Abstract
Background
The aim of this work was to study the Fabp4 and Pten gene expression and correlation in the liver, muscle, and adipose tissues of type 2 diabetes mellitus (T2DM) rats.
Material/Methods
Male Wistar rats (8 weeks old) were randomly divided into 2 groups (n=12/group): a control group fed a normal diet for 8 weeks and an experimental group fed a high-fat, high-sugar diet for 8 weeks and that received 25 mg/kg streptozotocin by intraperitoneal injection to induce T2DM. The random blood glucose, fasting blood glucose, and fasting insulin levels were measured. The expression of Pten and Fabp4 in the liver, muscle, and epididymal adipose tissues was estimated by real-time quantitative PCR. Pearson correlation coefficient analysis was used to investigate the expression correlation between Pten and Fabp4 in T2DM rats.
Results
The gene expressions of Pten and Fabp4 in the liver, muscle, and adipose tissues of T2DM rats were all significantly higher than those in the control group (P<0.05). Pten was highly expressed in the muscles and Fabp4 was highly expressed in muscle and adipose tissues. Furthermore, expressions of Fabp4 and Pten in the muscle and adipose tissues of T2DM rats were positively correlated (P<0.05), but not in the liver.
Conclusions
The increased expression of PTEN and FABP4 in the adipose and muscles of T2DM rats may play an important role in the insulin resistance of T2DM. However, the mechanism by which these 2 genes function in T2DM needs further study.
MeSH Keywords: Diabetes Mellitus, Type 2; Fatty Acid-Binding Proteins; PTEN Phosphohydrolase
Background
Type 2 diabetes mellitus (T2DM) is a pervasive metabolic syndrome and its pathogenesis remains unclear. The main characteristic of T2DM is considered to be insulin resistance (IR). To our knowledge, liver, adipose and muscle are major insulin responsive tissues. Under normal conditions, insulin stimulates the uptake of glucose and potently inhibits lipolysis in adipocytes. Under insulin-stimulated conditions, adipose and liver account for about 10% and 30% of the whole body glucose uptake, respectively [1]. The skeletal muscle accounts for about 75% of glucose disposal after glucose infusion [1]. In addition, the insulin-stimulated glucose uptake in adipose and muscle occurs via the intracellular PI3K/Akt pathway which is required for translocation of glucose transporter GLUT4 to the plasma membrane [1].
Phosphatase and tensin homolog deleted on chromosome ten (PTEN) was identified as a tumor suppressor with dual lipid and protein tyrosine phosphatase activity and frequently mutated/deleted in many human cancers [2]. It was also widely known as the negative regulator of insulin/phosphoinositide 3-kinase (PI3K) signaling pathway in which PTEN hydrolyzes PI(3,4,5)P3 to PI(4,5)P2, and suppresses cell growth and other PI3K/Akt-dependent processes [3]. Therefore, PTEN was considered as a promising target in T2DM and obesity treatment because of its negative effects on insulin resistance [4–6]. However, the specific role of PTEN in insulin target tissues and insulin resistance is not yet known.
Fatty acid-binding protein 4 (FABP4), known as adipocyte FABP (A-FABP) or aP2, is abundantly expressed in adipocytes and plays important roles in adipocyte differentiation and lipid metabolism [7]. Multiple studies have been showed that Fabp4 functions in the free fatty acid transport, regulation whole body insulin sensitivity and development of atherosclerosis [8–10].
Recently PTEN was found to be interacted with FABP4 [11], suggesting that PTEN may function in the regulation of lipid metabolism and adipocyte differentiation. Our previous study found that the plasma levels of FABP4 and PTEN were increased with more severe IR in women with gestational diabetes mellitus [12], indicating that both genes were important in the development of IR. Therefore, here we investigated the gene expression of Pten and Fabp4 in the liver, muscle and adipose tissues of T2DM rats to explore the expression and correlation between Pten and Fabp4 in T2DM.
Material and Methods
Ethics statement
All experimental protocols were performed in accordance with the approved national and international guidelines. All animal studies have been performed with the approval of the ethics committee of Inner Mongolia Medical University.
Animals, diets and sample preparation
Briefly, 24 male Wistar rats, 8 weeks old (weight, 200–220 grams) were purchased from the Experimental Animal Center of Inner Mongolia University and housed (3 animals per cage) under standard conditions. All animals were initially fed the normal diet for 1 week. Subsequently, all rats were randomly divided into 2 groups (n=12/group) fed 2 different diets: a control group fed normal diet (low-fat diet, LFD) containing 5% fat, 53% carbohydrate, and 23% protein, with total calorific value 25 kJ/kg, and the T2DM experimental group fed a high-fat, high-sugar diet (HFHSD) containing 22% fat, 48% carbohydrate, and 20% protein with total calorific value 44.3 kJ/kg. After 8 weeks, the experimental group rats received intraperitoneal (IP) injection of streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO, USA) 25 mg/kg of body weight, and the control rats were given vehicle citrate buffer (pH 4.4) 0.25 mL/kg IP. After 1 week, all the rats were fasted for 12 hours; blood glucose was measured from a tail vein using a glucometer (Roche Diagnostics GmbH, Germany). The rats with blood glucose <16.7 mmol/L were injected with STZ again (25 mg/kg). After 4 weeks, rats with blood glucose levels of ≥16.7 mmol/L were considered diabetic [13,14]. The insulin resistance index was calculated by the homeostasis model assessment (HOMA) (HOMA-IR=Fasting blood glucose levels (FBG, mmol/L) × Fasting insulin (FINS, mU/L)/22.5) [13,14]. The blood glucose level and body weight were measured every week. All rats were fasted for 12–16 hours and blood was obtained from the heart and separated by centrifugation (1500 rpm, 20 min) for serum. All the rats were killed and all the tissues were collected, flash frozen, and subsequently stored at −70°C.
Total RNA extraction and cDNA synthesis
The total RNA from muscle, liver, and epididymal adipose tissues were obtained with TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) following the manufacturer’s instructions. The total RNA concentration and purity were determined using a NanoDrop™ 2000c Spectrophotometer (Thermo Scientific, USA). The 1.5% agarose gel electrophoresis was performed to check the RNA integrity. One μg of total RNA was used for cDNA synthesis with a final volume of 20 μL, using PrimeScript™ RT reagent Kit (TaKaRa, Japan) following the manufacturer’s instructions.
Real-time quantitative PCR
To measure the relative mRNA expression, real-time qPCR was performed with an ABI 7500fast System (Applied Biosystems, Foster City, CA) with SYBR Premix Ex Taq™ reagent (TaKaRa, Japan). The housekeeping gene Gapdh was used as a reference gene for normalization. The primers sequences were as follows: Pten (NM_031606.1): 5′-CCCAGTTTGTGGTCTGCCAGC-3′ and 5′-ATGAGCTTGTCCTCCCGCCG-3′; Fabp4 (NM_053365.1): 5′-GTCCT GGTACATGTGCAGAA-3′ and 5′-CTCTTGTAGAAGTCACGCCT-3′; Gapdh (NM_017008.4): 5′-GGTGAAGGTCGGTGTGAACG-3′ and 5′-CTCGCTCCTGGAAGATGGTG-3′. The qPCR was performed with 12.5 ul 2X SYBR /ROX qPCR Mix and 10 pmol forward and reverse primers specific for the respective genes, in a total volume of 25 μl. The following reaction conditions were applied: 2 min at 95°C, 40 cycles of 15 s at 95°C and 30 s at 60°C, and a melting curve protocol (plates read when increased 0.5°C every 5 s from 65°C to 95°C) for amplicon specificity verification. All amplifications were run in triplicate, and any doubtful curves were excluded. The amplification efficiency for Pten, Fabp4 and Gapdh was estimated by real-time qPCR with different diluted cDNA template. The threshold cycle (Ct) values from all amplifications were measured.
Statistical analysis
The comparative 2−ΔΔCT method for relative quantitative analysis was used, and the results are expressed as a fold change of expression levels. The mean value of triplicates was applied for all calculations. Results are expressed as mean ±SD. All statistical calculations were performed with the SPSS 13.0 software package (SPSS Inc.). The t test was used for comparisons between 2 groups. Correlation between parameters was determined by Pearson’s correlation coefficient (r). A P value less than 0.05 was considered statistically significant.
Results
Animal model
The body weight of rats throughout the study is shown in Figure 1. At the beginning of the study, there were no significant differences in body weight between the groups. After high-fat diet feeding, the body weight of rats increased faster than in the control group with normal diet (P<0.01). The rats in the experimental group with blood glucose levels ≥16.7 mmol/L and HOMA-IR ≥2.69 (Table 1) were considered as diabetic and were used as the T2DM model for study. Insulin resistance was induced in the T2DM group as shown by hyperinsulinemia and increased HOMA-IR as compared with the control group (Table 1). Not surprisingly, the fasting blood glucose (FBG) in the T2DM group was significantly higher than that of the control group (all P<0.01), and the glucose level in the T2DM group was increased obviously compared to that of the control group (P<0.01) (Table 1), which indicated that the insulin sensitivity was remarkably decreased in the T2DM group compared to the control group. Phenotypically, T2DM rats appeared drowsy, with slow reaction, depression, loss of hair, and water intake and urine volume increased compared with the control group rats. Some diabetic rats’ tails were festered.
Figure 1.
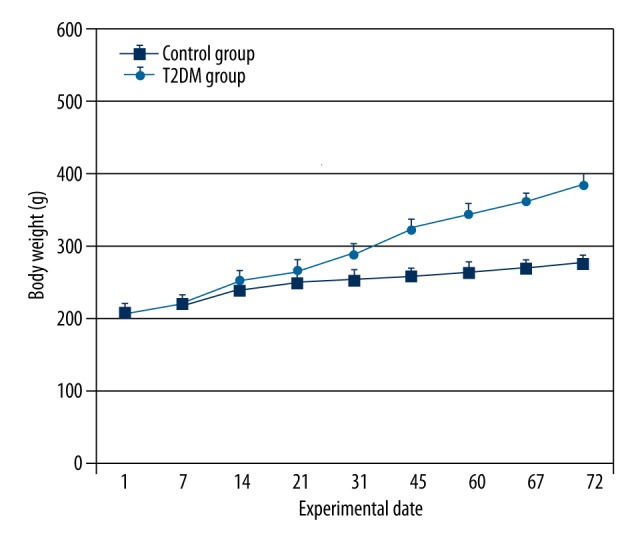
The body weight of rats. High-fat, high-sugar feeding resulted in greater increases in body weight in T2DM rats than in controls. Values are means ±SD. n=12/group.
Table 1.
Serum metabolic parameters in the 2 groups of rats (χ̄±S).
| Random blood glucose (mmol/L) | FPG (mmol/L) | FINS (mU/L) | HOMA-IR | |
|---|---|---|---|---|
| Experimental group (n=12) | 21.28±3.33 | 9.01±1.20 | 9.78±1.68 | 3.92±0.92 |
| Control group (n=12) | 6.93±0.46 | 5.59±0.81 | 7.49±1.31 | 1.87±0.47 |
| t | −14.753 | −8.161 | −3.715 | −6.857 |
| P | 0.000* | 0.000* | 0.001* | 0.000* |
p<0.01.
Pten and Fabp4 mRNA expression in liver, muscle, and adipose tissues of T2DM rats
The liver, muscle and adipose tissues were taken from T2DM and control rats and the relative expression of Pten mRNA was measured by real-time qPCR. The 2−ΔΔCT method for relative quantitative analysis was used since the amplification efficiency (in the range of 0.9–1.2) in Pten, Fabp4, and Gapdh genes is the same. The qPCR results showed that the Pten expression levels in liver, muscle, and adipose tissues of the T2DM group were significantly higher than those of the control group (2.25-, 6.59-, and 2.6-fold higher in liver, muscle, and adipose tissues, respectively p<0.05) (Figure 2, Supplementary Table 1). Pten was highly expressed in muscles of T2DM rats (Figure 2). Compared to the control group, Fabp4 mRNA relative expression in the T2DM group was also increased by 1.43-, 4.35-, and 5.17-fold in the liver, muscle, and adipose tissues of T2DM rats, respectively (P<0.01) (Figure 3, Supplementary Table 2). Interestingly, Fabp4 was also highly expressed in adipose tissues and muscles. The results indicate Pten and Fabp4 may be correlated and function in muscle and adipose tissues of T2DM rats.
Figure 2.
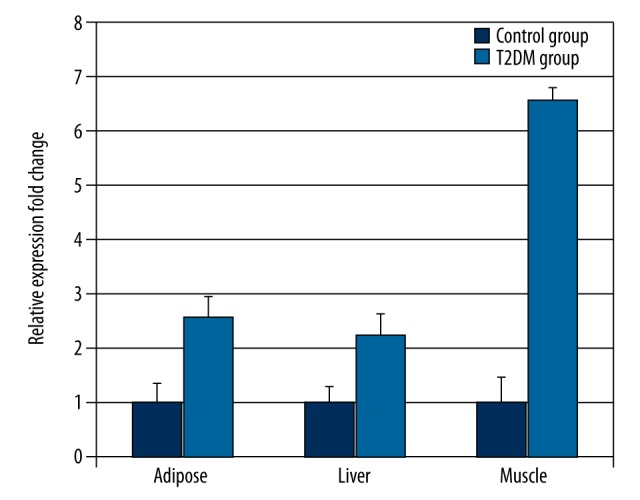
Pten mRNA expressions in the liver, muscle, and adipose tissues of the T2DM rats was determined by real-time PCR (n=12). Values were normalized to Gapdh mRNA expression. The mean change in liver, muscle, and adipose tissues was 2.25-, 6.59-, and 2.6-fold, respectively.
Figure 3.
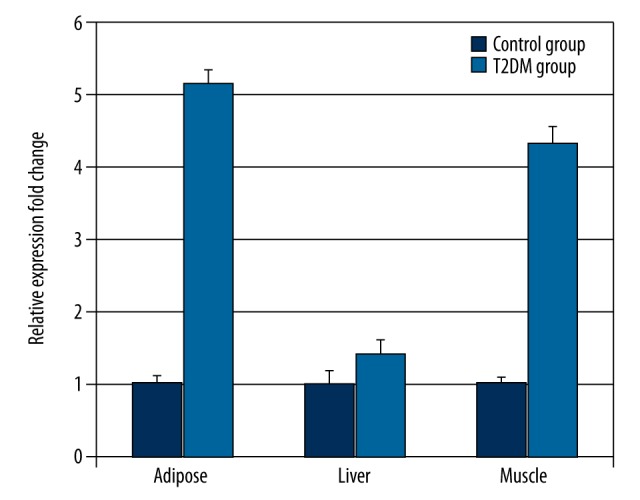
Fabp4 mRNA expressions in the liver, muscle and adipose tissues of the T2DM rats was determined by real-time PCR (n=12). Values were normalized to Gapdh mRNA expression. The mean change in liver, muscle, and adipose tissues was 1.43-, 4.35-, and 5.17-fold, respectively.
The gene expression correlation between Pten and Fabp4 in liver, muscle, and adipose tissues of T2DM rats
Since PTEN and FABP4 interacted each other, we analyzed the expression correlation in liver, muscle, and adipose tissues of T2DM rats by Pearson correlation coefficient, finding that the expression levels of Pten and Fabp4 in muscle and adipose tissues of T2DM rats were positively correlated (P<0.05) (Table 2), but in the liver tissue of T2DM rats no significant correlation was found (r=0.095, P=0.839) . The correlation study verified the result from qPCR by which Pten and Fabp4 were found to be highly expressed in adipose and muscles of T2DM rats.
Table 2.
The correlation between the expression of Pten and Fabp4 genes in T2DM rats.
| Tissue | Pearson correlation coefficient (r) | P value |
|---|---|---|
| Liver | 0.095 | 0.839 |
| Muscle | 0.667 | 0.018* |
| Adipose | 0.662 | 0.026* |
P<0.05.
Discussion
Type 2 diabetes is a complex and heterogeneous disease resulting from a combination of genetic and environmental factors. Many investigators developed the rat model by high-fat diet following low-dose STZ injection, closely mimicking the natural characteristics of T2DM [13–15]. Therefore, we used the T2DM rat model previously established to study the gene expression and correlation of Pten and Fabp4 in the liver, muscle, and adipose tissues. In the present paper, we intended, for the first time, to study the expression and correlation between Pten and Fabp4 in the liver, muscle, and adipose tissues of T2DM rats.
Adipose tissue, liver, and muscle are the principal responsive tissues in whole-body glucose homeostasis and their defects lead to insulin resistance and T2DM. A previous study demonstrated that Pten liver-specific deletion in mice could increase fatty acid synthesis, enhance liver insulin action, and improve systemic glucose tolerance [16]. Similarly, muscle- and adipose-specific PTEN deletions prevented the development of IR and diabetes induced by a high-fat diet in mice [17,18]. Overexpression Of PTEN in 3T3-L1 adipocytes significantly inhibits insulin-stimulated GLUT4 translocation, glucose uptake, and membrane ruffling, all of which are dependent on PI3K activity [19]. Few attempts have been made to fully investigate the changes of Pten in adipose tissue under insulin resistance conditions. Our results show that Pten was highly expressed in adipose, liver, and muscle tissues of T2DM rats, indicating that PTEN plays an important role in IR of T2DM rats. The increased expression of Pten was also observed in a diabetic mouse [20]. The result was consistent with our finding from T2DM rats, indicating that PTEN might suppress the PI3K activity, which induced IR in diabetic rats. It is necessary to further investigate the insulin signaling PI3K/Akt pathway in Pten highly expressed tissues of T2DM rats to define the mechanism of IR.
Fabp4 encodes the fatty acid binding protein found in adipocytes [21]. It is reported to function in fatty acid uptake, transport, and metabolism and plays a key role in obesity-induced insulin resistance [10]. A Fabp4 knockout mouse study showed that Fabp4 plays a key role in diabetes and atherosclerosis. Therefore, Fabp4 has been suggested as a potential therapeutic target for intervention in metabolic syndrome [22]. A previous study found that the serum FABP4 level was significantly increased in obese patients, and had a significant positive correlation with BMI and blood pressure. This observation suggests that FABP4 is involved in the development of metabolic syndrome and obesity [23]. Our previous study also found that the plasma level of FABP4 was also increased in women with gestational diabetes mellitus showing severe insulin resistance [12]. The deletion of Fabp4 in macrophages can lead to elevated glucose uptake in adipocytes and insulin signal conduction enhancement [24]. On the other hand, the loss of Fabp4 can improve peripheral insulin resistance and protect β cell function and thus glucose and lipid metabolism [24].
A recent study found that PTEN can interact with FABP4 using yeast 2-hybrid assay, and the interaction was confirmed by coimmunoprecipitation and gel-filtration assays [11]. The gene expression profile of PTEN-null keratinocytes analysis showed higher expression of FABP4, suggesting the importance of PTEN in the regulation of FABP4 expression at transcription level [25]. In addition, Ptenflox/flox-null hepatocytes showed increased Fabp4 expression along with elevated peroxisome proliferator-activated receptor γ (PPARγ) and PPARγ-regulated genes expression [26]. PPARγ, a transcription factor predominantly expressed in the adipose tissues, are important for adipogenesis and glucose metabolism [27]. The above evidence indicates that PTEN may function as a feedback down-regulator of Fabp4 and PPARγ activity via its direct interaction with Fabp4 [26].
Conclusions
The present study shows that the expression of Pten and Fabp4 was increased and was correlated in adipose and muscles of T2DM rats. Our study suggests a possible link between PTEN/FABP4 co-expression and pathogenesis of T2DM. The specific mechanism should be investigated further.
Supplementary materials
Supplementary Table 1.
PTEN expression value in adipose, liver, and muscle tissues by real-time PCR.
| PTEN CT | GAPDH CT | ΔCT | ΔΔCT | 2–ΔΔCT | std | |
|---|---|---|---|---|---|---|
| Adiposae | ||||||
| Control group | 23.12±0.4 | 17.41±0.21 | 5.71±0.35 | 0 | 1 | 0.35 |
| T2DM group | 23.1±0.42 | 18.77±0.15 | 4.33±0.36 | −1.38 | 2.6 | 0.36 |
| Liver | ||||||
| Control group | 25.11±0.28 | 18.26±0.3 | 6.85±0.29 | 0 | 1 | 0.29 |
| T2DM group | 23.43±0.35 | 17.75±0.41 | 5.68±0.38 | −1.17 | 2.25 | 0.38 |
| Muscle | ||||||
| Control group | 29.39±0.52 | 26.23±0.34 | 3.16±0.46 | 0 | 1 | 0.46 |
| T2DM group | 27.79±0.2 | 27.35±0.25 | 0.44±0.23 | −2.72 | 6.59 | 0.23 |
Supplementary Table 2.
FABP4 expression value in adipose, liver, and muscle tissues by real-time PCR.
| PTEN CT | GAPDH CT | ΔCT | ΔΔCT | 2–ΔΔCT | std | |
|---|---|---|---|---|---|---|
| Adiposae | ||||||
| Control group | 16.49±0.1 | 17.41±0.07 | −0.92 | 0 | 1 | 0.09 |
| T2DM group | 15.49±0.21 | 18.78±0.13 | −3.29 | −2.37 | 5.17 | 0.18 |
| Liver | ||||||
| Control group | 27.38±0.15 | 18.03±0.19 | 9.35 | 0 | 1 | 0.17 |
| T2DM group | 26.6±0.07 | 17.77±0.18 | 8.83 | −0.52 | 1.43 | 0.16 |
| Muscle | ||||||
| Control group | 26.5±0.11 | 22.08±0.07 | 4.42 | 0 | 1 | 0.1 |
| T2DM group | 30.47±0.25 | 28.17±0.16 | 2.3 | −2.12 | 4.35 | 0.22 |
Footnotes
Source of support: The study was supported by grants from the Inner Mongolia Autonomous Region Prairie Excellence Specialist Program and The Inner Mongolia Autonomous Region Key Laboratory Open Fund (2015)
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Smith U. Impaired (‘diabetic’) insulin signaling and action occur in fat cells long before glucose intolerance – is insulin resistance initiated in the adipose tissue? Int J Obes Relat Metab Disord. 2002;26(7):897–904. doi: 10.1038/sj.ijo.0802028. [DOI] [PubMed] [Google Scholar]
- 2.Myers MP, Stolarov JP, Eng C, et al. PTEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci USA. 1997;94:9052–57. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maehama T, Dixon JE. The tumor suppressor, Pten/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3, 4, 5-trisphosphate. J Biol Chem. 1998;273:13375–78. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 4.Tamguney T, Stokoe DJ. New insights into PTEN. Cell Sci. 2007;120:4071–79. doi: 10.1242/jcs.015230. [DOI] [PubMed] [Google Scholar]
- 5.Bulger DA, Conley J, Conner SH, et al. Role of PTEN in TNFα induced insulin resistance. Biochem Biophys Res Commun. 2015;461(3):533–36. doi: 10.1016/j.bbrc.2015.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pulido R. PTEN: a yin-yang master regulator protein in health and disease. Methods. 2015;77–78:3–10. doi: 10.1016/j.ymeth.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Xu A, Wang Y, Xu JY, et al. Adipocyte fatty acid binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem. 2006;52:405–13. doi: 10.1373/clinchem.2005.062463. [DOI] [PubMed] [Google Scholar]
- 8.Furuhashi M, Saitoh S, Shimamoto K, et al. Fatty acid-binding protein 4 (Fabp4): Pathophysiological insights and potent clinical biomarker of metabolic and cardiovascular diseases. Clin Med Insights Cardiol. 2015;8(Suppl 3):23–33. doi: 10.4137/CMC.S17067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Y, Luo N, Lopes-Virella MF, et al. The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis. 2002;165:259–69. doi: 10.1016/s0021-9150(02)00305-2. [DOI] [PubMed] [Google Scholar]
- 10.Makowski L, Hotamisligil GS. The role of fatty acid binding proteins in metabolic syndrome and atherosclerosis. Curr Opin Lipidol. 2005;16:543–48. doi: 10.1097/01.mol.0000180166.08196.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorbenko O, Panayotou G, Zhyvoloup A, et al. Identification of novel PTEN-binding partners: PTEN interaction with fatty acid binding protein FABP4. Mol Cell Biochem. 2010;337(1–2):299–305. doi: 10.1007/s11010-009-0312-1. [DOI] [PubMed] [Google Scholar]
- 12.Li YY, Xiao R, Li CP, et al. Increased plasma levels of FABP4 and PTEN is associated with more severe insulin resistance in women with gestational diabetes mellitus. Med Sci Monit. 2015;21:426–31. doi: 10.12659/MSM.892431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Lv XY, Li J, et al. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res. 2008;2008:704045. doi: 10.1155/2008/704045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reed MJ, Meszaros K, Entes LJ, et al. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism. 2000;49(11):1390–94. doi: 10.1053/meta.2000.17721. [DOI] [PubMed] [Google Scholar]
- 15.Komsuoglu Celikyurt I, Mutlu O, Ulak G, et al. Exenatide treatment exerts anxiolytic- and antidepressant-like effects and reverses neuropathy in a mouse model of type-2 diabetes. Med Sci Monit Basic Res. 2014;30(20):112–17. doi: 10.12659/MSMBR.891168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stiles B, Wang Y, Stahl A, et al. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity. Proc Natl Acad Sci USA. 2004;101:2082–87. doi: 10.1073/pnas.0308617100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wijesekara N, Konrad D, Eweida M, et al. Muscle-specific Pten deletion protects against insulin resistance and diabetes. Mol Cell Biol. 2005;25:1135–45. doi: 10.1128/MCB.25.3.1135-1145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurlawalla-Martinez C, Stiles B, Wang Y, et al. Insulin hypersensitivity and resistance to streptozotocin-induced diabetes in mice lacking PTEN in adipose tissue. Mol Cell Biol. 2005;25:2498–510. doi: 10.1128/MCB.25.6.2498-2510.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakashima N, Sharma PM, Imamura T, et al. The tumor suppressor Pten negatively regulates insulin signaling in 3T3-L1 adipocytes. J Biol Chem. 2000;275(17):12889–95. doi: 10.1074/jbc.275.17.12889. [DOI] [PubMed] [Google Scholar]
- 20.Zeng JB, Zhang Y, Sun Q, et al. Phosphatase and tension homolog overexpression in insulin resistant diabetic adipose tissue. Chin Med Sci J. 2014;29(3):167–73. doi: 10.1016/s1001-9294(14)60063-8. [DOI] [PubMed] [Google Scholar]
- 21.Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uysal KT, Scheja L, Wiesbrock SM, et al. Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology. 2000;141:3388–96. doi: 10.1210/endo.141.9.7637. [DOI] [PubMed] [Google Scholar]
- 23.Mankowska-Cyl A, Krintus M, Rajewski P, et al. A-FABP and its association with atherogenic risk profile and insulin resistance in young overweight and obese women. Biomark Med. 2013;7(5):723–30. doi: 10.2217/bmm.13.61. [DOI] [PubMed] [Google Scholar]
- 24.Furuhashi M, Tuncman G, Görgün CZ, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447:959–65. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuda M, Inoue-Narita T, Suzuki A, et al. Induction of gene encoding FABP4 in Ptennull keratinocytes. FEBS Lett. 2009;583:1319–22. doi: 10.1016/j.febslet.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 26.Horie Y, Suzuki A, Kataoka E, et al. Hepatocytespecific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Investig. 2004;113:1774–83. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawai M, Rosen CJ. PPARγ: a circadian transcription factor in adipogenesis and osteogenesis. Nat Rev Endocrinol. 2010;6(11):629–36. doi: 10.1038/nrendo.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
PTEN expression value in adipose, liver, and muscle tissues by real-time PCR.
| PTEN CT | GAPDH CT | ΔCT | ΔΔCT | 2–ΔΔCT | std | |
|---|---|---|---|---|---|---|
| Adiposae | ||||||
| Control group | 23.12±0.4 | 17.41±0.21 | 5.71±0.35 | 0 | 1 | 0.35 |
| T2DM group | 23.1±0.42 | 18.77±0.15 | 4.33±0.36 | −1.38 | 2.6 | 0.36 |
| Liver | ||||||
| Control group | 25.11±0.28 | 18.26±0.3 | 6.85±0.29 | 0 | 1 | 0.29 |
| T2DM group | 23.43±0.35 | 17.75±0.41 | 5.68±0.38 | −1.17 | 2.25 | 0.38 |
| Muscle | ||||||
| Control group | 29.39±0.52 | 26.23±0.34 | 3.16±0.46 | 0 | 1 | 0.46 |
| T2DM group | 27.79±0.2 | 27.35±0.25 | 0.44±0.23 | −2.72 | 6.59 | 0.23 |
Supplementary Table 2.
FABP4 expression value in adipose, liver, and muscle tissues by real-time PCR.
| PTEN CT | GAPDH CT | ΔCT | ΔΔCT | 2–ΔΔCT | std | |
|---|---|---|---|---|---|---|
| Adiposae | ||||||
| Control group | 16.49±0.1 | 17.41±0.07 | −0.92 | 0 | 1 | 0.09 |
| T2DM group | 15.49±0.21 | 18.78±0.13 | −3.29 | −2.37 | 5.17 | 0.18 |
| Liver | ||||||
| Control group | 27.38±0.15 | 18.03±0.19 | 9.35 | 0 | 1 | 0.17 |
| T2DM group | 26.6±0.07 | 17.77±0.18 | 8.83 | −0.52 | 1.43 | 0.16 |
| Muscle | ||||||
| Control group | 26.5±0.11 | 22.08±0.07 | 4.42 | 0 | 1 | 0.1 |
| T2DM group | 30.47±0.25 | 28.17±0.16 | 2.3 | −2.12 | 4.35 | 0.22 |


