Abstract
Objective
We investigated the effect of weight loss after bariatric surgery among patients with rheumatoid arthritis (RA).
Methods
We conducted a retrospective cohort study of RA patients who underwent bariatric surgery (Roux-en-Y gastric bypass, laparoscopic adjustable gastric banding, or sleeve gastrectomy) at two medical centers. We obtained anthropometrics, laboratory values, RA disease activity, and medication use at baseline (prior to surgery), at six and twelve months post-surgery, and at most recent follow-up visits. RA disease activity was determined by clinical or validated measures. At each post-surgical visit, characteristics were compared to baseline.
Results
We identified 53 RA patients who underwent bariatric surgery. At baseline prior to surgery, mean body mass index was 47.8 kg/m2 (SD 7.7), mean weight was 128.2 kg (SD 24.1), and 57% had moderate/high RA disease activity. Twelve months post-surgery, subjects lost a mean of 41.0 kg (SD 17.3), 70% (SD 24) of excess weight (P<0.001). RA disease activity significantly improved at post-surgical visits (P<0.001). At 12 months post-surgery, 6% had moderate/high disease activity compared to 57% at baseline (P<0.001). At most recent follow-up (mean 5.8 years [SD 3.2] after surgery), 74% were in remission compared to 26% at baseline (P<0.001). Subjects had significantly lower erythrocyte sedimentation rate, C-reactive protein, and RA-related medication use at follow-up visits compared to baseline (P<0.05).
Conclusion
After substantial weight loss from bariatric surgery, RA patients had lower disease activity, decreased serum inflammatory markers, and less RA-related medication usage. Weight loss may be an important non-pharmacologic strategy to reduce RA disease activity. However, other factors, such as improved efficacy of medications, improved physical activity, and metabolic changes, may also have contributed to these post-surgical improvements.
Introduction
Obesity, defined as a body mass index (BMI) greater than 30 kg/m2, is a serious public health problem that has been associated with many chronic diseases, including hypertension, diabetes mellitus, coronary heart disease, and cancer (1). Additionally, obesity causes a systemic inflammatory state due in part due to the secretion of adipokines by adipocytes and is associated with an elevation of serum inflammatory markers, such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) (2, 3). Overweight and obesity are associated with an increased risk of incident rheumatoid arthritis (RA) as well as increased RA disease severity and decreased treatment efficacy (4-8).
Bariatric surgery causes substantial weight loss that has been associated with improvement, and sometimes remission, of chronic diseases such as diabetes mellitus and hypertension (9, 10). Weight loss after bariatric surgery decreases serum uric acid levels in patients with diabetes and reduces serum inflammatory markers in the obese (11-13). However, the effect of substantial weight loss after bariatric surgery on systemic inflammatory rheumatic diseases has not been studied.
RA is a systemic, inflammatory autoimmune disease that affects about 1% of the population and causes polyarthritis (14). Elevation of acute phase reactants, including ESR and CRP, often correlate with RA disease activity (15). Disease-modifying antirheumatic drugs (DMARDs) are the mainstay of RA treatment, while glucocorticoids and non-steroidal anti-inflammatory drugs (NSAIDs) are used for symptomatic relief (16). Non-pharmacologic treatments such as dietary changes, physical activity, and weight loss, typically play a secondary role in RA management; it is unclear whether these interventions modify RA disease activity (17).
In this retrospective cohort study, we aimed to determine the effect of weight loss after bariatric surgery on RA. We hypothesized that weight loss after bariatric surgery would improve RA disease activity, decrease serum markers of inflammation, and reduce RA-related medication usage.
Methods
Study sample and data source
We conducted a retrospective cohort study of RA patients who underwent bariatric surgery using electronic medical record (EMR) data. To identify potential subjects, we performed electronic queries in the Partners HealthCare Research Patient Database Registry among patients with clinical data at Brigham and Women's Hospital and Massachusetts General Hospital (both located in Boston, Massachusetts, USA) between 1993 and 2013. Bariatric surgery was defined as Roux-en-Y gastric bypass (current procedural terminology [CPT] codes 43644, 43645, or 43864), sleeve gastrectomy (CPT code 43775), laparoscopic adjustable gastric banding (CPT codes 43659 or 43770), or other absorption limiting gastric procedures (CPT codes 43842, 43843, 43845, or 43847). We reviewed EMR notes from three months prior to surgery to July 2014 among subjects with at least two billing codes for RA (714.0) according to the 9th edition of the International Classification of Diseases (ICD-9). All subjects met the 1987 American College of Rheumatology RA classification criteria at time of bariatric surgery (18). Subjects who had adequate clinical data available in the EMR before and after bariatric surgery were included in the study (see Figure 1 for flow diagram defining the analyzed study sample). All aspects of this study were approved by the Partners HealthCare Institutional Review Board.
Figure 1.
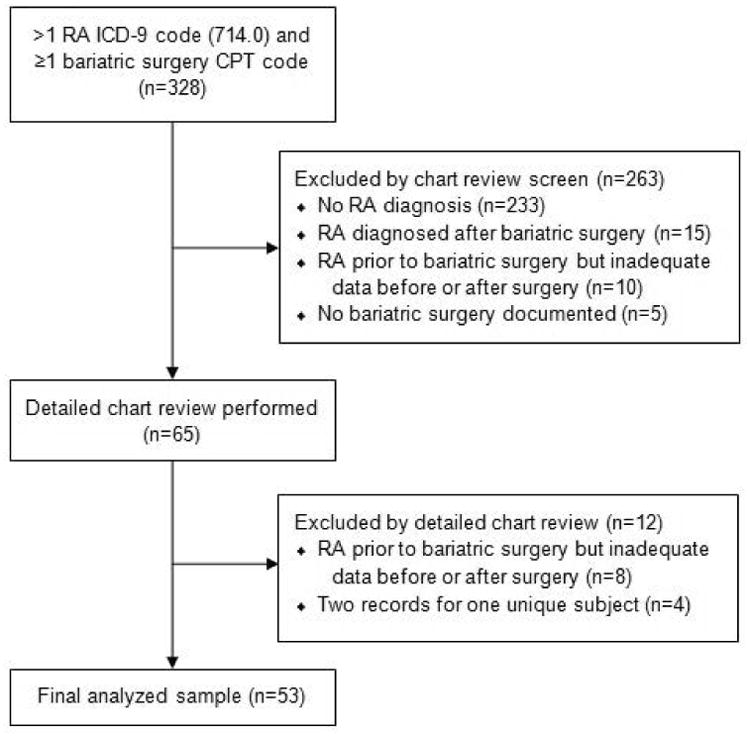
Flow diagram illustrating the identification of the final analyzed study sample (CPT, Current Procedural Terminology; ICD-9, International Classification of Diseases, 9th Revision; RA, rheumatoid arthritis).
Demographics, RA disease, anthropometrics, laboratory, and clinical measures
The index date was defined as the date of bariatric surgery. We collected data at the following time points: index date, baseline (closest clinical assessment prior to bariatric surgery), six months (±2) post-bariatric surgery, 12 months (±2) post-bariatric surgery, and most recent follow-up. Data were collected through routine medical care; medical records with assessments of joint signs, symptoms, and RA disease activity measures were prioritized for review. Data on demographics, comorbidities, and RA characteristics (serologic status, bone erosions on radiography, and disease duration) were evaluated as available at the index date.
BMI was calculated based on measured weight (in kilograms) divided by measured height (in meters-squared) (19). We calculated weight loss at each post-surgical time point, as well as percent excess weight loss (percent of baseline weight above BMI of 25 kg/m2 lost at each post-surgery point) (20). Laboratory values (CRP, ESR, white blood cell count [WBC], and platelet count) were measured in the routine clinical setting.
Two board-certified rheumatologists (JAS and BLB) reviewed medical records to determine RA disease activity, the second of whom was blinded to timing of bariatric surgery and amount of weight lost. RA disease activity was categorized according to accepted criteria into high, moderate, low, or remission (21, 22). Validated RA disease activity measures were available for 9/53 subjects at baseline and at least one follow-up point. Measures included: Disease Activity Score with 28-Joint Count with CRP (DAS28-CRP), DAS28-ESR, Clinical Disease Activity Index (CDAI), Simplified Disease Activity Index (SDAI), and Routine Assessment of Patient Index Data (RAPID).(23) Some subjects had RA disease activity measured by DAS28-CRP or CDAI in computerized modules designed for use in routine medical care (24). Since validated measures were unavailable in most patients, abstractors based assessment on patient symptoms, tender/swollen joint count, laboratory data, and treating clinician's global clinical assessment of RA disease activity according to an agreed upon a priori protocol (24). RA-related medications were collected at each time point and included DMARDs (further classified as biologic [bDMARD] or non-biologic [nbDMARD]), NSAIDs, and glucocorticoids.
Statistical analysis
We compared changes in anthropometrics, laboratory values, RA disease activity, and RA-related medication use between each post-surgical time point and baseline values for each subject using paired statistical tests. We used paired T-tests for normally distributed continuous variables, Wilcoxon signed-rank tests for non-normally distributed continuous variables, and McNemar's test for categorical variables. We compared measures at six and 12 months post-surgery and at most recent follow-up, to baseline measures. For all analyses, we considered a two-tailed P value <0.05 as statistically significant. Analyses were performed using IBM SPSS Statistics for Windows, Version 22.0 (Armonk, NY).
Results
We identified 53 RA patients who underwent bariatric surgery at two large academic medical centers with EMR data before and after surgery. Surgeries occurred between 2000 and 2012 for subjects in these analyses. The mean age at bariatric surgery was 47.9 years (standard deviation [SD] 10.5), 94% of subjects were female, and 72% were white (Table 1). Roux-en-Y gastric bypass was the most frequent type of bariatric surgery (81% of subjects). At bariatric surgery, all subjects were obese: mean weight was 128.2 kg (SD 24.1) and mean BMI was 47.9 kg/m2 (SD 7.7). The mean duration of RA was 8.6 years (SD 8.1), 51% were seropositive, and 40% had radiographic evidence of bone erosions. The most common comorbidities were osteoarthritis (26%), asthma (25%) and type 2 diabetes mellitus (25%).
Table 1.
Characteristics of subjects at bariatric surgery (N=53).
| Mean age, years (SD) | 47.9 (10.5) |
| Female sex, n (%) | 50 (94) |
| Race, n (%) | |
| White | 38 (72) |
| Black | 8 (15) |
| Hispanic | 7 (13) |
| Type of bariatric surgery, n (%) | |
| Roux-en-Y gastric bypass | 43 (81) |
| Laparoscopic adjustable gastric banding | 7 (13) |
| Sleeve gastrectomy | 3 (6) |
| Body mass index, kg/m2 (SD) | 47.9 (7.7) |
| Weight, kg (SD) | 128.4 (23.6) |
| WHO BMI category, n (%) | |
| Normal (18.5-24.9 kg/m2) | 0 (0) |
| Overweight (25-29.9 kg/m2) | 0 (0) |
| Obese I (30-34.9 kg/m2) | 2 (4) |
| Obese II (35-39.9 kg/m2) | 8 (15) |
| Obese III (≥40 kg/m2) | 43 (81) |
| Comorbidities, n (%) | |
| Osteoarthritis | 14 (26) |
| Asthma | 13 (25) |
| Type 2 diabetes mellitus | 13 (25) |
| Congestive heart failure | 4 (8) |
| Coronary artery disease | 3 (6) |
| Cancer | 2 (4) |
| Chronic obstructive pulmonary disease | 1 (2) |
| RA characteristics, n (%) | |
| Positive rheumatoid factor | 25 (47) |
| Positive anti-cyclic citrullinated peptide | 16 (30) |
| Seropositive RA* | 27 (51) |
| Erosive RA | 21 (40) |
| RA duration, years (SD) | 8.6 (8.1) |
| RA disease activity, n (%) | |
| Remission | 14 (26) |
| Low | 9 (17) |
| Moderate | 27 (51) |
| High | 3 (6) |
| RA-related medications**, n (%) | |
| On any DMARD | 49 (93) |
| On any bDMARD | 27 (51) |
| On any nbDMARD | 37 (70) |
| Number of DMARDs | |
| 0 | 4 (8) |
| 1 | 32 (60) |
| 2 | 13 (25) |
| 3 | 4 (8) |
| On NSAID | 24 (45) |
| On glucocorticoid | 9 (17) |
| On any DMARD, NSAID, or glucocorticoid | 52 (98) |
| Remission and no DMARDs, NSAIDs, or glucocorticoids | 1 (2) |
Seropositive RA was defined as positive rheumatoid factor or anti-cyclic-citrullinated peptide antibody.
Medications were as of baseline visit (mean of 96 days [SD 89] prior to bariatric surgery).
BMI, body mass index; bDMARDS, biologic disease-modifying anti-rheumatic drugs; DMARDs, disease-modifying anti-rheumatic drugs; nbDMARDs, non-biologic disease-modifying anti-rheumatic drugs; NSAIDs, non-steroidal anti-inflammatory drugs; RA, rheumatoid arthritis; SD, standard deviation; WHO, World Health Organization.
After bariatric surgery, subjects lost substantial weight (mean 32.5 kg [SD13.6] at six months post-surgery, 41.0 kg [SD 17.3] at 12 months post-surgery, and 35.4 kg [SD 23.0] at most recent follow-up; P<0.001 for all time points compared to baseline). Mean most recent follow-up visit was 5.8 years (SD 3.2) after bariatric surgery and occurred up to July 2014. Mean BMI at six months post-surgery was 35.7 kg/m2 (SD 6.9); 12 months post-surgery was 32.6 kg/m2 (SD 7.0); and most recent follow-up was 34.6 kg/m2 (SD 8.0) (P<0.001 for all time points compared to baseline BMI; mean 47.8 kg/m2 [SD 7.7]). Twelve months after bariatric surgery, subjects lost a mean of 70% (SD 24) of excess weight above a BMI of 25 kg/m2.
At baseline, 51% of subjects were classified as having moderate RA disease activity, 6% as high, 17% as low, and 26% were in remission. RA disease activity category measures were significantly lower at all follow-up time points compared to baseline (P<0.001, Table 2). Twelve months after bariatric surgery, only 6% of subjects were classified as having high or moderate RA disease activity at baseline, decreased from 57% at baseline (P<0.001). Twelve months after bariatric surgery, 68% of subjects were in remission compared to 26% at baseline (P<0.001).
Table 2.
Characteristics of subjects at baseline, six months post-bariatric surgery, 12 months post-bariatric surgery, and most recent follow-up visit (N=53).
| Baseline (mean 96 days [SD 89] before bariatric surgery) (Reference) | 6 months post-bariatric surgery | 12 months post-bariatric surgery | Most recent follow-up (mean 5.8 years [SD 3.2] post-bariatric surgery) | |
|---|---|---|---|---|
| Anthropometrics, mean (SD)1 | ||||
|
| ||||
| Body mass index, kg/m2 | 47.8 (7.7) | 35.7 (6.9)** | 32.6 (7.0)** | 34.6 (8.0)** |
| Change in body mass index, kg/m2 | N/A | -12.1 (4.8)** | -15.2 (6.1)** | -13.3 (8.3)** |
| Weight, kg | 128.2 (24.1) | 95.8 (22.0)** | 87.7 (20.0)** | 93.2 (24.0)** |
| Change in weight, kg | N/A | -32.5 (13.6)** | -41.0 (17.3)** | -35.4 (23.0)** |
| % Excess weight loss | N/A | 56 (21)** | 70 (24)** | 57 (47)** |
|
| ||||
| RA disease activity and medication use, N (%)1 | ||||
|
| ||||
| RA disease activity | ||||
| Remission | 14 (26) | 38 (72)** | 36 (68)** | 39 (74)** |
| Low | 9 (17) | 12 (23)** | 9 (17)** | 12 (23)** |
| Moderate | 27 (51) | 2 (4)** | 3 (6)** | 1 (2)** |
| High | 3 (6) | 1 (2)** | 0 (0)** | 0 (0)** |
| RA-related medications | ||||
| On any DMARD | 49 (92) | 41 (77)* | 31 (59)** | 33 (62)** |
| On any bDMARD2 | 27 (51) | 23 (43)** | 19 (36)** | 25 (47)** |
| On any nbDMARD3 | 37 (70) | 29 (55)* | 23 (43)* | 22 (42)* |
| Number of DMARDs | ||||
| 0 | 4 (8) | 12 (23)* | 17 (32)* | 19 (36)* |
| 1 | 32 (60) | 27 (51)* | 16 (30)* | 17 (32)* |
| 2 | 13 (25) | 12 (23)* | 12 (23)* | 12 (23)* |
| 3 | 4 (8) | 2 (4)* | 3 (6)* | 4 (8)* |
| On NSAID4 | 24 (45) | 8 (15)* | 8 (15)* | 8 (15)* |
| On Glucocorticoid | 9 (17) | 7 (13)* | 5 (9)* | 3 (6)* |
| On any DMARD, NSAID, or glucocorticoid | 52 (98) | 44 (83) | 35 (66) | 35 (66) |
| Remission and no DMARDs, NSAIDs, or glucocorticoids | 1 (2) | 8 (15) | 12 (23) | 15 (28) |
P<0.05 compared to baseline.
P<0.001 compared to baseline.
Changes in anthropometrics, laboratory values, RA disease activity, and medication usage are compared to baseline.
Biologic DMARDs used were etanercept, adalimumab, infliximab, golimumab, abatacept, rituximab, and tocilizumab.
Non-biologic DMARDs used were methotrexate, hydroxychloroquine, sulfasalazine, azathioprine, and mycophenolate mofetil.
Non-steroidal anti-inflammatory drugs used were ibuprofren, naproxen, celecoxib, rofecoxib, nabumetone, etodolac, diclofenac, and indomethacin.
bDMARDS, biologic disease-modifying anti-rheumatic drugs; DMARDs, disease-modifying anti-rheumatic drugs; ESR, erythrocyte sedimentation rate; N/A, not applicable; nbDMARDs, non-biologic disease-modifying anti-rheumatic drugs; NSAIDs, non-steroidal anti-inflammatory drugs; RA, rheumatoid arthritis.
CRP was significantly lower at six months post-surgery (10.1 mg/L [SD 13.1], P<0.05), 12 months post-surgery (5.9 mg/L [SD 8.2], P<0.001) and most recent follow-up (4.1 mg/L [SD 5·4], P<0.001), all compared to baseline (26.1 mg/L [SD 20.9], Figure 2). Similarly, ESR was significantly lower at six months post-surgery (35.4 mm/hr [SD 20.4], P<0.05), at 12 months post-surgery (26.1 mm/hr [SD 2.0], P<0.05), and at most recent follow-up (18.0 mm/hr [SD 12·6]; P<0.001) all compared to baseline (45.7 mm/hr [SD 26.2]). The use of DMARDs, NSAIDs, and glucocorticoids were decreased at all post-surgical time points compared to baseline (P<0.05). At baseline, 93% of subjects were on a DMARD compared to 59% at 12 months post-surgery (P<0.001). Biologic and non-biologic DMARD use was less common at all post-surgical time points (P<0.05). However, similar frequencies of subjects were taking two or three DMARDs at every time point assessed. Only one subject (2%) was in remission on no RA-related medications at baseline compared to 12 (23%) subjects at 12 months post-surgery, however this was not statistically significant (P=0.28). Figure 3 shows study subjects stratified by RA disease activity categories assessed at baseline. RA disease activity improved after substantial weight loss from bariatric surgery in subjects classified with high, moderate, and low RA disease activity, and remained stable for subjects in remission at baseline.
Figure 2.
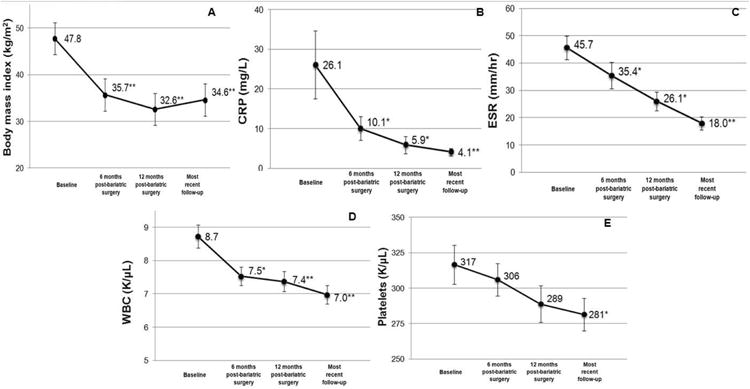
Mean values in subjects at baseline (prior to bariatric surgery), six months post-surgery, 12 months post-surgery, and most recent follow-up: A. body mass index; B. C-reactive protein; C. erythrocyte sedimentation rate; D. white blood cell count; E. platelet count. *P<0.05 compared to baseline; **P<0.001 compared to baseline.
Figure 3.
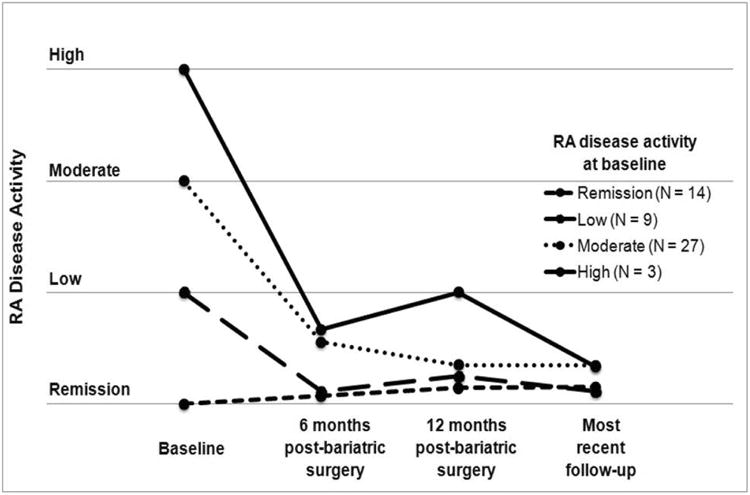
Rheumatoid arthritis disease activity in subjects after bariatric surgery stratified by RA disease activity classification at baseline prior to bariatric surgery.
Some subjects experienced serious adverse events after bariatric surgery (19/53 [36%] experienced at least one adverse event). Ten subjects (19%) underwent re-operation for lysis of adhesions, persistent nausea, malposition, dehiscence, or ventral hernia repair. Six subjects (11%) had post-operative serious infections (consisting of wound infection, pneumonia, and C. difficile colitis) and three subjects (6%) had intestinal obstructions. Pulmonary embolism occurred in one subject (2%) 94 days after initial Roux-en-Y gastric bypass and 28 days after exploratory laparoscopy for persistent nausea and gastrostomy tube placement. One subject (2%) died 111 days after Roux-en-Y gastric bypass; an autopsy was not performed.
Discussion
Our study describes reduced serum inflammatory markers, decreased RA disease activity and less RA medication use after bariatric surgery in patients with RA. Twelve months after bariatric surgery, subjects in our study lost a mean of 41 kg, corresponding to 70% mean excess weight loss. Prior to bariatric surgery, 57% had moderate/high RA disease activity, and this decreased to only 6% twelve months after surgery. This was not related to more aggressive medical management, as RA-related medication usage also significantly decreased after bariatric surgery, with 98% on medications at baseline compared to only 66% one year after surgery. We also observed significantly decreased serum inflammatory markers at all post-operative time points compared to baseline.
Only one prior study investigated the effect of weight loss on RA. Engelhart et al. enrolled 19 overweight RA patients in a non-surgical weight loss intervention; subjects lost a mean of 4.5 kg and their physical function significantly improved (25). No prior study has investigated the effects of more substantial weight loss on RA, the effects of bariatric surgery on RA, or examined changes in RA disease activity before and after bariatric surgery. While reductions in ESR and CRP after bariatric surgery have been previously reported, ESR and CRP changes in RA patients after bariatric surgery have not been studied (12, 13). A previous study evaluating CRP levels after bariatric surgery also noted improvements in CRP, though not as marked as the reduction in CRP that we report (26). In that study, CRP levels at baseline prior to bariatric surgery were 3.6 mg/L (compared to 26.1 mg/L in our study), 2.6 mg/L six months post-bariatric surgery (10.1 mg/L in our study), and 0.96 mg/L twelve months post-bariatric surgery (5.9 mg/L in our study) (26). Therefore, the reduction of CRP in the patients in our study was beyond what was expected from weight loss alone and thus may reflect an intrinsic reduction in systemic inflammation.
Excess body weight has been associated with higher risk of developing RA (4, 7, 27). Others have suggested that adipocyte-derived pro-inflammatory cytokines such as leptin, resistin, and visfatin contribute to ongoing inflammation that may eventually manifest in clinical RA and increase RA disease activity (28). In a high-risk population with arthralgias and positive RA-related antibodies, those who smoked and had increased BMI were more likely to develop classifiable RA (29).
Obesity may also contribute to a more severe clinical course among those with RA. Elevated BMI is associated with worse RA outcomes compared to those with normal BMI, including higher RA disease activity, poorer clinical response to treatment, worse quality of life, lower physical function, and more pain (5, 30-32). Among early RA patients however, obese patients are less likely to develop bone erosions compared to those with normal BMI, perhaps due to paradoxical anti-inflammatory effects of adiponectin on synoviocytes (33-35).
RA patients with obesity may have reduced response to DMARDs. In several studies, among those initiating tumor necrosis factor-α inhibitors, RA patients with obesity were less likely to be in remission after one year compared to those with normal BMI (8, 36-39). Other DMARDs may also be less effective in obese patients compared to those without obesity (40). Therefore, the high RA disease activity we observed prior to bariatric surgery may have been in part explained by decreased DMARD efficacy in those with more severe obesity. In our study, we note that similar frequencies of patients were on two or more DMARDs both before and after bariatric surgery, but the frequency of patients on a single DMARD markedly decreased. The overall markedly reduced need for DMARDs together with the increase in medication-free remission after bariatric surgery suggests that intrinsic changes in RA disease activity or response to DMARDs may have occurred.
The use of non-DMARD RA-related medications was also significantly reduced after bariatric surgery in our study. It is possible that the observed reduction in RA-related medication usage was in part due to perioperative medication adjustments as prior to bariatric surgery, patients are typically instructed to discontinue immunosuppressants, glucocorticoids, and NSAIDs due to the perioperative risk of gastrointestinal bleeding, poor wound healing, and infection. However, this is unlikely to be the sole explanation, as the reduction in RA-related medication persisted well after surgery, with 28% of subjects in remission off all RA-related medications at most recent follow-up (mean 5.8 years), accompanied by continued improvement in RA disease activity. This contrasts with prior studies in which only 12% of medically treated RA patients remain medication-free after two years of clinical remission (41). Our results suggest that bariatric surgery might significantly reduce the need for RA-related medications and even induce long-term RA remission requiring no medications in some patients.
Obesity is clearly associated with osteoarthritis, and 26% of subjects in our study had this diagnosis (42, 43). This association may be due in part to metabolic, in addition to mechanical, factors, as osteoarthritis is more prevalent in persons with obesity not only in weight-bearing joints, but also in the hands (43). Thus, it is possible that hand osteoarthritis contributed to some signs and symptoms of pain, tenderness, and swelling in our study, which might have also improved with weight loss (44). However, in our study the majority of subjects were seropositive and many had bone erosions, characteristic of RA but not osteoarthritis. Moreover, all subjects were assessed by experienced rheumatologists, who had expertise in differentiating between hand osteoarthritis and the findings of RA. Nonetheless, detecting synovitis in small joints in obese patients by physical examination alone is difficult even for experienced clinicians; therefore some of the reported improvements in joint examinations may have been due to reduced adiposity after bariatric surgery.
Some subjects in our study experienced serious adverse events after bariatric surgery, including re-operation, infection, pulmonary embolism, and death. Due to the limited sample size of our study, we are unable to determine whether adverse events from bariatric surgery in RA patients differ compared to the general population of patients undergoing bariatric surgery (10).
Our study has several limitations. The study design was a retrospective uncontrolled, observational cohort without a comparison group that utilized data collected in routine medical care. Ascertainment of RA disease activity was performed by blinded chart review when validated measures were not available. However, among a subset of subjects (N=9) with validated RA disease measures available at baseline and at least one follow-up time point, our findings were similar. Since validated measures were not collected consistently, we were not able to analyze whether individual components of RA disease activity measures, such as tender or swollen joints, were also improved after bariatric surgery. A potential source of bias in our study was that patients and providers were aware of bariatric surgery status, weight lost, and improvements in ESR and CRP. These factors might have influenced components of RA disease activity measures. We attempted to reduce this potential source of bias by blinded chart review to bariatric surgery status and weight loss.
Since we relied on data collected during routine clinical practice, not all data were available. For example, not all subjects had RF and anti-CCP tested at the institutions in this study as some had longstanding deforming RA diagnosed elsewhere and serologic markers were not routinely retested. Moreover, some subjects had longstanding RA diagnosed prior to the clinical availability of anti-CCP. This may explain a relatively low frequency of seropositive RA patients in this study. We note that obesity may have a stronger association with seronegative RA than seropositive RA, particularly in women (7). In our study, some subjects were excluded due to inadequate data for analysis, raising the possibility that our study sample may not be representative of patients with active RA (see Figure 1). However, given that both institutions included our study are large tertiary centers where RA patients with severe or refractory disease are routinely referred, our study sample likely represents RA patients with longstanding and active RA. In addition, almost all subjects in our study were women, likely reflecting the female predominance in both RA prevalence and bariatric surgery seekers. This raised the question of whether the effects of obesity in RA may be different for women and men (7, 45). However, the impact of weight loss after bariatric surgery on RA disease activity in our study was substantial, so that even a modest effect in men may still have clinical benefit.
Decreased mechanical strain on joints, subjective improvements in overall health, increased efficacy of medications at reduced weight, improved diet, and increased physical activity, may all have influenced RA disease activity measures without representing true changes in the underlying disease. While these factors may have played a role in the post-surgical improvements observed in our study, the substantial improvements in RA disease activity measures, reduced serum markers of inflammation, and decreased use of RA-related medications are all suggestive of reduced systemic inflammation.
Finally, we note that it is unclear whether the observed improvement in RA measures after bariatric surgery is related to the substantial weight loss itself, or to surgery-specific effects. Bariatric surgical procedures have profound metabolic effects, beyond those attributable to weight loss (46). In patients with type 2 diabetes who undergo Roux-en-Y gastric bypass, the need for glucose-lowering medications is significantly reduced within days, well before significant weight loss (47). The mechanisms responsible for these effects are only partially understood, and hypotheses include alterations in the microbiome, which may also influence RA (48-50). Alterations in gut hormones after bariatric surgery, particularly glucagon-like peptide-1, may further modulate inflammation and metabolic factors involved in RA disease activity (51). We were unable to investigate whether the specific surgical interventions, or the degree of weight lost, have differential effects on changes in RA measures in our study. Prospective studies of RA patients undergoing bariatric surgery with consistent measurement of RA disease activity, metabolic factors, and tissue samples for microbiome and translational studies are necessary to clarify the exact mechanisms for the clinical improvement we observed after bariatric surgery in RA patients.
In conclusion, we demonstrated that after bariatric surgery RA disease activity significantly improved, serum markers of inflammation decreased, and the usage of RA-related medications decreased. In this observational study, these effects were evident six months after surgery and persisted for years. Our findings suggest that weight loss may have an important role in the non-pharmacologic management of RA in patients with obesity. Whether the observed improvements in RA are attributable to substantial weight loss, specific effects of bariatric surgery, decreased inflammatory burden, reduced mechanical joint stress, improved quality of life, or a combination of these factors, warrants further study. Prospective, controlled studies are needed to elucidate the impact of weight loss and bariatric procedures on inflammation and disease activity in RA and to determine the mechanisms that mediate these effects.
Significance and Innovation.
Previous studies indicate that obesity worsens rheumatoid arthritis (RA) disease activity and contributes to worse clinical outcomes, but no study has evaluated the effect of substantial weight loss after bariatric surgery among patients with RA.
Among 53 RA patients who underwent bariatric surgery, there were significant improvements in RA disease activity, serum inflammatory markers, and RA-related medication usage.
One year after bariatric surgery, only 6% had moderate/high RA disease activity compared to 57% at baseline (P<0.001).
These findings suggest that substantial weight loss may have an important role in the non-pharmacologic management of RA in patients with obesity although factors specifically related to bariatric surgery may have contributed to the improvements described.
Acknowledgments
The authors would like to thank Nancy Shadick, MD, MPH, Christine Iannaccone, MPH, Michelle Frits, BA, the Brigham Rheumatoid Arthritis Sequential Study, and the Section of Clinical Sciences of the Division of Rheumatology, Immunology and Allergy at Brigham and Women's Hospital for helpful guidance.
Dr. Sparks is supported by the Rheumatology Research Foundation Scientist Development Award and the National Institutes of Health Loan Repayment Award. Research reported in this publication also was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award numbers AR049880, AR052403, and AR047782. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The funders had no role in study design, data collection, and analysis.
Footnotes
Financial disclosures: The authors report no other financial disclosures.
Author contributions: All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Sparks had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Sparks, Halperin, E. Karlson, Bermas.
Acquisition of data. Sparks, Halperin, E. Karlson, Bermas.
Analysis and interpretation of data. Sparks, Halperin, J. Karlson, E. Karlson, Bermas.
References
- 1.Overweight, obesity, and health risk. Arch Intern Med. 2000;160(7):898–904. doi: 10.1001/archinte.160.7.898. [DOI] [PubMed] [Google Scholar]
- 2.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen SB, Lee YC, Ser KH, Chen JC, Chen SC, Hsieh HF, et al. Serum C-reactive protein and white blood cell count in morbidly obese surgical patients. Obes Surg. 2009;19(4):461–6. doi: 10.1007/s11695-008-9619-3. [DOI] [PubMed] [Google Scholar]
- 4.Lu B, Hiraki LT, Sparks JA, Malspeis S, Chen CY, Awosogba JA, et al. Being overweight or obese and risk of developing rheumatoid arthritis among women: a prospective cohort study. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajeganova S, Andersson ML, Hafstrom I. Association of obesity with worse disease severity in rheumatoid arthritis as well as with comorbidities: a long-term followup from disease onset. Arthritis Care Res (Hoboken) 2013;65(1):78–87. doi: 10.1002/acr.21710. [DOI] [PubMed] [Google Scholar]
- 6.Sandberg ME, Bengtsson C, Kallberg H, Wesley A, Klareskog L, Alfredsson L, et al. Overweight decreases the chance of achieving good response and low disease activity in early rheumatoid arthritis. Ann Rheum Dis. doi: 10.1136/annrheumdis-2013-205094. [DOI] [PubMed] [Google Scholar]
- 7.Wesley A, Bengtsson C, Elkan AC, Klareskog L, Alfredsson L, Wedren S. Association between body mass index and anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis: results from a population-based case-control study. Arthritis Care Res (Hoboken) 2013;65(1):107–12. doi: 10.1002/acr.21749. [DOI] [PubMed] [Google Scholar]
- 8.Gremese E, Carletto A, Padovan M, Atzeni F, Raffeiner B, Giardina AR, et al. Obesity and reduction of the response rate to anti-tumor necrosis factor alpha in rheumatoid arthritis: an approach to a personalized medicine. Arthritis Care Res (Hoboken) 2013;65(1):94–100. doi: 10.1002/acr.21768. [DOI] [PubMed] [Google Scholar]
- 9.Sjostrom L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden A, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297–304. doi: 10.1001/jama.2014.5988. [DOI] [PubMed] [Google Scholar]
- 10.Oria HE, Moorehead MK. Updated Bariatric Analysis and Reporting Outcome System (BAROS) Surg Obes Relat Dis. 2009;5(1):60–6. doi: 10.1016/j.soard.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Dalbeth N, Chen P, White M, Gamble GD, Barratt-Boyes C, Gow PJ, et al. Impact of bariatric surgery on serum urate targets in people with morbid obesity and diabetes: a prospective longitudinal study. Ann Rheum Dis. 2014;73(5):797–802. doi: 10.1136/annrheumdis-2013-203970. [DOI] [PubMed] [Google Scholar]
- 12.Santos J, Salgado P, Santos C, Mendes P, Saavedra J, Baldaque P, et al. Effect of bariatric surgery on weight loss, inflammation, iron metabolism, and lipid profile. Scand J Surg. 2014;103(1):21–5. doi: 10.1177/1457496913490467. [DOI] [PubMed] [Google Scholar]
- 13.Illan-Gomez F, Gonzalvez-Ortega M, Orea-Soler I, Alcaraz-Tafalla MS, Aragon-Alonso A, Pascual-Diaz M, et al. Obesity and inflammation: change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes Surg. 2012;22(6):950–5. doi: 10.1007/s11695-012-0643-y. [DOI] [PubMed] [Google Scholar]
- 14.Gabriel SE, Crowson CS, O'Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955-1985. Arthritis Rheum. 1999;42(3):415–20. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 15.Mallya RK, de Beer FC, Berry H, Hamilton ED, Mace BE, Pepys MB. Correlation of clinical parameters of disease activity in rheumatoid arthritis with serum concentration of C-reactive protein and erythrocyte sedimentation rate. J Rheumatol. 1982;9(2):224–8. [PubMed] [Google Scholar]
- 16.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 17.Vliet Vlieland TP, Pattison D. Non-drug therapies in early rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2009;23(1):103–16. doi: 10.1016/j.berh.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 19.de Onis M, Habicht JP. Anthropometric reference data for international use: recommendations from a World Health Organization Expert Committee. Am J Clin Nutr. 1996;64(4):650–8. doi: 10.1093/ajcn/64.4.650. [DOI] [PubMed] [Google Scholar]
- 20.Oria HE. Reporting Results in Obesity Surgery: Evaluation of a Limited Survey. Obes Surg. 1996;6(4):361–8. doi: 10.1381/096089296765556719. [DOI] [PubMed] [Google Scholar]
- 21.Anderson J, Caplan L, Yazdany J, Robbins ML, Neogi T, Michaud K, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken) 2012;64(5):640–7. doi: 10.1002/acr.21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011;63(3):573–86. doi: 10.1002/art.30129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobbs KF, Cohen MD. Rheumatoid arthritis disease measurement: a new old idea. Rheumatology (Oxford) 2012;51(Suppl 6):vi21–7. doi: 10.1093/rheumatology/kes282. [DOI] [PubMed] [Google Scholar]
- 24.Collier DS, Grant RW, Estey G, Surrao D, Chueh HC, Kay J. Physician ability to assess rheumatoid arthritis disease activity using an electronic medical record-based disease activity calculator. Arthritis Rheum. 2009;61(4):495–500. doi: 10.1002/art.24335. [DOI] [PubMed] [Google Scholar]
- 25.Engelhart M, Kondrup J, Hoie LH, Andersen V, Kristensen JH, Heitmann BL. Weight reduction in obese patients with rheumatoid arthritis, with preservation of body cell mass and improvement of physical fitness. Clin Exp Rheumatol. 1996;14(3):289–93. [PubMed] [Google Scholar]
- 26.Holdstock C, Lind L, Engstrom BE, Ohrvall M, Sundbom M, Larsson A, et al. CRP reduction following gastric bypass surgery is most pronounced in insulin-sensitive subjects. Int J Obes (Lond) 2005;29(10):1275–80. doi: 10.1038/sj.ijo.0803000. [DOI] [PubMed] [Google Scholar]
- 27.Symmons DP, Bankhead CR, Harrison BJ, Brennan P, Barrett EM, Scott DG, et al. Blood transfusion, smoking, and obesity as risk factors for the development of rheumatoid arthritis: results from a primary care-based incident case-control study in Norfolk, England. Arthritis Rheum. 1997;40(11):1955–61. doi: 10.1002/art.1780401106. [DOI] [PubMed] [Google Scholar]
- 28.Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y, Kitas GD. Obesity in rheumatoid arthritis. Rheumatology (Oxford) 2011;50(3):450–62. doi: 10.1093/rheumatology/keq266. [DOI] [PubMed] [Google Scholar]
- 29.de Hair MJ, Landewe RB, van de Sande MG, van Schaardenburg D, van Baarsen LG, Gerlag DM, et al. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann Rheum Dis. 2013;72(10):1654–8. doi: 10.1136/annrheumdis-2012-202254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandberg ME, Bengtsson C, Kallberg H, Wesley A, Klareskog L, Alfredsson L, et al. Overweight decreases the chance of achieving good response and low disease activity in early rheumatoid arthritis. Ann Rheum Dis. 2014;73(11):2029–33. doi: 10.1136/annrheumdis-2013-205094. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Poma A, Segami MI, Mora CS, Ugarte MF, Terrazas HN, Rhor EA, et al. Obesity is independently associated with impaired quality of life in patients with rheumatoid arthritis. Clin Rheumatol. 2007;26(11):1831–5. doi: 10.1007/s10067-007-0583-4. [DOI] [PubMed] [Google Scholar]
- 32.Stavropoulos-Kalinoglou A, Metsios GS, Panoulas VF, Nevill AM, Jamurtas AZ, Koutedakis Y, et al. Underweight and obese states both associate with worse disease activity and physical function in patients with established rheumatoid arthritis. Clin Rheumatol. 2009;28(4):439–44. doi: 10.1007/s10067-008-1073-z. [DOI] [PubMed] [Google Scholar]
- 33.Westhoff G, Rau R, Zink A. Radiographic joint damage in early rheumatoid arthritis is highly dependent on body mass index. Arthritis Rheum. 2007;56(11):3575–82. doi: 10.1002/art.23033. [DOI] [PubMed] [Google Scholar]
- 34.van der Helm-van Mil AH, van der Kooij SM, Allaart CF, Toes RE, Huizinga TW. A high body mass index has a protective effect on the amount of joint destruction in small joints in early rheumatoid arthritis. Ann Rheum Dis. 2008;67(6):769–74. doi: 10.1136/ard.2007.078832. [DOI] [PubMed] [Google Scholar]
- 35.Visser AW, Ioan-Facsinay A, de Mutsert R, Widya RL, Loef M, de Roos A, et al. Adiposity and hand osteoarthritis: the Netherlands Epidemiology of Obesity study. Arthritis Res Ther. 2014;16(1):R19. doi: 10.1186/ar4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Gay MA, Gonzalez-Juanatey C. Rheumatoid arthritis: Obesity impairs efficacy of anti-TNF therapy in patients with RA. Nat Rev Rheumatol. 2012;8(11):641–2. doi: 10.1038/nrrheum.2012.158. [DOI] [PubMed] [Google Scholar]
- 37.Heimans L, van den Broek M, le Cessie S, Siegerink B, Riyazi N, Han KH, et al. Association of high body mass index with decreased treatment response to combination therapy in recent-onset rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2013;65(8):1235–42. doi: 10.1002/acr.21978. [DOI] [PubMed] [Google Scholar]
- 38.Klaasen R, Wijbrandts CA, Gerlag DM, Tak PP. Body mass index and clinical response to infliximab in rheumatoid arthritis. Arthritis Rheum. 2011;63(2):359–64. doi: 10.1002/art.30136. [DOI] [PubMed] [Google Scholar]
- 39.Klotz U, Teml A, Schwab M. Clinical pharmacokinetics and use of infliximab. Clin Pharmacokinet. 2007;46(8):645–60. doi: 10.2165/00003088-200746080-00002. [DOI] [PubMed] [Google Scholar]
- 40.Mirpourian M, Salesi M, Abdolahi H, Farajzadegan Z, Karimzadeh H. The association of body mass index with disease activity and clinical response to combination therapy in patients with rheumatoid arthritis. J Res Med Sci. 2014;19(6):509–14. [PMC free article] [PubMed] [Google Scholar]
- 41.van den Broek M, Huizinga TW, Dijkmans BA, Allaart CF. Drug-free remission: is it already possible? Curr Opin Rheumatol. 2011;23(3):266–72. doi: 10.1097/BOR.0b013e32834563e3. [DOI] [PubMed] [Google Scholar]
- 42.Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28(1):5–15. doi: 10.1016/j.berh.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Eaton CB. Obesity as a risk factor for osteoarthritis: mechanical versus metabolic. Med Health R I. 2004;87(7):201–4. [PubMed] [Google Scholar]
- 44.Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64(10):1471–9. doi: 10.1002/acr.21627. [DOI] [PubMed] [Google Scholar]
- 45.Katz PP, Yazdany J, Trupin L, Schmajuk G, Margaretten M, Barton J, et al. Sex differences in assessment of obesity in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65(1):62–70. doi: 10.1002/acr.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240(2):236–42. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238(4):467–84. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 84-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sweeney TE, Morton JM. The human gut microbiome: a review of the effect of obesity and surgically induced weight loss. JAMA Surg. 2013;148(6):563–9. doi: 10.1001/jamasurg.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol. 2011;7(10):569–78. doi: 10.1038/nrrheum.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halperin F, Goldfine AB. Metabolic surgery for type 2 diabetes: efficacy and risks. Curr Opin Endocrinol Diabetes Obes. 2013;20(2):98–105. doi: 10.1097/MED.0b013e32835edbb0. [DOI] [PubMed] [Google Scholar]
- 51.Chen CY, Tsai CY. From endocrine to rheumatism: do gut hormones play roles in rheumatoid arthritis? Rheumatology (Oxford) 2014;53(2):205–12. doi: 10.1093/rheumatology/ket255. [DOI] [PubMed] [Google Scholar]


