Abstract
The 90 kDa heat shock proteins (Hsp90) are responsible for the conformational maturation of nascent polypeptides and the rematuration of denatured proteins. Proteins dependent upon Hsp90 are associated with all six hallmarks of cancer. Upon Hsp90 inhibition, protein substrates are degraded via the ubiquitin-proteasome pathway. Consequentially, inhibition of Hsp90 offers a therapeutic opportunity for the treatment of cancer. Natural product inhibitors of Hsp90 have been identified in vitro, which have served as leads for the development of more efficacious inhibitors and analogs that have entered clinical trials. This review highlights the development of natural product analogs, as well as the development of clinically important inhibitors that arose from natural products.
Keywords: Hsp90, chaperone, geldanamycin, radicicol, natural product-based drug design
Introduction
Natural products cover a vast amount of chemical space and their unique, complex structures represent an excellent platform for the optimization of biological activities. Natural products or compounds derived from natural products comprise the majority of currently marketed drugs, in addition, there are others undergoing pre-clinical and/or clinical development.1-3 Recent advances in bioanalytical techniques, DNA sequencing, and bioinformatics have increased the rate at which new natural products can be discovered.4 Consequently, natural products remain a major source for lead compounds in drug discovery. Although natural products are common sources for leads, library and fragment based screens have gained popularity in recent years.5,6 Recently, Zeilinger and co-workers reported a microarray-based screening method for rapid screening that utilizes low protein and compound concentrations.7These methods have also resulted in drugs that have been FDA approved, as well as others undergoing clinical development. However, natural products continue to provide complex three-dimensional scaffolds that serve as leads for drug development. Such scaffolds manifest unique biological activities that continue to expand the number of validated therapeutic targets.8
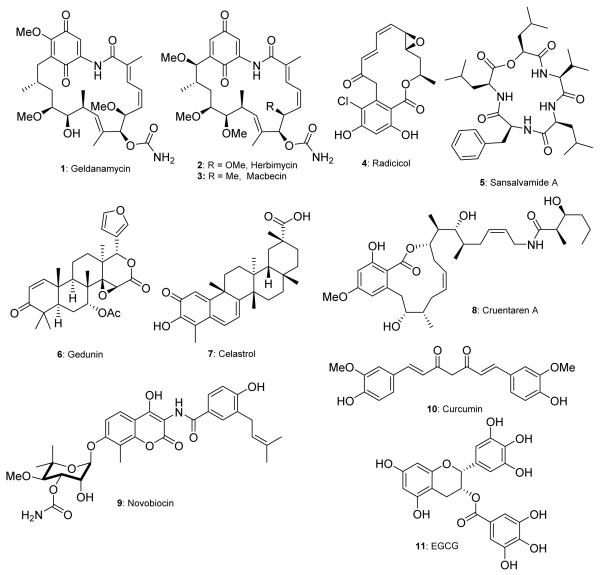
Geldanamycin (GDA), a member of the ansamycin class of antibiotics, is a classical example of natural product-based drug discovery in medicinal chemistry.9 Analysis of the biological activity elicited from the administration of GDA led to the discovery that its primary biological target is the 90 kDa heat shock protein, Hsp90.10 Many drug development campaigns with GDA have provided drug candidates that have entered clinical trials and provided biological probes that have increased the understanding of Hsp90 biology.11,12 In the last two decades, other natural products have also been shown to bind and inhibit the Hsp90 chaperone machinery (Figure 1).10,13-24 These natural products have served as a starting point for numerous drug development campaigns; which have led to the discovery of highly efficacious inhibitors that have now entered clinical trials.9,25-27 This review gives a synopsis of natural product-based Hsp90 N-terminal inhibitors.
Figure 1.
Natural product inhibitors of Hsp90. 1-4 bind Hsp90 in the N-terminal ATP-binding pocket. 5 is an allosteric modulator of Hsp90. 6-8 disrupt the interaction of Hsp90 and co-chaperones. 9-11 bind the C-terminal ATP-binding motif.
Molecular chaperones represent a class of proteins that are responsible for the conformational maturation of nascent polypeptides as well as the rematuration of denatured proteins.28-30 These chaperones work together with co-chaperones and partner proteins to facilitate the folding of substrate proteins (referred to as clients). Many of these chaperones are upregulated in response to conditions that cause cellular stress, such as exposure to heavy metals, hypoxia, acidosis, and increased/decreased temperature.31 The heat shock proteins were originally identified after cellular exposure to elevated temperatures, which led to their overexpression to refold proteins that denatured at such temperatures.32 Hsp90 is responsible for the maturation of more than 200 proteins, including several therapeutically sought after anticancer targets such as Her2, Raf, ALK, Src, and Akt.33,34 Hsp90 is highly conserved in eukaryotes and represents 1-2% of the total cellular protein at ambient temperatures, but can represent up to 6% in stressed cells.34-36 Hsp90 contains an ATP-binding site that is conserved within the GHKL superfamily of proteins, such as histidine kinase, DNA gyrase B, and MutL. The GHKL superfamily is characterized by the presence of Bergerat ATP-binding fold in which ATP binds in a unique, bent conformation that is in contrast to the typical extended conformation.17,37 In cells, Hsp90 exists as a homodimer and each monomer consists of an N-terminal ATP-binding domain, a middle domain connected to the N-terminus by a charged linker, and a C-terminal dimerization motif. ATP binds to the highly conserved N-terminus and its hydrolysis provides the requisite source of energy to facilitate client protein maturation. The middle domain plays a crucial role in client protein recognition, as well as interactions with co-chaperones. The C-terminus mediates dimerization and contains a second nucleotide binding motif.38. The Hsp90 family of proteins consists of four isoforms in mammals: Hsp90α and Hsp90β are localized in the cytosol, however, Hsp90β is constitutively expressed and Hsp90α is inducible upon exposure to cellular stress. Grp94 is found in the endoplasmic reticulum and Trap-1 resides in the mitochondria. 39
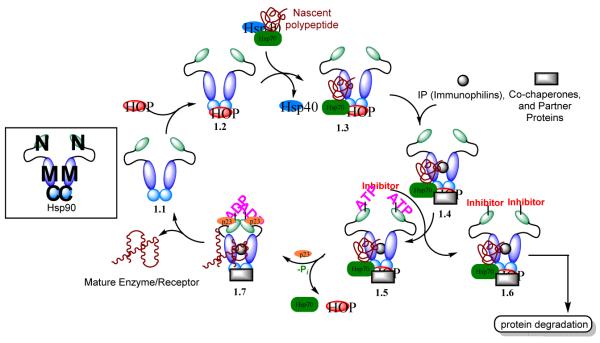
The Hsp90 chaperone cycle is complex, but advances in technology and small molecule probes have provided insights into the catalytic cycle. The Hsp90-mediated protein folding process is catalytic and protein maturation is driven by ATP-hydrolysis (Figure 2). The Hsp90 chaperone cycle begins with the delivery of nascent polypeptides to Hsp90 by Hsp70 (1.3). This process is mediated by the Hsp70-Hsp90 organizing co-chaperone, Hop (1.2). Upon delivery of the nascent polypeptide, immunophilins, co-chaperones, and partner proteins interact with the Hsp90 homodimer to form a heteroprotein complex (1.4).40 ATP is recruited to the N-terminus (1.5) and then hydrolyzed upon p23 association (1.7), which stabilizes the closed conformation of Hsp90.38 Following maturation, the client protein is released and the heteroprotein complex dissociates to regenerate the Hsp90 homodimer (1.1).41
Figure 2.
The Hsp90 chaperoning cycle highlighting the effect of inhibitors on the cycle (1.6)
Inhibition of either the N- or C-terminus can disrupt the Hsp90 chaperone cycle. The N-terminal binding site is very specific for ATP, however the C-terminus of Hsp90 binds both purine and pyrimidine nucleotides.42 Neckers and co-workers demonstrated that GDA does not bind to the C-terminus and is a specific, competitive inhibitor of ATP binding at the N-terminus.43 Alternatively, novobiocin binds exclusively at the C-terminus of Hsp90. Upon disruption of the chaperone cycle, the client protein is degraded primarily through the ubiquitin-proteasome pathway (1.6).44 Because malignant cells undergo a constant rate of proliferation, increased rates of protein synthesis and metabolism are required for survival. As a consequence of the increased dependency upon Hsp90, Hsp90 exists solely as the heteroprotein complex (1.4) in malignant cells. While in normal cells, Hsp90 exists as the homodimer (1.1). The heteroprotein complex possesses more than 200-fold greater affinity for ATP compared to the Hsp90 homodimer.27,45 Therefore, inhibitors that bind the ATP-binding pocket display an inherent selectivity for the heteroprotein complex, resulting in differential selectivities of ~200-fold for malignant verses normal cells. Not surprisingly, Hsp90 inhibitors accumulate at higher concentrations in tumor tissue than in normal tissue.45
Hsp90 inhibition exhibits therapeutic potential for the treatment of many different diseases; most notably Hsp90 inhibitors are sought after as cancer chemotherapeutics.27,31,45,46 Since many of the Hsp90-dependent clients are associated with signal transduction pathways that become hijacked during cancer transformation, Hsp90 inhibitors manifest a single drug combination approach toward the treatment of cancer.47 As shown in Table I, Hsp90 client proteins are present in every hallmark of cancer, therefore, inhibition of Hsp90 provides a unique opportunity to simultaneously target multiple oncogenic pathways, and thus provides a multidimensional attack on cancer.37,48,49 N-terminal inhibitors represent the most developed class of Hsp90 inhibitors and will be discussed in detail. In addition, Hsp90 C-terminal inhibitors have shown promise as therapeutic agents.50-54 Novobiocin, a member of the aminocoumarin class of antibiotics was identified as the first C-terminal Hsp90 inhibitor.43,55,56 In 2009, polyphenol epigallocatechin-3-gallate, EGCG, was shown to also bind the C-terminus and inhibit Hsp90 function.57 The tetranortriterpenoid, gedunin and cyclic peptide sansalvamide A bind at sites other than the N- or C-terminus and manifest anti-cancer activity through alternative modulation of the Hsp90 chaperone cycle.21,23,58-60 In addition, alternative approaches to target Hsp90’s interaction with partner proteins and co-chaperones have also emerged as novel strategies to target cancer.41,61 For example, Celestrol, disrupts the Hsp90-Cdc37 complex and leads to inhibition of the chaperone cycle.62 Cdc37 is a co-chaperone that mediates the loading of protein kinases to Hsp90. Additionally, the macrolide natural product, Cruentaren A, disrupts the interaction of F1F0 ATP synthase with Hsp90 and manifests low nanomolar efficacy against various cancer cell lines.16,63 Structure-activity relationship investigations of these natural products have produced highly efficacious anti-cancer agents.64,65
Table I.
The six hallmarks of cancer and Hsp90-dependent client proteins associated with each hallmark.
| Hallmarks of Cancer | Hsp90 Client Protein(s) |
|---|---|
| 1) Self-sufficient growth signals | Raf-1, AKT, Her2, MEK, Bcr-Abl |
| 2) Insensitive to anti-growth signals | Plk, Wee1, Myt1, CDK4, CDK6 |
| 3) Evasion of apoptosis | RIP, AKT, mutant p53,c-MET, Apaf-1, Survivin |
| 4) Limitless replicative potential | Telomerase (h-Tert) |
| 5) Sustained angiogenesis | FAK, AKT,Hif-1α, VEGFR, Flt-3 |
| 6) Tissue invasion and metastasis | C-MET |
Geldanamycin-based Inhibitors
GDA (1, Figure 1) was originally isolated from the geldanus variant of the filamentous soil bacterium, Streptomyces hygroscopicus, in 1970 and subsequently shown to manifest potent antibiotic and growth inhibitory activity against HeLa derived KB cancer cells.66 In subsequent years, GDA was shown to manifest potent anti-tumor activity against a variety of cancer cell lines. However, Hsp90 was not identified as the biological target of GDA until 1994, when Whitesell and co-workers demonstrated that GDA binds Hsp90 to induce the degradation of v-Src, an oncogenic client protein dependent upon Hsp90.10 Although GDA manifests excellent potency, its hepatotoxicity, low chemical stability, poor bioavailability and solubility, prevented GDA from advancing to clinical trials.67 Structure-activity relationship (SAR) studies on GDA have been pursued along with total synthesis, semi-synthesis, and genetic engineering of its biosynthetic pathway to generate new analogs. Such efforts have led to the development of improved derivatives that manifest superior pharmacokinetic and pharmacodynamic profiles.
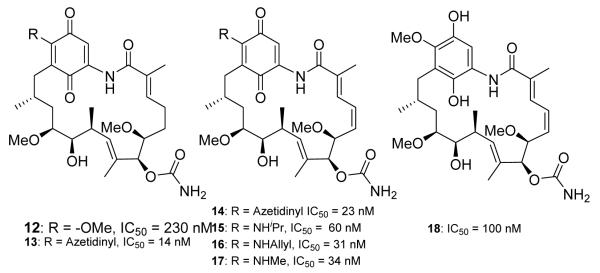
The first total synthesis of GDA was reported by Andrus and co-workers in 2002, followed by other groups that developed more succinct routes.68-72 Ultimately, the most efficacious analogs of GDA were produced by semi-synthesis that modified the 17-position. Due to the labile nature of the β-methoxy-α,β-unsaturated quinone, the methoxy could be easily replaced with various nucleophiles to provide C-17 substituted analogs. The greatest biological activities were observed by replacing the 17-methoxy with various alkyl amines, which further stabilized the reactive quinone moiety.11,73,74 Many of these analogs (12-17, Figure 3) manifest improved biological activity over GDA. The hydroquinone analog (18, Figure 3) of GDA was also synthesized by reducing GDA with sodium hydrosulfite, which was stable and isolable.75,76 The acetylated derivate of the hydroquinone was inactive (IC50 > 2.9 μM).76 The anti-cancer activity exhibited by these analogs was evaluated by measuring the degradation of the oncogenic, Her2 receptor. Among the analogs tested, 17-allylamino-GDA (17-AAG, 16) entered clinical trials.76,77
Figure 3.
GDA analogs reported from Pfizer. IC50’s determine by measuring depletion of p185 (an client protein of Hsp90) in SkBr3 cells. IC50 of GDA reported at 70 nM.
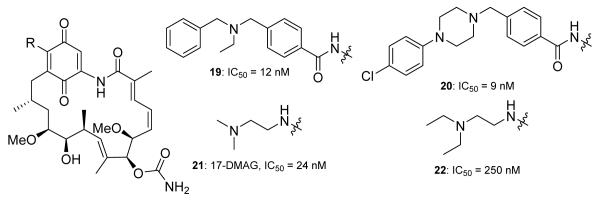
In 2004, Conforma Therapeutics further modified the 17-position of GDA and developed a cell lysate-based competition assay to evaluate the efficacy of new analogs. Among the reported analogs, 19 and 20 (Figure 4), manifested significant inhibition of cancer cell proliferation, however, they also manifested high clearance and low bioavailability.74 Kosan Biosciences reported the synthesis of more than 60 analogs of the 17-position as well as the C-7 carbamate during their pursuit of GDA analogs. Modifications to the carbamate led to a loss in potency, as the carbamate provides key hydrogen bond interactions with the N-terminal ATP-binding site of Hsp90 (see Figure 10).78 17-(2-dimethylaminoethyl)amino-17-demethoxygeldanamycin (17-DMAG, 21, Figure 4) was identified from these studies and was shown to manifest excellent potency (IC50 = 24 nM, in SkBr3 breast cancer cells) and solubility (1.4 mg/mL), and was selected to undergo clinical trial evaluation.78
Figure 4.
19-20 reported by Conforma Therapeutics. IC50 values were determined using a competitive binding assay measuring compounds binding to Hsp90 within the cell lysate of MCF-7 cells. The IC50 of 17-AAG was found to be 20 nM. 21-22 were reported by Kosan Biosciences. IC50 values were determined in SKBr3 cells and 17-AAG had a reported IC50 of 33 nM.
Figure 10.
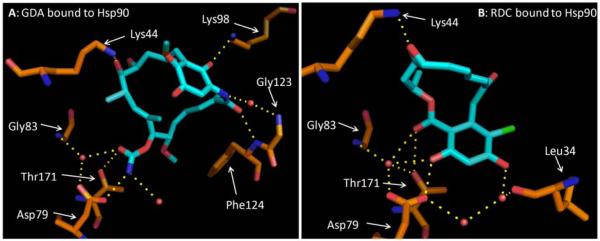
Binding modes of GDA (A) and RDC (B) to the Hsp90 N-terminal ATP-binding site. The hydrogen bonding network of the carbamate and resorcinol with the binding site has been proven to be critical for Hsp90 affinity. PDB Codes: 1A4H for GDA and 1AH6 for RDC.
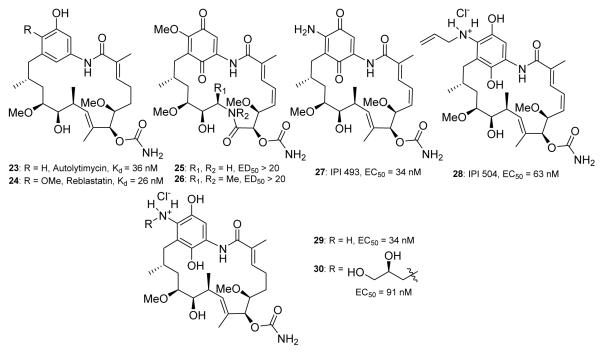
In 2008, Panek and co-workers reported a total synthesis of GDA and utilized this approach to prepare the phenolic ansamycins, 23-24 (Figure 5).72,79 The phenolic compounds were shown to exhibit increased affinity for Hsp90, compared to 17-AAG. Andrus and co-workers further simplified their total synthesis and reported the 8,9-amido-GDA derivatives, 25 and 26 (Figure 5), however, these compounds were determined inactive (ED50 > 20 μM in Her2 degradation assay).80
Figure 5.
GDA analogs prepared via semi-synthesis. Kd of 23-24 were measured in a competitive binding assay (17-AAG reported as 110 nM). ED50 values of 25-26 were determined via Her2 degradation in SkBr3 cells (GDA reported at 5 nM). EC50 of 27-30 were measured via fluorescence polarization with Hsp90α and BODIPY-GDA as a probe (17-AAG reported at 119 nM).
17-AAG undergoes cytochrome P450 3A4-mediated oxidation of the alpha methylene on the 17-amino substituent, resulting in subsequent hydrolysis of the C-N bond to produce 17-AG (27, Figure 5).81 This metabolite manifests growth inhibitory activity against breast cancer cells (SKBr3) at 33 nM, which is similar to that of 17-AAG.77 Consequently, Infinity Pharmaceuticals developed two water soluble GDA analogs, IPI-493(27)81 and hydroquinone 28 (IPI-504).11 It was determined that the hydroquinone of IPI-504 was more water-soluble than the corresponding quinone and upon subsequent preclinical studies, IPI-504 was found to exhibit excellent properties, which led to its clinical evaluation against various solid tumors, including non-small cell lung cancer, however IPI-504 is not currently being investigated in the clinic.73,82,83,84 Porter and co workers reported the hydroquinone analogs (29-30, Figure 5) to exhibit increased binding affinity for human Hsp90 compared to the quinone form, which paralleled prior studies with radicicol and geldanamycin chimeras.85
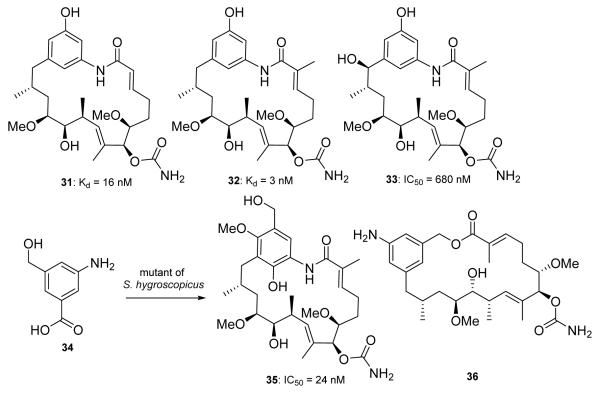
Throughout the last ten years, genetic engineering has provided additional GDA derivatives via biosynthetic manipulation.86,87 Kosan Biosciences reported the biosynthesis of GDA analogs by mutating the polyketide biosynthase genes present in the GDA biosynthetic pathway.88 The most significant derivative obtained from this work was 31 (Figure 6), which manifested a Kd of 16 nM against Hsp90, highlighting that the quinone/hydroquinone is not required for inhibitory activity. In 2008, Zhang and coworkers prepared derivatives of macbecin utilizing altered biosynthesis. Their efforts identified 32 (Figure 6), which was shown to exhibit improved Hsp90 binding affinity by ~80-fold compared to macbecin (3 nM v. 240 nM, respectively).89 32 also manifested a reduced toxicity profile; however, additional preclinical studies with this compound have not yet been reported.
Figure 6.
Kd of 31 was determine using Scintillation Proximity Assay for Hsp90 binding (GDA = 670 nM). Kd of 32 was determined using ITC. IC50 of 33 was determined using an ATPase inhibition assay (GDA = 3.19 μM). IC50 of 35 was determined in a competitive binding assay using FITC-GDA. Biological data was not reported for 36.
In 2007, Young-Soo Hong and co-workers reported a series of GDA analogs prepared from genetically engineered biosynthetic intermediates.90 Later in 2009, they reported the preparation of non-quinone GDA analogs by mutagenesis of GDA polyketide synthase.91 The lead compound, 33 (Figure 6), was shown to manifest greater affinity for Hsp90 and enhanced inhibition of Hsp90 ATPase activity than GDA. The 19-member macrocycle (35) and an unusual 20-membered macrocycle (36, Figure 6) of GDA were also prepared from an S. hygroscopicus strain that could not synthesize 3-amino-5-hydroxybenzoic acid, the initial substrate for GDA biosynthesis.92
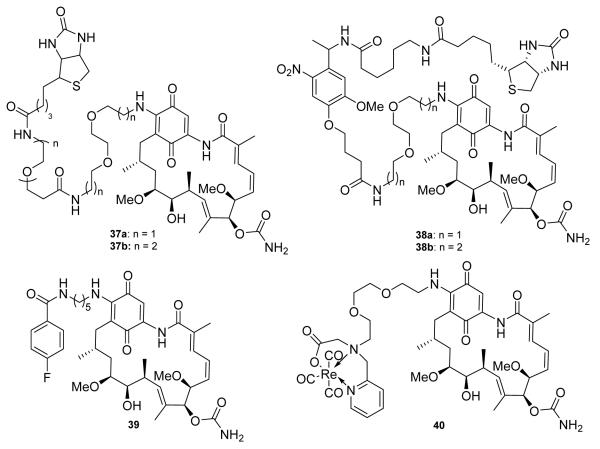
These synthetic/biosynthetic efforts led to discoveries in the biology of Hsp90 and the development of assays for detection of Hsp90 inhibitors. As stated earlier, Conforma Therapeutics developed a competition assay using immobilized GDA.74 However, other groups also contributed to the development of biological assays that could rapidly identify new inhibitors. Blagg and co-workers synthesized biotinylated GDA-analogs (37-38, Figure 7) that connected GDA and biotin via photolabile and non-photolabile linkers, which served as both a tool to understand Hsp90 biology as well as to identify other biological targets of GDA.93 More recently, Wuest and co-workers reported 18F-labeled and rhenium-containing GDA analogs as probes for imaging Hsp90 expression and early tumor detection.94 Their lead compound, 39 (Figure 7), manifests Hsp90 inhibitory activity comparable to GDA and the rhenium containing compound (40, Figure 7) is less active.
Figure 7.
Structure of probes synthesized to assess Hsp90 biology and function.
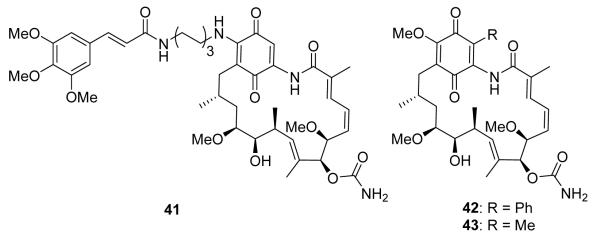
Recent synthetic efforts have attempted to address the issue of hepatotoxicity associated with GDA and its derivatives.95,96 Shen and co-workers modified the 17-postion and made compounds containing a diamine linker.97 The lead compound (41, Figure 8) produced lower levels of both aspartate transaminase and alanine transaminase in mice, as compared to GDA. 41 also manifests an IC50 of 190 nM against the MDA-MB-231 breast cancer cell line. This compound manifests increased in vivo tumor efficacy in a MDA-MB-231 xenograft model compared to 17-AAG and exhibits a MTD ≥ 250 mg/kg. In an alternative approach, Moody and co-workers envisioned the 19-position of the quinone ring to be susceptible to nucleophilic attack by biological thiols.98 Therefore, a library of GDA analogs was synthesized by modification of the 19-position. Two analogs, 19-phenyl-GDA (42) and 19-methyl-GDA (43, Figure 8), were shown to be significantly less toxic than both GDA and 17-AAG. However, these modifications also led to a decrease in potency, wherein 19-methyl-GDA binds Hsp90 with a Kd of 16.3 μM, which is 5-fold higher than GDA (Kd = 2.9 μM).
Figure 8.
Linker and 3,4,5-trimethoxycinnamyl group (41) provide decreased hepatotoxicity while maintaining efficacy similar to 17-AAG. 19-substitutions of GDA synthesized to mimic attack of biological nucleophiles, such as thiols (42-43).
The biological evaluation of GDA and its derivatives have provided significant insights into Hsp90 function, as well as to establish Hsp90 as a promising anti-cancer target.27,33,99-102 GDA has served as a starting point for several medicinal chemistry campaigns and several GDA analogs have advanced into clinical trials. The current focus of GDA research has centered on addressing the toxicities associated with the benzoquinone moiety, as well as the use of GDA analogs in combination with other therapies in the clinic.
Radicicol-based Inhibtors
The resorcinol lactone, radicicol (RDC, 4, Figure 9) was originally isolated from Monosporium bonorden in 1953.103 RDC was found to manifest antifungal properties and later determined to exhibit antitumor properties. Similar to GDA, RDC was believed to be an inhibitor of the v-Src and Ras-Raf-MAPK signaling pathways.104 RDC was known to exhibit a similar biological profile as GDA and in 1998, Schulte and co-workers demonstrated that RDC competes with GDA for binding Hsp90.18 Subsequent experiments showed that RDC binds the N-terminal ATP-binding site of Hsp90, similar to GDA, however, in a different orientation.105,106
Figure 9.
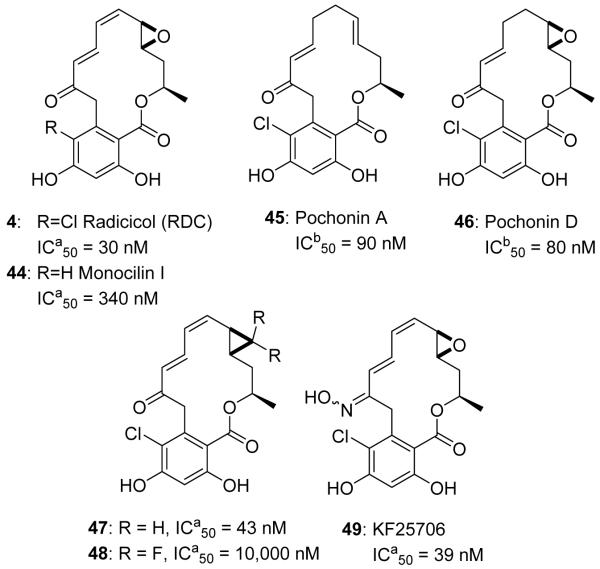
Natural product resorcinol-based inhibitors of Hsp90 (4, 44-46). 47-49 synthetic analogs of 4 to increase in vivo stability. ICa50 represents IC50 values of antiproliferative activity against the MCF-7 breast cancer (4, 44, 47-48) and the KNRK5.2 (49) cell line. ICb50 represents IC50 values of a time-resolved fluorescence resonance energy transfer (TR-FRET) assay.
RDC manifests greater affinity for Hsp90 than GDA in vitro (Kd in ATPase assay, 19 nM vs. 1.2 μM, respectively).107 Unfortunately, the administration of RDC in vivo does not produce anti-tumor activity.104,108,109 Because RDC is rapidly metabolized to inactive metabolites in vivo due to its electrophilic nature (allylic epoxide and α,β,γ,δ-unsaturated ketone), no in vivo activity has been observed with this natural product.14,110 Consequently, RDC was not considered a viable candidate for clinical evaluation. However, this scaffold allowed for the development of new analogs that do not exhibit the detriments associated with RDC or GDA. In addition to RDC, less electrophilic natural products (Monocillin I, 44, and Pochonins A, 45, and D, 46, Figure 9) have also been identified as Hsp90 inhibitors, although they manifest lower affinity.111-113
RDC attracted the attention of several synthetic groups beginning in the early 1990s, and the first total synthesis was reported by the Lett group in 1992.114,115 Subsequent routes toward the natural product were also developed by the Danishefsky and Winssinger laboratories.116-118 These total syntheses were developed for the preparation of analogs to reduce electrophilicity and to enhance metabolic stability in vivo. One strategy to reduce the metabolic susceptibility of RDC was to replace the epoxide with a cyclopropyl ring. Danishefsky and co-workers reported analogs that incorporated the cyclopropyl ring (47, Figure 9), which resulted in a two-fold loss in cellular efficacy.117,119 However, replacement with a difluorocyclopropane ring (48, Figure 9) resulted in a significant loss of activity, indicating the epoxide oxygen does not exhibit strong hydrogen bond accepting interactions. A second strategy to increase the stability of RDC was to convert the 2′-ketone of RDC to the corresponding oxime.25,120 The oxime analogs led to identification of the unsubstituted oxime analog, KF25706 (49, Figure 9), which manifested comparable potency to RDC against various human cancer cell lines.104 KF25706 was determined metabolically stable, and was administered in xenograft rodent models of cancer and reduced tumor growth over a 30 day period.25 Following this work, a series of substituted oximes was prepared and evaluated. The development of oxime derivatives was complicated by the mixture of E and Z isomers, however it was determined that the E isomer possesses higher affinity for Hsp90121. Metabolically stable RDC analogs represent a potential method to develop these natural product inhibitors, however, the complexity associated with this scaffold renders this compound difficult for large scale production. Fortunately, the data obtained from RDC analogs presented a new pharmacophore that could be utilized to design new Hsp90 inhibitors.105,109,111,113,122
As previously mentioned, GDA and RDC bind similarly within the N-terminus of Hsp90, however, the two natural products are not structurally similar. X-ray crystallographic studies have shown these structures to exhibit different interactions with Hsp90, which can be exploited for the development of more simplified scaffolds.105 For example, the resorcinol moiety mimics the hydrogen bonding interactions manifested by the adenine ring of ATP and is necessary for Hsp90 inhibition. These phenols compliment a conserved hydrogen bonding network between Leu34, Asp79, Gly83, Thr171, and two conserved water molecules (Figure 10a). Disruption of this network reduces the affinity of inhibitors for Hsp90.111 Hydrogen bonding interactions between Hsp90 and the RDC macrocycle are not critical, however the overall bent conformation appears important. While the hydrogen bonding network surrounding the resorcinol is important, RDC derives much of its affinity from entropic factors.14 Molecular dynamic studies with RDC led to the identification of three major conformations: the bioactive “c-shaped” conformation, a planar conformation, and a conformation in which the macrocycle is bent opposite the resorcinol ring. The bioactive conformation presents the lowest energy conformation by 2.4 and 3.3 kcal/mol, respectively.113 This analysis suggests that only a minimal entropic penalty exists when RDC binds Hsp90. Unfortunately, the RDC bioactive conformation does not exhibit differential selectivity for the Hsp90 heteroprotein complex.105,123
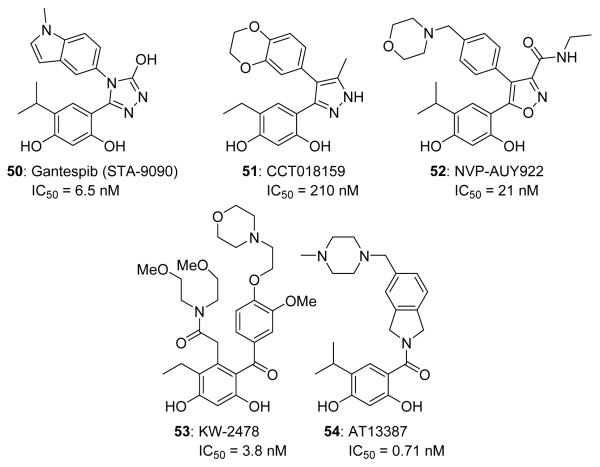
The resorcinol ring of RDC serves as a valuable pharmacophore for several inhibitors undergoing clinical evaluation.124,125 Ganetespib (STA-9090, 50, Figure 11) is currently in phase I-III evaluation for the treatment of cancer. STA-9090 manifests greater affinity for Hsp90 than 17-AAG against various lung cancer cell lines (average IC50 = 6.5 nM vs. 30.5 nM, respectively). STA-9090 also exhibits increased tumor penetration and an improved side effect profile in preclinical models.126,127 Currently, STA-9090 is undergoing nine different clinical trials for seven types of cancer.
Figure 11.
Resorcinol containing Hsp90 inhibitors currently in clinical trials. IC50 values represent antiproliferative activity against various cancer cell lines.
A high throughput screening campaign of 56,000 compounds led Workman and co-workers to identify the diaryl pyrazole resorcinol-containing compound, CCT018159 (51, IC50 0.21 μM, Figure 11), as an Hsp90 inhibitor.128,129 A medicinal chemistry campaign utilizing a structure-based approach led to the development of AUY922 (52, Figure 11), which is currently in phase II clinical trials for Her-2 positive and estrogen receptor positive metastatic breast cancer.130 AUY922 is also being evaluated in phase II trials for non-small cell lung cancer and has produced 20% response rate.131-133 Treatment of patients with multiple myeloma is also being evaluated with AUY922 in the presence and absence of the proteasome inhibitor, bortezomib.134 However, results have not been promising due to the lack of efficacy as a single agent. In addition, dose tolerance appears to be an issue when administered in combination with bortezomib.135
KW-2478 (53) and AT13387 (54, Figure 11) are additional resorcinol-derived inhibitors that have advanced into clinical trials. KW-2478 was developed by Kyowa Hakko Kirin Pharma in Japan through a unique lead optimization strategy that included the combination of microbial screening, structure-based drug design, cell-based screening, and in vivo models.67,125,136 This optimization strategy led to the identification of KW-2478, which manifests 3.8 nM affinity for Hsp90 and low nM antiproliferative IC50 values against various multiple myeloma cell lines. KW-2478 is currently being evaluated in patients with B-cell malignancies (phase I) and relapse or refractory multiple myeloma (phase I-II, in combination with bortezomib). Astex Pharmaceuticals developed AT13387 from a fragment-based screening approach. AT13387 manifests an IC50 of 0.71 nM via fluorescence polarization and antiproliferative IC50 of 48 nM against the HCT116 colon cancer cell line.137 AT13387 is currently in phase I trials for patients with metastatic solid tumors as well as phase II trials for the treatment of gastrointestinal stromal tumors with or without Imatinib.
Chimeric Inhibitors
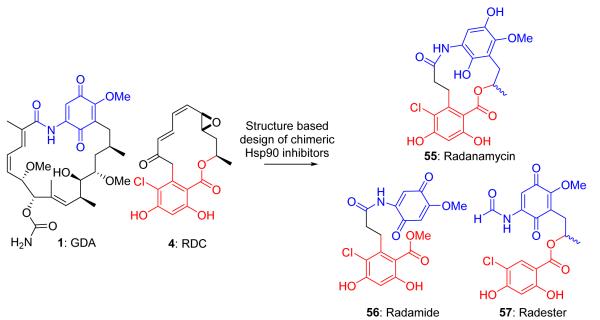
Toxicities associated with GDA and the lack of in vivo efficacy of RDC have proven difficult to overcome.67 However, one approach to develop new Hsp90 inhibitory scaffolds is to combine the structural features found in both GDA and RDC. This approach offers the potential to retain key interactions from both inhibitors with Hsp90, while simultaneously reducing the structural complexity, which enables rapid identification of structure-activity relationships for each natural product.
The first chimeric inhibitor of Hsp90 (radanamycin, 55, Figure 12) focused on maintenance of the resorcinol-mediated hydrogen bonding network.138 The quinone moiety manifests a key hydrogen bonding network near the solvent-exposed region of the ATP-binding pocket and allows for selective binding to the heteroprotein complex.105 Amino acids within this region are responsible for isomerization of the GDA amide bond, which dictates the high differential selectivity manifested by GDA.27,45,110
Figure 12.
Development of the first chimeric Hsp90 inhibitor (55) and the radanamycin seco agents, radamide and radester.
Molecular docking studies suggested two potential linkers to connect the quinone and resorcinol ring systems. The first approach connected the resorcinol ring to the quinone via a two carbon-linker containing an amide bond, which led to the radanamycin seco-agent, radamide (56, Figure 12).139 Radamide was found to inhibit Hsp90 ATPase activity at 5.9 μM compared to 2.5 μM for GDA.107 In vitro, radamide induced the degradation of Her2, an Hsp90-dependent client protein, in MCF-7 cells. The second approach maintained the ester linkage of RDC and through a two carbon linker, connected it to the quinone, termed radester (57, Figure 13).140 Antiproliferative activity of radester was determined in MCF-7 cells and was shown to manifest an IC50 of 13.9 μM and induced the degradation of Hsp90 client proteins, Raf and Her2.
Figure 13.
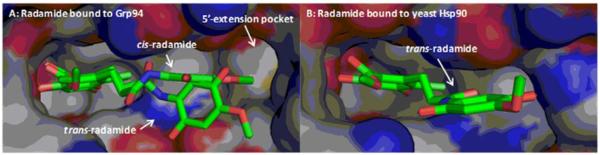
Radamide bound to Grp94 (A) and yeast Hsp90 (B). A depicts 5′-extension pocket present in Grp94. Cis-Radamide projects towards the unique pocket of Grp94.
Importantly, the hydroquinone derivatives of the seco agents of radanamycin were shown to manifest greater affinity for Hsp90 than the corresponding quinones.139,140 The hydroquinone of radamide inhibited Hsp90’s inherent ATPase activity at 1.8 μM and was shown to induce the degradation of Her-2 levels in MCF-7 cells. Similarly, the radester-hydroquinone manifested an antiproliferative IC50 of 7.1 μM and was also shown to induce the degradation of Her2 and Raf levels. Additionally, the macrocyclic chimera, radanamycin manifested antiproliferative activity against breast cancer cells at 1.2 μM and was shown to induce the degradation of Hsp90 client proteins, Her2 and Akt. These studies proved important as Infinity Pharmaceuticals was able to prepare a hydroquinone derivative of GDA, IPI-504 (see Figure 5). 11
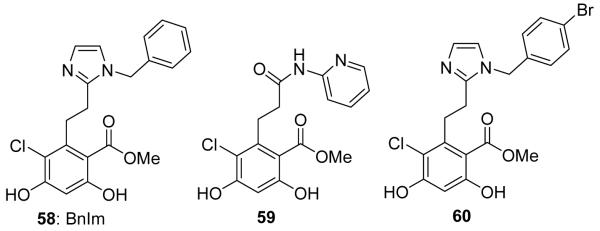
Co-crystallization studies of radamide bound to Grp94 and Hsp90 led to the discovery that radamide extends into a unique 5′-extension pocket present only in Grp94.141 Although the four Hsp90 isoforms share >85% identity in the N-terminal ATP-binding site, this 5′-extension pocket results from a 5-amino acid insertion into the primary sequence of Grp94.142-146 Extension into this pocket was shown with radamide, to result in Grp94-selectivity. As noted in the crystal structures, radamide projects into this region as a consequence of isomerization of the trans-amide to a cis-amide (Figure 13).141 Therefore, cis-amide bioisosteres were pursued for incorporation into the radamide scaffold to design Grp94-selective inhibitors. This led to the development of BnIm (58, Figure 14), which represents the first Grp94-selective inhibitor as determined by inhibition of Grp94-mediated Toll-like receptor trafficking to the cell surface and cytosolic client protein degradation dependent upon Hsp90α and Hsp90β.147
Figure 14.
Grp94-selective inhibitors. Incorporation of a cis-amide bioisostere led to the development of BnIm. 59 represents the most active compound from the Grp94-selective radamide analogs. 60 is an analog of BnIm that has been shown to reduce mutant myocilin aggregation in vitro.
BnIm was shown to exhibit no cytotoxic effects up to 100 μM, which is consistent with the non-essential nature of Grp94.148,149 In addition to BnIm, a series of radamide analogs was also investigated for Grp94 inhibition. These compounds were shown to inhibit the migration of the highly metastatic breast cancer cell line, MDA-MB-231 at ~1 μM, while exhibiting minimal toxicity at 100 μM.150 Grp94 is responsible for the trafficking of integrins and therefore Grp94 inhibition exhibits anti-migratory activity and represents a potential new target for the development of new anti-metastatic agents.151 Finally, an analog of BnIm (60) was shown to exhibit low μM inhibition of mutant myocilin aggregation, which occurs through disruption of the Grp94-mutant myocilin complex, which allows for the autophagic degradation of mutant myocilin and may provide a nontoxic approach towards the treatment of primary open angle glaucoma.152,153
Purine-based Inhibitors
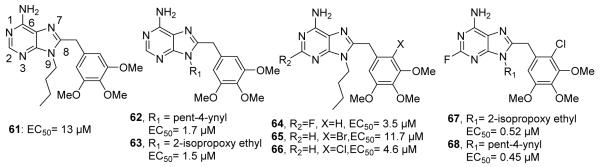
In addition to the natural products GDA and RDC, the natural substrate of Hsp90, ATP, has also been used as a starting point for the development of Hsp90 inhibitors.154 Chiosis and co-workers utilized the purine moiety of ATP as a template to design small molecule inhibitors that bind the N-terminal ATP-binding site.155 Using a structure-based approach, the purine moiety was linked to an aryl ring, which mimicked the unique shape adopted by ATP within the N-terminal binding pocket. The first inhibitor of this class, PU3 (61, Figure 15), was shown to inhibit Hsp90 with an EC50 of 15-20 μM, and manifested low micromolar anti-proliferative activity against various breast cancer cell lines.155-157 This early study identified this class of purine based Hsp90 inhibitors and further SAR studies resulted in a significant improvement in affinity.
Figure 15.
Structures of purine-based Hsp90 inhibitors. EC50 values were determined in a competitive assay with GDA on Affi-Gel resin.
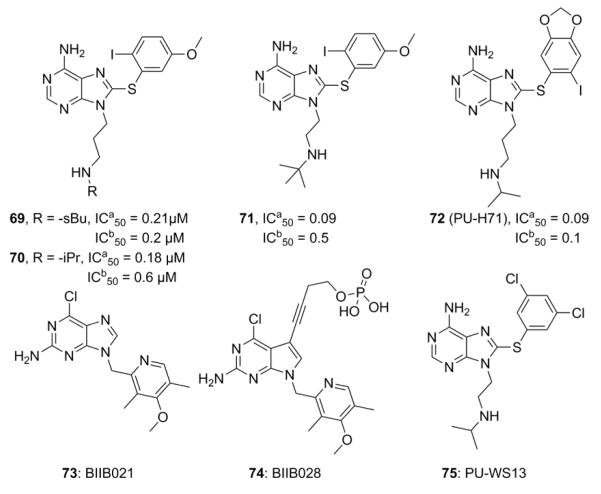
The first library of purine analogs probed alkyl substituents at the 2-position, and incorporated halogens on the trimethoxyphenyl group as well as the linker connecting these two rings.157 Various alkyl and aryl substitutions were also explored at the 9-position. Analogs containing pent-ynyl (62) and 2-isopropoxy-ethyl (63, Figure 15) linkers were most active and manifested inhibitory activities of 1.5 μM and 1.7 μM, respectively. Fluorine substitution at the 2-position (64, Figure 15) resulted in significant Hsp90 affinity.156 Its enhanced potency (IC50= 3.5 μM) correlated with its ability to increase hydrogen bonding of the C6-amine. Monobromo- (65) or monochloro-substitution (66, Figure 15) on the trimethoxyphenyl ring resulted in increased potency and the linker between the two rings was modified to contain a phenyl ether, secondary amine, sulfonyl, sulfinyl, benzyl ether and thioether.156,158 However, the sulfur linker proved most valuable. These optimized features were combined, and the resulting analogs (67 and 68, Figure 15) inhibited Hsp90 with submicromolar activity. Compound 68 manifests low micromolar anti-proliferative effects against breast, colon, lung, and prostate cancer cell lines. 68 also induced the degradation of Hsp90-dependent client proteins, Her2, Akt, Raf-1 and mutant p53.156 In vivo analysis of 68 revealed tumor specific inhibition of Hsp90, as this compound was retained in tumor tissues, but rapidly cleared from normal tissue. Administration of 68 resulted in a significant decrease in tumor mass upon dosing of 200mg/kg i.p. every other day for 30 days.159 Preliminary SAR studies and encouraging in vitro and in vivo studies led to the development of more efficacious, second generation purine analogs. This series of analogs included derivatives of the phenyl ring and concluded that substitutions at the 2-, 4- and 5-positions are important for Hsp90 inhibition.158 Researchers at Conforma Therapeutics addressed the low bioavailability associated with purine analogs and incorporated an amino group into the N-9 alkyl chain.160 The phosphoric acid salts of these amines were subsequently evaluated in murine tumor xenograft models. Ultimately, compounds 69-71 (Figure 16) became the first orally available Hsp90 inhibitors. Compound 71 manifests a 90 nM IC50 in Her2 degradation assays.160 Another important water soluble analog (72, PU-H71, Figure 16) was reported by Chiosis and co-workers, which contained a 3-isopropylamino-propyl chain. This compound was shown to manifest a 16 nM binding affinity for Hsp90 and an IC50 of 50 nM in Her2 degradation assays.161 This analog manifested significant efficacy in vivo and has advanced into clinical trials for the treatment of patients with low-grade non-Hodgkins lymphoma, as well as patients with advanced malignancies. Later, Conforma Therapeutics reported analogs with an amine at the 2-position and a chlorine at the 6-position. The most efficacious analog (73, Figure 16) also contained a pyridylmethylene group at the 9-position. Compound 73 (BIIB021) manifested 9 nM efficacy in Her2 degradation assays and exhibited 333-fold selectivity for tumor versus normal cells.162 After successful preclinical studies, this compound became the first rationally designed Hsp90 inhibitor to enter clinical trials and is currently being evaluated in Phase II trials for gastrointestinal stromal tumors.163 To increase tolerance, researchers at Conforma Therapeutics also reported the intravenously administered compound, 74 (BIIB028, Figure 16), a phosphate ester prodrug of the homopropargylic alcohol and it too, is currently under phase I clinical evaluation.164
Figure 16.
Second generation purine analogs with enhanced potency and solubility. IC50a values for 69-71 correspond to anti-proliferative activity in MCF-7 cells (MTS assay) and SkBr3 cells (sulforhodamine B assay) for 72. IC50b corresponds to the Her2 degradation assay 69-72 in the same cell lines used for determining anti-proliferative activity. 73-74 represent rationally designed purine analogs. 75 is a purine-based Grp94-selective inhibitor
A high throughput screen recently identified a purine-based compound that exhibits selective inhibition of Grp94.165 PU-WS13 (75, Figure 16) manifested Grp94 selectivity over other Hsp90 isoforms in purified protein assays. In vitro, 75 was shown to inhibit interactions between Her2 and Grp94 at the cell membrane, which resulted in the decreased stability of Her2, leading to its degradation via the lysosome. Additionally, inhibition of Grp94 resulted in the apoptosis of myeloma cells. Grp94 is significantly upregulated in multiple myeloma resulting from increased ER stress. Multiple myeloma cell death was found to result from disruption of Grp94 and LRP6, which lowered LRP6 expression on the cell surface.166,167 LRP6 is a co-receptor of Frizzled in the Wnt pathway and decreased interactions between these co-receptors results in caspase 9 activation and apoptosis.168
Conclusions & Future Directions
Hsp90 provides a unique opportunity to treat cancer due to the fact that Hsp90-dependent client proteins are associated with all six hallmarks of cancer. Consequently, Hsp90 has attracted the attention of research groups throughout the world. Beginning with the discovery of natural product inhibitors of Hsp90 (GDA and RDC), many analogs have been designed to probe the biological function of Hsp90, as well as to develop small molecule inhibitors that have advanced into clinical trials. Furthermore, key interactions within the N-terminal ATP-binding pocket have been identified, which has led to the discovery of new Hsp90 inhibitory classes. While GDA never advanced into clinical trials, 17-substituted analogs have proven superior candidates and exhibit fewer side effects while maintaining similar efficacy to GDA (16, 21, 27-28). Likewise, RDC did not advance into clinical trials, however the resorcinol ring of RDC emerged as a key pharmacophore. Several medicinal chemistry campaigns have used the resorcinol moiety as a starting point to produce candidates that are currently undergoing clinical evaluation. The natural substrate of Hsp90, ATP, has also been used to develop inhibitors that have advanced into clinical trials (72-74). Many Hsp90 inhibitors have shown promise in clinical trials, however, no compound has yet been approved by the FDA. Some concerns associated with Hsp90 inhibitors (ocular, cardio, and hepatotoxicities) have arisen during clinical trials that resulted in the termination of some trials, suggesting new approaches to Hsp90 inhibition may be needed.67,169 One method that may prove useful is the development of isoform-selective inhibitors.147,150,152,165 By developing selective inhibitors for each isoform, toxicities associated with individual isoforms may be overcome. Furthermore, upon identification of client proteins that are dependent upon each isoform, perhaps more personalized treatments can be developed via this approach.
Acknowledgements
The authors gratefully acknowledge financial support of this project by the NIH/NCI (CA109265 to BSJB).
Biosketches
Anuj Khandelwal earned a Bachelor’s degree in chemistry in 2006 and received a Masters degree in organic chemistry in 2008 from the University of Delhi. He began pursuing his PhD in medicinal chemistry at the University of Kansas in 2011. Anuj received a Masters degree in medicinal chemistry in 2013. Since then he has worked on the optimization of EGCG for Hsp90 inhibition as well as the development of isoform-selective inhibitors of Hsp90.
Vince Crowley received a BS in Chemistry in 2012 from Creighton University (Omaha, NE). He joined the Department of Medicinal Chemistry at the University of Kansas in 2012. He is currently working under the supervision of Dr. Brian Blagg in pursuing Hsp90 isoform-selective inhibitors. Vince received a Masters degree in medicinal chemistry in 2014.
Dr. Brian Blagg earned his PhD at the University of Utah (C. Dale Poulter) then trained as a postdoctoral fellow under the guidance of Dr. Dale Boger (The Scripps Research Institute). In 2002, Dr. Blagg became a faculty member in the Department of Medicinal Chemistry at the University of Kansas. Since then he has become a leader in Hsp90 inhibition and medicinal chemistry.
Footnotes
Disclosure of Financial or Competing Interests
The authors declare no competing interest regarding the material discussed.
References
- 1.Harvey AL. Natural products in drug discovery. Drug Discov Today. 2008;13(19-20):894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004;67(12):2141–2153. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 3.Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4(3):206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 4.Ngo LT, Okogun JI, Folk WR. 21st century natural product research and drug development and traditional medicines. Nat Prod Rep. 2013;30(4):584–592. doi: 10.1039/c3np20120a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li JW, Vederas JC. Drug discovery and natural products: end of an era or an endless frontier? Science. 2009;325(5937):161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- 6.Newman DJ. Natural products as leads to potential drugs: an old process or the new hope for drug discovery? J Med Chem. 2008;51(9):2589–2599. doi: 10.1021/jm0704090. [DOI] [PubMed] [Google Scholar]
- 7.Schax E, Walter JG, Marzhauser H, Stahl F, Scheper T, Agard DA, Eichner S, Kirschning A, Zeilinger C. Microarray-based screening of heat shock protein inhibitors. J Biotechnol. 2014;180:1–9. doi: 10.1016/j.jbiotec.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Lachance H, Wetzel S, Kumar K, Waldmann H. Charting, navigating, and populating natural product chemical space for drug discovery. J Med Chem. 2012;55(13):5989–6001. doi: 10.1021/jm300288g. [DOI] [PubMed] [Google Scholar]
- 9.Franke J, Eichner S, Zeilinger C, Kirschning A. Targeting heat-shock-protein 90 (Hsp90) by natural products: geldanamycin, a show case in cancer therapy. Nat Prod Rep. 2013;30(10):1299–1323. doi: 10.1039/c3np70012g. [DOI] [PubMed] [Google Scholar]
- 10.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91(18):8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian ZQ, Liu Y, Zhang D, Wang Z, Dong SD, Carreras CW, Zhou Y, Rastelli G, Santi DV, Myles DC. Synthesis and biological activities of novel 17-aminogeldanamycin derivatives. Bioorg Med Chem. 2004;12(20):5317–5329. doi: 10.1016/j.bmc.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 12.Waza M, Adachi H, Katsuno M, Minamiyama M, Tanaka F, Sobue G. Alleviating neurodegeneration by an anticancer agent: an Hsp90 inhibitor (17-AAG) Ann N Y Acad Sci. 2006;1086:21–34. doi: 10.1196/annals.1377.012. [DOI] [PubMed] [Google Scholar]
- 13.Bhat R, Tummalapalli SR, Rotella DP. Progress in the discovery and development of heat shock protein 90 (hsp90) inhibitors. J Med Chem. 2014;57(21):8718–8728. doi: 10.1021/jm500823a. [DOI] [PubMed] [Google Scholar]
- 14.Amolins MW, Blagg BS. Natural product inhibitors of Hsp90: potential leads for drug discovery. Mini Rev Med Chem. 2009;9(2):140–152. doi: 10.2174/138955709787316056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khandelwal A, Hall JA, Blagg BS. Synthesis and structure-activity relationships of EGCG analogues, a recently identified Hsp90 inhibitor. J Org Chem. 2013;78(16):7859–7884. doi: 10.1021/jo401027r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall JA, Kusuma BR, Brandt GE, Blagg BS. Cruentaren A binds F1F0 ATP synthase to modulate the Hsp90 protein folding machinery. ACS Chem Biol. 2014;9(4):976–985. doi: 10.1021/cb400906e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcu MG, Schulte TW, Neckers L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J Natl Cancer Inst. 2000;92(3):242–248. doi: 10.1093/jnci/92.3.242. [DOI] [PubMed] [Google Scholar]
- 18.Schulte TW, Akinaga S, Soga S, Sullivan W, Stensgard B, Toft D, Neckers LM. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones. 1998;3(2):100–108. doi: 10.1379/1466-1268(1998)003<0100:arbttn>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin CJ, Gaisser S, Challis IR, Carletti I, Wilkinson B, Gregory M, Prodromou C, Roe SM, Pearl LH, Boyd SM, Zhang MQ. Molecular characterization of macbecin as an Hsp90 inhibitor. J Med Chem. 2008;51(9):2853–2857. doi: 10.1021/jm701558c. [DOI] [PubMed] [Google Scholar]
- 20.Supko JG, Hickman RL, Grever MR, Malspeis L. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol. 1995;36(4):305–315. doi: 10.1007/BF00689048. [DOI] [PubMed] [Google Scholar]
- 21.Vasko RC, Rodriguez RA, Cunningham CN, Ardi VC, Agard DA, McAlpine SR. Mechanistic studies of Sansalvamide A-amide: an allosteric modulator of Hsp90. ACS Med Chem Lett. 2010;1(1):4–8. doi: 10.1021/ml900003t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amolins MW, Peterson LB, Blagg BS. Synthesis and evaluation of electron-rich curcumin analogues. Bioorg Med Chem. 2009;17(1):360–367. doi: 10.1016/j.bmc.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patwardhan CA, Fauq A, Peterson LB, Miller C, Blagg BS, Chadli A. Gedunin inactivates the co-chaperone p23 protein causing cancer cell death by apoptosis. J Biol Chem. 2013;288(10):7313–7325. doi: 10.1074/jbc.M112.427328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang T, Hamza A, Cao X, Wang B, Yu S, Zhan CG, Sun D. A novel Hsp90 inhibitor to disrupt Hsp90/Cdc37 complex against pancreatic cancer cells. Mol Cancer Ther. 2008;7(1):162–170. doi: 10.1158/1535-7163.MCT-07-0484. [DOI] [PubMed] [Google Scholar]
- 25.Soga S, Neckers LM, Schulte TW, Shiotsu Y, Akasaka K, Narumi H, Agatsuma T, Ikuina Y, Murakata C, Tamaoki T, Akinaga S. KF25706, a novel oxime derivative of radicicol, exhibits in vivo antitumor activity via selective depletion of Hsp90 binding signaling molecules. Cancer Res. 1999;59(12):2931–2938. [PubMed] [Google Scholar]
- 26.Usmani SZ, Bona R, Li Z. 17 AAG for HSP90 inhibition in cancer--from bench to bedside. Curr Mol Med. 2009;9(5):654–664. doi: 10.2174/156652409788488757. [DOI] [PubMed] [Google Scholar]
- 27.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10(8):537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Soroka J, Buchner J. The Hsp90 chaperone machinery: conformational dynamics and regulation by co-chaperones. Biochim Biophys Acta. 2012;1823(3):624–635. doi: 10.1016/j.bbamcr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Buchner J. Structure, function and regulation of the hsp90 machinery. Biomedical journal. 2013;36(3):106–117. doi: 10.4103/2319-4170.113230. [DOI] [PubMed] [Google Scholar]
- 30.Prodromou C, Pearl LH. Structure and functional relationships of Hsp90. Curr Cancer Drug Targets. 2003;3(5):301–323. doi: 10.2174/1568009033481877. [DOI] [PubMed] [Google Scholar]
- 31.Bagatell R, Whitesell L. Altered Hsp90 function in cancer: a unique therapeutic opportunity. Mol Cancer Ther. 2004;3(8):1021–1030. [PubMed] [Google Scholar]
- 32.Schlesinger MJ. Heat shock proteins. J Biol Chem. 1990;265(21):12111–12114. [PubMed] [Google Scholar]
- 33.Hong DS, Banerji U, Tavana B, George GC, Aaron J, Kurzrock R. Targeting the molecular chaperone heat shock protein 90 (HSP90): lessons learned and future directions. Cancer Treat Rev. 2013;39(4):375–387. doi: 10.1016/j.ctrv.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11(7):515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 35.Pratt WB. The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem. 1993;268(29):21455–21458. [PubMed] [Google Scholar]
- 36.Csermely P, Schnaider T, Soti C, Prohaszka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther. 1998;79(2):129–168. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 37.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5(10):761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 38.Prodromou C. The ‘active life’ of Hsp90 complexes. Biochim Biophys Acta. 2012;1823(3):614–623. doi: 10.1016/j.bbamcr.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sreedhar AS, Kalmar E, Csermely P, Shen YF. Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett. 2004;562(1-3):11–15. doi: 10.1016/s0014-5793(04)00229-7. [DOI] [PubMed] [Google Scholar]
- 40.Rohl A, Rohrberg J, Buchner J. The chaperone Hsp90: changing partners for demanding clients. Trends Biochem Sci. 2013;38(5):253–262. doi: 10.1016/j.tibs.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Hall JA, Forsberg LK, Blagg BS. Alternative approaches to Hsp90 modulation for the treatment of cancer. Future Med Chem. 2014;6(14):1587–1605. doi: 10.4155/fmc.14.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soti C, Vermes A, Haystead TA, Csermely P. Comparative analysis of the ATP-binding sites of Hsp90 by nucleotide affinity cleavage: a distinct nucleotide specificity of the C-terminal ATP-binding site. Eur J Biochem. 2003;270(11):2421–2428. doi: 10.1046/j.1432-1033.2003.03610.x. [DOI] [PubMed] [Google Scholar]
- 43.Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J Biol Chem. 2000;275(47):37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 44.Xu W, Marcu M, Yuan X, Mimnaugh E, Patterson C, Neckers L. Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc Natl Acad Sci U S A. 2002;99(20):12847–12852. doi: 10.1073/pnas.202365899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425(6956):407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 46.Moulick K, Ahn JH, Zong H, Rodina A, Cerchietti L, Gomes DaGama EM, Caldas-Lopes E, Beebe K, Perna F, Hatzi K, Vu LP, Zhao X, Zatorska D, Taldone T, Smith-Jones P, Alpaugh M, Gross SS, Pillarsetty N, Ku T, Lewis JS, Larson SM, Levine R, Erdjument-Bromage H, Guzman ML, Nimer SD, Melnick A, Neckers L, Chiosis G. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol. 2011;7(11):818–826. doi: 10.1038/nchembio.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyata Y, Nakamoto H, Neckers L. The therapeutic target Hsp90 and cancer hallmarks. Curr Pharm Des. 2013;19(3):347–365. doi: 10.2174/138161213804143725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 49.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 50.Shelton SN, Shawgo ME, Matthews SB, Lu Y, Donnelly AC, Szabla K, Tanol M, Vielhauer GA, Rajewski RA, Matts RL, Blagg BS, Robertson JD. KU135, a novel novobiocin-derived C-terminal inhibitor of the 90-kDa heat shock protein, exerts potent antiproliferative effects in human leukemic cells. Mol Pharmacol. 2009;76(6):1314–1322. doi: 10.1124/mol.109.058545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kusuma BR, Peterson LB, Zhao H, Vielhauer G, Holzbeierlein J, Blagg BS. Targeting the heat shock protein 90 dimer with dimeric inhibitors. J Med Chem. 2011;54(18):6234–6253. doi: 10.1021/jm200553w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao H, Donnelly AC, Kusuma BR, Brandt GE, Brown D, Rajewski RA, Vielhauer G, Holzbeierlein J, Cohen MS, Blagg BS. Engineering an antibiotic to fight cancer: optimization of the novobiocin scaffold to produce anti-proliferative agents. J Med Chem. 2011;54(11):3839–3853. doi: 10.1021/jm200148p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhatt T, Yang F, Pai YC. Learning from falling: retention of fall-resisting behavior derived from one episode of laboratory-induced slip training. J Am Geriatr Soc. 2011;59(12):2392–2393. doi: 10.1111/j.1532-5415.2011.03708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao H, Garg G, Zhao J, Moroni E, Girgis A, Franco LS, Singh S, Colombo G, Blagg BS. Design, synthesis and biological evaluation of biphenylamide derivatives as Hsp90 C-terminal inhibitors. Eur J Med Chem. 2015;89:442–466. doi: 10.1016/j.ejmech.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garnier C, Lafitte D, Tsvetkov PO, Barbier P, Leclerc-Devin J, Millot JM, Briand C, Makarov AA, Catelli MG, Peyrot V. Binding of ATP to heat shock protein 90: evidence for an ATP-binding site in the C-terminal domain. J Biol Chem. 2002;277(14):12208–12214. doi: 10.1074/jbc.M111874200. [DOI] [PubMed] [Google Scholar]
- 56.Soti C, Racz A, Csermely P. A Nucleotide-dependent molecular switch controls ATP binding at the C-terminal domain of Hsp90. N-terminal nucleotide binding unmasks a C-terminal binding pocket. J Biol Chem. 2002;277(9):7066–7075. doi: 10.1074/jbc.M105568200. [DOI] [PubMed] [Google Scholar]
- 57.Yin Z, Henry EC, Gasiewicz TA. (-)-Epigallocatechin-3-gallate is a novel Hsp90 inhibitor. Biochemistry. 2009;48(2):336–345. doi: 10.1021/bi801637q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brandt GE, Schmidt MD, Prisinzano TE, Blagg BS. Gedunin, a novel hsp90 inhibitor: semisynthesis of derivatives and preliminary structure-activity relationships. J Med Chem. 2008;51(20):6495–6502. doi: 10.1021/jm8007486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hieronymus H, Lamb J, Ross KN, Peng XP, Clement C, Rodina A, Nieto M, Du J, Stegmaier K, Raj SM, Maloney KN, Clardy J, Hahn WC, Chiosis G, Golub TR. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell. 2006;10(4):321–330. doi: 10.1016/j.ccr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 60.Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, Lerner J, Brunet JP, Subramanian A, Ross KN, Reich M, Hieronymus H, Wei G, Armstrong SA, Haggarty SJ, Clemons PA, Wei R, Carr SA, Lander ES, Golub TR. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313(5795):1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 61.Brandt GE, Blagg BS. Alternate strategies of Hsp90 modulation for the treatment of cancer and other diseases. Curr Top Med Chem. 2009;9(15):1447–1461. doi: 10.2174/156802609789895683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang T, Li Y, Yu Y, Zou P, Jiang Y, Sun D. Characterization of celastrol to inhibit hsp90 and cdc37 interaction. J Biol Chem. 2009;284(51):35381–35389. doi: 10.1074/jbc.M109.051532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kunze B, Sasse F, Wieczorek H, Huss M. Cruentaren A, a highly cytotoxic benzolactone from Myxobacteria is a novel selective inhibitor of mitochondrial F1-ATPases. FEBS Lett. 2007;581(18):3523–3527. doi: 10.1016/j.febslet.2007.06.069. [DOI] [PubMed] [Google Scholar]
- 64.Vintonyak VV, Cala M, Lay F, Kunze B, Sasse F, Maier ME. Synthesis and biological evaluation of cruentaren A analogues. Chemistry. 2008;14(12):3709–3720. doi: 10.1002/chem.200701673. [DOI] [PubMed] [Google Scholar]
- 65.Bindl M, Jean L, Herrmann J, Muller R, Furstner A. Preparation, modification, and evaluation of cruentaren A and analogues. Chemistry. 2009;15(45):12310–12319. doi: 10.1002/chem.200901817. [DOI] [PubMed] [Google Scholar]
- 66.DeBoer C, Meulman PA, Wnuk RJ, Peterson DH. Geldanamycin, a new antibiotic. J Antibiot. 1970;23(9):442–447. doi: 10.7164/antibiotics.23.442. [DOI] [PubMed] [Google Scholar]
- 67.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res. 2012;18(1):64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andrus MB, Meredith EL, Sekhar BB. Synthesis of the left-hand portion of geldanamycin using an anti glycolate aldol reaction. Org Lett. 2001;3(2):259–262. doi: 10.1021/ol0068997. [DOI] [PubMed] [Google Scholar]
- 69.Andrus MB, Meredith EL, Simmons BL, Soma Sekhar BB, Hicken EJ. Total synthesis of (+)-geldanamycin and (−)-o-quinogeldanamycin with use of asymmetric anti- and syn-glycolate aldol reactions. Org Lett. 2002;4(20):3549–3552. doi: 10.1021/ol0267432. [DOI] [PubMed] [Google Scholar]
- 70.Andrus MB, Hicken EJ, Meredith EL, Simmons BL, Cannon JF. Selective synthesis of the para-quinone region of geldanamycin. Org Lett. 2003;5(21):3859–3862. doi: 10.1021/ol035400g. [DOI] [PubMed] [Google Scholar]
- 71.Andrus MB, Meredith EL, Hicken EJ, Simmons BL, Glancey RR, Ma W. Total synthesis of (+)-geldanamycin and (−)-o-quinogeldanamycin: asymmetric glycolate aldol reactions and biological evaluation. J Org Chem. 2003;68(21):8162–8169. doi: 10.1021/jo034870l. [DOI] [PubMed] [Google Scholar]
- 72.Qin HL, Panek JS. Total synthesis of the Hsp90 inhibitor geldanamycin. Org Lett. 2008;10(12):2477–2479. doi: 10.1021/ol800749w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ge J, Normant E, Porter JR, Ali JA, Dembski MS, Gao Y, Georges AT, Grenier L, Pak RH, Patterson J, Sydor JR, Tibbitts TT, Tong JK, Adams J, Palombella VJ. Design, synthesis, and biological evaluation of hydroquinone derivatives of 17-amino-17-demethoxygeldanamycin as potent, water-soluble inhibitors of Hsp90. J Med Chem. 2006;49(15):4606–4615. doi: 10.1021/jm0603116. [DOI] [PubMed] [Google Scholar]
- 74.Le Brazidec JY, Kamal A, Busch D, Thao L, Zhang L, Timony G, Grecko R, Trent K, Lough R, Salazar T, Khan S, Burrows F, Boehm MF. Synthesis and biological evaluation of a new class of geldanamycin derivatives as potent inhibitors of Hsp90. J Med Chem. 2004;47(15):3865–3873. doi: 10.1021/jm0306125. [DOI] [PubMed] [Google Scholar]
- 75.Schnur RC, Corman ML, Gallaschun RJ, Cooper BA, Dee MF, Doty JL, Muzzi ML, DiOrio CI, Barbacci EG, Miller PE, et al. erbB-2 oncogene inhibition by geldanamycin derivatives: synthesis, mechanism of action, and structure-activity relationships. J Med Chem. 1995;38(19):3813–3820. doi: 10.1021/jm00019a011. [DOI] [PubMed] [Google Scholar]
- 76.Schnur RC, Corman ML, Gallaschun RJ, Cooper BA, Dee MF, Doty JL, Muzzi ML, Moyer JD, DiOrio CI, Barbacci EG, et al. Inhibition of the oncogene product p185erbB-2 in vitro and in vivo by geldanamycin and dihydrogeldanamycin derivatives. J Med Chem. 1995;38(19):3806–3812. doi: 10.1021/jm00019a010. [DOI] [PubMed] [Google Scholar]
- 77.Schulte TW, Neckers LM. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother Pharmacol. 1998;42(4):273–279. doi: 10.1007/s002800050817. [DOI] [PubMed] [Google Scholar]
- 78.Rastelli G, Tian ZQ, Wang Z, Myles D, Liu Y. Structure-based design of 7-carbamate analogs of geldanamycin. Bioorg Med Chem Lett. 2005;15(22):5016–5021. doi: 10.1016/j.bmcl.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 79.Wrona IE, Gozman A, Taldone T, Chiosis G, Panek JS. Synthesis of reblastatin, autolytimycin, and non-benzoquinone analogues: potent inhibitors of heat shock protein 90. J Org Chem. 2010;75(9):2820–2835. doi: 10.1021/jo1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andrus MB, Wong Y, Liu J, Beebe K, Neckers LM. Synthesis and evaluation of 8,9-amido analogs of geldanamycin. Tetrahedron Lett. 2009;50(48):6705–6708. [Google Scholar]
- 81.Egorin MJ, Rosen DM, Wolff JH, Callery PS, Musser SM, Eiseman JL. Metabolism of 17-(allylamino)-17-demethoxygeldanamycin (NSC 330507) by murine and human hepatic preparations. Cancer Res. 1998;58(11):2385–2396. [PubMed] [Google Scholar]
- 82.Maroney AC, Marugan JJ, Mezzasalma TM, Barnakov AN, Garrabrant TA, Weaner LE, Jones WJ, Barnakova LA, Koblish HK, Todd MJ, Masucci JA, Deckman IC, Galemmo RA, Jr., Johnson DL. Dihydroquinone ansamycins: toward resolving the conflict between low in vitro affinity and high cellular potency of geldanamycin derivatives. Biochemistry. 2006;45(17):5678–5685. doi: 10.1021/bi0524969. [DOI] [PubMed] [Google Scholar]
- 83.Floris G, Debiec-Rychter M, Wozniak A, Stefan C, Normant E, Faa G, Machiels K, Vanleeuw U, Sciot R, Schöffski P. The Heat Shock Protein 90 Inhibitor IPI-504 Induces KIT Degradation, Tumor Shrinkage, and Cell Proliferation Arrest in Xenograft Models of Gastrointestinal Stromal Tumors. Molecular cancer therapeutics. 2011;10(10):1897–1908. doi: 10.1158/1535-7163.MCT-11-0148. [DOI] [PubMed] [Google Scholar]
- 84.Siegel D, Jagannath S, Vesole DH, Borello I, Mazumder A, Mitsiades C, Goddard J, Dunbar J, Normant E, Adams J, Grayzel D, Anderson KC, Richardson P. A phase 1 study of IPI-504 (retaspimycin hydrochloride) in patients with relapsed or relapsed and refractory multiple myeloma. Leukemia & Lymphoma. 2011;52(12):2308–2315. doi: 10.3109/10428194.2011.600481. [DOI] [PubMed] [Google Scholar]
- 85.Porter JR, Ge J, Lee J, Normant E, West K. Ansamycin inhibitors of Hsp90: nature’s prototype for anti-chaperone therapy. Curr Top Med Chem. 2009;9(15):1386–1418. doi: 10.2174/156802609789895719. [DOI] [PubMed] [Google Scholar]
- 86.Lee K, Ryu JS, Jin Y, Kim W, Kaur N, Chung SJ, Jeon YJ, Park JT, Bang JS, Lee HS, Kim TY, Lee JJ, Hong YS. Synthesis and anticancer activity of geldanamycin derivatives derived from biosynthetically generated metabolites. Organic & biomolecular chemistry. 2008;6(2):340–348. doi: 10.1039/b713407j. [DOI] [PubMed] [Google Scholar]
- 87.Eichner S, Floss HG, Sasse F, Kirschning A. New, highly active nonbenzoquinone geldanamycin derivatives by using mutasynthesis. ChemBioChem. 2009;10(11):1801–1805. doi: 10.1002/cbic.200900246. [DOI] [PubMed] [Google Scholar]
- 88.Patel K, Piagentini M, Rascher A, Tian ZQ, Buchanan GO, Regentin R, Hu Z, Hutchinson CR, McDaniel R. Engineered biosynthesis of geldanamycin analogs for Hsp90 inhibition. Chem Biol. 2004;11(12):1625–1633. doi: 10.1016/j.chembiol.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 89.Zhang MQ, Gaisser S, Nur EAM, Sheehan LS, Vousden WA, Gaitatzis N, Peck G, Coates NJ, Moss SJ, Radzom M, Foster TA, Sheridan RM, Gregory MA, Roe SM, Prodromou C, Pearl L, Boyd SM, Wilkinson B, Martin CJ. Optimizing natural products by biosynthetic engineering: discovery of nonquinone Hsp90 inhibitors. J Med Chem. 2008;51(18):5494–5497. doi: 10.1021/jm8006068. [DOI] [PubMed] [Google Scholar]
- 90.Kim W, Lee JS, Lee D, Cai XF, Shin JC, Lee K, Lee CH, Ryu S, Paik SG, Lee JJ, Hong YS. Mutasynthesis of geldanamycin by the disruption of a gene producing starter unit: generation of structural diversity at the benzoquinone ring. ChemBioChem. 2007;8(13):1491–1494. doi: 10.1002/cbic.200700196. [DOI] [PubMed] [Google Scholar]
- 91.Kim W, Lee D, Hong SS, Na Z, Shin JC, Roh SH, Wu CZ, Choi O, Lee K, Shen YM, Paik SG, Lee JJ, Hong YS. Rational biosynthetic engineering for optimization of geldanamycin analogues. ChemBioChem. 2009;10(7):1243–1251. doi: 10.1002/cbic.200800763. [DOI] [PubMed] [Google Scholar]
- 92.Eichner S, Eichner T, Floss HG, Fohrer J, Hofer E, Sasse F, Zeilinger C, Kirschning A. Broad substrate specificity of the amide synthase in S. hygroscopicus--new 20-membered macrolactones derived from geldanamycin. J Am Chem Soc. 2012;134(3):1673–1679. doi: 10.1021/ja2087147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clevenger RC, Raibel JM, Peck AM, Blagg BS. Biotinylated geldanamycin. J Org Chem. 2004;69(13):4375–4380. doi: 10.1021/jo049848m. [DOI] [PubMed] [Google Scholar]
- 94.Wuest F, Bouvet V, Mai B, LaPointe P. Fluorine- and rhenium-containing geldanamycin derivatives as leads for the development of molecular probes for imaging Hsp90. Organic & biomolecular chemistry. 2012;10(33):6724–6731. doi: 10.1039/c2ob25744k. [DOI] [PubMed] [Google Scholar]
- 95.Li Z, Jia L, Wang J, Wu X, Hao H, Xu H, Wu Y, Shi G, Lu C, Shen Y. Design, synthesis and biological evaluation of 17-arylmethylamine-17-demethoxygeldanamycin derivatives as potent Hsp90 inhibitors. Eur J Med Chem. 2014;85:359–370. doi: 10.1016/j.ejmech.2014.07.101. [DOI] [PubMed] [Google Scholar]
- 96.Li Z, Jia L, Wang J, Wu X, Shi G, Lu C, Shen Y. Discovery of Novel 17-Phenylethylaminegeldanamycin Derivatives as Potent Hsp90 Inhibitors. Chem Biol Drug Des. 2014 doi: 10.1111/cbdd.12371. [DOI] [PubMed] [Google Scholar]
- 97.Li Z, Jia L, Wang J, Wu X, Hao H, Wu Y, Xu H, Wang Z, Shi G, Lu C, Shen Y. Discovery of diamine-linked 17-aroylamido-17-demethoxygeldanamycins as potent Hsp90 inhibitors. Eur J Med Chem. 2014;87C:346–363. doi: 10.1016/j.ejmech.2014.09.078. [DOI] [PubMed] [Google Scholar]
- 98.Kitson RR, Moody CJ. An improved route to 19-substituted geldanamycins as novel Hsp90 inhibitors--potential therapeutics in cancer and neurodegeneration. Chem Commun (Camb) 2013;49(76):8441–8443. doi: 10.1039/c3cc43457e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Taldone T, Ochiana SO, Patel PD, Chiosis G. Selective targeting of the stress chaperome as a therapeutic strategy. Trends Pharmacol Sci. 2014 doi: 10.1016/j.tips.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Centenera MM, Fitzpatrick AK, Tilley WD, Butler LM. Hsp90: still a viable target in prostate cancer. Biochim Biophys Acta. 2013;1835(2):211–218. doi: 10.1016/j.bbcan.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 101.Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med. 2002;8(4 Suppl):S55–61. doi: 10.1016/s1471-4914(02)02316-x. [DOI] [PubMed] [Google Scholar]
- 102.Whitesell L, Lin NU. HSP90 as a platform for the assembly of more effective cancer chemotherapy. Biochim Biophys Acta. 2012;1823(3):756–766. doi: 10.1016/j.bbamcr.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 103.Delmotte P, Delmotte-Plaque J. A new antifungal substance of fungal origin. Nature. 1953;171(4347):344. doi: 10.1038/171344a0. [DOI] [PubMed] [Google Scholar]
- 104.Soga S, Shiotsu Y, Akinaga S, Sharma SV. Development of radicicol analogues. Curr Cancer Drug Targets. 2003;3(5):359–369. doi: 10.2174/1568009033481859. [DOI] [PubMed] [Google Scholar]
- 105.Roe SM, Prodromou C, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42(2):260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 106.Schulte TW, Akinaga S, Murakata T, Agatsuma T, Sugimoto S, Nakano H, Lee YS, Simen BB, Argon Y, Felts S, Toft DO, Neckers LM, Sharma SV. Interaction of radicicol with members of the heat shock protein 90 family of molecular chaperones. Mol Endocrinol. 1999;13(9):1435–1448. doi: 10.1210/mend.13.9.0339. [DOI] [PubMed] [Google Scholar]
- 107.Duerfeldt AS, Brandt GE, Blagg BS. Design, synthesis, and biological evaluation of conformationally constrained cis-amide Hsp90 inhibitors. Org Lett. 2009;11(11):2353–2356. doi: 10.1021/ol900783m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kwon HJ, Yoshida M, Nagaoka R, Obinata T, Beppu T, Horinouchi S. Suppression of morphological transformation by radicicol is accompanied by enhanced gelsolin expression. Oncogene. 1997;15(21):2625–2631. doi: 10.1038/sj.onc.1201443. [DOI] [PubMed] [Google Scholar]
- 109.Proisy N, Sharp SY, Boxall K, Connelly S, Roe SM, Prodromou C, Slawin AM, Pearl LH, Workman P, Moody CJ. Inhibition of Hsp90 with synthetic macrolactones: synthesis and structural and biological evaluation of ring and conformational analogs of radicicol. Chem Biol. 2006;13(11):1203–1215. doi: 10.1016/j.chembiol.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 110.Hadden MK, Lubbers DJ, Blagg BS. Geldanamycin, radicicol, and chimeric inhibitors of the Hsp90 N-terminal ATP binding site. Curr Top Med Chem. 2006;6(11):1173–1182. doi: 10.2174/156802606777812031. [DOI] [PubMed] [Google Scholar]
- 111.Turbyville TJ, Wijeratne EM, Liu MX, Burns AM, Seliga CJ, Luevano LA, David CL, Faeth SH, Whitesell L, Gunatilaka AA. Search for Hsp90 inhibitors with potential anticancer activity: isolation and SAR studies of radicicol and monocillin I from two plant-associated fungi of the Sonoran desert. J Nat Prod. 2006;69(2):178–184. doi: 10.1021/np058095b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moulin E, Barluenga S, Winssinger N. Concise synthesis of pochonin A, an HSP90 inhibitor. Org Lett. 2005;7(25):5637–5639. doi: 10.1021/ol052263+. [DOI] [PubMed] [Google Scholar]
- 113.Moulin E, Zoete V, Barluenga S, Karplus M, Winssinger N. Design, synthesis, and biological evaluation of HSP90 inhibitors based on conformational analysis of radicicol and its analogues. J Am Chem Soc. 2005;127(19):6999–7004. doi: 10.1021/ja043101w. [DOI] [PubMed] [Google Scholar]
- 114.Lampilas M, Lett R. Convergent stereospecific total synthesis of monochiral Monocillin I related macrolides. Tetrahedron Lett. 1992;33(6):773–776. [Google Scholar]
- 115.Lampilas M, Lett R. Convergent stereospecific total synthesis of Monocillin I and Monorden (or Radicicol) Tetrahedron Lett. 1992;33(6):777–780. [Google Scholar]
- 116.Barluenga S, Moulin E, Lopez P, Winssinger N. Solution- and solid-phase synthesis of radicicol (monorden) and pochonin C. Chemistry. 2005;11(17):4935–4952. doi: 10.1002/chem.200500160. [DOI] [PubMed] [Google Scholar]
- 117.Yamamoto K, Garbaccio RM, Stachel SJ, Solit DB, Chiosis G, Rosen N, Danishefsky SJ. Total synthesis as a resource in the discovery of potentially valuable antitumor agents: cycloproparadicicol. Angew Chem Int Ed. 2003;42(11):1280–1284. doi: 10.1002/anie.200390329. [DOI] [PubMed] [Google Scholar]
- 118.Garbaccio RM, Stachel SJ, Baeschlin DK, Danishefsky SJ. Concise asymmetric syntheses of radicicol and monocillin I. J Am Chem Soc. 2001;123(44):10903–10908. doi: 10.1021/ja011364+. [DOI] [PubMed] [Google Scholar]
- 119.Yang ZQ, Geng X, Solit D, Pratilas CA, Rosen N, Danishefsky SJ. New efficient synthesis of resorcinylic macrolides via ynolides: establishment of cycloproparadicicol as synthetically feasible preclinical anticancer agent based on Hsp90 as the target. J Am Chem Soc. 2004;126(25):7881–7889. doi: 10.1021/ja0484348. [DOI] [PubMed] [Google Scholar]
- 120.Agatsuma T, Ogawa H, Akasaka K, Asai A, Yamashita Y, Mizukami T, Akinaga S, Saitoh Y. Halohydrin and oxime derivatives of radicicol: synthesis and antitumor activities. Bioorg Med Chem. 2002;10(11):3445–3454. doi: 10.1016/s0968-0896(02)00260-2. [DOI] [PubMed] [Google Scholar]
- 121.Ikuina Y, Amishiro N, Miyata M, Narumi H, Ogawa H, Akiyama T, Shiotsu Y, Akinaga S, Murakata C. Synthesis and antitumor activity of novel O-carbamoylmethyloxime derivatives of radicicol. J Med Chem. 2003;46(12):2534–2541. doi: 10.1021/jm030110r. [DOI] [PubMed] [Google Scholar]
- 122.Sgobba M, Rastelli G. Structure-based and in silico design of Hsp90 inhibitors. ChemMedChem. 2009;4(9):1399–1409. doi: 10.1002/cmdc.200900256. [DOI] [PubMed] [Google Scholar]
- 123.Cutler HG, Arrendale RF, Springer JP, Cole PD, Roberts RG, Hanlin RT. Monorden from a Novel Source, Neocosmospora tenuicristata: Stereochemistry and Plant Growth Regulatory Properties. Agric Biol Chem. 1987;51(12):3331–3338. [Google Scholar]
- 124.Messaoudi S, Peyrat JF, Brion JD, Alami M. Heat-shock protein 90 inhibitors as antitumor agents: a survey of the literature from 2005 to 2010. Expert Opin Ther Pat. 2011;21(10):1501–1542. doi: 10.1517/13543776.2011.594041. [DOI] [PubMed] [Google Scholar]
- 125.Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta. 2012;1823(3):742–755. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shimamura T, Perera SA, Foley KP, Sang J, Rodig SJ, Inoue T, Chen L, Li D, Carretero J, Li YC, Sinha P, Carey CD, Borgman CL, Jimenez JP, Meyerson M, Ying W, Barsoum J, Wong KK, Shapiro GI. Ganetespib (STA-9090), a nongeldanamycin HSP90 inhibitor, has potent antitumor activity in in vitro and in vivo models of non-small cell lung cancer. Clin Cancer Res. 2012;18(18):4973–4985. doi: 10.1158/1078-0432.CCR-11-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ying W, Du Z, Sun L, Foley KP, Proia DA, Blackman RK, Zhou D, Inoue T, Tatsuta N, Sang J, Ye S, Acquaviva J, Ogawa LS, Wada Y, Barsoum J, Koya K. Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy. Mol Cancer Ther. 2012;11(2):475–484. doi: 10.1158/1535-7163.MCT-11-0755. [DOI] [PubMed] [Google Scholar]
- 128.Cheung KM, Matthews TP, James K, Rowlands MG, Boxall KJ, Sharp SY, Maloney A, Roe SM, Prodromou C, Pearl LH, Aherne GW, McDonald E, Workman P. The identification, synthesis, protein crystal structure and in vitro biochemical evaluation of a new 3,4-diarylpyrazole class of Hsp90 inhibitors. Bioorg Med Chem Lett. 2005;15(14):3338–3343. doi: 10.1016/j.bmcl.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 129.Sharp SY, Boxall K, Rowlands M, Prodromou C, Roe SM, Maloney A, Powers M, Clarke PA, Box G, Sanderson S, Patterson L, Matthews TP, Cheung KM, Ball K, Hayes A, Raynaud F, Marais R, Pearl L, Eccles S, Aherne W, McDonald E, Workman P. In vitro biological characterization of a novel, synthetic diaryl pyrazole resorcinol class of heat shock protein 90 inhibitors. Cancer Res. 2007;67(5):2206–2216. doi: 10.1158/0008-5472.CAN-06-3473. [DOI] [PubMed] [Google Scholar]
- 130.Brough PA, Aherne W, Barril X, Borgognoni J, Boxall K, Cansfield JE, Cheung KM, Collins I, Davies NG, Drysdale MJ, Dymock B, Eccles SA, Finch H, Fink A, Hayes A, Howes R, Hubbard RE, James K, Jordan AM, Lockie A, Martins V, Massey A, Matthews TP, McDonald E, Northfield CJ, Pearl LH, Prodromou C, Ray S, Raynaud FI, Roughley SD, Sharp SY, Surgenor A, Walmsley DL, Webb P, Wood M, Workman P, Wright L. 4,5-diarylisoxazole Hsp90 chaperone inhibitors: potential therapeutic agents for the treatment of cancer. J Med Chem. 2008;51(2):196–218. doi: 10.1021/jm701018h. [DOI] [PubMed] [Google Scholar]
- 131.Garon EB, Finn RS, Hamidi H, Dering J, Pitts S, Kamranpour N, Desai AJ, Hosmer W, Ide S, Avsar E, Jensen MR, Quadt C, Liu M, Dubinett SM, Slamon DJ. The HSP90 inhibitor NVP-AUY922 potently inhibits non-small cell lung cancer growth. Mol Cancer Ther. 2013;12(6):890–900. doi: 10.1158/1535-7163.MCT-12-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ueno T, Tsukuda K, Toyooka S, Ando M, Takaoka M, Soh J, Asano H, Maki Y, Muraoka T, Tanaka N, Shien K, Furukawa M, Yamatsuji T, Kiura K, Naomoto Y, Miyoshi S. Strong anti-tumor effect of NVP-AUY922, a novel Hsp90 inhibitor, on non-small cell lung cancer. Lung Cancer. 2012;76(1):26–31. doi: 10.1016/j.lungcan.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 133.Perez CA, Velez M, Raez LE, Santos ES. Overcoming the resistance to crizotinib in patients with non-small cell lung cancer harboring EML4/ALK translocation. Lung Cancer. 2014;84(2):110–115. doi: 10.1016/j.lungcan.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 134.Stuhmer T, Zollinger A, Siegmund D, Chatterjee M, Grella E, Knop S, Kortum M, Unzicker C, Jensen MR, Quadt C, Chene P, Schoepfer J, Garcia-Echeverria C, Einsele H, Wajant H, Bargou RC. Signalling profile and antitumour activity of the novel Hsp90 inhibitor NVP-AUY922 in multiple myeloma. Leukemia. 2008;22(8):1604–1612. doi: 10.1038/leu.2008.111. [DOI] [PubMed] [Google Scholar]
- 135.Garcia-Carbonero R, Carnero A, Paz-Ares L. Inhibition of Hsp90 molecular chaperones: moving into the clinic. Lancet Oncol. 2013;14:e358–e369. doi: 10.1016/S1470-2045(13)70169-4. [DOI] [PubMed] [Google Scholar]
- 136.Nakashima T, Ishii T, Tagaya H, Seike T, Nakagawa H, Kanda Y, Akinaga S, Soga S, Shiotsu Y. New molecular and biological mechanism of antitumor activities of KW-2478, a novel nonansamycin heat shock protein 90 inhibitor, in multiple myeloma cells. Clin Cancer Res. 2010;16(10):2792–2802. doi: 10.1158/1078-0432.CCR-09-3112. [DOI] [PubMed] [Google Scholar]
- 137.Woodhead AJ, Angove H, Carr MG, Chessari G, Congreve M, Coyle JE, Cosme J, Graham B, Day PJ, Downham R, Fazal L, Feltell R, Figueroa E, Frederickson M, Lewis J, McMenamin R, Murray CW, O’Brien MA, Parra L, Patel S, Phillips T, Rees DC, Rich S, Smith DM, Trewartha G, Vinkovic M, Williams B, Woolford AJ. Discovery of (2,4-dihydroxy-5-isopropylphenyl)-[5-(4-methylpiperazin-1-ylmethyl)-1,3-dihydrois oindol-2-yl]methanone (AT13387), a novel inhibitor of the molecular chaperone Hsp90 by fragment based drug design. J Med Chem. 2010;53(16):5956–5969. doi: 10.1021/jm100060b. [DOI] [PubMed] [Google Scholar]
- 138.Wang M, Shen G, Blagg BS. Radanamycin, a macrocyclic chimera of radicicol and geldanamycin. Bioorg Med Chem Lett. 2006;16(9):2459–2462. doi: 10.1016/j.bmcl.2006.01.086. [DOI] [PubMed] [Google Scholar]
- 139.Clevenger RC, Blagg BS. Design, synthesis, and evaluation of a radicicol and geldanamycin chimera, radamide. Org Lett. 2004;6(24):4459–4462. doi: 10.1021/ol048266o. [DOI] [PubMed] [Google Scholar]
- 140.Shen G, Blagg BS. Radester, a novel inhibitor of the Hsp90 protein folding machinery. Org Lett. 2005;7(11):2157–2160. doi: 10.1021/ol050580a. [DOI] [PubMed] [Google Scholar]
- 141.Immormino RM, Metzger LEt, Reardon PN, Dollins DE, Blagg BS, Gewirth DT. Different poses for ligand and chaperone in inhibitor-bound Hsp90 and GRP94: implications for paralog-specific drug design. J Mol Biol. 2009;388(5):1033–1042. doi: 10.1016/j.jmb.2009.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen B, Piel WH, Gui L, Bruford E, Monteiro A. The HSP90 family of genes in the human genome: insights into their divergence and evolution. Genomics. 2005;86(6):627–637. doi: 10.1016/j.ygeno.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 143.Song HY, Dunbar JD, Zhang YX, Guo D, Donner DB. Identification of a protein with homology to hsp90 that binds the type 1 tumor necrosis factor receptor. J Biol Chem. 1995;270(8):3574–3581. [PubMed] [Google Scholar]
- 144.Felts SJ, Owen BA, Nguyen P, Trepel J, Donner DB, Toft DO. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J Biol Chem. 2000;275(5):3305–3312. doi: 10.1074/jbc.275.5.3305. [DOI] [PubMed] [Google Scholar]
- 145.Soldano KL, Jivan A, Nicchitta CV, Gewirth DT. Structure of the N-terminal domain of GRP94. Basis for ligand specificity and regulation. J Biol Chem. 2003;278(48):48330–48338. doi: 10.1074/jbc.M308661200. [DOI] [PubMed] [Google Scholar]
- 146.Taldone T, Patel PD, Patel M, Patel HJ, Evans CE, Rodina A, Ochiana S, Shah SK, Uddin M, Gewirth D, Chiosis G. Experimental and structural testing module to analyze paralogue-specificity and affinity in the Hsp90 inhibitors series. J Med Chem. 2013;56(17):6803–6818. doi: 10.1021/jm400619b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Duerfeldt AS, Peterson LB, Maynard JC, Ng CL, Eletto D, Ostrovsky O, Shinogle HE, Moore DS, Argon Y, Nicchitta CV, Blagg BS. Development of a Grp94 inhibitor. J Am Chem Soc. 2012;134(23):9796–9804. doi: 10.1021/ja303477g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Randow F, Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol. 2001;3(10):891–896. doi: 10.1038/ncb1001-891. [DOI] [PubMed] [Google Scholar]
- 149.Eletto D, Dersh D, Argon Y. GRP94 in ER quality control and stress responses. Semin Cell Dev Biol. 2010;21(5):479–485. doi: 10.1016/j.semcdb.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Muth A, Crowley V, Khandelwal A, Mishra S, Zhao J, Hall J, Blagg BS. Development of radamide analogs as Grp94 inhibitors. Bioorg Med Chem. 2014;22(15):4083–4098. doi: 10.1016/j.bmc.2014.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dejeans N, Glorieux C, Guenin S, Beck R, Sid B, Rousseau R, Bisig B, Delvenne P, Buc Calderon P, Verrax J. Overexpression of GRP94 in breast cancer cells resistant to oxidative stress promotes high levels of cancer cell proliferation and migration: implications for tumor recurrence. Free Radic Biol Med. 2012;52(6):993–1002. doi: 10.1016/j.freeradbiomed.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 152.Stothert AR, Suntharalingam A, Huard DJ, Fontaine SN, Crowley VM, Mishra S, Blagg BS, Lieberman RL, Dickey CA. Exploiting the interaction between Grp94 and aggregated myocilin to treat glaucoma. Hum Mol Genet. 2014;23(24):6470–6480. doi: 10.1093/hmg/ddu367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Suntharalingam A, Abisambra JF, O’Leary JC, 3rd, Koren J, 3rd, Zhang B, Joe MK, Blair LJ, Hill SE, Jinwal UK, Cockman M, Duerfeldt AS, Tomarev S, Blagg BS, Lieberman RL, Dickey CA. Glucose-regulated protein 94 triage of mutant myocilin through endoplasmic reticulum-associated degradation subverts a more efficient autophagic clearance mechanism. J Biol Chem. 2012;287(48):40661–40669. doi: 10.1074/jbc.M112.384800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007;1113:202–216. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- 155.Chiosis G, Lucas B, Shtil A, Huezo H, Rosen N. Development of a purine-scaffold novel class of Hsp90 binders that inhibit the proliferation of cancer cells and induce the degradation of Her2 tyrosine kinase. Bioorg Med Chem. 2002;10(11):3555–3564. doi: 10.1016/s0968-0896(02)00253-5. [DOI] [PubMed] [Google Scholar]
- 156.Wright L, Barril X, Dymock B, Sheridan L, Surgenor A, Beswick M, Drysdale M, Collier A, Massey A, Davies N, Fink A, Fromont C, Aherne W, Boxall K, Sharp S, Workman P, Hubbard RE. Structure-activity relationships in purine-based inhibitor binding to HSP90 isoforms. Chem Biol. 2004;11(6):775–785. doi: 10.1016/j.chembiol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 157.Chiosis G, Timaul MN, Lucas B, Munster PN, Zheng FF, Sepp-Lorenzino L, Rosen N. A small molecule designed to bind to the adenine nucleotide pocket of Hsp90 causes Her2 degradation and the growth arrest and differentiation of breast cancer cells. Chem Biol. 2001;8(3):289–299. doi: 10.1016/s1074-5521(01)00015-1. [DOI] [PubMed] [Google Scholar]
- 158.Llauger L, He H, Kim J, Aguirre J, Rosen N, Peters U, Davies P, Chiosis G. Evaluation of 8-arylsulfanyl, 8-arylsulfoxyl, and 8-arylsulfonyl adenine derivatives as inhibitors of the heat shock protein 90. J Med Chem. 2005;48(8):2892–2905. doi: 10.1021/jm049012b. [DOI] [PubMed] [Google Scholar]
- 159.Vilenchik M, Solit D, Basso A, Huezo H, Lucas B, He H, Rosen N, Spampinato C, Modrich P, Chiosis G. Targeting wide-range oncogenic transformation via PU24FCl, a specific inhibitor of tumor Hsp90. Chem Biol. 2004;11(6):787–797. doi: 10.1016/j.chembiol.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 160.Kasibhatla SR, Hong K, Biamonte MA, Busch DJ, Karjian PL, Sensintaffar JL, Kamal A, Lough RE, Brekken J, Lundgren K, Grecko R, Timony GA, Ran Y, Mansfield R, Fritz LC, Ulm E, Burrows FJ, Boehm MF. Rationally designed high-affinity 2-amino-6-halopurine heat shock protein 90 inhibitors that exhibit potent antitumor activity. J Med Chem. 2007;50(12):2767–2778. doi: 10.1021/jm050752+. [DOI] [PubMed] [Google Scholar]
- 161.He H, Zatorska D, Kim J, Aguirre J, Llauger L, She Y, Wu N, Immormino RM, Gewirth DT, Chiosis G. Identification of potent water soluble purine-scaffold inhibitors of the heat shock protein 90. J Med Chem. 2006;49(1):381–390. doi: 10.1021/jm0508078. [DOI] [PubMed] [Google Scholar]
- 162.Lundgren K, Zhang H, Brekken J, Huser N, Powell RE, Timple N, Busch DJ, Neely L, Sensintaffar JL, Yang YC, McKenzie A, Friedman J, Scannevin R, Kamal A, Hong K, Kasibhatla SR, Boehm MF, Burrows FJ. BIIB021, an orally available, fully synthetic small-molecule inhibitor of the heat shock protein Hsp90. Mol Cancer Ther. 2009;8(4):921–929. doi: 10.1158/1535-7163.MCT-08-0758. [DOI] [PubMed] [Google Scholar]
- 163.Dickson MA, Okuno SH, Keohan ML, Maki RG, D’Adamo DR, Akhurst TJ, Antonescu CR, Schwartz GK. Phase II study of the HSP90-inhibitor BIIB021 in gastrointestinal stromal tumors. Ann Oncol. 2013;24(1):252–257. doi: 10.1093/annonc/mds275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hong D, Said R, Falchook G, Naing A, Moulder S, Tsimberidou AM, Galluppi G, Dakappagari N, Storgard C, Kurzrock R, Rosen LS. Phase I study of BIIB028, a selective heat shock protein 90 inhibitor, in patients with refractory metastatic or locally advanced solid tumors. Clin Cancer Res. 2013;19(17):4824–4831. doi: 10.1158/1078-0432.CCR-13-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Patel PD, Yan P, Seidler PM, Patel HJ, Sun W, Yang C, Que NS, Taldone T, Finotti P, Stephani RA, Gewirth DT, Chiosis G. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat Chem Biol. 2013;9(11):677–684. doi: 10.1038/nchembio.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Weekes MP, Antrobus R, Talbot S, Hor S, Simecek N, Smith DL, Bloor S, Randow F, Lehner PJ. Proteomic plasma membrane profiling reveals an essential role for gp96 in the cell surface expression of LDLR family members, including the LDL receptor and LRP6. J Proteome Res. 2012;11(3):1475–1484. doi: 10.1021/pr201135e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu B, Staron M, Hong F, Wu BX, Sun S, Morales C, Crosson CE, Tomlinson S, Kim I, Wu D, Li Z. Essential roles of grp94 in gut homeostasis via chaperoning canonical Wnt pathway. Proc Natl Acad Sci U S A. 2013;110(17):6877–6882. doi: 10.1073/pnas.1302933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hua Y, White-Gilbertson S, Kellner J, Rachidi S, Usmani SZ, Chiosis G, Depinho R, Li Z, Liu B. Molecular chaperone gp96 is a novel therapeutic target of multiple myeloma. Clin Cancer Res. 2013;19(22):6242–6251. doi: 10.1158/1078-0432.CCR-13-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Peterson LB, Eskew JD, Vielhauer GA, Blagg BS. The hERG channel is dependent upon the Hsp90alpha isoform for maturation and trafficking. Mol Pharm. 2012;9(6):1841–1846. doi: 10.1021/mp300138n. [DOI] [PMC free article] [PubMed] [Google Scholar]