Abstract
Objectives:
Narcolepsy is a disabling disease with a delayed diagnosis. At least 3 years before the disorder identification, several comorbidities can be observed in patients with narcolepsy. The early recognition of narcolepsy symptoms may improve long-term prognosis of the patients. Thus, we aimed to investigate the prevalence of the symptoms associated with narcolepsy and its social and psychological association in a sample of Sao Paulo city inhabitants.
Methods:
We performed a cross-sectional evaluation with 1,008 individuals from the Sao Paulo Epidemiologic Sleep Study (EPISONO). Excessive daytime sleepiness (EDS) was assessed by the Epworth Sleepiness Scale. Volunteers were also asked about the occurrence of cataplectic-like, hypnagogic or hypnopompic hallucinations, and sleep paralysis symptoms. The participants underwent a full-night polysomnography and completed questionnaires about psychological, demographic, and quality of life parameters.
Results:
We observed a prevalence of 39.2% of EDS, 15.0% of cataplectic-like symptom, 9.2% of hypnagogic or hypnopompic hallucinations, and 14.9% of sleep paralysis in Sao Paulo city inhabitants. A frequency of 6.9% was observed when EDS and cataplectic-like symptoms were grouped. The other associations were EDS + hallucinations (4.7%) and EDS + sleep paralysis (7.5%). Symptomatic participants were predominantly women and younger compared with patients without any narcolepsy symptom (n = 451). Narcolepsy symptomatology was also associated with a poor quality of life and symptoms of depression, anxiety, and fatigue.
Conclusions:
Narcolepsy-related symptoms are associated with poor quality of life and worse psychological parameters.
Citation:
Kim LJ, Coelho FM, Hirotsu C, Araujo P, Bittencourt L, Tufik S, Andersen ML. Frequencies and associations of narcolepsy-related symptoms: a cross-sectional study. J Clin Sleep Med 2015;11(12):1377–1384.
Keywords: narcolepsy, symptomatology, epidemiological
Although narcolepsy may be considered a rare neurological condition, the repercussions of this disorder are substantial. Narcolepsy induces a 1.5-fold increase in the mortality rate compared with healthy individuals.1 As a consequence, narcoleptic patients have approximately 2-fold increased rates of hospital or other medical services admissions.2
Narcolepsy is often associated with a tetrad of symptoms, including, excessive daytime sleepiness (EDS), hypnagogic or hypnopompic hallucinations, sleep paralysis, and cataplexy. EDS is the most frequent symptom observed in narcoleptic patients.3 In the natural history of EDS, several cardiometabolic and psychiatric conditions are associated with the incidence and persistence of EDS.4
Patients with narcolepsy experience the symptoms over a long period until receiving the definitive diagnosis, ranging between 10 and 15 years.5,6 A possible factor associated with this delay could be the failure to recognize the characteristic clinical features.7 Indeed, Jennum et al. demonstrated that narcoleptic patients present a wide range of comorbidities at least 3 years before the narcolepsy diagnosis, including a higher frequency of neurological diseases and other sleep disorders.8 The recognition of the symptoms associated with narcolepsy seems to be an important approach for a better long-term prognosis of the disorder. Thus, we hypothesized that the presence of narcolepsy-related symptoms, without a confirmatory diagnosis, could be associated with a poor quality of life and psychological impairments.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Narcoleptic patients present a wide range of comorbidities at least 3 years before the narcolepsy diagnosis. The recognition of the symptoms associated with narcolepsy seems to be an important approach for a better long-term prognosis of the disorder.
Study Impact: We found that symptomatic patients, even without the confirmatory narcolepsy diagnosis, present higher scores of depression and anxiety symptoms, fatigue, and a poor self-perception of life quality. Identification of these symptoms could decrease the delay of narcolepsy diagnosis and improve the patient's prognosis right after the first years of the symptoms onset.
In the present study, we aimed to evaluate the prevalence of EDS, cataplectic-like symptoms, hypnagogic and hypnopompic hallucinations, and sleep paralysis in a sample of Sao Paulo city inhabitants. In addition, we purposed to characterize the demographic, psychological, and sleep parameters of individuals who presented EDS, the most prevalent symptom in narcolepsy, in association with another narcolepsy-related symptom.
METHODS
Study Design
We performed an observational cross-sectional analysis with the data obtained from the Sao Paulo Epidemiologic Sleep Study (EPISONO). The complete methodology was described in detail previously.9 Briefly, the sample was obtained by a probabilistic 3-stage cluster sampling technique.10 First, the sampling was performed according to the 4 socioeconomic regions of Sao Paulo as classified by the National Institute of Demographics and Statistics (IBGE) census. The next stages consisted of the selection of a given household and the random choose of 1 dweller of each picked house. An initial sample size of 1,056 participants was defined based on previous studies focused on the prevalence of obstructive sleep apnea (OSA), which was the primary objective of the EPISONO study. At that moment, most of the studies reported a prevalence of 2.2% to 4.5% of OSA in the general population.11–13 For this reason, the power of the study was based on 3% precision. A total of 1,101 volunteers were selected and, from this initial number, 1,042 volunteers (44.9% men; 55.1% women) signed the informed consent form and underwent a full-night polysomnography (PSG). The refusal and response rates were 5.4% and 94.6%, respectively. The study was approved by the University's Institutional Ethics Committee (CEP 0593/06) and was registered with ClinicalTrials.gov (Identifier NCT00596713).
Symptom Tetrad Assessment
Questionnaire
Excessive daytime somnolence: sleepiness was assessed using the Epworth Sleepiness Scale, which consists of an 8-question scale that measures the chance to fall asleep in daily situations.14 The cut-off for EDS was ≥ 10.
Closed Questions
All the questions below regarding narcolepsy symptoms were created by a panel of sleep specialists from the Sleep Institute (Sao Paulo, Brazil) and were not validated. Also, confirmatory clinical interview for assessment of narcolepsy features were not performed in the study due to methodological limitations.
Cataplectic-like symptom: the cataplectic-like symptom question covered the occurrence of an event in the last 6 months. The participants were asked if they had “any event of sudden weakness or difficultly in speaking in situations of strong emotion (such as joy, anger, fear, or surprise) without losing consciousness.”
Hypnagogic/hypnopompic hallucinations: the question assessing hypnagogic and hypnopompic hallucinations covered the occurrence of an event in the last 6 months, considering only visual hallucinations. The participants were asked if they had ever experienced an event of “viewing strange images immediately before sleep or waking up (such as a hallucination).”
Sleep paralysis: the sleep paralysis question covered the occurrence of events of “feeling paralyzed, unable to move, a few moments before falling asleep or just after awaking” without a time-frame, but including frequency ranging from daily to monthly. Thus, the sample was stratified into patients who had never experienced sleep paralysis and those with a frequency of at least 1 event per month.
Selection Criteria
We stratified the sample into 1 group of participants without narcolepsy symptoms and 3 groups of symptomatic patients. The inclusion and exclusion criteria for each group are described below.
Narcolepsy Symptom Grouping
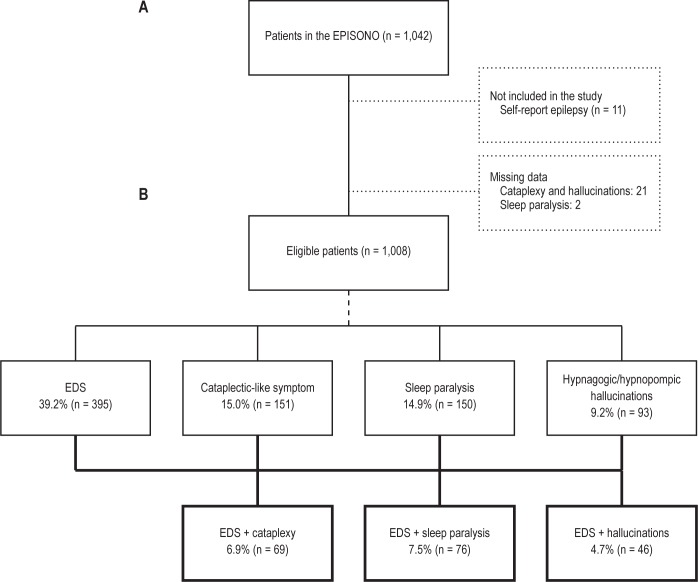
We excluded participants who reported they “have had seizures or epileptic seizures within the last 7 or the last 30 days” or were using anticonvulsant medication. Patients who showed missing data in the Epworth questionnaire or in the closed questions about the narcolepsy symptoms were also excluded from the analysis. As summarized in Figure 1, we grouped the sample according to the presence of EDS and 1 additional narcolepsy-related symptom (cataplectic-like symptom, sleep paralysis, or hypnagogic and hypnopompic hallucinations). Considering the study design, only the association of symptoms with a prevalence of at least 3% was included in the comparison analysis.
Figure 1. Flowchart of the study protocol and the frequencies of the narcolepsy associated symptoms in the sample.
(A) flowchart; (B) frequencies of the narcolepsy associated symptoms in the sample.
EDS + cataplexy group: patients with Epworth scores ≥ 10 reporting cataplectic-like symptoms in the last 6 months.
EDS + hallucinations group: patients with Epworth scores ≥ 10 reporting hypnagogic and/or hypnopompic hallucinations in the last 6 months.
EDS + sleep paralysis group: patients with Epworth scores ≥ 10 reporting at least 1 event of sleep paralysis per month.
Patients without Symptoms
To compare the demographic data and the sleep and psychological parameters, a group of patients without symptoms was selected as a control. As performed in the 3 symptomatic groups, we excluded participants with self-report of epilepsy or using anticonvulsant medication and those who showed missing data in the narcolepsy symptom assessment. In this group, the inclusion criteria for the comparisons consisted of patients without any of the 4 narcolepsy-related symptoms (EDS, cataplectic-like symptom, sleep paralysis, and hypnagogic or hypnopompic hallucinations). From the total amount of participants, 451 participants (44.7%) met the criteria for the group without symptoms.
Psychological Assessments
All questionnaire data were collected by a trained group of 10 psychologists, 3 physical education teachers, and 6 physicians.9
Demographic data: we assessed the age, gender, and ethnic classification of all sample. Body mass index (BMI) of each participant was calculated as body weight divided by square of the body height. Socioeconomic classification was obtained according to the Critério de Classificação Econômica Brasil (CCEB).15
To evaluate the possible influence of medication use and working period in the polysomnographic and psychological variables, we collected the data about central nervous system-acting medications (CNS-acting medication) and the proportion of shift-workers by UNIFESP Sleep Questionnaire.16 CNS-acting medication comprised antidepressants, benzodiazepines, and amphetamines.
Quality of life: patients filled the Portuguese version of the abbreviated World Health Organisation Quality of Life assessment (WHOQOL-BREF).17 This instrument comprises 26 questions distributed into 4 different domains associated with quality of life: physical health, psychological health, social relationships, and environment. The mean scores in each domain range from 0 to 100, according to the WHOQOL transformation table. Higher scores correspond to a better quality of life.
Psychological assessment: anxiety and depressive symptoms were evaluated by the Portuguese version18 of Beck Anxiety Inventory (BAI)19 and the Beck Depression Inventory (BDI),20 respectively. The final scores were classified as: < 11 (no anxiety or depression) and ≥ 11 (anxiety and depression).
Sleep quality and fatigue: to assess the perception of sleep quality and fatigue, the participants completed the Pittsburgh Sleep Quality Index (PSQI)21 and the Chalder Fatigue Scale,22 respectively. Cut-offs for PSQI were ≤ 5 (good sleepers) and > 5 (poor sleepers) and for fatigue were ≤ 4 (no fatigue) and > 4 (fatigue).
Polysomnography
Data from PSG were evaluated to compare the sleep parameters between the symptomatic and asymptomatic patients, and to control other possible sleep disorders from the analyses. The PSG procedures and criteria for sleep scoring have been previously described.9 To evaluate the possible association of the narcolepsy-related symptoms with the presence of other sleep disorders, we stratified the groups by apnea-hypopnea index (AHI) > 15 events/h and periodic limb movement index (PLMI) > 5 events/h.
Statistical Analysis
Qualitative variables were analyzed using χ2 test. To evaluate the effect of narcolepsy-related symptoms on the psychological and demographic parameters, the general linear model (ANCOVA) was performed. In the comparison between EDS + cataplexy group and patients without symptoms, the adjusted confounding factors were age, gender, and PLMI > 5 events/h. For the comparisons with EDS + hallucinations group, the adjusted confounding factors were age, gender, and ethnicity. For EDS + sleep paralysis group, the analyses were only controlled for age and gender. The data were represented as adjusted mean ± standard error of the mean (SEM) or percentage. Statistical significance was considered at a level of p < 0.05.
RESULTS
As shown in Figure 1A, 11 participants who reported having epilepsy were excluded from the analyses. Twenty-one patients did not correctly complete the cataplectic-like symptom and hallucinations questions. In the sleep paralysis question, there were 2 missing values.
Of the 1,008 individuals initially selected in the study, 39.2% (n = 395) had EDS. Sleep paralysis, cataplectic-like symptom, and hypnagogic or hypnopompic hallucinations were reported by 14.9% (n = 150), 15.0% (n = 151), and 9.2% (n = 93) of participants, respectively. When participants were grouped according to the presence of EDS and 1 more associated narcolepsy symptom, a frequency of 6.9% (n = 69) was observed for EDS + cataplexy (Figure 1B). The other associations were EDS + hallucinations (4.7%) and EDS + sleep paralysis (7.5%). When the 4 symptoms were grouped, a frequency of only 1.5% was observed. Regarding the design of the present study, this prevalence was not considered statistically precise. Thus, the grouping of 4 symptoms (narcolepsy tetrad) was not used in the analyses.
The 3 symptomatic groups (EDS + cataplexy, EDS + hallucinations, and EDS + sleep paralysis) showed similar demographic profiles when compared with the patients without symptoms (Table 1). On average, the 3 symptomatic groups were 6–9 years younger than the group without symptoms. A significant association with female gender was also observed. Approximately 67% of the participants with EDS + cataplexy (χ2 = 6.6; df = 1; p < 0.05), EDS + hallucinations (χ2 = 4.3; df = 1; p < 0.05), and EDS + sleep paralysis (χ2 = 6.4; df = 1; p < 0.05) were women. No significant effect of the narcolepsy symptoms was observed on the BMI, indicated by similar values between the symptomatic groups and patients without symptoms. The group EDS + hallucinations showed a significant association with a higher proportion of Afro-Brazilian and native self-declared ethnicities compared with patients without symptoms (χ2 = 17.6; df = 5; p < 0.05). The frequency of socioeconomic status, education level, CNS-acting medication use, and shift-workers were similar between the groups.
Table 1.
Sociodemographic characteristics of the study population.
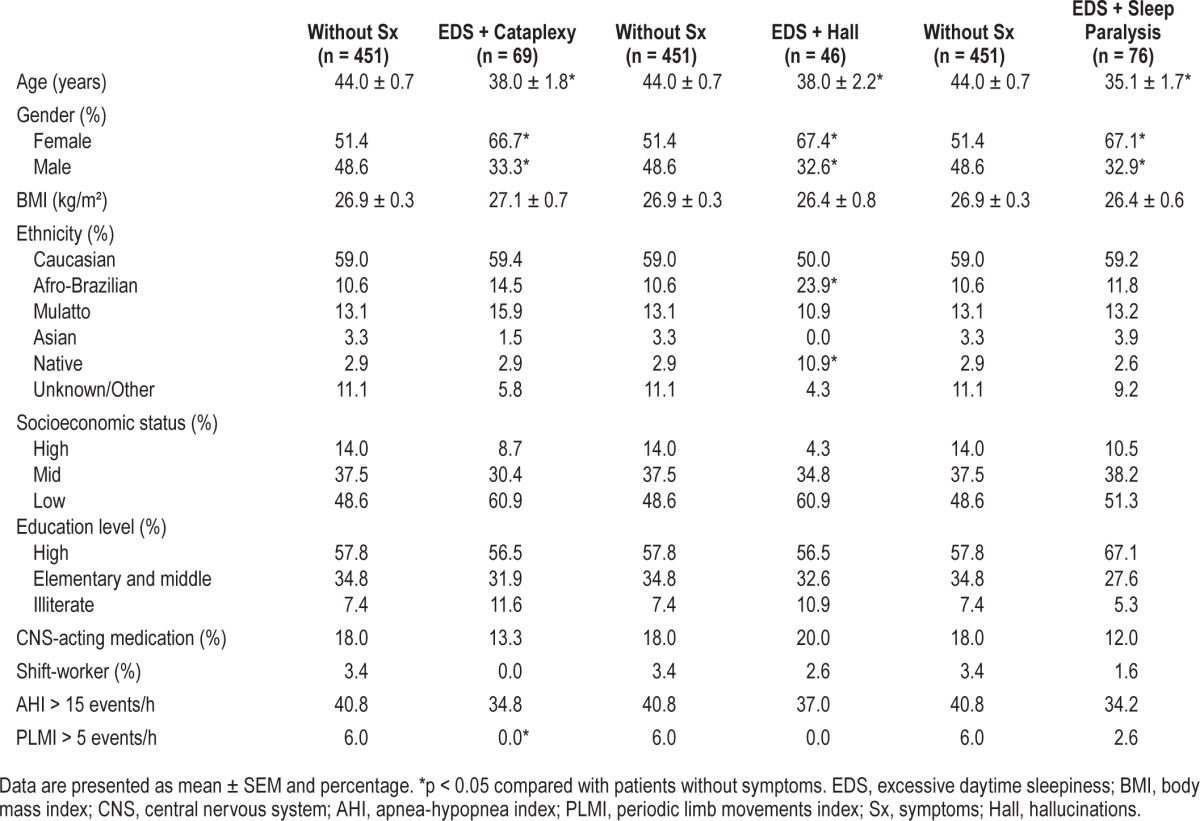
A significant association between a lower frequency of patients with PLMI > 5 events/h and the group EDS + cataplexy (χ2 = 4.4; df = 1; p < 0.05) was observed. To evaluate a possible association between the narcolepsy symptoms and OSA, we stratified the sample by AHI > 15 events/h. We observed no significant differences in the frequency of apneic patients between the 3 symptomatic groups (EDS + cataplexy, EDS + hallucinations, and EDS + sleep paralysis) and patients without symptoms.
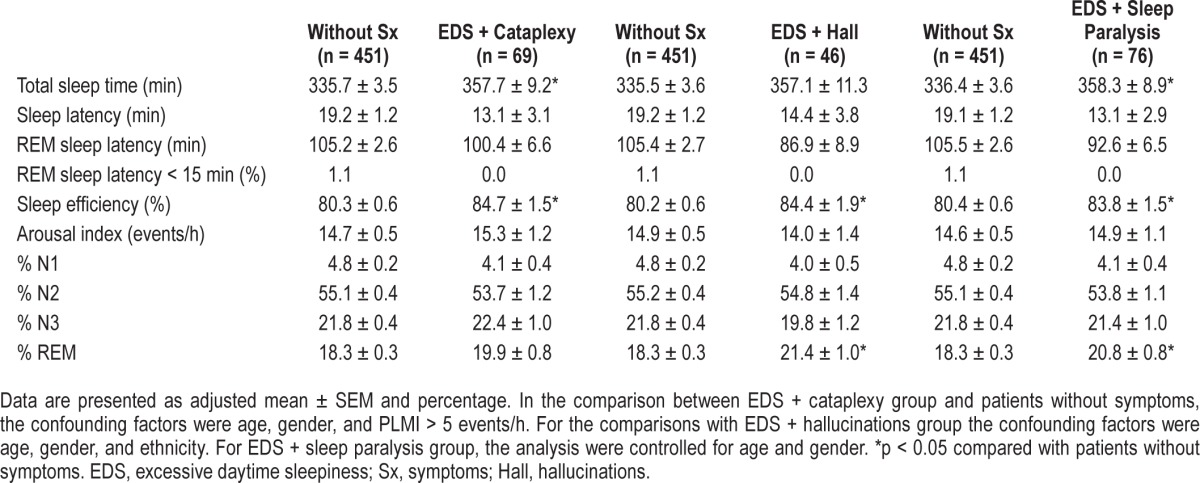
Sleep parameters obtained from the PSG exam are presented in Table 2. The 3 symptomatic groups (EDS + cataplexy, EDS + hallucinations, and EDS + sleep paralysis) showed higher sleep efficiencies compared with patients without symptoms. However, the mean values of sleep efficiency of all groups were lower than normal range (≥ 85%). A significant effect of the groups EDS + cataplexy (F1,518 = 5.0; p < 0.05) and EDS + sleep paralysis (F1,530 = 5.2; p < 0.05) were ob -served in the total sleep time. Patients with EDS + cataplexy and EDS + sleep paralysis showed longer total sleep time in the PSG compared with the group without symptoms. The percentage of REM sleep was also significantly higher in the EDS + hallucinations (F1,500 = 9.0; p < 0.05) and EDS + sleep paralysis (F1,530 = 8.8; p < 0.05) compared with the group without symptoms.
Table 2.
Polysomnographic variables among the participants with and without narcolepsy symptoms.
Narcolepsy symptoms were associated with a poor subjective sleep quality and psychiatric symptoms (Table 3). A higher frequency of poor sleepers was observed in the groups EDS + cataplexy (χ2 = 16.4; df = 1; p < 0.05) and EDS + hallucinations (χ2 = 20.3; df = 1; p < 0.05). Only 30.4% and 21.7% of the participants from EDS + cataplexy and EDS + hallucinations groups were classified as good sleepers. We observed a fatigue frequency of 75.4% in the EDS + cataplexy (χ2 = 60.0; df = 1; p < 0.05), 65.9% in EDS + hallucinations (χ2 2 8.7; df = 1; p < 0.05), and 58.9% in EDS + sleep paralysis (χ2 = 29.7; df = 1; p < 0.05) groups compared with 26.9% in patients without symptoms. Approximately 72%, 70%, and 55% of the participants from EDS + cataplexy (χ2 = 44.8; df = 1; p < 0.05), EDS + hallucinations (χ2 = 26.4; df = 1; p < 0.05), and EDS + sleep paralysis (χ2 = 18.5; df = 1; p < 0.05) groups were classified with depressive symptoms, respectively. The symptomatic groups also showed a significant association with anxiety symptoms in the BAI. Anxiety symptoms were observed in 75.8%, 69.2%, and 43.1% of the individuals with EDS + cataplexy (χ2 = 87.1; df = 1; p < 0.05), EDS + hallucinations (χ2 = 48.7; df = 1; p < 0.05), and EDS + sleep paralysis (χ2 = 19.3; df = 1; p < 0.05) groups, respectively. In the group without symptoms, only 19.5% of the participants showed anxiety symptoms. To exclude a possible effect of psychological symptoms on the sleep parameters, we reanalyzed the data including also the results of BDI and BAI as continuous covariates (data not shown). As the only alteration in the results was in the percentage of REM sleep of the EDS + hallucinations group that was no longer different from the patients without symptoms, we elected to keep our data without this control.
Table 3.
Association of quality of life and psychological parameters with narcolepsy symptoms.
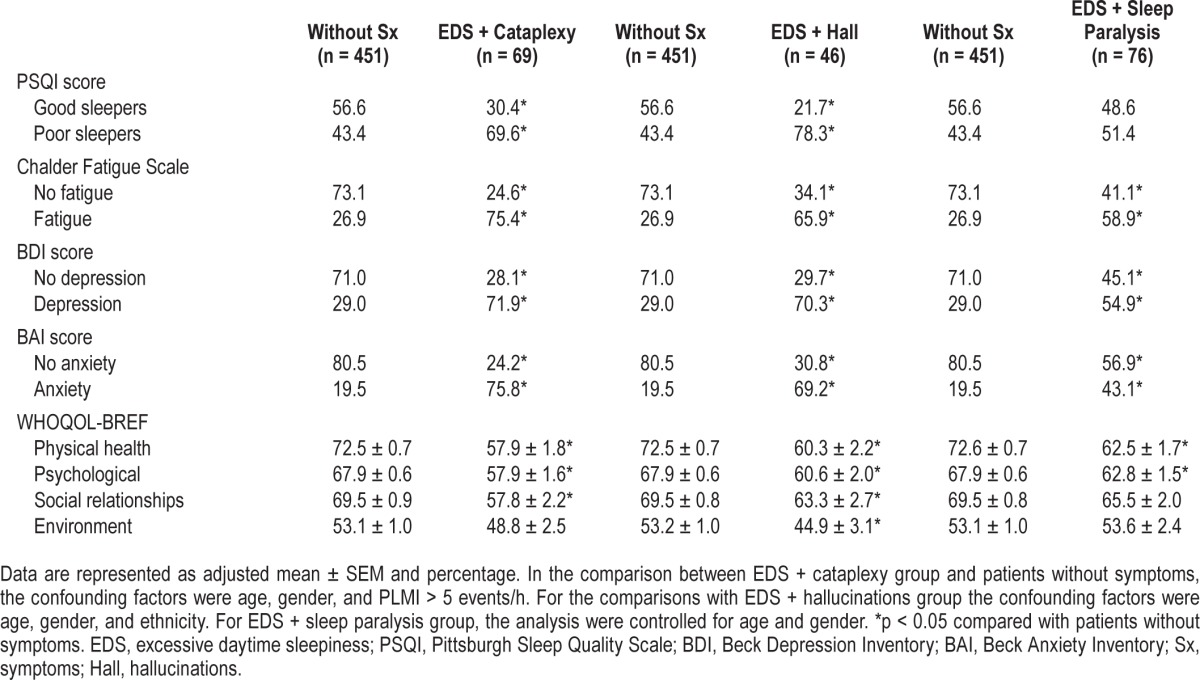
All the 3 symptomatic groups (EDS + cataplexy, EDS + hallucinations, and EDS + sleep paralysis) also showed poor quality of life in the physical health and psychological domains of WHOQOL-BREF, corroborating the findings of BAI and BDI. Only the EDS + hallucinations group showed a significant effect on the 4 domains of WHOQOL-BREF, presenting lower scores compared with the group without symptoms.
DISCUSSION
We investigated the frequencies of associated symptoms of narcolepsy in a sample of Sao Paulo city inhabitants. Symptomatic participants were predominantly women and younger compared with patients without symptoms. The presence of EDS in addition to cataplectic-like symptom, hallucinations, or sleep paralysis was associated with poor quality of life and worse psychological parameters.
EDS is the most prevalent symptom of narcolepsy and can be observed in up to 91% of the patients.3 In 40% of narcolepsy cases, EDS onset preceded cataplexy, and in 54% it was concomitant with cataplexy.23 Cataplexy may occur in 60% to 90% of the narcoleptic patients and hypnagogic or hypnopompic hallucinations in 30% to 60%.24,25 The occurrence of 2 or 3 symptoms is present in approximately 30% of narcoleptic patients.24 On the other hand, the complete symptom tetrad is observed in only 10% to 15% of narcolepsy cases.25
In the general population of Sao Paulo city, we observed a prevalence of 39.2% of EDS, 15.0% of cataplectic-like symptom, 9.2% of hypnagogic or hypnopompic hallucinations, and 14.9% of sleep paralysis. The frequency of cataplectic-like symptom in the present study was within the range of 7.6% to 29.3% previously reported in different ethnic populations.26–29 A study of five European countries by Ohayon et al.30 has investigated the prevalence of the narcolepsy tetrad in a general population. In the whole sample, daytime sleepiness, cataplexy, hypnagogic or hypnopompic hallucinations, and sleep paralysis were reported by 15%, 1.2%, 24%, and 6.2% of the participants, respectively. Some factors may explain these discrepancies in the symptom proportion between our results and the study of Ohayon et al.30 Firstly, we assessed EDS using only the Epworth Sleepiness Scale. Although this is the most widely used instrument for the evaluation of sleepiness, EDS comprises other clinical features in narcolepsy, such as the irresistible sleep attack and the relief of sleepiness provided by naps.31 Thus, a broad investigation of sleepiness could decrease the proportion of participants with EDS in our study. Secondly, in the study of Ohayon et al., narcolepsy symptoms differed considerably among the countries. For example, in Portugal, a lower frequency of 10.4% of hypnagogic or hypnopompic hallucinations was reported, corroborating the prevalence of 9.2% in our study.
The association between gender and narcolepsy is controversial. Some reports have suggested a higher frequency of narcolepsy in women,32 while others have indicated a 73% greater prevalence of the disease in men.33 In addition, gender seems to affect the interval between the symptom onset and diagnosis. Won et al.34 demonstrated that men are diagnosed on average 12 years earlier than women. The authors suggested that a possible factor involved in this delayed diagnosis is that women may be less forthcoming about their symptoms. However, our findings contradict this hypothesis, showing that women have higher frequency of reported narcolepsy-related symptoms. Thus, women seem to have a better perception of the symptoms associated with narcolepsy that could favor the early diagnosis of the disorder.
Indeed, it is not simply the patient's perception which affects the disease diagnosis, but also the difficulty that health professionals have in recognizing symptoms related to narcolepsy, that leads to delayed diagnosis.35 EDS and hallucinations are features commonly observed in other sleep, neurological, and psychiatric disorders.36 Although cataplexy is a phenomenon present only in narcolepsy, the variety of phenotypes can make it very difficult to diagnose.37 The severity of cataplexy ranges from partial muscle weakness to complete body paralysis with different frequencies, triggered by a variety of emotional conditions.37 In cases of narcolepsy without cataplexy, the diagnosis can be even more challenging due to the nonspecific nature of the symptoms.38
A full-night PSG followed by the multiple sleep latency test (MSLT) is mandatory for the narcolepsy diagnosis.39 However, these exams are expensive and need many resources, which may hinder their implementation in large-scale health services. In addition, the MSLT has been reported to have low sensitivity and specificity for EDS diagnosis,40 and a high false-positive and false-negative rate.41 Therefore, the assessment of the symptoms associated with narcolepsy may be a more effective initial way to increase diagnostic coverage. Indeed, structured interviews for sleep disorders present good agreement with PSG and MSLT, having an accuracy of 80% for narcolepsy with cataplexy.42
Patients with narcolepsy often experience more difficulties at their workplace than at home, especially because of their incapacity to remain awake during daytime.24 Consequently, these individuals show lower employment rates and income levels than healthy patients.43 Narcolepsy is also associated with poor quality of life44 and psychiatric disorders.45 In a case-control study, Droogleever et al.46 found that 53% of narcoleptic patients report anxiety or panic attacks, which were mainly related to frightening episodes during hypnagogic hallucinations. To our knowledge, we performed the first study that investigated the association of psychological and narcolepsy-related symptoms in the general population. We found that symptomatic patients, even without the confirmatory narcolepsy diagnosis, present higher scores of depression and anxiety symptoms, fatigue, and a poor perception of their quality of life. In this sense, the development of therapeutic approaches aiming to increase awareness about the importance of early recognition of associated symptoms of narcolepsy is necessary. Identification of these symptoms could decrease delay in narcolepsy diagnosis and improve quality of life of patients following symptom onset.
Study limitations include lack of a definitive narcolepsy diagnosis by MSLT after the full-night PSG. Thus, the prevalence of the associated symptoms of narcolepsy could have been overestimated by the presence of non-narcoleptic patients in the group. Regarding the study design, the results of the present study did not establish a causal relationship between narcolepsy-related symptoms and the psychological parameters. In addition, the questionnaire used for assessing catapletic-like symptom, hallucinations, and sleep paralysis symptoms were not validated. The evaluation of cataplectic-like symptom may also not represent a genuine cataplexy event in narcolepsy. Thus, further studies should be performed to elucidate this relationship and to evaluate the accuracy and effectiveness of the early recognition of the narcolepsy-related symptoms in the patients' life.
DISCLOSURE STATEMENT
This was not an industry-supported study. This study was supported by grants from the Associação Fundo de Incentivo à Pesquisa (AFIP) and São Paulo Research Foundation (FAPESP) [#2013/14420-1 to Lenise Jihe Kim, #2014/15259-2 to Dr. Hirotsu, and #2014/10255-9 to Dr. Araujo]. Dr. Tufik, Dr. Bittencourt, and Dr. Andersen received CNPq fellowships. The authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BAI
Beck Anxiety Inventory
- BDI
Beck Depression Inventory
- BMI
body mass index
- CCEB
Critério de Classificação Econômica Brasil
- CNS-acting medication
central nervous system acting medication
- EDS
excessive daytime sleepiness
- EPISONO
Sao Paulo Epidemiologic Sleep Study
- Hall
hallucinations
- IBGE
National Institute of Demographics and Statistics
- MSLT
multiple sleep latency test
- OSA
obstructive sleep apnea
- PLMI
periodic limb movement index
- PSG
polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- SEM
standard error of the mean
- Sx
symptoms
- WHOQOL-BREF
abbreviated World Health Organisation Quality of Life assessment
REFERENCES
- 1.Ohayon MM, Black J, Lai C, Eller M, Guinta D, Bhattacharyya A. Increased mortality in narcolepsy. Sleep. 2014;37:439–44. doi: 10.5665/sleep.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black J, Reaven NL, Funk SE, et al. The Burden of Narcolepsy Disease (BOND) study: health-care utilization and cost findings. Sleep Med. 2014;15:522–9. doi: 10.1016/j.sleep.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Carter LP, Acebo C, Kim A. Patients' journeys to a narcolepsy diagnosis: a physician survey and retrospective chart review. Postgrad Med. 2014;126:216–24. doi: 10.3810/pgm.2014.05.2769. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Mendoza J, Vgontzas AN, Kritikou I, Calhoun SL, Liao D, Bixler EO. Natural history of excessive daytime sleepiness: role of obesity, weight loss, depression, and sleep propensity. Sleep. 2015;38:351–60. doi: 10.5665/sleep.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flygare J, Parthasarathy S. Narcolepsy: let the patient's voice awaken us! Am J Med. 2015;128:10–3. doi: 10.1016/j.amjmed.2014.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med. 2004;5:37–41. doi: 10.1016/j.sleep.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med. 2014;15:502–7. doi: 10.1016/j.sleep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Jennum P, Ibsen R, Knudsen S, Kjellberg J. Comorbidity and mortality of narcolepsy: a controlled retro- and prospective national study. Sleep. 2013;36:835–40. doi: 10.5665/sleep.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santos-Silva R, Tufik S, Conway SG, Taddei JA, Bittencourt LR. Sao Paulo Epidemiologic Sleep Study: rationale, design, sampling, and procedures. Sleep Med. 2009;10:679–85. doi: 10.1016/j.sleep.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Kish I. New York: John Wiley & Sons Inc.; 1965. Survey sampling. [Google Scholar]
- 11.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 12.Marin JM, Gascon JM, Carrizo S, Gispert J. Prevalence of sleep apnoea syndrome in the Spanish adult population. Int J Epidemiol. 1997;26:381–6. doi: 10.1093/ije/26.2.381. [DOI] [PubMed] [Google Scholar]
- 13.Kim J, In K, Kim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170:1108–13. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 14.Bertolazi AN, Fagondes SC, Hoff LS, Pedro VD, Menna Barreto SS, Johns MW. Portuguese-language version of the Epworth sleepiness scale: validation for use in Brazil. J Bras Pneumol. 2009;35:877–83. doi: 10.1590/s1806-37132009000900009. [DOI] [PubMed] [Google Scholar]
- 15.Associação Brasileira de Empresas de Pesquisa - ABEP. Critério de Classificação Econômica Brasil (CCEB) 2003.
- 16.Braz S, Neumann BRB, Tufik S. Evaluation of sleep disorders: elaboration and validation of a questionnaire. Revista ABP-APL. 1987;9:9–14. [Google Scholar]
- 17.Fleck MP, Louzada S, Xavier M, et al. Aplicação da versão em português do instrumento abreviado de avaliação da qualidade de vida WHOQOL-BREF. Rev Saude Public. 2000;34:178–83. doi: 10.1590/s0034-89102000000200012. [DOI] [PubMed] [Google Scholar]
- 18.Gorenstein C, Andrade L. Validation of a Portuguese version of the Beck Depression Inventory and the State-Trait Anxiety Inventory in Brazilian subjects. Braz J Med Biol Res. 1996;29:453–7. [PubMed] [Google Scholar]
- 19.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Guth D, Steer RA, Ball R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav Res Ther. 1997;35:785–91. doi: 10.1016/s0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 21.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 22.Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. J Psychosom Res. 1993;37:147–53. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 23.Sturzenegger C, Bassetti CL. The clinical spectrum of narcolepsy with cataplexy: a reappraisal. J Sleep Res. 2004;13:395–406. doi: 10.1111/j.1365-2869.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- 24.Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the Innsbruck narcolepsy cohort. J Clin Sleep Med. 2013;9:805–12. doi: 10.5664/jcsm.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drakatos P, Leschziner GD. Update on hypersomnias of central origin. Curr Opin Pulm Med. 2014;20:572–80. doi: 10.1097/MCP.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 26.Honda Y. Census of narcolepsy, cataplexy and sleep life among teenagers in Fujisawa city. Sleep Res. 1979;8:191. [Google Scholar]
- 27.Billiard M, Alperovitch A, Perot C, Jammes A. Excessive daytime somnolence in young men: prevalence and contributing factors. Sleep. 1987;10:297–305. doi: 10.1093/sleep/10.4.297. [DOI] [PubMed] [Google Scholar]
- 28.Hublin C, Kaprio J, Partinen M, et al. The prevalence of narcolepsy: an epidemiological study of the Finnish Twin Cohort. Ann Neurol. 1994;35:709–16. doi: 10.1002/ana.410350612. [DOI] [PubMed] [Google Scholar]
- 29.Wing YK, Li RH, Lam CW, Ho CK, Fong SY, Leung T. The prevalence of narcolepsy among Chinese in Hong Kong. Ann Neurol. 2002;51:578–84. doi: 10.1002/ana.10162. [DOI] [PubMed] [Google Scholar]
- 30.Ohayon MM, Priest RG, Zulley J, Smirne S, Paiva T. Prevalence of narcolepsy symptomatology and diagnosis in the European general population. Neurology. 2002;58:1826–33. doi: 10.1212/wnl.58.12.1826. [DOI] [PubMed] [Google Scholar]
- 31.Alóe F, Alves RC, Araújo JF, et al. Brazilian guidelines for the diagnosis of narcolepsy. Ver Bras Psiquiatr. 2010;32:294–304. doi: 10.1590/s1516-44462010005000014. [DOI] [PubMed] [Google Scholar]
- 32.Longstreth WT, Jr, Ton TG, Koepsell T, Gersuk VH, Hendrickson A, Velde S. Prevalence of narcolepsy in King County, Washington, USA. Sleep Med. 2009;10:422–6. doi: 10.1016/j.sleep.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu H, Zhuang J, Stone WS, et al. Symptoms and occurrences of narcolepsy: a retrospective study of 162 patients during a 10-year period in eastern China. Sleep Med. 2014;15:607–13. doi: 10.1016/j.sleep.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Won C, Mahmoudi M, Qin L, Purvis T, Mathur A, Mohsenin V. The impact of gender on timeliness of narcolepsy diagnosis. J Clin Sleep Med. 2014;10:89–95. doi: 10.5664/jcsm.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueki Y, Hayashida K, Komada Y, et al. Factors Associated with Duration Before Receiving Definitive Diagnosis of Narcolepsy among Japanese Patients Affected with the Disorder. Int J Behav Med. 2014;21:966–70. doi: 10.1007/s12529-013-9371-5. [DOI] [PubMed] [Google Scholar]
- 36.Teeple RC, Caplan JP, Stern TA. Visual hallucinations: differential diagnosis and treatment. Prim Care Companion J Clin Psychiatry. 2009;11:26–32. doi: 10.4088/pcc.08r00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dauvilliers Y, Siegel JM, Lopez R, Torontali ZA, Peever JH. Cataplexy--clinical aspects, pathophysiology and management strategy. Nat Rev Neurol. 2014;10:386–95. doi: 10.1038/nrneurol.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baumann CR, Mignot E, Lammers GJ, et al. Challenges in diagnosing narcolepsy without cataplexy: a consensus statement. Sleep. 2014;37:1035–42. doi: 10.5665/sleep.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American Academy of Sleep Medicine. 3rd ed. Westchester, IL: American Academy of Sleep Medicine; 2014. International classification of sleep disorders. [Google Scholar]
- 40.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the Epworth Sleepiness Scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 41.Leschziner G. Narcolepsy: a clinical review. Pract Neurol. 2014;14:323–31. doi: 10.1136/practneurol-2014-000837. [DOI] [PubMed] [Google Scholar]
- 42.Merikangas KR, Zhang J, Emsellem H, et al. The structured diagnostic interview for sleep patterns and disorders: rationale and initial evaluation. Sleep Med. 2014;15:530–5. doi: 10.1016/j.sleep.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Jennum P, Ibsen R, Petersen ER, Knudsen S, Kjellberg J. Health, social, and economic consequences of narcolepsy: a controlled national study evaluating the societal effect on patients and their partners. Sleep Med. 2012;13:1086–93. doi: 10.1016/j.sleep.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Rovere H, Rossini S, Reimão R. Quality of life in patients with narcolepsy: a WHOQOL-bref study. Arq Neuropsiquiatr. 2008;66:163–7. doi: 10.1590/s0004-282x2008000200004. [DOI] [PubMed] [Google Scholar]
- 45.Zimerman L, Högl B, Delazera M, et al. Subjective deficits of attention, cognition and depression in patients with narcolepsy. Sleep Med. 2015;16:45–51. doi: 10.1016/j.sleep.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 46.Droogleever Fortuyn HA, Mulders PC, Renier WO, Buitelaar JK, Overeem S. Narcolepsy and psychiatry: an evolving association of increasing interest. Sleep Med. 2011;12:714–9. doi: 10.1016/j.sleep.2011.01.013. [DOI] [PubMed] [Google Scholar]