Abstract
Until recently, the general belief was that non-nutritive sweeteners (NNS) were healthy sugar substitutes because they provide sweet taste without calories or glycemic effects. However, data from several epidemiological studies have found that consumption of NNS, mainly in diet sodas, is associated with increased risk to develop obesity, metabolic syndrome, and type 2 diabetes. The main purpose of this article is to review recent scientific evidence supporting potential mechanisms that explain how “metabolically inactive” NNS, which have few, if any, calories, might promote metabolic dysregulation. Three potential mechanisms, which are not mutually exclusive, are presented: 1) NNS interfere with learned responses that contribute to control glucose and energy homeostasis, 2) NNS interfere with gut microbiota and induce glucose intolerance, and 3) NNS interact with sweet-taste receptors expressed throughout the digestive system that play a role in glucose absorption and trigger insulin secretion. In addition, recent findings from our laboratory showing an association between individual taste sensitivity to detect sucralose and sucralose’s acute effects on metabolic response to an oral glucose load are reported. Taken as a whole, data support the notion that NNS have metabolic effects. More research is needed to elucidate the mechanisms by which NNS may drive metabolic dysregulation and better understand potential effects of these commonly used food additives.
Keywords: artificial sweeteners, intense sweeteners, non-caloric sweeteners, sucralose, metabolism, glycemic control
1. Introduction
It is generally believed that non-nutritive sweeteners (NNS) are healthy substitutes for sugars because they provide sweet taste without calories or glycemic effects (1). Currently, six NNS (sucralose, aspartame, saccharin, acesulfame potassium, neotame and advantame) are approved to be used as a sweetener in food, and two (steviol glycosides, and Luo han guo extract) are generally recognized as safe and permitted for use in food by the US Food and Drug Administration (FDA) (2). Although these compounds have very different chemical structure, they all have in common that they very potently activate some of the multiple potential ligand binding sites of the heterodimeric T1R1+T1R3 sweet-taste receptor in human subjects (3). Before the FDA granted final approval of NNS, a battery of toxicology and clinical studies in a number of species, including humans, were conducted to demonstrate that NNS are generally safe and well-tolerated. In addition, the data from several studies, conducted in human subjects with and without diabetes, found that even extremely high doses of sucralose or aspartame (many times above estimated maximum intake), did not affect blood glucose, C-peptide, or HbA1c concentrations (e.g., 1, 4–6). However, data from several epidemiological studies have found that consumption of NNS, mainly in diet sodas, is not linked to better health outcomes (reviewed in 7 and 8). In fact, some studies found positive associations between NNS consumption and weight gain, metabolic syndrome, and type 2 diabetes (9–14), although other studies did not (e.g., 15, 16; reviewed in 17).
At least two hypotheses, not mutually exclusive, might explain the paradoxical association between consuming NNS and adverse metabolic outcomes: 1) reverse causation, i.e. individuals who are likely to develop metabolic disease or are gaining weight choose to consume NNS as a strategy to reduce sugar and caloric intake; and 2) NNS are not physiologically inert but affect biological processes involved in regulating energy and glucose homeostasis. This article reviews recent scientific evidence supporting potential mechanisms that explain how “metabolically inactive” NNS, which have few, if any, calories, might promote metabolic dysregulation and present some findings from our laboratory in which we explore associations between individual taste sensitivity to detect sucralose and sucralose acute effects on metabolic response to an oral glucose load.
2. Potential mechanism underlying the association between the use of nonnutritive sweetener and adverse metabolic outcomes
The list of potential mechanisms described below is not collectively exhaustive, nor mutually exclusive. In fact some of these mechanisms may act synergistically.
2.1. NNS interfere with learned responses that contribute to control glucose and energy homeostasis
Much of the evidence behind this concept derives from the seminal work by Swithers and Davidson in a rodent model (8, 18–20, reviewed in 21). Using the Pavlovian conditioning principles as the foundational context of their research, they hypothesize that the use of NNS weakens the ability of sweet taste to predict energy and evoke autonomic and endocrine learned responses that prepare the digestive tract for optimal process of ingested food, such as the cephalic response (19). In their elegant animal model, rats receive differential experience with a sweet taste that either predicts (glucose) or does not predict (saccharine, acesulfame K, or stevia) increased calories. Data from a series of experiments show that compared with rats that consume a diet always sweetened with glucose (i.e. sweet predicts calories), those consuming a diet where sweet taste does not reliable predict calories (i.e. sweetened with NNS) are heavier, accumulate more body fat, exhibit a diminished ability to compensate for calories ingested in a pre-meal, and have a reduced thermic response to eating a novel meal (18, 19, 22, 23). Consistent with their hypothesis that NNS weaken cephalic responses, compared with rats in the control group (i.e. sweet predicts calories), animals consuming a diet sweetened with NNS responded with relative hyperglycemia when given a novel sweet-tasting test meal or a standard glucose tolerance test (24). Importantly, this altered glucoregulatory response to a glucose load, which was associated with reduced circulating levels of the incretin hormone glucagon like peptide-1 (GLP-1), was observed when the glucose load was given orally but not when glucose was infused directly into the stomach by gavage (i.e. bypassing oral taste stimulation) (24). That previous experience with NNS affected glucoregulatory responses to a glucose load when glucose was tasted, but not when directly released in the stomach, further supports their hypothesis that it is disruptions in learned responses elicited by tasting sweetness, not in post-absorptive consequences of consuming sugar, that is altering glucose homeostasis in this rodent model.
Early studies by Deutsch (25) also strongly support the theory that in rodents, long term exposure to NNS ingestion weakens cephalic responses triggered by sweet taste. Following up from findings that saccharin ingestion potentiated hypoglycemic effects of exogenous administered insulin (26). Deutsch tested the hypothesis that the sweet taste of saccharin elicited a conditioned hypoglycemic response that could be extinguished by giving animals long term access to the non-caloric sweetener (25). He showed that, consistent with the conditioning theory, saccharin ingestion alone lead to relative hypoglycemia in animals with little to no prior experience with NNS. However, such a conditioned hypoglycemic response was extinguished after animals had long-term access to saccharin (i.e. the experience of tasting sweetness without the subsequent rise in blood sugar) (25).
The hypothesis that exposure to NNS weakens cephalic responses to sweet food has not been tested in human subjects, and future research in this area is warranted. There are important differences between human and rodents on the type of stimuli that elicit cephalic responses. Sweet liquids, either caloric or non-caloric, are good stimuli to elicit cephalic responses in rats (27–29) but generally do not elicit cephalic responses in human subjects (30–32). However, given that 1) classical or Pavlovian conditioning is one of the most basic forms of learning (demonstrated even in invertebrates such as the Aplysia (33), 2) cephalic responses are elicited when people taste and chew food (34) (reviewed in 35), and 3) studies in human subjects show that cephalic responses are required for a normal postprandial glucose tolerance (36, 37), there is great potential that the above theory, which posits that NNS interfere with learned responses that contribute to control glucose and energy homeostasis, is applicable to human subjects.
2.2. NNS interfere with gut microbiota and induce glucose intolerance
Perhaps the one unquestionable benefit of NNS is that they help reduce dental cavities (38). The anticavity effect of saccharin, sucralose, aspartame, and stevia is not only explained by the fact that these compounds are resistant to fermentation by oral bacteria, but also because of their demonstrated bacteriostatic effects (39–41). Data from studies in vitro (42), in animal models (43–45), and from a small study in human subjects (45), suggest that the effects of these NNS are not limited to the microbial inhabitants of the mouth, but extends to those in the gut, thereby affecting host metabolic phenotype and disease risk (46). Pioneer work from the group of Schiffman showed that 12 weeks of exposure to Splenda (a NNS compraising 1% w/w sucralose with glucose (1% w/w) and maltodextrin (94% w/w) as fillers) significantly altered gut microbiota composition by decreasing beneficial bacteria and was associated with weight gain in rats (43). In a recent work, Suez et al. confirmed and extend these findings by identifying a microbe-mediated mechanism by which NNS might influence metabolism (45). Suez et al. showed that 11 weeks exposure to saccharin, sucralose, or aspartame induced higher glucose excursions after a glucose load than those in control animals non exposed to NNS, and that such metabolic phenotype, at least for saccharin (which due to being the one NNS that more strongly affected glycemic responses in mice was studied further), was mediated by alteration of gut microbiota. Their elegant animal model truly determined causation because they demonstrated that saccharine induced hyperglycemia was transferable to germ-free mice that had never been exposed to saccharin in their life but that received a fecal transplant from saccharin-fed mice, or from microbiota incubated in vitro in the presence of saccharin (45). Further, they exposed seven young healthy volunteers who were not regular users of NNS, to one week of the FDA’s maximum acceptable daily saccharin intake and evaluated their responses to an oral glucose tolerance test daily. They found that regular saccharin exposure in most subjects (i.e. responders), but not in all of them, increased glycemic responses to a glucose load. Congruent with findings from their animal model, the transplant of stool from human subjects of the responders group induced glucose intolerance in recipient germ-free mice (45). Noteworthy, the inclusion of a control group for the exposure to saccharin in human subjects would have strengthened the conclusion of the study. Because such a control group was not included in the design, it is unclear whether some healthy individuals exposed to 7 consecutive oral glucose tolerance tests (i.e. daily consumption of 75 grams of glucose) would have developed changes in glucose metabolism in the absence of saccharin, and whether transplant of stools from such a group would have caused glucose intolerance in germ-free mice.
Consistent with the findings from Suez et al., Palmnas and collaborators showed that 8 weeks of aspartame exposure (in a dose equivalent to human subjects consuming ~ 2–3 diet soft drinks per day) perturbed gut microbiota and resulted in elevated fasting glucose levels and impaired insulin tolerance in rats (44). However, the mechanism by which aspartame perturbed gut microbiota is unclear, as aspartame is metabolized before reaching the colon by intestinal esterases and peptidases in to amino acids and methanol (47).
2.3. NNS interact with sweet-taste receptors in the digestive system that play a role in glucose absorption and trigger insulin secretion
2.3.1. Taste receptors are expressed in tissues beyond the tongue
One of the most exciting discoveries in recent years in the field of the chemical senses is the finding of taste receptors in non-taste tissues (48–50). Data obtained from studies in mouse models in vivo and in vitro and human duodenal L cells in vitro (48,49) strongly support the hypothesis that the sweet taste receptor subunit T1R3 coupled to the taste G protein alpha-gustducin, underlie at least one of the components of sugar sensing in the gut. Mice lacking alpha-gustducin or T1r3 show a severely blunted incretin response to glucose challenge (48, 51). Incretins (GLP-1 and glucose dependent insulinotropic peptide: GIP) are gut hormones that once released into the bloodstream stimulate pancreatic beta-cells to secrete insulin, among other effects (reviewed in 52). The so-called “incretin effect”, first described in the 60’s refers to the fact that an oral glucose load elicits a remarkably greater insulin response than an intravenous glucose load even when both loads are matched to cause identical blood glucose levels (53). That taste-signaling pathways in the gut intervene in the “incretin effect” is further supported by two observations. First, lactisole, a human sweet taste receptor antagonist, completely block GLP-1 release in vitro (48, 49), and significantly reduces GLP-1 secretion in response to intraduodenal or intagrastic glucose administration in human subjects (54, 55). Second, alpha-gustducin knockout mice have significantly disrupted glucose homeostasis both after a glucose challenge and after post-fasting feeding on chow (48).
In addition to its important function of regulating GLP-1 secretion, sweet-taste signaling pathways in the gut may play a key role in the regulation of glucose absorption from the intestinal lumen into enterocytes. Data obtained in rodents suggest that intestinal sweet taste receptors control both active glucose absorption, by modulating expression of sodium-dependent glucose transporter isoform 1 (SGLT1) (49), and passive glucose absorption, by modulating apical glucose transporter 2 (GLUT2) insertion to the intestine (50). Unlike wild type, knockout mice lacking either alpha-gustducin or T1r3 failed to up-regulate SGLT1 intestinal expression and glucose absorptive capacity when exposed to a high carbohydrate diet (70% sucrose) (49). In fact, recent data suggest that sweet taste receptor may contribute to the incretin response by activating SGLT1 (56). Consistent with findings from previous research that pharmacologically blocked SGLT1 activity (57), data from research studies using SGLT1 knock out mice determined that SGLT1 plays a critical role for intestinal glucose absorption and incretin release (56).
2.3.2. NNS and metabolic function in cell systems and animal models
The discovery of taste receptors in the gastrointestinal tract revived old speculations about the possibility that NNS could have post-ingestive effects (58). Supporting this hypothesis, results from studies conducted in cell systems and animal models show that NNS, like sugars, activate sweet taste receptors localized in enteroendocrine cells and pancreatic β-cells, which trigger the secretion of incretins (48, 49) and insulin (59–62), respectively. The sucralose dose-response for incretin release from L-cells is non-linear (0.004 mM to 5 mM sucralose stimulates GLP-1 release, but 20 mM sucralose does not), which might explain, at least in part, why studies that used sucralose doses many times above the estimated maximum intake (4 to 6 times) did not detect metabolic effects of sucralose ingestion. In addition, data from studies conducted in animal models demonstrate that the interaction of NNS with sweet taste receptors expressed in enteroendocrine cells increases both active and passive intestinal glucose absorption by upregulating the expression of sodium-dependent glucose transporter isoform 1 (SGLT1) (49, 63, 64) and increasing the translocation of glucose transporter 2 to the apical membrane of intestinal epithelia (50), and that NNS dietary supplementation increases body adiposity and cause hyperinsulinemia and insulin resistance in mice with diet-induced obesity (65).
3. NNS and metabolic function in human subjects
Data from four studies conducted in human subjects support the potential importance of NNS in regulating glucose homeostasis. The acute consumption of NNS, namely, a diet soda, or a small amount of sucralose (24 mg of sucralose in 200 ml of water) immediately before an oral glucose load significantly enhanced GLP-1 secretion in healthy children and young overweight/obese adults (66–68), but not in subjects with type 2 diabetes (T2D) (67, 68). Furthermore, we have recently found that the ingestion of sucralose, the most commonly used NNS, affects the glycemic response to an oral glucose load and increases both peak plasma glucose concentration and glucose-stimulated insulin secretion in subjects with obesity (69). We also found that sucralose ingestion tended to increase plasma GIP concentration (P=0.08), and, suggesting that acute sucralose intake could promote insulin resistance, we found that ~20% higher than normal concentrations of insulin were required to maintained same glycemia when obese subjects consumed sucralose than when they consumed water before glucose ingestion (69).
In contrast, the results from studies conducted in healthy lean adults have reported that sucralose does not affect glycemic or hormonal responses to the ingestion of glucose or other carbohydrates (70–74). The reason(s) for the discrepancy between the results from these studies and our own data (69) is not clear, but could be related to differences in study subjects and the inclusion of subjects who were regular users of NNS in the other studies. We specifically study subjects with obesity because 1) NNS are often promoted to help decrease calorie intake and facilitate weight management in this population; 2) the prevalence of NNS use is higher in this population than in lean subjects (36% vs. 22% (75)); and 3) data from animal models suggest that obese subjects may be the most affected by NNS consumption (20). In addition, we purposely tried to study a homogeneous group of subjects by only including those who were: i) “insulin sensitive” based on homeostasis model assessment of insulin resistance (HOMA-IR) ≤ 2.6, and ii) not regular users of NNS. Controlling for the use of NNS when evaluating potential “acute” metabolic effects of NNS is critical because, as described above, there is considerable evidence in support of the hypothesis that chronic NNS ingestion have biological activity. It has been shown that chronic NNS ingestion 1) upregulates the expression of SGLT1, which in turn increases the initial rate of Na+-dependent glucose uptake in three different mammalian species (mice (49), pigs (63), and cows (76)), and 2) increases the glycemic response to an oral glucose load in rodents (24, 44, 45, 65) and in human subjects, at least for the case of 7 days of exposure to maximum acceptable daily intake of saccharin (45).
4. Tongue and gut endocrine cells
In addition to the recent discovery that taste receptor-like cells are present in the digestive system, it has also been shown that functional gut hormones are expressed in the tongue of rodents (77–81) and macaques (80). For example, GLP-1 and its receptor (GLP-1R) are expressed in taste buds, and their secretion modulates sweet and savory taste sensitivity in mice (80). Furthermore, it has been proposed that a fraction of cephalic-phase rise in GLP-1 levels in rodents is directly released from taste cells into the bloodstream (79). A recent study in cultured human taste cells show that stimulation with very small concentrations of a free fatty acid, triggers GLP-1 release, just like observed in intestinal endocrine cells (82). These observation suggest that functional gut hormones are also expressed in the tongue of human subjects.
The similarities in the molecular mechanisms of taste signal transduction in the tongue and nutrient signal transduction in the gut suggest that the study of taste perception can provide novel insights into chemical sensing mechanisms in the gut that regulate metabolic function. For example, healthy individuals with a family history of type 2 diabetes have a significant impairment in taste detection that is specific to glucose (83). We recently tested the hypothesis that individual differences in the perception of sucralose sweetness would correlate with the effects of sucralose on metabolic responses to a glucose load (e.g., the higher the taste sensitivity to detect sucralose, the greater the effect of sucralose on glycemic responses). To test this hypothesis, we evaluated subjects’ taste sensitivity to detect sucralose and sucrose by using a two-alternative, forced-choice staircase procedure (84, 85) in 16 of the 17 subjects who completed the metabolic studies in which sucralose or water was consumed immediately before a glucose load (see 69). Two-alternative, forced-choice staircase procedure provides an accurate and reliable assessment of taste detection thresholds and is recommended as the method of choice to determine individual sensitivity to taste (86). It is important to note that a detection threshold is the lowest concentration of taste stimuli that a subject can detect, and is below an individual’s threshold for conscious perception (i.e. when performing this task, subjects detect that a taste stimulus is different than water, but do not recognize a sweet taste when sucrose or NNS detection thresholds are being measured). Therefore, taste detection thresholds are resistant to subjective response bias that could be originated by exposure to sucralose during the metabolic study. We found that, consistent with the literature, detection thresholds for sucralose were ~750 times lower than for sucrose (sucralose: 0.010± 0.001 mM vs. sucrose: 7.5± 2.2 mM; unpublished observation). Supporting our hypothesis, we found a significant correlation between individual differences in sucralose taste detection thresholds and the effects of sucralose on glycemic responses (r= −0.51, n=16, P=0.04; Figure 1; unpublished observation) such that the higher the sensitivity to detect sucralose taste (i.e., the smaller the amount of sucralose that is detectable as “a taste different than water”), the greater was the difference in the glucose peak between the sucralose and water (control) conditions (i.e. bigger the effect of sucralose on glucose peak responses to a glucose load). Although the inference of molecular mechanisms of taste perception from psychophysical data has major limitations, these data are consistent with the hypothesis that individual differences in signaling pathways in taste receptor (or taste receptor-like) cells affect, at least in part, both sensing of sucralose in the mouth and sucralose acute metabolic activity in the gut. These data, although indirectly, add to the evidence of a mechanistic link between taste perception and metabolism.
Figure 1.
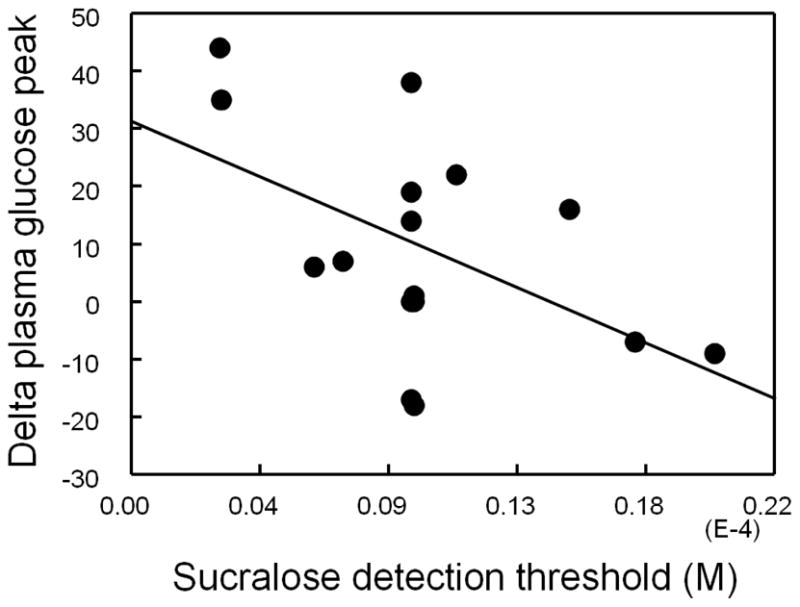
Correlation between sucralose detection thresholds and sucralose effects on plasma glucose peak concentration (i.e. difference between plasma glucose peak concentrations on the day that sucralose preceded the glucose load and the day that water preceded the glucose load) in 16 obese subjects.
CONCLUSION
Several potential mechanisms, which are not mutually exclusive, could explain the paradoxical association between the consumption of NNS with metabolic disorders observed in epidemiological studies. First, according with Pavlov’s’ theory, the dissociation of sweetness from calories could interfere with fundamental physiological responses that had evolved to control homeostasis (reviewed in 21). Second, NNS induce changes in the gastro-intestinal environment and thus of the gut microbiota (43–45), which can trigger glucose intolerance (44,45). Third, NNS interact with novel sweet taste receptors discovered in non-taste tissues including the gut and the pancreas, which can influence insulin secretion (48, 49, 59–62). However, to date, only the last two mechanisms have been evaluated in human subjects. The finding on the effects of NNS on gut microbiome in human subjects is limited to potential effects of saccharin. Although provocative, and highly congruent with findings from studies in rodent models, the results from this study in humans are limited, because of its small sample size and the lack of a control group for saccharin exposure (45). There are inconsistencies between findings from data from animal models and human subjects in regards to whether NNS can acutely affect glycemic responses in vivo, presumably by activating sweet taste receptors in the digestive system (48, 49, 66–74). The reasons for discrepancy between the results from different studies is unknown but could be related to differences in study subjects (e.g. lean vs. obese, frequent users of NNS vs. non users of NNS). Importantly, most of this research in human subjects has evaluated the effects of sucralose (or sucralose in combination with acesulfame-k), and therefore results from these studies should not be extrapolated to all NNS.
Taken as a whole, despite several epidemiological studies showing an association between NNS consumption and metabolic disorders (9–14), and strong data supporting causality between NNS exposure and metabolic disorders in animal models (18–24, 43–45), there is no irrefutable proof that NNS cause metabolic disorders in human subjects. However, data from at least five different mammalian species (rats, mice, pigs, cows, human) show that NNS can be metabolically active (49, 63, 65, 76, 66–69). Therefore, the old concept that NNS are invariable metabolically inert no longer holds true. More research is needed to elucidate the mechanisms by which NNS may drive metabolic effects and better understand potential effects of these commonly used food additives.
Highlights.
NNS use in humans is linked to weight gain and increase risk of developing type 2 diabetes
NNS in rodents disrupt learned responses that contribute to control glucose homeostasis
NNS in rodents increase glycemic responses to a glucose load by altering gut microbiota
NNS increase intestinal glucose transporter expression in three mammalian species
Acknowledgments
This manuscript is based on work presented during the 2014 Annual Meeting of the Society for the Study of Ingestive Behavior, July 29 – August 2, 2014. This work was supported by the National Institutes of Health (NIH) Clinical and Translational Sciences Award UL1 TR000448, sub award KL2 TR000450, and by NIH grants DK 56341 (Nutrition Obesity Research Center) and DK 02057937 (Diabetes Research Center).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nehrling JK, Kobe P, McLane MP, Olson RE, Kamath S, Horwitz DL. Aspartame use by persons with diabetes. Diabetes Care. 1985;8(5):415–7. doi: 10.2337/diacare.8.5.415. [DOI] [PubMed] [Google Scholar]
- 2. [last accessed on May 20, 2015]; http://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm.
- 3.Xu H, Staszewski L, Tang H, Adler E, Zoller M, Li X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc Natl Acad Sci U S A. 2004;101(39):14258–63. doi: 10.1073/pnas.0404384101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mezitis NH, Maggio CA, Koch P, Quddoos A, Allison DB, Pi-Sunyer FX. Glycemic effect of a single high oral dose of the novel sweetener sucralose in patients with diabetes. Diabetes Care. 1996;19(9):1004–5. doi: 10.2337/diacare.19.9.1004. [DOI] [PubMed] [Google Scholar]
- 5.Grotz VL, Henry RR, McGill JB, Prince MJ, Shamoon H, Trout JR, Pi-Sunyer FX. Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J Am Diet Assoc. 2003;103(12):1607–12. doi: 10.1016/j.jada.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 6.Baird IM, Shephard NW, Merritt RJ, Hildick-Smith G. Repeated dose study of sucralose tolerance in human subjects. Food Chem Toxicol. 2000;38(Suppl 2):S123–9. doi: 10.1016/s0278-6915(00)00035-1. [DOI] [PubMed] [Google Scholar]
- 7.Brown RJ, de Banate MA, Rother KI. Artificial sweeteners: a systematic review of metabolic effects in youth. Int J Pediatr Obes. 2010;5(4):305–12. doi: 10.3109/17477160903497027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swithers SE, Martin AA, Davidson TL. High-intensity sweeteners and energy balance. Physiol Behav. 2010;100(1):55–62. doi: 10.1016/j.physbeh.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D’Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116(5):480–8. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- 10.Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring) 2008;16(8):1894–900. doi: 10.1038/oby.2008.284. [DOI] [PubMed] [Google Scholar]
- 11.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117(6):754–61. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 12.Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR., Jr Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2009;32(4):688–94. doi: 10.2337/dc08-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93(6):1321–7. doi: 10.3945/ajcn.110.007922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fagherazzi G, Vilier A, Saes Sartorelli D, Lajous M, Balkau B, Clavel-Chapelon F. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the Etude Epidemiologique aupres des femmes de la Mutuelle Generale de l’Education Nationale-European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr. 2013;97(3):517–23. doi: 10.3945/ajcn.112.050997. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar- sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357(9255):505–8. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 16.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292(8):927–34. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 17.Swithers SE. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends in endocrinology and metabolism: TEM. 2013;24(9):431–41. doi: 10.1016/j.tem.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swithers SE, Baker CR, Davidson TL. General and persistent effects of high-intensity sweeteners on body weight gain and caloric compensation in rats. Behavioral neuroscience. 2009;123(4):772–80. doi: 10.1037/a0016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swithers SE, Davidson TL. A role for sweet taste: calorie predictive relations in energy regulation by rats. Behavioral neuroscience. 2008;122(1):161–73. doi: 10.1037/0735-7044.122.1.161. [DOI] [PubMed] [Google Scholar]
- 20.Swithers SE, Sample CH, Davidson TL. Adverse effects of high-intensity sweeteners on energy intake and weight control in male and obesity-prone female rats. Behavioral neuroscience. 2013;127(2):262–74. doi: 10.1037/a0031717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidson TL, Sample CH, Swithers SE. An application of Pavlovian principles to the problems of obesity and cognitive decline. Neurobiology of learning and memory. 2014;108:172–84. doi: 10.1016/j.nlm.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swithers SE, Martin AA, Davidson TL. High-intensity sweeteners and energy balance. Physiology & behavior. 2010;100(1):55–62. doi: 10.1016/j.physbeh.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson TL, Martin AA, Clark K, Swithers SE. Intake of high-intensity sweeteners alters the ability of sweet taste to signal caloric consequences: implications for the learned control of energy and body weight regulation. Quarterly journal of experimental psychology. 2011;64(7):1430–41. doi: 10.1080/17470218.2011.552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swithers SE, Laboy AF, Clark K, Cooper S, Davidson TL. Experience with the high- intensity sweetener saccharin impairs glucose homeostasis and GLP-1 release in rats. Behavioural brain research. 2012;233(1):1–14. doi: 10.1016/j.bbr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deutsch R. Conditioned hypoglycemia: a mechanism for saccharin-induced sensitivity to insulin in the rat. Journal of comparative and physiological psychology. 1974;86(2):350–8. doi: 10.1037/h0035948. [DOI] [PubMed] [Google Scholar]
- 26.Valenstein ES, Weber ML. Potentiation of insulin coma by saccharin. Journal of comparative and physiological psychology. 1965;60(3):443–6. doi: 10.1037/h0022560. [DOI] [PubMed] [Google Scholar]
- 27.Strubbe JH, Steffens AB. Rapid insulin release after ingestion of a meal in the unanesthetized rat. American Journal of Physiology. 1975;229:1019–22. doi: 10.1152/ajplegacy.1975.229.4.1019. [DOI] [PubMed] [Google Scholar]
- 28.Grill HJ, Berridge KC, Ganster DJ. Oral glucose is the prime elicitor of preabsorptive insulin secretion. Regulatory Integrative Comp Physiology. 1984;15:R88–R95. doi: 10.1152/ajpregu.1984.246.1.R88. [DOI] [PubMed] [Google Scholar]
- 29.Powley TL, Berthoud HR. Diet and cephalic phase insulin responses. American Journal of Clinical Nutrition. 1985;42:991–1002. doi: 10.1093/ajcn/42.5.991. [DOI] [PubMed] [Google Scholar]
- 30.Bruce DG, Storlien LH, Furler SM, Chisholm DJ. Cephalic phase metabolic responses in normal weight adults. Metabolism. 1987;36:721–25. doi: 10.1016/0026-0495(87)90106-5. [DOI] [PubMed] [Google Scholar]
- 31.Teff KL, Devine J, Engelman K. Sweet taste: effect on cephalic phase insulin release in men. Physiology and Behavior. 1995;57:1089–95. doi: 10.1016/0031-9384(94)00373-d. [DOI] [PubMed] [Google Scholar]
- 32.Abdallah L, Chabert M, Louis-Sylvestre J. Cephalic phase responses to sweet taste. American Journal of Clinical Nutrition. 1997;65:737–43. doi: 10.1093/ajcn/65.3.737. [DOI] [PubMed] [Google Scholar]
- 33.Walters ET, Carew TJ, Kandel ER. Classical conditioning in Aplysia californica. Proc Natl Acad Sci U S A. 1979;76(12):6675–9. doi: 10.1073/pnas.76.12.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teff KL, Mattes RD, Engelman K. Cephalic phase insulin release in normal weight males: verification and reliability. The American journal of physiology. 1991;261(4 Pt 1):E430–6. doi: 10.1152/ajpendo.1991.261.4.E430. [DOI] [PubMed] [Google Scholar]
- 35.Teff K. Nutritional implications of the cephalic-phase reflexes: endocrine responses. Appetite. 2000;34(2):206–13. doi: 10.1006/appe.1999.0282. [DOI] [PubMed] [Google Scholar]
- 36.Teff KL, Engelman K. Oral sensory stimulation improves glucose tolerance in humans: effects on insulin, C-peptide, and glucagon. The American journal of physiology. 1996;270(6 Pt 2):R1371–9. doi: 10.1152/ajpregu.1996.270.6.R1371. [DOI] [PubMed] [Google Scholar]
- 37.Ahren B, Holst JJ. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes. 2001;50(5):1030–8. doi: 10.2337/diabetes.50.5.1030. [DOI] [PubMed] [Google Scholar]
- 38.Fitch C, Keim KS Academy of N and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. Journal of the Academy of Nutrition and Dietetics. 2012;112(5):739–58. doi: 10.1016/j.jand.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Linke HA. Growth inhibition of glucose-grown cariogenic and other streptococci by saccharin in vitro. Zeitschrift fur Naturforschung Section C: Biosciences. 1977;32(9–10):839–43. doi: 10.1515/znc-1977-9-1029. [DOI] [PubMed] [Google Scholar]
- 40.Young DA, Bowen WH. The influence of sucralose on bacterial metabolism. Journal of dental research. 1990;69(8):1480–4. doi: 10.1177/00220345900690080601. [DOI] [PubMed] [Google Scholar]
- 41.Prashant GM, Patil RB, Nagaraj T, Patel VB. The antimicrobial activity of the three commercially available intense sweeteners against common periodontal pathogens: an in vitro study. The journal of contemporary dental practice. 2012;13(6):749–52. doi: 10.5005/jp-journals-10024-1222. [DOI] [PubMed] [Google Scholar]
- 42.Pfeffer M, Ziesenitz SC, Siebert G. Acesulfame K, cyclamate and saccharin inhibit the anaerobic fermentation of glucose by intestinal bacteria. Zeitschrift fur Ernahrungswissenschaft. 1985;24(4):231–5. doi: 10.1007/BF02023668. [DOI] [PubMed] [Google Scholar]
- 43.Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, McLendon RE, Schiffman SS. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome p-450 in male rats. Journal of toxicology and environmental health Part A. 2008;71(21):1415–29. doi: 10.1080/15287390802328630. [DOI] [PubMed] [Google Scholar]
- 44.Palmnas MS, Cowan TE, Bomhof MR, Su J, Reimer RA, Vogel HJ, Hittel DS, Shearer J. Low-dose aspartame consumption differentially affects gut microbiota-host metabolic interactions in the diet-induced obese rat. PloS one. 2014;9(10):e109841. doi: 10.1371/journal.pone.0109841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181–6. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 46.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–9. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 47.Ranney RE, Oppermann JA, Muldoon E, McMahon FG. Comparative metabolism of aspartame in experimental animals and humans. J Toxicol Environ Health. 1976;2:441–51. doi: 10.1080/15287397609529445. [DOI] [PubMed] [Google Scholar]
- 48.Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104(38):15069–74. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104(38):15075–80. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582(Pt 1):379–92. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kokrashvili Z, Mosinger B, Margolskee RF. T1r3 and alpha-gustducin in gut regulate secretion of glucagon-like peptide-1. Annals of the New York Academy of Sciences. 2009;1170:91–4. doi: 10.1111/j.1749-6632.2009.04485.x. [DOI] [PubMed] [Google Scholar]
- 52.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacological reviews. 2008;60(4):470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McIntyre N, Holdsworth CD, Turner DS. New Interpretation of Oral Glucose Tolerance. Lancet. 1964;2(7349):20–1. doi: 10.1016/s0140-6736(64)90011-x. [DOI] [PubMed] [Google Scholar]
- 54.Steinert RE, Gerspach AC, Gutmann H, Asarian L, Drewe J, Beglinger C. The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) Clinical nutrition. 2011;30(4):524–32. doi: 10.1016/j.clnu.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 55.Gerspach AC, Steinert RE, Schonenberger L, Graber-Maier A, Beglinger C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. American journal of physiology Endocrinology and metabolism. 2011;301(2):E317–25. doi: 10.1152/ajpendo.00077.2011. [DOI] [PubMed] [Google Scholar]
- 56.Gorboulev V, Schurmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61(1):187–96. doi: 10.2337/db11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moriya R, Shirakura T, Ito J, Mashiko S, Seo T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. American journal of physiology Endocrinology and metabolism. 2009;297(6):E1358–65. doi: 10.1152/ajpendo.00412.2009. [DOI] [PubMed] [Google Scholar]
- 58.Rogers PJ, Blundell JE. Separating the actions of sweetness and calories: effects of saccharin and carbohydrates on hunger and food intake in human subjects. Physiology & behavior. 1989;45(6):1093–9. doi: 10.1016/0031-9384(89)90093-0. [DOI] [PubMed] [Google Scholar]
- 59.Malaisse WJ, Vanonderbergen A, Louchami K, Jijakli H, Malaisse-Lagae F. Effects of artificial sweeteners on insulin release and cationic fluxes in rat pancreatic islets. Cellular signalling. 1998;10(10):727–33. doi: 10.1016/s0898-6568(98)00017-5. [DOI] [PubMed] [Google Scholar]
- 60.Nakagawa Y, Nagasawa M, Yamada S, Hara A, Mogami H, Nikolaev VO, Lohse MJ, Shigemura N, Ninomiya Y, Kojima I. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS ONE. 2009;4(4):e5106. doi: 10.1371/journal.pone.0005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kyriazis GA, Soundarapandian MM, Tyrberg B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci U S A. 2012;109(8):E524–32. doi: 10.1073/pnas.1115183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corkey BE. Banting lecture 2011: hyperinsulinemia: cause or consequence? Diabetes. 2012;61(1):4–13. doi: 10.2337/db11-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moran AW, Al-Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Daly K, Ionescu C, Bravo D, Shirazi-Beechey SP. Expression of Na+/glucose co-transporter 1 (SGLT1) is enhanced by supplementation of the diet of weaning piglets with artificial sweeteners. Br J Nutr. 2010;104:637–46. doi: 10.1017/S0007114510000917. [DOI] [PubMed] [Google Scholar]
- 64.Stearns AT, Balakrishnan A, Rhoads DB, Tavakkolizadeh A. Rapid upregulation of sodium-glucose transporter SGLT1 in response to intestinal sweet taste stimulation. Ann Surg. 2010;251(5):865–71. doi: 10.1097/SLA.0b013e3181d96e1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitsutomi K, Masaki T, Shimasaki T, Gotoh K, Chiba S, Kakuma T, Shibata H. Effects of a nonnutritive sweetener on body adiposity and energy metabolism in mice with diet-induced obesity. Metabolism. 2014;63(1):69–78. doi: 10.1016/j.metabol.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 66.Brown RJ, Walter M, Rother KI. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care. 2009;32(12):2184–6. doi: 10.2337/dc09-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown RJ, Walter M, Rother KI. Effects of diet soda on gut hormones in youths with diabetes. Diabetes Care. 2012;35(5):959–64. doi: 10.2337/dc11-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Temizkan S, Deyneli O, Yasar M, Arpa M, Gunes M, Yazici D, Sirikci O, Haklar G, Imeryuz N, Yavuz DG. Sucralose enhances GLP-1 release and lowers blood glucose in the presence of carbohydrate in healthy subjects but not in patients with type 2 diabetes. European journal of clinical nutrition. 2015;69(2):162–6. doi: 10.1038/ejcn.2014.208. [DOI] [PubMed] [Google Scholar]
- 69.Pepino MY, Tiemann CD, Patterson BW, Wice BM, Klein S. Sucralose Affects Glycemic and Hormonal Responses to an Oral Glucose Load. Diabetes Care. 2013;36(9):2530–5. doi: 10.2337/dc12-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma J, Chang J, Checklin HL, Young RL, Jones KL, Horowitz M, Rayner CK. Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br J Nutr. 2010;104(6):803–6. doi: 10.1017/S0007114510001327. [DOI] [PubMed] [Google Scholar]
- 71.Ford HE, Peters V, Martin NM, Sleeth ML, Ghatei MA, Frost GS, Bloom SR. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur J Clin Nutr. 2011;65(4):508–13. doi: 10.1038/ejcn.2010.291. [DOI] [PubMed] [Google Scholar]
- 72.Wu T, Zhao BR, Bound MJ, Checklin HL, Bellon M, Little TJ, Young RL, Jones KL, Horowitz M, Rayner CK. Effects of different sweet preloads on incretin hormone secretion, gastric emptying, and postprandial glycemia in healthy humans. Am J Clin Nutr. 2012;95(1):78–83. doi: 10.3945/ajcn.111.021543. [DOI] [PubMed] [Google Scholar]
- 73.Brown AW, Bohan Brown MM, Onken KL, Beitz DC. Short-term consumption of sucralose, a nonnutritive sweetener, is similar to water with regard to select markers of hunger signaling and short-term glucose homeostasis in women. Nutr Res. 2011;31(12):882–8. doi: 10.1016/j.nutres.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 74.Wu T, Bound MJ, Standfield SD, Bellon M, Young RL, Jones KL, Horowitz M, Rayner CK. Artificial sweeteners have no effect on gastric emptying, glucagon-like peptide-1, or glycemia after oral glucose in healthy humans. Diabetes Care. 2013;36(12):e202–3. doi: 10.2337/dc13-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sylvetsky AC, Welsh JA, Brown RJ, Vos MB. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr. 2012;96(3):640–6. doi: 10.3945/ajcn.112.034751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moran AW, Al-Rammahi M, Zhang C, Bravo D, Calsamiglia S, Shirazi-Beechey SP. Sweet taste receptor expression in ruminant intestine and its activation by artificial sweeteners to regulate glucose absorption. Journal of dairy science. 2014;97(8):4955–72. doi: 10.3168/jds.2014-8004. [DOI] [PubMed] [Google Scholar]
- 77.Herness S, Zhao FL, Lu SG, Kaya N, Shen T. Expression and physiological actions of cholecystokinin in rat taste receptor cells. J Neurosci. 2002;22:10018–29. doi: 10.1523/JNEUROSCI.22-22-10018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen T, Kaya N, Zhao FL, Lu SG, Cao Y, Herness S. Co-expression patterns of the neuropeptides vasoactive intestinal peptide and cholecystokinin with the transduction molecules alpha-gustducin and T1R2 in rat taste receptor cells. Neuroscience. 2005;130(1):229–38. doi: 10.1016/j.neuroscience.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 79.Kokrashvili Z, Yee KK, Ilegems E, Iwatsuki K, Li Y, Mosinger B, Margolskee RF. Endocrine taste cells. Br J Nutr. 2014;111 (Suppl 1):S23–9. doi: 10.1017/S0007114513002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shin YK, Martin B, Golden E, Dotson CD, Maudsley S, Kim W, Jang HJ, Mattson MP, Drucker DJ, Egan JM, et al. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem. 2008;106(1):455–63. doi: 10.1111/j.1471-4159.2008.05397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Geraedts MC, Munger SD. Gustatory stimuli representing different perceptual qualities elicit distinct patterns of neuropeptide secretion from taste buds. J Neurosci. 2013;33(17):7559–64. doi: 10.1523/JNEUROSCI.0372-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Ozdener MH, Subramaniam S, Sundaresan S, Sery O, Hashimoto T, Asakawa Y, Besnard P, Abumrad NA, Khan NA. CD36- and GPR120-mediated Ca2+ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology. 2014;146(4):995–1005. doi: 10.1053/j.gastro.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lawson WB, Zeidler A, Rubenstein A. Taste detection and preferences in diabetics and their relatives. Psychosom Med. 1979;41(3):219–27. doi: 10.1097/00006842-197905000-00005. [DOI] [PubMed] [Google Scholar]
- 84.Pepino MY, Finkbeiner S, Beauchamp GK, Mennella JA. Obese women have lower monosodium glutamate taste sensitivity and prefer higher concentrations than do normal-weight women. Obesity (Silver Spring) 2010;18(5):959–65. doi: 10.1038/oby.2009.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pepino MY, Mennella JA. Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol Clin Exp Res. 2007;31(11):1891–9. doi: 10.1111/j.1530-0277.2007.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Galindo-Cuspinera V, Waeber T, Antille N, Hartmann C, Stead N, Martin N. Reliability of Threshold and Suprathreshold Methods for Taste Phenotyping: Characterization with PROP and Sodium Chloride. Chemosensory perception. 2009;2(4):214–28. doi: 10.1007/s12078-009-9059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


