Abstract
Paraoxonase 1 (PON1), an antioxidant enzyme, is believed to play a critical role in many diseases, including cancer. PCBs are widespread environmental contaminants known to induce oxidative stress and cancer and to produce changes in gene expression of various pro- and antioxidant enzymes. Thus it appeared of interest to explore whether PCBs may modulate the activity and/or gene expression of PON 1 as well. In this study we compared the effects of dioxin-like and non-dioxin-like PCBs and of various aryl hydrocarbon receptor (AhR) ligands on PON1 regulation and activity in male and female Sprague Dawley rats. Our results demonstrate that i) the non-dioxin-like PCBs 154, 155 and 184 significantly reduced liver and serum PON1 activities, but only in male rats; ii) the non-dioxin-like PCB 153, the most abundant PCB in many matrices, did not affect PON1 mRNA level in the liver but significantly decreased serum PON1 activity in male rats; iii) PCB 126, an AhR ligand and dioxin-like PCB, increased both PON1 activities and gene expression; iv) even though three tested AhR ligands induced CYP1A in several tissues to a similar extent, they displayed differential effects on the three PONs and AhR, i.e. PCB 126 was an efficacious inducer of PON1, PON2, PON3, and AhR in the liver, while 3-methylcholantrene induced liver AhR and lung PON3, and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the most potent AhR agonist, increased only PON3 in the lung, at the doses and exposure times used in these studies. These results show that PCBs may have an effect on the antioxidant protection by paraoxonases in exposed populations and that regulation of gene expression through AhR is highly diverse.
Keywords: PON1, PON2, PON3, PCB126, TCDD, 3-MC, AhR, non-dioxin-like PCB
Introduction
The paraoxonase (PON) gene family has 3 members, PON1, PON2 and PON3, that share considerable structure homology resulting from gene duplication (Aviram & Rosenblat 2008). PON1 and PON3 are mainly synthesized in the liver and are secreted into the blood, while PON2, an intracellular protein, is ubiquitously expressed in many tissues including brain, liver and heart (Precourt et al. 2011). All PON enzymes are believed to have antioxidant properties (Furlong et al. 2005) and the recent discovery of unexpectedly wide distribution and expression of PON1 and PON3 in mouse and human tissues suggests a systemic role in anti-inflammatory protection (Rodriguez-Sanabria et al. 2010).
PON1, so far the most studied of the three PONs, is now recognized for its antioxidant and antiatherogenic properties besides the well-known detoxification of organophosphate pesticides. A low level of PON1 activity has been associated with various diseases such as cardiovascular disease, neurological disease, type II diabetes and cancer (Goswami et al. 2009, Precourt et al. 2011). Therefore PON1 status and factors affecting PON1 activity and expression take on added significance. Among these factors, environmental chemicals (e.g., some heavy metals, TCDD and 3-methylcholanthrene, 3-MC), drugs (e.g. statins, fibrates and aspirin), diet (e.g. dietary polyphenols, fat-rich food and alcohol), life-style (e.g., smoking), age, and gender were shown to modulate PON1 at transcriptional or post-translational levels in either direction (Costa et al. 2005).
Polychlorinated biphenyls are a group of 209 different congeners, defined by the number and position of chlorines on the biphenyl ring. They were used commercially in a multitude of applications and are now ubiquitous contaminants. PCBs increase intracellular oxidative stress which has been associated to a variety of disease processes mentioned above (Hennig et al. 2002, Hennig et al. 2005, Ludewig et al. 2008). The acute toxicity of individual PCB congeners follows structure-activity relationships in which the chlorination pattern determines potential for binding avidity to various cellular receptors (Hestermann et al. 2000, Luthe et al. 2008, Safe et al. 1985) with consequences for gene expression, metabolic activation or detoxification. Our earlier studies demonstrated that the dioxin-like PCB 126 (3,3′,4,4′,5-pentachlorobiphenyl) significantly increased PON1 activity in liver and serum of rats and PON1 gene expression in the liver (Shen et al. 2012). This increase was independent of the route of exposure (Aqil et al. 2014) or the diet (Shen et al. 2014). In this study, we asked whether non-dioxin-like PCBs or other AhR agonists affect the regulation of PON1. We report here that in contrast to the dioxin-like PCB 126, non-dioxin-like PCBs down-regulated PON1 expression and activity in the liver and serum of male but not female rats, that PCB 126 is upregulating all 3 PON genes in the liver, but not in the lung, and that other AhR agonists with similar CYP 1A1 inducing activity are less efficient in the induction of PONs than PCB 126.
Materials and Methods
Chemicals and reagents
PCB 126 (3,3′,4,4′,5-pentachlorobiphenyl) was synthesized by Dr. Gregor Luthe using a modified Suzuki-coupling method as previously reported (Luthe et al. 2009), purified by aluminum oxide column and flash silica gel column chromatography, and recrystallized from methanol. The final product purity (> 99.8%) and identity were confirmed by GC–MS and 13C NMR. PCB 153 (2,2′,4,4′,5,5′-hexachorobiphenyl), PCB 154 (2,2′,4,4′,5,6′-hexachlorobiphenyl), PCB155 (2,2′,4,4′,6,6′-hexachlorobiphenyl), and PCB184 (2,2′,3,4,4′,6,6′-heptachlorobiphenyl) were generously provided by Dr. Hans-Joachim Lehmler (University of Iowa Superfund Research Program). 3-methylcholanthrene (3-MC) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) were purchased from Sigma-Aldrich (St. Louis, MO). All other reagents were obtained from Fisher Scientific (Pittsburgh, PA), if not stated otherwise, and were the highest purity available.
Animals and treatment protocol
Female and male Sprague Dawley (SD) rats (75–100 g), purchased from Harlan Laboratories, Inc. (Indianapolis, IN), were housed in a controlled environment maintained at 22°C with a 12 hour dark-light cycle and fed a standard rat chow diet and water ad libitum. The animal protocols used were conducted with approval from the Institutional Animal Care and Use Committee of the University of Iowa. The doses of PCB 126, other PCBs and chemical inducers and exposure times were chosen based on a previous studies (Schramm et al. 1985, Shen et al. 2012).
For the study with non-dioxin-like PCBs, male and female SD rats received i.p. injections of PCB 154 (50 μmol/kg), PCB 154, PCB 155, or PCB 184 (100 μmol/kg each), or corn oil (vehicle control) on days 1 and 4, and were euthanized 6 days after first injection by carbon dioxide asphyxiation and cervical dislocation. Blood was processed to obtain the serum and livers were homogenized in sucrose solution. The liver homogenates were centrifuged at 10,000g for 20 min and the resulting supernatant again at 100,000g for 1h in ice-cold 0.25 M sucrose solution (pH 7.4). The pellets, containing the microsomes, were washed and resuspended in sucrose solution. All samples were stored at −80°C until use.
For the comparative study of dioxin and non-dioxin-like PCBs, male SD rats were administrated a single i.p. injection of corn oil, PCB 126 (5 μmol/kg) or PCB 153 (100 μmol/kg), and were euthanized 1 week after the injection by carbon dioxide asphyxiation and cervical dislocation. Blood was drawn immediately after the euthanasia for serum separation. Small samples of livers for total RNA isolation were placed immediately in liquid nitrogen. The remaining livers were homogenized in Tris-KCl buffer (20 mM Tris and 1.15% KCl, pH 7.4) to produce an approximate 10% (w/v) liver homogenate. Serum, liver samples and liver homogenate were stored at −80°C until use.
For the comparative study of different AhR ligands, male SD rats received a single i.p. injection of corn oil, PCB 126 (5 μmol/kg; euthanized 3 days later), TCDD (50 μg/kg equal to 0.16 μmol/kg; euthanized 2 days later) or 3 daily i.p. injections of 3-MC (300 μmol/kg; euthanized 1 day after the last injection). Blood and livers were harvested as described above for dioxin-like and non-dioxin-like PCBs and stored at −80°C until use.
Determination of liver and serum PON1 activity
Paraoxon and phenylacetate were used as two individual substrates in PON1 activity determinations. Briefly, the enzyme activities were determined spectrophotometrically by following the initial rate of substrate hydrolysis to p-nitrophenol (412 nm) or phenol (270 nm), respectively, in 1000 μL assay mixture (100 mM Tris-HCl, 2.0 mM CaCl2, 2.0 mM paraoxon or 4.0 mM phenylacetate, pH 8.0) at 25°C. A blank containing the assay buffer without sample was run simultaneously to correct for spontaneous substrate hydrolysis. The units (U) of enzyme activity were calculated from the molar extinction coefficients, E412 (18,290 M−1cm−1) and E270 (1310 M−1cm−1), respectively, and expressed as U/mL serum or U/mg protein in liver homogenate. Each unit of enzyme is defined as that hydrolyzing 1 nmol of paraoxon or 1 μmol of phenylacetate per min (Beltowski et al. 2005). The protein concentration in liver homogenates was determined using the Bradford protein assay regent (Bio-Rad Laboratories, Inc. CA).
Gene expression analyses
Total RNA was isolated from rat liver samples using the RNeasy Mini Kit from Qiagen, Inc. (Valencia, CA) according to the manufacturer’s protocol. RNA concentration and purity was determined by spectrophotometric measurement of A260/A280. cDNA templates were generated using 1 μg of total RNA per 20 μL reaction and a High-Capacity cDNA Reverse Transcription Kit from Applied Biosystems, Inc. (Foster City, CA) according to the manufacturer’s protocol. Real-time PCR was performed in a 20 μL reaction with 4 ng of cDNA template and 900 nM primer using a SYBR Green Master Mix kit from Applied Biosystems, Inc. (Foster City, CA) according to the manufacturer’s protocol.
Primers used here were from previous publications and synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). The specificity of these primers was further verified by the Primer-BLAST online program provided by the National Library of Medicine. The primers designed for rat (r) genes are listed as follows:
rPON1: forward 5′ TGCTGGCTCACAAGATTCAC3′; reverse 5′ TTCCTTTGTACACAGCAGCG3′ (Varatharajalu et al. 2009)
rPON2: forward 5′ CTAACGGCCAGAAGCTCTTCG 3′; reverse 5′ GATGTACACTGTCGTCACCGAT 3′ (Farid et al. 2010)
rPON3: forward 5′CTCTCGTCCACCTGAAAACC 3′; reverse 5′ GAAGTCCAGTGAGGGTCCAA3′ (Romani et al. 2009)
rRPL13a: forward 5′ CCCTCCACCCTATGACAAGA3′; reverse 5′ CCTTTTCCTTCCGTTTCTCC3′ (Gaub et al. 2010)
rCYP1A1: forward 5′ ATGTCCAGCTCTCAGATGATAAGGTC′3; reverse 5′ ATCCCTGCCAATCACTGTGTCTAAC′3 (Vondracek et al. 2006)
rCYP2B1/2: forward 5′ TGGTGGAGGAACTGCGGAAATC′3; reverse 5′ TGATGCACTGGAAGAGGAAGGT′3(Saito et al. 2010)
rApoA1: forward 5′ CCTGGATGAATTCCAGGAGA′3; reverse 5′ TCGCTGTAGAGCCCAAACTT′3 (Boesch-Saadatmandi et al. 2009)
rAhR: forward 5′ GGGCCAAGAGCTTCTTTGATG′3; reverse 5′ GCAAGTCCTGCCAGTCTCTGA′3 (Shipley & Waxman 2006)
The PCR program used started with 95° for 10 min followed by 40 cycles of 95° for 30 s, 55° for 30 s and 72° for 1 min performed on an Eppendorf RealPlex2 Mastercycler® (Hamburg, Germany). A melting curve analysis was carried out after the reaction to check for false amplification caused by primer dimers, non-specific binding or other contamination. The relative gene expression levels were calculated using the relative standard curve method. The target gene expression levels were adjusted to a house keeping gene, ribosomal protein L13a (RPL13a). Final results were presented as fold changes in which the adjusted expression level of each target gene in the treatment groups was divided by that in the corn oil control group.
Statistics
All data are expressed as means ± SEM. Statistical analysis was performed by one-way ANOVA General Linear Models (GLM) in SAS 9.2, (P<0.01), followed by Dunnett’s T comparison test. A P value of <0.05 was considered to be statistically significant.
Results
Effects of higher chlorinated PCBs on PON1 activities in rats
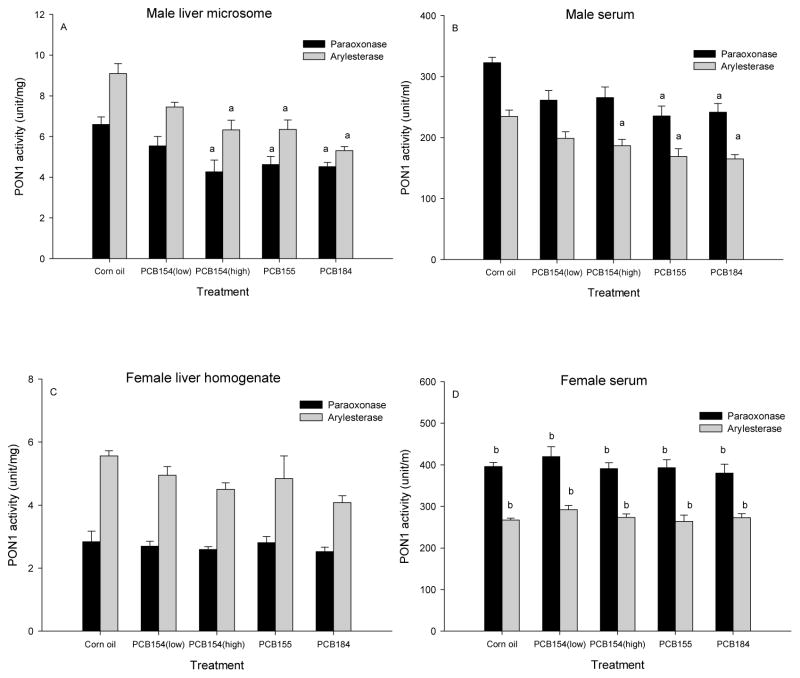
Two hexa- (PCBs 154 and 155) and one hepta- (PCB 184) chlorinated biphenyl with 3 or 4 chlorines in the ortho-positions were analyzed for effects on liver and serum PON1 activities in rats. As shown in Fig 1A, all three congeners significantly reduced liver microsomal PON1 activities, determined with two substrates, paraoxon and phenylacetate, after two injections of 100 μmol/kg each. The effect with the lower dose of PCB 154 (2 × 50 μmol/kg) did not reach significance. Fig 1B shows similar significant decreases in serum PON1 activities. In contrast, no significant change of PON1 activity in either liver homogenate (Fig 1C) or serum (Fig 1D) were found in female rats. PON1 serum activities in female were significantly (P<0.05) higher compared to those in male control and treatment rats. PON1 levels in female liver homogenate were similar to those in male control liver homogenates (Fig 2A, Fig 3A). PON1 levels per g protein were lower in liver homogenate (Fig 1C) than in male liver microsomes (Fig 1A). The corresponding female liver microsomes and male liver homogenates were unfortunately not available, but the similarity pattern with the two substrates suggested that both provided comparable results and therefore homogenate was used in all following studies. Also, since males were the more sensitive gender, all following studies were performed using males only.
Figure 1. Highly ortho- chlorinated PCBs 154, 155, and 184 reduce PON1 activity in rat liver and serum.
Male and female rats received injections of PCB154 low: 2× 50 μmol/kg, PCB154 high: 2× 100 μmol/kg, PCB155: 2× 100 μmol/kg), PCB184: 2× 100 μmol/kg. Results are expressed as mean ± SEM (n=6), Statistical significance: P<0.05, a. treatment vs corn oil, b. female vs corresponding male (serum only).
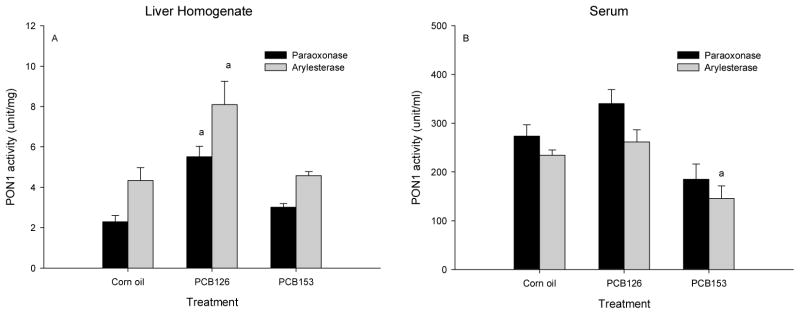
Figure 2. PCB 126 increased liver PON1 and PCB 153 lowered the serum PON1 activity.
Male rats received one ip injection of PCB 153 (100 μmol/kg) or PCB 126 (5 μmol/kg) and were euthanized 6 days later. Results are expressed as mean ± SEM (n=4), a. significance (p<0.05) of treatment vs corn oil.
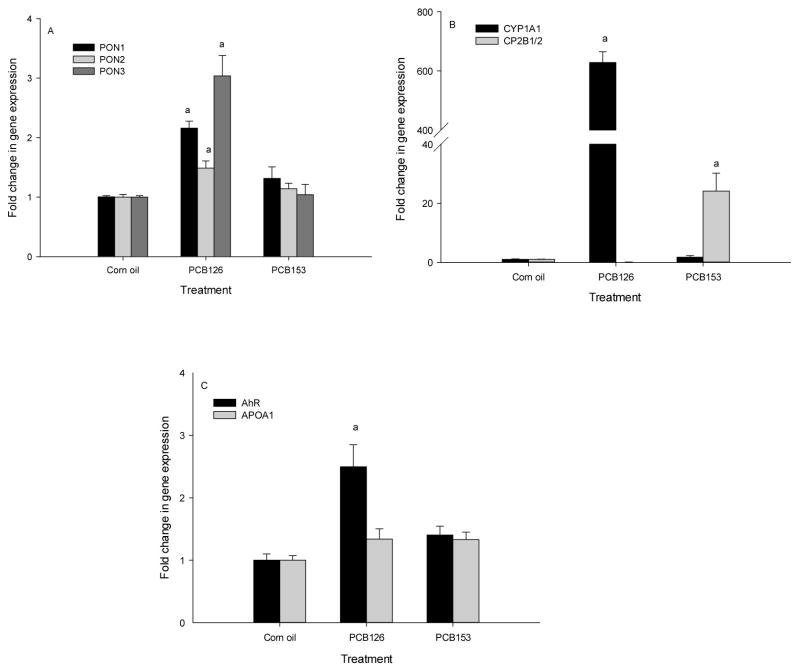
Figure 3. PCB 126 increased liver gene expression of PON1, 2, 3, and AhR while PCB 153 had no effect on these genes.
Male rats were treated with PCB 153 (100 μmol/kg) or PCB 126 (5 μmol/kg). Results are expressed as mean ± SEM (n=4), a: significance (P<0.05) of treatment vs corn oil.
Comparison of effects of dioxin- and non-dioxin-like PCBs on PON1 enzyme activity and PON 1/2/3 and CYP gene expression
To compare the effect of ortho-substituted non-dioxin-like PCBs with those of a dioxin-like PCB, PON1 activities in the liver homogenate and serum from male rats, injected with a single dose of either PCB 126 (dioxin-like) or PCB 153 (non-dioxin-like-PCB) and killed 7 days later were determined. Liver PON1 activities were significantly elevated by PCB 126, while PCB 153 treatment did not produce any significant change (Fig 2A). Serum PON1 activities were higher, but not significantly increased by PCB 126. However, a significant (P<0.05) decrease of PON1 arylesterase activity and visible decrease in paraoxonase activity in serum was seen with PCB 153 (Fig 2B).
We further analyzed the effects of PCB 153 and PCB 126 on gene expression of paraoxonases and other genes in male rat livers by quantitative RT-PCR. PCB 126 significantly induced liver PON1, PON2 and PON3 gene expression by about 2-, 1.5- and 3-fold, respectively, but no effect was detected with PCB 153 on any PON gene (Fig 3A). Exclusive induction of liver CYP1A1 and CYP2B1/2 genes by PCB 126 and PCB 153, respectively (Figs 3B) indicate that both AhR and CAR were activated in liver. PCB 126 but not PCB 153 also upregulated AhR mRNA levels (Fig 3C). No increase of APOA1 gene expression was observed with either congener (Fig 3C).
Examination of PON 1 gene expression and enzyme activity after exposure to different AHR ligands
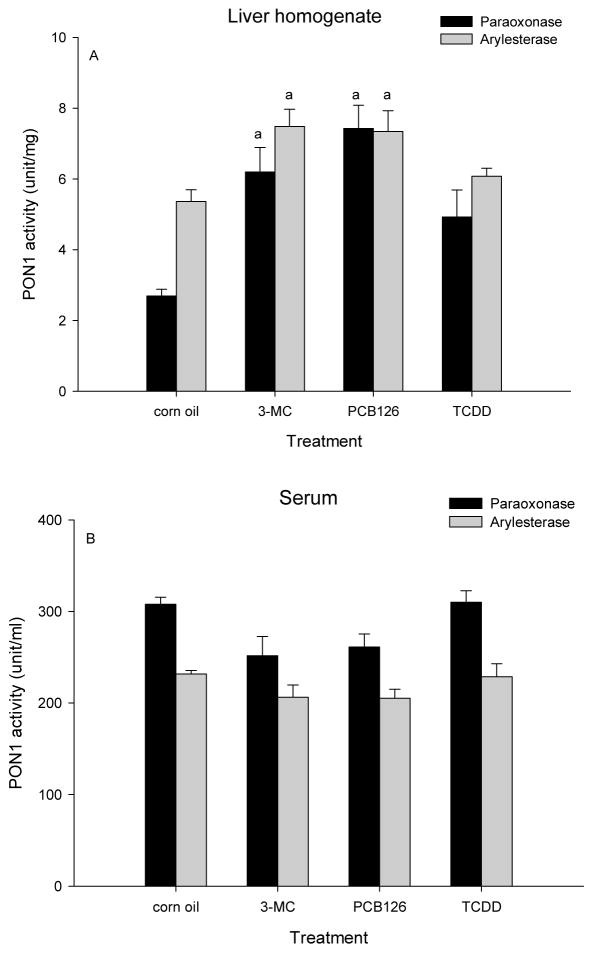
To evaluate whether PON induction is a general activity of AhR ligands, male rats were treated with three different types of AhR agonists by a single injection of PCB 126 or TCDD, or 3 daily injections of 3-MC and were killed as described. 3-MC and PCB 126 significantly increased PON1 activities measured with two different substrates (Fig 4A). TCDD caused a marginal increase which was not significant. Serum PON1 activities did not show significant changes with any of the AhR ligands (Fig 4B).
Figure 4. 3-MC and PCB 126, but not TCDD increased liver PON1 activity.
Male rats received ip injections of 3-MC (3 daily injections of 300 μmol/kg), PCB 126 (1× 5 μmol/kg) or TCDD (1× 0.16 μmol/kg) and were killed on day 4. Results are expressed as mean ± SEM (n=2–3). a. significant differences (P<0.05) of treatment vs corn oil.
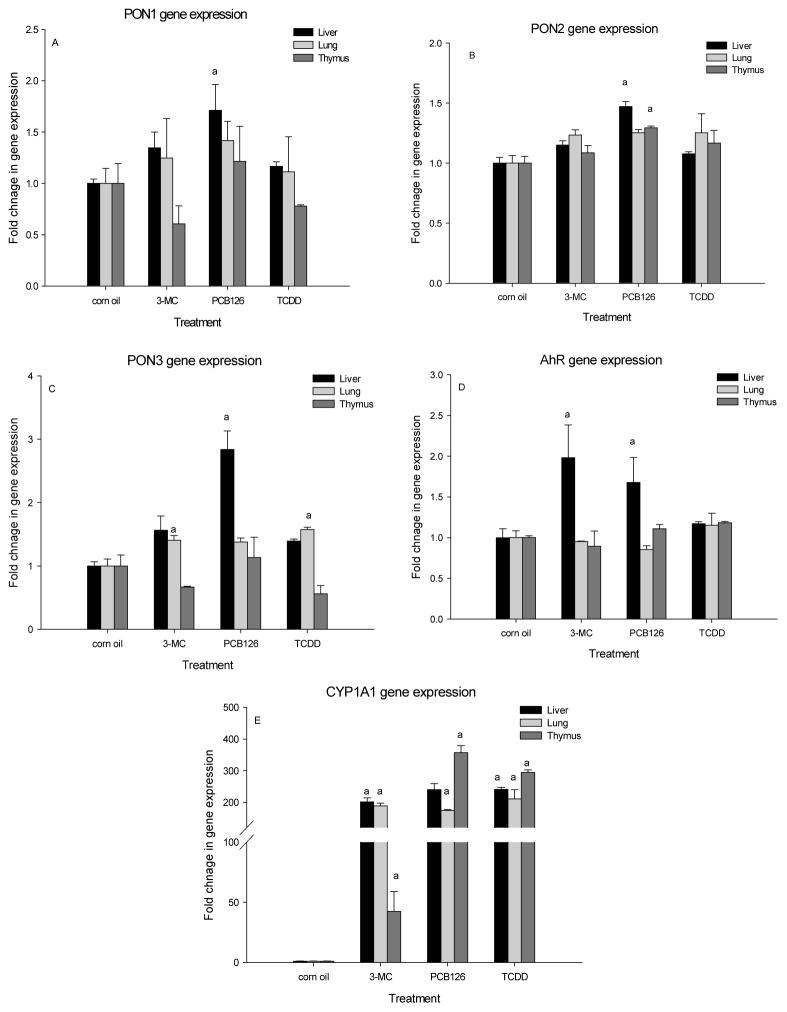
Fig 5A shows the PON1 gene expression in response to three different AhR ligands in liver, lung and thymus of male rats. Although a small increase in PON 1, 2, and 3 was seen in liver and lung with all three ligands, most of these effects were not statistically significant (Fig 5A-C). PON1 mRNA levels were significantly increased only by PCB 126 and only in the liver (Fig 5A). PCB 126 also significantly increased PON 2 in liver and thymus and PON 3 in liver (Fig 5B, 5C). 3-MC and TCDD even caused non-significant decreases of PON1 and PON 3 gene expression in the thymus, but they significantly increased PON 3 mRNA in the lung (Fig 5C). Interestingly, the AhR mRNA was significantly increased in liver by both 3-MC and PCB 126 (Fig 5D). The classical AhR inducible gene, CYP1A1, was upregulated about 200 – 300 fold by all three ligands in liver, lung and thymus, except with 3-MC in the thymus, where the increase was only 50-fold (Fig 5E).
Figure 5. Different AhR agonists (3-MC, PCB 126 and TCDD) have varying effect on PON1, PON2, PON3 gene expression in liver, lung, and thymus.
Male rats received 3-MC (3× 300 μmol/kg), PCB 126 (1× 5 μmol/kg) or TCDD (1× 0.16 μmol/kg). Results are expressed as mean ± SEM (n=2–3), a. significant (P<0.05) effect of treatment vs corn oil.
Discussion
Paraoxonases are important antioxidant enzymes in tissues and blood and our previous studies indicated that at least one PCB congener, PCB126, the most potent AhR agonist among all PCBs, can influence the PON1 gene expression and enzyme activity levels in rats (Shen et al. 2014, Shen et al. 2012). This raised two questions: 1. Are all PCB congeners inducers of PON1 activity and 2. If this is an AhR-related activity, can we see this effect with other AhR agonists?
We first examined whether other PCB congeners that are not dioxin-like and not AhR agonists would also induce PON1. To exclude potential effects due to weak AhR binding or metabolism, we chose 3 highly chlorinated congeners with 3 or 4 chlorines in the ortho-position, i.e. PCB 154, 155 and 184. These three congeners not only did not significantly increase PON1 activities in either male or female rats, but significantly reduced PON 1 enzyme activities in the livers and serum of male rats, but not female rats. The observation that the highly ortho-chlorinated congeners PCB 154, 155 and 184 not only did not increase, but actually reduced PON1 activity in male liver and serum, showed that these non-dioxin-like congeners act through a completely different mechanism with opposite effects compared to PCB126. Inflammation and pro-inflammatory cytokines such as IL-1β and TNF-α were reported to decrease PON1 activity by down-regulating PON1 expression in animals and in cells in culture (Feingold et al. 1998, Han et al. 2006, Kumon et al. 2002). Human clinical studies found that serum PON1 activity was exhausted in patients maintaining systemic low-grade inflammation (Kotur-Stevuljevic et al. 2008). We observed that male rats, but not females, treated with PCB154, PCB155 or PCB184 seemed to have higher thymus weights (data not shown and not confirmed in a second experiment), suggesting similar inflammatory actions as the mechanism of action of these congeners. Alternatively, various studies have demonstrated that PON1 activity was diminished by oxidative stress (Precourt et al. 2011). Studies indicate that PON’s protection against lipid oxidation is accompanied by partial inactivation of the enzyme, possibly through an interaction between the enzyme’s free sulfhydryl group and oxidized lipids (Aviram et al. 1999). Similarly, Fe/ascorbic acid-induced lipid peroxidation was quenched by PON1, resulting in decreased PON1 activity (Trudel et al. 2005). Thus the decreased PON1 activities in the liver and serum in male rats treated with PCB184, PCB 155 or PCB 154 may be due to oxidative inactivation as described above.
The regulation of PON1 activities involves both genetic and environmental factors. An interesting observation in our study was that the reduction in PON1 activity by these PCBs was only visible in male rats, not females. Our female rats had significantly higher constitutive PON1 activity levels in serum and liver compared to male rats. Other studies also reported that the PON1 gene is regulated in a gender-specific manner, with lower levels of serum PON1 activity and liver mRNA levels in male mice compared to females (bin Ali et al. 2003). In rats, serum PON1 protein and activity levels were shown to be about 30% higher in females than in males (Thomas-Moya et al. 2006, Thomas-Moya et al. 2008). This is similar to the about 15% (arylesterase) to 25% (paraoxonase) higher serum PON1 activity levels in females observed in our study. Liver PON1 activities were measured in homogenates in females and microsomes in males in this study, therefore absolute activity values are not comparable. However, male liver homogenate PON1 activity levels were always 10–30% lower than female levels in the following experiments (Fig 2A and 4A and other unpublished data). Similarly women have higher serum PON1 activities than men (Mueller et al. 1983). This higher baseline level may be the reason for the sex difference in effects in our study and suggests that men may be more vulnerable to attack on this antioxidant enzyme than women.
Lower doses of PCB 154 had no effect on PON1 in liver and serum in male rats, and female rats with their higher baseline PON1 activities showed only a marginal reduction in PON1 activity, suggesting that the effect of these PCB congeners is dose-dependent, opening the question whether additional dietary antioxidants could provide sufficient protection to avoid negative health effects. However this potential chemoprotection option needs to be carefully approached as our results with N-acetyl cysteine and selenium in PCB126 exposed rats have shown (Shen et al. 2014).
PCB154, 155, and 184 are considered agonists of the nuclear pregnane X receptor (PXR) s and inducers of CYP3A23 (Schuetz et al. 1998, Schuetz et al. 1986). In our next experiment we therefore analyzed the actions of a typical CAR agonist, PCB153 (2,2′,4,4′,5,5′-hexachlorobiphenyl; 2 ortho-chlorines) and compared them with those of a strong AhR agonist, PCB126 (3,3′,4,4′,5-pentachlorobiphenyl). Our results clearly reveal that the regulation of the PON1 gene is highly structure-dependent and possibly at least partially receptor mediated. Significant induction of CYP1A1 and CYP2B1/2 genes by PCB 126 and PCB 153, respectively, suggests that these two ligands were activating their specific receptors, AhR and CAR. PCB 153, a non AhR ligand, did not upregulate CYP1A and had no effect on liver PON1 gene expression, while PCB 126 up-regulated both, PON1 and CYP1A, in agreement with our hypothesis that the mechanism of PON1 upregulated is AhR dependent (Shen and Ludewig, unpublished results). The effect of PCB 153 and PCB 126 on liver gene expression highly correlated with liver PON1 activities, which indicates the modulation of activity was mainly due to alteration in liver gene expression of PON1. The dose of PCB 153 was only half (1 injection of 100 μmol/kg instead of 2) of that of the above discussed congeners so that no direct dose-effect comparison can be made. However, we did observe a significant decrease in serum PON1 activity with PCB 153. As discussed above, oxidative inactivation may be responsible for this effect (Fadhel et al. 2002). Since PCB 153 is also an agonist of PXR (Kopec et al. 2010), it cannot be distinguished whether the effect of PCB 153 on serum PON1 activity in these male rats was due to the activation of CAR- or PXR. PCB 126 is also able of inducing oxidative stress (Aqil et al. 2014), but the concomitant induction of PON1 gene expression and protein synthesis may counteract and/or overwhelm the inactivation effect resulting in an overall increase in PON1 activity due to PCB 126 exposure. This may be a natural antioxidant response to the oxidative stress. It remains to be determined whether this increase through PON1 induction is maintained during extending exposure times or higher doses. Under such circumstances, the exposure may produce damage that exceeds the body’s antioxidant resistance capacity.
In addition, PCB 126 induced PON2 and PON3, as well as the AhR gene, consistent with our previous study (Shen et al. 2012). In fact, considerably lower concentrations of PCB126, i.e. less than 1 μg/day administered for up to 45 days through subcutaneous implants (roughly 10 nmol/kg/day for male and 17 nmol/kg/day for female rats), produced significant gene induction of PON1, PON3 and AhR and PON1 activity increase (Aqil et al. 2014). This suggests that even very small amounts of AhR agonists may increase antioxidant protection by paraoxonases in liver and blood and other organs.
Induction of AhR transcription by AhR agonists has been reported before, for example for PCB 77, another AhR agonist (Mortensen & Arukwe 2008). But the mechanism of PON induction by AhR agonists is not clear. Existence of XRE-like sequence in rat PON promoter regions may explain the induction by AhR ligands such as PCB 126. We therefore decided to examine other AhR agonists for PON-inducing activity and to include the lung and thymus as potential target organs. Based on the similar induction capability towards the CYP1A1 gene, three AhR ligands were tested. However, these three ligands displayed differing characteristics, especially with respect to the induction of PON1, PON3 and AhR and tissue-specificity of effects. PCB 126 was the most potent and efficacious inducer of liver PON1, PON2 and PON3 as well as AhR gene. TCDD only weakly increased the expression of the genes tested, except for CYP1A1. 3-MC showed strong induction of the liver AhR gene, as did PCB 126. Our data that induction profiles of the AhR ligands are distinct with respect to the CYP1A1 and the PON 1 expression levels support the hypothesis of an involvement of AhR, since the PON genes only have XRE-like core sequences, whereas the CYP1A1 gene contains several consensus XREs. Therefore, the binding intensity of AhR to these response elements may well be different. Our results are also in agreement with the findings of a previous study (Gouedard et al. 2004). The finding that TCDD was only a very weak PON1 inducer compared to PCB126 may in part be explained by the much lower dose (0.16 vs 5 μmol/kg), but could also indicate that additional other, non-AhR-related pathways may be involved in the strong PON1-induction by PCB126. The effects on PON gene transcription were also tissue-dependent. By comparison, the liver genes showed the highest sensitivity in response to the AhR ligands for all three PON genes, but TCDD and 3-MC also increase PON2 expression in the lung and PCB126 the expression of PON2 in the thymus. These results suggest that more factors than the XRE-like sequence in the promoter determine the gene regulation of PON genes, resulting in compound, tissue, and gene-specific variability.
The most prevalent PCB congeners found in human blood and tissues are non-coplanar, with PCB 153 usually at the highest rate (Herrick et al. 2011, Marek et al. 2014). This would suggest that PON1 serum levels may be reduced in highly exposed individuals causing increased risk of adverse health effects. Indeed, recent human studies revealed an inverse relationship between serum PCB levels and PON 1 arylesterase activity and between decreased arylesterase activity and increased PCB levels and cardio-vascular disease (Ljunggren et al. 2014). On the other hand we could show that AhR agonists increase not only PON1 but also PON 2 and PON3. A full understanding of the tissue-specific regulatory factors of PON1/2/3 gene expression could open the door to the design of organ-specific PON inducers for chemoprotection and for medical applications.
Conclusions
These studies show that non-dioxin-like PCBs, in contrast to dioxin-like compounds, reduce PON1 activities, which may be due to oxidative inactivation. The up-regulation of all 3 PON genes in the livers appears to be AhR mediated, but the different induction capacities of various AhR ligands in different tissues, despite similar liver CYP 1A induction levels, suggest an influence of the different promoter sequence compared to the classical XRE binding site. In addition, differential recruitment of co-activators and/or modifications of the DNA superstructure may also be responsible for the variations in impact on transcription efficiency. This indicates that a better understanding of the effects of environmental chemicals on PON regulation and activity in liver, blood, and extrahepatic tissues is needed if we want to protect human health.
Acknowledgments
The authors thank members of the Ludewig and Robertson laboratories for help with the animal studies and special thanks go Dr. Bingxuan Wang for sharing the liver RNA samples. This study and HS were funded by Project 1 and the Training Core of the NIEHS-supported Iowa Superfund Research Program (P42 ES013661). Support is also recognized by NIEHS P30 ES05605. This work is part of the doctoral thesis of HS. The results, conclusions and opinions expressed here are solely those of the authors.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- Aqil F, Shen H, Jeyabalan J, Xin X, Lehmler H-J, Ludewig G, Robertson LW, Gupta RC. Sustained expression of CYPs and DNA adduct accumulation with continuous exposure to PCB126 and PCB153 through a new delivery method: Polmeric implants. Toxicology Reports. 2014;1 doi: 10.1016/j.toxrep.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviram M, Rosenblat M, Billecke S, Erogul J, Sorenson R, Bisgaier CL, Newton RS, La Du B. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic Biol Med. 1999;26:892–904. doi: 10.1016/s0891-5849(98)00272-x. [DOI] [PubMed] [Google Scholar]
- Aviram M, Rosenblat M. Paraoxonases (PON1, PON2, PON3) analyses in vitro and in vivo in relation to cardiovascular diseases. Methods Mol Biol. 2008;477:259–76. doi: 10.1007/978-1-60327-517-0_20. [DOI] [PubMed] [Google Scholar]
- Beltowski J, Jamroz-Wisniewska A, Borkowska E, Wojcicka G. Differential effect of antioxidant treatment on plasma and tissue paraoxonase activity in hyperleptinemic rats. Pharmacol Res. 2005;51:523–32. doi: 10.1016/j.phrs.2005.01.007. [DOI] [PubMed] [Google Scholar]
- bin Ali A, Zhang Q, Lim YK, Fang D, Retnam L, Lim SK. Expression of major HDL-associated antioxidant PON-1 is gender dependent and regulated during inflammation. Free Radic Biol Med. 2003;34:824–9. doi: 10.1016/s0891-5849(02)01436-3. [DOI] [PubMed] [Google Scholar]
- Boesch-Saadatmandi C, Pospissil RT, Graeser AC, Canali R, Boomgaarden I, Doering F, Wolffram S, Egert S, Mueller MJ, Rimbach G. Effect of quercetin on paraoxonase 2 levels in RAW264.7 macrophages and in human monocytes--role of quercetin metabolism. Int J Mol Sci. 2009;10:4168–77. doi: 10.3390/ijms10094168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Vitalone A, Cole TB, Furlong CE. Modulation of paraoxonase (PON1) activity. Biochem Pharmacol. 2005;69:541–50. doi: 10.1016/j.bcp.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Fadhel Z, Lu Z, Robertson LW, Glauert HP. Effect of 3,3′,4,4′-tetrachlorobiphenyl and 2,2′,4,4′,5,5′-hexachlorobiphenyl on the induction of hepatic lipid peroxidation and cytochrome P-450 associated enzyme activities in rats. Toxicology. 2002;175:15–25. doi: 10.1016/s0300-483x(02)00086-0. [DOI] [PubMed] [Google Scholar]
- Farid AS, Mido S, Linh BK, Hayashi T, Horii Y. An atherogenic lipid profile with low serum paraoxonase-1 activity during nematode infection in rats. Eur J Clin Invest. 2010;40:984–93. doi: 10.1111/j.1365-2362.2010.02352.x. [DOI] [PubMed] [Google Scholar]
- Feingold KR, Memon RA, Moser AH, Grunfeld C. Paraoxonase activity in the serum and hepatic mRNA levels decrease during the acute phase response. Atherosclerosis. 1998;139:307–15. doi: 10.1016/s0021-9150(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Furlong CE, Cole TB, Jarvik GP, Pettan-Brewer C, Geiss GK, Richter RJ, Shih DM, Tward AD, Lusis AJ, Costa LG. Role of paraoxonase (PON1) status in pesticide sensitivity: genetic and temporal determinants. Neurotoxicology. 2005;26:651–9. doi: 10.1016/j.neuro.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Gaub P, Tedeschi A, Puttagunta R, Nguyen T, Schmandke A, Di Giovanni S. HDAC inhibition promotes neuronal outgrowth and counteracts growth cone collapse through CBP/p300 and P/CAF-dependent p53 acetylation. Cell Death Differ. 2010;17:1392–408. doi: 10.1038/cdd.2009.216. [DOI] [PubMed] [Google Scholar]
- Goswami B, Tayal D, Gupta N, Mallika V. Paraoxonase: a multifaceted biomolecule. Clin Chim Acta. 2009;410:1–12. doi: 10.1016/j.cca.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Gouedard C, Barouki R, Morel Y. Dietary polyphenols increase paraoxonase 1 gene expression by an aryl hydrocarbon receptor-dependent mechanism. Mol Cell Biol. 2004;24:5209–22. doi: 10.1128/MCB.24.12.5209-5222.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CY, Chiba T, Campbell JS, Fausto N, Chaisson M, Orasanu G, Plutzky J, Chait A. Reciprocal and coordinate regulation of serum amyloid A versus apolipoprotein A-I and paraoxonase-1 by inflammation in murine hepatocytes. Arterioscler Thromb Vasc Biol. 2006;26:1806–13. doi: 10.1161/01.ATV.0000227472.70734.ad. [DOI] [PubMed] [Google Scholar]
- Hennig B, Hammock BD, Slim R, Toborek M, Saraswathi V, Robertson LW. PCB-induced oxidative stress in endothelial cells: modulation by nutrients. International journal of hygiene and environmental health. 2002;205:95–102. doi: 10.1078/1438-4639-00134. [DOI] [PubMed] [Google Scholar]
- Hennig B, Reiterer G, Majkova Z, Oesterling E, Meerarani P, Toborek M. Modification of environmental toxicity by nutrients: implications in atherosclerosis. Cardiovasc Toxicol. 2005;5:153–60. doi: 10.1385/ct:5:2:153. [DOI] [PubMed] [Google Scholar]
- Herrick RF, Meeker JD, Altshul L. Serum PCB levels and congener profiles among teachers in PCB-containing schools: a pilot study. Environmental health : a global access science source. 2011;10:56. doi: 10.1186/1476-069X-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestermann EV, Stegeman JJ, Hahn ME. Relative contributions of affinity and intrinsic efficacy to aryl hydrocarbon receptor ligand potency. Toxicol Appl Pharmacol. 2000;168:160–72. doi: 10.1006/taap.2000.9026. [DOI] [PubMed] [Google Scholar]
- Kopec AK, Burgoon LD, Ibrahim-Aibo D, Mets BD, Tashiro C, Potter D, Sharratt B, Harkema JR, Zacharewski TR. PCB153-elicited hepatic responses in the immature, ovariectomized C57BL/6 mice: comparative toxicogenomic effects of dioxin and non-dioxin-like ligands. Toxicology and applied pharmacology. 2010;243:359–71. doi: 10.1016/j.taap.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotur-Stevuljevic J, Spasic S, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V, Stefanovic A, Vujovic A, Memon L, Kalimanovska-Ostric D. PON1 status is influenced by oxidative stress and inflammation in coronary heart disease patients. Clin Biochem. 2008;41:1067–73. doi: 10.1016/j.clinbiochem.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Kumon Y, Nakauchi Y, Suehiro T, Shiinoki T, Tanimoto N, Inoue M, Nakamura T, Hashimoto K, Sipe JD. Proinflammatory cytokines but not acute phase serum amyloid A or C-reactive protein, downregulate paraoxonase 1 (PON1) expression by HepG2 cells. Amyloid. 2002;9:160–4. doi: 10.3109/13506120209114817. [DOI] [PubMed] [Google Scholar]
- Ljunggren SA, Helmfrid I, Salihovic S, van Bavel B, Wingren G, Lindahl M, Karlsson H. Persistent organic pollutants distribution in lipoprotein fractions in relation to cardiovascular disease and cancer. Environment international. 2014;65:93–9. doi: 10.1016/j.envint.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Ludewig G, Lehmann L, Esch H, Robertson LW. Metabolic Activation of PCBs to Carcinogens in Vivo - A Review. Environmental toxicology and pharmacology. 2008;25:241–6. doi: 10.1016/j.etap.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthe G, Jacobus JA, Robertson LW. Receptor interactions by polybrominated diphenyl ethers versus polychlorinated biphenyls: a theoretical Structure-activity assessment. Environ Toxicol Pharmacol. 2008;25:202–10. doi: 10.1016/j.etap.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthe GM, Schut BG, Aaseng JE. Monofluorinated analogues of polychlorinated biphenyls (F-PCBs) synthesis using the Suzuki-coupling, characterization, specific properties and intended use. Chemosphere. 2009;77:1242–8. doi: 10.1016/j.chemosphere.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Marek RF, Thorne PS, DeWall J, Hornbuckle KC. Variability in PCB and OH-PCB serum levels in children and their mothers in urban and rural U.S. communities. Environ Sci Technol. 2014;48:13459–67. doi: 10.1021/es502490w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen AS, Arukwe A. Estrogenic effect of dioxin-like aryl hydrocarbon receptor (AhR) agonist (PCB congener 126) in salmon hepatocytes. Mar Environ Res. 2008;66:119–20. doi: 10.1016/j.marenvres.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Mueller RF, Hornung S, Furlong CE, Anderson J, Giblett ER, Motulsky AG. Plasma paraoxonase polymorphism: a new enzyme assay, population, family, biochemical, and linkage studies. Am J Hum Genet. 1983;35:393–408. [PMC free article] [PubMed] [Google Scholar]
- Precourt LP, Amre D, Denis MC, Lavoie JC, Delvin E, Seidman E, Levy E. The three-gene paraoxonase family: physiologic roles, actions and regulation. Atherosclerosis. 2011;214:20–36. doi: 10.1016/j.atherosclerosis.2010.08.076. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sanabria F, Rull A, Beltran-Debon R, Aragones G, Camps J, Mackness B, Mackness M, Joven J. Tissue distribution and expression of paraoxonases and chemokines in mouse: the ubiquitous and joint localisation suggest a systemic and coordinated role. J Mol Histol. 2010;41:379–86. doi: 10.1007/s10735-010-9299-x. [DOI] [PubMed] [Google Scholar]
- Romani R, De Medio GE, di Tullio S, Lapalombella R, Pirisinu I, Margonato V, Veicsteinas A, Marini M, Rosi G. Modulation of paraoxonase 1 and 3 expression after moderate exercise training in the rat. J Lipid Res. 2009;50:2036–45. doi: 10.1194/jlr.M800493-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S, Bandiera S, Sawyer T, Robertson L, Safe L, Parkinson A, Thomas PE, Ryan DE, Reik LM, Levin W, et al. PCBs: structure-function relationships and mechanism of action. Environ Health Perspect. 1985;60:47–56. doi: 10.1289/ehp.856047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Kobayashi K, Mizuno Y, Fukuchi Y, Furihata T, Chiba K. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonists induce constitutive androstane receptor (CAR) and cytochrome P450 2B in rat primary hepatocytes. Drug Metab Pharmacokinet. 2010;25:108–11. doi: 10.2133/dmpk.25.108. [DOI] [PubMed] [Google Scholar]
- Schramm H, Robertson LW, Oesch F. Differential regulation of hepatic glutathione transferase and glutathione peroxidase activities in the rat. Biochem Pharmacol. 1985;34:3735–9. doi: 10.1016/0006-2952(85)90239-4. [DOI] [PubMed] [Google Scholar]
- Schuetz EG, Wrighton SA, Safe SH, Guzelian PS. Regulation of cytochrome P-450p by phenobarbital and phenobarbital-like inducers in adult rat hepatocytes in primary monolayer culture and in vivo. Biochemistry. 1986;25:1124–33. doi: 10.1021/bi00353a027. [DOI] [PubMed] [Google Scholar]
- Schuetz EG, Brimer C, Schuetz JD. Environmental xenobiotics and the antihormones cyproterone acetate and spironolactone use the nuclear hormone pregnenolone X receptor to activate the CYP3A23 hormone response element. Mol Pharmacol. 1998;54:1113–7. doi: 10.1124/mol.54.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Robertson LW, Ludewig G. Regulation of paraoxonase 1 (PON1) in PCB 126-exposed male Sprague Dawley rats. Toxicology letters. 2012;209:291–8. doi: 10.1016/j.toxlet.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Li M, Wang B, Lai IK, Robertson LW, Ludewig G. Dietary antioxidants (selenium and N-acetylcysteine) modulate paraoxonase 1 (PON1) in PCB 126-exposed rats. Environ Sci Pollut Res Int. 2014;21:6384–99. doi: 10.1007/s11356-013-1690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley JM, Waxman DJ. Aryl hydrocarbon receptor-independent activation of estrogen receptor-dependent transcription by 3-methylcholanthrene. Toxicol Appl Pharmacol. 2006;213:87–97. doi: 10.1016/j.taap.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Thomas-Moya E, Gianotti M, Llado I, Proenza AM. Effects of caloric restriction and gender on rat serum paraoxonase 1 activity. J Nutr Biochem. 2006;17:197–203. doi: 10.1016/j.jnutbio.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Thomas-Moya E, Gomez-Perez Y, Fiol M, Gianotti M, Llado I, Proenza AM. Gender related differences in paraoxonase 1 response to high-fat diet-induced oxidative stress. Obesity (Silver Spring) 2008;16:2232–8. doi: 10.1038/oby.2008.340. [DOI] [PubMed] [Google Scholar]
- Trudel K, Sinnett D, James RW, Delvin E, Amre D, Seidman E, Levy E. Iron-ascorbic acid-induced oxidant stress and its quenching by paraoxonase 1 in HDL and the liver: comparison between humans and rats. J Cell Biochem. 2005;96:404–11. doi: 10.1002/jcb.20542. [DOI] [PubMed] [Google Scholar]
- Varatharajalu R, Garige M, Leckey LC, Gong M, Lakshman MR. Betaine protects chronic alcohol and omega-3 PUFA-mediated down-regulations of PON1 gene, serum PON1 and homocysteine thiolactonase activities with restoration of liver GSH. Alcohol Clin Exp Res. 2009;34:424–31. doi: 10.1111/j.1530-0277.2009.01107.x. [DOI] [PubMed] [Google Scholar]
- Vondracek J, Svihalkova-Sindlerova L, Pencikova K, Krcmar P, Andrysik Z, Chramostova K, Marvanova S, Valovicova Z, Kozubik A, Gabelova A, Machala M. 7H-Dibenzo[c,g]carbazole and 5,9-dimethyldibenzo[c,g]carbazole exert multiple toxic events contributing to tumor promotion in rat liver epithelial ‘stem-like’ cells. Mutat Res. 2006;596:43–56. doi: 10.1016/j.mrfmmm.2005.11.005. [DOI] [PubMed] [Google Scholar]