Abstract
Objective
Emerging evidence suggests that methionine oxidation can directly affect protein function and may be linked to cardiovascular disease. The objective of this study was to define the role of the methionine sulfoxide reductase A (MsrA) in models of vascular disease and identify its signaling pathways.
Approach and Results
MsrA was readily identified in all layers of the vascular wall in human and murine arteries. Deletion of the MsrA gene did not affect atherosclerotic lesion area in apolipoprotein E-deficient mice, and had no significant effect on susceptibility to experimental thrombosis after photochemical injury. In contrast, the neointimal area after vascular injury due to complete ligation of the common carotid artery was significantly greater in MsrA-deficient compared to control mice. In aortic vascular smooth muscle cells (VSMC) lacking MsrA, cell proliferation was significantly increased due to accelerated G1/S transition. In parallel, cyclin D1 protein and cdk4/cyclin D1 complex formation and activity were increased in MsrA-deficient VSMC, leading to enhanced Rb phosphorylation and transcription of E2F. Finally, MsrA-deficient VSMC exhibited greater activation of ERK1/2 that was caused by increased activity of the Ras/Raf/MEK signaling pathway.
Conclusion
Our findings implicate MsrA as a negative regulator of VSMC proliferation and neointimal hyperplasia after vascular injury through control of the Ras/Raf/MEK/ERK1/2 signaling pathway.
Keywords: methionine sulfoxide reductase, oxidation, VSMC, neointima, proliferation, ERK1/2
INTRODUCTION
Methionine sulfoxide reductases (Msr) are evolutionarily conserved and ubiquitously expressed antioxidant enzymes that repair oxidized proteins by thioredoxin-dependent reduction of methionine sulfoxide residues. The cyclic oxidation-reduction of methionine residues has been proposed to be an antioxidant defense mechanism whereby oxidation of methionine serves as a “sink” for excess cellular reactive oxygen species (ROS).1 More recently, limited examples have emerged that methionine oxidation alters the function of specific proteins, including calmodulin,2 the calcium- and calmodulin-dependent kinase II3 and potassium channels.4 Methionine oxidation also has been observed to contribute to the redox regulation of several vascular proteins involved in thrombosis and atherosclerosis.5 These observations suggest that Msr may control specific signaling pathways via the regulation of protein methionine oxidation and reduction. However, despite the well-established role of oxidative stress in vascular diseases such as atherosclerosis, thrombosis and neointimal hyperplasia after mechanical injury,6 the distinct vascular phenotypes and signaling pathways affected by methionine oxidation in vivo have remained poorly defined.
The mammalian Msr system consists of two classes of proteins, MsrA and MsrB. MsrA is present in the nucleus, cytoplasm, and mitochondria in multiple cell types, including vascular smooth muscle cells (VSMC),7 and preferentially reduces the S-enantiomer of methionine sulfoxide. In contrast, MsrB comprises a class of three isoforms that preferentially reduce the R-enantiomer of peptide associated methionine sulfoxide,8 suggesting distinct roles for the two classes of Msr.
MsrA function has been studied in the context of aging and age-related neurodegenerative diseases such as Alzheimer's and Parkinson's diseases.9, 10 In the cardiovascular system, MsrA function has mainly been investigated in myocardial disease models that are characterized by high levels of oxidative stress. In particular, MsrA protects cardiomyocytes from hypoxia/reoxygenation injury in vitro and MsrA limits myocardial infarct size following ischemia/reperfusion injury of the heart ex vivo.11, 12 Myocardial-specific overexpression of MsrA in mice protects against wall rupture after myocardial infarction,13 whereas MsrA-deficient (MsrA−/−) mice display increased myocardial apoptosis, reduced survival, and impaired left ventricular ejection fraction.3 In contrast, the role of MsrA in ROS-dependent vascular disease remains less understood. Interestingly, two independent genome-wide association studies in humans have identified a polymorphism in intron 2 of the human MsrA gene that correlates with increased ischemic cardiovascular disease, though mechanistic insight is lacking.14, 15
Identification of the protein substrates of MsrA by systematic approaches has been hampered by the lack of specific tools to identify oxidized methionine residues in proteins.16-18 In addition, conventional sample preparation necessary for proteomic approaches may introduce artificial modifications due to the exquisite susceptibility of methionine residues to oxidation. For these reasons, only a few studies have applied a systematic proteomics-based approach to identify cellular proteins that are modified by methionine oxidation under conditions of increased oxidative stress.19, 20 There is limited agreement between the studies in terms of identified protein targets of methionine oxidation. Moreover, in most cases it remains unclear whether these modifications affect protein function. The alternative approach of treating cells with oxidants such as chloramine, which preferentially oxidizes methionine residues, has yielded a list of candidate target proteins with altered function. Examples with potential relevance to vascular disease include apolipoproteins, the extracellular matrix protein fibronectin21 and the actin-binding protein cofilin,22 which regulates VSMC migration and neointimal formation.23 However, it is unclear whether methionine modifications of these proteins occur under physiological or pathological conditions in vivo.
In this study, we applied an alternative strategy to define the in vivo contribution of MsrA to ischemic cardiovascular disease. In particular, we studied the impact of MsrA deletion in murine models of atherosclerosis, thrombosis, and mechanical injury, recapitulated relevant phenotypes in vitro and identified underlying signaling pathways.
RESULTS
Deficiency of MsrA does not alter susceptibility to experimental thrombosis or vascular lesion area in atherosclerotic mice
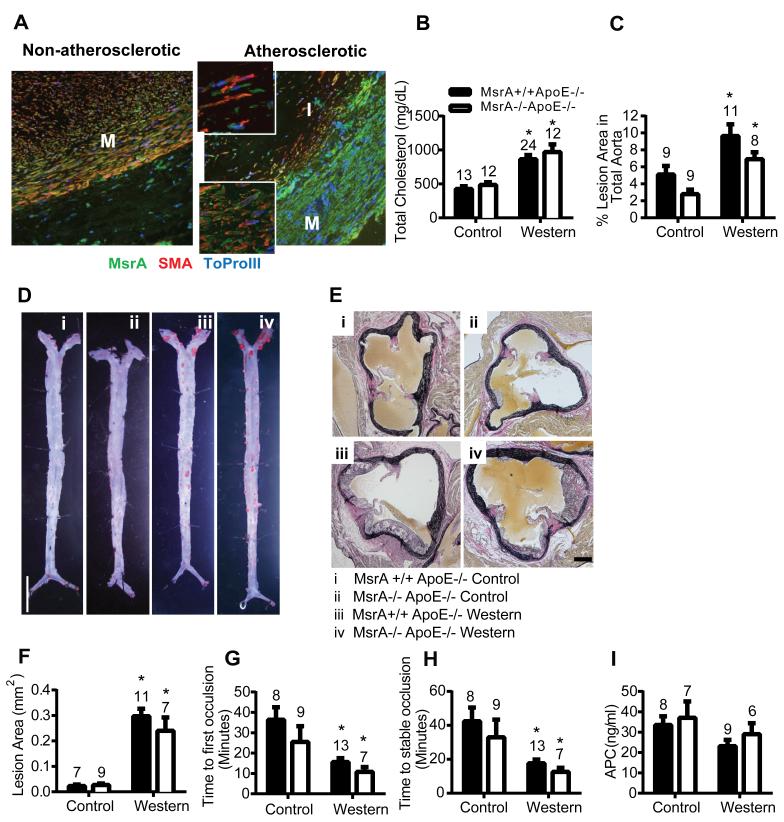
We first evaluated the expression of MsrA by immunofluorescence in non-atherosclerotic and atherosclerotic human coronary arteries. MsrA protein was distributed throughout the arterial wall in non-atherosclerotic samples. In the atherosclerotic plaque, MsrA was robustly expressed and most prominent in VSMC that were identified by co-labeling for α-smooth muscle actin (Figure 1A).
Figure 1. MsrA deletion does not affect atherosclerotic lesion size or susceptibility to experimental thrombosis.
A) Immunofluorescence for MsrA in human non-atherosclerotic and atherosclerotic coronary arteries showing expression in all layers. I=Intima, M=Media. Inset shows 63x magnification. MsrA=green, SMA (Smooth muscle actin)=red, ToPro-3 (Nucleus)=Blue B) Total cholesterol levels in MsrA+/+ or MsrA−/− mice in ApoE−/− background following 15-17 weeks of control or Western diet (n=12-24). C) and D) Quantification and representative images of percent lesion area (Oil red O area/total area; n=8-11 per group). Scale bar = 500 μm. E) and F) Representative images and quantification of atherosclerotic lesion area in aortic sinus. G) Time to first occlusion and H) stable occlusion of the carotid artery following photochemical injury (n=7-13). I) Activated protein C levels following thrombin infusion (n=6-9). *p<0.05 compared to control diet.
Because of its presence in atherosclerotic plaques, we hypothesized that deletion of MsrA increases atherosclerotic plaque formation. Thus, MsrA−/− mice were crossed with apolipoprotein E-deficient (ApoE−/−) mice and fed either a control diet or a Western diet to induce atherosclerosis. Total cholesterol levels were significantly increased by the Western diet as compared to the control diet regardless of genotype (Figure 1B). Similarly, HDL and LDL subfractions were not different between genotypes (Supplemental Figure IA). The Western diet significantly increased the cross-sectional atherosclerotic lesion area in the aortic sinus as well as the en face lesion area of the entire aorta as compared to the control diet (Figure 1C-F). Deficiency of MsrA did not significantly increase lesion area in ApoE−/− mice fed either the control diet or the Western diet. No differences in the number of macrophages in atherosclerotic plaque were detected between genotypes (Supplemental Figure IB, C).
Ischemic cardiovascular mortality is frequently caused by acute thrombosis at the site of an atherosclerotic lesion. Thus, we also examined whether MsrA deficiency alters the susceptibility to experimental thrombosis of the carotid artery induced by photochemical injury. As expected, mice fed the Western diet had shorter times to first occlusion and stable occlusion than mice fed the control diet (Figure 1G, H). However, the times to first occlusion or stable occlusion did not differ significantly between MsrA−/−ApoE−/− mice compared to MsrA+/+ ApoE−/− mice fed either the control diet or the Western diet.
Previous data have suggested that inactivation of thrombomodulin by methionine oxidation results in decreased generation of the anticoagulant activated protein C (APC) in vitro.24 Therefore, we measured plasma levels of APC after infusion of thrombin. Plasma APC levels were similar in MsrA+/+ ApoE−/− and MsrA−/−ApoE−/− mice (Figure 1I). Collectively, these findings suggest that MsrA does not protect against vascular lesion formation or susceptibility to experimental thrombosis in atherosclerotic mice.
Deficiency of MsrA increases neointimal formation through elevated proliferation of VSMC
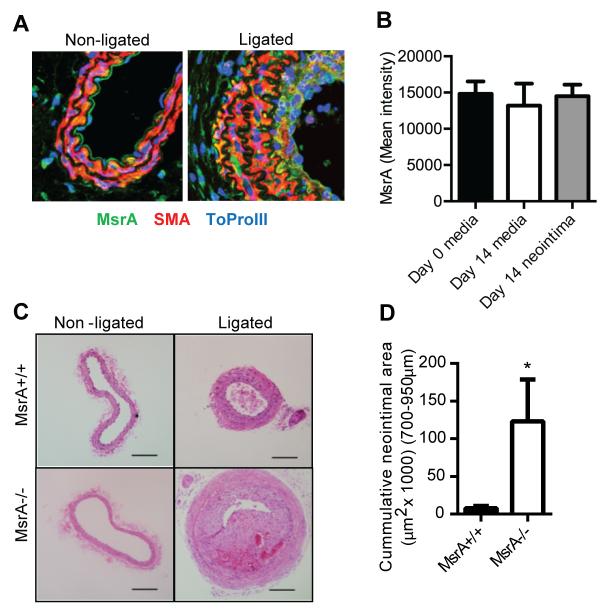
Since we detected abundant MsrA expression in α-smooth muscle actin-positive cells in human atherosclerotic lesions, we next examined expression of MsrA in murine vessel segments following carotid ligation, a model of vascular injury that induces a smooth muscle-rich neointima. Whereas MsrA was expressed throughout the arterial wall in non-ligated wild type (MsrA+/+) vessel segments, we observed robust MsrA staining in the neointima 14 days following carotid ligation (Figure 2A, B and Supplemental Figure II). Next, we assessed the effect of MsrA deletion on neointimal formation after carotid ligation injury using non-atherosclerotic MsrA−/− mice. No differences in vessel morphology between genotypes were present at baseline before carotid ligation (Figure 2C, Supplemental Figure II). In contrast, 14 days after ligation, the neointimal area was significantly increased in MsrA−/− mice compared to MsrA+/+ mice (Figure 2C, D, Supplemental Figure II). In addition, the medial area, total number of cells in the media, interior- and exterior-elastic lamina perimeters, and intimal/medial ratio were all significantly increased in MsrA−/− compared to MsrA+/+carotid arteries (Supplemental Figure II). Collectively, these data implicate MsrA in the protection against neointimal formation following vascular injury.
Figure 2. Deletion of MsrA significantly increases neointimal formation.
A) Expression of MsrA in MsrA+/+ mouse carotid arteries at Day 0 (Non-ligated) and Day 14 after ligation (Ligated). MsrA is detectable in the endothelium, vessel wall, adventitia and neointima. MsrA=green, SMA (Smooth muscle actin) = red, ToPro-3 (Nucleus) = blue. B) Quantification of MsrA signal intensity in the media at Day 0 and in the media and neointimal at Day 14. C) H&E staining and (D) quantification of neointimal area in arteries from MsrA+/+ or MsrA−/− mice. Scale bar = 200μm *p<0.05 vs. MsrA+/+ (n=6-9).
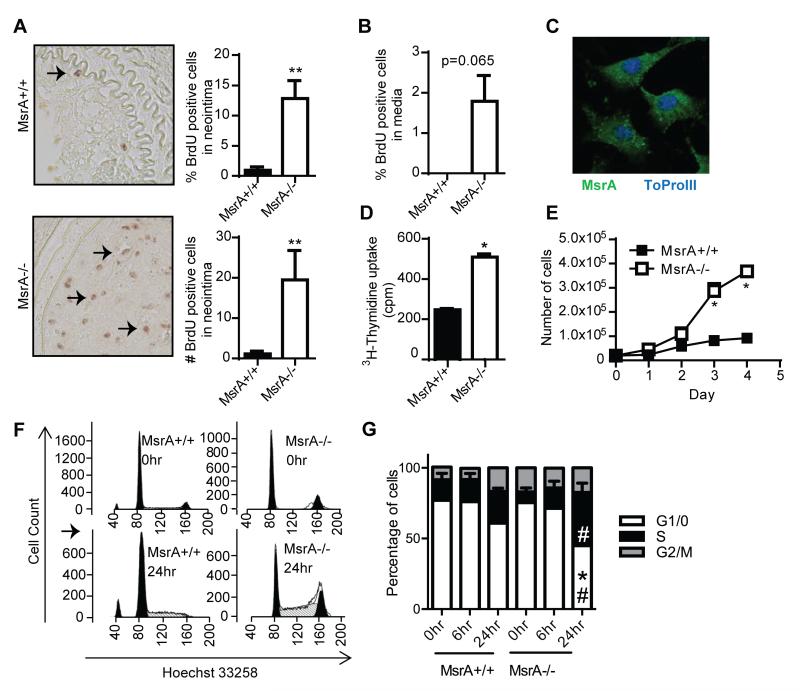
We next investigated proliferation in the neointima of mice 14 days after ligation and found that both the number and the percentage of BrdU-positive cells were significantly increased in MsrA−/− compared to MsrA+/+mice (Figure 3A and Supplemental Figure III). The number of BrdU-positive cells was also increased in the media (Figure 3B). Since the neointima primarily consists of VSMC that have migrated from the vessel wall into the lumen and proliferated, we isolated and cultured VSMC from aortas of MsrA+/+ and MsrA−/− mice to characterize the mechanisms by which MsrA deletion affects VSMC proliferation. We first confirmed the expression of MsrA in cultured MsrA+/+ VSMC by immunofluorescence (Figure 3C). We next examined proliferation of MsrA−/− and MsrA+/+ VSMC using 3H-thymidine uptake assays, which showed an increase in DNA synthesis, a surrogate marker of proliferation (Figure 3D). This was confirmed by a significant increase in the proliferation rate of MsrA−/− compared to MsrA+/+ VSMC grown in the presence of 10% serum (Figure 3E).
Figure 3. Deletion of MsrA accelerates proliferation of VSMC.
A) Left panels, representative images of BrdU staining of carotid artery sections from MsrA+/+ and MsrA−/− mice 14 days after carotid ligation. Right panels, quantification of number and percentage of BrdU positive cells within the neointima (n=6-10). B) Quantification of the number of BrdU positive cells in the medial layer 14 days after carotid ligation (n=5-9). C) MsrA expression by immunofluorescence in MsrA+/+ VSMC. MsrA= green, Nuclei (ToPro-3) = blue. D) 3H-Thymidine uptake in MsrA+/+ and MsrA−/− VSMC (n=12). E) Cell counts of MsrA+/+ and MsrA−/− VSMC (n=3). F) Cell cycle analysis of MsrA+/+ and MsrA−/− VSMC when growth arrested (0hr) and 6 hr and 24hr after release from growth arrest. Left panels, representative flow cytometry images; right panel, distribution of cells in phases of the cell cycle (n=6-11). * p<0.05 vs. MsrA+/+; ** p <0.01 vs. MsrA+/+. # = p<0.05 vs. growth arrested (0 hr).
Given the pronounced increase in proliferation of MsrA−/− VSMC, we next examined the impact of MsrA deficiency on cell cycle progression. Following synchronization in serum-free media for 48 hr, MsrA−/− and MsrA+/+ VSMC were stimulated with 10% serum and the cell cycle profile was determined 6 and 24 hr later by flow cytometry. MsrA−/− VSMC re-entered the cell cycle faster after cell cycle arrest, with significantly more cells in S-phase and fewer cells in G0/G1 phase as compared to MsrA+/+ VSMC 24 hours after release(Figure 3F and G). These data suggest a role for MsrA in cell cycle progression.
Since VSMC adhesion and migration contribute to the accumulation of VSMC in the neointima, we also evaluated whether MsrA deficiency impacts VSMC migration using Boyden chamber assays. We found that MsrA−/− VSMC had a 1.4-fold increase in migration compared to MsrA+/+ VSMC (Supplemental Figure IVA). Since the changes in migration were relatively modest, we sought to confirm the role of MsrA in migration by adenoviral MsrA overexpression in MsrA+/+ VSMC. Overexpression of MsrA decreased migration by 0.7-fold compared to control infected VSMC (Supplemental Figure IVB). Additionally, MsrA−/− VSMC displayed a decrease in adhesion (Supplemental Figure IVC). These data indicate the MsrA deletion has a modest effect on migration and impairs cell adhesion. Since these findings are unlikely to explain the large increase in neointimal size, we concentrated on the mechanisms by which MsrA deletion increases VSMC proliferation.
Deletion of MsrA increases cell cycle progression through post-transcription control of cyclin D1
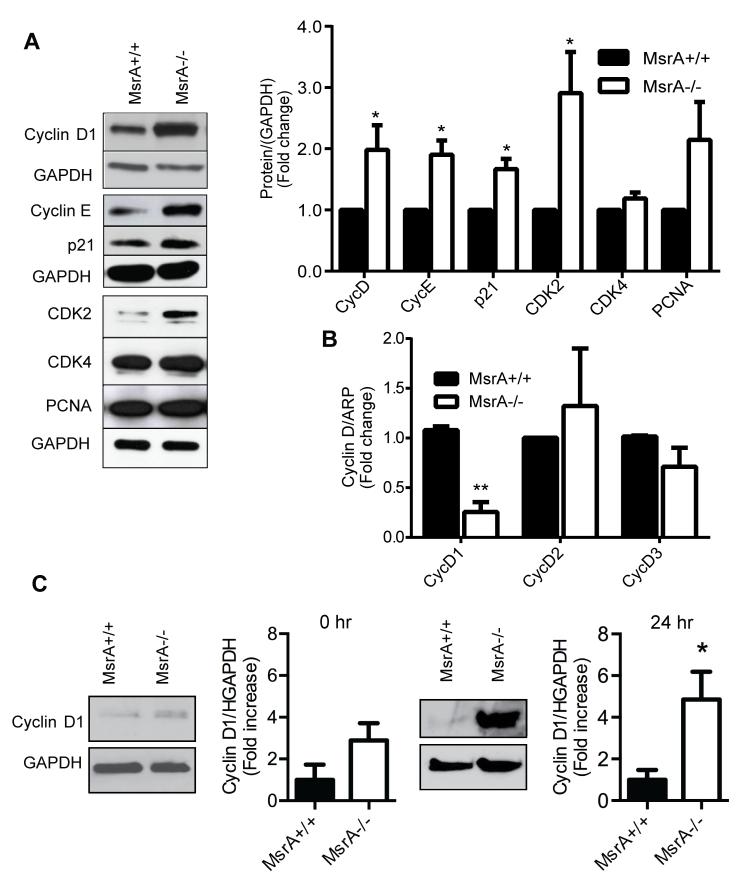
Based on data in Figure 3E that MsrA deletion accelerates progression through the cell cycle, we next evaluated protein levels of G0/G1 cell cycle regulators. In MsrA−/− VSMC, cyclin D1, cyclin E, CDK2 and were all significantly upregulated as compared to MsrA+/+ VSMC (Figure 4A). We also found that p21, which is traditionally considered a cell cycle inhibitor,25, 26 was upregulated in MsrA−/− compared to MsrA+/+ VSMC (Figure 4A). These data are consistent with recent publications indicating that p21 is required for cell proliferation through stabilization of the cyclin D/CDK4 complex.27-29 We also examined whether MsrA deficiency alters mRNA levels of cyclin D, the most upstream regulator of cell cycle progression. qRT-PCR demonstrated that, in contrast to protein expression, cyclin D1 mRNA levels were decreased rather than increased in MsrA−/− VSMC (Figure 4B). Cyclin D2 and D3 mRNA levels were similar between genotypes. Cyclin D1 protein expression was increased in growth-arrested cells and 24 hr after release from growth arrest (Figure 4C). These data suggest that MsrA post-transcriptionally regulates cyclin D1 expression.
Figure 4. MsrA controls expression of cell cycle regulators in proliferating VSMC.
A) Western blot and quantification of cell cycle regulators in proliferating MsrA+/+ and MsrA−/− VSMC. (n=7-12). B) mRNA levels of cyclin D isoforms in MsrA+/+ and MsrA−/− VSMC by qRT-PCR. (n=3-4) C) Western blot and densitometry of cyclin D1 protein in MsrA−/− and MsrA+/+ VSMC at growth arrest (left panel) and 24 h after release from growth arrest (right panel). *p < 0.05 **p<0.001 vs. MsrA+/+.
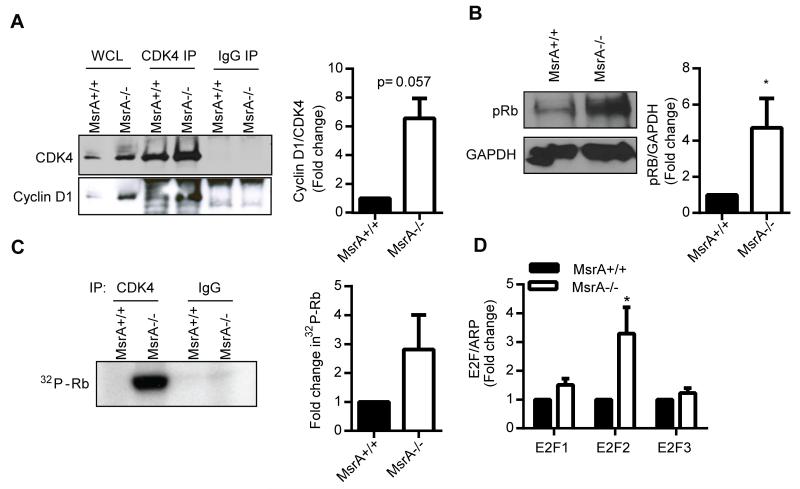
Cyclin D and CDK4 form a complex to phosphorylate and activate Rb, leading to cellular proliferation.30 To examine whether MsrA−/− VSMC have increased cyclin D1/CDK4 complex formation, CDK4 was immunoprecipitated from VSMC lysates and the amount of bound cyclin D1 determined by Western blotting. We detected an increase in the cyclin D1/CDK4 complex in MsrA−/− compared to MsrA+/+ VSMC (Figure 5A). Moreover, phospho-Rb levels were significantly elevated in MsrA−/− compared to MsrA+/+ VSMC (Figure 5B). These findings were confirmed using a radioactive in vitro cyclin D/CDK4 activity assay, in which MsrA−/− VSMC displayed an increase in phosphorylation of Rb (Figure 5C). It is well established that Rb binds the E2F family of transcription factors, repressing their activation.31 Upon phosphorylation of Rb, E2Fs are released and activate transcription of many genes, including E2F isoforms. Analysis of mRNA levels of E2F1, E2F2 and E2F3 in MsrA+/+ and MsrA−/− VSMC demonstrated that transcription of E2F is upregulated in MsrA−/− VSMC compared to MsrA+/+ controls (Figure 5D). Collectively, the data indicate that cyclin D1 activity is increased in VSMC lacking MsrA and promotes G0/G1 cycle progression through increased Rb phosphorylation and E2F2 transcription.
Figure 5. Cyclin D activity is increased in MsrA−/− VSMC.
A) Analysis of cyclin D1/CDK4 complex formation by immunoprecipitation with anti-CDK4 and Western blot with anti-cyclin D1. Left panel, representative blots; right panel, quantification of cyclin D1/CDK4 complex formation in MsrA−/− VSMC relative to MsrA+/+(normalized to total CDK4 levels; n=3). B) Western blot and quantification of Rb phosphorylation at S780 (pRb) in proliferating MsrA+/+ and MsrA−/− VSMC (n=9). C) In vitro kinase assay for CDK4 activity as determined by Rb phosphorylation (n=4). D) mRNA levels of E2F1, E2F2, and E2F3 in MsrA+/+ and MsrA−/− VSMC (n=6-15).
ERK1/2 activity is increased in MsrA−/− VSMC
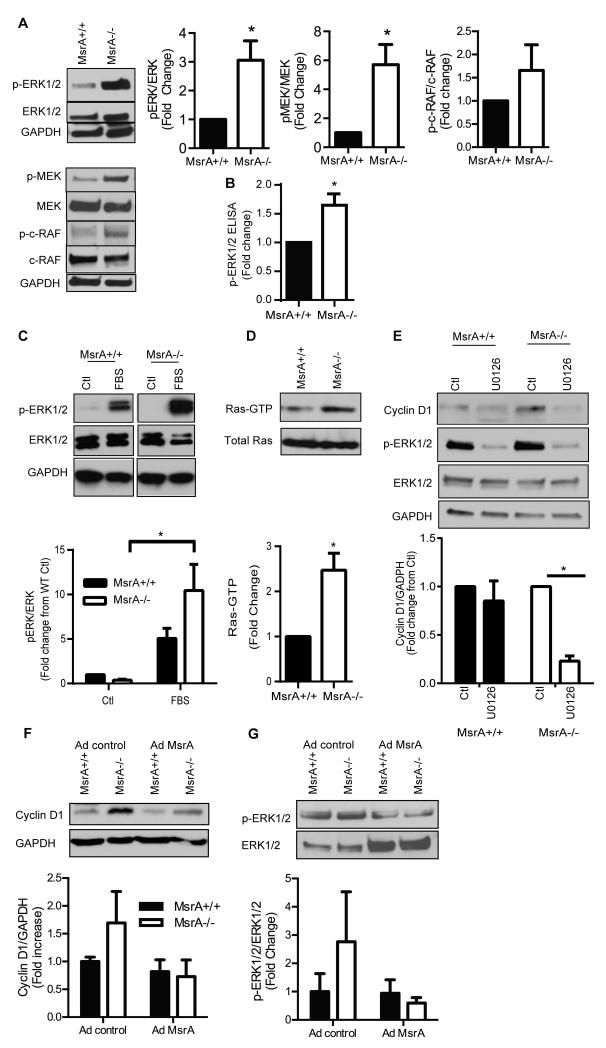
We next examined the mechanism underlying increased cyclin D levels. Canonical regulators of cell proliferation include ERK1/2 and Akt. We first examined the effect of MsrA deficiency on ERK1/2 activity in VSMC. In VSMC grown in 10% FBS, phosphorylation of ERK1/2 and its activating pathway was enhanced in MsrA−/− VSMC as compared to MsrA+/+ VSMC (Figure 6A). Increased ERK1/2 activity in MsrA−/− VSMC was confirmed by ELISA for phospho-ERK1/2 (Figure 6B). Next, we serum deprived VSMC followed by short-term treatment with 10% FBS and assessment of ERK1/2 activation. As compared to untreated control, ERK1/2 phosphorylation was increased in response to serum regardless of genotype (Figure 6C). However, the magnitude of the effect was more pronounced under MsrA deficiency. We also examined activation of Akt since a previous study in breast cancer cells demonstrated that MsrA knockdown reduces protein levels of PTEN, the negative master regulator of Akt.32 However, we did not detect changes in PTEN expression or Akt activation in MsrA−/− VSMC as compared to MsrA+/+ VSMC (Supplemental Figure V).
Figure 6. ERK activity is upregulated in MsrA−/− VSMC.
A) Activation of ERK1/2 pathway as determined by Western blotting (n=5-6). B) Activation of ERK1/2 as determined by ELISA for phospho-ERK (n=3). C) ERK1/2 activation following treatment of serum-starved VSMC (ctl) with 10% FBS for 15 min (n=6). D) Activation of Ras as determined by Raf-1 IP and Western blot for Ras. E) Inhibition of ERK1/2 activity decreases cyclin D1 protein levels in MsrA−/− VSMC. Cells were treated with 10 μM U0126 for 16hr to inhibit ERK1/2 (n=3). * p < 0.05 vs. MsrA+/+. F) Cyclin D1 protein levels and G) ERK1/2 activation 72 hr after adenoviral overexpression of MsrA (Ad MsrA) or control (Ad control) in MsrA−/− and MsrA+/+ VSMC
To hone in on the mechanism by which MsrA deletion results in increased ERK1/2 phosphorylation, we assessed activation of the upstream regulators of ERK1/2, c-Raf and MEK. As compared to MsrA+/+ VSMC, phosphorylation of MEK and c-Raf were increased in MsrA−/− compared to MsrA+/+ VSMC (Figure 6A). Additionally, MsrA deficient VSMC displayed an increase in the levels of activated Ras compared to MsrA+/+ VSMC (Figure 6D). To confirm that enhanced ERK activation in MsrA−/− VSMC is through MEK, cells were treated with the MEK inhibitor U0126. As expected, phospho-ERK1/2 was decreased by treatment with 10μM U0126 for 16 hours in both MsrA+/+ and MsrA−/− VSMC as compared to vehicle control (Figure 6E). Activation of ERK1/2 promotes cell cycle progression in part through upregulation of cyclin D1.33, 34 We therefore examined protein levels of cyclin D1 following MEK inhibition. As compared to MsrA+/+ VSMC, cyclin D1 protein levels were significantly decreased in MsrA−/− VSMC in response to MEK inhibition with U0126 (Figure 6E). To directly investigate the mechanistic effect of MsrA on the proposed pathway, we overexpressed MsrA in MsrA+/+ and MsrA−/− VSMC to determine its effect on cyclin D1 levels. As compared to MsrA−/− expressing control adenovirus, cyclin D1 levels and ERK1/2 phosphorylation were markedly lower with overexpression of MsrA (Figure 6F, G). Collectively, these data implicate MsrA in ERK1/2-dependent cyclin D1 regulation.
DISCUSSION
Here, we evaluated the role of MsrA in vascular pathology. MsrA was expressed in all layers of the arterial wall of humans and mice, with high levels in VSMC. MsrA deficiency did not affect susceptibility to atherosclerosis or thrombosis in established experimental mouse models. In contrast, neointimal area was significantly increased in MsrA−/− mice compared to MsrA+/+ mice following carotid ligation. Neointimal hyperplasia was primarily due to an increase in VSMC proliferation due to augmented G1/S transition. Parallel experiments in cultured VSMC revealed amplified cyclin D1 protein levels, cyclin D1/cdk4 complex formation, Rb phosphorylation, and increased E2F transcription. MsrA deletion increased activation of the Ras/Raf/MEK/ERK1/2 pathway, resulting in elevated cyclin D1 protein levels. Taken together, these data identify methionine oxidation as a key event in regulation of specific VSMC signaling pathways that mediate the vascular response to injury.
MsrA protein levels are downregulated in many types of human cancer in which cellular proliferation is increased, including leukemia and lymphoma cell lines and hepatocellular, breast, and colon tumors.32, 35, 36 In these studies, MsrA deficiency correlated with more aggressive and invasive growth and advanced tumor grade.32, 36 However, the signaling pathways by which MsrA downregulation mediates enhanced proliferation in malignant growth have not been conclusively identified. In one study that reported increased proliferation in breast cancer cells after knockdown of MsrA, protein levels of PTEN, a lipid phosphatase that antagonizes PI3K signaling, were decreased.32 In support of a mechanistic role for PI3K, treatment with a PI3K inhibitor blocked proliferation. Numerous studies have mechanistically implicated Akt in VSMC proliferation.37-40 Thus, we tested the hypothesis that Akt-dependent signaling is elevated in MsrA−/− VSMC. Unexpectedly, we did not detect a difference in PTEN expression or Akt signaling following deletion of MsrA. However, we found a significant increase in activation of ERK1/2, which has also been implicated in neointimal proliferation after mechanical vascular injury.41 These data are consistent with a recent study linking MsrA overexpression to inhibition of ERK1/2 in microglia.42 Together, these data support a role of MsrA in malignant and non-malignant cell proliferation.
Numerous previous reports have established that activation of Ras/MEK/ERK1/2 induces cyclin D,43 with the bulk of the studies identifying transcriptional regulation of cyclin D as a key driving mechanism. In our experiments, we detected increased cyclin D1 protein but not mRNA levels in MsrA−/− VSMC, and MEK inhibition restored cyclin D1 to MsrA+/+ levels. There is evidence that MEK1/ERK1/2 can regulate cyclin D post-transcriptionally, through promotion of the nucleocytoplasmic export of its mRNAs44-46 or through increased cyclin D1/Cdk4 complex assembly.47 ERK can also phosphorylate and activate p53, leading to cell cycle arrest.48 However, p53 stability is negatively regulated by methionine oxidation,49 thus deletion of MsrA may activate ERK but decrease p53 levels.
As the most upstream regulator of the proposed signaling pathway, we interrogated activation of the single-subunit small GTPase Ras. Ras mediates growth factor-dependent cell proliferation and differentiation.50 Consistently, the inhibition of Ras or its multiple regulators decreases neointimal proliferation after vascular injury.51-54 Ras activity is modulated directly by redox regulation of cysteine residues, as demonstrated for the conserved phosphoryl-binding loop or a nucleotide-binding domain at Cys 118.55 However, there are presently no data to implicate methionine modifications in modulating the activity of Ras or its binding partners. Alternatively, MsrA may promote the recruitment of Ras to the plasma membrane and thereby affect its activity as proposed in a recent study on MsrB1 regulation of the transient receptor potential melastatin type 6 TRPM6.56 Finally, multiple guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs) may be activated by methionine oxidation, a hypothesis that requires further in-depth investigation. Since Ras activation is a driver of accelerated proliferation in malignant diseases that are also characterized by lower MsrA activity, our data may provide further insight into MsrA function in other proliferative diseases.
In contrast to our findings in MsrA−/− VSMC, two earlier studies reported that acute knockdown of MsrA and MsrB3 in embryonic fibroblasts results in decreased cell proliferation.57, 58 Of note, the rate of proliferation in the fibroblast studies was mainly examined by MTT assay, which depends on the cellular metabolic activity due to NAD(P)H flux. Since MsrA is expressed in both cytoplasm and the mitochondria in VSMC,59 it may affect NAD(P)H flux, possibly independently from its role in proliferation. In fact, a proteomic analysis of 293T cells after MsrA knockdown revealed a subunit of NADH dehydrogenase 1β as target of methionine oxidation.20 For these reasons, we examined proliferation by cell counting, 3H-thymidine uptake, and cell cycle analysis, and found that all of these approaches confirmed increased proliferation of MsrA−/− VSMC. Another potential explanation for the discrepancy between our findings and the prior studies is that chronic MsrA deletion may induce alterations of the cellular redox state that affect cell proliferation, which may not be apparent with acute MsrA knockdown.
Since ischemic cardiovascular disease is typically caused by acute rupture of atherosclerotic plaque followed by thrombosis, we tested whether MsrA deletion affects the development of atherosclerotic lesions or the susceptibility to experimental thrombosis in murine models. Though corresponding in vivo studies are largely lacking, several recent in vitro studies have suggested that methionine oxidation may favor a pro-thrombotic state.5 First, the neutrophil oxidant hypochlorous acid inhibited von Willebrand Factor (VWF) proteolysis by oxidizing VWF at methionine 1606.60 Second, exposure of the VWF protease ADAMTS13 to hypochlorous acid induced methionine oxidation at several residues,61 which was accompanied by reduced enzyme activity. Third, methionine oxidation of fibrinogen was associated with a dense network of fibrin, which likely inhibits plasminogen diffusion.62 Finally, thrombomodulin in the membrane of endothelial cells is inactivated via oxidation of a key methionine residue under conditions of oxidative stress typical of vascular inflammation.24 Thrombomodulin binds thrombin and changes its substrate specificity, allowing it to efficiently generate APC, an endogenous circulating protease that has both antithrombotic and anti-inflammatory properties. However, we did not detect any differences in atherosclerotic lesion area, APC generation, or susceptibility to arterial thrombosis between MsrA+/+ApoE−/− and MsrA−/−ApoE−/− mice (Figure 1). These data suggest that methionine oxidation may not be a major mediator of atherosclerosis and thrombosis in vivo. Alternatively, it remains possible that MsrA is unable to reduce methionine sulfoxide residues in extracellular prothrombotic proteins because of its subcellular localization in the nucleus, cytoplasm, and mitochondria.
Likewise, in subjects with coronary artery disease, increased levels of methionine oxidation of apolipoprotein A-I correlate with disease severity.63 Since the protein modification also leads to decreased ATP-binding cassette transporter A1 (ABCA1)-mediated cholesterol efflux from HDL, methionine oxidation is expected to enhance the severity of atherosclerosis. A study in methionine-induced hyperhomocysteinemia reported similar findings with altered HDL oxidation and the ability to efflux cholesterol from macrophages.64 However, a direct proof of a causal relationship between methionine oxidation of HDL and atherosclerotic lesion size or instability is currently lacking. Of note, nearly all studies of HDL oxidation have been performed in vitro under oxidizing conditions that may not occur in vivo. An alternative explanation is that additional, unidentified anti-thrombotic or anti-atherosclerotic pathways are simultaneously activated by methionine oxidation. Lastly, murine disease models of thrombosis and atherosclerosis do not completely recapitulate the human pathology. Thus, based solely on the murine disease models examined, we cannot conclude that the protein targets that were identified in in vitro studies do not impact human disease.
Our results provide compelling evidence that MsrA deficiency increases proliferation of VSMC and ERK1/2-mediated cyclin D1 regulation. This suggests that MsrA expression, or activation, which is known to protect proteins from oxidation and which acts as a ROS scavenger, may protect against neointima formation. While treatments with untargeted antioxidants have failed to reduce cardiovascular events, targeted activation of MsrA may provide a therapeutic benefit. Others have demonstrated increased MsrA activity with dietary supplements, selenium compounds65 and S-methyl-L-cysteine.66 These compounds are potential therapeutics in the prevention of restenosis and should be further investigated.
Supplementary Material
SIGNIFICANCE.
Reactive oxygen species (ROS) play a role in the progression of cardiovascular diseases, such as atherosclerosis, thrombosis, and restenosis after injury, by altering protein function via oxidation of specific amino acids. While redox modifications of cysteines are well studied, methionine oxidation has been underappreciated as a potential regulator of redox-dependent signaling. Recent studies demonstrated that cell signaling can be controlled by methionine sulfoxide reductase A (MsrA), which reverses methionine oxidation. Our study examined the role of MsrA in the regulation of vascular pathologies. We found that deficiency of MsrA does not protect against atherosclerosis or thrombosis in mouse models. However, our data implicate MsrA in restenosis after injury through regulation of vascular smooth muscle cell growth. The identification of the precise signaling pathways may serve as a platform for the design of specific therapeutics for the treatment of restenosis.
ACKNOWLEDGEMENTS
We are indebted to Dr. Chantal Allamargot (Central Microscopy Research Facilities, University of Iowa) for expert technical support, to Dr. Kristina W. Thiel for assistance in the preparation of the manuscript, and to Lorie Leo for technical support.
SOURCES OF FUNDING
This work was supported by funding from the VA Office of Research and Development (1BX000163-01 to IMG), NIH (R01 HL108932 to IMG; T32 HL007121 to PJK; T32 GM007337 to SXG; R01 HL063943 and P01 HL062984 to SRL, R01HL118246 and R01HL118742 to AKC), American Heart Association (14POST19860006 to PJK; 12PRE940065 to SXG) and the Doris Duke Foundation to LZ. The content of this manuscript are solely the responsibility of the authors and do not necessarily represent the views of the granting agencies.
ABBREVIATIONS
- ADAMTS
a disintegrin and metalloproteinase with a thrombospondin type 1 motif
- ABCA1
ATP-binding cassette transporter A1
- ApoE−/− mice
Apolipoprotein E–deficient mice
- CaMKII
Calcium- and calmodulin-dependent kinase II
- CDK
Cyclin dependent kinase
- EDTA
Ethylenediaminetetraacetic acid
- ELISA
Enzyme-linked immunosorbent assay
- ERK
Extracellular signal-regulated kinase
- FBS
Fetal bovine serum
- GAP
GTPase-Activating Protein
- GEF
Guanine nucleotide exchange factor
- HDL
High-density lipoprotein
- MEK
Mitogen-activated protein kinase kinase
- MsrA
Methionine sulfoxide reductase A
- MsrB
Methionine sulfoxide reductase B
- NAD(P)H
Nicotinamide adenine dinucleotide phosphate
- PI3K
Phosphatidylinositol-3-kinase
- PTEN
Phosphatase and tensin homolog
- Raf
Rapidly Accelerated Fibrosarcoma
- Rb
Retinoblastoma protein
- ROS
Reactive oxygen species
- SMA
Smooth muscle actin
- TNF
Tumor necrosis factor
- TRPM6
Transient receptor potential ion channel
- U0126
MEK inhibitor
- VSMC
Vascular smooth muscle cell
- VWF
von Willebrand factor
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Levine RL, Mosoni L, Berlett BS, Stadtman ER. Methionine residues as endogenous antioxidants in proteins. Proc Natl Acad Sci U S A. 1996;93:15036–40. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robison AJ, Winder DG, Colbran RJ, Bartlett RK. Oxidation of calmodulin alters activation and regulation of CaMKII. Biochem Biophys Res Commun. 2007;356:97–101. doi: 10.1016/j.bbrc.2007.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–74. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciorba MA, Heinemann SH, Weissbach H, Brot N, Hoshi T. Modulation of potassium channel function by methionine oxidation and reduction. Proc Natl Acad Sci U S A. 1997;94:9932–7. doi: 10.1073/pnas.94.18.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu SX, Stevens JW, Lentz SR. Regulation of thrombosis and vascular function by protein methionine oxidation. Blood. 2015 doi: 10.1182/blood-2015-01-544676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madamanchi NR, Runge MS. Redox signaling in cardiovascular health and disease. Free Radic Biol Med. 2013;61:473–501. doi: 10.1016/j.freeradbiomed.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haenold R, Wassef R, Hansel A, Heinemann SH, Hoshi T. Identification of a new functional splice variant of the enzyme methionine sulphoxide reductase A (MSRA) expressed in rat vascular smooth muscle cells. Free Radic Res. 2007;41:1233–45. doi: 10.1080/10715760701642096. [DOI] [PubMed] [Google Scholar]
- 8.Lee BC, Dikiy A, Kim HY, Gladyshev VN. Functions and evolution of selenoprotein methionine sulfoxide reductases. Biochim Biophys Acta. 2009;1790:1471–7. doi: 10.1016/j.bbagen.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabbita SP, Aksenov MY, Lovell MA, Markesbery WR. Decrease in peptide methionine sulfoxide reductase in Alzheimer's disease brain. J Neurochem. 1999;73:1660–6. doi: 10.1046/j.1471-4159.1999.0731660.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu F, Hindupur J, Nguyen JL, Ruf KJ, Zhu J, Schieler JL, Bonham CC, Wood KV, Davisson VJ, Rochet JC. Methionine sulfoxide reductase A protects dopaminergic cells from Parkinson's disease-related insults. Free Radic Biol Med. 2008;45:242–55. doi: 10.1016/j.freeradbiomed.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prentice HM, Moench IA, Rickaway ZT, Dougherty CJ, Webster KA, Weissbach H. MsrA protects cardiac myocytes against hypoxia/reoxygenation induced cell death. Biochem Biophys Res Commun. 2008;366:775–778. doi: 10.1016/j.bbrc.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 12.Zhao H, Sun J, Deschamps AM, Kim G, Liu C, Murphy E, Levine RL. Myristoylated methionine sulfoxide reductase A protects the heart from ischemia-reperfusion injury. American journal of physiologyHeart and circulatory physiology. 2011;301:H1513–8. doi: 10.1152/ajpheart.00441.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He BJ, Joiner ML, Singh MV, Luczak ED, Swaminathan PD, Koval OM, Kutschke W, Allamargot C, Yang J, Guan X, Zimmerman K, Grumbach IM, Weiss RM, Spitz DR, Sigmund CD, Blankesteijn WM, Heymans S, Mohler PJ, Anderson ME. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med. 2011;17:1610–1618. doi: 10.1038/nm.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Bermudez M, Lopez-Mejias R, Gonzalez-Juanatey C, Castaneda S, Miranda-Filloy JA, Blanco R, Fernandez-Gutierrez B, Balsa A, Gonzalez-Alvaro I, Gomez-Vaquero C, Llorca J, Martin J, Gonzalez-Gay MA. Association of the methionine sulfoxide reductase A rs10903323 gene polymorphism with cardiovascular disease in patients with rheumatoid arthritis. Scand J Rheumatol. 2012;41:350–353. doi: 10.3109/03009742.2012.677063. [DOI] [PubMed] [Google Scholar]
- 15.Gu H, Chen W, Yin J, Chen S, Zhang J, Gong J. Methionine sulfoxide reductase A rs10903323 G/A polymorphism is associated with increased risk of coronary artery disease in a Chinese population. Clin Biochem. 2013;46:1668–1672. doi: 10.1016/j.clinbiochem.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Dun Y, Vargas J, Brot N, Finnemann SC. Independent roles of methionine sulfoxide reductase A in mitochondrial ATP synthesis and as antioxidant in retinal pigment epithelial cells. Free Radic Biol Med. 2013;65C:1340–1351. doi: 10.1016/j.freeradbiomed.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oien DB, Canello T, Gabizon R, Gasset M, Lundquist BL, Burns JM, Moskovitz J. Detection of oxidized methionine in selected proteins, cellular extracts and blood serums by novel anti-methionine sulfoxide antibodies. Arch Biochem Biophys. 2009;485:35–40. doi: 10.1016/j.abb.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wehr NB, Levine RL. Wanted and wanting: antibody against methionine sulfoxide. Free Radic Biol Med. 2012;53:1222–1225. doi: 10.1016/j.freeradbiomed.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghesquiere B, Jonckheere V, Colaert N, Van Durme J, Timmerman E, Goethals M, Schymkowitz J, Rousseau F, Vandekerckhove J, Gevaert K. Redox proteomics of protein-bound methionine oxidation. Mol Cell Proteomics. 2011;10:M110–006866. doi: 10.1074/mcp.M110.006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ugarte N, Ladouce R, Radjei S, Gareil M, Friguet B, Petropoulos I. Proteome alteration in oxidative stress-sensitive methionine sulfoxide reductase-silenced HEK293 cells. Free Radic Biol Med. 2013;65:1023–36. doi: 10.1016/j.freeradbiomed.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Vogt W. Oxidation of methionyl residues in proteins: tools, targets, and reversal. Free Radic Biol Med. 1995;18:93–105. doi: 10.1016/0891-5849(94)00158-g. [DOI] [PubMed] [Google Scholar]
- 22.Luo S, Uehara H, Shacter E. Taurine chloramine-induced inactivation of cofilin protein through methionine oxidation. Free Radic Biol Med. 2014;75:84–94. doi: 10.1016/j.freeradbiomed.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Torres RA, Drake DA, Solodushko V, Jadhav R, Smith E, Rocic P, Weber DS. Slingshot isoform-specific regulation of cofilin-mediated vascular smooth muscle cell migration and neointima formation. Arterioscler Thromb Vasc Biol. 2011;31:2424–31. doi: 10.1161/ATVBAHA.111.232769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carnemolla R, Greineder CF, Chacko AM, Patel KR, Ding BS, Zaitsev S, Esmon CT, Muzykantov VR. Platelet endothelial cell adhesion molecule targeted oxidant-resistant mutant thrombomodulin fusion protein with enhanced potency in vitro and in vivo. J Pharmacol Exp Ther. 2013;347:339–45. doi: 10.1124/jpet.113.205104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 26.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–25. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 27.Bond M, Sala-Newby GB, Wu YJ, Newby AC. Biphasic effect of p21Cip1 on smooth muscle cell proliferation: role of PI 3-kinase and Skp2-mediated degradation. Cardiovasc Res. 2006;69:198–206. doi: 10.1016/j.cardiores.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, Sherr CJ. The p21(Cip1) and p27(Kip1) CDK 'inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–83. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–62. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 30.Kato J, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–42. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 31.Weintraub SJ, Prater CA, Dean DC. Retinoblastoma protein switches the E2F site from positive to negative element. Nature. 1992;358:259–61. doi: 10.1038/358259a0. [DOI] [PubMed] [Google Scholar]
- 32.De Luca A, Sanna F, Sallese M, Ruggiero C, Grossi M, Sacchetta P, Rossi C, De Laurenzi V, Di Ilio C, Favaloro B. Methionine sulfoxide reductase A down-regulation in human breast cancer cells results in a more aggressive phenotype. Proc Natl Acad Sci U S A. 2010;107:18628–18633. doi: 10.1073/pnas.1010171107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villanueva J, Yung Y, Walker JL, Assoian RK. ERK activity and G1 phase progression: identifying dispensable versus essential activities and primary versus secondary targets. Mol Biol Cell. 2007;18:1457–63. doi: 10.1091/mbc.E06-10-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavoie JN, L'Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–16. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 35.Kuschel L, Hansel A, Schonherr R, Weissbach H, Brot N, Hoshi T, Heinemann SH. Molecular cloning and functional expression of a human peptide methionine sulfoxide reductase (hMsrA) FEBS Lett. 1999;456:17–21. doi: 10.1016/s0014-5793(99)00917-5. [DOI] [PubMed] [Google Scholar]
- 36.Lei KF, Wang YF, Zhu XQ, Lu PC, Sun BS, Jia HL, Ren N, Ye QH, Sun HC, Wang L, Tang ZY, Qin LX. Identification of MSRA gene on chromosome 8p as a candidate metastasis suppressor for human hepatitis B virus-positive hepatocellular carcinoma. BMC Cancer. 2007;7:172. doi: 10.1186/1471-2407-7-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WC. Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab. 2007;6:446–57. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maurel B, Chai F, Maton M, Blanchemain N, Haulon S. In stent restenosis and thrombosis assessment after EP224283 injection in a rat model. Atherosclerosis. 2013;229:462–8. doi: 10.1016/j.atherosclerosis.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 39.Xu XL, Ling DY, Zhu QY, Fan WJ, Zhang W. The effect of 2,3,4',5-tetrahydroxystilbene-2-0-beta-D glucoside on neointima formation in a rat artery balloon injury model and its possible mechanisms. Eur J Pharmacol. 2013;698:370–8. doi: 10.1016/j.ejphar.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 40.Yun SJ, Ha JM, Kim EK, Kim YW, Jin SY, Lee DH, Song SH, Kim CD, Shin HK, Bae SS. Akt1 isoform modulates phenotypic conversion of vascular smooth muscle cells. Biochim Biophys Acta. 2014;1842:2184–92. doi: 10.1016/j.bbadis.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 41.Izumi Y, Kim S, Namba M, Yasumoto H, Miyazaki H, Hoshiga M, Kaneda Y, Morishita R, Zhan Y, Iwao H. Gene transfer of dominant-negative mutants of extracellular signal-regulated kinase and c-Jun NH2-terminal kinase prevents neointimal formation in balloon-injured rat artery. Circ Res. 2001;88:1120–6. doi: 10.1161/hh1101.091267. [DOI] [PubMed] [Google Scholar]
- 42.Fan H, Wu PF, Zhang L, Hu ZL, Wang W, Guan XL, Luo H, Ni M, Yang JW, Li MX, Chen JG, Wang F. Methionine Sulfoxide Reductase A Negatively Controls Microglia-Mediated Neuroinflammation via Inhibiting ROS/MAPKs/NF-kappaB Signaling Pathways Through a Catalytic Antioxidant Function. Antioxidants & redox signaling. 2015;22:832–47. doi: 10.1089/ars.2014.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–39. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 44.Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL. eIF4E promotes nuclear export of cyclin D1 mRNAs via an element in the 3'UTR. J Cell Biol. 2005;169:245–56. doi: 10.1083/jcb.200501019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai HK, Borden KL. The promyelocytic leukemia (PML) protein suppresses cyclin D1 protein production by altering the nuclear cytoplasmic distribution of cyclin D1 mRNA. Oncogene. 2000;19:1623–34. doi: 10.1038/sj.onc.1203473. [DOI] [PubMed] [Google Scholar]
- 46.Topisirovic I, Capili AD, Borden KL. Gamma interferon and cadmium treatments modulate eukaryotic initiation factor 4E-dependent mRNA transport of cyclin D1 in a PML-dependent manner. Mol Cell Biol. 2002;22:6183–98. doi: 10.1128/MCB.22.17.6183-6198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ladha MH, Lee KY, Upton TM, Reed MF, Ewen ME. Regulation of exit from quiescence by p27 and cyclin D1-CDK4. Mol Cell Biol. 1998;18:6605–15. doi: 10.1128/mcb.18.11.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu GS. The functional interactions between the p53 and MAPK signaling pathways. Cancer Biol Ther. 2004;3:156–61. doi: 10.4161/cbt.3.2.614. [DOI] [PubMed] [Google Scholar]
- 49.Nomura T, Kamada R, Ito I, Chuman Y, Shimohigashi Y, Sakaguchi K. Oxidation of methionine residue at hydrophobic core destabilizes p53 tetrameric structure. Biopolymers. 2009;91:78–84. doi: 10.1002/bip.21084. [DOI] [PubMed] [Google Scholar]
- 50.Margolis B, Skolnik EY. Activation of Ras by receptor tyrosine kinases. J Am Soc Nephrol. 1994;5:1288–99. doi: 10.1681/ASN.V561288. [DOI] [PubMed] [Google Scholar]
- 51.Jin G, Chieh-Hsi Wu J, Li YS, Hu YL, Shyy JY, Chien S. Effects of active and negative mutants of Ras on rat arterial neointima formation. J Surg Res. 2000;94:124–32. doi: 10.1006/jsre.2000.6014. [DOI] [PubMed] [Google Scholar]
- 52.Ueno H, Yamamoto H, Ito S, Li JJ, Takeshita A. Adenovirus-mediated transfer of a dominant-negative H-ras suppresses neointimal formation in balloon-injured arteries in vivo. Arterioscler Thromb Vasc Biol. 1997;17:898–904. doi: 10.1161/01.atv.17.5.898. [DOI] [PubMed] [Google Scholar]
- 53.Work LM, McPhaden AR, Pyne NJ, Pyne S, Wadsworth RM, Wainwright CL. Short-term local delivery of an inhibitor of Ras farnesyltransferase prevents neointima formation in vivo after porcine coronary balloon angioplasty. Circulation. 2001;104:1538–43. doi: 10.1161/hc3801.095661. [DOI] [PubMed] [Google Scholar]
- 54.Zhang S, Ren J, Khan MF, Cheng AM, Abendschein D, Muslin AJ. Grb2 is required for the development of neointima in response to vascular injury. Arterioscler Thromb Vasc Biol. 2003;23:1788–93. doi: 10.1161/01.ATV.0000085015.49110.85. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell L, Hobbs GA, Aghajanian A, Campbell SL. Redox regulation of Ras and Rho GTPases: mechanism and function. Antioxidants & redox signaling. 2013;18:250–8. doi: 10.1089/ars.2012.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao G, Lee KP, van der Wijst J, de Graaf M, van der Kemp A, Bindels RJ, Hoenderop JG. Methionine sulfoxide reductase B1 (MsrB1) recovers TRPM6 channel activity during oxidative stress. J Biol Chem. 2010;285:26081–7. doi: 10.1074/jbc.M110.103655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi SH, Kim HY. Methionine sulfoxide reductase A regulates cell growth through the p53-p21 pathway. Biochem Biophys Res Commun. 2011;416:70–5. doi: 10.1016/j.bbrc.2011.10.145. [DOI] [PubMed] [Google Scholar]
- 58.Lee E, Kwak GH, Kamble K, Kim HY. Methionine sulfoxide reductase B3 deficiency inhibits cell growth through the activation of p53-p21 and p27 pathways. Arch Biochem Biophys. 2014;547:1–5. doi: 10.1016/j.abb.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 59.Haenold R, Wassef R, Brot N, Neugebauer S, Leipold E, Heinemann SH, Hoshi T. Protection of vascular smooth muscle cells by over-expressed methionine sulphoxide reductase A: role of intracellular localization and substrate availability. Free Radic Res. 2008;42:978–988. doi: 10.1080/10715760802566541. [DOI] [PubMed] [Google Scholar]
- 60.Chen J, Fu X, Wang Y, Ling M, McMullen B, Kulman J, Chung DW, Lopez JA. Oxidative modification of von Willebrand factor by neutrophil oxidants inhibits its cleavage by ADAMTS13. Blood. 2010;115:706–12. doi: 10.1182/blood-2009-03-213967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Chen J, Ling M, Lopez JA, Chung DW, Fu X. Hypochlorous acid generated by neutrophils inactivates ADAMTS13: an oxidative mechanism for regulating ADAMTS13 proteolytic activity during inflammation. J Biol Chem. 2015;290:1422–31. doi: 10.1074/jbc.M114.599084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weigandt KM, White N, Chung D, Ellingson E, Wang Y, Fu X, Pozzo DC. Fibrin clot structure and mechanics associated with specific oxidation of methionine residues in fibrinogen. Biophys J. 2012;103:2399–407. doi: 10.1016/j.bpj.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shao B, Tang C, Sinha A, Mayer PS, Davenport GD, Brot N, Oda MN, Zhao XQ, Heinecke JW. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ Res. 2014;114:1733–42. doi: 10.1161/CIRCRESAHA.114.303454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Julve J, Escola-Gil JC, Rodriguez-Millan E, Martin-Campos JM, Jauhiainen M, Quesada H, Renteria-Obregon IM, Osada J, Sanchez-Quesada JL, Blanco-Vaca F. Methionine-induced hyperhomocysteinemia impairs the antioxidant ability of high-density lipoproteins without reducing in vivo macrophage-specific reverse cholesterol transport. Mol Nutr Food Res. 2013;57:1814–24. doi: 10.1002/mnfr.201300133. [DOI] [PubMed] [Google Scholar]
- 65.Sagher D, Brunell D, Hejtmancik JF, Kantorow M, Brot N, Weissbach H. Thionein can serve as a reducing agent for the methionine sulfoxide reductases. Proc Natl Acad Sci U S A. 2006;103:8656–8661. doi: 10.1073/pnas.0602826103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moskovitz J. Prolonged selenium-deficient diet in MsrA knockout mice causes enhanced oxidative modification to proteins and affects the levels of antioxidant enzymes in a tissue-specific manner. Free Radic Res. 2007;41:162–71. doi: 10.1080/10715760600978823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.