Abstract
Antigen delivered within particulate materials leads to enhanced antigen-specific immunity compared to soluble administration of antigen. However, current delivery approaches for antigen encapsulated in synthetic particulate materials are limited by the complexity of particle production that affects stability and immunogenicity of the antigen. Herein, we describe a protein delivery system that utilizes plasma membrane vesicles (PMVs) derived from biological materials such as cultured cells or isolated tissues and a simple protein transfer technology. We show that these particulate PMVs can be easily modified within 4 h by a protein transfer process to stably incorporate a glycosylphosphatidylinositol (GPI)-anchored form of the breast cancer antigen HER-2 onto the PMV surface. Immunization of mice with GPI-HER-2-modified-PMVs induced strong HER-2-specific antibody responses and protection from tumor challenge in two different breast cancer models. Further incorporation of the immunostimulatory molecules IL-12 and B7-1 onto the PMVs by protein transfer enhanced tumor protection and induced beneficial Th1 and Th2-type HER-2-specific immune responses. Since protein antigens can be easily converted to GPI-anchored forms, these results demonstrate that isolated plasma membrane vesicles can be modified with desired antigens along with immunostimulatory molecules by protein transfer and used as a vaccine delivery vehicle to elicit potent antigen-specific immunity.
Keywords: Plasma membrane vesicle, protein transfer, breast cancer, tumor antigens, vaccine
Introduction
The identification of tumor-associated antigens (TAAs) has led to major advancements in the field of cancer immunotherapy (1). To elicit an immune response against TAA-bearing tumor cells, TAA-derived peptides and proteins have been delivered to the immune system by a variety of ways. These include direct administration of TAAs as vaccines in the presence of adjuvants, dendritic cells (DCs) loaded ex vivo with TAA proteins or peptides, and DNA encoding TAAs delivered directly or by viral vectors (2–4). However, poor immunogenicity and short half-life of soluble proteins and peptides, the need for standardization and extensive ex vivo preparation required for loading DCs (2, 3), the poor delivery of DNA vaccines, and safety issues with the use of viral vectors (4) have necessitated the development of new approaches to deliver TAAs to boost anti-tumor immune responses.
Successful delivery of antigens to develop effective antigen-specific immunity requires for the antigens to be delivered without degradation to antigen presenting cells (APCs) (5). Numerous studies have demonstrated the use of particle-based delivery systems for protein and peptide-based vaccines as a highly promising approach. Nanoparticles and microparticles that are particulate in nature and optimal in size for phagocytosis and uptake by APCs (6), also have the ability to increase antigen half-life of encapsulated or surface attached antigens by delivering antigens in a sustainable manner (7). These properties make particles an efficient vaccine delivery system (7–9). Many tumor, viral and parasitic antigens have been delivered using particles (10), which has resulted in augmented immunity against the antigen compared to soluble antigen administration (7, 9).
A variety of particle-based vaccines, such as lipid-based particles as well as natural and synthetic polymer-based biodegradable particles (11), have been used as antigen delivery vehicles to elicit an antigen-specific adaptive immune response. Nonetheless, these approaches require complex particle production. Encapsulating antigens during particle formation also often leads to exposure of antigens to toxic organic solvents (7). On the other hand, attachment of antigens to particle surfaces may involve chemical modification that can further affect antigen stability and immunogenicity, as well as alter particle formulation. Further, lack of complete biodegradability and biocompatibility of particles leads to further toxicity concerns (8), and production of reproducible large quantities of uniform particles may also raise cost issues (8). These caveats emphasize the need to develop biocompatible antigen delivery systems as vaccines.
In the present study, we describe the use of plasma membrane vesicles (PMVs) as a promising biological particle-based tumor antigen delivery system. These vesicles are distinct from exosomes secreted by cells which are approximately 110 nm in diameter (12, 13). PMVs are prepared from homogenization of cells or isolated tissues, followed by purification using sucrose gradient centrifugation (14–19). This method results in the formation of vesicles from plasma membranes; thus PMVs contain lipid bilayers, making them amenable to modification by protein transfer. Protein transfer uses glycosylphosphatidylinositol-anchored immunostimulatory molecules (GPI-ISMs) to modify cell or membrane surfaces in a simple, rapid process whereby cells or membranes are incubated with purified GPI-ISMs for 2–4 hours (16, 17, 19–21). Incubation results in the spontaneous incorporation of the GPI-ISMs onto cell membranes via the GPI-anchor in a concentration, time and temperature-dependent manner (16, 17, 19) and expression of incorporated GPI-ISMs on PMVs is not affected even after storage of the protein transferred vesicles (19).
Previously, ISMs such as the transmembrane co-stimulatory molecule B7-1 and the soluble cytokine IL-12 were converted to GPI-anchored forms and incorporated successfully onto PMVs to enhance immunity against antigens native to the PMVs (16, 17, 19). Protein transferred PMVs with incorporated GPI-B7-1 or GPI-IL-12 induced enhanced T-cell proliferation (16, 17, 19), and PMVs displaying GPI-B7-1 also induced tumor-specific T-cell mediated cytotoxicity, and protection from parental tumor challenge in mice (16). Although ISMs have been incorporated onto PMVs by protein transfer for elicitation of immunity against intrinsic antigens expressed on PMVs, the protein transfer of GPI-anchored antigens and the elicitation of immunity against incorporated foreign antigens has yet to be investigated. Such information will have implications in the delivery of a wide array of antigens to induce immunity against tumors and pathogens.
The ability to convert both transmembrane and soluble proteins into GPI-anchored forms suggests that TAAs can be converted into a GPI-anchored form, incorporated onto PMVs by protein transfer, and delivered to the immune system. Herein, we present a PMV-based adjuvanted co-delivery system of a breast cancer antigen, human epidermal growth factor receptor-2 (HER-2). HER-2 along with membrane-bound ISMs, B7-1 and IL-12, were incorporated onto breast cancer derived-PMVs by protein transfer. Vaccination with modified tumor tissue-derived PMVs induced complete protection against a HER-2-expressing tumor cell challenge along with delayed tumor growth and partial regression of established tumors. The protection induced was found to be mediated by both cellular and humoral immunity, in which the delivery of GPI-HER-2 on PMVs enhanced HER-2-specific serum IgG compared to soluble GPI-HER-2 administration and elicited both a T helper 1 (Th1) and Th2-type immune response.
Materials and Methods
Animals
BALB/c and C57BL/6 mice (6–8 weeks old) were purchased from Jackson Laboratories. All experiments were performed per Emory University approved IACUC guidelines.
DNA Constructs
To form GPI-anchored protein DNA constructs, the CD59 GPI-signal sequence was attached to the extracellular domain of transmembrane proteins HER-2 and B7-1 or to the C-terminal end of the soluble cytokine IL-12 as shown previously (22, 23). The GPI-HER-2 DNA construct was constructed as previously described (24). Mouse GPI-B7-1 was constructed by attaching the CD59 GPI-signal sequence nucleotides to the extracellular domain of mouse B7-1 (GenBank BC131959.1, nucleotides 45–800) via an EcoRI restriction enzyme site. Further, amino acid 253 was changed from a lysine to alanine by changing nucleotides AAG to GCG. To construct GPI-IL-12, the p35 subunit of mouse IL-12 was attached to the CD59 GPI-signal sequence. This sequence was then followed by an IRES along with the soluble p40 subunit of mouse IL-12. All three constructs were inserted into pUB6blast vectors (Invitrogen).
Cell lines
CHO-K1 cells, maintained in RPMI with 5% bovine calf serum (BCS), were transfected with HER-2-CD59, B7-1-CD59, or IL-12-a-CD59-b-sol DNA constructs using Lipofectamine 2000 (Invitrogen). The transfected CHO-K1 cells were selected with 20 μg/ml Blasticidin and maintained in RPMI with 5% BCS with 10 μg/ml Blasticidin. High expressing transfected CHO-K1 cells were selected for by panning and expression was analyzed by flow cytometry. 4TO7 cells are an aggressive, non-metastatic murine breast cancer cell line derived from a mammary tumor spontaneously grown in a Balb/cfC3H mouse (25). D2F2 and D2F2/E2 cells are murine breast cancer cell lines that were a kind gift from Dr. Wei-Zen Wei (Wayne State University) (26, 27). D2F2/E2 cells were established by transfecting D2F2 cells with full-length HER-2 DNA (26). E0771 cells were generated by Dr. Francis M. Sirotnak (Sloan-Kettering Institute) (28) and were a kind gift from Dr. Subra Malarkannan (The Med. College of Wisconsin). The cell line is derived from the mammary gland of C57BL/6 (29). E0771 cells are highly aggressive and metastatic (30).
Antibodies
Anti-HER-2 monoclonal antibody (mAb)-secreting hybridoma, TA1, was obtained from ATCC. Anti-HER-2 mAb was purified from the supernatant by a Gammabind-plus Sepharose column (GE Healthcare). Purified anti-mIL-12 p40 mAb, (C17.8) and anti-mB7-1 mAb (1G10) were obtained from Bio X Cell. Purified mAbs were coupled to CNBr-activated Sepharose beads (GE Healthcare) to create mAb-affinity columns for purification. Anti-CD4 hybridoma (GK1.5) and anti-CD8 hybridoma (H35) were a kind gift from Dr. Aaron Lukacher, Emory University. The anti-CD4 and anti-CD8 mAbs were purified from the hybridoma culture supernatant by a Gammabind-plus Sepharose column. Goat-anti-mouse IgG-FITC and Goat-anti-rat IgG-FITC (Jackson Immunoresearch) were used for flow cytometry analysis.
PI-PLC treatment
To determine if transfected CHO-K1 cells expressed the GPI-anchored form of each protein, transfected CHO-K1 cells were subjected to phosphatidylinositol-specific phospholipase C (PI-PLC) treatment. 1 ml of a 10 × 106 cell/ml cell suspension of transfected CHO-K1 cells in PBS containing 5mM EDTA and 0.1% ovalbumin was incubated with 1U of PI-PLC from Bacillus cereus (Invitrogen) at 37°C for 45 min. The cells were then washed three times and flow cytometry was used to determine surface protein expression.
Purification of GPI-anchored proteins
The GPI-anchored proteins (GPI-APs) were purified from lysates of CHO-K1 cell transfectants by affinity chromatography as previously described (17, 20, 24). GPI-HER-2 and GPI-B7-1 were eluted from the mAb-affinity chromatography column using 100mM Triethylamine, 1% n-octyl-β-D-glucopyranoside, pH 11.6, and GPI-IL-12 was eluted using 100 mM Glycine-HCl pH 2.8 containing 1% n-octyl-β-D-glucopyranoside, and 10 mM sodium iodoacetate. SDS PAGE followed by western blot and silver stain was performed to analyze the eluted fractions, which were then concentrated using polyvinylpyrrolidone (Sigma-Aldrich), followed by dialysis with three exchanges of PBS containing 0.01% n-octyl-β-D-glucopyranoside. A micro BCA kit (Thermo Scientific) was used to quantify concentrated purified GPI-HER-2 and GPI-B7-1. A direct ELISA with known concentrations of recombinant soluble mIL-12 p75 (eBioscience) as standards was used to quantify purified GPI-IL-12 concentrations.
Protein transfer of purified GPI-APs onto sheep RBCs
To test if purified GPI-APs can incorporate onto cell membrane lipid bilayers by protein transfer, sheep red blood cells (RBCs) were used. PBS-washed sheep RBCs were resuspended in PBS/0.1% ovalbumin at a concentration of 10 × 106 RBCs/ml. 200 μl of RBCs were incubated with purified GPI-AP for 4 h at 37°C under solution end-over-end rotation. Centrifugation with PBS was used to wash off unincorporated proteins and incorporation was analyzed by flow cytometry analysis.
PMV preparation and characterization
BALB/c mice were inoculated with 4 × 105 4TO7 regrowth (RG) or D2F2 cells and C57BL/6 mice were inoculated with 4 × 105 E0771 cells. After tumor growth, mice were sacrificed and tumor tissue was isolated and stored at −80°C until homogenization. For homogenizing tumor tissue into membrane vesicles, tumor tissue was thawed on ice and minced with scissors. 1 ml of ice cold homogenization buffer (20 mM Tris pH 8.0, 10 mM NaCl, 0.1 mM MgCl2, 0.1 mM PMSF) was added to 0.5 g of tumor tissue and the tissue was homogenized four times for 7–8 seconds with 1 min intervals between each homogenization. The homogenized solution was then centrifuged at 1,200 rpm for 5 min and the supernatant was collected. Homogenization of the remaining tumor tissue was repeated and the resulting supernatants were placed on a 41% sucrose gradient and ultracentrifuged at 23,000 rpm, 1 h, at 4°C using a SW-41 rotor. Purified PMVs were collected at the interphase and washed with PBS by centrifugation at 13,200 rpm, 1 h, 4°C. PMVs were similarly prepared from cultured tumor cells. PMVs were stored in PBS at 4°C short-term (3 months) and at −20°C long-term. A micro BCA kit (Thermo Scientific) was used to quantify PMV concentrations. Flow cytometry analysis was used to characterize protein expression on the TMVs and size distribution was assessed by Zetasizer analysis (Malvern Zetasizer Nano ZS Serial # MAL1047760). Electron microscopy imaging of the PMVs was performed at the Emory University Robert P. Apkarian Integrated Electron Microscopy Core facility.
Protein transfer of GPI-APs onto PMVs
In order to modify PMVs with GPI-APs by protein transfer, purified GPI-APs were added to PMVs in PBS. 250 μg GPI-HER-2, 50 μg GPI-IL-12 and 50 μg GPI-B7-1 were added to 1 mg 4TO7 PMVs in 8 ml total PBS for PMVs prepped for in vivo vaccination. The solution was rotated end-over-end at 37°C for 4 h. The PMVs were then centrifuged at 13,200 rpm, 1 h, at 4°C. The pelleted PMVs were resuspended in PBS and centrifuged again to wash off unincorporated proteins. Finally, the PMVs were resuspended in PBS and stored at 4°C until use. Western blot and flow cytometry analysis was used to determine incorporation. The amount of HER-2 incorporation on PMVs after protein transfer was calculated by western blot and Image J analysis using known concentrations of GPI-HER-2 as standards.
Experimental metastasis model
BALB/c mice were vaccinated and boosted with 25 μg of PMVs in 200 μl PBS intravenously (i.v.) 14 days apart. Vaccinated mice were then challenged with 1 × 105 4TO7-HER-2 cells i.v. 7 days post boost. 15 days post challenge, mice were sacrificed and lungs were isolated and homogenized using 1 mg/ml collagenase Type IV (Sigma-Aldrich) in HBSS (without calcium, magnesium and phenol red) for 2 h at 37°C. The homogenate was then passed through a cell strainer to create a single cell suspension. Lung cells were plated in tissue culture plates with 10 ml of DMEM containing 10% bovine calf serum, 1% penicillin/streptomycin, 0.8% nystatin, 5 mM L-glutamine, 5 mM HEPES, and 5 μg/ml 6-thioguanine. The cells were cultured at 37°C, 5% CO2 for 8 days. Media was removed and each plate was washed with PBS. Cells were detached with PBS containing 5 mM EDTA and centrifuged. Cell pellets were resuspended in equal volumes and cell counts for each sample were obtained by collecting the number of cellular events collected within 30 seconds using a Becton, Dickinson and Company FACSCalibur flow cytometer.
Tumor challenge studies
For prophylactic vaccination studies, female BALB/c mice were immunized subcutaneously (s.c.) with 25 μg of PMVs in 100 μl PBS on the left hind flank followed by a booster injection (s.c) with 25 μg of PMVs in 100 μl PBS on the left hind flank 14 days later. Mice were then challenged with 2 × 105 D2F2/E2 cells in 100 μl PBS s.c. on the contralateral flank 7 days after boost. Tumor area for each tumor was measured by multiplying tumor length by width. For therapeutic vaccination studies, BALB/c mice were challenged s.c. with 2 × 105 D2F2/E2 cells on the right hind flank on day 0. Day 3 and day 6 post challenge, mice were vaccinated s.c. with 100 μg of protein transferred-PMVs in 100 μl PBS on the left hind flank.
For cell depletion studies, mice were vaccinated and boosted s.c. as described above with 25 μg of PMVs in 100 μl PBS. Mice were given intraperitoneal i.p. administration of 200 μg anti-CD4 Ab (GK1.5), 200 μg anti-CD8 Ab (H35), or 200 μg of anti-CD4 with 200 μg anti-CD8 Ab in 200 μl PBS 3 days before challenge with D2F2/E2 cells, and days 2, 7, 12, and 18 post challenge.
Serum antibody analysis studies
As previously shown, flow cytometry was used to determine the presence of breast cancer-specific antibodies in serum from mice vaccinated with protein transferred-PMVs (24). Serum was collected 7 days after boost and incubated with 2.5 × 105 D2F2/E2, D2F2, or 4TO7 cells for 30 min at 4°C. Cells were then washed, and stained with FITC-goat-anti-mouse antibody (Jackson Immunoresearch).
Cell ELISA (31) was used to determine the presence of HER-2 specific IgG subtypes, in which 50,000 D2F2/E2 cells were incubated with 100 μl of 1:100 diluted serum in FACS buffer per well in triplicate for 30 min at 4°C. Cells were washed and then 50 μl of 1:2000 dilution of rat-anti-mouse-IgG1-HRP, rat-anti-mouse-IgG2a-HRP, or goat-anti-mouse-IgG2b-HRP (Southern Biotech) was added. 100 μl of TMB-1 substrate (BioLegend) was added to the cells to develop color. Color was stopped with the addition of 2N H2SO4 and absorbance was read at 450 nm.
Bioimaging studies to determine antigen persistence at the injection site
To monitor HER-2 persistence at the vaccination site in vivo, GPI-HER-2 was first labeled with IRDye 800CW (Licor), then incorporated onto 4TO7 tissue-derived PMVs by protein transfer and stored at 4°C until use. BALB/c mice (n = 3 to 4) were injected subcutaneously (s.c.) on the hind flank with 25 μg of the tagged GPI-HER-2-PMVs. Optical imaging was conducted using the Kodak In vivo FX imaging system (Carestream Health Inc) at the indicated time points (ex: 720 nm; em: 790 nm). Image J analysis was used to quantify the average fluorescence intensity at the injection sites.
Results
Production and characterization of PMVs
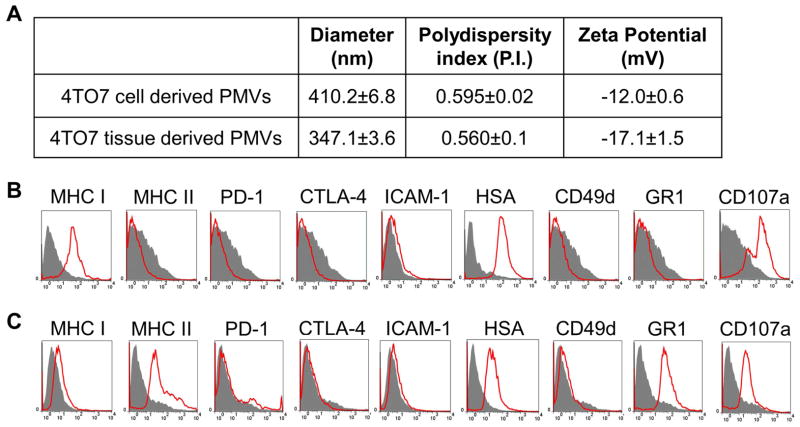
To generate sufficient quantities of PMVs for our studies we used the murine breast cancer cell line, 4TO7, as a source. Frozen pellets of in vitro cultured cells or tissues from tumors grown in mice were homogenized and PMVs were isolated by centrifugation over a 41% sucrose layer. The interphase containing PMVs (15) was collected and used for further studies. Our established protocol resulted in 1 mg of PMVs from homogenization of 2 × 108 cultured 4TO7 cells and 5 mg PMVs from 1 gram of 4TO7 tumor tissue. Zetasizer analysis showed that the PMVs derived from cultured cells were 410.2 ± 6.8 nm in diameter with a polydispersity index of 0.595 ± 0.02 and a zeta potential of −12.0 ± 0.6 mV (Figure 1A). 4TO7 tumor tissue derived PMVs were approximately 347 ± 3.6 nm in diameter with a polydispersity index of 0.560 ± 0.1, and a zeta potential of −17.1 ± 1.5 (Figure 1A). Further, electron microscopy imaging showed that the PMVs ranged in size from approximately 75–400 nm in diameter and contained lipid bilayer-like structures (Supplemental Figure 1). Flow cytometry analysis of cell surface markers expressed on 4TO7 cells showed that PMVs obtained from cultured cells and tissues (Figure 1B and 1C) expressed adhesion molecules, such as heat stable antigen (HSA), as well as major histocompatibility complex class I (MHC I) molecules. Also CD107a, a marker for degranulation on CD8+ T-cells and natural killer (NK) cells, was found to be expressed on both cell-derived and tissue-derived PMVs, although expression levels of all of these surface markers were higher in cell derived PMVs compared to tissue-derived PMVs (Figure 1B and 1C). Interestingly, PMVs obtained from tumor tissue expressed additional surface markers such as MHC Class II found on activated antigen presenting cells and GR1, usually expressed on neutrophils and myeloid derived suppressor cells (Figure 1B and 1C). The enhanced expression of these surface molecules on PMVs from tumor tissue may result from infiltration of immune cells into the tumor microenvironment. Overall, the size and particulate nature of these biological PMVs are ideal for uptake by APCs (6, 7) and surface expression of adhesion and immune molecules on the PMVs may further enhance binding with cognate receptors found on immune cells thus promoting the ability of PMVs to act as an efficient delivery vehicle.
Figure 1. Characterization of PMVs.
PMVs were produced from 4TO7 cultured cells or from 4TO7 tumor tissue obtained from subcutaneous inoculation in a BALB/c mice with 4TO7 cells. (A) Cultured cell derived and tissue derived 4TO7 PMVs portray similar size, polydispersity index and zeta potential. (B) Flow cytometry analysis of cultured 4TO7 cell derived PMVs. (C) Flow cytometry analysis of 4TO7 tumor tissue derived PMVs.
Construction and purification of GPI-HER-2
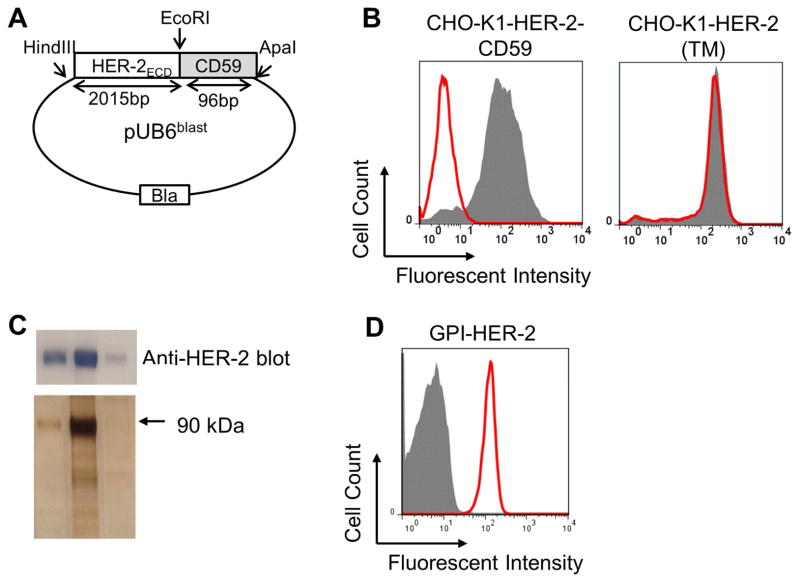
PMVs are derived from cellular membranes and thus contain a lipid bilayer making them amenable to protein transfer. In order to test whether the PMVs can be used as a scaffold to deliver TAAs by protein transfer we aimed to deliver the breast cancer antigen, HER-2. However, protein transfer-mediated modification of PMVs requires proteins to have a covalently attached lipid tail which spontaneously anchors the protein into the lipid bilayer through a lipid-lipid interaction (14, 18, 21). Therefore, we converted the transmembrane HER-2 antigen into GPI-anchored-HER-2 by attaching the GPI-anchor signal sequence DNA from CD59, a GPI-anchored protein, to the extracellular DNA domain of HER-2 in a pUB6blast vector (Figure 2A). CHO-K1 cells transfected with HER-2-CD59 plasmid were subjected to PI-PLC treatment in order to lead to the specific cleavage of the GPI-anchor. HER-2 expression was reduced by 99.99% on CHO-K1 cells transfected with HER-2-CD59 after PI-PLC treatment (Figure 2B, left panel), whereas the HER-2 expression level on CHO-K1 cells transfected with full-length transmembrane HER-2 was not affected by PI-PLC treatment (Figure 2B, right panel) suggesting that HER-2-CD59 (GPI-HER-2) is anchored onto the cell membrane via the GPI-anchor.
Figure 2. Construction, expression and purification of GPI-HER-2.
(A) Construction of GPI-HER-2. The extracellular domain (ECD) of HER-2 is attached to the GPI-anchor signal sequence from CD59 and cloned into a pUB6 vector (B) PI-PLC treatment of CHO-K1 cells transfected with HER-2. PI-PLC treatment was performed on CHO-K1 cells transfected with HER-2-CD59 (left panel) or on CHO-K1 cells transfected with full-length transmembrane (TM) HER-2 (right panel) and analyzed by flow cytometry. Grey – not treated with PI-PLC. Red – after PI-PLC treatment. (C) Purification of GPI-HER-2. GPI-HER-2 was purified from transfected CHO-K1 cells by mAb affinity chromatography. SDS PAGE and western blot (top panel) or silver staining (bottom panel) was performed on the purified fractions. (D) Protein transfer of purified GPI-HER-2 onto sheep red blood cells (RBCs). Sheep RBCs were incubated with purified GPI-HER-2 or buffer control for 4 h at 37°C. Flow cytometry analysis was performed to determine incorporation levels. Grey – buffer control. Red – GPI-HER-2 added during protein transfer.
GPI-HER-2 was purified from transfected CHO-K1 cells by affinity chromatography (Figure 2C), in which 13 g of GPI-HER-2-transfected-CHO-K1 cell pellet yielded approximately 2 mg of purified GPI-HER-2. Purified fractions were concentrated and dialyzed with PBS containing 0.01% octyl-β-glucopyranoside. Protein transfer was then performed on sheep red blood cells (RBCs) to evaluate if purified GPI-HER-2 could incorporate onto cell surfaces. Purified GPI-HER-2 incorporated onto sheep RBCs upon protein transfer suggesting that GPI-HER-2 retains an intact GPI-anchor after purification (Figure 2D).
Incorporation of GPI-HER-2 with GPI-ISMs onto 4TO7 PMVs by protein transfer
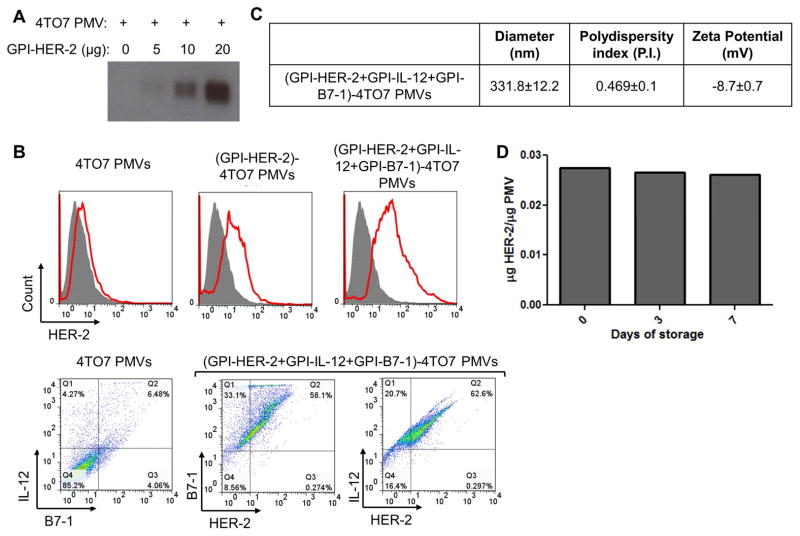
Since the presence of a lipid bilayer on PMVs makes them amenable to protein transfer of GPI-APs, we performed protein transfer of purified GPI-HER-2 onto PMVs derived from 4TO7 tumor tissue. Protein transfer resulted in GPI-HER-2 incorporation onto PMVs in a concentration dependent manner (Figure 3A). Similar results held true for protein transfer of GPI-HER-2 onto 4TO7 cultured cell derived PMVs (data not shown). Further, GPI-ISMs, GPI-IL-12 and GPI-B7-1, could be incorporated simultaneously along with GPI-HER-2 by protein transfer resulting in surface expression of HER-2 along with IL-12 and B7-1 on 4TO7 PMV surfaces (Figure 3B).
Figure 3. Protein transfer of GPI-HER-2 with GPI-ISMs onto PMVs derived from 4TO7 tumor tissue.
(A) Protein transfer mediated incorporation of GPI-HER-2 onto 4TO7 PMVs is concentration dependent. 20 μg of 4TO7 PMVs were protein transferred with increasing concentrations of GPI-HER-2. Incorporation of GPI-HER-2 was analyzed by SDS PAGE and western blot analysis. (B) Flow cytometry analysis of 4TO7 PMVs protein transferred with GPI-HER-2 +/− GPI-ISMs. 4TO7 PMVs were protein transferred with GPI-HER-2 along with GPI-IL-12 and/or GPI-B7-1. Then PMVs were stained with fluorescein conjugated mAbs and analyzed by flow cytometry. (C) Protein transfer of GPI-APs onto 4TO7 PMVs does not affect PMV size. The diameter, polydispersity index and zeta potential of unmodified or protein transferred 4TO7 PMVs were analyzed using a Zetasizer. (D) HER-2 incorporated onto 4TO7 PMVs by protein transfer remains stably expressed at room temperature. 4TO7 PMVs modified by protein transfer with GPI-HER-2 were stored at room temperature. Each time point, PMVs were washed by centrifugation with PBS and the presence of HER-2 was determined by SDS PAGE and western blot analysis by staining against HER-2.
To determine if protein transfer affected PMV size, Zetasizer analysis of 4TO7 PMVs before and after protein transfer was performed. 4TO7 PMVs simultaneously protein transferred with GPI-HER-2, GPI-IL-12, and GPI-B7-1 had a similar diameter (331.8 ± 12.2 nm) and polydispersity index (0.469 ± 0.1) (Figure 3C) compared to unmodified 4TO7 PMVs (347.1 ± 3.6 nm and 0.560 ± 0.1, respectively) (Figure 1A) suggesting that protein transfer mediated incorporation of new proteins onto PMVs does not alter the size distribution of PMVs (Figure 3C). The zeta potential was increased to −8.7 ± 0.7 after protein transfer however it still reflected particle stability.
Vaccines often require controlled storage conditions. However, cold storage is not always feasible. Therefore, we tested whether incorporated HER-2 by protein transfer remained bound to PMVs after storage at room temperature. As shown in Figure 3D, HER-2 remained stably expressed on protein transfer modified 4TO7 PMVs after storage for at least 7 days at room temperature.
Vaccination with protein transferred (GPI-HER-2)-4TO7 PMVs induces protection against HER-2-expressing tumor challenge in an experimental metastasis model
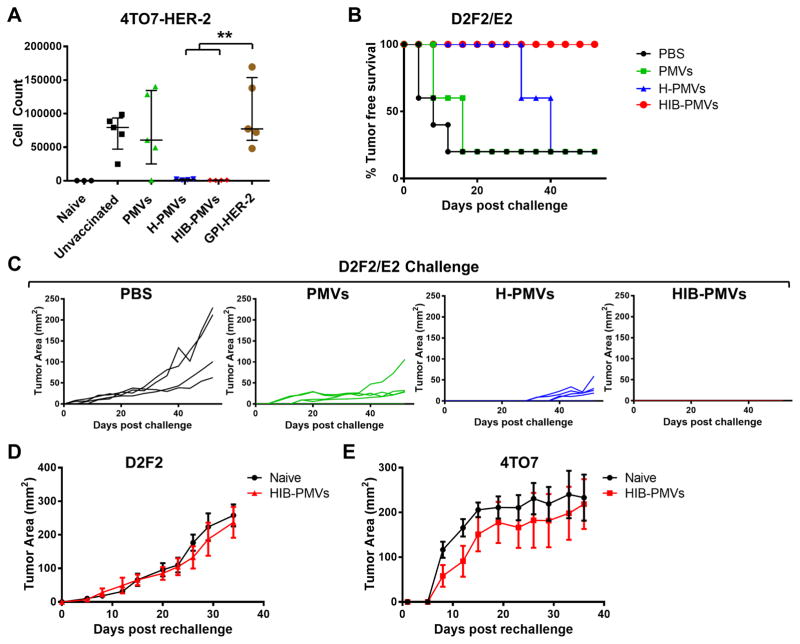
To determine whether delivering TAAs using PMVs to the immune system results in antitumor immunity, the in vivo efficacy of GPI-HER-2-protein transferred PMVs was assessed. We established 4TO7 cells expressing HER-2 by transfecting the cells with a gene encoding full-length HER-2 to test the protective antitumor immunity. However, subcutaneous (s.c.) challenge of BALB/c mice with 4TO7-HER-2 cells resulted in spontaneous tumor regression, whereas intravenous (i.v.) administration led to metastatic growth of the tumor cells in the lungs (data not shown). Therefore, we used the 4TO7-HER-2 experimental metastasis model to determine if vaccination with 4TO7 PMVs protein transferred with GPI-HER-2 conferred protection against metastatic growth in lungs. Mice were given two immunizations of either unmodified 4TO7 PMVs, GPI-HER-2-protein-transferred-4TO7 PMVs (H-PMVs), 4TO7 PMVs protein transferred simultaneously with GPI-HER-2, GPI-IL-12 and GPI-B7-1 (HIB-PMVs) or with similar concentrations of soluble GPI-HER-2 alone. The mice then received a challenge with live 4TO7-HER-2 cells i.v. 7 days post boost. Mice immunized with 4TO7 PMVs protein transferred with GPI-HER-2 had significantly decreased numbers of 4TO7-HER-2 cells in the lungs compared to control mice or mice vaccinated with unmodified 4TO7 PMVs (Figure 4A) although mice in all vaccinated groups did not show any differences in body weight (data not shown). Further, vaccination with 4TO7 PMVs protein transferred with GPI-HER-2 induced significantly less metastasis of 4TO7-HER-2 cells in the lungs compared to mice vaccinated with similar concentrations of soluble GPI-HER-2. However, addition of GPI-ISMs did not alter the protection induced by GPI-HER-2 PMVs. These results demonstrate that delivery of GPI-HER-2 using PMVs induces antitumor immunity sufficient to confer protection against metastatic tumor growth.
Figure 4. Vaccination with 4TO7 PMVs protein transferred simultaneously with GPI-HER-2, GPI-IL-12, and GPI-B7-1 protects mice against challenge with HER-2 expressing tumor cells.
(A) Immunization of mice with 4TO7 PMVs protein transferred with GPI-HER-2 confers protection against experimental metastasis of HER-2-expressing tumor cells. Groups of mice (n = 5) were vaccinated and boosted on day 14 with 25 μg protein transferred PMVs i.v and then challenged 7 days post boost with HER-2-transfected 4TO7 tumor cells i.v. to induce metastasis in the lungs. Mice were sacrificed day 15 post challenge and metastasis of 4TO7-HER-2 cells to the lungs were analyzed by counting the number of 6-thioguanine resistant cells after 8 days of selection. (B) Immunization of mice with 4TO7 PMVs protein transferred with GPI-HER-2 confers protection against D2F2/E2 tumor cells. Groups of BALB/c mice (n = 5) were vaccinated and boosted with 25 μg of protein transferred 4TO7 PMVs s.c. on day 14 and then challenged s.c. with 2 × 105 D2F2/E2 (HER-2 positive) tumor cells. Protein transferred PMVs expressed similar levels of HER-2 at 0.034 μg HER-2/μg PMV. Tumor incidence and (C) tumor area were measured. Tumor free mice from (GPI-HER-2+GPI-IL-12+GPI-B7-1)-4TO7 group were then rechallenged 65 days post boost with either (D) 2 × 105 D2F2 (HER-2 negative) cells or (E) 2 × 105 4TO7 (HER-2 negative) cells. Tumor area in each mouse was measured. (Statistical analysis: (A) Two-Way ANOVA – Tukey’s multiple comparisons test; ** p < 0.01).
Vaccination with protein transferred (GPI-HER-2)-4TO7 PMVs induces protection against subcutaneously administered syngeneic breast cancer cells
Due to the difficulty in longitudinal follow up of tumor growth in metastatic mouse models, we chose to use another syngeneic breast cancer model, the D2F2/E2 cell line that expresses HER-2. D2F2/E2 cells were derived from transfection of full-length HER-2 in the parental D2F2 breast cancer cell line (26, 27). Subcutaneous challenge with D2F2/E2 cells leads to steady tumor growth that can be measured longitudinally to follow the effect of immune responses on tumor growth without euthanizing mice. BALB/c mice immunized with protein transferred-4TO7 PMVs were challenged with D2F2/E2 tumor cells 7 days post boost. As shown in Figure 4B and 4C, mice vaccinated with GPI-HER-2-incorporated 4TO7 PMVs (H-PMVs) showed delayed D2F2/E2 tumor growth that developed 32 days post challenge compared to 4 or 8 days post challenge in control mice or mice vaccinated with unmodified 4TO7 PMVs, respectively.
Previous studies have shown that inoculation of 4TO7 cells that expressed both GPI-IL-12 and B7-1 led to better protection against a 4TO7 wild-type concomitant challenge compared to 4TO7 cells expressing either GPI-IL-12 or B7-1 (32). Therefore, to further enhance the immunity induced by GPI-HER-2 PMVs, mice were vaccinated with 4TO7 PMVs that were modified by protein transfer to express GPI-IL-12 and GPI-B7-1 on the surface along with GPI-HER-2 (HIB-PMVs). Mice vaccinated with HIB-PMVs were completely protected against challenge with D2F2/E2 cells (Figure 4B and 4C), suggesting that the inclusion of the ISMs, IL-12 and B7-1, on 4TO7 PMVs with GPI-HER-2 augmented the antitumor immune response. Interestingly, mice vaccinated with unmodified PMVs had reduced average tumor sizes compared to control mice suggesting that a polyclonal immune response induced during unmodified PMV vaccination affected tumor growth.
To further elucidate the specificity of immunity induced in mice vaccinated with HIB-PMVs, we examined whether vaccinated mice that remained tumor free after D2F2/E2 challenge were protected against the parental HER-2-negative tumor cell line D2F2. Upon challenge of tumor free mice with D2F2 cells 65 days post D2F2/E2 challenge, control and vaccinated mice observed similar D2F2 tumor growth (Figure 4D), suggesting that immunity induced with HIB- PMV vaccination was HER-2-specific. To determine if immunity was induced against antigens expressed on the PMVs used for vaccination other than HER-2, HIB-PMV vaccinated mice that remained tumor free after D2F2/E2 challenge were challenged with 4TO7 tumor cells. 4TO7 tumors grew steadily in control and vaccinated mice (Figure 4E), suggesting that the immunity induced is dominantly against the incorporated TAA, HER-2.
Since administration of soluble cytokines, such as IL-2 and IL-12, lead to systemic toxicity (33, 34), we determined whether membrane-bound IL-12 and B7-1 caused systemic toxicity when delivered attached to PMVs. One of the major indications of IL-12 induced toxicity is the release of inflammatory cytokines such as IFN-γ, which was increased to 700–900 ng/ml in mice administered with soluble IL-12 (35). Our results show that the serum concentration levels of IFN-γ in mice vaccinated with unmodified PMVs, H-PMVs or HIB-PMVs were all similar to IFN-γ levels in control mice (Supplemental Figure 2), suggesting that vaccination with PMVs modified by protein transfer to express HER-2 and membrane-bound ISMs does not lead to systemic increase in IFN-γ.
HIB-PMVs protect therapeutically against D2F2/E2 tumor growth
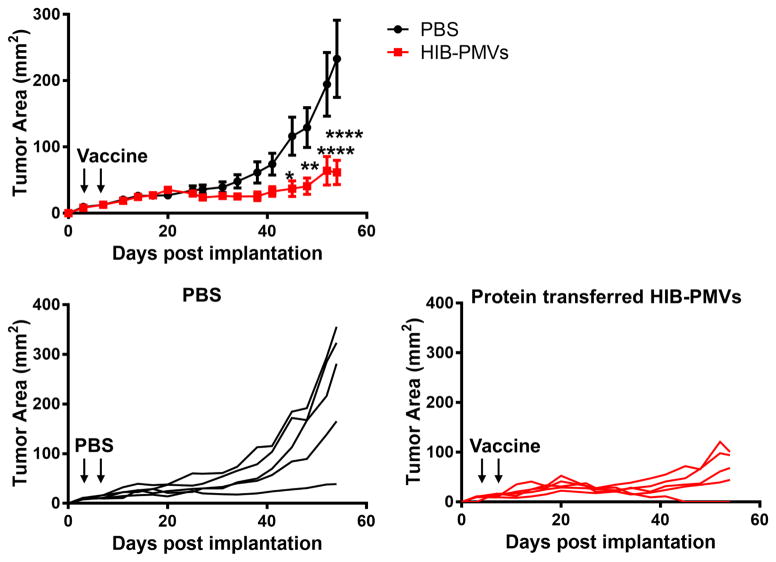
Although prophylactic vaccination with HIB-PMVs conferred protection against HER-2-expressing tumor challenge, therapeutic protection against an existing tumor is more clinically relevant. Therefore, we wanted to determine the therapeutic efficacy of vaccination with HIB- PMVs after challenge with D2F2/E2 tumor cells. Mice were implanted with tumor cells and then vaccinated on days 3 and 6. As shown in Figure 5, vaccination with HIB-PMVs showed a significant delay in tumor growth compared to control mice by day 45 post implant and tumors in 20% of mice fully regressed suggesting that HIB-PMV vaccination controls HER-2-expressing tumor growth therapeutically.
Figure 5. Vaccination with (GPI-HER-2+GPI-IL-12+GPI-B7-1)-4TO7 PMVs (HIB PMVs) controls the growth of established tumors.
Groups of mice (n = 5) were injected s.c. with 2 × 105 D2F2/E2 cells on the right hind flank on day 0. On days 3 and 6 (indicated by black arrows), the challenged mice were vaccinated with 100 μg of 4TO7 PMVs protein transferred with GPI-HER-2, GPI-IL-12, and GPI-B7-1 s.c. on the left hind flank. Tumor area was measured by multiplying the length and width of the tumor. (Statistical analysis: Repeated measures Two-Way ANOVA – Sidak’s multiple comparisons test; * p < 0.05, ** p < 0.01, **** p < 0.0001).
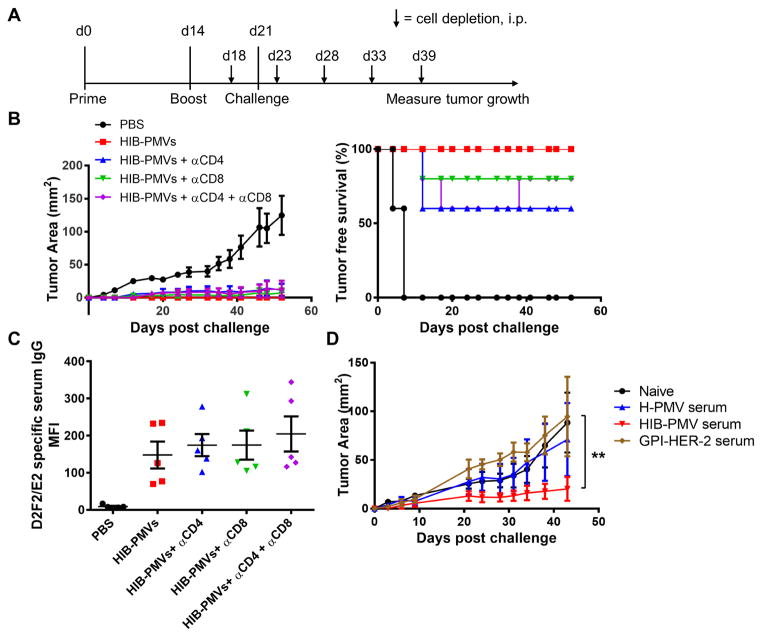
Role of CD4+ and CD8+ T lymphocytes induced by PMVs modified with GPI-HER-2 and GPI-ISMs in antitumor immunity
T-cells play a critical role in combating tumors. CD4+ T-cells prime an anti-tumor cytokine milieu to direct the type of immunity induced whereas CD8+ T-cells play a prominent role in killing tumor cells. Both are important in combating tumor growth (36–38). To elucidate the nature of HIB-PMV vaccine induced T-cell immunity responsible for protection against HER-2-expressing tumor growth, CD4+ and/or CD8+ cells were depleted in the effector phase after PMV vaccination as well as before and after D2F2/E2 challenge (Figure 6A). HIB-PMV vaccinated mice showed 0% tumor incidence upon D2F2/E2 challenge whereas control-PBS mice showed 100% tumor incidence. Mice depleted of CD4+ cells showed 40% tumor incidence and mice depleted of CD8+ cells showed 20% tumor incidence. Mice depleted of both CD4+ and CD8+ cells showed 40% tumor incidence during the depletion treatment, however after depletion was discontinued, tumor incidence reduced to 20% as tumors in 20% of the mice regressed (Figure 6B). These results suggest that CD4+ and CD8+ cells play a role in the effector phase of an anti-HER-2-specific immune response after vaccination with protein transferred HIB-PMVs, however the role is partial in terms of protection.
Figure 6. The role of cellular and humoral immunity in HIB-PMV mediated antitumor immunity.
To determine the role of CD4+ and CD8+ T-cells in HIB-PMV mediated antitumor immunity, groups of mice (n = 5) were first vaccinated with protein transferred 4TO7 PMVs derived from tumor tissue. Before and after challenge with 2 × 105 D2F2/E2 cells, mice were depleted of CD4+, CD8+ or both CD4+ and CD8+ cells. (A) Schematic of vaccination and depletion. (B) Tumor growth and incidence of D2F2/E2 after CD4+ and CD8+ cell depletion. (C) Anti-D2F2/E2 specific serum IgG responses in vaccinated mice before depletion. (D) The role of antibodies in HIB-PMV mediated antitumor protection. Serum was collected from mice vaccinated with protein transferred tumor tissue derived-4TO7 PMVs and adoptively transferred i.p. to naïve mice (n = 3) 1 day before and 3 days after challenge with 2 × 105 D2F2/E2 cells s.c. Tumor area was measured by multiplying the length and width of the tumor. (Statistical analysis: (D) Repeated measures Two-Way ANOVA – Tukey’s multiple comparisons test; ** p < 0.01).
The partial protection seen in the T-cell-depleted mice may be due to the presence of HER-2-specific antibodies against D2F2/E2 tumors. We observed that vaccinated mice depleted of CD4+ and/or CD8+ cells had similar amounts of anti-D2F2/E2 specific serum antibodies (Figure 6C), suggesting that the presence of anti-HER-2 antibodies could be involved in inhibiting D2F2/E2 tumor growth when treatment is administered in a prophylactic setting.
Vaccination using 4TO7 PMVs protein transferred with GPI-HER-2 induces HER-2-specific IgG
Since we noticed that HIB-PMV vaccination induced a D2F2/E2-specific serum antibody response, we wanted to test its efficacy in preventing D2F2/E2 tumor growth. We transferred serum collected from mice that were vaccinated with H-PMVs, HIB-PMVs, or similar concentrations of GPI-HER-2 into naïve mice one day before and three days after challenge with D2F2/E2 cells. Mice that received serum from HIB-PMV vaccinated mice had significantly smaller average tumor sizes compared to mice that received serum from H-PMV or GPI-HER-2 vaccinated mice (Figure 6D). These results suggest that vaccination with protein transfer-modified PMVs that express HER-2 along with ISMs, IL-12 and B7-1, also induced a protective humoral immune response.
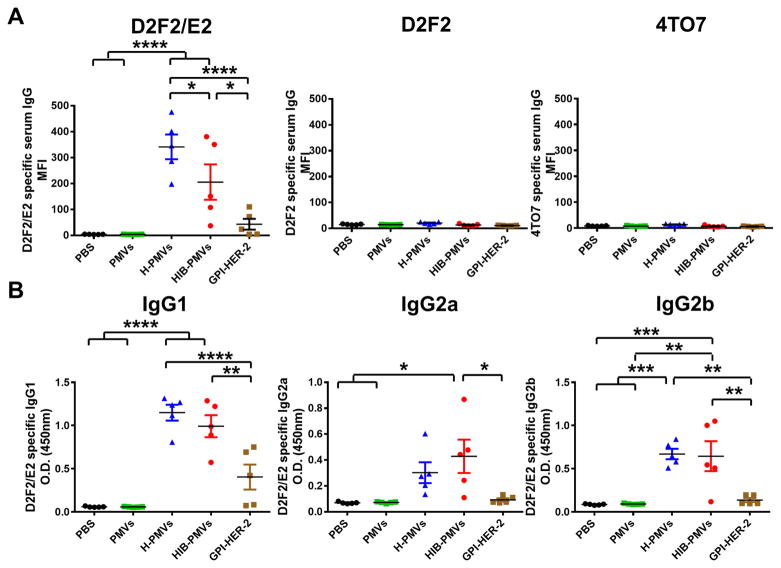
To further elucidate the mechanism of antitumor immunity induced with PMV vaccination, we determined the specificity and type of the antibody response after vaccination with PMVs. Serum was collected from mice vaccinated with 4TO7 PMVs protein transferred with GPI-HER-2 with or without GPI-ISMs. We included a group where mice were vaccinated with GPI-HER-2 alone. In this group we vaccinated mice with 0.85 μg GPI-HER-2 since the PMV vaccination dose of 25 μg expressed approximately 0.85 μg of GPI-HER-2. Flow cytometry analysis showed that serum from mice vaccinated with GPI-HER-2-protein transferred-4TO7 PMVs induced strong anti-D2F2/E2-specific antibodies (Figure 7A; left panel). Interestingly, mice vaccinated with soluble GPI-HER-2 produced significantly lower levels of anti-D2F2/E2 specific IgG in comparison. Serum IgG antibody binding to D2F2/E2 cells was HER-2-specific as no detectable response was seen against D2F2 or 4TO7 cells (Figure 7A; middle and right panel, respectively). Further, probing of antibodies produced upon vaccination with GPI-HER-2-PMVs via western blot analysis showed that antibodies were HER-2 specific and not against other antigens present on the PMVs (Supplemental Figure 3).
Figure 7. Vaccination of mice with 4TO7 PMVs protein transferred with GPI-HER-2 induces both Th1 and Th2-type HER-2-specific antibody production.
(A) Total anti-HER-2 specific serum IgG induced upon vaccination with 4TO7 PMVs protein transferred with GPI-HER-2. HER-2 expression was similar in all protein transferred samples at 0.034 μg HER-2/μg PMV. Serum was collected from groups of vaccinated mice (n = 5) day 7 post boost. Flow cytometry analysis using 1:200 diluted serum incubated with D2F2/E2, D2F2, or 4TO7 cells was performed. (B) IgG subtype analysis of anti-HER-2 antibodies in sera from vaccinated mice. 1:100 diluted serum collected on day 7 post boost from vaccinated mice (n = 5) was incubated with 50, 000 D2F2/E2 cells per well in triplicate in a cell ELISA. The subtype of bound antibody was detected by adding peroxidase conjugated subtype specific antibodies. (Statistical analysis: One-Way ANOVA – Tukey’s multiple comparisons test. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001).
T-helper 1 (Th1)-type immunity has been shown to be protective in combating tumors (39), however induction of both Th1 and Th2-type immunity is beneficial for tumor protection (40–42). To further determine the type of HER-2-specific immunity induced upon PMV vaccination, D2F2/E2-specific IgG subtypes were analyzed by cell ELISA. IgG1 subtypes correlate with a Th2-type immune response, whereas IgG2a and IgG2b correlate with a Th1-type immune response (43). Mice vaccinated with 4TO7 PMVs protein transferred with GPI-HER-2 alone (H-PMVs) or with GPI-IL-12 and GPI-B7-1 (HIB-PMVs) induced stronger anti-D2F2/E2 IgG1, IgG2a, and IgG2b responses compared to mice vaccinated with GPI-HER-2 (Figure 7B). Interestingly, mice vaccinated with GPI-HER-2 induced only detectable anti-D2F2/E2-specific IgG1 responses albeit at lower levels and no detectable IgG2a and IgG2b responses (Figure 7B). These results suggest that vaccination with soluble GPI-HER-2 induces only weak Th2-type immunity, whereas vaccination with GPI-HER-2 incorporated onto 4TO7 PMVs by protein transfer induces stronger Th1 and Th2-type immunity.
Since protein transfer-modified-PMVs that express HER-2 induced a Th1-type antibody response, we next determined if protein transfer-modified-PMVs also induced Th1-type cytokines. Splenocytes from mice vaccinated with protein transfer-modified-PMVs were stimulated with 4TO7 cells transfected to express full-length HER-2 for 2 days. Analysis of the supernatant obtained after 2 days of stimulation showed that splenocytes from mice vaccinated with 4TO7 PMVs protein transferred to express GPI-HER-2 with or without GPI-ISMs led to enhanced IFN-γ production compared to splenocytes from mice vaccinated with unmodified PMVs (Supplemental Figure 4). Further, inclusion of ISMs on PMVs led to enhanced IFN-γ production compared to H-PMVs suggesting that HER-2-expression on PMVs leads to a Th1-type immune response whereas inclusion of GPI-ISMs on PMVs further enhances Th1-type cytokine production.
Delivery of GPI-HER-2 using PMVs derived from syngeneic and allogeneic breast cancer tissues confers similar protection against D2F2/E2 tumor growth
We have shown that protein transfer of GPI-HER-2 onto PMVs derived from 4TO7 tumor tissue confers protection against HER-2-expressing tumor challenge, therefore we wanted to determine if similar protection was observed when protein transfer of GPI-HER-2 was performed on PMVs derived from syngeneic and allogeneic breast tumor tissues. PMVs were prepared from tumor tissue obtained from BALB/c mice challenged with D2F2 cells or from C57BL/6 mice challenged with E0771 cells. BALB/c mice were given two immunizations with 25 μg of GPI-HER-2-protein transferred-4TO7, D2F2, or E0771 PMVs, followed by challenge with D2F2/E2 cells 7 days post boost. Mice vaccinated with PMVs protein transferred with GPI-HER-2 had similar decreased levels of average tumor growth compared to control mice and mice vaccinated with unmodified PMVs by day 57 post challenge (Supplemental Figure 5). Therefore, protein transfer of GPI-HER-2 onto PMVs regardless of whether they are derived from syngeneic or allogeneic breast tumor tissues induces similar levels of protection against HER-2 expressing tumor cells.
Delivery of GPI-HER-2 using PMVs derived from cultured 4TO7 cells confers protection against D2F2/E2 tumor growth
Tumor tissues contain tumor infiltrating immune cells that include tumor-specific effector T-cells as well as many immunosuppressive cells such as regulatory T-cells and myeloid derived suppressor cells (MDSCs) (44, 45). Consequently, PMVs derived from tumor tissue may contain membrane vesicles from these immunosuppressive cells as well as express immunosuppressive molecules. Therefore, we next determined if PMVs derived from cultured tumor cells that are void of immunosuppressive cells leads to similar protection compared to PMVs derived from tumor tissue. Mice were immunized with 4TO7 cultured cell-derived PMVs that were protein transferred with GPI-HER-2, followed by challenge with D2F2/E2 cells 7 days post boost. Vaccination with GPI-HER-2-modified cell culture-derived 4TO7 PMVs also led to delayed tumor growth compared to control mice with 40% of mice remaining tumor-free by day 32 post challenge (Supplemental Figure 6A). Protection induced was HER-2-specific as challenge of immunized mice with HER-2-negative D2F2 tumor cells led to steady tumor growth (Supplemental Figure 6B).
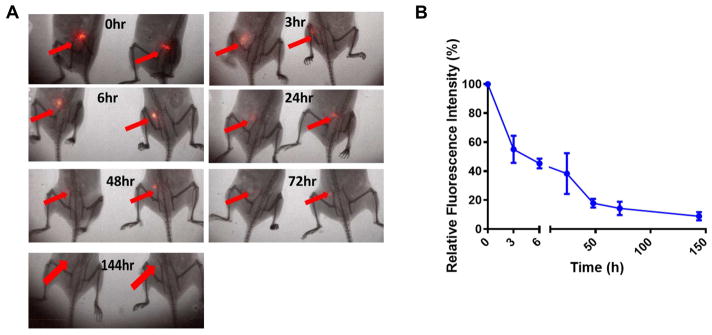
Vaccination using 4TO7 TMVs protein transferred to incorporate GPI-HER-2 results in prolonged antigen presence at the vaccination site
Vaccination with antigen encapsulated onto particles has been shown to enhance antigen persistence at the vaccination site for at least 7 days and thus prevents quick antigen clearance (within 6 h) that is seen with administration of soluble antigen (9). To test whether GPI-HER-2 incorporated onto PMVs persisted at the vaccination site, we performed in vivo imaging studies using fluorescently-labeled GPI-HER-2 that was incorporated onto 4TO7 PMVs by protein transfer. As shown in Figure 8A and 8B, incorporation of GPI-HER-2 onto PMVs led to antigen persistence at the vaccination site for at least 6 days, suggesting that enhanced HER-2-specific immunity induced by H-PMV vaccination may be due to the prolonged antigen exposure at the vaccination site.
Figure 8. Vaccination with H-PMVs leads to prolonged HER-2 persistence at the vaccination site.
Purified GPI-HER-2 was fluorescently labeled with IRDye 800CW and then protein transferred onto 4TO7 PMVs. BALB/c mice (n = 4) were injected s.c. on the hind flank with 25 μg of the resulting tagged H-PMVs. (A) Optical imaging was conducted using the Kodak In vivo FX imaging system (Carestream Health Inc.) at the indicated time points (ex: 720 nm; em: 790 nm). Representative images of mice are shown. (B) Image J analysis was used to quantify the average fluorescence intensity at the injection sites. Regions of Interests were selected for measuring the mean fluorescence intensity (MFI) of the protein at the vaccination site and corresponding body background. Signal to body background (S/B) ratio was calculated from the MFI of the injection area divided by the MFI of the body background area. Data shown is the mean S/B ratio ± standard error from three to four mice in which n = 4 was used for the initial time points and n = 3 was used for the 24 h time point onwards.
Discussion
The unique size and particulate structure of nano and microparticles are ideal for optimal engulfment and uptake by APCs. The engulfment process also leads to APC activation and antigen presentation, thus incorporation of antigen into these particles delivers them to the adaptive immune system. Further, inclusion of antigen onto particles enhances antigen stability and increases antigen presence at the vaccination site (9). Herein, we demonstrate the use of particles derived from biological material to deliver the tumor-associated antigen HER-2. In our approach, we used plasma membrane vesicles prepared from tumor cells or tumor tissue as a model system to show that biological plasma membrane vesicles can be used as a particle-based antigen delivery vehicle. Since these PMVs are derived directly from tissues, we expect that they will be highly biocompatible making them less toxic compared to commonly used synthetic lipid and polymer-based particles (8, 46). Homogenization of tumor cells or tissues resulted in vesicles of an average size range from 300–420 nm in diameter and thus the PMVs produced are of an optimal size to be taken up by APCs found in the vaccination site (6). More importantly, these PMVs were derived from cell membranes and thus contain a lipid bilayer making them susceptible to incorporation of new proteins by a simple protein transfer process using GPI-APs.
In the present study we show that the level of tumor antigen incorporation onto biological PMVs by protein transfer occurred in a concentration dependent manner, allowing antigen expression levels to be easily adjusted by varying the concentration of purified antigen incubated with the PMVs during protein transfer. Incorporation took place within a matter of hours and the incorporated antigen remained stably expressed on the PMVs for at least a week after protein transfer even after storage of the PMVs at room temperature. When injected into mice, GPI-HER-2 incorporated onto the PMVs was found to be present at the vaccination site for at least 6 days. Moreover, the simultaneous incorporation of ISMs, GPI-IL-12 and GPI-B7-1, along with tumor antigen HER-2 by protein transfer allows for controlled levels of antigen delivery along with targeting or immune activation molecules on the same vesicles.
Currently, the methods available to incorporate new antigens onto surfaces of synthetic and biological particles involve chemical modification of both antigen and particle. Often times such chemical modifications may alter antigenicity of the protein or nature of the particles (47–50). However, protein transfer-mediated-incorporation of GPI-HER-2 onto PMVs occurs by the interaction of the lipid moiety of the GPI-anchor with the lipid bilayer of the cell surface plasma membrane, avoiding such deleterious effects. GPI-anchor attachment to the protein is only added to the C-terminal end. Attachment does not alter the functionality of proteins as soluble and transmembrane proteins can be converted to GPI-anchored forms and remain functionally active (20, 22, 23). Our purification protocol described here allows for purification of these proteins with an intact GPI-anchor, which can then be used in protein transfer to decorate PMVs with desired protein molecules for targeted delivery.
Vaccination of mice with protein transfer-modified-PMVs led to complete protection against challenge with HER-2-expressing tumors prophylactically as well as delayed tumor growth therapeutically when GPI-HER-2 was incorporated alongside GPI-IL-12 and GPI-B7-1. Vaccination also protected mice against metastatic tumor growth in an experimental metastasis model. However, the ability to deliver antigen for an effective antitumor immune response was not restricted to PMVs from a single tumor. PMVs from many different tumor tissues, whether obtained from syngeneic or allogeneic mice, or from cultured 4TO7 cells, also were capable of being modified by protein transfer to express GPI-HER-2 and induce similar protection against a HER-2-expressing tumor challenge.
Administration of soluble proteins is known to induce a poor immune response perhaps due to soluble proteins being more prone to degradation in vivo or lack of activation of APCs during uptake of soluble proteins (7). However, a robust adaptive immune response is induced when antigens are delivered via particles in which the antigen persists longer in vivo (9), and by which phagocytosis of particulate materials can accompany APC activation (7, 8). Our results showed that vaccination with GPI-HER-2-protein transferred-PMVs increased HER-2 antigen persistence at the vaccination site and enhanced anti-HER-2 antibody responses compared to administration of soluble GPI-HER-2. Previous studies show that vaccination with soluble antigen leads to quick antigen clearance from the vaccination site where no detectable levels of antigen are seen at 6 hours post vaccination (9). However incorporation of GPI-HER-2 onto PMVs shown herein led to prolonged antigen persistence at the vaccination site with 45% of antigen being detectable at 6 hours post vaccination and detectable levels of antigen remaining at the vaccination site for at least 6 days post vaccination (Figure 8). Further, both Th1-type and Th2-type antibody responses were generated by vaccination with GPI-HER-2-protein transferred-PMVs whereas only Th2-type immune responses were detected upon soluble GPI-HER-2 vaccination. The induction of Th1-type immunity may be due to the particulate nature of the PMVs since particle-based antigen delivery has shown to enhance Th1-type immune responses (51, 52). Elicitation of tumor specific B-cell, Th1, and Th2-type immune responses have previously been shown to play important roles in the eradication of tumors (36); therefore, protein transferred-PMVs that induce tumor antigen-specific Th1 and Th2-type antibody responses as well as increased Th1-type cytokines are ideal candidates for an effective vaccine against cancer.
Moreover, protein transfer allows for simultaneous incorporation of more than one GPI-AP onto the PMVs, thus different ISMs can incorporate onto the PMVs along with the targeted TAA. We observed that inclusion of IL-12 with B7-1 on the PMVs alongside HER-2 led to long-lasting complete protection (up to at least 52 days) against D2F2/E2 challenge, suggesting that vaccination induced sufficient immune stimulation to eradicate HER-2-expressing tumor cells upon challenge (Figure 4). Both the cytokine IL-12 (17, 22, 53–58) and the costimulatory molecule B7-1 (59–62) have been widely studied for use in tumor immunotherapy, however, the combination of both has presented more promising results (16, 32, 63–65). The combination of membrane-bound IL-12 and B7-1 on tumor cells was shown to enhance anti-tumor immunity in vivo compared to individual ISM expression through the aid of both CD4+ and CD8+ cells and through the reduction of immunosuppressive regulatory T-cells and myeloid derived suppressor cells at the tumor site (32, 63). Reduced angiogenesis was also observed (32). In addition, vaccination with T-cell lymphoma-derived membranes that were protein transferred to express a GPI-anchored form of B7-1 induced minimal parental tumor-specific CTL lysis, however inclusion of soluble IL-12 along with B7-1-expressing membranes led to further enhanced specific lysis. Nonetheless, both vaccines protected against parental tumor growth (16). Enhanced production of IFN-γ also resulted after expression of both IL-12 and B7-1 on tumor cells (65). Therefore, the combination of both B7-1 and IL-12 enhances antitumor immunity and we showed herein that the co-expression of these ISMs along with the tumor antigen HER-2 on PMVs further enhanced HER-2-specific antitumor immunity. Also, the receptor for B7-1, CD28, is expressed on T-cells, NK cells, and mast cells, and the IL-12 receptor is expressed on NK cells, T-cells, and APCs (14). Therefore expression of B7-1 and IL-12 on PMVs along with HER-2 could lead to more efficient delivery of HER-2 to immune cells along with widespread immune activation. Further, attachment of cytokines, such as IL-12, to the membranes through the GPI-anchor may reduce deleterious effects of systemic toxicity seen with administration of soluble cytokines (35, 66).
This versatile biological membrane vesicle protein delivery approach may be further expanded to elicit an immune response against numerous TAAs including soluble and transmembrane antigens. Individual as well as multiple TAAs can be expressed on the PMVs simultaneously for delivery to broaden the immune response generated, and through the inclusion of different combinations of membrane-bound-ISMs along with TAAs, immunity can be skewed towards a desirable result allowing for a more controlled immune response depending on the disease. Therefore, the rapid, simultaneous protein transfer of GPI-TAAs along with GPI-ISMs onto PMVs can be used as an augmented vaccine in a tumor setting.
Conclusions
In summary, the ability of PMVs derived from biological materials, such as cells and tissues, to be rapidly modified by protein transfer to express antigens along with ISMs, allows for an attractive single antigen delivery system. This system not only enhances antigen immunogenicity but also broadens the scope of antigen-specific immunity induced thus providing broader implications in subunit vaccine production against various types of cancer.
Supplementary Material
Supplemental Figure 1. Electron microscopy imaging of 4TO7 PMVs. PMVs were derived from 4TO7 cells and resuspended in PBS before analyzing by transmission electron microscopy. Representative images of different fields are displayed.
Supplemental Figure 2. Vaccination with protein transfer-modified PMVs did not increase circulating IFN-γ in mice. The concentration of serum IFN-γ was analyzed in vaccinated mice (n = 5) 3 days after boost by a sandwich ELISA.
Supplemental Figure 3. Mice vaccinated with 4TO7 PMVs protein transferred with GPI-HER-2 induced HER-2 specific antibodies. Western blot analysis was performed after SDS PAGE of 4TO7 PMVs, D2F2 PMVs or purified GPI-HER-2 and blotting with 1:500 diluted pooled serum from mice vaccinated (n = 5) with protein transferred 4TO7 PMVs.
Supplemental Figure 4. Enhanced IFN-γ secretion by stimulated splenocytes from mice vaccinated with (GPI-HER-2-IL-12-B7-1)-4TO7 PMVs. Splenocytes from mice (n = 5) vaccinated and boosted with 25 μg protein transferred PMVs were stimulated for 2 days with mitomycin C-treated 4TO7-HER-2 cells. Supernatant was collected and IFN-γ concentrations were analyzed by sandwich ELISA in triplicate. (Statistical analysis: One-Way ANOVA – Tukey’s multiple comparisons test. *** p < 0.001, **** p < 0.0001).
Supplemental Figure 5. Vaccination with three different breast cancer derived PMVs protein transferred with GPI-HER-2 induce similar protection against HER-2-expressing tumor challenge. Groups of BALB/c mice (n = 5) were vaccinated and boosted with 25 μg of PMVs derived from 4TO7, D2F2, or E0771 tumor tissue and protein transferred with GPI-HER-2. Mice were then challenged 7 days post boost with 2 × 105 D2F2/E2 cells. Tumor area was measured and recorded as Average ± SD. Statistical Analysis: Two-Way ANOVA, multiple comparisons test; * p < 0.05.
Supplemental Figure 6. Vaccination with cell culture derived 4TO7 PMVs protein transferred with GPI-HER-2 protects mice against challenge with HER-2 expressing tumor cells. Groups of BALB/c mice (n = 5) were vaccinated and boosted with 25 μg of protein transferred 4TO7 PMVs derived from cultured 4TO7 cells on day 14. Protein transfer resulted in 0.01 μg HER-2/μg PMV. Vaccinated mice (n = 5) were challenged 7 days post boost with 2 × 105 (A) D2F2/E2 (HER-2 positive) cells or (B) D2F2 (HER-2 negative) cells. Tumor area (left panel) and tumor incidence (right panel) were measured. (Statistical analysis: Repeated measures Two-Way ANOVA – Tukey’s multiple comparisons test. * p < 0.05, ** p < 0.01, *** p < 0.001).
Acknowledgments
This work was supported in part by NIH grants R01 CA138993-01A1 (PS) and F31 CA165632-01 (JMP). We thank W.Z. Wei from Wayne State University for the D2F2 and D2F2/E2 tumor cell lines and Dr. Aaron Lukacher for the anti-CD4 and anti-CD8 mAb hybridomas. We also thank Grace Lovia Allotey-Babington at Mercer University for assistance with the Zetasizer and Hong Yi at the Emory University Robert P. Apkarian Integrated Electron Microscopy Core facility for assistance with electron microscopy.
Footnotes
Conflict of Interest:
Dr. Selvaraj is a co-founder & equity holder of Metaclipse Therapeutics Corporation, which is a startup company formed to develop therapeutic cancer vaccines for humans using the protein transfer technology described here for which he is a co-inventor.
Writing assistance was not utilized in the production of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nature reviews Cancer. 2014;14(2):135–46. doi: 10.1038/nrc3670. [DOI] [PubMed] [Google Scholar]
- 2.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nature medicine. 2004;10(5):475–80. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 3.Nestle FO, Farkas A, Conrad C. Dendritic-cell-based therapeutic vaccination against cancer. Current opinion in immunology. 2005;17(2):163–9. doi: 10.1016/j.coi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nature reviews Cancer. 2008;8(2):108–20. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 5.Bolhassani A, Safaiyan S, Rafati S. Improvement of different vaccine delivery systems for cancer therapy. Molecular cancer. 2011;10:3. doi: 10.1186/1476-4598-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. European journal of immunology. 2008;38(5):1404–13. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 7.Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Advanced drug delivery reviews. 2007;59(6):478–90. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Advanced drug delivery reviews. 2008;60(8):915–28. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Wang L, Liu Y, Chen X, Liu Q, Jia J, et al. Immune responses to vaccines involving a combined antigen-nanoparticle mixture and nanoparticle-encapsulated antigen formulation. Biomaterials. 2014;35(23):6086–97. doi: 10.1016/j.biomaterials.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Elamanchili P, Diwan M, Cao M, Samuel J. Characterization of poly(D,L-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine. 2004;22(19):2406–12. doi: 10.1016/j.vaccine.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 11.Sahdev P, Ochyl LJ, Moon JJ. Biomaterials for Nanoparticle Vaccine Delivery Systems. Pharmaceutical research. 2014 doi: 10.1007/s11095-014-1419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nature reviews Immunology. 2002;2(8):569–79. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 13.Sokolova V, Ludwig AK, Hornung S, Rotan O, Horn PA, Epple M, et al. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids and surfaces B, Biointerfaces. 2011;87(1):146–50. doi: 10.1016/j.colsurfb.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Cimino AM, Palaniswami P, Kim AC, Selvaraj P. Cancer vaccine development: protein transfer of membrane-anchored cytokines and immunostimulatory molecules. Immunologic research. 2004;29(1–3):231–40. doi: 10.1385/IR:29:1-3:231. [DOI] [PubMed] [Google Scholar]
- 15.Maeda T, Balakrishnan K, Mehdi SQ. A simple and rapid method for the preparation of plasma membranes. Biochimica et biophysica acta. 1983;731(1):115–20. doi: 10.1016/0005-2736(83)90404-2. [DOI] [PubMed] [Google Scholar]
- 16.McHugh RS, Nagarajan S, Wang YC, Sell KW, Selvaraj P. Protein transfer of glycosyl-phosphatidylinositol-B7-1 into tumor cell membranes: a novel approach to tumor immunotherapy. Cancer research. 1999;59(10):2433–7. [PubMed] [Google Scholar]
- 17.Nagarajan S, Selvaraj P. Human tumor membrane vesicles modified to express glycolipid-anchored IL-12 by protein transfer induce T cell proliferation in vitro: a potential approach for local delivery of cytokines during vaccination. Vaccine. 2006;24(13):2264–74. doi: 10.1016/j.vaccine.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 18.Poloso N, Nagarajan S, Bumgarner GW, Zampell JC, Selvaraj P. Designer cancer vaccines made easy: protein transfer of immunostimulatory molecules for use in therapeutic tumor vaccines. Frontiers in bioscience : a journal and virtual library. 2001;6:D760–75. doi: 10.2741/poloso. [DOI] [PubMed] [Google Scholar]
- 19.Poloso NJ, Nagarajan S, Bumgarner GW, Selvaraj P. Development of therapeutic vaccines by direct modification of cell membranes from surgically removed human tumor tissue with immunostimulatory molecules. Vaccine. 2001;19(15–16):2029–38. doi: 10.1016/s0264-410x(00)00424-2. [DOI] [PubMed] [Google Scholar]
- 20.McHugh RS, Ahmed SN, Wang YC, Sell KW, Selvaraj P. Construction, purification, and functional incorporation on tumor cells of glycolipid-anchored human B7-1 (CD80) Proceedings of the National Academy of Sciences of the United States of America. 1995;92(17):8059–63. doi: 10.1073/pnas.92.17.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel JMVVF, Selvaraj P. Lipid-Mediated Cell Surface Engineering. In: Zhao JKW, editor. Micro- and Nanoengineering of the Cell Surface Micro and Nano Technologies. USA: Elsevier Inc; 2014. pp. 121–41. [Google Scholar]
- 22.Nagarajan S, Selvaraj P. Glycolipid-anchored IL-12 expressed on tumor cell surface induces antitumor immune response. Cancer research. 2002;62(10):2869–74. [PubMed] [Google Scholar]
- 23.Poloso NJ, Nagarajan S, Mejia-Oneta JM, Selvaraj P. GPI-anchoring of GM-CSF results in active membrane-bound and partially shed cytokine. Molecular immunology. 2002;38(11):803–16. doi: 10.1016/s0161-5890(02)00005-6. [DOI] [PubMed] [Google Scholar]
- 24.Patel JM, Vartabedian VF, Kim MC, He S, Kang SM, Selvaraj P. Influenza virus-like particles engineered by protein transfer with tumor-associated antigens induces protective antitumor immunity. Biotechnology and bioengineering. 2015 doi: 10.1002/bit.25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heppner GH, Miller FR, Shekhar PM. Nontransgenic models of breast cancer. Breast cancer research : BCR. 2000;2(5):331–4. doi: 10.1186/bcr77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piechocki MP, Pilon SA, Kelly C, Wei WZ. Degradation signals in ErbB-2 dictate proteasomal processing and immunogenicity and resist protection by cis glycine-alanine repeat. Cellular immunology. 2001;212(2):138–49. doi: 10.1006/cimm.2001.1853. [DOI] [PubMed] [Google Scholar]
- 27.Pilon SA, Piechocki MP, Wei WZ. Vaccination with cytoplasmic ErbB-2 DNA protects mice from mammary tumor growth without anti-ErbB-2 antibody. Journal of immunology. 2001;167(6):3201–6. doi: 10.4049/jimmunol.167.6.3201. [DOI] [PubMed] [Google Scholar]
- 28.Otter GM, Sirotnak FM. Effective combination therapy of metastatic murine solid tumors with edatrexate and the vinca alkaloids, vinblastine, navelbine and vindesine. Cancer chemotherapy and pharmacology. 1994;33(4):286–90. doi: 10.1007/BF00685901. [DOI] [PubMed] [Google Scholar]
- 29.Sugiura K, Stock CC. Studies in a tumor spectrum. I. Comparison of the action of methylbis (2-chloroethyl)amine and 3-bis(2-chloroethyl)aminomethyl-4-methoxymethyl -5-hydroxy-6-methylpyridine on the growth of a variety of mouse and rat tumors. Cancer. 1952;5(2):382–402. doi: 10.1002/1097-0142(195203)5:2<382::aid-cncr2820050229>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Ewens A, Mihich E, Ehrke MJ. Distant metastasis from subcutaneously grown E0771 medullary breast adenocarcinoma. Anticancer research. 2005;25(6B):3905–15. [PubMed] [Google Scholar]
- 31.Cobern L, Selvaraj P. An enzymatic method to determine receptor-mediated endocytosis. Journal of biochemical and biophysical methods. 1995;30(4):249–55. doi: 10.1016/0165-022x(95)00013-3. [DOI] [PubMed] [Google Scholar]
- 32.Bozeman EN, Cimino-Mathews A, Machiah DK, Patel JM, Krishnamoorthy A, Tien L, et al. Expression of membrane anchored cytokines and B7-1 alters tumor microenvironment and induces protective antitumor immunity in a murine breast cancer model. Vaccine. 2013;31(20):2449–56. doi: 10.1016/j.vaccine.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305(5681):200–5. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 34.Chou SH, Shetty AV, Geng Y, Xu L, Munirathinam G, Pipathsouk A, et al. Palmitate-derivatized human IL-2: a potential anticancer immunotherapeutic of low systemic toxicity. Cancer immunology, immunotherapy : CII. 2013;62(3):597–603. doi: 10.1007/s00262-012-1364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90(7):2541–8. [PubMed] [Google Scholar]
- 36.Aly HA. Cancer therapy and vaccination. Journal of immunological methods. 2012;382(1–2):1–23. doi: 10.1016/j.jim.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. Journal of immunology. 2005;174(5):2591–601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer research. 2003;63(7):1555–9. [PubMed] [Google Scholar]
- 39.Nishimura T, Nakui M, Sato M, Iwakabe K, Kitamura H, Sekimoto M, et al. The critical role of Th1-dominant immunity in tumor immunology. Cancer chemotherapy and pharmacology. 2000;46 (Suppl):S52–61. doi: 10.1007/pl00014051. [DOI] [PubMed] [Google Scholar]
- 40.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. The Journal of experimental medicine. 1998;188(12):2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim HJ, Cantor H. CD4 T-cell subsets and tumor immunity: the helpful and the not-so-helpful. Cancer immunology research. 2014;2(2):91–8. doi: 10.1158/2326-6066.CIR-13-0216. [DOI] [PubMed] [Google Scholar]
- 42.Nishimura T, Iwakabe K, Sekimoto M, Ohmi Y, Yahata T, Nakui M, et al. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. The Journal of experimental medicine. 1999;190(5):617–27. doi: 10.1084/jem.190.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lefeber DJ, Benaissa-Trouw B, Vliegenthart JF, Kamerling JP, Jansen WT, Kraaijeveld K, et al. Th1-directing adjuvants increase the immunogenicity of oligosaccharide-protein conjugate vaccines related to Streptococcus pneumoniae type 3. Infection and immunity. 2003;71(12):6915–20. doi: 10.1128/IAI.71.12.6915-6920.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim R, Emi M, Tanabe K. Cancer immunosuppression and autoimmune disease: beyond immunosuppressive networks for tumour immunity. Immunology. 2006;119(2):254–64. doi: 10.1111/j.1365-2567.2006.02430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whiteside TL. Inhibiting the inhibitors: evaluating agents targeting cancer immunosuppression. Expert opinion on biological therapy. 2010;10(7):1019–35. doi: 10.1517/14712598.2010.482207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–7. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 47.Duncan G, Jess TJ, Mohamed F, Price NC, Kelly SM, van der Walle CF. The influence of protein solubilisation, conformation and size on the burst release from poly(lactide-co-glycolide) microspheres. Journal of controlled release : official journal of the Controlled Release Society. 2005;110(1):34–48. doi: 10.1016/j.jconrel.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Nam YS, Song SH, Choi JY, Park TG. Lysozyme microencapsulation within biodegradable PLGA microspheres: urea effect on protein release and stability. Biotechnology and bioengineering. 2000;70(3):270–7. doi: 10.1002/1097-0290(20001105)70:3<270::aid-bit4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 49.Sah H. Protein behavior at the water/methylene chloride interface. Journal of pharmaceutical sciences. 1999;88(12):1320–5. doi: 10.1021/js9900654. [DOI] [PubMed] [Google Scholar]
- 50.van de Weert M, Hoechstetter J, Hennink WE, Crommelin DJ. The effect of a water/organic solvent interface on the structural stability of lysozyme. Journal of controlled release : official journal of the Controlled Release Society. 2000;68(3):351–9. doi: 10.1016/s0168-3659(00)00277-7. [DOI] [PubMed] [Google Scholar]
- 51.Conway MA, Madrigal-Estebas L, McClean S, Brayden DJ, Mills KH. Protection against Bordetella pertussis infection following parenteral or oral immunization with antigens entrapped in biodegradable particles: effect of formulation and route of immunization on induction of Th1 and Th2 cells. Vaccine. 2001;19(15–16):1940–50. doi: 10.1016/s0264-410x(00)00433-3. [DOI] [PubMed] [Google Scholar]
- 52.Lutsiak ME, Kwon GS, Samuel J. Biodegradable nanoparticle delivery of a Th2-biased peptide for induction of Th1 immune responses. The Journal of pharmacy and pharmacology. 2006;58(6):739–47. doi: 10.1211/jpp.58.6.0004. [DOI] [PubMed] [Google Scholar]
- 53.Lucas ML, Heller L, Coppola D, Heller R. IL-12 plasmid delivery by in vivo electroporation for the successful treatment of established subcutaneous B16.F10 melanoma. Molecular therapy : the journal of the American Society of Gene Therapy. 2002;5(6):668–75. doi: 10.1006/mthe.2002.0601. [DOI] [PubMed] [Google Scholar]
- 54.Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. Journal of immunology. 1994;153(4):1697–706. [PubMed] [Google Scholar]
- 55.Tannenbaum CS, Tubbs R, Armstrong D, Finke JH, Bukowski RM, Hamilton TA. The CXC chemokines IP-10 and Mig are necessary for IL-12-mediated regression of the mouse RENCA tumor. Journal of immunology. 1998;161(2):927–32. [PubMed] [Google Scholar]
- 56.Tannenbaum CS, Wicker N, Armstrong D, Tubbs R, Finke J, Bukowski RM, et al. Cytokine and chemokine expression in tumors of mice receiving systemic therapy with IL-12. Journal of immunology. 1996;156(2):693–9. [PubMed] [Google Scholar]
- 57.Watkins SK, Egilmez NK, Suttles J, Stout RD. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. Journal of immunology. 2007;178(3):1357–62. doi: 10.4049/jimmunol.178.3.1357. [DOI] [PubMed] [Google Scholar]
- 58.Kanegane C, Sgadari C, Kanegane H, Teruya-Feldstein J, Yao L, Gupta G, et al. Contribution of the CXC chemokines IP-10 and Mig to the antitumor effects of IL-12. Journal of leukocyte biology. 1998;64(3):384–92. doi: 10.1002/jlb.64.3.384. [DOI] [PubMed] [Google Scholar]
- 59.Baskar S, Glimcher L, Nabavi N, Jones RT, Ostrand-Rosenberg S. Major histocompatibility complex class II+B7-1+ tumor cells are potent vaccines for stimulating tumor rejection in tumor-bearing mice. The Journal of experimental medicine. 1995;181(2):619–29. doi: 10.1084/jem.181.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen L, Ashe S, Brady WA, Hellstrom I, Hellstrom KE, Ledbetter JA, et al. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992;71(7):1093–102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 61.Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259(5093):368–70. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 62.Wu TC, Huang AY, Jaffee EM, Levitsky HI, Pardoll DM. A reassessment of the role of B7-1 expression in tumor rejection. The Journal of experimental medicine. 1995;182(5):1415–21. doi: 10.1084/jem.182.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan WY, Lo CH, Chen CC, Wu PY, Roffler SR, Shyue SK, et al. Cancer immunotherapy using a membrane-bound interleukin-12 with B7-1 transmembrane and cytoplasmic domains. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20(5):927–37. doi: 10.1038/mt.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Putzer BM, Hitt M, Muller WJ, Emtage P, Gauldie J, Graham FL. Interleukin 12 and B7-1 costimulatory molecule expressed by an adenovirus vector act synergistically to facilitate tumor regression. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(20):10889–94. doi: 10.1073/pnas.94.20.10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wen XY, Mandelbaum S, Li ZH, Hitt M, Graham FL, Hawley TS, et al. Tricistronic viral vectors co-expressing interleukin-12 (1L-12) and CD80 (B7-1) for the immunotherapy of cancer: preclinical studies in myeloma. Cancer gene therapy. 2001;8(5):361–70. doi: 10.1038/sj.cgt.7700321. [DOI] [PubMed] [Google Scholar]
- 66.Cohen J. IL-12 deaths: explanation and a puzzle. Science. 1995;270(5238):908. doi: 10.1126/science.270.5238.908a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Electron microscopy imaging of 4TO7 PMVs. PMVs were derived from 4TO7 cells and resuspended in PBS before analyzing by transmission electron microscopy. Representative images of different fields are displayed.
Supplemental Figure 2. Vaccination with protein transfer-modified PMVs did not increase circulating IFN-γ in mice. The concentration of serum IFN-γ was analyzed in vaccinated mice (n = 5) 3 days after boost by a sandwich ELISA.
Supplemental Figure 3. Mice vaccinated with 4TO7 PMVs protein transferred with GPI-HER-2 induced HER-2 specific antibodies. Western blot analysis was performed after SDS PAGE of 4TO7 PMVs, D2F2 PMVs or purified GPI-HER-2 and blotting with 1:500 diluted pooled serum from mice vaccinated (n = 5) with protein transferred 4TO7 PMVs.
Supplemental Figure 4. Enhanced IFN-γ secretion by stimulated splenocytes from mice vaccinated with (GPI-HER-2-IL-12-B7-1)-4TO7 PMVs. Splenocytes from mice (n = 5) vaccinated and boosted with 25 μg protein transferred PMVs were stimulated for 2 days with mitomycin C-treated 4TO7-HER-2 cells. Supernatant was collected and IFN-γ concentrations were analyzed by sandwich ELISA in triplicate. (Statistical analysis: One-Way ANOVA – Tukey’s multiple comparisons test. *** p < 0.001, **** p < 0.0001).
Supplemental Figure 5. Vaccination with three different breast cancer derived PMVs protein transferred with GPI-HER-2 induce similar protection against HER-2-expressing tumor challenge. Groups of BALB/c mice (n = 5) were vaccinated and boosted with 25 μg of PMVs derived from 4TO7, D2F2, or E0771 tumor tissue and protein transferred with GPI-HER-2. Mice were then challenged 7 days post boost with 2 × 105 D2F2/E2 cells. Tumor area was measured and recorded as Average ± SD. Statistical Analysis: Two-Way ANOVA, multiple comparisons test; * p < 0.05.
Supplemental Figure 6. Vaccination with cell culture derived 4TO7 PMVs protein transferred with GPI-HER-2 protects mice against challenge with HER-2 expressing tumor cells. Groups of BALB/c mice (n = 5) were vaccinated and boosted with 25 μg of protein transferred 4TO7 PMVs derived from cultured 4TO7 cells on day 14. Protein transfer resulted in 0.01 μg HER-2/μg PMV. Vaccinated mice (n = 5) were challenged 7 days post boost with 2 × 105 (A) D2F2/E2 (HER-2 positive) cells or (B) D2F2 (HER-2 negative) cells. Tumor area (left panel) and tumor incidence (right panel) were measured. (Statistical analysis: Repeated measures Two-Way ANOVA – Tukey’s multiple comparisons test. * p < 0.05, ** p < 0.01, *** p < 0.001).