SYNOPSIS
Since the discovery of Rapid Eye Movement (REM) sleep in the late 1950s, identification of the neural circuitry underlying wakefulness, sleep onset and the alternation between REM and non-REM (NREM) sleep has been an active area of investigation. Synchronization and desynchronization of cortical activity as detected in the electroencephalogram (EEG) is due to a corticothalamocortical loop, intrinsic cortical oscillators, monoaminergic and cholinergic afferent input to the thalamus, and the basal forebrain cholinergic input directly to the cortex. The monoaminergic and cholinergic systems are largely wake-promoting; the brainstem cholinergic nuclei are also involved in REM sleep regulation. These wake-promoting systems receive excitatory input from the hypothalamic hypocretin/orexin system. Sleep-promoting nuclei are GABAergic in nature and found in the preoptic area, brainstem and lateral hypothalamus. Although the pons is critical for the expression of REM sleep, recent research has suggested that melanin-concentrating hormone/GABAergic cells in the lateral hypothalamus "gate" REM sleep. The temporal distribution of sleep and wakefulness is due to interaction between the circadian system and the sleep homeostatic system. Although the hypothalamic suprachiasmatic nuclei contain the circadian pacemaker, the neural circuitry underlying the sleep homeostat is less clear. Prolonged wakefulness results in the accumulation of extracellular adenosine, possibly from glial sources, which is an important feedback molecule for the sleep homeostatic system. Cortical neuronal nitric oxide (nNOS) neurons may also play a role in propagating slow waves through the cortex in NREM sleep. Several neuropeptides and other neurochemicals likely play important roles in sleep/wake control. Although the control of sleep and wakefulness seemingly involves multiple redundant systems, each of these systems provides a vulnerability that can result in sleep/wake dysfunction that may predispose to physical and/or neuropsychiatric disorders.
Keywords: EEG, synchronization, homeostasis, slow wave activity, NREM sleep, REM sleep, neurotransmitter, hypocretin, orexin, adenosine
Our understanding of the neural control of sleep in large part parallels the history of the field of neuroscience. As new tools and methodologies have become available to the research community, sleep researchers have been quick to take advantage of such techniques. Thus, the description in the following sections progresses from relatively crude methods such as brain transections and lesion studies to the application of molecular biology and genetics to create transgenic models. The approach in this section will be largely historical, illustrating the insights and principles that have emerged as research has progressed.
The advent of inducible transgenic mouse strains and viral-mediated transfection has enabled the ability to target cell populations in a phenotype-, location- and time-specific manner. This added precision eliminates many of the caveats associated with more traditional lesion (impossible to isolate heterogeneous cell populations) and knockout methodologies (developmental confounds; lack of anatomical specificity). Recently, opto- and chemogenetic methods have enabled manipulation of specific cell populations with unprecedented precision using light or synthetic ligands, respectively. In optogenetics, neurons of interest are genetically engineered to express light-sensitive opsins that control specialized ion channels. Subsequent illumination of these cells (usually through surgically implanted optical fibers) by specific wavelengths of light can activate or inhibit the cells expressing that opsin without collateral activation of nearby cell types. For example, the blue light-sensitive channel rhodopsin-2 protein opens a Na+ channel and, when stimulated, will depolarize a cell. Conversely, the yellow/green light-sensitive halorhodopsin opens a Cl− channel and will thus inhibit cells when illuminated. Similarly, the DREADD (Designer Receptors Exclusively Activated by Designer Drugs) system relies on a modified G protein-coupled receptor that responds only to a (otherwise biologically inert) synthetic ligand. Following expression of the DREADD receptor in a cell population of choice, the ligand can be injected systemically to activate only that population. This combination of powerful tools for precisely manipulating neuronal activity with the specificity of genetic targeting is revolutionizing the study of neural circuits and their control of behavior.
Cortical Activity During Sleep and Wakefulness
The electrical activity of the cerebral cortex has been used to distinguish sleep vs. wakefulness since the earliest EEG studies of sleep.1 The firing rate of cortical neurons generally declines during non-Rapid Eye Movement (NREM) sleep relative to wakefulness and REM sleep,2, 3 although a few studies have reported cortical neurons with the opposite firing pattern.4, 5 EEG activity reflects the aggregate firing of large neuronal ensembles, and is conventionally referred to by bandwidths with the following approximate frequencies: alpha (9-12 Hz), beta (12-30 Hz), delta (0.5-4.0 Hz), low (30-60Hz) and high (60-100 Hz) gamma and theta (5-9 Hz). Several neural circuits have been implicated in the synchronization and desynchronization of cortical activity that distinguish NREM sleep from wakefulness and REM sleep. For example, input from the basal forebrain (BF), likely from both cholinergic and non-cholinergic neurons, is critical for the desynchronized EEG characteristic of wakefulness and REM.6, 7
Synchronization of the EEG during NREM depends on a corticothalamocortical loop8 as well as intrinsic cortical oscillators,9 whose activities are modulated by a number of subcortical systems. Here, our primary focus will be how interactions between these subcortical systems produce sleep and wake and their electrophysiological correlates at the cortical level.
Historical Overview
Classical brainstem transection studies
The first investigations relevant to the neurobiology of sleep and wakefulness were conducted in the 1930s. Bremer transected the cat brainstem, observing that sleep/wake cycles remained intact after a low medullary level transection (encephale isolé), whereas transection between the pons and midbrain (cerveau isolé) yielded chronic drowsiness.10 Conversely, electrical stimulation of the midbrain reticular formation caused alerting of the cortex.11 From these observations arose the concept that the forebrain was kept alert by tonic activity in the reticular formation. This ascending reticular activating system (ARAS) is comprised of cholinergic laterodorsal and pedunculopontine tegmentum (LDT/PPT), noradrenergic locus coeruleus (LC), serotonergic (5-HT) Raphe nuclei and dopaminergic ventral tegmental area (VTA), substantia nigra (SN) and periaqueductal gray projections that stimulate the cortex directly and indirectly via the thalamus, hypothalamus and BF.6, 12-18 These aminergic and catecholaminergic populations have numerous interconnections and parallel projections which likely impart functional redundancy and resilience to the system.6, 13, 19
In contrast, transecting the pons rostral to the trigeminal nerve induced constant wakefulness,20 suggesting that input from a sleep "center" in the lower pons or medulla inhibited a wakefulness "center" in the rostral pons. This result established that sleep is an active state of the brain;21 however, the identity of this lower brainstem “sleep center" remained a mystery. Although “sleep active” neurons were reported in the nucleus tractus solitarius,22 their location has proven to be elusive. More recently, the medullary parafacial zone (PZ) adjacent to the facial nerve was identified as a sleep-promoting center on the basis of anatomical, electrophysiological and chemo- and optogenetic studies.23, 24 GABAergic PZ neurons inhibit glutamatergic parabrachial (PB) neurons that project to the BF,25 thereby promoting NREM sleep at the expense of wakefulness and REM sleep. As we shall see below, these populations exert much of their effects via projections to the BF and hypothalamus.
Encephalitis lethargica: Insights into sleep/wake control from neuropathology
After World War I, a worldwide influenza epidemic claimed an estimated 25-40 million fatalities. One variant of this disease was encephalitis lethargica, in which patients entered a coma that often resulted in death. The neuropathologist Constantin von Economo identified distinct types of brain lesions associated with equally distinct effects on sleep and waking. Lesions in the posterior hypothalamus extending into the mesencephalic reticular formation were associated with persistent coma, whereas lesions in the anterior hypothalamus and the adjacent BF were associated with chronic insomnia. von Economo concluded that the posterior hypothalamus was important for the maintenance of wakefulness and the anterior hypothalamic/BF region important for sleep induction.26
Nauta subsequently demonstrated that anterior hypothalamic transections severely disrupted sleep and wakefulness,27 providing direct experimental evidence for von Economo’s clinical observations. Sterman, McGinty and others found that preoptic/basal forebrain lesions decreased sleep,28 whereas stimulation of this region facilitated sleep onset.29 Sleep-active neurons were later described in the BF, particularly the substantia innominata and the horizontal limb of the diagonal band of Broca.30 Subsequent electrophysiological, Fos activation, tract tracing and lesion studies identified the GABAergic ventrolateral preoptic area (VLPO) and median preoptic area (MnPO) as being sleep-active neuronal populations that project to and inhibit wake-active cell groups.31-38 These preoptic sleep-promoting groups are themselves inhibited by wake-active monoaminergic stimulation.39, 40 It should be noted that the BF also contains cortically-projecting cholinergic neurons distributed across the diagonal band of Broca, nucleus basalis and substantia innominata.41, 42 These neurons have been extensively studied for their role in promoting cortical activation and wakefulness. In addition, much research has examined the role of the BF, including the cholinergic neurons therein, in regulating sleep homeostasis; this work will be discussed in detail in a later section.
Also consistent with von Economo's earlier observations, lesions of the posterior lateral hypothalamus (PLH) were found to increase sleep in rats, cats and monkeys.27, 43, 44 Histaminergic (HA) cells were subsequently identified in the tuberomammillary nuclei (TM) and found to be wake-promoting.45 Antihistamines have long been known to be soporific, and knockout mice lacking the enzyme responsible for histamine synthesis are hypersomnolent.46, 47 Inhibitory VLPO neurons project to HA TM neurons48, and HA neurons project widely throughout the brain including to wake-promoting populations in the brainstem and to the cortex.49 Injections of the GABAA agonist muscimol into the posterior hypothalamus increased NREM sleep and suppressed REM sleep, whereas injections in the ventral PLH increased both NREM and REM sleep.50 Together, these results supported the hypothesis that sleep results from functional blockade of a posterior hypothalamic waking center. Although the neurons inactivated by muscimol were thought to be the HA cells, the PLH contains another wake-active neuronal population, the hypocretin/orexin cells, that were yet to be described.
REM sleep: The role of the Pons and Acetylcholine
REM sleep was first described by Aserinsky and Kleitman51-53 and was described in animals in 1959.54 As cellular neurophysiology entered the neurobiologist's toolbox in the '60s and early '70s,55-57 sleep physiologists characterized the firing rates of cells in specific brain regions across the arousal state continuum from wakefulness to NREM to REM sleep. These "arousal state profiles” showed that monoaminergic cell groups decrease their firing from wakefulness to REM and are thus called "REM-off" cells. In contrast, a smaller set of brainstem regions had maximal firing rates during REM ("REM-on” cells). Since these cell groups were anatomically localized, Hobson and McCarley58, 59 proposed that the NREM/REM cycle arises from a reciprocal interaction between these aminergic REM-off cell groups and cholinergic REM-on cell groups in the medial pons. More recent versions of this model recognize that the REM-on and REM-off cells are distributed in a variety of brain regions60-62 and include both glutamatergic and GABAergic populations.63, 64
The pons is both necessary and sufficient to generate REM sleep65, 66 and the dorsolateral pons, in particular, is crucial for the genesis of REM sleep.67, 68 Neurons in this region have a “REM-on” profile with their highest discharge rate occurring during REM sleep.69, 70 Microinjections of carbachol, a mixed cholinergic agonist, into the dorsolateral pons results in a prolonged REM-like state, and pontine acetylcholine levels are increased during REM sleep relative to NREM and wakefulness.71 Cholinergic input for REM sleep generation comes from the more rostral PPT and LDT, which project to the cholinoceptive subcoeruleus or sublaterodorsal tegmental nucleus (SLD) and the subjacent nucleus pontis oralis.72, 73 During REM sleep, activation of the dorsolateral pons regulates the varied physiological manifestations of REM sleep, e.g., EEG desynchronization and theta activity, ponto-geniculo-occipital (PGO) waves, rapid eye movements and atonia. More recent studies have implicated the melanin-concentrating hormone (MCH)/GABAergic neurons of the hypothalamus as providing critical input to the pontine generator of REM sleep.64, 74
Lesions of the dorsolateral pons produce REM sleep without atonia in cats: the cats orient, locomote, and engage in what appears to be prey-catching behavior - as if they were "acting out their dreams".75, 76 Cholinoceptive dorsolateral pontine neurons project to the ventral medulla, where they form synapses with inhibitory neuronal populations that, in turn, project to and inhibit spinal motorneurons, thereby preventing muscle movement during REM. This descending inhibition is thought to be mediated by glycinergic mechanisms;77 more recent work has implicated GABA78, 79 as well as local inhibition in the ventral horn.80 A similar condition, REM Behavior Disorder, exists in humans;81 interest in the neurophysiological basis of REM Behavior Disorder has been potentiated by the finding that REM Behavior Disorder may be a risk factor or predictor of Parkinson’s disease and other neurodegenerative synucleinopathies.82, 83
Narcolepsy/Cataplexy
The other sleep disorder related to the dorsolateral pons is narcolepsy. Narcoleptic patients suffer from excessive daytime sleepiness, abnormalities of REM sleep, and sudden attacks of muscle atonia during wakefulness known as cataplexy. Cataplexy is primarily triggered by “positive” emotional stimuli (laughter, surprise, sexual arousal) that are processed by the limbic system; these stimuli appear to converge on the REM atonia pathway, likely through the prefrontal cortex and the amygdala.84, 85 In narcoleptic dogs, cataplexy can be exacerbated by anticholinesterases, and the muscarinic cholinergic receptor is upregulated in the pons of these dogs.86, 87 Although this upregulation likely affects the same REM atonia pathway described above, narcoleptic dogs have been particularly valuable for the insights that they provided into the role of the hypocretin/orexin system in sleep/wake control and muscle atonia.88
The Hypocretin/orexin System
Discovery of the Hypocretins and the Orexins
Hypocretins 1 and 2 (Hcrt1 and Hcrt2), also known as orexins A and B, are excitatory hypothalamic neuropeptides that were independently described by two groups in 1998.89, 90 Since Sakurai et al.91 confirmed the common identity of the Hcrts and the orexins, we will use the term “Hcrt” here to refer to this system. While early studies emphasized the role of the Hcrt system in feeding and energy balance, subsequent research focused on sleep-wake regulation based, in part, on the discovery that Hcrt dysfunction underlies the sleep disorder narcolepsy.
Hcrt neurons are found exclusively in the PLH,89, 90, 92 numbering between 4,000-5,000 in the rat92-94 and 50,000-80,000 in humans.95 The two Hcrt peptides are derived from a single precursor molecule by proteolytic processing and Hcrt1 and Hcrt2 are largely co-localized in the same neurons.96 Hcrt neurons are co-extensive, but not co-localized, with MCH neurons.92, 97, 98 The Hcrt neurons project widely throughout the brain and spinal cord92, 96, 99, 100 including major projections to wake-promoting cell groups such as the HA cells of the TM,101 the 5-HT cells of the dorsal Raphe nuclei (DRN),101 the noradrenergic cells of the LC,102 and cholinergic cells in the LDT, PPT, and BF.101, 103 Afferent input to Hcrt neurons was mapped using a combination of retrograde and anterograde tract tracers;104 these studies described major projections from the lateral septal nucleus, bed nucleus of the stria terminalis, preoptic area, dorsomedial, ventromedial and posterior hypothalamic nuclei, substantia nigra and VTA, and the DRN. Using transgenic mice expressing transneuronal retrograde tracers linked to the Hcrt promoter,105 genetic tracing studies revealed a more circumscribed set of afferents to the Hcrt neurons from the amygdala, preoptic GABAergic neurons, and 5-HT neurons in the median/paramedian Raphe nuclei. Thus, the anatomy of the Hcrt system strongly suggests involvement in numerous physiological functions including sleep-wake, feeding, thermoregulation, blood pressure and neuroendocrine regulation.
To date, the Hcrt peptides have uniformly been reported as excitatory either by eliciting depolarization and/or increased spike frequency.89 Hcrt directly excites cellular systems involved in waking and arousal including the LC,102, 106, 107 DRN,108, 109 TM,110-112 LDT,113, 114 cholinergic BF,115 and both dopamine (DA) and non-DA neurons in the VTA.116, 117 The excitatory effects of Hcrt are mediated by multiple ionic mechanisms110, 118-125 which, combined with their capacity for neuromodulation,109, 113, 118, 126-129 suggest that Hcrt exerts potent direct and indirect effects on a variety of physiological systems, particularly arousal systems. Some of these effects appear to be mediated by colocalized transmitters and modulators including, but not limited to, dynorphin, galanin and glutamate.130-135 Cellular electrophysiological studies revealed that while Hcrt cells are excited by numerous substances,136-145 they are inhibited by the aminergic transmitters NE, DA, and 5-HT,136, 139, 146-148 as well as by GABA.136, 139, 149
Hcrt signaling is strongly associated with wakefulness. The region of the PLH containing the Hcrt cells has long been implicated in arousal state control.27 Hcrt neurons are wake-active as measured by Fos expression,150 electrophysiology,151-153 or brain/CSF peptide content.154-156 Hcrt1 increases arousal when infused into the brain,106, 157-162 and optogenetic stimulation or inhibition of Hcrt signaling increases or decreases wakefulness, respectively.163-167 Hcrt receptor antagonists promote sleep when administered systemically or directly into the brain.168-171 Indeed, newly-developed dual Hcrt receptor antagonists exhibit promise for the effective pharmacological promotion of sleep without adverse side effects such as cognitive performance deficits and dependence that are common to many sleep medications.172, 173
The Hcrt System and the Sleep Disorder Narcolepsy
Narcolepsy has a genetic component in both humans and dogs that has proven instrumental in identifying its unique pathology. In narcoleptic dogs, the canarc-1 gene that transmits narcolepsy was identified as a mutation in the hcrtr2 gene that results in a truncated, nonfunctional protein.88 In a remarkable convergence, Hcrt null mutant mice exhibited a narcoleptic phenotype, including cataplectic “behavioral arrests”, sleep-onset REM, and increased and fragmented NREM and REM sleep.101 Thus, dysfunction of either the Hcrt ligand or one of its receptors can result in narcolepsy. In humans, the HLA Class II antigen HLA DQB1*0602 is present in more than 85% of narcoleptic patients with cataplexy but only 12-38% of the general population.174 Such close association with the HLA system has led to the suggestion that narcolepsy may be an autoimmune disease.175, 176 Narcoleptic humans exhibit undetectable levels of Hcrt1 in cerebrospinal fluid (CSF).177 Postmortem studies revealed an absence of prepro-hcrt mRNA178 and an 85-95% reduction in the number of Hcrt-containing cells in human narcoleptic brains95 without any change in either MCH mRNA or the number of MCH cells. The presence of increased staining for glial fibrillary acid protein in the PLH of narcoleptic brains95, 178 suggests that degeneration of the Hcrt cells may cause human narcolepsy. Consistent with this, animal models in which the Hcrt neurons are ablated by selective neurotoxins179, 180 or engineered to degenerate postnatally181, 182 also present a narcoleptic phenotype.
Models for the Role of the Hcrt System in Arousal State Regulation
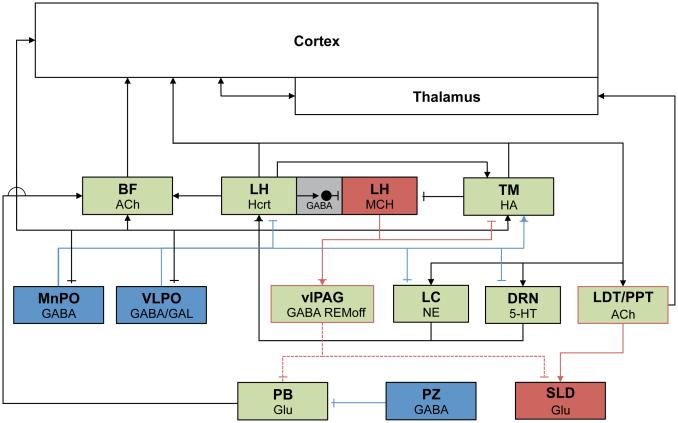
The balance between sleep and wakefulness has been proposed to be maintained by the relative activation of the wake-active systems found in the BF, LDT/PPT, LC, DRN and TM and the sleep-active systems found in the VLPO.13, 94 These relationships are summarized in Figure 1. In wakefulness, ascending monoaminergic projections from the ARAS nuclei activate wake-promoting cholinergic BF and histaminergic TM cells en route to the cerebral cortex, while inhibiting sleep-promoting VLPO and MnPO neurons. LDT/PPT cholinergic neurons ascend to the thalamus, which in turn stimulates the cortex. During NREM sleep, inhibitory, GABAergic output from the VLPO, MnPO and PZ inhibit these populations. REM sleep is driven by a combination of increased brainstem cholinergic (“REM-on”) activity and inhibition of “REM-off” populations; MCH neurons in the LH are proposed to be a part of the REM control mechanism as well. Hcrt signaling promotes waking by activating brainstem and forebrain wake-active populations and is, in turn, inhibited by ascending 5-HT and NE inputs. Hcrt has also been proposed to consolidate waking and sleep states by stabilizing transitions between sleep and wakefulness in the “flip-flop switch” model.13 When the Hcrt system is dysfunctional as occurs in human narcolepsy and transgenic mouse models, behavioral state instability results so that the affected individual cannot maintain extended periods of wakefulness of sleep and, instead, shifts rapidly between these states.183 This “flip-flop switch” concept was subsequently extended to account for the alternation between NREM and REM sleep.63
Figure 1.
Schematic depiction of major subcortical sleep-wake regulatory populations. Wake-promoting cell groups are green, NREM sleep-promoting cell groups are blue and REM sleep-related cell groups are red. Green boxes with red outlines indicated wake/REM-active populations. Gray boxes indicate local GABA interneurons. Excitatory connections are marked with arrowheads, and inhibitory connections are indicated by blunted terminals. Dotted lines indicate pathways that are inhibited during REM sleep.
Sleep Homeostasis and the Timing of Sleep and Wakefulness
Common knowledge, as well as scientific observations, suggest that sleep is a homeostatically regulated physiological response. The longer one is awake, the more likely one is to sleep or, at least, be sleepy. Sleep deprivation impairs cognition and prolonged sleep deprivation results in impaired physiological function, ultimately resulting in death.184 This homeostatic property has been incorporated into the "two-process model" of sleep regulation185, 186 which posits that the homeostatic sleep-related "Process S" integrates input from the circadian system ("Process C") to gate the occurrence of sleep and wakefulness across the day. Process S is proposed to be a neurochemical process(es) that begins to build up at the onset of wakefulness; once a threshold value is reached, sleep will ensue only if Process C is in the appropriate circadian phase. This seemingly simplistic model accounts remarkably well for the timing of sleep in humans and rodents.
EEG slow waves in the delta bandwidth (0.5-4.0 Hz) generated by thalamocortical interactions during NREM sleep increase in proportion to prior wake duration. The level of NREM delta power (NRD; also called EEG slow wave activity or EEG SWA) is highly dependent on the prior history of sleep and wakefulness: prolonged wakefulness dose-dependently increases NRD while both daytime naps and nighttime sleep decrease NRD, reflecting a diminution of Process S. Conversely, NRD itself is highly resistant to circadian modulation.187, 188 Thus, EEG NRD has been suggested to reflect the cortical manifestation of the recovery from prior waking activities185 and is commonly used as a quantitative measurement of Process S.
Anatomical substrates of the Two-Process model
The Suprachiasmatic Nuclei as the Basis for Process C
The hypothalamic suprachiasmatic nuclei (SCN) contain a master circadian pacemaker (or biological clock) in mammals,189-192 and are commonly recognized as the source of Process C.193-195 However, it remains unclear how SCN activity temporally organizes daily sleep-wake rhythms. Early studies relied on SCN lesions to functionally dissect circadian versus homeostatic regulation. The homeostatic response to sleep loss was intact in SCN-lesioned rats with no change in total sleep time per 24 h,196, 197 whereas similar studies in SCN-lesioned squirrel monkeys and mice and in behaviorally arrhythmic Siberian hamsters reported increased sleep time per 24 h.198-200 These results fueled ongoing debate over whether the SCN specifically promotes wakefulness (as in the “opponent process model”), sleep, or both.199, 201 More recently, Hcrt was proposed as a point of integration between circadian and homeostatic mechanisms based on CSF Hcrt1 levels assayed across the day and in conjunction with sleep deprivation. However, it is unclear to what extent changing Hcrt1 levels in the CSF result from active (e.g., circadian or homeostatic) regulation156, 202, 203 or are passively driven by increased locomotor activity.204 Integration of circadian and homeostatic signaling may also occur within the SCN. Indeed, SCN neuronal firing rates are modulated by sleep-wake state and by sleep deprivation,205, 206 and certain sleep-wake states (e.g., REM sleep)207-209 and EEG spectral signatures188 may be under stronger circadian control than others.
Circadian rhythms arise from interactions between a well-characterized set of dedicated “clock genes” found throughout the body and CNS.210, 211 Genetic disruption of the clock via knockout yields increased waking, increased sleep or no change of daily sleep-wake amounts depending on the particular gene targeted.212-216 Sleep deprivation can modulate extra-SCN clock gene expression and binding activity.217-221 Together, these findings suggest that different clock genes may play distinct roles in regulating or integrating circadian and homeostatic aspects of sleep.
In Search of Substrates for Process S
Whereas the anatomical substrate for Process C had been identified prior to the development of the two-process model, a similar substrate for Process S has proven more difficult to identify. GABAergic neurons in the MnPO are sleep-active,34, 222 project to wake-active brain regions including the LC, DRN, vlPAG and the Hcrt neurons,38 and Fos immunohistochemical studies indicate that MnPO activation occurs during sleep deprivation prior to sleep onset,223 suggesting that this region is responsive to homeostatic sleep pressure. MnPO neurons also exhibit increased Fos activation during REM sleep deprivation.224
A cortical neuronal population that expresses neuronal nitric oxide synthase (nNOS) has recently emerged as a candidate for involvement in Process S. Whereas the majority of cortical neurons express the immediate early gene product Fos during waking, cortical nNOS neurons express Fos during sleep, but not during wakefulness.225 Cortical nNOS neurons, which also coexpress the Substance P receptor NK1, represent the rarest subset of GABAergic interneurons and are anatomically and functionally quite distinctive: they are the only nNOS-synthesizing neuronal population reported to be sleep-active,226 they receive subcortical inputs from sleep-related cholinergic227 and serotonergic228 neurons, and send long-range rather than local circuit projections.229-232 Functionally, nNOS cortical neuron activation is positively correlated with NR bout duration and NRD energy233 and critically depends on elevated sleep pressure.234 Furthermore, loss of nNOS signaling in nNOS null mutant mice fragments NR sleep, attenuates NRD power while increasing delta power in wake, and increases sleepiness while attenuating response to sleep deprivation. Cortical nNOS neurons thus appear to be critical integrators in the neuronal network linking state-dependent afferent inputs, homeostatic sleep drive and EEG SWA.235
Other Neurochemicals Involved in Sleep/Wake Control
Although functional neuroanatomical approaches, especially when combined with electrophysiology, have led to many fundamental insights into the control of sleep and waking, there is an equally impressive literature on sleep substances and their contributions to behavioral state. These “sparks vs. soup” approaches are highly complementary and valuable insights into the control of sleep and wakefulness have arisen from both approaches.
Cytokines and Sleep
A number of lymphokines (e.g., interleukin-1, tumor necrosis factor alpha), inflammatory molecules and growth factors promote NREM sleep.236, 237 Interleukin-1 and tumor necrosis factor may regulate physiological sleep through direct, receptor-mediated modulation of the hypothalamus and serotonergic raphe nuclei. Other immune molecules, such as interleukin-6, promote NREM sleep and are elevated in sleep disorders with excessive daytime sleepiness as a symptom.
Peptides and Sleep
As indicated above, Hcrt has wake-promoting activity.106, 160 A number of other neuropeptides have also been found to promote wakefulness. Corticotrophin-releasing factor (CRF)238, 239 and adrenocorticotrophic hormone,240 two core components of the hypothalamo-pituitary adrenal axis, promote wakefulness, possibly mediated by CRF activation of CRF receptor-1 on Hcrt cells.141 Thyrotropin-releasing hormone (TRH) and TRH analogs are wake-promoting in rodents241, 242 but, in a clinical study, TRH only exerted a “weak” effect on sleep efficiency.243 Neuropeptide Y, a potent inducer of feeding behavior, exerts varied effects on rodent sleep ranging from sleep suppression to alterations in EEG spectral power.244-246 In humans, intravenous NPY reduced sleep latency in young men247 and older men and women.248 Neuropeptide S249 and urotensin250 are also reported to promote wakefulness in rodents.
Among sleep-promoting peptides, growth hormone releasing hormone (GHRH) has been extensively studied,238, 251, 252 in part because pharmacological stimulation of slow wave sleep (SWS) results in increased GH release.253 However, studies of peptides related to the GH system have produced varying results.246, 254-256 Intraperitoneal cholecystokinin-8 (CCK) reduced sleep latency and increased NREM sleep in rats and rabbits257-259 and centrally-administered CCK restored sleep in cats rendered insomniac by serotonin depletion.259, 260 α-melanocyte stimulating hormone is sleep-promoting as is corticotropin-like intermediate lobe peptide.240 Both peripheral261 and icv262 infusion of insulin increases SWS in rats. These effects could be related to postprandial sleepiness. Insulin also stimulates insulin-like growth factor-1 (IGF-1) receptors, although the molar doses of IGF-1 needed to promote sleep are much lower than that of insulin.263
As indicated above, the hypothalamic neuropeptide MCH is coextensive, but not co-localized, with Hcrt cells.92, 97, 98 MCH has profound effects on both SWS and REM sleep, in particular, when administered icv.264 Fos is activated in MCH neurons during recovery from REM sleep deprivation.264, 265 MCH neurons are inhibited directly by HA and indirectly by Hcrt via local GABA interneurons.266, 267 Optogenetic studies have implicated MCH neurons in the control of REM sleep268, 269 as well as sleep onset.270 Other peptides with REM-promoting activity include prolactin,271, 272 vasoactive intestinal polypeptide 272, 273 and pituitary adenylate cyclase-activating polypeptide.274-277
Extracellular Adenosine as an Indicator of Sleep Loss
Interest in adenosine (AD) as a potential modulator of sleep and wakefulness arose when the adenosine receptors were cloned and it was recognized that methylxanthines such as caffeine, a potent wake-promoting substance, were antagonists at AD receptors.278 AD is the ultimate breakdown product of ATP and, as such, there has also been great interest in a role for AD as a potential link between sleep and restoration of intracellular energy stores.279, 280 Injections of AD or AD analogs typically promote sleep and NREM EEG delta power;281-284 interestingly, such injections increase delta power even in sleep-satiated animals285. AD signaling regulates sleep and waking at targets including the BF, VLPO, Hcrt neurons, cortex and brainstem,280 primarily via inhibitory A1 receptors and excitatory A2A receptors.286-289
Levels of extracellular AD accumulate with time spent awake and decline during recovery sleep in the BF and cortex but weakly, if at all, in other brain regions.290, 291 In the BF, wake-related AD release appears to depend on cholinergic neurons, as cell-specific lesions of these neurons abolish AD increases induced by sleep deprivation.292, 293 Together, these data suggest that AD is an important endogenous regulator of sleep and waking in the brain, and that the BF, in particular, is important for adenosinergic influences on sleep homeostasis.294 While the source of AD in the BF has proven elusive, expression of inducible NOS (iNOS) in the cholinergic BF neurons appears to be important for the wake-related upregulation of AD.295-297 Astrocytes may be an important source for AD induced by waking, as abolishing vesicular release specifically in astrocytes attenuated the homeostatic sleep response and blocked the sleep-suppressing effect of an adenosine A1 receptor antagonist.298, 299 Given the important role played by astrocytes in regulating neuronal energy stores, it is tempting to speculate that glial-neuronal interactions may be a critical component of regulating sleep need.300-302
Melatonin
Melatonin is produced by the pineal gland during the night in both diurnal and nocturnal species. Specific receptors for melatonin are found in the cortex, SCN, and hypothalamic regions involved in thermoregulation. Exogenous melatonin is a popular hypnotic available in both physiological (0.03 mg) and pharmacological (1-10 mg) doses. Physiologic doses can help in sleep onset processes when sleep initiation is attempted at abnormal times, such as occurs following travel across time zones. Melatonin helps to synchronize circadian rhythms in totally blind individuals.303 Pharmacologic doses may work through non-melatonin receptors.
Prostaglandin D2 and Sleep/Wake Regulation
Intra-cerebral administration of prostaglandin D2 (PGD2) induces sleep, especially SWS, in rats and monkeys.304 Inhibition of the enzyme responsible for PGD2 synthesis, PGD synthase (PGDS),305, 306 markedly suppresses sleep and blockade of PGD2 receptors inhibits physiological sleep.307 CSF levels of PGD2 undergo significant modulation by time of day in rats, with a daytime peak and a nighttime trough.308 CSF levels of PGD2 in rats increase during sleep deprivation and tend to increase along with an increasing propensity toward sleep under normal conditions.309 The site of action for PGD2 has been identified as a sleep-promoting zone (PGD2-SZ) located on ventral surface of the rostral basal forebrain outside the brain parenchyma.310, 311 Administration of a selective adenosine A2a-R agonist (CGS21680), but not the selective adenosine A1-R agonist cyclohexyladenosine, markedly induces sleep when administered to the PGD2-SZ.312 The SWS-promoting effect of PGD2 is inhibited by pretreatment with KF17837, a highly selective A2a-R antagonist312 and is blunted in adenosine A2a-R-deficient mice.289 It is therefore hypothesized that PGD2 is coupled to A2a-R adenosinergic signaling via the brain parenchyma and that the PGD2-SZ plays an important role as an interface between these two systems.
Gonadal steroids and sleep
Sex and sex hormones have long been reported to modulate sleep and biological timing, but only recently have these phenomena begun to be studied on a more mechanistic level.313 Women exhibit increased subjective sleep disturbance, particularly insomnia,314 and increased spindle activity and SWA compared to men.315-318 Sex differences in SWA are amplified by sleep deprivation, aging and major depression.315, 319 Sleep spindle activity and REM sleep, but not SWA, also vary across the menstrual cycle.320-322 Female rodents exhibit increased wakefulness in the dark (active) phase compared to males,323-328 with the estrus cycle further modulating sleep in female rats,329-331 but not mice.325 Like humans, female mice exhibit increased spindle frequency activity and NRD compared to males.324, 326 Circulating ovarian steroids, particularly estradiol and progesterone, are important for maintaining many of these effects.327, 330, 332-334 Studies exploiting genetic tools to dissociate genetic and gonadal sex, along with use of classical neuroendocrine paradigms, recently showed that sex differences in sleep appear to be developmentally determined by a combination of genetic sex and gonadal hormone exposure.335, 336 Estradiol downregulates the synthetic enzyme for PGD2,337 increases Hcrt and Hcrt receptor expression levels,338, 339 and modulates Fos expression in the VLPO and TM.331
SUMMARY
Sleep is a regulated physiological state with clear implications for cognition, performance and overall well-being. Although beyond the scope of this review, numerous sleep disorders have been described that negatively impact these functions. Other chapters in this volume address the involvement of sleep disturbances in a number of psychiatric disorders. The neural substrates of sleep and wakefulness appear to be highly distributed and, to some extent, redundant systems distributed throughout the brain with monoaminergic and cholinergic systems largely promoting wakefulness and GABAergic systems in the preoptic area and brainstem promoting sleep. The hypocretin/orexin system appears to play a special role in the promotion of wakefulness and suppression of REM sleep by providing excitatory input to the monoaminergic and cholinergic systems. Sleep is not a unitary state but involves a cyclic alternation between NREM and REM sleep; the pons is critical for generating the multiple components (i.e., EEG synchronization, eye movements, muscle atonia, etc.) that characterize REM sleep. The timing of sleep and wakefulness is regulated by an interaction between the circadian pacemaker located in the hypothalamic SCN and a sleep homeostatic system whose anatomical location is yet to be convincingly identified. Among various neurochemicals, extracellular AD accumulates in the BF as wakefulness is extended and inhibits cortically-projecting cholinergic neurons, thereby influencing cortical activity. A corticothalamocortical loop plays a major role in generating SWA measured in the EEG; cortical nNOS/NK1 neurons may be important in coordinating and/or propagating SWA within the cortex. Since the control of sleep and wakefulness involves a complex orchestration of the activity of many neural systems, it is readily apparent that many nodes for dysfunction exist that can have implications for both physical and mental health.
KEY POINTS.
Sleep is not a unitary state; Rapid Eye Movement (REM) and non-REM (NREM) sleep recur with a 90 min cyclicity in humans and more rapidly in other mammals.
The monoaminergic systems of the brainstem, the cholinergic neuronal groups found in the brainstem and basal forebrain and the hypocretin/orexin cells of the hypothalamus are critical for the maintenance of wakefulness.
Sleep is regulated by GABAergic populations in both the preoptic area and the brainstem; increasing evidence suggests a role for the melanin-concentrating hormone cells of the lateral hypothalamus.
The pons has historically been viewed as critical for the production of REM sleep; recent research implicates descending projections from the hypothalamus and the periaqueductal gray as important for control of pontine REM generators.
The hypocretin/orexin cells of the posterior and lateral hypothalamus provide excitatory input to all wake-promoting monoaminergic and cholinergic brain nuclei and both promote wakefulness and suppress REM sleep; loss of these cells results in the sleep disorder narcolepsy.
The timing of sleep and wakefulness across the 24 h day/night cycle is due to the interaction between the circadian pacemaker located in the suprachiasmatic nuclei of the hypothalamus and a sleep homeostatic mechanism whose anatomical locus is yet to be conclusively defined.
Sleep is a homeostatically-regulated process in which adenosine plays an important feedback role; the underlying neural circuitry is incompletely understood but may involve cortical nNOS/NK1 neurons.
Synchonization of cortical activity as measured in the electroencephalogram (EEG) involves a corticothalamocortical loop and oscillators intrinsic to the cortex; EEG desynchronization results from monoaminergic and/or cholinergic input to the thalamus and cholinergic projections from the basal forebrain.
Acknowledgments
This work was supported by NIH R01 HL059658, R01 NS077408, R21 NS087550, R01 NS082876, R21 NS083639 and R21 NS085757. We thank Drs. Sarah Wurts Black, Stephen Morairty and Gregory Parks for valuable comments on the manuscript.
LIST OF ABBREVIATIONS
- 5-HT
5-hydroxytrytamine (serotonin)
- AD
Adenosine
- ARAS
Ascending reticular activating system
- BF
Basal forebrain
- CRF
Corticotrophin releasing factor
- CSF
Cerebrospinal fluid
- DA
Dopamine
- DRN
Dorsal raphe nuclei
- EEG
Electroencephalogram
- GABA
Gamma-aminobutyric acid
- GH
Growth hormone
- GHRH
Growth hormone-releasing hormone
- HA
Histaminergic
- Hcrt
Hypocretin (orexin)
- IGF-1
Insulin-like growth factor-1
- iNOS
Inducible Nitric Oxide Synthase
- LC
Locus coeruleus
- LDT
Laterodorsal tegmental nucleus
- LH
Lateral hypothalamus
- MCH
Melanin-concentrating hormone
- MnPO
Median preoptic area
- nNOS
Neuronal Nitric Oxide Synthase
- NREM
Non-rapid eye movement sleep
- NRD
NREM delta power
- PB
Parabrachial nucleus
- PGD2
Prostaglandin D2
- PGD2-SZ
Prostaglandin D2-sensitive zone
- PGO
Ponto-geniculo-occipital
- PLH
Posterolateral hypothalamus
- PPT
Pedunculopontine nuclei
- PZ
Parafacial zone
- REM
Rapid eye movement sleep
- SCN
Suprachiasmatic nuclei
- SLD
Sublaterodorsal tegmental nucleus
- SN
Substantia nigra
- SWA
Slow wave activity
- TM
Tuberomammillary nuclei
- TRH
Thyrotropin-releasing hormone
- VLPO
Ventrolateral preoptic area
- VTA
Ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Within the last 12 months, Dr. Kilduff has received research support from F. Hoffmann La-Roche and received honoraria from Merck Pharmaceuticals and Pfizer.
References
- 1.Loomis AL, Harvey EN, Hobart G. Potential rhythms of the cerebral cortex during sleep. Science. 1935;81(2111):597–598. doi: 10.1126/science.81.2111.597. [DOI] [PubMed] [Google Scholar]
- 2.Szymusiak R. Hypothalamic versus neocortical control of sleep. Current opinion in pulmonary medicine. 2010;16(6):530–535. doi: 10.1097/MCP.0b013e32833eec92. [DOI] [PubMed] [Google Scholar]
- 3.Siegel JM. The neurobiology of sleep. Seminars in neurology. 2009;29(4):277–296. doi: 10.1055/s-0029-1237118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rolls ET, Inoue K, Browning A. Activity of primate subgenual cingulate cortex neurons is related to sleep. J Neurophysiol. 2003;90(1):134–142. doi: 10.1152/jn.00770.2002. [DOI] [PubMed] [Google Scholar]
- 5.Rudolph M, Pospischil M, Timofeev I, et al. Inhibition determines membrane potential dynamics and controls action potential generation in awake and sleeping cat cortex. J Neurosci. 2007;27(20):5280–5290. doi: 10.1523/JNEUROSCI.4652-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown RE, Basheer R, McKenna JT, et al. Control of sleep and wakefulness. Physiological reviews. 2012;92(3):1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saper CB, Fuller PM, Pedersen NP, et al. Sleep state switching. Neuron. 2010;68(6):1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 9.Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci. 2010;13(1):9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bremer F. Cerveau “isole” et physiologie du sommeil. Comptes Rendus de la Societe de Biologie (Paris) 1935;118:1235–1241. [Google Scholar]
- 11.Moruzzi G, Magoun H. Brainstem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 12.Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends in pharmacological sciences. 2005;26(11):578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 14.Steriade M, McCarley RW. Brain Control of Wakefulness and Sleep. Kluwer Academic/Plenum Publishers; New York: 2005. [Google Scholar]
- 15.Carter ME, Yizhar O, Chikahisa S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13(12):1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito H, Yanase M, Yamashita A, et al. Analysis of sleep disorders under pain using an optogenetic tool: possible involvement of the activation of dorsal raphe nucleus-serotonergic neurons. Molecular brain. 2013;6:59. doi: 10.1186/1756-6606-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGinty D, Szymusiak R. Neural control of sleep in mammals. In: Siegel J, editor. Principles and Practice of Sleep Medicine. 5th St. Louis; Elsevier: 2011. pp. 76–91. [Google Scholar]
- 18.Van Dort CJ, Zachs DP, Kenny JD, et al. Optogenetic activation of cholinergic neurons in the PPT or LDT induces REM sleep. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(2):584–589. doi: 10.1073/pnas.1423136112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanco-Centurion C, Gerashchenko D, Shiromani PJ. Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake. J Neurosci. 2007;27(51):14041–14048. doi: 10.1523/JNEUROSCI.3217-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batini C, Moruzzi G, Palestini M, et al. Presistent patterns of wakefulness in the pretrigeminal midpontine preparation. Science. 1958;128(3314):30–32. doi: 10.1126/science.128.3314.30-a. [DOI] [PubMed] [Google Scholar]
- 21.Hess WR. Über die Wechselbeziehungen zwischen psychischen und vegetativen Funktionen. Schweiz Arch Neurol Psychiat. 1925;16:36–55. [Google Scholar]
- 22.Eguchi K, Satoh T. Characterization of the neurons in the region of solitary tract nucleus during sleep. Physiol Behav. 1980;24(1):99–102. doi: 10.1016/0031-9384(80)90020-7. [DOI] [PubMed] [Google Scholar]
- 23.Anaclet C, Ferrari L, Arrigoni E, et al. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat Neurosci. 2014;17(9):1217–1224. doi: 10.1038/nn.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anaclet C, Lin JS, Vetrivelan R, et al. Identification and characterization of a sleep-active cell group in the rostral medullary brainstem. J Neurosci. 2012;32(50):17970–17976. doi: 10.1523/JNEUROSCI.0620-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuller PM, Sherman D, Pedersen NP, et al. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519(5):933–956. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Economo C. Sleep as a problem of localization. J Nerv Ment Dis. 1930;71(3):249–259. [Google Scholar]
- 27.Nauta WJH. Hypothalamic regulation of sleep in rats. An experimental study. J Neurophysiol. 1946;9:285–316. doi: 10.1152/jn.1946.9.4.285. [DOI] [PubMed] [Google Scholar]
- 28.McGinty DJ, Sterman MB. Sleep suppression after basal forebrain lesions in the cat. Science. 1968;160(833):1253–1255. doi: 10.1126/science.160.3833.1253. [DOI] [PubMed] [Google Scholar]
- 29.Sterman MB, Clemente CD. Forebrain inhibitory mechanisms: sleep patterns induced by basal forebrain stimulation in the behaving cat. Exp Neurol. 1962;6:103–117. doi: 10.1016/0014-4886(62)90081-x. [DOI] [PubMed] [Google Scholar]
- 30.Szymusiak R, McGinty D. Sleep-related neuronal discharge in the basal forebrain of cats. Brain Res. 1986;370(1):82–92. doi: 10.1016/0006-8993(86)91107-8. [DOI] [PubMed] [Google Scholar]
- 31.Sherin JE, Shiromani PJ, McCarley RW, et al. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271(5246):216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 32.Szymusiak R, Alam N, Steininger TL, et al. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803(1-2):178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- 33.Gong H, Szymusiak R, King J, et al. Sleep-related c-Fos protein expression in the preoptic hypothalamus: effects of ambient warming. Am J Physiol Regul Integr Comp Physiol. 2000;279(6):R2079–2088. doi: 10.1152/ajpregu.2000.279.6.R2079. [DOI] [PubMed] [Google Scholar]
- 34.Suntsova N, Szymusiak R, Alam MN, et al. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol. 2002;543:665–677. doi: 10.1113/jphysiol.2002.023085. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu J, Greco MA, Shiromani P, et al. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20(10):3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chou TC, Bjorkum AA, Gaus SE, et al. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 2002;22(3):977–990. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu J, Bjorkum AA, Xu M, et al. Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. J Neurosci. 2002;22(11):4568–4576. doi: 10.1523/JNEUROSCI.22-11-04568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uschakov A, Gong H, McGinty D, et al. Efferent projections from the median preoptic nucleus to sleep- and arousal-regulatory nuclei in the rat brain. Neuroscience. 2007;150(1):104–120. doi: 10.1016/j.neuroscience.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bai D, Renaud LP. Median preoptic nucleus neurons: an in vitro patch-clamp analysis of their intrinsic properties and noradrenergic receptors in the rat. Neuroscience. 1998;83(3):905–916. doi: 10.1016/s0306-4522(97)00435-1. [DOI] [PubMed] [Google Scholar]
- 40.Gallopin T, Fort P, Eggermann E, et al. Identification of sleep-promoting neurons in vitro. Nature. 2000;404(6781):992–995. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- 41.Szymusiak R. Magnocellular nuclei of the basal forebrain: substrates of sleep and arousal regulation. Sleep. 1995;18(6):478–500. doi: 10.1093/sleep/18.6.478. [DOI] [PubMed] [Google Scholar]
- 42.Rye DB, Wainer BH, Mesulam MM, et al. Cortical projections arising from the basal forebrain: a study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience. 1984;13(3):627–643. doi: 10.1016/0306-4522(84)90083-6. [DOI] [PubMed] [Google Scholar]
- 43.Ranson SW. Somnolence caused by hypothalamic lesions in the monkey. Arch Neurol Psychiat. 1939;41(1):1–23. [Google Scholar]
- 44.Swett C, Hobson J. The effects of posterior hypothalamic lesions on behavioral and electrographic manifestations of sleep and waking in cats. Arch Ital Biol. 1968;106:270–282. [PubMed] [Google Scholar]
- 45.Watanabe T, Taguchi Y, Shiosaka S, et al. Distribution of the histaminergic neuron system in the central nervous system of rats: a fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res. 1984;295(1):13–25. doi: 10.1016/0006-8993(84)90811-4. [DOI] [PubMed] [Google Scholar]
- 46.Parmentier R, Ohtsu H, Djebbara-Hannas Z, et al. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J Neurosci. 2002;22(17):7695–7711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anaclet C, Parmentier R, Ouk K, et al. Orexin/hypocretin and histamine: distinct roles in the control of wakefulness demonstrated using knock-out mouse models. J Neurosci. 2009;29(46):14423–14438. doi: 10.1523/JNEUROSCI.2604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherin JE, Elmquist JK, Torrealba F, et al. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18(12):4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz JC, Arrang JM, Garbarg M, et al. Histaminergic transmission in the mammalian brain. Physiological reviews. 1991;71(1):1–51. doi: 10.1152/physrev.1991.71.1.1. [DOI] [PubMed] [Google Scholar]
- 50.Lin JS, Sakai K, Vanni-Mercier G, et al. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479(2):225–240. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- 51.Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118(3062):273–274. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- 52.Dement W, Kleitman N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr Clin Neurophysiol. 1957;9(4):673–690. doi: 10.1016/0013-4694(57)90088-3. [DOI] [PubMed] [Google Scholar]
- 53.Aserinsky E, Kleitman N. Two types of ocular motility occurring in sleep. J Appl Physiol. 1955;8(1):1–10. doi: 10.1152/jappl.1955.8.1.1. [DOI] [PubMed] [Google Scholar]
- 54.Jouvet M, Michel F, Courjon J. [On a stage of rapid cerebral electrical activity in the course of physiological sleep. C R Seances Soc Biol Fil. 1959;153:1024–1028. [PubMed] [Google Scholar]
- 55.Evarts EV, Bental E, Bihari B, et al. Spontaneous discharge of single neurons during sleep and waking. Science. 1962;135:726–728. doi: 10.1126/science.135.3505.726. [DOI] [PubMed] [Google Scholar]
- 56.McCarley RW, Hobson JA. Cortical unit activity in desynchronized sleep. Science. 1970;167(919):901–903. doi: 10.1126/science.167.3919.901. [DOI] [PubMed] [Google Scholar]
- 57.Siegel JM, McGinty DJ. Brainstem neurons without spontaneous unit discharge. Science. 1976;193(4249):240–242. doi: 10.1126/science.180599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189(4196):55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- 59.McCarley RW, Hobson JA. Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science. 1975;189(4196):58–60. doi: 10.1126/science.1135627. [DOI] [PubMed] [Google Scholar]
- 60.McCarley RW. Mechanisms and models of REM sleep control. Arch Ital Biol. 2004;142(4):429–467. [PubMed] [Google Scholar]
- 61.McCarley RW, Massaquoi SG. Neurobiological structure of the revised limit cycle reciprocal interaction model of REM cycle control. J Sleep Res. 1992;1(2):132–137. doi: 10.1111/j.1365-2869.1992.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 62.McCarley RW. Neurobiology of REM and NREM sleep. Sleep medicine. 2007;8(4):302–330. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 63.Lu J, Sherman D, Devor M, et al. A putative flip-flop switch for control of REM sleep. Nature. 2006;441(7093):589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 64.Luppi PH, Clement O, Fort P. Paradoxical (REM) sleep genesis by the brainstem is under hypothalamic control. Current opinion in neurobiology. 2013;23(5):786–792. doi: 10.1016/j.conb.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 65.Siegel JM, Nienhuis R, Tomaszewski KS. Rostral brainstem contributes to medullary inhibition of muscle tone. Brain Res. 1983;268(2):344–348. doi: 10.1016/0006-8993(83)90501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siegel JM, Nienhuis R, Tomaszewski KS. REM sleep signs rostral to chronic transections at the pontomedullary junction. Neurosci Lett. 1984;45(3):241–246. doi: 10.1016/0304-3940(84)90233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sastre JP, Sakai K, Jouvet M. Are the gigantocellular tegmental field neurons responsible for paradoxical sleep? Brain Res. 1981;229(1):147–161. doi: 10.1016/0006-8993(81)90752-6. [DOI] [PubMed] [Google Scholar]
- 68.Katayama Y, DeWitt DS, Becker DP, et al. Behavioral evidence for a cholinoceptive pontine inhibitory area: descending control of spinal motor output and sensory input. Brain Res. 1984;296(2):241–262. doi: 10.1016/0006-8993(84)90062-3. [DOI] [PubMed] [Google Scholar]
- 69.Steriade M, Datta S, Pare D, et al. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10(8):2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.el Mansari M, Sakai K, Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp Brain Res. 1989;76(3):519–529. doi: 10.1007/BF00248908. [DOI] [PubMed] [Google Scholar]
- 71.Kodama T, Takahashi Y, Honda Y. Enhancement of acetylcholine release during paradoxical sleep in the dorsal tegmental field of the cat brain stem. Neurosci Lett. 1990;114(3):277–282. doi: 10.1016/0304-3940(90)90576-u. [DOI] [PubMed] [Google Scholar]
- 72.Greene RW, Gerber U, McCarley RW. Cholinergic activation of medial pontine reticular formation neurons in vitro. Brain Res. 1989;476(1):154–159. doi: 10.1016/0006-8993(89)91549-7. [DOI] [PubMed] [Google Scholar]
- 73.Brown RE, Winston S, Basheer R, et al. Electrophysiological characterization of neurons in the dorsolateral pontine rapid-eye-movement sleep induction zone of the rat: Intrinsic membrane properties and responses to carbachol and orexins. Neuroscience. 2006;143(3):739–755. doi: 10.1016/j.neuroscience.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clement O, Sapin E, Libourel PA, et al. The lateral hypothalamic area controls paradoxical (REM) sleep by means of descending projections to brainstem GABAergic neurons. J Neurosci. 2012;32(47):16763–16774. doi: 10.1523/JNEUROSCI.1885-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jouvet M, Delorme F. Locus coeruleus et sommeil paradoxal. C R Seances Soc Biol Fil. 1965;159:895–899. [PubMed] [Google Scholar]
- 76.Henley K, Morrison AR. A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat. Acta Neurobiol Exp (Wars) 1974;34(2):215–232. [PubMed] [Google Scholar]
- 77.Chase MH, Soja PJ, Morales FR. Evidence that glycine mediates the postsynaptic potentials that inhibit lumbar motoneurons during the atonia of active sleep. J Neurosci. 1989;9(3):743–751. doi: 10.1523/JNEUROSCI.09-03-00743.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brooks PL, Peever JH. Glycinergic and GABA(A)-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia. J Neurosci. 2008;28(14):3535–3545. doi: 10.1523/JNEUROSCI.5023-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brooks PL, Peever JH. Identification of the transmitter and receptor mechanisms responsible for REM sleep paralysis. J Neurosci. 2012;32(29):9785–9795. doi: 10.1523/JNEUROSCI.0482-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krenzer M, Anaclet C, Vetrivelan R, et al. Brainstem and spinal cord circuitry regulating REM sleep and muscle atonia. PloS one. 2011;6(10):e24998. doi: 10.1371/journal.pone.0024998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schenck CH, Bundlie SR, Patterson AL, et al. Rapid eye movement sleep behavior disorder. A treatable parasomnia affecting older adults. JAMA. 1987;257(13):1786–1789. [PubMed] [Google Scholar]
- 82.Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–2788. doi: 10.1093/brain/awm056. Pt 11. [DOI] [PubMed] [Google Scholar]
- 83.McCarter SJ, St Louis EK, Boeve BF. REM sleep behavior disorder and REM sleep without atonia as an early manifestation of degenerative neurological disease. Current neurology and neuroscience reports. 2012;12(2):182–192. doi: 10.1007/s11910-012-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burgess CR, Oishi Y, Mochizuki T, et al. Amygdala lesions reduce cataplexy in orexin knock-out mice. J Neurosci. 2013;33(23):9734–9742. doi: 10.1523/JNEUROSCI.5632-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oishi Y, Williams RH, Agostinelli L, et al. Role of the medial prefrontal cortex in cataplexy. J Neurosci. 2013;33(23):9743–9751. doi: 10.1523/JNEUROSCI.0499-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boehme RE, Baker TL, Mefford IN, et al. Narcolepsy: cholinergic receptor changes in an animal model. Life Sci. 1984;34(19):1825–1828. doi: 10.1016/0024-3205(84)90675-1. [DOI] [PubMed] [Google Scholar]
- 87.Kilduff TS, Bowersox SS, Kaitin KI, et al. Muscarinic cholinergic receptors and the canine model of narcolepsy. Sleep. 1986;9:102–106. doi: 10.1093/sleep/9.1.102. 1 Pt 2. [DOI] [PubMed] [Google Scholar]
- 88.Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98(3):365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 89.de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 91.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(5) doi: 10.1016/s0092-8674(02)09256-5. 1 page following 696. [DOI] [PubMed] [Google Scholar]
- 92.Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Harrison TA, Chen CT, Dun NJ, et al. Hypothalamic orexin A-immunoreactive neurons project to the rat dorsal medulla. Neurosci Lett. 1999;273(1):17–20. doi: 10.1016/s0304-3940(99)00611-4. [DOI] [PubMed] [Google Scholar]
- 94.Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: Implications for sleep and sleep disorders. Trends Neurosci. 2000;23(8):359–365. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- 95.Thannickal T, Moore R,Y, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Date Y, Ueta Y, Yamashita H, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(2):748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Broberger C, De Lecea L, Sutcliffe JG, et al. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol. 1998;402(4):460–474. [PubMed] [Google Scholar]
- 98.Elias CF, Saper CB, Maratos-Flier E, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402(4):442–459. [PubMed] [Google Scholar]
- 99.Nambu T, Sakurai T, Mizukami K, et al. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827(1-2):243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- 100.van den Pol AN. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci. 1999;19(8):3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 102.Horvath TL, Peyron C, Diano S, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415(2):145–159. [PubMed] [Google Scholar]
- 103.Espana RA, Reis KM, Valentino RJ, et al. Organization of hypocretin/orexin efferents to locus coeruleus and basal forebrain arousal-related structures. J Comp Neurol. 2005;481(2):160–178. doi: 10.1002/cne.20369. [DOI] [PubMed] [Google Scholar]
- 104.Yoshida K, McCormack S, Espana RA, et al. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494(5):845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sakurai T, Nagata R, Yamanaka A, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46(2):297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 106.Hagan JJ, Leslie RA, Patel S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(19):10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Soffin EM, Evans ML, Gill CH, et al. SB-334867-A antagonises orexin mediated excitation in the locus coeruleus. Neuropharmacology. 2002;42(1):127–133. doi: 10.1016/s0028-3908(01)00156-3. [DOI] [PubMed] [Google Scholar]
- 108.Brown RE, Sergeeva O, Eriksson KS, et al. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology. 2001;40(3):457–459. doi: 10.1016/s0028-3908(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 109.Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002;22(21):9453–9464. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eriksson KS, Sergeeva O, Brown RS, et al. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21(23):9273–9279. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bayer L, Eggermann E, Serafin M, et al. Orexins (hypocretins) directly excite tuberomammillary neurons. Eur J Neurosci. 2001;14(9):1571–1575. doi: 10.1046/j.0953-816x.2001.01777.x. [DOI] [PubMed] [Google Scholar]
- 112.Yamanaka A, Tsujino N, Funahashi H, et al. Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem Biophys Res Commun. 2002;290(4):1237–1245. doi: 10.1006/bbrc.2001.6318. [DOI] [PubMed] [Google Scholar]
- 113.Burlet S, Tyler CJ, Leonard CS. Direct and indirect excitation of laterodorsal tegmental neurons by Hypocretin/Orexin peptides: implications for wakefulness and narcolepsy. J Neurosci. 2002;22(7):2862–2872. doi: 10.1523/JNEUROSCI.22-07-02862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Takahashi K, Koyama Y, Kayama Y, et al. Effects of orexin on the laterodorsal tegmental neurones. Psychiatry Clin Neurosci. 2002;56(3):335–336. doi: 10.1046/j.1440-1819.2002.00967.x. [DOI] [PubMed] [Google Scholar]
- 115.Eggermann E, Serafin M, Bayer L, et al. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108(2):177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- 116.Borgland SL, Taha SA, Sarti F, et al. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49(4):589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 117.Korotkova TM, Sergeeva OA, Eriksson KS, et al. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23(1):7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van den Pol AN, Gao XB, Obrietan K, et al. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18(19):7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ivanov A, Aston-Jones G. Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. Neuroreport. 2000;11(8):1755–1758. doi: 10.1097/00001756-200006050-00031. [DOI] [PubMed] [Google Scholar]
- 120.Hoang QV, Bajic D, Yanagisawa M, et al. Effects of orexin (hypocretin) on GIRK channels. J Neurophysiol. 2003;90(2):693–702. doi: 10.1152/jn.00001.2003. [DOI] [PubMed] [Google Scholar]
- 121.Hoang QV, Zhao P, Nakajima S, et al. Orexin (hypocretin) effects on constitutively active inward rectifier K+ channels in cultured nucleus basalis neurons. J Neurophysiol. 2004;92(6):3183–3191. doi: 10.1152/jn.01222.2003. [DOI] [PubMed] [Google Scholar]
- 122.Hwang LL, Chen CT, Dun NJ. Mechanisms of orexin-induced depolarizations in rat dorsal motor nucleus of vagus neurones in vitro. J Physiol. 2001;537:511–520. doi: 10.1111/j.1469-7793.2001.00511.x. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang B, Ferguson AV. Orexin-A depolarizes dissociated rat area postrema neurons through activation of a nonselective cationic conductance. J Neurosci. 2002;22(15):6303–6308. doi: 10.1523/JNEUROSCI.22-15-06303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang B, Ferguson AV. Orexin-A depolarizes nucleus tractus solitarius neurons through effects on nonselective cationic and K+ conductances. J Neurophysiol. 2003;89(4):2167–2175. doi: 10.1152/jn.01088.2002. [DOI] [PubMed] [Google Scholar]
- 125.Yang B, Samson WK, Ferguson AV. Excitatory effects of orexin-A on nucleus tractus solitarius neurons are mediated by phospholipase C and protein kinase C. J Neurosci. 2003;23(15):6215–6222. doi: 10.1523/JNEUROSCI.23-15-06215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Follwell MJ, Ferguson AV. Cellular mechanisms of orexin actions on paraventricular nucleus neurones in rat hypothalamus. J Physiol. 2002;545:855–867. doi: 10.1113/jphysiol.2002.030049. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Smith BN, Davis SF, Van Den Pol AN, et al. Selective enhancement of excitatory synaptic activity in the rat nucleus tractus solitarius by hypocretin 2. Neuroscience. 2002;115(3):707–714. doi: 10.1016/s0306-4522(02)00488-8. [DOI] [PubMed] [Google Scholar]
- 128.Xi MC, Fung SJ, Yamuy J, et al. Hypocretinergic facilitation of synaptic activity of neurons in the nucleus pontis oralis of the cat. Brain Res. 2003;976(2):253–258. doi: 10.1016/s0006-8993(03)02566-6. [DOI] [PubMed] [Google Scholar]
- 129.Davis SF, Williams KW, Xu W, et al. Selective enhancement of synaptic inhibition by hypocretin (orexin) in rat vagal motor neurons: implications for autonomic regulation. J Neurosci. 2003;23(9):3844–3854. doi: 10.1523/JNEUROSCI.23-09-03844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chou TC, Lee CE, Lu J, et al. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21(19):RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hakansson M, de Lecea L, Sutcliffe JG, et al. Leptin receptor- and STAT3-immunoreactivities in hypocretin/orexin neurones of the lateral hypothalamus. J Neuroendocrinol. 1999;11(8):653–663. doi: 10.1046/j.1365-2826.1999.00378.x. [DOI] [PubMed] [Google Scholar]
- 132.Risold PY, Griffond B, Kilduff TS, et al. Preprohypocretin (orexin) and prolactin-like immunoreactivity are coexpressed by neurons of the rat lateral hypothalamic area. Neurosci Lett. 1999;259(3):153–156. doi: 10.1016/s0304-3940(98)00906-9. [DOI] [PubMed] [Google Scholar]
- 133.Torrealba F, Yanagisawa M, Saper CB. Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience. 2003;119(4):1033–1044. doi: 10.1016/s0306-4522(03)00238-0. [DOI] [PubMed] [Google Scholar]
- 134.Henny P, Brischoux F, Mainville L, et al. Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience. 2010;169(3):1150–1157. doi: 10.1016/j.neuroscience.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schone C, Apergis-Schoute J, Sakurai T, et al. Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell reports. 2014;7(3):697–704. doi: 10.1016/j.celrep.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li Y, Gao XB, Sakurai T, et al. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36(6):1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 137.Yamanaka A, Beuckmann CT, Willie JT, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38(5):701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 138.Hara J, Gerashchenko D, Wisor JP, et al. Thyrotropin-releasing hormone increases behavioral arousal through modulation of hypocretin/orexin neurons. J Neurosci. 2009;29(12):3705–3714. doi: 10.1523/JNEUROSCI.0431-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yamanaka A, Muraki Y, Tsujino N, et al. Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem Biophys Res Commun. 2003;303(1):120–129. doi: 10.1016/s0006-291x(03)00299-7. [DOI] [PubMed] [Google Scholar]
- 140.Wollmann G, Acuna-Goycolea C, van den Pol AN. Direct excitation of hypocretin/orexin cells by extracellular ATP at P2X receptors. J Neurophysiol. 2005;94(3):2195–2206. doi: 10.1152/jn.00035.2005. [DOI] [PubMed] [Google Scholar]
- 141.Winsky-Sommerer R, Yamanaka A, Diano S, et al. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating the stress response. J Neurosci. 2004;24(50):11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Acuna-Goycolea C, van den Pol A. Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal. J Neurosci. 2004;24(37):8141–8152. doi: 10.1523/JNEUROSCI.1607-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tsujino N, Yamanaka A, Ichiki K, et al. Cholecystokinin activates orexin/hypocretin neurons through the cholecystokinin A receptor. J Neurosci. 2005;25(32):7459–7469. doi: 10.1523/JNEUROSCI.1193-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Burdakov D, Alexopoulos H. Metabolic state signalling through central hypocretin/orexin neurons. J Cell Mol Med. 2005;9(4):795–803. doi: 10.1111/j.1582-4934.2005.tb00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Burdakov D, Jensen LT, Alexopoulos H, et al. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50(5):711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 146.Li Y, van den Pol AN. Direct and indirect inhibition by catecholamines of hypocretin/orexin neurons. J Neurosci. 2005;25(1):173–183. doi: 10.1523/JNEUROSCI.4015-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Muraki Y, Yamanaka A, Tsujino N, et al. Serotonergic regulation of the orexin/hypocretin neurons through the 5-HT1A receptor. J Neurosci. 2004;24(32):7159–7166. doi: 10.1523/JNEUROSCI.1027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yamanaka A, Muraki Y, Ichiki K, et al. Orexin neurons are directly and indirectly regulated by catecholamines in a complex manner. J Neurophysiol. 2006;96(1):284–298. doi: 10.1152/jn.01361.2005. [DOI] [PubMed] [Google Scholar]
- 149.Xie X, Crowder TL, Yamanaka A, et al. GABA(B) receptor-mediated modulation of hypocretin/orexin neurones in mouse hypothalamus. J Physiol. 2006;574:399–414. doi: 10.1113/jphysiol.2006.108266. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Estabrooke IV, McCarthy MT, Ko E, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21(5):1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25(28):6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46(5):787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153(3):860–870. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 154.Kiyashchenko LI, Mileykovskiy BY, Maidment N, et al. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22(13):5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Salomon RM, Ripley B, Kennedy JS, et al. Diurnal variation of cerebrospinal fluid hypocretin-1 (Orexin-A) levels in control and depressed subjects. Biological psychiatry. 2003;54(2):96–104. doi: 10.1016/s0006-3223(02)01740-7. [DOI] [PubMed] [Google Scholar]
- 156.Zeitzer JM, Buckmaster CL, Parker KJ, et al. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23(8):3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bourgin P, Huitron-Resendiz S, Spier AD, et al. Hypocretin-1 modulates REM sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20(20):7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Espana RA, Baldo BA, Kelley AE, et al. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106(4):699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 159.Methippara MM, Alam MN, Szymusiak R, et al. Effects of lateral preoptic area application of orexin-A on sleep-wakefulness. Neuroreport. 2000;11(16):3423–3426. doi: 10.1097/00001756-200011090-00004. [DOI] [PubMed] [Google Scholar]
- 160.Piper DC, Upton N, Smith MI, et al. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur J Neurosci. 2000;12(2):726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 161.Xi M, Morales FR, Chase MH. Effects on sleep and wakefulness of the injection of hypocretin-1 (orexin-A) into the laterodorsal tegmental nucleus of the cat. Brain Res. 2001;901(1-2):259–264. doi: 10.1016/s0006-8993(01)02317-4. [DOI] [PubMed] [Google Scholar]
- 162.Morairty SR, Wisor J, Silveira K, et al. The wake-promoting effects of hypocretin-1 are attenuated in old rats. Neurobiology of aging. 2011;32(8):1514–1527. doi: 10.1016/j.neurobiolaging.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Adamantidis AR, Zhang F, Aravanis AM, et al. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450(7168):420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Carter ME, Adamantidis A, Ohtsu H, et al. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J Neurosci. 2009;29(35):10939–10949. doi: 10.1523/JNEUROSCI.1205-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Carter ME, Brill J, Bonnavion P, et al. Mechanism for Hypocretin-mediated sleep-to-wake transitions. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(39):E2635–2644. doi: 10.1073/pnas.1202526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tsunematsu T, Kilduff TS, Boyden ES, et al. Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice. J Neurosci. 2011;31(29):10529–10539. doi: 10.1523/JNEUROSCI.0784-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tsunematsu T, Tabuchi S, Tanaka KF, et al. Long-lasting silencing of orexin/hypocretin neurons using archaerhodopsin induces slow-wave sleep in mice. Behavioural brain research. 2013 doi: 10.1016/j.bbr.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 168.Vazquez-DeRose J, Schwartz MD, Nguyen AT, et al. Hypocretin/orexin antagonism enhances sleep-related adenosine and GABA neurotransmission in rat basal forebrain. Brain structure & function. 2014 doi: 10.1007/s00429-014-0946-y. [DOI] [PubMed] [Google Scholar]
- 169.Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. Journal of neurogenetics. 2011;25(1-2):52–61. doi: 10.3109/01677063.2011.566953. [DOI] [PubMed] [Google Scholar]
- 170.Brisbare-Roch C, Dingemanse J, Koberstein R, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13(2):150–155. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- 171.Morairty SR, Revel FG, Malherbe P, et al. Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PloS one. 2012;7(7):e39131. doi: 10.1371/journal.pone.0039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Morairty S, Wilk AJ, Lincoln WU, et al. The hypocretin/orexin antagonist almorexant promotes sleep without impairment of performance in rats. Front Neurosci. 2014;8:3. doi: 10.3389/fnins.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Uslaner JM, Tye SJ, Eddins DM, et al. Orexin receptor antagonists differ from standard sleep drugs by promoting sleep at doses that do not disrupt cognition. Science translational medicine. 2013;5(179):179ra144. doi: 10.1126/scitranslmed.3005213. [DOI] [PubMed] [Google Scholar]
- 174.Mignot E. Genetic and familial aspects of narcolepsy. Neurology. 1998;50(2 Suppl 1):S16–22. doi: 10.1212/wnl.50.2_suppl_1.s16. [DOI] [PubMed] [Google Scholar]
- 175.Mignot E, Tafti M, Dement WC, et al. Narcolepsy and immunity. Adv Neuroimmunol. 1995;5(1):23–37. doi: 10.1016/0960-5428(94)00043-n. [DOI] [PubMed] [Google Scholar]
- 176.Partinen M, Kornum BR, Plazzi G, et al. Narcolepsy as an autoimmune disease: the role of H1N1 infection and vaccination. The Lancet Neurology. 2014;13(6):600–613. doi: 10.1016/S1474-4422(14)70075-4. [DOI] [PubMed] [Google Scholar]
- 177.Nishino S, Ripley B, Overeem S, et al. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355(9197):39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 178.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6(9):991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 179.Gerashchenko D, Kohls MD, Greco MA, et al. Hypocretin-saporin induced lesion of the lateral hypothalamus produces narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21(18):7273–7283. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gerashchenko D, Blanco-Centurion C, Greco MA, et al. Effects of lateral hypothalamic lesion with the neurotoxin hypocretin-2-saporin on sleep in Long-Evans rats. Neuroscience. 2003;116(1):223–235. doi: 10.1016/s0306-4522(02)00575-4. [DOI] [PubMed] [Google Scholar]
- 181.Hara J, Beuckmann CT, Nambu T, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30(2):345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 182.Tabuchi S, Tsunematsu T, Black SW, et al. Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci. 2014;34(19):6495–6509. doi: 10.1523/JNEUROSCI.0073-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mochizuki T, Crocker A, McCormack S, et al. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24(28):6291–6300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rechtschaffen A, Gilliland MA, Bergmann BM, et al. Physiological correlates of prolonged sleep deprivation in rats. Science. 1983;221(4606):182–184. doi: 10.1126/science.6857280. [DOI] [PubMed] [Google Scholar]
- 185.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- 186.Daan S, Beersma DG, Borbely AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–183. doi: 10.1152/ajpregu.1984.246.2.R161. 2 Pt 2. [DOI] [PubMed] [Google Scholar]
- 187.Yasenkov R, Deboer T. Circadian regulation of sleep and the sleep EEG under constant sleep pressure in the rat. Sleep. 2010;33(5):631–641. doi: 10.1093/sleep/33.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yasenkov R, Deboer T. Interrelations and circadian changes of electroencephalogram frequencies under baseline conditions and constant sleep pressure in the rat. Neuroscience. 2011;180:212–221. doi: 10.1016/j.neuroscience.2011.01.063. [DOI] [PubMed] [Google Scholar]
- 189.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity are eliminated by suprachiasmatic lesions. Proc Natl Acad Sci USA. 1972;69(6):1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Moore RY, Eichler VB. Loss of circadian adrenal corticosterone rhythm following suprachiasmatic nucleus lesion in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 191.Ralph MR, Foster RG, Davis FC, et al. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 192.Inouye S-IT, Kawamura H. Persistence of circadian rhythmicity in a hypothalamic 'island' containing the suprachiasmatic nucleus. Proc Natl Acad Sci USA. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ibuka N, Inouye SI, Kawamura H. Analysis of sleep-wakefulness rhythms in male rats after suprachiasmatic nucleus lesions and ocular enucleation. Brain Res. 1977;122(1):33–47. doi: 10.1016/0006-8993(77)90660-6. [DOI] [PubMed] [Google Scholar]
- 194.Ibuka N, Kawamura H. Loss of circadian rhythm in sleep-wakefulness cycle in the rat by suprachiasmatic nucleus lesions. Brain Res. 1975;96(1):76–81. doi: 10.1016/0006-8993(75)90574-0. [DOI] [PubMed] [Google Scholar]
- 195.Mouret J, Coindet J, Debilly G, et al. Suprachiasmatic nuclei lesions in the rat: alterations in sleep circadian rhythms. Electroencephalogr Clin Neurophysiol. 1978;45(3):402–408. doi: 10.1016/0013-4694(78)90191-8. [DOI] [PubMed] [Google Scholar]
- 196.Tobler I, Borbely AA, Groos G. The effect of sleep deprivation on sleep in rats with suprachiasmatic lesions. Neurosci Lett. 1983;42(1):49–54. doi: 10.1016/0304-3940(83)90420-2. [DOI] [PubMed] [Google Scholar]
- 197.Mistlberger RE, Bergmann BM, Waldenar W, et al. Recovery sleep following sleep deprivation in intact and suprachiasmatic nuclei-lesioned rats. Sleep. 1983;6(3):217–233. doi: 10.1093/sleep/6.3.217. [DOI] [PubMed] [Google Scholar]
- 198.Easton A, Meerlo P, Bergmann B, et al. The suprachiasmatic nucleus regulates sleep timing and amount in mice. Sleep. 2004;27(7):1307–1318. doi: 10.1093/sleep/27.7.1307. [DOI] [PubMed] [Google Scholar]
- 199.Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13(3):1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Larkin JE, Yokogawa T, Heller HC, et al. Homeostatic regulation of sleep in arrhythmic Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2004;287(1):R104–111. doi: 10.1152/ajpregu.00676.2003. [DOI] [PubMed] [Google Scholar]
- 201.Mistlberger RE. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain research Brain research reviews. 2005;49(3):429–454. doi: 10.1016/j.brainresrev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 202.Deboer T, Overeem S, Visser NA, et al. Convergence of circadian and sleep regulatory mechanisms on hypocretin-1. Neuroscience. 2004;129(3):727–732. doi: 10.1016/j.neuroscience.2004.07.049. [DOI] [PubMed] [Google Scholar]
- 203.Fujiki N, Yoshida Y, Ripley B, et al. Changes in CSF hypocretin-1 (orexin A) levels in rats across 24 hours and in response to food deprivation. Neuroreport. 2001;12(5):993–997. doi: 10.1097/00001756-200104170-00026. [DOI] [PubMed] [Google Scholar]
- 204.Wu MF, John J, Maidment N, et al. Hypocretin release in normal and narcoleptic dogs after food and sleep deprivation, eating, and movement. Am J Physiol Regul Integr Comp Physiol. 2002;283(5):R1079–1086. doi: 10.1152/ajpregu.00207.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Deboer T, Detari L, Meijer JH. Long term effects of sleep deprivation on the mammalian circadian pacemaker. Sleep. 2007;30(3):257–262. doi: 10.1093/sleep/30.3.257. [DOI] [PubMed] [Google Scholar]
- 206.Deboer T, Vansteensel MJ, Detari L, et al. Sleep states alter activity of suprachiasmatic nucleus neurons. Nat Neurosci. 2003;6(10):1086–1090. doi: 10.1038/nn1122. [DOI] [PubMed] [Google Scholar]
- 207.Cambras T, Weller JR, Angles-Pujoras M, et al. Circadian desynchronization of core body temperature and sleep stages in the rat. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(18):7634–7639. doi: 10.1073/pnas.0702424104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lee ML, Swanson BE, de la Iglesia HO. Circadian timing of REM sleep is coupled to an oscillator within the dorsomedial suprachiasmatic nucleus. Current biology : CB. 2009;19(10):848–852. doi: 10.1016/j.cub.2009.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wurts SW, Edgar DM. Circadian and homeostatic control of rapid eye movement (REM) sleep: promotion of REM tendency by the suprachiasmatic nucleus. J Neurosci. 2000;20(11):4300–4310. doi: 10.1523/JNEUROSCI.20-11-04300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 211.Takahashi JS, Hong HK, Ko CH, et al. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nature reviews Genetics. 2008;9(10):764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dudley CA, Erbel-Sieler C, Estill SJ, et al. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science. 2003;301(5631):379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- 213.Laposky A, Easton A, Dugovic C, et al. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep. 2005;28(4):395–409. doi: 10.1093/sleep/28.4.395. [DOI] [PubMed] [Google Scholar]
- 214.Naylor E, Bergmann BM, Krauski K, et al. The circadian clock mutation alters sleep homeostasis in the mouse. J Neurosci. 2000;20(21):8138–8143. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Shiromani PJ, Xu M, Winston EM, et al. Sleep rhythmicity and homeostasis in mice with targeted disruption of mPeriod genes. Am J Physiol Regul Integr Comp Physiol. 2004;287(1):R47–57. doi: 10.1152/ajpregu.00138.2004. [DOI] [PubMed] [Google Scholar]
- 216.Wisor JP, O'Hara BF, Terao A, et al. A role for cryptochromes in sleep regulation. BMC Neurosci. 2002;3:20. doi: 10.1186/1471-2202-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Curie T, Maret S, Emmenegger Y, et al. In Vivo Imaging of the Central and Peripheral Effects of Sleep Deprivation and Suprachiasmatic Nuclei Lesion on PERIOD-2 Protein in Mice. Sleep. 2015 doi: 10.5665/sleep.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Franken P, Thomason R, Heller HC, et al. A non-circadian role for clock-genes in sleep homeostasis: a strain comparison. BMC Neurosci. 2007;8:87. doi: 10.1186/1471-2202-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mongrain V, La Spada F, Curie T, et al. Sleep loss reduces the DNA-binding of BMAL1, CLOCK, and NPAS2 to specific clock genes in the mouse cerebral cortex. PloS one. 2011;6(10):e26622. doi: 10.1371/journal.pone.0026622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Thompson CL, Wisor JP, Lee CK, et al. Molecular and anatomical signatures of sleep deprivation in the mouse brain. Front Neurosci. 2010;4:165. doi: 10.3389/fnins.2010.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wisor JP, Pasumarthi RK, Gerashchenko D, et al. Sleep deprivation effects on circadian clock gene expression in the cerebral cortex parallel electroencephalographic differences among mouse strains. J Neurosci. 2008;28(28):7193–7201. doi: 10.1523/JNEUROSCI.1150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Suntsova N, Guzman-Marin R, Kumar S, et al. The median preoptic nucleus reciprocally modulates activity of arousal-related and sleep-related neurons in the perifornical lateral hypothalamus. J Neurosci. 2007;27(7):1616–1630. doi: 10.1523/JNEUROSCI.3498-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gvilia I, Xu F, McGinty D, et al. Homeostatic regulation of sleep: a role for preoptic area neurons. J Neurosci. 2006;26(37):9426–9433. doi: 10.1523/JNEUROSCI.2012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gvilia I, Turner A, McGinty D, et al. Preoptic area neurons and the homeostatic regulation of rapid eye movement sleep. J Neurosci. 2006;26(11):3037–3044. doi: 10.1523/JNEUROSCI.4827-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gerashchenko D, Wisor JP, Burns D, et al. Identification of a population of sleep-active cerebral cortex neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(29):10227–10232. doi: 10.1073/pnas.0803125105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pasumarthi RK, Gerashchenko D, Kilduff TS. Further characterization of sleep-active neuronal nitric oxide synthase neurons in the mouse brain. Neuroscience. 2010;169(1):149–157. doi: 10.1016/j.neuroscience.2010.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Vaucher E, Linville D, Hamel E. Cholinergic basal forebrain projections to nitric oxide synthase-containing neurons in the rat cerebral cortex. Neuroscience. 1997;79(3):827–836. doi: 10.1016/s0306-4522(97)00033-x. [DOI] [PubMed] [Google Scholar]
- 228.Cauli B, Tong XK, Rancillac A, et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24(41):8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tomioka R, Okamoto K, Furuta T, et al. Demonstration of long-range GABAergic connections distributed throughout the mouse neocortex. Eur J Neurosci. 2005;21(6):1587–1600. doi: 10.1111/j.1460-9568.2005.03989.x. [DOI] [PubMed] [Google Scholar]
- 230.Tomioka R, Rockland KS. Long-distance corticocortical GABAergic neurons in the adult monkey white and gray matter. J Comp Neurol. 2007;505(5):526–538. doi: 10.1002/cne.21504. [DOI] [PubMed] [Google Scholar]
- 231.Higo S, Akashi K, Sakimura K, et al. Subtypes of GABAergic neurons project axons in the neocortex. Front Neuroanat. 2009;3:25. doi: 10.3389/neuro.05.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Higo S, Udaka N, Tamamaki N. Long-range GABAergic projection neurons in the cat neocortex. J Comp Neurol. 2007;503(3):421–431. doi: 10.1002/cne.21395. [DOI] [PubMed] [Google Scholar]
- 233.Morairty SR, Dittrich L, Pasumarthi RK, et al. A role for cortical nNOS/NK1 neurons in coupling homeostatic sleep drive to EEG slow wave activity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(50):20272–20277. doi: 10.1073/pnas.1314762110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dittrich L, Morairty SR, Warrier DR, et al. Homeostatic sleep pressure is the primary factor for activation of cortical nNOS/NK1 neurons. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40(3):632–639. doi: 10.1038/npp.2014.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kilduff TS, Cauli B, Gerashchenko D. Activation of cortical interneurons during sleep: an anatomical link to homeostatic sleep regulation? Trends Neurosci. 2011;34(1):10–19. doi: 10.1016/j.tins.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Krueger JM, Obal FJ, Fang J, et al. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci. 2001;933:211–221. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- 237.Majde JA, Krueger JM. Links between the innate immune system and sleep. J Allergy Clin Immunol. 2005;116(6):1188–1198. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 238.Ehlers CL, Reed TK, Henriksen SJ. Effects of corticotropin-releasing factor and growth hormone-releasing factor on sleep and activity in rats. Neuroendocrinology. 1986;42(6):467–474. doi: 10.1159/000124489. [DOI] [PubMed] [Google Scholar]
- 239.Opp M, Obal F, Jr., Krueger JM. Corticotropin-releasing factor attenuates interleukin 1-induced sleep and fever in rabbits. Am J Physiol. 1989;257:R528–535. doi: 10.1152/ajpregu.1989.257.3.R528. 3 Pt 2. [DOI] [PubMed] [Google Scholar]
- 240.Chastrette N, Cespuglio R, Jouvet M. Proopiomelanocortin (POMC)-derived peptides and sleep in the rat. Part 1--Hypnogenic properties of ACTH derivatives. Neuropeptides. 1990;15(2):61–74. doi: 10.1016/0143-4179(90)90042-w. [DOI] [PubMed] [Google Scholar]
- 241.Nishino S, Arrigoni J, Shelton J, et al. Effects of thyrotropin-releasing hormone and its analogs on daytime sleepiness and cataplexy in canine narcolepsy. J Neurosci. 1997;17(16):6401–6408. doi: 10.1523/JNEUROSCI.17-16-06401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Riehl J, Honda K, Kwan M, et al. Chronic oral administration of CG-3703, a thyrotropin releasing hormone analog, increases wake and decreases cataplexy in canine narcolepsy. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2000;23(1):34–45. doi: 10.1016/S0893-133X(99)00159-1. [DOI] [PubMed] [Google Scholar]
- 243.Hemmeter U, Rothe B, Guldner J, et al. Effects of thyrotropin-releasing hormone on the sleep EEG and nocturnal hormone secretion in male volunteers. Neuropsychobiology. 1998;38(1):25–31. doi: 10.1159/000026513. [DOI] [PubMed] [Google Scholar]
- 244.Zini I, Merlo Pich E, Fuxe K, et al. Actions of centrally administered neuropeptide Y on EEG activity in different rat strains and in different phases of their circadian cycle. Acta Physiol Scand. 1984;122(1):71–77. doi: 10.1111/j.1748-1716.1984.tb07483.x. [DOI] [PubMed] [Google Scholar]
- 245.Ehlers CL, Somes C, Lopez A, et al. Electrophysiological actions of neuropeptide Y and its analogs: new measures for anxiolytic therapy? Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1997;17(1):34–43. doi: 10.1016/S0893-133X(97)00001-8. [DOI] [PubMed] [Google Scholar]
- 246.Szentirmai E, Krueger JM. Central administration of neuropeptide Y induces wakefulness in rats. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R473–480. doi: 10.1152/ajpregu.00919.2005. [DOI] [PubMed] [Google Scholar]
- 247.Antonijevic IA, Murck H, Bohlhalter S, et al. Neuropeptide Y promotes sleep and inhibits ACTH and cortisol release in young men. Neuropharmacology. 2000;39(8):1474–1481. doi: 10.1016/s0028-3908(00)00057-5. [DOI] [PubMed] [Google Scholar]
- 248.Held K, Antonijevic I, Murck H, et al. Neuropeptide Y (NPY) shortens sleep latency but does not suppress ACTH and cortisol in depressed patients and normal controls. Psychoneuroendocrinology. 2006;31(1):100–107. doi: 10.1016/j.psyneuen.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 249.Xu YL, Reinscheid RK, Huitron-Resendiz S, et al. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43(4):487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 250.Huitron-Resendiz S, Kristensen MP, Sanchez-Alavez M, et al. Urotensin II modulates rapid eye movement sleep through activation of brainstem cholinergic neurons. J Neurosci. 2005;25(23):5465–5474. doi: 10.1523/JNEUROSCI.4501-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Obal F, Jr., Alfoldi P, Cady AB, et al. Growth hormone-releasing factor enhances sleep in rats and rabbits. Am J Physiol. 1988;255:R310–316. doi: 10.1152/ajpregu.1988.255.2.R310. 2 Pt 2. [DOI] [PubMed] [Google Scholar]
- 252.Steiger A. Neurochemical regulation of sleep. J Psychiatr Res. 2007;41(7):537–552. doi: 10.1016/j.jpsychires.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 253.Van Cauter E, Plat L, Scharf MB, et al. Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young Men. J Clin Invest. 1997;100(3):745–753. doi: 10.1172/JCI119587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Havlicek V, Rezek M, Friesen H. Somatostatin and thyrotropin releasing hormone: central effect on sleep and motor system. Pharmacol Biochem Behav. 1976;4(4):455–459. doi: 10.1016/0091-3057(76)90063-0. [DOI] [PubMed] [Google Scholar]
- 255.Rezek M, Havlicek V, Hughes KR, et al. Cortical administration of somatostatin (SRIF): effect on sleep and motor behavior. Pharmacol Biochem Behav. 1976;5(1):73–77. doi: 10.1016/0091-3057(76)90290-2. [DOI] [PubMed] [Google Scholar]
- 256.Szentirmai E, Kapas L, Krueger JM. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R575–585. doi: 10.1152/ajpregu.00448.2006. [DOI] [PubMed] [Google Scholar]
- 257.Rojas-Ramirez JA, Crawley JN, Mendelson WB. Electroencephalographic analysis of the sleep-inducing actions of cholecystokinin. Neuropeptides. 1982;3(2):129–138. doi: 10.1016/0143-4179(82)90008-7. [DOI] [PubMed] [Google Scholar]
- 258.Kapas L, Obal F, Jr., Alfoldi P, et al. Effects of nocturnal intraperitoneal administration of cholecystokinin in rats: simultaneous increase in sleep, increase in EEG slow-wave activity, reduction of motor activity, suppression of eating, and decrease in brain temperature. Brain Res. 1988;438(1-2):155–164. doi: 10.1016/0006-8993(88)91334-0. [DOI] [PubMed] [Google Scholar]
- 259.Kapas L, Obal F, Jr., Opp MR, et al. Intraperitoneal injection of cholecystokinin elicits sleep in rabbits. Physiol Behav. 1991;50(6):1241–1244. doi: 10.1016/0031-9384(91)90589-g. [DOI] [PubMed] [Google Scholar]
- 260.Prospero-Garcia O, Ott T, Drucker-Colin R. Cerebroventricular infusion of cholecystokinin (CCK-8) restores REM sleep in parachlorophenylalanine (PCPA)-pretreated cats. Neurosci Lett. 1987;78(2):205–210. doi: 10.1016/0304-3940(87)90634-3. [DOI] [PubMed] [Google Scholar]
- 261.Sangiah S, Caldwell DF, Villeneuve MJ, et al. Sleep: sequential reduction of paradoxical (REM) and elevation of slow-wave (NREM) sleep by a non-convulsive dose of insulin in rats. Life Sci. 1982;31(8):763–769. doi: 10.1016/0024-3205(82)90702-0. [DOI] [PubMed] [Google Scholar]
- 262.Danguir J, Nicolaidis S. Chronic intracerebroventricular infusion of insulin causes selective increase of slow wave sleep in rats. Brain Res. 1984;306(1-2):97–103. doi: 10.1016/0006-8993(84)90359-7. [DOI] [PubMed] [Google Scholar]
- 263.Obal F, Jr., Kapas L, Bodosi B, et al. Changes in sleep in response to intracerebral injection of insulin-like growth factor-1 (IFG-1) in the rat. Sleep Res Online. 1998;1(2):87–91. [PubMed] [Google Scholar]
- 264.Verret L, Goutagny R, Fort P, et al. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hanriot L, Camargo N, Courau AC, et al. Characterization of the melanin-concentrating hormone neurons activated during paradoxical sleep hypersomnia in rats. J Comp Neurol. 2007;505(2):147–157. doi: 10.1002/cne.21482. [DOI] [PubMed] [Google Scholar]
- 266.Apergis-Schoute J, Iordanidou P, Faure C, et al. Optogenetic Evidence for Inhibitory Signaling from Orexin to MCH Neurons via Local Microcircuits. J Neurosci. 2015;35(14):5435–5441. doi: 10.1523/JNEUROSCI.5269-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Parks GS, Olivas ND, Ikrar T, et al. Histamine inhibits the melanin-concentrating hormone system: implications for sleep and arousal. J Physiol. 2014;592:2183–2196. doi: 10.1113/jphysiol.2013.268771. Pt 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Jego S, Glasgow SD, Herrera CG, et al. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 2013;16(11):1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Tsunematsu T, Ueno T, Tabuchi S, et al. Optogenetic manipulation of activity and temporally controlled cell-specific ablation reveal a role for MCH neurons in sleep/wake regulation. J Neurosci. 2014;34(20):6896–6909. doi: 10.1523/JNEUROSCI.5344-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Konadhode RR, Pelluru D, Blanco-Centurion C, et al. Optogenetic stimulation of MCH neurons increases sleep. J Neurosci. 2013;33(25):10257–10263. doi: 10.1523/JNEUROSCI.1225-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Roky R, Valatx JL, Jouvet M. Effect of prolactin on the sleep-wake cycle in the rat. Neurosci Lett. 1993;156(1-2):117–120. doi: 10.1016/0304-3940(93)90453-r. [DOI] [PubMed] [Google Scholar]
- 272.Obal F, Jr., Opp M, Cady AB, et al. Prolactin, vasoactive intestinal peptide, and peptide histidine methionine elicit selective increases in REM sleep in rabbits. Brain Res. 1989;490(2):292–300. doi: 10.1016/0006-8993(89)90246-1. [DOI] [PubMed] [Google Scholar]
- 273.Riou F, Cespuglio R, Jouvet M. [Hypnogenic properties of the vasoactive intestinal polypeptide in rats] C R Seances Acad Sci III. 1981;293(12):679–682. [PubMed] [Google Scholar]
- 274.Fang J, Payne L, Krueger JM. Pituitary adenylate cyclase activating polypeptide enhances rapid eye movement sleep in rats. Brain Res. 1995;686(1):23–28. doi: 10.1016/0006-8993(95)00443-t. [DOI] [PubMed] [Google Scholar]
- 275.Aguilar-Roblero R, Verduzco-Carbajal L, Rodriguez C, et al. Circadian rhythmicity in the GABAergic system in the suprachiasmatic nuclei of the rat. Neurosci Lett. 1993;157:199–202. doi: 10.1016/0304-3940(93)90736-5. [DOI] [PubMed] [Google Scholar]
- 276.Ahnaou A, Basille M, Gonzalez B, et al. Long-term enhancement of REM sleep by the pituitary adenylyl cyclase-activating polypeptide (PACAP) in the pontine reticular formation of the rat. Eur J Neurosci. 1999;11(11):4051–4058. doi: 10.1046/j.1460-9568.1999.00811.x. [DOI] [PubMed] [Google Scholar]
- 277.Ahnaou A, Laporte AM, Ballet S, et al. Muscarinic and PACAP receptor interactions at pontine level in the rat: significance for REM sleep regulation. Eur J Neurosci. 2000;12(12):4496–4504. doi: 10.1046/j.0953-816x.2000.01345.x. [DOI] [PubMed] [Google Scholar]
- 278.Yanik G, Glaum S, Radulovacki M. The dose-response effects of caffeine on sleep in rats. Brain Res. 1987;403(1):177–180. doi: 10.1016/0006-8993(87)90141-7. [DOI] [PubMed] [Google Scholar]
- 279.Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Progress in neurobiology. 1995;45(4):347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 280.Scharf MT, Naidoo N, Zimmerman JE, et al. The energy hypothesis of sleep revisited. Progress in neurobiology. 2008;86(3):264–280. doi: 10.1016/j.pneurobio.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Dunwiddie TV, Worth T. Sedative and anticonvulsant effects of adenosine analogs in mouse and rat. J Pharmacol Exp Ther. 1982;220(1):70–76. [PubMed] [Google Scholar]
- 282.Feldberg W, Sherwood SL. Injections of drugs into the lateral ventricle of the cat. J Physiol. 1954;123(1):148–167. doi: 10.1113/jphysiol.1954.sp005040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Radulovacki M, Virus RM, Djuricic-Nedelson M, et al. Adenosine analogs and sleep in rats. J Pharmacol Exp Ther. 1984;228(2):268–274. [PubMed] [Google Scholar]
- 284.Radulovacki M, Virus RM, Rapoza D, et al. A comparison of the dose response effects of pyrimidine ribonucleosides and adenosine on sleep in rats. Psychopharmacology. 1985;87(2):136–140. doi: 10.1007/BF00431796. [DOI] [PubMed] [Google Scholar]
- 285.Benington JH, Kodali SK, Heller HC. Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Res. 1995;692(1-2):79–85. doi: 10.1016/0006-8993(95)00590-m. [DOI] [PubMed] [Google Scholar]
- 286.Bjorness TE, Kelly CL, Gao T, et al. Control and function of the homeostatic sleep response by adenosine A1 receptors. J Neurosci. 2009;29(5):1267–1276. doi: 10.1523/JNEUROSCI.2942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annual review of neuroscience. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 288.Huang ZL, Qu WM, Eguchi N, et al. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8(7):858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 289.Urade Y, Eguchi N, Qu WM, et al. Sleep regulation in adenosine A2A receptor-deficient mice. Neurology. 2003;61(11 Suppl 6):S94–96. doi: 10.1212/01.wnl.0000095222.41066.5e. [DOI] [PubMed] [Google Scholar]
- 290.Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99(3):507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 291.Porkka-Heiskanen T, Strecker RE, Thakkar M, et al. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276(5316):1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Blanco-Centurion C, Xu M, Murillo-Rodriguez E, et al. Adenosine and sleep homeostasis in the basal forebrain. J Neurosci. 2006;26(31):8092–8100. doi: 10.1523/JNEUROSCI.2181-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kalinchuk AV, McCarley RW, Stenberg D, et al. The role of cholinergic basal forebrain neurons in adenosine-mediated homeostatic control of sleep: lessons from 192 IgG-saporin lesions. Neuroscience. 2008;157(1):238–253. doi: 10.1016/j.neuroscience.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Strecker RE, Morairty S, Thakkar MM, et al. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behavioural brain research. 2000;115(2):183–204. doi: 10.1016/s0166-4328(00)00258-8. [DOI] [PubMed] [Google Scholar]
- 295.Kalinchuk AV, Lu Y, Stenberg D, et al. Nitric oxide production in the basal forebrain is required for recovery sleep. J Neurochem. 2006;99(2):483–498. doi: 10.1111/j.1471-4159.2006.04077.x. [DOI] [PubMed] [Google Scholar]
- 296.Kalinchuk AV, McCarley RW, Porkka-Heiskanen T, et al. The time course of adenosine, nitric oxide (NO) and inducible NO synthase changes in the brain with sleep loss and their role in the non-rapid eye movement sleep homeostatic cascade. J Neurochem. 2011;116(2):260–272. doi: 10.1111/j.1471-4159.2010.07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kalinchuk AV, Porkka-Heiskanen T, McCarley RW, et al. Cholinergic neurons of the basal forebrain mediate biochemical and electrophysiological mechanisms underlying sleep homeostasis. Eur J Neurosci. 2015;41(2):182–195. doi: 10.1111/ejn.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Halassa MM, Florian C, Fellin T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61(2):213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Schmitt LI, Sims RE, Dale N, et al. Wakefulness affects synaptic and network activity by increasing extracellular astrocyte-derived adenosine. J Neurosci. 2012;32(13):4417–4425. doi: 10.1523/JNEUROSCI.5689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Magistretti PJ. Neuron-glia metabolic coupling and plasticity. The Journal of experimental biology. 2006;209(Pt 12):2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- 301.Petit JM, Gyger J, Burlet-Godinot S, et al. Genes involved in the astrocyte-neuron lactate shuttle (ANLS) are specifically regulated in cortical astrocytes following sleep deprivation in mice. Sleep. 2013;36(10):1445–1458. doi: 10.5665/sleep.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Petit JM, Tobler I, Allaman I, et al. Sleep deprivation modulates brain mRNAs encoding genes of glycogen metabolism. Eur J Neurosci. 2002;16(6):1163–1167. doi: 10.1046/j.1460-9568.2002.02145.x. [DOI] [PubMed] [Google Scholar]
- 303.Lewy AJ. Melatonin and human chronobiology. Cold Spring Harbor symposia on quantitative biology. 2007;72:623–636. doi: 10.1101/sqb.2007.72.055. [DOI] [PubMed] [Google Scholar]
- 304.Onoe H, Ueno R, Fujita I, et al. Prostaglandin D2, a cerebral sleep-inducing substance in monkeys. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(11):4082–4086. doi: 10.1073/pnas.85.11.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Matsumura H, Takahata R, Hayaishi O. Inhibition of sleep in rats by inorganic selenium compounds, inhibitors of prostaglandin D synthase. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(20):9046–9050. doi: 10.1073/pnas.88.20.9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Takahata R, Matsumura H, Kantha SS, et al. Intravenous administration of inorganic selenium compounds, inhibitors of prostaglandin D synthase, inhibits sleep in freely moving rats. Brain Res. 1993;623(1):65–71. doi: 10.1016/0006-8993(93)90010-k. [DOI] [PubMed] [Google Scholar]
- 307.Qu WM, Huang ZL, Xu XH, et al. Lipocalin-type prostaglandin D synthase produces prostaglandin D2 involved in regulation of physiological sleep. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(47):17949–17954. doi: 10.1073/pnas.0608581103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Pandey HP, Ram A, Matsumura H, et al. Concentration of prostaglandin D2 in cerebrospinal fluid exhibits a circadian alteration in conscious rats. Biochem Mol Biol Int. 1995;37(3):431–437. [PubMed] [Google Scholar]
- 309.Ram A, Pandey HP, Matsumura H, et al. CSF levels of prostaglandins, especially the level of prostaglandin D2, are correlated with increasing propensity towards sleep in rats. Brain Res. 1997;751(1):81–89. doi: 10.1016/s0006-8993(96)01401-1. [DOI] [PubMed] [Google Scholar]
- 310.Gerashchenko D, Beuckmann CT, Kanaoka Y, et al. Dominant expression of rat prostanoid DP receptor mRNA in leptomeninges, inner segments of photoreceptor cells, iris epithelium, and ciliary processes. J Neurochem. 1998;71(3):937–945. doi: 10.1046/j.1471-4159.1998.71030937.x. [DOI] [PubMed] [Google Scholar]
- 311.Matsumura H, Nakajima T, Osaka T, et al. Prostaglandin D2-sensitive, sleep-promoting zone defined in the ventral surface of the rostral basal forebrain. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(25):11998–12002. doi: 10.1073/pnas.91.25.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Satoh S, Matsumura H, Suzuki F, et al. Promotion of sleep mediated by the A2a-adenosine receptor and possible involvement of this receptor in the sleep induced by prostaglandin D2 in rats. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(12):5980–5984. doi: 10.1073/pnas.93.12.5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Mong JA, Baker FC, Mahoney MM, et al. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. 2011;31(45):16107–16116. doi: 10.1523/JNEUROSCI.4175-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 315.Armitage R, Hoffmann R, Trivedi M, et al. Slow-wave activity in NREM sleep: sex and age effects in depressed outpatients and healthy controls. Psychiatry research. 2000;95(3):201–213. doi: 10.1016/s0165-1781(00)00178-5. [DOI] [PubMed] [Google Scholar]
- 316.Dijk DJ, Beersma DG, Bloem GM. Sex differences in the sleep EEG of young adults: visual scoring and spectral analysis. Sleep. 1989;12(6):500–507. doi: 10.1093/sleep/12.6.500. [DOI] [PubMed] [Google Scholar]
- 317.Manber R, Armitage R. Sex, steroids, and sleep: a review. Sleep. 1999;22(5):540–555. [PubMed] [Google Scholar]
- 318.Mourtazaev MS, Kemp B, Zwinderman AH, et al. Age and gender affect different characteristics of slow waves in the sleep EEG. Sleep. 1995;18(7):557–564. doi: 10.1093/sleep/18.7.557. [DOI] [PubMed] [Google Scholar]
- 319.Armitage R, Hoffmann RF. Sleep EEG, depression and gender. Sleep medicine reviews. 2001;5(3):237–246. doi: 10.1053/smrv.2000.0144. [DOI] [PubMed] [Google Scholar]
- 320.Driver HS, Dijk DJ, Werth E, et al. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. The Journal of clinical endocrinology and metabolism. 1996;81(2):728–735. doi: 10.1210/jcem.81.2.8636295. [DOI] [PubMed] [Google Scholar]
- 321.Baker FC, Driver HS, Paiker J, et al. Acetaminophen does not affect 24-h body temperature or sleep in the luteal phase of the menstrual cycle. Journal of applied physiology. 2002;92(4):1684–1691. doi: 10.1152/japplphysiol.00919.2001. [DOI] [PubMed] [Google Scholar]
- 322.Shechter A, Varin F, Boivin DB. Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep. 2010;33(5):647–656. doi: 10.1093/sleep/33.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Fang J, Fishbein W. Sex differences in paradoxical sleep: influences of estrus cycle and ovariectomy. Brain Res. 1996;734(1-2):275–285. [PubMed] [Google Scholar]
- 324.Franken P, Dudley CA, Estill SJ, et al. NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: genotype and sex interactions. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(18):7118–7123. doi: 10.1073/pnas.0602006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Koehl M, Battle S, Meerlo P. Sex differences in sleep: the response to sleep deprivation and restraint stress in mice. Sleep. 2006;29(9):1224–1231. doi: 10.1093/sleep/29.9.1224. [DOI] [PubMed] [Google Scholar]
- 326.Paul KN, Dugovic C, Turek FW, et al. Diurnal sex differences in the sleep-wake cycle of mice are dependent on gonadal function. Sleep. 2006;29(9):1211–1223. doi: 10.1093/sleep/29.9.1211. [DOI] [PubMed] [Google Scholar]
- 327.Paul KN, Laposky AD, Turek FW. Reproductive hormone replacement alters sleep in mice. Neurosci Lett. 2009;463(3):239–243. doi: 10.1016/j.neulet.2009.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Yamaoka S. Modification of circadian sleep rhythms by gonadal steroids and the neural mechanisms involved. Brain Res. 1980;185(2):385–398. doi: 10.1016/0006-8993(80)91076-8. [DOI] [PubMed] [Google Scholar]
- 329.Colvin GB, Whitmoyer DI, Lisk RD, et al. Changes in sleep-wakefulness in female rats during circadian and estrous cycles. Brain Res. 1968;7(2):173–181. doi: 10.1016/0006-8993(68)90095-4. [DOI] [PubMed] [Google Scholar]
- 330.Colvin GB, Whitmoyer DI, Sawyer CH. Circadian sleep-wakefulness patterns in rats after ovariectomy and treatment with estrogen. Exp Neurol. 1969;25(4):616–625. doi: 10.1016/0014-4886(69)90104-6. [DOI] [PubMed] [Google Scholar]
- 331.Hadjimarkou MM, Benham R, Schwarz JM, et al. Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur J Neurosci. 2008;27(7):1780–1792. doi: 10.1111/j.1460-9568.2008.06142.x. [DOI] [PubMed] [Google Scholar]
- 332.Deurveilher S, Rusak B, Semba K. Estradiol and progesterone modulate spontaneous sleep patterns and recovery from sleep deprivation in ovariectomized rats. Sleep. 2009;32(7):865–877. [PMC free article] [PubMed] [Google Scholar]
- 333.Schwartz MD, Mong JA. Estradiol suppresses recovery of REM sleep following sleep deprivation in ovariectomized female rats. Physiol Behav. 2011;104(5):962–971. doi: 10.1016/j.physbeh.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Schwartz MD, Mong JA. Estradiol modulates recovery of REM sleep in a time-of-day-dependent manner. Am J Physiol Regul Integr Comp Physiol. 2013;305(3):R271–280. doi: 10.1152/ajpregu.00474.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Cusmano DM, Hadjimarkou MM, Mong JA. Gonadal steroid modulation of sleep and wakefulness in male and female rats is sexually differentiated and neonatally organized by steroid exposure. Endocrinology. 2014;155(1):204–214. doi: 10.1210/en.2013-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Ehlen JC, Hesse S, Pinckney L, et al. Sex chromosomes regulate nighttime sleep propensity during recovery from sleep loss in mice. PloS one. 2013;8(5):e62205. doi: 10.1371/journal.pone.0062205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Mong JA, Devidze N, Frail DE, et al. Estradiol differentially regulates lipocalin-type prostaglandin D synthase transcript levels in the rodent brain: Evidence from high-density oligonucleotide arrays and in situ hybridization. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):318–323. doi: 10.1073/pnas.262663799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Silveyra P, Catalano PN, Lux-Lantos V, et al. Impact of proestrous milieu on expression of orexin receptors and prepro-orexin in rat hypothalamus and hypophysis: actions of Cetrorelix and Nembutal. American journal of physiology Endocrinology and metabolism. 2007;292(3):E820–828. doi: 10.1152/ajpendo.00467.2006. [DOI] [PubMed] [Google Scholar]
- 339.Silveyra P, Cataldi NI, Lux-Lantos V, et al. Gonadal steroids modulated hypocretin/orexin type-1 receptor expression in a brain region, sex and daytime specific manner. Regul Pept. 2009;158(1-3):121–126. doi: 10.1016/j.regpep.2009.08.002. [DOI] [PubMed] [Google Scholar]