of each GO term.
In contrast, the top enriched GO terms associated with those genes whose change in expression is unique to day 7 wounds in Acomys are those associated with the extracellular matrix reorganization, growth factors and hormone binding, including extracellular matrix structural constituent, metalloproteinase activity, carboxypeptidase activity, growth factor activity and binding, and peptide hormone binding (Fig 4B). The top enriched pathways in Acomys were those involved in protein digestion and absorption, extracellular matrix receptor interactions and focal adhesions (S4 Table).
A pathway analysis of the 1364 genes with significantly altered expression in day 14 wounds in Mus revealed an enrichment for genes associated with cell adhesion and migration, structural morphogenesis, muscle contraction and contractile fibers and myofibril components, as well as a lingering upregulation of cytokines, chemokines and interleukins (S2 Table). The results of a pathway analysis of the 327 altered genes in Acomys were more similar to Mus for day 14 than for day 7 wounds. Enriched pathways were observed for contractile fiber components and myofibril genes, as in Mus, as well genes associated with tissue development and morphogenesis (S2 Table).
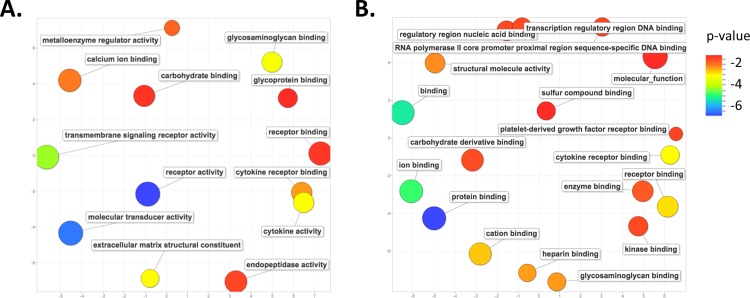
We again performed an analysis of GO terms for those genes whose altered expression is unique to either Mus (1254) or Acomys (217) at day 14 post-wounding (Fig 3D). The 1254 genes in Mus exhibit a persistence of enrichment for genes associated with the inflammation pathway, such as cytokine activity, cytokine receptor binding, and receptor binding and activity. There was also an enrichment for genes involved in the extracellular matrix organization, including metalloenzyme regulator activity and extracellular matrix structural constituent, as well as transmembrane signaling receptor activity and carbohydrate, glycosaminoglycan, glycoprotein and calcium ion binding (Fig 5A). A pathway analysis of these same genes reveals a large number of genes involved in cellular respiration, oxidative phosphorylation, fructose and mannose metabolism, metabolic pathways, as well as the genes involved with the lysosome and focal adhesions (S5 Table).
Fig 5. GO term enrichment analysis for differentially expressed genes unique to each species at day 14 post-wounding.
Scatter plot representing the summarized GO term analysis of differentially expressed genes at day 14 post wounding in (A) Mus and (B) Acomys. All labels, symbols and calculations are as those described in Fig 4.
An enrichment analysis for the GO terms associated with the 217 genes unique to Acomys at day 14 wounds reveals genes involved in the regulation of transcription, such as regulatory and transcription regulatory region binding and RNA-polII DNA binding. There was also an enrichment for carbohydrate, protein and ion binding, as well genes involved with cytokine receptor and platelet-derived growth factor receptor binding (Fig 5B). A pathway analysis for these same genes shows enrichment for growth factor pathways, including insulin, VEGF, toll-like receptor, adipocytokine and chemokine signaling pathways (S6 Table).
Discussion
The recent discovery of the remarkable capacity of Acomys cahirinus to regenerate full thickness excisional skin wounds in a scar free manner [21] provides a unique opportunity to compare scar free wound healing with scaring in two adult mammalian tissues. Previous studies were limited to comparing wound healing in adult tissues to fetal tissues, which have a limited ability for scar free healing. Although much has been learned from these studies, there are inherent differences between fetal and adult tissues which imposes limits on these comparative studies and as such, very little has translated into improved clinical outcomes. In this study, we have used RT-PCR and microarray analyses to generate gene expression profiles of full thickness excisional skin wounds in both Mus and Acomys to compare scar free wound healing to scaring in adult tissue and to determine if there are any similarities to scar free healing observed in fetal tissues. We have identified changes in gene expression patterns for several aspects of wound healing between Mus and Acomys, particularly for those genes involved in the inflammatory pathway and the deposition and digestion of the extracellular matrix, and have identified similarities to fetal wounds.
There are several notable differences between fetal and adult wounds, which are predominately in the inflammatory pathway and the collagen profile of the ECM and in expression levels of various growth factors. Previous studies have shown that the inflammatory response is quite blunted in fetal wounds [4, 5]. Additionally, it has been shown that ectopically induced inflammation in fetal wounds increases fibrosis and results in scar formation [38, 39]. Our data indicates that wounding initiates a strong inflammatory response in Mus, typical of the adult mammalian response, while the inflammatory response in Acomys appears to be modest, similar to fetal wounds.
Immediately after wounding platelets enter the wound bed and release Pdgfa and Tgf-β1/2 [40–42]. Studies have shown that Pdgfa and Tgf-β1/2 levels are lower in fetal compared to adult wounds [43]. In contrast to fetal wounds, our data indicate no difference in Pdgfa expression in day 3 and 5 wounds, and an increase in Tgf-β1 expression in Acomys.
Shortly after platelets enter the wound, neutrophils are recruited by neutrophil attracting chemokines and activated by neutrophil activating cytokines (reviewed extensively in [44–46]). Studies have shown that neutrophil levels are higher in adult wounds compared to fetal wounds [9]. Counts of circulating leukocytes, from non-wounded animals, revealed that neutrophils are present in Acomys at only 20% the levels observed in Mus (Brant et al. manuscript under review). The impact of reduced circulating neutrophil levels is unknown at this time but certainly warrants further study. By day 2 after wounding, macrophages constitute the predominant blood-derived cell type in the wound bed and initiate release of pro inflammatory cytokines and growth factors [47]. Our data show a striking difference in the expression levels of several genes thought to play important roles in inflammation. The expression of the chemokine Cxcl3 is only slighter higher in Acomys wounds, compared to normal skin, and Cxcl1 and Cxcl5 are barely detectable by RT-PCR, in contrast to the high levels of induction observed in Mus. As the c-x-c like family of chemokines is expressed by macrophages and neutrophils, this observed difference in expression could in part be explained by the observed reduction in circulating neutrophils in Acomys. Additionally, the induction of granulocyte stimulating factor Csf3 mRNA is much lower in Acomys as well, suggesting an overall diminished role of neutrophils in Acomys. We have additional data that shows that while macrophages are present in Acomys in very early wounds, they are confined to the underlying fascial connective tissue and are not present in the wound bed at later time points (Brant et al. manuscript in review). The increased upregulation of Tgf-β1 is intriguing in light of the seemingly blunted immune response observed in Acomys, given its role in promoting inflammation, among others, during wound healing. It is also interesting to note that while differences have been observed for various growth factors between fetal and adult wounds [34, 48–50], our data indicate that there were no statistically significant changes observed in expression levels of the growth factors Csf2, Ctgf, Egf, Fgf2, Fgf10 and Vegfa between Mus and Acomys wounds (S1 Table).
Interleukin 4 expression is significantly downregulated in Acomys, while there is no significant change in expression for Mus. As Il4 is thought to promote alternative activation of macrophages [51–53], the downregulation of Il4 seems to run counter to the observed blunted inflammatory response. Il4 has also been shown to be involved in the stimulation of production of components of the ECM [30–32]. Perhaps lower levels of Il4 prevent an overproduction of collagen formation and may help to prevent scar formation. Interleukin 10 (Il10) is known to be a potent anti-inflammatory pleiotropic cytokine produced by a number of cell types, including T cells, B cells, monocytes and alternatively activated macrophages (reviewed extensively in [54] and [55]). While Il10 is upregulated approximately 6 fold in Mus day 3 and 5 wounds, there is no detectable expression of Il10 in Acomys. This could be due to an absence of those cells that produce Il10 in the wound, as we have shown with F4/80 and cd206 +ve macrophages (Brant et al. manuscript in review). Alternatively, it may be that the Mus Il10 PCR primers do not efficiently anneal to Acomys Il10 sequence. Given the importance of Il10 function in dampening the inflammatory response, this will need to be studied further going forward. Acomys also exhibits much lower levels of Il1β expression in wounds than in Mus. Il1β is a potent proinflammatory cytokine produced by monocytes and activated macrophages [56, 57]. Il1β is also known to induce Ptgs2 expression [58], although the expression of Ptgs2 is much higher in Acomys wounds compared to normal skin than in Mus wounds. The high levels of Ptgs2 in Acomys wounds is interesting, in that it also seems contrary to data suggesting Ptgs2 levels are correlated with scar formation in skin wounds. Wilgus et al. showed that the Ptgs2 inhibitor Celecoxib® decreased inflammation in incisional wounds and reduced scar formation [35] and have also shown that high levels of Ptgs2 promote scarring and delay wound closure in fetal wounds [34].
The collagen composition of fetal skin has been shown to differ from adult skin [12–14], with fetal skin having higher levels of collagen type III and V than adult skin, among other differences. The expression of collagens in Acomys wounds suggests that they have a profile similar to fetal skin, with higher levels of expression for collagens 3a1, 5a1, 5a2, and lower levels of expression of collagens 5a3 and 14a1. In addition to differences in the composition of the ECM, there are notable differences in the expression of matrix metalloproteinases involved in the degradation of the ECM. Fetal skin wounds have been shown to have a higher matrix metalloproteinase to tissue inhibitor of metalloproteinase ratio [16, 17]. Similar to fetal wounds, Acomys wounds have significantly higher levels of expression of Mmp2 and Mmp9, and lower levels of expression of Timp1 in day 3 and 5 wounds compared to Mus. This suggests that there is a higher turnover of ECM components and an increase in cell migration through the wound bed in Acomys. Fetal wounds have also been shown to have essentially no myofibroblasts, which are thought to interact with the ECM and aid in wound closure through contraction, while both scaring fetal and adult wounds have high levels of contractile myofibroblasts appearing 7 days post wounding [59]. Although Acomys wounds heal in a scar-free manner, similar to fetal wounds, our data indicate there is no difference in the expression of smooth muscle actin, a marker of myofibroblasts, between Mus and Acomys wounds from days 3, 5 (S1 Table), 7 and 14 (S2 Table).
We considered the possibility that some of the observed differences in expression could be the result of inefficient cross species hybridization of oligos used in the RT-PCR arrays. Although the primers used in the arrays were designed for Mus, there was detectable amplification for 54/84 genes in Acomys, compared to 79/84 for Mus. This could be due to the fact that not all genes from the array are expressed in skin or wounds in both species. Alternatively, some genes may have sufficiently diverged from the mouse, such that Mus specific primers do not anneal. In either case, the majority of genes have detectable levels of amplification, suggesting a fairly high degree of conservation, at least for coding regions. In order to confirm that we are in fact amplifying the correct mRNA, we have cloned and sequenced the Tgf-β1, Timp1 and Mmp9 RT-PCR amplicons from Acomys. A BLAST using the resulting sequences against the Mus transcript database reveals high identity matches for all three sequences (S1A–S1C Fig). The percent identity for Tgf-β1 and Timp1 was 98% and 100%, respectively. Although the percent identity for Mmp9 was 74% for Mus, there were higher identity matches in other rodent species, e.g. Peromyscus maniculatus (Deer mouse, 91%).
Microarray analysis was performed for both Acomys and Mus wounds at day 7 and 14 post wounding. The expression data for day 7 wounds are similar to the day 3 and 5 wound data, confirming the observation that the inflammatory response in Acomys is quite blunted and further validates our RT-PCR data. We appreciate the fact that the reduced number of differentially expressed genes observed in Acomys wounds, as compared to Mus, could be an issue of cross-species hybridization, as we used microarrays designed for use with Mus RNA. Therefore, part of our analysis was focused on those genes whose changes in expression are unique to Acomys. We felt that these would be of the highest confidence, giving the limits of the technology, and could provide the most significant biological insights into scar free healing. Pathway analysis and GO term enrichment analysis of these unique genes gave the same results as analyses using the whole dataset, i.e. using both unique and common sets of differentially expressed genes, suggesting that the observed enriched pathways are largely driven by those genes which are uniquely differentially expressed in each species and not necessarily by those whose change is common to both species.
In summary, the data presented here provide insights into scar free healing of full-thickness excisional wounds of Acomys, and provides a starting point of potential gene candidates that may be further studied, in hopes of devising potential strategies for improved clinical outcomes in preventing scarring in humans.
Supporting Information
BLAST results of cloned and sequenced Acomys RT-PCR amplicons against the Mus transcript database for (A) Tgf-β1, (B) Timp1, and (C) Mmp9.
(PDF)
List of genes analyzed by Wound-Healing RT2 Profiler Array in day 3 and 5 wounds, compared to normal skin, within each species. Bold entries are those with a p-value ≤0.01.
(DOCX)
Tables of p-values and fold-change in expression with each analysis in its own worksheet.
(XLSX)
Pathway analysis of differentially expressed genes between day 7 wounds and normal skin in Mus.
(DOCX)
Pathway analysis of differentially expressed genes between day 7 wounds and normal skin in Acomys.
(DOCX)
Pathway analysis of differentially expressed genes between day 14 wounds and normal skin in Mus.
(DOCX)
Pathway analysis of differentially expressed genes between day 14 wounds and normal skin in Acomys.
(DOCX)
Acknowledgments
This work was funded by a grant from the WM Keck Foundation. Based in Los Angeles, the W. M. Keck Foundation was established in 1954 by the late W. M. Keck, founder of the Superior Oil Company. The Foundation’s grant making is focused primarily on pioneering efforts in the areas of medical, science and engineering research. The Foundation also maintains an undergraduate education program that promotes distinctive learning and research experiences for students in the sciences and in the liberal arts, and a Southern California Grant Program that provides support for the Los Angeles community, with a special emphasis on children and youth from low-income families, special needs populations and safety-net services. For more information visit www.wmkeck.org
Data Availability
All relevant data are within the paper and its Supporting Information files. The microarray dataset can be found at the NCBI's Gene Expression Omnibus under the accession number GSE74387.
Funding Statement
This work was funded by a grant from the WM Keck Foundation, grant: The Transformative Role of the Regenerating Spiny Mouse to MM and BB (http://www.wmkeck.org/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Clark RAF, Henson PM. The Molecular and cellular biology of wound repair New York: Plenum Press; 1988. xxii, 597 p. p. [Google Scholar]
- 2. Rolfe KJ, Grobbelaar AO. A review of fetal scarless healing. ISRN dermatology. 2012;2012:698034 Epub 2012/06/08. 10.5402/2012/698034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Satish L, Kathju S. Cellular and Molecular Characteristics of Scarless versus Fibrotic Wound Healing. Dermatology research and practice. 2010;2010:790234 Epub 2011/01/22. 10.1155/2010/790234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liechty KW, Crombleholme TM, Cass DL, Martin B, Adzick NS. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. The Journal of surgical research. 1998;77(1):80–4. Epub 1998/08/12. 10.1006/jsre.1998.5345 . [DOI] [PubMed] [Google Scholar]
- 5. Liechty KW, Adzick NS, Crombleholme TM. Diminished interleukin 6 (IL-6) production during scarless human fetal wound repair. Cytokine. 2000;12(6):671–6. Epub 2000/06/14. 10.1006/cyto.1999.0598 . [DOI] [PubMed] [Google Scholar]
- 6. Olutoye OO, Barone EJ, Yager DR, Cohen IK, Diegelmann RF. Collagen induces cytokine release by fetal platelets: implications in scarless healing. Journal of pediatric surgery. 1997;32(6):827–30. Epub 1997/06/01. . [DOI] [PubMed] [Google Scholar]
- 7. Cowin AJ, Brosnan MP, Holmes TM, Ferguson MW. Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Developmental dynamics: an official publication of the American Association of Anatomists. 1998;212(3):385–93. Epub 1998/07/22. . [DOI] [PubMed] [Google Scholar]
- 8. Adzick NS, Harrison MR, Glick PL, Beckstead JH, Villa RL, Scheuenstuhl H, et al. Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations. Journal of pediatric surgery. 1985;20(4):315–9. Epub 1985/08/01. . [DOI] [PubMed] [Google Scholar]
- 9. Jennings RW, Adzick NS, Longaker MT, Duncan BW, Scheuenstuhl H, Hunt TK. Ontogeny of fetal sheep polymorphonuclear leukocyte phagocytosis. Journal of pediatric surgery. 1991;26(7):853–5. Epub 1991/07/01. . [DOI] [PubMed] [Google Scholar]
- 10. Sullivan KM, Lorenz HP, Meuli M, Lin RY, Adzick NS. A model of scarless human fetal wound repair is deficient in transforming growth factor beta. Journal of pediatric surgery. 1995;30(2):198–202; discussion -3. Epub 1995/02/01. . [DOI] [PubMed] [Google Scholar]
- 11. Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009;17(2):153–62. Epub 2009/03/27. 10.1111/j.1524-475X.2009.00466.x . [DOI] [PubMed] [Google Scholar]
- 12. Smith LT, Holbrook KA, Madri JA. Collagen types I, III, and V in human embryonic and fetal skin. The American journal of anatomy. 1986;175(4):507–21. Epub 1986/04/01. 10.1002/aja.1001750409 . [DOI] [PubMed] [Google Scholar]
- 13. Hallock GG, Rice DC, Merkel JR, DiPaolo BR. Analysis of collagen content in the fetal wound. Annals of plastic surgery. 1988;21(4):310–5. Epub 1988/10/01. . [DOI] [PubMed] [Google Scholar]
- 14. Cuttle L, Nataatmadja M, Fraser JF, Kempf M, Kimble RM, Hayes MT. Collagen in the scarless fetal skin wound: detection with picrosirius-polarization. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2005;13(2):198–204. Epub 2005/04/15. 10.1111/j.1067-1927.2005.130211.x . [DOI] [PubMed] [Google Scholar]
- 15. Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. The international journal of biochemistry & cell biology. 2008;40(6–7):1334–47. Epub 2007/12/18. 10.1016/j.biocel.2007.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peled ZM, Phelps ED, Updike DL, Chang J, Krummel TM, Howard EW, et al. Matrix metalloproteinases and the ontogeny of scarless repair: the other side of the wound healing balance. Plastic and reconstructive surgery. 2002;110(3):801–11. Epub 2002/08/13. . [DOI] [PubMed] [Google Scholar]
- 17. Dang CM, Beanes SR, Lee H, Zhang X, Soo C, Ting K. Scarless fetal wounds are associated with an increased matrix metalloproteinase-to-tissue-derived inhibitor of metalloproteinase ratio. Plastic and reconstructive surgery. 2003;111(7):2273–85. Epub 2003/06/10. 10.1097/01.PRS.0000060102.57809.DA . [DOI] [PubMed] [Google Scholar]
- 18. Levesque M, Villiard E, Roy S. Skin wound healing in axolotls: a scarless process. Journal of experimental zoology Part B, Molecular and developmental evolution. 2010;314(8):684–97. Epub 2010/08/19. 10.1002/jez.b.21371 . [DOI] [PubMed] [Google Scholar]
- 19. Seifert AW, Monaghan JR, Voss SR, Maden M. Skin regeneration in adult axolotls: a blueprint for scar-free healing in vertebrates. PLoS ONE. 2012;7(4):e32875 Epub 2012/04/10. 10.1371/journal.pone.0032875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steppan S, Adkins R, Anderson J. Phylogeny and divergence-date estimates of rapid radiations in muroid rodents based on multiple nuclear genes. Systematic biology. 2004;53(4):533–53. Epub 2004/09/17. 10.1080/10635150490468701 . [DOI] [PubMed] [Google Scholar]
- 21. Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature. 2012;489(7417):561–5. Epub 2012/09/29. 10.1038/nature11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. Epub 2002/02/16. 10.1006/meth.2001.1262 . [DOI] [PubMed] [Google Scholar]
- 23. Zhang B, Kirov S, Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33(Web Server issue):W741–8. Epub 2005/06/28. 10.1093/nar/gki475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang J, Duncan D, Shi Z, Zhang B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res. 2013;41(Web Server issue):W77–83. Epub 2013/05/25. 10.1093/nar/gkt439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Supek F, Bosnjak M, Skunca N, Smuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE. 2011;6(7):e21800 Epub 2011/07/27. 10.1371/journal.pone.0021800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Welte K, Platzer E, Lu L, Gabrilove JL, Levi E, Mertelsmann R, et al. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci U S A. 1985;82(5):1526–30. Epub 1985/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Begley CG, Lopez AF, Nicola NA, Warren DJ, Vadas MA, Sanderson CJ, et al. Purified colony-stimulating factors enhance the survival of human neutrophils and eosinophils in vitro: a rapid and sensitive microassay for colony-stimulating factors. Blood. 1986;68(1):162–6. Epub 1986/07/01. . [PubMed] [Google Scholar]
- 28. Frampton JE, Lee CR, Faulds D. Filgrastim. A review of its pharmacological properties and therapeutic efficacy in neutropenia. Drugs. 1994;48(5):731–60. Epub 1994/11/01. . [DOI] [PubMed] [Google Scholar]
- 29. Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39(6):1003–18. Epub 2013/12/18. 10.1016/j.immuni.2013.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fertin C, Nicolas JF, Gillery P, Kalis B, Banchereau J, Maquart FX. Interleukin-4 stimulates collagen synthesis by normal and scleroderma fibroblasts in dermal equivalents. Cellular and molecular biology. 1991;37(8):823–9. Epub 1991/01/01. . [PubMed] [Google Scholar]
- 31. Gillery P, Fertin C, Nicolas JF, Chastang F, Kalis B, Banchereau J, et al. Interleukin-4 stimulates collagen gene expression in human fibroblast monolayer cultures. Potential role in fibrosis. FEBS letters. 1992;302(3):231–4. Epub 1992/05/18. . [DOI] [PubMed] [Google Scholar]
- 32. Salmon-Ehr V, Ramont L, Godeau G, Birembaut P, Guenounou M, Bernard P, et al. Implication of interleukin-4 in wound healing. Laboratory investigation; a journal of technical methods and pathology. 2000;80(8):1337–43. Epub 2000/08/19. . [DOI] [PubMed] [Google Scholar]
- 33. Futagami A, Ishizaki M, Fukuda Y, Kawana S, Yamanaka N. Wound healing involves induction of cyclooxygenase-2 expression in rat skin. Laboratory investigation; a journal of technical methods and pathology. 2002;82(11):1503–13. Epub 2002/11/14. . [DOI] [PubMed] [Google Scholar]
- 34. Wilgus TA, Bergdall VK, Tober KL, Hill KJ, Mitra S, Flavahan NA, et al. The impact of cyclooxygenase-2 mediated inflammation on scarless fetal wound healing. The American journal of pathology. 2004;165(3):753–61. Epub 2004/08/28. 10.1016/S0002-9440(10)63338-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wilgus TA, Vodovotz Y, Vittadini E, Clubbs EA, Oberyszyn TM. Reduction of scar formation in full-thickness wounds with topical celecoxib treatment. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2003;11(1):25–34. Epub 2003/02/13. . [DOI] [PubMed] [Google Scholar]
- 36. Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–28. Epub 1999/02/20. . [DOI] [PubMed] [Google Scholar]
- 37. Annes JP, Rifkin DB, Munger JS. The integrin alphaVbeta6 binds and activates latent TGFbeta3. FEBS letters. 2002;511(1–3):65–8. Epub 2002/02/01. . [DOI] [PubMed] [Google Scholar]
- 38. Ozturk S, Deveci M, Sengezer M, Gunhan O. Results of artificial inflammation in scarless foetal wound healing: an experimental study in foetal lambs. British journal of plastic surgery. 2001;54(1):47–52. Epub 2000/12/21. 10.1054/bjps.2000.3460 . [DOI] [PubMed] [Google Scholar]
- 39. Dixon JB. Inflammation in the foetal and neonatal rat: the local reactions to skin burns. The Journal of pathology and bacteriology. 1960;80:73–82. Epub 1960/07/01. . [DOI] [PubMed] [Google Scholar]
- 40. Ross R, Raines EW, Bowen-Pope DF. The biology of platelet-derived growth factor. Cell. 1986;46(2):155–69. Epub 1986/07/18. . [DOI] [PubMed] [Google Scholar]
- 41. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiological reviews. 2003;83(3):835–70. Epub 2003/07/05. 10.1152/physrev.00031.2002 . [DOI] [PubMed] [Google Scholar]
- 42. Plasari G, Calabrese A, Dusserre Y, Gronostajski RM, McNair A, Michalik L, et al. Nuclear factor I-C links platelet-derived growth factor and transforming growth factor beta1 signaling to skin wound healing progression. Mol Cell Biol. 2009;29(22):6006–17. Epub 2009/09/16. 10.1128/MCB.01921-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Olutoye OO, Barone EJ, Yager DR, Uchida T, Cohen IK, Diegelmann RF. Hyaluronic acid inhibits fetal platelet function: implications in scarless healing. Journal of pediatric surgery. 1997;32(7):1037–40. Epub 1997/07/01. . [DOI] [PubMed] [Google Scholar]
- 44. Nathan C. Neutrophils and immunity: challenges and opportunities. Nature reviews Immunology. 2006;6(3):173–82. Epub 2006/02/25. 10.1038/nri1785 . [DOI] [PubMed] [Google Scholar]
- 45. Wilgus TA, Roy S, McDaniel JC. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Advances in wound care. 2013;2(7):379–88. Epub 2014/02/15. 10.1089/wound.2012.0383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lo DD, Zimmermann AS, Nauta A, Longaker MT, Lorenz HP. Scarless fetal skin wound healing update. Birth defects research Part C, Embryo today: reviews. 2012;96(3):237–47. Epub 2012/10/31. 10.1002/bdrc.21018 . [DOI] [PubMed] [Google Scholar]
- 47. Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. The American journal of pathology. 1975;78(1):71–100. Epub 1975/01/01. [PMC free article] [PubMed] [Google Scholar]
- 48. Cowin AJ, Holmes TM, Brosnan P, Ferguson MW. Expression of TGF-beta and its receptors in murine fetal and adult dermal wounds. European journal of dermatology: EJD. 2001;11(5):424–31. Epub 2001/08/30. . [PubMed] [Google Scholar]
- 49. Dang CM, Beanes SR, Soo C, Ting K, Benhaim P, Hedrick MH, et al. Decreased expression of fibroblast and keratinocyte growth factor isoforms and receptors during scarless repair. Plastic and reconstructive surgery. 2003;111(6):1969–79. Epub 2003/04/25. 10.1097/01.PRS.0000054837.47432.E7 . [DOI] [PubMed] [Google Scholar]
- 50. Haynes JH, Johnson DE, Mast BA, Diegelmann RF, Salzberg DA, Cohen IK, et al. Platelet-derived growth factor induces fetal wound fibrosis. Journal of pediatric surgery. 1994;29(11):1405–8. Epub 1994/11/01. . [DOI] [PubMed] [Google Scholar]
- 51. Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10(2):137–42. Epub 1999/03/11. . [DOI] [PubMed] [Google Scholar]
- 52. Varin A, Mukhopadhyay S, Herbein G, Gordon S. Alternative activation of macrophages by IL-4 impairs phagocytosis of pathogens but potentiates microbial-induced signalling and cytokine secretion. Blood. 2010;115(2):353–62. Epub 2009/11/03. 10.1182/blood-2009-08-236711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC immunology. 2002;3:7 Epub 2002/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunological reviews. 2008;226:205–18. 10.1111/j.1600-065X.2008.00706.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews Immunology. 2008;8(12):958–69. 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kurt-Jones EA, Beller DI, Mizel SB, Unanue ER. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci U S A. 1985;82(4):1204–8. Epub 1985/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annual review of immunology. 2009;27:519–50. Epub 2009/03/24. 10.1146/annurev.immunol.021908.132612 . [DOI] [PubMed] [Google Scholar]
- 58. O'Banion MK, Miller JC, Chang JW, Kaplan MD, Coleman PD. Interleukin-1 beta induces prostaglandin G/H synthase-2 (cyclooxygenase-2) in primary murine astrocyte cultures. Journal of neurochemistry. 1996;66(6):2532–40. Epub 1996/06/01. . [DOI] [PubMed] [Google Scholar]
- 59. Estes JM, Vande Berg JS, Adzick NS, MacGillivray TE, Desmouliere A, Gabbiani G. Phenotypic and functional features of myofibroblasts in sheep fetal wounds. Differentiation; research in biological diversity. 1994;56(3):173–81. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BLAST results of cloned and sequenced Acomys RT-PCR amplicons against the Mus transcript database for (A) Tgf-β1, (B) Timp1, and (C) Mmp9.
(PDF)
List of genes analyzed by Wound-Healing RT2 Profiler Array in day 3 and 5 wounds, compared to normal skin, within each species. Bold entries are those with a p-value ≤0.01.
(DOCX)
Tables of p-values and fold-change in expression with each analysis in its own worksheet.
(XLSX)
Pathway analysis of differentially expressed genes between day 7 wounds and normal skin in Mus.
(DOCX)
Pathway analysis of differentially expressed genes between day 7 wounds and normal skin in Acomys.
(DOCX)
Pathway analysis of differentially expressed genes between day 14 wounds and normal skin in Mus.
(DOCX)
Pathway analysis of differentially expressed genes between day 14 wounds and normal skin in Acomys.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. The microarray dataset can be found at the NCBI's Gene Expression Omnibus under the accession number GSE74387.
The microarray dataset discussed in this publication has been deposited in NCBI’s Gene Expression Omnibus and is accessible through GEO Series accession number GSE74387. (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE74387)