Abstract
The concept of ‘Successful Aging’ has long intrigued the scientific community. Despite this long-standing interest, a consensus definition has proven to be a difficult task, due to the inherent challenge involved in defining such a complex, multi-dimensional phenomenon. The lack of a clear set of defining characteristics for the construct of successful aging has made comparison of findings across studies difficult and has limited advances in aging research. The domain in which consensus on markers of successful aging is furthest developed is the domain of physical functioning. For example, walking speed appears to be an excellent surrogate marker of overall health and predicts the maintenance of physical independence, a cornerstone of successful aging. The purpose of the present article is to provide an overview and discussion of specific health conditions, behavioral factors, and biological mechanisms that mark declining mobility and physical function and promising interventions to counter these effects. With life expectancy continuing to increase in the United States and developed countries throughout the world, there is an increasing public health focus on the maintenance of physical independence among all older adults.
Keywords: age, mobility, obesity, sarcopenia, healthspan, longevity
1. Introduction
The concept of “Successful Aging” has intrigued philosophers and scientists for hundreds of years. For example, in the ancient treatise De Senectute, Cicero contemplates the extent to which individuals can remain active and experience vitality in later life. Despite this long standing interest, scientific attempts to define the phenomenon of successful aging have primarily occurred during the past century. This term was first introduced in the scientific literature by Robert J. Havighurst in 1961(Havighurst 1961) in which he states that the science of gerontology has the practical purpose of “adding life to the years” with the goal of increasing enjoyment and satisfaction during the latter stages of an individual’s lifespan. To achieve such an objective, he argues that it is essential to have a theory of successful aging. However, this has proven to be a difficult task, due to the inherent challenge involved in defining this complex, multi-dimensional phenomenon which comprises biological, physical, cognitive, affective, and social components.
Many of the initial definitions of successful aging were in line with the biomedical model, which conceptualizes health, and by extension successful aging, largely based on the absence of chronic disease conditions and reduction in risk factors for disease. Within this model, individuals are typically classified into distinct dichotomous categories of healthy or diseased, such that successful aging is represented by good health, independence, and high levels of cognitive and physical functioning. The role of lifestyle and/or psychosocial factors in contributing to good or poor health, however, is not recognized by this model. Due to the increasing recognition of the complex interplay among biological, psychological, and social factors in affecting an individual’s health status and disease risk, a new model for understanding the development of disease, termed the biopsychosocial model, was proposed in the 1970s.(Engel 1977;Schwartz 1982) As applied to the construct of successful aging, this model views an individual’s health and functional status later in life not as a dichotomous classification of healthy or diseased but rather as occurring along a spectrum across multiple dimensions.(Coyne and Downey 1991)
Consistent with the biopsychosocial approach, biological and psychosocial concepts have been successfully integrated in many recent models of successful aging.(Bowling 2007;Glass 2003;Hung, Lee, Chen, and Huang 2010;Pruchno, Wilson-Genderson, and Cartwright 2010;Pruchno, Wilson-Genderson, Rose, and Cartwright 2010) For example, Rowe and Kahn’s model of successful aging, (Rowe and Kahn 1997) one of the most widely accepted and applied models,(Dillaway and Byrnes 2009) views “better than average” aging as a combination of three components: avoiding disease and disability, high cognitive and physical function, and engagement with life. Recent models continue to support the multidimensional nature of this construct and have incorporated both objective and subjective dimensions in their definitions of successful aging.(Pruchno, Wilson-Genderson, Rose, and Cartwright 2010) For example, Pruchno and colleagues (2010) have proposed a two-factor model of successful aging, which incorporates both objective criteria (i.e., functional abilities, pain, and diagnosed health conditions) and subjective criteria (i.e., perceptions of quality of life and successful aging).(Pruchno, Wilson-Genderson, and Cartwright 2010)
In order to determine the most suitable theory of successful aging, the majority of experts in aging and gerontology would need to agree on an operational definition and methods to measure the degree to which an individual fits this definition. Such a consensus definition does not currently exist, and the construct of successful aging remains an evolving concept without a clear set of metrics to define this phenomenon.(Cosco, Prina, Perales, Stephan, and Brayne 2014) In the absence of an accepted definition and commonly used metrics, a diverse array of measures are being used to assess different aspects the construct of successful aging.(Depp and Jeste 2006) This has had the unintended consequence of limiting advances in aging research since comparisons of findings across studies are not possible without clearly defined constructs and standardized measures to assess them.(Depp and Jeste 2006)
To determine the effectiveness of treatment approaches targeting agreed upon markers of successful aging, outcome measures are needed that can be directly translated to measureable improvements in both health and functional abilities. The strongest evidence of such markers can be found within the physical domain of the multidimensional construct of successful aging. Within this domain, there is a growing consensus of indicators of successful aging (i.e., mobility performance and physical function), measures to assess these markers, and metrics for evaluating performance on such measures. For example, mobility performance (i.e., walking speed) has emerged as a surrogate marker of overall health and functional ability among older adults.(Cesari 2011) Mobility performance is frequently measured based on time to complete a distance walk ranging from 4 meters to 400 meters or distance covered within a pre-specified time period, typically 6 min. Accumulating evidence indicates slow walking speed (<1 m/sec indicating a high risk and < 0.8 m/sec indicating a very high risk), as well as reductions in walking speed, strongly predict functional decline, major health outcomes, and mortality in older adults.(Abellan Van, Rolland, Andrieu, Bauer, Beauchet, Bonnefoy, Cesari, Donini, Gillette, Inzitari, Nourhashemi, Onder, Ritz, Salva, Visser, and Vellas 2009;Cesari, Kritchevsky, Penninx, Nicklas, Simonsick, Newman, Tylavsky, Brach, Satterfield, Bauer, Visser, Rubin, Harris, and Pahor 2005;den Ouden, Schuurmans, Arts, and van der Schouw 2011;Menant, Schoene, and Lord 2014;Studenski, Perera, Patel, Rosano, Faulkner, Inzitari, Brach, Chandler, Cawthon, Connor, Nevitt, Visser, Kritchevsky, Badinelli, Harris, Newman, Cauley, Ferrucci, and Guralnik 2011) In line with this clinical evidence, a close relationship between mobility performance and longevity has been documented in preclinical models.(Carter, Sonntag, Onder, and Pahor 2002;Demontis and Perrimon 2010)
Another commonly used and well validated measure of physical function in older adults is the Short Physical Performance Battery (SPPB).(Guralnik, Simonsick, Ferrucci, Glynn, Berkman, Blazer, Scherr, and Wallace 1994) The SPPB assesses multiple aspects of lower extremity function including balance, strength, and walking speed and incorporates performance on these three dimensions into an overall physical function score (range = 0 – 12 points). Scores on the SPPB and walking speed have been found to predict onset of difficulty in activities of daily living (Wennie Huang, Perera, VanSwearingen, and Studenski 2010) and disability in community-dwelling older adults.(Albert, Bear-Lehman, and Anderson 2014;Carter, Sonntag, Onder, and Pahor 2002;Demontis and Perrimon 2010;Guralnik, Ferrucci, Pieper, Leveille, Markides, Ostir, Studenski, Berkman, and Wallace 2000;Guralnik, Ferrucci, Simonsick, Salive, and Wallace 1995;Guralnik, Simonsick, Ferrucci, Glynn, Berkman, Blazer, Scherr, and Wallace 1994) Moreover, small improvements in walking speed (Δ > 0.05 m/sec) and performance on the SPPB (Δ > 0.5 points) have been found to translate to meaningful improvements in performance of activities of daily living (e.g., stair climbing, walking a block).(Perera, Mody, Woodman, and Studenski 2006) Such findings suggest that performance on these objective and easy to administer measures of physical function (e.g., SPPB) and mobility (e.g., 400 meter walk) constitute useful indicators of health status and risk for disease and functional decline in older adults.(Bua, Johnson, Herbst, Delong, McKenzie, Salamat, and AIKEN 2006)
With the growing population of older adults in the United States and developed countries throughout the world, there is an increasing public health focus on the maintenance of physical independence in aging. An increased understanding of the biological and behavioral mechanisms contributing to functional decline can aid in the development of future interventions designed to improve mobility and function or attenuate declines in physical function in older adults.(Lonergan and Krevans 1991) As such, the purpose of the present article is to provide an overview of specific health conditions, behavioral factors, and biological mechanisms that are strongly related to mobility and physical function during aging. In addition, we summarize promising interventions that have been shown to enhance mobility and physical function in older adults by targeting one or more biological or behavioral factors contributing to functional decline (see Conceptual Figure 1). This review is divided into four main sections. In the first section, we review specific health conditions that contribute to functional decline. Next, we describe key behavioral and social factors that can affect physical function and risk for functional decline in older adults. We then discuss the biological mechanisms that show a strong link with physical function (see Figure 2). In the final section, we discuss promising interventions for enhancing mobility and physical function in older adults (see Figure 3 and Table 1), as well as important statistical considerations in evaluating such interventions.
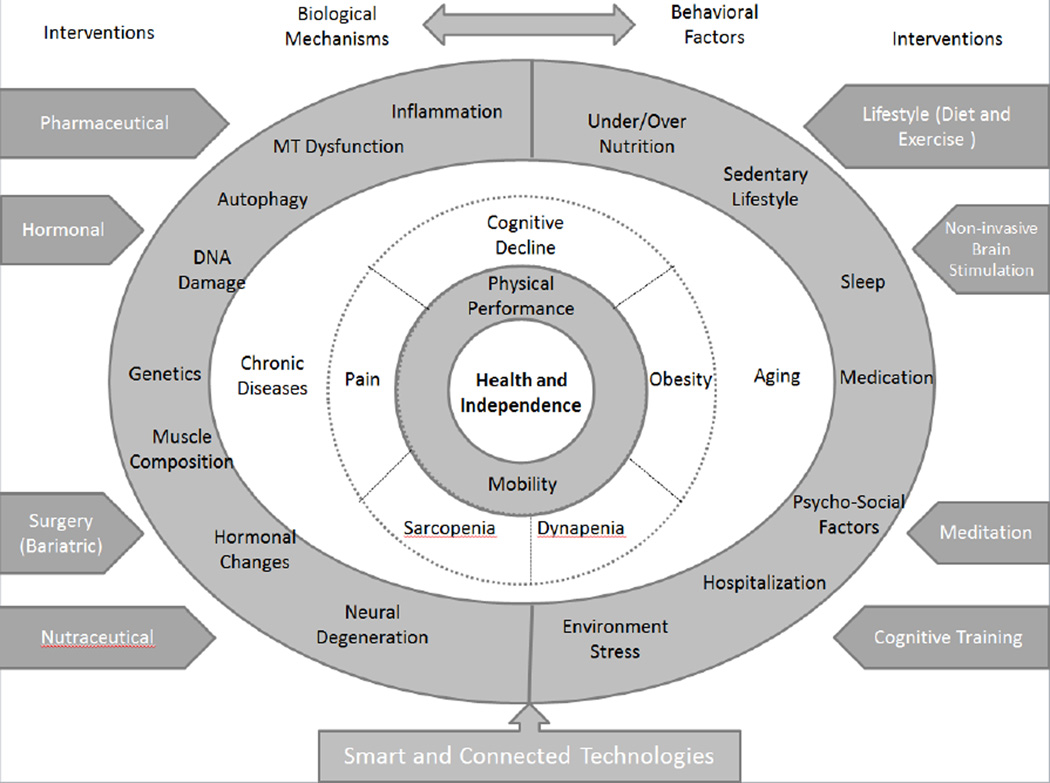
Figure 1.
The conceptual model illustrates important factors that can affect physical function during aging and ultimately maintenance of health and independence in older adults. Biological mechanisms and behavioral factors associated with reductions in physical function are illustrated in the outer ring. Specific health conditions that contribute to reductions in mobility and physical performance during aging are displayed in the middle ring. These conditions include but are not limited to cognitive decline, dynapenia, obesity, pain, and sarcopenia. Promising interventions for enhancing mobility and physical function, which target one or more biological mechanisms, behavioral factors, or health conditions contributing to functional decline, are shown in the outer edges. The ultimate goal is maintenance of health and independence, displayed in the inner most ring, through enhancement of mobility and/or physical function. This figure is not intended to be exhaustive but rather to highlight key biological mechanisms, behavioral factors, and health conditions that can contribute to functional decline in older adults, as well as promising interventions to attenuate declines in mobility and physical function during aging. MT: Mitochondria.
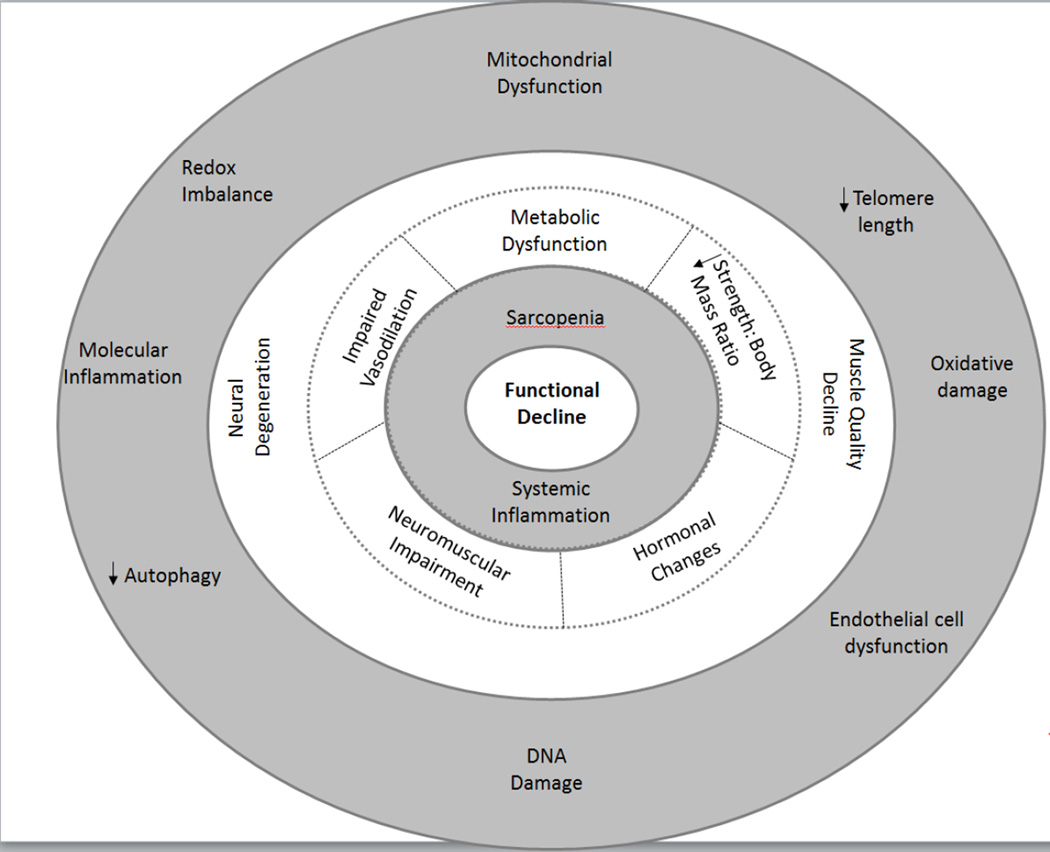
Figure 2.
The pathophysiology model illustrates key biological mechanisms that contribute to functional decline. Cellular mechanisms are displayed in the outer ring, tissue specific mechanisms are displayed in the next ring, and systemic mechanisms are displayed in the two inner rings.
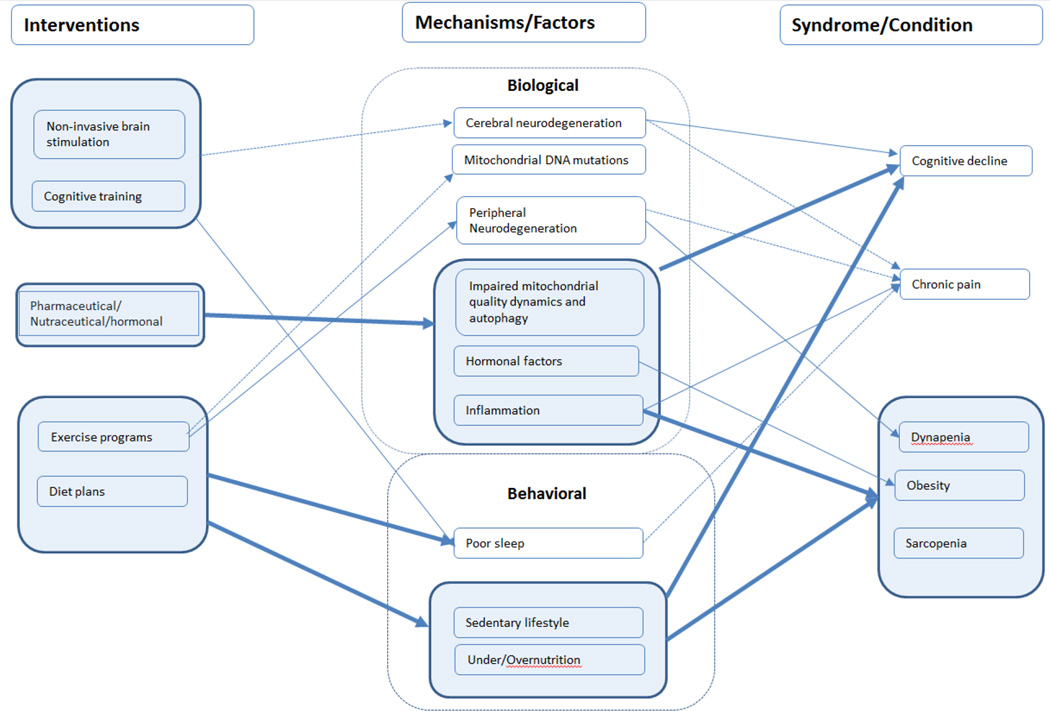
Figure 3.
The intervention model displays the effects different interventions have on biological and behavioral factors contributing to functional decline, as well as the interconnection between biological and behavioral factors with health conditions known to contribute to functional decline. Connections that have clear empirical support are displayed with a solid line, and theoretical connections are displayed with a dotted line.
Table 1.
Effects of Lifestyle Interventions on Change in Walking Speed in Mobility-Limited Older Adults.
| Study | N | Age Range (years) |
Intervention Type |
Length (weeks) |
Walking Speed Measure |
Baseline Walking Speed (m/s) |
Δ in Walking Speed Pre-to Post- intervention (m/s) |
P-value |
|---|---|---|---|---|---|---|---|---|
| Barnett et al. (2003) | 83 | ≥60 | PA | 6 | 6-m Walk | 0.95±0.3 | 0.03 | n/a |
| Beling et al. (2009) | 12 | ≥65 | PA | 12 | GAITRite | 0.88±0.2 | 0.07 | n/a |
| Chmelo et al. (2015) | 40 | ≥65 | PA | 20 | 400-m Walk | 0.81±0.16 | 0.08 | 0.001 |
| Fiatarone et al. (1994) | 25 | ≥70 | PA | 10 | 6-m Walk | 0.51±0.04 | 0.04 | <0.01 |
| Freiberger et al. (2012) | 64 | >70 | PA | 10 | 6-m Walk | 0.98±0.18 | 0.02 | n/a |
| Gine-Garriage et al. (2010) | 26 | 70–90 | PA | 12 | 8-m Walk | 0.82±0.04 | 0.12 | n/a |
| Lazowski et al. (1999) | 35 | ≥65 | PA | 16 | 7-m Walk | 0.69±0.28 | 0.04 | n/a |
| Manini et al. (2010) | 424 | 70–88 | PA | 52 | 400-m Walk | 0.88±0.18 | 0.04 | n/a |
| Phillips et al. (2010) | 213 | ≥65 | PA | 57 | 400-m Walk | 0.86±0.18 | 0.08 | 0.03 |
| Rosendahl et al. (2006) | 45 | ≥65 | PA | 24 | 2.4-m Walk | 0.35±0.17 | 0.05 | 0.02 |
| Van Swearingen et al. (2011) | 47 | ≥65 | PA | 12 | 4-m Walk | 0.88±0.13 | 0.21 | <0.001 |
| Van Swearingen et al. (2009) | 47 | ≥65 | PA | 12 | 4-m Walk | 0.88±0.13 | 0.14 | <0.001 |
| Villareal et al. (2006) | 27 | ≥65 | CR+PA | 26 | 25ft Walk | 0.84±0.15 | 0.08 | 0.05 |
| Villareal et al. (2011) | 107 | ≥65 | CR | 52 | 25ft Walk | 0.68±0.12 | 0.08 | ns |
| PA | 0.79±0.19 | 0.14 | ns | |||||
| CR+PA | 0.82±0.17 | 0.28 | 0.01 |
CR= Caloric Restriction; PA= Physical Activity; m/s = meters per second; Δ= change; ft = feet. Change data are defined as the follow-up minus the baseline value.
2. Discussion
2.1 Contribution of Specific Health Conditions to Reductions in Physical Function
A number of health conditions which we briefly review in this section have been shown to contribute to reductions in physical function during aging and may affect successful aging.(Anton, Karabetian, Naugle, and Buford 2013;Buford, Anton, Judge, Marzetti, Wohlgemuth, Carter, Leeuwenburgh, Pahor, and Manini 2010a) Specifically, a growing body of evidence indicates specific conditions including cognitive decline, obesity, sarcopenia, dynapenia, chronic pain, are strongly linked to physical function. In this section, we briefly review the contribution of each of these factors to reductions in physical function during aging.
2.1.1 Cognitive Decline
A broad range of cognitive functions decline with advanced age, such as memory, attention, and executive functions. (Salthouse 2012;Woods, Cohen, and Pahor 2013;Woods, Mark, Pitts, and Mennemeier 2011) Yet, other elements of cognitive function, like vocabulary, remain stable over the lifespan.(Botwinick and Storandt 1973;Salthouse 2010) In addition to experiencing changes in cognitive function over time, many older adults may also be susceptible to transient episodes of cognitive decline (e.g., delirium). A growing body of evidence suggests that age-related and transient declines in cognitive function significantly contribute to functional decline and eventual development of physical disability.(Atkinson, Cesari, Kritchevsky, Penninx, Fried, Guralnik, and Williamson 2005;Brummel, Jackson, Pandharipande, Thompson, Shintani, Dittus, Gill, Bernard, Ely, and Girard 2014;Deshpande, Metter, Bandinelli, Guralnik, and Ferrucci 2009;Dodge, Mattek, Austin, Hayes, and Kaye 2012;Fitzpatrick, Buchanan, Nahin, Dekosky, Atkinson, Carlson, and Williamson 2007;Jedrziewski, Lee, and Trojanowski 2007;Raji, Kuo, Snih, Markides, Peek, and Ottenbacher 2005;Woods, Cohen, and Pahor 2013;Woods, Mark, Pitts, and Mennemeier 2011) Given the association between cognitive decline and increased rates of injury, hospitalization, dependence on assisted living, and mortality,(Boockvar, Signor, Ramaswamy, and Hung 2013;Brummel, Jackson, Pandharipande, Thompson, Shintani, Dittus, Gill, Bernard, Ely, and Girard 2014;Woods, Mark, Pitts, and Mennemeier 2011) an improved understanding of the link between cognitive and physical function is important for the development of multidisciplinary interventions that are effective at combating age-related physical decline.
2.1.2 Obesity
The prevalence of obesity has increased dramatically during the past few decades, particularly among older adults.(Main, Rao, and O'Keefe 2010;Ogden, Carroll, and Flegal 2014) Obese, older adults currently represent 33 percent of American older adults (age > 60 years).(Flegal, Carroll, Kit, and Ogden 2012) Obesity is a multifactorial, complex chronic disease, including age-related or adult-onset obesity, and its burden will likely increase as the population of older American adults increases over the coming decades.(Manson and Bassuk 2003;Vastag 2004) Although the initial onset and progression of functional decline is typically associated with the progressive loss of skeletal muscle mass (i.e., sarcopenia),(Hyatt, Whitelaw, Bhat, Scott, and Maxwell 1990;Janssen, Heymsfield, and Ross 2002) age-related changes in muscle composition appear to be even more pronounced in obese individuals. (Barbat-Artigas, Pion, Leduc-Gaudet, Rolland, and Aubertin-Leheudre 2014;Koster, Ding, Stenholm, Caserotti, Houston, Nicklas, You, Lee, Visser, Newman, Schwartz, Cauley, Tylavsky, Goodpaster, Kritchevsky, and Harris 2011) The combination of muscle loss and fat gain may act synergistically to increase risk of physical disability in obese, older adults.(Baumgartner 2000b;Blaum, Xue, Michelon, Semba, and Fried 2005a;Cesari, Kritchevsky, Baumgartner, Atkinson, Penninx, Lenchik, Palla, Ambrosius, Tracy, and Pahor 2005) Moreover, obesity has been identified as a contributor to the progression of sarcopenia (Buford, Anton, Judge, Marzetti, Wohlgemuth, Carter, Leeuwenburgh, Pahor, and Manini 2010a) and directly linked to an increased risk of physical disability.(Figaro, Kritchevsky, Resnick, Shorr, Butler, Shintani, Penninx, Simonsick, Goodpaster, Newman, Schwartz, and Harris 2006) Excess adiposity leads to an increased production of reactive oxygen species and inflammatory cytokines, damaging mitochondria and adversely affecting cellular quality control processes, (Bayeva, Gheorghiade, and Ardehali 2013;Verdejo, del, Troncoso, Gutierrez, Toro, Quiroga, Pedrozo, Munoz, Garcia, Castro, and Lavandero 2012) which can further accelerate the process of functional decline in older adults.(Naugle, Higgins, and Manini 2012;Peeters, Bonneux, Nusselder, Laet, and Barendregt 2004;Rillamas-Sun, LaCroix, Waring, Kroenke, LaMonte, Vitolins, Seguin, Bell, Gass, and Manini 2014) Obesity also contributes to cognitive decline,(Bhargava, Weiner, Hynan, Diaz-Arrastia, and Lipton 2006;Riekse, Leverenz, McCormick, Bowen, Teri, Nochlin, Simpson, Eugenio, Larson, and Tsuang 2004) which in turn contributes to increased risk of disability.(Deshpande, Metter, Bandinelli, Guralnik, and Ferrucci 2009;Dodge, Mattek, Austin, Hayes, and Kaye 2012)
2.1.3 Sarcopenia
Individuals tend to lose muscle mass at a rate of 1–2% per year after the age of 50 years.(Hiona and Leeuwenburgh 2008;Lauretani, Russo, Bandinelli, Bartali, Cavazzini, Di, Corsi, Rantanen, Guralnik, and Ferrucci 2003;Marcell 2003) The age-related loss of muscle mass and quality, otherwise known as sarcopenia, is primarily due to the progressive atrophy and loss of type II muscle fibers and motor neurons.(Larsson, Sjodin, and Karlsson 1978) However, other morphological changes occur during the atrophy process including increased variability in fiber size, accumulation of non-grouping, scattered and angulated fibers, expansion of extracellular space, and deposition of protein aggregates within the interstitial matrix.(Kim, Kwak, Leeuwenburgh, and Lawler 2008) These morphological changes occur in conjunction with increased infiltration of non-contractile material such as adipose and connective tissues.(Brooks and Faulkner 1994;Goldspink, Fernandes, Williams, and Wells 1994;Goodpaster, Chomentowski, Ward, Rossi, Glynn, Delmonico, Kritchevsky, Pahor, and Newman 2008;McNeil, Doherty, Stashuk, and Rice 2005;Petersen, Befroy, Dufour, Dziura, Ariyan, Rothman, DiPietro, Cline, and Shulman 2003) These changes contribute to declines in functional capacity of the muscle, which in turn contribute to functional disability.(Evans 1997;Janssen, Heymsfield, and Ross 2002;Muhlberg and Sieber 2004;Rolland, Czerwinski, Abellan Van, Morley, Cesari, Onder, Woo, Baumgartner, Pillard, Boirie, Chumlea, and Vellas 2008;Visser, Goodpaster, Kritchevsky, Newman, Nevitt, Rubin, Simonsick, and Harris 2005)
Sarcopenia is a major healthcare concern for older adults, (Buford, Anton, Judge, Marzetti, Wohlgemuth, Carter, Leeuwenburgh, Pahor, and Manini 2010a) as it is associated with the development of functional disability (Janssen, Shepard, Katzmarzyk, and Roubenoff 2004;Visser, Goodpaster, Kritchevsky, Newman, Nevitt, Rubin, Simonsick, and Harris 2005) and may lead to the loss of independence. Because of the costs associated with caring for an individual with compromised function, sarcopenia has been linked to elevated healthcare costs.(Janssen, Shepard, Katzmarzyk, and Roubenoff 2004) Moreover, the absolute costs associated with sarcopenia are likely to rise sharply in the coming decades considering that the total number of persons over 65 years is expected to double over the next 25 years.(Federal Interagency Forum on Aging-Related Statistics. 2009)
While impaired locomotion is certainly the hallmark concern of sarcopenia, muscle atrophy may impair other physiological functions including glucose regulation, hormone production, and cellular communication.(Moon 2014) Moreover, muscle tissue provides the body’s only major “reserve” of readily available amino acids. Thus, inadequate muscle mass prior to the onset of a disease condition may be dangerous in patients who need a large protein reservoir to recover. As a result, patients with sarcopenia prior to disease diagnosis may face impaired recovery from surgery (Rutan and Herndon 1990) or increased risk of mortality.(Prado, Baracos, McCargar, Reiman, Mourtzakis, Tonkin, Mackey, Koski, Pituskin, and Sawyer 2009) While the contributions of sarcopenia to functional impairments are well documented, data regarding the importance of skeletal muscle in the recovery from life-threatening situations, such as severe burns or traumatic surgeries, are limited.
Currently, there are several recommendations for criteria and the inclusion of cut-points for defining low muscle mass and low grip strength. The European Working Group on Sarcopenia and The Society of Sarcopenia, Cachaexia and Wasting Disorders both define sarcopenia using a multifactorial approach of combining low walking speed and low muscle mass.(Cruz-Jentoft, Baeyens, Bauer, Boirie, Cederholm, Landi, Martin, Michel, Rolland, Schneider, Topinkova, Vandewoude, and Zamboni 2010;Morley, Abbatecola, Argiles, Baracos, Bauer, Bhasin, Cederholm, Coats, Cummings, Evans, Fearon, Ferrucci, Fielding, Guralnik, Harris, Inui, Kalantar-Zadeh, Kirwan, Mantovani, Muscaritoli, Newman, Rossi-Fanelli, Rosano, Roubenoff, Schambelan, Sokol, Storer, Vellas, von, Yeh, and Anker 2011) These definitions take advantage of the strong association between low walking speed and health outcomes in elders and thus are expected to serve as a primary indicator of sarcopenia.
2.1.4 Dynapenia
Dynapenia, a term coined by Manini and Clark (Manini and Clark 2012), is the age-related loss of muscle strength.(Clark and Manini 2008;Manini, Hong, and Clark 2013) In contrast to sarcopenia, dynapenia may or may not be related to age-related changes in muscle mass. Rather, dynapenia can be multifactorial including deficits in muscle quality and neuromuscular control. The presence of dynapenia can be assessed by measurements of isometric maximal voluntary force, one-repetition maximum force, rate of force production and other related measures.(Clark and Manini 2008)
While muscle mass seems to have little relationship to mobility impairments (Cruz-Jentoft, Baeyens, Bauer, Boirie, Cederholm, Landi, Martin, Michel, Rolland, Schneider, Topinkova, Vandewoude, and Zamboni 2010;McLean, Shardell, Alley, Cawthon, Fragala, Harris, Kenny, Peters, Ferrucci, Guralnik, Kritchevsky, Kiel, Vassileva, Xue, Perera, Studenski, and Dam 2014), dynapenia has been shown to be independently associated with loss of mobility function.(Bassey, Fiatarone, O'Neill, Kelly, Evans, and Lipsitz 1992;Bean, Kiely, Herman, Leveille, Mizer, Frontera, and Fielding 2002;Bean, Leveille, Kiely, Bandinelli, Guralnik, and Ferrucci 2003;Clark, Patten, Reid, Carabello, Phillips, and Fielding 2010;Clark, Patten, Reid, Carabello, Phillips, and Fielding 2011;Foldvari, Clark, Laviolette, Bernstein, Kaliton, Castaneda, Pu, Hausdorff, Fielding, and Singh 2000;Suzuki, Bean, and Fielding 2001). For example, older adults with muscle loss, and no strength loss, do not have mobility impairment. (McLean, Shardell, Alley, Cawthon, Fragala, Harris, Kenny, Peters, Ferrucci, Guralnik, Kritchevsky, Kiel, Vassileva, Xue, Perera, Studenski, and Dam 2014) This association only becomes more consistent when loss of muscle mass co-occurs with loss of muscle strength. (McLean, Shardell, Alley, Cawthon, Fragala, Harris, Kenny, Peters, Ferrucci, Guralnik, Kritchevsky, Kiel, Vassileva, Xue, Perera, Studenski, and Dam 2014;Studenski, Peters, Alley, Cawthon, McLean, Harris, Ferrucci, Guralnik, Fragala, Kenny, Kiel, Kritchevsky, Shardell, Dam, and Vassileva 2014) For example, grip strength less than 26kg or 16kg for men and women respectively is highly predictive of mobility disability. (Alley, Shardell, Peters, McLean, Dam, Kenny, Fragala, Harris, Kiel, Guralnik, Ferrucci, Kritchevsky, Studenski, Vassileva, and Cawthon 2014) A ten year follow-up with participants revealed that muscle weakness is also associated with higher mortality rates in both older men and women, to a degree much higher than a cohort who only has low lean mass. In fact, this study indicated that low lean mass in women was not associated with elevated mortality rates. (McLean, Shardell, Alley, Cawthon, Fragala, Harris, Kenny, Peters, Ferrucci, Guralnik, Kritchevsky, Kiel, Vassileva, Xue, Perera, Studenski, and Dam 2014) This suggests that at some level, there is a disconnect between muscle mass and weakness that may theoretically be explained by other factors such as age-dependent inter- and intramuscular fat infiltration (Goodpaster, Carlson, Visser, Kelley, Scherzinger, Harris, Stamm, and Newman 2001;Visser, Kritchevsky, Goodpaster, Newman, Nevitt, Stamm, and Harris 2002), excitation-contraction coupling, (Delbono 2000) or dysfunctions of the central nervous system or neuromuscular junctions. (Vandervoort 2002) In support of this, evidence suggests that neither the measurement of muscle mass nor muscle index (appendicular lean mass relative to BMI) is consistently associated with adverse outcomes.
2.1.5 Chronic Pain
Chronic pain is a highly prevalent condition in older adults with estimates as high as 70% in community settings and up to 80% in nursing homes.(Ferrell, Whedon, and Rollins 1995;Helme and Gibson 2001;Karttunen, Turunen, Ahonen, and Hartikainen 2014) Of the types of pain experienced, knee osteoarthritis represents the most common chronic pain condition and the leading cause of disability among older adults.(Neogi and Zhang 2013) Chronic pain significantly contributes to functional decline and activity limitation, (Fletcher, Guthrie, Berg, and Hirdes 2010;Pisters, Veenhof, van Dijk, Heymans, Twisk, and Dekker 2012;Rossi, Pereira, Driusso, Rebelatto, and Ricci 2013;White, Felson, Niu, Nevitt, Lewis, Torner, and Neogi 2011) adversely affects quality of life, and is associated with increased morbidity and mortality.(Andersson 2004;Bruckenthal, Reid, and Reisner 2009;Torrance, Elliott, Lee, and Smith 2010) Multiple factors inevitably influence age-related increases in pain vulnerability, including greater frequency of chronic medical conditions as well as multiple changes in biological and psychosocial functioning.(Cruz-Almeida, King, Goodin, Sibille, Glover, Riley, Sotolongo, Herbert, Schmidt, Fessler, Redden, Staud, Bradley, and Fillingim 2013) Indeed, as has been proposed for gerontology in general (Alkema and Alley 2006), age-related changes in pain are best conceptualized in the context of the biopsychosocial model, which posits that the experience of pain is sculpted by dynamic interactions between biological and psychosocial forces.(Kerns, Sellinger, and Goodin 2011) Complicating matters, older adults are also at increased risk for under treatment of pain and for adverse effects when pain treatment is provided.(Helme and Gibson 2001) (Karttunen, Turunen, Ahonen, and Hartikainen 2014) Despite the prevalence and adverse impact of pain among older adults, the mechanisms underlying age-related increases in pain and their contribution to functional decline remain poorly understood, and treatments designed to reduce pain and pain-related disability among older adults are woefully underdeveloped.
Given the relationship between pain and aging is multifaceted and dynamic, research efforts are needed across numerous pathways and from multiple directions. Age-related changes in sensory (i.e., increased central sensitization, pain facilitation, reduced pain inhibition)(Cruz-Almeida, Sibille, Goodin, Petrov, Bartley, Riley, III, King, Glover, Sotolongo, Herbert, Schmidt, Fessler, Staud, Redden, Bradley, and Fillingim 2014;Riley, III, Cruz-Almeida, Glover, King, Goodin, Sibille, Bartley, Herbert, Sotolongo, Fessler, Redden, Staud, Bradley, and Fillingim 2014) and immune (i.e., increased pro-inflammatory profiles) function,(Naugle, Cruz-Almeida, Vierck, Mauderli, and Riley, III 2015) as well as central and peripheral changes in structure of the nervous system (i.e., decreased number of myelinated afferents, decreased cortical and subcortical connectivity) can contribute to both pain and functional decline among older adults.(Cruz-Almeida, Black, Christou, and Clark 2014) Moreover, premorbid cognitive dysfunction contributes to the development and/or persistence of chronic pain, (Lavand'homme 2011;Solberg, Roach, and Segerstrom 2009;Williams 2010) although few studies have examined age-specific differences.(Oosterman, Gibson, Pulles, and Veldhuijzen 2013) Additionally, the contribution of pain to the development of sarcopenia and/or dynapenia is not currently known. Accumulating evidence indicates that recurring pain (via its associated somatosensory dysfunction) can induce long-term adaptation of the motor system, including reorganization of neural motor control (Tsao, Galea, and Hodges 2008;Tsao, Galea, and Hodges 2010) as well as biochemical and morphological changes to muscle tissue.(Gerdle, Lemming, Kristiansen, Larsson, Peolsson, and Rosendal 2008;Gerdle, Soderberg, Salvador, Rosendal, and Larsson 2010;Rosendal, Larsson, Kristiansen, Peolsson, Sogaard, Kjaer, Sorensen, and Gerdle 2004) Further research is needed to elucidate the complex interactions between pain, functional decline, age related disability, sarcopenia, and dynapenia. Additionally, living with chronic pain is stressful further burdening biological system functioning, and ultimately contributing to allostatic overload, accelerated cellular aging, and increased morbidity and mortality.(Andersson 2004;Sibille, Witek-Janusek, Mathews, and Fillingim 2012;Szanton, Allen, Seplaki, Bandeen-Roche, and Fried 2009;Torrance, Elliott, Lee, and Smith 2010) Identifying risk factors, including resilience and vulnerability factors, that impact the onset and/or consequences of chronic pain will permit development of targeted interventions for reducing pain, improving physical functioning and enhancing quality of life in our aging population.
2.2 Emerging Evidence of Behavioral Risk Factors for Functional Decline
A number of behavior factors have been associated with the pathogenesis of functional decline and physical disability (in non-acute disease conditions) in older adults.(Anton, Karabetian, Naugle, and Buford 2013;Buford, Anton, Judge, Marzetti, Wohlgemuth, Carter, Leeuwenburgh, Pahor, and Manini 2010a) An increased understanding of such factors can assist in identifying at risk older adults. In this section, we will briefly review the contribution of each of these factors to reductions in physical function during aging.
2.2.1 Under/Over Nutrition
Both humans and rodents demonstrate a steady increase in body weight and adiposity through early senescence followed by a decline in later life. The development of leptin resistance, as seen in senescent rats, may be one factor contributing to increased adiposity with age.(Scarpace and Tumer 2001) Leptin behaves as a potent anorexic and energy-enhancing hormone in most young or lean animals, but its effects are diminished or lacking in the aged or obese state. Emerging evidence suggests that leptin resistance predisposes animals to exacerbated diet-induced obesity.(Scarpace and Zhang 2009) Recent studies have found that aged rats consume a greater amount of highly palatable high-fat (HF) diet than young rats, leading to exacerbated weight gain mostly in the form of fat. These finding indicate that susceptibility to diet-induced obesity increases with age.(Petervari, Rostas, Soos, Tenk, Miko, Furedi, Szekely, and Balasko 2014)
The findings described are concerning as epidemiological studies indicate that the per capita energy intake has significantly increased by approximately 300 kcal/d from 1985 to 2000, despite remaining fairly constant for the previous 75 years.(Finkelstein, Ruhm, and Kosa 2005) If left unchecked, a constant oversupply of energy and nutrients can contribute to increased adiposity during aging, which can accelerate functional decline and significantly increases risk for disability.(Baumgartner 2000a;Blaum, Xue, Michelon, Semba, and Fried 2005b) For example, higher fat mass has been associated with reductions in functional abilities in both healthy, postmenopausal women and older men.(Broadwin, Goodman-Gruen, and Slymen 2001;Lebrun, van der Schouw, de Jong, Pols, Grobbee, and Lamberts 2006) These changes occur even when body weight remains stable (Delmonico, Harris, Visser, Park, Conroy, Velasquez-Mieyer, Boudreau, Manini, Nevitt, Newman, and Goodpaster 2009) and are associated with increased fat depots within the muscle, as well as loss of muscle strength (Delmonico, Harris, Visser, Park, Conroy, Velasquez-Mieyer, Boudreau, Manini, Nevitt, Newman, and Goodpaster 2009) and power.(Tuttle, Sinacore, and Mueller 2012) Compared to older adults with a body mass index (BMI; kg/m2) in the healthy range (i.e., 20 – 24.9 kg/m2), obese, older adults have been found to be significantly more likely to experience impairments in activities of daily living.(Peeters, Bonneux, Nusselder, Laet, and Barendregt 2004) Such findings suggest that obese, older adults appear are at greater risk of functional decline compared to peers with a BMI in the healthy range, and therefore represent a high-risk group.(Vincent, Horodyski, Gearen, Vlasak, Seay, Conrad, and Vincent 2012)
2.2.2 Sedentary Lifestyle
In the US, comprehensive reports indicate daily energy expenditure in work-related physical activity has fallen by more than 100 calories per day during the past 50 years.(Brownson, Boehmer, and Luke 2005) Most adults in affluent countries live sedentary lifestyles and spend more than half their waking hours in sedentary pursuits. (Cohen, Matthews, Signorello, Schlundt, Blot, and Buchowski 2013;Maher, Mire, Harrington, Staiano, and Katzmarzyk 2013) Moreover, the amount of time engaged in sedentary behavior appears to increase with age.(Troiano, Berrigan, Dodd, Masse, Tilert, and McDowell 2008) These trends are concerning as physical inactivity is currently the most common behavioral risk factor for obesity in the United States.(2008;Physical activity guidelines for Americans 2008) Despite a growing body of evidence indicating time spent in sedentary pursuits represents an independent disease risk factor,(Henson, Yates, Biddle, Edwardson, Khunti, Wilmot, Gray, Gorely, Nimmo, and Davies 2013) most adults are currently unaware of the potential insidious health risks associated with high levels of sedentary behavior.(Shuval, Hebert, Siddiqi, Leonard, Lee, Tiro, McCallister, and Skinner 2013) Such findings raise a new concern regarding the health consequences of sedentary time that potentially have major clinical and public health significance.
Individuals have traditionally been defined as ‘active’ or ‘inactive’ based on whether or not they achieve minimum physical activity recommendations (i.e., 150 min of moderate intensity or 90 min of vigorous intensity exercise per week) as described by physical activity guidelines. (Physical activity guidelines for Americans 2008)
Although health risks associated with lack of engagement in moderate-to-vigorous intensity physical activity are clear,(WHO 2009) current definitions comingle the health consequences associated with lack of moderate-to-vigorous intensity activity with potential adverse health effects of extended periods of physical inactivity (sitting, reclining).(Sedentary Behaviour 2012) An important consequence of the current approach for classifying individuals as ‘active’ or ‘inactive’ is that the potential physiological effects of the types of activities that most adults engage in during the vast majority of their day, namely sedentary (sitting) and non-exercise light activities (standing, walking), are not well understood.(Hamilton, Hamilton, and Zderic 2007)
For over a quarter century, experts have differing opinions regarding the contribution of a sedentary lifestyle to age-related muscle loss.(Faulkner, Larkin, Claflin, and Brooks 2007) These differences in opinion are not surprising given inherent difficulties understanding the heterogeneity of physical activity patterns across the lifespan as well as challenges in measuring free-living activity. These challenges can be addressed through the development and standardization of assessment techniques for measuring the energy expended during physical activity. Recently, Manini et al. utilized the doubly-labeled water technique to measure energy expenditure during free-living activity in older adults.(Manini, Everhart, Anton, Schoeller, Cummings, Mackey, Delmonico, Bauer, Simonsick, Colbert, Visser, Tylavsky, Newman, and Harris 2009) Participants were divided into equal thirds based on energy expended through physical activity, and individuals in the highest third of daily activity energy expenditure had the greatest amount of lean mass. The rate of muscle loss across the most active individuals over five years was similar to those in the lowest third of free-living activity. These data suggest that high levels of physical inactivity may not directly accelerate the trajectory of sarcopenia; however, a sedentary lifestyle certainly adds to the risk of numerous pathological conditions, many of which do appear to accelerate sarcopenia progression.(Buford, Anton, Judge, Marzetti, Wohlgemuth, Carter, Leeuwenburgh, Pahor, and Manini 2010a)
2.2.3 Poor Sleep
Sleep problems are commonly underdiagnosed (Roth, Coulouvrat, Hajak, Lakoma, Sampson, Shahly, Shillington, Stephenson, Walsh, and Kessler 2011) and are a significant source of concern in older adults.(Ory, Smith, Ahn, Jiang, Lorig, and Whitelaw 2014) In older adults poor sleep has been associated with reduced physical function.(Foley, Ancoli-Israel, Britz, and Walsh 2004) Several diverse factors may contribute to sleep disturbances in a large percentage of older adults including retirement, health problems, death of spouse/family members,(Jackowska, Dockray, Hendrickx, and Steptoe 2011;Luo, Zhu, Zhao, Guo, Meng, Hong, and Ding 2013) and changes in circadian rhythm. Changes in sleep patterns may be part of the normal aging process; older adults generally have decreased sleep efficiency (time asleep divided by time in bed), stable or decreased total sleep time, and increased sleep latency (time to fall asleep).(Grandner, Martin, Patel, Jackson, Gehrman, Pien, Perlis, Xie, Sha, Weaver, and Gooneratne 2012) Older adults also report an earlier bedtime and earlier morning awakening, more awakenings during the night, more wakefulness during the night, and more daytime napping.(Grandner, Martin, Patel, Jackson, Gehrman, Pien, Perlis, Xie, Sha, Weaver, and Gooneratne 2012;Pace-Schott EF and Spencer RMC 2013) Abnormal sleep patterns were reported by over 15% of older adults who also reported limitations in ambulatory function. (Rantanen, Volpato, Ferrucci, Heikkinen, Fried, and Guralnik 2003) Notably, many of these disturbances may be related to pathological processes that are not considered a normal part of aging.(Orozco-Solis and Sassone-Corsi 2014) Epidemiologic studies in older adults have demonstrated an association between sleep complaints and risk factors for sleep disturbance (e.g., chronic illness, multiple medical problems, mood disturbance, less physical activity, physical disability) but little association with older age, suggesting that these risk factors, rather than aging per se, account for much of the increase in insomnia with age.(Luo, Zhu, Zhao, Guo, Meng, Hong, and Ding 2013) Functional limitations related to poor sleep have been reported as difficulty performing 6 instrumental activities of daily living (IADLs) which included walking 2–3 blocks, walking up or down 10 steps, preparing meals, heavy housework, and shopping.(Gregg, Mangione, Cauley, Thompson, Schwartz, Ensrud, and Nevitt 2002) In addition to affecting quality of life because of excessive daytime sleepiness, as well as physical, psychological, and cognitive problems, sleep disorders have been associated with increased mortality.(Kripke, Langer, Elliott, Klauber, and Rex 2011) There is a lower mortality risk when individuals sleep 6–7 hours per night.(Patel, Ayas, Malhotra, White, Schernhammer, Speizer, Stampfer, and Hu 2004) The relationship between sleep and physical limitation based on neuromuscular performance is complex, but it has been shown that poor sleep as measured by actigraphy may be associated with functional limitations.(Goldman, Stone, Ancoli-Israel, Blackwell, Ewing, Boudreau, Cauley, Hall, Matthews, and Newman 2007) In addition, the number of medications used tends to increase with age, which can lead to increased morbidity, mortality, and side effects such as falls, cognitive impairment, and even sleep disturbances.(Huang, Mallet, Rochefort, Eguale, Buckeridge, and Tamblyn 2012)
2.2.4 Environmental Stress
Environmental stressors are a major contributor to mortality and declining health in older individuals.(Leahy and Crews 2012) Among numerous environmental stressors, three have been extensively researched and appear to have the greatest impact: air pollution, extreme temperature fluctuations, and mobility barriers within living environments.
In the last 10 years there has been a call to focus on the impact of air pollution research in older adults.(Sandstrom, Frew, Svartengren, and Viegi 2003) Outdoor air pollution (i.e., particulate and chemical), in major population centers around the world, is directly responsible for increased mortality and declining health, especially with regards to respiratory illness.(Bentayeb, Simoni, Baiz, Norback, Baldacci, Maio, Viegi, and Annesi-Maesano 2012;Kunzli, Kaiser, Medina, Studnicka, Chanel, Filliger, Herry, Horak, Jr., Puybonnieux-Texier, Quenel, Schneider, Seethaler, Vergnaud, and Sommer 2000;Pope, III, Ezzati, and Dockery 2009;Saldiva, Pope, III, Schwartz, Dockery, Lichtenfels, Salge, Barone, and Bohm 1995;Wen and Gu 2012;Zhang, Mauzerall, Zhu, Liang, Ezzati, and Remais 2010) Older individuals are particularly susceptible given their compromised immune systems (Bentayeb, Simoni, Baiz, Norback, Baldacci, Maio, Viegi, and Annesi-Maesano 2012) and predisposition to respiratory infections, and as such are vulnerable to increased risk for chronic obstructive pulmonary disease (COPD).(Bentayeb, Simoni, Baiz, Norback, Baldacci, Maio, Viegi, and Annesi-Maesano 2012) There are limited studies measuring mobility issues and more globally disability; however, due to the scope of the problem in developing countries such China, where there are fewer regulations for monitoring and controlling air pollution, this population has been better characterized. Using the Chinese Longitudinal Health Longevity Survey cohort, Wen & Gu designed a multilevel prospective cohort study based on a nationally representative sample of older Chinese men and women, comparing those individuals living in provinces with good versus poor air quality. Whereas women were shown to have a higher mortality rate than men in poor air quality zones, both sexes experienced a 42–47% odds increase for being deficient in at least one of six measured activities of daily living (ADLs) (Wen and Gu 2012). Indeed, ADL was the most affected health outcome measured (among 20) in this study.
Acute temperature extremes experienced in the context of heat/cold waves are responsible for increased mortality in a variety of vulnerable subgroups including older adults.(Pan, Li, and Tsai 1995) The real threat of global climate change has hastened the need for evaluation and prediction of societal healthcare costs and particularly in vulnerable populations including the elderly.(Keatinge and Donaldson 2004;McGeehin and Mirabelli 2001;O'Neill and Ebi 2009;Zanobetti, O'Neill, Gronlund, and Schwartz 2012) Regardless of race and gender, individuals over the age of 65 are more susceptible than their younger counterparts to heat-related mortality.(Astrom, Forsberg, and Rocklov 2011;McGeehin and Mirabelli 2001) Fewer studies address the relationship to declining physical function; however, this was internationally highlighted by the heat wave experienced in August 2003 across Europe. A study conducted in Modena, Italy (Foroni, Salvioli, Rielli, Goldoni, Orlandi, Zauli, Guerzoni, Maccaferri, Daya, and Mussi 2007) demonstrated that older individuals with deficiencies in ADLs were more likely to succumb to the effects of the heat wave than those with no deficiencies in ADLs. The relationship between extremely cold winters is inconsistent with some studies showing increased mortality in the elderly and others not finding this relationship. (Conlon, Rajkovich, White-Newsome, Larsen, and O'Neill 2011) The Chinese Longitudinal Health Longevity Survey demonstrated that very low seasonal temperature increased the odds of ADL disability by 44% and increased mortality by 32%.(The CLHLS Research Team 2008) There was no effect of high temperatures on ADLs, however. These data demonstrate that environmental temperature regulation (i.e., air conditioning in the summer; heating in the winter) may serve as a frontline intervention for preventing and/or protecting older adults with or at risk of disability.
Finally, community mobility barriers and facilitators may influence the ability of older individuals to ambulate in their social environment and hence remain independent/dependent in their activities of daily living. Much of this research has come from the Multi-center Osteoarthritis Study (MOST), a prospective study of community-dwelling adults with or at risk for developing systemic knee osteoarthritis.(Keysor, Jette, LaValley, Lewis, Torner, Nevitt, and Felson 2010) The Home and Community Survey was used to ascertain daily activity limitation and daily activity frequency with the Late-Life Disability Instrument. Approximately one third of individuals in this cohort were more likely to have high mobility barriers and low transportation facilitators. Interestingly, individuals with functional limitations living in restrictive communities felt limited in their daily activities but did not perform tasks less frequently. Furthermore, investigators demonstrated that those individuals living in neighborhoods with no parks or walking areas engaged less frequently in regular fitness whereas those with neighborhoods with adequate handicap parking were more likely to engage in social and work activities.(Gray, Hollingsworth, Stark, and Morgan 2008;White, Jette, Felson, LaValley, Lewis, Torner, Nevitt, and Keysor 2010) Thus, social engineering of communities and neighborhoods that provide walking areas adequate handicap parking and public transportation may prevent and/or provide access to older individuals with or at risk for mobility disability.
2.2.5 Depression and Other Psychosocial Factors
Aging is associated with an increased risk for depressive symptoms (Alexopoulos 2005), which have been linked with functional decline. In fact, the World Health Organization estimates that depression is the leading cause of functional disability worldwide. A recent review identified a positive, and potentially bidirectional, association between depressive symptoms and functional impairment. (Mezuk, Edwards, Lohman, Choi, and Lapane 2012) In particular, cross-sectional studies provide evidence that older adults with functional deficits experience more depressive symptoms than robust individuals. (Chang and Chueh 2011;Ni Mhaolain, Fan, Romero-Ortuno, Cogan, Cunningham, Lawlor, and Kenny 2012)
The effect may be linear, such that increasing severity of depressive symptoms is associated with increasing functional deficits (St John, Tyas, and Montgomery 2013), and functional ability scores improve as depressive symptoms remit. (Ormel, Oldehinkel, Brilman, and vanden Brink 1993). In addition, longitudinal investigations have shown that depression predicts future frailty (Fried, Tangen, Walston, Newman, Hirsch, Gottdiener, Seeman, Tracy, Kop, Burke, and McBurnie 2001;Woods, LaCroix, Gray, Aragaki, Cochrane, Brunner, Masaki, Murray, and Newman 2005) and functional disability. (Carbonare LD, Maggi S, Giannini S, Rozzini R, Cascio VL, Crepaldi G, and ILSA Working Group 2009;Hybels, Pieper, and Blazer 2009) In turn, there is evidence that onset of functional decline leads to increased depressive symptoms over time in older adults. (Kennedy, Kelman, and Thomas 1990;Lyness, Chapman, McGriff, Drayer, and Duberstein 2009;Roberts, Kaplan, Shema, and Strawbridge 1997;Yang and George 2005)
The relationship between depression and functional decline does not appear to be fully explained by somatic comorbidities. In an investigation of symptom dimensions of depression, which included symptoms of negative affect, lack of positive affect, somatic symptoms, and interpersonal difficulties, all symptoms were associated with frailty except for interpersonal difficulties. (St John, Tyas, and Montgomery 2013) This suggests that affective symptoms of depression contribute to the depression–functional ability relationship. There also is evidence that anhedonia and apathy, common non-somatic symptoms of depression that are also prevalent in non-clinically depressed populations (Ishii, Weintraub, and Mervis 2009;Ritchie, Hearld, Gross, Allman, Sawyer, Sheppard, Salanitro, Locher, Brown, and Roth 2013) confer risks for physical disability, functional impairment, and death independent of depression (Covinsky, Cenzer, Yaffe, O'Brien, and Blazer 2014;Holtta, Laakkonen, Laurila, Strandberg, Tilvis, and Pitkala 2012). Evidence that depression increases the risk for functional decline independent of the presence or severity of medical illness (Ng, Niti, Zaw, and Kua 2009;Patten 1999;Steffens, Hays, and Krishnan 1999) further suggests that somatic comorbidities do not fully explain the relationship between depressive symptoms and functional ability.
The mechanisms underlying the relationship between depressive symptoms and functional decline have not been fully elucidated, but likely involve a complex interrelationship of psychosocial, cognitive, and brain changes. Depressed individuals may be at risk for functional decline due to poor self-care, including poor nutritional intake and disengagement from daily activities. (Katon 2011) Conversely, functional decline may lead to depression by limiting participation in social, leisure, and physical activities, thereby increasing chronic stress and social isolation. (Carriere, Villebrun, Peres, Stewart, Ritchie, and Ancelin 2009;James, Wilson, Barnes, and Bennett 2011) This hypothesis is supported by evidence that the diversity of social networks, availability of confidants, satisfaction with social support, sense of control and self-esteem mediate the relationship between depression and functional decline. (Escobar-Bravo, Puga-Gonzalez, and Martin-Baranera 2012)
Biological factors might also have an important role. For example, depression-related changes in the hypothalamic–pituitary–adrenal (HPA) axis and the immunological system may aggravate medical illnesses, leading to functional decline. (Furtado and Katzman 2015). Furthermore, depression is also associated with cognitive and brain changes, (Dotson, Resnick, and Zonderman 2008;Dotson, Szymkowics, Kirton, McLaren, Green, and Rohani 2014;Dotson, Zonderman, Davatzikos, Kraut, and Resnick 2009;Dotson, Zonderman, Kraut, and Resnick 2013) which are also known to impact functional abilities.
In addition to depression, psychosocial influences pertaining to self-regulatory and emotion-regulatory strategies, personality, social isolation, stress, and psychiatric symptoms and disorders have recently been identified as contributing to risk for functional decline. (Baltes and Baltes 1993;Dark-Freudeman, Ebner, and West 2014;Ebner and Freund 2007;Freund and Ebner 2005) Psychosocial influences may become particularly relevant in old age possibly due to the experience of increasing physical ailments, dependency, and age-related social losses.(Ebner, Maura, Macdonald, Westberg, and Fischer 2013) For example, self-efficacy and control beliefs are associated with fear of falling, gait speed, balance, worse pain management, and limitations in ADLs.(Dark-Freudeman, Ebner, and West 2014) Heightened levels of negative emotions predict worse cardiovascular health, (Suls and Bunde 2005) and inefficient emotionregulatory strategies are associated with increased levels of inflammation.(Appleton, Buka, Loucks, Gilman, and Kubzansky 2013) Goal orientation and selection appears to affect well-being with goal orientation towards maintenance being associated with well-being in older adults.(Ebner, Freund, and Baltes 2006)
2.3 Evolving Concepts on Biological Mechanisms Underlying Functional Decline
Several biological factors have been associated with the pathogenesis of functional decline during aging and physical disability (in non-acute disease conditions), but the exact mechanisms contributing to age-related functional decline remain largely undefined. In this section, we will briefly review specific biological mechanisms that have been directly linked with physical function and appear to play an important role in maintenance of physical function in older adults. These mechanisms, which are described below, include mitochondrial function and dynamics, autophagy, oxidative stress, chronic inflammation, muscle composition, hormonal factors, and neurodegeneration.
As diverse as the etiologies of physical disability are, a growing body of evidence strongly implicates the mitochondria (Mt) as playing a key role in the initial onset and progression of functional decline in many individuals.(Garnier, Fortin, Zoll, N'Guessan, Mettauer, Lampert, Veksler, and Ventura-Clapier 2005a;Safdar, Hamadeh, Kaczor, Raha, and Tarnopolsky 2010) Mitochondria are dynamic organelles responsible for the plasticity of skeletal muscle and its ability to adapt to the ever changing metabolic demands of the cell. Given that Mt are the main energy producing organelles of the body, it is not surprising that they play a central role in the maintenance of muscle mass and that their dysfunction may contribute to sarcopenia. Abundant evidence points to mitochondrial dysregulation in aging tissues including altered gene expression pathways, aberrant quality control processes, and activation of myonuclear cell death.(Calvani, Joseph, Adhihetty, Miccheli, Bossola, Leeuwenburgh, Bernabei, and Marzetti 2013) Additionally, accumulating evidence from both preclinical studies and recent human clinical trials strongly suggests that Mt have a key role in the pathogenesis of age-related functional decline, marked by slower walking speed.(Hiona and Leeuwenburgh 2008;Studenski, Perera, Patel, Rosano, Faulkner, Inzitari, Brach, Chandler, Cawthon, Connor, Nevitt, Visser, Kritchevsky, Badinelli, Harris, Newman, Cauley, Ferrucci, and Guralnik 2011) Abnormalities in mitochondrial enzyme activity, (Rooyackers, Adey, Ades, and Nair 1996;Short, Bigelow, Kahl, Singh, Coenen-Schimke, Raghavakaimal, and Nair 2005a) lower mitochondrial protein synthesis rates,(Rooyackers, Adey, Ades, and Nair 1996) oxidative capacity, and adenosine triphosphate (ATP) synthesis(Short, Bigelow, Kahl, Singh, Coenen-Schimke, Raghavakaimal, and Nair 2005a) have been observed in the skeletal muscle of older persons. In addition, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), a powerful regulator of mitochondrial oxidative metabolism,(Liang and Ward 2006;Russell 2005;Spiegelman 2007) shows marked declines with aging.(Corton and Brown-Borg 2005;Ling, Poulsen, Carlsson, Ridderstrale, Almgren, Wojtaszewski, Beck-Nielsen, Groop, and Vaag 2004) Specifically, declines in PGC-1α have been associated with reductions in mitochondrial oxidative metabolism which is associated with poor muscle quality and function.(Garnier, Fortin, Zoll, N'Guessan, Mettauer, Lampert, Veksler, and Ventura-Clapier 2005b;Little, Safdar, Bishop, Tarnopolsky, and Gibala 2011;Safdar, Hamadeh, Kaczor, Raha, Debeer, and Tarnopolsky 2010)
To date, reasons for the decrease in mitochondrial function with aging remain under debate and the molecular details are still not well understood; however, a growing body of evidence implicates reactive oxygen species (ROS) in contributing to Mt damage.(Vermulst, Wanagat, Kujoth, Bielas, Rabinovitch, Prolla, and Loeb 2008) In line with this, the mitochondrial theory of aging proposes that cumulative damage caused by the production of oxidants can alter MtDNA (e.g., point mutations and increase deletions). According to this theory, an overproduction of ROS from the electron transport chain (ETC) within the inner mitochondrial membrane are the driving force for the mitochondrial dysfunction associated with age-related muscle atrophy.(Harman 1972;Miquel, Economos, Fleming, and Johnson Jr 1980) The mitochondrial genome is particularly susceptible to free radical (oxidant) damage because of its close proximity to ROS generation and the lack of protective histones. In fact, there is substantial evidence showing increased ROS production and associated oxidative stress-induced damage to mitochondrial DNA (mtDNA) and ETC complexes in aged rodents and non-human primates (Figueiredo, Powers, Ferreira, Appell, and Duarte 2009;Lee, Choi, Birkenfeld, Alves, Jornayvaz, Jurczak, Zhang, Woo, Shadel, and Ladiges 2010;Lee, Wang, and Fanburg 1998;WANAGAT, CAO, PATHARE, and AIKEN 2001), as well as in the vastus lateralis muscle of humans.(Bua, Johnson, Herbst, Delong, McKenzie, Salamat, and AIKEN 2006) Increased damage to mtDNA can lead to abberrations in mtDNA-encoded ETC subunits affecting their assembly function and ultimately ATP production. Moreover, these impairments can further exacerbate the production of ROS leading to a vicious cycle. Additionally, damage to mitochondrial proteins and lipids may also contribute to mitochondrial dysfunction by not only affecting oxidative phosphorylation and ETC activity but also by altering the susceptibility of Mt to apoptosis.(Conley, Marcinek, and Villarin 2007;Dirks and Leeuwenburgh 2002;Gonzalvez and Gottlieb 2007;Paradies, Petrosillo, Pistolese, and Ruggiero 2002;Shidoji, Hayashi, Komura, Ohishi, and Yagi 1999) Recently, the use of a mitochondrial-targeted antioxidant peptide (SS-31) has been shown to inhibit ROS production and skeletal muscle atrophy in a variety of experimental animal models of inactivity (Min, Smuder, Kwon, Kavazis, Szeto, and Powers 2011;Powers, Hudson, Nelson, Talbert, Min, Szeto, Kavazis, and Smuder 2011) suggesting the importance of mitochondrially-produced ROS in skeletal muscle atrophy.
2.3.1 Mitochondrial Quality Control Processes; Dynamics and Autophagy
Mitochondrial dynamics are another important determinant of organelle quality control and the maintenance of genome integrity by facilitating the renewal of healthy mitochondria and the removal of unhealthy/damaged organelles. (Busch, Kowald, and Spelbrink 2014) Mitochondrial morphology is governed by fusion and fission proteins including the dynamin-related GTPases mitofusin 1 and 2 (Mfn1 and Mfn2) and optic atrophy protein 1 (Opa1),as well as dynamin-related protein 1 (Drp1) and fission protein 1 (Fis1) respectively.(Seo, Joseph, Dutta, Hwang, Aris, and Leeuwenburgh 2010) Fusion is responsible for the formation of an interconnected mitochondrial network and the distribution of metabolites, proteins, and mtDNA, while fission facilitates the removal of damaged organelles by lysosomal degradation pathways known as autophagy.(Twig, Elorza, Molina, Mohamed, Wikstrom, Walzer, Stiles, Haigh, Katz, and Las 2008) Muscle-specific deletion of Mfn1 and Mfn2 results in the accumulation of mtDNA mutations and deletions, reduced mtDNA content, as well as muscle atrophy.(Chen, Vermulst, Wang, Chomyn, Prolla, McCaffery, and Chan 2010) Moreover, enlarged mitochondria indicative of dyshomeostasis between fusion and fission (i.e. greater fusion and/or lower fission events) have previously been documented in aging muscles.(Tandler and Hoppel 1986) Similarly, increased fission via overexpression of fission proteins Drp1 and Fis1 leads to fragmented mitochondria and increased autophagy culminating in muscle atrophy.(Romanello, Guadagnin, Gomes, Roder, Sandri, Petersen, Milan, Masiero, Del, Foretz, Scorrano, Rudolf, and Sandri 2010)
Aging is characterized by a reduction in efficiency of cellular maintenance, repair, and turnover mechanisms resulting in the accumulation of lipids and damaged biomolecules and organelles.(Singh and Cuervo 2011;Weber and Reichert 2010;Wohlgemuth, Calvani, and Marzetti 2014;Yang, Li, Fu, Calay, and Hotamisligil 2010) The cellular quality control (QC) process of autophagy represents an important but underexplored biological process affecting age-related changes in muscle composition and functional decline.(Baumgartner 2000b;Blaum, Xue, Michelon, Semba, and Fried 2005a;Cesari, Kritchevsky, Baumgartner, Atkinson, Penninx, Lenchik, Palla, Ambrosius, Tracy, and Pahor 2005) It is generally accepted that ROS can trigger the induction of macroautophagy,(Scherz-Shouval and Elazar 2011) and the selective removal of damaged mitochondria, a major source and target of ROS, following oxidative stress has been reported.(Kim, Rodriguez-Enriquez, and Lemasters 2007) Impairments in autophagy can result in the accumulation of damaged mitochondria,(Brunk and Terman 2002;Terman and Brunk 2005) reductions in the bioenergetic status of the cell,(Marzetti, Lees, Wohlgemuth, and Leeuwenburgh 2009;Pyo, Yoo, and Jung 2013) and in myocyte apoptosis,(Wohlgemuth, Calvani, and Marzetti 2014) which may be reflected in decreased muscle strength(Buford, Anton, Judge, Marzetti, Wohlgemuth, Carter, Leeuwenburgh, Pahor, and Manini 2010b;Buford, Lott, Marzetti, Wohlgemuth, Vandenborne, Pahor, Leeuwenburgh, and Manini 2012) and slower walking speed observed with aging.(Marzetti, Calvani, Bernabei, and Leeuwenburgh 2012;Nocera, Buford, Manini, Naugle, Leeuwenburgh, Pahor, Perri, and Anton 2011) Thus, evidence to date strongly implicates the mitochondria as having a pivotal role in the pathogenesis of age-related functional decline,(Coen, Jubrias, Distefano, Amati, Mackey, Glynn, Manini, Wohlgemuth, Leeuwenburgh, Cummings, Newman, Ferrucci, Toledo, Shankland, Conley, and Goodpaster 2013) and reductions in autophagy appear to be a critical biological mechanism affecting the collective function of the mitochondrial pool.(Weber and Reichert 2010;Zhu, Wang, and Chu 2013)
A growing body of evidence indicates DNA oxidative damage is also associated with impairments in bioenergetics and key defects in mitochondrial quality control processes including biogenesis, morphology, and autophagy.(Joseph, Adhihetty, Buford, Wohlgemuth, Lees, Nguyen, Aranda, Sandesara, Pahor, and Manini 2012;Short, Bigelow, Kahl, Singh, Coenen-Schimke, Raghavakaimal, and Nair 2005b) The majority of proteins required for mitochondrial biogenesis are encoded in the nucleus, targeted to mitochondria, and assembled into subcompartments where they form functional holoenzyme complexes.(Joseph, Pilegaard, Litvintsev, Leick, and Hood 2006) Improper gene expression, translation, import, and assembly can lead to the impaired assembly of ETC subunits and deficits in energy production. Of particular importance is PGC-1α, known as the master regulator of mitochondrial biogenesis because of its ability to coactivate and upregulate the coordinated expression of a number of genes required for substrate metabolism and biogenesis.(Handschin and Spiegelman 2006) PGC-1α protein and its downstream molecular targets are reduced in aging animals (Baker, Betik, Krause, and Hepple 2006) and humans.(Joseph, Adhihetty, Buford, Wohlgemuth, Lees, Nguyen, Aranda, Sandesara, Pahor, and Manini 2012;Safdar, Hamadeh, Kaczor, Raha, and Tarnopolsky 2010) In fact, forced overexpression of this gene in muscle attenuates mitochondrial dysfunction and the associated muscle atrophy in aging animals.(Wenz, Rossi, Rotundo, Spiegelman, and Moraes 2009) Thus, the ability of the cell to repair the damage and maintain genome stability may be impaired with age leading to a greater rate of damage formation than damage removal.
2.3.2 Mitochondrial DNA Mutations
The mammalian mitochondrial genome consists of 37 genes, encoding 13 proteins of the electron transport oxidative phosphorylation system.(Anderson, Bankier, Barrell, de Bruijn, Coulson, Drouin, Eperon, Nierlich, Roe, Sanger, Schreier, Smith, Staden, and Young 1981) Since mtDNA encoded proteins play a critical role in energy metabolism, mtDNA mutations have been hypothesized to accelerate the aging processes and contribute to age-related diseases. Indeed, specific mutations in mtDNA cause a number of mitochondrial disorders in humans including mitochondrial encephalomyopathy, lactic acidosis, stroke-like episodes (MELAS), and myoclonic epilepsy and ragged red fibers (MERRF). Noteworthy, more than 100 different deletions of mtDNA have been associated with mitochondrial disorders.(Chinnery, Elliott, Green, Rees, Coulthard, Turnbull, and Griffiths 2000;Kujoth, Bradshaw, Haroon, and Prolla 2007;Someya and Prolla 2010) Nuclear-encoded POLG is the only known DNA polymerase in mitochondria and has conserved polymerase and exonuclease domains, which repair mtDNA mutations.(Kujoth, Bradshaw, Haroon, and Prolla 2007) There are over 80 pathogenic mutations in POLG in humans, including progressive external ophthalmoplegia (PEO), Alpers syndrome, and ataxia. Most reported mutations are recessive, commonly found in combination with other mutations in POLG, and associated with a variety of symptoms including ophthalmoplegia, cataracts, progressive muscle weakness, parkinsonism, premature ovarian failure, male infertility, hearing loss, and cardiac dysfunction.(Kujoth, Bradshaw, Haroon, and Prolla 2007;Mancuso, Filosto, Bellan, Liguori, Montagna, Baruzzi, DiMauro, and Carelli 2004) Taken together, these observations strongly suggest that accumulation of mtDNA mutations plays a key role in the development of a variety of age-related disorders and impairment.
2.3.3 Inflammation
A consistent observation of aging in humans and animals is a persistent low level increase in serum markers of inflammation, such as C-reactive protein and interleukin-6.(Chung, Cesari, Anton, Marzetti, Giovannini, Seo, Carter, Yu, and Leeuwenburgh 2009) Markers of inflammation in the blood and in specific tissues are associated with impaired motor and cognitive function (Blalock, Chen, Sharrow, Herman, Porter, Foster, and Landfield 2003;Speisman, Kumar, Rani, Foster, and Ormerod 2013;Zeier, Madorsky, Xu, Ogle, Notterpek, and Foster 2011) suggesting that the pro-inflammatory phenotype impairs the ability of older individuals to cope with age-related stressors and thus contributes to functional decline. Interestingly, anti-inflammatory treatments or behavioral therapies that reduce inflammatory markers in the blood are associated with improved cognitive performance and immune function.(Speisman, Kumar, Rani, Foster, and Ormerod 2013)
Growing evidence shows that low-grade chronic inflammation, characterized by elevations in plasma C-reactive protein (CRP), tumor necrosis factor Alpha (TNF-α), and particularly interleukin-6 (IL-6),(Brinkley, Leng, Miller, Kitzman, Pahor, Berry, Marsh, Kritchevsky, and Nicklas 2009;Cesari, Penninx, Pahor, Lauretani, Corsi, Guralnik, and Ferrucci 2004;Ferrucci, Harris, Guralnik, Wacholder, Tracy, Corti, Penninx, Pahor, Wallace, and Havlik 1999;Hsu, Kritchevsky, Liu, Kanaya, Newman, Perry, Visser, Pahor, Harris, and Nicklas 2009;Penninx, Kritchevsky, Newman, Nicklas, Simonsick, Rubin, Nevitt, Visser, Harris, and Pahor 2004;Sanders, Ding, Arnold, Kaplan, Cappola, Kizer, Boudreau, Cushman, and Newman 2014;Singh and Newman 2011) is an independent risk factor for disability, impaired mobility, and slow walking speed.(Brinkley, Leng, Miller, Kitzman, Pahor, Berry, Marsh, Kritchevsky, and Nicklas 2009;Cesari, Marzetti, Laudisio, Antonica, Pahor, Bernabei, and Zuccala 2010;Hsu, Kritchevsky, Liu, Kanaya, Newman, Perry, Visser, Pahor, Harris, and Nicklas 2009;McDermott, Liu, Ferrucci, Tian, Guralnik, Green, Tan, Liao, Pearce, Schneider, McCue, Ridker, Rifai, and Criqui 2008;Penninx, Abbas, Ambrosius, Nicklas, Davis, Messier, and Pahor 2004;Penninx, Kritchevsky, Newman, Nicklas, Simonsick, Rubin, Nevitt, Visser, Harris, and Pahor 2004;Verghese, Holtzer, Lipton, and Wang 2012) The age-related dysregulation of immune function may have direct adverse consequences on physical function and promote disability by causing fatigue and loss of muscle strength.(Brinkley, Leng, Miller, Kitzman, Pahor, Berry, Marsh, Kritchevsky, and Nicklas 2009;Ferrucci, Penninx, Volpato, Harris, Bandeen-Roche, Balfour, Leveille, Fried, and Md 2002;Schaap, Pluijm, Deeg, Harris, Kritchevsky, Newman, Colbert, Pahor, Rubin, Tylavsky, and Visser 2009) The sources and consequences of chronic inflammation are important for identifying promising interventions (Figure 3.a.1.). Inflammation in older persons is affected by a numbers of factors including genes and their modifiers, the environment, and the in situ biochemical environment. Important exogenous environmental exposures include smoking, moderate (beneficial) and excessive alcohol consumption, and an anti-oxidative diet (beneficial). Pro-inflammatory endogenous factors include adiposity (especially visceral adiposity), subclinical disease burden, and oxidative stress, among others.
Regarding direct consequences, activation of NF-κB signaling increases muscle protein degradation and inhibits myogenesis,(Li, Malhotra, and Kumar 2008;Merritt, Stec, Thalacker-Mercer, Windham, Cross, Shelley, Craig, Kosek, Kim, and Bamman 2013) except in very late differentiation where it promotes homeostasis.(Li, Malhotra, and Kumar 2008) Low-grade chronic inflammation is a modifiable risk factor; however, it is unknown whether interventions that reduce the levels of inflammatory markers per se can improve mobility, or avert decline in mobility, in older adults.
2.3.4 Hormonal Factors
Successful aging is highly influenced by the interaction of genetic programming with endogenous and exogenous (i.e., hormonal replacement) hormonal changes.(Bean, Ianov, and Foster 2014;Foster 2012) In general, hormones are secreted from specific tissues in the body and the brain and transported in the blood to influence metabolic functions at close or distant sites. There is evidence that hormonal dysregulation during aging impacts multiple physiological systems including the muscle, brain, and immune system. This dysregulation includes an age-related decrease in some trophic hormones such as the gonadal steroids, estrogen and testosterone (Bean, Ianov, and Foster 2014), age-related increase in stress-related hormones such as cortisol(Bizon, Helm, Han, Chun, Pucilowska, Lund, and Gallagher 2001), and age-related changes in neuropeptides such as oxytocin.(Ebner, Maura, Macdonald, Westberg, and Fischer 2013) These hormones are crucially involved in the regulation of physiological and psychological processes related to aging.(Ebner, Kamin, Diaz, Cohen, and Macdonald 2014) For example, chronically high levels of cortisol have been shown to exert neurotoxic effects on muscles and the brain that contribute to age-related impairments. (Saleem 2011) In turn, estrogen and testosterone replacement may reduce risk for osteoporosis, cardiovascular disease, and Alzheimer’s disease.(Nguyen, Dolomie-Fagour, Georges, and Corcuff 2008) More recent work has revealed that delivery of oxytocin can have beneficial effects on socio-affective functioning. (Bean, Ianov, and Foster 2014;Ebner, Horta, Lin, Feifel, Fisher, and Cohen 2015;Fekete, Seay, Antoni, Mendez, Fletcher, Szeto, and Schneiderman 2014;Scheele, Wille, Kendrick, Stoffel-Wagner, Becker, Gunturkun, Maier, and Hurlemann 2013;Schneiderman, Zagoory-Sharon, Leckman, and Feldman 2012) We therefore propose that hormonal changes represent an important contribution to the multi-factorial processes underlying functional decline during aging.
2.3.5 Cerebral Neurodegeneration
Accumulating evidence indicates that age-related loss of function is strongly linked to degeneration of cerebral structure and function.(Rosso, Studenski, Chen, Aizenstein, Alexander, Bennett, Black, Camicioli, Carlson, Ferrucci, Guralnik, Hausdorff, Kaye, Launer, Lipsitz, Verghese, and Rosano 2013) Cerebral gray matter (primarily consisting of neuronal cell bodies) has been shown to atrophy with advancing age, (Hoffstaedter, Grefkes, Roski, Caspers, Zilles, and Eickhoff 2014) and gray matter volume has been linked to a variety of movement deficits. Bradykinesia has been shown to be associated with gray matter volume of the primary sensorimotor area.(Rosano, Bennett, Newman, Venkatraman, Yaffe, Harris, Kritchevsky, and Aizenstein 2012) Gait disturbances, such as slow speed, shorter steps and longer double support time, have been associated with reduced gray matter volume of the prefrontal, medial temporal, frontoparietal, and sensorimotor areas.(Rosano, Aizenstein, Brach, Longenberger, Studenski, and Newman 2008;Rosano, Aizenstein, Studenski, and Newman 2007;Rosano, Bennett, Newman, Venkatraman, Yaffe, Harris, Kritchevsky, and Aizenstein 2012) Cerebral white matter (i.e., myelinated axons) is also affected by aging. Most notably, an increase in size and intensity of white matter hyperintensities indicates the presence of demyelination and/or dilated perivascular spaces. White matter hyperintensity has been linked to mobility limitation, marked by slow walking speed,(Moscufo, Wolfson, Meier, Liguori, Hildenbrand, Wakefield, Schmidt, Pearlson, and Guttmann 2012;Rosano, Sigurdsson, Siggeirsdottir, Phillips, Garcia, Jonsson, Eiriksdottir, Newman, Harris, van Buchem, Gudnason, and Launer 2010;Viana-Baptista, Bugalho, Jordao, Ribeiro, Esperanca-Pina, and Ferro 2011;Wakefield, Moscufo, Guttmann, Kuchel, Kaplan, Pearlson, and Wolfson 2010;Willey, Scarmeas, Provenzano, Luchsinger, Mayeux, and Brickman 2013) and may attenuate the potential for rehabilitation induced gains in walking speed.(Nadkarni, Studenski, Perera, Rosano, Aizenstein, Brach, and Van Swearingen 2013) Altered connectivity in neuronal networks, as measured by transcranial magnetic stimulation, has also been associated with improvements in physical function in older adults.(McGinley, Hoffman, Russ, Thomas, and Clark 2010;Papegaaij, Taube, Baudry, Otten, and Hortobagyi 2014;Plow, Varnerin, Cunningham, Janini, Bonnett, Wyant, Hou, Siemionow, Wang, Machado, and Yue 2014) Emerging evidence suggests that chronic physical activity is a neuroprotective factor that may prevent or slow the course of cerebral neurodegeneration with advancing age.(Baezner, Blahak, Poggesi, Pantoni, Inzitari, Chabriat, Erkinjuntti, Fazekas, Ferro, Langhorne, O'Brien, Scheltens, Visser, Wahlund, Waldemar, Wallin, and Hennerici 2008;Gow, Bastin, Munoz, Valdes Hernandez, Morris, Murray, Royle, Starr, Deary, and Wardlaw 2012)
2.3.6 Peripheral Neurodegeneration
Weakness and functional decline have been linked to impairment of the peripheral nervous system including both motor (Resnick, Vinik, Schwartz, Leveille, Brancati, Balfour, and Guralnik 2000;Strotmeyer, de, Schwartz, Faulkner, Resnick, Goodpaster, Shorr, Vinik, Harris, and Newman 2008;Strotmeyer, de, Schwartz, Resnick, Goodpaster, Faulkner, Shorr, Vinik, Harris, and Newman 2009) and sensory (Buchman, Wilson, Leurgans, and Bennett 2009;Cruz-Almeida, Black, Christou, and Clark 2014;Deshpande, Ferrucci, Metter, Faulkner, Strotmeyer, Satterfield, Schwartz, and Simonsick 2008;Mold, Vesely, Keyl, Schenk, and Roberts 2004;Resnick, Vinik, Schwartz, Leveille, Brancati, Balfour, and Guralnik 2000) nerve function. A variety of factors can contribute to decline of nerve health in older adults including chronic inflammation, oxidative stress, diabetes, chronic infections, prolonged smoking, prolonged alcohol use, and prolonged vitamin deficiency.(Di, Cherubini, Volpato, Sparvieri, Lauretani, Franceschi, Senin, Abate, Paganelli, Martin, Andres-Lacueva, and Ferrucci 2006;Mallik and Weir 2005;Manini, Hong, and Clark 2013) Motor neuron degeneration is evidenced by a decline in conduction velocity and in the amplitude of the maximum evoked compound muscle action potential.(Di, Cherubini, Volpato, Sparvieri, Lauretani, Franceschi, Senin, Abate, Paganelli, Martin, Andres-Lacueva, and Ferrucci 2006;Rivner, Swift, and Malik 2001) Another well-documented change with aging is increased rates of apoptosis of the large diameter, fast conducting motor neurons that innervate powerful Type II muscle fibers,(Kawamura, O'Brien, Okazaki, and Dyck 1977;Mittal and Logmani 1987) which can affect functional capacity. This process begins in middle-adulthood and accelerates with increasing age.(Larsson, Grimby, and Karlsson 1979;Mittal and Logmani 1987) Thus, peripheral neurodegeneration appears to represent an important factor underlying age-related decline in physical function.
Sensory nerve degeneration also contributes to mobility deficits. For example, impaired somatosensation in the lower extremities has been linked to the age-related decline of balance and walking speed.(Buchman, Wilson, Leurgans, and Bennett 2009;Cruz-Almeida, Black, Christou, and Clark 2014;Deshpande, Ferrucci, Metter, Faulkner, Strotmeyer, Satterfield, Schwartz, and Simonsick 2008;Mold, Vesely, Keyl, Schenk, and Roberts 2004;Resnick, Vinik, Schwartz, Leveille, Brancati, Balfour, and Guralnik 2000) In order to enhance somatosensory input to lower extremity somatosensory receptors, a number of researchers have investigated shoe insole devices that use vibration or augmented texture. The evidence shows that a vibrating insole can improve sway parameters during standing balance in older adults.(Priplata, Niemi, Harry, Lipsitz, and Collins 2003) Similarly, a textured insole improves sway parameters during walking and standing balance in older adult.(Palluel, Nougier, and Olivier 2008;Palluel, Olivier, and Nougier 2009) Recent evidence suggests that enhancing somatosensory feedback can restore a more normal motor control strategy during walking.(Clark, Christou, Ring, Williamson, and Doty 2014) Thus, peripheral neurodegeneration appears to represent an important factor underlying age-related decline in physical function.
2.4 Promising Interventions to Enhance Physical Function and Mobility
Few therapies have been identified to improve functional performance in human aging, and there are currently no FDA-approved medications for the treatment of functional decline in older adults. Empirically supported treatments are urgently needed to improve functional ability and prevent mobility disability in older adults. This section will review promising interventions for functional enhancement in older adults including lifestyle, smart and connected technologies, cognitive training, exergaming, non-invasive brain stimulation, pharmaceutical, nutraceutical, and hormone-based interventions.
2.4.1 Lifestyle (Diet)
Dietary restriction (DR) may confer significant functional benefits by reducing the structural and biological consequences of excess adiposity. Dietary weight loss can directly impact physical function by improving muscle quality and reducing intramuscular adipose tissue(Manini, Buford, Lott, Vandenborne, Daniels, Knaggs, Patel, Pahor, Perri, and Anton 2014) and decreasing joint load (Messier, Loeser, Miller, Morgan, Rejeski, Sevick, Ettinger, Pahor, and Williamson 2004). Weight reduction in overweight individuals can also impact physical function indirectly by reducing pain(McCarthy, Bigal, Katz, Derby, and Lipton 2009) and fatigue.(Avlund 2010) In addition, DR can promote proper redox status (Hofer, Fontana, Anton, Weiss, Villareal, Malayappan, and Leeuwenburgh 2008;Ivanova and Yankova 2013), anti-inflammatory action(Chung, Cesari, Anton, Marzetti, Giovannini, Seo, Carter, Yu, and Leeuwenburgh 2009), and cellular quality control, as well as maintain a healthy population of mitochondria through biogenesis.(Aris, Alvers, Ferraiuolo, Fishwick, Hanvivatpong, Hu, Kirlew, Leonard, Losin, and Marraffini 2013;Dutta, Calvani, Bernabei, Leeuwenburgh, and Marzetti 2012) Furthermore, DR has been shown to be a potent stimulant of autophagy in preclinical models (Cavallini, Donati, Gori, Pollera, and Bergamini 2001;Donati, Cavallini, Paradiso, Vittorini, Pollera, Gori, and Bergamini 2001;Wohlgemuth, Julian, Akin, Fried, Toscano, Leeuwenburgh, and Dunn 2007), which may be a key mediator through which DR improves physical performance.(Jensen, Roy, Buchanan, and Berg 2004;Villareal, Chode, Parimi, Sinacore, Hilton, Armamento-Villareal, Napoli, Qualls, and Shah 2011)
Accumulating evidence indicates that DR leading to weight loss can improve physical function among obese older adults, independent of changes in physical activity.(Anton, Karabetian, Naugle, and Buford 2013) Yet, a concern associated with diet-induced weight loss is that it is comprised of reductions in both fat mass and fat free mass (Pourhassan, Bosy-Westphal, Schautz, Braun, Gluer, and Muller 2014;Wadstrom, Muller-Suur, and Backman 1991), and thus may exacerbate the progression of sarcopenia and further impair physical function. Therefore, a current challenge is finding ways to minimize the loss of fat-free mass that typically occurs during weight loss interventions targeting obese, older adults. Despite the promise of DR for improved physical function, few clinical trials have been conducted to support the efficacy of DR alone in obese, older adults. Jensen et al. examined changes in physical function after a three month dietary weight loss program for obese older women.(Jensen, Roy, Buchanan, and Berg 2004) At follow up, participants achieved a significant reduction in body weight and body composition parameters, in both fat and fat-free mass, and overall physical performance, as measured by a battery of physical tasks including 400-meter walk and stair climb, improved significantly. In line with these findings, self-reported physical functioning also improved significantly. In a one-year randomized controlled trial examining DR weight loss alone, exercise alone, and weight loss plus exercise compared to an educational control group, significant benefits related to physical function were found for all intervention groups.(Villareal, Chode, Parimi, Sinacore, Hilton, Armamento-Villareal, Napoli, Qualls, and Shah 2011) Although the weight loss plus exercise group showed the greatest improvements, the DR weight loss only group performed significantly better than the control group on measures of physical function.
Based on the findings of these studies, DR alone appears to be effective for improving physical functioning levels in obese older adults. More research is needed as the use of DR in older adult populations remains an area of significant debate due to concerns about lean muscle loss following DR. Limited evidence demonstrates that changes in fat mass following intentional weight loss may be more closely related to change in physical function compared to changes in lean mass.(Santanasto, Glynn, Newman, Taylor, Brooks, Goodpaster, and Newman 2010) Thus, reductions in body fat and change in body composition may be key physiological changes leading to improved physical function in this group. Importantly, little is known regarding the effects of DR on changes in physical function among healthy or overweight older adults, thus DR should be limited to obese older adults until this is better understood.
2.4.2 Lifestyle (Exercise)
To date, physical exercise is the only intervention consistently demonstrated to attenuate functional decline among older adults.(Brown, Sinacore, Ehsani, Binder, Holloszy, and Kohrt 2000;Cress, Buchner, Questad, Esselman, deLateur, and Schwartz 1999;Miszko, Cress, Slade, Covey, Agrawal, and Doerr 2003;Nelson, Layne, Bernstein, Nuernberger, Castaneda, Kaliton, Hausdorff, Judge, Buchner, Roubenoff, and Fiatarone Singh 2004;Pahor, Blair, Espeland, Fielding, Gill, Guralnik, Hadley, King, Kritchevsky, Maraldi, Miller, Newman, Rejeski, Romashkan, and Studenski 2006) Regardless of dependent outcome, most studies in older adults show some degree of benefit to exercise when based on changes in the mean score of a given performance metric. However, these benefits are not observed in all individuals and the change in performance is quite variable.(Kohrt, Malley, Coggan, Spina, Ogawa, Ehsani, Bourey, Martin, III, and Holloszy 1991) A variety of participant-specific factors may limit gains in functional performance. For example, Manini et al. recently reported that obesity attenuated exercise-induced improvements in physical function among older adults in the Lifestyle Interventions for Independence Pilot Study.(Manini, Newman, Fielding, Blair, Perri, Anton, Goodpaster, Katula, Rejeski, Kritchevsky, Hsu, Pahor, and King 2010) In the same cohort, Buford et al. observed that participants who took ACE inhibitors derived greater physical benefit from the exercise than non-users, independent of obesity status.(Buford, Manini, Hsu, Cesari, Anton, Nayfield, Stafford, Church, Pahor, and Carter 2012) Importantly, each of these findings was independent of differences in confounding characteristics as well as the volume of exercise performed. Accordingly, phenotype (i.e. obesity) and medication use each had significant yet independent influences on the responsiveness of the participants to training. These findings suggest that exercise may be necessary, but insufficient, for preserving physical function and preventing disability among many older adults.(Keysor and Brembs 2011) Consequently, alternative or adjuvant strategies appear necessary to optimize the functional benefits of exercise. Table 1 summarizes the results of major lifestyle intervention trials for improving walking speed in mobility-limited older adults (defined by walking speed of < 1.0 m/s).
2.4.3 Smart and Connected Technologies
Long-term maintenance of lifestyle interventions can be difficult. The rapid pace of sensor and mobile technology along with recent advances in machine learning and intelligent system techniques have provided new opportunities for improving older adults’ health and well-being through innovative smart and connected health solutions, such as real-time monitoring of mobility information, temperature and pulse information over time. (Rashidi and Mihailidis 2013;van, Kort, Rutten, and Duijnstee 2011) Today, even most smart phones are equipped with various sensors such as accelerometer, gyroscope, proximity sensor, and global positioning system (GPS), which can be used for detecting user activity and mobility. Additionally, recent advances in epidermal electronics and Micro-Electro-Mechanical Systems or MEMS technology promise a new era of health-related sensor technology that will allow for capturing temperature and pulse information. Furthermore, the Internet of things (IoT) (Hoy 2015) will allow for everyday objects to be sensed and controlled remotely, which will provide many additional opportunities for healthy aging, e.g. by detecting and recognizing ADLs and IADLs (Bookman, Harrington, Pass, and Reisner E. 2007) in older adults as a daily measure of their cognitive and physical function. These technologies, when paired with lifestyle intervention hold great promise for informing older adults about their own adherence to recommended/prescribed lifestyle strategies in their daily lives.
Already the concept of “aging in place” has prompted interest in real time monitoring of physical and cognitive health of older adults via “smart homes.” (Rantz, Skubic, Koopman, Alexander, Phillips, Musterman, Back, Aud, Galambos, Guevara, and Miller 2012;Rashidi, Cook, Holder, and Schmitter-Edgecombe 2011) A smart home is a regular home that has been augmented with various sensors and actuators. For example, smart homes have been used for monitoring falls in older adults using ambient motion sensors, pressure sensors placed in floor tiles, as well as floor vibration detection through audio analysis (Alwan, Rajendran, Kell, Mack, Dalal S, Wolfe, and Felder 2006). Some researchers also have investigated providing interventions and means for overcoming physical limitations, e.g. by integrating assistive robots within the environments. (Graf, Heyer, Klein, and Wallhoff 2013)
Large data sets collected from smart and connected health solutions, often transmitted wirelessly, poses many unique data analysis challenges due to its inherent complexity and size. Advanced machine learning and other computational data analysis techniques (Bishop 2006) can be used to transform data into actionable knowledge. For example, abnormal mobility patterns can be extracted from longitudinal raw accelerometer data to identify current and potential future mobility complications. Additionally, it is possible to obtain valuable knowledge about the environment, one’s habits, activities, emotional well-being, as well as cognitive and physical health status over time.
This data from ambient and wearable sensors and analyzing it using advanced computational techniques (Bishop 2006) promises a new generation of health monitoring solutions for the older adults. The extracted knowledge will be used to inform the healthcare providers to enable them to provide better care and empower the elderly and their family members to interact with a wealth of knowledge in an interactive fashion using customizable interfaces. Ultimately, it will facilitate targeted initiation of intervention and preventive measures by using a cost effective and time saving method. (Rashidi and Mihailidis 2013)
2.4.4 Cognitive Training
While lifestyle interventions using physical exercise and diet have been found to attenuate declines in physical function and health, interventions aimed at slowing or preventing age-related decline in thinking and memory skills also hold promise for improving physical function.(Ball, Berch, Helmers, Jobe, Leveck, Marsiske, Morris, Rebok, Smith, Tennstedt, Unverzagt, and Willis 2002;Ball and Owsley 1993;Marsiske, Dzierzewski, Thomas, Kasten, Jones, Johnson, Willis, Whitfield, Ball, and Rebok 2013;Rebok, Ball, Guey, Jones, Kim, King, Marsiske, Morris, Tennstedt, Unverzagt, and Willis 2014) These cognitive abilities play an important role in functional decline in older adults, specifically related to fall injuries, loss of independence, and hospitalization. Cognitive training interventions typically involve computerized “games” aimed at progressively training specific cognitive skills (e.g., working memory, attentions, etc.) using a series of challenging tasks. Recent years have seen a proliferation of marketed cognitive training tasks available to older adults. While the validity of many of the products remain untested, the science of cognitive training has proven effective in laboratory settings across a number of large clinical trials (e.g., NIA-funded ACTIVE study).(Rebok, Ball, Guey, Jones, Kim, King, Marsiske, Morris, Tennstedt, Unverzagt, and Willis 2014) The ACTIVE study showed significant improvement in driving performance and training measures lasting as long as ten years after the initial training targeting processing speed and attention in over 1500 older adults.(Rebok, Ball, Guey, Jones, Kim, King, Marsiske, Morris, Tennstedt, Unverzagt, and Willis 2014) Unfortunately, many other cognitive training measures have failed to demonstrate this level of transfer to real-world tasks, with effects limited to the specific training tasks.(Li, Li, Li, Li, Wang, and Zhou 2011;Papp, Walsh, and Snyder 2009;Reijnders, van, and van 2013) Thus, future work aimed at enhancing the effectiveness of cognitive training will be important for maximizing it effectiveness in preventing functional decline. Regardless, these methods hold great promise for enhancement of declining cognitive function in older adults. One area of emerging investigation is that of home-based cognitive training using gamified platforms (Edwards, Valdes, Peronto, Castora-Binkley, Alwerdt, Andel, and Lister 2015), which shows evidence of enhancing targeted cognitive functions and transferring to IADLs like driving (Ross, Edwards, O'Connor, Ball, Wadley, and Vance 2015). Such findings more broadly support the concept of deploying home based interventions, especially in computer-assisted and gamified forms.
2.4.5 Exergaming: Combining smart technologies, exercise, and cognitive stimulation
One area of intervention that may combine lifestyle/exercise and cognitive interventions is that of video games and exergames (i.e., video games in which the user engages the gaming interface through the use of physical activity). (Bamidis, Vivas, Styliadis, Frantzidis, Klados, Schlee, Siountas, and Papageorgiou 2014) Exergames hold appeal as an intervention delivery mechanism because they are widely available off-the-shelf, are relatively low cost (legacy/older systems can be deployed with an existing television, can obtained for under $200 US), and can be placed in home and congregate environments with essentially no redesign/retrofitting. In addition, there is clear evidence that older adults can adhere to long-term (e.g., 3 month) home-based interventions while maintaining and improving high levels of engagement. (Belchior, Marsiske, Sisco, Yam, and Mann 2012) Video games without exercise have been shown to improve executive and cognitive control, and selective attention in older adults. (Anguera, Boccanfuso, Rintoul, Al-Hashimi, Faraji, Janowich, Kong, Larraburo, Rolle, Johnston, and Gazzaley 2013;Basak, Boot, Voss, and Kramer 2008;Belchior, Marsiske, Sisco, Yam, and Mann 2012) Exergames are a broad category, and can range from simple seated tasks of dexterity and eye-hand coordination (e.g., tennis, golf, bowling, shooting) through full-body engagement using sensor/force plate/accelerometer technology (e.g., gamified “coaches” to encourage walking, running, stretching, yoga). There is evidence that, via engaging in exergames, middle aged and older adults can improve cardiovascular risk indices, energy expenditure, walk time, and balance. (Agmon, Perry, Phelan, Demiris, and Nguyen 2011;Guderian, Borreson, Sletten, Cable, Stecker, Probst, and Dalleck 2010;Studenski, Perera, Hile, Keller, Spadola-Bogard, and Garcia 2010) A secondary benefit of video games seems to be mood improvement (Rosenberg, Depp, Vahia, Reichstadt, Palmer, Kerr, Norman, and Jeste 2010;Studenski, Perera, Hile, Keller, Spadola-Bogard, and Garcia 2010), with reduced levels of subsyndromal depression likely to further promote engagement in subsequent health behaviors. To date, benefits of exergaming for cognitive enhancement and/or reducing functional limitations have been relatively understudied, but initial evidence suggests both fitness and broad cognitive improvements with custom games. (Bamidis, Vivas, Styliadis, Frantzidis, Klados, Schlee, Siountas, and Papageorgiou 2014;Maillot, Perrot, and Hartley 2012) Thus, the exergaming environment holds great promise as a cost-effective implementation of widespread, community-based interventions to impact physical and cognitive health. Risks associated with unmonitored participation (e.g., increase probability of injury, social isolation) must also be studied.
2.4.5 Non-Invasive Brain Stimulation
While all human behaviors can be considered to require changes in brain activity (e.g., remembering a phone number, planning to walk across a room, etc.), a variety of intervention techniques specifically aim to modulate activity in the brains a means to enhance brain function. This general class of intervention can be referred to as “non-invasive brain stimulation,” and parsed further into direct and indirect stimulation methods. Transcranial direct current stimulation (tDCS) is one example of direct non-invasive brain stimulation with potential for impact on function in older adults. tDCS involves application of a weak electrical current through electrodes placed on the scalp.(Kessler, Minhas, Woods, Rosen, Gorman, and Bikson 2013;Minhas, Bikson, Woods, Rosen, and Kessler 2012;Woods, Hamilton, Kranjec, Minhaus, Bikson, Yu, and Chatterjee 2014) The resulting electrical field directly stimulates underlying cortical and subcortical tissue in the brain.(Bikson, Datta, and Elwassif 2009;Bikson, Name, and Rahman 2013) Using specific stimulation parameters, this technique can change the resting membrane potential of stimulated neurons, resulting in a net increase or decrease in the firing rate of these neurons.(Batsikadze, Moliadze, Paulus, Kuo, and Nitsche 2013;Nitsche, Muller-Dahlhaus, Paulus, and Ziemann 2012;Nitsche and Paulus 2011) Effects of stimulation last beyond the period of stimulation and facilitate neuroplastic response of the stimulated tissue.(Nitsche and Paulus 2000) When paired with neuro-behavioral interventions, such as motor skill training or cognitive training, tDCS can enhance the effectiveness and duration of paired interventions.(Berryhill and Jones 2012;Bolognini, Pascual-Leone, and Fregni 2009;Nitsche 2002;Nitsche, Schauenburg, Lang, Liebetanz, Exner, Paulus, and Tergau 2003;Rosen, Ramkumar, Nguyen, and Hoeft 2009) Furthermore, tDCS has shown effectiveness in reducing chronic pain and symptoms of depression, factors contributing to decreased mobility in older adults.(Kaye, Baluch, and Scott 2010) Thus, tDCS may serve as either an independent and/or adjunctive therapy that has the potential to alleviate factors limiting mobility and improve interventions aimed at enhancing physical and cognitive function in older adults.
2.4.6 Pharmaceutical
Pharmaceutical interventions represent another form of therapeutic strategy with significant promise for enhancing physical function and mobility in older adults. In recent years, studies have evaluated the use of pharmacologic agents for the prevention and treatment of age-related sarcopenia and functional decline. This approach has the benefit of requiring minimal effort on the part of patients, an important point given that the initial effort required to begin an intervention program is a primary barrier to lifestyle-based treatments.(Lees, Clarkr, Nigg, and Newman 2005) Yet, evidence from studies evaluating the effects of mono-modal pharmacologic strategies on physical function have been mixed at best. Despite these equivocal findings, the potential use of pharmacotherapy for improving physical function in older adults should not be abandoned as the efficacy of such medications may be at least partially dependent on the lifestyle habits of the individual. For example, the popular drug metformin could potentially improve function and reduce frailty risk in older adults with type 2 diabetes.(Wang, Lorenzo, and Espinoza 2014) While the underlying mechanisms of metformin remain to be fully elucidated, biologically, it makes sense that one of the major proposed mechanism of metformin is to activate the enzyme AMP- activated protein kinase (AMPK), a key sensor of cellular energy status,(Zhou, Myers, Li, Chen, Shen, Fenyk-Melody, Wu, Ventre, Doebber, Fujii, Musi, Hirshman, Goodyear, and Moller 2001) which can further suppress m-TOR pathway. It has also been suggested that metformin recapitulates the effects of dietary restriction.(Onken and Driscoll 2010)
Others have proposed that exercise may stimulate adaptations to pharmaceuticals that are not observed in response to the drug alone.(Booth and Laye 2010) Findings from pre-clinical models provide initial support for this approach. For example, despite showing no effect when given to mice in isolation, an oral PPARδ agonist increased exercise tolerance when given in conjunction with an exercise-training regimen.(Narkar, Downes, Yu, Embler, Wang, Banayo, Mihaylova, Nelson, Zou, Juguilon, Kang, Shaw, and Evans 2008) Thus, the strategy of combining potentially beneficial medications with chronic exercise or other non-invasive interventions may be more efficacious than either intervention alone.
The use of some pharmaceutical agents as adjunctive therapies to exercise may enhance the effectiveness of each intervention. In particular, interest has been high regarding the potential utility of ACE inhibitors as therapeutic agents for preventing sarcopenia and functional decline.(Cranney 2007;Sumukadas, Struthers, and McMurdo 2006) Epidemiologic evidence from the Women’s Health and Aging Study and the Systematic Assessment of Geriatric drug use via Epidemiology Study indicated that, compared to non-users, older adults using ACE inhibitors displayed attenuated declines in walking speed and fewer limitations in ADLs.(Gambassi, Lapane, Sgadari, Carbonin, Gatsonis, Lipsitz, Mor, and Bernabei 2000;Onder, Penninx, Balkrishnan, Fried, Chaves, Williamson, Carter, Di, Guralnik, and Pahor 2002) Other epidemiologic and animal evidence suggests that these outcomes may have been due to attenuated declines in skeletal muscle mass and strength.(Carter, Marzetti, Leeuwenburgh, Manini, Foster, Groban, Scarpace, and Morgan 2012;Di, van de Poll-Franse LV, Onder, Kritchevsky, Newman, Harris, Williamson, Marchionni, and Pahor 2004;Onder, Penninx, Balkrishnan, Fried, Chaves, Williamson, Carter, Di, Guralnik, and Pahor 2002) Despite these promising findings, the results of subsequent RCTs have been mixed regarding the impact of ACE inhibitor use on functional decline.(Cesari, Pedone, Incalzi, and Pahor 2010;Hutcheon, Gillespie, Crombie, Struthers, and McMurdo 2002;Sumukadas, Witham, Struthers, and McMurdo 2007;Zi, Carmichael, and Lye 2003) Thus, the benefits of ACE inhibitors on functional outcomes may be limited when utilized as an isolated treatment. However, Buford et al. recently reported that older adults (age > 70 years) who took ACE inhibitors displayed significant improvements in physical function in response to a 12-month exercise program compared to peers who did not take these medications.(Buford, Manini, Hsu, Cesari, Anton, Nayfield, Stafford, Church, Pahor, and Carter 2012) These data demonstrate the potential impact of this adjunctive strategy in preserving function and mobility in older adults, and helps to highlight the value of adjunctive intervention strategies.
2.4.7 Nutraceutical
Natural compounds may also represent an important source of potential new interventions for older individuals. Similar to pharmaceutical agents, these compounds would likely be most effectively used as an adjunctive treatment with hypocaloric diets, behavioral self- management programs, physical exercise, or cognitive interventions. Estimates indicate that Americans spend over 12 billion dollars per year on nutritional supplements (Blanck, Khan, and Serdula 2001), and sales of these products are projected to continue to increase by approximately 10% per year. (Goldman 2001) Unfortunately, the vast majority of these products have little supportive data from well-designed randomized trials.(Bray 2008) Given the increasing popularity of these products among middle-age and older adults,(Mehlman, Binstock, Juengst, Ponsaran, and Whitehouse 2004) studies are needed to examine their safety and efficacy.
Among the various compounds, resveratrol, a polyphenol found in the skins of grapes and red wine, may have unique promise to improve age-related functional decline because it appears to activate similar pathways as DR without leading to muscle loss. These beneficial biological effects have been demonstrated across multiple species, and have been associated with improvements on a variety of functional tasks and health-span measures. In several species, resveratrol has even been found to extend healthy lifespan.(Baur, Pearson, Price, Jamieson, Lerin, Kalra, Prabhu, Allard, Lopez-Lluch, Lewis, Pistell, Poosala, Becker, Boss, Gwinn, Wang, Ramaswamy, Fishbein, Spencer, Lakatta, Le, Shaw, Navas, Puigserver, Ingram, de, and Sinclair 2006;Greer and Brunet 2009;Long, Gao, Sun, Liu, and Zhao-Wilson 2009) These findings are not unexpected given that resveratrol has been found to activate a wide variety of targets, and produces broad, systemic effects that are very similar to caloric restriction,(Marzetti, Wohlgemuth, Anton, Bernabei, Carter, and Leeuwenburgh 2009;Testa, Biasi, Poli, and Chiarpotto 2014) the only method to date that has been found to increase healthy lifespan in multiple species. The critical question of whether resveratrol works in humans and by what mechanisms, however, has not been answered, and very few studies have examined the effects of resveratrol in older adults.
2.4.8 Hormonal
There are a multiple hormonal approaches that may lead to improvements in physical function and mobility in at risk older adults, including testosterone, estrogen, oxytocin, DHEA, and growth hormone supplementation.
At present, there is not conclusive evidence to support one approach over another. Below we highlight recent studies related to novel administration of the hormone oxytocin to the administration of the hormone oxytocin, which can be delivered to the human brain by the use of nasal spray, tapping into central functions.(Born, Lange, Kern, McGregor, Bickel, and Fehm 2002) A number of experimental studies using acute or chronic oxytocin treatment have revealed intriguing effects on a wide spectrum of processes related to health, cognition, and psychosocial functioning.(Ebner, Maura, Macdonald, Westberg, and Fischer 2013) In particular, going beyond its classic role in labor and lactation (Pedersen 1997), the hormone oxytocin has modulatory effects on higher-order cognitive processing (Meyer-Lindenberg, Domes, Kirsch, and Heinrichs 2011) and effects on the cardiovascular system in that it has been found to improve the vasculature, possibly in interaction with sex hormones (Gimpl and Fahrenholz 2001), and benefits weight control and insulin sensitivity.(Zhang, Wu, Chen, Chen, Xu, Wu, and Cai 2013) In addition, there is increasing evidence of pain amelioration, improved wound healing, and anti-inflammatory effects associated with oxytocin.(Clodi, Vila, Geyeregger, Riedl, Stulnig, Struck, Luger, and Luger 2008) The few existing studies on oxytocin administration in human aging provide first evidence that increased oxytocin levels ameliorate age-related physical decline and reduce fatigue (Barraza, Grewal, Ropacki, Perez, Gonzalez, and Zak 2013) as well as benefit psychosocial functioning.(Campbell, Ruffman, Murray, and Glue 2014;Ebner, Horta, Lin, Feifel, Fisher, and Cohen 2015) Thorough examination of oxytocin’s cardiovascular and anti-inflammatory effects in aging constitutes a cutting-edge avenue for future research on interventions promoting physical function and mobility.
2.5 Statistical Considerations in Evaluating Interventions to Enhance Physical Function
Clinical trials are becoming increasingly expensive while the success rate remains low. (DiMasi, Hansen, and Grabowski 2003;Walker and Newell 2009) This is also true for evaluating interventions to enhance function in older adults. The success rate could be improved with more efficient clinical trial designs. Below we review the potential of adaptive designs to improve quality, speed and efficiency of decision making in clinical trials.
Group sequential designs became practical in the final quarter of the 20th century.(Jennison and Turnbull B.W. 2000) Such designs permit early termination of the study for efficacy or futility with flexibility to alter number and spacing of interim analyses. Group sequential designs with unblinded sample size re-estimation were introduced in the new millennium. (Cui, Hung, and Wang 1999;Lehmacher and Wassmer 1999;Proschan and Hunsberger 1995) Currently, many adaptive designs have been developed to use accumulating data to guide the subsequent modification of the study design without undermining the validity and integrity of the trial. Adaptions can include adding or dropping treatment arms/doses, modifying treatment allocation ratios, switching between non-inferiority and superiority hypothesis, changing in study population and trial phase. For example, an enrichment design attempts to reduce trial costs by targeting from the original heterogeneous study population a patient subpopulation that might optimally benefits from the treatment; (Wu, Tu, and He 2014) a seamless design combines the phase II trial and the confirmatory phase III trial, which improves efficiency through joint analysis of data and eliminates the gap between the two phases. (Wu, Wang, and Yang 2010)
Statistics will also play an important role in dealing with the challenges posed by two major changes in health care. The first one is connected health, a model for remote care or self-care through computer and cellular technologies, which allow patients to receive healthcare information electronically and enable clinicians to monitor patients’ conditions remotely. Due to the increasingly widespread availability of Internet access and wellness devices, connected health has been flourishing into a multi-billion market. (Pai A 2014) The second revolution is “big data”, which refers to a massive volume of both structured and unstructured data that traditional database and software techniques are inadequate. Challenges include analysis, capture, curation, search, sharing, storage, transfer, visualization, and information privacy. Major factors driving this approach are the skyrocketing cost of medical care (17.9% of US GDP in 2012) and the increasing emphasis on evidence-based medicine. (Kayyali B, Knott D, and Van Kuiken S 2013) Modern digital and mobile technologies make it much easier to collect information from patients on a large-scale, which has resulted in a vast amount of medical data accumulating at numerous medical facilities throughout the world. Tremendous additional values can be uncovered if medical data from different sources, including individual patients, can be streamed together and made readily available. The resulting big data will give new, expanded meanings to connected health; it will serve as a nourishing base for new medical/ statistical/social technologies that connect patients, medical researchers, clinical practitioners, and health and pharmaceutical companies, in a concerted effort to lower medical cost, enable large-scale studies, and encourage preventive medicine at a community level.
3. Conclusions
In this review, we have highlighted the importance of developing a consensus definition of multi-faceted construct of "Successful Aging," in order to advance aging research and the development of strategies that promote independence in aging populations. Our theoretical reflections in this paper are in line with Bowling and Dieppe (2005), who argued that successful aging “needs to be viewed not only multidimensionally, but as an ideal state to be aimed for, and the concept itself should be placed on a continuum of achievement rather than subject to simplistic normative assessments of success and failure.”
Despite the inherent difficulties in defining this multidimensional phenomenon, we believe that it is worth the effort to move toward this objective for the following reasons. First, in the absence of an accepted definition and commonly agreed on metrics, a diverse array of measures have been used to assess various dimensions of this construct. This has made it difficult to compare findings across studies. Second and perhaps most important, the adoption of a consensus definition that takes into account factors from multiple domains could provide much value for older adults themselves. Rather than classifying successful aging based on function within a single domain, a broader definition can provide critical information on level of function within and across multiple domains. As discussed in the current paper, interventional approaches can be targeted to improving function within domains in which an individual’s functional level is lower relative to their functioning in other domains. Given the strong inter-relationship of functioning level across many domains (e.g., cognitive and physical function) that comprise this construct, an added benefit of the type of interventional approaches discussed in the current paper is that they are likely to lead to functional improvements in domains not specifically targeted.
To achieve the ambitious objective of developing a consensus definition of successful aging, an inter-disciplinary approach is needed, as adopted in the current paper, in which experts within each domain provide evidence-based recommendations regarding markers of successful aging within their domain. In this manner, scientists from multiple domains work together towards a shared goal of having evidence-based markers of successful aging. Currently, there appears to be a sufficient body of evidence to move towards such a consensus definition on markers of successful aging within the physical domain. Evidence from multiple disciplines has converged on the use of common outcome measures, specifically walking speed (i.e., distance completed divided by time) and performance on a standardized measure of physical function (i.e., SPPB), as excellent surrogate markers of both functional ability and health status in older adults. By extension, we believe the evidence is now sufficient to propose that walking speed and performance on the SPPB should be considered surrogate markers of successful aging within the physical domain.
There remains a strong need, however, for clear and accepted criteria to assess functioning within other domains of successful aging. Once accepted definitions, metrics, and measures to evaluate such constructs are agreed upon, the effectiveness of treatment approaches targeting these outcomes can be better assessed. An increased understanding of the contribution of function within each of these domains would not only enhance our ability to define successful aging, but can also lead to the development of future interventions designed to improve mobility and physical function or attenuate declines in physical function in older adults.
To this end, we have provided an overview of specific health conditions, behavioral factors, and biological mechanisms that have been directly linked with physical function, and therefore appear to play an important role in contributing to declines in mobility and physical function during aging. We have also reviewed the role of promising interventions that can enhance mobility and physical function or attenuate functional decline in older adults. A continuing focus on interdisciplinary research is recommended to help move towards an accepted definition of successful aging that can be used to promote independence in the growing population of older adults.
Highlights.
A consensus definition is needed for the multi-faceted construct of "Successful Aging" to advance the development of strategies that promote independence in an aging population.
Once accepted definitions, metrics, and measures to evaluate dimensions of the construct of Successful Aging are agreed upon, the effectiveness of treatment approaches targeting these outcomes can be better assessed.
A number of health conditions, behavioral factors, and biological mechanisms have been strongly linked to the pathogenesis of functional decline during aging.
Few therapies have been identified to improve functional performance in human aging, and there are currently no FDA-approved medications for the treatment of functional decline in older adults.
Empirically-supported treatments are urgently needed to improve mobility and prevent disability in older adults.
Acknowledgements
Support was provided by the University of Florida’s Claude D. Pepper Older Americans Independence Center (NIH/NIA P30AG028740), and Clinical and Translational Science Institute (NIH/NCRR UL1TR000064). Stephen Anton was previously supported by the Thomas H. Maren Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Stephen D. Anton, Email: santon@ufl.edu.
Adam J. Woods, Email: ajwoods@ufl.edu.
Tetso Ashizawa, Email: tetsuo.ashizawa@neurology.ufl.edu.
Diana Barb, Email: diana.barb@medicine.ufl.edu.
Thomas W. Buford, Email: tbuford@ufl.edu.
Christy S. Carter, Email: cartercs@ufl.edu.
David J. Clark, Email: davidclark@ufl.edu.
Ronald A. Cohen, Email: roncohen@ufl.edu.
Duane B. Corbett, Email: dcorbett@ufl.edu.
Yenisel Cruz-Almeida, Email: cryeni@ufl.edu.
Vonetta Dotson, Email: vonetta@ufl.edu.
Natalie Ebner, Email: natalie.ebner@ufl.edu.
Philip A. Efron, Email: Philip.efron@surgery.ufl.edu.
Roger B. Fillingim, Email: RFillingim@ufl.edu.
Thomas C. Foster, Email: foster1@ufl.edu.
David M. Gundermann, Email: dgunderm@ufl.edu.
Anna-Maria Joseph, Email: amjoseph@ufl.edu.
Christy Karabetian, Email: ckarabetian@phhp.ufl.edu.
Christiaan Leeuwenburgh, Email: cleeuwen@ufl.edu.
Todd M. Manini, Email: tmanini@ufl.edu.
Michael Marsiske, Email: marsiske@phhp.ufl.edu.
Robert T. Mankowski, Email: r.mankowski@ufl.edu.
Heather L. Mutchie, Email: hmutchie@ufl.edu.
Michael G. Perri, Email: mperri@phhp.ufl.edu.
Sanjay Ranka, Email: ranka@cise.ufl.edu.
Parisa Rashidi, Email: parisa.rashidi@bme.ufl.edu.
Bhanuprasad Sandesara, Email: bsandesara@ufl.edu.
Philip J. Scarpace, Email: scarpace@ufl.edu.
Kimberly T. Sibille, Email: ksibille@ufl.edu.
Laurence M. Solberg, Email: lmsolberg@ufl.edu.
Shinichi Someya, Email: someya@ufl.edu.
Connie Uphold, Email: upholdc@ufl.edu.
Stephanie Wohlgemuth, Email: steffiw@ufl.edu.
Samuel Shangwu Wu, Email: samwu@biostat.ufl.edu.
Marco Pahor, Email: mpahor@ufl.edu.
Literature Cited
- 1.Prevalence of self-reported physically active adults--United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57:1297–1300. [PubMed] [Google Scholar]
- 2.Abellan Van KG, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette GS, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 3.Agmon M, Perry CK, Phelan E, Demiris G, Nguyen HQ. A pilot study of Wii Fit exergames to improve balance in older adults. J Geriatr Phys Ther. 2011;34:161–167. doi: 10.1519/JPT.0b013e3182191d98. [DOI] [PubMed] [Google Scholar]
- 4.Albert SM, Bear-Lehman J, Anderson SJ. Declines in Mobility and Changes in Performance in the Instrumental Activities of Daily Living Among Mildly Disabled Community-Dwelling Older Adults. J Gerontol A Biol Sci Med Sci. 2014 doi: 10.1093/gerona/glu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- 6.Alkema GE, Alley DE. Gerontology's future: An integrative model for disciplinary advancement. Gerontologist. 2006;46:574–582. doi: 10.1093/geront/46.5.574. [DOI] [PubMed] [Google Scholar]
- 7.Alley DE, Shardell MD, Peters KW, McLean RR, Dam TT, Kenny AM, Fragala MS, Harris TB, Kiel DP, Guralnik JM, Ferrucci L, Kritchevsky SB, Studenski SA, Vassileva MT, Cawthon PM. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:559–566. doi: 10.1093/gerona/glu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alwan M, Rajendran P, Kell S, Mack D, Dalal S, Wolfe M, Felder R. A smart and passive floor vibration based fall detector for elderly. Proc 2nd IEEE Inf Commun Technol. 2006;1:1003–1007. [Google Scholar]
- 9.Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 10.Andersson HI. The course of non-malignant chronic pain: a 12-year follow-up of a cohort from the general population. Eur J Pain. 2004;8:47–53. doi: 10.1016/S1090-3801(03)00064-8. [DOI] [PubMed] [Google Scholar]
- 11.Anguera JA, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, Kong E, Larraburo Y, Rolle C, Johnston E, Gazzaley A. Video game training enhances cognitive control in older adults. Nature. 2013;501:97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anton SD, Karabetian C, Naugle K, Buford TW. Obesity and diabetes as accelerators of functional decline: can lifestyle interventions maintain functional status in high risk older adults? Exp Gerontol. 2013;48:888–897. doi: 10.1016/j.exger.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Appleton AA, Buka SL, Loucks EB, Gilman SE, Kubzansky LD. Divergent associations of adaptive and maladaptive emotion regulation strategies with inflammation. Health Psychol. 2013;32:748–756. doi: 10.1037/a0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aris JP, Alvers AL, Ferraiuolo RA, Fishwick LK, Hanvivatpong A, Hu D, Kirlew C, Leonard MT, Losin KJ, Marraffini M. Autophagy and leucine promote chronological longevity and respiration proficiency during calorie restriction in yeast. Experimental gerontology. 2013;48:1107–1119. doi: 10.1016/j.exger.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Astrom DO, Forsberg B, Rocklov J. Heat wave impact on morbidity and mortality in the elderly population: a review of recent studies. Maturitas. 2011;69:99–105. doi: 10.1016/j.maturitas.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson HH, Cesari M, Kritchevsky SB, Penninx BW, Fried LP, Guralnik JM, Williamson JD. Predictors of combined cognitive and physical decline. J Am Geriatr Soc. 2005;53:1197–1202. doi: 10.1111/j.1532-5415.2005.53362.x. [DOI] [PubMed] [Google Scholar]
- 17.Avlund K. Fatigue in older adults: an early indicator of the aging process? Aging clinical and experimental research. 2010;22:100–115. doi: 10.1007/BF03324782. [DOI] [PubMed] [Google Scholar]
- 18.Baezner H, Blahak C, Poggesi A, Pantoni L, Inzitari D, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM, Langhorne P, O'Brien J, Scheltens P, Visser MC, Wahlund LO, Waldemar G, Wallin A, Hennerici MG. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008;70:935–942. doi: 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- 19.Baker DJ, Betik AC, Krause DJ, Hepple RT. No decline in skeletal muscle oxidative capacity with aging in long-term calorically restricted rats: effects are independent of mitochondrial DNA integrity. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006;61:675–684. doi: 10.1093/gerona/61.7.675. [DOI] [PubMed] [Google Scholar]
- 20.Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Rebok GW, Smith DM, Tennstedt SL, Unverzagt FW, Willis SL. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ball K, Owsley C. The useful field of view test: a new technique for evaluating age-related declines in visual function. J Am Optom Assoc. 1993;64:71–79. [PubMed] [Google Scholar]
- 22.Baltes PB, Baltes MM. Psychological perspectives on successful aging: The model of selective optimization with compensation. In: Baltes PB, Baltes MM, editors. Successful Aging: Perspectives from the Behavioral Sciences. Cambridge University Press; 1993. pp. 1–34. [Google Scholar]
- 23.Bamidis PD, Vivas AB, Styliadis C, Frantzidis C, Klados M, Schlee W, Siountas A, Papageorgiou SG. A review of physical and cognitive interventions in aging. Neurosci Biobehav Rev. 2014;44:206–220. doi: 10.1016/j.neubiorev.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Barbat-Artigas S, Pion CH, Leduc-Gaudet JP, Rolland Y, Aubertin-Leheudre M. Exploring the role of muscle mass, obesity, and age in the relationship between muscle quality and physical function. J Am Med Dir Assoc. 2014;15:303–320. doi: 10.1016/j.jamda.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Barraza JA, Grewal NS, Ropacki S, Perez P, Gonzalez A, Zak PJ. Effects of a 10-day oxytocin trial in older adults on health and well-being. Exp Clin Psychopharmacol. 2013;21:85–92. doi: 10.1037/a0031581. [DOI] [PubMed] [Google Scholar]
- 26.Basak C, Boot WR, Voss MW, Kramer AF. Can training in a real-time strategy video game attenuate cognitive decline in older adults? Psychol Aging. 2008;23:765–777. doi: 10.1037/a0013494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bassey EJ, Fiatarone MA, O'Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 1992;82:321–327. doi: 10.1042/cs0820321. [DOI] [PubMed] [Google Scholar]
- 28.Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol. 2013;591:1987–2000. doi: 10.1113/jphysiol.2012.249730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumgartner RN. Body composition in healthy aging. Annals of the New York Academy of Sciences. 2000a;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 30.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000b;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 31.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le CD, Shaw RJ, Navas P, Puigserver P, Ingram DK, de CR, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayeva M, Gheorghiade M, Ardehali H. Mitochondria as a therapeutic target in heart failure. Journal of the American College of Cardiology. 2013;61:599–610. doi: 10.1016/j.jacc.2012.08.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bean JF, Kiely DK, Herman S, Leveille SG, Mizer K, Frontera WR, Fielding RA. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50:461–467. doi: 10.1046/j.1532-5415.2002.50111.x. [DOI] [PubMed] [Google Scholar]
- 34.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci. 2003;58:728–733. doi: 10.1093/gerona/58.8.m728. [DOI] [PubMed] [Google Scholar]
- 35.Bean LA, Ianov L, Foster TC. Estrogen receptors, the hippocampus, and memory. Neuroscientist. 2014;20:534–545. doi: 10.1177/1073858413519865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belchior P, Marsiske M, Sisco S, Yam A, Mann W. Older adults' engagement with a video game training program. Act Adapt Aging. 2012;36:269–279. doi: 10.1080/01924788.2012.702307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bentayeb M, Simoni M, Baiz N, Norback D, Baldacci S, Maio S, Viegi G, Annesi-Maesano I. Adverse respiratory effects of outdoor air pollution in the elderly. Int J Tuberc Lung Dis. 2012;16:1149–1161. doi: 10.5588/ijtld.11.0666. [DOI] [PubMed] [Google Scholar]
- 38.Berryhill ME, Jones KT. tDCS selectively improves working memory in older adults with more education. Neurosci Lett. 2012;521:148–151. doi: 10.1016/j.neulet.2012.05.074. [DOI] [PubMed] [Google Scholar]
- 39.Bhargava D, Weiner MF, Hynan LS, Diaz-Arrastia R, Lipton AM. Vascular disease and risk factors, rate of progression, and survival in Alzheimer's disease. J Geriatr Psychiatry Neurol. 2006;19:78–82. doi: 10.1177/0891988706286505. [DOI] [PubMed] [Google Scholar]
- 40.Bikson M, Datta A, Elwassif M. Establishing safety limits for transcranial direct current stimulation. Clin Neurophysiol. 2009;120:1033–1034. doi: 10.1016/j.clinph.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bikson M, Name A, Rahman A. Origins of specificity during tDCS: anatomical, activity-selective, and input-bias mechanisms. Front Hum Neurosci. 2013;7:688. doi: 10.3389/fnhum.2013.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bishop C. Pattern recognition and machine learning. Springer; 2006. [Google Scholar]
- 43.Bizon JL, Helm KA, Han JS, Chun HJ, Pucilowska J, Lund PK, Gallagher M. Hypothalamic-pituitary-adrenal axis function and corticosterone receptor expression in behaviourally characterized young and aged Long-Evans rats. Eur J Neurosci. 2001;14:1739–1751. doi: 10.1046/j.0953-816x.2001.01781.x. [DOI] [PubMed] [Google Scholar]
- 44.Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blanck HM, Khan LK, Serdula MK. Use of nonprescription weight loss products: results from a multistate survey. JAMA. 2001;286:930–935. doi: 10.1001/jama.286.8.930. [DOI] [PubMed] [Google Scholar]
- 46.Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP. The association between obesity and the frailty syndrome in older women: the Women's Health and Aging Studies. J Am Geriatr Soc. 2005a;53:927–934. doi: 10.1111/j.1532-5415.2005.53300.x. [DOI] [PubMed] [Google Scholar]
- 47.Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP. The association between obesity and the frailty syndrome in older women: the Women's Health and Aging Studies. Journal of the American Geriatrics Society. 2005b;53:927–934. doi: 10.1111/j.1532-5415.2005.53300.x. [DOI] [PubMed] [Google Scholar]
- 48.Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J Neuroeng Rehabil. 2009;6:8. doi: 10.1186/1743-0003-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boockvar K, Signor D, Ramaswamy R, Hung W. Delirium during acute illness in nursing home residents. J Am Med Dir Assoc. 2013;14:656–660. doi: 10.1016/j.jamda.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Bookman A, Harrington M, Pass L, Reisner E. Family Caregiver Handbook. Cambridge: Massachusetts Institute of Technology; 2007. [Google Scholar]
- 51.Booth FW, Laye MJ. The future: genes, physical activity and health. Acta physiologica. 2010;199:549–556. doi: 10.1111/j.1748-1716.2010.02117.x. [DOI] [PubMed] [Google Scholar]
- 52.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 53.Botwinick J, Storandt M. Speed functions, vocabulary ability, and age. Percept Mot Skills. 1973;36:1123–1128. doi: 10.2466/pms.1973.36.3c.1123. [DOI] [PubMed] [Google Scholar]
- 54.Bowling A. Aspirations for older age in the 21st century: what is successful aging? Int J Aging Hum Dev. 2007;64:263–297. doi: 10.2190/L0K1-87W4-9R01-7127. [DOI] [PubMed] [Google Scholar]
- 55.Bray GA. Are non-prescription medications needed for weight control? Obesity (Silver Spring) 2008;16:509–514. doi: 10.1038/oby.2007.100. [DOI] [PubMed] [Google Scholar]
- 56.Brinkley TE, Leng X, Miller ME, Kitzman DW, Pahor M, Berry MJ, Marsh AP, Kritchevsky SB, Nicklas BJ. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455–461. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Broadwin J, Goodman-Gruen D, Slymen D. Ability of fat and fat-free mass percentages to predict functional disability in older men and women. J Am Geriatr Soc. 2001;49:1641–1645. doi: 10.1046/j.1532-5415.2001.t01-1-49273.x. [DOI] [PubMed] [Google Scholar]
- 58.Brooks SV, Faulkner JA. Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc. 1994;26:432–439. [PubMed] [Google Scholar]
- 59.Brown M, Sinacore DR, Ehsani AA, Binder EF, Holloszy JO, Kohrt WM. Low-intensity exercise as a modifier of physical frailty in older adults. Arch Phys Med Rehabil. 2000;81:960–965. doi: 10.1053/apmr.2000.4425. [DOI] [PubMed] [Google Scholar]
- 60.Brownson RC, Boehmer TK, Luke DA. Declining rates of physical activity in the United States: what are the contributors? Annu Rev Public Health. 2005;26:421–443. doi: 10.1146/annurev.publhealth.26.021304.144437. [DOI] [PubMed] [Google Scholar]
- 61.Bruckenthal P, Reid MC, Reisner L. Special issues in the management of chronic pain in older adults. Pain Med. 2009;10(Suppl 2):S67–S78. doi: 10.1111/j.1526-4637.2009.00667.x. [DOI] [PubMed] [Google Scholar]
- 62.Brummel NE, Jackson JC, Pandharipande PP, Thompson JL, Shintani AK, Dittus RS, Gill TM, Bernard GR, Ely EW, Girard TD. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42:369–377. doi: 10.1097/CCM.0b013e3182a645bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brunk UT, Terman A. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem. 2002;269:1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- 64.Bua E, Johnson J, Herbst A, Delong B, McKenzie D, Salamat S, AIKEN JM. Mitochondrial DNAGÇôdeletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. The American Journal of Human Genetics. 2006;79:469–480. doi: 10.1086/507132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buchman AS, Wilson RS, Leurgans S, Bennett DA. Vibratory thresholds and mobility in older persons. Muscle Nerve. 2009;39:754–760. doi: 10.1002/mus.21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buford TW, Anton SD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS, Leeuwenburgh C, Pahor M, Manini TM. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev. 2010a;9:369–383. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buford TW, Anton SD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS, Leeuwenburgh C, Pahor M, Manini TM. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev. 2010b;9:369–383. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buford TW, Lott DJ, Marzetti E, Wohlgemuth SE, Vandenborne K, Pahor M, Leeuwenburgh C, Manini TM. Age-related differences in lower extremity tissue compartments and associations with physical function in older adults. Exp Gerontol. 2012;47:38–44. doi: 10.1016/j.exger.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buford TW, Manini TM, Hsu FC, Cesari M, Anton SD, Nayfield S, Stafford RS, Church TS, Pahor M, Carter CS. Angiotensin-converting enzyme inhibitor use by older adults is associated with greater functional responses to exercise. J Am Geriatr Soc. 2012;60:1244–1252. doi: 10.1111/j.1532-5415.2012.04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Busch KB, Kowald A, Spelbrink JN. Quality matters: how does mitochondrial network dynamics and quality control impact on mtDNA integrity? Philos Trans R Soc Lond B Biol Sci. 2014;369:20130442. doi: 10.1098/rstb.2013.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calvani R, Joseph AM, Adhihetty PJ, Miccheli A, Bossola M, Leeuwenburgh C, Bernabei R, Marzetti E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biological chemistry. 2013;394:393–414. doi: 10.1515/hsz-2012-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campbell A, Ruffman T, Murray JE, Glue P. Oxytocin improves emotion recognition for older males. Neurobiol Aging. 2014;35:2246–2248. doi: 10.1016/j.neurobiolaging.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 73.Carbonare LD, Maggi S, Giannini S, Rozzini R, Cascio VL, Crepaldi G, ILSA Working Group Physical disability and depressive symptomatology in an elderly population: A complex relationship - The Italian Longitudinal Study on Aging (ILSA) American Journal of Geriatric Psychiatry. 2009;17:144–154. doi: 10.1097/jgp.0b013e31818af817. [DOI] [PubMed] [Google Scholar]
- 74.Carriere I, Villebrun D, Peres K, Stewart R, Ritchie K, Ancelin ML. Modelling complex pathways between late-life depression and disability: evidence for mediating and moderating factors. Psychol Med. 2009;39:1587–1590. doi: 10.1017/S0033291709005273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carter CS, Marzetti E, Leeuwenburgh C, Manini T, Foster TC, Groban L, Scarpace PJ, Morgan D. Usefulness of preclinical models for assessing the efficacy of late-life interventions for sarcopenia. J Gerontol A Biol Sci Med Sci. 2012;67:17–27. doi: 10.1093/gerona/glr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carter CS, Sonntag WE, Onder G, Pahor M. Physical performance and longevity in aged rats. J Gerontol A Biol Sci Med Sci. 2002;57:B193–B197. doi: 10.1093/gerona/57.5.b193. [DOI] [PubMed] [Google Scholar]
- 77.Cavallini G, Donati A, Gori Z, Pollera M, Bergamini E. The protection of rat liver autophagic proteolysis from the age-related decline co-varies with the duration of anti-ageing food restriction. Experimental gerontology. 2001;36:497–506. doi: 10.1016/s0531-5565(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 78.Cesari M. Role of gait speed in the assessment of older patients. JAMA. 2011;305:93–94. doi: 10.1001/jama.2010.1970. [DOI] [PubMed] [Google Scholar]
- 79.Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BW, Lenchik L, Palla SL, Ambrosius WT, Tracy RP, Pahor M. Sarcopenia, obesity, and inflammation--results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr. 2005;82:428–434. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- 80.Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, Tylavsky FA, Brach JS, Satterfield S, Bauer DC, Visser M, Rubin SM, Harris TB, Pahor M. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 81.Cesari M, Marzetti E, Laudisio A, Antonica L, Pahor M, Bernabei R, Zuccala G. Interaction of HDL cholesterol concentrations on the relationship between physical function and inflammation in community-dwelling older persons. Age Ageing. 2010;39:74–80. doi: 10.1093/ageing/afp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cesari M, Pedone C, Incalzi RA, Pahor M. ACE-inhibition and physical function: results from the Trial of Angiotensin-Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN) study. J Am Med Dir Assoc. 2010;11:26–32. doi: 10.1016/j.jamda.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Guralnik JM, Ferrucci L. Inflammatory markers and physical performance in older persons: the InChianti study. J Gerontol A Biol Sci Med Sci. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 84.Chang TY, Chueh KH. Relationship between elderly depression and health status in male veterans. J Nurs Res. 2011;19:298–304. doi: 10.1097/JNR.0b013e318236cf89. [DOI] [PubMed] [Google Scholar]
- 85.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chinnery PF, Elliott C, Green GR, Rees A, Coulthard A, Turnbull DM, Griffiths TD. The spectrum of hearing loss due to mitochondrial DNA defects. Brain. 2000;123(Pt 1):82–92. doi: 10.1093/brain/123.1.82. [DOI] [PubMed] [Google Scholar]
- 87.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing research reviews. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–834. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 89.Clark DJ, Christou EA, Ring SA, Williamson JB, Doty L. Enhanced somatosensory feedback reduces prefrontal cortical activity during walking in older adults. J Gerontol A Biol Sci Med Sci. 2014;69:1422–1428. doi: 10.1093/gerona/glu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Impaired voluntary neuromuscular activation limits muscle power in mobility-limited older adults. J Gerontol A Biol Sci Med Sci. 2010;65:495–502. doi: 10.1093/gerona/glq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Muscle performance and physical function are associated with voluntary rate of neuromuscular activation in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:115–121. doi: 10.1093/gerona/glq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clodi M, Vila G, Geyeregger R, Riedl M, Stulnig TM, Struck J, Luger TA, Luger A. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. Am J Physiol Endocrinol Metab. 2008;295:E686–E691. doi: 10.1152/ajpendo.90263.2008. [DOI] [PubMed] [Google Scholar]
- 93.Coen PM, Jubrias SA, Distefano G, Amati F, Mackey DC, Glynn NW, Manini TM, Wohlgemuth SE, Leeuwenburgh C, Cummings SR, Newman AB, Ferrucci L, Toledo FG, Shankland E, Conley KE, Goodpaster BH. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68:447–455. doi: 10.1093/gerona/gls196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cohen SS, Matthews CE, Signorello LB, Schlundt DG, Blot WJ, Buchowski MS. Sedentary and physically active behavior patterns among low-income African-American and white adults living in the southeastern United States. PLoS One. 2013;8:e59975. doi: 10.1371/journal.pone.0059975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Conley KE, Marcinek DJ, Villarin J. Mitochondrial dysfunction and age. Current Opinion in Clinical Nutrition & Metabolic Care. 2007;10:688–692. doi: 10.1097/MCO.0b013e3282f0dbfb. [DOI] [PubMed] [Google Scholar]
- 96.Conlon KC, Rajkovich NB, White-Newsome JL, Larsen L, O'Neill MS. Preventing cold-related morbidity and mortality in a changing climate. Maturitas. 2011;69:197–202. doi: 10.1016/j.maturitas.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Corton JC, Brown-Borg HM. Peroxisome proliferator-activated receptor gamma coactivator 1 in caloric restriction and other models of longevity. J Gerontol A Biol Sci Med Sci. 2005;60:1494–1509. doi: 10.1093/gerona/60.12.1494. [DOI] [PubMed] [Google Scholar]
- 98.Cosco TD, Prina AM, Perales J, Stephan BC, Brayne C. Operational definitions of successful aging: a systematic review. Int Psychogeriatr. 2014;26:373–381. doi: 10.1017/S1041610213002287. [DOI] [PubMed] [Google Scholar]
- 99.Covinsky KE, Cenzer IS, Yaffe K, O'Brien S, Blazer DG. Dysphoria and anhedonia as risk factors for disability or death in older persons: implications for the assessment of geriatric depression. Am J Geriatr Psychiatry. 2014;22:606–613. doi: 10.1016/j.jagp.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Coyne JC, Downey G. Social factors and psychopathology: stress, social support, and coping processes. Annu Rev Psychol. 1991;42:401–425. doi: 10.1146/annurev.ps.42.020191.002153. [DOI] [PubMed] [Google Scholar]
- 101.Cranney A. Is there a new role for angiotensin-converting-enzyme inhibitors in elderly patients? Canadian Medical Association Journal. 2007;177:891–892. doi: 10.1503/cmaj.071062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cress ME, Buchner DM, Questad KA, Esselman PC, deLateur BJ, Schwartz RS. Exercise: effects on physical functional performance in independent older adults. J Gerontol A Biol Sci Med Sci. 1999;54:M242–M248. doi: 10.1093/gerona/54.5.m242. [DOI] [PubMed] [Google Scholar]
- 103.Cruz-Almeida Y, Black ML, Christou EA, Clark DJ. Site-specific differences in the association between plantar tactile perception and mobility function in older adults. Front Aging Neurosci. 2014;6:68. doi: 10.3389/fnagi.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cruz-Almeida Y, King CD, Goodin BR, Sibille KT, Glover TL, Riley JL, Sotolongo A, Herbert MS, Schmidt J, Fessler BJ, Redden DT, Staud R, Bradley LA, Fillingim RB. Psychological profiles and pain characteristics of older adults with knee osteoarthritis. Arthritis Care Res (Hoboken) 2013;65:1786–1794. doi: 10.1002/acr.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cruz-Almeida Y, Sibille KT, Goodin BR, Petrov ME, Bartley EJ, Riley JL, III, King CD, Glover TL, Sotolongo A, Herbert MS, Schmidt JK, Fessler BJ, Staud R, Redden D, Bradley LA, Fillingim RB. Racial and ethnic differences in older adults with knee osteoarthritis. Arthritis Rheumatol. 2014;66:1800–1810. doi: 10.1002/art.38620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cui L, Hung HM, Wang SJ. Modification of sample size in group sequential clinical trials. Biometrics. 1999;55:853–857. doi: 10.1111/j.0006-341x.1999.00853.x. [DOI] [PubMed] [Google Scholar]
- 108.Dark-Freudeman AR, Ebner NC, West RL. In: Psychosocial aspects of aging. Light KE, editor. F.A. Davis: Geriatric Rehabilitation; 2014. [Google Scholar]
- 109.Delbono O. Regulation of excitation contraction coupling by insulin-like growth factor-1 in aging skeletal muscle. J Nutr Health Aging. 2000;4:162–164. [PubMed] [Google Scholar]
- 110.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143:813–825. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.den Ouden ME, Schuurmans MJ, Arts IE, van der Schouw YT. Physical performance characteristics related to disability in older persons: a systematic review. Maturitas. 2011;69:208–219. doi: 10.1016/j.maturitas.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 113.Depp CA, Jeste DV. Definitions and predictors of successful aging: a comprehensive review of larger quantitative studies. Am J Geriatr Psychiatry. 2006;14:6–20. doi: 10.1097/01.JGP.0000192501.03069.bc. [DOI] [PubMed] [Google Scholar]
- 114.Deshpande N, Ferrucci L, Metter J, Faulkner KA, Strotmeyer E, Satterfield S, Schwartz A, Simonsick E. Association of lower limb cutaneous sensitivity with gait speed in the elderly: the health ABC study. Am J Phys Med Rehabil. 2008;87:921–928. doi: 10.1097/PHM.0b013e31818a5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Deshpande N, Metter EJ, Bandinelli S, Guralnik J, Ferrucci L. Gait speed under varied challenges and cognitive decline in older persons: a prospective study. Age Ageing. 2009;38:509–514. doi: 10.1093/ageing/afp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Di BM, van de Poll-Franse LV, Onder G, Kritchevsky SB, Newman A, Harris TB, Williamson JD, Marchionni N, Pahor M. Antihypertensive medications and differences in muscle mass in older persons: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:961–966. doi: 10.1111/j.1532-5415.2004.52265.x. [DOI] [PubMed] [Google Scholar]
- 117.Di IA, Cherubini A, Volpato S, Sparvieri E, Lauretani F, Franceschi C, Senin U, Abate G, Paganelli R, Martin A, Andres-Lacueva C, Ferrucci L. Markers of inflammation, vitamin E and peripheral nervous system function: the InCHIANTI study. Neurobiol Aging. 2006;27:1280–1288. doi: 10.1016/j.neurobiolaging.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dillaway HE, Byrnes M. Reconsidering Successful Aging: A Call for Renewed and Expanded Academic Critiques and Conceptualizations. Journal of Applied Gerontology. 2009;28:702–722. [Google Scholar]
- 119.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22:151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 120.Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol. 2002;282:R519–R527. doi: 10.1152/ajpregu.00458.2001. [DOI] [PubMed] [Google Scholar]
- 121.Dodge HH, Mattek NC, Austin D, Hayes TL, Kaye JA. In-home walking speeds and variability trajectories associated with mild cognitive impairment. Neurology. 2012;78:1946–1952. doi: 10.1212/WNL.0b013e318259e1de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Donati A, Cavallini G, Paradiso C, Vittorini S, Pollera M, Gori Z, Bergamini E. Age-Related Changes in the Autophagic Proteolysis of Rat Isolated Liver Cells Effects of Antiaging Dietary Restrictions. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2001;56:B375–B383. doi: 10.1093/gerona/56.9.b375. [DOI] [PubMed] [Google Scholar]
- 123.Dotson VM, Resnick SM, Zonderman AB. Differential association of concurrent, baseline, and average depressive symptoms with cognitive decline in older adults. Am J Geriatr Psychiatry. 2008;16:318–330. doi: 10.1097/JGP.0b013e3181662a9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dotson VM, Szymkowics SM, Kirton JW, McLaren ME, Green ML, Rohani JY. Unique and interactive effort of anxiety and depressive symptoms on cognitive and brain function in young and older adults. J. Depress Anxiety. 2014 doi: 10.4172/2167-1044.S1-003. Ref Type: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dotson VM, Zonderman AB, Davatzikos C, Kraut MA, Resnick SM. Frontal Atrophy and Attention Deficits in Older Adults with a History of Elevated Depressive Symptoms. Brain Imaging Behav. 2009;3:358. doi: 10.1007/s11682-009-9078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dotson VM, Zonderman AB, Kraut MA, Resnick SM. Temporal relationships between depressive symptoms and white matter hyperintensities in older men and women. Int J Geriatr Psychiatry. 2013;28:66–74. doi: 10.1002/gps.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dutta D, Calvani R, Bernabei R, Leeuwenburgh C, Marzetti E. Contribution of Impaired Mitochondrial Autophagy to Cardiac Aging Mechanisms and Therapeutic Opportunities. Circulation research. 2012;110:1125–1138. doi: 10.1161/CIRCRESAHA.111.246108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ebner NC, Freund AM. Handbook of Gerontology. New York: John Wiley & Sons, Inc; 2007. Personality Theories of Successful Aging; pp. 87–116. [Google Scholar]
- 129.Ebner NC, Freund AM, Baltes PB. Developmental changes in personal goal orientation from young to late adulthood: from striving for gains to maintenance and prevention of losses. Psychol Aging. 2006;21:664–678. doi: 10.1037/0882-7974.21.4.664. [DOI] [PubMed] [Google Scholar]
- 130.Ebner NC, Horta M, Lin T, Feifel D, Fisher H, Cohen RA. Oxytocin modulates meta-mood as a function of age and sex. Frontiers in Aging Neuroscience. 2015 doi: 10.3389/fnagi.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ebner NC, Kamin H, Diaz V, Cohen RA, Macdonald K. Hormones as "difference makers" in cognitive and socioemotional aging processes. Front Psychol. 2014;5:1595. doi: 10.3389/fpsyg.2014.01595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ebner NC, Maura GM, Macdonald K, Westberg L, Fischer H. Oxytocin and socioemotional aging: Current knowledge and future trends. Front Hum Neurosci. 2013;7:487. doi: 10.3389/fnhum.2013.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Edwards JD, Valdes EG, Peronto C, Castora-Binkley M, Alwerdt J, Andel R, Lister JJ. The Efficacy of InSight Cognitive Training to Improve Useful Field of View Performance: A Brief Report. J Gerontol B Psychol Sci Soc Sci. 2015;70:417–422. doi: 10.1093/geronb/gbt113. [DOI] [PubMed] [Google Scholar]
- 134.Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 135.Escobar-Bravo MA, Puga-Gonzalez D, Martin-Baranera M. Protective effects of social networks on disability among older adults in Spain. Arch Gerontol Geriatr. 2012;54:109–116. doi: 10.1016/j.archger.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 136.Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127:998S–1003S. doi: 10.1093/jn/127.5.998S. [DOI] [PubMed] [Google Scholar]
- 137.Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol. 2007;34:1091–1096. doi: 10.1111/j.1440-1681.2007.04752.x. [DOI] [PubMed] [Google Scholar]
- 138.Federal Interagency Forum on Aging-Related Statistics. Statistical Data of Older Americans 2008: Key Indicators of Well-Being. Washington, DC: Government Printing Office; 2009. [10-14-2009]. Federal Interagency Forum on Aging-Related Statistics. [Google Scholar]
- 139.Fekete EM, Seay J, Antoni MH, Mendez AJ, Fletcher MA, Szeto A, Schneiderman N. Oxytocin, social support, and sleep quality in low-income minority women living with HIV. Behav Sleep Med. 2014;12:207–221. doi: 10.1080/15402002.2013.791297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ferrell B, Whedon M, Rollins B. Pain and quality assessment/improvement. J Nurs Care Qual. 1995;9:69–85. doi: 10.1097/00001786-199504000-00010. [DOI] [PubMed] [Google Scholar]
- 141.Ferrucci L, Harris TB, Guralnik JM, Wacholder S, Tracy RP, Corti MC, Penninx BWJH, Pahor M, Wallace RB, Havlik RJ. Inflammation, a novel risk factor for disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 142.Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, Leveille SG, Fried LP, Md JM. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 143.Figaro MK, Kritchevsky SB, Resnick HE, Shorr RI, Butler J, Shintani A, Penninx BW, Simonsick EM, Goodpaster BH, Newman AB, Schwartz AV, Harris TB. Diabetes, inflammation, and functional decline in older adults: findings from the Health, Aging and Body Composition (ABC) study. Diabetes Care. 2006;29:2039–2045. doi: 10.2337/dc06-0245. [DOI] [PubMed] [Google Scholar]
- 144.Figueiredo PA, Powers SK, Ferreira RM, Appell HJ, Duarte JA. Aging impairs skeletal muscle mitochondrial bioenergetic function. J Gerontol A Biol Sci Med Sci. 2009;64:21–33. doi: 10.1093/gerona/gln048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Finkelstein EA, Ruhm CJ, Kosa KM. Economic causes and consequences of obesity. Annu Rev Public Health. 2005;26:239–257. doi: 10.1146/annurev.publhealth.26.021304.144628. [DOI] [PubMed] [Google Scholar]
- 146.Fitzpatrick AL, Buchanan CK, Nahin RL, Dekosky ST, Atkinson HH, Carlson MC, Williamson JD. Associations of gait speed and other measures of physical function with cognition in a healthy cohort of elderly persons. J Gerontol A Biol Sci Med Sci. 2007;62:1244–1251. doi: 10.1093/gerona/62.11.1244. [DOI] [PubMed] [Google Scholar]
- 147.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 148.Fletcher PC, Guthrie DM, Berg K, Hirdes JP. Risk factors for restriction in activity associated with fear of falling among seniors within the community. J Patient Saf. 2010;6:187–191. doi: 10.1097/pts.0b013e3181f1251c. [DOI] [PubMed] [Google Scholar]
- 149.Foldvari M, Clark M, Laviolette LC, Bernstein MA, Kaliton D, Castaneda C, Pu CT, Hausdorff JM, Fielding RA, Singh MA. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000;55:M192–M199. doi: 10.1093/gerona/55.4.m192. [DOI] [PubMed] [Google Scholar]
- 150.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 151.Foroni M, Salvioli G, Rielli R, Goldoni CA, Orlandi G, Zauli SS, Guerzoni A, Maccaferri C, Daya G, Mussi C. A retrospective study on heat-related mortality in an elderly population during the 2003 heat wave in Modena, Italy: the Argento Project. J Gerontol A Biol Sci Med Sci. 2007;62:647–651. doi: 10.1093/gerona/62.6.647. [DOI] [PubMed] [Google Scholar]
- 152.Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus. 2012;22:656–669. doi: 10.1002/hipo.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Freund AM, Ebner NC. The aging self: Shifting from promoting gains to balancing losses. In: Greve W, Rothermund K, Wentura D, editors. The adaptive self: Personal continuity and intentional self-development. Ashland: Hogrefe & Huber Publishers; 2005. pp. 185–202. [Google Scholar]
- 154.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 155.Furtado M, Katzman MA. Examining the role of neuroinflammation in major depression. Psychiatry Res. 2015;229:27–36. doi: 10.1016/j.psychres.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 156.Gambassi G, Lapane KL, Sgadari A, Carbonin P, Gatsonis C, Lipsitz LA, Mor V, Bernabei R. Effects of angiotensin-converting enzyme inhibitors and digoxin on health outcomes of very old patients with heart failure. SAGE Study Group. Systematic Assessment of Geriatric drug use via Epidemiology. Arch Intern Med. 2000;160:53–60. doi: 10.1001/archinte.160.1.53. [DOI] [PubMed] [Google Scholar]
- 157.Garnier A, Fortin D, Zoll J, N'Guessan B, Mettauer B, Lampert E, Veksler V, Ventura-Clapier R. Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. FASEB J. 2005a;19:43–52. doi: 10.1096/fj.04-2173com. [DOI] [PubMed] [Google Scholar]
- 158.Garnier A, Fortin D, Zoll J, N'Guessan B, Mettauer B, Lampert E, Veksler V, Ventura-Clapier R. Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. FASEB J. 2005b;19:43–52. doi: 10.1096/fj.04-2173com. [DOI] [PubMed] [Google Scholar]
- 159.Gerdle B, Lemming D, Kristiansen J, Larsson B, Peolsson M, Rosendal L. Biochemical alterations in the trapezius muscle of patients with chronic whiplash associated disorders (WAD)--a microdialysis study. Eur J Pain. 2008;12:82–93. doi: 10.1016/j.ejpain.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 160.Gerdle B, Soderberg K, Salvador PL, Rosendal L, Larsson B. Increased interstitial concentrations of pyruvate and lactate in the trapezius muscle of patients with fibromyalgia: a microdialysis study. J Rehabil Med. 2010;42:679–687. doi: 10.2340/16501977-0581. [DOI] [PubMed] [Google Scholar]
- 161.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 162.Glass TA. Assessing the success of successful aging. Ann Intern Med. 2003;139:382–383. doi: 10.7326/0003-4819-139-5_part_1-200309020-00015. [DOI] [PubMed] [Google Scholar]
- 163.Goldman EL. Industry self-regulation in the manufacture of dietary supplements and botanical medicines. Clin Obstet Gynecol. 2001;44:789–800. doi: 10.1097/00003081-200112000-00017. [DOI] [PubMed] [Google Scholar]
- 164.Goldman SE, Stone KL, Ancoli-Israel S, Blackwell T, Ewing SK, Boudreau R, Cauley JA, Hall M, Matthews KA, Newman AB. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep. 2007;30:1317–1324. doi: 10.1093/sleep/30.10.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Goldspink G, Fernandes K, Williams PE, Wells DJ. Age-related changes in collagen gene expression in the muscles of mdx dystrophic and normal mice. Neuromuscul Disord. 1994;4:183–191. doi: 10.1016/0960-8966(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 166.Gonzalvez F, Gottlieb E. Cardiolipin: setting the beat of apoptosis. Apoptosis. 2007;12:877–885. doi: 10.1007/s10495-007-0718-8. [DOI] [PubMed] [Google Scholar]
- 167.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 168.Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, Kritchevsky SB, Pahor M, Newman AB. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol (1985) 2008;105:1498–1503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gow AJ, Bastin ME, Munoz MS, Valdes Hernandez MC, Morris Z, Murray C, Royle NA, Starr JM, Deary IJ, Wardlaw JM. Neuroprotective lifestyles and the aging brain: activity, atrophy, and white matter integrity. Neurology. 2012;79:1802–1808. doi: 10.1212/WNL.0b013e3182703fd2. [DOI] [PubMed] [Google Scholar]
- 170.Graf B, Heyer T, Klein B, Wallhoff F. [Service robots in elderly care. Possible application areas and current state of developments] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:1145–1152. doi: 10.1007/s00103-013-1755-9. [DOI] [PubMed] [Google Scholar]
- 171.Grandner MA, Martin JL, Patel NP, Jackson NJ, Gehrman PR, Pien G, Perlis ML, Xie D, Sha D, Weaver T, Gooneratne NS. Age and sleep disturbances among American men and women: data from the U.S. Behavioral Risk Factor Surveillance System. Sleep. 2012;35:395–406. doi: 10.5665/sleep.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gray DB, Hollingsworth HH, Stark S, Morgan KA. A subjective measure of environmental facilitators and barriers to participation for people with mobility limitations. Disabil Rehabil. 2008;30:434–457. doi: 10.1080/09638280701625377. [DOI] [PubMed] [Google Scholar]
- 173.Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gregg EW, Mangione CM, Cauley JA, Thompson TJ, Schwartz AV, Ensrud KE, Nevitt MC. Diabetes and incidence of functional disability in older women. Diabetes Care. 2002;25:61–67. doi: 10.2337/diacare.25.1.61. [DOI] [PubMed] [Google Scholar]
- 175.Guderian B, Borreson LA, Sletten LE, Cable K, Stecker TP, Probst MA, Dalleck LC. The cardiovascular and metabolic responses to Wii Fit video game playing in middle-aged and older adults. J Sports Med Phys Fitness. 2010;50:436–442. [PubMed] [Google Scholar]
- 176.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 177.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 179.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 180.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 181.Harman D. The biologic clock: the mitochondria? Journal of the American Geriatrics Society. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 182.Havighurst RJ. Successful aging. The Gerontologist. 1961 [Google Scholar]
- 183.Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med. 2001;17:417–31. v. doi: 10.1016/s0749-0690(05)70078-1. [DOI] [PubMed] [Google Scholar]
- 184.Henson J, Yates T, Biddle SJ, Edwardson CL, Khunti K, Wilmot EG, Gray LJ, Gorely T, Nimmo MA, Davies MJ. Associations of objectively measured sedentary behaviour and physical activity with markers of cardiometabolic health. Diabetologia. 2013;56:1012–1020. doi: 10.1007/s00125-013-2845-9. [DOI] [PubMed] [Google Scholar]
- 185.Hiona A, Leeuwenburgh C. The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondrial vicious cycle theory of aging. Exp Gerontol. 2008;43:24–33. doi: 10.1016/j.exger.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hofer T, Fontana L, Anton SD, Weiss EP, Villareal D, Malayappan B, Leeuwenburgh C. Longterm effects of caloric restriction or exercise on DNA and RNA oxidation levels in white blood cells and urine in humans. Rejuvenation research. 2008;11:793–799. doi: 10.1089/rej.2008.0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hoffstaedter F, Grefkes C, Roski C, Caspers S, Zilles K, Eickhoff SB. Age-related decrease of functional connectivity additional to gray matter atrophy in a network for movement initiation. Brain Struct Funct. 2014 doi: 10.1007/s00429-013-0696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Holtta EH, Laakkonen ML, Laurila JV, Strandberg TE, Tilvis RS, Pitkala KH. Apathy: prevalence, associated factors, and prognostic value among frail, older inpatients. J Am Med Dir Assoc. 2012;13:541–545. doi: 10.1016/j.jamda.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 189.Hoy MB. The "Internet of Things": What It Is and What It Means for Libraries. Med Ref Serv Q. 2015;34:353–358. doi: 10.1080/02763869.2015.1052699. [DOI] [PubMed] [Google Scholar]
- 190.Hsu FC, Kritchevsky SB, Liu Y, Kanaya A, Newman AB, Perry SE, Visser M, Pahor M, Harris TB, Nicklas BJ. Association Between Inflammatory Components and Physical Function in the Health, Aging, and Body Composition Study: A Principal Component Analysis Approach. J Gerontol A Biol Sci Med Sci. 2009;64:581–589. doi: 10.1093/gerona/glp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Huang AR, Mallet L, Rochefort CM, Eguale T, Buckeridge DL, Tamblyn R. Medication-related falls in the elderly: causative factors and preventive strategies. Drugs Aging. 2012;29:359–376. doi: 10.2165/11599460-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 192.Hung YC, Lee JH, Chen HM, Huang GS. Effects of static magnetic fields on the development and aging of Caenorhabditis elegans. J Exp Biol. 2010;213:2079–2085. doi: 10.1242/jeb.039768. [DOI] [PubMed] [Google Scholar]
- 193.Hutcheon SD, Gillespie ND, Crombie IK, Struthers AD, McMurdo ME. Perindopril improves six minute walking distance in older patients with left ventricular systolic dysfunction: a randomised double blind placebo controlled trial. Heart. 2002;88:373–377. doi: 10.1136/heart.88.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hyatt RH, Whitelaw MN, Bhat A, Scott S, Maxwell JD. Association of muscle strength with functional status of elderly people. Age Ageing. 1990;19:330–336. doi: 10.1093/ageing/19.5.330. [DOI] [PubMed] [Google Scholar]
- 195.Hybels CF, Pieper CF, Blazer DG. The complex relationship between depressive symptoms and functional limitations in community-dwelling older adults: the impact of subthreshold depression. Psychol Med. 2009;39:1677–1688. doi: 10.1017/S0033291709005650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ishii S, Weintraub N, Mervis JR. Apathy: a common psychiatric syndrome in the elderly. J Am Med Dir Assoc. 2009;10:381–393. doi: 10.1016/j.jamda.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 197.Ivanova DG, Yankova TM. The Free Radical Theory of Aging In Search of a Strategy for Increasing Life Span. Folia medica. 2013;55:33–41. doi: 10.2478/folmed-2013-0003. [DOI] [PubMed] [Google Scholar]
- 198.Jackowska M, Dockray S, Hendrickx H, Steptoe A. Psychosocial factors and sleep efficiency: discrepancies between subjective and objective evaluations of sleep. Psychosom Med. 2011;73:810–816. doi: 10.1097/PSY.0b013e3182359e77. [DOI] [PubMed] [Google Scholar]
- 199.James BD, Wilson RS, Barnes LL, Bennett DA. Late-life social activity and cognitive decline in old age. J Int Neuropsychol Soc. 2011;17:998–1005. doi: 10.1017/S1355617711000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 201.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 202.Jedrziewski MK, Lee VM, Trojanowski JQ. Physical activity and cognitive health. Alzheimers Dement. 2007;3:98–108. doi: 10.1016/j.jalz.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jennison C, Turnbull BW. Group Sequential Tests with Applications to Clinical Trials. London: Chapman & Hill/CRC; 2000. [Google Scholar]
- 204.Jensen GL, Roy MA, Buchanan AE, Berg MB. Weight loss intervention for obese older women: improvements in performance and function. Obes Res. 2004;12:1814–1820. doi: 10.1038/oby.2004.225. [DOI] [PubMed] [Google Scholar]
- 205.Joseph A, Pilegaard H, Litvintsev A, Leick L, Hood D. Control of gene expression and mitochondrial biogenesis in the muscular adaptation to endurance exercise. Essays Biochem. 2006;42:13–29. doi: 10.1042/bse0420013. [DOI] [PubMed] [Google Scholar]
- 206.Joseph A, Adhihetty PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguyen L, Aranda JM, Sandesara BD, Pahor M, Manini TM. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high and low functioning elderly individuals. Aging Cell. 2012;11:801–809. doi: 10.1111/j.1474-9726.2012.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Karttunen NM, Turunen J, Ahonen R, Hartikainen S. More attention to pain management in community-dwelling older persons with chronic musculoskeletal pain. Age Ageing. 2014;43:845–850. doi: 10.1093/ageing/afu052. [DOI] [PubMed] [Google Scholar]
- 208.Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13:7–23. doi: 10.31887/DCNS.2011.13.1/wkaton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kawamura Y, O'Brien P, Okazaki H, Dyck PJ. Lumbar motoneurons of man II: the number and diameter distribution of large- and intermediate-diameter cytons in "motoneuron columns" of spinal cord of man. J Neuropathol Exp Neurol. 1977;36:861–870. doi: 10.1097/00005072-197709000-00010. [DOI] [PubMed] [Google Scholar]
- 210.Kaye AD, Baluch A, Scott JT. Pain management in the elderly population: a review. Ochsner J. 2010;10:179–187. [PMC free article] [PubMed] [Google Scholar]
- 211.Kayyali B, Knott D, Van Kuiken S. The big-data revolution in US health care: Acceleration value and innovation. [1-21-0015];2013 [Google Scholar]
- 212.Keatinge WR, Donaldson GC. The impact of global warming on health and mortality. South Med J. 2004;97:1093–1099. doi: 10.1097/01.SMJ.0000144635.07975.66. [DOI] [PubMed] [Google Scholar]
- 213.Kennedy GJ, Kelman HR, Thomas C. The emergence of depressive symptoms in late life: the importance of declining health and increasing disability. J Community Health. 1990;15:93–104. doi: 10.1007/BF01321314. [DOI] [PubMed] [Google Scholar]
- 214.Kerns RD, Sellinger J, Goodin BR. Psychological treatment of chronic pain. Annu Rev Clin Psychol. 2011;7:411–434. doi: 10.1146/annurev-clinpsy-090310-120430. [DOI] [PubMed] [Google Scholar]
- 215.Kessler SK, Minhas P, Woods AJ, Rosen A, Gorman C, Bikson M. Dosage considerations for transcranial direct current stimulation in children: a computational modeling study. PLoS One. 2013;8:e76112. doi: 10.1371/journal.pone.0076112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Keysor JJ, Brembs A. Exercise: necessary but not sufficient for improving function and preventing disability? Curr Opin Rheumatol. 2011;23:211–218. doi: 10.1097/BOR.0b013e3283432c41. [DOI] [PubMed] [Google Scholar]
- 217.Keysor JJ, Jette AM, LaValley MP, Lewis CE, Torner JC, Nevitt MC, Felson DT. Community environmental factors are associated with disability in older adults with functional limitations: the MOST study. J Gerontol A Biol Sci Med Sci. 2010;65:393–399. doi: 10.1093/gerona/glp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kim JH, Kwak HB, Leeuwenburgh C, Lawler JM. Lifelong exercise and mild (8%) caloric restriction attenuate age-induced alterations in plantaris muscle morphology, oxidative stress and IGF-1 in the Fischer-344 rat. Exp Gerontol. 2008;43:317–329. doi: 10.1016/j.exger.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kohrt WM, Malley MT, Coggan AR, Spina RJ, Ogawa T, Ehsani AA, Bourey RE, Martin WH, III, Holloszy JO. Effects of gender, age, and fitness level on response of VO2max to training in 60–71 yr olds. J Appl Physiol (1985) 1991;71:2004–2011. doi: 10.1152/jappl.1991.71.5.2004. [DOI] [PubMed] [Google Scholar]
- 221.Koster A, Ding J, Stenholm S, Caserotti P, Houston DK, Nicklas BJ, You T, Lee JS, Visser M, Newman AB, Schwartz AV, Cauley JA, Tylavsky FA, Goodpaster BH, Kritchevsky SB, Harris TB. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci. 2011;66:888–895. doi: 10.1093/gerona/glr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kripke DF, Langer RD, Elliott JA, Klauber MR, Rex KM. Mortality related to actigraphic long and short sleep. Sleep Med. 2011;12:28–33. doi: 10.1016/j.sleep.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kujoth GC, Bradshaw PC, Haroon S, Prolla TA. The role of mitochondrial DNA mutations in mammalian aging. PLoS Genet. 2007;3:e24. doi: 10.1371/journal.pgen.0030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kunzli N, Kaiser R, Medina S, Studnicka M, Chanel O, Filliger P, Herry M, Horak F, Jr, Puybonnieux-Texier V, Quenel P, Schneider J, Seethaler R, Vergnaud JC, Sommer H. Public-health impact of outdoor and traffic-related air pollution: a European assessment. Lancet. 2000;356:795–801. doi: 10.1016/S0140-6736(00)02653-2. [DOI] [PubMed] [Google Scholar]
- 225.Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol Respir Environ Exerc Physiol. 1979;46:451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- 226.Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand. 1978;103:31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 227.Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di IA, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985) 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 228.Lavand'homme P. The progression from acute to chronic pain. Curr Opin Anaesthesiol. 2011;24:545–550. doi: 10.1097/ACO.0b013e32834a4f74. [DOI] [PubMed] [Google Scholar]
- 229.Leahy R, Crews DE. Physiological dysregulation and somatic decline among elders: modeling, applying and re-interpreting allostatic load. Coll Antropol. 2012;36:11–22. [PubMed] [Google Scholar]
- 230.Lebrun CE, van der Schouw YT, de Jong FH, Pols HA, Grobbee DE, Lamberts SW. Relations between body composition, functional and hormonal parameters and quality of life in healthy postmenopausal women. Maturitas. 2006;55:82–92. doi: 10.1016/j.maturitas.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 231.Lee HY, Choi CS, Birkenfeld AL, Alves TC, Jornayvaz FR, Jurczak MJ, Zhang D, Woo DK, Shadel GS, Ladiges W. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell metabolism. 2010;12:668–674. doi: 10.1016/j.cmet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lee SL, Wang WW, Fanburg BL. Superoxide as an intermediate signal for serotonin-induced mitogenesis. Free Radical Biology and Medicine. 1998;24:855–858. doi: 10.1016/s0891-5849(97)00359-6. [DOI] [PubMed] [Google Scholar]
- 233.Lees FD, Clarkr PG, Nigg CR, Newman P. Barriers to exercise behavior among older adults: a focus-group study. J Aging Phys Act. 2005;13:23–33. doi: 10.1123/japa.13.1.23. [DOI] [PubMed] [Google Scholar]
- 234.Lehmacher W, Wassmer G. Adaptive sample size calculations in group sequential trials. Biometrics. 1999;55:1286–1290. doi: 10.1111/j.0006-341x.1999.01286.x. [DOI] [PubMed] [Google Scholar]
- 235.Li H, Li J, Li N, Li B, Wang P, Zhou T. Cognitive intervention for persons with mild cognitive impairment: A meta-analysis. Ageing Res Rev. 2011;10:285–296. doi: 10.1016/j.arr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 236.Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med (Berl) 2008;86:1113–1126. doi: 10.1007/s00109-008-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30:145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 238.Ling C, Poulsen P, Carlsson E, Ridderstrale M, Almgren P, Wojtaszewski J, Beck-Nielsen H, Groop L, Vaag A. Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins. J Clin Invest. 2004;114:1518–1526. doi: 10.1172/JCI21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Little JP, Safdar A, Bishop D, Tarnopolsky MA, Gibala MJ. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1alpha and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1303–R1310. doi: 10.1152/ajpregu.00538.2010. [DOI] [PubMed] [Google Scholar]
- 240.Lonergan ET, Krevans JR. A national agenda for research on aging. N Engl J Med. 1991;324:1825–1828. doi: 10.1056/NEJM199106203242527. [DOI] [PubMed] [Google Scholar]
- 241.Long J, Gao H, Sun L, Liu J, Zhao-Wilson X. Grape extract protects mitochondria from oxidative damage and improves locomotor dysfunction and extends lifespan in a Drosophila Parkinson's disease model. Rejuvenation Res. 2009;12:321–331. doi: 10.1089/rej.2009.0877. [DOI] [PubMed] [Google Scholar]
- 242.Luo J, Zhu G, Zhao Q, Guo Q, Meng H, Hong Z, Ding D. Prevalence and risk factors of poor sleep quality among Chinese elderly in an urban community: results from the Shanghai aging study. PLoS One. 2013;8:e81261. doi: 10.1371/journal.pone.0081261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lyness JM, Chapman BP, McGriff J, Drayer R, Duberstein PR. One-year outcomes of minor and subsyndromal depression in older primary care patients. Int Psychogeriatr. 2009;21:60–68. doi: 10.1017/S1041610208007746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Maher CA, Mire E, Harrington DM, Staiano AE, Katzmarzyk PT. The independent and combined associations of physical activity and sedentary behavior with obesity in adults: NHANES 2003–06. Obesity (Silver Spring) 2013;21:E730–E737. doi: 10.1002/oby.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Maillot P, Perrot A, Hartley A. Effects of interactive physical-activity video-game training on physical and cognitive function in older adults. Psychol Aging. 2012;27:589–600. doi: 10.1037/a0026268. [DOI] [PubMed] [Google Scholar]
- 246.Main ML, Rao SC, O'Keefe JH. Trends in obesity and extreme obesity among US adults. JAMA. 2010;303:1695–1696. doi: 10.1001/jama.2010.517. [DOI] [PubMed] [Google Scholar]
- 247.Mallik A, Weir AI. Nerve conduction studies: essentials and pitfalls in practice. J Neurol Neurosurg Psychiatry. 2005;76(Suppl 2):ii23–ii31. doi: 10.1136/jnnp.2005.069138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Mancuso M, Filosto M, Bellan M, Liguori R, Montagna P, Baruzzi A, DiMauro S, Carelli V. POLG mutations causing ophthalmoplegia, sensorimotor polyneuropathy, ataxia, and deafness. Neurology. 2004;62:316–318. doi: 10.1212/wnl.62.2.316. [DOI] [PubMed] [Google Scholar]
- 249.Manini TM, Buford TW, Lott DJ, Vandenborne K, Daniels MJ, Knaggs JD, Patel H, Pahor M, Perri MG, Anton SD. Effect of dietary restriction and exercise on lower extremity tissue compartments in obese, older women: a pilot study. J Gerontol A Biol Sci Med Sci. 2014;69:101–108. doi: 10.1093/gerona/gls337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67:28–40. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Manini TM, Everhart JE, Anton SD, Schoeller DA, Cummings SR, Mackey DC, Delmonico MJ, Bauer DC, Simonsick EM, Colbert LH, Visser M, Tylavsky F, Newman AB, Harris TB. Activity energy expenditure and change in body composition in late life. Am J Clin Nutr. 2009;90:1336–1342. doi: 10.3945/ajcn.2009.27659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Manini TM, Hong SL, Clark BC. Aging and muscle: a neuron's perspective. Curr Opin Clin Nutr Metab Care. 2013;16:21–26. doi: 10.1097/MCO.0b013e32835b5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Manini TM, Newman AB, Fielding R, Blair SN, Perri MG, Anton SD, Goodpaster BC, Katula JA, Rejeski WJ, Kritchevsky SB, Hsu FC, Pahor M, King AC. Effects of exercise on mobility in obese and nonobese older adults. Obesity (Silver Spring) 2010;18:1168–1175. doi: 10.1038/oby.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Manson JE, Bassuk SS. Obesity in the United States: a fresh look at its high toll. JAMA. 2003;289:229–230. doi: 10.1001/jama.289.2.229. [DOI] [PubMed] [Google Scholar]
- 255.Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003;58:M911–M916. doi: 10.1093/gerona/58.10.m911. [DOI] [PubMed] [Google Scholar]
- 256.Marsiske M, Dzierzewski JM, Thomas KR, Kasten L, Jones RN, Johnson KE, Willis SL, Whitfield KE, Ball KK, Rebok GW. Race-related disparities in 5-year cognitive level and change in untrained ACTIVE participants. J Aging Health. 2013;25:103S–127S. doi: 10.1177/0898264313497794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Marzetti E, Calvani R, Bernabei R, Leeuwenburgh C. Apoptosis in skeletal myocytes: a potential target for interventions against sarcopenia and physical frailty - a mini-review. Gerontology. 2012;58:99–106. doi: 10.1159/000330064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Marzetti E, Lees HA, Wohlgemuth SE, Leeuwenburgh C. Sarcopenia of aging: underlying cellular mechanisms and protection by calorie restriction. Biofactors. 2009;35:28–35. doi: 10.1002/biof.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Marzetti E, Wohlgemuth SE, Anton SD, Bernabei R, Carter CS, Leeuwenburgh C. Cellular mechanisms of cardioprotection by calorie restriction: state of the science and future perspectives. Clin Geriatr Med. 2009;25:715–32. ix. doi: 10.1016/j.cger.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.McCarthy LH, Bigal ME, Katz M, Derby C, Lipton RB. Chronic pain and obesity in elderly people: results from the Einstein aging study. J Am Geriatr Soc. 2009;57:115–119. doi: 10.1111/j.1532-5415.2008.02089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.McDermott MM, Liu K, Ferrucci L, Tian L, Guralnik JM, Green D, Tan J, Liao Y, Pearce WH, Schneider JR, McCue K, Ridker P, Rifai N, Criqui MH. Circulating blood markers and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2008;56:1504–1510. doi: 10.1111/j.1532-5415.2008.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.McGeehin MA, Mirabelli M. The potential impacts of climate variability and change on temperature-related morbidity and mortality in the United States. Environ Health Perspect. 2001;109(Suppl 2):185–189. doi: 10.1289/ehp.109-1240665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.McGinley M, Hoffman RL, Russ DW, Thomas JS, Clark BC. Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Exp Gerontol. 2010;45:671–678. doi: 10.1016/j.exger.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.McLean RR, Shardell MD, Alley DE, Cawthon PM, Fragala MS, Harris TB, Kenny AM, Peters KW, Ferrucci L, Guralnik JM, Kritchevsky SB, Kiel DP, Vassileva MT, Xue QL, Perera S, Studenski SA, Dam TT. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci. 2014;69:576–583. doi: 10.1093/gerona/glu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young old, and very old men. Muscle Nerve. 2005;31:461–467. doi: 10.1002/mus.20276. [DOI] [PubMed] [Google Scholar]
- 266.Mehlman MJ, Binstock RH, Juengst ET, Ponsaran RS, Whitehouse PJ. Anti-aging medicine: can consumers be better protected? Gerontologist. 2004;44:304–310. doi: 10.1093/geront/44.3.304. [DOI] [PubMed] [Google Scholar]
- 267.Menant JC, Schoene D, Lord SR. Single and dual task tests of gait speed are equivalent in the prediction of falls in older people: A systematic review and meta-analysis. Ageing Res Rev. 2014 doi: 10.1016/j.arr.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 268.Merritt EK, Stec MJ, Thalacker-Mercer A, Windham ST, Cross JM, Shelley DP, Craig TS, Kosek DJ, Kim JS, Bamman MM. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J Appl Physiol (1985) 2013;115:937–948. doi: 10.1152/japplphysiol.00019.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, Ettinger WH, Pahor M, Williamson JD. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis & Rheumatism. 2004;50:1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 270.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 271.Mezuk B, Edwards L, Lohman M, Choi M, Lapane K. Depression and frailty in later life: a synthetic review. Int J Geriatr Psychiatry. 2012;27:879–892. doi: 10.1002/gps.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Min K, Smuder AJ, Kwon OS, Kavazis AN, Szeto HH, Powers SK. Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy. J Appl Physiol (1985) 2011;111:1459–1466. doi: 10.1152/japplphysiol.00591.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Minhas P, Bikson M, Woods AJ, Rosen AR, Kessler SK. Transcranial direct current stimulation in pediatric brain: a computational modeling study. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:859–862. doi: 10.1109/EMBC.2012.6346067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Miquel J, Economos AC, Fleming J, Johnson JE., Jr Mitochondrial role in cell aging. Experimental gerontology. 1980;15:575–591. doi: 10.1016/0531-5565(80)90010-8. [DOI] [PubMed] [Google Scholar]
- 275.Miszko TA, Cress ME, Slade JM, Covey CJ, Agrawal SK, Doerr CE. Effect of strength and power training on physical function in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2003;58:171–175. doi: 10.1093/gerona/58.2.m171. [DOI] [PubMed] [Google Scholar]
- 276.Mittal KR, Logmani FH. Age-related reduction in 8th cervical ventral nerve root myelinated fiber diameters and numbers in man. J Gerontol. 1987;42:8–10. doi: 10.1093/geronj/42.1.8. [DOI] [PubMed] [Google Scholar]
- 277.Mold JW, Vesely SK, Keyl BA, Schenk JB, Roberts M. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older patients. J Am Board Fam Pract. 2004;17:309–318. doi: 10.3122/jabfm.17.5.309. [DOI] [PubMed] [Google Scholar]
- 278.Moon SS. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocr J. 2014;61:61–70. doi: 10.1507/endocrj.ej13-0244. [DOI] [PubMed] [Google Scholar]
- 279.Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJ, Cummings SR, Evans WJ, Fearon K, Ferrucci L, Fielding RA, Guralnik JM, Harris TB, Inui A, Kalantar-Zadeh K, Kirwan BA, Mantovani G, Muscaritoli M, Newman AB, Rossi-Fanelli F, Rosano GM, Roubenoff R, Schambelan M, Sokol GH, Storer TW, Vellas B, von HS, Yeh SS, Anker SD. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–409. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Moscufo N, Wolfson L, Meier D, Liguori M, Hildenbrand PG, Wakefield D, Schmidt JA, Pearlson GD, Guttmann CR. Mobility decline in the elderly relates to lesion accrual in the splenium of the corpus callosum. Age (Dordr) 2012;34:405–414. doi: 10.1007/s11357-011-9242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Muhlberg W, Sieber C. Sarcopenia and frailty in geriatric patients: implications for training and prevention. Z Gerontol Geriatr. 2004;37:2–8. doi: 10.1007/s00391-004-0203-8. [DOI] [PubMed] [Google Scholar]
- 282.Nadkarni NK, Studenski SA, Perera S, Rosano C, Aizenstein HJ, Brach JS, Van Swearingen JM. White matter hyperintensities, exercise, and improvement in gait speed: does type of gait rehabilitation matter? J Am Geriatr Soc. 2013;61:686–693. doi: 10.1111/jgs.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Naugle KM, Cruz-Almeida Y, Vierck CJ, Mauderli AP, Riley JL., III Age-related differences in conditioned pain modulation of sensitizing and desensitizing trends during response dependent stimulation. Behav Brain Res. 2015;289:61–68. doi: 10.1016/j.bbr.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Naugle KM, Higgins TJ, Manini TM. Obesity and use of compensatory strategies to perform common daily activities in pre-clinically disabled older adults. Archives of gerontology and geriatrics. 2012;54:e134–e138. doi: 10.1016/j.archger.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Nelson ME, Layne JE, Bernstein MJ, Nuernberger A, Castaneda C, Kaliton D, Hausdorff J, Judge JO, Buchner DM, Roubenoff R, Fiatarone Singh MA. The effects of multidimensional home-based exercise on functional performance in elderly people. J Gerontol A Biol Sci Med Sci. 2004;59:154–160. doi: 10.1093/gerona/59.2.m154. [DOI] [PubMed] [Google Scholar]
- 287.Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2013;39:1–19. doi: 10.1016/j.rdc.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Ng TP, Niti M, Zaw MH, Kua EH. Depressive symptoms and incident cognitive impairment in cognitively well-functioning older men and women. J Am Geriatr Soc. 2009;57:1058–1063. doi: 10.1111/j.1532-5415.2009.02262.x. [DOI] [PubMed] [Google Scholar]
- 289.Nguyen TD, Dolomie-Fagour L, Georges A, Corcuff JB. What about bioavailable estradiol? Ann Biol Clin (Paris) 2008;66:493–497. doi: 10.1684/abc.2008.0259. [DOI] [PubMed] [Google Scholar]
- 290.Ni Mhaolain AM, Fan CW, Romero-Ortuno R, Cogan L, Cunningham C, Lawlor B, Kenny RA. Depression: a modifiable factor in fearful older fallers transitioning to frailty? Int J Geriatr Psychiatry. 2012;27:727–733. doi: 10.1002/gps.2780. [DOI] [PubMed] [Google Scholar]
- 291.Nitsche MA. Transcranial direct current stimulation: a new treatment for depression? Bipolar Disord. 2002;4(Suppl 1):98–99. doi: 10.1034/j.1399-5618.4.s1.43.x. [DOI] [PubMed] [Google Scholar]
- 292.Nitsche MA, Muller-Dahlhaus F, Paulus W, Ziemann U. The pharmacology of neuroplasticity induced by non-invasive brain stimulation: building models for the clinical use of CNS active drugs. J Physiol. 2012;590:4641–4662. doi: 10.1113/jphysiol.2012.232975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Nitsche MA, Paulus W. Transcranial direct current stimulation--update 2011. Restor Neurol Neurosci. 2011;29:463–492. doi: 10.3233/RNN-2011-0618. [DOI] [PubMed] [Google Scholar]
- 295.Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci. 2003;15:619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- 296.Nocera J, Buford TW, Manini TM, Naugle K, Leeuwenburgh C, Pahor M, Perri MG, Anton SD. The impact of behavioral intervention on obesity mediated declines in mobility function: implications for longevity. J Aging Res. 2011;2011:392510. doi: 10.4061/2011/392510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.O'Neill MS, Ebi KL. Temperature extremes and health: impacts of climate variability and change in the United States. J Occup Environ Med. 2009;51:13–25. doi: 10.1097/JOM.0b013e318173e122. [DOI] [PubMed] [Google Scholar]
- 298.Ogden CL, Carroll MD, Flegal KM. Prevalence of obesity in the United States. JAMA. 2014;312:189–190. doi: 10.1001/jama.2014.6228. [DOI] [PubMed] [Google Scholar]
- 299.Onder G, Penninx BW, Balkrishnan R, Fried LP, Chaves PH, Williamson J, Carter C, Di BM, Guralnik JM, Pahor M. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359:926–930. doi: 10.1016/s0140-6736(02)08024-8. [DOI] [PubMed] [Google Scholar]
- 300.Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Oosterman JM, Gibson SJ, Pulles WL, Veldhuijzen DS. On the moderating role of age in the relationship between pain and cognition. Eur J Pain. 2013;17:735–741. doi: 10.1002/j.1532-2149.2012.00235.x. [DOI] [PubMed] [Google Scholar]
- 302.Ormel J, Oldehinkel T, Brilman E, vanden Brink W. Outcome of depression and anxiety in primary care. A three-wave 3 1/2-year study of psychopathology and disability. Arch Gen Psychiatry. 1993;50:759–766. doi: 10.1001/archpsyc.1993.01820220009001. [DOI] [PubMed] [Google Scholar]
- 303.Orozco-Solis R, Sassone-Corsi P. Circadian clock: linking epigenetics to aging. Curr Opin Genet Dev. 2014;26:66–72. doi: 10.1016/j.gde.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Ory MG, Smith ML, Ahn S, Jiang L, Lorig K, Whitelaw N. National study of chronic disease selfmanagement: age comparison of outcome findings. Health Educ Behav. 2014;41:34S–42S. doi: 10.1177/1090198114543008. [DOI] [PubMed] [Google Scholar]
- 305.Pace-Schott EF, Spencer RMC. Sleep Lossin Older Adults: Effects on Waking Performance and Sleep-Dependant Memory Consolidation with Healthy Aging and Insomnia. In: Bianchi MT, editor. Sleep Deprivation and Disease: Effects on the Body, Brain, and Behavior. New York: Springer; 2013. pp. 185–197. [Google Scholar]
- 306.Pahor M, Blair SN, Espeland M, Fielding R, Gill TM, Guralnik JM, Hadley EC, King AC, Kritchevsky SB, Maraldi C, Miller ME, Newman AB, Rejeski WJ, Romashkan S, Studenski S. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 307.Pai A. Connected health market will reach $8B in 2018. [1-21-2015];mobihealthnews. 2014 [Google Scholar]
- 308.Palluel E, Nougier V, Olivier I. Do spike insoles enhance postural stability and plantar-surface cutaneous sensitivity in the elderly? Age (Dordr) 2008;30:53–61. doi: 10.1007/s11357-008-9047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Palluel E, Olivier I, Nougier V. The lasting effects of spike insoles on postural control in the elderly. Behav Neurosci. 2009;123:1141–1147. doi: 10.1037/a0017115. [DOI] [PubMed] [Google Scholar]
- 310.Pan WH, Li LA, Tsai MJ. Temperature extremes and mortality from coronary heart disease and cerebral infarction in elderly Chinese. Lancet. 1995;345:353–355. doi: 10.1016/s0140-6736(95)90341-0. [DOI] [PubMed] [Google Scholar]
- 311.Papegaaij S, Taube W, Baudry S, Otten E, Hortobagyi T. Aging causes a reorganization of cortical and spinal control of posture. Front Aging Neurosci. 2014;6:28. doi: 10.3389/fnagi.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Papp KV, Walsh SJ, Snyder PJ. Immediate and delayed effects of cognitive interventions in healthy elderly: a review of current literature and future directions. Alzheimers Dement. 2009;5:50–60. doi: 10.1016/j.jalz.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 313.Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene. 2002;286:135–141. doi: 10.1016/s0378-1119(01)00814-9. [DOI] [PubMed] [Google Scholar]
- 314.Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, Stampfer MJ, Hu FB. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 315.Patten SB. Depressive symptoms and disorders, levels of functioning and psychosocial stress: an integrative hypothesis. Med Hypotheses. 1999;53:210–216. doi: 10.1054/mehy.1998.0747. [DOI] [PubMed] [Google Scholar]
- 316.Pedersen CA. Oxytocin control of maternal behavior. Regulation by sex steroids and offspring stimuli. Ann N Y Acad Sci. 1997;807:126–145. doi: 10.1111/j.1749-6632.1997.tb51916.x. [DOI] [PubMed] [Google Scholar]
- 317.Peeters A, Bonneux L, Nusselder WJ, Laet C, Barendregt JJ. Adult obesity and the burden of disability throughout life. Obesity Research. 2004;12:1145–1151. doi: 10.1038/oby.2004.143. [DOI] [PubMed] [Google Scholar]
- 318.Penninx BW, Abbas H, Ambrosius W, Nicklas BJ, Davis C, Messier SP, Pahor M. Inflammatory Markers and Physical Function Among Older Adults with Knee Osteoarthritis. J Rheumatol. 2004;31:2027–2031. [PubMed] [Google Scholar]
- 319.Penninx BW, Kritchevsky SB, Newman AB, Nicklas BJ, Simonsick EM, Rubin S, Nevitt M, Visser M, Harris T, Pahor M. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x. [DOI] [PubMed] [Google Scholar]
- 320.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 321.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Petervari E, Rostas I, Soos S, Tenk J, Miko A, Furedi N, Szekely M, Balasko M. Age versus nutritional state in the development of central leptin resistance. Peptides. 2014;56:59–67. doi: 10.1016/j.peptides.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 323.Physical activity guidelines for Americans. Physical activity guidelines for Americans. Okla Nurse. 2008;53:25. [PubMed] [Google Scholar]
- 324.Pisters MF, Veenhof C, van Dijk GM, Heymans MW, Twisk JW, Dekker J. The course of limitations in activities over 5 years in patients with knee and hip osteoarthritis with moderate functional limitations: risk factors for future functional decline. Osteoarthritis Cartilage. 2012;20:503–510. doi: 10.1016/j.joca.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 325.Plow EB, Varnerin N, Cunningham DA, Janini D, Bonnett C, Wyant A, Hou J, Siemionow V, Wang XF, Machado AG, Yue GH. Age-related weakness of proximal muscle studied with motor cortical mapping: a TMS study. PLoS One. 2014;9:e89371. doi: 10.1371/journal.pone.0089371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Pope CA, III, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med. 2009;360:376–386. doi: 10.1056/NEJMsa0805646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Pourhassan M, Bosy-Westphal A, Schautz B, Braun W, Gluer CC, Muller MJ. Impact of body composition during weight change on resting energy expenditure and homeostasis model assessment index in overweight nonsmoking adults. Am J Clin Nutr. 2014;99:779–791. doi: 10.3945/ajcn.113.071829. [DOI] [PubMed] [Google Scholar]
- 328.Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness*. Critical care medicine. 2011;39:1749–1759. doi: 10.1097/CCM.0b013e3182190b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, Mackey JR, Koski S, Pituskin E, Sawyer MB. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15:2920–2926. doi: 10.1158/1078-0432.CCR-08-2242. [DOI] [PubMed] [Google Scholar]
- 330.Priplata AA, Niemi JB, Harry JD, Lipsitz LA, Collins JJ. Vibrating insoles and balance control in elderly people. Lancet. 2003;362:1123–1124. doi: 10.1016/S0140-6736(03)14470-4. [DOI] [PubMed] [Google Scholar]
- 331.Proschan MA, Hunsberger SA. Designed extension of studies based on conditional power. Biometrics. 1995;51:1315–1324. [PubMed] [Google Scholar]
- 332.Pruchno RA, Wilson-Genderson M, Cartwright F. A two-factor model of successful aging. J Gerontol B Psychol Sci Soc Sci. 2010;65:671–679. doi: 10.1093/geronb/gbq051. [DOI] [PubMed] [Google Scholar]
- 333.Pruchno RA, Wilson-Genderson M, Rose M, Cartwright F. Successful aging: early influences and contemporary characteristics. Gerontologist. 2010;50:821–833. doi: 10.1093/geront/gnq041. [DOI] [PubMed] [Google Scholar]
- 334.Pyo JO, Yoo SM, Jung YK. The Interplay between Autophagy and Aging. Diabetes Metab J. 2013;37:333–339. doi: 10.4093/dmj.2013.37.5.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Raji MA, Kuo YF, Snih SA, Markides KS, Peek MK, Ottenbacher KJ. Cognitive status, muscle strength, and subsequent disability in older Mexican Americans. J Am Geriatr Soc. 2005;53:1462–1468. doi: 10.1111/j.1532-5415.2005.53457.x. [DOI] [PubMed] [Google Scholar]
- 336.Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51:636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 337.Rantz MJ, Skubic M, Koopman RJ, Alexander GL, Phillips L, Musterman K, Back J, Aud MA, Galambos C, Guevara RD, Miller SJ. Automated technology to speed recognition of signs of illness in older adults. J Gerontol Nurs. 2012;38:18–23. doi: 10.3928/00989134-20120307-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Rashidi P, Cook DJ, Holder LB, Schmitter-Edgecombe M. Discovering Activities to Recognize and Track in a Smart Environment. IEEE Trans Knowl Data Eng. 2011;23:527–539. doi: 10.1109/TKDE.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Rashidi P, Mihailidis A. A survey on ambient-assisted living tools for older adults. IEEE J Biomed Health Inform. 2013;17:579–590. doi: 10.1109/jbhi.2012.2234129. [DOI] [PubMed] [Google Scholar]
- 340.Rebok GW, Ball K, Guey LT, Jones RN, Kim HY, King JW, Marsiske M, Morris JN, Tennstedt SL, Unverzagt FW, Willis SL. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62:16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Reijnders J, van HC, van BM. Cognitive interventions in healthy older adults and people with mild cognitive impairment: a systematic review. Ageing Res Rev. 2013;12:263–275. doi: 10.1016/j.arr.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 342.Resnick HE, Vinik AI, Schwartz AV, Leveille SG, Brancati FL, Balfour J, Guralnik JM. Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age: the Women's Health and Aging Study. Diabetes Care. 2000;23:1642–1647. doi: 10.2337/diacare.23.11.1642. [DOI] [PubMed] [Google Scholar]
- 343.Riekse RG, Leverenz JB, McCormick W, Bowen JD, Teri L, Nochlin D, Simpson K, Eugenio C, Larson EB, Tsuang D. Effect of vascular lesions on cognition in Alzheimer's disease: a community-based study. J Am Geriatr Soc. 2004;52:1442–1448. doi: 10.1111/j.1532-5415.2004.52405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Riley JL, III, Cruz-Almeida Y, Glover TL, King CD, Goodin BR, Sibille KT, Bartley EJ, Herbert MS, Sotolongo A, Fessler BJ, Redden DT, Staud R, Bradley LA, Fillingim RB. Age and race effects on pain sensitivity and modulation among middle-aged and older adults. J Pain. 2014;15:272–282. doi: 10.1016/j.jpain.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Rillamas-Sun E, LaCroix AZ, Waring ME, Kroenke CH, LaMonte MJ, Vitolins MZ, Seguin R, Bell CL, Gass M, Manini TM. Obesity and late-age survival without major disease or disability in older women. JAMA internal medicine. 2014;174:98–106. doi: 10.1001/jamainternmed.2013.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Ritchie CS, Hearld KR, Gross A, Allman R, Sawyer P, Sheppard K, Salanitro A, Locher J, Brown CJ, Roth DL. Measuring symptoms in community-dwelling older adults: the psychometric properties of a brief symptom screen. Med Care. 2013;51:949–955. doi: 10.1097/MLR.0b013e3182a53d1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Rivner MH, Swift TR, Malik K. Influence of age and height on nerve conduction. Muscle Nerve. 2001;24:1134–1141. doi: 10.1002/mus.1124. [DOI] [PubMed] [Google Scholar]
- 348.Roberts RE, Kaplan GA, Shema SJ, Strawbridge WJ. Does growing old increase the risk for depression? Am J Psychiatry. 1997;154:1384–1390. doi: 10.1176/ajp.154.10.1384. [DOI] [PubMed] [Google Scholar]
- 349.Rolland Y, Czerwinski S, Abellan Van KG, Morley JE, Cesari M, Onder G, Woo J, Baumgartner R, Pillard F, Boirie Y, Chumlea WM, Vellas B. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del PP, Foretz M, Scorrano L, Rudolf R, Sandri M. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 2010;29:1774–1785. doi: 10.1038/emboj.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93:15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1380–1388. doi: 10.1093/gerona/63.12.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Rosano C, Aizenstein HJ, Studenski S, Newman AB. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62:1048–1055. doi: 10.1093/gerona/62.9.1048. [DOI] [PubMed] [Google Scholar]
- 354.Rosano C, Bennett DA, Newman AB, Venkatraman V, Yaffe K, Harris T, Kritchevsky S, Aizenstein HJ. Patterns of focal gray matter atrophy are associated with bradykinesia and gait disturbances in older adults. J Gerontol A Biol Sci Med Sci. 2012;67:957–962. doi: 10.1093/gerona/glr262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Rosano C, Sigurdsson S, Siggeirsdottir K, Phillips CL, Garcia M, Jonsson PV, Eiriksdottir G, Newman AB, Harris TB, van Buchem MA, Gudnason V, Launer LJ. Magnetization transfer imaging, white matter hyperintensities, brain atrophy and slower gait in older men and women. Neurobiol Aging. 2010;31:1197–1204. doi: 10.1016/j.neurobiolaging.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Rosen AC, Ramkumar M, Nguyen T, Hoeft F. Noninvasive transcranial brain stimulation and pain. Curr Pain Headache Rep. 2009;13:12–17. doi: 10.1007/s11916-009-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Rosenberg D, Depp CA, Vahia IV, Reichstadt J, Palmer BW, Kerr J, Norman G, Jeste DV. Exergames for subsyndromal depression in older adults: a pilot study of a novel intervention. Am J Geriatr Psychiatry. 2010;18:221–226. doi: 10.1097/JGP.0b013e3181c534b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Rosendal L, Larsson B, Kristiansen J, Peolsson M, Sogaard K, Kjaer M, Sorensen J, Gerdle B. Increase in muscle nociceptive substances and anaerobic metabolism in patients with trapezius myalgia: microdialysis in rest and during exercise. Pain. 2004;112:324–334. doi: 10.1016/j.pain.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 359.Ross LA, Edwards JD, O'Connor ML, Ball KK, Wadley VG, Vance DE. The Transfer of Cognitive Speed of Processing Training to Older Adults' Driving Mobility Across 5 Years. J Gerontol B Psychol Sci Soc Sci. 2015 doi: 10.1093/geronb/gbv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Rossi AL, Pereira VS, Driusso P, Rebelatto JR, Ricci NA. Profile of the elderly in physical therapy and its relation to functional disability. Braz J Phys Ther. 2013;17:77–85. doi: 10.1590/s1413-35552012005000060. [DOI] [PubMed] [Google Scholar]
- 361.Rosso AL, Studenski SA, Chen WG, Aizenstein HJ, Alexander NB, Bennett DA, Black SE, Camicioli R, Carlson MC, Ferrucci L, Guralnik JM, Hausdorff JM, Kaye J, Launer LJ, Lipsitz LA, Verghese J, Rosano C. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci. 2013;68:1379–1386. doi: 10.1093/gerona/glt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Roth T, Coulouvrat C, Hajak G, Lakoma MD, Sampson NA, Shahly V, Shillington AC, Stephenson JJ, Walsh JK, Kessler RC. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 363.Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997;37:433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- 364.Russell AP. PGC-1alpha and exercise: important partners in combating insulin resistance. Curr Diabetes Rev. 2005;1:175–181. doi: 10.2174/1573399054022811. [DOI] [PubMed] [Google Scholar]
- 365.Rutan RL, Herndon DN. Growth delay in postburn pediatric patients. Arch Surg. 1990;125:392–395. doi: 10.1001/archsurg.1990.01410150114021. [DOI] [PubMed] [Google Scholar]
- 366.Safdar A, Hamadeh MJ, Kaczor JJ, Raha S, Debeer J, Tarnopolsky MA. Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PLoS One. 2010;5:e10778. doi: 10.1371/journal.pone.0010778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Safdar A, Hamadeh MJ, Kaczor JJ, Raha S, Tarnopolsky MA. Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PloS one. 2010;5:e10778. doi: 10.1371/journal.pone.0010778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Saldiva PH, Pope CA, III, Schwartz J, Dockery DW, Lichtenfels AJ, Salge JM, Barone I, Bohm GM. Air pollution and mortality in elderly people: a time-series study in Sao Paulo, Brazil. Arch Environ Health. 1995;50:159–163. doi: 10.1080/00039896.1995.9940893. [DOI] [PubMed] [Google Scholar]
- 369.Saleem M. Comparing Memory and Executive Function Performance in Coronary Artery Disease Patients Dischotomized into Low and High Cortisol Groups over 1 year of Cardiac Rehabilitation. 2011 [Google Scholar]
- 370.Salthouse TA. Influence of age on practice effects in longitudinal neurocognitive change. Neuropsychology. 2010;24:563–572. doi: 10.1037/a0019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Salthouse TA. Robust cognitive change. J Int Neuropsychol Soc. 2012;18:749–756. doi: 10.1017/S1355617712000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Sanders JL, Ding V, Arnold AM, Kaplan RC, Cappola AR, Kizer JR, Boudreau RM, Cushman M, Newman AB. Do changes in circulating biomarkers track with each other and with functional changes in older adults? J Gerontol A Biol Sci Med Sci. 2014;69:174–181. doi: 10.1093/gerona/glt088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Sandstrom T, Frew AJ, Svartengren M, Viegi G. The need for a focus on air pollution research in the elderly. Eur Respir J Suppl. 2003;40:92s–95s. doi: 10.1183/09031936.03.00403503. [DOI] [PubMed] [Google Scholar]
- 374.Santanasto AJ, Glynn NW, Newman MA, Taylor CA, Brooks MM, Goodpaster BH, Newman AB. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. Journal of obesity. 2010;2011 doi: 10.1155/2011/516576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Scarpace PJ, Tumer N. Peripheral and hypothalamic leptin resistance with age-related obesity. Physiol Behav. 2001;74:721–727. doi: 10.1016/s0031-9384(01)00616-3. [DOI] [PubMed] [Google Scholar]
- 376.Scarpace PJ, Zhang Y. Leptin resistance: a prediposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R493–R500. doi: 10.1152/ajpregu.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, Visser M. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–1189. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Gunturkun O, Maier W, Hurlemann R. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci U S A. 2013;110:20308–20313. doi: 10.1073/pnas.1314190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 380.Schneiderman I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin during the initial stages of romantic attachment: relations to couples' interactive reciprocity. Psychoneuroendocrinology. 2012;37:1277–1285. doi: 10.1016/j.psyneuen.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Schwartz GE. Testing the biopsychosocial model: the ultimate challenge facing behavioral medicine? J Consult Clin Psychol. 1982;50:1040–1053. doi: 10.1037//0022-006x.50.6.1040. [DOI] [PubMed] [Google Scholar]
- 382.Sedentary Behaviour RN. Letter to the editor: standardized use of the terms "sedentary" and "sedentary behaviours". Appl Physiol Nutr Metab. 2012;37:540–542. doi: 10.1139/h2012-024. [DOI] [PubMed] [Google Scholar]
- 383.Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. Journal of cell science. 2010;123:2533–2542. doi: 10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Shidoji Y, Hayashi K, Komura S, Ohishi N, Yagi K. Loss of Molecular Interaction between Cytochrome< i> c</i> and Cardiolipin Due to Lipid Peroxidation. Biochemical and biophysical research communications. 1999;264:343–347. doi: 10.1006/bbrc.1999.1410. [DOI] [PubMed] [Google Scholar]
- 385.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005a;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair K. Decline in skeletal muscle mitochondrial function with aging in humans. Proceedings of the National Academy of Sciences of the United States of America. 2005b;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Shuval K, Hebert ET, Siddiqi Z, Leonard T, Lee SC, Tiro JA, McCallister K, Skinner CS. Impediments and facilitators to physical activity and perceptions of sedentary behavior among urban community residents: the Fair Park Study. Prev Chronic Dis. 2013;10:E177. doi: 10.5888/pcd10.130125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Sibille KT, Witek-Janusek L, Mathews HL, Fillingim RB. Telomeres and epigenetics: potential relevance to chronic pain. Pain. 2012;153:1789–1793. doi: 10.1016/j.pain.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab. 2011;13:495–504. doi: 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Solberg NL, Roach AR, Segerstrom SC. Executive functions, self-regulation, and chronic pain: a review. Ann Behav Med. 2009;37:173–183. doi: 10.1007/s12160-009-9096-5. [DOI] [PubMed] [Google Scholar]
- 392.Someya S, Prolla TA. Mitochondrial oxidative damage and apoptosis in age-related hearing loss. Mech Ageing Dev. 2010;131:480–486. doi: 10.1016/j.mad.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Speisman RB, Kumar A, Rani A, Foster TC, Ormerod BK. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain Behav Immun. 2013;28:25–43. doi: 10.1016/j.bbi.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Spiegelman BM. Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. Novartis Found Symp. 2007;287:60–63. [PubMed] [Google Scholar]
- 395.St John PD, Tyas SL, Montgomery PR. Depressive symptoms and frailty. Int J Geriatr Psychiatry. 2013;28:607–614. doi: 10.1002/gps.3866. [DOI] [PubMed] [Google Scholar]
- 396.Steffens DC, Hays JC, Krishnan KR. Disability in geriatric depression. Am J Geriatr Psychiatry. 1999;7:34–40. [PubMed] [Google Scholar]
- 397.Strotmeyer ES, de RN, Schwartz AV, Faulkner KA, Resnick HE, Goodpaster BH, Shorr RI, Vinik AI, Harris TB, Newman AB. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults: the Health, Aging, and Body Composition (Health ABC) study. Diabetes Care. 2008;31:1767–1772. doi: 10.2337/dc08-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Strotmeyer ES, de RN, Schwartz AV, Resnick HE, Goodpaster BH, Faulkner KA, Shorr RI, Vinik AI, Harris TB, Newman AB. Sensory and motor peripheral nerve function and lower-extremity quadriceps strength: the health, aging and body composition study. J Am Geriatr Soc. 2009;57:2004–2010. doi: 10.1111/j.1532-5415.2009.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Studenski S, Perera S, Hile E, Keller V, Spadola-Bogard J, Garcia J. Interactive video dance games for healthy older adults. J Nutr Health Aging. 2010;14:850–852. doi: 10.1007/s12603-010-0119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam TT, Vassileva MT. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 403.Sumukadas D, Struthers AD, McMurdo ME. Sarcopenia--a potential target for Angiotensinconverting enzyme inhibition? Gerontology. 2006;52:237–242. doi: 10.1159/000093656. [DOI] [PubMed] [Google Scholar]
- 404.Sumukadas D, Witham MD, Struthers AD, McMurdo ME. Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial. CMAJ. 2007;177:867–874. doi: 10.1503/cmaj.061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Suzuki T, Bean JF, Fielding RA. Muscle power of the ankle flexors predicts functional performance in community-dwelling older women. J Am Geriatr Soc. 2001;49:1161–1167. doi: 10.1046/j.1532-5415.2001.49232.x. [DOI] [PubMed] [Google Scholar]
- 406.Szanton SL, Allen JK, Seplaki CL, Bandeen-Roche K, Fried LP. Allostatic load and frailty in the women's health and aging studies. Biol Res Nurs. 2009;10:248–256. doi: 10.1177/1099800408323452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Tandler B, Hoppel CL. Studies on giant mitochondria. Annals of the New York Academy of Sciences. 1986;488:65–81. doi: 10.1111/j.1749-6632.1986.tb46548.x. [DOI] [PubMed] [Google Scholar]
- 408.Terman A, Brunk UT. The aging myocardium: roles of mitochondrial damage and lysosomal degradation. Heart Lung Circ. 2005;14:107–114. doi: 10.1016/j.hlc.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 409.Testa G, Biasi F, Poli G, Chiarpotto E. Calorie restriction and dietary restriction mimetics: a strategy for improving healthy aging and longevity. Curr Pharm Des. 2014;20:2950–2977. doi: 10.2174/13816128113196660699. [DOI] [PubMed] [Google Scholar]
- 410.The CLHLS Research Team. Specification of the coding system for the 1998–2005 longitudinal dataset (including the 1998 baseline survey, 2000, 2002, and 2005 follow-up surveys of both survivors and died persons) Durham NC: Duke University; 2008. pp. 1–318. [Google Scholar]
- 411.Torrance N, Elliott AM, Lee AJ, Smith BH. Severe chronic pain is associated with increased 10 year mortality. A cohort record linkage study. Eur J Pain. 2010;14:380–386. doi: 10.1016/j.ejpain.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 412.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 413.Tsao H, Galea MP, Hodges PW. Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain. 2008;131:2161–2171. doi: 10.1093/brain/awn154. [DOI] [PubMed] [Google Scholar]
- 414.Tsao H, Galea MP, Hodges PW. Driving plasticity in the motor cortex in recurrent low back pain. Eur J Pain. 2010;14:832–839. doi: 10.1016/j.ejpain.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 415.Tuttle LJ, Sinacore DR, Mueller MJ. Intermuscular adipose tissue is muscle specific and associated with poor functional performance. J Aging Res. 2012;2012:172957. doi: 10.1155/2012/172957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. The EMBO journal. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.van HJ, Kort HS, Rutten PG, Duijnstee MS. Ageing-in-place with the use of ambient intelligence technology: perspectives of older users. Int J Med Inform. 2011;80:310–331. doi: 10.1016/j.ijmedinf.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 418.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 419.Vastag B. Obesity Is Now on Everyone's Plate. JAMA. 2004;291:1186–1188. doi: 10.1001/jama.291.10.1186. [DOI] [PubMed] [Google Scholar]
- 420.Verdejo HE, del CA, Troncoso R, Gutierrez T, Toro B, Quiroga C, Pedrozo Z, Munoz JP, Garcia L, Castro PF, Lavandero S. Mitochondria, myocardial remodeling, and cardiovascular disease. Curr Hypertens Rep. 2012;14:532–539. doi: 10.1007/s11906-012-0305-4. [DOI] [PubMed] [Google Scholar]
- 421.Verghese J, Holtzer R, Lipton RB, Wang C. High-sensitivity C-reactive protein and mobility disability in older adults. Age Ageing. 2012;41:541–545. doi: 10.1093/ageing/afs038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Vermulst M, Wanagat J, Kujoth GC, Bielas JH, Rabinovitch PS, Prolla TA, Loeb LA. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet. 2008;40:392–394. doi: 10.1038/ng.95. [DOI] [PubMed] [Google Scholar]
- 423.Viana-Baptista M, Bugalho P, Jordao C, Ribeiro O, Esperanca-Pina JA, Ferro J. Motor dysfunction correlates with frontal white matter ischemic changes in patients with leukoaraiosis. J Aging Res. 2011;2011:950341. doi: 10.4061/2011/950341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K. Weight loss, exercise, or both and physical function in obese older adults. New England Journal of Medicine. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Vincent HK, Horodyski M, Gearen P, Vlasak R, Seay AN, Conrad BP, Vincent KR. Obesity and long term functional outcomes following elective total hip replacement. J Orthop Surg Res. 2012;7:16. doi: 10.1186/1749-799X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 427.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 428.Wadstrom C, Muller-Suur R, Backman L. Influence of excessive weight loss on respiratory function. A study of obese patients following gastroplasty. Eur J Surg. 1991;157:341–346. [PubMed] [Google Scholar]
- 429.Wakefield DB, Moscufo N, Guttmann CR, Kuchel GA, Kaplan RF, Pearlson G, Wolfson L. White matter hyperintensities predict functional decline in voiding, mobility, and cognition in older adults. J Am Geriatr Soc. 2010;58:275–281. doi: 10.1111/j.1532-5415.2009.02699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Walker I, Newell H. Do molecularly targeted agents in oncology have reduced attrition rates? Nat Rev Drug Discov. 2009;8:15–16. doi: 10.1038/nrd2758. [DOI] [PubMed] [Google Scholar]
- 431.Wanagat Jona, Cao Zhen, Pathare Pran, Aiken JM. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. The FASEB Journal. 2001;15:322–332. doi: 10.1096/fj.00-0320com. [DOI] [PubMed] [Google Scholar]
- 432.Wang CP, Lorenzo C, Espinoza SE. Frailty Attenuates the Impact of Metformin on Reducing Mortality in Older Adults with Type 2 Diabetes. J Endocrinol Diabetes Obes. 2014;2 [PMC free article] [PubMed] [Google Scholar]
- 433.Weber TA, Reichert AS. Impaired quality control of mitochondria: aging from a new perspective. Exp Gerontol. 2010;45:503–511. doi: 10.1016/j.exger.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 434.Wen M, Gu D. Air pollution shortens life expectancy and health expectancy for older adults: the case of China. J Gerontol A Biol Sci Med Sci. 2012;67:1219–1229. doi: 10.1093/gerona/gls094. [DOI] [PubMed] [Google Scholar]
- 435.Wennie Huang WN, Perera S, VanSwearingen J, Studenski S. Performance measures predict onset of activity of daily living difficulty in community-dwelling older adults. J Am Geriatr Soc. 2010;58:844–852. doi: 10.1111/j.1532-5415.2010.02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 437.White DK, Felson DT, Niu J, Nevitt MC, Lewis CE, Torner JC, Neogi T. Reasons for functional decline despite reductions in knee pain: the Multicenter Osteoarthritis Study. Phys Ther. 2011;91:1849–1856. doi: 10.2522/ptj.20100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.White DK, Jette AM, Felson DT, LaValley MP, Lewis CE, Torner JC, Nevitt MC, Keysor JJ. Are features of the neighborhood environment associated with disability in older adults? Disabil Rehabil. 2010;32:639–645. doi: 10.3109/09638280903254547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.WHO. Geneva: World Health Organization; 2009. Global health risks: mortality and burden of disease attributable to selected major risks. [Google Scholar]
- 440.Willey JZ, Scarmeas N, Provenzano FA, Luchsinger JA, Mayeux R, Brickman AM. White matter hyperintensity volume and impaired mobility among older adults. J Neurol. 2013;260:884–890. doi: 10.1007/s00415-012-6731-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Williams PG. Self-regulation, executive functioning, and neurovisceral integration. Pain. 2010;151:5–6. doi: 10.1016/j.pain.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 442.Wohlgemuth SE, Calvani R, Marzetti E. The interplay between autophagy and mitochondrial dysfunction in oxidative stress-induced cardiac aging and pathology. J Mol Cell Cardiol 71C. 2014:62–70. doi: 10.1016/j.yjmcc.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 443.Wohlgemuth SE, Julian D, Akin DE, Fried J, Toscano K, Leeuwenburgh C, Dunn J. Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation research. 2007;10:281–292. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
- 444.Woods AJ, Cohen RA, Pahor M. Cognitive frailty: frontiers and challenges. J Nutr Health Aging. 2013;17:741–743. doi: 10.1007/s12603-013-0398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Woods AJ, Hamilton RH, Kranjec A, Minhaus P, Bikson M, Yu J, Chatterjee A. Space, time, and causality in the human brain. Neuroimage. 2014;92:285–297. doi: 10.1016/j.neuroimage.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Woods AJ, Mark VW, Pitts AC, Mennemeier M. Pervasive cognitive impairment in acute rehabilitation inpatients without brain injury. PM R. 2011;3:426–432. doi: 10.1016/j.pmrj.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 448.Wu SS, Tu YH, He Y. Testing for efficacy in adaptive clinical trials with enrichment. Stat Med. 2014;33:2736–2745. doi: 10.1002/sim.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Wu S, Wang W, Yang M. Interval estimation for drop-the-loser designs. Biometrika. 2010;97:405–418. [Google Scholar]
- 450.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Yang Y, George LK. Functional disability, disability transitions, and depressive symptoms in late life. J Aging Health. 2005;17:263–292. doi: 10.1177/0898264305276295. [DOI] [PubMed] [Google Scholar]
- 452.Zanobetti A, O'Neill MS, Gronlund CJ, Schwartz JD. Summer temperature variability and longterm survival among elderly people with chronic disease. Proc Natl Acad Sci U S A. 2012;109:6608–6613. doi: 10.1073/pnas.1113070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Zeier Z, Madorsky I, Xu Y, Ogle WO, Notterpek L, Foster TC. Gene expression in the hippocampus: regionally specific effects of aging and caloric restriction. Mech Ageing Dev. 2011;132:8–19. doi: 10.1016/j.mad.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Zhang H, Wu C, Chen Q, Chen X, Xu Z, Wu J, Cai D. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS One. 2013;8:e61477. doi: 10.1371/journal.pone.0061477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Zhang J, Mauzerall DL, Zhu T, Liang S, Ezzati M, Remais JV. Environmental health in China: progress towards clean air and safe water. Lancet. 2010;375:1110–1119. doi: 10.1016/S0140-6736(10)60062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Zhu J, Wang KZ, Chu CT. After the banquet: mitochondrial biogenesis, mitophagy, and cell survival. Autophagy. 2013;9:1663–1676. doi: 10.4161/auto.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Zi M, Carmichael N, Lye M. The effect of quinapril on functional status of elderly patients with diastolic heart failure. Cardiovasc Drugs Ther. 2003;17:133–139. doi: 10.1023/a:1025387702212. [DOI] [PubMed] [Google Scholar]