Abstract
Dysconnectivity hypothesis posits that schizophrenia is a disorder with dysconnectivity of the cortico-cerebellar-thalamic-cortical circuit (CCTCC). However, it remains unclear to the changes of the cerebral connectivity with the cerebellum in schizophrenia patients and unaffected siblings. Forty-nine patients with first-episode, drug-naive schizophrenia patients, 46 unaffected siblings of schizophrenia patients and 46 healthy controls participated in the study. Seed-based resting-state functional connectivity approach was employed to analyze the data. Compared with the controls, the patients and the siblings share increased default-mode network (DMN) seed – right Crus II connectivity. The patients have decreased right dorsal attention network (DAN) seed – bilateral cerebellum 4,5 connectivity relative to the controls. By contrast, the siblings exhibit increased FC between the right DAN seed and the right cerebellum 6 and right cerebellum 4,5 compared to the controls. No other abnormal connectivities (executive control network and salience network) are observed in the patients/siblings relative to the controls. There are no correlations between abnormal cerebellar-cerebral connectivities and clinical variables. Cerebellar-cerebral connectivity of brain networks within the cerebellum are differently affected in first-episode, drug-naive schizophrenia patients and unaffected siblings. Increased DMN connectivity with the cerebellum may serve as potential endophenotype for schizophrenia.
Schizophrenia is a serious mental disorder with onset commonly during early adulthood and adolescence. In recent decades, schizophrenia is postulated to be a disorder with abnormalities in neuronal connectivity as dysconnectivity hypothesis, which suggests that schizophrenia is a disorder with dysconnectivity of the cortico-cerebellar-thalamic-cortical circuit (CCTCC)1,2. Dysconnectivity hypothesis posits that abnormal neural connectivity is due to genetic and environmental factors that affect neurodevelopmental process in schizophrenia3,4. Unaffected siblings have a higher risk to develop schizophrenia than general population, and share similar brain functional abnormalities with the patients5,6,7,8,9,10,11, which can serve as potential endophenotypes for schizophrenia. An endophenotype is heritable and segregates with the disorder within families12.
Resting-state functional magnetic resonance imaging (fMRI) has observed that intrinsic neural activity across brain regions is organized into functional connectivity (FC) networks13. Several high-order networks have been revealed by resting-state fMRI13,14,15,16,17,18. They are: 1) the default-mode network (DMN), a well-known network consisting of the medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC)/precuneus and lateral posterior cortices; 2) dorsal attention network (DAN) including the frontal eye fields, intraparietal sulcus/superior parietal lobule, and extrastriate visual regions; 3) executive control network (ECN) comprising of the dorsolateral prefrontal cortex – parietal regions; and 4) salience network (SN), which includes the anterior cingulate cortex (ACC) and inferior frontal cortex/anterior insular cortex.
Among these networks, the DMN is the most examined network in schizophrenia with mixed findings: increased connectivity19,20 and decreased connectivity21,22,23 or both24,25. By contrast, evidence of abnormal connectivity from other networks is limited. For example, several studies have revealed abnormal connectivity within the ECN in schizophrenia23,26,27, whereas Lui et al.28 reported no changes of the ECN connectivity in first-episode, drug-naive schizophrenia patients. Reduced connectivity in the DMN, DAN and ECN has been found in medicated patients, whereas the SN did not exhibit abnormal connectivity in schizophrenia patients29. The mixed findings may result from sample heterogeneity in addition to sample size, scanners and analysis methods. Most of the above-mentioned studies recruited chronic and/or medicated patients. Long illness duration and medication use may have biased their results30,31,32. Therefore, it is important to select first-episode, drug-naive patients as a starting point to reveal the naive connectivity of these networks in schizophrenia.
Furthermore, a critical issue to be settled is whether the connectivity between the above-mentioned networks and the cerebellum is affected in schizophrenia. The cerebellum is traditionally considered as a coordinator of motor function33. Evidence of the cerebellum participating high-order brain function has accumulated34. Through the CCTCC, the cerebellum connects with widespread cerebral regions35. The cerebellum is divided into 26 regions in the Anatomical Automatic Labeling template. Several cerebellar regions have been revealed to link with the cortical networks, i.e., cerebellar Crus I and Crus II with the ECN, lobule VI with the SN, and Crus I, Crus II and lobule IX with the DMN36. Reduced cerebellar function and metabolism have been revealed in schizophrenia by neuroimaging studies33. Emotional dysregulation and cognitive deficits present in the patients are linked to cerebellar dysfunction, especially with abnormal cerebellar-cerebral connections37,38. The cerebellum may play a key role in cognition and emotion in schizophrenia patients via the CCTCC38. However, it remains unclear to the changes of the cerebral connection with the cerebellum in schizophrenia, especially the cerebellar-cerebral dysconnectivity shared by the patients and the siblings.
In the present study, we explored the cerebellar-cerebral connectivity in a relatively large sample of first-episode, drug-naive schizophrenia patients and unaffected siblings. The FC method was employed to analyze the data using the seeds of the four networks (DMN, DAN, ECN and SN). The aim of this study was to examine the cerebellar-cerebral connectivity shared by the patients and the siblings, which could be used as potential endophenotypes for schizophrenia. According to dysconnectivity hypothesis of schizophrenia, reduced cerebellar-cerebral connectivity was expected to be present in the patients and the siblings. In addition, we examined the correlations between abnormal cerebellar-cerebral connectivity and clinical variables (i.e. symptom severity) in the patients.
Methods and Materials
Participants
Forty-nine patients with first-episode, drug-naive schizophrenia patients, 46 unaffected siblings of schizophrenia patients and 46 healthy controls participated in the study. All participants were right-handed, and aged from 16 to 30 years with more than 9 years of formal education. Fourteen patients and 14 unaffected siblings were sib pairs, and the other participants were from different families. The study was conducted in accordance with the Helsinki Declaration39. The local ethics committee of the First Affiliated Hospital, Guangxi Medical University approved this study, and all participants gave their written informed consent.
The patients and the siblings were recruited from the Mental Health Center, the First Affiliated Hospital, Guangxi Medical University, China, and the controls were recruited from the community. The siblings had brothers or sisters diagnosed as schizophrenia. The diagnosis of schizophrenia was made based on the Structured Clinical Interview of the Diagnostic and Statistical Manual of Mental Disorders-IV (SCID) criteria, patient edition40. The duration of untreated psychosis (DUP) of the patients was less than 3 years, and symptom severity was assessed by Positive and Negative Symptom Scale (PANSS). All participants shared the following exclusion criteria: neurological disorders, severe medical disorders, substance abuse, or any contraindications for MRI scan. The potential controls had a first-degree relative with psychiatric disorders were also excluded.
Scan acquisition
Scans were acquired on a Siemens 3T scanner. Participants were required to keep still and remain awake with their eyes closed. Soft earplugs and foam pads were applied to reduce scanner noise and head movement. The following parameters with a gradient-echo echo-planner imaging (EPI) sequence were used to obtain resting-state functional scans: repetition time/echo time = 2000 ms/30 ms, 30 slices, 64 × 64 matrix, 90° flip angle, 24 cm field of view, 4 mm slice thickness, 0.4 mm gap, and 250 volumes (500 s).
Scan preprocessing
Data Processing Assistant for Resting-State fMRI41 were employed to preprocess functional scans. After the correction of slice timing and head movement, no participants had more than 2° of maximal rotation and 2 mm of maximal translation. The scans were subsequently normalized to the standard Montreal Neurological Institute (MNI) EPI space in SPM8 and resampled to 3 × 3 × 3 mm3. After that, the scans were smoothed with a 4 mm full width at half maximum Gaussian kernel, bandpass filtered (0.01–0.08 Hz), and linearly detrended. Several spurious covariates were regressed, including 24 head motion parameters, signal from a ventricular region of interest (ROI), and signal from a region centered in the white matter. We did not regress out the global signal as previously suggested42.
FC analyses
Seed-based FC method was used to create seed-to-voxel maps for each participant. The seeds were defined as 6 mm radius sphere centered on MNI coordinates applied to detect the corresponding networks in previous studies18,29,43,44,45. The seeds included: the DMN (1, −55, 17), DAN (left/right: −25, −53, 52/25, −57, 52); ECN (left/right: −42, 34, 20/44, 36, 20), and SN (left/right: −32, 26, −14/38, 22, −10). Software REST46 was used to conduct the seed-based FC analyses. Pearson correlation coefficients were computed between the seeds and the voxels of the whole cerebellum to create the correlation maps for each seed and each participant. Finally, the correlation maps were z-transformed with Fisher’s r-to-z transformation.
As described in a previous study47, the framewise displacement (FD) was computed for each participant since head micromotion might affect the FC results. The formula for computing the FD was provided in the previous study47. For each seed, analyses of covariance (ANCOVA), followed by post hoc t-tests, were conducted with the mean FD as a covariate to identify significant differences between each pair of groups. The significance level was set at p < 0.005 corrected for multiple comparisons using the Gaussian Random Field (GRF) theory (min z > 2.807, cluster significance: p < 0.005).
Correlation analyses
The mean z values were extracted from the clusters with abnormal cerebellar-cerebral FC in the patients. Pearson correlations were performed to examine the correlations between the mean z values and clinical variables (i.e., symptom severity) in the patients after assessing the normality of the data.
Results
Participants
Participant demographics are present in Table 1. The 3 groups do not differ in age, sex ratio, education level, and FD values.
Table 1. Demographic characteristics of the participants.
| Patients (n = 49) | Siblings (n = 46) | Controls (n = 46) | p value | |
|---|---|---|---|---|
| Sex (male/female) | 30/19 | 29/17 | 23/23 | 0.39a |
| Age (years) | 22.69 ± 4.62 | 22.96 ± 4.01 | 23.30 ± 2.30 | 0.52b |
| Years of education (years) | 10.94 ± 2.40 | 11.50 ± 2.21 | 11.34 ± 1.78 | 0.80b |
| FD (mm) | 0.06 ± 0.05 | 0.06 ± 0.03 | 0.05 ± 0.02 | 0.15b |
| DUP (months) | 22.45 ± 6.71 | |||
| PANSS | ||||
| Positive scores | 22.27 ± 5.33 | |||
| Negative scores | 22.82 ± 6.86 | |||
| Total scores | 91.31 ± 10.98 | |||
FD = framewise displacement, DUP = duration of untreated psychosis, PANSS = Positive and Negative Symptom Scale.
aThe p value for sex distribution was obtained by chi-square test.
bThe p values were obtained by analysis of variance (ANOVA).
Seed-based FCs: Group differences
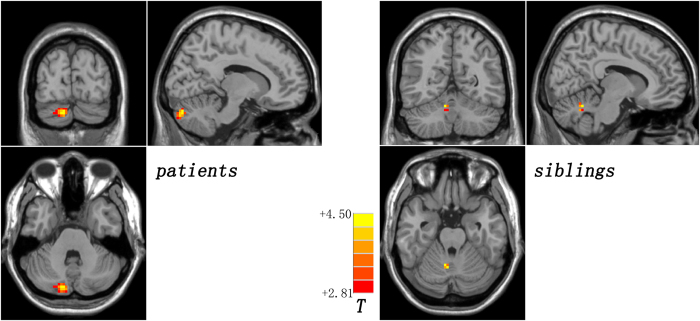
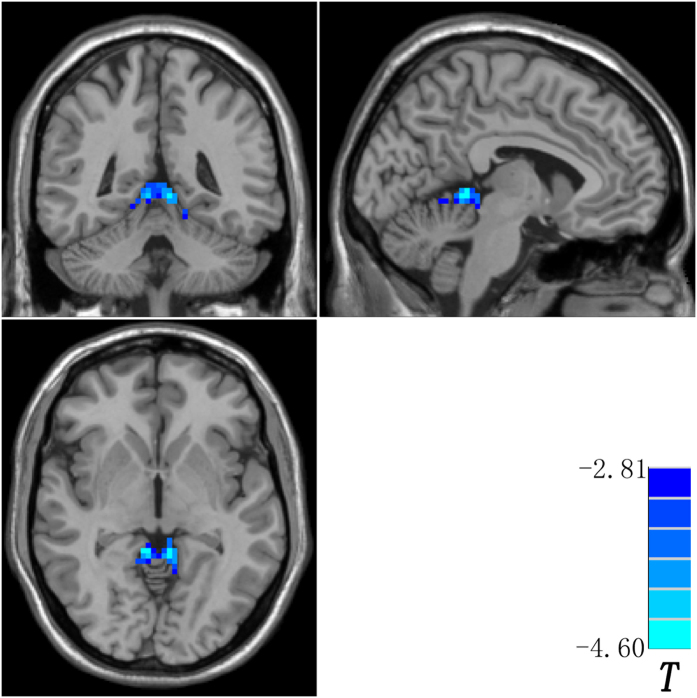
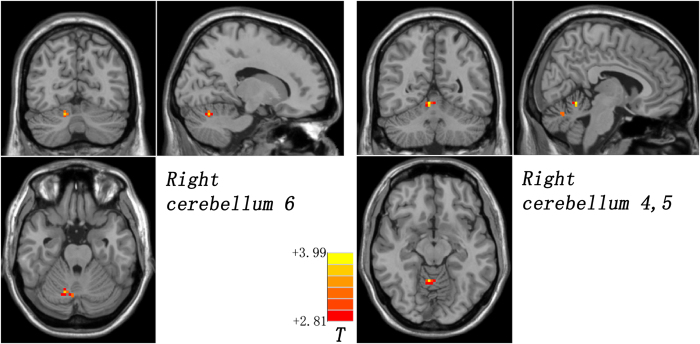
Compared with the controls, the patients and the siblings share increased DMN seed – right Crus II connectivity by using post hoc t-tests (Fig. 1 and Table 2). The patients have decreased right DAN seed – bilateral cerebellum 4,5 connectivity relative to the controls (Fig. 2 and Table 2). By contrast, the siblings exhibit increased FC between the right DAN seed and the right cerebellum 6 and right cerebellum 4,5 compared to the controls (Fig. 3 and Table 2). No other abnormal connectivities (ECN and SN) are observed in the patients/siblings relative to the controls.
Figure 1. Increased default-mode network seed – right Crus II connectivity shared by the patients and the siblings.
Red denotes increased connectivity in the patients/siblings relative to the controls and the color bar indicates T values from post hoc t-tests.
Table 2. Cerebellar regions with abnormal functional connectivity with the cerebral seeds in the patients and the siblings.
| Cluster location | Peak (MNI) |
Number of voxels | T value* | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Seed: Default-mode network (1, −55, 17) | |||||
| Patients > Controls | |||||
| Right Crus II | 12 | −84 | −33 | 47 | 4.5032 |
| Siblings > Controls | |||||
| Right Crus II | 9 | −57 | −24 | 11 | 3.7188 |
| Seed: Right dorsal attention network (25, −57, 52) | |||||
| Patients < Controls | |||||
| Bilateral Cerebellum 4,5 | −6 | −45 | −3 | 114 | −4.6002 |
| Siblings > Controls | |||||
| Right Cerebellum 6 | 6 | −54 | −12 | 10 | 3.9930 |
| Right Cerebellum 4,5 | 15 | −66 | −24 | 11 | 3.7159 |
| Seed: Left dorsal attention network (−25, −53, 52) | |||||
| None | |||||
| Seed: Executive control network (−42, 34, 20/44, 36, 20) | |||||
| None | |||||
| Seed: Salience network (−32, 26, −14/38, 22, −10) | |||||
| None | |||||
*A positive/negative T value represents an increased/decreased functional connectivity; MNI = Montreal Neurological Institute.
Figure 2. Decreased right dorsal attention network seed – bilateral cerebellum 4,5 connectivity in the patients.
Blue denotes decreased connectivity in the patients relative to the controls and the color bar indicates T values from post hoc t-tests.
Figure 3. Increased right dorsal attention network seed – right cerebellum 6/right cerebellum 4,5 connectivity in the siblings.
Red denotes increased connectivity in the siblings relative to the controls and the color bar indicates T values from post hoc t-tests.
Correlation results in the patients
No correlations are found between the mean z values of the clusters with abnormal cerebellar-cerebral connectivities and clinical variables (DUP and PANSS scores) in the patient group. There are also no correlations between the mean z values of the clusters with abnormal cerebellar-cerebral connectivities and age or education level in the patients.
Reproducibility of the shared increased DMN seed – right Crus II connectivity in the patients and the siblings
To examine the reproducibility of the shared increased DMN seed – right Crus II connectivity in the patients and the siblings, the connectivities between the DMN seed, a 6 mm radius sphere centered on the MNI coordinates (1, −55, 17), and other voxels of the whole brain were calculated. Voxel-based one-sample t-tests revealed that the DMN seed had links with the cerebellum and the cerebrum in the patients, siblings, and controls (p < 0.005, GRF corrected; Figure S1). Then, a DMN mask (Figure S2) was generated from the union of the results of one-sample t-tests from 3 groups. Voxel-wise network homogeneity (NH) was calculated with the equation which can be found in a previous study48, and the mean NH of each voxel in the DMN mask was obtained and z-transformed for standardization purpose. Finally, ANCOVA, followed by post hoc t-tests, were computed with the mean FD as a covariate to detect significant NH differences between each pair of groups. Similar results (p < 0.005, GRF corrected; Figure S3 and Table S1) were obtained as the original results (Fig. 1 and Table 2).
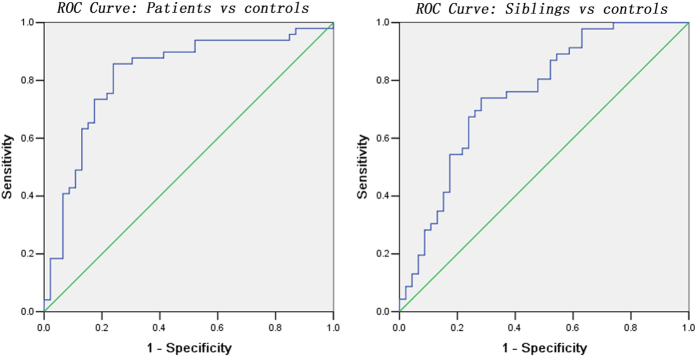
In addition, mean z values of the clusters with shared increased DMN seed – right Crus II connectivity were extracted for further receiver operating characteristic curves (ROC) analysis. As shown in Fig. 4 and Table 3, the areas under the curves were high, which indicated that z values of this connectivity might be used as markers to differentiate the patients/siblings from the controls with relatively high sensitivity and specificity.
Figure 4. Receiver operating characteristic (ROC) curves using the mean the z values of the clusters with shared increased DMN seed – right Crus II connectivity to separate the patients/siblings from the controls.
DMN = default mode network.
Table 3. ROC analysis for differentiating the patients/siblings from the controls.
| Connectivity | Area Under the Curve | Cut-off point | Sensitivity | Specificity |
|---|---|---|---|---|
| Separating patients from controls | ||||
| DMN seed – right Crus II | 0.818 | 0.2209a | 85.71% (42/49) | 76.09% (35/46) |
| Separating controls from controls | ||||
| DMN seed – right Crus II | 0.751 | 0.1403 | 73.91% (34/46) | 71.74% (33/46) |
ROC = receiver operating characteristic, DMN = default mode network.
aBy this cut-off point, the z values of the clusters with shared increased DMN seed – right Crus II connectivity could correctly classify 42 of 49 patients and 35 of 46 controls, resulted in a sensitivity of 85.71% and a specificity of 76.09%. The meaning of other cut-off point was similar.
Discussion
In the present study, we first examined the abnormalities of the cerebellar-cerebral connectivities in first-episode, drug-naive schizophrenia patients and unaffected siblings using cerebral seeds connecting with the corresponding networks. The main findings were that the patients and the siblings shared increased DMN seed – right Crus II connectivity relative to the controls. Compared with the controls, the patients exhibited decreased right DAN seed – bilateral cerebellum 4,5 connectivity, whereas the siblings had increased right DAN – cerebellum connectivities. No significant correlations were found between the z values of abnormal connectivities and clinical variables.
According to the definition, potential endophenotype can be neuroanatomical, neurophysiological, biochemical, endocrine or cognitive parameters. In this study, we observed increased DMN seed – right Crus II connectivity shared by the patients and the siblings. No correlations were observed between the z values of this connectivity and clinical variables in the patients, which indicate that this increased connectivity may be a trait alteration for schizophrenia independently of symptom severity and illness duration. Further ROC analysis revealed that z values of this connectivity could be used as markers to differentiate the patients/siblings from the controls. From the definition of endophenotype, no correlations to clinical variables and ROC results, increased DMN seed – right Crus II connectivity shared by the patients and the siblings can be used as potential endophenotype for schizophrenia.
At first glance, increased DMN seed – right Crus II connectivity seems inconsistent with the dysconnectivity hypothesis of schizophrenia. The dysconnectivity hypothesis is proposed based on studies with chronic and/or medicated patients4. When first-episode, drug-naive schizophrenia patients were recruited in this study, it is not surprised that the present findings were different from those obtained from chronic and/or medicated patients. Increased connectivity can be interpreted from the neurodevelopmental perspective. Previously, the FC differences of the prefrontal-thalamic-cerebellar circuit were examined in healthy children, adolescents, and adults, and the findings showed that the connectivities of the prefrontal-thalamic-cerebellar circuit present an inverted U-curve with maximal position in healthy adolescents49. Since our patients are at the developmental stage from adolescents to adults (aged from 16 to 30 years), the development process of the DMN seed – right Crus II connectivity is supposed to be disrupted by the disease, and thereby remain at a relatively high position of the inverted U-curve. This perspective is supported by previous studies revealing increased frontal FCs in early-course, drug-naive patients50,51.
Increased connectivity is also meaningful from the physiological perspective of FC. Increased connectivity is usually explained as compensatory reallocation or dedifferentiation52,53,54,55. Inflammation may modulate the compensatory reallocation in the early course of the disease50. In the early course of schizophrenia, proinflammatory cytokines (i.e., interleukin-6) can activate the astrocytes, and exhibit increased metabolism and blood flow (hyperfunction) to the astrocytes56. The regional hypofunction can result in increased regional activity and connectivity. This perspective is supported by a previous study that observed increased connectivities across the DMN areas in early-course patients with schizophrenia50. Although we speculated that increased DMN seed – right Crus II connectivity was a compensatory reallocation related to inflammation in the patients, further studies are needed to warrant or to refute this speculation.
The present results are generally consistent with a previous study that reported differentially affected functional resting-state networks in schizophrenia29. However, the two studies are different in details. In the previous study, Woodward et al.29 reported reduced cerebral connectivity in the DMN, DAN and ECN in a group of medicated patients with schizophrenia and schizoaffective disorder. The cerebellum was “cut off” in their study, and we could not compare the two studies directly. In this study, we focused on the cerebral connectivity with the cerebellum, and found increased DMN seed – right Crus II connectivity and decreased right DAN seed – bilateral cerebellum 4,5 connectivity in the patients. In addition to sample size, scanners and analysis method, sample heterogeneity may account for the inconsistency. However, the two studies are consistent when conceived from neurodevelopmental perspective. The patients of Woodward et al. had the mean age of 36.9 years and medicated, and the brain connectivity is expected to be at the relatively low position of the inverted U-curve. Hence, it is no wonder for their patients to show decreased connectivities.
Interestingly, the siblings exhibit increased DMN and DAN connectivities with the cerebellum. The siblings have a higher risk to develop schizophrenia than the general population although they are unaffected at the recruitment time. The changes of brain connectivity in the siblings suggest that these changes occur earlier than illness onset57. Together with the findings from the patients and the siblings and from a previous study29, we can portray a picture of the connectivity changes of brain networks for schizophrenia. Increased DMN and DAN connectivities appears earlier than disease onset (as observed in the siblings), and decreases with illness duration. The DAN connectivities decrease immediately after disease onset as reported in our patients.
Previously, associations between abnormal brain function and symptom severity is inconsistent. Positive and negative correlations are reported23,58, and some studies reported no correlations59,60. In line with the previous studies59,60, the present results found no correlations between abnormal cerebellar-cerebral connectivities and clinical variables (DUP and PANSS scores) in the patients. One possibility for no correlations is that abnormal cerebellar-cerebral connectivities are inherent characteristics for the patients independent of the symptom severity and illness duration.
The study has certain limitations. First, we focused on the cerebral connectivities with the cerebellum. This method can enhance the specificity of cerebellar contribution to the neurobiology of schizophrenia. For the same reason, other meaningful information from the cerebrum has been overlooked. Second, only 14 patients and 14 siblings are sib pairs, and the other participants are from different families. This recruitment criterion is critical for the reason that it is different from the traditional sib pair approach. As mentioned in a previous study61, recruiting participants from different families can reduce the confounding effect from genes unrelated to schizophrenia and shared early environment. Therefore, the recruitment criterion enhances the specificity of identifying potential endophenotypes for schizophrenia. Finally, this study is cross-sectional. A longitudinal study is needed to precisely portray the connectivity changes of brain networks in the patients and the siblings.
Despite the limitations, this study first observed that the cerebral connectivity of brain networks with the cerebellum are differently affected in first-episode, drug-naive schizophrenia patients and unaffected siblings. Increased DMN connectivity with the cerebellum can serve as potential endophenotype for schizophrenia.
Additional Information
How to cite this article: Guo, W. et al. Resting-state cerebellar-cerebral networks are differently affected in first-episode, drug-naive schizophrenia patients and unaffected siblings. Sci. Rep. 5, 17275; doi: 10.1038/srep17275 (2015).
Supplementary Material
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81260210 and 81571310), the Natural Science Foundation of Guangxi Province for Distinguished Young Scientists (Grant No. 2014GXNSFGA118010), and the Natural Science Foundation of Guangxi Province (Grant No. 2013GXNSFAA019107).
Footnotes
Author Contributions Drs. G.W. and Z.J. designed the study. Drs. Y.M., X.C. and Z.Z. collected the original imaging data. Drs. G.W., L.F., C.J. and W.R. managed and analyzed the imaging data. Drs. G.W. and L.F. wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
References
- Friston K. J. Schizophrenia and the disconnection hypothesis. Acta psychiatrica Scandinavica. Supplementum 395, 68–79 (1999). [DOI] [PubMed] [Google Scholar]
- Stephan K. E., Friston K. J. & Frith C. D. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull 35, 509–527 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard T. M., Sikich L., Lieberman J. A. & LaMantia A. S. Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr Bull 27, 457–476 (2001). [DOI] [PubMed] [Google Scholar]
- Karlsgodt K. H. et al. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Dev Psychopathol 20, 1297–1327 (2008). [DOI] [PubMed] [Google Scholar]
- Guo W. et al. Decreased resting-state interhemispheric functional connectivity in unaffected siblings of schizophrenia patients. Schizophr Res 152, 170–175 (2014). [DOI] [PubMed] [Google Scholar]
- Jang J. H. et al. Reduced prefrontal functional connectivity in the default mode network is related to greater psychopathology in subjects with high genetic loading for schizophrenia. Schizophr Res 127, 58–65 (2011). [DOI] [PubMed] [Google Scholar]
- MacDonald A. W. 3rd, Thermenos H. W., Barch D. M. & Seidman L. J. Imaging genetic liability to schizophrenia: systematic review of FMRI studies of patients’ nonpsychotic relatives. Schizophr Bull 35, 1142–1162 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson-Yeo W., Allen P., Benetti S., McGuire P. & Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev 35, 1110–1124 (2011). [DOI] [PubMed] [Google Scholar]
- van Buuren M., Vink M. & Kahn R. S. Default-mode network dysfunction and self-referential processing in healthy siblings of schizophrenia patients. Schizophr Res 142, 237–243 (2012). [DOI] [PubMed] [Google Scholar]
- van Buuren M., Vink M., Rapcencu A. E. & Kahn R. S. Exaggerated brain activation during emotion processing in unaffected siblings of patients with schizophrenia. Biol Psychiatry 70, 81–87 (2011). [DOI] [PubMed] [Google Scholar]
- Lyu H. et al. Regional white matter abnormalities in drug-naive, first-episode schizophrenia patients and their healthy unaffected siblings. Aust N Z J Psychiatry 49, 246–254 (2015). [DOI] [PubMed] [Google Scholar]
- Gottesman I. I. & Gould T. D. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 160, 636–645 (2003). [DOI] [PubMed] [Google Scholar]
- Fox M. D. et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102, 9673–9678 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Hudetz A. G., Yetkin F. Z., Haughton V. M. & Hyde J. S. Hypercapnia reversibly suppresses low-frequency fluctuations in the human motor cortex during rest using echo-planar MRI. J Cereb Blood Flow Metab 17, 301–308 (1997). [DOI] [PubMed] [Google Scholar]
- Biswal B., Yetkin F. Z., Haughton V. M. & Hyde J. S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34, 537–541 (1995). [DOI] [PubMed] [Google Scholar]
- Li S. J. et al. Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med 43, 45–51 (2000). [DOI] [PubMed] [Google Scholar]
- Greicius M. D., Krasnow B., Reiss A. L. & Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100, 253–258 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J. L., Kahn I., Snyder A. Z., Raichle M. E. & Buckner R. L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100, 3328–3342 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res 97, 194–205 (2007). [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S. et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA 106, 1279–1284 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm R. L. et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull 33, 1004–1012 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J., MacDonald A. W. 3rd, Bell C., Mueller B. A. & Lim K. O. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull 37, 640–650 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotarska-Jagiela A. et al. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res 117, 21–30 (2010). [DOI] [PubMed] [Google Scholar]
- Ongur D. et al. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res 183, 59–68 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingoia G. et al. Default mode network activity in schizophrenia studied at resting state using probabilistic ICA. Schizophr Res 138, 143–149 (2012). [DOI] [PubMed] [Google Scholar]
- Zhou Y. et al. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett 417, 297–302 (2007). [DOI] [PubMed] [Google Scholar]
- Skudlarski P. et al. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry 68, 61–69 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S. et al. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry 166, 196–205 (2009). [DOI] [PubMed] [Google Scholar]
- Woodward N. D., Rogers B. & Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res 130, 86–93 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J. A. et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry 62, 361–370 (2005). [DOI] [PubMed] [Google Scholar]
- Lui S. et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry 67, 783–792 (2010). [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B. et al. Caudate nucleus volume and its clinical and cognitive correlations in first episode schizophrenia. Schizophr Res 91, 87–96 (2007). [DOI] [PubMed] [Google Scholar]
- Andreasen N. C. & Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry 64, 81–88 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath R. G., Franklin D. E. & Shraberg D. Gross pathology of the cerebellum in patients diagnosed and treated as functional psychiatric disorders. J Nerv Ment Dis 167, 585–592 (1979). [DOI] [PubMed] [Google Scholar]
- Chen Y. L. et al. Resting-state fMRI mapping of cerebellar functional dysconnections involving multiple large-scale networks in patients with schizophrenia. Schizophr Res 149, 26–34 (2013). [DOI] [PubMed] [Google Scholar]
- Habas C. et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29, 8586–8594 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarski J. Z., McIntyre R. S., Grupp L. A. & Kennedy S. H. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? J Psychiatry Neurosci 30, 178–186 (2005). [PMC free article] [PubMed] [Google Scholar]
- Phillips J. R., Hewedi D. H., Eissa A. M. & Moustafa A. A. The cerebellum and psychiatric disorders. Frontiers in public health 3, 66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. E. Orthodontic Clinical Trials III: reporting of ethical issues associated with clinical trials published in three orthodontic journals between 1989 and 1998. Journal of orthodontics 32, 115–121 (2005). [DOI] [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M. & Williams J. B. W. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). Washington, DC, American Psychiatric Press (1997). [Google Scholar]
- Yan C. & Zang Y. DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci 4, 13 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahamy A. et al. Save the global: global signal connectivity as a tool for studying clinical populations with functional magnetic resonance imaging. Brain Connect 4, 395–403 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W. W. et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27, 2349–2356 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J. L. et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol 96, 3517–3531 (2006). [DOI] [PubMed] [Google Scholar]
- Yang Z. et al. Brain network informed subject community detection in early-onset schizophrenia. Scientific reports 4, 5549 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X. W. et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 6, e25031 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J. D., Barnes K. A., Snyder A. Z., Schlaggar B. L. & Petersen S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L. Q. et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods 169, 249–254 (2008). [DOI] [PubMed] [Google Scholar]
- Fair D. A. et al. Maturing thalamocortical functional connectivity across development. Front Syst Neurosci 4, 10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A. et al. Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. J Neurosci 35, 267–286 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W. et al. Abnormal Causal Connectivity by Structural Deficits in First-Episode, Drug-Naive Schizophrenia at Rest. Schizophr Bull 41, 57–65 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., Anderson N. D., Locantore J. K. & McIntosh A. R. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage 17, 1394–1402 (2002). [DOI] [PubMed] [Google Scholar]
- Grady C. L., McIntosh A. R. & Craik F. I. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia 43, 1466–1481 (2005). [DOI] [PubMed] [Google Scholar]
- Guo W. et al. Is there a cerebellar compensatory effort in first-episode, treatment-naive major depressive disorder at rest? Prog Neuropsychopharmacol Biol Psychiatry 46, 13–18 (2013). [DOI] [PubMed] [Google Scholar]
- Su Q. et al. Increased functional connectivity strength of right inferior temporal gyrus in first-episode, drug-naive somatization disorder. Aust N Z J Psychiatry 49, 74–81 (2015). [DOI] [PubMed] [Google Scholar]
- Liberto C. M., Albrecht P. J., Herx L. M., Yong V. W. & Levison S. W. Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem 89, 1092–1100 (2004). [DOI] [PubMed] [Google Scholar]
- Cannon T. D. et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry 65, 28–37 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman A., Whitford T. J., Westin C. F., Golland P. & Kubicki M. Whole brain resting state functional connectivity abnormalities in schizophrenia. Schizophr Res 139, 7–12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. et al. Schizophrenic patients and their unaffected siblings share increased resting-state connectivity in the task-negative network but not its anticorrelated task-positive network. Schizophr Bull 38, 285–294 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W. et al. Increased short-range and long-range functional connectivity in first-episode, medication-naive schizophrenia at rest. Schizophr Res 166, 144–150 (2015). [DOI] [PubMed] [Google Scholar]
- Guo W. et al. Decreased gray matter volume in the left middle temporal gyrus as a candidate biomarker for schizophrenia: a study of drug naive, first-episode schizophrenia patients and unaffected siblings. Schizophr Res 159, 43–50 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.