Abstract
Objective
To evaluate the rate of weight loss maintenance, defined as a 10% loss of initial weight maintained beyond 1 year, among patients with BMI > 25 kg/m2 who had been managed by primary care physicians practicing behavioral nutrition (moderately high-protein diet, carbohydrate restriction, and behavioral therapy).
Methods
Restrospective analysis of anthropometric characteristics, weight loss, and its determinants was conducted in 14,256 patients.
Results
26.7% of subjects met the success criterion (successful maintenance group; SM), 25.7% did not maintain their weight loss (unsuccessful maintenance group; UM), and 47.6% did not lose 10% of their initial weight (failure group; F). At inclusion, patients in the SM group had a greater BMI and fat mass percentage (40.5% in SM, 38.5% in UM, and 37.0% in F). These patients lost more weight (−14.1% vs. −4.59%) and fat mass (−24.7% vs. −8.21%) than patients in the UM group, and contribution of adiposity to their weight loss was 75.1%. Follow-up of patients in the SM group was characterized by a greater frequency of consultations.
Conclusions
Management by primary care providers with behavioral nutrition facilitates weight loss maintenance in patients with overweight and obesity. The determinants of success are frequency of consultations, initial BMI, and initial weight loss.
Introduction
Overweight and obesity are currently considered as a worldwide epidemic. The prevalence of overweight and obesity has increased continuously in many developed and developing countries rising by 27.5% for adults and 47.1% for children between 1980 and 2013 (1). Obesity is a significant public health issue in the USA with 35% of adults with a body mass index (BMI) ≥ 30 kg/m2 (2). The prevalence of obesity in Europe is estimated at 17.2% of the overall population, with a range of 11-26% depending on countries (3). Increased prevalence of obesity is recognized as an independant cardiovascular risk factor for hypertension, type 2 diabetes, and dyslipidemia but is also associated with nonalcoholic fatty liver disease and depression (2). Moreover, BMI increase is associated with the appearance of different kinds of cancer (4). As a result, obesity is associated with increased health care costs and higher mortality rates (2). As shown in Look AHEAD, DPP, or SOS studies, weight loss is associated with the improvement of hypertension, systolic and diastolic blood pressure, triglycerides, and HDL cholesterol levels (5) and with a decrease in the incidence of diabetes (6), cancer, cardiovascular events, and overall mortality (7). Losing 5-10% of initial body weight is a public health recommendation in several countries, including France and the USA (8,9). But most people tend to regain weight within 1 year after intervention (10). Mild degrees of weight regain (2-6%) can induce plasma lipids, blood pressure, fasting glucose, and insulin concentrations to revert back to baseline (10).
Weight loss interventions generally consist of caloric restriction, modification of macronutrients distribution, and glycemic index reduction (11). However, maintenance of weight loss does not rest only on dietary intervention; it depends also on other parameters, such as keeping the behavior changes learned during an intervention (12) and associating physical activity (11). Although interventional trials demonstrated the efficacy of these associations, studies on weight loss maintenance in primary care practice are scarce. Behavioral nutrition is a weight loss management program provided by a network of primary care physicians (PCPs) who receive an 8-h initial training and regularly participate in training sessions on nutrition and dietary counseling, obesity and comorbidities, patient motivation, and eating behavior. It combines a moderately high-protein diet and carbohydrate restriction with cognitive behavioral therapy.
This retrospective study was performed in a large cohort of patients who had been managed for weight loss by PCPs practicing behavioral nutrition. The primary aim was to assess the success rate of such management, the success criterion being defined as at least 10% loss of initial weight maintained beyond 1 year (12), and the secondary aim was to identify the determinants of success. Sustained weight loss has been defined as 10% intentional loss of initial weight and its maintainance for at least 1 year in the National Weight Control Registry (NWCR) (12). Although there is no consensus on this definition, it has the advantage to take into account a percentage of weight loss that is beneficial for health and a period during which most subjects regain the weight they have lost.
Methods
This is an observational retrospective study based on the patient files of 478 general practitioners collected between 2000 and 2012. The study was approved by the Commission Nationale de l'Informatique et des Libertés (CNIL) (National Comission on Informatics and Liberty) (DE-2014-59).
Inclusion criteria
Patients eligible for analysis were aged over 18 and under 65 years, had overweight or obesity (BMI>25 kg/m2), managed for weight loss by a PCP practicing behavioral nutrition, followed up for a minimum of 12 months after weight loss, and registered in the SPI-OPENweb® software between January 6, 2000 and November 28, 2013.
Weight loss management in behavioral nutrition
Behavioral management, dietary counseling, and weight loss follow-up are provided by PCP practicing behavioral nutrition. The number of consultations is decided by mutual agreement between the patient and his/her PCP. Dietary counseling in behavioral nutrition is based on a moderately high-protein diet providing an intake of 1.2 or 1.5 g protein/kg ideal body weight/day for a BMI>25 kg/m2 or >30 kg/m2, respectively. The diet consists of three phases, which duration varies according to practitioner discretion. During the first phase, proteins are provided five times per day by INSUDIET foods developed to provide 18-20 g of protein and less than 5 g of carbohydrate per serving. Vegetables providing less than 5 g of carbohydrate per serving are allowed. During the second phase, proteins are provided four times per day by at least 2 INSUDIET foods progressively associated with animal proteins (at least 150 g of meat, fish, or eggs per serving). A wider range of vegetables and fruits is allowed (vegetables: approximately 200 g cooked and 120 g raw). During the third phase, proteins are provided once a day by 1 INSUDIET food and by animal proteins (3 serving per day), vegetables as desired, fruits, dairy products, and cereals are allowed. The first, second, and third phases provide approximately 900, 1,150 and 1,500 kcal per day, respectively. During the first, second, and third phases, 15, 22, and 31% of daily caloric intake, respectively, are provided by carbohydrates. Cognitive behavioral therapy is based on validated behavioral questionnaires not detailed here and aim to enhance therapeutic alliance and long-term behavioral modifications.
Monitoring with the SPI-OPENweb® software
Monitoring of weight loss by PCPs was carried out using the SPI-OPENweb® software, which allowed PCPs to collect all necessary data to support patient care throughout follow-up. This included identity (name, age, and gender), anthropometric data (weight, height, hip, and waist sizes), body composition (fat mass [FM], fat-free mass [FFM]), and a food questionnaire evaluating protein intake.
Data collection
Weight and body composition were measured using bioelectrical impedance (Tanita TBF 300, Tanita BC 1000.1 or Tanita BC 420). Values including height were recorded by the PCP in the SPI-OPENweb® software that directly infer BMI and FM/FFM ratio.
Success rate
Success criterion was the minimum loss of 10% of initial weight and its maintenance beyond 12 months. Accordingly, patients were divided into three groups: patients who failed to lose 10% of their initial weight, Failure (F) group; patients who lost at least 10% of their initial weight but failed to maintain it beyond 12 months, Unsuccessful Maintenance (UM) group; and patients who lost at least 10% of their initial weight and succeeded in maintaining it at least beyond 12 months, Successful Maintenance (SM) group. Maintenance of the weight loss was the percentage of patients meeting the success criterion after the median follow-up after the 10% initial weight loss.
Determinants of weight loss
Data analyzed to identify the determinants of weight loss were follow-up characteristics: number of consultations (total or per month), duration of follow-up (months), time required to reach the 10% initial weight loss goal (months), duration of follow-up after weight loss (months), and anthropometric characteristics at inclusion: weight (kg), BMI (kg/m2), FM (kg), FFM (kg), fat percentage (%), and FM/FFM ratio. Changes between first consultation and last consultation were expressed as weight difference (kg) or percentage of initial weight loss, BMI difference (kg/m2) or BMI variation (%), FM difference (kg) or FM variation (%).
Contribution of adiposity to weight loss
The contribution of adiposity to weight loss was calculated as the ratio between FM variation (kg) and weight variation (kg) in patients who lost weight between first and last consultation. For patients who had a gain of FM, the contribution of adiposity to weight loss was considered as null.
Statistical analysis
Patients having a follow-up over the 95th centile of follow-up distribution were excluded to remove outliers that may be found in a large database like the present one. The distribution of the final sample was tested according to gender, age class (18-30; 31-40; 41-50; 51-65 years) and BMI range (25-29.9; 30-34.9; 35-39.9; over 40 kg/m2) using the non-parametric chi-square test. Sample distributions according to gender, age class, BMI, and year of inclusion were compared between the F, UM, and SM groups using a chi-square test. Both follow-up and anthropometric data were compared between groups using a general linear model test (crude and adjusted for gender, age class, BMI, and year of inclusion), as well as the absolute and relative changes in weight, BMI, and FM between first and last consultation. The contribution of FM to weight loss between first and last consultation was also compared between groups. Finally, the average change in weight (absolute and relative) was calculated every month. A P-value lower than 0.05 was considered statistically significant. Statistical analyses were performed using SAS software v9.4 (SAS Institute, Cary, NC).
Results
From January 2000 to November 2012, 17,543 eligible patients were retrieved from the database. Among them, 3,287 were excluded from the analysis for the following reasons: a single consultation not allowing to measure weight loss (n = 607), duration of follow-up less than 12 months after the 10% loss of initial weight not corresponding to analysis criteria (n = 1,844), more than 90 months (>95th centile follow-up distribution) of follow-up according to statistical analysis (n = 812), or a BMI>60 kg/m2 at inclusion or last consultation requiring special care (n = 24) (Figure 1). Consequently, data from 14,256 patients were analyzed. The large majority were women (84.6%; Table 1). The proportion of patients aged between 51 and 65 years were significantly higher than that of patients aged between 18 and 30 years (37.2% vs. 13.3%; P < 0.001). Among the patients analyzed, 49.9% were overweight and 50.1% suffered from obesity, split on 30.9% obesity, 13.0% severe obesity, and 6.17% morbid obesity (Table 1).
Figure 1.
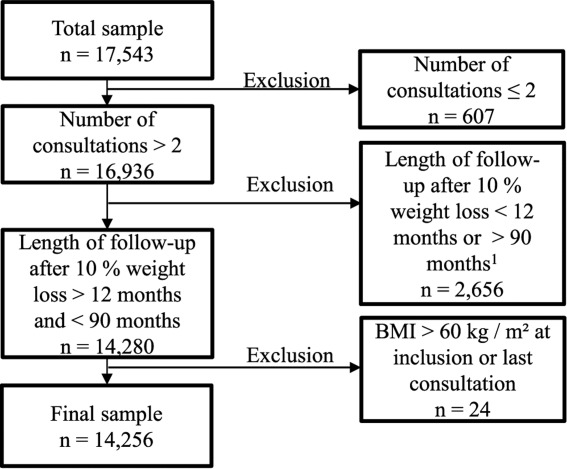
Data set flow through the screening process leading to the final sample. 190 months was the 95th centile of the distribution when the length of follow-up was calculated among the 17,543 patients.
TABLE 1.
Distribution of patients according to gender, age, and BMI
| N | Percentage, % | Pa | |
|---|---|---|---|
| Total | 14 256 | 100.00 | |
| Gender | <0.001 | ||
| Female | 12 054 | 84.55 | |
| Male | 2 202 | 15.45 | |
| Age classes (y) | <0.001 | ||
| 18-30 | 1 894 | 13.29 | |
| 31-40 | 3 292 | 23.03 | |
| 41-50 | 3 763 | 26.40 | |
| 51-65 | 5 307 | 37.23 | |
| BMI levels (kg/m2) | <0.001 | ||
| 25-29.9 | 7 114 | 49.90 | |
| 30-34.9 | 4 410 | 30.93 | |
| 35-39.9 | 1 852 | 12.99 | |
| >= 40 | 880 | 6.17 |
Non-parametric chi-square test was significant (P < 0.001) showing that the sample was not homogeneously distributed.
Success rate
About 47.6% (n = 6,784) of patients failed to lose at least 10% of their initial weight (F group) and 52.4% (n = 7,472) of patients lost at least 10% of their initial weight. Among the latest, 26.7% (n = 3,803) succeeded in maintaining the 10% initial weight loss over at least 12 months (SM group) and 25.7% (n = 3,669) did not (UM group) (Figure 2). At median follow-up (29 months) after the 10% of initial weight loss, 1,819 patients of the initial SM group were still visiting their PCPs. Among them, 63.4% met the success criterion and had still a 12% decrease in their initial weight and a 23% decrease in adiposity (data not shown).
Figure 2.
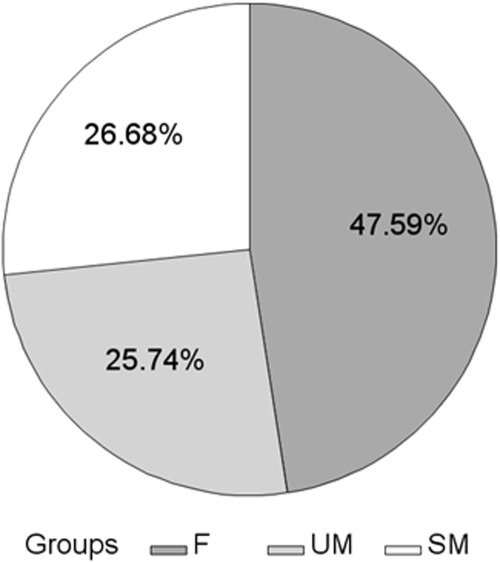
Distribution of subjects according to success in the final sample. Results are expressed as percentages of the final sample (n = 14,256). F: failure group; SM: successful maintenance group; UM: unsuccessful maintenance group.
Determinants of weight loss
Initial weight and BMI of patients were significantly higher in the SM group than in the UM and F groups (P < 0.001) (Table 2 and Figure 3). This was associated with a FM percentage significantly greater in the SM group (40.5%) than in the F (37.0%) and UM (38.5%; P < 0.001) groups (Table 3). No difference in FFM was observed at inclusion. The SM group consisted mainly of women with a mean age of 45.1 years (Table 2). On average, in this group, patients met their PCP 19.6 times over 34.2 months. There was a significantly greater number of consultations in the SM group than in the F and UM groups (P < 0.001). The mean follow-up duration was significantly shorter in the SM group than in the UM group (P < 0.001). Consequently, patients in the SM group had a higher frequency of consultations (P < 0.001) than patients in the two other groups. The achievement of the 10% initial weight loss required a shorter period in the SM group than in the UM group (P < 0.001).
TABLE 2.
Follow-up characteristics and anthropometric measures at inclusion and variation between first and last consultation in the overall population and the three success groups
| Overall (n = 14,256) |
F group (n = 6,784) |
UM group (n = 3,669) |
SM group (n = 3,803) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nmiss | Mean | SD | Nmiss | Mean | SD | Nmiss | Mean | SD | Nmiss | Mean | SD | Crude P1 | Adjusted P2 | |
| Patient characteristics | ||||||||||||||
| Females (%) | 0 | 84.55 | . | 0 | 82.8 | . | 0 | 85.61 | . | 0 | 86.67 | . | <0.001 | |
| Age (y) | 0 | 44.97 | 11.71 | 0 | 45.45 | 11.89 | 0 | 43.89 | 11.43 | 0 | 45.15 | 11.6 | <0.001 | |
| Follow-up characteristics | ||||||||||||||
| Number of consultations | 0 | 14.4 | 10.34 | 0 | 10.82a | 8.57 | 0 | 15.58b | 9.6 | 0 | 19.64c | 11.38 | <0.001 | <0.001 |
| Number of consultations3 | 0 | 12 | 0 | 9a | 0 | 13b | 0 | 17c | <0.001 | |||||
| Follow-up period, months | 0 | 35.41 | 19.34 | 0 | 34.47a | 19.63 | 0 | 38.35b | 19.48 | 0 | 34.25a | 18.39 | <0.001 | <0.001 |
| Number of consultations per month | 0 | 0.48 | 0.33 | 0 | 0.39a | 0.29 | 0 | 0.48b | 0.3 | 0 | 0.65c | 0.33 | <0.001 | <0.001 |
| Follow-up period after a weight loss of 10%, months | 6784 | 31.73 | 18.18 | 6784 | . | . | 0 | 33.36a | 18.66 | 0 | 30.16b | 17.57 | <0.001 | <0.001 |
| Number of months necessary to lose 10% of initial weight | 6784 | 4.53 | 6.4 | 6784 | . | . | 0 | 4.99a | 6.91 | 0 | 4.09b | 5.83 | <0.001 | <0.001 |
| Measures at inclusion | ||||||||||||||
| Weight (kg) | 0 | 84.26 | 16.08 | 0 | 81.9a | 14.98 | 0 | 83.81b | 15.51 | 0 | 88.89c | 17.5 | <0.001 | <0.001 |
| BMI (kg/m2) | 0 | 31.15 | 4.99 | 0 | 30.22a | 4.68 | 0 | 31.02b | 4.63 | 0 | 32.93c | 5.4 | <0.001 | <0.001 |
| Variation between first and last consultation | ||||||||||||||
| Weight, kg | 41 | −4.18 | 8.52 | 0 | 0.49a | 5.24 | 11 | −3.89b | 5.92 | 30 | −12.85c | 8.7 | <0.001 | <0.001 |
| Weight, % | 41 | −4.6 | 9.22 | 0 | 0.66a | 6.45 | 11 | −4.59b | 6.77 | 30 | −14.07c | 7.85 | <0.001 | <0.001 |
| BMI, kg/m2 | 40 | −1.54 | 3.13 | 0 | 0.19a | 1.95 | 11 | −1.43b | 2.17 | 29 | −4.75c | 3.15 | <0.001 | <0.001 |
| BMI, % | 40 | −4.6 | 9.25 | 0 | 0.66a | 6.47 | 11 | −4.58b | 6.79 | 29 | −14.08c | 7.92 | <0.001 | <0.001 |
Mean values with different superscript letters were significantly different (P < 0.05).
GLM test was used for all variables except for gender for which a chi-square was used and for the number of consultations for which median values were compared using the non-parametric Kruskal–Wallis test.
GLM tests were adjusted for age, gender, year of recruitment, and BMI level at inclusion, except for the BMI at the inclusion which was adjusted for age, gender, and year of recruitment.
Median values.
F: failure group; SM: successful maintenance group; UM: unsuccessful maintenance group.
Figure 3.
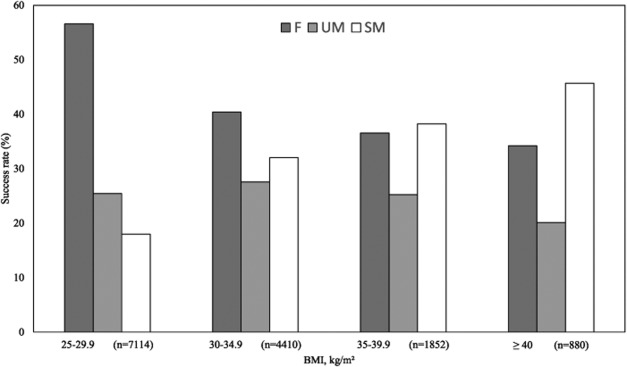
Success rate according to BMI range. All chi-square tests were significant. F: failure group; SM: successful maintenance group; UM: unsuccessful maintenance group.
TABLE 3.
Variation of body composition between first and last consultation in the overall population and the three groups
| Overall (n = 14,256) |
F group (n = 6,189) |
UM group (n = 3,473) |
SM group (n = 3,488) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nmiss | Mean | SD | Nmiss | Mean | SD | Nmiss | Mean | SD | Nmiss | Mean | SD | Crude P1 | Adjusted P2 | |
| FM, kg | 0 | 32.74 | 9.09 | 0 | 30.99a | 8.59 | 0 | 32.54b | 8.63 | 0 | 36.03c | 9.51 | <0.001 | <0.001 |
| FM, % | 0 | 38.32 | 6.74 | 0 | 36.98 | 7.01 | 0 | 38.52 | 6.16 | 0 | 40.53 | 6.2 | <0.001 | <0.001 |
| FFM, kg | 0 | 50.59 | 8.96 | 0 | 50.09a | 8.89 | 0 | 50.57a | 8.94 | 0 | 51.52a | 9.05 | <0.001 | 0.3130 |
| FM/FFM ratio | 0 | 0.65 | 0.15 | 0 | 0.62a | 0.15 | 0 | 0.65b | 0.15 | 0 | 0.70c | 0.15 | <0.001 | <0.001 |
| FM variation, kg | 0 | −2.9 | 6.38 | 0 | 0.45 | 4.29 | 0 | −2.7 | 4.89 | 0 | −9.05 | 6.26 | <0.001 | <0.001 |
| FM variation, % | 0 | −7.81 | 18.3 | 0 | 1.92 | 14.83 | 0 | −8.21 | 14.7 | 0 | −24.69 | 14.3 | <0.001 | <0.001 |
| Contribution of adiposity in the weight loss | 3,850 | 84.67 | 116.6 | 3016 | 99.32 | 176.37 | 722 | 79.54 | 89.2 | 112 | 75.07 | 37.73 | <0.001 | <0.001 |
Mean values with different superscript letters were significantly different (P < 0.05).
GLM tests.
GLM tests adjusted for age, gender, year of recruitment and BMI level at inclusion. F: failure group; SM: successful maintenance group; UM: unsuccessful maintenance group.
Weight change
The mean weight loss in the SM group was significantly greater than in the UM group (14.1% vs. 4.59% of their initial weight at last consultation; P < 0.001) (Table 2). This decrease in weight corresponds to 4.75 BMI units for patients in the SM group and 1.43 BMI units for patients in the UM group (P < 0.001). The FM decrease was significantly greater in the SM group than in the UM group (−24.7% vs. −8.21%; P < 0.001) (Table 3). Contribution of adiposity to the weight loss was of 75.1% in the SM group and 79.5% in the UM group. For all groups, weight variations followed very similar patterns over time (Figure 4). Patients of the SM group had a greater initial weight loss, expressed as percentage of initial body weight, than patients of the UM group (Figure 4B). Over time, a trend to regain weight was observed in each group. The weight curve of the UM group crossed the −10% initial weight threshold 10 months after inclusion, while the weight curve of the SM group remained below this threshold throughout the study.
Figure 4.
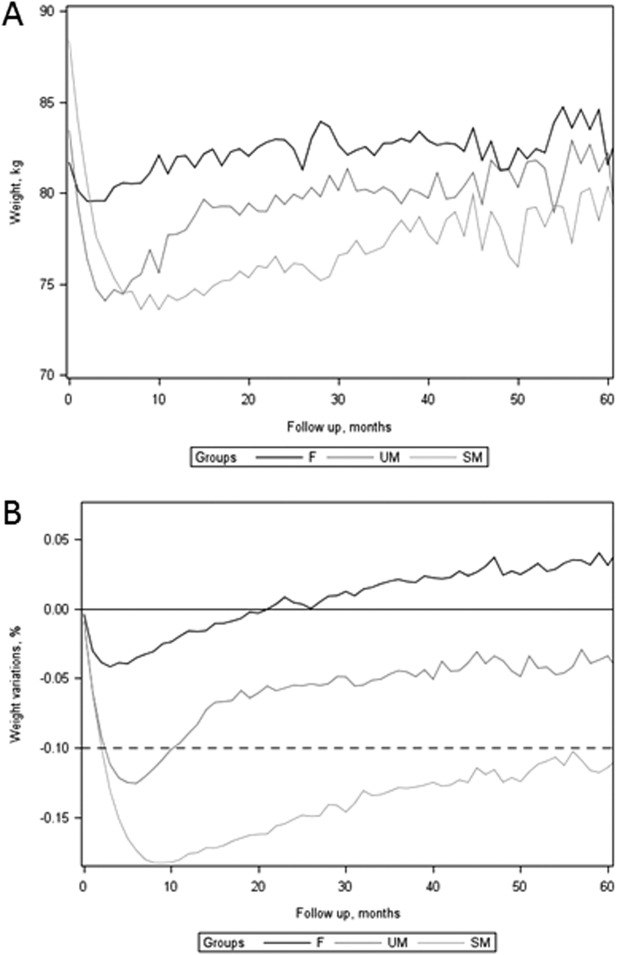
(A) Absolute weight and (B) weight variation during follow-up in the three groups. F: failure group; SM: successful maintenance group; UM: unsuccessful maintenance group.
Discussion
In this retrospective study, behavioral nutrition allowed 26.7% of patients with overweight or obesity to lose at least 10% of their initial weight and to maintain the weight loss for at least 12 months. This weight loss was associated with a significant decrease in adiposity. In a previous retrospective study, it was shown that ∼21% of overweight subjects (BMI>27 kg/m2) reporting intentional weight loss were maintainers at 1 year (13). In the National Health and Nutrition Examination Survey (prospective study), 20% of adults with obesity achieved to lose 10% of their initial weight for 12 months (14). Association of intervention diet and behavioral treatment is known to enable ∼10% reduction of body weight as shown in the Look AHEAD study at 12 (15) and 48 months (16) or in the IDEA study at 18 months (17). In view of all these results, with more than 20% of success, behavioral nutrition care combining dietary changes (carbohydrate restriction, moderately high protein consumption supported by supplementary foods) with individual behavioral support belongs to the pool of effective weight loss strategies currently available.
In our study, patients with a higher initial BMI were more likely to maintain a 10% initial weight loss. A recent meta-analysis (18) investigated the effect of lifestyle intervention (diet and exercise) on weight change according to BMI classes. Although overweight patients loss less absolute weight than patients with obesity, no significant differences in the weight change percentage among BMI classes was described (18). Furthermore, in the Weight Loss Maintenance Trial, a higher initial weight was a predictor of the weight loss (19) as was shown in other weight loss interventions (20,21). For now, we can only be speculative about factors that could explain the better results obtained in patients with a high BMI: intensity of caloric restriction with respect to resting metabolism, number of attempts to lose weight, stronger motivation or a more intense support by relatives could be involved. At any rate, our results suggest that behavioral nutrition enables a successful weight loss in patients with obesity and severe obesity.
The second determinant of success identified in our study was the greater consultation frequency for successful patients. Previously, a meta-analysis had shown that a more frequent scheduled support meeting was an independent predictors of greater weight loss (22). In the Look AHEAD study, subjects randomized in the intensive lifestyle intervention were advised to follow both group and individual meetings devoted to dietary counseling and behavior modification. At 12 months, participants having attended a higher number of sessions showed a greater weight loss (15). Later in this study, patients having maintained at least a 10% weight loss were characterized by greater contacts with care providers during years 2-4 (16). Similarly, in the Diabetes Prevention Program, subjects receiving dietary training, physical exercise, and behavior modification were those achieving a greater weight loss as well as a greater reduction of diabetes incidence, compared with those in the metformin or placebo groups (6). Transposition of this clinical program to primary care confirmed the impact of coach follow-up to weight loss at 15 (23) and 24 months (24). Most of the trials associating dietary restriction and behavioral treatment offer individual and group sessions. Although it has been shown that group sessions for weight loss are more effective than individual sessions at 6 months (25), this asset was not confirmed at 12 or 36 months (26). As seen above, we observed that an individual follow-up alone is effective also. In most of the interventional studies managing weight loss, subjects received care provided by coaches with training in nutrition, exercise, or behavior modification but not by PCPs in charge of the patients. In addition to dietary advice, the behavioral nutrition program has the particularity to educate PCPs. They are trained by expert colleagues and follow course of increasing levels to become more specialized. Nevertheless, PCPs were able to manage patients with overweight or obesity immediately after the first training session. This is in agreement with methods of the POWER-UP study whose aim is to manage obesity in primary care practice and in which PCPs received a 6-8 h training (27). PCPs education is of paramount importance since they are encouraged by authorities to manage obesity in routine clinical care whereas most of them consider being not well prepared for it (28). In our study, based on the experience of PCPs practicing behavioral nutrition, we obtained a long-term weight loss at least as efficiently as in multicentric interventional studies. Thus, coaching by PCPs could allow patients to maintain a healthier weight.
Analysis of the weight loss dynamic showed that successful subjects were those who achieved the 10% weight loss at a faster rate (mean: 4.09 months). This is in agreement with previous studies showing that significant individual weight loss differences already exist after the first 2 weeks of diet (29) and that initial weight loss at 1 month is a strong predictor of weight loss percentage at 12 months (30). Moreover, in the TOURS study, patients who lost faster obtained a greater weight reduction and a longer term maintenance (31). As in numerous studies, a trend of weight regain was observed in the different groups (weight regain was not quantified). However, a great percentage of patients were still successful at 29 months. Maintenance may be related to the dietary counseling not providing a very-low-calorie diet known to promote weight regain after fast initial weight loss (32) as well as the long-term follow-up period facilitating the behavioral modification maintenance (31).
Dietary counseling in behavioral nutrition consists of carbohydrate restriction and moderately high protein intake. The efficacy of this kind of dietary recommendations for weight loss maintenance has been shown in the DIOGENE study (33,34). Recently, a meta-analysis comparing weight loss among named diet programs in interventional studies showed that low-carbohydrate diets and low-fat diets had a high probability to induce long-term weight loss, this effect being enhanced by the integration of behavioral management (35). Diet for weight loss should be designed in order to limit loss of FFM that cannot be avoided, although subjects aim at losing FM. The FFM must be preserved since non-adipose tissues are implicated in resting metabolic rate, regulation of body temperature, preservation of skeletal integrity, and maintenance of function and quality of life (36). In the present study, for patients meeting the success criterion, FM contributed for 75% of the weight loss, meaning a 25% FFM loss. This is consistent with most weight loss methods using caloric restriction that show a FFM loss closed to 25% (37,38).
To our knowledge, our study focusing on overweight and obesity managed by PCPs in real life is one of the first dealing with such a large sample. Since obesity studies are characterized by a high dropout (39), a large sample is often required to observe the effectiveness of a program care. In our study, the median follow-up for the entire population was 29 months, while most studies are conducted in shorter periods. Thus, obesity care with behavioral nutrition allowed maintaining patients’ motivation in the long term. In the observational NWCR in which data were recorded by patients themselves and not by PCPs, determinants of weight loss mainly concerned patient behavior: low-calorie and low-fat diets, high level of physical activity, frequent weight monitoring, breakfast every day or 2.5 meals/week in restaurants (40). In complement of this, determinants identified in the present study were initial BMI, consultation frequency, speed of weight loss, and follow-up duration. With time, we observed a dropout effect with a slow weight regain, suggesting that it could be of great interest to stimulate patients’ involvement in the long term. The retrospective design of our study presents some limitations. First, since our analyses were based on health records that had previously been collected, some data that would have been of interest for our study i.e., comorbidities, biological variables, quality of life, were not available. Second, the large number of PCPs implicated might have increased the chance of missing data. Moreover, the statistical analysis performed was descriptive and thus, did not allow us to establish causation between determinants of weight loss and success. Another limitation was the lack of food record and especially proteins quantification allowing the comparison of our results to other diets.
In conclusion, overweight and obesity care by PCPs with behavioral nutrition facilitates weight loss maintenance. Slightly more than a quarter of the patients included in this large cohort maintained a 10% initial weight loss for more than 12 months. It appears that initial BMI, frequency of visits to PCPs, and intensity of initial weight loss are all determinants of success. Further clinical studies will be needed to evaluate the efficacy of behavioral nutrition in terms of metabolic parameters such as glycemia and insulinemia.
References
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121:21–33. doi: 10.3810/pgm.2009.11.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Vliet-Ostaptchouk JV, Nuotio ML, Slagter SN, et al. The prevalence of metabolic syndrome and metabolically healthy obesity in europe: a collaborative analysis of ten large cohort studies. BMC Endocr Disord. 2014;14:1–13. doi: 10.1186/1472-6823-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sjostrom L. Review of the key results from the swedish obese subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–234. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 8. Haute Autorité de Santé. Surpoids et obésité de l'adulte: prise en charge médicale de premier recours. 2011.
- 9. National Institutes of Health. The practical guide, identification, evaluation, and treatment of overweight and obesity in adults. 2000.
- 10.Kroeger CM, Hoddy KK, Varady KA. Impact of weight regain on metabolic disease risk: a review of human trials. J Obes. 2014;2014:1–8. doi: 10.1155/2014/614519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramage S, Farmer A, Eccles KA, McCargar L. Healthy strategies for successful weight loss and weight maintenance: a systematic review. Appl Physiol Nutr Metab. 2014;39:1–20. doi: 10.1139/apnm-2013-0026. [DOI] [PubMed] [Google Scholar]
- 12.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–341. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 13.McGuire MT, Wing RR, Hill JO. The prevalence of weight loss maintenance among american adults. Int J Obes Relat Metab Disord. 1999;23:1314–1319. doi: 10.1038/sj.ijo.0801075. [DOI] [PubMed] [Google Scholar]
- 14.Nicklas JM, Huskey KW, Davis RB, Wee CC. Successful weight loss among obese U.S. adults. Am J Prev Med. 2012;42:481–485. doi: 10.1016/j.amepre.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadden TA, West DS, Neiberg RH, et al. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring) 2009;17:713–722. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011;19:1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barte JC, Veldwijk J, Teixeira PJ, Sacks FM, Bemelmans WJ. Differences in weight loss across different BMI classes: a meta-analysis of the effects of interventions with diet and exercise. Int J Behav Med. 2014;21:784–793. doi: 10.1007/s12529-013-9355-5. [DOI] [PubMed] [Google Scholar]
- 19.Hollis JF, Gullion CM, Stevens VJ, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008;35:118–126. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Packianathan I, Sheikh M, Boniface D, Finer N. Predictors of programme adherence and weight loss in women in an obesity programme using meal replacements. Diabetes Obes Metab. 2005;7:439–447. doi: 10.1111/j.1463-1326.2004.00451.x. [DOI] [PubMed] [Google Scholar]
- 21.Rautio N, Jokelainen J, Saaristo T, et al. Predictors of success of a lifestyle intervention in relation to weight loss and improvement in glucose tolerance among individuals at high risk for type 2 diabetes: the FIN-D2D project. J Prim Care Community Health. 2013;4:59–66. doi: 10.1177/2150131912444130. [DOI] [PubMed] [Google Scholar]
- 22.Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EM. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med. 2007;147:41–50. doi: 10.7326/0003-4819-147-1-200707030-00007. [DOI] [PubMed] [Google Scholar]
- 23.Xiao L, Yank V, Wilson SR, Lavori PW, Ma J. Two-year weight-loss maintenance in primary care-based diabetes prevention program lifestyle interventions. Nutr Diabetes. 2013;3:3–1. doi: 10.1038/nutd.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma J, Yank V, Xiao L, et al. Translating the diabetes prevention program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med. 2013;173:113–121. doi: 10.1001/2013.jamainternmed.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renjilian DA, Perri MG, Nezu AM, McKelvey WF, Shermer RL, Anton SD. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol. 2001;69:717–721. [PubMed] [Google Scholar]
- 26.Cresci B, Tesi F, La FT, et al. Group versus individual cognitive-behavioral treatment for obesity: results after 36 months. Eat Weight Disord. 2007;12:147–153. doi: 10.1007/BF03327591. [DOI] [PubMed] [Google Scholar]
- 27.Wadden TA, Volger S, Tsai AG, et al. Managing obesity in primary care practice: an overview with perspective from the POWER-up study. Int J Obes (Lond) 2013;37(Suppl 1):S3–S11. doi: 10.1038/ijo.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thuan JF, Avignon A. Obesity management: attitudes and practices of french general practitioners in a region of france. Int J Obes (Lond) 2005;29:1100–1106. doi: 10.1038/sj.ijo.0803016. [DOI] [PubMed] [Google Scholar]
- 29.Postrach E, Aspalter R, Elbelt U, et al. Determinants of successful weight loss after using a commercial web-based weight reduction program for six months: cohort study. J Med Internet Res. 2013;15:e219. doi: 10.2196/jmir.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortner HM, Mucalo I, Hrabac P, Matic T, Rahelic D, Bozikov V. Factors predictive of drop-out and weight loss success in weight management of obese patients. J Hum Nutr Diet. 2014;28:24–32. doi: 10.1111/jhn.12270. [DOI] [PubMed] [Google Scholar]
- 31.Nackers LM, Ross KM, Perri MG. The association between rate of initial weight loss and long-term success in obesity treatment: does slow and steady win the race? Int J Behav Med. 2010;17:161–167. doi: 10.1007/s12529-010-9092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGuire MT, Wing RR, Klem ML, Lang W, Hill JO. What predicts weight regain in a group of successful weight losers? J Consult Clin Psychol. 1999;67:177–185. doi: 10.1037//0022-006x.67.2.177. [DOI] [PubMed] [Google Scholar]
- 33.Aller EE, Larsen TM, Claus H, et al. Weight loss maintenance in overweight subjects on ad libitum diets with high or low protein content and glycemic index: the DIOGENES trial 12-month results. Int J Obes (Lond) 2014;38:1511–1517. doi: 10.1038/ijo.2014.52. [DOI] [PubMed] [Google Scholar]
- 34.Larsen TM, Dalskov SM, van BM, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102–2113. doi: 10.1056/NEJMoa1007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014;312:923–933. doi: 10.1001/jama.2014.10397. [DOI] [PubMed] [Google Scholar]
- 36.Marks BL, Rippe JM. The importance of fat free mass maintenance in weight loss programmes. Sports Med. 1996;22:273–281. doi: 10.2165/00007256-199622050-00001. [DOI] [PubMed] [Google Scholar]
- 37.Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev. 2010;68:375–388. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 38.Chaston TB, Dixon JB, O'Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond) 2007;31:743–750. doi: 10.1038/sj.ijo.0803483. [DOI] [PubMed] [Google Scholar]
- 39.Colombo O, Ferretti VV, Ferraris C, et al. Is drop-out from obesity treatment a predictable and preventable event? Nutr J. 2014;13:1–7. doi: 10.1186/1475-2891-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]


